Abstract
Human immunodeficiency virus type 1 (HIV-1) and human T-lymphotropic virus type 1 (HTLV-1) are complex retroviruses mainly infecting CD4+ T lymphocytes. In addition, antigen-presenting cells such as dendritic cells (DCs) are targeted in vivo by both viruses, although to a lesser extent. Interaction of HIV-1 with DCs plays a key role in viral dissemination from the mucosa to CD4+ T lymphocytes present in lymphoid organs. While similar mechanisms may occur for HTLV-1 as well, most HTLV-1 data were obtained from T-cell studies, and little is known regarding the trafficking of this virus in DCs. We first compared the efficiency of cell-free versus cell-associated viral sources of both retroviruses at infecting DCs. We showed that both HIV-1 and HTLV-1 cell-free particles are poorly efficient at productively infecting DCs, except when DC-SIGN has been engaged. Furthermore, while SAMHD-1 accounts for restriction of cell-free HIV-1 infection, it is not involved in HTLV-1 restriction. In addition, cell-free viruses lead mainly to a nonproductive DC infection, leading to trans-infection of T-cells, a process important for HIV-1 spread but not for that of HTLV-1. Finally, we show that T-DC cell-to-cell transfer implies viral trafficking in vesicles that may both increase productive infection of DCs (“cis-infection”) and allow viral escape from immune surveillance. Altogether, these observations allowed us to draw a model of HTLV-1 and HIV-1 trafficking in DCs.
INTRODUCTION
Human T-lymphotropic virus type 1 (HTLV-1) and human immunodeficiency virus type 1 (HIV-1) infect 5 to 10 million (1) and 30 million (2) individuals worldwide, respectively. HTLV-1 is present in clusters of high endemicity such as in Japan, intertropical Africa, the Caribbean, and South America (3), whereas HIV-1 is pandemic. Interestingly, while both viruses infect CD4+ T cells in vivo, the consequences of infection are opposite. After a long period of clinical latency, HTLV-1 infection leads either to adult T-cell leukemia (ATL) (4), an uncontrolled CD4+ T lymphocyte proliferation with a very poor prognosis, or to an inflammatory disorder named HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in a fraction of infected individuals (5). On the other hand, HIV-1 is associated with CD4+ T-lymphocyte death and causes AIDS (for a review, see reference 6).
Interestingly, both viruses also infect antigen-presenting cells (APCs) to a lesser extent, among which are different subtypes of dendritic cells (DCs), such as myeloid DCs, monocyte-derived DCs (MDDCs), and plasmacytoid DCs (pDCs) (7–10). After viral entry in mucosal tissues during sexual intercourse or breastfeeding (11, 12), DCs can be used as viral carriers, thus allowing the virus to reach lymphoid organs, where it infects CD4+ T lymphocytes (13). Thus, both retroviruses may hijack (i) the ability of DCs to capture pathogens and (ii) their vesicular traffic pathways in order to be transmitted to target cells without requiring a productive viral cycle (i.e., by trans-infection of T cells). trans-infection is probably the main route of HIV-1 transfer to T cells, since in vitro, MDDCs are poorly permissive to HIV-1 replication. This is due to several restriction factors, among which is SAM domain- and HD domain-containing protein 1 (SAMHD-1) (14) (Fig. 1A), which depletes cellular deoxynucleoside triphosphates (dNTPs) and prevents HIV-1 reverse transcription (15).
FIG 1.
Cell-free virus entry determines the fate of infection in DCs. Cell-free viruses can use at least 3 nonexclusive pathways to enter DCs. (A) In the absence of DC-SIGN, viral binding in enriched lipid raft areas could lead to viral membrane fusion at the plasma membrane. Restriction factors in the cytoplasm will prevent viral replication. (B) In the presence of DC-SIGN in lipid rafts, its interaction with viral glycoproteins leads to signaling (shown as a dashed arrow) favoring the productive infection. DC-SIGN triggering leads to viral internalization in ill-identified vesicles (VCCs) (see the text for details), in which viral fusion could occur. (C) If viral capture occurs in the absence of a coreceptor and DC-SIGN, virions are internalized in clathrin-rich endosomes and directed toward degradation.
In contrast, SAMHD-1 does not restrict HTLV-1 in monocytes in vivo (9, 16, 17), although it does in vitro (18), and also does not prevent MDDC infection (19). Importantly, cis-infection (i.e., productive infection of DCs) seems to be required for HTLV-1 transfer to T cells, in vitro (20) as well as in experimentally infected macaques (17).
The first step of viral infection relies on envelope binding to specific receptors, followed by fusion and entry. In the case of HTLV-1, the gp46 envelope protein successively binds to heparan sulfate proteoglycans (HSPGs), neuropilin-1 (NRP-1), and GLUT-1 (21), while HIV-1 gp120 requires CD4 and CXCR4 (or CCR5). Fusion requires HTLV-1 gp21 and HIV-1 gp41. After reverse transcription, the preintegration complex is translocated into the nucleus, where viral cDNA integrates into the cell genome (for a review, see reference 22). Later, viral transcription and translation lead to expression of viral proteins (23, 24). Finally, viral components eventually assemble and egress as new particles.
This model has been established in CD4+ T cells. Except for binding and reverse transcription, none of these steps has been investigated in DCs in the case of HTLV-1 infection. Here, we review the current knowledge on the puzzling HTLV-1 cycle in DCs and compare it to that of HIV-1. We also discuss how DC cis-infection and/or trans-infection of T cells may allow viral transfer to CD4+ T cells. We provide a focus on the source of viral inoculum, i.e., cell-free particles or cell-associated viruses, and analyze how these distinct viral sources may drive distinct viral trafficking modalities in DCs and determine distinct infection outcomes.
CELL-FREE OR CELL-ASSOCIATED VIRUSES USE SPECIFIC ENTRY AND TRAFFICKING ROUTES IN IMMATURE DCs AND DETERMINE THE OUTCOME OF INFECTION
DC infection may occur after capture of cell-free particles present in fluids or after cell-cell contact with an infected CD4+ T cell (for reviews, see references 13 and 25). Cell-free HIV-1 infects both T cells and MDDCs, but infection is more efficient after cell-to-cell transfer (26, 27). Using mathematical models, Iwami et al. estimated that cell-to-cell transfer of HIV-1 contributes to 60% of viral infection (28). In contrast, cell-free HTLV-1 is poorly capable of infecting either CD4+ T cells or MDDCs (20, 29, 30). It is worth noting that cell-free HTLV-1 is extremely rare in vivo. This may explain why the risk of becoming infected by HTLV-1 with cell-free blood products is negligible (1).
Most HTLV-1 particles released in the supernatant of infected cells display an incomplete capsid shell, suggesting a defect in viral assembly (31). In addition, HTLV-1 particles are unstable, with a half-life decrease in infectivity of 0.6 h (32). In light of this observation, one may hypothesize that viral assembly of cell-free HTLV-1 may not allow production of a significant percentage of infectious particles, in contrast to viral assembly during cell-to-cell transmission.
Entering DCs as cell-free virus.
Both HIV-1 (co)receptors (CD4 and CCR5) are expressed at the surface of DCs (33). Similarly, HTLV-1 receptors NRP-1 and GLUT-1 are also both expressed in MDDCs (34, 35). Binding to CD4 and CCR5 was also described during cell-free HIV-1 infection of DCs (36). Binding to HSPGs allows cell-free HTLV-1 docking on the DC membrane (34). Then, NRP-1/HTLV-1 gp46 interaction occurs in a process that is partially dependent upon HSPG/HTLV-1 gp46 interaction (35). The different modes of cell-free particle entry into DCs are shown in Fig. 1. Besides specific receptors, a number of viruses use DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin), a C-type lectin receptor, as an attachment factor (37). DC-SIGN is expressed in all DC subsets, including MDDCs, but is absent from T cells. Both HTLV-1 and HIV-1 bind to DC-SIGN (34, 38, 39). DC-SIGN binds the soluble form of HTLV-1 SU (gp46) (34) but does not interact with the HTLV-1 receptor binding domain (RBD), which is involved in the interaction of HTLV-1 SU with NRP-1 and GLUT-1 (38). Thus, the molecular basis of DC-SIGN interaction with HTLV-1 remains to be defined. DC-SIGN ectopic expression in THP-1 cells results in their productive infection by cell-free HTLV-1 (34). Conversely, silencing DC-SIGN in MDDCs or neutralizing DC-SIGN with antibodies prevents HTLV-1 binding and infection (34). Finally, efficient infection of MDDCs with cell-free virus is dependent upon the presence of GLUT-1 and DC-SIGN but does not require HSPG or NRP-1 (34), suggesting that DC-SIGN might be sufficient for HTLV-1 to bind MDDCs. This could expose the GLUT-1 binding domain present in gp46 and promote viral fusion. These results illustrate one major difference in receptor requirement between DC and CD4+ T-cell infection. It is worth noting that the cell-free viruses used in these experiments may have in fact been biofilm-entrapped viruses rather than true free viral particles (see “Entering DCs after cell-cell contact” below). Indeed, we have recently shown that HTLV-1 free particles purified from the supernatant of chronically infected cells are poorly capable of infecting MDCCs, in contrast to purified biofilm (20).
