ABSTRACT
The Zn-dependent membrane-located protease YvjB has previously been shown to serve as a target receptor for LsbB, a class II leaderless lactococcal bacteriocin. Although yvjB is highly conserved in the genus Lactococcus, the bacteriocin appears to be active only against the subspecies L. lactis subsp. lactis. Comparative analysis of the YvjB proteins of a sensitive strain (YvjBMN) and a resistant strain (YvjBMG) showed that they differ from each other in 31 positions. In this study, we applied site-directed mutagenesis and performed directed binding studies to provide biochemical evidence that LsbB interacts with the third transmembrane helix of YvjB in susceptible cells. The site-directed mutagenesis of LsbB and YvjB proteins showed that certain amino acids and the length of LsbB are responsible for the bacteriocin activity, most probably through adequate interaction of these two proteins; the essential amino acids in LsbB responsible for the activity are tryptophan (Trp25) and terminal alanine (Ala30). It was also shown that the distance between Trp25 and terminal alanine is crucial for LsbB activity. The crucial region in YvjB for the interaction with LsbB is the beginning of the third transmembrane helix, particularly amino acids tyrosine (Tyr356) and alanine (Ala353). In vitro experiments showed that LsbB could interact with both YvjBMN and YvjBMG, but the strength of interaction is significantly less with YvjBMG. In vivo experiments with immunofluorescently labeled antibody demonstrated that LsbB specifically interacts only with cells carrying YvjBMN.
IMPORTANCE The antimicrobial activity of LsbB bacteriocin depends on the correct interaction with the corresponding receptor in the bacterial membrane of sensitive cells. Membrane-located bacteriocin receptors have essential primary functions, such as cell wall synthesis or sugar transport, and it seems that interaction with bacteriocins is suicidal for cells. This study showed that the C-terminal part of LsbB is crucial for the bacteriocin activity, most probably through adequate interaction with the third transmembrane domain of the YvjB receptor. The conserved Tyr356 and Ala353 residues of YvjB are essential for the function of this Zn-dependent membrane-located protease as a bacteriocin receptor.
INTRODUCTION
Bacteriocins are small, ribosomally synthesized, cationic, and hydrophobic peptides produced by various bacteria, and they are often found to be active against bacteria closely related to the producers. However, some also have broader inhibitory spectra, including pathogens and problematic bacteria. Producer organisms are immune to their own bacteriocin(s), a property that is mediated by specific immunity proteins (1).
Bacteriocins from Gram-positive bacteria are generally classified into two main groups: the class I lantibiotics, containing posttranslationally modified peptides with ring-forming lanthionine or methyllanthionine residues, and class II, composed of nonmodified or minimally modified peptide bacteriocins (1–3). Class II bacteriocins are further subdivided into pediocin-like bacteriocins (class IIa), two-peptide bacteriocins (class IIb), circular bacteriocins (class IIc), and nonpediocin one-peptide bacteriocins (class IId) (2).
Bacteriocins have been much studied from a fundamental and scientific perspective and also for their potential applications as food preservatives, and in veterinary and human medicine as alternatives to antibiotics or as synergists (1, 4).
Bacteriocins have a number of positive attributes that have made them especially attractive for various applications (5). Some of them exhibit a broad spectrum of activity, inhibiting microorganisms belonging to different genera and species, including many bacterial pathogens that cause human, animal, or plant infections (6). For clinical applications, bacteriocins have been presented as a viable alternative to antibiotics due to the high specificity of certain bacteriocins against clinical pathogens, including multidrug-resistant (MDR) strains (4). Therefore, these substances have various potential applications in the food industry and medicine, either alone or in combination with other chemicals or methods (7).
The multiplicity and diversity of bacteriocins and the resultant effects of their interactions with targeted bacteria on microbial ecology have been thoroughly studied and remain an area of investigation, attracting many researchers (8).
The mechanisms involved in the inhibitory activity of bacteriocins produced by Gram-positive bacteria toward target cells have been shown to be diverse. Lipid II, along with related cell wall precursors, has been identified as both the receptor and the target for several class I bacteriocins from the lantibiotic subgroup (9–11), as well as for the class II bacteriocin lactococcin 972 (12). The mannose phosphotransferase system (man-PTS) has been found to be involved in the sensitivity to some bacteriocins, but it was not until 2007 that it was finally established that man-PTS serves as a receptor for the class IIa (i.e., pediocin-like) and some class IId bacteriocins (lactococcin A and lactococcin B) (13, 14). In recent years, several other receptors have been identified through a genome sequencing approach. A maltose-ABC transporter was found to be required in target cells for sensitivity to garvicin ML, a circular bacteriocin (class IIc) (15). By screening of a cosmid library and genome sequencing of resistant mutants, a Zn-dependent metallopeptidase was found to be the target for the LsbB (class IId) and related bacteriocins (16). In addition, UppP has been found to be the bacteriocin receptor for lactococcin G and enterocin 1071 (both class IIb) (17). Although all these bacteriocins differ greatly from each other in many aspects, including physicochemical properties, composition, target specificity, width of spectrum, and mode of action, they all share a feature, which is the fact that they all target components of the bacterial membrane.
LsbB is a 30-amino-acid (aa) leaderless class IId bacteriocin produced by Lactococcus lactis subsp. lactis BGMN1-5 (18). It has a narrow inhibitory spectrum containing mostly lactococcal strains. The target receptor for LsbB is YvjB, a member of the highly conserved Zn-dependent membrane-located protease M50 protein family (16). Some members of this protein family are known to be involved in gene regulation in response to stress in Escherichia coli and Enterococcus faecalis (19–22). However, the role of YvjB in Lactococcus is presently unknown. It seems that LsbB interacts with YvjB with its C-terminal part (23). In this study, we further analyzed the residues of LsbB and its membrane receptor YvjB that are essential for their interaction.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains, their derivatives, and plasmids used in this study are listed in Table 1. Lactococcal and enterococcal strains were grown in M17 medium (Merck GmbH, Darmstadt, Germany) supplemented with d-glucose (0.5% [wt/vol]) (GM17) at 30°C. Escherichia coli DH5α, TOP10, M15, and BL21(DE3) were used for cloning and propagation of constructs and were grown in Luria-Bertani (LB) broth aerobically at 37°C. To each medium, agar (1.5% [wt/vol]; Torlak, Belgrade, Serbia) was added for use as a solid medium. Transformants of lactococci and enterococci were selected on GM17 plates containing 10 μg/ml erythromycin (Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) or 7.5 μg/ml chloramphenicol (final concentration). E. coli transformants were selected on LB plates containing 300 μg/ml erythromycin, 100 μg/ml ampicillin, or 100 μg/ml kanamycin, depending on the plasmids used. When necessary, 5-bromo-4-chloro-3-indolyl-d-galactoside (X-gal) (Fermentas, Vilnius, Lithuania) was added to LB or GM17 medium plates at a final concentration of 50 μg/ml, for blue/white screening of colonies carrying vectors with clones.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Lactococcus lactis subsp. lactis | ||
| BGMN1-596 | Plasmid-free derivative of L. lactis subsp. lactis BGMN1-5 | 18 |
| BGMN1-596R2/23 | Mutant of BGMN1-596 semiresistant to LsbB up to 625 μg/ml | 16 |
| BGMN1-596T | BGMN1-596 transformed with pMN5 plasmid | 18 |
| Lactococcus lactis subsp. cremoris | ||
| MG7284 | MG1363, Prt− Lac− Bacr Fusr Spcr | 41 |
| MG7284/pAZIL-lsbB | MG7284 transformed with pAZIL-lsbB | 16 |
| Enterococcus faecalis | ||
| BGZLS10-27 | Natural isolate, Agg− | 42 |
| BGZLS10-27/pAZIL | BGZLS10-27 transformed with pAZIL | This study |
