Abstract
Particulate matter (PM), a component of air pollution, has been shown to enhance allergen-mediated airway hypersensitivity and inflammation. Surprisingly, exposure to PM during the sensitization to allergen is sufficient to produce immunological changes that result in heightened inflammatory effects upon future allergen exposures (challenge) in the absence of PM. This suggests that PM has the ability to modulate the allergic immune response, thereby acting as an adjuvant by enhancing the immunological memory formed during the adaptive immune response; however, the mechanisms through which this occurs remain elusive. Establishing a reproducible animal model to study the PM-mediated immunotoxicological effects that enhance allergy, may provide insights to understand how air pollution activates the immune system and thereby modulates the pathophysiology of asthma. The protocol detailed below can be used to study various characteristics of air pollution, such as PM size, source, or chemical composition, to help elucidate how such features may affect the allergic response in a mouse model of asthma. Using a BALB/c model of acute exposure (14 days) mice are first sensitized with allergen and PM then subsequently challenged with allergen only. The endpoints of this protocol include the assessment of inflammation via cells recovered from broncho-alveolar lavage (BAL), histopathological analysis, gene expression profiles, and protein quantification of inflammatory markers.
Keywords: Allergic Mouse Model, Particulate Matter (PM), Air Pollution, Allergen, Ovalbumin (OVA), House Dust Mite (HDM), Airway Inflammation
INTRODUCTION
Allergic asthma is characterized by airway inflammation and hypersensitivity in individuals that become sensitized to innocuous allergens. Common human allergens that trigger asthmatic attacks are proteins derived from sources such as pollen, dust mites, animal dander, and mold spores. Allergic asthma develops when the immune system fails to develop tolerance towards an inhaled protein antigen and subsequently develops immunological memory towards that antigen. Primarily, this occurs through antigen-specific IgE antibodies and T helper type 2 (Th2) immune responses. Upon subsequent encounters with the antigen (i.e., allergen), IgE-allergen complex binding to FcεRI-receptors on mast cells and basophils triggers these cells to become activated and release inflammatory mediators, such as cytokines and chemokines as well as broncho-constricting mediators, such as histamine, leukotrienes, and prostaglandins. Mast cell- and eosinophil-mediated cytokine release induces inflammation by recruiting additional immune cells into the airways as well as reinforcing Th2-cell differentiation. Collectively, these events elicit coughing, wheezing, and shortness of breath commonly observed during asthmatic attacks.
The immunotoxicity that develops from exposure to particulate matter may manifest itself as hyperactivation and/or misregulation of the immune response. Clinically, this may predispose individuals to disease or enhance morbidity and mortality of existing conditions, such as asthma, COPD, pneumonia, and cardiovascular disease (Anderson et al., 2012; Samet et al., 2000). Interestingly, the inflammatory range of PM may extend beyond the lung, the organ of primary deposition. In recent years PM exposure has been linked to autism, ischemic stroke, schizophrenia, and cancer; however, these relationships are correlative and casual relationships have yet to be established (Kalkbrenner et al., 2015; Pedersen et al., 2004; Turner et al., 2011; Wellenius et al., 2012).
The incidence of asthma and allergic diseases has steadily risen in the past few decades (Moorman et al., 2012). With respect to asthma, epidemiological studies have demonstrated a higher incidence of disease in areas of high air pollution, particularly traffic related pollution (Bowatte et al., 2015). This relationship has been extensively modeled in animals, primarily rodents, where either single particles, laboratory generated particles, or ambient particles are given in conjunction with an allergen to study how PM modulates the allergic immune response. Nonetheless, the immuno-toxicological mechanisms by which PM modulates the immune system still remain unclear. It should be noted that PM mixtures are highly complex in chemical composition as they differ with source and thus, differentially affect the immune mediated inflammatory response with some fractions of PM enhancing the allergic response and others producing no change (Carosino et al., 2015).
The protocol described can be used to investigate the in-vivo immunotoxicological mechanisms through which PM exacerbates the pulmonary allergic immune response. A key component of this model is the ability to assess the potential toxicity of PM that has been characterized by particle size (PM10, PM2.5, PM0.1), ambient source (urban, rural, vehicular emissions, etc.), predominant composition (major/trace metals, inorganic ions, polyaromatic hydrocarbons, organic compounds, or elemental/organic carbons), or time collection points (seasonal, day/night). The Basic Protocol describes the sensitization of mice (days 1, 3 and 5) with either allergen alone or the combination of allergen with PM. The appropriate time is allotted for the formation of immunological memory (days 6-11), and mice are challenged (days 12-14) with allergen alone to induce an allergic response. Exposure to PM during allergen sensitization is sufficient to alter immunological memory formation as the subsequent allergen challenge (in the absence of PM) enhances the inflammatory response compared to mice only sensitized and challenged with allergen. This protocol, therefore, allows the investigator to assess how various types of PM modulate the formation of immunological memory against the allergen.
To characterize PM-mediated inflammatory changes during the allergic response this protocol outlines various assays including broncho-alveolar lavage (BAL) to assess airway inflammation via recovered immune cells, gene expression analysis via qPCR to determine upregulation of genes that mediate inflammation, and collection of total lung protein to perform enzyme-linked immunosorbent assay (ELISA) or other protein based assays to confirm gene expression changes. Furthermore, the reader is referred to a Support Protocol (Zeller, 2001) for histopathological analysis to determine pulmonary inflammation and assessment of PM immunotoxicity.
BASIC PROTOCOL
INTRANASAL SENSITIZATION (ALLERGEN AND PARTICULATE MATTER) AND CHALLENGE (ALLERGEN ALONE)
The mouse model used for this protocol is the BALB/c mouse, a strain that is Th2-dominant as opposed to the C57BL6 mouse that is Th1-dominant (Nials and Uddin, 2008). BALB/c mice develop the classical characteristics of an allergic response, including Th2-immune responses, allergen-specific IgE, eosinophilic airway inflammation, and airway hyperresponsiveness (AHR). An investigator may choose to use a different mouse strain than the BALB/c to accommodate their scientific question, but note that the allergic response may differ.
Mice are intranasally sensitized with allergen on days 1, 3 and 5. This is followed by a seven day rest period to allow for the formation of the adaptive immune response towards the allergen. Mice are intranasally challenged with allergen on days 12, 13, and 14 to elicit an allergic inflammatory response. Finally, on day 15, mice are euthanized to collect BAL and lung tissue for assessment of inflammation. Proper controls should be utilized for this study and should include a control group (administration of the delivery vehicle-only for sensitization and challenge periods) and a PM control treatment group (administration of PM-only during the sensitization period and delivery vehicle-only for the challenge period).