In the HIV-1 model, DC-SIGN binding to gp120 results in stabilization of the gp120/CD4 complex (36). This contributes to faster conformational changes in gp120, leading to the formation and exposition of an helix involved in the binding to CCR5. Thus, not only does DC-SIGN increase HIV-1 capture at the plasma membrane, but it also promotes infection through the stabilization of the gp120/CD4 complex and subsequent CCR5-dependent membrane fusion.
In addition to attachment and fusion enhancement, HIV-1 gp120 binding to DC-SIGN induces signal transduction leading to Raf1 activation, phosphorylation of p65/RelA, and recruitment of transcription elongation factor b (p-TEFb) on the HIV-1 promoter, thus allowing viral expression (40). Interestingly, blocking gp120/DC-SIGN interaction, Raf1 silencing, or inhibition of Raf1 activation during HIV-1 exposure abolishes DC infection (40), suggesting that DC-SIGN signaling may also alleviate the intracellular restriction. Interestingly, SAMHD-1 phosphorylation overcomes HIV-1 restriction (41, 42). SAMHD-1 phosphorylation can be induced by myeloid cell activation (41) or by opsonized HIV-1 particles (43). In addition, coculturing MDDCs with T cells downregulates SAMHD-1, thus allowing HIV-1 replication in MDDCs (44). Thus, DC-SIGN signaling may induce SAMHD-1 phosphorylation, that would then relieve restriction. Finally, HIV-1 reverse transcriptase (RT) has evolved to efficiently synthesize DNA in the presence of low dNTP concentrations (45, 46), suggesting that even in the presence of SAMHD-1, HIV-1 can replicate in DCs, although with a delayed kinetics. Although not described during HTLV-1 infection, such signaling events after gp46 binding to DC-SIGN cannot be excluded and may also activate HTLV-1 long terminal repeat (LTR) transcription (Fig. 1B). However, a few incoming HIV-1 particles are present in vesicles (47) after internalization through clathrin-independent CD4-rich raft domains (48), thus allowing a pH-independent fusion from these virus-containing compartments (VCCs), leading to productive infection (47–49) (Fig. 1B).
Cell-free HIV-1 is also internalized by endocytosis in MDDCs (49) or by macropinocytosis in macrophages (47). Most viral particles that enter through macropinocytosis are directed toward endolysosomal compartments and degraded (Fig. 1C).
Entering DCs after cell-cell contact.
Compared to cell-free infection, DC infection with HIV-1 is more efficient after cell-to-cell contact (50). This is likely due to the fact that coculture with T cells can overcome SAMHD-1 restriction (44). Nevertheless, there are few data describing viral entry in DCs after contact with infected cells. One can hypothesize that increased DC infection efficiency could result from signaling either through DC-SIGN after its interaction with HIV-1 gp120 (40, 51) or through intercellular adhesion molecule 1 (ICAM-1) after its interaction with lymphocyte function-associated antigen 1 (LFA-1) on infected CD4+ T lymphocytes (52) during the formation of the viral synapse (VS), although these structures have been observed only in the case of T-cell infection (33, 53, 54). The VS interface was imaged using electron tomography in cell lines (53, 55), revealing a very complex architecture with multiple membrane invaginations and projections.
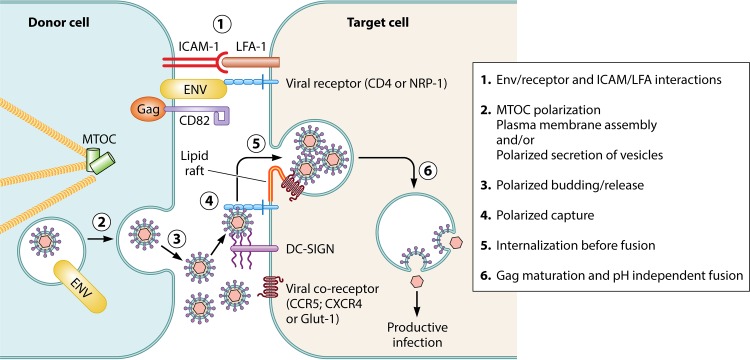
The viral synapse is formed after engagement of HIV-1 or HTLV-1 Env proteins present at the plasma membrane of infected cells with their cognate receptors on target cells. This is followed by engagement of adhesion molecules through protein complexes involving ICAM (LFA-1) (52, 56) and tetraspanins such as CD81 and CD82. These proteins are also required for the establishment of immune synapses (57). They interact with Gag proteins in tetraspanin-enriched membrane domains (58, 59) (Fig. 2). VSs are stabilized or surrounded by virally induced cellular protrusions, i.e., filopodia in the case of HIV-1 (60, 61), or conduits for HTLV-1 (62).
FIG 2.
Infection after cell-cell contact: the viral synapse. The viral synapse is characterized by an intimate contact between the infected donor cell (left) and the target cell (right). The formation of the VS can be arbitrarily divided into 6 steps: 1, cell-cell contact is established through interactions between fusion-incompetent viral Env proteins (represented in yellow) and ICAM-1 on the donor cell side and viral receptor (represented in blue) and LFA-1 on the target cell; 2, adhesion leads to MTOC polarization and virion assembly at the cell-cell contact in the donor cell; 3, newly synthesized virions are released in the synaptic cleft; 4, polarized capture of virions by the target cell is driven by Env-receptor interaction; 5, captured virions are internalized through endocytosis in the target cell; and 6, Gag maturation in endosomes leads to Env-mediated viral fusion and release of viral capsids in the cytosol, allowing productive infection.
Both VS formation and filopodium-dependent stabilization involve cytoskeleton remodeling in infected cells. In the context of DC-to-T-cell transmission, HIV-1 triggers actin polymerization through the use of the formin 2 Rho-GTPase, CDC42, and Env, Nef, and Gag viral proteins (51). HTLV-1 alters actin polymerization through p8, which is responsible for increasing conduit formation (63), and through Tax by upregulating Gem (64). In HTLV-1-infected cells, Gem colocalizes with actin and strongly increases both formation of conjugates between infected and uninfected lymphocytes and viral transfer (64). In contrast to the case for HIV-1, CDC42 is not involved in HTLV-1-induced actin polymerization, although it interacts with Tax in CD4+ T lymphocytes (65). Both retroviruses are also known to act on the microtubule network by inducing microtubule-organizing center (MTOC) polarization during VS formation (66, 67). MTOC polarization is a hallmark of the immune synapse. Importantly, it occurs in the donor cell and not in the target cell in the case of the VS (68), thus implying a direct role of viral proteins independently of TcR triggering. HTLV-1 Tax protein localization close to the MTOC in infected CD4+ T cells suggests a role for Tax in microtubule manipulation leading to MTOC polarization (67). Virions can be released in the synaptic cleft (53–55, 69, 70) after polarized assembly and budding at the cell-cell contact. After a VS is established, HTLV-1 Env and Gag and the HTLV-1 viral RNA accumulate at the site of contact and are rapidly transferred to the target cell (53) (Fig. 2). Both receptor expression and coreceptor expression in recipient cells are necessary for productive infection. NRP-1 and GLUT-1 have been shown to colocalize at the site of contact in uninfected cells during HTLV-1 cell-to-cell transfer and are also likely to promote VS formation (71, 72). During HIV-1 infection, visualization of cell-to-cell transfer using live imaging showed a random localization of Gag followed by its rapid polarization at the contact zone after the formation of the VS (73, 74). This leads to Gag multimerization and viral budding within the close connection between the donor cell and the target cell (73). In contrast, the viral protein(s) involved in MTOC polarization during HIV-1-induced VS formation remains unknown. Multiple synapses can also occur, leading to concomitant viral delivery to several target cells (75).
