Abstract
Nano titanium dioxide (nTiO2) is the most abundantly released engineered nanomaterial (ENM) in aquatic environments. Therefore, it is prudent to assess its fate and its effects on lower trophic-level organisms in the aquatic food chain. A predator-and-prey-based laboratory microcosm was established using Paramecium caudatum and Escherichia coli to evaluate the effects of nTiO2. The surface interaction of nTiO2 with E. coli significantly increased after the addition of Paramecium into the microcosm. This interaction favoured the hetero-agglomeration and co-sedimentation of nTiO2. The extent of nTiO2 agglomeration under experimental conditions was as follows: combined E. coli and Paramecium > Paramecium only > E. coli only > without E. coli or Paramecium. An increase in nTiO2 internalisation in Paramecium cells was also observed in the presence or absence of E. coli cells. These interactions and nTiO2 internalisation in Paramecium cells induced statistically significant (p < 0.05) effects on growth and the bacterial ingestion rate at 24 h. These findings provide new insights into the fate of nTiO2 in the presence of bacterial-ciliate interactions in the aquatic environment.
Engineered nanomaterials (ENMs) are used in diverse applications, owing to their unique optical, chemical, mechanical, thermal, magnetic and catalytic properties1. Currently, more than 1800 nano-based consumer products derived from 45 different ENMs are manufactured globally2. ENMs can enter into the environment at various stages in their life cycle: production, manufacturing, transportation, consumer use and product disposal3,4,5.
Nano titanium dioxide (nTiO2) is one of the most abundant materials in products such as cosmetics, paints, food additives, pharmaceuticals, electronics, and textiles as well as in construction and wastewater treatment6,7,8. Moreover, the unique photocatalytic and UV-reflecting properties of nTiO2 have enhanced the exponential growth of low-cost and safer consumer products9,10,11. Risk assessment studies have predicted nTiO2 to be the most abundant ENM in the environment [21–10000 ng/L in surface water, 1–100 μg/L in waste water treatment plant (WWTP) effluent, 100–2000 mg/kg in WWTP sludge]12.
Aquatic environments act as a sinks for chemicals as well as emerging metal pollutants such as ENMs13. Aquatic bodies contain a dominant and ubiquitous community of bacteria (~106 cells/ ml) as well as the bacterial predators ciliated protozoans (102–104 cells/ml)14,15. ENMs affect the organisms within and across trophic levels in the aquatic food chain. Poor water solubility and long-term persistence of ENMs in aquatic systems16,17 facilitate their bioaccumulation and biomagnification in aquatic organisms such as bacteria, ciliated protozoans, rotifers, algae, crustaceans, zebrafish, and mussels18,19,20,21,22,23,24. The accumulation of ENMs can also affect the growth, reproduction, ingestion and digestion behaviour of aquatic organisms18,20,21. Factors such as surface interactions (adsorption or hetero-agglomeration), internalisation, oxidative stress, membrane damage and mitochondrial perturbations have been reported to be responsible for the acute toxicity of ENMs in microorganisms, cell lines and eukaryotic organisms25. The surface interactions of ENMs with microbial cells, the first step in ENM toxicity, are predominantly governed by charge interactions between ENMs and microbes26,27. ENMs with positive surface charges have been found to have higher toxicities than ENMs with negative charges. This finding has been attributed to the negative charges of cell surfaces28,29. In the natural environment, bacterial cells are ubiquitously present and have a high ratio of surface area to their volume; thus, the cells interact with and absorb high levels of ENMs15,30. Additionally, the presence of exopolymeric substances (EPS) on the outer membranes of bacterial cells complements the adsorption of ENMs from the aquatic environment15,31,32. Ciliated protozoans such as Tetrahymena secrete mucus from their mucous membranes under stress conditions, and this surface coating affects the fate of ENMs in the medium33.
To understand the actual behaviours and toxicities of ENMs in aquatic systems, it is necessary to study the surface interactions, such as adsorption and hetero-agglomeration, of ENMs with microorganisms. For instance, the physical properties of E. coli cells are affected by exposure to hematite nanoparticles (NPs)34. The adsorption of ENMs on the E. coli surface is dependent on size: large hematite NPs adsorb faster than smaller NPs do32. In another study conducted in Paramecium multimicronucleatum, nTiO2 has been found not to cause toxicity, owing to weak surface interaction energy35. Furthermore, different pH and ionic strength conditions play roles in the hetero-agglomeration and co-sedimentation of discharged oxide nanoparticles with chlorella cells36. Studies examining the surface adsorption and hetero-agglomeration of ENMs with biotic factors have been limited to the single organism level.
No studies have examined the adsorption, hetero-agglomeration and co-sedimentation of ENMs in the presence of a predator-prey interaction model of a real-world environmental situation. Such an interaction model, involving two organisms in lower trophic levels of the food chain, can be represented by a bacterium and a ciliated protozoan. E. coli has been used extensively as a model bacterium in toxicity assessments of ENMs because it divides rapidly and is easily cultured32,37. Paramecium caudatum, a ubiquitous single-celled ciliated protozoan that feeds on bacteria, is a significant ecological link between microbes and multicellular organisms33,35.
Therefore, in the present study, an experimental aquatic microcosm involving Escherichia coli as prey and Paramecium caudatum as a predator was established to understand the hetero-agglomeration and co-sedimentation of nTiO2 in the presence of predator-prey interactions. The microcosm was also used to determine the bioavailability, trophic transfer and effect of nTiO2 on the food chain.
Results and Discussion
Characteristics of nTiO2
The commercial nTiO2 used in the present study was heterogeneously distributed, with a particle distribution ranging from 100 nm to 400 nm, as determined by dynamic light scattering (DLS) analysis (SI-Fig. S2a). Transmission electron microscopy (TEM) indicated that most particles ranged from 10 to 70 nm, with an average size of 40 nm (SI-Fig. S2c). The zeta potential of nTiO2 in Dryl’s buffer was −31 mV (SI-Fig. S2b). The size of nTiO2 determined by DLS was higher because of the formation of the hydrodynamic layer on its surface.
Rationale for selection of sampling time points
In all the experiments, the initial time point was 1 h to allow optimum adsorption of nTiO2 on the surface of test organisms. The final time point was selected as 24 h, coinciding with the life cycle of Paramecium38. This duration was sufficient to allow recurrent ingestion and digestion of bacteria. It also ensured that nanoparticle agglomeration achieved a steady state39.
Adsorption and hetero-agglomeration of nTiO2 in the microcosm
The average hydrodynamic diameters of nTiO2 agglomerates observed in different groups (nTiO2, nTiO2 + E. coli, nTiO2 + Paramecium and nTiO2 + E. coli + Paramecium) at 1 h and 24 h are presented in Table 1. A concomitant and significant (p < 0.001) increase in the size of nTiO2 agglomerates was observed because of the presence of test organisms (E. coli and/or Paramecium) in the exposure medium. The agglomerate size of an individual nTiO2 suspension at the initial time point (1 h) was uniform across all characterised zones (upper zone, UZ; middle zone, MZ and lower zone, LZ) of the simple microcosm. However, a statistically significant (p < 0.001) increase in agglomerate size was observed after the addition of E. coli and Paramecium cells, individually or in combination.
Table 1. Hydrodynamic diameter (d-nm) of nTiO2 in the microcosm, reflecting agglomeration.
| Groups | 1 h | 24 h |
|---|---|---|
| nTiO2-UZ | 395 ± 8 | 299 ± 44 |
| nTiO2-MZ | 411 ± 6 | 318 ± 41 |
| nTiO2-LZ | 411 ± 9 | 402 ± 28 |
| Ec + nTiO2-UZ | 699 ± 9** | 425 ± 10 |
| Ec + nTiO2-MZ | 521 ± 6** | 530 ± 4* |
| Ec + nTiO2-LZ | 601 ± 6** | 814 ± 15** |
| Pm + nTiO2-UZ | 742 ± 8** | 734 ± 6** |
| Pm + nTiO2-MZ | 657 ± 11** | 790 ± 19** |
| Pm + nTiO2-LZ | 781 ± 10** | 1211 ± 47** |
| Ec + Pm + nTiO2-UZ | 1036 ± 27** | 1138 ± 46** |
| Ec + Pm + nTiO2-MZ | 967 ± 6** | 1400 ± 68** |
| Ec + Pm + nTiO2-LZ | 875 ± 17** | ND |
Pm, Paramecium, Ec, E. coli. ND, not detected.