Mice are sensitized using either ovalbumin (OVA) or house dust mite (HDM). Based on work from our lab, we have shown that ambient PM enhances both OVA and HDM allergic responses (unpublished data) (Carosino et al., 2015). OVA has been used extensively in rodent models of human asthma. Specifically in BALB/c mice OVA elicits many of the features of human asthma although the limitations are modest pulmonary inflammation and mild AHR. Although OVA can cause occupational asthma in individuals working in egg processing facilities, house dust mite is a more humanly relevant allergen and is one of the most common allergens to trigger asthma. Similar to OVA, HDM mouse models of human asthma also elicit many of the features commonly seen in human asthma. Unlike OVA, which is a single protein, commercially available HDM allergen (Greer® Laboratories) is composed of whole ground dust mites (D. farinae or D. pteronyssinus) that contain a much more diverse array of allergens and pattern recognition receptor ligands (proteases, lipopolysaccharides/endotoxin, β-glucans, and chitin) (Gregory and Lloyd, 2011). The use of whole dust mites in HDM allergen preparation results in an allergen rich in endotoxin and more closely mimics natural human exposure. Additionally, endotoxin in HDM may have differential inflammatory effects via Toll-like receptors, OVA on the contrary is usually relatively low in endotoxin. As a result of the spectrum of allergens in HDM and endotoxin contamination, HDM produces a more robust inflammatory response in the lung compared to OVA when both are administered intranasally (sensitization and challenge) on an equal mass basis (unpublished data). Since endotoxin enhances inflammation, the amount of endotoxin should be screened with a limulous amoebocyte lysate test (discussed later). OVA sensitization may be performed via intraperitoneal (i.p.) administration with alum and challenged by OVA inhalation to produce more robust pulmonary inflammation; however, we have had inconclusive results using this method as the robust allergic response masks the inflammatory PM-mediates effects. For this reason, and for the fact that i.p. exposure to OVA is not a normal method of allergy development in humans (i.e., systemic immune responses differ from mucosal immune responses), we recommend the administration of HDM intranasally as it is a humanly relevant allergen that produces substantial inflammatory effects that resemble many of the underlying features of human asthma.
The investigator may choose which source of PM to utilize for their experiment. Commercially available PM is available through the National Institute of Standards and Technology Standard Reference Materials (http://www.nist.gov/srm), with many sources having a chemical characterization profile. Alternatively, ambient PM may be collected by the investigator. We refer the reader to various publications that outline the collection of ambient PM (Ayres et al., 2008; Kulkarni et al., 2011). Furthermore, we strongly emphasize the need to sonicate PM immediately before administration, as once PM is resuspended in solution particle agglomeration occurs rapidly and has the potential to diminish the inflammatory response.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC). Researchers must abide by IACUC guidelines when handling laboratory animals.
NOTE: The investigator should consult the manufacturer’s instruction manual for specific information regarding the operation of specialized instrumentation, equipment, and software as well as appropriate protocol procedures for assays.
Materials
Mice
Allergen (Ovalbumin or House Dust Mite Allergen, Greer® Laboratories)
Particulate matter
Microcentrifuge tubes
Ultrasonic cleaner water bath
Delivery vehicle: saline, phosphate buffered saline (PBS), or Hank’s balanced salt solution (HBSS); use the same delivery vehicle for all further support protocols
Veterinary anesthesia machine with anesthesia induction chamber
Isoflurane
Timer
20μL, 200μL, and 1000μL pipettes with appropriate tips
Balance for weighing mice
Data sheets for recording animal weights and dosing schedule
Prepare Allergen
- Dissolve 1 mg/mL OVA (Grade V; Sigma) or 1 mg/mL HDM allergen (Greer Laboratories) into a sterile and endotoxin-free delivery vehicle (saline, PBS, or HBSS). Gently vortex this “stock solution” and then allow it to dissolve for 1 hr or overnight at 4°C. The solution may be dispensed into aliquots or stored as is at −20°C or −80°C (avoid freeze-thaw cycles).A 25μL dose/per mouse/day at a concentration of 1mg/mL allergen solution will deliver 25 μg of allergen. Common doses used in allergy models in the literature for both OVA and HDM range between 10ug to100ug/per mouse/day. The user may wish to change the dose based on their scientific investigation by re-calculating the “stock solution” to achieve the desired allergen mass while maintaining a dosing volume of 25-30μL as this is an optimal volume to deliver intranasally to mice between 6-8 weeks of age. Avoid exceeding an intranasal delivery volume greater than 50μL.
Prepare Particulate Matter
2. Dissolve 1 mg/mL of PM into the sterile, endotoxin-free delivery vehicle solution to create a stock solution.
3. Place the glass vial or microcentrifuge containing the PM solution in an ultrasonic cleaner water bath at 60 sonics/min for 10-15 min to eliminate particle agglomerates.
- 4. After sonication, immediately aliquot the solution into microcentrifuge tubes for each day of dosing. Freeze the microcentrifuge tubes that will not be used that day at −20°C.Sonication is an essential step that is necessary to prevent PM agglomeration prior to dosing. Failing to sonicate particles each day prior to dosing can result in no change or even a suppression of the allergic response.
- 5. On day 1, administer the PM immediately after sonication as particle agglomeration can occur as quickly as 30 min.Typically, three aliquots (one for each day of dosing) are sufficient for dosing. However when working with a large number of animals, more than 1 aliquot per day may need to be prepared if dosing takes longer than 30 min. The aliquots may have to be sonicated in a staggered time fashion to prevent particle agglomeration.
6. On days 3 and 5, thaw the frozen PM aliquots. Aliquots that are thawed each day for dosing will need to be sonicated 10-15 min to eliminate particle agglomeration.
Intranasal Sensitization with Particulate Matter and Allergen (Days 1, 3, and 5)
Animals will be dosed in two separate rounds. The first round will consist of anesthetizing and administering the PM dose to the appropriate treatment groups (PM-only and allergen+PM groups). The allergen+PM group will be allowed a short recovery period. The second round consists of anesthetizing and administering the delivery vehicle or allergen to the appropriate groups (delivery vehicle control, allergen, and allergen+PM groups). Separating the delivery of PM and allergen in the allergen+PM group ensures that the PM does not bind or agglomerate with the allergen.
