Abstract
Purpose
Most well-differentiated neuroendocrine tumors (WD-NETs) of the enteropancreatic system are low-intermediate grade (G1,G2). Elevated proliferation demonstrated by either a brisk mitotic rate (>20/10 high power fields) or high Ki67 index (>20%) defines a group of aggressive neoplasms designated as high grade (G3) neuroendocrine carcinoma (NEC). High grade NEC is equated with poorly-differentiated NEC (PD-NEC) and is associated with a dismal outcome. Progression of WD-NETs to a high grade neuroendocrine neoplasm very rarely occurs and their clinicopathologic and molecular features need to be characterized.
Methods
We investigated the 31 cases of WD-NETs with evidence of component of a high grade neoplasm. The primary sites included pancreas, small bowel, bile duct, and rectum. Histopathology of the cases was retrospectively reviewed and selected immunohistochemistry and gene mutation analyses performed.
Results
The high grade component occurred either within the primary tumor (48%) or at metastatic sites (52%). The clinical presentation, radiographic features, biomarkers, and the genotype of these WD-NETs with high grade component remained akin to those of G1-G2 WD-NETs. The median disease specific survival (DSS) was 55 months (16-119 months), and 2-year and 5-year DSS was 88% and 49%, respectively – significantly better than that of a comparison group of true PD-NEC (DSS 11 months).
Conclusion
Mixed grades can occur in WD-NETs, which are distinguished from PDNECs by their unique phenotype, proliferative indices, and the genotype. This phenomenon of mixed grade in WD-NET provides additional evidence to the growing recognition that the current WHO G3 category contains both WD-NETs as well as PDNECs.
INTRODUCTION
Well-differentiated neuroendocrine tumors of the gastroenteropancreatic system exhibit pathological heterogeneity and a spectrum of clinical behavior. They have been stratified by certain clinical and histopathological features1,2. However, the criteria for predicting prognosis within a uniformly staged tumors have unsatisfactory. Grading based on tumor proliferative activity, as assessed by mitotic rate and Ki67 index, can stratify prognostic subgroups of WD-NETs 3-5. Tumor grade has thus been used as the basis for prognostic classification systems, including those proposed by European Neuroendocrine Tumor Society6 and the World Health Organization (WHO)7.
In 2010, the revised WHO classification of neuroendocrine neoplasms defined three grades based on the mitotic rate and Ki67 index (G1: <2 mitoses/10 HPF and Ki67 <3%; G2: 2-20 mitoses/10HPF or Ki67 3-20%; G3: >20 mitoses/10 HPF or Ki67 >20%). The G1/G2 grade NETs are regarded as well-differentiated. High grade (G3) neoplasms have been regarded as synonymous with PD-NECs, which include small cell and large cell subtype. The well-differentiated and poorly-differentiated groups of neuroendocrine neoplasms have different etiologies and exhibit different genetic alterations8. The outcome and treatment of WD-NETs are also strikingly different from those of PD-NECs9.
PD-NEC may have a combined component of a conventional carcinoma, such as squamous cell carcinoma or adenocarcinoma10,11, but they do not typically contain a lower grade WD-NET. In contrast, WD-NETs, although mostly low/intermediate grade, can uncommonly contain regions with increased proliferation that place them in the WHO G3 category12. Cases of PD-NEC are morphologically homogeneous. Thus, it appears unlikely that PD-NECs arise via progression from WD-NETs with any frequency. It is increasingly apparent that the current WHO G3 category includes neuroendocrine neoplasms of two distinct types: 1) a highly proliferative group of WD-NETs and 2) PD-NEC as represented by small and large cell NEC9,12,13
WD-NETs can show progression from G1 primary tumors to G2 metastases, and heterogeneity between grades can occur with individual tumor or between sites of metastasis14. However, documentation of progression of a G1/G2 WD-NET to a G3 neoplasm has been rare, and the histologic and molecular features of the tumors remain to be described.
Material and Methods
Patient Information
Retrospective and prospective review of well-differentiated neuroendocrine neoplasms with increased proliferative activity (G3) was performed using the pathology files at the authors’ institutions with IRB approval. Some patients were captured because of the tumor grade discrepancy in different specimens (i.e. primary vs. metastasis) during the course of clinical follow up. All patients were evaluated clinically at our institutions with appropriate radiological and laboratory studies and surgical or oncological management. Follow-up information was obtained in all cases.
