Abstract
Chromothripsis is a recently observed phenomenon in cancer cells in which one or several chromosomes shatter into pieces with subsequent inaccurate reassembly and clonal propagation. This type of event generates a potentially vast number of mutations within a relatively short-time period, and has been considered as a new paradigm in cancer development. Despite recent advances, much work is still required to better understand the molecular mechanisms of this phenomenon, and thus an easy-to-use tool is in urgent need for automatically detecting and annotating chromothripsis. Here we present CTLPScanner, a web server for detection of chromothripsis-like pattern (CTLP) in genomic array data. The output interface presents intuitive graphical representations of detected chromosome pulverization region, as well as detailed results in table format. CTLPScanner also provides additional information for associated genes in chromothripsis region to help identify the potential candidates involved in tumorigenesis. To assist in performing meta-data analysis, we integrated over 50 000 pre-processed genomic arrays from The Cancer Genome Atlas and Gene Expression Omnibus into CTLPScanner. The server allows users to explore the presence of chromothripsis signatures from public data resources, without carrying out any local data processing. CTLPScanner is freely available at http://cgma.scu.edu.cn/CTLPScanner/.
INTRODUCTION
Chromothripsis is a type of complex chromosome rearrangement that was initially found and described in cancer cells (1). This phenomenon is characterized by the shattering of one or a few chromosomes and a random reassembly of the DNA fragments to form a derivative chromosome. The process may result in a very large number of somatic mutations and genome structural variations including duplications, deletions, inversions and translocations (2,3). It may also lead to the amplification of oncogenes or the deletion of tumor suppressors, which play essential roles in promoting tumorigenesis (4–6). The most notable feature is that all the events are likely to occur in a single catastrophic event rather than a series of subsequent alterations. The chromothripsis phenomenon reveals a new paradigm of oncogenic transformation, in contrast to the multistep model of cancer development (1).
The discovery of chromothripsis has drawn an increasing attention in the research community due to its functional impact on cancer progression. Since the initial report of chromothripsis, this phenomenon has been observed in many tumor types (7–11). Although the molecular mechanisms leading to this event are not fully characterized, several hypotheses have been proposed, such as micronuclei formation (12,13), breakage-fusion-bridge cycles (1,11,14–16), premature chromosome compaction (17), abortive apoptosis (18,19), ionizing radiation (1,20) and telomere-based mechanism (21). To further elucidate the potential mechanisms, it is essential to identify chromothripsis events and locations. However, there is no formal or universal tool available for automatically detecting and annotating shattered chromosomal regions. Although Korbel and Campbell have introduced six rigorous criteria for chromothripsis (22), the complex rearrangements and the diversity of copy number patterns make it challenging to infer chromothripsis reliably (23). Most studies identified chromothripsis by visual inspection or reported chromothripsis phenomenon on the basis of operational definitions (4,8,9,24). Hitherto, there has been only one algorithm developed for detecting chromothripsis from next-generation sequencing (NGS) data (25). As such, user-friendly and flexible tools are still needed to assist researchers in identifying and annotating chromothripsis in an intuitive manner.
To provide a powerful method for detection of chromothripsis-like pattern (CTLP), we recently introduced an algorithm for large-scale data analysis (10). The algorithm is a scan-statistic model-based method and is able to detect the chromosomal location of chromothripsis events. However, the usage of the algorithm can be a cumbersome process. More importantly, it lacks the visualization and annotation of the results. Here, we introduce a web-based implementation of the algorithm, called CTLPScanner, which is designed for users to screen chromosome pulverization regions and obtain annotations. It assesses the most striking features of chromothripsis, i.e. clustering of chromosomal breakpoints and oscillating copy number changes in specific regions (1,4). The web interface is capable of processing input segmented genomic array data with flexible visualization options and screening parameters. Moreover, a key functionality of CTLPScanner is that besides the private data processing module, it contains more than 50 000 pre-processed public oncogenomic arrays, mainly derived from The Cancer Genome Atlas (TCGA) (26) and NCBI Gene Expression Omnibus (GEO) (27) (Table 1). Since the incidence of chromothripsis is relatively low in most cancer types (probably occurs in only 2–3% of all cancers) (1,9,24,28), public databases are invaluable resources for identifying this phenomenon. The web server allows users to easily explore the chromothripsis-like genome patterns with array accession IDs and self-defined constraints. CTLPScanner provides a platform to perform large-scale genomic data analysis to unravel the molecular mechanisms underlying chromothripsis phenomenon.