7. Mice should be randomly divided into each control/treatment group and tail marked.
8. A veterinary anesthetic machine is used to sedate mice. The system should contain an oxygen tank that feeds into the anesthetic vaporizer, followed by a flowmeter that feeds the vaporized isoflurane into an anesthesia induction chamber. Finally an activated charcoal adsorption filter is attached to the anesthetic chamber to collect isoflurane fumes.
9. Ensure that the anesthetic vaporizer reservoir is filled with isoflurane and is set to “On” with an output of 2%. Open the oxygen tank pressure valve and set the flow rate to 1 L/min. Allow 2-3 min for the anesthesia induction chamber to become saturated with isoflurane.
10. Begin with the PM administration (first round) for the appropriate treatment groups. Ensure that the PM is sonicated immediately before its administration to animals (see Steps 4-5).
- 11. Place a mouse into the anesthesia induction chamber, closing the lid once the animal is inside, and begin the timer. Mice will become unconscious within 1-1.5 min. Allow the animal to remain in the chamber for a total of 3-5 min, this will allow the mouse to undergo a deep pane of anesthesia that will allow the researcher to have the appropriate time to administer the dose intranasally before the animal regains consciousness.The amount of time the mouse should be left in the anesthesia induction chamber depends on weight and age, with older and heavier mice taking longer time to become unconscious. Begin with 3 min and increase the time as necessary.
12. Prior to removing the animal from the anesthetic chamber, pre-load the pipette tip with 15μL of PM solution.
- 13. Remove the unconscious mouse from the chamber and quickly administer the PM dose intranasally. Hold the mouse in the non-dominant hand and using the dominant hand carefully and quickly bring the pipette tip 1-2mm above the nares of the mouse, and in a slow drop-wise manner, alternate administering the drops between each nare. As the animal naturally inspires, the solution will be inhaled into the lungs.The animal will begin to regain partial consciousness within 15-30 sec awake. Therefore, it is important to administer the dose quickly, ideally within 5 sec.
14. Once the dose is administered, return the animal to its cage, preferably in a position that does not allow the dose to reflux into the trachea/nares. If time suffices, the animal can be weighed while under anesthesia. Alternatively, the mice can be weighed prior to dosing. The animal will regain full consciousness within 1-2 min after being removed from the chamber.
15. Reset the timer and repeat the procedure with the remaining mice (Steps 11-14).
16. Administer the delivery vehicle or allergen dose intranasally (second round) to the appropriate treatment groups by repeating Steps 11-15 with the expectation of using 25μL of delivery vehicle or allergen solution, respectively. For animals that receive the combination of allergen and PM, allow the animals to recover at least 15 min after the isoflurane-induced anesthesia before animals are sedated again for allergen administration.
17. Keep a record of the dosed animals or notes if issues arise during the procedure.
Intranasal Challenge with Allergen (Days 12-14)
18. Repeat steps 8-17 (with the exception of not administering PM) using delivery vehicle or allergen-only for intranasal administration on days 12-14. During the challenge period, mice sensitized with PM only will receive the delivery vehicle and mice sensitized with the allergen+PM combination will receive allergen-only (i.e., no PM is administered during the challenge period).
Necropsy and Tissue Collection
Necropsy occurs 24 hr after the final challenge. Mice are euthanized and BAL and lung tissue are collected to assess pulmonary inflammation. The investigator may wish to collect various tissues beyond those outlined in this protocol if systemic PM effects are being assessed or suspected. Ideally, a minimum of 3 people are recommended to perform the necropsy in a day since SUPPORT PROTOCOL 1: Assessment of broncho-alveolar lavage fluid for pulmonary inflammatory cellular profiles will need to be performed on the same day as necropsy.
Materials
Pentobarbital sodium diluted in sterile saline to 65mg/mL
Balance for weighing mice
Absorbent paper (inscribe with animal ID numbers)
Surgical dissecting scissors (blunt)
Forceps (serrated, curved tip, and blunt)
Gauze
Cotton swabs
1mL syringe (optional for cardiac puncture) with 21-gauge hypodermic needle
EDTA coated tubes (optional for cardiac puncture; BD Microtainer®)
Surgical hemostats (serrated and non-serrated)
Braided silk suture (USP sizes 1 and 2-0)
Razor blade
Cannula (21-guage, 1”, blunt end for tracheal cannulation)
1mL syringe (for broncho-alveolar lavage)
Sterile saline, PBS, or HBSS (same as delivery vehicle; for broncho-alveolar lavage)
5mL polystyrene round bottom tubes (BD Falcon™, Fisher Scientific)
Container with ice
Reservoir with liquid nitrogen
Cryogenic tubes (Nunc, Thermo Scientific™)
RNAlater Solution© (optional; Ambion)
Weight boats
4% paraformaldehyde made in PBS
Tissue perfusion system
20mL glass scintillation vials for storing fixed lungs (Sigma-Aldrich)
Data sheets for project date, treatment, animal ID numbers, anesthetic amounts, BAL volume recovery, and lung fixation time
Biohazard disposal bag for animals
Collection of Broncho-alveolar Lavage Fluid and Blood (Optional)
Inject the mouse with 0.3mL of the 65mg/mL pentobarbital solution i.p. and place the animal back in its cage. Once the mouse is incapacitated (1-2 min), remove it from its cage and weigh it.
Position the mouse in a supine position onto a 6”×9” absorbent paper. Stretch and tape all four legs and tail onto the paper.
Ensure the mouse has been euthanized by lightly pinching the toes; there should be no reflex.
Use surgical dissecting scissors to create an incision on the skin from the abdomen up to the throat.
With the non-dominant hand, use tweezers to pull the xiphoid process up, and with the dominant hand, use the scissors to slowly cut up the sternum to open the thoracic cavity. Be specifically careful not to puncture the lungs (begin with small incisions, the lungs will quickly retract once the cavity is punctured).
-
Perform cardiac puncture if blood will be collected and place it in EDTA coated tubes.
To process and analyze plasma, see section ASSESSMENT OF PLASMA IgE.
Secure the diaphragm with a hemostat. Pull the diaphragm downward to expose the thoracic cavity.
Using two tweezers, separate the muscles/fat/connective tissue surrounding the trachea. Once the trachea is isolated, place a 3” suture (USP size 1) underneath the trachea (Fig. 1).
