Abstract
Rationale
Operant self-administration (SA) is an important model of motivation to consume ethanol (EtOH), but low rates of voluntary consumption in rats are thought to necessitate water deprivation and saccharin/sucrose fading for acquisition of responding.
Objectives
Here, we sought to devise an effective model of SA that does not use water deprivation or saccharin/sucrose fading.
Methods
First, we tested if Wistar rats would acquire and maintain SA behavior of a 20% EtOH under two conditions, water deprived (WD) and not water deprived (NWD). Secondly, we tested the efficacy of our SA procedure by confirming a prior study which found that the NK1 antagonist L822429 specifically blocked stress-induced reinstatement of EtOH seeking but not SA. Finally, we assessed the effect of naltrexone, an FDA-approved medication for alcohol dependence that has been shown to suppress EtOH SA in rodents.
Results
Lever presses (LPs) and rewards were consistent with previous reports that utilized WD and saccharin/sucrose fading. Similar to previous findings, we found that L822429 blocked stress-induced reinstatement, but not baseline SA of 20% EtOH. Moreover, naltrexone dose-dependently decreased alcohol intake and motivation to consume alcohol for rats self-administering 20 % EtOH.
Conclusions
Our findings provide a method for voluntary oral EtOH SA in rats that is convenient for experimenters and eliminates the potential confound of sweeteners in EtOH operant SA studies. Unlike models that use intermittent access to 20% EtOH, this method does not induce escalation, and based on pharmacological experiments, appears to be driven by the positive reinforcing effects of EtOH.
Keywords: EtOH, self-administration, addiction
INTRODUCTION
Operant self-administration (SA) is a widely used behavioral model that is one of the most effective means for assessing primary reinforcement for an orally consumed ethanol (EtOH) solution. However, this method suffers from relatively low throughput due to extended training times required to promote stable levels of SA. A standard protocol requires water deprivation and/or saccharin or sucrose fading for the acquisition and maintenance of SA (Koob and Weiss 1990), and generally 3 weeks of training are required just to initiate responding for 10% EtOH in water. This approach is problematic, as it requires extended periods of training before investigators can begin to measure EtOH SA. In contrast, intravenous SA of cocaine or heroin requires 0–1 days of pre-drug training on a food-delivering lever in food restricted animals, and stable responding for drug is often achieved in 10–12 days. Importantly, saccharin and sucrose are highly rewarding to rats and elicit similar brain activation patterns to drugs of abuse, thus introducing the potential for confounds in EtOH SA studies (Lenoir et al. 2007, Spangler et al. 2004, Cantin et al. 2010, Augier et al. 2012). Although a previous study by Simms and colleagues found that rats would indeed self-administer a 20% EtOH in water solution without the use of sucrose fading or water deprivation, their modified procedure still required extended access training before SA could be measured and was suggested to model escalated EtOH seeking rather than primary reinforcement (Simms et al. 2010). Furthermore, it is unclear whether the facilitated responding in that study was primarily due to intermittent access schedules or to the concentration of EtOH used. Consistent with our second hypothesis, a recent investigation by Carnicella and colleagues found that SA for oral EtOH displays an inverted U shaped dose response curve with highest EtOH intake during SA at a 20% EtOH concentration, thus providing support for the optimal efficiency of our experimental design (Carnicella et al. 2011). Here, we tested whether Wistar rats would self-administer a 20% EtOH in water solution without the use of water deprivation, extended access training, or saccharin fading.