Table 1. Summary of public array data integrated in CTLPScanner.
| Data type | The cancer genome atlas | Gene expression omnibus |
|---|---|---|
| Series | 33 | 457 |
| Arrays | 22 419 | 29 334 |
| Cancer types | 33 | 159 |
| Platforms | 1 | 223 |
| Publications | na | 352 |
na, not available.
WEB SERVER CONSTRUCTION
CTLPScanner consists of two main modules: (i) detection and visualization of chromothripsis, and annotation of the detected chromosome pulverization region and the associated genes; (ii) public array data exploration. The web interface was developed using HTML, PHP and JavaScript. The back-end statistical calculations and data visualization were implemented using R and Perl programming language.
Detection and annotation of chromothripsis
The most striking and array detectable features of chromothripsis are the clustering of chromosomal breakpoints and oscillating copy number changes in a relatively small region (1,4). CTLPScanner implements a scan-statistic based algorithm to identify the clustering of copy number status changes, as described in our previous work (10). Based on the segmented genomic array data, a fixed-size window is moved along the genome, and for each window the likelihood ratio is computed by counting the observed and expected copy number status change times. The algorithm finds the region that is most likely to be a cluster. Due to lack of prior knowledge about the chromosomal pulverization size, a set of sliding windows with different sizes are applied to screen the genome. The window that maximizes the likelihood ratio defines the most probable chromothripsis region. In this way, our algorithm is able to detect both the location and the size of chromosome fragmentation region. The visualization of called copy number aberrations (CNAs) and detected chromothripsis regions was carried out using our in-house developed R-based software. The information used for the annotation of chromosome shattering regions was collected from multiple sources. The gene symbols and location information were downloaded from Ensembl (genome assembly versions from hg16 to hg38) (29). The cancer associated genes were collected from the Catalogue of Somatic Mutations in Cancer (COSMIC) database (30).
Public data collection and curation
The public data exploration module of CTLPScanner gives users the ability to interactively explore over 50 000 pre-processed genomic array data. We collected these data from two of the most comprehensive public array repositories: TCGA (26) and NCBI GEO (27). For TCGA datasets, segmentation data (level 3) of single nucleotide polymorphism (SNP) arrays were downloaded from the TCGA data portal. In total, we collected 22 419 tumor samples, which were classified into 33 cancer types. The cancer type and array information of TCGA datasets integrated in CTLPScanner are given in Supplementary Table S1 and 2, respectively. For arrays in GEO, we collected re-analyzed array data from arrayMap database (31), which is a curated reference database providing copy number profiling data in human cancer. All the data collected from arrayMap were human malignancies analyzed by genomic array platforms. We downloaded segmentation data of about 30 000 tumor samples in 159 cancer types. Detailed information of the GEO datasets is provided in Supplementary Table S3. The arrayMap database implemented a pipeline for raw array data processing. For array comparative genomic hybridization (aCGH) arrays, in-house scripts were used to process the raw probe signal intensity. For Affymetrix SNP arrays, a re-analysis of raw CEL files was performed by the R package aroma.affymetrix with the CRMAv.2 method (32). The genome positions of probes were mapped to the human reference genome assembly hg18. All the copy number data were segmented by the circular binary segmentation (CBS) algorithm (33).