Pathological Assessment
Hematoxylin and eosin stained sections were examined with an average of 4 slides per case (2-21 sections/case). The criteria for further classification and grading were based upon the morphological features of the tumor and the proliferation rate. WD- NETs with HG component were defined as tumors with areas (at least 50%) showing conventional well-differentiated features and a low proliferative rate of < 20 mitoses/10 HPFs and Ki67 < 20%, and topographically recognizable separable regions, which constituted at last 20% of the tumor volume, showing increased nuclear atypia, a confluent growth pattern, necrosis, and increased mitotic activity. We acknowledge that the 20% was chosen arbitrarily to ensure that both components were sufficiently well-represented to be able to separately recognize them, but there is no biological basis for this cut-point.
A minimal mitotic rate to define the regions of HG component was not specified (although all cases had a mitotic rate of >10/10 HPFs), but the Ki67 index in these regions was > 20%. The mitotic count was derived from evaluation of multiple sections in 50 HPFs and expressed as mitoses/10 HPFs. Small tumor biopsies with <10 HPFs were excluded. Each high power field was defined as 0.24 mm2 using Olympus BX41 microscope. The excluded PD-NECs were defined by: 1)confluent growth pattern of a typical small cell or large cell NEC without a recognizable component of G1/G2 grade WD-NET; 2)the presence of a combined conventional non-neuroendocrine carcinoma; or 3) a metastasis of high grade neuroendocrine neoplasm from a previously documented non-NEC.
Immunohistochemistry
Standard ABC peroxidase techniques were used for immunohistochemistry performed on 4 μ paraffin sections of formalin-fixed, paraffin-embedded tissue. Antigen retrieval in heated citrate buffer at pH 6.0 was applied for all antibodies. The Ki67 monoclonalantibody (1:100), Rb monoclonal-antibody (1:400), p53 monoclonal-antibody (1:500) Chromogranin-A polyclonal-antibody (1:8000), and synaptophysin (1:500) were obtained from Dako (Carpinteria, CA). Immunohistochemistry was performed on BenchMark XT automated equipment (Ventana Medical System Inc., Tucson, AZ). Positive control tissue was stained in parallel with all the study cases. Ki67 immunoreactivity was expressed as the percentage of tumor cells with nuclear staining, based on counting >2000 cells in the regions with the highest labeling recognizable on scanning magnification. The Ki67 indices for the regions with G1/G2 morphology and high grade morphology were recorded separately. p53 immunoreactivity, with strong intensity in >25% tumor cells, was regarded as abnormal, and complete loss of Rb protein expression was regarded as abnormal for this marker.
Gene Mutation Analysis
Exome gene mutation analyses of RB1, DAXX, ATRX, and MEN1 were performed on Illumina miSeq platform (Illumina Inc. San Diego, CA). Briefly, specimens were obtained from institutional tissue bank with Human Biospecimen Utilization Committee approval. Hematoxylin and Eosin sections were evaluated for tumor quantity and quality. Components of WD-NET with different histologic grades were macrodissected before DNA extraction. Frozen tumor tissue with cellularity > 80% and without necrosis were macro-dissected (20-25 mg) and DNA extraction was carried out using DNeasy Tissue Kit (Qiagen, Valencia, CA). All mutations detected on Illumina miSeq were validated by Sanger sequencing.
Statistical Analysis
Data are represented as mean ± standard deviation. Graphpad Prism 6 (Graphpad Software Inc, La Jolla, Ca) was used for statistical and survival analyses. Survival analysis p values (2-sided) were based on log-rank tests. T-tests and Fisher's exact/chi squared tests were used to determine differences between groups. Significance was defined as P < 0.05. Cox proportional hazards model (SAS9.3) was used to analyze disease specific modality for patients with pancreatic primary neuroendocrine tumors and neuroendocrine carcinomas. Model included patients with complete data for age, tumor size and stage.
Results
Clinical Presentation of WD-NET with HG component (Table 1)
Table 1.
Clinical features of NET with high grade transformation.