| BGZLS10-27/pAZIL-YvjBMN | BGZLS10-27 transformed with pAZIL-YvjBMN | This study |
| BGZLS10-27/pAZIL-YvjBMG | BGZLS10-27 transformed with pAZIL-YvjBMG | This study |
| Escherichia coli | ||
| DH5α | supE44 ΔlacU169 (ϕ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 43 |
| EC101 | JM101 containing repA gene of pWV01 in chromosome | 44 |
| TOP10 | F− mcrA (mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 lacX74 recA1 ara139 (ara-leu)7697 galU galK rpsL (Strr) andA1 nupG | Invitrogen |
| M15(pREP4) | Nals Strs Rifs Thi− Lac− Ara+ Gal+ Mtl− F− RecA+ Uvr+ Lon+ | Qiagen |
| BL21(DE3) | B F− dcm ampT hsdS (rB− mB−) gal λ(DE3) | Agilent Technologies |
| BL21/p-GEX-6P-3/LsbB | p-GEX-6P-3/LsbB transformant of BL21 | This study |
| BL21/p-GEX-6P-3 | p-GEX-6P-3 transformant of BL21 | This study |
| Plasmids | ||
| pMN5 | Natural plasmid carrying lsbB operon | 18; accession no. NC_004922.1 |
| pAZIL | 7,109 bp, Emr, shuttle cloning vector | 45; accession no. LMBP9596b |
| pAZIL-lsbB | pAZIL carrying lsbB gene | 16 |
| pAZIL-lsbB-W25F | pAZIL carrying lsbB gene with amino acid change Trp25Phe | This study |
| pAZIL-lsbB-A30-STOP | pAZIL carrying lsbB gene with deleted Ala30 amino acid | This study |
| pAZIL-lsbB-K29A-STOP | pAZIL carrying lsbB gene with amino acid change Lys29Ala and deleted Ala30 amino acid | This study |
| pAZIL-lsbB-A30+A31 | pAZIL carrying lsbB gene with addition of Ala31 amino acid | This study |
| pAZIL-lsbB-A30V | pAZIL carrying lsbB gene with amino acid change Ala30Val | This study |
| pAZIL-lsbB-A30G | pAZIL carrying lsbB gene with amino acid change Ala30Gly | This study |
| pAZIL-lsbB-A30S | pAZIL carrying lsbB gene with amino acid change Ala30Ser | This study |
| pAZIL-lsbB-A30P | pAZIL carrying lsbB gene with amino acid change Ala30Pro | This study |
| pAZIL-lsbB-K2N-T3S | pAZIL carrying lsbB gene with amino acid changes Lys2Asn and Thr3Ser | This study |
| pAZIL-lsbB-K17A-K19A | pAZIL carrying lsbB gene with amino acid changes Lys17Ala and Lys19Ala | This study |
| pAZIL-YvjBMN | pAZIL carrying yvjB gene from BGMN1-596 | This study |
| pAZIL-YvjBMG | pAZIL carrying yvjB gene from MG7284 | This study |
| pAZIL-YvjBMGB | pAZIL carrying yvjB gene from MG7284 with amino acid changes Ser257Gly and Asn259Lys | This study |
| pAZIL-YvjBMNB | pAZIL carrying yvjB gene from BGMN1-596 with insertion of BamHI restriction site | This study |
| pAZIL-YvjB1/2MN-1/2MG | pAZIL carrying first half of yvjB gene from BGMN1-596 and second half of yvjB gene from MG7284 (YvjBMN1-259-YvjBMG260-428 aa) | This study |
| pAZIL-YvjB1/2MG-1/2MN | pAZIL carrying first half of yvjB gene from MG7284 and second half of yvjB gene from BGMN1-596 (YvjBMG1-256-YvjBMN257-428 aa) | This study |
| pAZIL-YvjBMGE | pAZIL carrying yvjB gene from MG7284 with insertion of EagI restriction site at position of aa 327 without amino acid changes | This study |
| pAZIL-YvjBMNE | pAZIL carrying yvjB gene from BGMN1-596 with insertion of EagI restriction site at position of aa 327 without amino acid changes | This study |
| pAZIL-YvjB3/4MG-1/4MN | pAZIL carrying first 3/4 of yvjB gene from MG7284 and 1/4 of yvjB gene from BGMN1-596 (YvjBMG1-327-YvjBMN328-428 aa) | This study |
| pAZIL-YvjB1/2MG-1/4MN-1/4MG | pAZIL carrying first half of yvjB gene from MG7284, 1/4 of yvjB gene from BGMN1-596, and last and 1/4 of yvjB gene from MG7284 (YvjBMG1-256-YvjBMN257-327-YvjBMG328-428 aa) | This study |
| pAZIL-YvjBMG-L351F | pAZIL carrying yvjB gene from MG7284 with amino acid change Leu351Phe | This study |
| pAZIL-YvjBMG-T353A | pAZIL carrying yvjB gene from MG7284 with amino acid change Thr353Ala | This study |
| pAZIL-YvjBMG-Q356Y | pAZIL carrying yvjB gene from MG7284 with amino acid change Gln356Tyr | This study |
| pAZIL-YvjBMG-P396Q | pAZIL carrying yvjB gene from MG7284 with amino acid change Pro396Gln | This study |
| pAZIL-YvjBMG-L351F-T353A | pAZIL carrying yvjB gene from MG7284 with amino acid changes Leu351Phe and Thr353Ala | This study |
| pAZIL-YvjBMG-L351F-Q356Y | pAZIL carrying yvjB gene from MG7284 with amino acid changes Leu351Phe and Gln356Tyr | This study |
| pAZIL-YvjBMG-T353A-Q356Y | pAZIL carrying yvjB gene from MG7284 with amino acid changes Thr353Ala and Gln356Tyr | This study |
| pAZIL-YvjBMG-T353A-P396Q | pAZIL carrying yvjB gene from MG7284 with amino acid changes Thr353Ala and Pro396Gln | This study |
| pAZIL-YvjBMG-L351F-T353A-Q356Y | pAZIL carrying yvjB gene from MG7284 with amino acid changes Leu351Phe, Thr353Ala, and Gln356Tyr | This study |
| pAZIL-YvjBMG-L351F-T353A-P396Q | pAZIL carrying yvjB gene from MG7284 with amino acid changes Leu351Phe, Thr353Ala, and Pro396Gln | This study |
| pAZIL-YvjBMG-L351F-T353A-Q356Y-P396Q | pAZIL carrying yvjB gene from MG7284 with amino acid changes Leu351Phe, Thr353Ala, Gln356Tyr, and Pro396Gln | This study |
| pAZIL-YvjBMG-L351F-T353A-Q356Y-T314A | pAZIL carrying yvjB gene from MG7284 with amino acid changes Leu351Phe, Thr353Ala, Gln356Tyr, and Thr314Ala | This study |
| pBluescript | 2,958 bp, Ampr, cloning vector | Stratagene |
| pGEM-T-Easy | 3,015 bp, Ampr, PCR cloning vector | Promega |
| pGEM-T-YvjBex1 | PCR-amplified fragment carrying region of YvjB protein between first and second transmembrane domains cloned into pGEM-T-Easy | This study |
| pGEM-T-YvjBex2 | PCR-amplified fragment carrying region of YvjB protein between second and third transmembrane domains cloned into pGEM-T-Easy | This study |
| pGEX-6P-3 | 4,900 bp, Ampr, GST expression vector | GE Healthcare Life Sciences |
| pGEX-6P-3/GST-LsbB | Cloned lsbB gene into pGEX-6P-3 vector, GST-LsbB fusion protein | This study |
| pQE30 | Ampr, ColE1 replicon, 6×His expression vector | Qiagen |
| pQE30-YvjBex1 | PCR-amplified fragment carrying region of YvjB protein between first and second transmembrane domains cloned as SacI/HindIII into pQE30 | This study |
| pQE30-YvjBex2 | PCR-amplified fragment carrying region of YvjB protein between second and third transmembrane domains cloned as BamHI/HindIII into pQE30 | This study |
| pNZ8150lacZ1PlcnB | PlcnB promoter cloned into pNZ8150lacZ1 vector | 28 |
Agg−, aggregative protein negative; Prt−, proteinase negative; Lac−, lactose utilization negative; Bacr, bacteriocin resistant; Fusr, fusaric acid resistant; Spcr, spectinomycin resistant; Strr, streptomycin resistant; Nals, nalidixic acid susceptible; Strs, streptomycin susceptible; Rifs, rifampin susceptible; Emr, erythromycin resistant; Ampr, ampicillin resistant; aa, amino acid.
LMBP9596, BCCM plasmid collection accession number.
Test for bacteriocin activity.
For detection of bacteriocin activity of lactococci, recombinant strains, and mutants, an agar well diffusion assay was performed as described previously by Kojic et al. (24). To test the level of LsbB production, zones of inhibition were compared by size and intensity with zones formed by synthetic LsbB (known concentrations) and two control strains, L. lactis BGMN1-596T and MG7284 carrying pAZILlsbB, in order to quantify production by different producers.
DNA manipulations.
Electrocompetent L. lactis subsp. cremoris MG7284 and Enterococcus faecalis BGZLS10-27 cells were prepared as described by Holo and Nes (25). Transformations were done by electroporation using an Eppendorf electroporator (Eppendorf, Hamburg, Germany), except E. coli DH5α, EC101, TOP10, M15, and BL21, which were transformed by heat shock. Appropriate agar plates with antibiotics were used for the selection of transformants.
Plasmid DNA from E. coli DH5α, EC101, TOP10, M15, and BL21 was isolated using the QIAprep Spin miniprep kit (Qiagen GmBH, Hilden, Germany). Digestion with restriction enzymes was conducted according to the supplier's instructions (Fermentas). DNA fragments were purified from agarose gels using a QIAquick gel extraction kit, as described by the manufacturer (Qiagen).
DNA was ligated with T4 DNA ligase (Agilent Technologies, USA), according to the manufacturer's recommendations.
The sets of specific primers used in this study are listed in Table 2. Kapa Taq DNA polymerase (Kapa Biosystems, Inc., Boston, MA, USA) was used to amplify DNA fragments by PCR using a GeneAmp PCR system 2700 thermal cycler (Applied Biosystems, Foster City, CA, USA). PCR products were purified with a QIAquick PCR purification kit (Qiagen), according to the protocol of the supplier, and sequenced by the Macrogen Sequencing Service (Macrogen, The Netherlands). The DNA Strider program was used for open reading frame (ORF) prediction. Commercial pGEM-T-Easy (Promega, Madison, WI, USA) or laboratory vector pBS-TA (26) was used for cloning of PCR products.
