Abstract
High grade neuroendocrine neoplasms (WHO G3) of the pancreas include both well differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC). According to the WHO classification scheme, the diagnosis of this group of tumors is based on both the histopathology of the tumor and the assessment of proliferation fraction. However, the former can be challenging due to the lack of well-defined histological criteria and the latter alone (i.e., >20 mitoses/10 high power fields or Ki67 >20%) may not sufficiently distinguish WD-NETs from PD-NECs. Given the considerable differences in treatment strategies and clinical outcome, additional practical modalities are required to facilitate the accurate diagnosis of high grade pancreatic neuroendocrine neoplasms.
We examined 33 cases of WHO G3 neuroendocrine neoplasms of the pancreas and attempted to classify them into WD-NET, small cell PD-NEC (PD-NEC-SCC), and large cell PD-NEC (PD-NEC-LCC), or to designate them as “ambiguous” when an uncertain diagnosis was rendered by any of the observers or there was any disagreement in classification among the 3 observers. To simplify the interpretation, both PD-NEC-SCC and PD-NEC-LCC were considered together as PD-NECs in the final analysis. The initial approach was to assess microscopically a single morphologically challenging H&E section from each case without the knowledge of Ki67 values, performed independently by three pathologists to assess the degree of diagnostic concordance, and then evaluate immunohistochemical staining for surrogate biomarkers of known genotypes of WD-NET and PD-NEC, respectively, and lastly, complete a clinicopathologic review to establish a final definitive classification. Loss of DAXX or ATRX protein expression defined WD-NET and abnormal p53, Rb, SMAD4 expression signified PD-NEC. When the chosen section displayed an element of WD histopathology, or other tumor sections contained WHO G1/G2 components, or there had been a prior established diagnosis of a primary WD-NET, the final diagnosis was rendered as a WD-NET with high grade (G3) progression. If a component of conventional adenocarcinoma was present (in slides not seen in the initial review), the diagnosis was established as a combined adenocarcinoma and PD-NEC.
All three pathologists agreed on the morphological classification of 33% of the cases (6 WD-NET, 3 PD-NEC-SCC, and 2 PD-NEC-LCC), were conflicted on 2 cases between PD-NEC-SCC and PD-NEC-LCC, and disagreed or were uncertain on the classification for the remaining 20 cases (61%), which were therefore categorized as ambiguous. In the group of cases where all pathologists agreed on the classification, the six WD-NET cases had either loss of DAXX or ATRX or had evidence of a WD-NET based on additional or prior pathology slides. The seven PD-NEC cases had abnormal expression of p53, Rb, and/or SMAD4 or a coexisting adenocarcinoma. In the ambiguous group (n=20), 14 cases were established as WD-NETs, based upon loss of DAXX or ATRX in 7 cases and additional pathology evidence of high grade progression from WD-NET in the other 7 cases; 5 cases were established as PD-NEC based upon abnormal expression of p53, Rb, and/or SMAD4; one case remained undetermined with normal expression of all markers and no evidence of entity-defining histologic findings in other slides. Based on the final pathologic classifications, the disease specific survival was 75 months and 11 months for the WD-NET and PD-NEC groups, respectively.
Thus, we conclude that morphologic diagnosis of high grade pancreatic neuroendocrine neoplasms is challenging, especially when limited pathologic materials are available, and necessitates better defined criteria. The analysis of both additional sections and prior material, along with an immunohistochemical evaluation, can facilitate accurate diagnosis in the majority of cases and guide the appropriate clinical management and prognosis.
Keywords: Pancreas, Neuroendocrine tumor, Neuroendocrine carcinoma, WHO G3, High grade
Introduction
Recent investigations have indicated that there exist uncommon pancreatic well differentiated NETs that can exhibit characteristic morphologic features of a low or intermediate grade neoplasm but a proliferative rate that breaches the threshold for the WHO classification of a high grade (G3) neuroendocrine neoplasm.1 Some cases may be morphologically homogeneous and appear well differentiated throughout, with the high grade nature only revealed by assessment of the mitotic rate or, more commonly, the Ki67 index. Other cases have components of a low or intermediate grade NET, with a low proliferative rate, either admixed with the high grade neuroendocrine neoplasm or in a different focus or prior sample from the patient; such cases have been interpreted as high grade progression of a WD-NET. In both of these scenarios of WD-NETs with a G3 proliferative rate, the tumors do not possess the clinical, pathological, and genotypical features of a true PD-NEC2-6. Mutations in TP53, RB1, and SMAD4, found in PD-NECs, are absent, and loss of DAXX or ATRX can occur, as in other WD-NETs of the pancreas. Thus, these neoplasms are increasingly being classified as high grade (G3) WD-NETs, rather than PD-NECs. While this phenomenon is generally rare in WD-NETs, the prevalence is higher in pancreatic primaries3. In the absence of pertinent clinical information (such as symptoms at the initial presentation, results of radiographic assessment, and blood biomarkers) and without evidence of a lower grade counterpart (WHO G1/G2), the distinction between a high grade WD-NET and PD-NEC may be challenging, particularly in common scenario of suboptimal biopsy material or limited tumor sections. The difficulty is enhanced when the morphologic features are not those of classic small cell carcinoma, as pancreatic WD-NETs can particularly resemble large cell PD-NECs. In addition to applying classic but rather inconsistent morphologic criteria, some pathologists may use a combination of their intuition from personal experience and available clinical information to distinguish WD-NET from PD-NEC; others simply use a rigid mitotic count or Ki67 index cut-point to assign the classification. Given the significant difference in treatment strategies and outcome for WD-NET and PD-NEC, better defined morphologic criteria, ancillary studies, and clinical information are crucial to facilitate the accurate interpretation of these two distinct neoplasms7, 8. The present study was conducted to determine the utility of a selected panel of immunohistochemical stains to improve the classification of G3 pancreatic neuroendocrine neoplasms.