| Transformed NET | Elevated NE markers | Abnormal carcinoma markers | Octreoscan® Avid | PET positive | Generally well at the initial presentation | NET related symptoms | Medical Treatment | Surgical Procedure |
|---|---|---|---|---|---|---|---|---|
| Total = 31 Pancreas = 21 Small Bowel = 6 Bile Duct = 2 Rectum = 2 |
19/23 (83%) Chromo = 13 Gastrin = 3 Insulin = 2 Serotonin = 2 Glucagon = 1 |
2/19 (11%) CEA =1 CA19.9 = 1 |
22/25 (88%) | 10/10 | 14/21 (67%) Incidental or non-related symptoms |
12/29 (41%) | Chemo 21/31 (68%) Octreotide alone 3/31 Embolization 2/31 No treatment = 4/31 |
Biopsy 6/31 Resection 25/31 |
Thirty-one cases satisfied the criteria for WD-NETs with HG component. The mean age was 54.5±2.6 years with a female prevalence of 68%. The primary sites included pancreas, small bowel, bile duct, and rectum (Table 1). Most patients were generally well at the time of initial presentation - either asymptomatic or presenting with unrelated symptoms. Neuroendocrine tumor-related symptoms occurred in 41%, which included carcinoid syndrome and other symptoms characteristic of functional PanNETs (insulinoma, glucagonoma) were evident in pancreatic primaries. Plasma neuroendocrine markers were elevated in 83% patients who had the tests performed. In contrast, plasma carcinoma-related biomarkers were abnormal in only 11% patients tested. Somatostatin receptor scintigraphy (Octreoscan™) was performed in 25 patients and 88% demonstrated avidity in the tumors. Fluorodeoxyglucose (18F)-positron emission tomography (FDG-PET) was positive in all 10 patients who had the test performed with an average standardized uptake value (SUV) is 2.9 (2.2 – 5.9).
Pathological Features of WD-NET with HG component (Table 2)
Table 2.
Pathologic features of NET with high grade transformation.
| Transformed NET | Average mitosis/10HPF | Average Ki67% | Site of transformation | Time of Transformation | Metastasis |
|---|---|---|---|---|---|
| Total = 31 Pancreas = 21 Small Bowel = 6 Bile Duct = 2 Rectum = 2 |
Low: 2.9±2.8 (0-10) High: 20.4±6.4 (11-40) |
Low: 7.3±5.3 (1-20) High: 50.2±17.2 (25-80) |
Primary = 12 Local/LN = 3 Distant = 16 |
Synchronous 74% Metachronous 26% Time to Transform: 50±37 mons (10-135) |
29/31 (94%) No Met = 2 Local/LN = 3 Distant = 26/31 |
Resection specimens constituted 25 of 31 cases, and the remaining 6 were biopsies of metachronous metastases. Regardless of the grade of the tumors, all cases exhibited diffuse positive immunoreactivity for both synaptophysin and chromogranin. The staining intensity and extent did not appear to be reduced in the high grade regions.