Virus may also be transferred from biofilm-like structures present at the surface of HTLV-1 infected cells (76). Biofilm-like structures consist of an extracellular carbohydrate-rich matrix in which viral particles are embedded together with extracellular matrix proteins such as collagen, agrin, and linker proteins (for instance, galectin-3 or tetherin) (76, 77). Interestingly, expression of most of these proteins is induced or increased during HTLV-1 infection via Tax expression (78). Immunofluorescence data revealed that viral particles entrapped in this structure are able to rapidly reach uninfected lymphocytes, through contacts both outside and within the viral synapse (76). This suggests that VS formation and virus transfer within the VS may not be concomitant with viral assembly at the synapse and that already-assembled viruses entrapped in biofilms could also be transferred to uninfected cells during intimate cell-cell contacts. It was suggested that viruses stored in biofilm at the plasma membrane could account for 80% of the infectious capacity of HTLV-1-infected lymphocytes (76). In addition, we have shown that purified biofilm-like structures are sufficient for infecting primary T lymphocytes and DCs (20). Biofilm production in the context of HIV-1 infection has not yet been described.
Does budding in the infected donor cell occur at the plasma membrane or in vesicles during cell-to-cell transfer?
VS formation does not lead to cellular membrane fusion, suggesting that plasma membrane-associated Env proteins may be fusion incompetent. These proteins would be necessary during the early steps of VS formation through interaction with their receptors CD4 or Glut1 but not for budding. Following interaction with Gag, the formation of HIV-1 nascent virions requires a functional secretion vesicle pathway. This suggests that plasma membrane-associated Env proteins are not involved in viral budding during VS formation (79–82). Escape from antibody neutralization, which is a common feature of cell-to-cell viral transmission (26, 27, 83), could be the consequence of an immature HIV-1 Env conformation at the plasma membrane. Interestingly, blocking HIV-1 Env trafficking through the secretion vesicular pathway has no effect on infectious cell-free virus production (79), emphasizing that viral budding of cell-free particles differs from that of cell-cell-transmitted virus. This hypothesis is further supported by studies showing that different Env-Gag interactions govern cell-free or cell-to-cell transmission (81, 84, 85). Interestingly, colocalization of HIV-1 virions with late endosomes in chronically infected cells (86) led to the hypothesis that viral assembly and budding may also occur inside the cell rather than at the plasma membrane. In contrast, budding at the plasma membrane may be the preferred mechanism of viral production in newly infected cells (86). HIV-1 virions could bud preferentially in the multivesicular body (MVB) and be released in the synaptic cleft after fusion of the MVB with the plasma membrane (87, 88). Thus, viral assembly may initiate at the plasma membrane, but viral budding could occur in the MVB. Viral release would then use the endosomal sorting pathway in a way similar to that of exosomes.
The same mechanisms may also occur for HTLV-1 assembly before VS transmission. Interactions between both HTLV-1 Gag proteins and proteins belonging to the ESCRT (endosomal sorting complexes required for transport) pathway (89–91), as well as Gag trafficking through the MVB, were reported (92, 93). This suggests that viral budding requires MVB machinery. However, these results are difficult to reconcile with viral assembly at the plasma membrane. This may be linked to different mechanisms of viral release that occur either in a cell-free context or during cell-cell contacts. As exemplified in the HIV-1 model (86), different budding mechanisms, i.e., at plasma membrane or inside vesicles, may occur in newly infected T cells or in chronically infected T cells, respectively.
Does viral capture occur in vesicles during cell-to-cell transfer?
A significant number of virions are able to enter the target cell during transmission through the VS (94). This could account for the resistance against antiviral drugs that is observed during cell-to-cell spread (for a review, see reference 95). Alternatively, efficient cell-to-cell transmission could be the consequence of a different viral entry pathway, although one still dependent on the production of infectious virus particles (96), as described for the HIV-1 VS between T cells (for a review, see reference 25). After viral delivery through a VS between infected and uninfected T cells, viral fusion proceeds after the internalization of the particles in vesicles whose nature is still debated (73, 97, 98). This could rely on macropinocytosis or dynamin-dependent endocytosis (98). This entry process may also be independent of Env-CD4 interaction at the plasma membrane (80) (see “Does budding in the infected donor cell occur at the plasma membrane or in vesicles during cell-to-cell transfer?” above). However, once in the vesicles, immature HIV-1 Gag proteins undergo endosomal maturation, allowing modification of Env conformation and coreceptor-dependent fusion that induces the release of HIV-1 capsids into the cytoplasm, thus leading to productive infection (80) (Fig. 2). DCs are specialized for antigen uptake via endocytosis. Since viral capture occurs via endocytosis during VS formation between T cells, it is conceivable that viral entry will also occur through endocytosis if DCs form a VS with an infected T cells.
However, little is known about the mechanism that facilitates HTLV-1 uptake after its transmission across the VS or after contact with biofilm, although it is conceivable that this would also occur via endocytosis. This hypothesis is supported by electron microscopy analyses of MDDCs cocultured with HTLV-1-infected lymphocytes, which show some particles internalized in vesicles (38). Whether these vesicles result from macropinocytosis or endocytosis after synaptic transfer of HTLV-1 and whether this process leads to productive HTLV-1 infection are currently unknown.
Viral transfer to T cells after DC cis-infection.
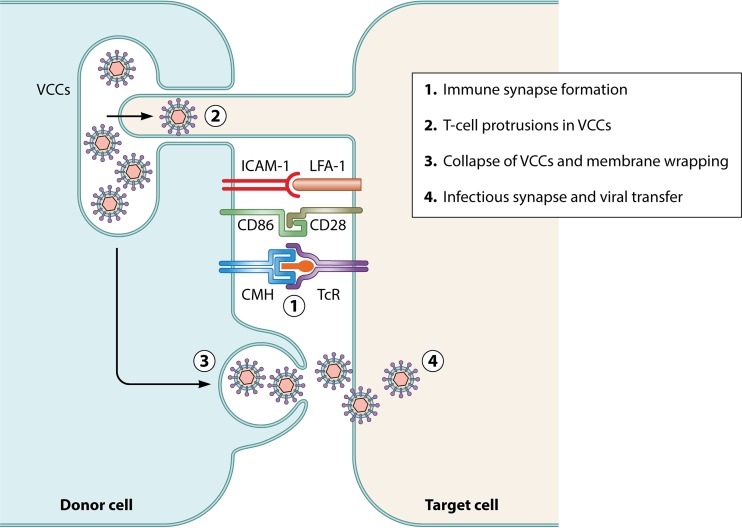
Since HIV-1-infected DCs are rare and HTLV-1 release in the supernatant of infected DCs seems to be limited (8), information regarding HIV-1 or HTLV-1 budding in productively infected DCs is lacking. Nevertheless, productively infected DCs are capable of transferring virus to T cells after cell-cell contact (8, 99–101). Whether transfer occurs via formation of a VS, involving MTOC relocalization in infected DCs and polarized budding, or via an infectious synapse, i.e., independently of Env protein recognition, is unclear (102) (Fig. 3).
FIG 3.
The infectious synapse between either cis-infected DCs or mature DCs that have captured virions and T cells. In contrast to the VS, the infectious synapse depends first on the formation of an immune synapse (1). This initial contact, which is independent of viral protein engagement, induces protrusions of T-cell membrane filopodia inside VCCs where viruses are stored, allowing virus capture at the tips of the protrusions (2), and/or VCC collapse (3) and release of viruses at the synapse, leading to T-cell infection (4).
In infected macrophages, HIV-1 is found in large intracytoplasmic vacuoles that are often referred to as virus-containing compartments (VCCs) (87, 88, 103–109). Interestingly, these membrane structures also exist in DCs (106). Electron microscopy and tomography revealed that HIV-1 buds at the limiting membrane of the VCCs (87, 88, 106). VCCs are distinct from endosomes since they lack classical markers such as EE1A and are inaccessible to bovine serum albumin (BSA)-gold beads (103) and show unique vesicular structures (104–106). VCCs also contain tetraspanin proteins such as CD81, CD82, CD63, and CD36 (110, 111), a hallmark of the MVB, that were also shown to be critical for viral release in T cells. Finally, these VCCs contain GM3 sphingolipid-rich lipid raft domains that are also found in plasma membrane lipid rafts (for a review, see reference 112). Thus, VCCs have been suggested to represent viral assembly platforms in infected macrophages and can be related to the MVB observed in DCs. VCC-contained HIV-1 remains infectious for long periods of time (113), and rapid release of cell-free HIV-1 from the VCCs into the extracellular milieu can be induced by external addition of ATP (114). In addition, infected macrophages can rapidly transfer HIV-1 to target T cells after a cell-to-cell contact (115), leading to the collapse of the VCCs toward the point of contact in a process resembling an infectious synapse (see “HIV transfer to T cells after capture by mature DCs,” below) (106) (Fig. 3). Finally, indirect evidence suggests that DC-to-T-cell viral transfer is mediated by a VS (101).