UTILITY AND WEB INTERFACE
Preparation of input files
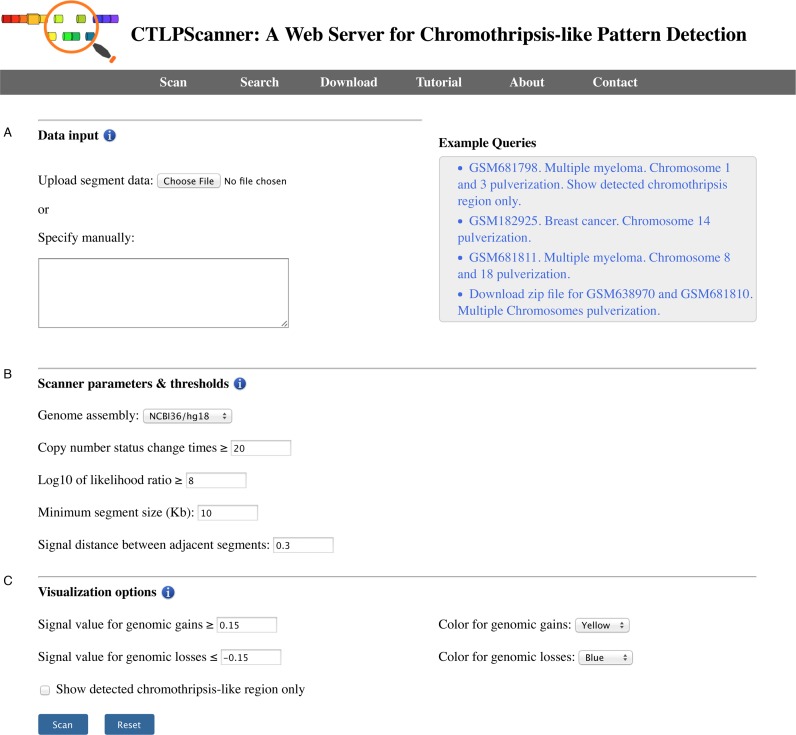
The screening of chromothripsis event is carried out based on DNA copy number segmentation data, which can be generated by aCGH or SNP array platforms. The minimum required data contents include the sample name, chromosome number, start and stop positions of each segment and log normalized signal intensity. The input file should be tab-delimited with an optional first line identifying the columns. All required data fields can be generated by using CBS algorithm (33). The server allows users to ‘copy and paste’ segmentation data into the web interface directly or upload a compressed file (.zip) for batch processing (Figure 1A). If the uploaded zip archive file contains multiple sample files, it will be automatically decompressed and all samples will be processed. The file names will be used as sample identifiers in the results page.
Figure 1.
The snapshot of CTLPScanner start page. (A) The user can input genomic segmentation data directly or upload a compressed file for batch processing. (B) The thresholds and parameters for screening chromothripsis event. (C) The parameters for copy number aberration calling and data visualization. The checkbox switches between a simplified and a more detailed representation of the results.
Parameters and thresholds
CTLPScanner offers a set of parameters for the effective detection of chromothripsis. The web server provides optimized default values for all parameters, which may also be adjusted for customized screening (Figure 1B). To start the detection process, users need to select the genome build corresponding to the input data. Currently, the server supports multiple versions of the genome assembly (from hg16 to hg38). Following this, users have an option to set the thresholds for copy number status change times and likelihood ratio score, which represent the strength of the evidence for the detected chromothripsis event. In addition, several parameters can be tuned by users to process data derived from different array platforms. Users may also specify the parameters for CNA calling and results visualization (Figure 1C). Once all the parameters are set, users can click the Scan button to begin the screening. In most cases, it takes several tens of seconds to process a single sample. The textual processing progress bar indicates the completed samples and the total sample number.
Result visualization and annotation
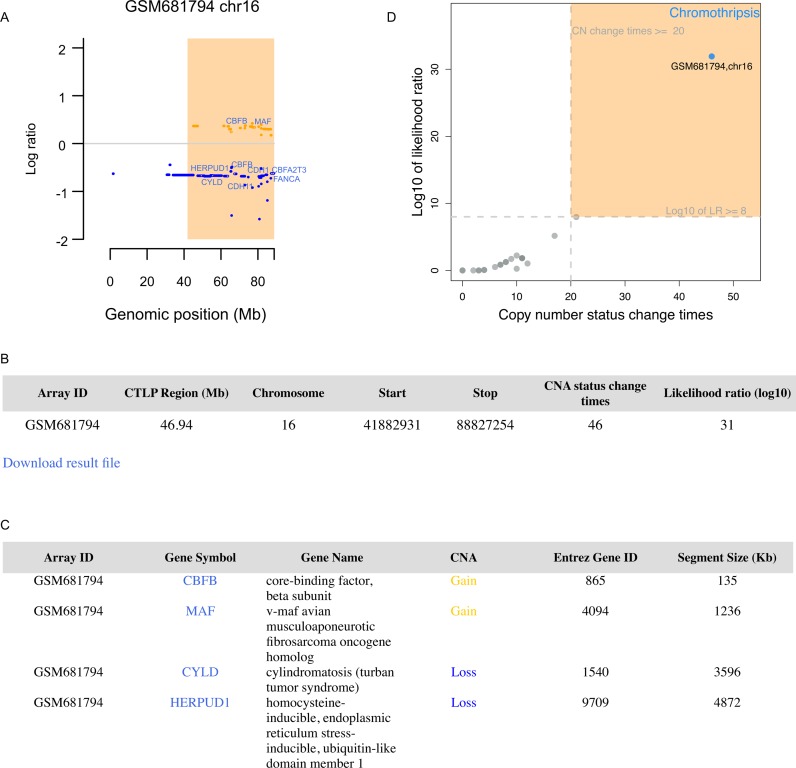
Once a task is completed, the results page will show the parameters and thresholds for the detection of chromothripsis and display genomic CNA profile of each sample. If a chromosome shattering event is detected, the chromothripsis region will be highlighted with a light orange rectangle (Figure 2A). To facilitate identification of chromothripsis specific genes, the COSMIC database (30) annotated cancer genes located in chromothripsis regions will be presented in the plot. Users can click on gene symbols or CNA segments for detailed information. Users can also zoom in specific genomic regions by inputting the start and stop locations. Moreover, recent studies revealed a potential link between chromothripsis and telomere dysfunction (15,34–36). The patterns and distribution of copy number state switches indicate the emergence and temporal order of the major genomic rearrangement events (15,34). The web server provides a histogram of copy number segment switches to facilitate the identification of chromothripsis