ABSTRACT
Several innate sensing pathways contribute to the control of early cytomegalovirus (CMV) infection, leading to a multiphasic type I interferon (IFN-I) response that limits viral replication and promotes host defenses. Toll-like receptor (TLR)-dependent pathways induce IFN-I production in CMV-infected plasmacytoid dendritic cells; however, the initial burst of IFN-I that occurs within the first few hours in vivo is TLR independent and emanates from stromal cells. Here we show that primary human endothelial cells mount robust IFN-I responses to human CMV that are dependent upon cyclic GMP-AMP synthase (cGAS), STING, and interferon regulatory factor 3 (IRF3) signaling. Disruption of STING expression in endothelial cells by clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 revealed that it is essential for the induction of IFN-I and restriction of CMV replication. Consistently, STING was necessary to mount the first phase of IFN-I production and curb CMV replication in infected mice. Thus, DNA sensing through STING is critical for primary detection of both human and mouse CMV in nonhematopoietic cells and drives the initial wave of IFN-I that is key for controlling early viral replication in vivo.
IMPORTANCE Cytomegalovirus (CMV) is one of the most common viral pathogens, with the majority of people contracting the virus in their lifetime. Although acute infection is mostly asymptomatic in healthy persons, significant pathology is observed in immunocompromised individuals, and chronic CMV infection may exacerbate a myriad of inflammatory conditions. Here we show that primary human endothelial cells mount robust IFN-I responses against CMV via a cGAS/STING/IRF3 pathway. Disruption of STING expression by CRISPRs revealed an essential role in eliciting IFN-I responses and restricting CMV replication. Consistently, in mice, STING is necessary for the first phase of IFN-I production that limits early CMV replication. Our results demonstrate a pivotal role for the cGAS-STING pathway in the initial detection of CMV infection.
INTRODUCTION
Cytomegalovirus (CMV) (human herpesvirus 5 [HHV-5], a betaherpesvirus) is one of the most common etiological agents of chronic viral infection in humans, with primary infection developing in the majority of people at relatively young ages and lasting for life (1). Although acute CMV infection is mostly asymptomatic in healthy individuals, debilitating and even fatal complications arise in those with weakened immune systems, including pregnant women, infants, transplant recipients, and HIV patients. Remarkably, a recent analysis of monozygotic twin pairs revealed that CMV infection was the primary factor driving nonheritable diversity in the immune system (2). Consistent with the dramatic impact of CMV on the host immune system, infection has been associated with multiple chronic inflammatory conditions, including high blood pressure, heart disease/atherosclerosis, cancer, and aging-related immunodeficiencies (3, 4). Despite the clinical importance of human CMV (HCMV), there is currently no vaccine, and antiviral drugs are relatively toxic and cannot restrict viral persistence/latency.
Both mouse CMV (MCMV) and HCMV contain a linear double-stranded DNA (dsDNA) genome of ∼230 kb encoding hundreds of viral open reading frames (ORFs) (5, 6). CMV harbors an arsenal of immunoregulatory genes that allow the virus to evade or dampen host immune defenses. However, despite these numerous strategies, the virus still induces significant levels of type I interferon (IFN-I) from infected cells both in vitro and in vivo. We have previously shown that MCMV induces a biphasic IFN-I response in vivo, with the first wave of IFN-I being completely independent of Toll-like receptor (TLR), Nalp3, and MAVS and emanating from virally infected stromal cells during the first 12 h (7, 8). This “first burst” of IFN-I is followed by a later wave produced at 36 to 44 h by plasmacytoid dendritic cells (DCs) (pDCs) and conventional DCs (cDCs) in response to viral particles released from the infected stroma (7, 9–11). It is the first wave of IFN-I that is critical to limit viral spread in the peripheral organs (7), while the second pDC-derived wave is mostly dispensable for restricting MCMV replication levels (12). Despite our previous work showing that lymphotoxin (LT) signaling is required to promote an optimal first-phase IFN-I response to MCMV in vivo (7, 8, 11, 13, 14), the specific innate sensing pathway required for this initial production has remained uncharacterized, although several have been implicated in regulating later phases (e.g., DAI/ZBP [Z-DNA binding protein] and IFI16 [IFN-γ-inducible protein 16]) (15–18).
Recent studies showed that both IFI16 and STING contribute to the innate detection of herpes simplex virus 1 (HSV-1) (an alphaherpesvirus) through a cGAS-dependent pathway (19, 20), and IFI16/STING signaling has also been implicated in sensing HCMV in fibroblasts (16, 19). Upon binding to dsDNA, the activation of cGAS enzymatic activity produces cyclic GMP-AMP (cGAMP) from ATP and GTP, an atypical second messenger that directly binds to STING. Once activated by cyclic dinucleotides, STING then recruits TANK-binding kinase 1 (TBK1), which phosphorylates and activates the transcription factor interferon regulatory factor 3 (IRF3) (21, 22), a critical component of the IFN-β enhanceosome (23). Notably, a role for the cGAS-STING pathway in innate sensing of CMV has not yet been formally investigated. Here we show that HCMV infection of primary human vascular endothelial cells induces robust IRF3 nuclear translocation within 3 h, resulting in IFN-β and antiviral chemokine expression. Using small interferon RNA (siRNA) knockdowns, we show that the activation of the IRF3–IFN-I axis by HCMV is dependent upon cGAS, STING, and TBK1. The essential function of STING in the innate detection of HCMV in endothelial cells was confirmed by using clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9-mediated gene disruption and also resulted in enhanced viral replication. Using mice deficient for functional STING, we confirmed that this pathway is key for restricting viral replication by promoting the first phase of IFN-I induced by MCMV in vivo. In conclusion, we demonstrate an essential role for innate DNA sensing through a cGAS-STING-TBK1-IRF3 pathway in driving the initial IFN-I response that limits early CMV infection.
