Abstract
Objective
Enhanced serum and glucocorticoid-inducible kinase 1 (SGK1) activity contributes to the pathogenesis of vascular disease. We evaluated SGK1 modulation in vascular smooth muscle cells by the adipokine, resistin and in aortic tissue in a murine model of diet-induced obesity (DIO).
Methods
Modulation of SGK1 by resistin was assessed in human aortic smooth muscle cells (HAoSMC) in vitro by quantitative RT-PCR and western blot analyses. To induce the lean or obese phenotype, mice were fed a 10 kcal% low-fat or 60 kcal% high-fat diet, respectively for eight weeks. Upon study completion, plasma resistin was assessed and aortic tissue was harvested to examine the effect of DIO on regulation of SGK1 in vivo.
Results
Resistin increased SGK1 mRNA, total protein abundance and its activation as determined by phosphorylation of its serine 422 residue (pSGK1) in HAoSMC. Resistin-mediated SGK1 phosphorylation was dependent upon phosphatidylinositol-3-kinase and Toll-like receptor 4. Furthermore, inhibition of SGK1 attenuated resistin-induced proliferation in HAoSMC. DIO led to upregulation of total SGK1 protein levels and pSGK1 in association with increased plasma resistin.
Conclusions
These data suggest that high levels of resistin observed during obesity may activate SGK1 in the vasculature and contribute to the development of obesity-related vascular disease.
Keywords: Resistin, SGK1, TLR4, vascular smooth muscle cell, obesity
Introduction
Obesity is a growing global epidemic in which excess body fat expansion is associated with adverse cardiovascular health complications such as insulin resistance, diabetes, atherosclerosis, hypertension and stroke (1). Studies have highlighted that the basis for these clinical sequelae is vascular disease. However, the mechanisms underlying obesity-related vascular dysfunction have not been fully defined.
Serum and glucocorticoid-inducible kinase 1, (SGK1) is a serine/threonine protein kinase that participates in a wide variety of physiological functions including regulation of metabolism, cell proliferation, apoptosis, and inflammation (2). This kinase is activated by both transcriptional and post-translational mechanisms. For instance, SGK1 mRNA can be up-regulated by various stimuli including serum, glucocorticoids, insulin, and glucose (3). Moreover, SGK1 is activated by phosphatidylinositol-3-kinase-(PI3K) and mammalian target of rapamycin complex C2-dependent phosphorylation of serine 422 (pS422) and threonine 256 (4;5). Multiple lines of evidence point to a role for SGK1 in the pathogenesis of vascular disease. Several studies established a critical role for SGK1 in vascular remodeling in response to vein grafts and in pulmonary hypertension (6). Accordingly, our lab defined a molecular role for elevated SGK1 activity in growth factor-dependent stimulation of aortic smooth muscle cell (SMC) proliferation, an important event in the pathogenesis of vascular dysfunction (7). Other studies implicated a role for SGK1 in the pathophysiology of obesity-associated disorders such atherosclerosis, hypertension, and diabetes; all hallmarks of metabolic syndrome associated with enhanced cardiovascular disease morbidity and mortality(8). In this regard, a gain of function polymorphism in intron 6 [I6CC] and exon 8 [E8CC/CT] of the SGK1 gene shows a strong positive correlation with increased body mass index, elevated blood pressure, and type 2 diabetes (2;9). Of note, a recent study demonstrated that SGK1 was important for the development of vascular inflammation and atherogenesis in ApoE/SGK1 double knockout mice (10). In addition, SGK1 participates in activation of the pro-inflammatory transcription factor, NFκB (11). Thus, SGK1 may contribute to obesity-induced vascular dysfunction. However, even though expression of SGK1 has been shown to be elevated during obesity in adipose tissue and kidneys; (12–14) its regulation in the vasculature during obesity is unknown.
Derangement in the secretion of adipokines from adipocytes and macrophages is reported to influence the expression of different genes and contribute to obesity-related organ dysfunction. Plasma levels of the pro-inflammatory adipokine, resistin is elevated upon high fat feeding in rodents and in people with obesity (15–17). Moreover, hyperresistinemia is linked to obesity-related insulin resistance and diabetes (15). Like SGK1, resistin plays a permissive role in the uncontrolled proliferation of vascular smooth muscle cells (VSMC), a hallmark of vascular remodeling (18). High plasma resistin concentration is implicated in clinical and experimental models of cardiovascular diseases. A positive correlation between plasma resistin and the severity of coronary artery disease was observed in a recent clinical study (19). Further, overexpression of resistin was observed to increase atherosclerotic lesion progression in the ldlr knockout mice (20). In addition, resistin is markedly expressed and participates in the inflammatory response in human atherosclerotic lesions. Toll-like receptor 4 (TLR4) is shown to mediate the effects of resistin in VSMC and other cell types (21–25). Moreover, resistin interacts physically with TLR4, suggesting that it is indeed a bona fide receptor for resistin. Resistin and SGK1 regulate similar functions in the vasculature; however, to date, a biological interaction between resistin and SGK1 in the vasculature has not been defined.
Increased understanding of the molecular mechanisms that contribute to obesity-associated vascular disorders is a key prerequisite to the identification of novel therapeutic targets to treat or prevent the clinical manifestations of diabetes, atherosclerosis or myocardial infarctions. Thus, in this study, we examined the effects of diet-induced obesity and resistin on regulation of SGK1 in the vasculature in vivo and in VSMC in vitro. Our results suggest that high levels of resistin may lead to activation of SGK1 in the vasculature and point to inhibition of resistin/SGK1 signaling as a potential therapeutic target for obesity-associated vascular dysfunction.
Methods and Methods
Animal Studies
All experimental procedures of our animals were approved by the Institutional Animal Care and Use Committee at Morehouse School of Medicine and were performed in accordance with the Committee’s Guidelines and Regulations for Animal Care. Male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and acclimated to their new environment for 3 days before experimentation. At eight weeks of age, mice were randomized to a 10%kcal (Research Diets No. D12450J) or 60%kcal (Research Diet No. D12492) fat diet for eight weeks ad libitum. Body weight and food intake were measured weekly. At the end of the study, the mice were euthanized and plasma resistin was analyzed with a commercially available resistin ELISA kit (Abcam). Aortic tissue was collected and immediately frozen in liquid nitrogen to later assess phosphorylated and total SGK1 via immunoblotting.
Human Aortic Smooth Muscle (HAoSMC) Cell Culture
HAoSMC purchased from Lonza were cultured in basal medium supplemented with growth factors (Cell Applications, Inc.) at 37°C and 5% CO2. Cells were used at passages 5–8 and serum-starved using OPTI-MEM (Gibco) for 48h prior to stimulation with vehicle or human recombinant resistin (BioVision).