TABLE 2.
Primers used in this study
| Primer name | Sequence (5′ to 3′)a | Template |
|---|---|---|
| lsbB-F | CTCCAAGAATTCCTAAAAAAATAGG | pMN5 |
| lsbB-R | TGATATCTTAAGCTTTTCCACGTTCCCATGG | pMN5 |
| lsbB-A30-STOP-R | TGATATCTTATTATTTTCCACGTTCCCATGG | pMN5 |
| lsbB-K29A-STOP-R | TGATATCTTATTATGCTCCACGTTCCC | pMN5 |
| lsbB-A30+A31-R | TGATATCTTAAGCAGCTTTTCCACGTTCCCATGG | pMN5 |
| lsbB-A30V-R | TGATATCTTAAACTTTTCCACGTTCCCATGG | pMN5 |
| lsbB-A30G-R | TGATATCTTAACCTTTTCCACGTTCCCATGG | pMN5 |
| lsbB-A30S-R | TGATATCTTAAGATTTTCCACGTTCCCATGG | pMN5 |
| lsbB-A30P-R | TGATATCTTAAGGTTTTCCACGTTCCCATGG | pMN5 |
| lsbB-SN-F | ATGAATTCAATCCTACGTTTGTTGCTTGC | pMN5 |
| lsbB-AKA-R | TGATATCTTAAGCTTTTCCACGTTCCCATGGATAGCCGCCAGTTGCCTTTGCATGAC | pMN5 |
| LsbB-tag (GST)-F | GGATCCATGAAAACAATCCTACG | pMN5 |
| LsbX-SalI-R | GTTGTCGACTAATCAATATGTTCC | pMN5 |
| ORFF | GGCGTAAAAGATTCAGG | Total DNA BGMN1-596 or MG7284 |
| ORFR | GAAGGGTTGGTATAAGC | Total DNA BGMN1-596 or MG7284 |
| MGBH257G-F | GAAATTTCAGGATCCAATGGAAAAG | pAZIL-YvjBMN pAZIL-YvjBMG |
| MGBH257G-R | CCATTGGATCCTGAAATTTCTGTGACC | pAZIL-YvjBMN pAZIL-YvjBMG |
| MGBH257G-259K-F | GAAATTTCAGGATCCAAAGGAAAAG | pAZIL-YvjBMN pAZIL-YvjBMG |
| MGBH257G-259K-R | CCTTTGGATCCTGAAATTTCTGTGACC | pAZIL-YvjBMN pAZIL-YvjBMG |
| Eag-F | GATTGCACGGCCGAGTCTTG | pAZIL-YvjBMN pAZIL-YvjBMG |
| Eag-R | CAAGACTCGGCCGTGCAATC | pAZIL-YvjBMN pAZIL-YvjBMG |
| YvjBIV-TA-F | GCAGGACAAGCGGCCACAGCAATTTTCAGAGC | pAZIL-YvjBMG |
| YvjBIV-TA-R | GCTCTGAAAATTGCTGTGGCCGCTTGTCCTGC | |
| YvjBIV-LF-F | GGCAAGAGCAGGTTTTCCAACAATTATTCAGTTGTTAGC | pAZIL-YvjBMG |
| YvjBIV-LF-R | GCTAACAACTGAATAATTGTTGGAAAACCTGCTCTTGCC | |
| YvjBIV-TA2-F | GCAGGTTTGCCAGCAATTATTCAGTTGTTAGC | pAZIL-YvjBMG |
| YvjBIV-TA-R | GCTAACAACTGAATAATTGCTGGCAAACCTGC | |
| YvjBIV-QY-F | GCCAACAATTATTTATTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-QY-R | GCATAGCTAACAAATAAATAATTGTTGGC | |
| YvjBIV-PQ-F | GGCAAAGCACTTTCGCAAGAGAAAGAATC | pAZIL-YvjBMG |
| YvjBIV-PQ-R | GATTCTTTCTCTTGCGAAAGTGCTTTGCC | |
| YvjBIV-LF-TA-QY-F | GCGGCAAGAGCAGGTTTTCCAGCAATTATTTATTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-LF-TA-QY-R | GCATAGCTAACAAATAAATAATTGCTGGAAAACCTGCTCTTGCCGC | |
| YvjBIV-LF-TA-F | GCGGCAAGAGCAGGTTTTCCAGCAATTATTCAGTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-LF-TA-R | GCATAGCTAACAACTGAATAATTGCTGGAAAACCTGCTCTTGCCGC | |
| YvjBIV-TA-QY-F | GCGGCAAGAGCAGGTTTGCCAGCAATTATTTATTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-TA-QY-R | GCATAGCTAACAAATAAATAATTGCTGGCAAACCTGCTCTTGCCGC | |
| YvjBIV-LF-QY-F | GCGGCAAGAGCAGGTTTTCCAACAATTATTTATTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-LF-QY-R | GCATAGCTAACAAATAAATAATTGTTGGAAAACCTGCTCTTGCCGC | |
| YvjBIV-LF-TA-QY-F | GCGGCAAGAGCAGGTTTTCCAGCAATTATTTATTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-LF-TA-QY-R | GCATAGCTAACAAATAAATAATTGCTGGAAAACCTGCTCTTGCCGC | |
| YvjBIV-TA-QY-F | GCGGCAAGAGCAGGTTTGCCAGCAATTATTTATTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-TA-QY-R | GCATAGCTAACAAATAAATAATTGCTGGCAAACCTGCTCTTGCCGC | |
| YvjBIV-LF-QY-F | GCGGCAAGAGCAGGTTTTCCAACAATTATTTATTTGTTAGCTATGC | pAZIL-YvjBMG |
| YvjBIV-LF-QY-R | GCATAGCTAACAAATAAATAATTGTTGGAAAACCTGCTCTTGCCGC | |
| Ex1/SacIF | GACTGAGCTCAAAAAAGGACAAGC | pAZIL-YvjBMN |
| Ex1/HindIIIR | GGACCACCGAAGCTTGTCAAC | pAZIL-YvjBMN |
| Ex2/BamHIF | GGATCCCCCGCTTACAATGCAGGC | pAZIL-YvjBMN |
| Ex2/HindIIIR | CCTGTAAGCTTATCAAAGAAACC | pAZIL-YvjBMN |
Restriction sites are underlined; changed amino acid codons are indicated by bold type.
β-Galactosidase activity assay.
Membrane damage of lactococcal cells caused by LsbB was determined using a modified β-galactosidase assay described by O'Neill and coauthors (27). The activity of β-galactosidase was determined from the logarithmic phase of MG7284, BGMN1-596, and BGMN1-596R2/23 (16) carrying pNZ8150lacZ1PlcnB (28) by assaying the degradation of ortho-nitrophenyl-β-galactoside (ONPG) (Sigma-Aldrich Co., St. Louis, MO) at 30°C using a modified method described by Miller (29). Cells from 1 ml of logarithmic-phase (optical density at 600 nm [OD600], ∼0.6) cultures were collected by centrifugation, resuspended in 500 μl of PP buffer (0.5 M sucrose, 40 mM NH4-acetate, 10 mM Mg-acetate [pH 7]) containing 4 mg/ml lysozyme, and incubated 30 min at 37°C. Protoplasts were harvested by centrifugation (1,500 × g) and resuspended in 200 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). Suspensions were divided into two parts of 100 μl and to each was added 500 μl of Z buffer (60 mM Na2HPO4·7H2O, 40 mM NaH2PO4·H2O, 10 mM KCl, 1 mM MgSO4·7H2O, 50 mM β-mercaptoethanol). To one mixture, 20 μl of chloroform and 20 μl of 0.1% SDS were added, and to a second mixture, LsbB (100 μg/ml) was added. These mixtures were incubated for 10 min at room temperature (RT) before centrifuging at 16,000 × g for 3 min to pellet undisrupted protoplasts. The supernatants were transferred to new tubes, and 200 μl of ONPG (4 mg/ml) was added. After the appearance of yellow color, the reactions were stopped by adding 250 μl of 1 M Na2CO3, and A420 values were measured for the supernatants. β-Galactosidase activity in Miller units was calculated as 1,000 × A420/(t × v × OD600), where t is time in minutes, v is the volume of culture used in the assay in milliliters, and OD600 is the optical density of the culture at 600 nm. The efficiency of membrane damage caused by LsbB was calculated as the percentage of β-galactosidase activity obtained by treatment of cells with LsbB (100 μg/ml) compared to those obtained by SDS and chloroform (activity obtained by treatment with SDS and chloroform was taken as 100%).
Site-directed mutagenesis.
Desired mutations were introduced through the QuikChange site-directed mutagenesis protocol (Agilent Technologies, USA) with the oligonucleotide primers (and their reverse complements) listed in Table 2. After PCR for site-directed mutagenesis, the methylated template strands were digested by 1 μl (10 U) of DpnI restriction enzyme per reaction (at 37°C for 2 h) and purified by Thermo Scientific GeneJET PCR purification kit, as described by the manufacturer (Thermo Scientific, Lithuania). Subsequently, 4 μl of the purified DNA was used to transform 40 μl of E. coli TOP10 high-competency cells by heat shock treatment. The Thermo Scientific GeneJET plasmid miniprep kit was used, according to the manufacturer's recommendations (Thermo Scientific), to obtain and purify the plasmids from the selected colonies, after which sequencing (Macrogen Europe, The Netherlands) was done to confirm the introduction of the desired mutations.
