ABSTRACT
Following infection of epithelial tissues, herpes simplex virus 1 (HSV-1) virions travel via axonal transport to sensory ganglia and establish a lifelong latent infection within neurons. Recent studies have revealed that, following intraganglionic or intrathecal injection, recombinant adeno-associated virus (rAAV) vectors can also infect sensory neurons and are capable of stable, long-term transgene expression. We sought to determine if application of rAAV to peripheral nerve termini at the epithelial surface would allow rAAV to traffic to sensory ganglia in a manner similar to that seen with HSV. We hypothesized that footpad or ocular inoculation with rAAV8 would result in transduction of dorsal root ganglia (DRG) or trigeminal ganglia (TG), respectively. To test this, we inoculated the footpads of mice with various amounts of rAAV as well as rAAV capsid mutants. We demonstrated that this method of inoculation can achieve a transduction rate of >90% of the sensory neurons in the DRG that innervate the footpad. Similarly, we showed that corneal inoculation with rAAV vectors in the rabbit efficiently transduced >70% of the TG neurons in the optic tract. Finally, we demonstrated that coinfection of mouse footpads or rabbit eyes with rAAV vectors and HSV-1 resulted in colocalization in nearly all of the HSV-1-positive neurons. These results suggest that rAAV is a useful tool for the study of HSV-1 infection and may provide a means to deliver therapeutic cargos for the treatment of HSV infections or of dysfunctions of sensory ganglia.
IMPORTANCE Adeno-associated virus (AAV) has been shown to transduce dorsal root ganglion sensory neurons following direct intraganglionic sciatic nerve injection and intraperitoneal and intravenous injection as well as intrathecal injection. We sought to determine if rAAV vectors would be delivered to the same sensory neurons that herpes simplex virus (HSV-1) infects when applied peripherally at an epithelial surface that had been treated to expose the underlying sensory nerve termini. For this study, we chose two well-established HSV-1 infection models: mouse footpad infection and rabbit ocular infection. The results presented here provide the first description of AAV vectors transducing neurons following delivery at the skin/epithelium/eye. The ability of AAV to cotransduce HSV-1-infected neurons in both the mouse and the rabbit provides an opportunity to experimentally explore and disrupt host and viral proteins that are integral to the establishment of HSV-1 latency, to the maintenance of latency, and to reactivation from latency in vivo.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) establishes a lifelong latent infection within the neurons of sensory ganglia. These ganglia are highly specialized structures composed of a diverse assemblage of neuronal and nonneuronal cells. Ganglionic neurons detect a wide variety of sensory inputs, including temperature, touch, and pain, and relay this information into the central nervous system (CNS). Immunohistochemical analyses of infected trigeminal ganglia (TG) have indicated that HSV-1 preferentially establishes latency within certain neuronal subpopulations (1, 2). Historically, such studies have required the use of animal models such as the rabbit eye to establish latent infections in TG neurons or the mouse footpad to establish latent infections in dorsal root ganglia (DRG) neurons. However, the fact that HSV-1 establishes latency within a heterogeneous population of cells with such a complex anatomical structure often makes mechanistic studies of HSV-1 latency challenging. In addition, the fact that most latency studies have to be performed in in vivo models, as well as the fact that there are no good techniques available to deliver RNA or plasmids to the sensory ganglia in vivo, makes it virtually impossible to use standard techniques such as small interfering RNA (siRNA) knockdowns or overexpression of proteins to study gene function.
In an effort to overcome some of these challenges, we sought to employ a viral vector approach as a method of delivery of genes into sensory neurons. Many features of adeno-associated virus (AAV) make it an ideal choice for this application. Recombinant AAV (rAAV) vectors are widely considered to be nonpathogenic, can infect a wide variety of cell types, and are capable of long-term gene expression in nonmitotic, terminally differentiated cells (3, 4). These vectors have a transgene carrying capacity of about 4.5 kb within their single-stranded DNA (ssDNA) genomes; however, single-stranded AAV (ssAAV) genomes require second-strand synthesis before the transgene can be transcribed, effectively slowing the onset of transgene expression (5, 6). To overcome this limitation, modified self-complementary AAV (scAAV) genomes have been generated (7). These genomes encode a half-size genome dimer that base pairs to form a double-stranded DNA template that does not require second-strand synthesis. The cost of this modification is that the transgene capacity is reduced by half, although this still represents ample space for promoters and open reading frames (ORFs) encoding small interfering RNAs, as well as for relatively small reporter genes such as the gene encoding green fluorescent protein (GFP).
In addition to modifications of the genome which allow more-efficient transgene expression, the AAV capsid proteins can also be modified to allow greater transduction efficiency, as the capsid serotype is a major determinant of cell tropism (8). New serotypes are constantly being discovered or engineered and tested for efficacy in cell culture and animal models of disease (9, 10). Studies of intracellular vector trafficking revealed that phosphorylation of exposed tyrosine residues on the AAV capsid surface leads to vector degradation and reduced transgene expression (11). When certain capsid tyrosine residues were mutated to phenylalanine (Y733F), transduction efficiency was shown to be greatly increased relative to that seen with vectors with wild-type capsids (12–20). Most relevant to the current study, AAV is capable of transducing DRG sensory neurons via direct intraganglionic sciatic nerve injection (21, 22) and intraperitoneal and intravenous injection (23), as well as via intrathecal injection following lumbar puncture (24–27). Therefore, we sought to determine if rAAV vectors would be delivered to the same sensory neurons that HSV-1 infects when applied at an epithelial surface that has been treated to expose the underlying sensory nerve termini. For this study, we chose two well-established HSV-1 infection models: mouse footpad infection and rabbit ocular infection. We also compared the efficacies of delivery of several different types of rAAV vectors, including ssAAV and scAAV, as well as of tyrosine capsid mutants. The results presented here provide the first description of AAV vectors transducing neurons following delivery at the epithelium. The ability of AAV to cotransduce HSV-1-infected neurons in both the mouse and the rabbit provides an opportunity to experimentally explore and disrupt host and viral proteins that are integral to the establishment of HSV-1 latency, to the maintenance of latency, and to reactivation from latency in vivo.
