Abstract
OBJECTIVE
The antifibrinolytic agent tranexamic acid (TXA) has demonstrated clinical benefit in trauma patients with severe bleeding but its effectiveness in patients with traumatic brain injury (TBI) is unclear. We conducted a systematic review to evaluate the following research question: In ED patients with or at risk of intracranial hemorrhage secondary to TBI, does TXA compared to placebo improve patients’ outcomes?
METHODS
MEDLINE, EMBASE, CINAHL and other databases were searched for randomized (RCT) or quasi-RCT studies that compared the effect of TXA to placebo on outcomes of TBI patients. The main outcomes of interest included mortality, neurological function, hematoma expansion, and adverse effects. We used “Grading quality of evidence and strength of recommendations” (GRADE) to assess the quality of trials. Two authors independently abstracted data using a data collection form. Results from studies were pooled when appropriate.
RESULTS
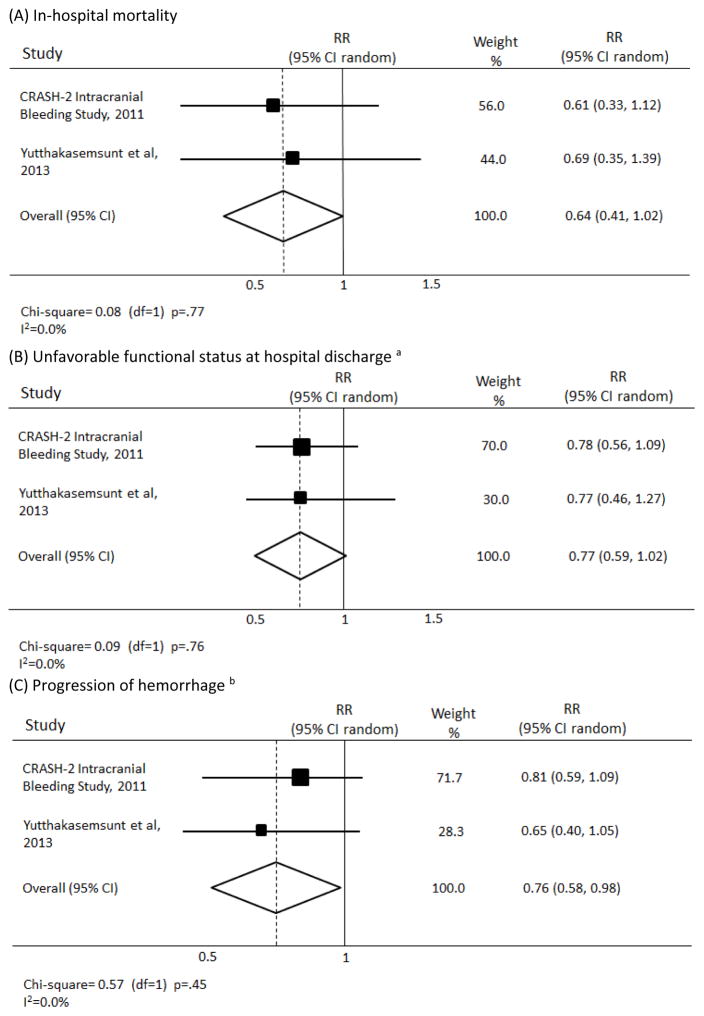
Out of 1030 references identified through the search, two high-quality RCTs met inclusion criteria. The effect of TXA on mortality had a pooled relative risk (RR) of 0.64 (95%CI, 0.41–1.02), on unfavorable functional status a RR of 0.77 (95%CI, 0.59–1.02), and on intracranial hemorrhage progression a RR of 0.76 (95%CI, 0.58–0.98). No serious adverse effects (such as thromboembolic events) associated with TXA group were reported in the included trials.
CONCLUSION
Pooled results from the two RCTs demonstrated statistically significant reduction in intracranial hemorrhage progression with TXA and a non-statistically significant improvement of clinical outcomes in ED patients with TBI. Further evidence is required to support its routine use in patients with TBI.