Preparation of bacterial cell membrane.
The method for extracting cell membrane enzymes from selected lactococcal strains was applied according to Attri et al. (30), with modifications.
Cells from the bacterial log cultures (MG7284, BGMN1-596, and BGMN1-596/23) were harvested by centrifugation at 3,600 × g for 10 min and the cell pellets washed in TEN buffer (50 mM Tris, 10 mM EDTA, 50 mM NaCl [pH 8.0]). Cell pellets (∼5 g) were suspended in 2 to 3 ml of PP buffer (see above) with addition of lysozyme (5 mg/ml) and stirred for 1 h at 37°C. Lysozyme-treated cells were harvested by centrifugation at 1,000 × g at 4°C for 5 min, and pellets were resuspended in A buffer (50 mM sodium phosphate, 100 mM NaCl, 0.5% Triton X-100 supplemented with the protease inhibitor cocktail at a 1:200 [vol/vol] ratio [pH 8.0]). The cells were subjected to sonication for 1 min at a frequency of 8 kHz while being kept in an ice bath. The homogenate was centrifuged at 16,000 × g for 30 min to obtain the supernatant. In the next step, the supernatant was centrifuged at 100,000 × g for 18 h at 4°C to pellet the cell membranes. Pellets containing cellular membranes were resuspended in B buffer (20 mM Tris, 5 mM MgCl2, 0.01 mM Zn2+ supplemented with the protease inhibitor cocktail at a 1:200 [vol/vol] ratio [pH 6.8]).
In order to obtain more pure membrane fractions, homogenates were centrifuged at 45,000 × g for 5 h at 4°C. Supernatants representing cell cytoplasm and pellets representing the membrane fraction were stored in an ice bath (up to 7 days).
Production of polyclonal antibody.
Synthetic LsbB (ChinaPeptides Co., Ltd., Shanghai, China) was used for production of anti-LsbB polyclonal antibody.
Two regions of YvjB protein (YvjBex1 from 80th to 185th amino acids and YvjBex2 from 225th to 300th amino acids) that do not contain intermembrane domains were expressed using the pQE30 6×His tag (Qiagen) expression system for the production of anti-YvjB polyclonal antibody. First, using total DNA BGMN1-596 and designed primers ex1 SacI/HindIII for YvjBex1, and ex2 BamHI/HindIII for YvjBex2, corresponding fragments were amplified by PCR. The PCR products were purified and cloned first to pGEM-T-Easy vector, confirmed by sequencing, and recloned as SacI/HindIII or BamHI/HindIII in frame with 6×His tag to pQE30 expression vector. Fusion His-tagged proteins were expressed in E. coli M15 cells.
His tag affinity purification of YvjBex1 and YvjBex2 proteins was conducted under denaturing conditions. The refolding method using urea to disrupt noncovalent bonds and increase protein solubility was used to solubilize and make the His-tagged YvjBex1 and YvjBex2 more accessible to the nickel-nitrilotriacetic acid (Ni-NTA) resin. The recombinant proteins were purified according to the protocol recommended by The QIAexpressionist (Qiagen). The eluted proteins were dialyzed by ultrafiltration (centrifugal filter units; Amicon Ultra-15 centrifugal filter devices, 3K; Millipore). Polyclonal antibodies were produced by immunization of mice with the synthetic or purified fusion proteins in the animal house of the International Centre for Genetic Engineering and Biotechnology (ICGEB), Trieste, Italy.
Construction of GST-LsbB-tag protein and GST pulldown assay.
Specific sets of primers [LsbB-tag(GST)-F and LsbX-Sal-R; Table 2] were designed for amplification of selected a part of the lsbB gene from pMN5 plasmid DNA. The PCR product was purified and cloned to pGEM-T-Easy vector and confirmed by sequencing. The lsbB gene was transferred from pGEM-T-Easy as a BamHI/SalI fragment into the expression vector pGEX-6P-3 to obtain glutathione S-transferase (GST)-LsbB fusion protein. After transforming the expression vector into competent E. coli BL21 cells, BL21/p-GEX-6P-3/LsbB and BL21/p-GEX-6P-3 (control strain with empty vector) were grown in 50 ml of LB broth (containing 100 μg/ml ampicillin) to an OD600 (A600) of 0.6, with vigorous agitation at 37°C. Before the next step, 1 ml of the cultures was collected (noninduced samples). Fusion protein expression was induced by 0.1 mM isopropyl-β-d-thiogalactoside (IPTG) (Sigma-Aldrich) by incubating the cultures for an additional for 2 h at 28°C. One milliliter of the cultures was collected (induced samples) and analyzed. Noninduced and induced samples for both strain BL21/p-GEX-6P-3/LsbB and BL21/p-GEX-6P-3 were boiled for 2 min and checked by protein gel electrophoresis (12.5% gel) prior to Coomassie blue staining. The rest of the BL21/p-GEX-6P-3/LsbB and BL21/p-GEX-6P-3 (control strain) bacterial cultures were harvested by centrifugation at 4,500 × g for 10 min, and the cell pellets were suspended in 3 ml of lysis buffer containing 0.5% Triton X-100, 0.2% SDS, 0.5% NP-40, and 0.1% Tween 20. The cells were lysed in the ice for 30 min with gentle agitation. The lysates were subjected to sonication for 1 min (6 times 10 s) at a frequency of 10 kHz while being kept in an ice bath. The homogenate was centrifuged at 10,000 × g for 30 min at 4°C to obtain the supernatant.
In order to bind the engineered tagged protein to the matrix, supernatants of BL21/p-GEX-6P-3/LsbB and BL21/p-GEX-6P-3 were incubated with glutathione Sepharose 4 Fast Flow (GE Healthcare, Germany) for 2 h at 4°C with continuous mixing.
Beads of glutathione Sepharose (200 μl) with bound proteins were centrifuged briefly and washed with the same lysis buffer at 4°C. The quantity of GST-LsbB fusion protein and GST protein (20 μl per each) was tested by SDS-PAGE and Western blot analysis using anti-LsbB antibody. Equal amounts of beads with bound GST-LsbB and GST (50 μl each) proteins were homogeneously mixed with the membrane fractions of strains MG7284, BGMN1-596, and BGMN1-596R2/23 (50 μl each) and made up to a volume of 600 μl with supplement buffer (50 mM Tris, 120 mM NaCl [pH 6.8]). The mixture was incubated overnight at 4°C with gentle agitation, washed five times for 30 min each in phosphate-buffered saline (PBS) buffer (10 mM Na2HPO4, 1 mM KH2PO4, 140 mM NaCl, 3 mM KCl [pH 7.1]), and protein complexes were pulled down.
Western blotting.
Protein(s) associated with the GST-LsbB fusion protein and GST protein was analyzed by SDS-PAGE and Western blot assays using anti-YvjB and anti-LsbB antibodies (independently). Samples were loaded into a 12.5% polyacrylamide gel, subjected to electrophoresis, and transferred to a polyvinylidene difluoride (PVDF) membrane (Merck Millipore, Darmstadt, Germany) for 1 h at appropriate voltage (constant voltage). Membranes were incubated with 10% skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) overnight at 4°C in order to block nonspecific binding. Following blocking, the membranes were incubated for 1 h at RT with gentle agitation in appropriate dilutions of primary antibodies, as follows: mouse polyclonal antibody anti-YvjB, 1:3,000 dilution, and mouse polyclonal antibody anti-LsbB, 1:5,000 dilution (independently). Primary antibodies were diluted in 5% skim milk in TBST. After washing three times in TBST for 15 min, membranes were incubated for 1 h with horseradish peroxidase-labeled anti-mouse IgG (A9044 anti-mouse; Sigma) at a 1:10,000 dilution in 5% skim milk in TBST. The blots were washed three times in TBST for 15 min. Bands of target proteins were detected using the CN/DAB substrate kit (Thermo Scientific), according to the manufacturer's instructions.
Immunocytochemistry.
After plating on coverslips, bacterial cells were fixed in 4% paraformaldehyde (PFA) for 20 min at RT. Cells were blocked in 5% bovine serum albumin (BSA) in PBS for 1 h at RT. Primary antibodies were diluted in PBS containing 1% BSA and incubated overnight at 4°C with mouse polyclonal anti-LsbB (this study, diluted 1:50). Coverslips were washed three times for 10 min in PBS and incubated with biotinylated goat anti-mouse IgG (Vector, Burlingame, CA, USA) for 1 h at RT in 1% BSA, followed by Cy3-streptavidin (diluted 1:5,000; Jackson ImmunoResearch, West Grove, PA, USA) diluted in PBS for 1 h at RT. Bacterial DNA was stained with 0.1 mg/ml 4′,6-diamino-2-phenylindole (DAPI; Sigma-Aldrich). Samples were visualized under an Olympus BX51 fluorescence microscope with appropriate filters and analyzed using the CytoVision 3.1 software (Applied Imaging Corporation, USA).