Resistin, Inhibitor Compounds, and Cell Treatments
To examine the dose and temporal response relationship of SGK1 to resistin 70–80% confluent HAoSMc were serum-starved in Opti-MEM for 48h. For all dose response studies, cells were then stimulated with increasing doses (0–100ng/ml) of human recombinant resistin for 1h. The dose of resistin that produced the maximal change in SGK1 expression was then used in subsequent temporal studies (0–24h). To examine the involvement of the PI3K pathway in resistin-mediated effects, HAoSMC were pre-treated with 50µM LY294002, the PI3K inhibitor (Enzo) or vehicle for 1h and then for 3h in the presence of 40ng/ml resistin.
Cell Proliferation Assay
A colorimetric Quick Cell Proliferation Assay (Abcam) based on the activity of mitochondrial dehydrogenase as a measurement of viable cells, was used to examine a role for SGK1 in resistin-induced HAoSMC proliferation. Briefly, 0.5 × 104 HAoSMC were cultured in a flat bottom 96-well plate. Following 24h serum-starvation in Opti-MEM HAoSMC were pre-treated for 1h with varying doses (0–10µM) of the SGK1 inhibitor, GSK650394 (Tocris). Cells were then stimulated with vehicle or 40ng/ml human resistin in the presence of GSK650394 (0–10µM) for 48h. Color development was performed according to manufacturer’s instructions. Signal detection was performed on a SpectraMax (Roche) plate reader.
Transfection of HAoSMC
To determine whether resistin mediates its effects via the TLR4, HAoSMC were transfected with appropriate scramble or siRNA oligonucleotide, using the electroporator Nucleofection device (Amaxa) according to the manufacturer’s protocols. Briefly, 0.75 × 106 cells were re-suspended in nucleofector solution containing 75nM non-specific scrambled sequence or TLR4 siRNA (Dharmacon) and nucleofected using program U-025. After 16h, fresh growth medium was added to the cells. The next day cells were serum-starved for 24h then treated with vehicle or resistin (40ng/ml) for 3h.
Quantitative RT-PCR
To assess the effects of resistin on SGK1 mRNA expression, total RNA was isolated from HAoSMC stimulated with vehicle or resistin as described above using an RNeasy mini kit (Qiagen) according to the manufacturer’s protocol. cDNA was generated by reverse transcription from mRNA using the iScript cDNA synthesis kit (BioRad). RT-PCR was performed using the 480 Roche Light Cycler. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal loading control to account for variations in mRNA loading. SGK1 mRNA expression was normalized to GAPDH.
Immunoblotting
Western blotting was performed on protein from aortic tissue lysed in T-PER Mammalian Protein Tissue Extraction Reagent (Thermo Scientific) supplemented with Halt protease inhibitor cocktail (Pierce, Inc) containing 500µM phenylmethylsulfonyl fluoride, 5µg/ml each of leupeptin, aprotinin, and pepstatin, 2mM sodium orthovanadate, 50mM NaF and 10mM β-glycerophosphonate. Protein concentration was determined using Bradford Assay (Bio-Rad). Equal amounts of total protein (18–25 µg) in 1× laemmli loading buffer with β-mercaptoethanol were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. PVDF membranes were blocked using 5% milk in Tris-buffered saline with 1% Tween 20 (TBS-T) for 1h. Membranes were incubated overnight at 4 °C using the following primary antibodies: phosphoSGK-1(S422) (Santa Cruz), total SGK-1 (Abcam). After washing, the PVDF membrane was incubated with appropriate secondary HRP-conjugated anti-rabbit antibodies (Santa Cruz) for 1h and the immune complexes were visualized using enhanced chemiluminescence according to manufacturer’s protocol (Pierce, Inc). To verify equal loading, PVDF membrane were stripped with Restore Stripping Buffer (Thermo Scientific) and reprobed with β-actin. Signal was quantified by densitometry using the NIH software Image J. Normalization of phosphorylated SGK1 was determined by its total protein levels.
Statistical Analysis
Data are presented as the mean of three independent experiments ± the standard error of the mean (SEM). Statistical analysis was evaluated by Student’s t test for comparison between two groups, or One-Way or Two-Way analysis of variance (ANOVA) followed by the appropriate post hoc test for multiple comparisons. For body weight analysis, Two-way ANOVA Repeated Measures with appropriate post hoc test was used. Statistical significance was set at a probability value p< 0.05.
Results
Resistin increases SGK1 mRNA and protein expression in HAoSMC
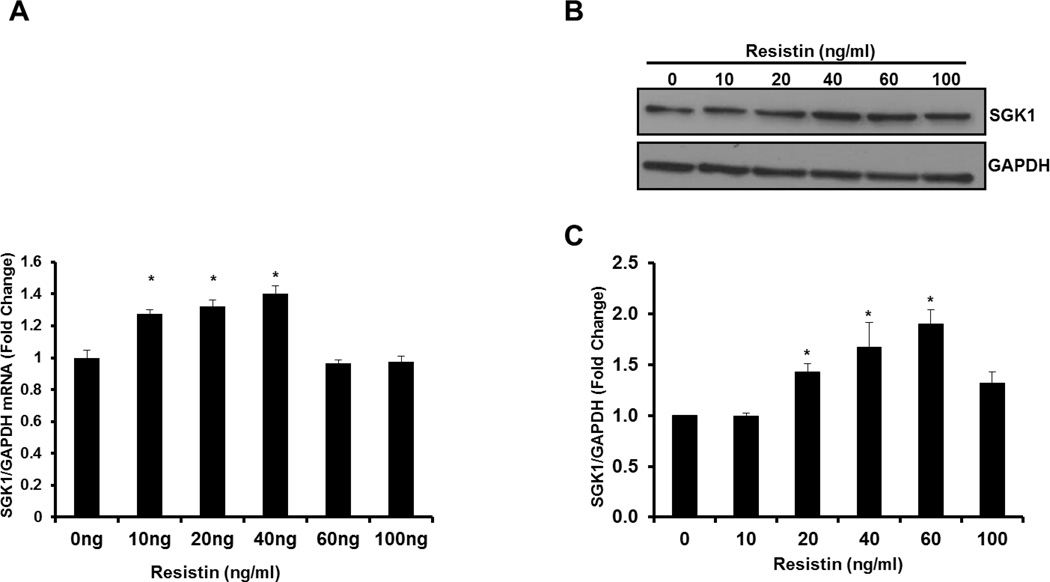
To determine whether resistin treatment can increase SGK1 mRNA levels in SMC in vitro, HAoSMC were treated with increasing concentrations of recombinant resistin (0–100ng/ml) for 1h and SGK1 mRNA expression was examined by quantitative RT-PCR. We found that resistin treatment significantly increased SGK1 mRNA expression at 10, 20 and 40ng/ml with a maximal increase of approximately 40% at the 40ng/ml dose (Figure 1A). Next, we examined whether SGK1 protein levels were also increased in HAoSMC stimulated with resistin as described above. Our results indicated that increasing doses of resistin significantly up-regulated SGK1 protein levels up to 80%. Significant up-regulation of SGK1 protein was observed at a dose of 20–60ng/ml (Figure 1B). Based on these results subsequent resistin stimulation experiments were conducted at a dose of 40ng/ml.