Keywords: Brain injuries, traumatic brain injury, intracranial hemorrhage, tranexamic Acid
INTRODUCTION
Traumatic Brain Injury (TBI) is a major cause of death and disability in the United States, accounting for an estimated 1.4 million emergency department (ED) visits, 275,000 hospitalizations and 52,000 deaths each year.1 It also exerts substantial burden on the cost of health care in the United States with an estimated cost of 60 billion dollars annually.2
Secondary brain injury from progressive intracranial bleeding, cerebral edema, increased intracranial pressure, and subsequent cerebral ischemia is the primary cause of morbidity and mortality following TBI.3,4,5,6 Secondary brain injury is worsened by post-traumatic coagulopathy, which occurs in a third of brain injured patients and is associated with a ten-fold increase in risk of death.4,7,8
Recently, the antifibrinolytic agent tranexamic acid (TXA) demonstrated improved mortality compared to placebo in severely bleeding trauma patients in the CRASH-2 trial, which enrolled 20,211 patients in 40 countries.9 In addition to the robust data demonstrating clinical benefit in trauma patients with severe bleeding, TXA also has an excellent safety profile10 and has been shown to be cost-effective.11 Because of the mechanistic potential for TXA to decrease secondary brain injury it has been considered as a possible therapy to improve clinically important outcomes in patients with TBI.
The objective of this systematic review was to address the following research question: In ED patients with or at risk of TBI (Patients) does administration of tranexamic acid (Intervention) compared to placebo (Comparison) improves patients’ outcomes such as reduction in mortality, neurological function, and hemorrhage progression (Outcome)?
METHODS
This systematic review was conducted following the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA) recommendation.12 The authors followed a pre-designed protocol for literature search, trial selection, data abstraction, quality assessment of trials, and reporting the results.
The following inclusion/exclusion criteria were applied for selecting eligible trials:
Participants: ED patients with or at risk of intracranial hemorrhage (ICH) secondary to TBI. We considered any plausible definition for TBI and “at risk of ICH” used by original articles.
Intervention: Intervention consisted of tranexamic acid administration at any dose, route, and time after TBI.
Control: Placebo administration.
Outcomes: The primary outcome measures were a. death due to any cause following TBI assessed at the end of the follow-up period scheduled by original studies (e.g. in-hospital, 30-day, 6-month, etc.). b. Neurological outcomes measured by any criteria proposed by the original studies such as Glasgow Outcome Score (GOS) and discharge status. The secondary outcomes included hemorrhage progression, transfusion requirement, the need for neurosurgical intervention, and adverse effects (such as thromboembolic events) associated with the use of tranexamic acid. We also considered additional radiologic outcomes such as development of new hemorrhage, mass effect, midline shift, if reported by the original studies.
Study Designs: Only randomized or quasi-randomized controlled trials that compared the impact of tranexamic acid compared to placebo on outcomes of TBI patients were considered for inclusion.
Literature search
Using a pre-designed search strategy developed by an expert medical librarian (LF), databases including MEDLINE (1946 to March 2014), EMBASE (1980 to March 2014), CINAHL (1981 to March 2014), and the Cochrane Library were searched. Additional databases searched included Web of Science, Google Scholar, and clinicaltrials.gov. The authors also searched the proceedings of emergency medicine, hematology, trauma, neurology, and neurosurgery conferences to look for relevant presented abstracts. In addition we reviewed the bibliographies of pertinent articles for citations of eligible studies not identified in the electronic databases. Lastly, the experts in the field were contacted to solicit information about possible ongoing, unpublished studies. The MEDLINE and EMBASE search strategies are presented in the Appendix.