With respect to HTLV-1 transfer from infected DCs to T cells, a vesicular localization of the virus was reported in infected DCs (38), suggesting that budding could also take place in vesicles. Viral transfer to target T-cell might then occur via an infectious synapse.
CELL-FREE VIRUS CAPTURE BY MATURE DCs CAN LEAD TO TRANS-INFECTION OF T CELLS
DC-mediated HIV-1 trans-infection of T cells occurs within the first 24 h of viral capture (100). It is characterized by the transfer of infectious viral particles without viral replication. In this case, viral transfer occurs after the formation of an immune synapse between DCs and T cells (102), by a mechanism that is thought to be very similar to that for the infectious synapse observed between macrophages and T cells (Fig. 3). In DCs, the mechanism of DC-mediated cell-free HIV-1 “trans-infection” of T cells is well documented, while HTLV-1 trans-infection (after cell-to-cell contact) is barely described (8). Below, we review how HIV-1 can use DCs as Trojan horses for trans-infection and discuss how HTLV-1 might use the same pathway.
Surfing of HIV-1 cell-free particles and viral “sac-like” components.
How cell-free HIV-1 virus is captured upon trans-infection of T cells and whether it traffics through specific compartments or whether it only “surfs” along the plasma membrane before being delivered to T cells is still a matter of debate. Indeed, conflicting results have been reported regarding the nature of the compartments where HIV-1 particles accumulate after cell-free virus capture.
Initially, trans-infection was related to capture of HIV-1 cell-free particles after DC-SIGN engagement (116, 117). Virus accumulation in an intracellular nonacidic, tetraspanin-enriched compartment suggested a localization within the MVB (118), similar to the situation in productively infected macrophages (see “Viral transfer to T cells after DC cis-infection,” above). However, plasma membrane-bound HIV-1 cell-free particles have also been described in Toll-like receptor 4 (TLR4) agonist-matured MDDCs (lipopolysaccharide [LPS]-matured MDDCs), where DC-SIGN expression is low (119, 120). The virus then “surfs” outside the plasma membrane using actin-dependent polarized movements and accumulates in invaginated domains continuous with the plasma membrane (121). Viral capture is highly dynamic in LPS-matured MDDCs and involves a continuous exchange of particles with the extracellular milieu. Indeed, constant remodeling of the compartment content could favor the capture of viruses but also the release of entrapped ones (50). These “sac-like” compartments were shown to be distinct from classical endosomal, lysosomal, or antigen-presenting compartments. They are CD63 and CD81 tetraspanin positive (121), a feature reminiscent of VCCs observed in macrophages. Since the connection of sac-like structures to plasma membrane is very thin (119), it is likely that HIV-1-positive MVB-like compartments and sac-like compartments connected to the plasma membrane represent identical structures described in different experimental settings. Finally, sac-like structures containing viruses were also observed at the contact sites formed between MDDCs and T cells, suggesting that they may be involved in viral transfer (122) in a process that differs from the VS formation described with productively infected cells.
HIV-1 receptors for trans-infection.
Until recently, the nature of the molecules involved in cell-free HIV-1 capture upon trans-infection remained undetermined. Although DC-SIGN was reported to bind HIV-1 in immature DCs (116, 117), inhibition of DC-SIGN expression did not affect trans-infection (123). In addition, and unlike the situation for immature DCs, HIV-1 capture by mature MDDCs is independent of gp120 (120). Finally, DC maturation results in a reduced macropinocytosis (124), while HIV-1 capture and transfer to T cells are enhanced (119, 125, 126). Altogether, these observations exclude DC-SIGN as an important player in HIV-1 trans-infection. Interestingly, HIV-1 capture is dramatically dependent upon the sphingolipid contents of incoming viral particles (120, 127). Moreover, incorporation of membrane gangliosides during budding of viruses within plasma membrane lipid rafts of infected T cells strictly controls the ability of mature MDDCs to transfer HIV-1 to T cells (128, 129). Incorporation of α2,3-GM3 ganglioside into HIV-1 particles is responsible for viral capture and trans-infection of T cells by mature MDDCs (130). Transcriptomic analysis of mature MDDCs displaying different abilities to capture and transfer HIV-1 to T cells allowed the identification of Siglec-1 as an important molecule for HIV-1 trans-infection (122). Direct interaction of GM3 with Siglec-1 was further demonstrated (122, 131). Siglec-1 colocalizes with HIV-1 in sac-like compartments and accumulates at the infectious synapse when LPS-matured MDDCs are cocultured with T cells (122, 132, 133), further demonstrating that DC-mediated trans-infection of T cells relies on Siglec-1. However, these studies also highlighted the fact that trans-infection is not per se dependent upon viral component recognition. Indeed, Siglec-1 has also been identified as the receptor for exosomes (131, 134). This finding shed light on earlier studies showing that HIV-1 trafficking in infected cells or in mature MDDCs was very similar to that of exosomes (120). Thus, trans-infection of T cells by HIV-1 after HIV-1 capture by mature MDDCs could represent a hijacked process of cell-to-cell communication based on exosome capture.
HIV-1 transfer to T cells after capture by mature DCs.
Once captured in VCCs, the MVB, or sac-like membrane invaginations, viruses may be transferred to target T cells. As already mentioned for cis-infected DCs, transfer of viral particles stored in intracellular compartments is not triggered by the virus, as it is the case during VS formation. It is rather related to immune synapse hijacking and its switch into an infectious synapse (102) (Fig. 3). In contrast to the VS, infectious synapses between HIV-1-containing DCs and uninfected T cells do not rely upon Env-CD4 interactions but are dependent on LFA–ICAM-1 and are enhanced by TcR triggering (102). Thus, blocking CD4 on the target T cell does not decrease the number of conjugates with DCs but decreases the number of HIV-1 viruses bound to the T-cell membrane at the synapse, thus resulting in a decreased number of infected T cells (102, 135). Cell-cell contacts consist of large sheet-like membrane structures derived from DCs that wrap around T cells, resulting in large cell-cell contacts at the infectious synapse (135). In addition, membrane protrusions originating from T cells were also found within the virus sac-like compartment of trans-infected DCs, allowing viruses to bind both at the tips of the T-cell protrusions and along the length of the protrusions, probably after viral surfing (135) (Fig. 3).
Does HTLV-1 also trans-infect T cells after capture by DCs?
HTLV-1 transfer to T cells after DC-mediated trans-infection has not been documented but is very likely to exist. Several lines of evidence support this hypothesis.
First, HTLV-1 transfer from DCs to T cells occurs in two waves: during the first hours after viral capture and after several days of infection (8). This is reminiscent of trans- and cis-infection described for HIV-1, respectively. Short exposure of MDDCs or pDCs to cell-free HTLV-1 in the presence of zidovudine (AZT) does not prevent subsequent viral transfer to T cells, suggesting trans-infection. In contrast, AZT treatment prevents DCs from transmitting the virus to T cells when DCs are treated for longer periods with AZT along with cell-free HTLV-1 before being exposed to target T cells (8), thus demonstrating productive cis-infection of DCs.
Second, HTLV-1 egress likely parallels the exosome secretion pathway (136), suggesting that the lipid composition of HTLV-1 particles might also allow viral capture by Siglec-1 in DCs.
Third, productive infection of DCs by HTLV-1 is prevented in LPS- and prostaglandin E2-matured MDDCs (35). Such a maturation induces vascular endothelial growth factor (VEGF) expression in MDDCs, which competes with HTLV-1 Env protein binding on the NRP-1/HSPG complex, thus resulting in a decrease in viral capture and to a subsequent lack of infection. This is in sharp contrast with data showing that DC maturation increases HIV-1 capture and trans-infection of T cells but reduces cis-infection (119, 125, 126). It suggests that different DC maturation processes may have different outcomes. Similarly, LPS-matured MDDCs could have a different susceptibility to cis-infection by HTLV-1 than immature MDDCs, despite efficient viral capture. Finally, since capture of small vesicles by LPS-matured MDDCs is restricted not to viral components but to lipid components (134), HTLV-1 capture by LPS-matured MDDCs is expected to be increased compared to that by immature MDDCs. Viral capture as well as susceptibility to HTLV-1 infection of MDDCs matured under different conditions remains to be investigated.