event arising in conjunction with telomere crises. The detailed information and related features of the detected chromothripsis region will be summarized in a table and can be downloaded as a flat file (Figure 2B). All the cancer genes located in chromothripsis regions will be shown in a table, along with mutation types and links to more detailed information (Figure 2C). The ‘segment size’ data item provides the information about whether the mutation is driven by a focal copy number alteration or a large chromosomal rearrangement. All the genes involved in chromothripsis regions will be listed in a flat file, which allows users to perform further in-depth analysis. Furthermore, CTLPScanner creates a scatter plot to compare the two decision parameters, i.e. copy number status change times and likelihood ratio (Figure 2D). Each point in the plot represents a chromosome, and the candidates falling in the upper right area are inferred chromothripsis cases. The closer the point is to the top-right corner, the more accurately the algorithm can distinguish chromothripsis from non-chromothripsis cases.
Figure 2.
The CTLPScanner output. (A) An example of detected chromothripsis event in chromosome 16. The light orange rectangle represents the chromosomal pulverization region. Yellow and blue lines represent genomic gains and losses respectively. The cancer genes located in chromothripsis region are shown. (B) The tabular representation gives an overview of identified chromothripsis region. (C) An example of COSMIC database annotated cancer genes that are located in chromothripsis region. (D) The scatter plot shows the likelihood ratio and copy number status change times of each chromosome. The user-defined thresholds are indicated with dashed lines. Each point represents a chromosome, and the point falling in the upper right area indicates the chromothripsis event.
Public data exploration
The public data exploration module allows users to explore potential chromothripsis events from public data sources with self-defined constraints. CTLPScanner integrates more than 50 000 pre-processed genomic arrays, and all the data were converted into segmentation data format. The server allows users to search either by a single array or by a data series. For search by array, users only need to specify the array accession ID to initiate the screening process. For search by series, users can select tumor type in the selection box, which contains over 40 different cancer types as classified by tumor loci. The content of the batch accession list in the next selection box is dynamically determined by user's selection of cancer type. The entire list of array and series accession IDs can be downloaded from the web interface. The public data exploration interface provides all the required parameters for chromothripsis detection and visualization. The results page will display all detected chromothripsis samples and regions, and provide more detailed information, including links to the related publication and raw datasets. Recently, hyperploidy is identified as a risk factor for chromothripsis (34). The genomic instability in the context of hyperploidy may promote chromothripsis. To facilitate the identification of chromothripsis in hyperploidy samples, the data exploration module provides the B allele frequency plot for SNP arrays. All the chromothripsis data and the corresponding involved genes can be downloaded for further study. The scan parameters and thresholds will be integrated into the downloadable result file.