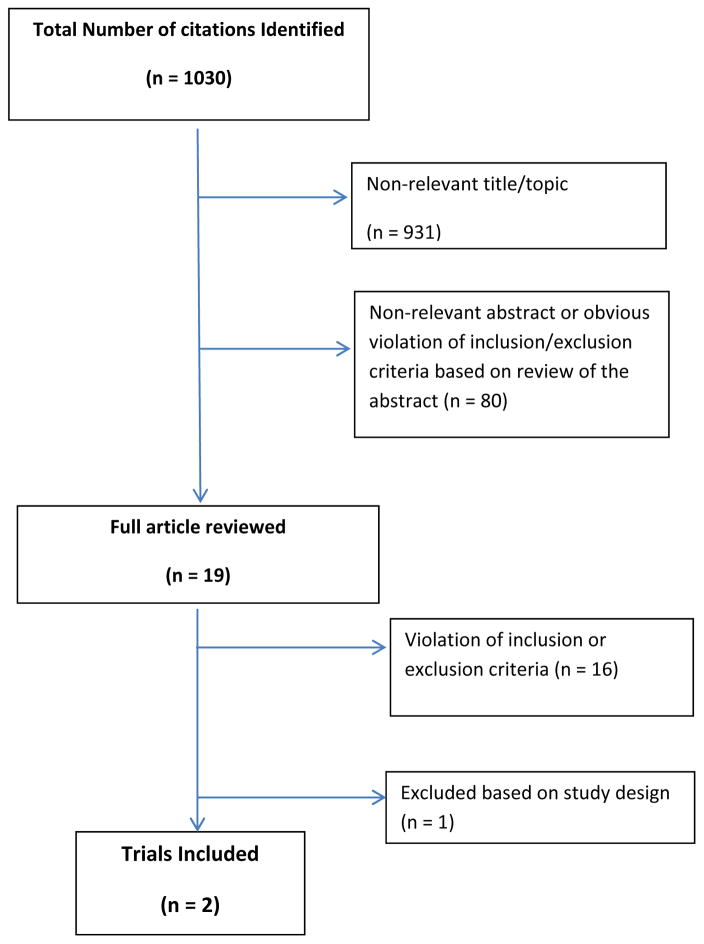
Two authors (SZ and SGA), working independently, reviewed all references and obtained the full text of potentially relevant articles. Disagreements were resolved by consensus. The search identified a total of 1,030 citations from the databases. After excluding non-relevant articles based on their titles and abstracts (Figure 1), 19 full text articles were reviewed. Two randomized controlled trials met the eligibility criteria.13,14 Searching the gray literature identified no additional studies. However, contacting the experts and searching the clinical trials registration website revealed an ongoing large multicenter trial (CRASH-3) on the topic with estimated completion date of 2016.15
Figure 1.
Flow diagram of study selection process for systematic review.
Data Extraction
Data from the identified studies were abstracted independently by two of the authors (SZ and SGA) using a standardized form. When more than one technique was used to measure an outcome; data on all measures used were extracted separately. For studies with incomplete quantitative information available, attempts were made to obtain data from the study authors.
The dichotomous outcomes were reported by percentages and 95% confidence intervals and relative risks. Data reported as continuous variables (e.g., size of hematoma) were summarized as mean with standard deviation or medians with quartiles, whichever used in the original studies.
Quality Assessment
We used “Grading quality of evidence and strength of recommendations” (GRADE criteria) to assess the quality of the included trial and rate the level of evidence.16 Two authors (SZ and DKN) independently assessed the quality of the included trials. Their agreement on the assessment criteria was measured by kappa.
Quantitative Data Synthesis
The effect of TXA on dichotomous outcomes was assessed using a random effects model since the trials were expected to be heterogeneous in their design and patient populations. Relative risk and 95% confidence intervals were calculated. We quantitatively synthesized three outcome measures from the two randomized trials -- in-hospital mortality, unfavorable functional status, and significant hemorrhage growth. The definitions of significant intracranial hemorrhage and in-hospital mortality differed slightly between the two trials.13,14 Because both trials used slightly different measures of functional status at hospital discharge, we defined the outcome of unfavorable functional status as death, vegetative statue, fully dependent requiring constant attention, or dependent but not requiring constant attention.
Statistical heterogeneity was examined using the chi-square and I2 tests for heterogeneity. Data were analyzed using STATA 11.0 statistical software (STAT Corp, College Station, TX) with weighting for size of the trial.
RESULTS
Two randomized controlled trials met the inclusion criteria.13,14 The characteristics of the included trials are listed in table 1.
Table 1.
Characteristics of the included trials.