MATERIALS AND METHODS
Mice.
C57BL/6 and Stinggt/gt mice were obtained from the Jackson Laboratory. Stinggt/gt mice harbor a T596A mutation in exon 6 of the STING gene, which was identified in an N-ethyl-N-nitrosourea forward-genetics screen (24). Mice were housed under specific-pathogen-free conditions at the La Jolla Institute. All experiments were approved by the IACUC and performed according to institutional guidelines for animal use.
Cell culture.
Primary human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell growth Medium 2 (EGM2) and growth factors (Lonza) at 37°C with 5% CO2 and were kept in culture for up to 10 doublings. Neonatal healthy dermal fibroblasts (NHDFs; Clonetics) and HEK-Blue-IFN-α/β cells (InvivoGen) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin-streptomycin (100 U/ml), l-glutamine (2 mM), and HEPES (10 mM; pH 7.4). Insulin (5 μg/ml; Sigma-Aldrich) and basic fibroblast growth factor (bFGF) (1 ng/ml; Sigma-Aldrich) were included for NHDF medium. HUVECs were immortalized by retroviral transduction with pBABE-hygro-hTERT (catalog number 1773; Addgene). NIH 3T3 and primary mouse embryonic fibroblasts (MEF) were cultured in DMEM supplemented with 10% FBS, penicillin-streptomycin (100 U/ml), l-glutamine (2 mM), and HEPES (10 mM; pH 7.4).
Reagents and antibodies.
Monoclonal mouse anti-HCMV antibody (clone CCH2) was obtained from Dako, monoclonal mouse anti-HCMV IE1 was a kind gift from Bill Britt (University of Alabama at Birmingham), polyclonal rabbit anti-IRF3 was obtained from Santa Cruz, polyclonal rabbit anti-phospho-TBK1 (Ser172) and monoclonal rabbit anti-STING were obtained from Cell Signaling Technology, polyclonal rabbit anti-cGAS was obtained from Abgent, monoclonal mouse anti-TBK1 was obtained from Abcam, and monoclonal mouse anti-β-actin was obtained from Sigma. Alexa Fluor 647 AffiniPure goat anti-rabbit IgG(H+L) was obtained from Jackson Immunological Research. An IFN-β enzyme-linked immunosorbent assay (ELISA) kit was purchased from PBL Interferon Source.
siRNA transfection.
Nontargeting control and cGAS-, STING-, TBK1-, IFI16-, type I IFN receptor (IFNAR)-, STAT1-, and STAT2-specific siRNAs (On-Target Plus [OTP] and SmartPool siGenome) were obtained from GE Dharmacon. siRNAs were transfected into HUVECs by using DharmaFECT 4 (GE Dharmacon) at a final concentration of 20 nM, according to the manufacturer's instructions. Briefly, for transfection in 6-well plates, HUVECs were trypsinized and plated at 5 × 105 cells per well into 6-well plates the day before transfection. Fresh medium was replaced 1 h before transfection. siRNA and DharmaFECT 4 were each diluted with Opti-MEM (Life Technologies) and incubated at room temperature for 5 min before mixing. Lipid-siRNA complexes were incubated for an additional 20 min and added dropwise to cells. Medium was replaced at 24 h posttransfection, cells were trypsinized and plated onto 96-well plates after 48 h (1 × 104 cells per well), and cells were infected with HCMV after 72 h.
Immunofluorescence microscopy.
A total of 1 × 104 cells were plated onto black-rim, clear-bottom, 96-well plates (Corning) the day before HCMV infection. Three hours after infection, cells were fixed with 4% paraformaldehyde (Affymetrix), permeabilized with 0.2% Triton X-100–phosphate-buffered saline (PBS), and blocked with 5% FBS–PBS. For IRF3 and phospho-TBK1 detection, cells were stained with primary antibodies, washed, and subsequently stained with Alexa 647 secondary antibody (see below). DNA was counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Cells were imaged on an ImageXpress Micro instrument (Molecular Device), and ∼3,000 individual cells were acquired for each well, with three wells for each treatment per experiment. Images were analyzed by using the enhanced translocation module of MetaXpress (Molecular Devices), as previously described (25). Cells were scored as positive for IRF3 nuclear translocation if IRF3 fluorescence significantly overlapped that of DAPI with a correlation coefficient of between 0.6 and 0.8, adjusted according to the background fluorescence signal in untreated cells. For quantification of phosphorylated TBK1, cells with a significant fluorescence signal in the cytosol (DAPI-negative area) were scored as positive. In both cases, uninfected cells were used to establish the baseline for quantification.
Quantification of IFN-I.
Human IFN-I (IFN-β) produced by HUVECs was detected by using HEK-Blue-IFN-α/β reporter cells according to the manufacturer's instructions (InvivoGen). Briefly, 20 μl of the HUVEC culture supernatant was cultured with 5 × 104 reporter cells in 96-well plates with a final volume of 200 μl. A standard curve was established by serial dilutions of recombinant IFN-β. After 24 h, secreted embryonic alkaline phosphatase activity was quantified with QuantiBlue (InvivoGen). Levels of mouse IFN-β in sera of wild-type (WT) and Stinggt/gt mice were quantified by an ELISA according to the manufacturer's instructions (PBL Interferon Source).
Generation of STING-deficient cells with CRISPRs.
A single guide RNA (sgRNA) targeting STING exon 5 (sgRNASTING) (5′-AATATGACCATGCCAGCCCA-3′) was cloned into the LentiCRISPR vector (catalog number 49535; Addgene) (26) to generate pLVCR-sgSTING. Human telomerase reverse transcriptase (hTERT)-immortalized HUVECs (tHUVECs) were transduced with either the empty LentiCRISPR vector (control) or pLVCR-sgSTING (STING knockout [STINGKO]) and selected with puromycin (1 μg/ml) for 7 days. To obtain single-cell clones, cells were plated at 0.6 cells per well into 96-well plates, and all STINGKO clones used in this study (clones 9 and 11) were verified by Sanger sequencing for deletion and by Western blotting for STING expression.