DISCUSSION
Identification and characterization of chromosome shattering is a key step toward a better understanding of molecular mechanisms underlying chromothripsis phenomenon. CTLPScanner is an intuitive and user-friendly interface that provides chromothripsis screening, visualization and annotation in a single step without requiring any additional manual processes. The original chromothripsis detection algorithm was published in our previous work (10). In this study, we have implemented this algorithm as a web server and added several new modules. Firstly, the annotation of detected chromothripsis region is provided, which allows users to investigate affected genes in detail. The most obvious mechanism by which chromothripsis can drive tumorigenesis is by activating oncogenes or interrupting tumor suppressor gene functions (6,37). The large number of chromosome rearrangements associated with chromothripsis also reflects that it may have a high frequency of generating oncogenic gene fusions (38). The annotation of chromothripsis region facilitates the discovery of mutated genes that contributed to the rapid karyotype evolution. Secondly, we enhanced our data visualization and presentation capability. The web server interface provides multiple plotting options and CNA calling thresholds for more flexible data visualization. Thus, users can easily interpret the results at a glance and export the required data. Thirdly, we integrated a large number of pre-processed array datasets into our web server. These data were assembled from the most comprehensive and significant public repositories of microarray data. This module allows users to interactively explore extremely large-scale datasets by providing array or series accession identifiers.
Currently, besides CTLPScanner, there is another tool named ShatterProof (25), which was developed to detect chromothripsis event in a straightforward manner. It quantifies the criteria for identifying chromothripsis and produces a description of the evaluation metrics used. However, the two algorithms are different in several aspects. ShatterProof is designed to process NGS data, whereas CTLPScanner focuses on array data analysis. While NGS technologies are now widely used for identifying mutations, genomic array platforms are commonly employed for detecting CNAs. The advantages of microarrays compared to NGS technology include lower costs, faster turnaround time and lower computational complexity. Given the low incidence of chromothripsis observed to date, the large number of public arrays integrated in CTLPScanner is especially valuable for metadata analysis. In addition, ShatterProof is implemented as a Perl module, while CTLPScanner is available as a web server. Although both tools allow bioinformaticians to perform automated screening of large-scale datasets, CTLPScanner can be easily used by researchers without any programming skills.
There are some limitations of CTLPScanner. The strict definition of chromothripsis includes six criteria (22). However, only a subset of the criteria can be tested based on microarray data, and this may produce false positive and false negative results in chromothripsis detection. For example, the orientation of breakpoint junctions can only be obtained using a sequencing approach. Thus, chromothripsis with copy number balanced profile, which is often observed in congenital disorders (39), are likely to be missed. Furthermore, recent studies revealed that the combination of chromothripsis and breakage-fusion-bridge (BFB) cycles may generate very complex genomic aberration patterns (15,34). In this case, it is difficult to distinguish chromothripsis events from other forms of complex chromosomal rearrangements.
Recent advances in chromothripsis research have attracted much attention and increased our understanding of molecular mechanisms involved in the catastrophic DNA rearrangement process. Chromothripsis event identification is gradually becoming a routine part of cancer genome research. CTLPScanner will help reveal more general features of this new paradigm in tumorigenesis, and thus eventually improving cancer classification. We will continue to improve our web server and provide raw array data submission interface in the future. Furthermore, as the costs of sequencing continue to fall, we expect that NGS will become increasingly prevalent in the field of cancer research and many more tumor samples will be sequenced. We will extend the web server to support NGS data processing.
Supplementary Material
Acknowledgments
We would like to thank Yang Cao, Michael Baudis and Mark D. Robinson for their valuable comments and help in manuscript writing.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Natural Science Foundation of China [31571314, 81402496]. Funding for open access charge: National Natural Science Foundation of China [31571314].
Conflict of interest statement. None declared.
