ABSTRACT
Several species of the bacterial genus Shewanella are well-known dissimilatory reducers of manganese under anaerobic conditions. In fact, Shewanella oneidensis is one of the most well studied of all metal-reducing bacteria. In the current study, a number of Shewanella strains were tested for manganese-oxidizing capacity under aerobic conditions. All were able to oxidize Mn(II) and to produce solid dark brown manganese oxides. Shewanella loihica strain PV-4 was the strongest oxidizer, producing oxides at a rate of 20.3 mg/liter/day and oxidizing Mn(II) concentrations of up to 9 mM. In contrast, S. oneidensis MR-1 was the weakest oxidizer tested, producing oxides at 4.4 mg/liter/day and oxidizing up to 4 mM Mn(II). Analysis of products from the strongest oxidizers, i.e., S. loihica PV-4 and Shewanella putrefaciens CN-32, revealed finely grained, nanosize, poorly crystalline oxide particles with identical Mn oxidation states of 3.86. The biogenic manganese oxide products could be subsequently reduced within 2 days by all of the Shewanella strains when culture conditions were made anoxic and an appropriate nutrient (lactate) was added. While Shewanella species were detected previously as part of manganese-oxidizing consortia in natural environments, the current study has clearly shown manganese-reducing Shewanella species bacteria that are able to oxidize manganese in aerobic cultures.
IMPORTANCE Members of the genus Shewanella are well known as dissimilatory manganese-reducing bacteria. This study shows that a number of species from Shewanella are also capable of manganese oxidation under aerobic conditions. Characterization of the products of the two most efficient oxidizers, S. loihica and S. putrefaciens, revealed finely grained, nanosize oxide particles. With a change in culture conditions, the manganese oxide products could be subsequently reduced by the same bacteria. The ability of Shewanella species both to oxidize and to reduce manganese indicates that the genus plays a significant role in the geochemical cycling of manganese. Due to the high affinity of manganese oxides for binding other metals, these bacteria may also contribute to the immobilization and release of other metals in the environment.
INTRODUCTION
Members of the genus Shewanella are facultatively anaerobic, Gram-negative bacteria that are found in a wide range of environments but predominately in marine sediments and in association with fish (1, 2). The genus comprises mesophiles, psychrotrophs, and psychrophiles, some of which have been the subject of detailed studies due to their ability to use a wide range of electron acceptors in anaerobic respiration processes (1, 3). In particular, numerous Shewanella species are capable of reducing metals such as Mn(IV), Fe(III), V(V), Cr(VI), and U(VI), coupled to oxidation of organic and inorganic compounds (4–7).
Metal-reducing and metal-oxidizing bacteria are well recognized as playing important roles in the cycling of metals and organic matter in many environments (8–11). The majority of studies of metal reducers have centered on members of the Shewanella and Geobacter genera, even though metal reducers are generally a phylogenetically diverse group of bacteria. Fe(III) and Mn(IV) reducers like Shewanella oneidensis have been investigated extensively, due to their importance in carbon turnover in anoxic environments and their potential in biotechnology processes such as bioremediation (12–14). A wide range of Fe(III) and Mn(IV) forms, from soluble chelated types to poorly crystalline solid minerals, can be both reduced and produced by bacteria, depending on culture conditions. Electron transfer mechanisms in reductive processes have been investigated in detail, revealing a variety of mechanisms that can involve c-type cytochromes, extracellular electron shuttles, and direct interspecies electron transfer (15, 16).
Manganese exists as soluble or particulate Mn(II), Mn(III), or Mn(IV) compounds, with prevailing states being influenced by redox conditions and the presence of manganese-transforming microorganisms (17, 18). Understanding the role of manganese-transforming microorganisms in environmental manganese cycling is important because manganese is the second most abundant metal in the earth's crust, directly influences the cycling of other elements, and is an essential trace element for all living organisms. Similar to reductive processes, bacteria are known to be major contributors to manganese oxidation. The oxidation of Mn(II) to Mn(III/IV) is carried out by both phylogenetically diverse bacteria and fungi originating in a wide range of environments (18).
Numerous studies have indicated the requirement for multicopper oxidase (MCO)-like enzymes in the oxidation of Mn(II) (19, 20). Several model bacteria have been studied in detail, revealing the involvement of genes encoding MCOs, mnxG in spores of Bacillus strain SG-1 (21), cumA in Pseudomonas putida GB-1 (22), mofA in Leptothrix discophora SS-1 (23), and moxA in Pedomicrobium sp. ACM 3067 (24). In addition, laccases and peroxidases have been found to oxidize Mn(II) in fungi and several other bacteria (25–27).
In addition to their involvement in manganese cycling, Mn(II)-oxidizing bacteria are thought to have contributed in large part to the formation of natural manganese deposits around the world, including deep-sea manganese nodules (28, 29) and ore bodies (30, 31). Biogenic manganese oxides also have considerable potential biotechnological applications. The disordered oxide structures, with defects and cation vacancies, can act as depolarizers in electrochemical cells (32). The high affinity of biogenic manganese oxides for binding metals has led to the proposal of such oxides as a means of bioremediating metal contamination in waters and wastewaters (33–36). The current study extends the versatility of Shewanella species and describes manganese oxidation as a trait within the genus. Rates of oxidation, Mn(II) toxicity, and characteristics of the products were determined in the current study.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used for the current study were Shewanella oneidensis MR-1, Shewanella putrefaciens CN-32, Shewanella putrefaciens 200, Shewanella loihica PV-4, and Shewanella denitrificans OS217. Isolates were kindly provided by Kenneth Nealson at the University of Southern California. All strains are psychrotrophic facultative anaerobes and were maintained aerobically in PYE medium (37); PYE medium contained 1.0 g/liter peptone, 1.5 g/liter yeast extract, 7.5 g/liter NaCl, and 1.0 g/liter (NH4)2SO4 in distilled H2O, with 10 mM HEPES buffer (pH 7.5) added after autoclaving. All cultures were incubated on an orbital shaker (120 rpm) under aerobic conditions unless otherwise stated and were grown at 27°C.
Oxidation-reduction experiments.