CMV stock preparation.
The HCMV MOLD clinical strain (27) (a kind gift from Martin Raftery) was used throughout the study, and in our experience, this strain infects HUVECs at the highest efficiency of any strain that we have tested, including a modestly higher efficiency than that of the TB40E strain. MOLD stocks were prepared by infecting primary HUVECs at a multiplicity of infection (MOI) of 0.1 and letting the virus spread throughout the culture until monolayers were ∼100% cytopathic (∼12 to 14 days), and supernatant and cell-associated virus (released by sonication of infected cells for 5 min in a water bath) fractions were pooled, followed by pelleting through a 20% sorbitol cushion and resuspension of the viral pellet in PBS. HCMV stock titers in both HUVECs and NHDFs were determined by flow cytometry using an anti-UL44 antibody, and 2-fold limiting dilutions were performed to achieve ∼50% cell infection in order to ensure that titer determinations were done in cells infected in the linear range.
Salivary gland (SG) stocks of MCMV were utilized for all experiments and were generated from the bacterial artificial chromosome (BAC)-cloned genome of the K181 strain (28). The K181 BAC was first electroporated into NIH 3T3 cells, replication was allowed to proceed until 100% cytopathogenic effect was achieved, and viral supernatants were harvested. Limiting-dilution cloning of this supernatant was then performed in 96-well dishes to allow selection of single MCMVK181-BAC clones that had lost the BAC green fluorescent protein (GFP) expression cassette and had recombined correctly through the m07 genomic locus as described previously (28). Three separate, verified clones were then expanded by passaging twice through NIH 3T3 cells (generating “1°” stocks and “seed” stocks) and expanded to make “working stocks” in primary C57BL/6 MEFs, and titers were then determined on NIH 3T3 cells as described previously (8). Viral DNA was isolated from working-stock preparations of several BAC-derived clones, and all samples were verified to have an identical EcoRI restriction fragment length polymorphism (RFLP) digestion pattern. This pattern was also identical to that of the K181Perth non-BAC-cloned strain (gift from Alec Redwood). Replications of the 3 BAC-derived K181 clones and K181Perth were shown to be similar at days 2 and 4 in spleen and liver of infected C57BL/6 and BALB/c mice, and clone 1 was picked to continue experiments. Three-week-old BALB/c mice were then infected with 5 × 104 PFU of clone 1 K181, and salivary glands were harvested at day 13 for isolation of the virus. This first in vivo passage of MEF-derived K181 was used to infect 3-week-old BALB/c mice again (5 × 103 PFU), and SG working stocks were generated from these mice as described previously (SGs from 30 mice yielded ∼6 × 107 total PFU) (8). Replication of this MCMVK181-BAC SG stock was compared with that of an SG stock of K181Perth and was shown to have identical replication levels in the spleen and liver of C57BL/6 and BALB/c mice at days 2 and 4 and in the SG at days 12 and 30. Mice were infected via the intraperitoneal route, and MCMV replication levels in spleen and liver of C57BL/6 WT and Stinggt/gt mice were determined by titration in NIH 3T3 cells 36 h later, as described previously (8).
HCMV infection.
Monolayers of HUVECs were plated into 96- or 24-well dishes and were infected the following day with HCMV diluted in EGM2 at the indicated MOIs. Cells were spin infected with HCMV, or mock infected, at 1,600 × g for 10 min at room temperature and subsequently incubated in a 37°C incubator with 5% CO2 for a total of 3 h before analysis.
RNA isolation and real-time quantitative PCR.
Total RNA was isolated by using an RNeasy minikit (Qiagen). Residual DNA was removed by using a Turbo DNase-free kit (Life Technologies). Equal amounts of total RNA (∼200 ng) were used to synthesize cDNA by using SuperScript III reverse transcriptase (Life Technologies) and oligo(dT) primers. Gene expression was analyzed by quantitative reverse transcription-PCR (qRT-PCR) with a StepOne real-time PCR machine (Life Technologies), using FastStart SYBR green Master (Roche) with primer pairs for CXCL10 (forward [F] primer GTGGCATTCAAGGAGTACCTC and reverse [R] primer TGATGGCCTTCGATTCTGGATT) CCL5 (F primer CCAGCAGTCGTCTTTGTCAC and R primer CTCTGGGTTGGCACACACTT), IFNB1 (F primer GCTTGGATTCCTACAAAGAAGCA and R primer ATAGATGGTCAATGCGGCGTC), and ACTB (F primer TGAAGTGTGACGTGGACATC and R primer GGAGGAGCAATGATCTTGAT). TaqMan assays (Thermo Fisher) were performed with primer pairs for cGAS (catalog number Hs03835606_m1), IFI16 (catalog number Hs00194261_m1), and HPRT (catalog number Hs02800695_m1).
Statistical analysis.
Statistical analyses were performed with Excel (Microsoft) and Prism (GraphPad). At least two independent experiments were performed, and error bars represent means ± standard deviations. Statistical comparisons were evaluated by using Student's t test (unpaired, two tailed), with P values indicated in the figure legends.
RESULTS
HCMV triggers a robust IFN-I response in endothelial cells.