| Study | Patients | Intervention | Comparison | Outcomes |
|---|---|---|---|---|
| CRASH-2 Intracranial Bleeding Study, 2011, 13 |
Institution: 10 hospitals in India and Colombia Population: 270 adult (≥16 years old) Inclusion criteria: CRASH-2 inclusion criteria (trauma with significant hemorrhage [SBP <90 mmHg or heart rate>110 or both], or at risk of significant hemorrhage, within 8 hours of injury ) plus TBI (GCS≤14 and a brain CT compatible with TBI) Exclusion criteria: pregnant women and patients for whom a second brain CT was not possible |
TXA 1 gram intravenously (IV) over 10 minutes followed by an IV infusion of 1 gram over 8 hours | Matching Placebo |
Primary: Total hemorrhage growth from the first (before randomization) to second CT scan (24–48 hours later) Secondary: (1) Significant of hemorrhage growth defined as an increase by ≥ 25% of total hemorrhage in relation to its initial volume, (2) New intracranial hemorrhage, (3)change in subarachnoid hemorrhage grade, (4) mass effect, (5) new focal cerebral ischemia, and (6) clinical outcomes (death from any cause, dependency, and need for neurosurgical intervention) Clinical outcomes were measured upon discharge, at 28 days, or at death, whichever came first CT outcomes were measured in 249 patients who had first and second CT. Clinical outcomes were measured in all patients (n=270) |
| Yutthakasemsunt et al, 2013 14 |
Institution: single center study in Thailand Population: 240 adult (>16 years old) Inclusion criteria: non-penetrating TBI (GCS score 4 – 12) within 8 hours of onset and with no indication for emergency neurosurgical intervention Exclusion criteria: patients with coagulopathy and elevated serum creatinine (>2mg/dl) |
TXA 1 gram IV over 30 minutes followed by an IV infusion of 1 g over 8 hours | Matching Placebo |
Primary: 1. Progression of intracranial hemorrhage revealed by CT scan at 24 hours (defined as new ICH on second CT, expansion of existing ICH by 25% or more; 2. increase in pressure effect (increase in midline shift of greater than 1 mm or increase in basal cistern between first and second CT) Secondary: In-hospital mortality, Glasgow Outcome Scale at hospital discharge, blood transfusion requirement, neurosurgical intervention, and any in-hospital thromboembolic events |
Abbreviations: TBI, traumatic brain injury; GCS, Glasgow Coma Scale, CT, computed tomography; TXA, tranexamic acid, SBP, systolic blood pressure.
Both studies were high of high quality. Kappa representing the agreement of the two authors on elements of quality assessment was 1.0. The details of the quality assessment of the two trials are listed in table 2.
Table 2.
Quality assessment of the included trials.
| Criteria | CRASH-2, 2011 13 | Yutthakasemsunt et al, 2013 14 |
|---|---|---|
| Randomization | Yes, patient randomization was balanced by center and with an allocation sequence based on a block size of eight, generated with a computer random number generator. | Yes, computer randomization with random block size. |
| Concealment | Yes, allocations were masked. Both randomization and allocation assignments were kept in a different city by an international coordinating center. The study drug and the placebo ampoules were indistinguishable. | Yes, group assignments were kept in opaque sealed envelopes. |
| Intention-to-treat analysis | Yes | Yes |
| Blinding | Yes, double-blinded (subjects and investigators). | Yes, subjects, caregivers, and outcome assessors were blinded. |
| Follow-up | 270/270 (100%) with clinical follow up 249/270 (92.2%) for CT outcomes (21 missed: 10 in treatment group and 11 in placebo group) |
227/238 (95.4%) had primary outcome analyzed (11 missed [5 in treatment group and 6 in placebo]: 2 inappropriate consent, 7 dead, 1 agitated, 1 refused) |
| Outcome Reporting bias | None identified | None identified |
| Quality of evidence | High | High |
The results of the included trials are summarized in table 3. CRASH-2 Intracranial Bleeding Study (ICB)13 also adjusted the outcomes by Glasgow Coma Scale (GCS) score, age, time from injury to first computed tomography, time from injury to second computed tomography, and initial hemorrhage volume. The results of the adjusted outcome analysis are presented in table 4.
Table 3.
Summary of the reported outcomes by the included trials comparing tranexamic acid to placebo in ED patients with traumatic brain injury.