HG component of WD-NET occurred locally in 15/31 cases; in the remaining 16/31 cases, high grade regions were found in distant metastatic sites, with liver being the most common location (75%) followed by ovary, bone, and lung. The majority (74%) of the cases presented with HG component at the time of initial diagnosis and the remainder occurred metachronously with a mean time to progression of 50±37 months (10-135 months) following the initial diagnosis (Table 2). It is of note that 68% of tumors at the site of metastasis had both the low/intermediate grade and the high grade components; six cases with mixed grades at the primary site had high grade tumor only in metastases; in one case the G1/G2 tumor had exclusively metastasized to the distant location although approximately 40% of the primary tumor was high grade.
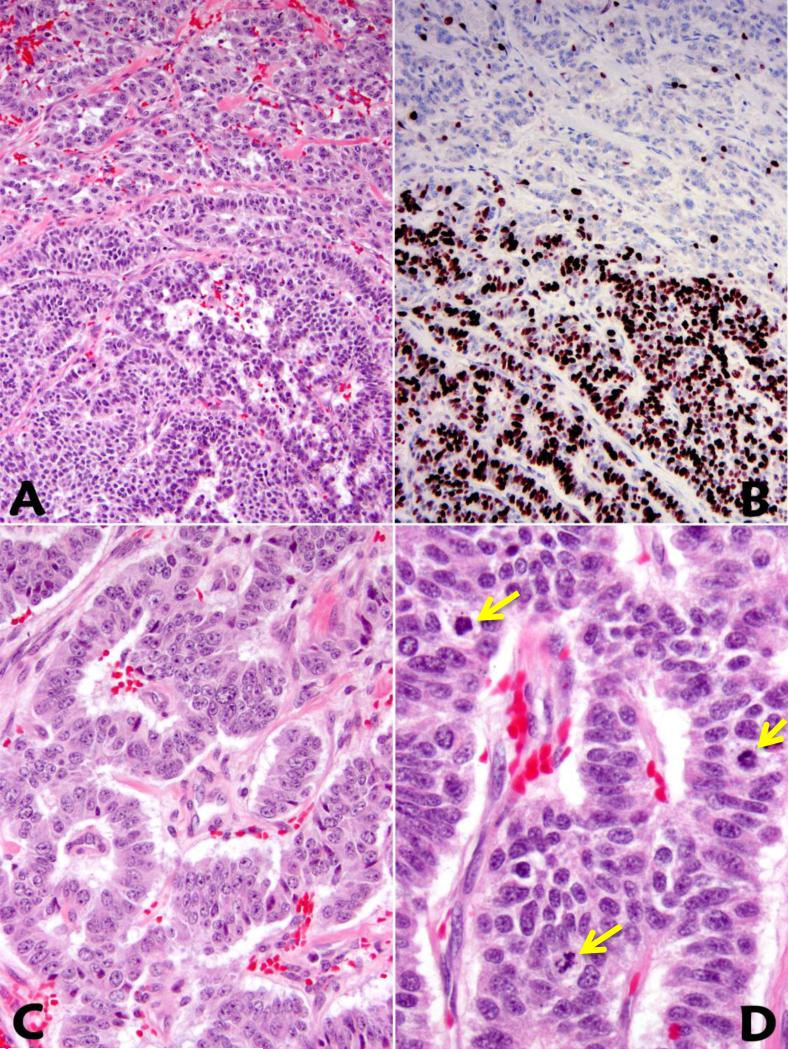
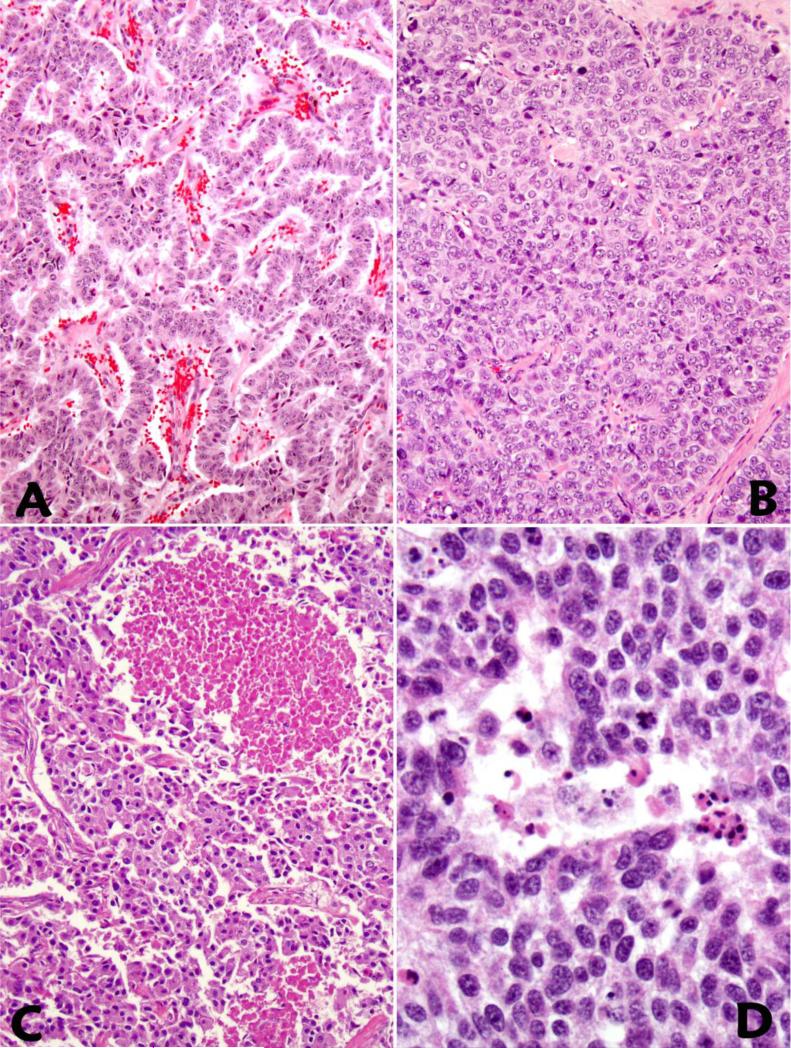
The hallmark of WD-NETs with HG component was the presence of a significant component of the tumor with low to intermediate grade in resection specimens. The morphologically distinct high grade areas had increased mitotic activity and Ki67 indices (Figure 1A-D and Table 2). It is of note that WD-NETs with high grade component were more than just microscopic foci, and in most cases they constituted at least 20% of the entire tumor. Both the mitotic rate and the Ki67 index were rather heterogeneous in the high grade areas, although focal homogenous high Ki67 indices were observed (Figure 1). While the G1/G2 component of the tumor maintained the histologic phenotype of a WD-NET, areas of HG component revealed architectural alterations including: 1)confluent growth pattern with reduced tumor stroma and vasculature; 2)ischemic-type tumor necrosis; 3)increased nuclear size and atypia, nuclear membrane abnormalities, and chromatin clumping (Figure 2A-D). In none of the cases did the high grade components display classic histologic features of small cell carcinoma, although there was some degree of histologic overlap between the high grade portion in WD-NET with large cell NEC. Nevertheless, the presence of a lower grade counterpart or a clinical history of a lower grade WD-NET confirmed in a previous specimen separated this group of WDNET with HG component from PD-NEC. While the evidence of high grade component could be seen on microscopic scanning by architectural alterations and the presence of tumor necrosis (Figure 1A, Figure 2B, 2C), the grade transition from low to high was better appreciated on Ki67 immunohistochemical stains (Figure 1B).