Material and Methods
Patient Information
Pancreatic neuroendocrine neoplasms with increased proliferative activity (WHO G3 category, mitosis >20/10 high power field or Ki67>20%) were identified retrospectively and prospectively using the pathology files at the authors' institution, with IRB approval. These included primary surgical resection specimens, core biopsies, and resections of recurrent or metastatic tumors. Of the thirty-three cases selected for the study, all patients, except one, were evaluated clinically at our institution with appropriate radiological and laboratory studies and surgical or oncologic management. Follow-up information was available for all cases, except one.
Pathological Assessment
A single representative H&E slide was selected from each case to represent the high grade region of the tumor (in cases where other material may have displayed lower grade components). Initially, three pathologists specialized in gastrointestinal and hepato-pancreatobiliary pathology independently assessed the selected sections from each case. The cases were blinded to the reviewers (LHT, OB, and DSK) by a 4th individual with regard to the patient's identification, the histopathology of additional tumor sections and prior diagnoses, any clinical information, and results of any ancillary studies, including the Ki67 index. Initially, the cases were classified into the following categories: WD-NET, small cell PD-NEC (PD-NEC-SCC), large cell PD-NEC (PD-NEC-LCC), and uncertain, when the subtype could not be definitively assigned on the morphologic findings alone. For purposes of further analysis the PD-NEC-SCC and PD-NEC-LCC groups were considered together as PD-NECs. A consensus diagnosis was achieved when all 3 reviewers agreed. In cases with disagreement among reviewers as to WD-NET versus PD-NEC, or when any individual reviewer considered a diagnosis to be uncertain, the consensus diagnosis was regarded as ambiguous. The secondary evaluation included incorporating the analysis of immunohistochemistry with surrogate biomarkers of known genotypes for WD-NET and PD-NEC, respectively, and a final complete clinicopathologic review of the cases, including assessment of other slides and prior specimens, for a definitive final classification. The contribution of each type of data to the establishment of the final classification was assessed.
Immunohistochemistry
Standard ABC peroxidase techniques were used for immunohistochemistry performed on 4 μ sections of formalin-fixed and paraffin-embedded tissue. Antigen retrieval in heated citrate buffer at pH 6.0 was applied for all antibodies. The Ki67 monoclonal-antibody (1:100), Rb monoclonal-antibody (1:400), p53 monoclonal-antibody (1:500), Chromogranin-A polyclonal-antibody (1:8000), and synaptophysin (1:500) were obtained from Dako (Carpentaria, CA). The SMAD4 monoclonal-antibody (1:800) was acquired from Santa Cruz Bio (Santa Cruz, CA). The ATRX polyclonal-antibody (1:500) and DAXX (1:100) polyclonal-antibody were obtained from Sigma-Aldrich Corporation (St. Louis, MO). Immunohistochemistry was performed on BenchMark XT automated equipment (Ventana Medical System Inc., Tucson, AZ). Positive control tissue was stained in parallel with each study case. The Ki67 immunoreactivity was expressed as the percentage of tumor cells with nuclear staining, which was based upon digital counting >2,000 tumor cells in regions with the highest labeling recognizable on scanning magnification. p53 immunoreactivity with strong staining intensity in >25% tumor cells was regarded as abnormal (positive), and complete loss of SMAD4, DAXX, ATRX, and Rb protein expression (negative), in the presence of positive staining in non-neoplastic cells, was regarded as abnormal.
Results
Patient Information
Of thirty-three cases chosen for this study, the mean age ± SD was 57±16 years old (ranging from 13-81 years) with a male to female ratio of 17:16, and a median follow up of 18.6±35 months (ranging from 1-120 months). Primary pancreatic tumors constituted 20/33 cases and metastases made up 13/33 cases. Every case had a proliferative index (Ki67 index) greater than 20% with a mean of 60±20% (26-93%). The mitotic rate was assessed in all cases, and 15/33 (45%) had a mitotic rate in the G3 range (>20 per 10HPF), 17/33 (52%) were in the G2 range (2-20 per 10HPF), and one case was in the G1 range (<2 per 10HPF).
Morphologic Assessment of High Grade Pancreatic Neuroendocrine Neoplasm
Of the 33 slides reviewed independently by three pathologists, 8 were core biopsy specimens and 25 were surgical resections or excisional biopsies. The results of the morphologic assessment are shown in Table 1.