Values represented are the mean ± SE of three independent experiments.
*p < 0.05 was considered significant compared with the control.
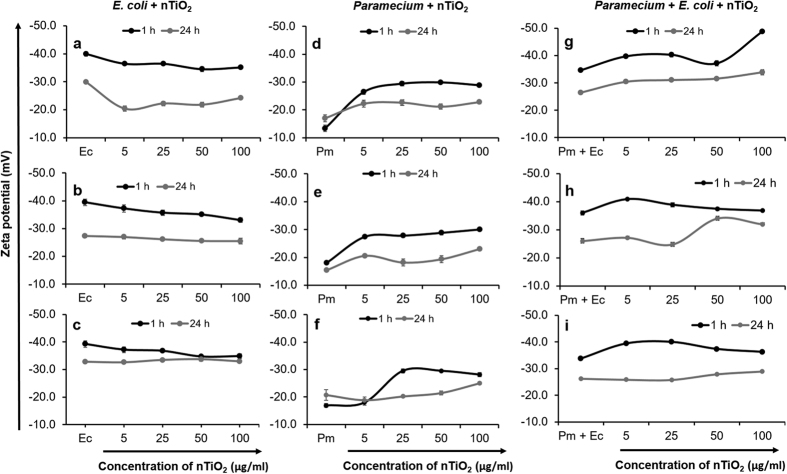
At the final time point (24 h), the size of nTiO2 agglomerates in the LZ of the microcosm increased significantly (p < 0.001), with sedimentation of larger agglomerates in all the groups (nTiO2 + E. coli, nTiO2 + Paramecium and nTiO2 + E. coli + Paramecium) (Table 1). Sedimentation occurred because of the increased adsorption of nTiO2 on the surface of the E. coli and the release of mucus exudates by Paramecium cells33. The agglomerate size observed in the lower zone was maximised at 24 h in the following order: nTiO2 + E. coli + Paramecium > nTiO2 + Paramecium > nTiO2 + E. coli. The agglomerate size of nTiO2 in the Paramecium co-incubation group was greater than that in the E. coli co-incubation group because of differences in binding interactions between nTiO2 and organisms. The negatively charged surfaces of nTiO2 (−32 mV) and E. coli (−39 mV) do not permit electrostatic interactions (SI-Figure S3 and Fig. 1). A weak electrostatic association may be possible between nTiO2 (−32 mV) and Paramecium (−18 mV), given the extreme difference in the intensity of negative charges of both surfaces (Fig. 1d–f). Further, the combination of Paramecium with E. coli cells in the microcosm decreased the overall zeta-potential of E. coli in the absence of nTiO2 but increased slightly after the addition of nTiO2, possibly because of adsorption on the E. coli surface. This observation shows that poor electrostatic interactions between nTiO2 and E. coli in the presence of Paramecium are possible (Fig. 1i). At 24 h, these interactions were negligible, owing to the equilibration of the overall charges between E. coli and nTiO2 (Fig. 1c,i).
Figure 1. Zeta potential of nTiO2 in different zones of the experimental tube at 1 h and 24 h.
(a–c) nTiO2 incubated with E. coli, (d–f) nTiO2 incubated with Paramecium, (g–i) nTiO2 incubated with Paramecium and E. coli. (a,d,g) = upper zone; (b,e,h) = middle zone and (c,f,i) = lower zone. Ec, E. coli, Pm, Paramecium. Values represented are the mean ± SE of three independent experiments.
Previous studies have shown that E. coli cells produce exopolymeric substances that are involved in nTiO2 adhesion40,41,42. Jucker et al.43 and Host et al.41 suggested that numerous bacterial exopolymers, such as lipopolysaccharides (LPS) and siderophores (in Pseudomonas aeruginosa), participate in the adsorption of nTiO2 and other metals on the bacterial surface. Li and Logan44 have observed that long-chain LPS in E. coli cells adheres more widely to nTiO2 than short chain LPS. Hahn et al.45 and Pernthaler14 have also shown that the predation of planktonic bacteria by a ciliated protozoan in the aquatic environment induces the biosynthesis of exopolymeric substances in bacteria, which protect against predation by facilitating biofilm formation. In the present study, a similar mechanism may be responsible, thus facilitating the adsorption of nTiO2 onto the E. coli surface in the presence of Paramecium.
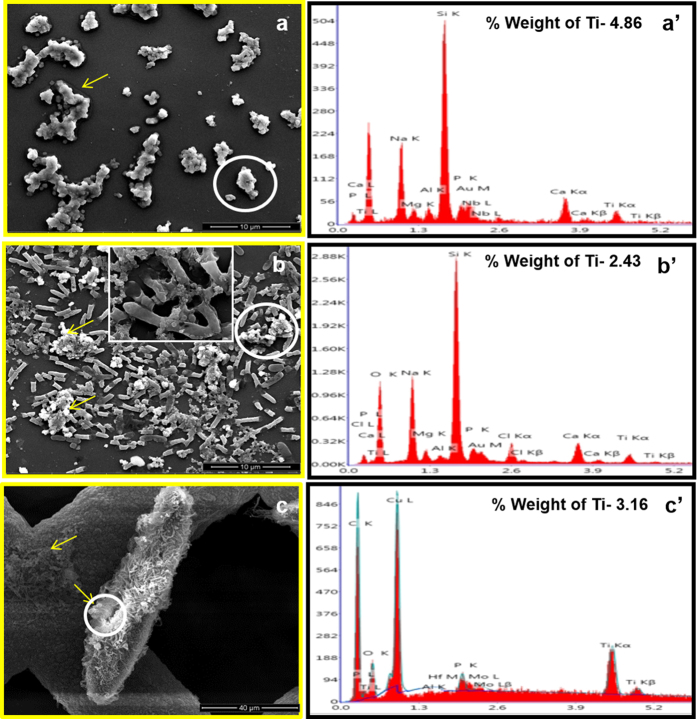
The adsorption and hetero-agglomeration of nTiO2 on the surface of organisms in the microcosm was further assessed through dark field (SI-Fig. S4) and scanning electron microscopy coupled with EDS (SEM-EDS) (Fig. 2). Dark field microscopy revealed the hetero-agglomeration of nTiO2 after 1 h of incubation with E. coli and Paramecium cells (SI-Fig. S4). SEM-EDS observations further confirmed the hetero-agglomeration of nTiO2 in the presence or absence of E. coli and Paramecium at 1 h (SI Figs S5 and S6) and 24 h (Fig. 2). The images showed the adsorption and agglomeration of nTiO2 on the surface of E. coli and Paramecium cells within 1 h. Moreover, at 24 h, the size of the nTiO2 agglomerates increased in co-incubated and individual suspensions (Table 1). The increased size of individual nTiO2 agglomerates in suspension was attributable to homo-agglomeration in the presence of Na+ and Ca++ ions in Dryl’s buffer, which was used as an exposure medium throughout the experiments36,46,47.
Figure 2. Adsorption and homo- and hetero-agglomeration of nTiO2 in an aquatic microcosm.
SEM images with corresponding EDS spectra after 24 h of incubation with and without organisms. (a,a’) nTiO2 in Dryl’s buffer, (b,b’) nTiO2 incubated with E. coli in Dryl’s buffer, (c,c’) nTiO2 incubated with E. coli and Paramecium in Dryl’s buffer. The white circle corresponds to the point where the EDS spectrum analysis was performed. The yellow arrow indicates nTiO2 agglomerates. The inset image in (b) shows the surface interaction of nTiO2 with E. coli.