On the anterior side of the trachea, make a 1-2 mm incision above the suture using a razor blade being careful not to severe the trachea as it will retract into the thoracic cavity. Insert a 21-guage blunt-end cannula into the trachea and tie the suture tightly to secure the cannula to the trachea (Fig. 1).
- Use a 1mL syringe loaded with 0.6 mL sterile saline, PBS, or HBSS to perform whole lung lavage. Attach the syringe onto the cannula and slowly instill the solution; the lungs will begin to expand in the thoracic cavity. Once all fluid has been instilled, recover the fluid by slowly drawing the fluid out of the lungs. Without removing the syringe from the cannula, instill and recover the lavage 2 more times for a total of 3 instillations and 3 recoveries using the original 0.6mL solution. After the final recovery, remove the syringe from the cannula and place the lavage fluid in a 5mL round bottom tube (keep on ice).To process broncho-alveolar lavage fluid, refer to section ASSESSMENT OF BRONCHO-ALVEOLAR LAVAGE FLUID FOR PULMONARY INFLAMMATORY CELLULAR PROFILES.It is unlikely to recover the same volume of solution instilled in the lung. When removing the fluid from the lungs, stop pulling on the syringe when there is resistance in the syringe plunger as pulling on the plunger beyond this point may lead to rupturing of the lungs.Optional: Using a non-serrated hemostat, clamp the left primary bronchus prior to broncho-alveolar lavage. This will leave the left lung undisturbed for a more accurate assessment of histology. Use half the volume for lavage (0.3mL). The lavaged right lung lobes can still be used for protein assays, gene expression analysis, or other assays to assess inflammation.
Figure 1.
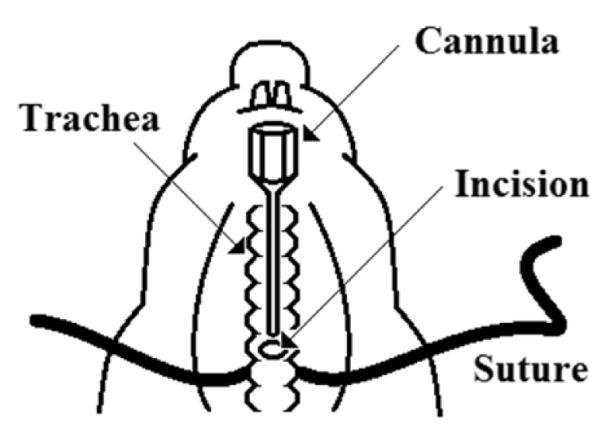
Illustration of how to position the suture behind the trachea, create an incision and cannulate the trachea.
Collection of Lung Tissue
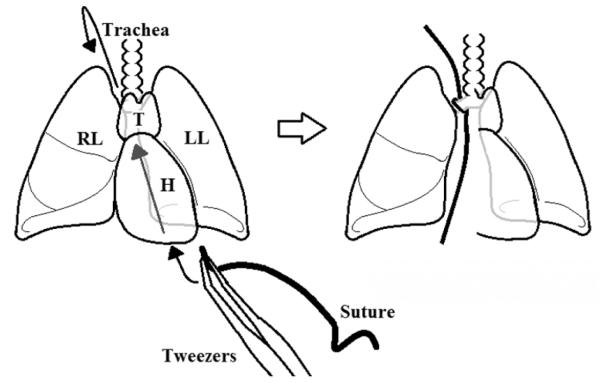
11. Using a suture, tie the right lung where the primary right bronchus exits off the trachea (if the left lung was clamped during lavage, remove the hemostat). Using curved tweezers, grab a 3” suture. Carefully guide the tweezers in the closed position, holding the suture beneath the lungs in a diagonal direction beginning at the bottom right of the thoracic cavity and exiting on the top left of the thoracic cavity. Open the tweezer slightly and grasp the small suture with a second set of tweezers. Carefully pull back the first set of tweezers back down beneath the lungs while being careful not to puncture the lungs. Tie the suture tightly to isolate the right lung lobes (Fig. 2).
- 12. Cut the cranial, middle, caudal, and accessory lobes and place them in the appropriate cryovials.For RNA isolation it is essential that the lung lobe is immediately flash frozen in liquid nitrogen followed by storage at -80°C to prevent enzymatic degradation of RNA. Alternatively, the lung lobe may be infused with RNAlater Solution© immediately after being cut by using a dissecting microscope to locate the bronchus and injecting 0.1-0.2mL RNAlater Solution© with a 1mL syringe attached to a blunt ended cannula.To process one of the right lung lobes for gene expression analysis, refer to section ASSESSMENT OF PULMONARY INFLAMMATION VIA GENE EXPRESSION.To process one of the right lung lobes for protein/ELISA based assays, refer to section ASSESSMENT OF PULMONARY INFLAMMATION VIA PROTEIN LEVELS.
- 13. Remove the left lung with the attached trachea and cannula for inflation fixation of the lung in 4% paraformaldehyde. Turn the absorbent paper 180° and hold the cannula directly up with the non-dominant hand in a manner that does not cause the cannula to tear the trachea. Carefully and slowly cut the muscles/connective tissue attached to the trachea. Once the thoracic cavity is reached, be careful not to puncture the left lungs while cutting. Continue cutting connective tissue, the esophagus, and the aorta to free the left lung/trachea/heart.Use a small tugging motion on the canula while pulling directly up to visualize tissue that are attached to the trachea/left lung and require cutting. Use small incisions while keeping the scissors pointed downward at roughly a 45° angle.
- 14. The left lung is fixed using a tissue perfusion system. Fix the left lung lobe for 1 hour at 30cm hydrostatic pressure using 4% paraformaldehyde. After an hour, submerge the inflated lung in 4% paraformaldehyde in a 20mL scintillation vial and store at 4°C.To process the left lung for histopathology, refer to section ASSESSMENT OF PULMONARY INFLAMMATION VIA HISTOPATHOLOGY.
15. Continue collecting other organs if other endpoints will be measured. Once completed, properly discard the carcasses in biohazard bags following all proper institutional procedures.
Figure 2.
Depiction of securing the right lung by using a suture to tie the primary right bronchus. Use tweezers to carefully guide the suture behind the lungs and heart. Tie the suture tightly around the right bronchus. H= heart, LL= left lung, RL= right lung, T= thymus.