Table 1. Morphologic Assessment of High Grade Pancreatic Neuroendocrine Neoplasms.
| Consensus | Reviewer 1 | Reviewer 2 | Reviewer 3 | Specimen Type |
|---|---|---|---|---|
| WD-NET | WD-NET | WD-NET | WD-NET | Resection |
| WD-NET | WD-NET | WD-NET | WD-NET | Resection |
| WD-NET | WD-NET | WD-NET | WD-NET | Resection |
| WD-NET | WD-NET | WD-NET | WD-NET | Resection |
| WD-NET | WD-NET | WD-NET | WD-NET | Resection |
| WD-NET | WD-NET | WD-NET | WD-NET | Resection |
| Ambiguous | WD-NET | Ambiguous | WD-NET | Biopsy |
| Ambiguous | WD-NET | WD-NET | Ambiguous | Resection |
| Ambiguous | Ambiguous | WD-NET | WD-NET | Biopsy |
| Ambiguous | WD-NET | WD-NET | Ambiguous | Resection |
| Ambiguous | WD-NET | WD-NET | Ambiguous | Resection |
| Ambiguous | WD-NET | WD-NET | Ambiguous | Resection |
| Ambiguous | WD-NET | WD-NET | Ambiguous | Biopsy |
| Ambiguous | WD-NET | WD-NET | PD-NET-LCC | Resection |
| Ambiguous | WD-NET | WD-NET | PD-NET-LCC | Biopsy |
| Ambiguous | Ambiguous | Ambiguous | Ambiguous | Biopsy |
| Ambiguous | Ambiguous | Ambiguous | PD-NEC-SCC | Resection |
| Ambiguous | PD-NEC-SCC | Ambiguous | PD-NEC-SCC | Resection |
| PD-NEC-LCC | PD-NEC-LCC | PD-NEC-LCC | PD-NEC-LCC | Resection |
| Ambiguous | Ambiguous | Ambiguous | Ambiguous | Biopsy |
| PD-NEC-LCC | PD-NEC-LCC | PD-NEC-LCC | PD-NEC-LCC | Resection |
| PD-NEC-LCC | PD-NEC-LCC | PD-NEC-LCC | PD-NEC-LCC | Resection |
| PD-NEC-SCC | PD-NEC-SCC | PD-NEC-SCC | PD-NEC-SCC | Resection |
| PD-NEC-SCC | PD-NEC-SCC | PD-NEC-SCC | PD-NEC-SCC | Resection |
| PD-NEC-SCC | PD-NEC-SCC | PD-NEC-SCC | PD-NEC-SCC | Resection |
| PD-NEC | PD-NEC-LCC | PD-NEC-SCC | PD-NEC-LCC | Resection |
| PD-NEC | PD-NEC-SCC | PD-NEC-SCC | PD-NEC-LCC | Resection |
| Ambiguous | WD-NET | PD-NEC-LCC | PD-NEC-LCC | Resection |
| Ambiguous | PD-NEC-LCC | PD-NEC-LCC | Ambiguous | Resection |
| Ambiguous | Ambiguous | Ambiguous | PD-NEC-SCC | Resection |
| Ambiguous | Ambiguous | PD-NEC-SCC | Ambiguous | Biopsy |
| Ambiguous | Ambiguous | PD-NEC-LCC | Ambiguous | Biopsy |
| Ambiguous | Ambiguous | PD-NEC-LCC | PD-NEC-LCC | Resection |
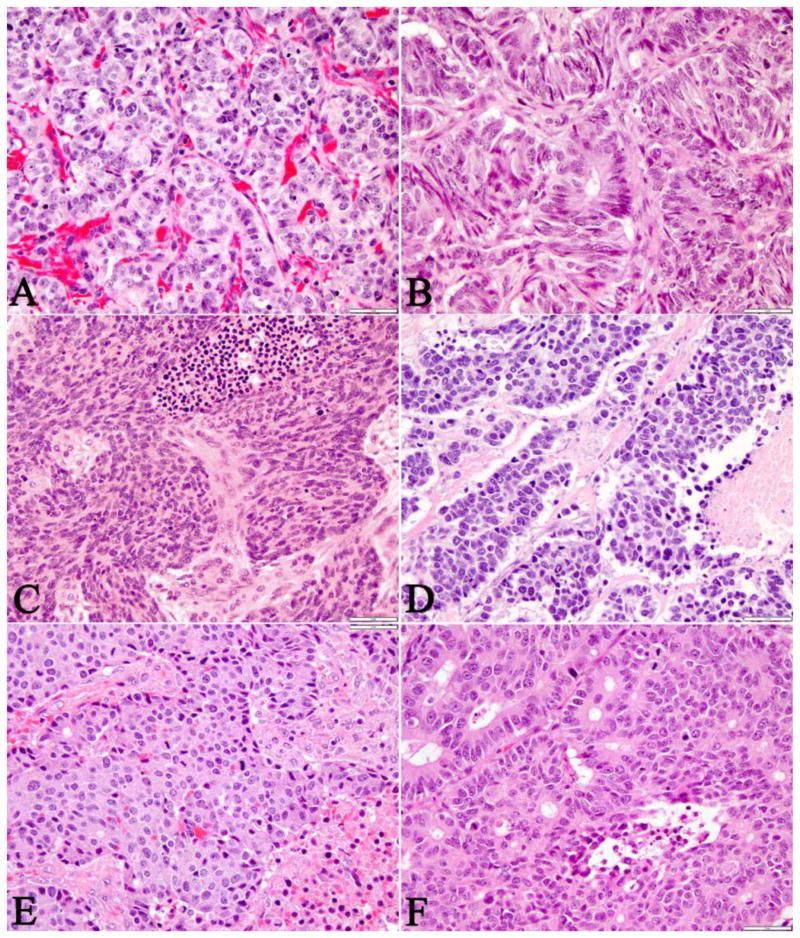
Approximately one-third (11/33) of the cases, which were all surgical resections, achieved diagnostic consensus by all three reviewers and 61% of the cases were regarded to be ambiguous, because an uncertain diagnosis was rendered by any of the observers or there was disagreement between WD-NET and PD-NEC among the 3 observers. Every biopsy specimen (n=8) in this cohort failed to achieve consensus among the reviewers. All of the six WD-NET cases that achieved consensus revealed some classic histopathologic and cytologic features of WD-NET, which included an organoid, trabecular architecture, a regular intratumoral vascular pattern, abundant granular cytoplasm, and stippled nuclei with inconspicuous nucleoli (Figure 1A-B). Three cases of PD-NEC that reached consensus demonstrated features of small cell carcinoma, such as geographic tumor necrosis, spindled or fusiform cell morphology, minimal cytoplasm, finely granular, hyperchromatic nuclei with inconspicuous nucleoli, and nuclear molding (Figure 1C-D). The morphologic features of PD-NEC-LCC appeared to be the least specific and reproducible, as they overlapped with both WD-NET and PD-NEC-SCC. In fact, one of the three cases classified as PD-NEC-LCC by all three reviewers, was re-classified as WD-NET in the final assessment (see below, table 2). The other two PD-NEC-LCC cases had a large expansile growth pattern with subtle peripheral nuclear palisading, rosette/tubule forming structures within the large nests, irregular large vessels, and tumor necrosis (Figure 1E-F). The ambiguous cases either shared overlapping morphologic features with WD-NET, PD-NEC-SCC, and PD-NEC-LCC subtypes or were present in suboptimal small biopsies with varying degrees of histologic processing artifact (Figure 2A-F).
Figure 1. Typical Morphologic Features of Pancreatic WD-NET, PD-NEC-SCC, and PD-NEC-LCC.
WD-NETs (A-B) revealed nested/organoid and trabecular architecture, a regular intratumoral vascular pattern, abundant granular cytoplasm, and stippled nuclei with inconspicuous nucleoli. PD-NEC-SCC (C-D) demonstrated stromal desmoplasia (C), tumor necrosis (C), fusiform (“oat cell”) nuclei lacking nucleoli, and nuclear molding. PD-NEC-LCC (E-F) displayed tumor necrosis, expansile and irregular nests with peripheral palisading, and rosettes/tubular structures within the large nests.