For manganese oxidation testing, the Shewanella strains were grown to mid-late log phase and 1 ml of each culture, diluted to an optical density at 600 nm (OD600) of 0.1, was inoculated into a 100-ml volume of PYE medium amended with 3 mM MnCl2·4H2O, in 250-ml Erlenmeyer flasks, and incubated at 27°C. Cultures were sampled after 0, 1, 2, 3, 4, 5, 7, 10, 15, and 20 days. Rates of oxide production were determined by measuring the amount of oxide produced at each time point. The extent of oxidation was the conversion of Mn(II) to Mn(IV) at each sampling time. Cell numbers were determined using the most probable number technique. Controls were set up as uninoculated medium and inoculated medium with 15 mM NaN3 added. Controls were sampled for abiotic oxidation at the same time as inoculated cultures. All tests were done in triplicate. After 20 days of incubation, the manganese oxide products were recovered and analyzed. In separate cultures, all seven Shewanella strains were grown for 20 days in PYE medium amended with 3 mM Mn(II). After the manganese oxide was produced, cultures were tested to determine whether the bacteria were able to reduce the oxides. The cultures were transferred aseptically to sterile 125-ml serum bottles, 15 mM sodium lactate was added, and anoxic conditions were created by gassing the cultures and headspaces with sterile N2. Manganese reduction was monitored, and the time taken for complete disappearance of the brown color was noted and confirmed using the leucoberbelin blue assay (38).
The effects of the initial Mn(II) concentration on oxidation by each of the Shewanella strains were tested in PYE medium amended with 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 mM MnCl2·4H2O. The bacteria were grown to mid-late log phase in PYE medium and 1 ml of each culture was inoculated into a 100-ml volume of PYE medium amended with the appropriate concentration of Mn(II), in 250-ml Erlenmeyer flasks. After 20 days of incubation, the amount of manganese oxide produced was measured.
Recovery of oxide products.
The dark brown-black oxides produced were recovered by filtration. The solids were washed once in distilled water and then three times in dilute 0.03 M H2SO4, to remove inorganic residual matter as well as residual Mn(II) that might interfere with further analyses. The oxides were then washed with deionized water until filtrates were acid free.
Physical analyses.
Fourier transform infrared (FTIR) spectroscopy (Spectrum Two FTIR spectrometer; PerkinElmer) was used to tentatively characterize biogenic oxides by comparison with standard analytical reagent-grade MnO2 (Sigma-Aldrich). The FTIR spectroscopy technique for characterization of manganese oxides was well documented in previous studies (39–41).
A JSM-6510LV scanning electron microscope (JEOL USA) was used to visualize the biogenic oxides and analytical reagent-grade MnO2, to compare the structures and to visualize detectable bacteria in the biological samples. Samples were processed with the assistance of Glenn Walker at the Australian National Fabrication Facility, Griffith University (Brisbane, Australia).
Chemical analyses.
The presence of Mn(IV) was detected using the leucocrystal violet assay (42) and the leucoberbelin blue assay (38). Aliquots of 0.1 ml were taken, diluted as necessary, and analyzed using a Shimazdu UV-2550 UV spectrometer, at the appropriate wavelength for each assay. Absorbance values were plotted against a standard curve of known concentrations of potassium permanganate.
The O/Mn ratio of manganese oxide products was determined according to a modified iodometric method described by Murray et al. (43). Approximately 10 mg of each preprepared sample was weighed into a 50-ml beaker. A composite mixture of 10 ml demineralized water, 1 ml 20% (vol/vol) H2SO4, and 1 ml alkaline NaI solution (comprising 32 g NaOH and 60 g NaI in 100 ml demineralized water) was added to the sample and left for 18 h. Following this, the sample was filtered into a 100-ml beaker using a Millex GS 0.45-μm microfilter. A 1-ml aliquot of 1% (wt/vol) starch solution was added to the filtrate. To determine the total oxidizing equivalents, the I2 liberated from each sample was titrated with 5 mM sodium thiosulfate, using a 50-ml burette. The titration was deemed complete when the blue color dissipated. The solution was brought up to 100 ml with demineralized water in a volumetric flask, and the manganese concentration was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) (Optima 8300 ICP-OES system; PerkinElmer). The average oxidation state was calculated and presented as MnOx, where x is the aggregate average across all oxide states.
RESULTS
Manganese oxidation by Shewanella species.
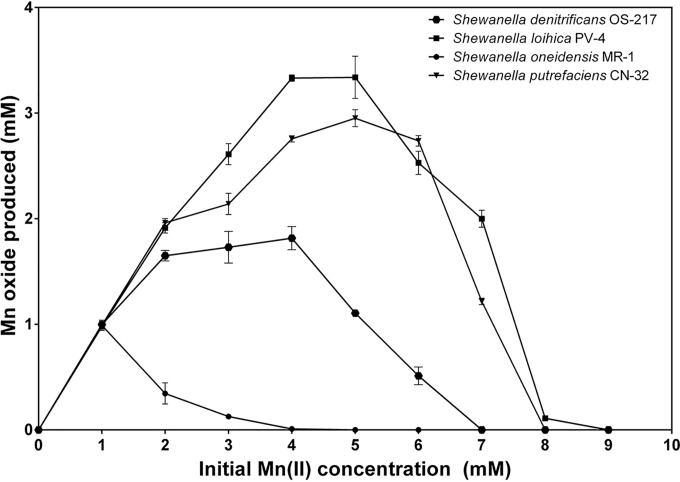
Oxidation of Mn(II) was investigated in five strains of Shewanella at 27°C (Table 1). All strains tested were able to oxidize Mn(II) to Mn(IV) under aerobic conditions, producing finely grained dark brown precipitates. The rates of oxidation varied considerably, with S. loihica being the most efficient and producing oxide at an average of 20.3 mg/liter/day. This was followed by the S. putrefaciens strains CN-32 and 200, which produced 13.9 and 15.9 mg/liter/day, respectively, and S. denitrificans, which produced 8.7 mg/liter/day. The least efficient oxidizer was the extensively studied metal reducer S. oneidensis MR-1, which produced 4.4 mg/liter/day. The maximum concentration of Mn(II) oxidized also varied between species. Generally, bacteria that oxidized higher maximum concentrations of Mn(II) were more rapid oxidizers of manganese; for example, S. loihica oxidized Mn(II) concentrations up to 9 mM but S. oneidensis only up to 4 mM. In addition, the efficiency of oxidation by S. oneidensis decreased substantially at concentrations above 1 mM. Figure 1 shows the optimal concentration of Mn(II) oxidized by the four Shewanella species. S. loihica and S. putrefaciens had optima around 3 to 5 mM, S. denitrificans 2 to 4 mM, and S. oneidensis ≤1 mM. After oxidation occurred, the Shewanella species were tested, under anaerobic conditions, to determine whether they could reduce the preformed manganese oxides. In all cases, the oxides were reduced rapidly and completely within 2 days.