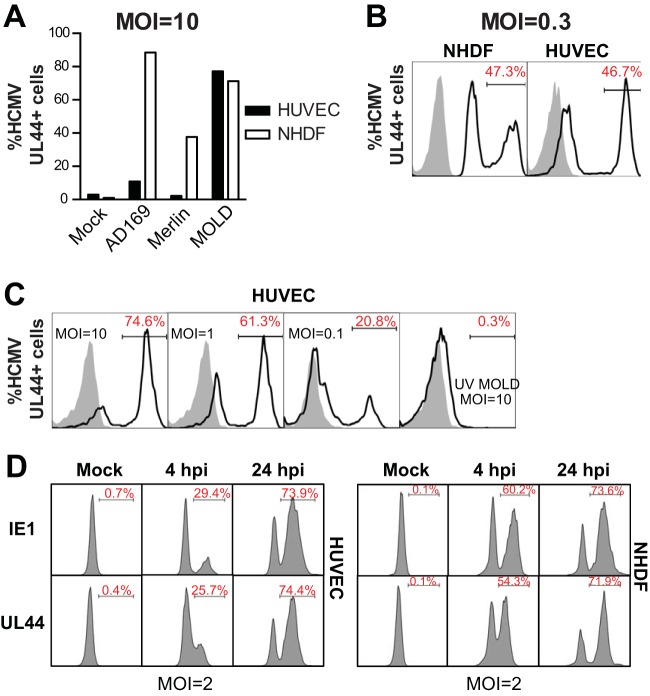
For both mouse and human CMV, vascular endothelial cells represent an important cellular reservoir for acute and persistent/latent infection (29). Consequently, we set out to examine the cell-intrinsic innate IFN-I response to HCMV infection using primary HUVECs. Since different HCMV strains and isolates vary significantly in their abilities to infect any nonfibroblast cell type, we tested several HCMV strains, including AD169, MERLIN, and MOLD (Fig. 1A). As expected, at an MOI of 10, each strain readily infected NDHFs, as determined by analysis of HCMV antigen expression at 24 h postinfection (hpi) (Fig. 1A). However, of these three strains, only MOLD efficiently infected HUVECs (Fig. 1A to C), likely due to the fact that MOLD has been propagated solely in endothelial cells since its original isolation from patients (27), thus retaining a functional pentameric envelope protein complex required for infection of nonfibroblastic cells (30). To confirm productive infection of HUVECs by HCMV MOLD, we monitored the expression of both immediate early and early-late HCMV antigens (IE1 and UL44), and both antigens showed virtually identical levels of expression in NHDFs and HUVECs (Fig. 1D). Together, these results show that the MOLD strain of HCMV productively infects primary human endothelial cells.
FIG 1.
HCMV MOLD productively infects primary human endothelial cells. (A) A total of 1 × 104 primary HUVECs or NHDF cells were plated into 96-well plates and infected the following day with the indicated HCMV strains at an MOI of 10. The percentage of HCMV-infected cells was determined by intracellular staining and flow cytometry using anti-HCMV UL44 antibody. (B) HUVECs or NHDFs were infected with the same volume of HCMV MOLD (MOI = 0.3) to compare relative infection efficiencies. (C) HUVECs were infected with HCMV MOLD at the indicated MOIs and analyzed 24 h later by intracellular staining and flow cytometry for UL44 antigen expression. UV HCMV was inactivated with UV light prior to infection. (D) HUVECs or NHDFs were infected with the same volume of HCMV MOLD (MOI = 2), and the percentage of HCMV-infected cells was determined by intracellular staining and flow cytometry using anti-HCMV UL44 and anti-HCMV IE1 antibodies. Results are representative of data from experiments performed 3 to 4 times.
In order to examine the initial, cell-intrinsic response to HCMV in endothelial cells, analyses of IFN-I pathway activation were performed 3 h after infection of HUVECs with HCMV MOLD at an MOI of 5. At this time, inducible nuclear translocation of IRF3 was quantified in >90% of cells, compared to 2% of uninfected control cells (Fig. 2A and B). Additionally, autophosphorylation of TBK1, an IRF3 kinase, was detectable in ∼70% of cells, compared to <5% of controls (Fig. 2C and D). Consistent with the robust levels of activation of IRF3 and TBK1 in HCMV-infected HUVECs, several well-characterized IRF3- and IFN-I-regulated genes were significantly upregulated at the mRNA level, including IFNB, CCL5, and CXCL10 (Fig. 2E). These results clearly demonstrate that HCMV triggers a robust and rapid innate immune response in HUVECs.
FIG 2.

HCMV infection triggers a robust IFN-I response in primary human endothelial cells. (A to D) HUVECs were infected with HCMV at an MOI of 5 for 3 h, and IRF3 nuclear translocation (A and B) and TBK1 autophosphorylation (pTBK1) (red) (C and D) were quantified by immunofluorescence microscopy, with the percentage of cells scored positive being plotted. DNA was counterstained with DAPI (blue). (E) HUVECs were infected with HCMV at an MOI of 5 for 3 h, and mRNA expression levels of IFNB (left), CCL5 (middle), and CXCL10 (right) were analyzed by qRT-PCR. Relative expression levels were calculated by normalization to the values for the housekeeping gene ACTB. Triplicate wells were used for each experiment, and results are from at least 2 independent experiments. Values are shown as means ± standard errors of the means. ***, P ≤ 0.001; ND, not detected.
The cGAS-STING-TBK1-IRF3 pathway drives the early interferon response to HCMV.