| Study | Outcomes | TXA n/N % (95% CI) |
Placebo n/N % (95% CI) |
Relative Risk (95%CI) |
|---|---|---|---|---|
| CRASH-2 ICB Study, 2011 13 | Total hemorrhage growth (mean ± SD) a | 5.9 ml (± 27) | 8.1 ml (± 29) | b |
| Significant hemorrhage growth c | 44/123; 36% (28 – 45%) | 56/126; 44% (36 – 53%) | 0.80 (0.59 – 1.09) | |
| Area of new hemorrhage on repeat CT (not seen on initial CT) | 13/123; 11% (6 – 17%) | 20/126; 16% (11 – 23%) | 0.66 (0.35 – 1.28) | |
| Change in subarachnoid hemorrhage grade | − 0.11 | − 0.12 | d | |
| Presence of mass effect on CT | 58/123; 47% (39 – 56%) | 76/126; 60% (52 – 68%) | 0.78 (.06 – 0.99) | |
| New focal cerebral ischemia | 6/123; 5% (2 – 10%) | 12/126; 10% (6 – 16%) | 0.51 (0.19 – 1.32) | |
| Mortality at discharge or 28 days (whichever came first) | 14/133; 11% (6 – 17%) | 24/137; 18% (12 – 26%) | 0.60 (0.33 – 1.11) | |
| Dependency in survivors e | 26/119; 22% (15 – 30%) | 29/113; 26% (19 – 34%) | 0.85 (0.54 – 1.35) | |
| Need for neurosurgical intervention at discharge or 28 days | 20/133; 15% (10 – 22%) | 21/137; 15% (10 – 22%) | 0.98 (0.56 – 1.72) | |
| Composite outcome f | 60/133 45% (36 – 54%) | 80/137; 58% (50 – 67%) | 0.77 (0.61 – 0.98) | |
| Yutthakasemsunt et al, 2013 14 | Significant hemorrhage growth g | 21/120; 18% (12 – 25%) | 32/118; 27% (20 – 36%) | 0.65 (0.39 – 1.05) |
| Increase in intracranial pressure effect h | 11/114; 10% (5 – 16%) | 12/115; 11% (6 – 17%) | 0.92 (0.60 – 1.40) | |
| Improved Glasgow Coma Scale motor score at 24 hours | 37/120; 31% (23 – 40%) | 37/118; 31% (23 – 41%) | 0.98 (0.67 – 1.40) | |
| In-hospital mortality | 12/120; 10% (6 – 17%) | 17/118; 14% (9 – 22%) | 0.69 (0.35 – 1.39) | |
| Unfavorable Glasgow Outcome Scale at hospital discharge i | 21/120; 18% (12 – 25%) | 27/118; 23% (16 – 31%) | 0.76 (0.46 – 1.27) | |
| Blood transfusion requirement | 31/120; 26% (19 – 34%) | 33/118; 28% (21 – 37%) | 0.92 (0.60 – 1.40) | |
| Need for neurosurgical intervention | 3/120; 3% (1 – 7%) | 0/118; 0% (0 – 3%) j | 5.95 (0.30 – 117) | |
| In-hospital thromboembolic events | 0/120; 0% (0 – 3%) j | 4/118; 3% (1 – 8%) | 0.12 (0.01 – 2.28) |
Defined as change in total volume from all hemorrhagic lesions between initial and repeat CT (at 24–48 hours)
Unadjusted : reduction of −2.1 ml, 95% CI −9.8 to 5.6; adjusted: reduction of −3.8 ml, 95% CI, −11.5 to 3.9, p= 0.33
Defined as ≥ 25% increase in total volume from all hemorrhagic lesions between initial and repeat CT (at 24–48 hours)
p= .93
Measured using the five point modified Oxford Handicap Score (mOHS) and dichotomized into dependent (fully dependent required attention day and night or dependent not requiring constant attention) or independent (some restriction in lifestyle but independent, minor symptoms, or no symptoms)
Significant hemorrhage growth, area of new hemorrhage, new focal cerebral ischemic lesion, need for neurosurgical intervention, death
Defined as ≥ 25% increase of intracranial hemorrhage in any dimension (height, length, or width) or new intracranial hemorrhage between initial and repeat CT (at 24 +/− 8 hours)
Defined as increase in midline shift of greater than 1 mm or an increase in basal cistern between the first and second CT scan
Defined as death, persistent vegetative state, and severe disability
0.5 added to both cells to obtain confidence intervals
Abbreviations: TXA (tranexamic acid); SD, standard deviation; CI, confidence interval; CT, computed tomography
Table 4.