TABLE 1.
Manganese-oxidizing properties of Shewanella strainsa
| Species and strain | Maximum Mn(II) concentration (mM)b | Rate of oxide production (mg/liter/day)c | Reduction of bioformed oxided |
|---|---|---|---|
| S. denitrificans OS217 | 7 | 8.7 ± 2.7 | + |
| S. loihica PV-4 | 9 | 20.3 ± 1.6 | + |
| S. oneidensis MR-1 | 4 | 4.4 ± 1.2 | + |
| S. putrefaciens 200 | 7 | 15.9 ± 3.9 | + |
| S. putrefaciens CN-32 | 8 | 13.9 ± 3.1 | + |
Cultures were incubated at 27°C.
Mn(II) concentration at which complete inhibition of oxidation occurred.
Oxidation at 3 mM Mn(II) (average of replicates ± standard deviation).
Cultures were switched to anoxic conditions and replenished with 15 mM lactate.
FIG 1.
Optimal concentrations of Mn(II) oxidized by the Shewanella species S. loihica, S. putrefaciens, S. oneidensis, and S. denitrificans. No manganese oxides were formed in sterile controls or in poisoned controls with any of the Shewanella species.
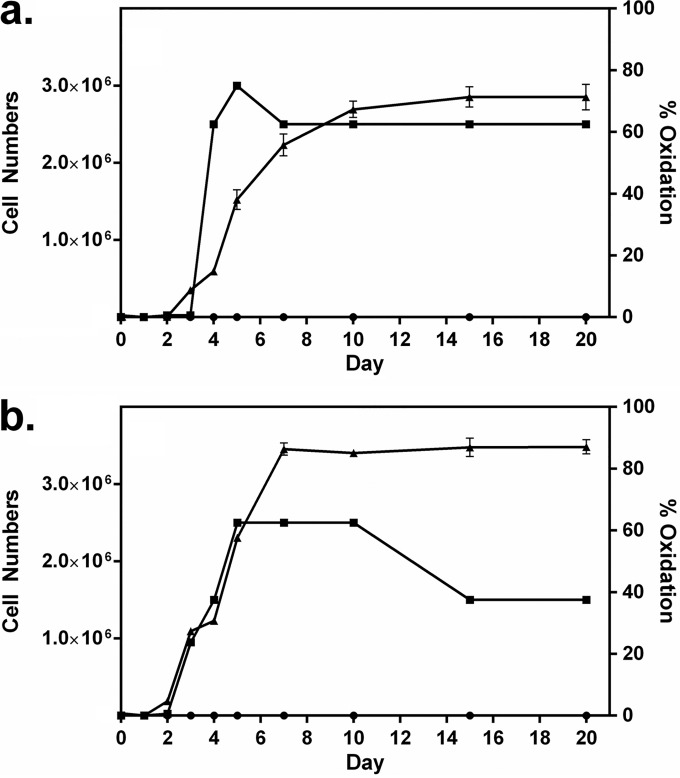
Figure 2 shows the time courses of oxidation and cell growth for two of the best oxidizers under the growth and incubation conditions used, namely, S. putrefaciens CN-32 and S. loihica PV-4. Log-phase growth for both bacteria commenced after 2 to 3 days; oxide formation began shortly thereafter and continued for several days, into the stationary phase of growth. Most oxide production by S. putrefaciens occurred after rapid growth began to slow, while oxide production was more closely aligned with growth for S. loihica. For both bacteria, oxide production slowed when around 70 to 90% of Mn(II) had been converted to an oxide. In a separate experiment, both S. putrefaciens and S. loihica were again shown to oxidize manganese (Fig. 3). Once oxidation had ceased after 10 days, cultures were transferred to anaerobic vessels, gassed with N2, and sealed, and 15 mM lactate was provided. The bioformed manganese oxide products were reduced rapidly by the bacteria. After 1 to 2 days of incubation, there was no oxide evident in the cultures and there were concomitant increases in cell numbers.
FIG 2.
Extent of manganese oxidation (▲) and increases in cell numbers (cells per milliliter) (■) over time for Shewanella putrefaciens CN-32 (a) and Shewanella loihica PV-4 (b). Cultures were grown with 3 mM Mn(II). The extent of manganese oxidation in poisoned controls is also shown (●).
FIG 3.
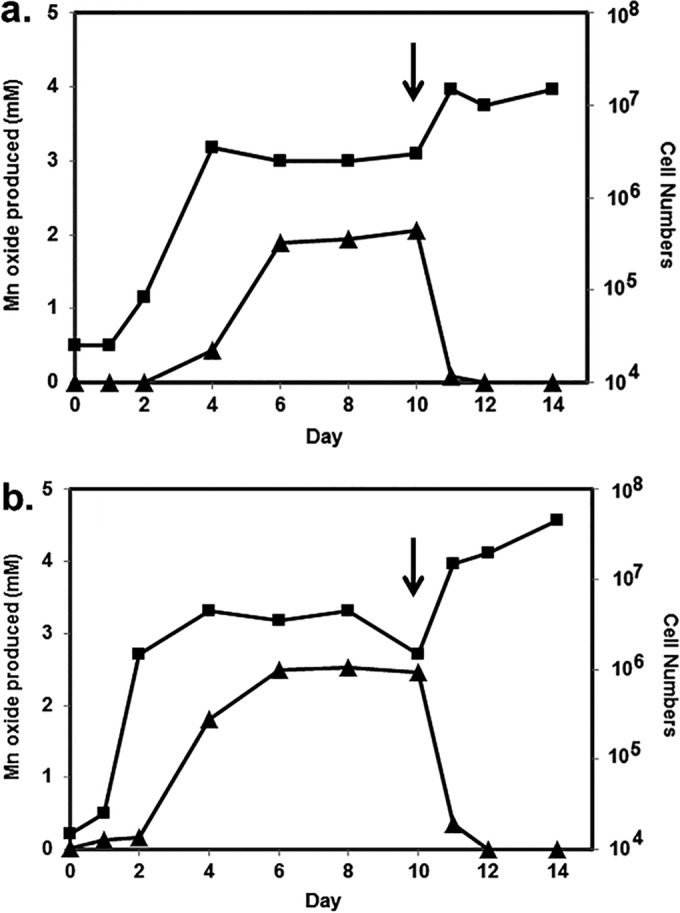
Manganese oxide formation (▲) and cell number (cells per milliliter) (■) variations over time for Shewanella putrefaciens CN-32 (a) and Shewanella loihica PV-4 (b), initially under aerobic conditions. After 10 days of incubation, conditions were changed to anaerobic (N2 gas) with the addition of 15 mM lactate (arrows).