MATERIALS AND METHODS
Viruses and cells.
For colocalization, immunofluorescence (IF) and immunohistochemistry (IHC) were performed on sections of dorsal root ganglia (DRG) from mice infected with HSV-1 vector or with rAAV vector or both. KOS/62, an avirulent HSV-1 latency-associated-transcript-negative (LAT−) recombinant that expresses β-galactosidase (β-Gal) from the LAT loci (28, 29), was used for colocalization. KOS/62 was grown using rabbit skin cells, and titers were determined using Eagle's minimal essential medium (Life Technologies) supplemented with 5% bovine serum, 250 U penicillin/ml, 250 μg streptomycin/ml, and 292 μg l-glutamine/ml (Life Technologies). A variety of rAAV vectors were used, including single-stranded (ssAAV) and self-complementary (scAAV) vectors, as well as wild-type (WT) or modified (Y733F) capsids (11). All rAAV vectors expressed GFP driven by a chicken β-actin promoter and cytomegalovirus (CMV) immediate-early enhancer and were diluted in saline solution.
Mouse footpad infections.
Six-week-old female ND4 Swiss mice were anesthetized with isoflurane and subcutaneously injected with 50 μl of a 10% sterile saline solution into both rear footpads. Three hours after the saline treatment, mice were anesthetized by intramuscular injection of 20 μl of a cocktail of ketamine (30 to 45 mg/kg of body weight), xylazine (7.5 to 11.5 mg/kg), and acepromazine (2.5 to 3.75 mg/kg). Both rear footpads were abraded with an emery board to remove the keratinized epithelium. A total volume of 50 μl of virus per mouse was applied to the exposed dermis, and virus was allowed to adsorb for 1 h while mice remained under anesthesia. The compositions of the applied virus differed by treatment group as described below.
Treatment groups for colocalization experiments.
The mice in groups A to C were infected as described below and were sacrificed at 14 days postinoculation (dpi). The mice in groups D to G, infected as described below, were sacrificed at 4 dpi.
Group A: 1010 viral genomes (vg) ssAAV8-GFP-WT only.
Group B: 5,000 PFU KOS/62 only.
Group C: 5,000 PFU KOS/62 plus 1010 vg ssAAV8-GFP-WT.
Group D: 5,000 PFU KOS/62 plus 1010 vg scAAV8-GFP-WT.
Group E: 5,000 PFU KOS/62 plus 1010 vg scAAV8-GFP-Y733F.
Group F: 5,000 PFU KOS/62 plus 109 vg scAAV8-GFP-Y733F.
Group G: 5,000 PFU KOS/62 plus 108 vg scAAV8-GFP-Y733F.
Rabbit ocular infections.
New Zealand White rabbits were anesthetized using intramuscular injections of ketamine (30 to 45 mg/kg body weight) and xylazine (7.5 to 11.5 mg/kg body weight). Using a blunt-tip 27-gauge needle, a 3-by-3 crosshatch pattern was made on the corneal surface. ssAAV8-GFP-Y733 was applied to each eye at an initial inoculum of 1 ×1010 vg/eye in a total volume of 20 μl. For coinfections with ssAAV8-GFP-Y733 and KOS/62, rabbits first received ssAAV8-GFP-Y733 at a titer of 1 ×1010 vg/eye followed by rescarification of the corneal surface and infection with KOS/62 at a titer of 200,000 PFU/eye (20 μl) 17 days after the application of AAV to the corneal surface. Mice were euthanized by isoflurane overdose, and death was ensured by cervical dislocation. For coinfected rabbits, rabbits were anesthetized with ketamine and then euthanized using an overdose of pentobarbital and ganglia were harvested at 31 days following the KOS infection. Prior to euthanasia, all rabbits underwent slit lamp examination to ensure that no ocular lesions remained. Controls for experiments were either naive rabbits or rabbits with latent HSV-1 strain KOS/62 infections.
Mouse DRG tissue processing/sectioning.
On the appropriate day after infection, mice were deeply anesthetized by intramuscular injection of 40 μl of a cocktail of ketamine (60 to 90 mg/kg of body weight), xylazine (15 to 23 mg/kg), and acepromazine (5 to 7.5 mg/kg). Cardiac perfusion was immediately performed with 15 ml of 0.1 M phosphate-buffered saline (PBS; pH 7.4) followed by 15 ml of 4% paraformaldehyde (PFA)–0.1 M phosphate buffer (PB; pH 7.4). DRG from each mouse were fixed in ice-cold 4% PFA for 1 h and then transferred to 70% ethanol and stored at 4°C. Tissue was dehydrated in a graded series of ethanol baths and xylene and embedded in paraffin, and 6-μm sections were prepared and applied to glass microscope slides.