In the interest of using this method as a standard procedure for assessing primary reinforcement, one issue that should be resolved is whether SA of 20% EtOH is more akin to baseline SA observed in non-dependent animals, or if increased EtOH concentrations promote rates of responding that are increased relative to baseline levels and represent escalated motivation for EtOH. To directly assess this issue, we used the neurokinin-1 receptor (NK1R) antagonist L822429. Recent studies have shown that a specific subset of alcohol-related behaviors are sensitive to pharmacological blockade of the NK1R. For example, Schank and colleagues have demonstrated that while alcohol SA in non-dependent Wistar rats is unaffected by L822429, escalated EtOH SA in alcohol preferring (P) rats is suppressed by even moderate doses of the compound (15 mg/kg, i.p.; Schank et al., 2011, Schank et al., 2013). In contrast, stress-induced reinstatement of EtOH seeking is attenuated by L822429 pretreatment in non-dependent Wistars at a dose of 30 mg/kg (Schank et al., 2011). The studies cited above all used oral SA of 10% EtOH in water with saccharin fading and WD procedures. Therefore, we administered a 15 mg/kg dose of L822429 to determine if SA of 20% EtOH would be affected. If so, this would suggest that oral SA of 20% EtOH is more likely to model escalated SA. Furthermore, we assessed the effect of 30 mg/kg L822429 on stress-induced reinstatement to confirm that reinstatement behavior was not differentially influenced by access to 20% versus 10% EtOH.
Finally, to further test the validity of our SA model on a clearer measure of motivation to obtain EtOH, we trained a group of Wistar rats under an extended fixed-ratio (FR3) reinforcement schedule and verified the efficacy of naltrexone, an FDA-approved medication for alcohol dependence. Naltrexone is an opiate receptor antagonist that has shown promising results in both animal models of alcohol intake and clinical trials. In animal models, naltrexone is efficient in reducing alcohol consumption and SA in non-dependent animals (Gonzales and Weiss, 1998, Stromberg et al., 1998, Czachowski and Delory, 2009).
METHODS
Drugs
L822429 was synthesized in the laboratory of Dr. K. Rice at NIDA. L822429 was dissolved in 45% w/v 2-hydroxypropyl β-cyclodextrin and pH was adjusted as necessary. The compound was injected at a volume of 1.5–3 ml/kg. Naltrexone (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline and pH was adjusted as necessary. The drug was injected at a volume of 1.0 ml/kg. EtOH solutions were prepared volume/volume in tap water from 95 % EtOH. Doses for L822429 (15 mg/kg and 30 mg/kg) were chosen in accordance with a previous investigation conducted in our lab (Schank et al., 2011). For naltrexone, the literature provides good evidence for a dose choice between the ranges of 0.1–1mg/kg, so 0.1, 0.3 and 1 mg/kg were tested (Czachowski and Delory, 2009)
Subjects
A total of 48 adult male Wistar rats (Charles River, Frederick, MD, USA) weighing 300–500g were housed in pairs in a temperature (21°C) and humidity-controlled environment with reversed 12 hour light-dark cycle. Rats were given free access to chow and tap water for the duration of the experiment, except on days of water deprivation during experiment 1. All behavioral testing was conducted during the dark phase of the light-dark cycle. The studies were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Apparatus
All behavioral training and testing were conducted in sixteen identical operant chambers (Med Associates Inc., St Albans, VT, USA; 30.5×29.2×24.1 cm) housed in sound-attenuating cubicles. Each operant chamber was equipped with two retractable levers positioned laterally to a liquid cup receptacle.
Behavior
Experiment 1
In the first experiment, 16 rats were divided in two groups and trained to self-administer 20% (v/v) EtOH under two different conditions. One rat was excluded because it did not acquire SA (defined as a minimum of 10 LPs during the last 3 stabilized sessions). In one condition, rats were water deprived for 22 hours per day during the first 3 days of SA (n=7, water-deprived (WD) Group). In the other condition, rats were not water deprived (n=8, non-water-deprived (NWD) group). For days 1–4 of SA, rats were reinforced on a fixed-ratio 1 (FR1) schedule. Following this, rats were on an FR1 reinforcement schedule with a cue light paired with each active lever press (LP). SA sessions were 30 minutes long and started at 10:00 AM 5 days/week for 13 days. Other than the aforementioned alterations in conditions and timeline, all aspects of the SA were based on established operant SA methods used in previous studies (Samson 1986). Briefly, rats were placed individually in light and sound-proof chambers containing one active and one inactive lever separated by a drinking well where EtOH rewards were given. Presses on the active lever resulted in the delivery of 100 microliters of EtOH in water paired with a cue-light, while presses on the inactive lever did not result in cue presentation or EtOH delivery. A 5-second timeout period where no EtOH rewards could be given followed each EtOH delivery. On day 10 of baseline SA, blood was collected from the lateral tail vein of WD and NWD rats immediately following the session. Blood EtOH concentrations (BECs) were assayed using a nicotinamide adenine dinucleotide phosphate dehydrogenase/spectrophotometric assay kit (Sigma Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions.
