ABSTRACT
Adenovirus is the most prevalent enteric virus in waters worldwide due to its environmental stability, which leads to public health concerns. Mitigation strategies are therefore required. The aim of this study was to assess the inactivation of human adenovirus type 5 (HAdV-5) by gamma radiation in aqueous environments. Various substrates with different organic loads, including domestic wastewater, were inoculated with HAdV-5 either individually or in a viral pool (with murine norovirus type 1 [MNV-1]) and were irradiated in a Cobalt-60 irradiator at several gamma radiation doses (0.9 to 10.8 kGy). The infectivity of viral particles, before and after irradiation, was tested by plaque assay using A549 cells. D10 values (dose required to inactivate 90% of a population or the dose of irradiation needed to produce a 1 log10 reduction in the population) were estimated for each substrate based on virus infectivity inactivation exponential kinetics. The capability of two detection methods, nested PCR and enzyme-linked immunosorbent assay (ELISA), to track inactivated viral particles was also assessed. After irradiation at 3.5 kGy, a reduction of the HAdV-5 titer of 4 log PFU/ml on substrates with lower organic loads was obtained, but in highly organic matrixes, the virus titer reduction was only 1 log PFU/ml. The D10 values of HAdV-5 in high organic substrates were significantly higher than in water suspensions. The obtained results point out some discrepancies between nested PCR, ELISA, and plaque assay on the assessments of HAdV-5 inactivation. These results suggest that the inactivation of HAdV-5 by gamma radiation, in aqueous environments, is significantly affected by substrate composition. This study highlights the virucidal potential of gamma radiation that may be used as a disinfection treatment for sustainable water supplies.
IMPORTANCE Human adenovirus (HAdV) is the most prevalent of the enteric viruses in environmental waters worldwide. The purposes of this study are to provide new insights on the inactivation of enteric virus by gamma irradiation and to introduce new concepts and reinforce the benefits and utility of radiation technologies as disinfection processes. This may be an effective tool to guarantee the reduction of viral pathogens and to contribute to public health and sustainable water supplies.
INTRODUCTION
Human enteric viruses infect and replicate in the gastrointestinal tract of their hosts. Individuals suffering from viral gastroenteritis may excrete about 105 to 1011 viral particles per gram of stool, consisting of various genera of viruses, such as adenovirus (AdV), norovirus (NV), enterovirus, or rotavirus (1). The discharge of inadequately treated sewage effluents is the most common source of enteric viral pathogens in aquatic environments. The persistence of enteric viruses in environmental waters and their tolerance to disinfection often lead to outbreaks of human infections through the contamination of food and drinking and recreational waters (1).
The detection and control of enteric viruses in waters remain challenging. As one of the few enteric viruses with a double-stranded DNA (dsDNA) genome, human adenovirus (HAdV) is known for its high stability and persistence in aquatic environments compared to other viruses (2, 3). The adenovirus is recognized as the most prevalent enteric virus in waters worldwide, which can cause serious implications for public health (4). Adenoviruses are nonenveloped viruses and are about 65 to 80 nm in diameter. The viral genome is a single linear dsDNA molecule (34 to 48 kb) that is surrounded by a complex icosahedral capsid and a nucleoprotein core (5). There are 51 adenovirus serotypes, divided into 6 subgroups (A to F), that can cause infection in humans, including clinical manifestations in the gastrointestinal tract, respiratory tract, and urinary tract as well as in the eyes (6, 7). Viral infections can occur worldwide throughout the year (1). Adenoviruses in drinking and recreational waters are considered to constitute a potential health risk because water may play a meaningful role in the transmission of many of the serotypes, specifically the enteric human adenovirus (HAdV), which is typically transmitted by the fecal-oral route (6, 7, 8). The study of enteric adenoviruses, types 40 and 41, is limited by the complications associated with in vitro conditions. Adenovirus types 2 and 5 (nonenteric types) are more often used in laboratory research. HAdV-2 and HAdV-5 are attractive model systems for laboratory studies since they are more easily assayed in cell culture-based systems and can be grown to higher titers (9, 10).
HAdV can be infectious at low concentrations, and it is documented to be the most UV-resistant virus. HAdV is also resistant in water to monochloramine and other common chemical disinfection methods. Adenoviruses are waterborne opportunistic pathogens that have been detected in tap and treated drinking water, surface water, coastal seawater, swimming pool water, and treated and untreated wastewater (6, 11, 12, 13, 14). Adenovirus outbreaks have been largely associated with water contamination and human consumption. Therefore, HAdVs have emerged as waterborne pathogens of health concern (10, 15).
The development of alternative or complementary disinfection technologies with enhanced efficacy against viruses is important for sustainable water supplies (9). Ionizing radiation has emerged as an alternative method to ensure the safety of drinking water and to reduce the wastewater-linked contamination of fresh food products (16, 17). The explanation for the effect of gamma radiation on viruses is still unknown. It is believed that the mechanism of virus inactivation is based on the damage of nucleic acid strands and viral coat proteins (18). Despite the existence of different molecular and structural targets, the radiosensitivity of viruses seems to be also related to several external factors, such as the composition of the medium. The efficiency of gamma rays seems to be impaired by the presence of solutes (scavengers), which react with free radicals (indirect effect of gamma rays on aqueous solutions), so ionizing irradiation of viruses is less effective in high-protein-content substances (19).
The present work was carried out to contribute to the body of knowledge on the inactivation patterns by gamma irradiation of human adenovirus type 5 (HAdV-5) in different aqueous environments. Seven aqueous irradiation matrices, with different organic loads, were tested to study the influence of the substrate composition on the inactivation of human adenovirus. The substrates were chosen to mimic potential scenarios where gamma radiation may be used as a disinfection tool for human enteric viruses, namely, in drinking, recreational, and wastewater treatments. Furthermore, we investigated the effect of gamma radiation on the cell culture assay of HAdV-5 and the capabilities of two detection methods, nested PCR and enzyme-linked immunosorbent assay (ELISA), to detect the presence of infectious viral particles after inactivation. The intended purposes of this study are to provide new insights on the inactivation of enteric viruses by gamma irradiation and to introduce new concepts on the utility of radiation technologies as effective tools to guarantee the reduction of viral pathogens. This study reports the inactivation patterns of human adenoviruses in different aqueous matrices by gamma radiation.