Rabbit TG tissue processing/sectioning.
Immediately upon removal, TGs were placed in 10% neutral buffered formalin, stored at 4°C overnight, and then transferred to 70% ethanol. All samples were then paraffin embedded and serially sectioned to 10 μm in thickness, with 3 to 4 sections per each slide.
Dual-immunofluorescence analysis of AAV/HSV-1 colocalization in mouse DRG.
Tissue sections were deparaffinized in xylene and rehydrated through a graded series of ethanol baths to double-distilled water (ddH2O). Antigens that may have been obscured by fixation were exposed using heat-mediated epitope retrieval by heating tissue sections to 90 to 95°C for 25 min in 0.01 M citrate buffer (pH 6.0). A 1.5% normal goat serum blocking solution (Vector Laboratories) was applied for 20 min to prevent nonspecific antibody interactions. All incubations were performed in a dark moist chamber. Rabbit anti-GFP and chicken anti-β-galactosidase primary antibodies (Abcam) were incubated on tissue sections overnight at 4°C at a dilution of 1:200. Fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (Vector Laboratories) and Texas Red-labeled goat anti-chicken IgG (Abcam) secondary antibodies were applied for 30 min at room temperature at dilutions of 1:50 and 1:200, respectively. Sections were washed for 2 min in water, and then coverslips were mounted with PBS. Sections were washed with TBS-T (1% Tween 20–Tris HCl-buffered saline; pH 7.5) between steps. Slides were viewed immediately with a Zeiss Axioskop 2 microscope, and dark-field fluorescence images were captured using a SPOT digital camera and software package version 1.2.1 (Diagnostic Instruments). Twelve tissue sections from each mouse, each comprising profiles through 1 to 4 ganglia, were analyzed for fluorescence. As a negative control for primary antibody specificity, four sections from each mouse were treated as described, except that no primary antibodies were applied.
Immunohistochemical analysis of AAV reporter gene expression in mouse DRG.
Tissue sections were deparaffinized in xylene and rehydrated and then soaked in a 3% hydrogen peroxide–70% methanol solution for 10 min to block endogenous peroxidase activity. Epitope retrieval was carried out by heating tissue sections to 90 to 95°C for 25 min in 0.01 M citrate buffer (pH 6.0). A solution of 1.5% normal goat serum and 10% Avidin D (Vector Laboratories) was applied for 20 min to prevent nonspecific antibody and avidin interactions with tissue. Slides were then placed in a moisture chamber to prevent evaporation of solutions. A solution of 10% biotin (Vector Laboratories) and a 1:1,000 dilution of rabbit anti-GFP primary antibody (Abcam) was applied for 1 h. Further processing was performed with a Vectastain ABC Elite kit (Vector Laboratories). A biotinylated anti-rabbit secondary antibody was applied for 30 min at a dilution of 1:200, followed by incubation with avidin-biotin-conjugated horseradish peroxidase (HRP) enzyme for 30 min and, finally, development of DAB (3,3′-diaminobenzidine) chromogen substrate for 8 min. All incubations were performed at room temperature, and sections were washed with TBS-T between steps. Tissue sections were counterstained with hematoxylin, dehydrated in a graded series of ethanol baths, and cleared in xylene. Glass coverslips were permanently mounted with Cytoseal XYL (Richard-Allan Scientific). Slides were allowed to dry overnight before being viewed with a Zeiss Axioskop 2 microscope. Bright-field images were captured using a SPOT digital camera and software package version 1.2.1 (Diagnostic Instruments). Eight tissue sections from each mouse, each comprising profiles through 1 to 4 ganglia, were analyzed for brown DAB staining. Neuron cell bodies were identified by analysis of cell morphology and large blue-stained nuclei. The percentage of transduced neurons was calculated by dividing the number of stained neurons by the total number of neurons counted. Differences in staining intensity between treatment groups were observed visually as a measure of reporter gene expression. As a control for primary antibody specificity, two sections from each mouse were treated as described above, except that no primary antibody was applied.
Immunohistochemistry—rabbit TG.
Slides containing serial sections of rabbit TG were deparaffinized, hydrated, and peroxidase blocked through a series of washes that included (i) two xylene washes performed for 5 min for each wash, (ii) two 100% ethanol washes for 2 min each, (iii) one 3% hydrogen peroxide wash (30% hydrogen peroxide was diluted 1:10 in 100% methanol) for 10 min, (iv) one 95% ethanol wash for 3 min, (v) one 70% ethanol wash for 1 min, and (vi) one ddH2O wash for 1 min. Epitope retrieval was done by microwaving the slides in citrate buffer using a Coplin jar that was filled with 10 mM citrate buffer (pH 6.0) and then placed inside a beaker filled with ddH2O. The apparatus was brought to a light boil by microwaving at a high temperature setting for 8 min and allowed to sit at room temperature for 25 min such that the samples would remain at 90°C. The slides were removed from the citrate buffer, rinsed in water, and washed in TBS-T. A serum and avidin block, followed by a biotin block, was performed using normal serum from the species in which the secondary antibody was raised and avidin/biotin (Vector Laboratories Avidin-Biotin blocking kit) diluted in Zymed antibody diluent according to the manufacturer's instructions. Sections were then incubated with primary antibody (1:200 of either mouse anti-GFP or mouse anti-β-galactosidase antibody [Abcam]) overnight at 4°C, followed by incubation with goat anti-mouse biotinylated secondary antibody (1:200 dilution in Zymed diluent) at room temperature for 2 h. Following secondary antibody incubation, slides were developed for IHC using ABC Elite reagent and DAB chromogen substrate according to the protocols of the manufacturer (Vector Laboratories). Counterstaining was done with hematoxylin QS (Vector Laboratories), and then the reaction mixtures were dehydrated and cleared in xylene using another series of washes: one 1-min water wash, one 1-min 70% ethanol wash, one 1-min 95% ethanol wash, two 1-min 100% ethanol washes, and two 1-min xylene washes. The slides were then allowed to dry at room temperature and were mounted with Vecta Mount (Vector Laboratories). Slides containing 4 serial sections per rabbit (with a total of 5 rabbits per treatment group) were used for each experiment. Sections were imaged on a Zeiss microscope equipped with AxioVision Rel 4.6. Negative controls included either naive rabbit TG subjected to the full IHC protocol described above with antibody incubation or rabbit TG with latent KOS/62 infection subjected to the above-described IHC protocol without incubation with the primary antibody.