REFERENCES
- 1.Stephens P.J., Greenman C.D., Fu B., Yang F., Bignell G.R., Mudie L.J., Pleasance E.D., Lau K.W., Beare D., Stebbings L.A., et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu P., Erez A., Nagamani S.C., Dhar S.U., Kolodziejska K.E., Dharmadhikari A.V., Cooper M.L., Wiszniewska J., Zhang F., Withers M.A., et al. Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell. 2011;146:889–903. doi: 10.1016/j.cell.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kloosterman W.P., Tavakoli-Yaraki M., van Roosmalen M.J., van Binsbergen E., Renkens I., Duran K., Ballarati L., Vergult S., Giardino D., Hansson K., et al. Constitutional chromothripsis rearrangements involve clustered double-stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep. 2012;1:648–655. doi: 10.1016/j.celrep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Rausch T., Jones D.T., Zapatka M., Stutz A.M., Zichner T., Weischenfeldt J., Jager N., Remke M., Shih D., Northcott P.A., et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. 2012;148:59–71. doi: 10.1016/j.cell.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloosterman W.P., Hoogstraat M., Paling O., Tavakoli-Yaraki M., Renkens I., Vermaat J.S., van Roosmalen M.J., van Lieshout S., Nijman I.J., Roessingh W., et al. Chromothripsis is a common mechanism driving genomic rearrangements in primary and metastatic colorectal cancer. Genome Biol. 2011;12:R103. doi: 10.1186/gb-2011-12-10-r103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forment J.V., Kaidi A., Jackson S.P. Chromothripsis and cancer: causes and consequences of chromosome shattering. Nat. Rev. Cancer. 2012;12:663–670. doi: 10.1038/nrc3352. [DOI] [PubMed] [Google Scholar]
- 7.Molenaar J.J., Koster J., Zwijnenburg D.A., van Sluis P., Valentijn L.J., van der Ploeg I., Hamdi M., van Nes J., Westerman B.A., van Arkel J., et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 8.Kim T.M., Xi R., Luquette L.J., Park R.W., Johnson M.D., Park P.J. Functional genomic analysis of chromosomal aberrations in a compendium of 8000 cancer genomes. Genome Res. 2013;23:217–227. doi: 10.1101/gr.140301.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zack T.I., Schumacher S.E., Carter S.L., Cherniack A.D., Saksena G., Tabak B., Lawrence M.S., Zhsng C.Z., Wala J., Mermel C.H., et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013;45:1134–1140. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai H., Kumar N., Bagheri H.C., von Mering C., Robinson M.D., Baudis M. Chromothripsis-like patterns are recurring but heterogeneously distributed features in a survey of 22,347 cancer genome screens. BMC Genomics. 2014;15:82. doi: 10.1186/1471-2164-15-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nones K., Waddell N., Wayte N., Patch A.M., Bailey P., Newell F., Holmes O., Fink J.L., Quinn M.C., Tang Y.H., et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat. Commun. 2014;5:5224. doi: 10.1038/ncomms6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crasta K., Ganem N.J., Dagher R., Lantermann A.B., Ivanova E.V., Pan Y., Nezi L., Protopopov A., Chowdhury D., Pellman D. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53–58. doi: 10.1038/nature10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C.Z., Spektor A., Cornils H., Francis J.M., Jackson E.K., Liu S., Meyerson M., Pellman D. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorzano C.O., Pascual-Montano A., Sanchez de Diego A., Martinez A.C., van Wely K.H. Chromothripsis: breakage-fusion-bridge over and over again. Cell Cycle. 2013;12:2016–2023. doi: 10.4161/cc.25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Schwab C., Ryan S.L., Papaemmanuil E., Robinson H.M., Jacobs P., Moorman A.V., Dyer S., Borrow J., Griffiths M., et al. Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature. 2014;508:98–102. doi: 10.1038/nature13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garsed D.W., Marshall O.J., Corbin V.D., Hsu A., Di Stefano L., Schroder J., Li J., Feng Z.P., Kim B.W., Kowarsky M. The architecture and evolution of cancer neochromosomes. Cancer Cell. 2014;26:653–667. doi: 10.1016/j.ccell.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Meyerson M., Pellman D. Cancer genomes evolve by pulverizing single chromosomes. Cell. 2011;144:9–10. doi: 10.1016/j.cell.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Tubio J.M., Estivill X. Cancer: when catastrophe strikes a cell. Nature. 2011;470:476–477. doi: 10.1038/470476a. [DOI] [PubMed] [Google Scholar]