Table 2. Classification of High Grade Pancreatic Neuroendocrine Neoplasms by Secondary Evidence.
| Initial Consensus | Immunohistochemical Abnormalities | Ki67% | Other Histologic Components | Confirmed Classification |
|---|---|---|---|---|
| WD-NET | 50 | G1/G2 WD-NET | WD-NET | |
| WD-NET | DAXX | 70 | G1/G2 WD-NET | WD-NET |
| WD-NET | ATRX | 50 | G1/G2 WD-NET | WD-NET |
| WD-NET | 40 | G1/G2 WD-NET | WD-NET | |
| WD-NET | DAXX | 35 | G1/G2 WD-NET | WD-NET |
| WD-NET | 32 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | 35 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | 65 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | DAXX | 50 | G1/G2 WD-NET | WD-NET |
| Ambiguous | ATRX | 35 | G1/G2 WD-NET | WD-NET |
| Ambiguous | DAXX | 30 | G1/G2 WD-NET | WD-NET |
| Ambiguous | 60 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | ATRX | 40 | WD-NET | |
| Ambiguous | DAXX | 80 | G1/G2 WD-NET | WD-NET |
| Ambiguous | DAXX | 49 | G1/G2 WD-NET | WD-NET |
| Ambiguous | 38 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | 60 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | 50 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | 70 | G1/G2 WD-NET | WD-NET | |
| Ambiguous | p53/Rb | 88 | PD-NEC | |
| Ambiguous | p53/SMAD4 | 38 | Ductal adenocarcinoma | PD-NEC |
| Ambiguous | p53/Rb | 70 | PD-NEC | |
| Ambiguous | p53/Rb | 85 | PD-NEC | |
| Ambiguous | p53 | 60 | PD-NEC | |
| Ambiguous | 70 | Undetermined | ||
| PD-NEC-LCC | DAXX | 66 | G1/G2 WD-NET | WD-NET |
| PD-NEC-LCC | Rb | 44 | PD-NEC | |
| PD-NEC-LCC | 26 | Ductal adenocarcinoma | PD-NEC | |
| PD-NEC-SCC | p53 | 80 | Ductal adenocarcinoma | PD-NEC |
| PD-NEC-SCC | Rb | 90 | PD-NEC | |
| PD-NEC-SCC | p53/Rb | 94 | Ductal adenocarcinoma | PD-NEC |
| PD-NEC | Rb | 84 | PD-NEC | |
| PD-NEC | p53 | 88 | PD-NEC |
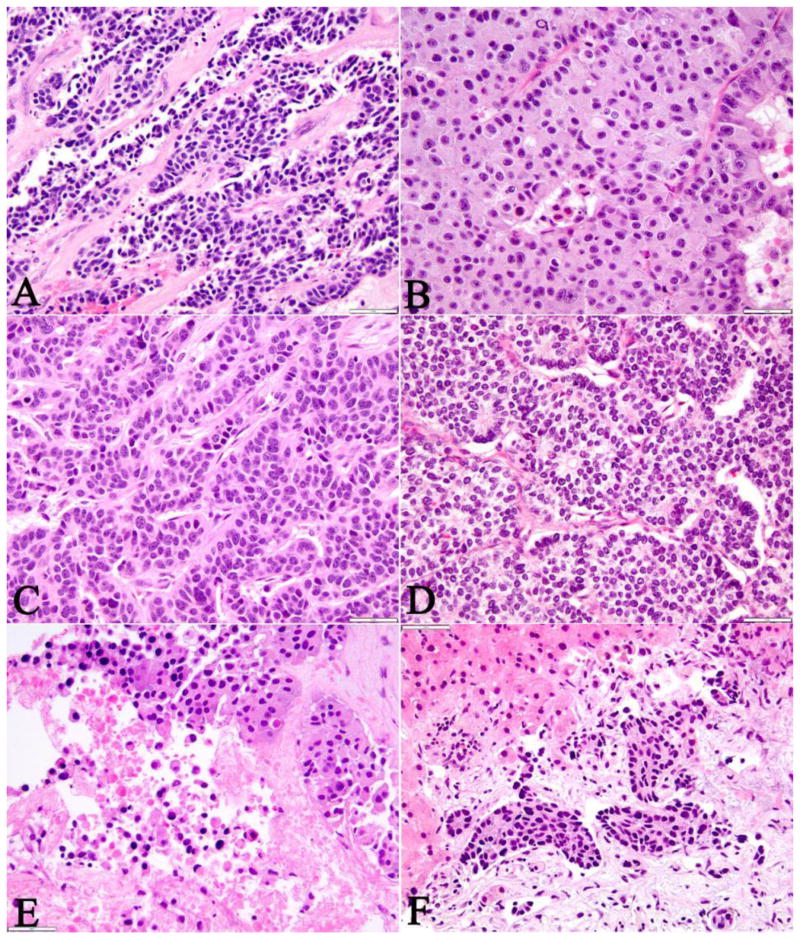
Figure 2. Morphologically Ambiguous Pancreatic Neuroendocrine Neoplasms.
Two cases of WD-NET (A-B) were initially considered as morphologically ambiguous due to an infiltrative growth pattern with irregular architecture, significant intratumoral fibrosis, single cell (A) and punctate (B) tumor necrosis, and brisk mitotic activity. However, upon retrospective review, the tumors appeared to retain some morphologic features of WD-NETs such as a hyalinized type of fibrosis (A), delicate vascular pattern (B), and abundant granular cytoplasm and low nuclear to cytoplasm (N/C) ratio (B).
The two cases of PD-NEC (C-D) shared some morphologic features of WD-NET such as the vascular patterns and nested or trabecular architecture. Although the cytologic features such as large nuclei, high N/C ratio, and minimal cytoplasm might have suggested PD-NEC.
The distinction between WD-NET (E) and PD-NEC (F) was especially difficult in small biopsies where the architecture of the tumor could not be fully appreciated. While the cytologic features of the tumor (abundant cytoplasm and low N/C ratio) were suggestive of a WD-NET (E), the presence of extensive tumor necrosis rendered the tumor as ambiguous. Similarly, the small nested structures in a PD-NEC (F) without the context of the global architecture of the tumor could culminate in an incorrect classification of this neuroendocrine neoplasm.