Immunofluorescence—rabbit TG.
Slides were processed in a manner identical to that described for immunohistochemistry for rabbit TG through the primary antibody incubation. Secondary antibody incubation was then performed using 1:1,000 goat anti-chicken Alexa Fluor 548 (Invitrogen) or 1:1,000 goat anti-mouse Alexa Fluor 488 (Invitrogen) for 2 h at room temperature. The slides were mounted using ProLong gold mounting media (Invitrogen) and imaged using a Leica deconvolution microscope equipped with Slidebook 5.0. Five slides per rabbit were subjected to the IF protocol described above, and a total of 5 rabbits per treatment group were used.
RESULTS
rAAV vectors transduce a high percentage of murine DRG neurons.
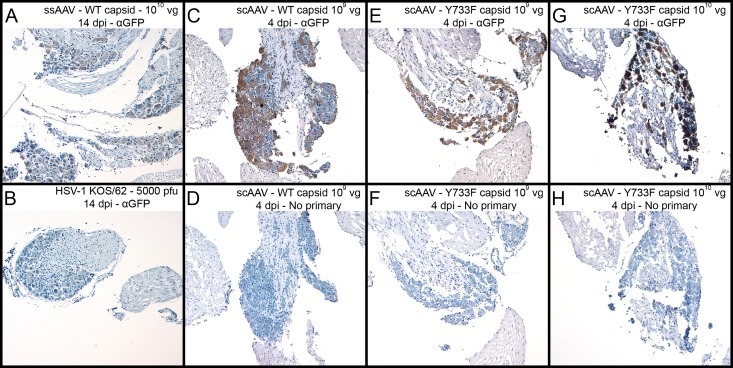
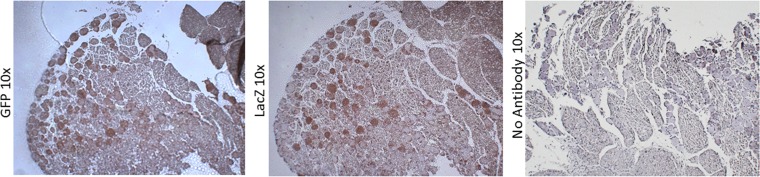
To determine if recombinant AAV vectors could transduce DRG sensory neurons following application of virus at the abraded epidermis, we inoculated mouse footpads with several different types of rAAV vectors. Mice were sacrificed at 4 and 14 dpi, and DRG were analyzed by immunohistochemistry (IHC) for AAV reporter gene (GFP) expression (Fig. 1). All AAV-transduced ganglia exhibited brown DAB staining, indicating the presence of GFP. Staining was not apparent in tissues infected with KOS/62 alone (Fig. 1B). Tissue sections that did not receive primary anti-GFP antibody also had no apparent staining (Fig. 1D, F, and H). These data suggest that the staining was specific for GFP expression and that the rAAV vectors did indeed transduce DRG sensory neurons following external application. Counts of stained neurons indicated that nearly all of the sensory neurons were transduced (data not shown).
FIG 1.
Following footpad inoculation, rAAV8 vectors transduce nearly 100% of sensory neurons in mouse DRG. Vectors with modified capsids are at least 10-fold more efficient than wild-type vectors. To analyze the immunohistochemistry of infected mouse DRG, mice were infected with rAAV or KOS/62 at the given dose and tissues were harvested as shown. Anti-GFP primary antibody was incubated with sections A, B, C, E, and G to detect the presence of GFP. No primary antibody was incubated with sections D, F, and H. All sections were stained by HRP enzymatic cleavage of DAB substrate (brown) for 8 min. Hematoxylin (blue) was used as a counterstain.
Modified capsids increase transduction efficiency.
Regardless of the rAAV vector used for infection, the percentages of stained neurons were the same across treatment groups. However, staining intensities were notably different depending upon the type of genome (single stranded versus self-complementary). The staining intensity was greater at 4 dpi for scAAV (Fig. 1C) than at 14 dpi for ssAAV (Fig. 1A). Moreover, the staining intensity was affected by the type of capsid (wild type versus Y733F mutation). Sections of tissue infected with 109 vg scAAV with the mutant capsid (Fig. 1E) had levels of expression similar to those of sections of tissue infected with 1010 vg scAAV with wild-type capsids (Fig. 1C). The most intense staining was observed in sections from tissue infected with 1010 vg scAAV8-GFP-Y733F (Fig. 1G). While staining intensity is not a perfect quantitative measure of reporter gene expression, the results strongly suggest that use of modified capsids will be beneficial in further experiments.