Co-sedimentation of nTiO2 in the microcosm
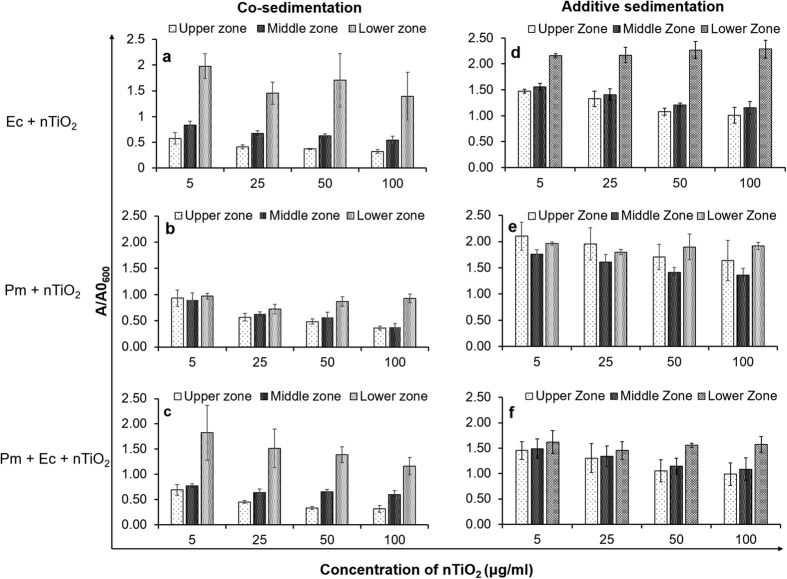
Figure 3 shows that the co-sedimentation of nTiO2 occurred only with E. coli and not with Paramecium in the microcosm. This result may be attributed to the low motility of E. coli cells (25 μm/s) compared with the high motility of Paramecium cells in the water column (1.7 mm/s)48,49. The adsorption of nTiO2 on the E. coli surface led to co-sedimentation because the presence of titanium was detected by EDS scanning of bacterial cells recovered from the LZ of the microcosm (Fig. 2).
Figure 3. Co-sedimentation (a–c) and additive-sedimentation (d–f) plots of nTiO2 in the presence of E. coli and Paramecium in an aquatic microcosm.
The ratio of initial to final absorbance at 600 nm in the upper, middle and lower zones of the microcosm depicting the effects of E. coli and Paramecium individually and in combination on the sedimentation of nTiO2. Ec, E. coli, Pm, Paramecium. Values represented are the mean ± SE of three independent experiments.
nTiO2 and E. coli
The most significant co-sedimentation of nTiO2 with E. coli was obtained at 5 μg/ml nTiO2, a concentration at which hetero-agglomeration is the favourable option, because of the availability of an optimum ratio between the number of nanoparticles and the surface area of E. coli cells (SI-Table S1). However, at higher concentrations (50 and 100 μg/ml) homo-agglomeration was favoured, owing to dominant nanoparticles-nanoparticle interactions that reduced the co-sedimentation of NPs with E. coli cells (SI-Fig. S9b). These observations are consistent with results from previous studies examining the homo- and hetero-agglomeration of ENMs39,40,47,50. Furthermore, the analysis of additive sedimentation of E. coli and nTiO2 permitted the calculation of nanoparticle-cell interactions, thus supporting our findings regarding hetero-agglomeration (Fig. 3d)36. The maximum interaction of E. coli with nTiO2 was observed at the lowest concentration of nTiO2 (5 μg/ml), as evident in the discrepancy (ΔOD) between nTiO2-bacteria co-sedimentation and additive settling in the LZ (Table 2). ΔOD > 0 represents the occurrence of NP−cell hetero-agglomeration and co-sedimentation, whereas zero or negative ΔOD values indicate no or very weak agglomeration between NPs and cells36.
Table 2. Differences (∆OD) in the A/A0 values of co- and additive sedimentation of nTiO2 in an aquatic microcosm.
| Concentration of nTiO2 (μg/ml) |
E. coli + nTiO2 |
Paramecium + nTiO2 |
Paramecium + E. coli + nTiO2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| UpperZone | MiddleZone | LowerZone | UpperZone | MiddleZone | LowerZone | UpperZone | MiddleZone | LowerZone | |
| 5 | −0.90 | −0.73 | −0.18 | −1.17 | −0.87 | −1.00 | −0.76 | −0.72 | 0.20 |
| 25 | −0.91 | −0.74 | −0.71 | −1.39 | −0.98 | −1.08 | −0.85 | −0.70 | 0.06 |
| 50 | −0.71 | −0.59 | −0.56 | −1.22 | −0.85 | −1.04 | −0.72 | −0.49 | −0.16 |
| 100 | −0.69 | −0.61 | −0.89 | −1.27 | −0.98 | −0.99 | −0.67 | −0.49 | −0.41 |
Values represented are the mean ± SE of three independent experiments.
A/A0 = Final absorbance/Initial absorbance.
nTiO2 and Paramecium
Co-sedimentation of nTiO2 was not observed with Paramecium in the absence of E. coli because the ΔOD value was highly negative (>−0.85; Table 2) for all concentrations of nTiO2 (Fig. 3b). Slight sedimentation of nTiO2 in LZ was observed at higher concentrations (50 and 100 μg/ml; Fig. 3b). This observation may be attributable to the continuous release of larger nTiO2-loaded vesicles from the cytoproct region of the Paramecium, which is a normal physiological process to egest un-digested material (SI-Fig. S10c,d). These vesicles are covered with the mucous membrane, which may further influence agglomeration in exposure medium33,51.
nTiO2 in combination with Paramecium and E. coli
A significant increase in the co-sedimentation of nTiO2 with E. coli cells was observed in the presence of Paramecium, as evidenced by the high positive ΔOD (0.20) value at the lowest concentration of nTiO2 (5 μg/ml), although the ΔOD value was close to zero (0.06) at 25 μg/ml (Table 2; Fig. 3c). This result indicated that the interaction of nTiO2 with E. coli was stronger in the presence of Paramecium at lower concentrations. The enhanced interaction and hetero-agglomeration of nTiO2 on the surface of E. coli in the presence of a predator species (Paramecium) indicated that the biotic interactions of nTiO2 in aquatic systems influence the fate as well as toxicity of this NP in bacteria and other organisms. These observations are supported by an earlier report examining the effects of surface interactions between nanoparticles and bacteria on toxicity27. In addition, co-sedimentation of nTiO2 may adversely affect sediment dwelling organisms52.
The interaction of nTiO2 with Paramecium in the presence of E. coli was also observed by using dark field and scanning electron microscopy. The dark field microscopy demonstrated that nTiO2 was taken up by Paramecium in the form of vesicles (SI-Fig. S4c,d), which were later released from the cytoproct in the form of aggregates. Further, SEM-EDS revealed the presence of nTiO2 in the oral groove regions of Paramecium cells, thus indicating its internalisation via phagocytosis (Fig. 2c,c’ & SI-Fig. S8c). Phagocytosis is a well-defined mechanism for NP uptake in ciliates such as Tetrahymena33.
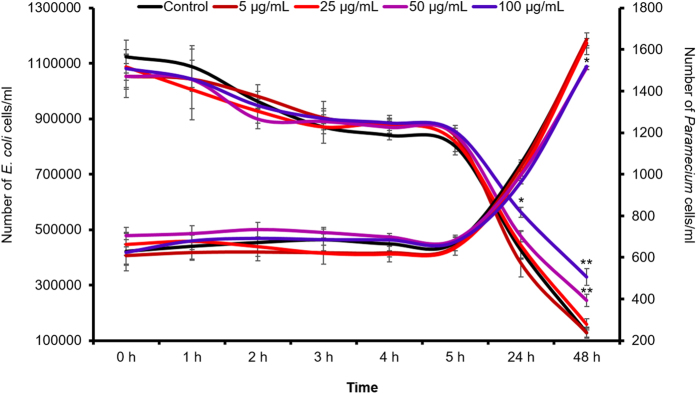
The behaviour of the test organisms in the presence or absence of nTiO2 in the microcosm was also determined. The observations revealed that Paramecium cells remained alive throughout the experiment as there were no signs of mortality, such as membrane leakage or loss of motility, at the tested time points. Furthermore, measurements revealed compromised bacterial ingestion and growth of Paramecium at 24 h after exposure to a higher dose of nTiO2 (100 μg/ml; Fig. 4). The Paramecium ingestion rate was ~1600 bacterial cells/ciliate/h. The morphological observation of E. coli cells by SEM revealed the shrinking size of E. coli cells recovered at 24 h from the LZ of the microcosm (SI-Figs S7a and S8a,b). This effect was also observed in the DLS analysis, which showed a decrease in the size of E. coli cells (SI- Table S2).