Figure 1.
Well-differentiated neuroendocrine tumor with HG component is characterized (in the direction from upper to lower) by subtle architectural alterations (A) and a markedly increased Ki67 proliferative index (B). In comparison with the lower grade component (C), areas with HG component within the same tumor reveal increased nuclear to cytoplasmic ratio and brisk mitotic activity (D).
Figure 2.
Compared to the lower grade regions (A), a WD-NET with HG component shows a more solid and confluent growth pattern with loss of stroma and vasculature (B), and tumor necrosis can be present as either geographic (C) or punctuate patterns or as single cell necrosis (D).
When the high grade features of WD-NETs were seen in small biopsy specimens, the morphologic evidence of grade progression was difficult to assess in the absence of the lower grade component. However, all the patients in this setting had previously established diagnoses of WD- NETs of G1/G2 and subsequently developed metastases in which biopsies revealed increased mitotic activity to the level of G3.
Treatment of WD-NET with HG component
Twenty-one of 31 patients received chemotherapy, including platinum-based regimens as neoadjuvant therapy, adjuvant therapy, or at the time of disease progression (Table 1). Eleven-percent received no adjuvant therapy following resection of the primary tumor. Given the diversity of the therapeutic regimens and primary sites of origin, it was difficult to compare the tumor response between different treatment groups. Nevertheless, of all the patients who received chemotherapy, 30% had partial responses, 10% had stable disease or no evidence of recurrence, and 60% had disease progression while on chemotherapy. Of eleven patients who were treated with platinum-based chemotherapy, a)one had adjuvant therapy after the complete surgical removal of the primary tumor and did recur; b)five patients had either stable disease or an initial partial response at the primary site and subsequent tumor progression in the liver metastasis; c)and the remaining five patients had disease progression while on therapy.
Outcome of WD-NET with HG component
Clinical follow-up information was available for all 31 patients (mean follow-up of 55 ± 5 months, range of 16-119 months). The median disease specific survival (DSS) for the entire cohort of WD-NETs with HG component was 55 months, with 2-year and 5-year DSSs of 89% and 49%, respectively.
Comparison of WD-NET with HG component and PD-NEC of the Pancreas
Since the majority cases of NETs with HG component in this series were pancreatic primaries, we compared their clinicopathological features with those of WD-NETs of low/intermediate grade (n=329) and PD-NECs of the pancreas (n=35); data related to some cases have been previously published4,12. The onset age was similar between the two groups of WD-NETs, 56±1 years in the low/intermediate grade group and 52±3 years in the group with HG component, respectively (Table 3). In contrast, patients with PD-NECs were one decade older (65±6 years). WD- NETs with HG component were larger than low/intermediate grade NETs. In the absence of HG component, 34% of pancreatic WD-NETs had distant metastatic disease, whereas 81% of WD-NET with HG component demonstrated either synchronous (82%) or metachronous (18%) distant metastases. The incidence of distant metastasis observed in PD-NEC was 100%.
Table 3.