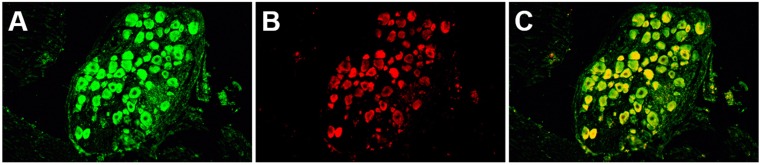
rAAV vectors and HSV-1 coinfect murine sensory neurons.
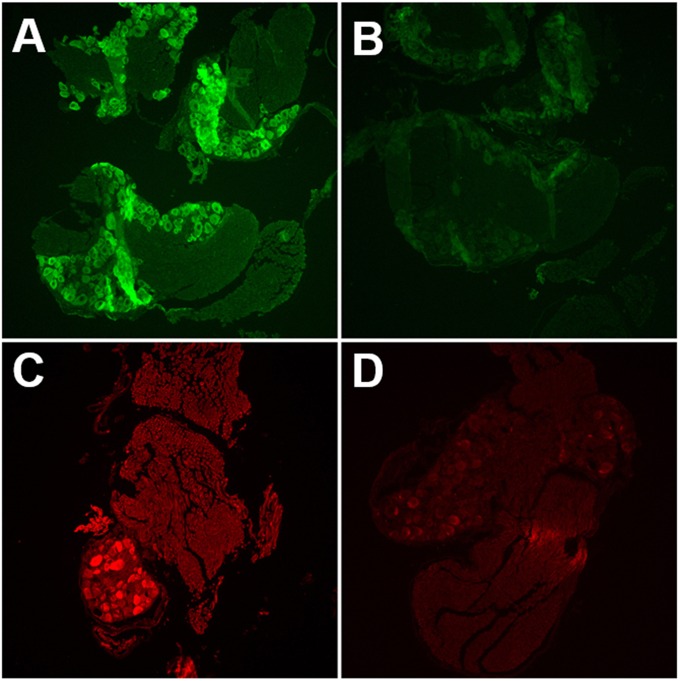
To determine if the rAAV vectors were capable of transducing HSV-1-infected DRG neurons, we coinfected mouse footpads and harvested DRG for IF analysis. Similarly to IHC results, GFP expression from AAV vectors was widespread within neurons (Fig. 2A). β-Gal expression from KOS/62 was limited to a smaller subset of neurons (Fig. 2C). This result is consistent with previous findings demonstrating that not all DRG neurons are infected with HSV-1 following mouse footpad infection (28). Colocalization was observed in those neurons expressing β-Gal (Fig. 3). Tissues transduced with rAAV8 alone did not stain for β-Gal, and tissues infected with HSV-1 alone did not stain for GFP (not shown). Tissue sections not receiving primary antibodies displayed only nonspecific background fluorescence, demonstrating that antibody binding was specific for the intended targets, i.e., the GFP and β-Gal reporters (Fig. 2B and D).
FIG 2.
Single-color immunofluorescence detects both rAAV8 and HSV-1 in mouse DRG. (A and B) Mice were infected with ssAAV-GFP only. DRG were harvested 14 dpi. Tissue sections were incubated with anti-GFP primary antibody (A) or with no primary antibody (B). Primary antibody was detected by the use of FITC-labeled secondary antibody. (C and D) Mice were infected with KOS/62 only. DRG were harvested 4 dpi. Tissue sections were incubated with anti-β-Gal primary antibody (C) or with no primary antibody (D). Primary antibody was detected by Texas Red-labeled secondary antibody.
FIG 3.
Dual-color immunofluorescence demonstrates colocalization of rAAV8 and HSV-1 within a subset of sensory neurons in mouse DRG. Mice were infected with both 1010 particles of scAAV-GFP-WT and 5,000 PFU of KOS/62. DRG were harvested 4 dpi. Tissue sections were incubated with both anti-GFP and anti-β-Gal primary antibodies. (A) Green channel image. Anti-GFP primary antibody was detected by FITC-labeled secondary antibody. Green cells were transduced by AAV. (B) Red channel image. Anti-β-Gal primary antibody was detected by Texas Red-labeled secondary antibody. Red cells were infected by HSV-1. (C) Merged image representing the red and green images. Yellow indicates colocalization of GFP and β-Gal expression from cells coinfected with both AAV and HSV-1.
rAAV vectors transduce a high percentage of rabbit TG neurons.