SUPPORT PROTOCOL 1
ASSESSMENT OF BRONCHO-ALVEOLAR LAVAGE FLUID FOR PULMONARY INFLAMMATORY CELLULAR PROFILES
A characteristic feature of mouse models of allergic asthma is the influx of inflammatory cells into the lung. Specifically, eosinophils are indicators of allergic responses. Additionally, there is also a large influx of monocytes and neutrophils into the airspace of the lungs when HDM allergen is administered. The profile of immune cells in the lung can indicate the extent and type of inflammatory response. To obtain and quantify white blood cells, the lungs are lavaged (generally in the same delivery vehicle used for allergen and PM) by cannulation of the animal trachea followed by instillation of the appropriate solution using a syringe and re-collection for microscopic analysis.
Materials
Centrifuge (refrigerated)
1.5mL microcentrifuge tubes
Sterile saline, PBS, or HBSS
0.5mL microcentrifuge tubes
Trypan blue solution (0.4%)
20μL, 200μL, and 1000μL pipettes with appropriate tips
Hemocytometer
Light microscope
Shandon Cytospin 4 centrifuge
Shandon filter cards (for samples with <0.4mL volume)
Aqua color frost glass slides (Fisher Scientific)
Microscope cover glasses
ClearMount mounting media
100% methanol
Hematoxylin and eosin stain
Data sheet to count/calculate cells
Centrifuge the 5mL round bottom tubes containing BAL fluid at 500 × g for 15 min at 4°C.
Remove the supernatant and re-suspend the cell pellet in 0.5mL of the appropriate solvent (sterile saline, PBS, or HBSS). The supernatant may be aliquoted into microcentrifuge tubes, flash frozen in liquid nitrogen and stored at −80°C for further analysis of BAL (via Lowry Protein Assay and/or ELISA).
Trypan Blue Solution is used to check cellular viability; dead cells turn blue as a result of membrane permeability. In a 0.5mL microcentrifuge tube combine 10μL of Trypan Blue Solution and 100μL volume from the re-suspended cells. Gently vortex and incubate for 1 min at room temperature.
Gently vortex and add 10μL of the cell/trypan cell mixture into the hemocytometer counting chamber.
Using a light microscope, count the total number of viable cells (also count non-viable cells separately) in each of the 4 hemocytometer chamber quadrants (each quadrant has a length and width of 1mm and a depth of 0.1mm). Count cells that touch the upper and left perimeters and exclude cells that touch the lower and right perimeters. Count only white blood cells. Do not count epithelial cells or red blood cells.
- Use the following equation to calculate the total white blood cells recovered per mL of lavage fluid (cells/mL):Where 104 is equivalent to the conversion factor from the volume of 1 quadrant of the hemocytometer (1mm × 1mm × 0.1mm = 0.1mm3) to a volume of 1mL (0.1mm3=10−4cm3 since 1cm3=1mL; therefore, 10−4cm3=104/mL)The dilution factor of 1.1 represents the fraction of Trypan Blue Solution volume plus the volume of cell suspension, over the volume of cell suspension (10 μL of Trypan Blue Solution plus 100 μL cell suspension, divided by 100 μL, corresponding to the volume of cell suspension alone, is equal to 1.1)*The same equation can be used to determine the number non-viable cells/mL (non-viable cells are counted separately from viable cells) as a measure of cell death/inflammation
Prepare each cytospin slide by using approximately 1.5 × 103 cells in a volume of 100μL. Dilute the cells if necessary in the appropriate solvent. Add the cell suspension to a single cytospin funnel attached to a glass slide containing absorbent paper. Centrifuge for 5 min at 1,500 rpm. It is recommended that slides be made in duplicate or triplicate for each animal.
- Air dry the slides for 10 min and fix in 100% methanol for 10 sec. Air dry the slides in a fume hood.The slides may be stored at this point for staining at a different time.
Stain the slides with hematoxylin and eosin as recommend by the manufacture’s protocol. Coverslip the slides.
Using a light microscope, count 500 white blood cells per slide. Differentiate between eosinophils, lymphocytes, macrophages and neutrophils. The data can be expressed as number of cells for each cell type or percent cell type.
SUPPORT PROTOCOL 2
ASSESSMENT OF PLASMA IgE
Immunoglobulin E is indicative of allergic responses. Both OVA and HDM allergens can stimulate class switching in B cells to produce allergen-specific IgE that can be detected in the lung and systemically (in the blood). The detection of IgE can be used to assess the degree and the extent of the allergic response.
Materials
Centrifuge (refrigerated)
1.5mL microcentrifuge tubes
1000μL pipette with appropriate tips
Mouse IgE ELISA kit (various manufacturers)
To collect plasma, centrifuge the blood collected in ETDA-coated tubes at 1000 × g for 10 min at 4°C, ideally within 30 min after blood collection.
Transfer the plasma (top aqueous layer) to newly labeled 1.5 mL microcentrifuge tubes and freeze at −80°C.
Using a commercially available IgE ELISA kit, follow the manufacturer’s instructions to determine IgE levels in blood plasma. A dilution of plasma is recommended, but will be dependent on the range of the ELISA kit; perform an optimization assay to calculate the optimal dilution ratio.
SUPPORT PROTOCOL 3
ASSESSMENT OF PULMONARY INFLAMMATION VIA HISTOPATHOLOGY
We recommend lung histopathological assessment to determine the overall level of pulmonary inflammation, specifically to investigate pathological changes produced by PM treatment. We refer the reader to Current Protocols in Molecular Biology, UNIT 14.1, Fixation, Embedding, and Sectioning of Tissues, Embryos, and Single Cells (Zeller, 1989).
Embed lung tissue following the protocol detailed in “Paraformaldehyde Fixation and Paraffin Wax Embedding of Tissues and Embryos” (Zeller, 1989).
Section lung tissue following the protocol detailed in “Sectioning Samples in Wax Blocks” (Zeller, 2001).
- Sectioned lung tissues can be assessed with various stains. We recommend the use of a hematoxylin and eosin (H&E) stain, a combined eosinophil and mast cell (CEM) stain, and an alcian blue periodic acid Schiff (ABPAS) stain.Morphometric analysis may be used to quantify intraepithelial mucosubstance content as a method to characterize the allergic response. We refer the reader to (Carosino et al., 2015) for a description of the protocol.
Tissue sections may also be used to perform immunohistochemistry of proteins of interest.