HCMV can activate multiple innate sensing pathways in diverse cell types, including TLR2 and TLR9 (15, 18, 31, 32). In addition to TLRs, the HCMV dsDNA genome directly engages cytosolic DNA-sensing pathways, as the depletion of the cytosolic DNA sensor ZBP/DAI or IFI16 decreases the induction of interferon-stimulated genes (ISGs) by HCMV in fibroblasts and macrophages, respectively (15, 33). Recent studies using transfected dsDNA and HSV-1 (an alphaherpesvirus) have uncovered two important new players in cytosolic DNA sensing, cGAS and STING (19, 34). To investigate whether the STING pathway regulates the innate immune response to HCMV in endothelial cells, siRNA transfections were performed. Significant depletion of cGAS, STING, and TBK1 protein expression (Fig. 3A) resulted in near-complete ablation of inducible IRF3 nuclear translocation and TBK1 autophosphorylation upon HCMV infection (Fig. 3B and C), with a commensurate ∼90% decrease in IRF3-dependent IFN-I production (Fig. 3D). The depletion of STING also significantly reduced the HCMV-induced expression of IFNB, CCL5, and CXCL10 (Fig. 3E), while the depletion of IFNAR, STAT1, or STAT2 selectively reduced the expression of CXCL10 (Fig. 3E). In fibroblasts, IFI16 has also been implicated as an innate sensor of DNA viruses (20), with studies indicating that the HCMV tegument protein UL83/pp65 dampens IFN-I induction by suppressing IFI16 activity (16). However, near-complete depletion of IFI16 mRNA levels in HUVECs not only failed to diminish HMCV-induced IRF3 activation but also, in fact, significantly enhanced it (Fig. 3F), which is consistent with recently reported results showing that IFI16-deficient THP-1 monocytes produce increased levels of IFN-β after infection with HCMV (35). Taken together, these results definitively show that HCMV infection of primary endothelial cells activates a cytosolic DNA-sensing pathway involving a cGAS-STING-TBK1-IRF3 axis, leading to the transcription of innate antiviral genes, including IFN-I and IFN-I-inducible genes.
FIG 3.
STING and cGAS are required for the HCMV-induced IFN-I response in endothelial cells. (A to D) Primary HUVECs were transfected with siRNAs targeting cGAS, STING, and TBK1 or a nontargeting control siRNA (siCTRL). Seventy-two hours posttransfection, protein levels were assessed in cell lysates by Western blotting (A), or cells were infected with HCMV at an MOI of 2 or 5, the percentages of cells containing nuclear IRF3 (B) and pTBK1 (C) were quantified after 3 h, and levels of IFN-I in supernatants were quantified after 12 h (D). IB, immunoblotting. (E) Primary HUVECs were transfected with siRNAs targeting STING, IFNAR, STAT1, or STAT2 or with a nontargeting siRNA. Seventy-two hours later, cells were infected with HCMV at an MOI of 5 for 3 h, and mRNA expression levels of IFNB (left), CCL5 (middle), and CXCL10 (right) were analyzed by qRT-PCR. mRNA expression levels were first normalized to the values for ACTB, and percent expression was calculated from values for cells transfected with nontargeting siRNA. (F) Primary HUVECs were transfected with siRNAs targeting cGAS or IFI16 or a nontargeting siRNA. Seventy-two hours later, cGAS and IFI16 mRNA expression levels were analyzed by qRT-PCR, and nuclear IRF3 was analyzed at 3 h post-HCMV infection, as described above for panel B. cGAS and IFI16 mRNA expression levels were first normalized to ACTB, and percent expression was calculated from values for cells transfected with nontargeting siRNA. Triplicate wells were used for each experiment, and results from at least 2 independent experiments are shown. Values are shown as means ± standard errors of the means, with P values indicating comparisons between the indicated siRNA and the control nontargeting siRNA at the same MOI. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ND, not detected.
STING signaling restricts HCMV replication in endothelial cells.
To confirm a requirement for STING in the cell-intrinsic innate response to HCMV in endothelial cells, targeted disruption of the STING gene was performed in telomerase-immortalized HUVECs using CRISPR-Cas9. HUVEC clones harboring nonsynonymous deletions in both alleles of exon 5 of the STING gene were established (STINGKO), resulting in a C-terminally truncated STING mutant whose expression could not be detected at the protein level (Fig. 4A). As expected, IRF3 nuclear translocation (Fig. 4B) and IFNB gene expression (Fig. 4C) were essentially abolished in STINGKO HUVECs after infection with HCMV (with 22.8- and 14.4-fold decreases in STINGKO cells at MOIs of 2 and 5, respectively). Together, these results show that STING is essential for the HCMV-induced IFN-I response in endothelial cells.
FIG 4.
STING restricts HCMV replication in endothelial cells. (A) tHUVECs were transduced with an empty Cas9-expressing lentivirus (control) or a lentivirus containing sgRNASTING targeting the complementary strand of exon 5 of the STING gene (STINGKO) (sequence shown in the blue box). Monoclonal cells were selected for each group, and DNA near the sgRNA-targeting site was amplified by PCR and sequenced. The predicted location of the Cas9-induced double-strand break (DSB) is annotated by a red arrowhead. Chromatograms show a 10-bp deletion (indicated by the red box and dotted line) observed in the STINGKO cell clone compared to the control, suggesting that a single STING mutation was repaired by homologous recombination with the mutated allele. The 10-bp deletion causes a frameshift, resulting in a premature stop codon. Amino acid sequences are shown for wild-type STING (379 amino acids) and truncated STING predicted from the STINGKO clone (ΔSTING) (166 amino acids), abolishing protein detection by Western blotting. (B and C) Control and STINGKO tHUVECs were infected with HCMV, and 3 h later, levels of nuclear IRF3 and IFNB1 mRNA were analyzed. (D and E) Control and STINGKO tHUVECs were seeded into 96-well plates the day before infection with HCMV at an MOI of 0.1. (D) Percentages of cells infected with HCMV were quantified by flow cytometry at 7 days postinfection (dpi). Cells were collected from at least 3 separate wells for analysis. (E and F) Levels of HCMV produced from infected cells were determined as described in Materials and Methods (n = 3 each). Results are representative of data from 2 independent experiments. Values are shown as means ± standard errors of the means. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001; ND, not detected.