Manganese oxide characterization.
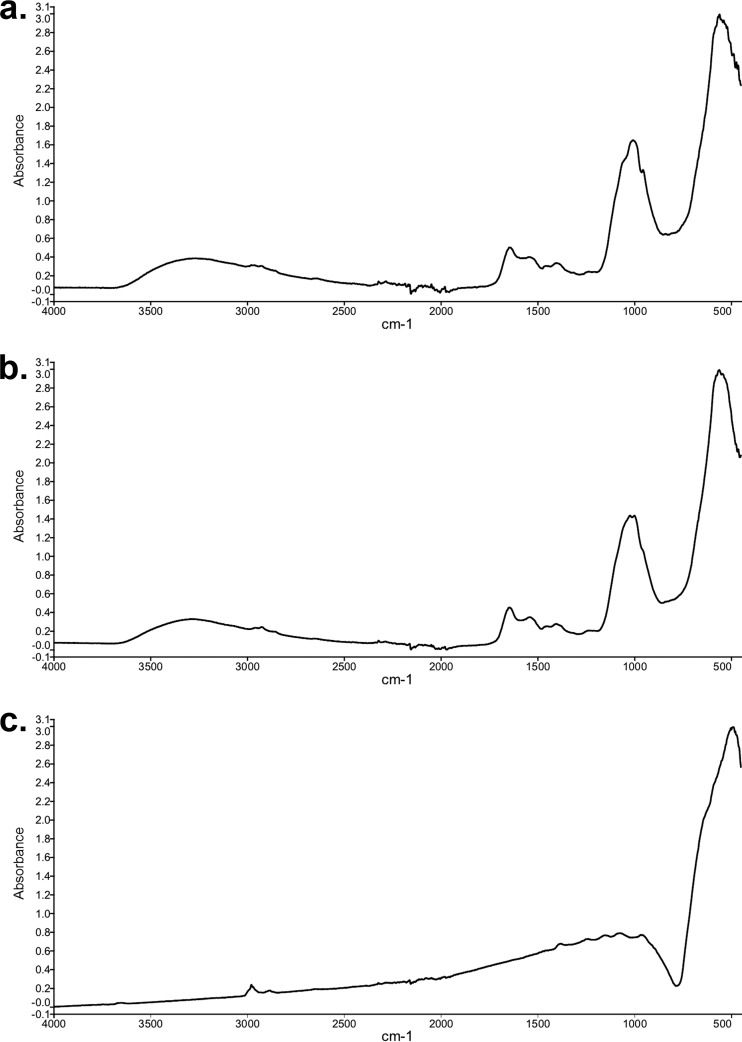
The manganese oxide products formed by S. putrefaciens CN-32 and S. loihica PV-4 were characterized. FTIR spectra revealed that both oxides had a major broad peak at around 530 wavenumbers (Fig. 4), which was typical of microbially formed disordered MnO2 (32). Additional smaller peaks occurred at 1,600 and 1,000 wavenumbers, which were consistent with organic contamination (bacterial cells) and inorganic compounds such as medium precipitates. The control, i.e., standard analytical reagent-grade MnO2, had a similarly broad peak at a slightly lower wavenumber, which was consistent with natural MnO2 ores (44). Chemical analysis of oxide products revealed that, for both bacteria, the manganese had an oxidation state of 3.86 (Table 2), equating to a manganese oxide of MnO1.93. The products were 92.0% and 91.6% manganese oxide for S. putrefaciens CN-32 and S. loihica PV-4, respectively. The non-manganese oxide component remaining was likely a combination of organic and inorganic impurities, as reflected in the FTIR spectra. The oxidation state of the analytical reagent-grade oxide was very close to complete Mn(IV) at 3.98, equating to MnO1.99.
FIG 4.
FTIR spectroscopy of manganese oxides formed by Shewanella putrefaciens CN-32 (a), manganese oxides formed by Shewanella loihica PV-4 (b), and analytical reagent-grade MnO2 (c).
TABLE 2.
Characteristics of manganese oxides produced by Shewanella putrefaciens CN-32 and Shewanella loihica PV-4, compared with analytical reagent-grade MnO2
| Manganese oxide origin | Oxidation state | Manganese oxide content (%) |
|---|---|---|
| S. putrefaciens CN-32 | 3.86 | 92.0 |
| S. loihica PV-4 | 3.86 | 91.6 |
| Analytical reagent-grade MnO2 | 3.98 | 100 |
Scanning electron microscopy revealed finely grained, nanosize, amorphous, round manganese oxide particles produced by both Shewanella strains (Fig. 5). Even after the precipitates were washed, bacteria were still seen clearly in the micrographs (Fig. 5, arrows). In contrast, the analytical reagent-grade MnO2 particles were generally much larger crystalline particles, with no bacteria evident.
FIG 5.