Experiment 2
For the L822429 experiments, a separate group of operant- and drug naive rats (n=32) were trained on a FR1 schedule to self-administer 20% EtOH without water deprivation during 30 minutes sessions. Similar to the above experiment, two levers were extended to mark the onset of the session and to signal alcohol availability. Pressing once on the lever associated with EtOH (active) was rewarded by the delivery of a volume of 100 microliters of 20 % EtOH in water in the adjacent drinking well and initiated a concomitant 5-s time-out period signaled by the illumination of the cue-light above the lever. Responses on the other lever (inactive) were recorded but had no behavioral consequences. During the time-out period, responding had no scheduled consequences. Sessions were conducted 5/6 days a week until stabilization of performance (defined as a minimum of 15 sessions and no change greater than 15 % in the total number of rewards earned during the last 3 sessions). A total of 19 sessions of SA were performed. On day 20, rats were treated with L822429 (15 mg/kg, IP, n=8; 30 mg/kg, IP, n=8) or vehicle (IP, n=16) 1 hour before the SA session in a between-subject design. Rats were given saline injections on days 16–19, one hour before SA, to habituate them to the injection. Then, a subgroup of rats (n=16) underwent 23 days of extinction followed by treatment with L822429 (30 mg/kg) or vehicle 1 hour preceding stress-induced reinstatement. This dose was chosen in accordance with a previous investigation (Schank et al., 2011) showing that it decreases significantly stress-induced reinstatement, but not the lower dose. The other 16 rats were used to test the effect of naltrexone on EtOH seeking in our protocol (see experiment 3). During extinction, conditions were identical to baseline SA except that active LPs did not result in EtOH delivery. To prepare for stress-induced reinstatement, rats were habituated for 15 minutes in SA chambers immediately preceding extinction sessions for the last 7 days of extinction. Rats were given saline injections 1 hour preceding extinction sessions for the last 2 days of extinction. Rats were tested for reinstatement if they decreased their active LPs to fewer than 10 during the last 3 stabilized sessions of extinction. All rats in the study achieved this criterion. For stress-induced reinstatement, rats received 15 minutes of intermittent footshock (0.5 s shock, 0.6 mA, mean intershock interval 40 s) immediately preceding the reinstatement test, in accordance with previous experiments by Schank and colleagues (Schank et al. 2011).
Experiment 3
After returning to baseline during 4 consecutive days on FR1, a subgroup of rats from experiment 2 (n=16) were trained to self-administer 20% EtOH under a fixed-ratio 3 schedule of reinforcement. Responses on the other lever (inactive) were recorded but had no behavioral consequences. During the 5s time-out period, responding had no scheduled consequences. Similarly to experiment 2, sessions were conducted 5/6 days a week until stabilization of performance. A total of 19 sessions of SA were performed. Rats were given subcutaneous saline injections on days 15–19 thirty minutes prior SA to habituate them to the injection. Starting on day 20, rats were tested in a balanced/random order in a between-subjects design across one of the four naltrexone dosing cycles (0, 0.1, 0.3 and 1 mg/kg) 30 minutes before the SA session. Between each dosing cycle, rats were allowed to washout the drug with two consecutive SA sessions. As a result, at the end of the test, all rats had been injected with each of the four doses. After this phase, we selected the most efficient dose (1mg/kg) and tested the effect of naltrexone on the motivation of the animals to consume alcohol using a progressive ratio (PR) schedule (Richardson and Roberts, 1996). All experimental conditions were identical to those used in the FR schedule, except that the response requirement or cost was increased within-session according to the following formula: 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 28, 32…. The PR session terminated after 30 minutes had elapsed without a reward. The breakpoint was defined as the last completed response requirement during the PR session. Rats were similarly tested in a between-subjects design across one of the two doses (0 and 1 mg/kg). Between two dosing cycles, rats were allowed to washout the drug with two consecutive SA sessions.