Figure 1. Resistin-mediated increase in SGK1 expression is dose-dependent.
A: mRNA. HAoSMC were serum-starved for 48h and stimulated with increasing doses of resistin (0–100ng/ml) for 1h and SGK1 mRNA was quantified by qRT-PCR. n=3. B: Representative western blot image of protein extracted from HAoSMC serum-starved for 48h and stimulated with increasing doses of resistin (0–100ng/ml) for 1h C: SGK1 protein levels were normalized to GAPDH and quantified using NIH Image J. Data were analyzed by One-Way ANOVA followed by appropriate post hoc test for multiple comparisons and expressed as ± SEM, *P<0.05 vs 0ng/ml control. (n=3 independent studies).
Resistin enhances phosphosphorylation of SGK1 at S422
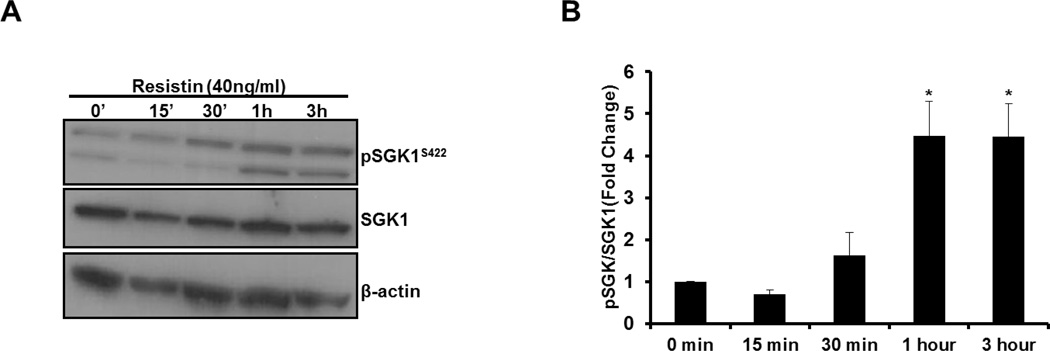
SGK1 kinase activity can be activated by phosphorylation of serine 422 (Ser422) within its C-terminal hydrophobic motif (2). Therefore, to determine whether resistin can activate SGK1, we examined Ser422 phosphorylation of SGK1 in HAoSMC treated with resistin for 0–3h by Western blot using phospho-specific antibodies for Ser422. As observed in Figure 2A–B, resistin treatment significantly increased net Ser422 phosphorylation by more than 350% with peak phosphorylation occurring within 1h of resistin stimulation. Thus, these results indicate that in addition to increasing SGK1 mRNA and protein levels, resistin stimulates activation of SGK1 in HAoSMC.
Figure 2. Resistin increases the phosphorylation of SGK1 at Ser422.
A: Representative western blot image of protein extracted from HAoSMC serum-starved for 48h and stimulated with 40ng/ml of resistin for the indicated times. B: pSGK1 protein levels were normalized to total SGK1 and quantified using NIH Image J. Data were analyzed by One-Way ANOVA followed by appropriate post hoc test for multiple comparisons and expressed as ± SEM, *P< 0.0005 vs 0min control. (n=4 independent studies).
Resistin-mediated SGK1 phosphorylation is PI3K-dependent
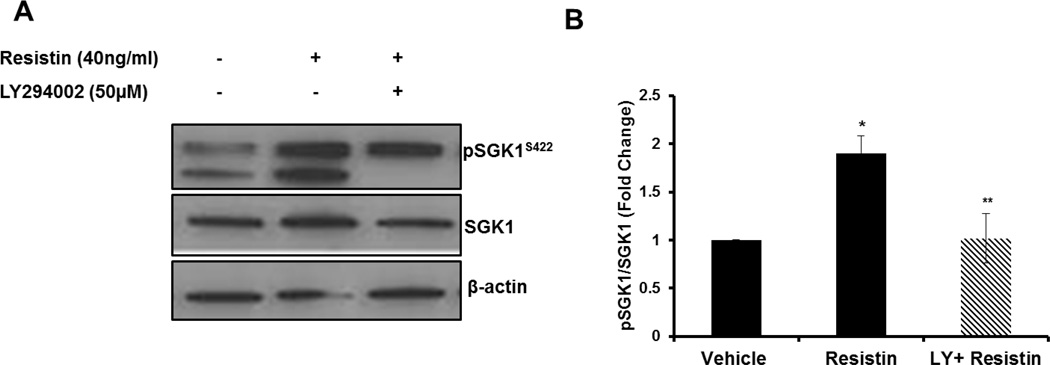
Next, we explored the downstream signaling pathway that participates in resistin-mediated activation of SGK1. Resistin stimulation of VSMC leads to activation of the PI3K pathway (18), therefore, we examined S422 phosphorylation in HAoSMC stimulated with resistin in the presence or absence of the PI3K inhibitor, LY294002. PI3K inhibition with LY294002 completely and significantly abolished resistin-mediated up-regulation of SGK1 phosphorylation (Figure 3A–B).
Figure 3. Resistin-mediated SGK1 phosphorylation is PI3K-dependent.
A: HAoSMC were pre-treated with 50µM LY294002 or vehicle for 1h. Media was replaced with fresh LY294002 and incubated in the presence or absence of 40ng/ml resistin for 3h. SGK1 phosphorylation at Ser422 was examined by western blot. B: phospho-SGK1 protein levels were normalized to total SGK1 and quantified using NIH Image J. Data were analyzed by One-Way ANOVA followed by appropriate post hoc test for multiple comparisons and expressed as ± SEM *P< 0.05 vs Vehicle; *P< 0.05 vs Resistin, *P<0.05. (n=4 independent studies).
