Abstract
Background
The patterns of regional metastasis in adenoid cystic carcinoma (ACC) of the head and neck and its association with outcome is not established.
Methods
We conducted a retrospective multicentered multivariate analysis of 270 patients who underwent neck dissection.
Results
The incidence rate of neck metastases was 29%. The rate observed in the oral cavity is 37%, and in the major salivary glands is 19% (p = .001). The rate of occult nodal metastases was 17%. Overall 5-year survival rates were 44% in patients undergoing therapeutic neck dissections, and 65% and 73% among those undergoing elective neck dissections, with and without nodal metastases, respectively (p = .017). Multivariate analysis revealed that the primary site, nodal classification, and margin status were independent predictors of survival.
Conclusion
Our findings support the consideration of elective neck treatment in patients with ACC of the oral cavity.
Keywords: incidence, lymph node, metastasis, survival, adenoid cystic carcinoma
INTRODUCTION
Adenoid cystic carcinoma (ACC) accounts for 3% to 5% of all head and neck malignancies. ACC is characterized by an intermediate growth rate, low probability of lymphatic spread, and frequent lung metastases.1 The presence of cervical lymph node metastases is one of the most significant prognostic factors in patients with carcinomas of the head and neck. Because the survival rate of patients with neck metastases decreases, according to the number and level of the nodes involved, treatment of the neck is crucial in the management of these patients.2–4 Clinical evidence of nodal metastases clearly requires therapeutic neck dissection in any head and neck cancer. However, in the absence of clinical or radiological evidence of nodal metastases, the decision regarding an elective neck dissection should be based on the incidence of occult lymph node metastases and on the expectation of the impact of treatment on survival. A number of clinical series of patients with head and neck squamous cell carcinoma describe the pattern of lymphatic drainage and the incidence of neck metastases.5–7 Previous publications questioned the need for elective neck dissection in patients with salivary gland carcinoma, because the rate of occult neck metastasis in low and intermediate grade tumors is relatively small.8,9 Early reports and a recent comprehensive review of the literature indicated that the general incidence of cervical lymph node metastasis in ACC is approximately 10%.10,11 However, because of the scarcity of data regarding the incidence of neck metastases and its association with outcomes of patients with ACC, the association between the presence of lymph node metastases and the overall survival rate of ACC is inconclusive. Therefore, the advisability of an elective neck treatment in these patients is unclear.
The main purposes of this study were to assess the incidence of lymph node metastases in patients with ACC and to assess its association with survival.
PATIENTS AND METHODS
Patients
The International Study Group of the ACC cohort comprised all patients treated for ACC of the head and neck between 1985 and 2011 at 9 cancer centers worldwide. Data were recorded retrospectively using uniform database templates to ensure consistent collection. Eligible patients had undergone primary surgery for head and neck ACC, with or without adjuvant chemoradiotherapy. Of the 495 patients in the cohort, 270 (55%) had undergone a neck dissection involving levels I to III, I to IV, or I to V, as described by the American Head and Neck Society.12 The decision whether to perform an elective neck dissection was based on the surgeon’s preference. The duration of follow-up ranged from 7 to 258 months (median, 51 months).
Histopathological analysis
After neck dissection, specimens were subjected to pathological analysis by a certified head and neck pathologist in each center. Specimen dissection and tissue sampling of the primary tumor were carried out according to the guidelines for the histopathological assessment of head and neck cancer carcinoma.13
Statistical analysis
Five-year overall survival (OS), disease-specific survival (DSS), disease-free survival, local control, regional control, and distant metastasis rates were calculated using the Kaplan–Meier method for patients undergoing therapeutic neck dissection, elective neck dissection without nodal metastasis (cN0/pN0), and elective neck dissection with occult metastasis (cN0/pN+). The difference in survival rate was assessed by the log-rank test.14 OS was measured from the date of surgery to the date of death or last follow-up. The DSS was calculated from the time of diagnosis to death resulting from ACC. The association between individual clinical features and survival was determined with the log-rank test.15 A multivariate analysis using the Cox proportional hazards regression model was performed to compare the factors with prognostic potential as indicated by univariate analyses.16,17 The seventh edition of the TNM staging system for head and neck cancer was used for TNM staging. The limit of significance for all analyses was defined as p < .05. Two-sided statistical tests were used in all calculations. Statistical analysis of the distribution of the lymph node metastases was performed, and a correlation between distribution and primary sites was established. Calculations were performed with JMP version 10 (SAS Institute, Cary, NC). The Fisher exact test (StatCalc 2.0; University of Louisiana, Lafayette, LA) was used when the number of events was <10.