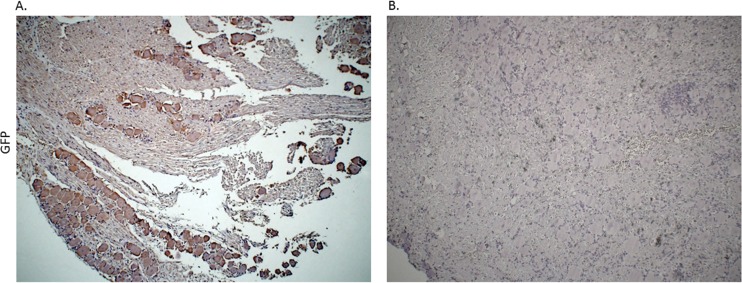
On the basis of the efficiency of delivery of rAAV to the murine DRG via footpad infection, we sought to extend this concept to the HSV-1 ocular infection model. HSV-1 infection of mouse eyes is commonly used to study latency in the TG neurons, but the rabbit ocular model provides a robust model of induced reactivation. Therefore, we chose to ask if corneal delivery of AAV might transduce TG neurons. Rabbit eyes were abraded, and the ssAAV8-GFP-Y733 vector was applied to the corneas. At 17 days postinoculation, the TG were harvested and tissue sections were stained with an anti-GFP antibody to assess AAV reporter gene (GFP) expression (Fig. 4). Regions of neurons in the TG corresponding to the ophthalmic branch of the trigeminal nerve exhibited the most DAB staining, indicating the presence of GFP (Fig. 4). It should be noted that applying AAV vector to unabraided or unscarified eyes and without saline solution does not result in efficient transduction of the TG (data not shown). While a large number of neurons in this region stained for GFP, the number of transduced neurons approached 70%, compared with the >90% seen in the murine DRG. The difference may reflect less-efficient delivery to the sensory neuron fibers of the cornea than to the sensory nerve termini of the mouse footpad. Nevertheless, these results demonstrate that the AAV vector efficiently transduced the neurons in the ophthalmic branch following delivery to the cornea.
FIG 4.
Immunohistochemistry analysis of rabbit TGs following corneal delivery of AAV8-GFP capsid mutant confirmed that AAV8 efficiently transduces neurons through a corneal route of delivery. Immunohistochemistry was done using rabbit TG following corneal inoculation with ssAAV8-GFP-Y733. All TG were harvested on postinoculation day 16. (A) Primary antibody incubation was done using mouse anti-GFP (Abcam), followed by incubation with a biotinylated HRP secondary antibody (Vector Laboratories). (B) The control slide was prepared from the TG of naive rabbit without AAV treatment and was subjected to both primary and secondary antibody incubation.
rAAV vectors and HSV-1 coinfect rabbit sensory neurons.
In order to determine if rAAV could transduce HSV-1-infected neurons in the rabbit TG as it did in the mouse, the ssAAV-GFP-Y733 vector was applied to the rabbit cornea at the same time as the HSV-1 lacZ recombinant, KOS/62. At 28 days after treatment and infection, DAB staining for either GFP or lacZ demonstrated the presence of a large number of neurons infected with HSV and transduced with AAV (Fig. 5).
FIG 5.
Immunohistochemistry using rabbit TGs following corneal delivery of ssAAV8-GFP-Y733 and a KOS/62 demonstrates colocalization of both the vector and HSV-1 in sensory neurons. Serial sections of TG from rabbits inoculated with ssAAV8-GFP-Y733 and KOS/62 were used to demonstrate that the vector and HSV-1 colocalize in neurons. Serial sections of <5 μm are presented at magnifications of ×10. IHC to detect the presence of KOS/62 was done using a mouse anti-β-galactosidase primary antibody (Abcam), followed by a biotinylated HRP secondary antibody (Vector Laboratories). To detect AAV8-GFP in neurons, sections were incubated with mouse anti-GFP antibody (Abcam), followed by incubation with a biotinylated HRP secondary antibody (Vector Laboratories). IHC was done to detect either HSV-1 or AAV8 using serial sections to demonstrate that neurons harbor both the vector and the virus. Control slides were prepared from coinfected rabbit TG, subjected to IHC with no primary antibody.
rAAV vectors display sustained expression in the coinfected rabbit sensory neurons.
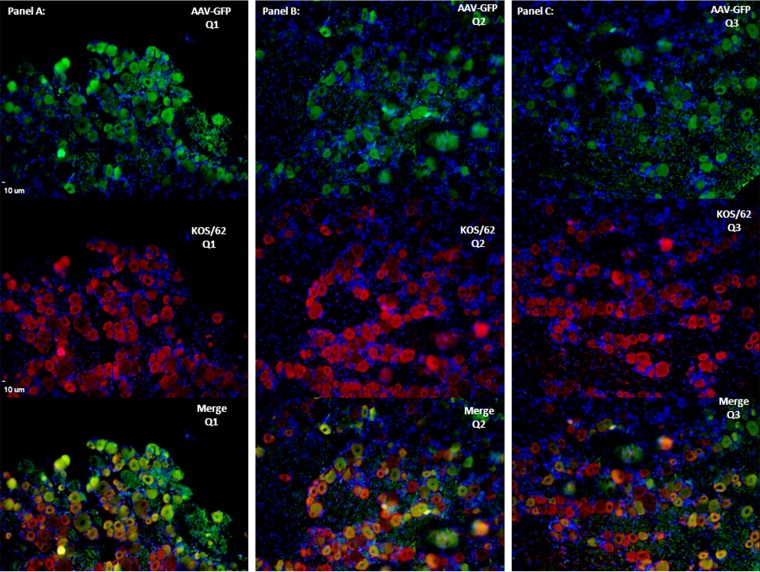
In order to more directly assess the colocalization of AAV-transduced neurons with HSV-infected ones and to establish long-term AAV expression in latently infected rabbits, ssAAV-GFP-Y733 was applied to the surface of the rabbit cornea at a titer of 1 × 1010 vg/eye. The eyes were infected with KOS/62 17 days after AAV application. Rabbits were allowed to establish a latent infection (∼30 days post-KOS/62 infection). TG were harvested, and indirect immunofluorescence analyses of the same sections stained for both GFP and ß-galactosidase were performed (Fig. 6). These results clearly demonstrate the presence of AAV-driven GFP expression in the majority of the same neurons that were expressing lacZ from the HSV-1 recombinant. It is striking that whereas the AAV does not transduce as high of a percentage of the neurons in the TG as in the murine DRG, the majority of HSV-1-infected neurons were also transduced by AAV, suggesting that these neurons were accessible to both viruses at the site of infection or that the propensity of these neurons for the transgene expression is shared. These results demonstrate the ability of AAV to efficiently transduce TG neurons when applied to the treated eye surface and that it is possible to efficiently transduce HSV-infected neurons with this vector. Furthermore, this result confirms stable AAV expression at 6 weeks posttreatment.