CONCLUSION
HIV-1 and HTLV-1 can enter and/or replicate in DCs and T cells. However, few studies have compared side by side the efficiencies of the viral sources in infecting DCs and/or T cells. Virus delivery as cell-free particles or through cell-cell contact leads to different modes of viral capture, interaction with different receptors, different routes of trafficking, and additional signaling. In addition, DC exposure to the virus results in both cis-infection of DCs and/or DC-mediated trans-infection of T cells, adding a higher level of complexity to the system. Current data are consistent with a model where both productive infection (cis-infection) and trans-infection coexist in immature and mature DCs. cis-infection of immature DCs by HIV-1 or HTLV-1 occurs at a low level. DC maturation enhances viral capture and the ability to transfer virus, while diminishing productive infection. An enhanced ability of mature DCs to trans-infect T cells would be involved in rapid viral spread, a process that could happen in lymph nodes, where both naive T cells and mature DCs are present.
Information on many pieces of the HTLV-1 cycle in DCs is still missing, and further investigations are needed to allow the identification of cellular and viral actors involved in this process and the role of DC cis- and/or trans-infection in HTLV-1 pathogenesis.
ACKNOWLEDGMENTS
We thank Louis-Marie Bloyet and Patrick Lécine for critical readings of the manuscript and helpful discussions. We thank Patrick Lane from ScEYEnce Studios for graphical enhancement of the figures.
This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM) and by La Ligue Contre le Cancer (Program Équipes Labéllisées). R.M. is supported by ENS and by a PHRT program (AP-HP).
Biographies

Hélène Dutartre is a senior scientist from INSERM. She obtained a Ph.D. in 1997 in immunology from the Université de la Méditérannée (Marseille, France) at the research center for cancerology of Marseille (CRCL, Marseille). She has a background in HIV-1 and HCV virus-host interactions at the interface of immunology and virology, acquired from her training in the teams of Professor Daniel Olive (CRCL, Marseille France), Dr. Bruno Canard (AFMB, Marseille, France), and Dr. Jacques Banchereau (BIIR, Dallas, TX, USA). As a member of Professor Renaud Mahieux's team since 2012, her current interest focuses on understanding the interaction between the HTLV-1 oncovirus and innate cells to provide new strategies for anticancer treatment.

Mathieu Clavière is a Ph.D. student at the Center for International Research in Infectiology (CIRI) in the team of Professor M. Faure. He received his master's degree in 2014 at the Ecole Normale Supérieure de Lyon in Professor Renaud Mahieux's team under the supervision of Dr. Hélène Dutartre, where he studied HTLV-1 dendritic cell trafficking. His current interest covers the contribution of autophagic cellular process to viral and bacterial infections.

Chloé Journo is an Assistant Professor teaching virology and immunology at the Ecole Nationale Supérieure de Lyon. She obtained her Ph.D. in virology in 2010 from ENS-Lyon after receiving a master's degree from Institut Pasteur in Paris. Her research activity focuses on the molecular mechanisms leading to HTLV-1-induced cell transformation. She studies the properties of two viral proteins, Tax and the antisense HBZ protein, and investigates how interactions of these viral proteins with cellular partners affect the NF-κB pathway, cell cycle checkpoints, and gene expression.

Renaud Mahieux obtained his Ph.D. from Paris 6 University in 1997. He is Professor of Virology at the Ecole Normale Supérieure de Lyon, France, is Head of the ENS-Lyon biology department, and leads the retroviral oncogenesis laboratory at the Center for International Research in Infectiology (CIRI, Lyon, France). The main objectives of the team are to understand the physiopathology of human T-cell leukemia virus type 1 (HTLV-1) infection and to develop new regimens that could either prevent the occurrence of adult T-cell leukemia or allow treatment of patients. To achieve this goal, his team takes advantage of the combined expertise of its members and of several in vitro or in vivo models. The team also benefits from ongoing collaborations with clinicians, epidemiologists, biochemists, and chemists.
Funding Statement
This work was funded by Ligue Contre le Cancer (EL2013-3). Hélène Dutartre is supported by INSERM. Chloé Journo and Renaud Mahieux are supported by ENS.
REFERENCES
- 1.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. 2013. AIDS by the numbers. www.unaids.org UNAIDS, Washington, DC. [Google Scholar]
- 3.Bruhn R, Mahieux R, Murphy EL. Human lymphotropic viruses: HTLV-1 and HTLV-2. In clinical virology, 4th ed, in press ASM Press, Washington, DC. [Google Scholar]
- 4.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481–492. [PubMed] [Google Scholar]
- 5.Gessain A, Barin F, Vernant J, Gout O, Maurs L, Calender A, de Thé G. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407–410. [DOI] [PubMed] [Google Scholar]
- 6.Gallo RC, Montagnier L. 2003. The discovery of HIV as the cause of AIDS. N Engl J Med 349:2283–2285. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham AL, Donaghy H, Harman AN, Kim M, Turville SG. 2010. Manipulation of dendritic cell function by viruses. Curr Opin Microbiol 13:524–529. doi: 10.1016/j.mib.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. 2008. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med 14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 9.Macatonia SE, Cruickshank JK, Rudge P, Knight SC. 1992. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses 8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, KewalRamani VN. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blattner WA, Nomura A, Clark JW, Ho GY, Nakao Y, Gallo R, Robert-Guroff M. 1986. Modes of transmission and evidence for viral latency from studies of human T-cell lymphotrophic virus type I in Japanese migrant populations in Hawaii. Proc Natl Acad Sci U S A 83:4895–4898. doi: 10.1073/pnas.83.13.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hino S, Katamine S, Kawase K, Miyamoto T, Doi H, Tsuji Y, Yamabe T. 1994. Intervention of maternal transmission of HTLV-1 in Nagasaki, Japan. Leukemia 8(Suppl 1):S68–S70. [PubMed] [Google Scholar]
- 13.Pique C, Jones KS. 2012. Pathways of cell-cell transmission of HTLV-1. Front Microbiol 3:378. doi: 10.3389/fmicb.2012.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nascimento CR, Lima MA, d A Serpa MJ, Espindola O, Leite ACC, Echevarria-Lima J. 2011. Monocytes from HTLV-1-infected patients are unable to fully mature into dendritic cells. Blood 117:489–499. doi: 10.1182/blood-2010-03-272690. [DOI] [PubMed] [Google Scholar]
- 17.Valeri V, Hryniewicz A, Andresen V, Jones K, Fenizia C, Bialuk I, Chung H, Fukumoto R, Parks R, Ferrari M, Nicot C, Cecchinato V, Ruscetti F, Franchini G. 2010. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood 116:3809–3817. doi: 10.1182/blood-2010-05-284141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sze A, Belgnaoui SM, Olagnier D, Lin R, Hiscott J, van Grevenynghe J. 2013. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 14:422–434. doi: 10.1016/j.chom.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Gramberg T, Kahle T, Bloch N, Wittmann S, Müllers E, Daddacha W, Hofmann H, Kim B, Lindemann D, Landau NR. 2013. Restriction of diverse retroviruses by SAMHD1. Retrovirology 10:26. doi: 10.1186/1742-4690-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alais S, Mahieux R, Dutartre H. 2015. Viral source-independent high susceptibility of dendritic cells to human T-cell leukemia virus type 1 infection compared to that of T lymphocytes. J Virol 89:10580–10590. doi: 10.1128/JVI.01799-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones KS, Lambert S, Bouttier M, Benit L, Ruscetti FW, Hermine O, Pique C. 2011. Molecular aspects of HTLV-1 entry: functional domains of the HTLV-1 surface subunit (SU) and their relationships to the entry receptors. Viruses 3:794–810. doi: 10.3390/v3060794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marx A, Alian A. 2015. The road less traveled: HIV's use of alternative routes through cellular pathways. J Virol 89:5204–5212. doi: 10.1128/JVI.03684-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Journo C, Douceron E, Mahieux R. 2009. HTLV gene regulation: because size matters, transcription is not enough. Future Microbiol 4:425–440. doi: 10.2217/fmb.09.13. [DOI] [PubMed] [Google Scholar]