Features of pancreatic NET and NEC
| Tumor Type | Age | Tumor Size |
Average Mitotic Rate |
Average Ki67% |
P53 by IHC |
RB1 Mutation |
Rb Protein loss by IHC |
Daxx/ATRX/MEN1 Mutation |
Distant Met |
Median Survival (months) |
2 Year DDS |
5 Year DDS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WD Pancreatic NET (Low-Intermediate grade), n=329 | 56±1 | 3.6±3 | <1/10 HPF (3/50 HPF) | <20% | 0 | 0/63 | 0 | 35/63 | 34% | 162 | 97% | 90% |
| Transformed WD pancreatic NET (mixed grade), n=21 | 52±3 | 5.5±0.7 | 20/10HPF | 50% | 0 | 0/4 | 0 | 3/4 | 84% | 55 | 88% | 48% |
| PD NEC of pancreas (High grade), n=35 | 65±6 | 4.7±0.5 | 42/10HPF | 75% | 56% | 5/7* | 59% | 0/28* | 100% | 16 | 24% | 24% |
Yachida et al8
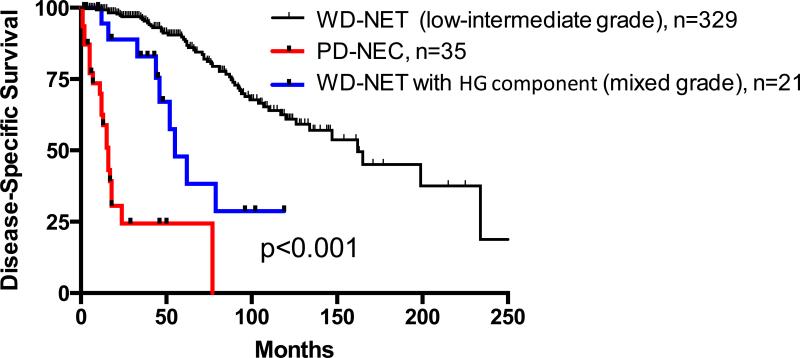
The median DSS of all stages of pancreatic WD-NET of G1/G2, WD-NET with HG component, and PD-NEC were 162 months, 55 months, and 16 months, respectively (p<0.001, CI 95%); and the 2-year and 5-year DSSs for the three groups were 97%, 88%, 24%, and 90%, 48%, 24%, respectively (Table 3, Figure 3). While the presence of high grade component in WD-NET was associated with an unfavorable clinical outcome, but its prognosis was not nearly as dismal as that of a true PD-NEC. However, in stage-matched (stage IV) analysis, pancreatic WD-NET of G1/G2 and with HG component revealed no statistical significance in DSS; and as a group, they demonstrated survival advantage over that of pancreatic PD-NEC (Median survival of 61 months vs. 16 months, p<0.001, CI 95%). Furthermore, Cox proportional hazards model showed similar results in which WD-NET of G1/G2 group and with HG component group of had a hazard ratios of 0.17 and 0.16, respectively, relative to the PD-NEC group with a reference of 1 (p<0.001).
Figure 3.
Disease Specific Survival of stage-matched pancreatic WD-NET with or without HG component and pancreatic PD-NEC4,12.
Assessment of tumor genotype in the pancreatic cases revealed that DAXX/ATRX/MEN1 mutations were detected in three of four pancreatic WD-NETs in the HG component as well as its lower grade counterpart, and this frequency was comparable to the counterpart low/intermediate grade WD-NETs (57%) (Table 3). Immunohistochemical studies reveal loss of DAXX/ATRX protein expression in cases with corresponding gene mutation (data not shown). In contrast, RB1 gene mutation and loss of Rb protein expression by immunohistochemistry were not detected in WD-NETs of any grade; but Rb protein loss was found in 59% of PD-NECs (Table 3). While mutational analysis of TP53 was not performed in this study, p53 immunoreactivity, as a surrogate measure of p53 mutation, was negative in WD-NETs of low/intermediate grades, as well as in those with HG component; in contrast, it was positive in 56% of PD-NECs (Table 3).
Discussion
We have documented that there exists uncommon WD- NETs that can exhibit low/intermediate grade neoplasm and a higher grade phenotype, breaching the threshold for the WHO classification of a high grade (G3) NEC, but not possessing the clinical, pathological, and genotypical features of a true PD- NEC.
WD- NET and PD-NEC represent distinct groups of neoplasms from their clinical presentations to their pathological characteristics. Although they exhibit a neuroendocrine phenotype, PD-NECs in the enteropancreatic system are commonly (up to 50%) associated with a conventional carcinoma 10,11; these combinations are extremely rare in WD-NETs. This phenomenon suggests that PD-NECs represent a neoplastic transformation from conventional carcinoma counterparts or their precursor lesions. Furthermore, recent genomic investigation has established that DAXX/ATRX and MEN1 gene mutations are present in 43% and 44% of pancreatic WD- NETs 15, respectively; but they are not identified in pancreatic PD-NECs 8. In contrast RB1 and other commonly mutated genes in conventional adenocarcinomas are frequently seen in PD-NECs but not in WD-NETs 8. The data from this study support these findings and further suggest that PD-NEC represents a neoplastic entity that is genetically more closely related to a conventional carcinoma than to a WD-NET. Therefore, from histogenetic point of view, it appears that WD-NETs have a neuroendocrine/endocrine cell lineage16,17; in contrast, PD-NECs are likely of either squamous or glandular cell origin. Thus, in most cases, PD-NEC does not represent genetic progression of a low or intermediate grade WD-NET.