MATERIALS AND METHODS
Viruses and cell cultures.
Human adenovirus type 5 (HAdV-5; ATCC VR-1516) was propagated in confluent monolayers of human lung carcinoma cells A549 (ATCC CCL-185). Murine norovirus type 1 (MNV-1) strain P3 (kindly provided by Christiane E. Wobus at the University of Michigan Medical School, USA) was propagated in confluent monolayers of mouse macrophages Raw 264.7 (ATCC TIB-71). Cells were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle medium (DMEM; Gibco, Life Technologies, Paisley, United Kingdom) supplemented with 1 mM l-glutamine, 10% fetal bovine serum (FBS) (heat inactivated; Gibco, Life Technologies, Carlsbad, CA, USA), 1 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 1 mM HEPES buffer.
To prepare HAdV-5 and MNV-1 stocks, confluent A549 cells and Raw 264.7 macrophages, respectively, were infected with inocula containing 109 PFU/ml. After 1 h of incubation at 37°C with mild agitation, the cellular monolayer was washed twice with phosphate-buffered saline (PBS) solution, and supplemented DMEM was added. The viruses were harvested after 7 days and 3 days postinfection for HAdV-5 and MNV-1, respectively, by three freeze-thaw cycles at a low centrifugation of 3,000 rpm (Beckman J2-21M, rotor J20-1) for 30 min at 18°C. The resulting supernatants were aliquoted and stored at −80°C.
Sample preparation.
Tap water (pH 7.6) was collected from the laboratory, and deionized filtered water (DI) (pH 8.0) was produced by the Milli-Q system (Millipore). Ten percent and 50% solutions of heat-inactivated fetal bovine serum (FBS) (pH 7.7; Gibco, Life Technologies, Carlsbad, CA, USA) were prepared in phosphate-buffered saline solutions (pH 7.2). Wastewater (pH 5.8) was collected before tertiary treatment from a municipal wastewater treatment plant (Barreiro, Portugal).
Chemical oxygen demand (COD), a routine parameter used to indicate the total organics in water and wastewaters, was measured for tap water, FBS, and wastewater samples before inoculation according to the closed reflux titrimetric method described in reference 20. All of the tested solutions were filter sterilized using a nitrocellulose membrane of 0.2 μm pore size (Sartorius).
HAdV-5 virus stock (1012 PFU/ml) was inoculated into 1 ml of PBS, deionized filtered water, tap water, FBS, aqueous solutions of 10% and 50% of FBS, and wastewater to achieve an inoculation level between 105 and 106 PFU/ml. For the viral pool samples, HAdV-5 virus stock (1012 PFU/ml) and MNV-1 virus stock (108 PFU/ml) were inoculated into 1 ml of tap water, FBS, and wastewater to achieve an inoculation level between 105 and 106 PFU/ml.
Irradiation process.
The irradiations were performed at room temperature in a Cobalt-60 experimental chamber (Precisa 22; Graviner Manufacturing Company Ltd., United Kingdom; 1971) with an activity of 165 TBq (4.45 kCi) and a dose rate of 1.6 kGy/h at Campus Tecnológico e Nuclear (Bobadela, Portugal). The dose rate was determined by Fricke dosimetry (21).
For plaque assays and nested PCR, the spiked samples of deionized filtered water, tap water, and PBS were irradiated to gamma radiation doses of 0.9 kGy to 3.5 kGy; FBS virus suspension samples (100%, 50%, and 10%) were irradiated at 3.2 kGy to 10.8 kGy; and spiked wastewater samples were exposed to gamma radiation doses of 1.0 kGy to 4.5 kGy.
For enzyme-linked immunosorbent assay, the spiked tap water and wastewater samples were irradiated to gamma radiation doses of 1.8 kGy and 3.6 kGy, and FBS virus suspensions were irradiated to gamma radiation doses of 3.6 kGy and 10.7 kGy at a dose rate of 1.6 kGy/h.
Absorbed doses were measured by routine dosimeters (batch X; Amber Perspex Harwell, London) with nominal uncertainty limits of about 2.5% (22). For each set of assayed conditions, one sample was irradiated per gamma radiation dose, and two independent irradiation batches were performed. An average uniformity of dose (maximum dose [Dmax]/minimum dose [Dmin]) of 1.1 was obtained. Nonirradiated spiked samples (0 kGy) followed all of the assays.
HAdV-5 plaque assay.
The HAdV-5 plaque assay was performed in A549 cells. Briefly, cells were seeded into 60-mm plates at a density of 7.5 × 105 cells per plate. After 24 h of incubation at 37°C and 5% CO2, cellular monolayers were infected with 300 μl of 10-fold serial dilutions of nonirradiated and irradiated HAdV-5 samples. Triplicates were made for each sample. Then, they were incubated for 1 h at 37°C and 5% CO2, with mild agitation every 15 min. After removal of the inoculum, cells were overlaid with 3 ml of overlay medium (2× DMEM) with 0.5% agarose (SeaKem ME; Lonza, Rockland, ME, USA). After incubation for 72 h, a second 1.5-ml overlay of 2× DMEM with 0.5% agarose was added. Plaques were subsequently counted 8 to 24 h after a third agarose overlay (1.5 ml) with 1% of a neutral red solution (3.3 g/liter; Sigma, St. Louis, MO, USA). Virus titer was expressed in PFU per milliliter of substrate (PFU/ml).
Adenovirus DNA extraction and nested PCR.