The adjusted analysis of the reported outcomes in the CRASH-2 Intracranial Bleeding Study, comparing tranexamic acid to placebo in ED patients with traumatic brain injury.
| Study | Outcomes | TXA n/N % (95% CI) |
Placebo n/N % (95% CI) |
Adjusted Odds Ratio a (95%CI) |
|---|---|---|---|---|
| CRASH-2 ICB Study, 2011 13 | Total hemorrhage growth (mean ± SD) b | 5.9 ml (± 27) | 8.1 ml (± 29) | c |
| Significant hemorrhage growth d | 44/123; 36% (28 – 45%) | 56/126; 44% (36 – 53%) | 0.67 (0.40 – 1.13) | |
| Area of new hemorrhage on repeat CT (not seen on initial CT) | 13/123; 11% (6 – 17%) | 20/126; 16% (11 – 23%) | 0.62 (0.28 – 1.35) | |
| Presence of mass effect on CT | 58/123; 47% (39 – 56%) | 76/126; 60% (52 – 68%) | 0.59 (0.35 – 0.97) e | |
| New focal cerebral ischemia | 6/123; 5% (2 – 10%) | 12/126; 10% (6 – 16%) | 0.49 (0.18 – 1.44) | |
| Mortality at discharge or 28 days (whichever came first) | 14/133; 11% (6 – 17%) | 24/137; 18% (12 – 26%) | 0.47 (0.21 – 1.04) | |
| Dependency in survivors f | 26/119; 22% (15 – 30%) | 29/113; 26% (19 – 34%) | 0.66 (0.32 – 1.36) | |
| Need for neurosurgical intervention at discharge or 28 days | 20/133; 15% (10 – 22%) | 21/137; 15% (10 – 22%) | 0.98 (0.45 – 1.93) | |
| Composite outcome g | 60/133 45% (36 – 54%) | 80/137; 58% (50 – 67%) | 0.57 (0.33 – 0.98) |
Adjusted by Glasgow Coma Scale score, time from injury to first CT, time from injury to second CT, and initial hemorrhage volume
Defined as change in total volume from all hemorrhagic lesions between initial and repeat CT (at 24–48 hours)
Reduction of −3.8 ml, 95% CI, −11.5 to 3.9, p= 0.33
Defined as ≥ 25% increase in total volume from all hemorrhagic lesions between initial and repeat CT (at 24–48 hours)
Adjusted by Glasgow Coma Scale score, time from injury to first CT, time from injury to second CT, initial hemorrhage volume, and initial mass effect
Measured using the five point modified Oxford Handicap Score (mOHS) and dichotomized into dependent (fully dependent required attention day and night or dependent not requiring constant attention) or independent (some restriction in lifestyle but independent, minor symptoms, or no symptoms)
Significant hemorrhage growth, area of new hemorrhage, new focal cerebral ischemic lesion, need for neurosurgical intervention, death
Abbreviations: TXA, Tranexamic acid; SD, standard deviation; CI, confidence interval; CT, computed tomography
The forest plots representing the pooled analysis of data pertaining to the main outcomes are shown in Figure 2.
Figure 2.
Forest plots representing the effect of tranexamic acid on outcome of patients with traumatic brain injury.
a- defined as death, vegetative state, or fully dependent requiring attention day and night or dependent but not requiring constant attention
b- defined as ≥ 25% increase in total volume from all hemorrhagic lesions between initial and repeat CT (at 24–48 hours)
Abbreviations: RR, relative risk; CI, confidence intervals; df, degrees of freedom
No adverse events related to tranexamic acid was reported in CRASH-2 ICB.13 In the study by Yutthakasemsunt et al,14 four cases of in-hospital thromboembolic events were documented in the placebo group but none was reported in the tranexamic acid group.
We were not able to examine the impact of tranexamic acid on outcome of TBI patients with isolated head injury alone. In Yutthakasemusunt’s study, 17% (n=20) and 14% (n=16) of patients in tranexamic acid and placebo groups had isolated head injury, respectively.14 The authors did not evaluate the outcomes in this subgroup because of the small sample size. Similarly, the CRASH-2 ICB13 authors also indicated that only a small number of patients in their study had isolated head injury and the outcomes could not be assessed in this particular subgroup.