Figure 4. Growth and bacterial ingestion rate of Paramecium cells after exposure to nTiO2 at different concentrations.
Values represented are the mean ± SE of the three independent experiments. *p < 0.05 was considered significant compared with control.
The shrinking and co-sedimentation of E. coli cells in the presence of nTiO2 may be attributable to the adsorption of NPs on the E. coli surface, thus affecting their physico-mechanical properties and possibly play a critical role in maintaining the cellular morphology and motility of bacterial cells. In an earlier study, Zhang et al.34 have shown that the adsorption of hematite nanoparticles on the E. coli surface exerts multiple effects on physiological and mechanical properties, such as the loss of adhesiveness, elasticity, hardness, electrical potential and deformities in appendages such as flagella. These effects have the potential to inflict numerous consequences on bacterial cells, such as the loss of cellular motility, adhesion to surfaces and stability. The compromised motility and stability of bacterial cells due to the adsorption of nanoparticles may underlie the co-sedimentation of E. coli cells with nTiO2. Protistan grazing on bacterial cells in the environment has been shown to affect the morphology, physiological behaviour and activity of bacterial cells53. In general, bacterivorous protists preferentially feed on medium-size bacterial cells rather than smaller or larger bacteria. In this context, del George et al.54 have suggested that bacterial cells become inactive and thus adapt to powerful grazing pressure generated by the efficient grazing of bacteria by protists. Further, Gasol et al.55 have shown that inactive bacterial cells are smaller than active cells. Therefore, the presence of Paramecium in the microcosm facilitates the shrinking and co-sedimentation of E. coli cells with nTiO2.
The natural assemblage contains diverse communities that include motile, less-motile and sessile organisms in similar or diverse habitats. Therefore, under natural conditions, sessile organisms may also promote the co-sedimentation of NPs after interactions. To test this hypothesis, the adsorption of nTiO2 on sessile embryos (4 h post-fertilisation) of an established zebrafish ecotoxicity model was evaluated. Preliminary observations using optical and scanning electron microscopy revealed the extensive binding of nTiO2 on the chorion, which protects early stage embryos (SI-Figs S11 and 12). The presence of titanium on the chorion surface was further confirmed by EDS analysis (SI-Fig. S12). Therefore, it can be reasoned that interactions and co-sedimentation in water columns are mediated by members of the natural assemblage, including the highly motile Paramecium and less-motile organisms such as E. coli or sessile zebrafish embryos.
Previous studies have demonstrated that the fate and toxicity of ENMs may change because of the presence of natural colloids and organic matter in the environment56. The present study elucidated the role of predator-prey interactions in the hetero-agglomeration and co-sedimentation of ENMs in an aquatic environment. The findings also suggest that the adsorption and internalisation of nTiO2 on highly motile Paramecium cells may further increase bioavailability to higher trophic level organisms in the food chain.
Real-time monitoring of nTiO2 intracellular interactions in Paramecium
Internalisation of nTiO2 in Paramecium in the absence of E. coli
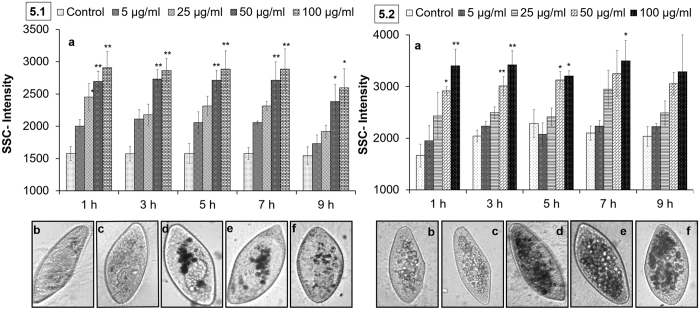
Flow cytometry is a well-established technique to measure the cellular density of cells and microbes. The side scattering intensity (SSC-intensity) parameter correlates with changes in cellular granularity due to the uptake of nanoparticles57,58. The real-time monitoring of nTiO2-exposed Paramecium cells in a flow cytometer revealed a dose-dependent and statistically significant (p < 0.05) increase in SSC-intensity, thus demonstrating the cellular internalisation of nTiO2 (Fig. 5.1a). The SSC-intensity was ~0.8-fold higher at an exposure concentration of 100 μg/ml compared with control conditions at 1 h. Further, the intensity of SSC remained constant up to 5 h, possibly because of the saturated uptake of nTiO2 within 1 h, as represented by the dark vesicles packed into the cytoplasm of nTiO2-treated Paramecium (Fig. 5.1b–e). At 7 h, a decrease in intracellular levels of nTiO2 was observed, evidenced by a reduction in the number of dark cytoplasmic vesicles and a consistent decrease in the SSC-intensity of Paramecium cells (Fig. 5.1a,f). The reduction in the intracellular levels of nTiO2 in Paramecium cells was attributable to the egestion of NPs loaded with undigested food vacuoles from the cytoproct and was confirmed by images captured through dark (SI-Figure S4d) and bright field microscopy (SI-Fig. S10c). The uptake rate of nTiO2 was also decreased after 7 h because of agglomeration and co-sedimentation, as evidenced by DLS measurements (Table 1) and observations under bright field microscopy (SI-Fig. S10d).
Figure 5. Real-time monitoring of nTiO2 intracellular interactions in Paramecium.
(5.1) Direct exposure of Paramecium cells to nTiO2 up to 9 h. (a) A concentration-dependent and statistically significant (p < 0.05) increase in the internalisation of nTiO2 in cells determined by flow cytometry. (b–f) Internalisation of nTiO2 confirmed by bright field microscopy: (b,c) controls at 1 and 9 h, (d–f) 50 μg/ml-treated cells captured at 1, 7 and 9 h. (5.2) Exposure of Paramecium cells to nTiO2 through Dryl’s buffer and E. coli up to 9 h. (a) A concentration dependent and significant (p < 0.05) increase in the internalisation and retention of nTiO2 in cells determined by flow cytometry. (b–f) Internalisation of nTiO2 confirmed by bright field microscopy: (b,c) controls at 1 and 9 h, (d–f) 50 μg/ml-treated cells captured at 1, 7 and 9 h. (All bright field microscopy images were captured at 200x magnification). Values represented are the mean ± SE of three independent experiments. *p < 0.05 was considered significant compared to the control.
Internalisation of nTiO2 in Paramecium through exposure medium and E. coli
Figure 5.2 shows a concentration-dependent (5, 10, 50 and 100 μg/ml), statistically significant (p < 0.05) increase in the SSC-intensity of Paramecium cells exposed to nTiO2 in the presence of E. coli cells. The Paramecium cellular density increased because of the internalisation of nanoparticles. The SSC-intensity of the Paramecium cells exposed to 100 μg/ml nTiO2 was 1.04-fold higher than that of the control. This result demonstrated that the density of nanoparticles within Paramecium cells was slightly higher in the presence of E. coli than direct exposure (Paramecium + nTiO2). Thus, additional exposure through feeding was involved in the uptake of nanoparticles into Paramecium cells. The internalisation of nTiO2 was maximal at 1 h and remained constant up to 9 h in contrast to 7 h after direct exposure in the absence of E. coli (Fig. 5.2a and 5.1a). Under co-exposure (Paramecium + E. coli + nTiO2) conditions, the retention of nTiO2 in Paramecium cells was extended because of the presence of nTiO2 and E. coli cells (Fig. 5.2b–f).