RESULTS
LsbB causes membrane damage of sensitive cells.
To determine whether LsbB causes membrane damage, different lactococcal strains with different levels of sensitivity to LsbB were exposed to synthetic LsbB. These were the resistant strain MG7284, the sensitive strain BGMN1-596, and the semisensitive mutant BGMN1-596R2/23 (see Table 1). The strains were transformed with pNZ8150lacZ1PlcnB encoding β-galactosidase, which was used as a reporter system for the detection of membrane damage. The results showed that the level of β-galactosidase activity (and thus membrane damage) is directly correlated with the level of sensitivity to LsbB (see Fig. S1 in the supplemental material), i.e., more β-galactosidase activity was found in sensitive cells than in less-sensitive cells. The results provide evidence that LsbB kills target cells by membrane damage.
Identification of essential amino acid residues in LsbB bacteriocin involved in antimicrobial activity.
We have previously demonstrated that the last C-terminal 8 amino acids of the LsbB bacteriocin are involved in the interaction with the receptor protein YvjB (23). The residue Trp25 plays a particularly important role, as the substitution Trp25 to Ala caused a total loss of antimicrobial activity. To further study the importance of Trp25 and other amino acids in receptor binding, additional amino acid substitutions were made in LsbB (Fig. 1). It was found that the substitution of Trp25 with Phe, an amino acid belonging to the same aromatic group, caused a small reduction in bacteriocin activity, indicating the involvement of specific groups on amino acid 25 in specific interactions with YvjB (Fig. 1B). Previous work indicates that the last residue, Ala30, is important for bacteriocin activity. As expected, the deletion of the terminal Ala30, the addition of another alanine at the end (Ala31), or the substitution of Ala30 with Pro caused a total loss of antimicrobial activity of LsbB, whereas substitutions of Ala30 with other residues, such as Gly and Val, had no apparent impact on the bacteriocin activity, while substitution with Ser enhanced bacteriocin activity (Fig. 1A). In our previous work (23), we demonstrated that the Lys29Ala mutation increased bacteriocin activity. To assess whether this substitution (Lys29Ala) can replace the importance of the terminal alanine Ala30, we created a double mutant with Lys29Ala and ΔAla30; thus, the resulting peptide was only 29 residues, with alanine as the last residue. As shown in Fig. 1E, this double mutant did not exert any antimicrobial activity at all. These results confirmed that the residues Trp25 and Ala30 are both important for LsbB's antimicrobial activity, although Ala30 appeared to tolerate to some extent a substitution with another aliphatic amino acid or serine. The results might also indicate that the distance between Trp25 and the last alanine is crucial, as shortening (termination with Ala29 instead of Ala30) or elongation (addition of Ala31) of this distance seriously affected the antimicrobial activity.
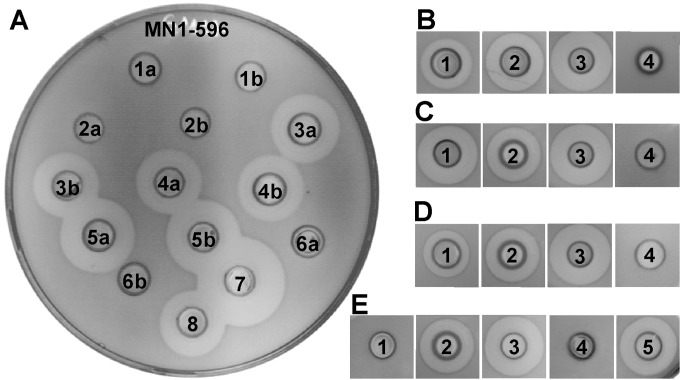
FIG 1.
Antimicrobial activity of LsbB-derived mutants. (A) Modifications at or near residue Ala30. 1, ΔAla30; 2, Ala30 plus Ala31; 3, Ala30Val; 4, Ala30Gly; 5, Ala30Ser; 6, Ala30Pro; 7, BGMN1-596T; 8, MG7284/pAZIL-lsbB (a and b are two colonies from the same transformation). (B) Trp25Phe substitution. (C) Lys17Ala plus Lys19Ala substitution. (D) Lys2Asn plus Thr3Ser substitutions. (E) Lys29Ala plus ΔAla30 substitutions. (B to E) 1, mutated LsbB; 2, MG7284/pAZIL-lsbB; 3, BGMN1-596T; 4, BGMN1-596; 5, mutated LsbB with one Lys29Ala substitution. Cultures of transformants were introduced into wells made in soft agar inoculated with indicator strain L. lactis BGMN1-596. The size of the zone is referred to the clone containing the wild-type lsbB gene (MG7284/pAZIL-lsbB). Inhibition is seen as clear zones around the wells.
To assess the importance of the N-terminal half, we created a double mutant; Lys2 and Thr3 were changed to Asn2 and Ser3, respectively. The activity of the modified peptide did not change drastically (Fig. 1D), indicating that the N-terminal part of LsbB seems to be less sensitive to changes/modifications.
One of the peptides (LcnG-α) of the two-peptide bacteriocin lactococcin G (like LsbB) contains a series of cationic residues (Arg35Lys36Lys37Lys38His39) at the C-terminal part, and it has been proposed that this feature functions as a means to force the peptide to the target cell membrane by electrostatic interaction (31). LsbB contains a similar string of basic residues in its C-terminal half (His16Lys17Lys18Lys19Thr20). To examine whether this feature has a similar function, the number of cationic residues in this feature was reduced by changing His16Lys17Lys18Lys19Thr20 to His16Ala17Lys18Ala19Thr20, thus reducing the pI from 10.99 to 10.50. The resulting mutant peptide did not have any apparent adverse effect on bacteriocin activity; in fact, it even slightly enhanced the activity (Fig. 1C).
Based on all these amino acid substitutions, deletions, and additions, it is possible to conclude that the C-terminal part of the LsbB bacteriocin is most important for bacteriocin activity, likely through interaction with the receptor protein YvjB.
Mapping of the region on YvjB involved in LsbB interaction.
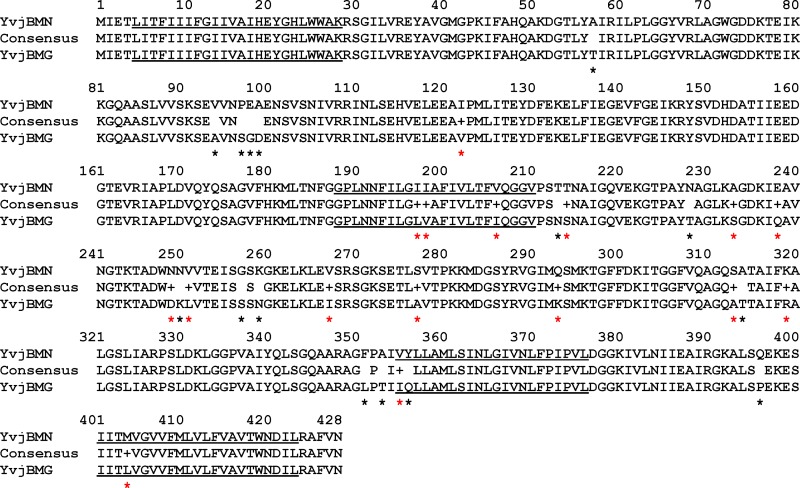
It has previously been established that among lactococci, there exist both sensitive and resistant strains to LsbB, such as the resistant strain MG7284 and the sensitive strain BGMN1-596, both of lactococcal genotype. The LsbB-resistant strain MG7284 possesses a homolog of YvjB (YvjBMG) that differs in 31 residues from the YvjB of the sensitive strain BGMN1-596 (YvjBMN). These differences appear to be randomly distributed over the entire protein length (Fig. 2). To search for the regions in YvjBMN and YvjBMG that cause their different sensitivity to LsbB, hybrid proteins containing portions of both proteins were constructed (see Fig. S2 in the supplemental material). A hybrid protein containing the C-terminal half of YvjBMN and the N-terminal half of YvjBMG (YvjB1/2MG-1/2MN) was able to confer sensitivity to LsbB when expressed in strain MG7284 (naturally resistant to LsbB), indicating that the C-terminal half of YvjBMN is involved in target specificity. Further, a hybrid protein (YvjB3/4MG-1/4MN) with an even smaller part of YvjBMN at the C terminus was still capable of conferring sensitivity to LsbB. Different hybrid combinations, as depicted in Fig. S2, further confirmed that the last section of the C terminus of YvjB is most likely involved in direct interaction with LsbB and consequently in sensitivity to the bacteriocin.
FIG 2.
Comparison of the amino acid sequences of YvjBMN and YvjBMG using Strider software. All amino acid differences are indicated by red stars for ones belonging to the same physicochemical group and by black stars for ones belonging to different physicochemical groups. Underlined amino acids represent transmembrane domains.