Statistics
For experiment 1, the total number of active LPs and rewards earned during sessions 1 to 13 for the WD and NWD groups were analyzed using a two-way repeated measures analysis of variance (Two-way RM ANOVA). Correlational significance was calculated using the Pearson Correlation Coefficient value. Mean body weight and consumption during days 7–13 were compared using a one-way analysis of variance (One-way ANOVA). For experiment 2, a between-subject design was used to test the effect of L822429 on LPs and rewards. Data were therefore analyzed using a one-way ANOVA. Responding during baseline, the 23 days of extinction as well as the stress-induced reinstatement for the vehicle and L822429 treated group were analyzed using a two-way RM ANOVA. For experiment 3, a within-subject design was used to test the effect of multiple doses of naltrexone on the SA of 20 % EtOH in the same 16 rats. Data were therefore analyzed using a one-way RM ANOVA. The effect of a dose of 1 mg/kg of naltrexone on progressive ratio was analyzed using a one-way ANOVA. All post hoc analyses were conducted when appropriate using a Tukey-HSD test.
RESULTS
Experiment 1: Wistar rats acquire SA of 20 % EtOH without saccharin fading
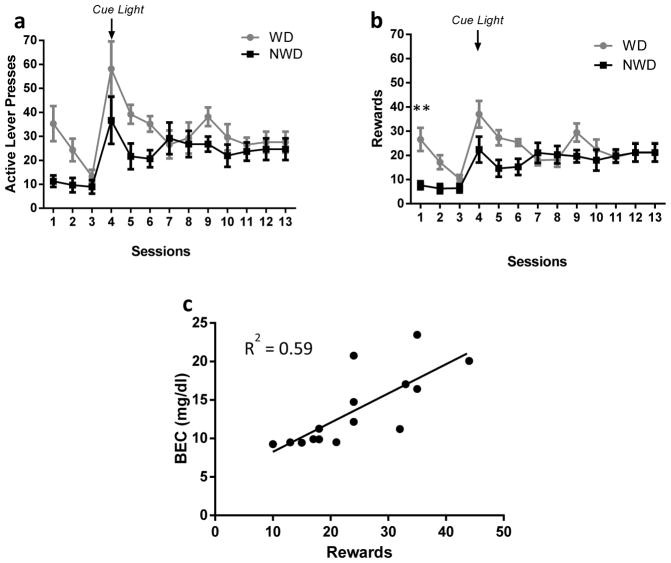
A RM ANOVA of LPs over sessions 1 to 13 (Figure 1a) revealed that there was a main effect of water deprivation (F1,13=4.89, p=0.046), a main effect of sessions (F12,156=6.93, p <.0001) but only a trend level of significance for a water deprivation/sessions interaction (F12,156=1.72, p =0.068). A RM ANOVA of the number of EtOH rewards earned over sessions 1 to 13 (Figure 1b) revealed that there was a trend for water deprivation (F1,13=4.62, p=0.051), a main effect of sessions (F12,156=6.44, p <.0001) and a significant water deprivation/sessions interaction (F12,156=2.96, p = 0.001). However, post hoc analyses revealed that the WD group obtained significantly more rewards than the NWD group only on day 1 (p=0.041). Despite this significant increase in acquisition of EtOH SA during the first day of training in the water deprived rats, both groups reached stable responding rates on the active lever by day 7 of SA, with average active LPs of 29 ± 3.0 for the WD group and 25 ± 3.8 for the NWD group (days 7–13). Water deprivation did not result in differences in body weight between groups (average weight WD=426.5 ± 9.9 g, NWD=427.6 ± 9.9 g, F1,13=0.007, p=0.94). For days 7–13, there were no significant differences between the groups in lever presses (p ≥ 0.99) and rewards earned (p ≥ 0.95) (Figures 1a and 1b). Average consumption was 0.80 ± 0.09 g/kg for the WD group and 0.76 ± 0.12 g/kg for the NWD group for days 7–13 of SA, and consumption was not significantly different between groups (F1,13=0.055, p=0.81). There was a positive correlation between BEC and EtOH rewards on day 10 of SA (R2=0.59, p <.001, Figure 1c).