Subclassification of PD-NECs into PD-NEC-SCC and PD-NEC-LCC also revealed poor interobserver concordance, and the three observers did not agree or determine the sub-classification on 8/13 (62%) cases (Table 1).
Secondary Evidence for the Classification of Pancreatic Neuroendocrine Neoplasms
While all cases had a Ki67 index of >20% in this cohort, 35% (7/20) of the confirmed WD-NET case had Ki67 >55% and 33% (4/12) of the confirmed PD-NEC case had Ki67 <55% (Table 2). Thus, both the morphology and the Ki67 could not accurately distinguish these two pathologic entities. Following the initial morphologic assessment, immunohistochemistry was performed using surrogate biomarkers of known genotypes for WD-NET (i.e., DAXX and ATRX loss)9 and PD-NEC (i.e. p53 overexpression; Rb or SMAD4 loss),3, 10 respectively. Loss of DAXX or ATRX protein expression was mutually exclusive and occurred in WD-NETs in 10/33 cases. Abnormal p53, Rb, or SMAD4 expression characterized PD-NECs and was found in 11/33 cases (Table 2 and Figure 3). In no cases were there concurrent abnormalities in DAXX/ATRX along with p53, Rb, or SMAD4. Thus, immunohistochemistry confirmed 3/6 of WD-NET and 6/7 of PD-NEC cases that had reached consensus; and 60% (12/20) cases with no consensus (including one with incorrect classification) were defined as WD-NET or PD-NEC based upon the results of immunohistochemistry. Nonetheless, 8/20 of the ambiguous cases remained unclassified after immunohistochemical analysis.
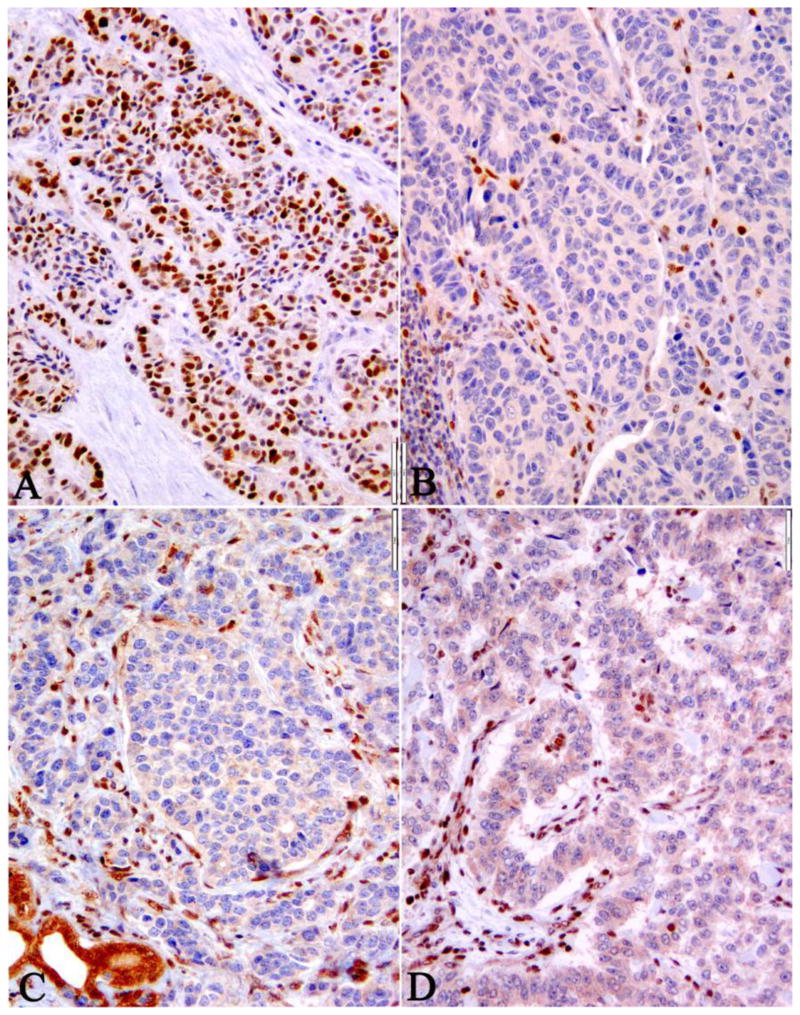
Figure 3. Abnormal p53, Rb, SMAD4, and DAXX expression by Immunohistochemistry in High Grade Pancreatic WD-NET and PD-NEC.
The expression of abnormal p53 served as a surrogate biomarker of TP53 gene mutation, which was observed in 67% of PD-NECs (A). Similarly, loss of Rb (B) and SMAD4 (C) protein expression were associated with PD-NEC. In contrast, loss of DAXX (D) or ATRX (data not shown) expression was seen in 40-50% WD-NET, but not in PD-NEC.
Additional pathologic and clinical information was further acquired to facilitate the classification of this group of high grade neuroendocrine neoplasms. When a case either contained WHO G1/G2 areas in other tumor sections within the same neoplasm (8/19) or had a prior pathologic diagnosis of a G1/G2 WD-NET (11/19), the final diagnosis of the high grade neoplasm in the study cohort was rendered as WD-NET, reflecting high grade progression from G1/G2 to G3; 19 cases fulfilled this criterion (Table 2), including 13 in which the morphologic diagnosis was ambiguous (uncertain diagnosis rendered by any of the observers or disagreement among the 3 observers) or wrong and 10 cases in which IHC failed to demonstrate abnormalities in the markers examined.
Therefore, the combined immunoprofile and clinicopathologic assessment confirmed 20 WD-NET in the cohort of 33 high grade cases; 50% (10/20) of the WD-NET cases had loss of DAXX or ATRX expression, and 95% (19/20) had evidence of a concurrent or prior G1/G2 WD-NET (high grade progression). Twelve of the 33 cases were confirmed as PD-NEC, of which the majority (11/12) had abnormal Rb, p53, or SMAD4 expression, and 4/12 had a component of ductal carcinoma present on other sections of the tumor (Table 2). The distinction between WD-NET and PD-NEC could not be established for one case in this cohort: the morphologic assessment of the case was categorized as ambiguous, the clinical information and the prior pathology were not available because the patient was not seen at our institution, and the selected biomarkers did not demonstrate an abnormal immunoprofile.