To address whether the disruption of STING-mediated IFN-I responses resulted in a reduced control of HCMV replication, control and STINGKO HUVECs were infected at a relatively low multiplicity of infection (MOI = 0.1), and viral spread was assessed after 7 days. Significantly higher numbers of HCMV-infected cells were observed in STINGKO HUVECs than in control cells at day 7 (53.2% of STINGKO cells and 19.5% of control cells) (Fig. 4D). Accordingly, HCMV growth curves performed over this time course showed enhanced replication in STINGKO endothelial cells at multiple time points (76.1-, 13.4-, and 9.9-fold at days 4, 5, and 7, respectively) (Fig. 4E), a result that was replicated by using 2 different STINGKO clones (Fig. 4F). These results demonstrate a critical role for STING in restricting HCMV replication in vitro.
Induction of the initial IFN-I response to MCMV infection is STING dependent.
Our studies with HCMV reveal an essential role for the STING pathway in controlling HCMV infection in primary human vascular endothelial cells. Previously, we showed that MCMV infection triggers a biphasic, systemic IFN-I response in vivo that peaks at ∼12 and 36 h, with the initial burst of IFN-I being produced by lymphotoxin-dependent splenic stromal cells present in the marginal zone (MZ) (11). Notably, it is this first burst of IFN-I that is essential for restricting CMV production in the spleen and liver (8), with TLRs, Nalp3, and MAVS playing no protective role (7, 36). Consequently, we tested whether STING-dependent DNA sensing was required for this initial IFN-I production from MCMV-infected stromal cells in vivo. Infection of STING-deficient mice (Stinggt/gt) (24) with MCMV (K181 strain) resulted in significantly more weight loss than infection of WT mice (Fig. 5A). Consistent with this increased viral susceptibility, serum IFN-β levels were also severely diminished in MCMV-infected Stinggt/gt mice compared to WT mice at 12 h, the peak of the first-phase response (1.3 versus 18.9 pg/ml for Stinggt/gt versus WT mice) (Fig. 5B). In addition, enhanced virus replication was also seen in both the spleen and liver of Stinggt/gt mice at 36 hpi (5.6- and 4.3-fold increases versus the WT for spleen and liver, respectively) (Fig. 5C), a time point corresponding to the first burst of MCMV production in vivo (8). These results clearly demonstrate that STING-dependent induction of IFN-I contributes to restricting the first round of MCMV replication in vivo. Interestingly, serum IFN-β levels were ∼6-fold higher in Stinggt/gt mice at 36 hpi than in control mice (Fig. 5D). This marked increase parallels the higher levels of MCMV production at this time (Fig. 5C), which can then trigger an enhanced second wave of IFN-I from pDCs via a TLR9-MyD88 pathway (11).
FIG 5.
Sting is required for initial IFN-I production and control of MCMV infection in vivo. (A) Control C57BL/6 (B6) and Stinggt/gt mice were infected with the indicated doses of MCMV K181. Body weights were measured daily from the time of challenge until 7 days postinfection. The percent loss of initial body weight is shown. Each symbol represents the mean ± standard error of the mean (n = 5), with statistical analysis between Stinggt/gt and wild-type mice being calculated with Student's unpaired t test. (B) Control C57BL/6 and Stinggt/gt mice were infected with 2 × 105 PFU of MCMV as described above for panel A, and the amount of IFN-β in sera was quantified by an ELISA after 12 h. (C) Increased MCMV titer in the absence of Sting. At 36 h postinfection, mice from panel B were sacrificed, and MCMV titers in spleen (left) and liver (right) were quantified by a plaque assay. (D) Positive correlation between increased virus burden and IFN-β. Sera were collected from MCMV-infected mice as described above for panel B, and the IFN-β level was quantified by an ELISA at 36 h postinfection (n = 4, except for Stinggt/gt mice at 0 hpi in panel B [n = 3] and C57BL/6 mice in panel C [n = 5]). Data shown in panels B and D were obtained from an IfnB ELISA. Data shown are representative of results from at least 2 independent experiments. Values are shown as means ± standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, not significant.
DISCUSSION
To ensure the rapid and efficient detection of impending infection, the innate immune system relies on germ line-encoded pattern recognition receptors that sense highly conserved pathogen-associated molecular patterns (PAMPs) or motifs. Atypical or mislocalized nucleic acids are highly immunostimulatory PAMPs that can engage multiple overlapping but distinct innate immune pathways to trigger IFN-I downstream production and the establishment of the cellular “antiviral” state (37). TLR9 is a receptor for unmethylated CpG DNA motifs present in the endolysosomal lumen of phagocytic cells such as macrophages, dendritic cells, and other professional innate immune cells. Signaling through TLR9 is known to induce HCMV- and MCMV-induced IFN-I responses in pDCs, and pDCs produce the majority of this cytokine at ∼36 h in MCMV-infected mice, but this source of IFN-I has a limited impact on viral replication (12). In contrast, the first burst of IFN-I during MCMV infection emanates from stromal cells at ∼12 h of infection and markedly restricts the levels of acute infection (7, 8), which is independent of TLR9 and likely involves one or more cytosolic DNA-sensing pathways. Of these, the pathway involving cGAS and the essential downstream effector STING is quickly emerging as the dominant cytosolic sensing pathway for several DNA viruses, including vaccinia virus, HSV-1, and adenovirus (34, 38, 39).
Using a clinical isolate of HCMV that is able to efficiently infect primary human endothelial cells (i.e., MOLD), we showed that this betaherpesvirus potently activates the cGAS-STING pathway and induces IFN-I by 3 h after infection in this cell type (Fig. 2). Ablation of STING expression by siRNA-mediated mRNA depletion or CRISPR-mediated gene disruption substantially decreased the initial IFN-I response and resulted in a marked increase in HCMV replication (Fig. 3 and 4). Furthermore, activation of STING by HCMV required cGAS, as cGAS knockdown also curtailed the early IFN-I response (Fig. 3). Finally, bringing physiological significance to these observations, infection of STING-deficient mice with MCMV revealed that the initial phase of IFN-I production in vivo is STING dependent and key for controlling viral replication (Fig. 5). Taken together, these results clearly demonstrate that DNA sensing through the STING pathway is critical for initial innate sensing of CMV infection, limiting the replication of both human and mouse CMV.