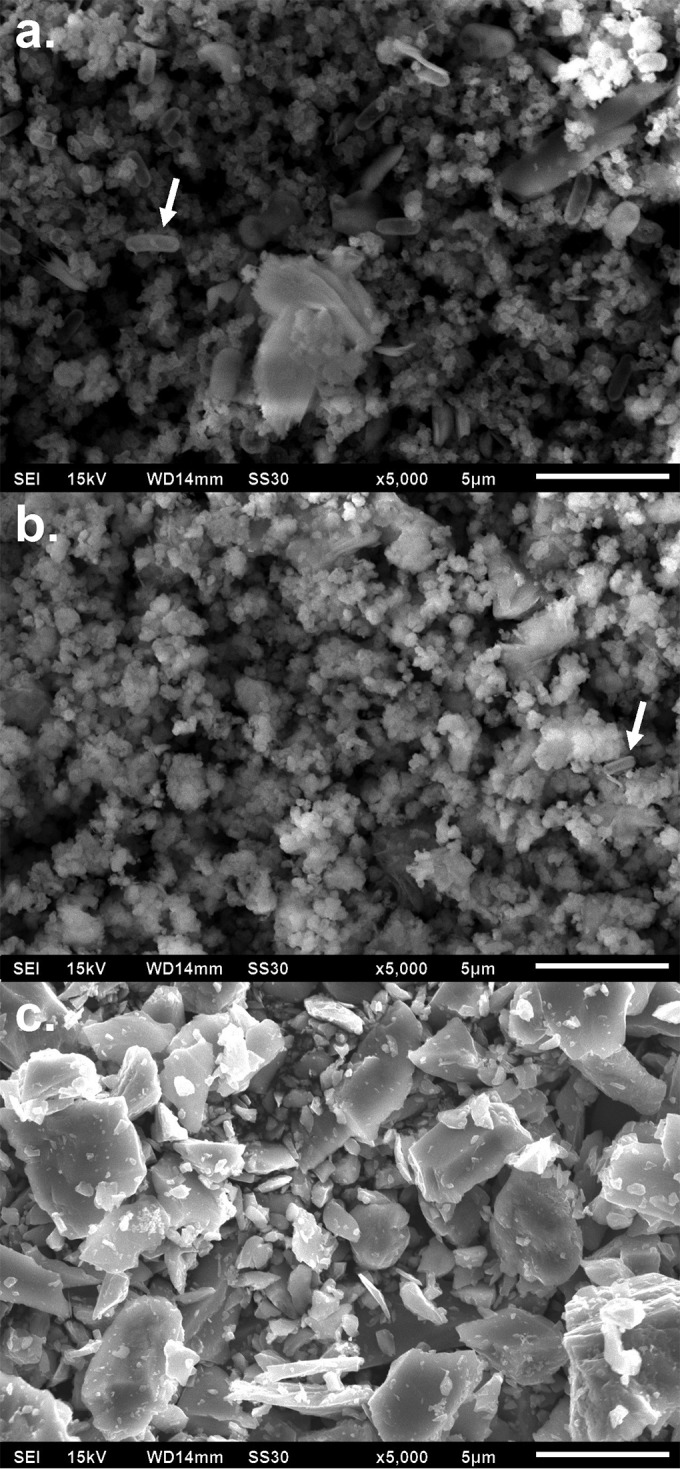
Scanning electron microscopy of manganese oxides formed by Shewanella putrefaciens CN-32 (a), manganese oxides formed by Shewanella loihica PV-4 (b), and analytical reagent-grade MnO2 (c). Arrows indicate bacteria.
DISCUSSION
Bacteria play an important role in the cycling of manganese in a wide range of natural environments. The oxidation state of manganese in an environment is dependent on redox conditions, with oxidation being favored under oxic conditions and reduction being favored under anoxic conditions. Many bacteria are known either to oxidize or to reduce manganese, and there has been little investigation of bacteria capable of doing both. Members of the genus Shewanella have been known for some time to be involved in the anaerobic reduction of metals, including manganese, in a wide variety of environments (45). Unlike many dissimilatory metal-reducing bacteria, Shewanella species are able to use oxygen as a terminal electron acceptor in respiration. While manganese reduction in the Shewanella genus has been investigated extensively, the possibility of manganese oxidation has not been investigated in any detail. However, Shewanella species have been found as part of manganese-oxidizing communities and were recognized as being important in manganese cycling in the Columbia River in the United States (46, 47). Furthermore, Bräuer and colleagues (47) found that the manganese-oxidizing Shewanella isolates were most closely related to S. denitrificans, a moderate oxidizer in our studies. Similarly, manganese-oxidizing Shewanella isolates were found as part of the microbial community at Vailulu'u Seamount, Samoa (48). Blöthe and colleagues (49) found Shewanella isolates as dominant members of deep-sea manganese nodule communities and suggested that they had a key role in manganese cycling, including oxidation.
All Shewanella strains tested in the current study were able to oxidize manganese. The rates of oxidation by the Shewanella species compared favorably with those for species isolated previously from manganese and nonmanganese environments (40). In contrast to the current study, Chubar and colleagues (50) found that S. putrefaciens strain 200R precipitated Mn(II) as phosphates but not oxides. However, testing was performed in the absence of nutrients and cell growth. Studies in our laboratory have shown that the types and concentrations of nutrients directly influence the ability of the Shewanella strains to oxidize Mn(II) (A. C. Greene, unpublished data).
In the present study, S. loihica could oxidize Mn(II) concentrations up to 9 mM. Similarly, Mesorhizobium australicum strain T-G1 was found to oxidize Mn(II) concentrations up to 10 mM (51). In some of the earlier studies of manganese oxidation, Bromfield and David (52) found that an Arthrobacter soil isolate oxidized Mn(II) at concentrations up to 30 mM, with the maximum rate occurring between 0.5 and 6 mM. Xuezheng and coworkers (53) found several Shewanella isolates among 40 psychrotrophic and psychrophilic manganese bacteria isolated from the Arctic Ocean. The same authors found that the Shewanella species tolerated Mn(II) concentrations up to 10 mM with minimal inhibition.
Once the oxides had formed, changing conditions to anaerobic and providing an appropriate carbon source (15 mM lactate) resulted in all Shewanella species reducing these biogenic oxides. Indeed, the ability of Shewanella species not only to reduce and to oxidize manganese but also to readily utilize biogenic oxides as terminal electron acceptors indicates that the genus is likely to play a significant role in the geochemical cycling of the metal in environments that members inhabit. Several older studies reported bacteria that can both reduce and oxidize manganese. Bromfield and David (52) found that their Arthrobacter strain oxidized manganese under aerobic conditions and reduced it in deep static cultures, which presumably created anaerobic conditions. Indeed, the most studied manganese oxidizer, Bacillus strain SG-1, was reported to reduce Mn(IV) as well (54). The difference with respect to the Shewanella species tested in our study is that, for Bacillus strain SG-1, the spores oxidized manganese whereas the vegetative cells reduced it.