Fig. 1.
a: Average active lever presses completed during 30 minute SA sessions (FR1) of 20% EtOH for water deprived (WD) and non-water deprived (NWD) rats b: Average rewards during 30 minute SA sessions (FR1) of 20% EtOH for water deprived (WD) and non-water deprived (NWD) rats (** = p<0.01). c: Number of rewards earned during one 30 minute SA session versus BEC (mg/dl) (R2=0.59, p= 0.0009)
Experiment 2: L822429 selectively suppresses stress-induced reinstatement
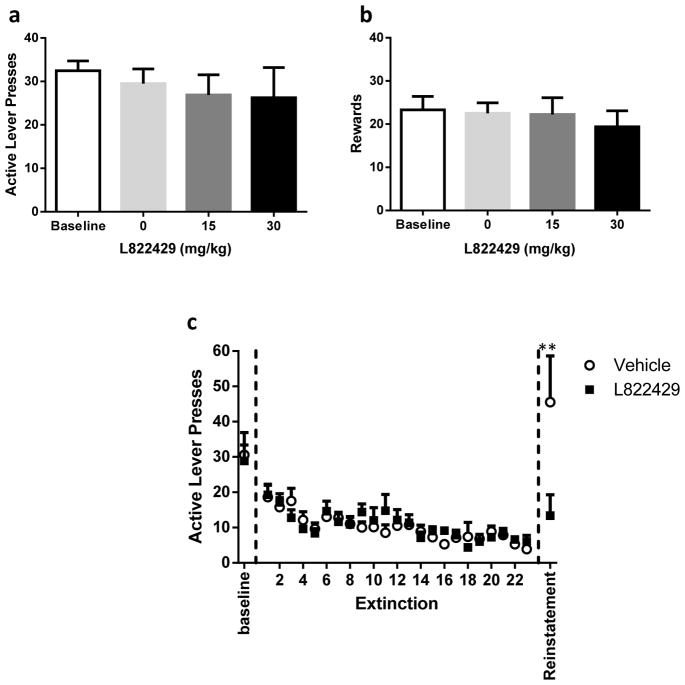
As water deprivation did not affect stabilized levels of SA, Wistar rats were not water deprived in the following experiments. Baseline responding before the test was similar between the three groups (One-way ANOVA, F2,29=0.65, p=0.53) and similar to Schank et al. (2011), L822429 did not significantly alter active LPs (One-way ANOVA, F2,29=0.15, p=0.86, Figure 2a) or rewards (One-way ANOVA, F2,29=0.26, p=0.77, Figure 2b) compared to vehicle neither at the 15 mg/kg nor at the 30 mg/kg dose. Following baseline testing, rats successfully extinguished their SA behavior and maintained sufficiently low active LPs (RM ANOVA, F1,24=10.61, p <.0001), reaching an average of 6.3 ± 0.7 LPs through the 3 last sessions of extinction. Both vehicle and treated groups showed similar extinction levels (RM ANOVA, F1,24=0.17, p =0.69). In further agreement with Schank et al. (2011), post hoc reveals that 30 mg/kg L822429 blocked stress-induced reinstatement for 20% EtOH (p<0.001, Figure 2c) without altering inactive LPs (p=0.89). While the vehicle group completed an average of 45.5±13.1 presses on the active lever following the stress-induced reinstatement, this apparent large increase in responses compared to baseline responding (30.6±6.4) was mainly due to a higher variability during the stress-induced reinstatement. However, a statistical comparison between LPs during baseline and stress-induced reinstatement revealed that this apparent increase was not significant (p=0.26).
Fig 2.
a: Mean active lever presses (± SEM) completed during a 30 minute SA session (FR1) of 20% EtOH following either vehicle (n=16) or L822429 treatment (15 or 30 mg/kg) in Wistar rats (n=8 for each dose) (p=0.86). b: Mean rewards (±SEM) earned during a 30 minute SA session (FR1) of 20% EtOH following either vehicle (n=16) or L822429 treatment (15 or 30 mg/kg) in Wistar rats (n=8 for each dose) (p=0.77). c: Mean baseline, extinction, and reinstatement active lever presses (± SEM) following either vehicle or L822429 treatment (30 mg/kg) in Wistar rats (n=8 in each group) (**= p<0.01).