- 24.Brady J, Kashanchi F. 2005. Tat gets the “green” light on transcription initiation. Retrovirology 2:69. doi: 10.1186/1742-4690-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez RA, Barria MI, Chen BK. 2014. Unique features of HIV-1 spread through T cell virological synapses. PLoS Pathog 10:e1004513. doi: 10.1371/journal.ppat.1004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong P, Agosto LM, Munro JB, Mothes W. 2013. Cell-to-cell transmission of viruses. Curr Opin Virol 3:44–50. doi: 10.1016/j.coviro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazurov D, Ilinskaya A, Heidecker G, Lloyd P, Derse D. 2010. Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors. PLoS Pathog 6:e1000788. doi: 10.1371/journal.ppat.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwami S, Takeuchi JS, Nakaoka S, Mammano F, Clavel F, Inaba H, Kobayashi T, Misawa N, Aihara K, Koyanagi Y, Sato K. 2015. Cell-to-cell infection by HIV contributes over half of virus infection. eLife 4. doi: 10.7554/eLife.08150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuroda Y, Kurohara K, Fujiyama F, Takashima H, Endo C, Matsui M, Neshige R, Kakigi R. 1992. Systemic interferon-alpha in the treatment of HTLV-I-associated myelopathy. Acta Neurol Scand 86:82–86. doi: 10.1111/j.1600-0404.1992.tb08059.x. [DOI] [PubMed] [Google Scholar]
- 30.de Rossi A, Aldovini A, Franchini G, Mann D, Gallo RC, Wong-Staal F. 1985. Clonal selection of T lymphocytes infected by cell-free human T-cell leukemia/lymphoma virus type I: parameters of virus integration and expression. Virology 143:640–645. doi: 10.1016/0042-6822(85)90405-2. [DOI] [PubMed] [Google Scholar]
- 31.Cao S, Maldonado JO, Grigsby IF, Mansky LM, Zhang W. 2015. Analysis of human T-cell leukemia virus type 1 particles by using cryo-electron tomography. J Virol 89:2430–2435. doi: 10.1128/JVI.02358-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinagawa M, Jinno-Oue A, Shimizu N, Roy BB, Shimizu A, Hoque SA, Hoshino H. 2012. Human T-cell leukemia viruses are highly unstable over a wide range of temperatures. J Gen Virol 93:608–617. doi: 10.1099/vir.0.037622-0. [DOI] [PubMed] [Google Scholar]
- 33.Coleman CM, Gelais CS, Wu L. 2013. Cellular and viral mechanisms of HIV-1 transmission mediated by dendritic cells. Adv Exp Med Biol 762:109–130. doi: 10.1007/978-1-4614-4433-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain P, Manuel SL, Khan ZK, Ahuja J, Quann K, Wigdahl B. 2009. DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. J Virol 83:10908–10921. doi: 10.1128/JVI.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert S, Bouttier M, Vassy R, Seigneuret M, Cari P-S, Janvier S, Heveker N, Ruscetti FW, Perret G, Jones KS, Pique C. 2009. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood 113:5176–5185. doi: 10.1182/blood-2008-04-150342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hijazi K, Wang Y, Scala C, Jeffs S, Longstaff C, Stieh D, Haggarty B, Vanham G, Schols D, Balzarini J, Jones IM, Hoxie J, Shattock R, Kelly CG. 2011. DC-SIGN increases the affinity of HIV-1 envelope glycoprotein interaction with CD4. PLoS One 6:e28307. doi: 10.1371/journal.pone.0028307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercer J, Greber UF. 2013. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol 21:380–388. doi: 10.1016/j.tim.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Ceccaldi P-EE, Delebecque F, Prevost M-CC, Moris A, Abastado J-PP, Gessain A, Schwartz O, Ozden S. 2006. DC-SIGN facilitates fusion of dendritic cells with human T-cell leukemia virus type 1-infected cells. J Virol 80:4771–4780. doi: 10.1128/JVI.80.10.4771-4780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee B, Leslie G, Soilleux E, OD U, Baik S, Levroney E, Flummerfelt K, Swiggard W, Coleman N, Malim M, Doms R. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol 75:12028–12038. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. 2010. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol 11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- 41.Cribier A, Descours B, Valadao AL, Laguette N, Benkirane M. 2013. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 3:1036–1043. doi: 10.1016/j.celrep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Arnold LH, Kunzelmann S, Webb MR, Taylor IA. 2015. A continuous enzyme-coupled assay for triphosphohydrolase activity of HIV-1 restriction factor SAMHD1. Antimicrob Agents Chemother 59:186–192. doi: 10.1128/AAC.03903-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posch W, Steger M, Knackmuss U, Blatzer M, Baldauf HM, Doppler W, White TE, Hortnagl P, Diaz-Griffero F, Lass-Florl C, Hackl H, Moris A, Keppler OT, Wilflingseder D. 2015. Complement-opsonized HIV-1 overcomes restriction in dendritic cells. PLoS Pathog 11:e1005005. doi: 10.1371/journal.ppat.1005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su B, Biedma ME, Lederle A, Peressin M, Lambotin M, Proust A, Decoville T, Schmidt S, Laumond G, Moog C. 2014. Dendritic cell-lymphocyte cross talk downregulates host restriction factor SAMHD1 and stimulates HIV-1 replication in dendritic cells. J Virol 88:5109–5121. doi: 10.1128/JVI.03057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenzi GM, Domaoal RA, Kim DH, Schinazi RF, Kim B. 2015. Mechanistic and kinetic differences between reverse transcriptases of Vpx coding and non-coding lentiviruses. J Biol Chem 290:30078–30086. doi: 10.1074/jbc.M115.691576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenzi GM, Domaoal RA, Kim DH, Schinazi RF, Kim B. 2014. Kinetic variations between reverse transcriptases of viral protein X coding and noncoding lentiviruses. Retrovirology 11:111. doi: 10.1186/s12977-014-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gobeil L-A, Lodge R, Tremblay MJ. 2013. Macropinocytosis-like HIV-1 internalization in macrophages is CCR5 dependent and leads to efficient but delayed degradation in endosomal compartments. J Virol 87:735–745. doi: 10.1128/JVI.01802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Wilgenburg B, Moore MD, James WS, Cowley SA. 2014. The productive entry pathway of HIV-1 in macrophages is dependent on endocytosis through lipid rafts containing CD4. PLoS One 9:e86071. doi: 10.1371/journal.pone.0086071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janas AM, Dong C, Wang JH, Wu L. 2008. Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry. Virology 375:442–451. doi: 10.1016/j.virol.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izquierdo-Useros N, Esteban O, Rodriguez-Plata MT, Erkizia I, Prado JG, Blanco J, García-Parajo MF, Martinez-Picado J. 2011. Dynamic imaging of cell-free and cell-associated viral capture in mature dendritic cells. Traffic (Copenhagen, Denmark) 12:1702–1713. doi: 10.1111/j.1600-0854.2011.01281.x. [DOI] [PubMed] [Google Scholar]
- 51.Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, Piguet V. 2011. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood 118:4841–4852. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jolly C, Mitar I, Sattentau QJ. 2007. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol 81:13916–13921. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 54.Nejmeddine M, Bangham CR. 2010. The HTLV-1 virological synapse. Viruses 2:1427–1447. doi: 10.3390/v2071427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jolly C. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med 199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnard AL, Igakura T, Tanaka Y, Taylor GP, Bangham CR. 2005. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood 106:988–995. doi: 10.1182/blood-2004-07-2850. [DOI] [PubMed] [Google Scholar]
- 57.Lagaudriere-Gesbert C, Lebel-Binay S, Hubeau C, Fradelizi D, Conjeaud H. 1998. Signaling through the tetraspanin CD82 triggers its association with the cytoskeleton leading to sustained morphological changes and T cell activation. Eur J Immunol 28:4332–4344. doi:. [DOI] [PubMed] [Google Scholar]