Typically, bioformed manganese oxides are poorly crystalline (18, 32, 34, 55). Electron micrographs confirmed the precipitation of finely grained particles similar to those formed by other bacteria (55, 56). Furthermore, the relatively low O/Mn ratio suggests that the bioformed oxides from Shewanella species are consistent with poorly crystalline, disordered manganese oxides (32). Reduction of the bioformed manganese oxides by Shewanella species was quick, usually completed within 1 to 2 days. The high rate of reduction was consistent with the much higher rate of reduction of poorly crystalline manganese oxides, compared with natural crystalline oxides (57). In fact, Burdige and colleagues (58) found that S. oneidensis MR-1 reduced highly crystalline pyrolusite substantially more slowly than amorphous, structurally disordered δ-MnO2 (vernadite). It is likely that the characteristics of the poorly crystalline oxides, including greater surface area and structural defects, allowed more rapid transfer of electrons.
The mechanisms of oxidation have been investigated for numerous manganese-oxidizing bacteria. Evidence suggests that multicopper oxidase enzymes mediate manganese oxidation, and genomic investigations have indicated that the presence of multicopper oxidases is important for oxidation to occur (19, 20, 59). Laccases belong to the multicopper oxidase family and have been found to be present in S. putrefaciens (60) and S. oneidensis (61). In fact, laccases from fungi have been shown to oxidize Mn(II) enzymatically (25). The reason why bacteria oxidize Mn(II) is not clear. Although it has long been suggested to be an energy-yielding process, no definitive evidence has ever been presented (19). It would make sense for Shewanella species to oxidize manganese as a strategy for storing electron acceptors that can be used when oxygen is depleted. Glasauer and colleagues (62) observed the synthesis of manganese nanogranules in the cytoplasm of S. putrefaciens during anaerobic growth.
The presence of Mn(II) and Mn(III/IV) in aquatic environments is largely influenced by interactions with the microbial flora and surrounding environmental conditions (57, 63). The ability of Shewanella species both to oxidize and to reduce manganese indicates that the genus plays a significant role in the geochemical cycling of the metal. Due to the facultative nature of the Shewanella species, it is likely that these bacteria contribute to the release of not only manganese but also other elements and metals in the environment, such as copper, cobalt, nickel, lead, iron, radium, uranium, and rare earth elements.
Funding Statement
This research was supported by an Australian Postgraduate Award for Mitchell H. Wright.
REFERENCES
- 1.Nealson KH, Scott J. 2006. Ecophysiology of the genus Shewanella. Prokaryotes 6:1133–1151. [Google Scholar]
- 2.Wright MH, Matthews B, Arnold MSJ, Greene AC, Cock IE. 2016. The prevention of fish spoilage by high antioxidant Australian culinary plants: Shewanella putrefaciens growth inhibition. Int J Food Sci Technol 51:801–813. doi: 10.1111/ijfs.13026. [DOI] [Google Scholar]
- 3.Vogel BF, Venkateswaran K, Satomi M, Gram L. 2005. Identification of Shewanella baltica as the most important H2S-producing species during iced storage of Danish marine fish. Appl Environ Microbiol 71:6689–6697. doi: 10.1128/AEM.71.11.6689-6697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers CR, Nealson KR. 1990. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J Bacteriol 172:6232–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truex MJ, Peyton BM, Valentine NB, Gorby YA. 1997. Kinetics of U(VI) reduction by a dissimilatory Fe(III)-reducing bacterium under non-growth conditions. Biotechnol Bioeng 55:490–496. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Guha H, Jayachandran K, Maurrasse F. 2001. Kinetics of chromium (VI) reduction by a type strain Shewanella alga under different growth conditions. Environ Pollut 115:209–218. doi: 10.1016/S0269-7491(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 7.Myers JM, Antholine WE, Myers CR. 2004. Vanadium(V) reduction by Shewanella oneidensis MR-1 requires menaquinone and cytochromes from the cytoplasmic and outer membranes. Appl Environ Microbiol 70:1405–1412. doi: 10.1128/AEM.70.3.1405-1412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovley DR. 1993. Dissimilatory metal reduction. Annu Rev Microbiol 47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 9.Nealson KH. 2002. Breathing metals as a way of life: geobiology in action. Antonie Van Leeuwenhoek 81:215–222. doi: 10.1023/A:1020518818647. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd JR. 2003. Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425. doi: 10.1016/S0168-6445(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 11.Kappler C, Straub KL. 2005. Geomicrobiological cycling of iron. Rev Mineral Geochem 59:85–108. doi: 10.2138/rmg.2005.59.5. [DOI] [Google Scholar]
- 12.Lovley DR. 2002. Dissimilatory metal reduction: from early life to bioremediation. ASM News 68:231–237. [Google Scholar]
- 13.Gadd GM. 2010. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156:609–643. doi: 10.1099/mic.0.037143-0. [DOI] [PubMed] [Google Scholar]
- 14.Watts MP, Lloyd JP. 2013. Bioremediation via microbial metal reduction, p 161–201. In Gescher J, Kappler A (ed), Microbial metal respiration. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 15.Lovley DR, Holmes DE, Nevin KP. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286. doi: 10.1016/S0065-2911(04)49005-5. [DOI] [PubMed] [Google Scholar]