Experiment 3: Naltrexone decreases both SA and motivation to consume alcohol
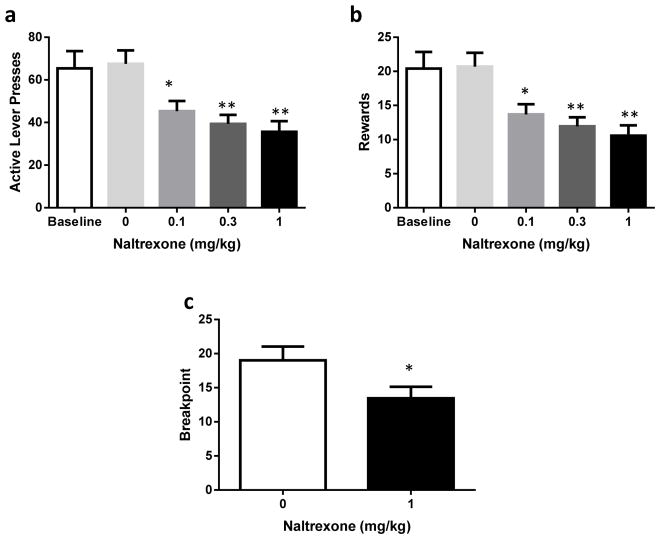
Finally, we tested the effect of naltrexone on rats trained on a FR3 schedule with this new protocol. Once stabilized, rats produced an average of 65.4 (± 8.1) responses on the active lever while presses on the inactive lever stabilized at a low level (4.3 ± 0.7). They obtained an average of 20.4 (± 2.4) rewards, reaching a similar level of consumption to rats trained on FR1 in experiments 1 and 2 (see Figure 1 and 2). One-way RM ANOVA revealed that naltrexone caused a dose-dependent decrease in both active LPs (F4,60=13.84, p < .0001, Figure 3a) and rewards (F4,60=15.16, p <.0001, Figure 3b) while inactive LPs remained low and unaffected (F4,60=1.07, p=0.38). Moreover, post hoc analyses confirmed that all three doses of naltrexone significantly reduced active LPs and rewards compared to vehicle (p<.01 for 0.1 mg/kg, p<.001 for 0.3mg/kg and 1 mg/kg) and that the vehicle had no effect compared to baseline responding (p= 0.996 for active LPs and p=0.999 for rewards). Finally, we assessed the effect of the highest dose (1 mg/kg) of naltrexone on the motivation of the animals to consume alcohol using a progressive ratio schedule. Naltrexone significantly reduced breakpoint compared to vehicle (F1,30=4.4, p=0.044, Figure 3c).
Fig. 3.
a: Mean active lever presses (± SEM) completed during a 30 minute SA session (FR3) of 20% EtOH following either saline or naltrexone treatment (0.1, 0.3 or 1 mg/kg) in Wistar rats (n=16) (** = p<0.01, *** = p<0.001) b: Mean rewards (± SEM) earned during a 30 minute SA session (FR3) of 20% EtOH following either saline or naltrexone treatment (0.1, 0.3 or 1 mg/kg) in Wistar rats (n=16) (** = p<0.01, *** = p<0.001). c: Mean breakpoint (± SEM) reached during a PR session of 20% EtOH following either saline or naltrexone treatment (1 mg/kg) in Wistar rats (n=16) (* = p<0.05)
DISCUSSION
Contrary to classic models of EtOH SA, we found that Wistar rats acquire and maintain stable SA of 20% EtOH without the use of water deprivation, extended access training, or saccharin/sucrose fading. A total of 48 operant- and drug-naive Wistar rats were used to perform this study and only one rat was excluded from the analysis because it did not acquire SA. In addition, BECs and rewards were positively correlated for a 30 minute SA session. Moreover, we verified the validity of our new SA paradigm by confirming the findings of Schank and colleagues (Schank et al., 2011, Schank et al., 2013), which used a classic WD and saccharin fading procedure to illustrate that 30 mg/kg of the NK1 antagonist L822429 blocks stress-induced reinstatement, but not baseline SA, of 10% EtOH. This distinction is important, as it demonstrates the ability of our model to specifically assess primary reinforcement for EtOH. The NK1R has been shown to impact alcohol-related behaviors only following the recruitment of stress systems, such as during stress-induced reinstatement or escalated EtOH SA that is induced by dependence or genetic selection. A similar effect is seen with compounds that block the activity of corticotropin releasing hormone (CRH) receptors. CRH is largely thought of as the prototypical stress-sensitive neuropeptide and this system has identical effects to NK1R on alcohol SA and reinstatement. Therefore, it is predicted that CRH antagonists would not suppress SA in our model, but would retain their ability to attenuate stress-induced reinstatement.