DISCUSSION
We identified two, high-quality clinical trials that tested the hypothesis that administration of TXA to patients with TBI would reduce hematoma growth compared to placebo.13,14 Both trials were powered to detect a difference in intracranial hemorrhage progression (initial and repeat head CTs) but also evaluated clinical outcomes as secondary outcome measures.13,14
While not statistically significant, both trials did demonstrate a trend towards decreased intracranial hemorrhage progression in the TXA cohort compared to placebo. 13,14 This trend was noted in a number of different measures of intracranial hemorrhage progression including total volumetric growth, proportion with significant (25%) hemorrhage growth, new area of hemorrhage, and the presence of mass effect. Both trials also demonstrated a slight trend (non-statistically significant) towards improved mortality in the TXA cohort. 13,14
After pooling the data pertaining to the three outcomes of in-hospital mortality, functional status, and hemorrhage progression, the meta-analysis revealed a statistically significant reduction in hemorrhage progression in TBI patients receiving tranexamic acid. The pooled relative risks for in-hospital mortality and functional status were not statistically significant.
The use of TXA for TBI to improve clinical outcomes is based on the theory that TXA may limit secondary brain injury through two mechanisms. First, TXA, as an antifibrinolytic agent, may limit fibrinolysis and thus intracranial hemorrhage progression. Fibrinolysis is common in TBI and has been shown to be a strong independent predictor of intracranial hemorrhage progression.17 Second, TXA may inhibit the effect of tissue plasminogen activator (tPA), which plays a role in peri-lesional edema.18
The results of these two trials suggesting a trend towards decreased intracranial hemorrhage progression with early administration of TXA should be viewed with caution. Hematoma expansion has been associated with poor outcome in patients with TBI.19 Although this outcome likely lies on the causal pathway to clinical outcomes such as functional status and mortality, surrogate outcomes do not always translate into actual clinical outcomes.20 For example, while an initial phase II clinical trial demonstrated reduction in the hematoma growth and mortality after administration of activated factor VII to patients with non-traumatic intracranial hemorrhage,21 subsequent phase III trial confirmed the reduction in hematoma growth but failed to show improved survival or functional outcomes.22
LIMITATIONS
Some limitations exist in the current body of evidence. We did not identify any studies that were adequately powered to detect any clinical outcomes. There was some heterogeneity between identified studies, particularly in the inclusion criteria. The CRASH-2 Intracranial Bleeding Study13 enrolled a broader range of TBI, with 45% having mild TBI [GCS score 13–15]), compared to Yutthakasemsunt et al,14 which enrolled patients with moderate to severe TBI (GCS score 4–12). Almost all patients in the CRASH-2 Intracranial Bleeding Study13 had significant extracranial injuries. Because TXA has proven mortality benefit in patients with significant hemorrhage,9 the trend towards improved mortality in this study may be a result of limiting extracranial hemorrhage progression by tranexamic acid and the benefits might not be the same in patients with isolated TBI. CRASH-2 ICB13 study also had a protocol deviation leading to enrollment of 31 (11%) patients with a GCS score of 15 and 7 (3%) patients with a normal initial head CT. TXA would unlikely have any clinical benefit in these patients. This issue could have diluted the results of study.
Neither trial was able to examine the outcomes in patients with isolated head injury due to the small number of such patients in both trials. Therefore, it is difficult to distinguish the effects of tranexamic acid in TBI from that of polytrauma.
The included studies did not account for patients receiving anticoagulants or antiplatelet agents. Such therapies can significantly increase hemorrhage progression post TBI and thus interfere with TXA effects. However, the mean age of the patients recruited in both studies was less than 38 years and, therefore a population less frequently treated with antiplatelet and anticoagulant therapy.13,14
While the study by Yutthakasemsunt et al14 only enrolled non-penetrating TBI, the CRASH-2 ICB13 didn’t categorize TBI into blunt or penetrating. The mechanism of injury could be a confounder that needs to be examined in future trials.
Lastly, the meta-analysis was performed with only two trials. While this fact limits the generalizability of the findings, the high quality of the included trials and absence of significant heterogeneity validates the analysis.
CONCLUSION
Pooled results from the two RCTs demonstrated statistically significant reduction in intracranial hemorrhage progression with TXA and a non-statistically significant improvement of clinical outcomes in ED patients with TBI. Despite an excellent safety profile, further evidence is required to support the routine use of tranexamic acid in patients with TBI. An ongoing, international, multicenter, phase III trial (CRASH-3)15 evaluating the use of TXA on death and disability in patients with TBI with a planned enrollment of 10,000 patients will certainly shed light on this particular question.