TLR4 mediates resistin-induced SGK1 phosphorylation
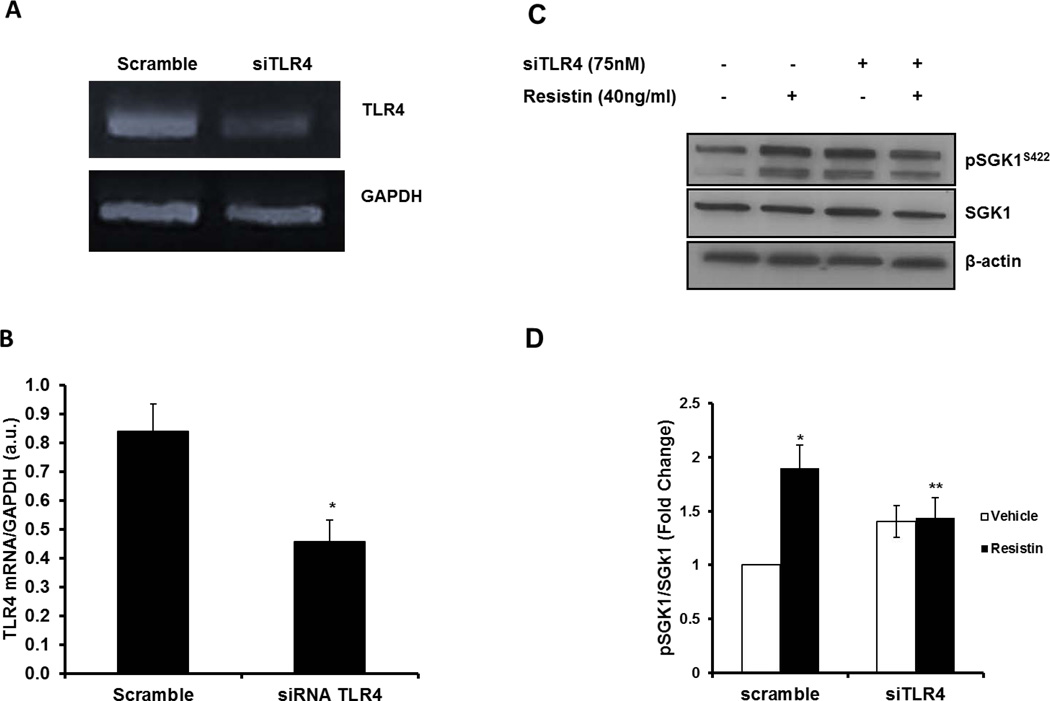
As the TLR4 receptor is reported to transmit signals from resistin, we investigated the possible involvement of TLR4 in resistin-induced Ser422 phosphorylation of SGK1 in HAoSMC. To this end, HAoSMC were transfected with non-targeting scrambled or TLR4 siRNA prior to stimulation with resistin. As shown in Figure 4A, we achieved greater than 50% reduction in TLR4 mRNA expression. As shown previously, resistin stimulated SGK1 phosphorylation by greater than 90% in cells transfected with scrambled oligonucleotide (p<0.05). However, stimulation of SGK1 phosphorylation was significantly blocked in siRNA TLR4 -transfected HAoSMC treated with resistin (Figure 4C–D).
Figure 4. Resistin-mediated SGK1 phosphorylation is TLR4-dependent.
A: PCR product verification on agarose gel of siRNA TLR4 knockdown. B: mRNA quantification of HAoSMC transfected with scramble or siRNA TLR4. C: Representative western blot image of phospho- and total SGK1 protein levels from HAoSMC transfected with scramble or siRNA TLR4 in the presence or absence of 40ng/ml resistin. D: Quantitative analysis of phospho- and total SGK1 were performed with NIH Image J. Data were analyzed by Student’s t-test or Two-Way ANOVA followed by appropriate post hoc test for multiple comparisons and expressed as ± SEM *P<0.01 vs scramble control; **P<0.05 vs scramble resistin. (n=3–5 independent studies).
SGK1 is Required for Resistin-Induced Proliferation
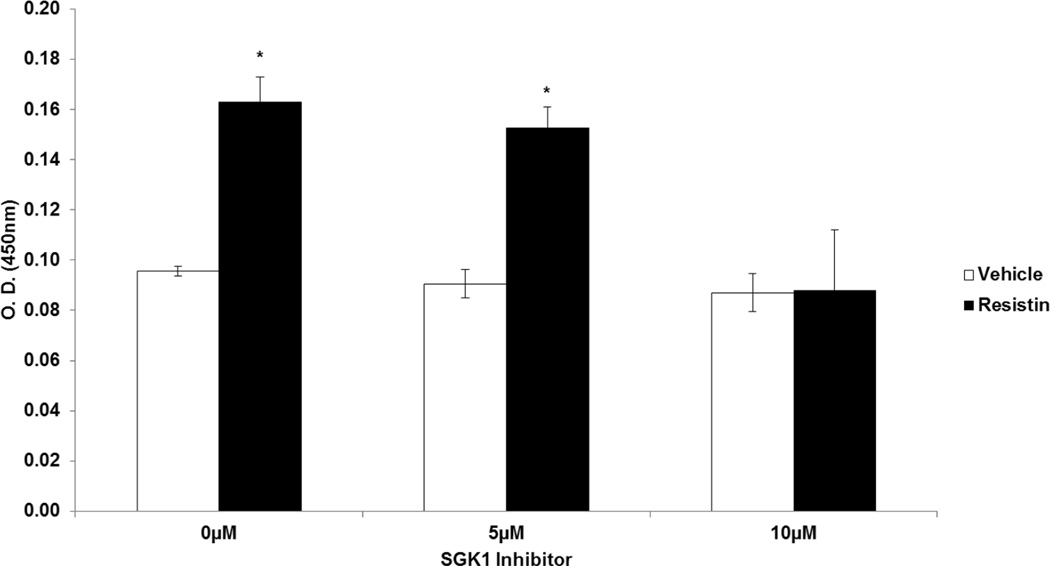
To test the hypothesis that resistin stimulates VSMC proliferation via activation of SGK1, we treated HAoSMC with vehicle or resistin in the presence or absence of the pharmaceutical SGK1 inhibitor, GSK650394 and assessed VSMC proliferation. Our results indicate that treatment of HAoSMC with resistin increased proliferation by 70%. However, inhibition of SGK1 significantly attenuated resistin-induced VSMC proliferation in vitro (Figure 5).
Figure 5. SGK1 is Required for Resistin-Induced Proliferation.
Serum-starved HAoSMC were pre-treated for 1h with varying doses (0–10µM) of the SGK1 inhibitor, GSK650394. Cells were then stimulated with vehicle or 40ng/ml human resistin in the presence of the inhibitor for 48h. Sample absorbance was measured at OD 450nm. Data were analyzed by Two-Way ANOVA followed by appropriate post hoc test for multiple comparisons and expressed as ± SEM, *P<0.001 vs inhibitor control. (n=3 independent studies).