FIG 6.
Immunofluorescence results showed long-term expression and colocalization of AAV8-GFP and KOS/62 in coinfected rabbit TG. IF analysis was performed to demonstrate neuronal colocalization of vector and virus. Sections were incubated with either mouse anti-GFP or mouse anti-β-galactosidase primary antibodies. Alexa Fluor 548 (Invitrogen) or goat anti-mouse Alexa Fluor 488 (Invitrogen) was used as the secondary antibody for immunofluorescent visualization using a Leica deconvolution microscope with Slidebook 5.0. The TG section on the slide was divided into three equal sections (Q1 to Q3 [A to C]) to represent the colocalization of virus and vector over the entire TG cross-section. DAPI (4′,6-diamidino-2-phenylindole) staining (in blue) represents nuclei of satellite cells present in the sections. All images are shown in ×10 magnification.
DISCUSSION
The ability to knockdown or augment cellular and viral gene products within sensory neurons would be invaluable to the study of HSV-1 latency. HSV-1 does not establish latency in standard tissue culture lines, and HSV-1 latency is established only in early stages of development in in vitro models. Studies of the mechanisms silencing viral lytic gene expression will require in vivo animal models, in which it is difficult or impossible to perform techniques such as transfections or knockdowns. The primary aim of the experiments described here was to develop a method of performing these experiments in vivo.
Results of IHC experiments demonstrated that, following footpad inoculation, AAV vectors transduced DRG sensory neurons (Fig. 1). These results are in agreement with many previous studies of AAV and its tropism for various nervous system tissues following intravenous (i.v.), intraperitoneal (i.p.), and intrathecal inoculation. To our knowledge, rAAV application at the foot, or at the peripheral epithelium, innervated by the DRG or at the eye innervated by the TG has not been previously demonstrated. Axonal transport of AAV is serotype dependent in the CNS (30, 31), and AAV9 transport following brain injection is driven by cytoplasmic dynein and kinesin proteins (32). AAV8 selectively transduces sensory neurons, but not motor neurons, in the peripheral nervous system (PNS) (23). It is likely that the vectors enter the neurons at nerve termini and are trafficked to cell bodies using the same transport machinery as HSV-1, and this conclusion is supported by our findings. Most neurons showed positive staining for GFP expression, indicating that AAV8 did not preferentially infect any particular neuronal subtype. Expression from scAAV vectors was detected at 4 dpi, and staining intensity was greater than that observed with ssAAV vectors at 14 dpi. We infer that the self-complementary genomes bypassed the usual rate-limiting step of second-strand synthesis, resulting in earlier turning on of transgenes. The staining intensity increased with the dose and was also affected by the capsid type. Consistent with previous reports of capsid tyrosine mutants (16, 17), the Y733F single mutation resulted in an approximately 10-fold increase in transduction efficiency. Additional studies were performed in the rabbit ocular model to ensure that coinfection and application of the AAV vector with virulent strains of HSV-1 did not enhance corneal lesions during the acute infection or alter the HSV-1 reactivation efficiency of strain 17syn+. Neither corneal lesion score nor reactivation frequency was altered by the coapplication of the AAV vector (data not shown).
Results of IF experiments indicated that, after application to the mouse footpad or rabbit cornea, AAV and HSV-1 coinfected the same DRG sensory neurons. From these data, we conclude that AAV is an effective gene delivery vector for sensory neurons and can be delivered efficiently from the periphery, making it a very useful tool for in vivo studies of not only HSV-1 latency but also reactivation from latency as well. Further studies using AAV to deliver short hairpin RNA (shRNA) or to express other gene products such as signaling pathway agonists or chromatin modifiers will allow us to test this methodology in the relevant in vivo models, targeting genes or proteins in neurons that are colocalized with the AAV vector rather than globally inhibiting or upregulating gene expression. In addition, it is possible that these AAV vectors could provide a useful tool to target HSV latency itself by delivering repressors or genome editing machinery such as transcription activator-like effector nucleases (TALENs) or clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9.
ACKNOWLEDGMENTS
This work was supported in part by grants R01 AI48633 and R56 AI101174 from the NIH and by an unrestricted grant from Research to Prevent Blindness. The Center for Vision Research Vector Core laboratory is supported by a core grant from the National Eye Institute (P30 EY02172).
We thank William Hauswirth and the Center for Vision Research Vector Core laboratory for providing some of the AAV vectors used in these experiments. We also thank E. Barrozo, A. Dhummakupt, T. Edwards, C. Fisher, and D. Phelan for helpful comments on the manuscript.