Viral genomic DNA was extracted from nonirradiated and irradiated HAdV-5 suspensions using the PureLink Viral RNA/DNA kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations. A total of 200 μl of unpurified virus sample suspensions (nonirradiated and irradiated) was used for DNA extraction, and the purified nucleic acids were eluted in 15 μl of RNase/DNase-free water. Nested PCR was performed using MyTaq HS Red DNA polymerase (Bioline, London, United Kingdom). Two primers were used to flank an outer 301-bp fragment of the adenovirus hexon gene, Hex1deg 5′-GCC SCA RTG GKC WTA CAT GCA CAT C-3′ and Hex2deg 5′-CAG CAC SCC ICG RAT GTC AAA-3′. The nested primer pair, nehex3deg (5′-GCC CGY GCM ACI GAI ACS TAC TTC-3′) and nehex4deg (5′-CCY ACR GCC AGI GTR WAI CGM RCY TTG TA-3′), produced a 171-bp amplimer (23). The 1st-round reaction was performed in a 50-μl reaction mixture containing 10 μl of reaction buffer 5×, 0.5 μM each primer, 1 U of MyTaq polymerase and 5 μl of DNA template. One-tenth of the PCR mixture was subjected to nested PCR in an identical mixture but with nested primers. The first-round and second-round cycling conditions were as follows: initial temperature of 95°C for 5 min followed by 40 cycles of 95°C for 30 s, 56°C for 30 s, 72°C for 30 s, and a final temperature of 72°C for 5 min. The amplified products were analyzed individually by 2% agarose gel (stained with gel red; Biotium, Hayward, CA, USA) electrophoresis at 80 V.
Enzyme-linked immunosorbent assay.
Enzyme-linked immunosorbent assay (ELISA) was performed using the Ridascreen adenovirus kit (R-Biopharm, Darmstadt, Germany) according to the manufacturer's recommendations. Briefly, HAdV-5 stock was inoculated into 1 ml of tap water, FBS, and wastewater to achieve an inoculation level of approximately 109 PFU/ml. Aliquots of 100 μl of nonirradiated and irradiated unpurified virus suspension samples were used for ELISA protocol. The samples were tested in duplicated. Absorbance was measured by a microplate reader, EZ Read 800 (Biochrom), at 405 nm, using 620 nm as a reference.
Data analysis.
Origin software version 7.5 (OriginLab Corporation, Northampton, MA, USA) was used for data analysis. Virus infectivity determined by plaque assay was expressed as the mean log titer plus or minus the standard error. D10 (measured in kGy), which is the gamma radiation dose required to reduce a virus titer by 1 log10, was calculated from the linear regression model of the log of the surviving fractions. Statistically significant differences for D10 values were evaluated by a parallelism test (24) at a 0.05 significance level. ELISA results were expressed as the mean percentages of the relative binding of antibody HAdV related to nonirradiated samples plus or minus the standard error. These data were subjected to analysis of variance (ANOVA), and significant differences among the means were determined by Tukey's post hoc test at a P of <0.05 significance level.
RESULTS
The inactivation of HAdV-5 by gamma radiation in different aqueous substrates was tracked using three methods: plaque assay, nested PCR, and enzyme-linked immunosorbent assay (ELISA).
Plaque assay infectivity assessment.
When a suspension of a microorganism is irradiated at incremental doses, the number of surviving microorganisms after each incremental dose may be used to construct a dose survival curve, that is, a log variation of the surviving fractions in function of the absorbed radiation dose (kGy). Such radiation survival curves in most cases follow exponential kinetics. Figure 1 shows the logarithmical target viruses' titer reduction measured by plaque assay after irradiation at several gamma radiation doses for each tested substrate.
FIG 1.
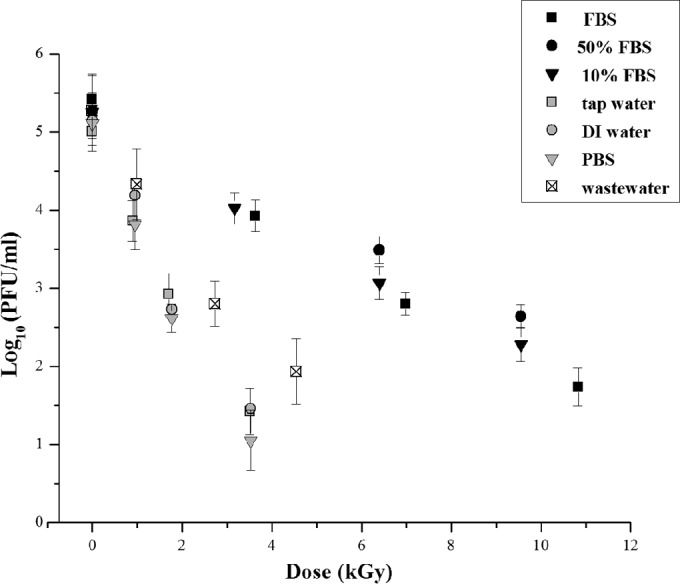
Survival curves to gamma radiation of human adenovirus type 5 (HAdV-5) for several suspension matrices: phosphate-buffered saline (PBS), deionized filtered water (DI water), tap water, 100% solution of fetal bovine serum (FBS), 50% solution of FBS in PBS (50% FBS), 10% solution of FBS in PBS (10% FBS), and wastewater collected from a municipal wastewater treatment plant before tertiary treatment. Error bars correspond to the standard errors about the mean values (n = 6).
Seven aqueous matrices, with different organic loads (<50 mg O2/liter to 60,532 mg O2/liter) (Table 1), were tested in order to find out the influence of substrate composition on the inactivation of human adenovirus. This approach intended to mimic potential application scenarios where gamma radiation can be used as a disinfection process for human enteric viruses, namely, in drinking, recreational (pH 7.2 to 7.8), and wastewater treatment.
TABLE 1.
D10 values of human adenovirus, individually or pooled, in different substrates
| Substrate | COD (mg O2/liter) | D10 values (HAdV-5) ± standard error (kGy)a |
|---|---|---|
| Phosphate-saline buffer, pH 7.2 | <50 | 0.9 ± 0.1 A |
| Deionized filtered water, pH 8.0 | <50 | 0.9 ± 0.1 AB |
| Tap water, pH 7.6 | <50 | 1.0 ± 0.1 AB |
| Tap water + 105 PFU/ml of MNV-1 | NDb | 0.8 ± 0.1 A |
| Wastewater, pH 5.8 | 76 | 1.3 ± 0.1 BC |
| Wastewater + 105 PFU/ml of MNV-1 | ND | 1.6 ± 0.1 C |
| 100% FBS, pH 7.7 | 60,532 | 2.9 ± 0.2 D |
| 100% FBS + 105 PFU/ml of MNV-1 | ND | 2.9 ± 0.1 D |
| 10% FBS | ND | 3.2 ± 0.2 D |
| 50% FBS | ND | 3.6 ± 0.1 D |
The D10 values that have the same letter are not considered significantly different (P < 0.05).
ND, not determined.