Supplementary Material
Footnotes
Conflict of interest: SZ, LF, and DKN have no conflict of interest to report. SGA owns stock options in Bio-Signal Group Corporation and is a co-inventor on US patents pending 61/554,743 and 13/284, and 886. He is also the principal investigator of an NIH funded trial 5R41HD072881-02.
Contributor Information
Shahriar Zehtabchi, Email: Shahriar.zehtabchi@downstate.edu, Department of Emergency Medicine, State University of New York, Downstate Medical Center, Brooklyn, NY.
Samah G Abdel Baki, Email: samahabdulbaki@gmail.com, Bio-Signal Group Corporation., Brooklyn, NY.
Louise Falzon, Email: af2215@cumc.columbia.edu, Center for Behavioral Cardiovascular Health, Department of Medicine, Columbia University Medical Center, New York, NY.
Daniel K Nishijima, Email: daniel.nishijima@ucdmc.ucdavis.edu, Department of Emergency Medicine, University of California, Davis, Sacramento, CA.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2.Olin GLRJ. AHRQ, editor. The five most costly medical conditions, 1997 and 2002: estimates for the US civilian noninstitutionalized populations. Rockville, MD: 2006. [Google Scholar]
- 3.Shackford SR, Mackersie RC, Davis JW, Wolf PL, Hoyt DB. Epidemiology and pathology of traumatic deaths occurring at a Level I Trauma Center in a regionalized system: the importance of secondary brain injury. J Trauma. 1989;29:1392–7. doi: 10.1097/00005373-198910000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Narayan RK, Maas AI, Servadei F, Skolnick BE, Tillinger MN, Marshall LF. Progression of traumatic intracerebral hemorrhage: a prospective observational study. J Neurotrauma. 2008;25:629–639. doi: 10.1089/neu.2007.0385. [DOI] [PubMed] [Google Scholar]
- 5.Graham DI, Ford I, Adams JH, Doyle D, Teasdale GM, Lawrence AE, McLellan DR. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52:346–50. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall LFGT, Klauber MR. The outcome of severe closed head injury. J Neurosurg. 1991;75:S28–36. [Google Scholar]
- 7.Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien) 2008;150:165–75. doi: 10.1007/s00701-007-1475-8. [DOI] [PubMed] [Google Scholar]
- 8.Talving P, Benfield R, Hadjizacharia P, Inaba K, Chan LS, Demetriades D. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma. 2009;66:55–61. doi: 10.1097/TA.0b013e318190c3c0. [DOI] [PubMed] [Google Scholar]
- 9.CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 10.Roberts I, Shakur H, Ker K, Coats T. CRASH-2 Trial collaborators. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2012;12:CD004896. doi: 10.1002/14651858.CD004896.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Guerriero C, Cairns J, Perel P, Shakur H, Roberts I CRASH 2 trial collaborators. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One. 2011;6:e18987. doi: 10.1371/journal.pone.0018987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Last accessed April 17, 2014]; Available at: http://www.prisma-statement.org.
- 13.CRASH-2 Collaborators, Intracranial Bleeding Study. Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study) BMJ. 2011;343:354. doi: 10.1136/bmj.d3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yutthakasemsunt S, Kittiwatanagul W, Piyavechvirat P, Thinkamrop B, Phuenpathom N, Lumbiganon P. Tranexamic acid for patients with traumatic brain injury: a randomized, double-blinded, placebo-controlled trial. BMC Emerg Med. 2013;13:20. doi: 10.1186/1471-227X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewan Y, Komolafe EO, Mejía-Mantilla JH, Perel P, Roberts I, Shakur H CRASH-3 Collaborators. CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012;13:87. doi: 10.1186/1745-6215-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Bayir A, Kalkan E, Koçak S, Ak A, Cander B, Bodur S. Fibrinolytic markers and neurologic outcome in traumatic brain injury. Neurol India. 2006;54:363–5. doi: 10.4103/0028-3886.28106. [DOI] [PubMed] [Google Scholar]
- 18.Figueroa BE, Keep RF, Betz AL, Hoff JT. Plasminogen activators potentiate thrombin-induced brain injury. Stroke. 1998;29:1202–7. doi: 10.1161/01.str.29.6.1202. [DOI] [PubMed] [Google Scholar]
- 19.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–13. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 21.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 22.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T FAST Trial Investigators. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.