Abstract
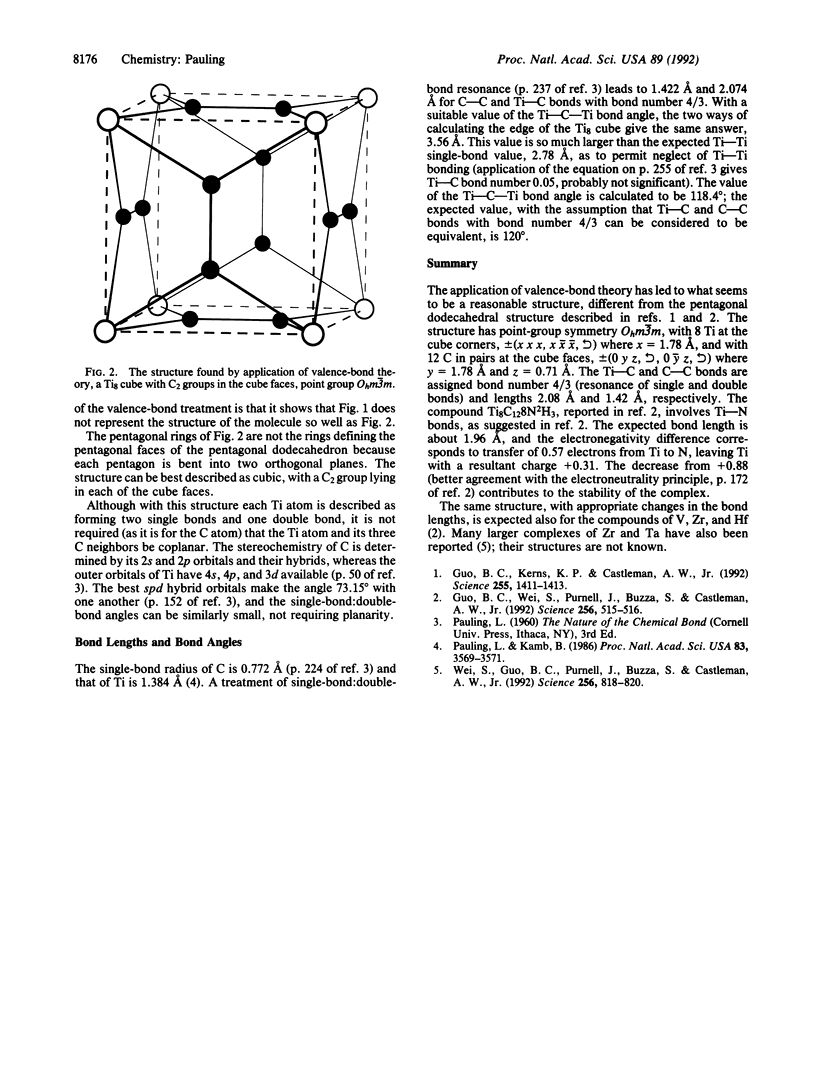
Application of valence-bond theory leads to the assignment to the molecule Ti8C12 of a cubic structure, point group Ohm3m, with 8 Ti at the cube corners, +/-(x x x, x, x x [symbol, see text]) where x = 1.78 A, and with 12 C in pairs in the cube faces, +/-(0 y z, [symbol, see text], 0, y z [symbol, see text]) where y = 1.78 A and z = 0.71 A. The Ti-C and C-C bonds have bond number 4/3, corresponding to resonance of single and double bonds in 2:1 ratio.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Guo B. C., Kerns K. P., Castleman A. W., Jr Ti8C12+-Metallo-Carbohedrenes: A New Class of Molecular Clusters? Science. 1992 Mar 13;255(5050):1411–1413. doi: 10.1126/science.255.5050.1411. [DOI] [PubMed] [Google Scholar]
- Guo B. C., Wei S., Purnell J., Buzza S., Castleman A. W., Jr Metallo-Carbohedrenes [M8C12+ (M = V, Zr, Hf, and Ti)]: A Class of Stable Molecular Cluster Ions. Science. 1992 Apr 24;256(5056):515–516. doi: 10.1126/science.256.5056.515. [DOI] [PubMed] [Google Scholar]
- Pauling L., Kamb B. A revised set of values of single-bond radii derived from the observed interatomic distances in metals by correction for bond number and resonance energy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3569–3571. doi: 10.1073/pnas.83.11.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]