The inactivation of HAdV-5 by gamma radiation in the analyzed matrices appeared to follow exponential inactivation kinetics, although the virucidal point turned out to be quite different among the matrices. A maximum reduction of 4 log PFU/ml of HAdV-5 titers was obtained after irradiation of PBS, deionized filtered water, and tap water viral suspensions at 3.5 kGy. At this radiation dose, only 1 log PFU/ml reduction of HAdV-5 infectivity was observed for the inoculated FBS solutions. It was necessary to increase the dose to 10 kGy to obtain a 4 log PFU/ml decrease in the virus titer. However, HAdV-5 in wastewater presented an intermediate radioresistance corresponding to a reduction of 2 log PFU/ml after irradiation at 3 kGy. Therefore, the virus demonstrated distinct patterns of radioresistance due to the different matrices: tap water (sensitive), wastewater (intermediate), and FBS (resistant).
In line with simulating potential actual scenarios and because the virus can be mixed together with other enteric viruses in the environment, the infectivity of HAdV-5 was evaluated in the presence of murine norovirus type 1 (MNV-1) as a human norovirus surrogate (viral pool) (25). According to the literature, human adenoviruses (HAdVs) are present at a higher frequency in sewage than are other enteric viruses (26, 27). Considering this data, a potential worst-case scenario was assayed where other enteric viruses coexist at the same concentration as HAdVs. As such, survival of gamma radiation doses of adenovirus strain pooled with MNV-1 was analyzed. The pooled viral suspensions were inoculated in tap water, wastewater, and FBS, which demonstrated distinct patterns of radioresistance for the nonpool cases.
When HAdV-5 and MNV-1 were inoculated together into the different matrices, the level of inactivation of HAdV-5 was similar to that seen in the samples spiked with HAdV-5 alone (Fig. 2). Namely, after irradiation at 3.5 kGy, reductions of 4 log PFU/ml, 2 log PFU/ml, and 1 log PFU/ml were obtained for tap water, wastewater, and FBS suspensions, respectively. Therefore, the irradiation of adenovirus particles in a viral pool with MNV-1 seems to have no significant influence on the resistance of HAdV-5 to gamma radiation.
FIG 2.
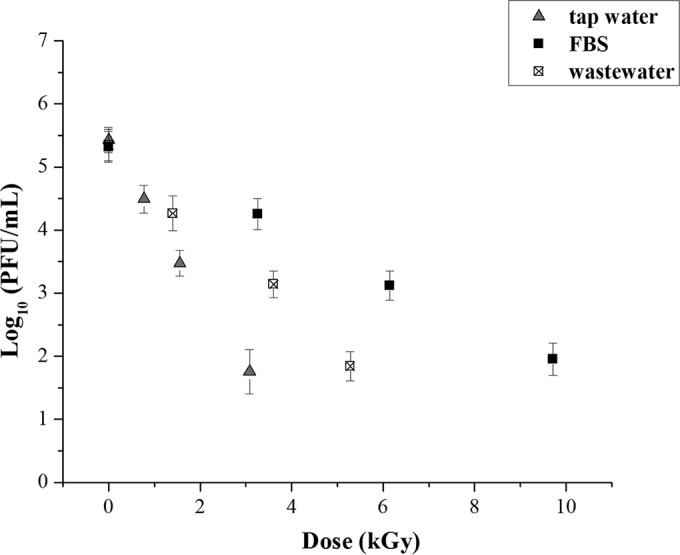
Survival curves to gamma radiation of human adenovirus type 5 (HAdV-5) in the presence of murine norovirus type 1 (MNV-1, human norovirus surrogate) for three suspension substrates: tap water, fetal bovine serum (FBS), and wastewater before tertiary treatment from a municipal wastewater treatment plant. Error bars correspond to the standard errors about the mean values (n = 6).
In order to characterize organisms by their radiation sensitivity, the D10 value is used. This is defined as the dose required to inactivate 90% of a population or the dose of irradiation needed to produce a 1 log10 reduction in the population. It can be calculated using the reciprocal of the slope of the regression linear model of the survival curve. Table 1 shows the calculated D10 values for the human adenovirus strain in the different analyzed conditions.
The calculated D10 values of HAdV-5 ranged from 0.8 kGy (tap water + MNV-1) to 3.6 kGy (50% FBS). The performed statistical analyses of survival curves using the parallelism test indicated that the D10 values of HAdV-5 were not significantly different (P < 0.05) between PBS, deionized filtered water, and tap water. However, the obtained D10 values for these matrices differ significantly (P > 0.05) from those estimated for FBS. The D10 values of HADV-5 in FBS suspensions were not significantly affected by FBS concentration. The D10 values determined for spiked wastewater were significantly different (P > 0.05) from the PBS and FBS suspensions. The presence of another enteric virus (MNV-1) did not appear to have a significant sensitizing or protective effect on the HAdV-5 response to gamma radiation in the matrices tested.
Nested PCR assessment.
PCR has become the most well-established method for detecting or identifying enteric viruses in a very large set of biological and environmental samples (23). In this study, PCR detection of HAdV-5 was assessed to track the inactivation of the virus in suspensions treated by gamma irradiation. Figure 3 presents the representative results of the final products of the nested PCR, which amplifies part of the coding region of the hexon gene. As shown in Fig. 3A, in the 1st round of nested PCR, the intensity of the amplified fragment (301 bp) is similar for all of the nonirradiated samples (untreated) despite the inoculated substrate. This fact can indicate the absence of inhibitory effects of the technique in the tested matrices. With an increase in the absorbed gamma radiation dose, a decrease was observed in the intensity of the amplicon compared with the untreated controls. However, with FBS viral suspensions, the intensity of the amplification signal seems to be equivalent for spiked FBS suspensions irradiated at 3 kGy and 10 kGy. Moreover, the signal was only detected in the first round of nested PCR in nonirradiated tap water samples containing virus.
FIG 3.