- 16.Rotaru AE, Shrestha PM, Liu F, Ueki T, Nevin K, Summers ZM, Lovley DR. 2012. Interspecies electron transfer via hydrogen and formate rather than direct electrical connections in cocultures of Pelobacter carbinolicus and Geobacter sulfurreducens. Appl Environ Microbiol 78:7645–7651. doi: 10.1128/AEM.01946-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuetz RM, Greene AC, Madgwick JC. 1996. Microalgal-facilitated bacterial oxidation of manganese. J Ind Microbiol 16:267–273. doi: 10.1007/BF01570033. [DOI] [Google Scholar]
- 18.Tebo BM, Bargar JR, Clement B, Dick G, Murray KJ, Parker D, Verity R, Webb S. 2004. Biogenic manganese oxides: properties and mechanisms of formation. Annu Rev Earth Planet Sci 32:287–328. doi: 10.1146/annurev.earth.32.101802.120213. [DOI] [Google Scholar]
- 19.Tebo BM, Johnson HA, McCarthy JK, Templeton AS. 2005. Geomicrobiology of manganese(II) oxidation. Trends Microbiol 13:421–428. doi: 10.1016/j.tim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Soldatova AV, Butterfield C, Oyerinde OF, Tebo BM, Spiro TG. 2012. Multicopper oxidase involvement in both Mn(II) and Mn(III) oxidation during bacterial formation of MnO2. J Biol Inorg Chem 17:1151–1158. doi: 10.1007/s00775-012-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Waasbergen LG, Hildebrand M, Tebo BM. 1996. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J Bacteriol 178:3517–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwers GJ, de Vrind JPM, Corstjens PLAM, Cornelis P, Baysse C, de Vrind-de Jong EW. 1999. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl Environ Microbiol 65:1762–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corstjens PLAM, de Vrind JPM, Goosen T, de Vrind-de Jong EW. 1997. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol J 14:91–108. doi: 10.1080/01490459709378037. [DOI] [Google Scholar]
- 24.Ridge JP, Lin M, Larsen EI, Fegan M, McEwan AG, Sly LI. 2007. A multicopper oxidase is essential for manganese oxidation and laccase-like activity in Pedomicrobium sp. ACM 3067. Environ Microbiol 9:944–953. [DOI] [PubMed] [Google Scholar]
- 25.Hofer C, Schlosser D. 1999. Novel enzymatic oxidation of Mn2+ to Mn3+ catalyzed by a fungal laccase. FEBS Lett 451:186–190. doi: 10.1016/S0014-5793(99)00566-9. [DOI] [PubMed] [Google Scholar]
- 26.Palma C, Martinez AT, Lema JM, Martinez MJ. 2000. Different fungal manganese-oxidizing peroxidases: a comparison between Bjerkandera sp. and Phanerochaete chrysosporium. J Biotechnol 77:235–245. doi: 10.1016/S0168-1656(99)00218-7. [DOI] [PubMed] [Google Scholar]
- 27.Dick GJ, Torpey JW, Beveridge TJ, Tebo BM. 2008. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase mnxG, from spores of several different marine Bacillus species. Appl Environ Microbiol 74:1527–1534. doi: 10.1128/AEM.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Wiens M, Divekar M, Grebenjuk VA, Schröder HC, Batel R, Müller WEG. 2011. Isolation and characterization of a Mn(II)-oxidizing Bacillus strain from the demosponge Suberites domuncula. Mar Drugs 9:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahaney WC, Krinsley DH, Allen CCR, Ditto J, Langworthy K, Batchelor AD, Lecompte M, Milner MW, Hart K, O'Reilly SS, Kelleher BP, Hancock RGV. 2015. Reassessment of the microbial role in Mn-Fe nodule genesis in Andean paleosols. Geomicrobiol J 32:27–41. doi: 10.1080/01490451.2014.920939. [DOI] [Google Scholar]
- 30.Schweisfurth R, Jung W, Gundlach H. 1980. Manganese-oxidizing microorganisms and their importance for the genesis of manganese ore deposits, p 279–283. In Varentsov IM, Grasselly G (ed), Geology and geochemistry of manganese, vol III. Manganese on the bottom of recent basins. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart, Germany. [Google Scholar]
- 31.Du Q, Yi H, Hui B, Li S, Xia G, Yang W, Wu X. 2013. Recognition, genesis and evolution of manganese ore deposits in southeastern China. Ore Geol Rev 55:99–109. doi: 10.1016/j.oregeorev.2013.05.001. [DOI] [Google Scholar]
- 32.Greene AC, Madgwick JC. 1991. Microbial formation of manganese oxides. Appl Environ Microbiol 57:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuetz RM, Greene AC, Madgwick JC. 1996. The potential use of manganese oxidation in treating metal effluents. Miner Eng 9:1253–1261. doi: 10.1016/S0892-6875(96)00120-3. [DOI] [Google Scholar]
- 34.Miyata N, Sugiyama D, Tani Y, Tsuno H, Seyama H, Sakata M, Iwahori K. 2007. Production of biogenic manganese oxides by repeated-batch cultures of laboratory microcosms. J Biosci Bioeng 103:432–439. doi: 10.1263/jbb.103.432. [DOI] [PubMed] [Google Scholar]
- 35.Hennebel T, Gusseme BD, Verstraete W. 2009. Biogenic metals in advanced water treatment. Trends Biotechnol 27:90–98. doi: 10.1016/j.tibtech.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Santelli CM, Pfister DH, Lazarus D, Sun L, Burgos WD, Hansel CM. 2010. Promotion of Mn(II) oxidation and remediation of coal mine drainage in passive treatment systems by diverse fungal and bacterial communities. Appl Environ Microbiol 76:4871–4875. doi: 10.1128/AEM.03029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright MH, Matthews B, Greene AC, Cock IE. 2015. Growth inhibition of the zoonotic bacteria Bacillus anthracis by high antioxidant Australian plants: new leads for the prevention and treatment of anthrax. Pharmacognosy Commun 5:173–189. doi: 10.5530/pc.2015.3.3. [DOI] [Google Scholar]
- 38.Krumbein WE, Altman HJ. 1973. A new method for detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol Meeresunters 25:347–356. doi: 10.1007/BF01611203. [DOI] [Google Scholar]
- 39.Potter RM, Rossman GR. 1979. The tetravalent manganese oxides: identification, hydration and structural relationships by infrared spectroscopy. Am Mineral 64:1199–1218. [Google Scholar]