- 19.Ichim G., Lopez J., Ahmed S.U., Muthalagu N., Giampazolias E., Delgado M.E., Haller M., Riley J.S., Mason S.M., Athineos D., et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell. 2015;57:860–872. doi: 10.1016/j.molcel.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morishita M., Muramatsu T., Suto Y., Hirai M., Konishi T., Hayashi S., Shigemizu D., Tsunoda T., Moriyama K., Inazawa J. Chromothripsis-like chromosomal rearrangements induced by ionizing radiation using proton microbeam irradiation system. Oncotarget. 2016;7:10182–10192. doi: 10.18632/oncotarget.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maciejowski J., Li Y., Bosco N., Campbell P.J., de Lange T. Chromothripsis and kataegis induced by telomere crisis. Cell. 2015;163:1641–1654. doi: 10.1016/j.cell.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korbel J.O., Campbell P.J. Criteria for inference of chromothripsis in cancer genomes. Cell. 2013;152:1226–1236. doi: 10.1016/j.cell.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Kinsella M., Patel A., Bafna V. The elusive evidence for chromothripsis. Nucleic Acids Res. 2014;42:8231–8242. doi: 10.1093/nar/gku525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra A., Lindberg M., Faust G.G., Leibowitz M.L., Clark R.A., Layer R.M., Quinlan A.R., Hall I.M. Breakpoint profiling of 64 cancer genomes reveals numerous complex rearrangements spawned by homology-independent mechanisms. Genome Res. 2013;23:762–776. doi: 10.1101/gr.143677.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govind S.K., Zia A., Hennings-Yeomans P.H., Watson J.D., Fraser M., Anghel C., Wyatt A.W., van der Kwast T., Collins C.C., McPherson J.D., et al. ShatterProof: operational detection and quantification of chromothripsis. BMC Bioinformatics. 2014;15:78. doi: 10.1186/1471-2105-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein J.N., Collisson E.A., Mills G.B., Shaw K.R., Ozenberger B.A., Ellrott K., Shmulevich I., Sander C., Stuart J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M., et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magrangeas F., Avet-Loiseau H., Munshi N.C., Minvielle S. Chromothripsis identifies a rare and aggressive entity among newly diagnosed multiple myeloma patients. Blood. 2011;118:675–678. doi: 10.1182/blood-2011-03-344069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yates A., Akanni W., Amode M.R., Barrell D., Billis K., Carvalho-Silva D., Cummins C., Clapham P., Fitzgerald S., Gil L., et al. Ensembl 2016. Nucleic Acids Res. 2016;44:D710–D716. doi: 10.1093/nar/gkv1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes S.A., Beare D., Gunasekaran P., Leung K., Bindal N., Boutselakis H., Ding M., Bamford S., Cole C., Ward S., et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H., Gupta S., Rath P., Ai N., Baudis M. arrayMap 2014: an updated cancer genome resource. Nucleic Acids Res. 2015;43:D825–D830. doi: 10.1093/nar/gku1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bengtsson H., Wirapati P., Speed T.P. A single-array preprocessing method for estimating full-resolution raw copy numbers from all Affymetrix genotyping arrays including GenomeWideSNP 5 & 6. Bioinformatics. 2009;25:2149–2156. doi: 10.1093/bioinformatics/btp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olshen A.B., Venkatraman E.S., Lucito R., Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5:557–572. doi: 10.1093/biostatistics/kxh008. [DOI] [PubMed] [Google Scholar]
- 34.Mardin B.R., Drainas A.P., Waszak S.M., Weischenfeldt J., Isokane M., Stutz A.M., Raeder B., Efthymiopoulos T., Buccitelli C., Segura-Wang M., et al. A cell-based model system links chromothripsis with hyperploidy. Mol. Syst. Biol. 2015;11:828. doi: 10.15252/msb.20156505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernst A., Jones D.T., Maass K.K., Rode A., Deeg K.I., Jebaraj B.M., Korshunov A., Hovestadt V., Tainsky M.A., Pajtler K.W., et al. Telomere dysfunction and chromothripsis. Int. J. Cancer. 2016;138:2905–2914. doi: 10.1002/ijc.30033. [DOI] [PubMed] [Google Scholar]
- 36.Harrison C.J. Blood Spotlight on iAMP21 acute lymphoblastic leukemia (ALL), a high-risk pediatric disease. Blood. 2015;125:1383–1386. doi: 10.1182/blood-2014-08-569228. [DOI] [PubMed] [Google Scholar]
- 37.Leibowitz M.L., Zhang C.Z., Pellman D. Chromothripsis: a new mechanism for rapid karyotype evolution. Annu. Rev. Genet. 2015;49:183–211. doi: 10.1146/annurev-genet-120213-092228. [DOI] [PubMed] [Google Scholar]
- 38.Kloosterman W.P., Koster J., Molenaar J.J. Prevalence and clinical implications of chromothripsis in cancer genomes. Curr. Opin. Oncol. 2014;26:64–72. doi: 10.1097/CCO.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 39.Kloosterman W.P., Guryev V., van Roosmalen M., Duran K.J., de Bruijn E., Bakker S.C., Letteboer T., van Nesselrooij B., Hochstenbach R., Poot M., et al. Chromothripsis as a mechanism driving complex de novo structural rearrangements in the germline. Hum. Mol. Genet. 2011;20:1916–1924. doi: 10.1093/hmg/ddr073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.