Given that the potential LsbB-binding domain is located at the C-terminal end of YvjB, we are interested in finding the membrane topology of this part. Using Phobius (http://phobius.binf.ku.dk/index.html) or TMHMM server version 2.0 for prediction of transmembrane (TM) helices in proteins (http://www.cbs.dtu.dk/services/TMHMM/), YvjB was predicted to possess four transmembrane (TM) domains: TMI from amino acids (aa) 5 to 27, TMII from aa 188 to 210, TMIII from aa 354 to 376, and TMIV from aa 401 to 423 (Fig. 2; see also Fig. S3 in the supplemental material). Based on this prediction, the LsbB-binding domain is likely located within the region containing two transmembrane helices connected by a noncytoplasmic hinge.
Further defining the amino acids involved in receptor function by site-directed mutagenesis.
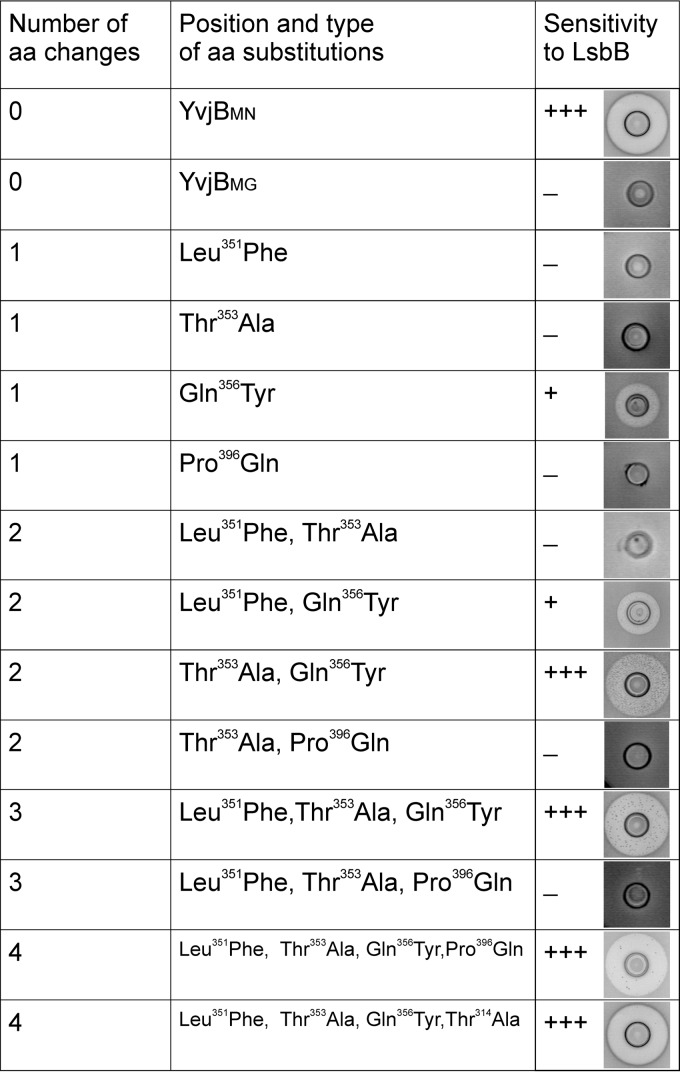
The hybrid molecule YvjB3/4MG-1/4MN contains the last part of the YvjBMN protein, which differs from YvjBMG in six positions (amino acids Phe351, Ala353, Val354, Tyr356, Gln396, and Met404 in YvjBMN are different in comparison to those present at the same positions Leu351, Thr353, Ile354, Gln356, Pro396, and Leu404 in YvjBMG) (Fig. 2). Some of these differences are likely responsible for the observed difference between YvjBMG and YvjBMN in terms of sensitivity to LsbB. Four of YvjBMG's six amino acids belong to different physicochemical groups from the ones in YvjBMN. These were therefore mutated separately and in combination, in both the YvjBMG and YvjB3/4MN-1/4MG proteins (in constructs pAZILyvjBMG and pAZILyvjB3/4MN-1/4MG, respectively), into the residues present in YvjBMN in order to determine which amino acid(s) is directly responsible for the sensitivity to LsbB. In the first round of mutagenesis (single amino acid changes), it is worth noting that Tyr356 was able to transform carrier cells from a resistant to a sensitive phenotype (Fig. 3). This sensitivity occurred, in fact, in both tested YvjB proteins (MG7284/YvjBMG and MG7284/YvjB3/4MN-1/4MG), although the zone of inhibition by BGMN1-596T as an LsbB producer was smaller than for YvjBMN. This smaller zone of inhibition indicated a possible additional effect of other amino acids in the interaction with LsbB. In the second round of site-directed mutagenesis (when different combinations of two, three, or four amino acids were changed), some interesting results were obtained, as depicted in Fig. 3. First, only the changes in combination with Gln356Tyr could cause sensitivity of the carriers. However, the zones of inhibition of these mutants were different in size and transparency; for example, adding the change Leu351Phe to Gln356Tyr slightly increased the clear inhibition zone (Fig. 3). Additionally, the change Gln356Tyr plus Thr353Ala drastically increased the zone of inhibition, which was even larger than with YvjBMN: however, it contained a high number of revertant-like resistant colonies (Fig. 3; see also Fig. S4 in the supplemental material). Triple and quadruple amino acid changes to YvjBMG and YvjB3/4MN-1/4MG showed that additional changes reduced the number of revertant-like resistant bacteria in the inhibition zone and restored the size of the zone to the size obtained with YvjBMN. In summary, it was concluded that the amino acid in the YvjB receptor most responsible for the interaction with LsbB and the sensitivity of cells to LsbB is Tyr356, whereas the other amino acids are also important but to a lesser extent than Tyr356.
FIG 3.
Antimicrobial activity of LsbB on different mutants obtained by site-directed mutagenesis of YvjBMG protein. Cultures of MG7284 transformants carrying different amino acid substitutions of YvjB protein were inoculated as indicator strains into soft agar in which wells were loaded with L. lactis BGMN1-596T. The size of the zone is referred to the clone containing the wild-type yvjB gene (L. lactis BGMN1-596). Inhibition is seen as clear zones around the wells. −, no inhibition; +, inhibition zone up to 3 mm; +++, inhibition zone >5 mm; aa, amino acid.
It is worth noting that the residue Tyr356 is located at the beginning of the predicted third transmembrane helix (from residue 355 to residue 376), with the C-terminal end pointing toward the cytoplasm. More important, Tyr356 is closely flanked by three residues that differentiate YvjBMG from YvjBMN in this region, suggesting that these residues together probably form a site to which LsbB binds to kill target cells.
LsbB interacts directly with YvjB.
It was of interest to determine if the LsbB bacteriocin interacts directly with the YvjB receptor.
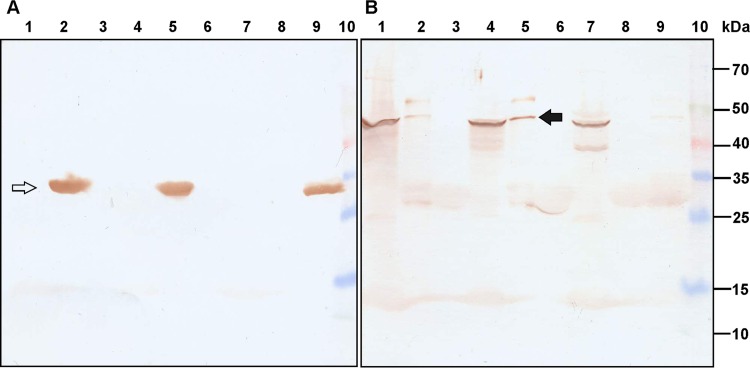
In a GST pulldown assay, LsbB coupled to GST (it was decided to fuse the GST tag at the N terminus of LsbB, as our previous studies determined that the C terminus was important for the interaction with the target receptor [23]) was able to bind YvjBMN protein from membrane fractions and to retain it during stringent washing (Fig. 4). Similar experiments determined that YvjBMG and YvjBMNR2/23 proteins were able to bind LsbB but much less efficiently than YvjBMN (Fig. 4B, lanes 2, 9, and 5, respectively). In the control reaction with GST alone, no unspecific binding was detected (Fig. 4B, lanes 3, 6, and 8, respectively).
FIG 4.
GST pulldown assay with purified cell membranes. (A) Western blot with anti-LsbB antibody. (B) Western blot with anti-YvjB antibody. Lane 1, total cell membrane fraction of MG7284; lane 2, proteins that interacted with GST-LsbB from the cell membrane fraction of MG7284; lane 3, proteins that interacted with GST from the cell membrane fraction of MG7284; lane 4, total cell membrane fraction of BGMN1-596; lane 5, proteins that interacted with GST-LsbB from the cell membrane fraction of BGMN1-596; lane 6, proteins that interacted with GST from the cell membrane fraction of BGMN1-596; lane 7, total cell membrane fraction of BGMN1-596R2/23; lane 8, proteins that interacted with GST from the cell membrane fraction of BGMN1-596R2/23; lane 9, proteins that interacted with GST-LsbB from the cell membrane fraction of BGMN1-596R2/23; lane 10, Spectra multicolor broad range protein ladder (Thermo Fisher Scientific). The quantities of proteins of membrane fractions of MG7284, BGMN1-596, and BGMN1-596R2/23 were normalized to 100 μg per reaction. The white arrow indicates the GST-LsbB fusion protein, while the black arrow indicates YvjBMN protein from the GST pulldown assay.