SGK1 Expression and Phosphorylation are Increased in the Vasculature of DIO Mice
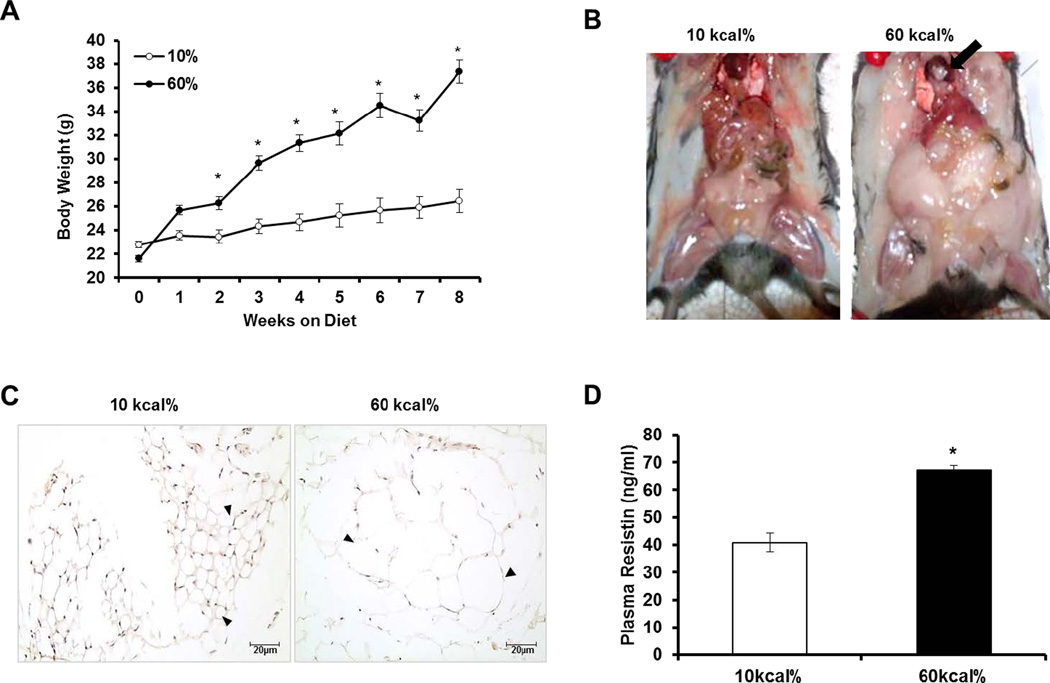
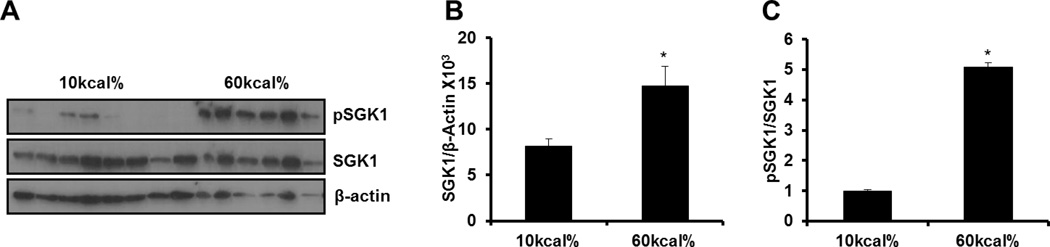
As expected, high fat (HF) feeding caused a steady increase in body weight throughout the course of the study. At the end of eight weeks, body weight significantly increased greater than 70% in HF fed mice compared to low fat (LF) fed mice (Figure 6A). Gross examination indicated enhanced adipose tissue deposition throughout the entire body cavity including the heart (arrow) in HF fed mice compared to LF fed mice (Figure 6B). Further, mesenteric adipose tissue was hypertrophied (arrowhead) in obese mice compared to their lean counterparts (Figure 6C). Consistent with previous reports, (15) fasting plasma levels of resistin were statistically elevated approximately 70% in HF fed mice compared to LF fed mice (Figure 6D). SGK1 is implicated in the development of obesity-related complications(14). Therefore, we examined SGK1 expression and phosphorylation status in the vasculature in response to diet-induced obesity (DIO). Western blotting analysis revealed that SGK1 was up-regulated over 2-fold in the aortas of obese mice (Figure 7A–B). Moreover, we observed a significant increase in the ratio of SGK1-Ser422 phosphorylation to total SGK1 (p<0.05) in the aortas of DIO mice compared to lean mice (Figure 7A and C).
Figure 6. Metabolic parameters in C57B6/J mice fed a low (LF) or high fat (HF) diet.
A: Body weight throughout the course of the 8-week period of the study. B: Fat distribution throughout body cavity of representative mice fed a LF or HF diet. C: Representative hemotoxylin and eosin staining of mesenteric adipose tissue from mice fed a LF or HF diet. Scale bar is 20µm. D: Plasma resistin levels measured by ELISA in LF and HF mice. Data were analyzed by Student’s t-test when comparing two groups or by Two-Way ANOVA with Repeated Measures followed by appropriate post hoc test for multiple comparisons and expressed as ± SEM, *P<0.001vs 10 kcal% fat. (n=7–12 mice).
Figure 7. Activation of SGK1 is increased in the vasculature following DIO.
A: Western blot of total and SGK1 phosphorylation at Ser422 (pSGK1) in the aortas of mice fed a LF and HF diet. B: SGK1 abundance in the vasculature of lean and obese mice. C: Ratio of pSGK1/SGK1 in the vasculature of lean and obese mice. Quantitative analysis of pSGK1 and total SGK1 were performed with NIH Image J. Data were analyzed by Student’s t-test and expressed as ± SEM, *P<0.05 vs 10%kcal. (n=6–8 mice).
Discussion
In the current study, we demonstrate a permissive role for resistin in transcriptional and post-translational regulation of SGK1 and define the molecular mechanisms involved in this process. Specifically, we established that resistin significantly increased phosphorylation of SGK1 at S422 via a TLR4-PI3K-dependent mechanism. We also demonstrated a role for SGK1 in resistin-induced VSMC proliferation. Consistent with these findings, we found that elevated plasma levels of resistin correlated with an increase in total SGK1 abundance and phosphorylation in the aortas of DIO mice.
Resistin Regulates SGK1 via a PI3K- and TLR4-dependent mechanism in vitro
Resistin is secreted by abdominal adipocytes, inflammatory cells and the perivascular adipose tissue surrounding the vasculature (1). In response to increasing adiposity, resistin secretion is exaggerated and can lead to vascular dysfunction and the progression of atherosclerosis. Our results which show that resistin can significantly increase SGK1 expression in a dose- and time-dependent manner in VSMC in vitro establish a biological interaction between resistin and SGK1 in the vasculature. We also demonstrate that resistin can increase SGK1 phosphorylation at Ser-422 in HAoSMC, an effect which was blocked by treatment of cells with the PI3K inhibitor, LY294002. Post-translational phosphorylation of SGK1 is an important regulator of SGK1 activity. Phosphorylation of SGK1 at Ser-422, in particular, stimulates its activation and downstream signaling. Thus, our findings demonstrate that resistin stimulation leads to activation of SGK1 and that the PI3K pathway is critical for resistin-mediated activation of SGK1. More importantly, our data implicates SGK1 as an effector molecule that mediates the actions of resistin in VSMC.