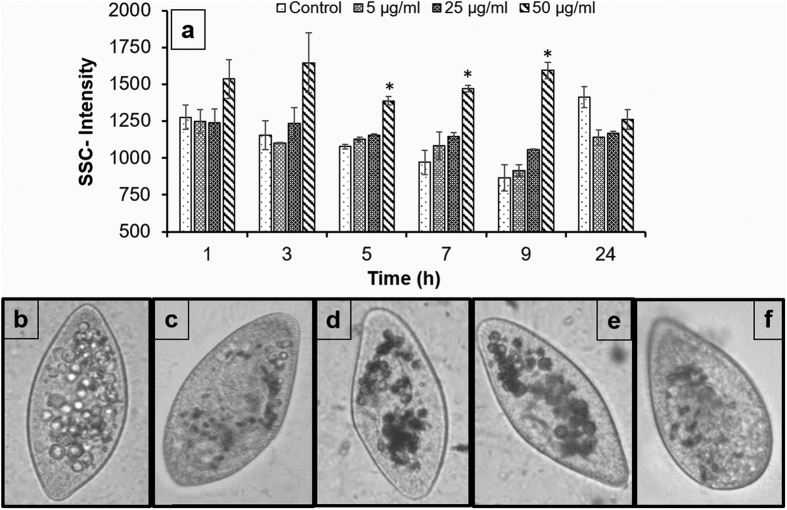
In addition, nTiO2 bioavailability through E. coli was observed after the exposure of Paramecium cells to nTiO2-pre-exposed E. coli. Figure 6 shows an increase in the SSC-intensity of Paramecium within 1 h of exposure to nTiO2-pre-exposed E. coli and was significantly (p < 0.05) constant for more than 9 h. An increase in the cellular density of Paramecium cells was attributable to the transfer of nTiO2 from E. coli cells. E. coli cells adsorb a mass of nTiO2 within 1 h of exposure that is further transferred to their predator protozoan via phagocytosis33. Further, the digestion of ingested bacteria in Paramecium cells is complete within 1 h, and thus nTiO2 is released from E. coli59. There is increased biocompatibility between nanoparticles and Paramecium cells, owing to the presence of bacterial components on the surface that prevent immediate release from the cytoproct of Paramecium cells. Here, the SSC-intensity of Paramecium cells was normalised to control levels at 24 h compared to 7 and 9 h under direct and co-exposure conditions. The cytoplasmic vacuoles of treated Paramecium cells also returned to control levels at 24 (Fig. 5b–f). Overall, the presence of a prey species (E. coli) plays significant role in the bioavailability and persistence of nTiO2 in a predator (Paramecium). These observations also provide evidence, supporting the trophic transfer of nTiO2 during prey-predator interactions.
Figure 6. Trophic transfer of nTiO2 in Paramecium relative to E. coli.
(a) A statistically significant (p < 0.05) increase in the cellular granularity of Paramecium cells fed with nTiO2-contaminated E. coli as determined by flow cytometry. (b–f) Trophic transfer of nTiO2 further confirmed by bright field microscopy: (b,c) controls at 1 and 24 h, (d–f) 50 μg/ml-treated cells captured at 1, 7 and 24 h. (All bright field microscopy images were captured at 200x magnification). Values represented are the mean ± SE of the three independent experiments. *p < 0.05 was considered significant compared with control.
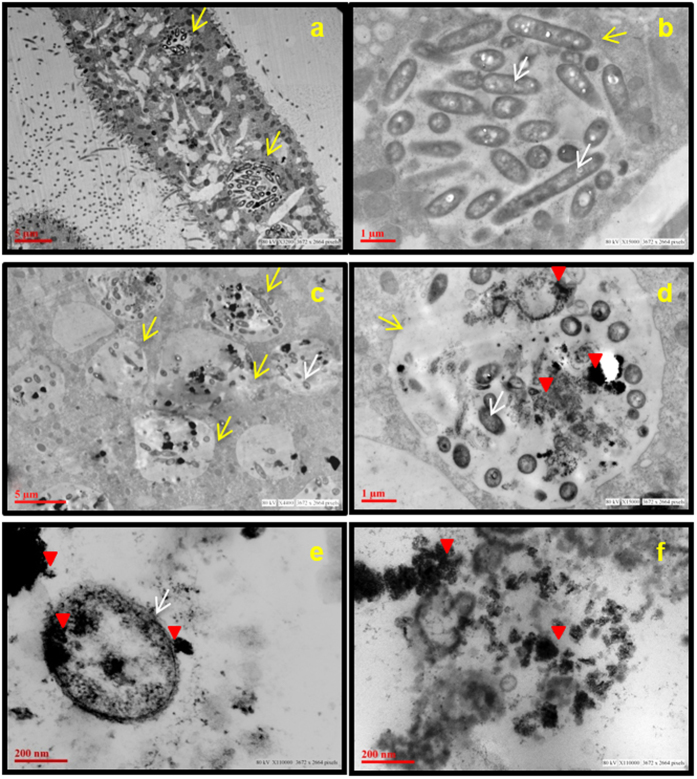
Transmission electron microscopy further confirmed the presence of nTiO2, E. coli and nTiO2-containing E. coli in the food vacuoles of Paramecium (Fig. 7a–f). TEM images clearly revealed the presence of nTiO2 inside and outside the E. coli cells as well as those lodged in the food vacuoles of Paramecium cells, thus indicating that NPs were internalised into the cells either directly from Dryl’s buffer or through E. coli (Fig. 7e,f). The role of E. coli as a vehicle for the transfer of nTiO2 to Paramecium cells establishes the trophic transfer of nTiO2 from the lower to the upper trophic levels in the microbial food chain. The trophic transfer of nTiO2 was further supported by the presence of packaged bacteria and nTiO2 in the egested Paramecium food vacuoles (SI-Fig. S10a).
Figure 7. The interaction of Paramecium with E. coli and nTiO2 after 1 h of incubation.
Transmission electron microscopy images, (a,b) control, and (c–f) treated cells (25 μg/ml) showing the presence of E. coli and nTiO2 in food vacuoles (c,d). nTiO2 is present inside (e) and outside E. coli cells (f). Red arrowhead represents nTiO2, yellow arrow represents food vacuoles and white arrow represents bacteria.
Conclusion
Bacterial abundance and their high surface-area-to-volume ratio increases the probability of interactions with ENMs in the environment. The magnitude of interaction between ENMs and the bacterial surface can significantly alter the environmental fate and consequent toxicity of ENMs. Bacteria are the simplest organisms in the lower trophic levels linked to ciliated protozoans in the upper trophic levels of the food chain. The present study demonstrates that the presence of a predator, Paramecium, significantly influences the surface interactions between nTiO2 and its prey, E. coli. The increased surface interactions between nTiO2 and E. coli further enhanced the hetero-agglomeration and co-sedimentation of nTiO2 in the modelled predator-prey-based microcosm. Although co-sedimentation reduces the bioavailability of nTiO2 to organisms present in surface water columns, sediment-dwelling organisms may be at a higher risk of exposure to nTiO2. The strong adsorption and internalisation of nTiO2 in Paramecium cells increases the bioavailability of nTiO2 via Paramecium to organisms such as rotifers and fish larvae, which are present at higher trophic levels in the food chain. The present study addresses a one-consumer (protozoan), one-resource (bacterium) system to assess the fate of nTiO2. However, in a real-world situation, the effects of predation and interactions with prey species may be much greater than those observed in composite natural communities.
Methods
Chemicals and biological materials
Titanium dioxide nanopowder (anatase; 99.7%; CAS No. 1317-70-0) was purchased from Sigma Aldrich (St. Louis, USA). The E. coli K12 substrain DH10B was purchased from the Microbial Type Culture Collection Centre (MTCC, Chandigarh, India). Paramecium caudatum and protozoan pellets were purchased from Carolina Biological Supply Co. (Burlington, USA). Details regarding the use of E. coli and Paramecium as model organisms are provided in the supplementary information (SI-method section 1). All other chemicals were of analytical reagent grade and purchased from HiMedia Pvt. Ltd. (Mumbai, India).