SUPPORT PROTOCOL 4
ASSESSMENT OF PULMONARY INFLAMMATION VIA GENE EXPRESSION
Inflammation can be rapidly assessed by isolating RNA for the generation of CDNA to perform quantitative PCR. A variety of genes can be tested, include those associated with immunological/inflammatory changes (chemokines and cytokines), phase I and II metabolism enzymes, and transcription factors. The advantage of this method is that a large number of genes can be assessed at a relatively lower cost compared to protein based techniques. Furthermore, nearly any gene can be assessed by generating the appropriate primers from the mRNA nucleotide sequence using the National Center for Biotechnology Information’s Nucleotide search engine. This allows the investigator to tailor their study to specific genes of interest. However, we note the limitation of this technique, in that changes in mRNA do not always reflect changes in protein levels, and therefore key genes that are studied by the investigator should further examined using protein detection based assays (ELISA or Western Blot). We refer the investigator to Sigma-Aldrich’s TRI Reagent® Protocol for the extraction RNA from lung tissue. Below, Steps 2-9, are an adaptation of the TRI Reagent® Protocol for this experiment.
Materials
1.5mL microcentrifuge tubes
2mL round bottom microcentrifuge tubes
20μL, 200μL, and 1000μL pipettes with appropriate tips
TRI Reagent® (Sigma-Aldrich)
Stainless steel beads, 5 mm (Qiagen)
TissueLyser (Qiagen)
Chloroform
Centrifuge (refrigerated)
RNA extraction kit (various manufactures)
Ultraviolet-visible light spectrophotometer (Nanodrop, Thermo Scientific)
Nuclease-free water
Reverse transcription PCR kit (various manufactures)
SYBR® Green Master Mix (Applied Biosystems™, Thermo Scientific)
Real-time PCR instrument (various manufactures)
Gene specific primers (species-specific)
RNA Isolation
- Use only one of the right lung lobes. If lung tissue was frozen at −80°C, thaw cryovials on ice. Alternatively, if the lung tissue was stored in RNAlater Solution© (room temperature or 4°C) the tissue is ready to be processed.If the lung tissue was not stored in a RNA preservative solution but rather flash frozen at −80°C, be prepared to immediately place the tissue in TRI Reagent® as soon as it thaws. Failure to do so will result in degradation of RNA material.
In a clean 2mL round bottom microcentrifuge tube, place a clean stainless steel bead and 1mL of TRI Reagent® (perform this step and subsequent steps involving TRI Reagent® in a fume hood as this reagent is toxic, please refer to the MSDS). Transfer the lung lobes into the appropriately labeled 2mL microcentrifuge tubes and ensure the tissue is completely submerged in the TRI Reagent®.
Place the microcentrifuge tubes in a TissueLyser and homogenize the lung tissue for 30 sec at a frequency of 27 Hz (1/s). Determine if the tissue is homogenized. This step may be repeated once or twice if the tissue is not completely homogenized. If repeated, wait 1 min between each homogenization step to prevent RNA degradation via excessive heat.
Incubate the homogenate for 5 min at room temperature. This step is important for the TRI Reagent® to deactivate RNases and dissociate RNA complexes.
After 5 min, transfer the entire 1mL homogenate lysate to a newly labeled 1.5mL tube (in the fume hood). The aim of this step is to remove the bead from the solution without contaminating the solution.
Add 200μL of chloroform to each tube. Mix the tubes gently by inverting for 15 seconds. Incubate the samples for 3 min at room temperature.
Centrifuge the samples at 12,000 × g for 15 min at 4°C.
Label clean 1.5mL tubes and transfer 350μL of the top clear aqueous layer containing RNA from the centrifuged samples to the newly labeled tubes. Avoid contamination from the middle white layer (DNA) or the bottom pink organic layer (proteins & lipids).
Use a commercially available RNA extraction kit according to the manufacturer’s instructions to obtain RNA for gene analysis.
- Once RNA is eluted with water, measure the RNA concentration of each sample using a spectrophotometer.Always keep RNA on ice during all procedures. Store RNA at −20°C or −80°C to prevent degradation and avoid freeze-thaw cycles.
Reverse Transcription PCR and Quantitative PCR
11. Perform reverse transcription polymerase chain reaction (RT-PCR) of the RNA to generate stable complementary DNA (cDNA) using a commercially available RT-PCR kit according to the manufacturer’s instructions.
12. Once cDNA is obtained, use a SYBR® Green-based detection system to perform quantitative polymerase chain reaction (qPCR) according to the manufacturer’s instructions. Analyze Ct-values using the ΔΔ-Ct method to determine fold change of gene expression for genes of interest in treated animals vs. control animals that have been standardized to a housekeeping control gene.
SUPPORT PROTOCOL 5
ASSESSMENT OF PULMONARY INFLAMMATION VIA PROTEIN LEVELS
The assessment of specific proteins should be reserved for the study of key targets of interest. Gene changes that are found to be biologically significant to the allergic or toxicological response can be confirmed by assessing protein levels using assays such as ELISA or Western Blot.
Materials
2mL round bottom microcentrifuge tubes
Balance
20μL, 200μL, and 1000μL pipettes with appropriate tips
Tissue/cell lysis kit (various manufactures)
Lowry protein assay kit (various manufactures)
Spectrophotometer (to measure protein content)
Mouse enzyme-linked immunoabsorbant assay (ELISA) kit (various manufactures)
Data sheets for recording lung mass and dilution volumes
Use only one of the right lung lobes. Remove the cryovials from −80°C and thaw on ice.
Label 2mL round bottom microcentrifuge tubes and place the appropriate lung samples in each tube. Keep the tubes on ice during this procedure.
Homogenize the lung tissue using a tissue/cell lysis kit following the manufacture’s protocol. Aliquots should be made of lung homogenate and immediately frozen at −80°C for future experiments. Avoid freeze-thaw cycles. From this point forward, always keep the lung homogenate on ice during all future experiments.
Using the supernatant collected after homogenization, perform a Lowry protein assay to measure protein content using the manufacture’s protocol.
- Use the lung homogenate to perform the ELISA according to the manufacturer’s protocol on proteins of interest. Standardize the ELISA readout (ng/mL or pg/mL) to the total protein content per mL obtained in Step 4. Standardized values should be expressed as ng/mg or pg/mg.Lung homogenate must be diluted to concentrations that fall within the protein standards for each ELISA assay. The dilution factor will depend on the sensitivity of the ELISA (standard range) as well as the content of specific proteins of interest in the lung sample. We suggest the user perform an optimization assay with various concentrations of the lung homogenate (1:1, 1:10, 1:100, etc.) using a small sample size to determine the optimal lung homogenate dilution factor.