In general, studies pointing to the identity of the receptor which transmits resistin’s signals are limited. Nonetheless, several studies have shown that the TLR4 receptor mediates some of the downstream effects of resistin in myeloid cells, epithelial cells, leukocytes, monocytes, neuronal, lymphocytes, and adipocytes (22;31). However, not much is known about the receptor that mediate resistin’s actions in VSMC. Recently, two independent studies have shown that TLR4 functions as a receptor for resistin. TLR4 was found to mediate resistin-induced inflammatory responses in VSMC by mechanisms involving the chemokine fractalkine (24;25). In addition, both resistin and TLR4 are markedly expressed in human atherosclerotic lesions and participate in the inflammatory response of atherosclerosis (25;32). Thus, we hypothesized a potential crosstalk between resistin and TLR4 in the regulation of SGK1 phosphorylation in HAoSMC. TLR4 silencing blocked resistin-mediated SGK1 phosphorylation suggesting that resistin binding to the TLR4 receptor leads to SGK1 activation. In this study, we observed that PI3K inhibitor, LY294002 did not completely abolish SGK1 phosphorylation induced by resistin. Perhaps, other signaling pathways are activated in VSMC upon resistin stimulation. Resistin is reported to engage other membrane receptors such as the Gi-protein receptor and to activate other intracellular signaling pathways. Also, the adenylyl cyclase-associated protein 1 has been shown to bind resistin and mediate inflammatory actions in monocytes (33). Recently, NFκB and MAPK pathways have been shown to work downstream of TLR4 (21). Similarly, ERK and p38MAPK have already been shown to be phosphorylated upon resistin stimulation of vascular cell lines (18;34). Moreover, PI3K signaling is redox sensitive (35). Thus, it would be interesting to determine in future studies whether NFκB, ERK, p38, or reactive oxidative stress is also involved in resistin-mediated phosphorylation of SGK1 in VSMC.
Resistin-Induced Stimulation of VSMC Proliferation is SGK1-Dependent
Aberrant proliferation of VSMCs can lead to pathological changes within the vessel wall (26). Indeed, resistin has been shown to induce both HAoSMC and rat aortic smooth muscle cell proliferation in a dose-dependent manner via both the MAPK and AKT signaling pathways (18;27). Moreover, resistin also promotes VSMC migration (28;29). Uncontrolled proliferation and migration of VSMC occur as a consequence of vascular injury and can contribute to neointimal formation. Notably, the importance of resistin in neointimal thickening after mechanical injury has been established (30). Our data indicate that blocking SGK1 signaling abolishes resistin-mediated VSMC proliferation. Thus, our findings here support the notion that crosstalk between resistin and SGK1 leads to VSMC hyperplasia and neointimal development in individuals that are obese.
A High-Fat Diet Induces Vascular SGK1 Expression and Activation
Epidemiological and animal studies implicate SGK1 in the pathogenesis of obesity-related complications (13). Recent studies highlight that SGK1 is up-regulated in adipose tissue of individuals with obesity and in murine models of DIO. However, regulation of SGK1 in the vasculature in the setting of obesity is unknown. In this study, we demonstrated that a high-fat diet increased both SGK1 abundance and Ser-422 phosphorylation in the vasculature of DIO mice. Previous studies from our laboratory and others demonstrate that enhanced expression or activation of SGK1 exacerbate cellular processes that lead to pathological vascular remodeling (7;36;37). Thus, our findings suggest that SGK1 is activated in the vasculature with the onset of obesity and may participate in cellular processes that lead to vascular dysfunction. Several studies have highlighted resistin’s role in activation of the NFkβ pathway and its association with inflammatory markers such as TNF-α, IL-6, C-reactive protein thus supporting a pro-inflammatory role for resistin in the vasculature (1). Activated SGK1 phosphorylates IκB kinase alpha up-regulating the activity of the pro-inflammatory transcriptional regulator, NFκB (38). Taken together, our results suggest that SGK1 may function downstream of resistin to enhance obesity-related vascular inflammation. SGK1 may also participate in resistin-mediated perpetuation of atherosclerosis. In particular, resistin derived from subendothelial atherosclerotic plaques has been shown to enhance macrophage recruitment and transmigration in endothelial and smooth muscle cell co-cultures via fractalkine and MCP-1(25). Given our results, it is possible that SGK1 may participate in resistin-related development of atherosclerosis by increasing macrophage recruitment and accumulation within the vessel wall.
The mechanisms underlying enhanced vascular SGK1 phosphorylation are unknown, but may be related to altered secretion of adipokines into the plasma or perivascular adipose tissue (39;40) during the development of obesity. In this regard, we found that plasma resistin levels were elevated in our DIO mice. Resistin concentrations are in the range of 3–13ng/ml in healthy subjects with levels approaching 40ng/ml in individuals with obesity-associated diabetes and cardiovascular disease that correlates with inflammation (1;16;17). Notably, 40ng/ml was the optimal dose that increased SGK1 abundance and phosphorylation in our human VSMC line. Thus, resistin along with insulin and other pro-inflammatory mediators may participate in vascular activation of SGK1 in humans.
Conclusion
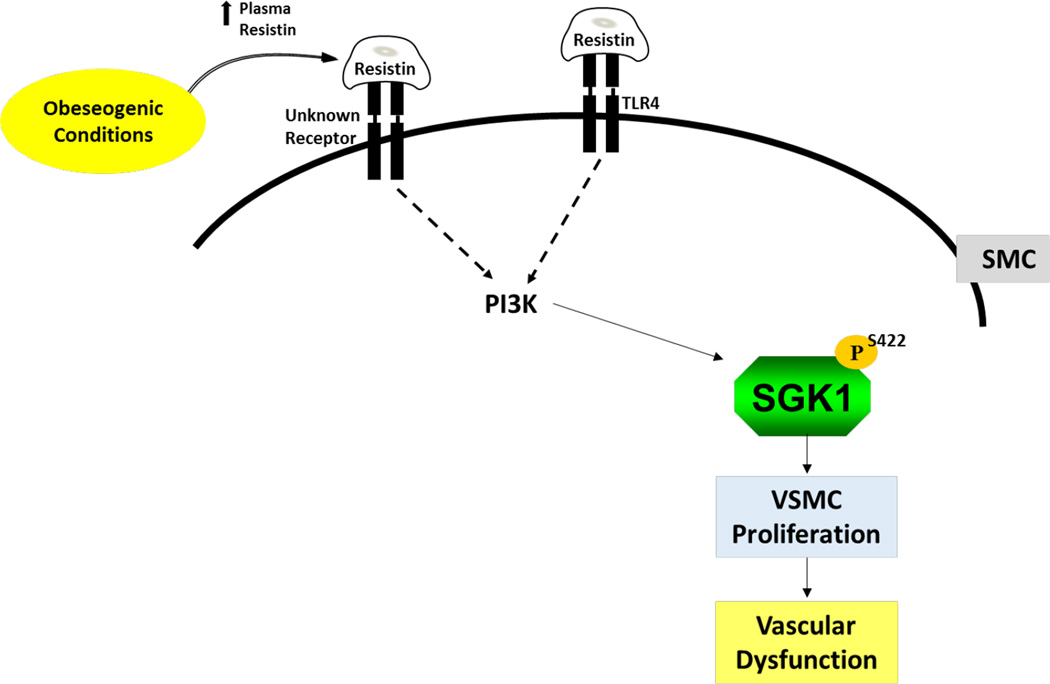
In summary, while the role of resistin in the vasculature has been an area of intense study, the downstream signaling pathways that mediate resistin’s actions in VSMC during obesity are not fully understood. An increasing body of evidence points to aberrant levels of adipokines as a contributor to the molecular processes that promote that pathogenesis of obesity-mediated cardiovascular disease. Data from this study provide compelling evidence that the elevated plasma resistin profile that occur in individuals with obesity may lead to activation of SGK1, aberrant VSMC proliferation and ultimately the development of vascular dysfunction (Figure 8). These findings point to inhibition of the resistin/SGK1 pathway as a novel therapeutic target for the treatment obesity-associated vascular diseases such as atherosclerosis.