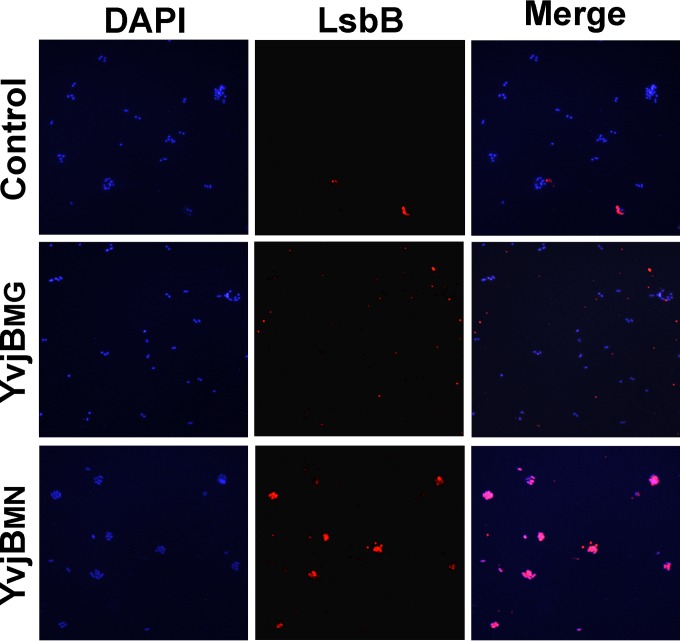
The GST pulldown assay is an in vitro assay. Thus, to determine the interaction in vivo, an analysis with fluorescent antibodies against YvjB and LsbB proteins was performed in live cells. The immunocytochemistry assay was performed with transformants of E. faecalis BGZLS10-27 harboring the pAZIL vector carrying different yvjB genes (yvjBMN and yvjBMG). We used the enterococcal strain as a host, because there was no lactococcal strain without endogenous YvjB protein to serve as a negative control (the negative control was strain BGZLS10-27 transformed with empty pAZIL vector). In addition, this particular enterococcal strain was used because it was resistant to LsbB and nonreactive with the antibodies used (data not shown). Before beginning this assay, the sensitivity of BGZLS10-27 transformants carrying different constructs was confirmed by an antimicrobial assay (see Fig. S5 in the supplemental material). The in vivo immunocytochemistry assay showed that LsbB interacts specifically with the cells expressing YvjBMN (Fig. 5). Full matching of the color that locates the presence of transformed cells (DAPI) and fluorescent antibody that locates LsbB bacteriocin (LsbB) is obtained only when transformants BGZLS10-27/pAZIL-YvjBMN (merge) were used. In experiments that used the transformants carrying empty plasmid BGZLS10-27/pAZIL (control) or cloned YvjB gene from the strain MG7284 (BGZLS10-27/pAZIL-YvjBMG), colocalization of these two signals was not obtained.
FIG 5.
Immunocytochemistry assay with anti-LsbB antibodies was performed on transformants of Enterococcus faecalis BGZLS10-27 with pAZIL vector (control), pAZIL-YvjBMG (YyjBMG), and pAZIL-YvjBMN (YvjBMN). Bacterial DNA was stained with 4′,6-diamino-2-phenylindole (DAPI).
DISCUSSION
In recent years, two important needs have emerged: the increasing consumer demand for natural food preservatives, such as bacteriocins produced by lactic acid bacteria, and the demand for new antimicrobial compounds in response to the continuing emergence of antibiotic-resistant bacteria, in the face of declining numbers of new antibiotics (32–34). Consequently, the research and application of bacteriocins are ever-more-attractive propositions. Until now, it has been shown that the antimicrobial activity of bacteriocins is directly related to the presence of specific receptor proteins in the membranes of target bacteria. Thus, information on the receptors to which bacteriocins bind is potentially of great importance (4).
In our previous studies, we identified the Zn-dependent metallopeptidase YvjB as the receptor for the bacteriocin LsbB (16) and further established that the C-terminal part of LsbB is involved in the interaction with the receptor protein YvjB (23). In this work, we combined site-directed mutagenesis with the construction of hybrid protein molecules to define the residues of LsbB and YvjB involved in the interactions that eventually lead to the killing of target cells. We demonstrated that the essential amino acids are Trp25 and Ala30 in the LsbB C terminus and Tyr356 and Ala353 in the penultimate transmembrane domain of YvjB receptor.
Such a site-directed mutagenesis approach has been used for amino acid substitution in different bacteriocins of lactic acid bacteria to analyze function or to improve antimicrobial activity (35–40). The results obtained by site-directed mutagenesis of the LsbB bacteriocin are consistent with data obtained by other laboratories, where most amino acid substitutions cause a reduction in or complete loss of activity. It is interesting, however, that some substitutions, such as alanine to serine (Ala30Ser), enhanced bacteriocin activity, most likely due to better interaction and positioning in the YvjB receptor. In addition, we suggest that not only residues Trp25 and Ala30, but also the distance between them, play an essential role in the antimicrobial activity of LsbB.
The construction of hybrid molecules and site-directed mutagenesis with structure-function assays enabled us to define essential domains responsible for the interaction of the target receptor YvjB with LsbB. It is interesting that all key amino acids involved in the interaction with LsbB are located in the beginning of the penultimate transmembrane domain of YvjB (Fig. 2; see also Fig. S3 in the supplemental material). The Gly188Ser mutant obtained by random mutagenesis (16) also includes changes in the beginning of the second transmembrane domain and shows a semiresistant phenotype. From the results presented here, it is possible to conclude that these regions are most likely accessible for interaction with LsbB. It is interesting that the beginning of the third transmembrane domain (amino acids Ala353, Val354, and Tyr356) seems to be very close to the outside surface of the membrane, possibly making it accessible for LsbB interaction (see Fig. S3). In contrast, the beginning of the second transmembrane domain is located closer to the cytoplasmic side, indicating that both sides of the membrane are involved in the interaction of YvjB with LsbB, causing membrane damage of cells. It could be postulated that the third transmembrane domain is involved in the first interaction with LsbB, but the second transmembrane domain is also important for complex formation and membrane damage of sensitive cells. Mutants with an amino acid substitution in the second transmembrane domain, such as Gly188Ser, are semiresistant, meaning that they can interact with LsbB on the surface but require higher concentrations of LsbB for complex formation and membrane damage of the cells. Given that LsbB in vitro was able to interact with all three YvjB proteins (from resistant strain MG7284, semiresistant mutant BGMN1-596R2/23, and sensitive strain BGMN1-596) but not in vivo indicates the involvement of other factors present in cell wall in the control of the interaction. Any changes to the essential amino acids in the bacteriocin receptor protein cause partial or complete resistance to certain bacteriocins. For this reason, it is important to identify specific receptors for bacteriocins and the domains involved in the interaction. The identification of new bacteriocin receptors present in potential target bacteria and understanding of their mechanisms of action are of great importance for the design of target-specific engineered antimicrobials.
Supplementary Material
ACKNOWLEDGMENTS
The Ministry of Education and Science of the Republic of Serbia, Republic of Serbia (grant 173019), supported this work.
We thank the personnel of the Animal House of ICGEB for excellent technical assistance during the immunization of animals and blood sampling.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01293-16.