Lung homogenate can be used to perform other downstream applications, such as immunoprecipitation, western blots, electrophoretic motility shift assay, or other assays that assess protein activity.
COMMENTARY
Background Information
Much of the early work that studied the toxicology of PM found that certain components had the potential to produce free radicals, which through mechanisms, such as redox cycling and Fenton chemistry, would generate oxidative stress. The activation of oxidative stress pathways was, therefore, responsible for the observed biological effects, mainly cellular damage and inflammation. It is important to note that PM-mediated oxidative stress occurs exogenously, that is directly from components of the PM, as well as endogenously via activated macrophages that release reactive chemical species as a result of PM phagocytosis. This has been the most widely accepted hypothesis to explain the toxicity of PM. The toxicity of PM has been characterized into three general processes (or Tiers) that occur in a concerted fashion: 1) free radicals generate oxidative stress that leads to an upregulation of phase I and II enzymes for detoxification; 2) free radicals overwhelm detoxification processes and trigger inflammation via the activation of epithelial and immune cells that release of pro-inflammatory cytokines and chemokines; and 3) the failure to clear PM and long term exposure to PM leads to chronic inflammation that initiates disease or enhances ongoing disease states (Ayres et al., 2008).
With respect to allergy, and as demonstrated through our experimental animal model, oxidative stress may not be the sole explanation for the enhancement of inflammation seen in subsequent encounters with the allergen (challenge). The fact that PM is not administered in the challenge period in this model, yet heightened inflammatory responses are observed in animals exposed to PM during allergen sensitization suggest that specific components of PM may modulate immunological memory during the formation of the adaptive immune response. In recent years, polyaromatic hydrocarbons, components of fossil fuel and organic matter combustion, have gained attention for their ability to alter the adaptive immune response through the aryl hydrocarbon receptor (AhR). The AhR is a cytosolic receptor that binds both endogenous and exogenous ligands. The AhR plays a pivotal role in T cell differentiation, with some ligands promoting regulatory T cell (Treg) differentiation and others ligands promoting Th17 differentiation. Kynurenine, a byproduct of tryptophan metabolism by the enzyme indoleamine 2,3-dioxygenase (IDO1) in dendritic cells promotes Treg differentiation in an AhR-dependent manner (Mezrich et al., 2010). Conversely, exogenous ligands, such polyaromatic hydrocarbons in air pollution, appear to act as “danger signals” that promote Th17 differentiation and inflammation through the AhR, as mice lacking the AhR fail to develop Th17 responses (van Voorhis et al., 2013). Although it is unclear how AhR ligands lead to differential biological outcomes, ligand binding strength, duration, and method of exposure are thought to dictate the delicate balance between Treg and Th17 differentiation. It is postulated that high levels of air pollution exposure impede bacterial clearance mechanisms in the lung (i.e., mucocillary apparatus and alveolar macrophage-mediated clearance), thereby enhancing susceptibility to infection. Th17 responses are, therefore, heightened to recruit neutrophils into the lung via IL-17 and promote epithelial cell repair via IL-22 to mitigate these effects (Julliard et al., 2014). These findings support an alternative method through which PM may promote inflammation by modulating the immune system, independent of oxidative stress.
Critical Parameters and Troubleshooting
Allergen Preparation
HDM allergen contains high levels of endotoxin, which influences the allergic response, typically producing a more neutrophilic response. The allergen can be purified by using endotoxin-removal kits, such as Detoxi-Gel™ Endotoxin Removing Gel Columns (Thermo Scientific), that significantly reduce endotoxin levels, although protein is also lost. After purification, the amount of endotoxin in the allergen can be measured using a Limulus Amebocyte Lysate assay (Kinetic-QCL; Lonza), and the total protein content can be measured using a Lowry assay. It is important to use endotoxin free pipette tips, glassware, and plastic ware; glass can be treated overnight at 175°C to destroy endotoxin and plastic ware can be submerged in 1M NaOH overnight to destroy endotoxin.
Allergen and Particulate Matter Administration
During the intranasal administration, the mouse may temporarily halt its breathing pattern, preventing the dose from being inhaled. Three techniques can be used to ensure the entire dose is inhaled: 1) temporarily stop drop-wise dosing until the animal begins breathing again; 2) gently massage the chest of the mouse using the thumb to expand/compress the thoracic cavity to assist inhalation; or 3) if the mouse begins to regain consciousness, place it back in the anesthetic chamber for 1-2 min.
Anticipated Results
Total cells recovered by BAL should be elevated in allergen and allergen+PM groups compared to PBS and PM controls. A comparison between allergen and allergen+PM groups can give a general indication of overall inflammatory potential of PM. As stated previously, not all types of PM can enhance allergic inflammation. Alternatively, no changes between the allergen and allergen+PM groups does not necessarily imply PM does not have inflammatory effects, as these effects can occur at sub-cellular levels that may not result in significant influx of leukocytes into the lung. The frequency of cell types found in the lung, from most to least common, in allergen±PM treated mice is: monocytes, macrophages, neutrophils, eosinophils, and lymphocytes. The lungs of PBS and PM control mice typically are composed entirely of macrophages (~90%). PM treated mice do not exhibit an inflammatory profile because PM is administered 12 days prior to the analysis and PM by itself, at the dose administered, does not produce significant inflammatory effects relative to allergen treatment.
Histopathological analysis of the lung is done to determine the anatomical locations of inflammation. We note here that performing a whole-lung lavage may significantly alter the location of leukocytes and focal points of inflammation that occur in-vivo. Therefore, we recommend the use of split lung lavage, as noted in the protocol. The HDM-allergic mouse model exhibits marked to severe sub-epithelial inflammation due to an influx of predominantly monocytes, neutrophils and eosinophils. Inflammatory cells are also elevated in perivascular regions with occasional focal points of inflammation commonly seen in the parenchyma. HDM allergen also elicits significant intra-epithelial mucosubstance production in the airways, a feature that is absent in an OVA allergy model if the allergen is administered intranasally during sensitization (in contrast to i.p. delivery).
Gene expression of inflammatory cytokines that drive allergic reactions are elevated, particularly in the allergen+PM group. These include the following cytokines: IL-4, IL-5, IL-6, IL-13, IL-25, and IL-33. Other genes of interest that investigators may wish to examine include oxidative stress enzymes, chemokines, and genes involved in AhR signaling. Protein levels of elevated genes can be confirmed via ELISA.