Another important pharmacological validation of our model is provided by our results with naltrexone. Specifically, we found that naltrexone successfully decreased alcohol SA, as well as the motivation to consume alcohol of Wistar rats trained with our protocol. This result provides an important validation of our protocol as it is consistent with previous reports showing that naltrexone is effective in reducing alcohol consumption in non-dependent rats, primarily by decreasing the immediate rewarding value of alcohol.
Our results indicate that it is possible to achieve levels of alcohol consumption and BECs similar to previous EtOH SA studies by utilizing a markedly simpler SA procedure that features a shorter training/acquisition period. The similarities in stabilized consumption levels and BECs strongly validate our methods and support the compatibility of our model with previous experimental results that examine the positive reinforcing effects of EtOH. For example, the Schank et al. study replicated here found that Wistar rats trained with WD and saccharin fading achieved an average consumption of 0.83 g/kg during 30 minute FR1 sessions with 10% EtOH (2011). In agreement with this, our 30 minute FR1 sessions with 20% EtOH resulted in an average consumption of 0.80 g/kg. This relative level of consumption has been verified in numerous other studies using WD and/or saccharin/sucrose fading SA procedures (Carnicella et al. 2011, Samson et al. 1988, De Bruin et al. 2013, Shulteis et al. 1996). In addition, the BEC range reached in our procedure (10–25 mg/dl) is comparable to the range reached in a study by Shulteis et al. using 10% EtOH, WD, and sucrose fading (1996, 15–30 mg/dl), as well as in other similarly designed EtOH SA experiments (Weiss et al. 1993, Weiss et al. 1990, Weiss and Koob 1991).
To our knowledge, the present investigation is the first to provide support for a model of baseline EtOH SA that removes the need for water deprivation, extended access training, or saccharin/sucrose fading. There are numerous important advantages to such a model. First, it allows rats to reach active LP rates for relevant levels of EtOH (Samson and Pfeffer 2006) in one week. Previous paradigms necessitate up to six weeks of training before animals reach sufficient baseline EtOH SA (Koob and Weiss 1990, Samson 1986), thus wasting valuable time and resources. Secondly, our model eliminates the marked decrease in EtOH consumption commonly seen following the removal of sweeteners, therefore possibly decreasing the rate of attrition in EtOH SA studies (Koob and Weiss 1990). Furthermore, our model simplifies the interpretation of EtOH SA studies by removing confounds due to the inherently rewarding properties of saccharin and sucrose in rats. These properties have been heavily studied in recent years and ultimately emphasize the need for a more precise model of EtOH SA. For example, there is evidence to support the idea that sucrose may itself be addictive in rats, as rats display behaviors such as bingeing, seeking, and withdrawal in response to excessive sucrose intake (Avena et al. 2008, 2010, Morgan and Sizemore 2011). In addition, sucrose and saccharin are more potent reinforcers than drugs of abuse such as cocaine and opiates and similarly alters Fos activation in brain regions associated with the reinforcing effects of these drugs (and subsequently EtOH), including the nucleus accumbens (Lenoir et al. 2007, Spangler et al. 2004, Cantin et al. 2010, Augier et al. 2012). Rats bred to have high preference for saccharin have been shown to be more vulnerable to negative affect during opiate withdrawal, therefore suggesting problematic overlaps in reward mechanisms between saccharin and EtOH (Radke et al. 2013).