- 58.Jolly C, Sattentau QJ. 2007. Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J Virol 81:7873–7884. doi: 10.1128/JVI.01845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jolly C, Sattentau QJ. 2005. Human immunodeficiency virus type 1 virological synapse formation in T cells requires lipid raft integrity. J Virol 79:12088–12094. doi: 10.1128/JVI.79.18.12088-12094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aggarwal A, Iemma TL, Shih I, Newsome TP, Samantha M, Cunningham AL, Turville SG. 2012. Mobilization of HIV spread by diaphanous 2 dependent filopodia in infected dendritic cells. PLoS Pathog 8:e1002762. doi: 10.1371/journal.ppat.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol 10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 62.Van Prooyen N, Gold H, Andresen V, Schwartz O, Jones K, Ruscetti F, Lockett S, Gudla P, Venzon D, Franchini G. 2010. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc Natl Acad Sci U S A 107:20738–20743. doi: 10.1073/pnas.1009635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Prooyen N, Andresen V, Gold H, Bialuk I, Cynthia P-M, Franchini G. 2010. Hijacking the T-cell communication network by the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) p12 and p8 proteins. Mol Aspects Med 31:333–343. doi: 10.1016/j.mam.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chevalier SA, Turpin J, Cachat A, Afonso PV, Gessain A, Brady JN, Pise-Masison CA, Mahieux R. 2014. Gem-induced cytoskeleton remodeling increases cellular migration of HTLV-1-infected cells, formation of infected-to-target T-cell conjugates and viral transmission. PLoS Pathog 10:e1003917. doi: 10.1371/journal.ppat.1003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu K, Bottazzi ME, de la Fuente C, Deng L, Gitlin SD, Maddukuri A, Dadgar S, Li H, Vertes A, Pumfery A, Kashanchi F. 2004. Protein profile of tax-associated complexes. J Biol Chem 279:495–508. doi: 10.1074/jbc.M310069200. [DOI] [PubMed] [Google Scholar]
- 66.Jolly C. 2010. T cell polarization at the virological synapse. Viruses 2:1261–1278. doi: 10.3390/v2061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nejmeddine M, Barnard AL, Tanaka Y, Taylor GP, Bangham CR. 2005. Human T-lymphotropic virus, type 1, tax protein triggers microtubule reorientation in the virological synapse. J Biol Chem 280:29653–29660. doi: 10.1074/jbc.M502639200. [DOI] [PubMed] [Google Scholar]
- 68.Lehmann M, Nikolic DS, Piguet V. 2011. How HIV-1 takes advantage of the cytoskeleton during replication and cell-to-cell transmission. Viruses 3:1757–1776. doi: 10.3390/v3091757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 70.Majorovits E, Nejmeddine M, Tanaka Y, Taylor GP, Fuller SD, Bangham CR. 2008. Human T-lymphotropic virus-1 visualized at the virological synapse by electron tomography. PLoS One 3:e2251. doi: 10.1371/journal.pone.0002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghez D, Lepelletier Y, Lambert S, Fourneau JM, Blot V, Janvier S, Arnulf B, van Endert PM, Heveker N, Pique C, Hermine O. 2006. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J Virol 80:6844–6854. doi: 10.1128/JVI.02719-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takenouchi N, Jones KS, Lisinski I, Fugo K, Yao K, Cushman SW, Ruscetti FW, Jacobson S. 2007. GLUT1 is not the primary binding receptor but is associated with cell-to-cell transmission of human T-cell leukemia virus type 1. J Virol 81:1506–1510. doi: 10.1128/JVI.01522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. 2009. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Groppelli E, Starling S, Jolly C. 2015. Contact-induced mitochondrial polarization supports HIV-1 virological synapse formation. J Virol 89:14–24. doi: 10.1128/JVI.02425-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol 83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pais-Correia AM, Sachse M, Guadagnini S, Robbiati V, Lasserre R, Gessain A, Gout O, Alcover A, Thoulouze MI. 2010. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat Med 16:83–89. doi: 10.1038/nm.2065. [DOI] [PubMed] [Google Scholar]
- 77.Rizkallah G, Mahieux R, Dutartre H. 2015. Intercellular transmission of HTLV-1: not all mechanisms have been revealed. Med Sci (Paris) 31:629–637. doi: 10.1051/medsci/20153106016. [DOI] [PubMed] [Google Scholar]
- 78.Chevalier SA, Durand S, Dasgupta A, Radonovich M, Cimarelli A, Brady JN, Mahieux R, Pise-Masison CA. 2012. The transcription profile of Tax-3 is more similar to Tax-1 than Tax-2: insights into HTLV-3 potential leukemogenic properties. PLoS One 7:e41003. doi: 10.1371/journal.pone.0041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jolly C, Welsch S, Michor S, Sattentau QJ. 2011. The regulated secretory pathway in CD4(+) T cells contributes to human immunodeficiency virus type-1 cell-to-cell spread at the virological synapse. PLoS Pathog 7:e1002226. doi: 10.1371/journal.ppat.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dale BM, McNerney GP, Thompson DL, Hubner W, de Los Reyes K, Chuang FY, Huser T, Chen BK. 2011. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe 10:551–562. doi: 10.1016/j.chom.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Durham ND, Chen BK. 2015. HIV-1 cell-free and cell-to-cell infections are differentially regulated by distinct determinants in the Env gp41 cytoplasmic tail. J Virol 89:9324–9337. doi: 10.1128/JVI.00655-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. 2012. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog 8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schiffner T, Sattentau QJ, Duncan CJ. 2013. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine 31:5789–5797. doi: 10.1016/j.vaccine.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 84.Beaumont E, Vendrame D, Verrier B, Roch E, Biron F, Barin F, Mammano F, Brand D. 2009. Matrix and envelope coevolution revealed in a patient monitored since primary infection with human immunodeficiency virus type 1. J Virol 83:9875–9889. doi: 10.1128/JVI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brandenberg OF, Rusert P, Magnus C, Weber J, Boni J, Gunthard HF, Regoes RR, Trkola A. 2014. Partial rescue of V1V2 mutant infectivity by HIV-1 cell-cell transmission supports the domain's exceptional capacity for sequence variation. Retrovirology 11:75. doi: 10.1186/s12977-014-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grigorov B, Arcanger F, Roingeard P, Darlix JL, Muriaux D. 2006. Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines. J Mol Biol 359:848–862. doi: 10.1016/j.jmb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 87.Pelchen-Matthews A, Kramer B, Marsh M. 2003. Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol 162:443–455. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raposo G, Moore M, Innes D, Leijendekker R, Leigh-Brown A, Benaroch P, Geuze H. 2002. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic 3:718–29. doi: 10.1034/j.1600-0854.2002.31004.x. [DOI] [PubMed] [Google Scholar]
- 89.Sundquist WI, Krausslich HG. 2012. HIV-1 assembly, budding, and maturation. Cold Spring Harb Perspect Med 2:a006924. doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Urata S, Yokosawa H, Yasuda J. 2007. Regulation of HTLV-1 Gag budding by Vps4A, Vps4B, and AIP1/Alix. Virol J 4:66. doi: 10.1186/1743-422X-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorjbal B, Derse D, Lloyd P, Soheilian F, Nagashima K, Heidecker G. 2011. The role of ITCH protein in human T-cell leukemia virus type 1 release. J Biol Chem 286:31092–31104. doi: 10.1074/jbc.M111.259945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dorweiler IJ, Ruone SJ, Wang H, Burry RW, Mansky LM. 2006. Role of the human T-cell leukemia virus type 1 PTAP motif in Gag targeting and particle release. J Virol 80:3634–3643. doi: 10.1128/JVI.80.7.3634-3643.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blot V, Perugi F, Gay B, Prevost MC, Briant L, Tangy F, Abriel H, Staub O, Dokhelar MC, Pique C. 2004. Nedd4.1-mediated ubiquitination and subsequent recruitment of Tsg101 ensure HTLV-1 Gag trafficking towards the multivesicular body pathway prior to virus budding. J Cell Sci 117:2357–2367. doi: 10.1242/jcs.01095. [DOI] [PubMed] [Google Scholar]
- 94.Russell RA, Martin N, Mitar I, Jones E, Sattentau QJ. 2013. Multiple proviral integration events after virological synapse-mediated HIV-1 spread. Virology 443:143–149. doi: 10.1016/j.virol.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Agosto LM, Uchil PD, Mothes W. 2015. HIV cell-to-cell transmission: effects on pathogenesis and antiretroviral therapy. Trends Microbiol 23:289–295. doi: 10.1016/j.tim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Monel B, Beaumont E, Vendrame D, Schwartz O, Brand D, Mammano F. 2012. HIV cell-to-cell transmission requires the production of infectious virus particles and does not proceed through env-mediated fusion pores. J Virol 86:3924–3933. doi: 10.1128/JVI.06478-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bosch B, Grigorov B, Senserrich J, Clotet B, Darlix JL, Muriaux D, Este JA. 2008. A clathrin-dynamin-dependent endocytic pathway for the uptake of HIV-1 by direct T cell-T cell transmission. Antiviral Res 80:185–193. doi: 10.1016/j.antiviral.