Using the final immunohistochemistry and clinicopathologic classification (and omitting the single without a definitive diagnosis), the mean Ki67 index for the WD-NETs (46±14%, ranging 30-80%) was significantly lower than that for the PD-NECs (72±20%, ranging 26-93%) (p=0.012). However, there was significant overlap, and 7/20 WD-NETs had a Ki67 above 55% whereas 8/12 of PD-NECs were below 55%.
In the group confirmed as WD-NETs (n=20) after every level of assessment, thirteen were categorized as ambiguous and one as PD-NEC-LCC in the initial morphologic assessment (Table 2); we confirmed the correct classification by the loss of DAXX/ATRX expression (Figure 3 D) in 7/14 cases and with the clinical and pathologic evidence of WD-NET with high grade progression in the remaining 7 cases. Similarly, in the group of confirmed PD-NECs, all 5 cases initially rendered as ambiguous had abnormal p53, Rb, or SMAD4 protein expression (Figure 3 A-C) (Table 2).
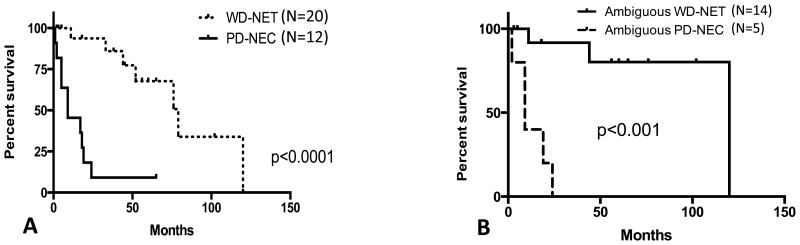
The median disease specific survival for the entire cohort based upon the final classification of WD-NET (n=20) and PD-NEC (n=12) was 75 months and 11 months, respectively (p<0.0001) (Figure 4A). Similarly, the median disease specific survival for the morphologically ambiguous cases upon the final classification of WD-NET (n=14) and PD-NEC (n=5) was 120 months and 11 months, respectively (p<0.001) (Figure 4B).
Figure 4. Disease Specific Survival of High Grade Pancreatic Neuroendocrine Neoplasm.
Disease specific survival of the entire cohort (A) and disease specific survival of morphologically ambiguous cases (B)
Discussion
In this study, we simulated a practical scenario and challenged our morphologic intuition for diagnosing difficult cases of high grade WD-NET and PD-NEC. As demonstrated by the results, pathologists with extensive experience in neuroendocrine neoplasms could not reach consensus on approximately two-thirds of these highly selected cases in the absence of an ancillary workup and the pertinent clinicopathologic information on the patients, particularly those with suboptimal or limited tumor tissue in biopsies.
Certain morphologic features, although not entirely specific, might have helped to distinguish between WD-NETs and PD-NEC. A geographic pattern of necrosis, while more commonly seen in PD-NECs, can be present in WD-NETs with high grade transformation3. Despite having high grade transformation, WD-NETs may retain certain organoid histologic patterns, such as nested, trabecular, or loosely cohesive architecture with a relatively organized vascular network and a hyalinized type of intratumoral fibrosis (Figure 1A-B, Figure 2 A-B, E). In contrast, PD-NECs have expansile large and irregular nests, an infiltrative growth pattern with randomly oriented large vascular structures, and desmoplastic type fibrosis (Figure 1C-F). Cytologically, WD-NETs, particularly pancreatic primaries, usually have abundant granular cytoplasm, which results in a low nuclear-to-cytoplasmic (N/C) ratio, and stippled chromatin (Figure 1 A-B, Figure 2 B, E); conversely, PD-NECs have less granular cytoplasm and a higher N/C ratio with either open chromatin with conspicuous nucleoli (large cell NEC) or hyperchromatic and molded nuclei lacking nucleoli (small cell carcinoma) (Figure 1 C-F, Figure 2 C-D). Nevertheless, the classic description of small and large cell neuroendocrine carcinoma in the pulmonary system11, 12 does not perfectly translate to the gastrointestinal tract and the pancreatobiliary system12, 13. Thus, there are overlapping features between both WD-NET and PD-NEC, and between PD-NEC-SCC and PD-NEC-LCC; in fact, the overlap between PD-NEC-SCC and PD-NEC-LCC was such that these two entities were combined as simply “PD-NEC” for purposes of the analysis.
The marked differences in proliferative rate between WD-NETs and PD-NECs suggest that this feature may be sufficient for their distinction. Re-review of the cases for which we did reach agreement showed that mitotic activity appears to have influenced the classification of G3 WD-NETs (mean mitoses 11.7±10/10 HPF) and PD-NETs (mean mitoses 47/10±19 HPF); however, this does not seem to be the case in the ambiguous group, which had mean mitoses of 13.6±9/10 HPF and 33±2/10 HPF in the cases ultimately classified as WD-NET and PD-NEC, respectively. Thus additional morphologic characteristics might have played a role to place them in the ambiguous category. In previous investigations, a cut-off Ki67 index of 55% has been suggested to separate G3 WD-NETs from PD-NECs14. While the Ki67 indices were unknown to the reviewers at the initial morphologic assessment, 7/20 (32%) WD-NETs and 8/12 (67%) PD-NECs (based on the final classification) had a Ki67 index greater than 55%. Thus, it is apparent that although PD-NECs generally have a higher average proliferative index (72±20%, ranging 26-93%) than WD-NETs (46±14%, ranging 30-80%), there is no absolute cut-off value that can sufficiently distinguish these two neoplasms15. Based on the inadequacy of pure morphologic or proliferation rate criteria to distinguish G3 WD-NET and PD-NEC, it is clear that assessment of additional biomarkers and clinical features is necessary to improve histopathologic diagnosis.