Figure 8. Model illustrating a crosstalk between resistin-TLR4-PI3K in resistin-mediated phosphorylation of SGK1.
Resistin binds to TLR4 activating the PI3K pathway. This in turn phosphorylates SGK1 at S422 leading to its activation and the initiation of VSMC processes that promote VSMC dysfunction and the perpetuation of vascular disease.
Novelty/Significance.
What is already known about this subject
A gain of function polymorphism in intron 6 [I6CC] and exon 8 [E8CC/CT] of the SGK1 gene shows a strong positive correlation with increased body mass index, elevated blood pressure, and type 2 diabetes.
Sgk1 expression has been shown to be elevated during obesity in adipose tissue, kidney and liver.
Hyperresistinemia is linked to obesity-related insulin resistance and diabetes and has been implicated in clinical and experimental models of cardiovascular diseases.
What does your study add
For the first time we have shown that expression and activation of SGK1 is increased in the arteries during obesity supporting a role of SGK1 in the pathogenesis of obesity-related cardiovascular diseases.
Our results indicate that the adipokine resistin promotes activation of SGK1 via a TLR4-PI3K-dependent mechanism and that SGK1 participates in resistin-mediated stimulation of vascular smooth muscle cell proliferation.
Targeting the mechanisms that regulate this pathway could be a novel therapeutic approach for vascular diseases such as diabetes, atherosclerosis, and hypertension.
Acknowledgments
None.
Funding: This work was supported by NIH grants SC2:HL094341, COE:HL003676, SC1:HL107236, and T32HL103104
Footnotes
Author contributions:
Takara A. Scott contributed in the design and conduct of the study, data collection and analysis, animal experiments, and manuscript writing. Oguljahan Babayeva conducted all animal experiments including feeding, body weight measurements and tissue harvest, Saswati Banerjee contributed to siRNA RT-PCR experiments and agarose gel data collection, Wei Zhong contributed to data collection. Sharon C. Francis contributed to the design and conduct of the study, data interpretation, and manuscript writing.
Disclosure: None.
Reference List
- 1.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart. J. 2008 Dec;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 2.Lang F, Artunc F, Vallon V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr. Opin. Nephrol. Hypertens. 2009 Sep;18(5):439–448. doi: 10.1097/MNH.0b013e32832f125e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng J, Wang Y, Ma Y, Chan BT, Yang M, Liang A, Zhang L, Li H, Du J. The mechanical stress-activated serum-, glucocorticoid-regulated kinase 1 contributes to neointima formation in vein grafts. Circ. Res. 2010 Nov 12;107(10):1265–1274. doi: 10.1161/CIRCRESAHA.110.222588. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem. J. 2008 Dec 15;416(3):375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999 Jun 1;18(11):3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belaiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, Hess J, Gorlach A. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ. Res. 2006 Mar 31;98(6):828–836. doi: 10.1161/01.RES.0000210539.54861.27. [DOI] [PubMed] [Google Scholar]
- 7.Zhong W, Oguljahan B, Xiao Y, Nelson J, Hernandez L, Garcia-Barrio M, Francis SC. Serum and glucocorticoid-regulated kinase 1 promotes vascular smooth muscle cell proliferation via regulation of beta-catenin dynamics. Cell Signal. 2014 Dec;26(12):2765–2772. doi: 10.1016/j.cellsig.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006 Oct;86(4):1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 9.Lang F, Gorlach A, Vallon V. Targeting SGK1 in diabetes. Expert. Opin. Ther. Targets. 2009 Nov;13(11):1303–1311. doi: 10.1517/14728220903260807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borst O, Schaub M, Walker B, Schmid E, Munzer P, Voelkl J, Alesutan I, Rodriguez JM, Vogel S, Schoenberger T, et al. Pivotal role of serum- and glucocorticoid-inducible kinase 1 in vascular inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2015 Mar;35(3):547–557. doi: 10.1161/ATVBAHA.114.304454. [DOI] [PubMed] [Google Scholar]
- 11.Tai DJ, Su CC, Ma YL, Lee EH. SGK1 phosphorylation of IkappaB Kinase alpha and p300 Up-regulates NF-kappaB activity and increases N-Methyl-D-aspartate receptor NR2A and NR2B expression. J. Biol. Chem. 2009 Feb 13;284(7):4073–4089. doi: 10.1074/jbc.M805055200. [DOI] [PubMed] [Google Scholar]
- 12.Huang DY, Boini KM, Osswald H, Friedrich B, Artunc F, Ullrich S, Rajamanickam J, Palmada M, Wulff P, Kuhl D, et al. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am. J. Physiol Renal Physiol. 2006 Dec;291(6):F1264–F1273. doi: 10.1152/ajprenal.00299.2005. [DOI] [PubMed] [Google Scholar]
- 13.Schernthaner-Reiter MH, Kiefer F, Zeyda M, Stulnig TM, Luger A, Vila G. Strong association of serum- and glucocorticoid-regulated kinase 1 with peripheral and adipose tissue inflammation in obesity. Int. J. Obes. (Lond) 2015 Mar 26; doi: 10.1038/ijo.2015.41. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Pan F, Hao Y, Feng W, Song H, Zhu D. SGK1 is regulated by metabolic-related factors in 3T3-L1 adipocytes and overexpressed in the adipose tissue of subjects with obesity and diabetes. Diabetes Res. Clin. Pract. 2013 Oct;102(1):35–42. doi: 10.1016/j.diabres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001 Jan 18;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 16.Pfutzner A, Langenfeld M, Kunt T, Lobig M, Forst T. Evaluation of human resistin assays with serum from patients with type 2 diabetes and different degrees of insulin resistance. Clin. Lab. 2003;49(11–12):571–576. [PubMed] [Google Scholar]