REFERENCES
- 1.Margolis TP, Dawson CR, LaVail JH. 1992. Herpes simplex viral infection of the mouse trigeminal ganglion. Immunohistochemical analysis of cell populations. Invest Ophthalmol Vis Sci 33:259–267. [PubMed] [Google Scholar]
- 2.Yang L, Voytek CC, Margolis TP. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J Virol 74:209–217. doi: 10.1128/JVI.74.1.209-217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell DW, Miller AD, Alexander IE. 1994. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci U S A 91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovric J, Mano M, Zentilin L, Eulalio A, Zacchigna S, Giacca M. 2012. Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins. Mol Ther 20:2087–2097. doi: 10.1038/mt.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari FK, Samulski T, Shenk T, Samulski RJ. 1996. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol 70:3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. 1996. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol 70:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarty DM, Monahan PE, Samulski RJ. 2001. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 8.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. 2008. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 9.Grieger JC, Samulski RJ. 2012. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol 507:229–254. doi: 10.1016/B978-0-12-386509-0.00012-0. [DOI] [PubMed] [Google Scholar]
- 10.Grimm D, Zolotukhin S. 2015. E pluribus unum: 50 years of research, millions of viruses, and one goal—tailored acceleration of AAV evolution. Mol Ther 23:1819–1831. doi: 10.1038/mt.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L, Agbandje-McKenna M, Srivastava A. 2007. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther 15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 12.Qi YF, Li QH, Shenoy V, Zingler M, Jun JY, Verma A, Katovich MJ, Raizada MK. 2013. Comparison of the transduction efficiency of tyrosine-mutant adeno-associated virus serotype vectors in kidney. Clin Exp Pharmacol Physiol 40:53–55. doi: 10.1111/1440-1681.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song L, Li X, Jayandharan GR, Wang Y, Aslanidi GV, Ling C, Zhong L, Gao G, Yoder MC, Ling C, Tan M, Srivastava A. 2013. High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS One 8:e58757. doi: 10.1371/journal.pone.0058757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, Dyka FM, Kasuga D, Ayala AE, Van Vliet K, Agbandje-McKenna M, Hauswirth WW, Boye SL, Boye SE. 2013. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 8:e62097. doi: 10.1371/journal.pone.0062097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalkara D, Byrne LC, Lee T, Hoffmann NV, Schaffer DV, Flannery JG. 2012. Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Ther 19:176–181. doi: 10.1038/gt.2011.163. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Jayandharan GR, Li B, Ling C, Ma W, Srivastava A, Zhong L. 2010. High-efficiency transduction of fibroblasts and mesenchymal stem cells by tyrosine-mutant AAV2 vectors for their potential use in cellular therapy. Hum Gene Ther 21:1527–1543. doi: 10.1089/hum.2010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M, Jayandharan GR, Ling C, Zolotukhin I, Ma W, Zolotukhin S, Srivastava A, Zhong L. 2010. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 18:2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao C, Zhang W, Yuan Z, Shin JH, Li J, Jayandharan GR, Zhong L, Srivastava A, Xiao X, Duan D. 2010. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum Gene Ther 21:1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, Zhong L, Zolotukhin S, Srivastava A, Lewin AS, Hauswirth WW. 2009. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther 17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. 2008. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A 105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Fischer G, Hogan QH. 2016. AAV-mediated gene transfer to dorsal root ganglion. Methods Mol Biol 1382:251–261. doi: 10.1007/978-1-4939-3271-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer G, Kostic S, Nakai H, Park F, Sapunar D, Yu H, Hogan Q. 2011. Direct injection into the dorsal root ganglion: technical, behavioral, and histological observations. J Neurosci Methods 199:43–55. doi: 10.1016/j.jneumeth.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foust KD, Poirier A, Pacak CA, Mandel RJ, Flotte TR. 2008. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum Gene Ther 19:61–70. doi: 10.1089/hum.2007.093. [DOI] [PubMed] [Google Scholar]
- 24.Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS, Morrison JH, Beutler AS. 2008. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A 105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beutler AS, Banck MS, Walsh CE, Milligan ED. 2005. Intrathecal gene transfer by adeno-associated virus for pain. Curr Opin Mol Ther 7:431–439. [PubMed] [Google Scholar]
- 26.Wang X, Wang C, Zeng J, Xu X, Hwang PY, Yee WC, Ng YK, Wang S. 2005. Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther 12:314–320. doi: 10.1016/j.ymthe.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 27.Vulchanova L, Schuster DJ, Belur LR, Riedl MS, Podetz-Pedersen KM, Kitto KF, Wilcox GL, McIvor RS, Fairbanks CA. 2010. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain 6:31. doi: 10.1186/1744-8069-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis TP, Sedarati F, Dobson AT, Feldman LT, Stevens JG. 1992. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology 189:150–160. doi: 10.1016/0042-6822(92)90690-Q. [DOI] [PubMed] [Google Scholar]
- 29.Sawtell NM, Thompson RL. 1992. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol 66:2157–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aschauer DF, Kreuz S, Rumpel S. 2013. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, Beyer J, Forsayeth J, Bankiewicz KS. 2013. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther 20:348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castle MJ, Perlson E, Holzbaur EL, Wolfe JH. 8 October 2013. Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment. Mol Ther doi: 10.1038/mt.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]