Genomic investigations have discovered recurrent and mutually exclusive DAXX and ATRX mutations, which culminate in loss of corresponding protein expression in tumor cells, in approximately 44% of pancreatic WD-NETs9. This genotype is specific for WD-NET and has not been seen in other pancreatic neoplasms, including PD-NECs3, 10. In contrast, pancreatic PD-NECs share some of the genotypic alterations of conventional pancreatic ductal adenocarcinoma including frequent gene mutations in TP53, and less commonly KRAS, p16, and SMAD4, which have not been identified in pancreatic WD-NETs in a number of investigations3, 10. Furthermore, RB1 gene mutations and the associated loss of Rb protein expression are commonly observed in high grade PD-NECs, with a frequency in the small cell subtype of >91%16 and in the large cell subtype of 50-60% , regardless of the anatomical site of tumor origin17, 18. RB1 and TP53 mutations have not been identified in WD-NETs3, 10. In the current study, we have demonstrated that these genotypes and corresponding phenotypes for pancreatic WD-NET (DAXX and ATRX) and for PD-NEC (p53, SMAD4, and Rb) as assessed by immunohistochemistry are indeed very useful to aid in the differential diagnosis. For the WD-NETs in this study, the DAXX/ATRX immunoprofile facilitated the correct interoperation in 50% (7/14) cases that were morphologically ambiguous. Furthermore, the p53/SMAD4/Rb immunophenotype exhibited even better efficacy (particularly p53 and Rb), and abnormal expression of at least one of these proteins supported the diagnosis of PD-NEC in all except one morphologically ambiguous case. Of note, the loss of SMAD4 expression was only present in one case of PD-NEC which also had a p53 abnormality; thus inclusion of SMAD4 may not provide supplementary value for the diagnosis. Similar results were observed in the consensus cases (3/6 in WD-NETs and 5/6 in PD-NETs). However, in the absence of these mutations (∼50% of DAXX/ATRX and ∼10% of p53/SMAD4/Rb) the classification of a high grade neuroendocrine neoplasm with ambiguous morphology cannot be established by immunohistochemistry.
We have previously demonstrated that despite high proliferative index and overlapping morphologic features between G3 WD-NET and PD-NEC, there are certain clinical and pathologic characteristics that can assist in distinguishing the two neoplasms3. In this study, we have further emphasized that when dealing with metastatic high grade neuroendocrine neoplasms, the consideration of WD-NET with high grade progression is frequently supported by a co-existing or prior lower grade WD-NET component in the sample at hand or at another site of disease (e.g., the G1/G2 WD-NET primary pancreatic tumor in the face of a high grade liver metastasis). In fact, every biopsy specimen (n=6), except for one, with a metastatic high grade NE neoplasm, initially rendered as morphologically ambiguous and ultimately confirmed as a WD-NET with high grade progression, had a previously documented lower grade pancreatic WD-NET in other specimens. In resection specimens, the lower grade component may be overt and usually constitutes a significant component (>50%) of the tumor. The sections chosen for inclusion in the current study were specifically selected to exclude any lower grade regions known to exist elsewhere within primary WD-NETs. In small biopsies, the heterogeneous tumor grades may not be well appreciated. Similarly, in the presence of a co-existing conventional carcinoma (i.e., squamous cell carcinoma or adenocarcinoma), a high grade neuroendocrine neoplasm would be considered a PD-NEC, since the combination with a non-neuroendocrine carcinoma component is extraordinarily rare in WD-NETs.
In the absence of a co-existing lower grade WD-NET or a conventional carcinoma component, additional clinical information (history of the disease, symptoms at the presentation, serum biomarkers, and radiographic assessment) can play an important role in the establishment of the correct classification of a high grade neuroendocrine neoplasm, particularly when dealing with recurrence or metastasis. Given the relatively protracted clinical course, the primary diagnosis of a WD-NET may have taken place several years earlier (up to 10 years before recurrence)19, 20. In fact, most cases (10/11) of metastatic WD-NETs with high grade progression in this cohort had a previous history of a lower grade (WHO G1/G2) tumor; this facilitated the correct classification of morphologically ambiguous cases in the absence of abnormal immunohistochemical biomarker expression tested in this study. Therefore, WD-NETs can be heterogeneous in grade, and they are unlikely to have an exclusively high grade component in a resection specimen, and a lower grade component almost inevitably can be identified in one of the tumor sections or in a prior specimen. On the contrary, patients with PD-NECs have rapid clinical deterioration8, and they are unlikely to have a prior similar malignancy in the extended history. In contrast to WD-NET, PD-NECs are homogeneously high grade in any type of specimen although some tumors may reveal paradoxical reduction of Ki67 after chemotherapy.
Additional clinical data, as discussed in our previous investigation3, such as onset age (younger for WD-NET), initial clinical presentations (often asymptomatic in WD-NETs), in vivo biomarkers (i.e. chromogranin, CEA, CA19.9 ect) and radiographic studies (i.e. Octreotide Scintigraphy, FDG-PET-CT) are also helpful in providing supplementary information for classifying these high grade neoplasms.
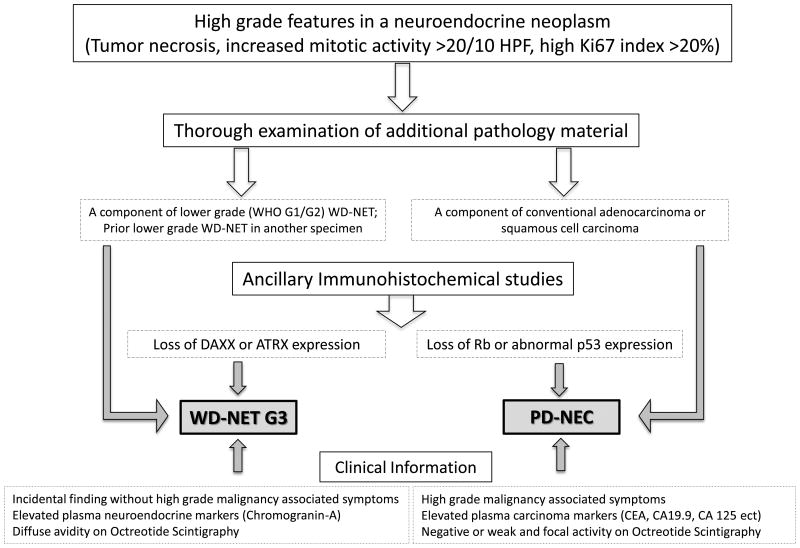
We have thus proposed a diagnostic algorithm for high grade neuroendocrine neoplasms. This algorithm is mostly useful for pancreatic primaries for their known mutated DAXX and ATRX genotype in WD-NETs (Figure 5). While DAXX and ATRX are not involved in substantial numbers of extrapancreatic WD-NETs, data exist verifying the restriction of p53 and Rb abnormalities to the PD-NEC category for non-pancreatic primaries.