However, it is important to note that our protocol, contrary to the one reported by Simms and colleagues (2010) does not engender high level of EtOH SA and high BECs, thus not inducing physical dependence in rats. Simms and colleagues succeeded in designing an effective SA paradigm for 20% EtOH without the use of water deprivation or saccharin or sucrose fading in Long-Evans rats (2010). Long-Evans rats trained according to this protocol reached average BEC levels of approximately 60 mg/dl after 38 SA sessions. However, the acquisition phase of their paradigm was 18 days, while ours is 7. In addition, it required 14 overnight, 14 hour, SA sessions, which can be argued models escalated motivation to consume EtOH at levels above baseline SA and not baseline drinking in non-dependent animals. Our model is thus valuable primarily because it retains the objectives of previous SA paradigms to measure baseline SA in non-dependent animals yet also manages to enhance convenience and throughput.
Overall, we have identified that Wistar rats will acquire and maintain SA behavior with a 20% ETOH solution without water deprivation, saccharin or sucrose fading, and extended access training. Importantly, the model eliminates the use of caloric and non-caloric sweeteners that are a major confound in ETOH behavioral studies. We believe this model will be extremely useful for behavioral labs that want to decrease the amount of time and resources spent on behavioral experiments and increase total SA capacity.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
References
- Augier E, Vouillac C, Ahmed SH. Diazepam promotes choice of abstinence in cocaine self-administering rats. Addiction Biology. 2012;17:378–391. doi: 10.1111/j.1369-1600.2011.00368.x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavior effects of excessive intermittent sugar intake. Neuroscience Behavior Reviews. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM. The study of food addiction using animal models of binge eating. Appetite. 2011;55:734–737. doi: 10.1016/j.appet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PloS One. 2010;5(7):e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcinella L, Yowell QV, Ron D. Regulation of operant oral ethanol self-administration: a dose-response curve study in rats. Alcoholism, clinical and experimental research. 2011;35:116–125. doi: 10.1111/j.1530-0277.2010.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology. 2009;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin NM, McCreary AC, van Loevezijn A, de Vries TJ, Venhorst J, van Drimmelen M, Kruse CG. A novel highly selective 5-HT6 receptor antagonist attenuates ethanol and nicotine seeking but does not affect inhibitory response control in Wistar rats. Behav Brain Res. 2013;236(1):157–165. doi: 10.1016/j.bbr.2012.08.048. [DOI] [PubMed] [Google Scholar]
- Ebner K, Muigg P, Singewald G, Singewald N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Annals of the New York Academy of Sciences. 2008;1144:61–73. doi: 10.1196/annals.1418.018. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- Koob G, Weiss F. Pharmacology of drug self-administration. Alcohol. 1990;7(3):193–197. doi: 10.1016/0741-8329(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Sizemore GM. Animal models of addiction: fat and sugar. Curr Pharm Des. 2011;17:1168–1172. doi: 10.2174/138161211795656747. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of EtOH reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Pfeffer AO, Tolliver GA. Oral EtOH self-administration in rats: models of EtOH-seeking behavior. ACER. 1988;12:591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology. 2011;218(1):111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Tapocik JD, Barbier E, Damadzic R, Eskay RL, Sun H, Rowe KE, King CE, Yao M, Flanigan ME, Solomon MG, Karlsson C, Cheng K, Rice KC, Heilig M. Tacr1 gene variation and neurokinin 1 receptor expression is associated with antagonist efficacy in genetically selected alcohol-preferring rats. Biological psychiatry. 2013;73:774–781. doi: 10.1016/j.biopsych.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon J, Chatterjee S, Bartlett S. Long-evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Radke AK, Holtz NA, Gewirtz JC, Carroll ME. Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Psychopharmacology. 2013;227:116–126. doi: 10.1007/s00213-012-2945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Weiss F, Mitchiner M, Bloom FE, Koob GF. Free-choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology. 1990;101(2):178–186. doi: 10.1007/BF02244123. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]