E. coli and Paramecium culture
E. coli cells were cultured in Luria Bertani (LB) broth (HiMedia Pvt. Ltd., Mumbai, India) at 37 °C in an environmental shaker incubator. Paramecium caudatum was cultured in a protozoan pellet medium at 22 °C in a BOD incubator (Model LBI-500M, Daihan Labtech, India). A detailed protocol for medium preparation and E. coli and Paramecium culture is given in the supplementary information (SI-method section 2).
Nanoparticle preparation and characterisation
A stock suspension of 200 μg/ml nTiO2 was prepared by adding 8 mg of nTiO2 powder (anatase) to 40 ml of filtered Dryl’s buffer (0.22-μm membrane filter). The suspension was then sonicated for 10 min using a probe sonicator (Sonics Vibra Cell, Sonics & Material Inc., New Town, USA) at 30% amplitude and 30 watts at a pulse rate of 50 sec on and 10 sec off. Before use, the suspension was maintained in a BOD incubator for 10 min to optimise the temperature to 22 °C. Subsequently, it was diluted to four different working concentrations of 5, 25, 50 and 100 μg/ml. A detailed description of the selection of concentrations is given in the supplementary information (SI-results section 2.0).
Hydrodynamic size and zeta potential were measured by dynamic light scattering (DLS) and phase analysis light scattering (PALS) using a Zeta-sizer Nano-ZS equipped with a 4.0-mW, 633-nm laser (Model ZEN3600; Malvern Instruments, UK). The distribution of different sizes of nTiO2 was also observed by transmission electron microscopy (TEM). The samples for TEM analysis were prepared as described by Shukla et al.58. Briefly, a drop of nTiO2 suspension (25 μg/ml) was placed on a formvar-coated copper grid. The film on the TEM grid was allowed to dry in the dark at room temperature. Images were captured using a TecnaiTM G2 Spirit electron microscopy (FEI, The Netherlands) at an accelerating voltage of 80 kV.
Microcosm setup
A simple aquatic microcosm was designed in a 50-ml polystyrene tube to assess the effects of biotic factors on the fate and stability of nTiO2. The experiments were conducted in Dryl’s buffer in seven different groups at 22 °C in a BOD incubator (LabTeK, India). Each group contained a 40-ml suspension of nTiO2 or Dryl’s buffer as well as predator-prey organisms and was marked into three zones, an upper zone (UZ), a middle zone (MZ) and a lower zone (LZ), as shown in the supplementary information (SI Fig. S1). One millilitre of sample was withdrawn carefully from each zone at 1 h and 24 h for analysis. Dryl’s buffer was used because of its unique chemical composition [sodium citrate (2 mM), NaH2PO4•H2O (1 mM), Na2HPO4 (1 mM), CaCl2 (1.5 mM), pH 7–7.2], which helps organisms to survive without stress for long durations, and the presence of citrate ions maintains the environmental relevance of the study because citrate is present in abundance in the natural environment39. Detailed information on the microcosm setup is given in the supplementary information (SI-method section 3).
Group 1: nTiO2
Group 2: E. coli
Group 3: E. coli + nTiO2
Group 4: Paramecium
Group 5: Paramecium + nTiO2
Group 6: Paramecium + E. coli
Group 7: Paramecium + E. coli + nTiO2
Measurement and analysis of nTiO2 agglomeration and adsorption onto organisms in the microcosm
The agglomeration of nTiO2 in the microcosm was measured by dynamic light scattering, which was further validated by dark field microscopy and scanning electron microscopy (SEM). The SEM was equipped with an energy-dispersive X-ray spectroscope (EDS), which complemented the qualitative images with compositional analysis of the adsorption of nTiO2.
Sample preparation and analysis of hydrodynamic diameter and electro-kinetic measurements
Nanoparticle suspensions at different working concentrations were incubated with organism/s according to the experimental setup described for groups 2, 5 and 7 to measure the hetero-agglomeration.
Samples for analysis were withdrawn from each group at the initial (1 h) and final time points (24 h) from three different zones (upper, middle and lower) in the microcosm. The samples were analysed immediately using a Zeta-sizer Nano-ZS equipped with a 4.0-mW, 633-nm laser (Model ZEN3600, Malvern instruments Ltd., Malvern, UK). The zeta-sizer was equipped with a He–Ne laser (wavelength 633 nm) with a scattering angle of 173° and a constant temperature of 25 ± 1 °C. Water was selected as the experimental medium, with a refractive index of 1.330, and the refractive index of nTiO2 was 0.200. A minimum of ten runs per analysis was carried out in automatic mode, and the data were analysed using the Zetasizer Software version 7.01 (Malvern, UK).
Measurement of hydrodynamic diameter
Measurements of the z-average intensity-based hydrodynamic size and size distribution were carried out with the DLS on the basis of the Stokes-Einstein equation. The modality of nTiO2 size measurement by DLS was also verified by running E. coli and Paramecium. The multi-modal peaks of the individual organisms before and after incubation with nTiO2 did not interfere with the measurement of nTiO2 agglomeration, as shown in the supplementary information (SI Fig. S13). Given that the detection limit of the DLS equipment was ~8 μm, Paramecium cells could not be detected because of their large size (length ~110 μm and width ~40 μm) (SI-Fig. S7b and Table S2). E. coli was represented by a small peak in the size range of 2–6 μm by DLS (SI-Fig. S13 and Table S2) and 3–5 μm in length and ~0.7 μm in width by SEM (SI-Fig. S7a). The addition of E. coli did not interfere with the measurement of nTiO2, owing to differences in the refractive index of E. coli. In an earlier study examining hematite nanoparticles, similar measurements were performed by DLS for bacterial cells34. The data are presented as the mean ± standard error of three independent experiments.
Zeta potential measurements
Zeta potential measures the total surface charge on particles and is independent of nanoparticle size and shape.42Zeta potential changes if the chemistry of the exposure medium is altered. The zeta potential of nTiO2 and E. coli was measured in Dryl’s buffer. Briefly, 1 ml of sample was injected into a capillary zeta cell (Folded Capillary Cell, DTS 1060, Malvern, UK). During injection of the sample, caution was taken so that bubbles did not form in the capillary cells and interfere with the analysis. Data were generated in the form of electrophoretic mobility, which was further converted to zeta potential by application of the Smoluchowki equation41.
Microscopic observation and sample preparation
Samples from different zones of the microcosm were taken at 1 h and 24 h and analysed for homo- and hetero-agglomerates using dark field microscopy as well as scanning electron microscopy linked with energy dispersive X-ray spectroscopy (SEM-EDS).
Detailed protocols for sample preparation for dark field microscopy and SEM are given in the supplementary information (SI method section 4.1 and 4.2).
Measurement and analysis of nTiO2 co-sedimentation and hetero-agglomeration in the microcosm
The experiments were carried out in the established microcosm as described above, with seven distinct groups. Four different concentrations of nTiO2 (5, 25, 50 and 100 μg/ml) were used to determine the effects of nTiO2 dilution on co-sedimentation.
Samples from each group and all zones were withdrawn at the initial (1 h) and final time points (24 h), immediately transferred to a quartz cuvette with a 1-cm path-length, and the absorbance was recorded at 600 nm using a UV-Vis spectrophotometer (Synergy HT, BioTek, Winooski, USA).
Co-sedimentation
Individual sedimentation and co-sedimentation patterns of nTiO2 were measured and plotted using the method described by Ma et al.36 with minor modifications. Briefly, the ordinate was calculated as the ratio of absorbance at a given time point (A) to the initial absorbance (A0), and the graph was plotted against the concentrations of the nTiO2. Independently, an additive sedimentation curve was also plotted as the sum of the individual absorbance of two different groups.
Hetero-agglomeration
The hetero-agglomeration of nTiO2 with E. coli in the microcosm was measured as described by Ma et al. in algae36. The difference (ODmix-ODsum) between co-sedimentation [co-(ODmix)] and additive sedimentation [additive-(ODsum)] was calculated to determine the intensities of NP-cell interactions.