The appearance of morphologically recognizable high grade components in well differentiated NETs can be explained in several ways. Commonly, higher grade regions in epithelial neoplasms are regarded to reflect neoplastic progression, implying that, over time, additional molecular and genetic events have occurred in the higher grade region. An alternative explanation is that regional variations in morphology reflect epigenetic variations or multiclonality. These explanations can be explored by the ongoing genomic analysis of the regions of different tumor grades within WD-NETs.
Clinically, WD-NET and PD-NEC are also distinct based on their presenting symptoms, serum biomarkers, radiographic characteristics, and prognosis18,19. Most WD-NETs (>85%) are evident on somatostatin receptor scintigraphy imaging (Octreoscan™) 20,21,22. In contrast, given their low proliferative activity, WD-NETs of low/intermediate grade are usually negative on FDG-PET scans 23. Patients with PD-NEC may present with neoplastic syndromes secondary to ectopic hormone production, such as ACTH, but they uncommonly exhibit carcinoid syndrome or conditions associated functional hormone hypersecretion; they may have elevated serum carcinoma-associated markers but uncommonly have measurable chromogranin-A. PD-NECs are detectable on FDG-PET scans with a high standardized uptake value (SUV) and are uncommonly avid on Octreoscan™. Patients with PD-NEC require cytotoxic chemotherapy, usually with platinum-based regimens, and they are likely to have at least a transient response, particularly those with small cell carcinomas 24.
One particular issue with the current WHO classification is the so-called high grade NEC category, designated as G3. It has been increasingly recognized and our data have illustrated that G3 tumors include, in addition to PD-NEC of small cell or large cell type, a group of WD-NETs, particularly pancreatic primaries, in which the proliferative rate (usually the Ki67 index) crosses the threshold of high grade25. Our currently reported cases of WD-NETs with HG component also fit into the category of high grade WD-NETs. Even when the high grade regions may resemble large cell neuroendocrine carcinomas, the association with a low grade component and the genetic features we describe clearly relate these neoplasms to the WD-NET group, rather than the PD-NEC category. Thus, it must be acknowledged that classification of a high grade neuroendocrine neoplasm based on proliferative activity alone may fail to reveal the underlying pathologic basis of different neoplastic entities.
Without consideration of other relevant clinical and pathological features, a tumor with either a mitotic rate of >20/10 HPF or a Ki67 index of > 20% could be classified a high-grade neuroendocrine neoplasm, which may be clinically assumed to be synonymous with a PD-NEC, and the patient may be subjected to platinum-based chemotherapy. It is thus not unexpected that G3 neuroendocrine neoplasms exhibit diverse clinical behavior and mixed responses to chemotherapy regimens9. In fact, results from a number of investigations including data in this study suggest that patients with WD-NETs, even with HG component, are unlikely to have long term benefit from platinum-based chemotherapy 9,24.
It has been well recognized that grade heterogeneity exists within WD-NETs14,26,27. There is also clinical evidence supporting the concept of grade progression in WD-NETs. Some patients with relatively stable metastatic disease experience rapid growth of one or more metastases. Although the pathological findings of the more rapidly enlarging foci have not been rigorously documented, it is possible they have undergone a high grade transformation.
It should be noted that in the absence of a lower grade counterpart, the component of the WD-NET with HG component can be morphologically indistinguishable from a large cell PD-NEC. This issue is of clinical significance when dealing with biopsies in which the comprehensive features of the tumor cannot be appreciated. Genotyping or immunoprofiling of this group of tumors may provide a more definitive classification, although lack of gene mutations or altered protein expression (e.g., DAXX/ATRX, MEN1, TP53, KRAS, RB1) would not be helpful. With the evolving molecular and genetic/epigenetic information additional genomic investigation of WD-NET with HG component and PD-NEC has already been initiated. We anticipate the establishment of the “gold standard” to separate the pathologic distinct entities of well-differentiated and poorly differentiated neoplasms particularly those which are difficult to assess on the morphologic basis alone. Furthermore, as delineated in this study, the combined systematic evaluation of clinical history, laboratory data, radiology, and pathologic assessment can facilitate the correct diagnosis of these two pathologically and therapeutically distinct diseases.
It is important to emphasize that tumor grading is only one component of disease assessment in neuroendocrine malignancies, and clinical management of the disease requires multidisciplinary input. Grading of WD-NETs is necessary for the projection of clinical outcome, although there is currently no indication for a specific chemotherapy regimen based on tumor grade alone within group of WD- NETs. In contrast, PD-NEC has clear differences in outcome and therapeutic approach that justify its separation from the well-differentiated group. The recognition that WD- NETs can achieve a proliferative rate in the G3 range complicates the therapeutic stratification of neuroendocrine neoplasms and suggests that modification of the WHO grading scheme would be necessary.