- 17.Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012 Feb;165(3):622–632. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004 Nov 23;110(21):3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 19.Joksic J, Sopic M, Spasojevic-Kalimanovska V, Kalimanovska-Ostric D, Andjelkovic K, Jelic-Ivanovic Z. Circulating resistin protein and mRNA concentrations and clinical severity of coronary artery disease. Biochem. Med. (Zagreb.) 2015;25(2):242–251. doi: 10.11613/BM.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asterholm IW, Rutkowski JM, Fujikawa T, Cho YR, Fukuda M, Tao C, Wang ZV, Gupta RK, Elmquist JK, Scherer PE. Elevated resistin levels induce central leptin resistance and increased atherosclerotic progression in mice. Diabetologia. 2014 Jun;57(6):1209–1218. doi: 10.1007/s00125-014-3210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh YY, Shen CH, Huang WS, Chin CC, Kuo YH, Hsieh MC, Yu HR, Chang TS, Lin TH, Chiu YW, et al. Resistin-induced stromal cell-derived factor-1 expression through Toll-like receptor 4 and activation of p38 MAPK/ NFkappaB signaling pathway in gastric cancer cells. J. Biomed. Sci. 2014;21:59. doi: 10.1186/1423-0127-21-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, He Y, Zhang J, Sun S, Sun B. Lipopolysaccharide regulates toll-like receptor 4 expression in human aortic smooth muscle cells. Cell Biol. Int. 2007 Aug;31(8):831–835. doi: 10.1016/j.cellbi.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Otsui K, Inoue N, Kobayashi S, Shiraki R, Honjo T, Takahashi M, Hirata K, Kawashima S, Yokoyama M. Enhanced expression of TLR4 in smooth muscle cells in human atherosclerotic coronary arteries. Heart Vessels. 2007 Nov;22(6):416–422. doi: 10.1007/s00380-007-1001-1. [DOI] [PubMed] [Google Scholar]
- 24.Gan AM, Butoi ED, Manea A, Simion V, Stan D, Parvulescu MM, Calin M, Manduteanu I, Simionescu M. Inflammatory effects of resistin on human smooth muscle cells: up-regulation of fractalkine and its receptor, CX3CR1 expression by TLR4 and Gi-protein pathways. Cell Tissue Res. 2013 Jan;351(1):161–174. doi: 10.1007/s00441-012-1510-9. [DOI] [PubMed] [Google Scholar]
- 25.Pirvulescu MM, Gan AM, Stan D, Simion V, Calin M, Butoi E, Manduteanu I. Subendothelial resistin enhances monocyte transmigration in a co-culture of human endothelial and smooth muscle cells by mechanisms involving fractalkine, MCP-1 and activation of TLR4 and Gi/o proteins signaling. Int. J. Biochem. Cell Biol. 2014 May;50:29–37. doi: 10.1016/j.biocel.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J. Clin. Invest. 2009 Sep;119(9):2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirai H, Satoh H, Kudoh A, Watanabe T. Interaction between resistin and adiponectin in the proliferation of rat vascular smooth muscle cells. Mol. Cell Endocrinol. 2013 Feb 5;366(1):108–116. doi: 10.1016/j.mce.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Jiang C, Zhang H, Zhang W, Kong W, Zhu Y, Zhang H, Xu Q, Li Y, Wang X. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am. J. Physiol Cell Physiol. 2009 Dec;297(6):C1466–C1476. doi: 10.1152/ajpcell.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, Kim SJ, Kim SY, Lee HK, Park KS. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc. Res. 2006 Jan;69(1):76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Shyu KG, Lien LM, Wang BW, Kuan P, Chang H. Resistin contributes to neointimal formation via oxidative stress after vascular injury. Clin. Sci. (Lond) 2011 Feb;120(3):121–129. doi: 10.1042/CS20100226. [DOI] [PubMed] [Google Scholar]
- 31.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J. Cell Mol. Med. 2010 Jun;14(6B):1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsui K, Inoue N, Kobayashi S, Shiraki R, Honjo T, Takahashi M, Hirata K, Kawashima S, Yokoyama M. Enhanced expression of TLR4 in smooth muscle cells in human atherosclerotic coronary arteries. Heart Vessels. 2007 Nov;22(6):416–422. doi: 10.1007/s00380-007-1001-1. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, Lee S, Kim JY, Lee J, Yang HM, et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014 Mar 4;19(3):484–497. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu WY, Chao YW, Tsai YL, Lien CC, Chang CF, Deng MC, Ho LT, Kwok CF, Juan CC. Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J. Cell Physiol. 2011 Aug;226(8):2181–2188. doi: 10.1002/jcp.22555. [DOI] [PubMed] [Google Scholar]
- 35.Silva A, Girio A, Cebola I, Santos CI, Antunes F, Barata JT. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia. 2011 Jun;25(6):960–967. doi: 10.1038/leu.2011.56. [DOI] [PubMed] [Google Scholar]
- 36.BelAiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, Hess J, Gorlach A. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ. Res. 2006 Mar 31;98(6):828–836. doi: 10.1161/01.RES.0000210539.54861.27. [DOI] [PubMed] [Google Scholar]
- 37.Catela C, Kratsios P, Hede M, Lang F, Rosenthal N. Serum and glucocorticoid-inducible kinase 1 (SGK1) is necessary for vascular remodeling during angiogenesis. Dev. Dyn. 2010 Aug;239(8):2149–2160. doi: 10.1002/dvdy.22345. [DOI] [PubMed] [Google Scholar]
- 38.Tai DJ, Su CC, Ma YL, Lee EH. SGK1 phosphorylation of IkappaB Kinase alpha and p300 Up-regulates NF-kappaB activity and increases N-Methyl-D-aspartate receptor NR2A and NR2B expression. J. Biol. Chem. 2009 Feb 13;284(7):4073–4089. doi: 10.1074/jbc.M805055200. [DOI] [PubMed] [Google Scholar]
- 39.Lastra G, Manrique C. Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease. Horm. Mol. Biol. Clin. Investig. 2015 Apr 1;22(1):19–26. doi: 10.1515/hmbci-2015-0010. [DOI] [PubMed] [Google Scholar]
- 40.Molica F, Morel S, Kwak BR, Rohner-Jeanrenaud F, Steffens S. Adipokines at the crossroad between obesity and cardiovascular disease. Thromb. Haemost. 2015 Mar;113(3):553–566. doi: 10.1160/TH14-06-0513. [DOI] [PubMed] [Google Scholar]