Figure 5. Recommended Diagnostic Algorithm for Pancreatic High Grade Neuroendocrine Neoplasms.
In summary, due to the lack of easily recognized morphologic criteria, pathologists are challenged when trying to distinguish a high grade (G3) WD-NET from a PD-NEC, which is critical for clinical treatment decisions. The combined morphologic features, with knowledge of the histology of prior specimens or other sites of disease, and ancillary immunohistochemistry can facilitate the accurate diagnosis in the majority of cases and can provide guidance for the appropriate clinical management and prognosis. While at this time, the distinction between PD-NEC-SCC and PD-NEC-LCC may not have a direct clinical impact, future molecular investigations may reveal differences in treatment response and novel diverse therapeutic regimens. The distinction of these two subtypes of PD-NEC remains challenging and somewhat subjective, and none of the markers evaluated in the current study appeared to be helpful. Further molecular and genomic investigation may provide insights on these two related but phenotypically diverse carcinomas.
Supplementary Material
References
- 1.Bosmon F, Carneiro F, Hruban RH, Theise N. World Health Organization (WHO) Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010. [Google Scholar]
- 2.Velayoudom-Cephise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D, Debaere T, Caramella C, Schlumberger M, Planchard D, Elias D, Ducreux M, Scoazec JY, Baudin E. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20:649–57. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 3.Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–90. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basturk O, Yang Z, Tang L, et al. Increased (>20%) Ki67 Proliferation index in morphologically well differentiated pancreatic neuroendocrine tumors (PanNETs) correlates with decreased overall survival. Mod Pathol. 2013;26:423A. [Google Scholar]
- 6.Crippa S, Partelli S, Bassi C, Berardi R, Capelli P, Scarpa A, Zamboni G, Falconi M. Long-term outcomes and prognostic factors in neuroendocrine carcinomas of the pancreas: Morphology matters. Surgery. 2016;159:862–71. doi: 10.1016/j.surg.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Fazio N, Spada F, Giovannini M. Chemotherapy in gastroenteropancreatic (GEP) neuroendocrine carcinomas (NEC): a critical view. Cancer Treat Rev. 2013;39:270–4. doi: 10.1016/j.ctrv.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814–23. doi: 10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]
- 9.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA, Jr, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010;21(Suppl 7):vii65–71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
- 12.Shia J, Tang LH, Weiser MR, Brenner B, Adsay NV, Stelow EB, Saltz LB, Qin J, Landmann R, Leonard GD, Dhall D, Temple L, Guillem JG, Paty PB, Kelsen D, Wong WD, Klimstra DS. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32:719–31. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirabayashi K, Zamboni G, Nishi T, Tanaka A, Kajiwara H, Nakamura N. Histopathology of gastrointestinal neuroendocrine neoplasms. Front Oncol. 2013;3:2. doi: 10.3389/fonc.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 15.Liu TC, Hamilton N, Hawkins W, Gao F, Cao D. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2013;37:853–9. doi: 10.1097/PAS.0b013e31827fcc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, Leenders F, Lu X, Fernandez-Cuesta L, Bosco G, Muller C, Dahmen I, Jahchan NS, Park KS, Yang D, Karnezis AN, Vaka D, Torres A, Wang MS, Korbel JO, Menon R, Chun SM, Kim D, Wilkerson M, Hayes N, Engelmann D, Putzer B, Bos M, Michels S, Vlasic I, Seidel D, Pinther B, Schaub P, Becker C, Altmuller J, Yokota J, Kohno T, Iwakawa R, Tsuta K, Noguchi M, Muley T, Hoffmann H, Schnabel PA, Petersen I, Chen Y, Soltermann A, Tischler V, Choi CM, Kim YH, Massion PP, Zou Y, Jovanovic D, Kontic M, Wright GM, Russell PA, Solomon B, Koch I, Lindner M, Muscarella LA, la Torre A, Field JK, Jakopovic M, Knezevic J, Castanos-Velez E, Roz L, Pastorino U, Brustugun OT, Lund-Iversen M, Thunnissen E, Kohler J, Schuler M, Botling J, Sandelin M, Sanchez-Cespedes M, Salvesen HB, Achter V, Lang U, Bogus M, Schneider PM, Zander T, Ansen S, Hallek M, Wolf J, Vingron M, Yatabe Y, Travis WD, Nurnberg P, Reinhardt C, Perner S, Heukamp L, Buttner R, Haas SA, Brambilla E, Peifer M, Sage J, Thomas RK. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takizawa N, Ohishi Y, Hirahashi M, Takahashi S, Nakamura K, Tanaka M, Oki E, Takayanagi R, Oda Y. Molecular characteristics of colorectal neuroendocrine carcinoma; similarities with adenocarcinoma rather than neuroendocrine tumor. Hum Pathol. 2015;46:1890–900. doi: 10.1016/j.humpath.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Li AF, Li AC, Tsay SH, Li WY, Liang WY, Chen JY. Alterations in the p16INK4a/cyclin D1/RB pathway in gastrointestinal tract endocrine tumors. Am J Clin Pathol. 2008;130:535–42. doi: 10.1309/TLLVXK9HVA89CHPE. [DOI] [PubMed] [Google Scholar]
- 19.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, Klimstra DS, Allen PJ. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–15. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 20.La Rosa S, Klersy C, Uccella S, Dainese L, Albarello L, Sonzogni A, Doglioni C, Capella C, Solcia E. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol. 2009;40:30–40. doi: 10.1016/j.humpath.2008.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.