Both stromal and hematopoietic cells are critical first responders in the initiation and amplification of innate defenses (40). For instance, reticular stromal cells located in the splenic MZ are the main producers of the first wave of IFN-I during MCMV infection (7, 41). Notably, these MZ stromal cells are anatomically positioned to encounter the majority of blood-borne agents, as >90% of blood enters the spleen through the MZ sinus and can also promote both innate and adaptive immune responses (42). IFN-I functions both directly and indirectly to promote antiviral defense, with the activation of NK cell effector functions and promotion of splenocyte survival being two key roles in the case of MCMV (11). Additionally, in order for MZ stromal cells to mount a robust IFN-I response to MCMV, they must cross talk with MZ B cells, which constitutively express LTαβ (a tumor necrosis factor [TNF] family cytokine) and signal to LTβ receptor-expressing stroma (7). This supports a model where constitutive cross talk between lymphoid tissue stromal cells and LTαβ-expressing hematopoietic cells promotes cGAS-STING-dependent sensing of MCMV infection in vivo, perhaps by facilitating STING-mediated NF-κB activation (43).
Despite an extensive repertoire of immunomodulatory strategies employed by CMV, STING signaling plays the major role in restricting early MCMV replication. However, it is very common for this largely nonpathogenic virus to employ mechanisms that target key host innate defense pathways. Recent work indicates that the M48 tegument protein of MCMV contains a deubiquitinase activity that inhibits STING sensing of MCMV in myeloid cells (44) and, taken together with our results, suggests that M48 dampens this pathway in vivo but does not completely block it. The effectiveness of STING sensing of MCMV despite M48 counteraction may be due to cGAMP, which has been shown to traffic to neighboring cells (45). Two recent reports show that cGAMP can also be directly incorporated into MCMV virions, serving to activate STING virtually immediately upon virus entry into the cytosol (46, 47), and perhaps, this pathway supersedes the M48 blockade (or an analogous/parallel viral strategy targeting STING). Taken together, our results and those of others highlight cGAS-STING as a fundamental mechanism for cell-intrinsic sensing of various herpesviruses and the downstream control of viral infection and pathogenesis.
ACKNOWLEDGMENTS
This work was supported by NIH grants CA199376 (to S.S.) and AI101423 (to C.A.B.) and a Cancer Research Institute Irvington Fellowship (to C.-W.J.L.).
REFERENCES
- 1.Cannon MJ, Schmid DS, Hyde TB. 2010. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 2.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT, Davis MM. 2015. Variation in the human immune system is largely driven by non-heritable influences. Cell 160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobbs CS. 2013. Cytomegalovirus and brain tumor: epidemiology, biology and therapeutic aspects. Curr Opin Oncol 25:682–688. doi: 10.1097/CCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 4.Herbein G, Kumar A. 2014. The oncogenic potential of human cytomegalovirus and breast cancer. Front Oncol 4:230. doi: 10.3389/fonc.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern-Ginossar N, Weisburd B, Michalski A, Le VT, Hein MY, Huang SX, Ma M, Shen B, Qian SB, Hengel H, Mann M, Ingolia NT, Weissman JS. 2012. Decoding human cytomegalovirus. Science 338:1088–1093. doi: 10.1126/science.1227919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawlinson WD, Farrell HE, Barrell BG. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol 70:8833–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. 2008. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma S, Wang Q, Chodaczek G, Benedict CA. 2013. Lymphoid-tissue stromal cells coordinate innate defense to cytomegalovirus. J Virol 87:6201–6210. doi: 10.1128/JVI.00113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Brière F, Trinchieri G, Biron CA. 2002. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med 195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma S, Benedict CA. 2011. Sources and signals regulating type I interferon production: lessons learned from cytomegalovirus. J Interferon Cytokine Res 31:211–218. doi: 10.1089/jir.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. 2010. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedict CA, Banks TA, Senderowicz L, Ko M, Britt WJ, Angulo A, Ghazal P, Ware CF. 2001. Lymphotoxins and cytomegalovirus cooperatively induce interferon-b, establishing host-virus détente. Immunity 15:617–626. doi: 10.1016/S1074-7613(01)00222-9. [DOI] [PubMed] [Google Scholar]
- 14.Banks TA, Rickert S, Benedict CA, Ma L, Ko M, Meier J, Ha W, Schneider K, Granger SW, Turovskaya O, Elewaut D, Otero D, French AR, Henry SC, Hamilton JD, Scheu S, Pfeffer K, Ware CF. 2005. A lymphotoxin-IFN-beta axis essential for lymphocyte survival revealed during cytomegalovirus infection. J Immunol 174:7217–7225. doi: 10.4049/jimmunol.174.11.7217. [DOI] [PubMed] [Google Scholar]