Measurement of bacterial ingestion rate of Paramecium cells
The assay was performed by counting the numbers of bacterial and Paramecium cells/ml in the control (Paramecium + E. coli) and treated (Paramecium + E. coli + nTiO2 at 5, 25, 50 and 100 μg/ml) groups at 0, 1, 2, 3, 4, 5, 24 and 48 h after incubation. At each time point, 2 ml of culture was withdrawn from each group and divided into two separate 1-ml Eppendorf tubes. Separate tubes were used to count Paramecium and bacterial cells. The detailed protocol for bacterial and Paramecium cell counting is given in the supplementary information (SI-Method 2.1). To show the growth of Paramecium cells and the reduction in E. coli cells over time, a graph was plotted with the number of E. coli and Paramecium cells/ml on a double y-axis and time (h) on the x-axis.
The ingestion rate of Paramecium was determined by applying the formula suggested by Ali & Saleh60.
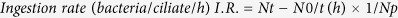 |
N0 = number of prey (bacteria) at 0 h; Nt = number of prey (bacteria) at time (t);
Np = number of predators (bacterivorous ciliate).
Uptake of nTiO2 in Paramecium cells via direct exposure and co-exposure
An experiment was designed to determine the intracellular fate of nTiO2 in Paramecium cells under three different exposure conditions: (1) direct exposure of Paramecium cells to nTiO2 in Dryl’s buffer, (2) co-exposure of Paramecium cells to nTiO2 in the presence of E. coli, and (3) feeding of Paramecium cells with nTiO2-preloaded E. coli.
The experimental setup included: Paramecium alone, Paramecium + E. coli and nTiO2 without cells (Control; Group 1), Paramecium + nTiO2 and Paramecium + E. coli + nTiO2 (Treatment; Group 2) and Paramecium + nTiO2-preloaded E. coli (Treatment; Group 3).
The experiments were performed in 6-well plates, and each well contained 3 ml of Dryl’s buffer with Paramecium (600–800 cells/ml) and/or E. coli (OD600 0.4). Paramecium in the treatment group was exposed to different concentrations (5, 25, 50 and 100 μg/ml) of nTiO2. The cellular internalisation of nTiO2 was assessed using flow cytometry and validated by both bright field and transmission electron microscopy. Each sample was analysed after 1 h of exposure and subsequently every 2 h until experimental completion (9 h). Additionally, samples from Group 3 were also analysed at 24 h to verify the dilution of NPs in the cells because the dilution was not evaluated at 9 h.
The details regarding sample preparation and flow cytometry data analysis are given in the supplementary information (SI method section 5).
Paramecium sample preparation for dark field, bright field and transmission electron microscopy
Detailed protocols for the sample preparation of Paramecium for dark field, bright field and transmission electron microscopy are given in the supplementary information (SI method section 4.1 and 4.3).
Statistical analysis
The three independent experiments were performed for all assays, and the results were expressed as the mean ± standard error (SE). Statistical analysis was carried out using Microsoft Excel 2007 and GraphPad Prism version 3.02. The significance of the data were analysed using one-way analysis of variance (ANOVA) with Dunnett’s post hoc test as well as t-tests (non-parametric). In all cases, p < 0.05 was considered significant.
Additional Information
How to cite this article: Gupta, G. S. et al. Assessment of agglomeration, co-sedimentation and trophic transfer of titanium dioxide nanoparticles in a laboratory-scale predator-prey model system. Sci. Rep. 6, 31422; doi: 10.1038/srep31422 (2016).
Supplementary Material
Acknowledgments
The authors acknowledge funding from CSIR Network Projects NWP35 and BSC0112 (NanoSHE), India, and the EU-FP7/2007-2013/Grant Agreement 263147 (NanoValid-Development of reference methods for hazard identification, risk assessment and LCA of engineered nanomaterials), Europe. Funding received from the GICT, India, under the CENTRA project Grant Agreement (ILS/GICT/2013/003) is also acknowledged. GSG acknowledges UGC, India, for a Junior Research Fellowship. The CSIR-IITR and DBLS-Ahmedabad University manuscript communication numbers are 3345 and DBLS 65, respectively.
Footnotes
Author Contributions R.S. conceptualised the experimental design. A.D. discussed the experimental design and critically evaluated the manuscript. G.S.G. executed the experiments. A.K. helped with flow cytometry analysis. All authors contributed to the writing of the manuscript.
References
- Lazareva A. & Keller A. A. Estimating potential life cycle releases of engineered nanomaterials from wastewater treatment plants. ACS Sustainable Chem. Eng 2, 1656–1665 (2014). [Google Scholar]
- Vance M. E. et al. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6, 1769–1780 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. H., Bilal M., Lazareva A., Keller A. & Cohen Y. Simulation tool for assessing the release and environmental distribution of nanomaterials. Beilstein J Nanotechnol 6, 938–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk F. & Nowack B. The release of engineered nanomaterials to the environment. J Environ Monit 13, 1145–55 (2011). [DOI] [PubMed] [Google Scholar]
- Holden P. A. et al. Ecological nanotoxicology: integrating nanomaterial hazard considerations across the subcellular, population, community, and ecosystems levels. Acc Chem Res 46, 813–22 (2013). [DOI] [PubMed] [Google Scholar]
- Weir A., Westerhoff P., Fabricius L., Hristovski K. & von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46, 2242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Magaye R., Castranova V. & Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10, 15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Mahendra S. & Alvarez P. J. J. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS-Nano 4, 3580–3590 (2010). [DOI] [PubMed] [Google Scholar]
- Gnanaprakasam A., Sivakumar V. M., Sivayogavalli P. L. & Thirumarimurugan M. Characterization of TiO2 and ZnO nanoparticles and their applications in photocatalytic degradation of azodyes. Ecotoxicol Environ Saf, doi: 10.1016/j.ecoenv (2015). [DOI] [PubMed] [Google Scholar]
- Morabito K., Shapley N. C., Steeley K. G. & Tripathi A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int J Cosmet Sci 33, 385–90 (2011). [DOI] [PubMed] [Google Scholar]
- Gulson B., McCall M. J., Bowman D. M. & Pinheiro T. A review of critical factors for assessing the dermal absorption of metal oxide nanoparticles from sunscreens applied to humans, and a research strategy to address current deficiencies. Arch Toxicol, doi: 10.1007/s00204-015-1564-z (2015). [DOI] [PubMed] [Google Scholar]
- Maurer-Jones M. A., Gunsolus I. L., Murphy C. J. & Haynes C. L. Toxicity of engineered nanoparticles in the environment. Anal Chem 85, 3036–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk F., Ort C., Scholz R. W. & Nowack B. Engineered nanomaterials in rivers-exposure scenarios for Switzerland at high spatial and temporal resolution. Environ Pollut 159, 3439–45 (2011). [DOI] [PubMed] [Google Scholar]
- Pernthaler J. Predation on prokaryotes in the water column and its ecological implications. Nat Rev Microbiol 3, 537–46 (2005). [DOI] [PubMed] [Google Scholar]
- Mullen M. D., Wolf D. C., Ferris F. G., Beveridge T. J., Flemming C. A. & Bailey G. W. Bacterial sorption of heavy metals. Appl Environ Microbiol 55, 3143–9 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batley G. E., Kirby J. K. & McLaughlin M. J. Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46, 854–62 (2013). [DOI] [PubMed] [Google Scholar]
- Hoet P. H., Nemmar A. & Nemery B. Health impact of nanomaterials? Nat Biotechnol 22, 19 (2004). [DOI] [PubMed] [Google Scholar]
- Werlin R. et al. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat Nanotechnol 6, 65–71 (2011). [DOI] [PubMed] [Google Scholar]
- Holbrook R. D., Murphy K. E., Morrow J. B. & Cole K. D. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat Nanotechnol 3, 352–355 (2008). [DOI] [PubMed] [Google Scholar]