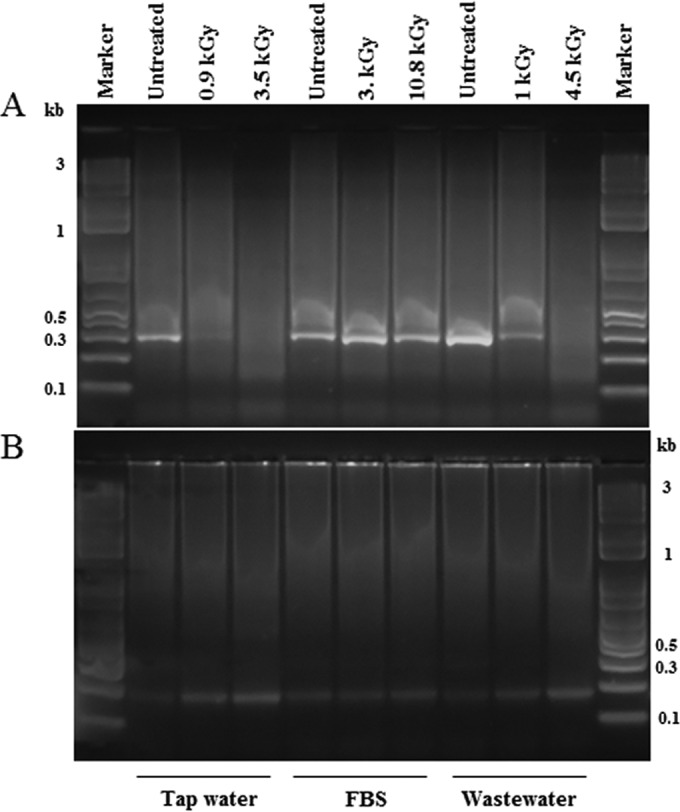
Amplification products of the nested PCR of extracted genomic DNA from HAdV-5 suspensions in tap water, FBS, and wastewater untreated and treated with several doses of gamma radiation (0.9 kGy to 10.8 kGy). (A) Detection of a 301-bp fragment of the hexon gene (1st round of amplification) of HAdV-5. (B) Detection of a 171-bp fragment of the hexon gene (2nd round of amplification) of HAdV-5.
After the 2nd round of nested PCR, amplification yielded positive results for all of the tested samples with the amplification of a 171-bp hexon fragment (Fig. 3B). All of the amplified products appeared to have similar intensities, including the ones resulting from irradiated samples.
ELISA assessment.
The presence and the infectious potential of HAdV on irradiated suspensions were also assessed by a commercial ELISA, which employs a monoclonal antibody (MAb) to the hexon antigen of human adenovirus types. The results are shown in Fig. 4. The relative binding of the HAdV monoclonal antibody (HAdV MAb) was calculated based on the obtained values for nonirradiated control samples, which were considered to correspond with 100% of binding HAdV MAbs. The detection of HAdV-5 viral particles in irradiated FBS suspensions was significantly different (P > 0.05) from the control. This result may indicate the inactivation of HAdV-5 virions by gamma radiation, although no significant difference (P < 0.05) was observed between the detectable signals of FBS-spiked suspensions irradiated at either 3.6 kGy and 10.7 kGy. The recognition signals of HAdV-5 decreased significantly (P > 0.05) with the gamma radiation dose applied to tap water and wastewater viral suspensions and were not detected after irradiation at 3.6 kGy (negative result according to the manufacturer's instructions). Moreover, the detection signals of HAdV-5 viral particles by ELISA for the same dose of 3.6 kGy were significantly different (P > 0.05) between the three tested irradiation matrices. The wastewater substrate seemed to provide an intermediate level of protection to the viral hexon protein against radiolysis compared to tap water and FBS, which presented sensitizing and radioprotective effects, respectively.
FIG 4.
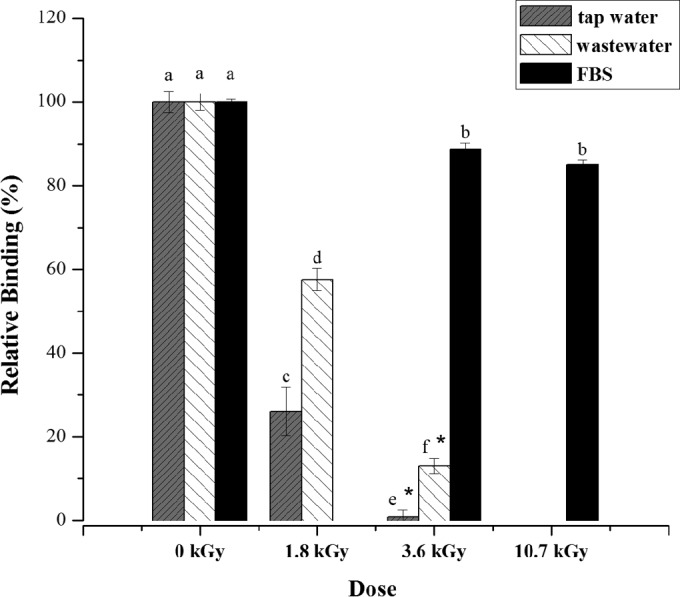
Qualitative detection by ELISA using a monoclonal antibody to the hexon antigen of human adenovirus types of HAdV-5 suspensions on FBS, tap water, and wastewater that were untreated (0 kGy control) and treated with different doses of gamma radiation (1.8 kGy, 3.6 kGy, and 10.7 kGy). Bars represent the relative percentage to control HAdV-5 antibody binding. Error bars correspond to the standard errors about the mean values of three replicates (n = 3). *, negative result according to the manufacturer's instructions. Values with the same letter are not significantly different at a P value of <0.05.
DISCUSSION
Inactivation patterns of human adenovirus.
In this study, we document new evidence about the reduction of viral contaminants in aqueous environments by gamma radiation, which is to be further used as a disinfection treatment. Specifically, this work generated data about the inactivation of human adenovirus in different aqueous matrices by gamma radiation. The selected matrices were used as potential application scenarios, where irradiation can potentially be used to mitigate the presence of enteric viruses, specifically in drinking and recreational waters as well as in wastewaters. However, at the same time, the matrices should not be very complex in order to correctly identify the factors that influence the inactivation of the target virus. The results from the infectivity assessment indicated that, for all of the irradiated viral suspensions, the number of infectious virions decreased linearly with an increasing gamma radiation dose (Fig. 1). Gamma rays have direct and indirect effects on target molecules. They can directly “hit” the molecule or interfere indirectly via free oxygen radicals formed after water radiolysis (28, 29). Radioresistance is assumed to be inversely proportional to the size and complexity of the organism. A large target is more sensitive to ionizing radiation than a smaller one. Viruses have very small genomes (compared to bacteria and fungi), resulting in higher resistance to ionizing radiation than bacterial pathogens (17). To the best of our knowledge, there is only one study that cites D10 values for adenoviruses (30). That study demonstrated that adenoviruses 3, 5, and 12 suspended in Eagle's minimal medium (MEM) plus 2% FBS required 4.9, 4.4, and 4.6 kGy, respectively, to achieve 1 log reduction. These D10 values are much higher than the ones that we document in this study. This difference may be attributed to the fact that the former experiments were conducted at much lower temperatures (0.5°C). Low temperatures are known to have an increasing effect on radiation resistance (31).