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Sloan RD, Kuhl BD, Mesplede T, Munch J, Donahue DA, Wainberg MA. 2013. Productive entry of HIV-1 during cell-to-cell transmission via dynamin-dependent endocytosis. J Virol 87:8110–8123. doi: 10.1128/JVI.00815-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Do T, Murphy G, Earl LA, Del Prete GQ, Grandinetti G, Li GH, Estes JD, Rao P, Trubey CM, Thomas J, Spector J, Bliss D, Nath A, Lifson JD, Subramaniam S. 2014. Three-dimensional imaging of HIV-1 virological synapses reveals membrane architectures involved in virus transmission. J Virol 88:10327–10339. doi: 10.1128/JVI.00788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stossel H, Romani N, Piatak M Jr, Lifson JD, Pope M, Cunningham AL. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 101.Clotet-Codina I, Bosch B, Senserrich J, Fernandez-Figueras MT, Pena R, Ballana E, Bofill M, Clotet B, Este JA. 2009. HIV endocytosis after dendritic cell to T cell viral transfer leads to productive virus infection. Antiviral Res 83:94–98. doi: 10.1016/j.antiviral.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez-Plata MT, Puigdomenech I, Izquierdo-Useros N, Puertas MC, Carrillo J, Erkizia I, Clotet B, Blanco J, Martinez-Picado J. 2013. The infectious synapse formed between mature dendritic cells and CD4(+) T cells is independent of the presence of the HIV-1 envelope glycoprotein. Retrovirology 10:42. doi: 10.1186/1742-4690-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jouve M, Sol-Foulon N, Watson S, Schwartz O, Benaroch P. 2007. HIV-1 buds and accumulates in “nonacidic” endosomes of macrophages. Cell Host Microbe 2:85–95. doi: 10.1016/j.chom.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 104.Gaudin R, Berre S, Cunha de Alencar B, Decalf J, Schindler M, Gobert FX, Jouve M, Benaroch P. 2013. Dynamics of HIV-containing compartments in macrophages reveal sequestration of virions and transient surface connections. PLoS One 8:e69450. doi: 10.1371/journal.pone.0069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benaroch P, Billard E, Gaudin R, Schindler M, Jouve M. 2010. HIV-1 assembly in macrophages. Retrovirology 7:29. doi: 10.1186/1742-4690-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Welsch S, Groot F, Krausslich HG, Keppler OT, Sattentau QJ. 2011. Architecture and regulation of the HIV-1 assembly and holding compartment in macrophages. J Virol 85:7922–7927. doi: 10.1128/JVI.00834-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kramer B, Pelchen-Matthews A, Deneka M, Garcia E, Piguet V, Marsh M. 2005. HIV interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol Dis 35:136–142. doi: 10.1016/j.bcmd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Finzi A, Brunet A, Xiao Y, Thibodeau J, Cohen EA. 2006. Major histocompatibility complex class II molecules promote human immunodeficiency virus type 1 assembly and budding to late endosomal/multivesicular body compartments. J Virol 80:9789–9797. doi: 10.1128/JVI.01055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Finzi A, Perlman M, Bourgeois-Daigneault MC, Thibodeau J, Cohen EA. 2013. Major histocompatibility complex class-II molecules promote targeting of human immunodeficiency virus type 1 virions in late endosomes by enhancing internalization of nascent particles from the plasma membrane. Cell Microbiol 15:809–822. doi: 10.1111/cmi.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berre S, Gaudin R, Cunha de Alencar B, Desdouits M, Chabaud M, Naffakh N, Rabaza-Gairi M, Gobert FX, Jouve M, Benaroch P. 2013. CD36-specific antibodies block release of HIV-1 from infected primary macrophages and its transmission to T cells. J Exp Med 210:2523–2538. doi: 10.1084/jem.20130566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. 2007. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol 177:329–341. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mariani C, Desdouits M, Favard C, Benaroch P, Muriaux DM. 2014. Role of Gag and lipids during HIV-1 assembly in CD4(+) T cells and macrophages. Front Microbiol 5:312. doi: 10.3389/fmicb.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sharova N, Swingler C, Sharkey M, Stevenson M. 2005. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J 24:2481–2489. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graziano F, Desdouits M, Garzetti L, Podini P, Alfano M, Rubartelli A, Furlan R, Benaroch P, Poli G. 2015. Extracellular ATP induces the rapid release of HIV-1 from virus containing compartments of human macrophages. Proc Natl Acad Sci U S A 112:E3265–E3273. doi: 10.1073/pnas.1500656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groot F, Welsch S, Sattentau QJ. 2008. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 116.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 117.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135–144. doi: 10.1016/S1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 118.Garcia E, Pion M, Pelchen-Matthews A, Collinson L, Arrighi JF, Blot G, Leuba F, Escola JM, Demaurex N, Marsh M, Piguet V. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 119.Wang JH, Janas AM, Olson WJ, Wu L. 2007. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol 81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Izquierdo-Useros N, Mar N-G, Archer J, Hatch SC, Erkizia I, Blanco J, Borràs FE, Puertas MC, Connor JH, Maria T F-F, Moore L, Clotet B, Gummuluru S, Javier M-P. 2009. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu HJ, Reuter MA, McDonald D. 2008. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog 4:e1000134. doi: 10.1371/journal.ppat.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti A, Kräusslich H-G, Martinez-Picado J. 2012. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol 10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boggiano C, Manel N, Littman DR. 2007. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J Virol 81:2519–2523. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Austyn JM. 1998. Dendritic cells. Curr Opin Hematol 5:3–15. doi: 10.1097/00062752-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 125.Dong C, Janas AM, Wang J-HH, Olson WJ, Wu L. 2007. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virol 81:11352–11362. doi: 10.1128/JVI.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Izquierdo-Useros N, Blanco J, Erkizia I, Fernandez-Figueras MT, Borras FE, Naranjo-Gomez M, Bofill M, Ruiz L, Clotet B, Martinez-Picado J. 2007. Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J Virol 81:7559–7570. doi: 10.1128/JVI.02572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hatch SC, Archer J, Gummuluru S. 2009. Glycosphingolipid composition of human immunodeficiency virus type 1 (HIV-1) particles is a crucial determinant for dendritic cell-mediated HIV-1 trans-infection. J Virol 83:3496–3506. doi: 10.1128/JVI.02249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen DH, Hildreth JE. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol 74:3264–3272. doi: 10.1128/JVI.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ono A, Freed EO. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A 98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S. 2012. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc Natl Acad Sci U S A 109:7475–7480. doi: 10.1073/pnas.1201104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S. 2013. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog 9:e1003291. doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Akiyama H, Ramirez NG, Gudheti MV, Gummuluru S. 2015. CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog 11:e1004751. doi: 10.1371/journal.ppat.1004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pino M, Erkizia I, Benet S, Erikson E, Fernandez-Figueras MT, Guerrero D, Dalmau J, Ouchi D, Rausell A, Ciuffi A, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J, Izquierdo-Useros N. 2015. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology 12:37. doi: 10.1186/s12977-015-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Izquierdo-Useros N, Lorizate M, Contreras FX, Rodriguez-Plata MT, Glass B, Erkizia I, Prado JG, Casas J, Fabrias G, Krausslich HG, Martinez-Picado J. 2012. Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1. PLoS Biol 10:e1001315. doi: 10.1371/journal.pbio.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Felts RL, Narayan K, Estes JD, Shi D, Trubey CM, Fu J, Hartnell LM, Ruthel GT, Schneider DK, Nagashima K, Bess JW Jr, Bavari S, Lowekamp BC, Bliss D, Lifson JD, Subramaniam S. 2010. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A 107:13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jaworski E, Narayanan A, Van Duyne R, Shabbeer-Meyering S, Iordanskiy S, Saifuddin M, Das R, Afonso PV, Sampey GC, Chung M, Popratiloff A, Shrestha B, Sehgal M, Jain P, Vertes A, Mahieux R, Kashanchi F. 2014. Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J Biol Chem 289:22284–22305. doi: 10.1074/jbc.M114.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]