- Zhu X., Chang Y. & Chen Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 78, 209–215 (2009). [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang J., Zhang X., Chang Y. & Chen Y. Trophic transfer of TiO(2) nanoparticles from Daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 79, 928–933 (2010). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Bioaccumulation of CdTe quantum dots in a freshwater alga Ochromonas danica: a kinetics study. Environ Sci Technol 47, 10601–10610 (2014). [DOI] [PubMed] [Google Scholar]
- Bouldin J. L. et al. Aqueous toxicity and food chain transfer of Quantum DOTs in freshwater algae and Ceriodaphnia dubia. Environ Toxicol Chem 27, 1958–1963 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J. R., Hanna S. K., Lenihan H. S. & Keller A. A. Effects and implications of trophic transfer and accumulation of CeO2 nanoparticles in a marine mussel. Environ Sci Technol 48, 1517–1524 (2014). [DOI] [PubMed] [Google Scholar]
- Manke A., Wang L. & Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013, 942916 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Stack A. G. & Chen Y. Interaction force measurement between E. coli cells and nanoparticles immobilized surfaces by using AFM. Colloids Surf B Biointerfaces 82, 316–324 (2011). [DOI] [PubMed] [Google Scholar]
- Pagnout C. et al. Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli. Colloids Surf B Biointerfaces 92, 315–321 (2012). [DOI] [PubMed] [Google Scholar]
- El Badawy A. M. et al. Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45, 283–287 (2011). [DOI] [PubMed] [Google Scholar]
- Arvizo R. R. et al. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett 10, 2543–2548 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden P. A., Schimel J. P. & Godwin H. A. Five reasons to use bacteria when assessing manufactured nanomaterial environmental hazards and fates. Curr Opin Biotechnol 27, 73–78 (2014). [DOI] [PubMed] [Google Scholar]
- Chojnacka K. Biosorption and bioaccumulation–the prospects for practical applications. Environ Int 36, 299–307 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang W., Rittmann B. & Chen Y. Size effects on adsorption of hematite nanoparticles on E. coli cells. Environ Sci Technol 45, 2172–8 (2011). [DOI] [PubMed] [Google Scholar]
- Ghafari P. et al. Impact of carbon nanotubes on the ingestion and digestion of bacteria by ciliated protozoa. Nature Nanotech 3, 347–351 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang W., Hughes J. & Chen Y. Impacts of hematite nanoparticle exposure on biomechanical, adhesive, and surface electrical properties of Escherichia coli cells. Appl Environ Microbiol 78, 3905–3915 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. et al. Surface interactions affect the toxicity of engineered metal oxide nanoparticles toward Paramecium. Chem Res Toxicol 25, 1675–1681 (2012). [DOI] [PubMed] [Google Scholar]
- Ma S., Zhou K., Yang K. & Lin D. Hetero-agglomeration of oxide nanoparticles with algal cells: effects of particle type, ionic strength and pH. Environ Sci Technol 49, 932–9 (2015). [DOI] [PubMed] [Google Scholar]
- Brayner R. et al. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6, 866–870 (2006). [DOI] [PubMed] [Google Scholar]
- Rao J. V., Srikanth K., Arepalli S. K. & Gunda V. G. Toxic effects of acephate on Paramecium caudatum with special emphasis on morphology, behaviour, and generation time. Pesticide biochemistry and physiology 86, 131–137 (2006). [Google Scholar]
- Mudunkotuwa I. A. & Grassian V. H. Citric acid adsorption on TiO2 nanoparticles in aqueous suspensions at acidic and circumneutral pH: surface coverage, surface speciation, and its impact on nanoparticle-nanoparticle interactions. J. Am. Chem. Soc. 132, 14986–14994 (2010). [DOI] [PubMed] [Google Scholar]
- Planchon M. et al. Exopolysaccharides protect Synechocystis against the deleterious effects of titanium dioxide nanoparticles in natural and artificial waters. J Colloid Interface Sci 405, 35–43 (2013). [DOI] [PubMed] [Google Scholar]
- Horst A. M. et al. Dispersion of TiO2 nanoparticle agglomerates by Pseudomonas aeruginosa. Appl Environ Microbiol 76, 7292–7298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. S., Srivatsan P., Vaishnavi N. Mukherjee A. & Chandrasekaran N. Interaction of silver nanoparticles (SNPs) with bacterial extracellular proteins (ECPs) and its adsorption isotherms and kinetics. J Hazard Mater 192, 299–306 (2011). [DOI] [PubMed] [Google Scholar]
- Jucker B. A., Zehnder A. J. & Harms H. Quantification of polymer interactions in bacterial adhesion. Envron Sci Technol 32, 2909–2915 (1998). [Google Scholar]
- Li B. K. & Logan B. E.. Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surf. B Biointerfaces 36, 81–90 (2004). [DOI] [PubMed] [Google Scholar]
- Hahn M. W., Lünsdorf H. & Janke L. Exopolymer production and microcolony formation by planktonic freshwater bacteria: defence against protistan grazing. Aquatic microbial ecology 35, 297–308 (2004). [Google Scholar]
- French R. A., Jacobson A. R., Kim B., Isley S. L., Penn R. L. & Baveye P. C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ Sci Technol 43, 1354–9 (2009). [DOI] [PubMed] [Google Scholar]
- Afrooz A. R., Khan I. A., Hussain S. M. & Saleh N. B. Mechanistic heteroaggregation of gold nanoparticles in a wide range of solution chemistry. Environ Sci Technol 47, 1853–60 (2013). [DOI] [PubMed] [Google Scholar]
- Roberts A. M. The mechanics of gravitaxis in Paramecium. J Exp Biol 213, 4158–4162 (2010). [DOI] [PubMed] [Google Scholar]
- Ford R. M. & Harvey R. W. Role of chemotaxis in the transport of bacteria through saturated porous media. Advances in Water Resources 30, 1608–1617 (2007). [Google Scholar]
- Labille J., Harns C., Bottero J. Y. & Brant J. Heteroaggregation of titanium dioxide nanoparticles with natural clay colloids. Environ Sci Technol 49, 6608–16 (2015). [DOI] [PubMed] [Google Scholar]
- Chan T. S. et al. Carbon nanotube compared with carbon black: effects on bacterial survival against grazing by ciliates and antimicrobial treatments. Nanotoxicology 7, 251–8 (2013). [DOI] [PubMed] [Google Scholar]
- Cross R. K., Tyler C. & Galloway T. S. Transformations that affect fate, form and bioavailability of inorganic nanoparticles in aquatic sediments. Environmental Chemistry 12, 627–642 (2015). [Google Scholar]
- Hahn M. W. & Hofle M. G. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol Ecol 35, 113–121 (2001). [DOI] [PubMed] [Google Scholar]
- del Giorgio P. A., Gasol J. M., Vaque D., Mura P., Agusti S. & Duarte C. M. Bacterioplankton community structure: protists control net production and the proportion of active bacteria in a coastal marine community. Limnology and Oceanography 41, 1169–1179 (1996). [Google Scholar]
- Gasol J. M., Del Giorgio P. A., Massana R. & Duarte C. M. Active versus inactive bacteria: size-dependence in a coastal marine plankton community. Marine Ecology Progress Series 128, 91–97 (1995). [Google Scholar]
- Neale P. A., Jamting A. K., O’Malley E., Herrmann J. & Escher B. I. Behaviour of titanium dioxide and zinc oxide nanoparticles in the presence of wastewater-derived organic matter and implications for algal toxicity. Environ Sci: Nano, doi: 10.1039/C4EN00161C (2014). [DOI] [Google Scholar]
- Kumar A., Pandey A. K., Singh S. S., Shanker R. & Dhawan A. A flow cytometric method to assess nanoparticle uptake in bacteria. Cytometry A 9, 707–712 (2011). [DOI] [PubMed] [Google Scholar]
- Shukla R. K., Sharma V., Pandey A. K., Singh S., Sultana S. & Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro 25, 231–41 (2011). [DOI] [PubMed] [Google Scholar]
- Fok A. K., Lee Y. & Allen R. D. The correlation of digestive vacuole pH and size with the digestive cycle in Paramecium caudatum. The Journal of Protozoology, 29, 409–414 (1982). [Google Scholar]
- Ali T. H. & Saleh D. S. A simplified experimental model for clearance of some pathogenic bacteria using common bacterivorous ciliated spp. in Tigris river. Applied Water Science 4, 63–71 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.