As shown in Fig. 1, the survival rates of HAdV-5 subjected to gamma radiation differ among the tested virus suspension matrices. These differences indicate the critical influence of the matrix on the radioprotection of the viral particle. The results obtained for matrices with higher organic loads (highly scavenged systems) like FBS solutions (COD of 60,532 mg O2/liter) suggested that these matrices offer a more protective environment for the viral particles with respect to gamma radiation. The organic load of the substrate may play a protective role, acting like a scavenger, mainly by diminishing the indirect effect of gamma rays on the viral particle. In water viral suspensions, the effect of gamma radiation in decreasing the infectivity of HAdV-5 was more pronounced. This can be explained by considering that tap water, deionized filtered water, and PBS represent substrates with organic matter at levels that are too low (COD < 50 mg O2/liter) to allow scavenging or protect against radiolysis.
Inadequately treated wastewaters are among the most important means of environmental contamination with enteric viruses like HAdV (32, 33). Adenoviruses are resistant to the disinfection methods that are widely used in municipal wastewater treatment plants (6, 10, 13, 34). It is fundamental to understand the behavior of these viruses under alternative disinfection technologies, such as gamma radiation. With this aim, the infectivity of HAdV-5 was assessed following increasing gamma radiation doses in municipal wastewater collected before tertiary treatment (since gamma radiation is intended to be applied as a disinfection tertiary treatment). Also, in an attempt to mimic a real scenario, the variation of HAdV-5 radioresistance in a viral pool with MNV-1, as a human norovirus surrogate (25), was studied. The intermediate organic load of wastewater (75.7 mg O2/liter), compared with FBS and deionized filtered water, creates an in-between context of viral sensitivity to gamma radiation as well. The obtained results indicate that the presence of another enteric virus seems to have no influence on the HAdV-5 response to gamma radiation.
Viral concentrations of 5,000 to 100,000 PFU/liter are commonly reported in raw sewage and may be decreased during treatment by only 2 log10 to 3 log10 (35). Despite this reduction in the viral load of wastewater, the infectious potential of the remaining enteric virus and its ability to persist in the environment do not diminish its risk. The present study indicates that it is possible to achieve higher viral reduction rates with gamma radiation. The criterion normally set for virucidal efficacy is a 99.9%, 99.99%, or 99.999% reduction in viral load (36). Extrapolating to a potential application as a disinfection treatment for tap water and wastewater, the data obtained showed that virus titers can be effectively reduced by 4 log10 PFU/ml (virucidal efficacy of 99.99%) using gamma radiation doses of 4 kGy and 6 kGy, respectively. Based on previous studies, the 6-kGy dose may also attain the elimination of the coliform load in municipal wastewater (37). Studies on other irradiation disinfection technologies have shown that a UV dose (UV fluence) of about 200 mJ/cm2 from a low pressure (LP) UV source (emitting at 253.7 nm) or the addition of 10 mg/liter H2O2 enhancing the UV-induced inactivation with a dose of 120 mJ/cm2 would also achieve a 4 log reduction in adenovirus. This 4-log inactivation of adenovirus (set by U.S. authorities as the required goal for groundwater under LP UV) (38) can be reached by using irradiation at a dose of 6 kGy as a single treatment process without adding any chemicals, with the added benefit of increasing the water quality (39).
Detection methods to track adenovirus inactivation.
Virion detection methods are important to ensure our wellbeing, investigate outbreaks, and devise preventive measures (40). Cell culture-based methods, such as plaque assays, are classical techniques commonly used to detect and quantify infectious viruses in environmental samples as well as to analyze the viability of viruses after treatment with disinfection agents. However, they are time-consuming, and not all viruses produce clear cytopathic effects (CPEs) or plaques as occurs with some adenovirus serotypes (41). Alternative methods to quantify and detect infectious enteric viruses in environmental samples should be assessed. The applied PCR and ELISA detection methods are used as molecular detection methods in diagnostic and viral identification from environmental samples (13, 14, 42, 43). In this study, the capabilities of these simpler methods to track the inactivation of an adenovirus strain by gamma radiation were evaluated. The results of this study point out some discrepancies between nested PCR and plaque assay assessments. For example, negative results in the first round of nested PCR were obtained for tap water and wastewater viral suspensions irradiated at 3.5 kGy and 4.5 kGy, respectively, that the plaque assay was able to detect at an approximately 1 log10 PFU/ml viral titer. Nevertheless, the implementation of a second round of amplification (2nd-round nested PCR) showed this PCR assay to be more specific and to increase the accuracy of the method in the detection of HAdV in aqueous samples. Despite the gradual inactivation of HAdV by gamma radiation detected by plaque assay, the final PCR results do not show any difference between nonirradiated and irradiated samples, even at the highest applied gamma radiation doses. Still, our results confirm the utility of nested PCR techniques as tools to determine the presence of adenoviruses in final effluent samples (33). However, the detection of enteric viruses in the environment by PCR must be performed with caution because the presence of genomic copies may not be necessarily associated with infectious virus particles (27) as was observed in this study.