Statement of Translational Relevance.
This manuscript makes two very important contributions to the field of neuroendocrine tumor practice and research: 1) this is the first time that a thoroughly combined clinicopathologic and genetic investigations are performed to distinguish the 2 related but distinct high grade neuroendocrine neoplasms. The pathologic and the scientific basis of the study will provide guidance for the clinical management of specific neuroendocrine disease entities ; 2) The phenomenon of high grade component in WD-NETs provides evidence that the current WHO G3 category contains both WD-NETs as well as PDNECs. Thus this study will contribute to the future edition of WHO classification of neuroendocrine tumors.
We have chosen to publish these results in Clinical Cancer Research because we believe that our investigation will have direct impact on both scientific understanding and clinical management or neuroendocrine disease.
Acknowledgments
Funding: This work was partially supported by the Raymond and Beverley Sackler Research Foundation and the Mushett Family Foundation, as well as being funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Part of this study was presented at United States and Canadian Academy of Pathology (USCAP) annual meeting, March 2010, Washington D.C., USA.
Disclosure: The authors have no conflicts of interest.
REFERENCES
- 1.Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. ERC. 2010;17:909–18. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 2.Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. ERC. 2011;18(Suppl 1):S1–16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- 3.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. JCO. 2002;20:2633–42. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? JCO. 2007;25:5609–15. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 5.Liu TC, Hamilton N, Hawkins W, Gao F, Cao D. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. AJSP. 2013;37:853–9. doi: 10.1097/PAS.0b013e31827fcc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosman FTCF, Hruban RH, Theise ND. World Health Organization (WHO) Classification of Tumours of the Digestive System. WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 8.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. AJSP. 2012;36:173–84. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 10.Shia J, 1, Tang LH, Weiser MR, Brenner B, Adsay NV, Stelow EB, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? AJSP. 2008;32:719–31. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PubMed] [Google Scholar]
- 11.Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol. 2007;34:43–50. doi: 10.1053/j.seminoncol.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. AJSP. 2014;38:437–47. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vélayoudom-Céphise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? ECR. 2013;20:649–57. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. AJSP. 2011;35:853–60. doi: 10.1097/PAS.0b013e31821a0696. [DOI] [PubMed] [Google Scholar]
- 15.Jiao Y, 1, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science (New York, NY) 2011;331:1199–203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masson P. Carcinoids (Argentaffin-Cell Tumors) and Nerve Hyperplasia of the Appendicular Mucosa. AJP. 1928;4:181–212.19. [PMC free article] [PubMed] [Google Scholar]
- 17.Rosai J. The origin of neuroendocrine tumors and the neural crest saga. Mod Pathol. 2011;24(Suppl 2):S53–7. doi: 10.1038/modpathol.2010.166. [DOI] [PubMed] [Google Scholar]
- 18.Oberg K. Circulating biomarkers in gastroenteropancreatic neuroendocrine tumours. ECC. 2011;18(Suppl 1):S17–25. doi: 10.1530/ERC-10-0280. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:111–34. viii. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Reubi JC, Schär JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. EJNMMI. 2000;27:273–82. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 21.Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42:80–7. doi: 10.1007/s12020-012-9631-1. [DOI] [PubMed] [Google Scholar]
- 22.Velikyan I, Sundin A, Sörensen J, Lubberink M, Sandström M, Garske-Román U, et al. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014;55:204–10. doi: 10.2967/jnumed.113.126177. [DOI] [PubMed] [Google Scholar]
- 23.Toumpanakis C, Kim MK, Rinke A, Bergestuen DS, Thirlwell C, Khan MS, et al. Combination of Cross-Sectional and Molecular Imaging Studies in the Localization of Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology. 2014 doi: 10.1159/000358727. [DOI] [PubMed] [Google Scholar]
- 24.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014 doi: 10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]
- 25.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The High-grade (WHO G3) Pancreatic Neuroendocrine Tumor Category Is Morphologically and Biologically Heterogenous and Includes Both Well Differentiated and Poorly Differentiated Neoplasms. AJSP. 2015;39:683–90. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCall CM, 1, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. AJSP. 2013;37:1671–7. doi: 10.1097/PAS.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. AJSP. 2012;36:1761–70. doi: 10.1097/PAS.0b013e318263207c. [DOI] [PubMed] [Google Scholar]