- 40.Swinkels DAJ, Fredericks PM, Anthony KE. 1985. FTIR studies on MnO2 structure and electrochemical behaviour, p 158–179. In Schumm B Jr, Middaugh RL, Grotheer MP, Hunter JC (ed), Manganese dioxide electrode theory and practice for electrochemical applications. Electrochemical Society, Pennington, NJ. [Google Scholar]
- 41.Greene AC, Madgwick JC. 1988. Heterotrophic manganese-oxidizing bacteria from Groote Eylandt, Australia. Geomicrobiol J 6:119–127. doi: 10.1080/01490458809377829. [DOI] [Google Scholar]
- 42.Spratt HG Jr, Siekmann EC, Hodson RE. 1994. Microbial manganese oxidation in saltmarsh surface sediments using a leuco crystal violet manganese oxide detection technique. Estuar Coast Shelf Sci 38:91–112. doi: 10.1006/ecss.1994.1006. [DOI] [Google Scholar]
- 43.Murray JW, Balistrieri LS, Paul B. 1984. The oxidation state of manganese in marine sediments and ferromanganese nodules. Geochim Cosmochim Acta 48:1237–1247. doi: 10.1016/0016-7037(84)90058-9. [DOI] [Google Scholar]
- 44.Fredericks PM, Osborn PR, Swinkels DAJ. 1983. Materials characterisation by Fourier transform infrared spectroscopy. BHP Tech Bull 27:26–32. [Google Scholar]
- 45.Nealson KH, Saffarini D. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol 48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- 46.Anderson CR, Davis RE, Bandolin NS, Baptista AM, Tebo BM. 2011. Analysis of in situ manganese(II) oxidation in the Columbia River and offshore plume: linking Aurantimonas and the associated microbial community to an active biogeochemical cycle. Environ Microbiol 13:1561–1576. doi: 10.1111/j.1462-2920.2011.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bräuer SL, Adams C, Kranzler K, Murphy D, Xu M, Zuber P, Simon HM, Baptista AM, Tebo BM. 2011. Culturable Rhodobacter and Shewanella species are abundant in estuarine turbidity maxima of the Columbia River. Environ Microbiol 13:589–603. doi: 10.1111/j.1462-2920.2010.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staudigel H, Hart SR, Pile A, Bailey BE, Baker ET, Brook S, Connelly DP, Haucke L, German CR, Hudson I, Jones D, Koppers AAP, Konter J, Lee R, Pietsch TW, Tebo BM, Templeton AS, Zierenberg R, Young CM. 2006. Vailulu'u Seamount, Samoa: life and death on an active submarine volcano. Proc Natl Acad Sci U S A 103:6448–6453. doi: 10.1073/pnas.0600830103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blöthe M, Wegorzewski A, Müller C, Simon F, Kuhn T, Schippers A. 2015. Manganese-cycling microbial communities inside deep-sea manganese nodules. Environ Sci Technol 49:7692–7700. doi: 10.1021/es504930v. [DOI] [PubMed] [Google Scholar]
- 50.Chubar N, Visser T, Avramut C, de Waard H. 2013. Sorption and precipitation of Mn2+ by viable and autoclaved Shewanella putrefaciens: effect of contact time. Geochim Cosmochim Acta 100:232–250. doi: 10.1016/j.gca.2012.09.051. [DOI] [Google Scholar]
- 51.Bohu T, Santelli CM, Akob DM, Neu TR, Ciobota V, Rösch P, Popp J, Nietzsche S, Küsel K. 2015. Characterization of pH dependent Mn(II) oxidation strategies and formation of a bixbyite-like phase by Mesorhizobium australicum T-G1. Front Microbiol 6:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bromfield SM, David DJ. 1976. Sorption and oxidation of manganous ions and reduction of manganese oxide by cell suspensions of a manganese oxidizing bacterium. Soil Biol Biochem 8:37–43. doi: 10.1016/0038-0717(76)90019-5. [DOI] [Google Scholar]
- 53.Xuezheng L, Aiguo G, Haowen C. 2008. Isolation and phylogenetic analysis of cultivable manganese bacteria in sediments from the Arctic Ocean. Acta Ecol Sin 28:6364–6370. doi: 10.1016/S1872-2032(09)60017-2. [DOI] [Google Scholar]
- 54.de Vrind JPM, Boogerd FC, de Vrind-de Jong EW. 1986. Manganese reduction by a marine Bacillus species. J Bacteriol 167:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villalobos M, Toner B, Bargar J, Sposito G. 2003. Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1. Geochim Cosmochim Acta 67:2649–2662. doi: 10.1016/S0016-7037(03)00217-5. [DOI] [Google Scholar]
- 56.Spiro TG, Bargar JR, Sposito G, Tebo BM. 2010. Bacteriogenic manganese oxides. Acc Chem Res 43:2–9. doi: 10.1021/ar800232a. [DOI] [PubMed] [Google Scholar]
- 57.Burdige DJ. 1993. The biogeochemistry of manganese and iron reduction in marine sediments. Earth Sci Rev 35:249–284. doi: 10.1016/0012-8252(93)90040-E. [DOI] [Google Scholar]
- 58.Burdige DJ, Dhakar SP, Nealson KH. 1992. Effects of manganese oxide mineralogy on microbial and chemical manganese reduction. Geomicrobiol J 10:27–48. doi: 10.1080/01490459209377902. [DOI] [Google Scholar]
- 59.Tebo BM, Ghiorse WC, van Waasbergen LG, Siering PL, Caspi R. 1997. Bacterially mediated mineral formation: insights into manganese(II) oxidation from molecular genetic and biochemical studies. Rev Mineral Geochem 35:225–266. [Google Scholar]
- 60.Sinirlioglu ZA, Sinirlioglu D, Akbas F. 2013. Preparation and characterization of stable cross-linked enzyme aggregates of novel laccase enzyme from Shewanella putrefaciens and using malachite green decolorization. Bioresour Technol 146:807–811. doi: 10.1016/j.biortech.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 61.Wu J, Kim KS, Sung NC, Kim CH, Lee YC. 2009. Isolation and characterization of Shewanella oneidensis WL-7 capable of decolorizing azo dye Reactive Black 5. J Gen Appl Microbiol 55:51–55. doi: 10.2323/jgam.55.51. [DOI] [PubMed] [Google Scholar]
- 62.Glasauer S, Langley S, Beveridge T. 2004. Intracellular manganese granules formed by a subsurface bacterium. Environ Microbiol 6:1042–1048. doi: 10.1111/j.1462-2920.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 63.Aguilar C, Nealson KH. 1998. Biogeochemical cycling of manganese in Oneida Lake, New York: whole lake studies of manganese. J Great Lakes Res 24:93–104. doi: 10.1016/S0380-1330(98)70802-0. [DOI] [PubMed] [Google Scholar]