The results obtained by ELISA through the detection of the viral capsid hexon protein, which are the negative results (indicative of absence of viral particles) measured for tap water and wastewater samples irradiated at 3.6 kGy, did not correspond to those achieved by plaque assays or nested PCR. The results suggested a structural alteration of the hexon protein of the HAdV capsid by gamma radiation treatment of the water viral suspensions. This damage can have a major influence on the external and more exposed regions of the viral proteins that disable interactions with the anti-hexon antibodies, but it does not interfere with the infectious potential of the virus, detected by plaque assay. Human adenoviruses are very complex particles. The viral nucleocapsid is composed mostly by hexon, penton, and fiber proteins organized in an intricate network. Each one of these proteins plays an important role in adenovirus replication (9). Generally, damage to the viral capsid can result in the loss of its capacity to protect the viral genome and its ability to replicate in the host (44). Alteration in the adenovirus hexon protein may alter the shape of the nucleocapsid and interfere negatively with the virus' life cycle (2). The potential alterations of the capsid proteins detected by ELISA seem like they do not correlate with the loss of infectivity with the increase of gamma radiation dose estimated by plaque assay. Since ELISA signals are semiquantitative, the estimated relative binding (%) may not be linearly correlated with the infectivity loss measured by plaque assay, although the influence of the suspending matrices on the HAdV-5 response to gamma radiation was noticed in both methods.
Conclusions.
This study shows that gamma radiation has virucidal activity against HAdV-5 in the tested conditions. Moreover, among the tested methods of plaque assay, nested PCR, and ELISA, the first technique (plaque assay) was the only one that allowed tracking of the inactivation of the adenovirus strain by gamma radiation. Considering the tested conditions, the adenovirus strain turned out to be significantly more sensitive to radiation when suspended in water (radiosensitizing matrices) than in organic-loaded aqueous solutions (radioprotective matrices). Since part of the effect of ionizing radiation on an organism is due to indirect action mediated through radicals (resultant from the radiochemical reactions in the suspending matrices), the nature of the medium in which the virus is suspended can play an important role in determining the dose required for a given biocidal effect. This study of HAdV inactivation patterns in different matrices can open new insights into the virucidal mechanisms of gamma radiation, with outcomes for safe, improved, and unique applications for virus inactivation (e.g., wastewater treatment, vaccines, and drug deliverables). Furthermore, this work may introduce new concepts to reinforce the benefits of radiation technologies as an effective mitigation tool to contribute to public health and sustainable water supplies.
REFERENCES
- 1.Okoh AI, Sibanda T, Gusha SS. 2010. Inadequately treated wastewater as a source of human enteric viruses in the environment. Int J Environ Res Public Health 7:2620–2637. doi: 10.3390/ijerph7062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosshard F, Armand F, Hamelin R, Kohn T. 2013. Mechanisms of human adenovirus inactivation by sunlight and UVC light as examined by quantitative PCR and quantitative proteomics. Appl Environ Microbiol 79:1325–1332. doi: 10.1128/AEM.03457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Lázaro D, Cook N, Ruggeri FM, Sellwood J, Nasser A, Nascimento MS, D'Agostino M, Santos R, Saiz JC, Rzeżutka A, Bosch A, Gironés R, Carducci A, Muscillo M, Kovač K, Diez-Valcarce M, Vantarakis A, von Bonsdorff CH, de Roda Husman AM, Hernández M, van der Poel WH. 2012. Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev 36:786–814. doi: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslan A, Xagoraraki I, Simmons FJ, Rose JB, Dorevitch S. 2011. Occurrence of adenovirus and other enteric viruses in limited-contact freshwater recreational areas and bathing waters. J Appl Microbiol 111:1250–1261. doi: 10.1111/j.1365-2672.2011.05130.x. [DOI] [PubMed] [Google Scholar]
- 5.Nemerow GR, Pache L, Reddy V, Stewart PL. 2009. Insights into adenovirus host cell interactions from structural studies. Virology 384:380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Heerden J, Ehlers MM, Grabow WO. 2005. Detection and risk assessment of adenoviruses in swimming pool water. J Appl Microbiol 99:1256–1264. doi: 10.1111/j.1365-2672.2005.02607.x. [DOI] [PubMed] [Google Scholar]
- 7.van Heerden J, Ehlers MM, Vivier JC, Grabow WO. 2005. Risk assessment of adenoviruses detected in treated drinking water and recreational water. J Appl Microbiol 99:926–933. doi: 10.1111/j.1365-2672.2005.02650.x. [DOI] [PubMed] [Google Scholar]
- 8.Pedley S, Pond K. 2003. Emerging issues in water and infectious diseases. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Page MA, Shisler JL, Mariñas BJ. 2010. Mechanistic aspects of adenovirus serotype 2 inactivation with free chlorine. Appl Environ Microbiol 76:2946–2454. doi: 10.1128/AEM.02267-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eischeid AC, Meyer JN, Linden KG. 2009. UV disinfection of adenoviruses: molecular indications of DNA damage efficiency. Appl Environ Microbiol 75:23–28. doi: 10.1128/AEM.02199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong TT, Griffin DW, Lipp EK. 2005. Molecular assays for targeting human and bovine enteric viruses in coastal waters and their application for library-independent source tracking. Appl Environ Microbiol 71:2070–2078. doi: 10.1128/AEM.71.4.2070-2078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang SC. 2006. Human adenoviruses in water: occurrence and health implications: a critical review. Environ Sci Technol 40:7132–7140. doi: 10.1021/es060892o. [DOI] [PubMed] [Google Scholar]
- 13.Papapetropoulou M, Vantarakis AC. 1998. Detection of adenovirus outbreak at a municipal swimming pool by nested PCR amplification. J Infect 36:101–103. doi: 10.1016/S0163-4453(98)93414-4. [DOI] [PubMed] [Google Scholar]
- 14.Vantarakis AC, Papapetropoulou M. 1999. Detection of enteroviruses, adenoviruses and hepatitis A viruses in raw sewage and treated effluents by nested-PCR. Water Air Soil Pollut 114:85–93. doi: 10.1023/A:1005065326395. [DOI] [Google Scholar]