REFERENCES
- 1.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 2.Nes IF, Yoon SS, Diep DB. 2007. Ribosomally synthesized antimicrobial peptides (bacteriocins) in lactic acid bacteria: a review. Food Sci Biotechnol 16:675–690. [Google Scholar]
- 3.Rea MC, Cotter PD, Hill C, Ross RP. 2011. Classification of bacteriocins from Gram-positive bacteria, p 29–53. In Drider D, Rebuffat S (ed), Prokaryotic antimicrobial peptides–from genes to applications. Springer, New York, NY. [Google Scholar]
- 4.Cotter PD. 2014. An ‘Upp’-turn in bacteriocin receptor identification. Mol Microbiol 92:1159–1163. doi: 10.1111/mmi.12645. [DOI] [PubMed] [Google Scholar]
- 5.Perez RH, Zendo T, Sonomoto K. 2014. Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact 13(Suppl 1):S3. doi: 10.1186/1475-2859-13-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastos MDC, Coelho ML, Santos OC. 2015. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 161:683–700. doi: 10.1099/mic.0.082289-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Hoover DG. 2003. Bacteriocins and their food applications. Compr Rev Food Sci Food Saf 2:82–100. doi: 10.1111/j.1541-4337.2003.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 8.Cavera VL, Arthur TD, Kashtanov D, Chikindas ML. 2015. Bacteriocins and their position in the next wave of conventional antibiotics. Int J Antimicrob Agents 46:494–501. doi: 10.1016/j.ijantimicag.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Martin NI, Breukink E. 2007. Expanding role of lipid II as a target for lantibiotics. Future Microbiol 2:513–525. doi: 10.2217/17460913.2.5.513. [DOI] [PubMed] [Google Scholar]
- 10.Müller A, Ulm H, Reder-Christ K, Sahl HG, Schneider T. 2012. Interaction of type A lantibiotics with undecaprenol-bound cell envelope precursors. Microb Drug Resist 18:261–270. doi: 10.1089/mdr.2011.0242. [DOI] [PubMed] [Google Scholar]
- 11.Münch D, Muller A, Schneider T, Kohl B, Wenzel M, Bandow JE, Maffioli S, Sosio M, Donadio S, Wimmer R, Sahl HG. 2014. The lantibiotic NAI-107 binds to bactoprenol bound cell wall precursors and impairs membrane functions. J Biol Chem 289:12063–12076. doi: 10.1074/jbc.M113.537449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez B, Bottiger T, Schneider T, Rodriguez A, Sahl HG, Wiedemann I. 2008. Specific interaction of the unmodified bacteriocin lactococcin 972 with the cell wall precursor lipid II. Appl Environ Microbiol 74:4666–4670. doi: 10.1128/AEM.00092-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF. 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc Natl Acad Sci U S A 104:2384–2389. doi: 10.1073/pnas.0608775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjos M, Salehian Z, Nes IF, Diep DB. 2010. An extracellular loop of the mannose phosphotransferase system component IIC is responsible for specific targeting by class IIa bacteriocins. J Bacteriol 192:5906–5913. doi: 10.1128/JB.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrielsen C, Brede DA, Hernández PE, Nes IF, Diep DB. 2012. The maltose ABC transporter in Lactococcus lactis facilitates high-level sensitivity to the circular bacteriocin garvicin ML. Antimicrob Agents Chemother 56:2908–2915. doi: 10.1128/AAC.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzelac G, Kojic M, Lozo J, Aleksandrzak-Piekarczyk T, Gabrielsen C, Kristensen T, Nes IF, Diep DB, Topisirovic L. 2013. A Zn-dependent metallopeptidase is responsible for sensitivity to LsbB, a class II leaderless bacteriocin of Lactococcus lactis subsp. lactis BGMN1-5. J Bacteriol 195:5614–5621. doi: 10.1128/JB.00859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjos M, Oppegård C, Diep DB, Nes IF, Veening JW, Nissen-Meyer J, Kristensen T. 2014. Sensitivity to the two-peptide bacteriocin lactococcin G is dependent on UppP, an enzyme involved in cell-wall synthesis. Mol Microbiol 92:1177–1187. doi: 10.1111/mmi.12632. [DOI] [PubMed] [Google Scholar]
- 18.Kojic M, Strahinic I, Fira D, Jovcic B, Topisirovic L. 2006. Plasmid content and bacteriocin production by five strains of Lactococcus lactis isolated from semi-hard homemade cheese. Can J Microbiol 52:1110–1120. doi: 10.1139/w06-072. [DOI] [PubMed] [Google Scholar]
- 19.Kroos L, Akiyama Y. 2013. Biochemical and structural insights into intramembrane metalloprotease mechanisms. Biochim Biophys Acta 1828:2873–2885. doi: 10.1016/j.bbamem.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama K, Mizuno S, Hizukuri Y, Mori H, Nogi T, Akiyama Y. 2015. Roles of the membrane-reentrant β-hairpin-like loop of RseP protease in selective substrate cleavage. eLife 4:e08928. doi: 10.7554/eLife.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hizukuri Y, Oda T, Tabata S, Tamura-Kawakami K, Oi R, Sato M, Takagi J, Akiyama Y, Nogi T. 2014. A structure-based model of substrate discrimination by a noncanonical PDZ tandem in the intramembrane-cleaving protease RseP. Structure 22:326–336. doi: 10.1016/j.str.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Varahan S, Iyer VS, Moore WT, Hancock LE. 2013. Eep confers lysozyme resistance to Enterococcus faecalis via the activation of the extracytoplasmic function sigma factor SigV. J Bacteriol 195:3125–3134. doi: 10.1128/JB.00291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ovchinnikov K, Kristiansen PE, Uzelac G, Topisirovic L, Kojic M, Nissen-Meyer J, Nes IF, Diep DB. 2014. Defining the structure and receptor binding domain of the leaderless bacteriocin LsbB. J Biol Chem 289:23838–23845. doi: 10.1074/jbc.M114.579698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojic M, Svircevic J, Banina A, Topisirovic L. 1991. Bacteriocin-producing strain of Lactococcus lactis subsp. diacitilactis S50. Appl Environ Microbiol 57:1835–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holo H, Nes IF. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol 55:3119–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uzelac G, Miljkovic M, Lozo J, Radulovic Z, Tosic N, Kojic M. 2015. Expression of bacteriocin LsbB is dependent on a transcription terminator. Microbiol Res 179:45–53. doi: 10.1016/j.micres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill AJ, Miller K, Oliva B, Chopra I. 2004. Comparison of assays for detection of agents causing membrane damage in Staphylococcus aureus. J Antimicrob Chemother 54:1127–1129. doi: 10.1093/jac/dkh476. [DOI] [PubMed] [Google Scholar]
- 28.Vukotic G, Mirkovic N, Jovcic B, Miljkovic M, Strahinic I, Fira D, Radulovic Z, Kojic M. 2015. Proteinase PrtP impairs lactococcin LcnB activity in Lactococcus lactis BGMN1-501: new insights into bacteriocin regulation. Front Microbiol 6:92. doi: 10.3389/fmicb.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JM. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 30.Attri P, Jodha D, Singh J, Dhanda S. 2012. An improved protocol for rapid extraction of membrane enzymes from Gram positive bacteria. Anal Methods 4:2574–2577. doi: 10.1039/c2ay05931b. [DOI] [Google Scholar]
- 31.Oppegård C, Rogne P, Kristiansen PE, Nissen-Meyer J. 2010. Structure analysis of the two-peptide bacteriocin lactococcin G by introducing d-amino acid residues. Microbiology 156:1883–1889. doi: 10.1099/mic.0.038430-0. [DOI] [PubMed] [Google Scholar]
- 32.Braine T. 2011. Race against time to develop new antibiotics. Bull World Health Organ 89:88–89. doi: 10.2471/BLT.11.030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebhart G, Lok S, Clare S, Tomas M, Stares M, Scholl D, Donskey C, Lawley TD, Govoni GR. 2015. A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. mBio 6(2):e02368-14. doi: 10.1128/mBio.02368-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Shea EF, O'Connor PM, O'Sullivan O, Cotter PD, Ross RP, Hill C. 2013. Bactofencin A, a new type of cationic bacteriocin with unusual immunity. mBio 4(6):e00498-13. doi: 10.1128/mBio.00498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Kraaij C, Breukink E, Rollema HS, Siezen RJ, Demel RA, De Kruijff B, Kuipers OP. 1997. Influence of charge differences in the C-terminal part of nisin on antimicrobial activity and signaling capacity. Eur J Biochem 247:114–120. doi: 10.1111/j.1432-1033.1997.00114.x. [DOI] [PubMed] [Google Scholar]
- 36.Quadri LE, Yan LZ, Stiles ME, Vederas JC. 1997. Effect of amino acid substitutions on the activity of carnobacteriocin B2. Overproduction of the antimicrobial peptide, its engineered variants, and its precursor in Escherichia coli. J Biol Chem 272:3384–3388. [DOI] [PubMed] [Google Scholar]
- 37.Kazazic M, Nissen-Meyer J, Fimland G. 2002. Mutational analysis of the role of charged residues in target-cell binding, potency and specificity of the pediocin-like bacteriocin sakacin P. Microbiology 148:2019–2027. doi: 10.1099/00221287-148-7-2019. [DOI] [PubMed] [Google Scholar]
- 38.Haugen HS, Kristiansen PE, Fimland G, Nissen-Meyer J. 2008. Mutational analysis of the class IIa bacteriocin curvacin A and its orientation in target cell membranes. Appl Environ Microbiol 74:6766–6773. doi: 10.1128/AEM.01068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haugen HS, Fimland G, Nissen-Meyer J. 2011. Mutational analysis of residues in the helical region of the class IIa bacteriocin pediocin PA-1. Appl Environ Microbiol 77:1966–1972. doi: 10.1128/AEM.02488-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plat A, Kluskens LD, Kuipers A, Rink R, Moll GN. 2011. Requirements of the engineered leader peptide of nisin for inducing modification, export, and cleavage. Appl Environ Microbiol 77:604–611. doi: 10.1128/AEM.01503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gasson MJ. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol 154:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valenzuela AS, ben Omar N, Abriouel H, López RL, Veljovic K, Canamero MM, Kojic M, Topisirovic L, Gálvez A. 2009. Virulence factors, antibiotic resistance, and bacteriocins in enterococci from artisan foods of animal origin. Food Control 20:381–385. doi: 10.1016/j.foodcont.2008.06.004. [DOI] [Google Scholar]
- 43.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 44.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol 177:7011–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojic M, Jovcic B, Strahinic I, Begovic J, Lozo J, Veljovic K, Topisirovic L. 2011. Cloning and expression of a novel lactococcal aggregation factor from Lactococcus lactis subsp. lactis BGKP1. BMC Microbiol 11:265–274. doi: 10.1186/1471-2180-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.