- 15.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. 2010. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol 84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, Chen J, Cristea IM. 2013. Human cytomegalovirus tegument protein pUL83 inhibits IFI16-mediated DNA sensing for immune evasion. Cell Host Microbe 14:591–599. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dell'Oste V, Gatti D, Gugliesi F, De Andrea M, Bawadekar M, Lo Cigno I, Biolatti M, Vallino M, Marschall M, Gariglio M, Landolfo S. 2014. Innate nuclear sensor IFI16 translocates into the cytoplasm during the early stage of in vitro human cytomegalovirus infection and is entrapped in the egressing virions during the late stage. J Virol 88:6970–6982. doi: 10.1128/JVI.00384-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gariano GR, Dell'Oste V, Bronzini M, Gatti D, Luganini A, De Andrea M, Gribaudo G, Gariglio M, Landolfo S. 2012. The intracellular DNA sensor IFI16 gene acts as restriction factor for human cytomegalovirus replication. PLoS Pathog 8:e1002498. doi: 10.1371/journal.ppat.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orzalli MH, Knipe DM. 2014. Cellular sensing of viral DNA and viral evasion mechanisms. Annu Rev Microbiol 68:477–492. doi: 10.1146/annurev-micro-091313-103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 23.Maniatis T, Falvo JV, Kim TH, Kim TK, Lin CH, Parekh BS, Wathelet MG. 1998. Structure and function of the interferon-beta enhanceosome. Cold Spring Harb Symp Quant Biol 63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- 24.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun 79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. 2013. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature 499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. 2014. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997–1009. doi: 10.1016/S1074-7613(01)00239-4. [DOI] [PubMed] [Google Scholar]
- 28.Redwood AJ, Messerle M, Harvey NL, Hardy CM, Koszinowski UH, Lawson MA, Shellam GR. 2005. Use of a murine cytomegalovirus K181-derived bacterial artificial chromosome as a vaccine vector for immunocontraception. J Virol 79:2998–3008. doi: 10.1128/JVI.79.5.2998-3008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis MA, Nelson JA. 2002. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr Opin Microbiol 5:403–407. doi: 10.1016/S1369-5274(02)00334-X. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Kamil JP. 2016. Viral regulation of cell tropism in human cytomegalovirus. J Virol 90:626–629. doi: 10.1128/JVI.01500-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juckem LK, Boehme KW, Feire AL, Compton T. 2008. Differential initiation of innate immune responses induced by human cytomegalovirus entry into fibroblast cells. J Immunol 180:4965–4977. doi: 10.4049/jimmunol.180.7.4965. [DOI] [PubMed] [Google Scholar]
- 33.Horan KA, Hansen K, Jakobsen MR, Holm CK, Soby S, Unterholzner L, Thompson M, West JA, Iversen MB, Rasmussen SB, Ellermann-Eriksen S, Kurt-Jones E, Landolfo S, Damania B, Melchjorsen J, Bowie AG, Fitzgerald KA, Paludan SR. 2013. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. J Immunol 190:2311–2319. doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paijo J, Doring M, Spanier J, Grabski E, Nooruzzaman M, Schmidt T, Witte G, Messerle M, Hornung V, Kaever V, Kalinke U. 2016. cGAS senses human cytomegalovirus and induces type I interferon responses in human monocyte-derived cells. PLoS Pathog 12:e1005546. doi: 10.1371/journal.ppat.1005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes LD, Verma S, McDonald B, Wu R, Tacke R, Nowyhed HN, Ekstein J, Feuvrier A, Benedict CA, Hedrick CC. 2015. Cardif (MAVS) regulates the maturation of NK cells. J Immunol 195:2157–2167. doi: 10.4049/jimmunol.1402060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Chen ZJ. 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol 32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 38.Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, Dabelic R, Manicassamy B, Aitchison JD, Aderem A, Elliott RM, Garcia-Sastre A, Racaniello V, Snijder EJ, Yokoyama WM, Diamond MS, Virgin HW, Rice CM. 2014. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam E, Falck-Pedersen E. 2014. Unabated adenovirus replication following activation of the cGAS/STING-dependent antiviral response in human cells. J Virol 88:14426–14439. doi: 10.1128/JVI.02608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malhotra D, Fletcher AL, Turley SJ. 2013. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev 251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu KM, Pratt JR, Akers WJ, Achilefu SI, Yokoyama WM. 2009. Murine cytomegalovirus displays selective infection of cells within hours after systemic administration. J Gen Virol 90:33–43. doi: 10.1099/vir.0.006668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baptista AP, Roozendaal R, Reijmers RM, Koning JJ, Unger WW, Greuter M, Keuning ED, Molenaar R, Goverse G, Sneeboer MM, den Haan JM, Boes M, Mebius RE. 2014. Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. eLife 3:e04433. doi: 10.7554/eLife.04433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiscott J, Grandvaux N, Sharma S, Tenoever BR, Servant MJ, Lin R. 2003. Convergence of the NF-kappaB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann N Y Acad Sci 1010:237–248. doi: 10.1196/annals.1299.042. [DOI] [PubMed] [Google Scholar]
- 44.Sun C, Schattgen SA, Pisitkun P, Jorgensen JP, Hilterbrand AT, Wang LJ, West JA, Hansen K, Horan KA, Jakobsen MR, O'Hare P, Adler H, Sun R, Ploegh HL, Damania B, Upton JW, Fitzgerald KA, Paludan SR. 2015. Evasion of innate cytosolic DNA sensing by a gammaherpesvirus facilitates establishment of latent infection. J Immunol 194:1819–1831. doi: 10.4049/jimmunol.1402495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. 2013. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503:530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gentili M, Kowal J, Tkach M, Satoh T, Lahaye X, Conrad C, Boyron M, Lombard B, Durand S, Kroemer G, Loew D, Dalod M, Thery C, Manel N. 2015. Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science 349:1232–1236. doi: 10.1126/science.aab3628. [DOI] [PubMed] [Google Scholar]
- 47.Bridgeman A, Maelfait J, Davenne T, Partridge T, Peng Y, Mayer A, Dong T, Kaever V, Borrow P, Rehwinkel J. 2015. Viruses transfer the antiviral second messenger cGAMP between cells. Science 349:1228–1232. doi: 10.1126/science.aab3632. [DOI] [PMC free article] [PubMed] [Google Scholar]