- 15.EPA. 1998. Drinking water contamination candidate list. Notice Fed Reg 63:10274–10287. [Google Scholar]
- 16.Carter MJ. 2005. Enterically infecting viruses: pathogenicity, transmission and significance for food and waterborne infection. J Appl Microbiol 98:1354–1380. doi: 10.1111/j.1365-2672.2005.02635.x. [DOI] [PubMed] [Google Scholar]
- 17.Stefanova R, Vasilev NV, Spassov SL. 2010. Irradiation of food, current legislation framework, and detection of irradiated foods. Food Anal Methods 3:225–252. doi: 10.1007/s12161-009-9118-8. [DOI] [Google Scholar]
- 18.Feng K, Divers E, Ma Y, Li J. 2011. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl Environ Microbiol 77:3507–3517. doi: 10.1128/AEM.00081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limoli CL, Kaplan MI, Giedzinski E, Morgan WF. 2001. Attenuation of radiation-induced genomic instability by free radical scavengers and cellular proliferation. Free Radic Biol Med 31:10–19. doi: 10.1016/S0891-5849(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 20.American Public Health Association/American Water Works Association/Water Environment Federation. Aggregate organic constituents, p 5.13–5.14. In Clesceri LS, Greenberg AE, Eaton AD (ed), Standard methods for the examination of water and wastewater, 20th ed American Public Health Association Press, Washington, DC. [Google Scholar]
- 21.American Society for Testing and Materials. 1992. Practice for using the Fricke reference standard dosimetry system. ASTM E1026 In Annual Book of ASTM Standards; ASTM, Philadelphia, PA. [Google Scholar]
- 22.Whittaker B, Watts M. 2001. The influence of dose rate, ambient temperature and time on the radiation response of Harwell PMMA dosimeters. Radiat Phys Chem 60:101–110. doi: 10.1016/S0969-806X(00)00316-9. [DOI] [Google Scholar]
- 23.Allard A, Albinsson B, Wadell G. 2001. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J Clin Microbiol 39:498–505. doi: 10.1128/JCM.39.2.498-505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottschalk PG, Dunn JR. 2005. Measuring parallelism, linearity, and relative potency in bioassay and immunoassay data. J Biopharm Stat 15:437–463. doi: 10.1081/BIP-200056532. [DOI] [PubMed] [Google Scholar]
- 25.Wobus CE, Thackray LB, Virgin HW IV. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong T-T, Phanikumar MS, Irene Xagoraraki I, Rose JB. 2010. Quantitative detection of human adenoviruses in wastewater and combined sewer overflows influencing a Michigan river. Appl Environ Microbiol 76:715–723. doi: 10.1128/AEM.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pina S, Puig M, Lucena F, Jofre J, Girones R. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl Environ Microbiol 64:3376–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milhøj BO, Sauer SP. 2015. Insight into the mechanism of the initial reaction of an OH radical with DNA/RNA nucleobases: a computational investigation of radiation damage. Chemistry 21:17786–17799. doi: 10.1002/chem.201503107. [DOI] [PubMed] [Google Scholar]
- 29.Sommer R, Pribil W, Appelt S, Gehringer P, Eschweiler H, Leth H, Cabaj A, Haider T. 2001. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: a comparative approach. Water Res 35:3109–3116. doi: 10.1016/S0043-1354(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan R, Fassolitis AC, Larkin EP, Read RB Jr, Peeler JT. 1971. Inactivation of thirty viruses by gamma radiation. Appl Microbiol 22:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen JM, Shaffer HL. 2001. Sterilization and preservation by radiation sterilization, p 729–746. In Block SS. (ed), Disinfection, sterilization, and preservation, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 32.Silva HD, García-Zapata MTA, Anunciacão CE. 2011. Why the use of adenoviruses as water quality virologic marker? Food Environ Virol 3:138–140. doi: 10.1007/s12560-011-9069-2. [DOI] [Google Scholar]
- 33.Symonds EM, Griffin DW, Breitbart M. 2009. Eukaryotic viruses in wastewater samples from the United States. Appl Environ Microbiol 75:1402–1409. doi: 10.1128/AEM.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cromeans TL, Kahler AM, Hill VR. 2010. Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Appl Environ Microbiol 76:1028–1033. doi: 10.1128/AEM.01342-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosh A, Pintó RM, Abad FX. 2006. Survival and transport of enteric viruses in the environment, p 151–187. In Goyal SM. (ed), Viruses in food. Springer, New York, NY. [Google Scholar]
- 36.Poschetto LF, Ike A, Papp T, Mohn U, Böhm R, Marschang RE. 2007. Comparison of the sensitivities of noroviruses and feline calicivirus to chemical disinfection under field-like conditions. Appl Environ Microbiol 73:5494–5500. doi: 10.1128/AEM.00482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabo Verde S, Silva T, Matos P. 2016. Effects of gamma radiation on wastewater microbiota. Radiat Environ Biophys 55:125–131. doi: 10.1007/s00411-015-0617-2. [DOI] [PubMed] [Google Scholar]
- 38.USEPA. 2006. National primary drinking water regulation, long-term 2 enhanced surface water treatment rule. Fed Reg 71:653. [PubMed] [Google Scholar]
- 39.Melo R, Cabo Verde S, Branco J, Botelho ML. 2008. Gamma radiation induced effects on slaughterhouse wastewater treatment. Radiat Phys Chem 77:98–100. doi: 10.1016/j.radphyschem.2007.03.006. [DOI] [Google Scholar]
- 40.Yadav R, Dwivedi S, Kumar S, Chaudhury A. 2010. Trends and perspectives of biosensors for food and environmental virology. Food Environ Virol 2:53–63. doi: 10.1007/s12560-010-9034-5. [DOI] [Google Scholar]
- 41.Calgua B, Barardi CRM, Bofill-Mas S, Rodriguez-Manzano J, Girones R. 2011. Detection and quantitation of infectious human adenoviruses and JC polyomaviruses in water by immunofluorescence assay. J Virol Methods 171:1–7. doi: 10.1016/j.jviromet.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Park SH, Kim EJ, Yun TH, Lee JH, Kim CK, Seo YH, Oh SA, Choi SS, Cho SJ, Kim MS, Han GY, Kim MY, Jeong HS, Cheon DS, Kim HS. 2010. Human enteric viruses in groundwater. Food Environ Virol 2:69–73. [Google Scholar]
- 43.Tao CW, Hsu BM, Kao PM, Huang WC, Hsu TK, Ho YN, Lu YJ, Fan CW. 2016. Seasonal difference of human adenoviruses in a subtropical river basin based on 1-year monthly survey. Environ Sci Pollut Res 23:2928–2936. doi: 10.1007/s11356-015-5501-8. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez RA, Pepper IL, Gerba CP. 2009. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental samples. Appl Environ Microbiol 75:297–307. doi: 10.1128/AEM.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