Further measurements can be performed to characterize the immunotoxicological effects of PM on the allergic response in this mouse model of asthma. For example, quantifying the ratio of reduced to oxidized glutathione (GSH/GSSG) or performing a DTT assay can provide insights into the oxidative potential of PM. Gas chromatography-mass spectrometry can also be used to quantify polyaromatic hydrocarbon content in collected atmospheric PM. Plasma samples can be further assessed for other antibody responses, such as IgG4. BAL fluid can be further examined for protein content (Lowry protein assay), specific proteins (ELISA) or evidence of cellular injury (lactate dehydrogenase). Phenotyping of immune cells via flow cytometry can be a powerful tool to meticulously characterize in-vivo mechanisms. In-vivo lung-derived immune cells may also be cultured to further delineate mechanisms by re-challenging cells with allergen and characterizing their responses. Lastly, measurements in lung function can be performed to determine airway hyperresponsiveness, including the use of methylcholine challenge.
Time Considerations
Mice should be ordered with sufficient time to allow at least a one week acclimation period after delivery. The dosing schedule of the mice will take 2 weeks: sensitization on days 1, 3, and 5; challenge on days 12-14; and tissue collection performed on day 15. On day 15, lavage and lung tissue collection for each mouse will take approximately 15-20 min, and perfusion fixation of the lung tissue will take 1 hour per lung. Total BAL cell counts, cytospin slide preparation, and staining can be completed by the end of day 15. The time to complete the necropsy and tissue collection on day 15 will depend on the number of animals as well as the size and efficiency of the staff. Large sample-size experiments may be split into multiple day necropsies; however, the dosing schedule for the mice will need to be altered respectively.
Preparation of lung tissue sections from paraformaldehyde fixed tissue will take approximately one week. Fixation of the lung tissue in 4% paraformaldehyde is done on day 15 of the protocol. The following day, the fixation solution is replaced with ethanol for tissue dehydration for 24 hours. Allot two days for paraffin embedding of tissue followed by 1 day for sectioning of tissue (depending on number of samples). The sectioned lung tissue can then be stained with various histological stains or can be used for immunohistochemistry, which can be performed in a single day.
BAL differential cell count analysis (500 cells/slide) will take an experienced counter 15-20 minutes to count each slide. Histopathological assessment of the lung will depend on the analysis performed and the number of slides to be analyzed. Semi-quantitative histopathological analysis and intra-epithelial mucosubstance quantification can be time-intensive techniques.
To perform gene expression analysis allow one day for complete trizol extraction of RNA, RNA purification, and RNA concentration measurements. Conversation of RNA to cDNA via RT-PCR can be completed the following day as well as a qPCR reaction. The number of days to perform subsequent qPCR reactions will depend on the battery of genes being tested.
When performing protein analysis of the total lung, schedule two days to perform lung homogenization. On day one, the lungs are homogenized and frozen over night to promote cell membrane fracture. On day two, the lung homogenate is thawed and the supernatant is collected after centrifugation. A Lowry protein assay is then used to quantify total lung protein to standardize downstream assay values. The samples can then be processed using protein analysis assays, such as immunostaining (ELISA) or immunoblot (western blot), each of which may take 2-3 days per each protein of interest analyzed.
Significance Statement.
The protocol is designed to allow investigators to assess the immunotoxicological mechanisms of particulate matter, a component of air pollution, during development of the immune response in the context of a mouse model of allergic airway inflammation. Using an acute exposure model of 14 days, mice are sensitized with allergen and PM and challenged with allergen only to determine how PM modulates the development of the adaptive immune response and subsequent allergic inflammatory response after allergen challenge. The unique aspect of this protocol is that it can be adapted to study how various characteristics of air pollution, such as PM size, PM source, or chemical composition, shape the allergic response in a mouse model of asthma.
Acknowledgements
We thank Ms. Alexa Pham for her help in determining the equation used to calculate the total white blood cells recovered per mL of lavage. We also thank Ms. Pham and Mr. Dale Uyeminami for their feedback on proper methodological and technical procedures.
The development of the methodology described in this protocol was supported under the following grants and training programs: NIOSH OH07550, P30 ESO23513, and P51 OD011107. Alejandro R. Castaneda was supported by a NIGMS-funded Pharmacology Training Program (T32GM099608).
Footnotes
The National Institute of Standards and Technology Standard Reference Materials provides a variety of commercially available PM sources that can be used for this protocol.
Conflicts of Interest
The authors report no conflicts of interest.
Literature Cited
- Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8:166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal Toxicol. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70:245–256. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- Carosino CM, Bein KJ, Plummer LE, Castaneda AR, Zhao Y, Wexler AS, Pinkerton KE. Allergic airway inflammation is differentially exacerbated by daytime and nighttime ultrafine and submicron fine ambient particles: heme oxygenase-1 as an indicator of PM-mediated allergic inflammation. J Toxicol Environ Health A. 2015;78:254–266. doi: 10.1080/15287394.2014.959627. [DOI] [PubMed] [Google Scholar]
- Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliard W, Fechner JH, Mezrich JD. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front Immunol. 2014;5:458. doi: 10.3389/fimmu.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, Thayer BP, Daniels JL. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26:30–42. doi: 10.1097/EDE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- Kulkarni P, Baron PA, Willeke K. Aerosol Measurement: Principles, Techniques, and Applications. 3rd Edition John Wiley & Sons; New York: 2011. [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. Journal of immunology. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, Liu X. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. 2012;3:1–58. [PubMed] [Google Scholar]
- Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CB, Raaschou-Nielsen O, Hertel O, Mortensen PB. Air pollution from traffic and schizophrenia risk. Schizophr Res. 2004;66:83–85. doi: 10.1016/s0920-9964(03)00062-8. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, Schwartz J, Zanobetti A. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst. 2000;94:5–70. discussion 71-79. [PubMed] [Google Scholar]
- Turner MC, Krewski D, Pope CA, 3rd, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med. 2011;184:1374–1381. doi: 10.1164/rccm.201106-1011OC. [DOI] [PubMed] [Google Scholar]
- van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, Mezrich JD. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545. doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, Schlaug G, Gold DR, Mittleman MA. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R. Fixation, embedding, and sectioning of tissues, embryos, and single cells. Curr Protoc Molec Biol. 1989:14.1.1–14.1.18. doi: 10.1002/0471142727.mb0101s07. [DOI] [PubMed] [Google Scholar]