The study was approved by the local institutional review board committees of the participating centers.
RESULTS
All 270 patients of the International Study Group of the ACC cohort who underwent neck dissection for ACC were included in this study. The study was comprised of 140 men (52%) and 130 women (48%), with a median age of 56 years (range, 20–88 years). The primary tumor sites were minor salivary glands of the oral cavity in 148 patients (55%), parotid gland in 54 (20%), submandibular glands in 39 (14%), sublingual glands in 12 (4%), sinonasal salivary glands in 25 (9%), and larynx in 2 (1%). Preoperative clinical and radiological workup revealed ipsilateral nodal metastases in 44 patients (16%), consisting of 33 in levels I to III and 11 in levels IV to V (all on the ipsilateral side).
Elective neck dissection was performed in 226 patients (84%) and therapeutic neck dissection in 44 patients (16%; Figure 1). Patients’ demographic and clinical characteristics, according to elective and therapeutic neck dissection, are summarized in Table 1. In 28 patients (10%), the contralateral neck was dissected. Adjuvant radiation was given to 125 patients (46%) and chemoradiation to an additional 58 (21%).
FIGURE 1.
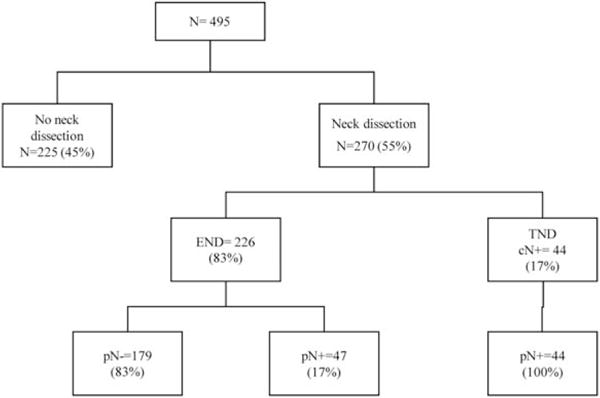
Management of the 495 patients of the International Study Group of the adenoid cystic carcinoma (ACC) cohort. cN, clinical nodal status; pN, pathological nodal status; END, elective neck dissection; TND, therapeutic neck dissection.
TABLE 1.
Patient demographic and clinical characteristics.
| Variables | Elective neck dissection (%) | Therapeutic neck dissection (%) | p value |
|---|---|---|---|
| No. of patients | 226 (84) | 44 (16) | |
| Age, y, mean ± SD | 56 ± 14 | 58 ± 14 | .31 |
| Sex | .73 | ||
| Male | 105 (46) | 25 (56) | |
| Female | 121 (54) | 19 (43) | |
| Primary site | .01 | ||
| Oral cavity | 116 (51) | 32 (73) | |
| Parotid | 44 (20) | 0 (0) | |
| Submandibular | 31 (14) | 8 (18) | |
| Sublingual | 10 (4) | 2 (5) | |
| Paranasal sinuses | 24 (10) | 1 (2) | |
| Larynx | 1 (1) | 1 (2) | |
| pT classification | < .001 | ||
| 1 | 62 (28) | 3 (5) | |
| 2 | 89 (39) | 12 (27) | |
| 3 | 41 (18) | 8 (17) | |
| 4 | 34 (15) | 21 (51) | |
| pN classification | < .001 | ||
| N0 | 179 (79) | 0 (0) | |
| N1 | 35 (15) | 16 (36) | |
| N2a | 7 (3) | 14 (32) | |
| N2b | 4 (2) | 11 (25) | |
| N2c | 1 (<1) | 1 (2) | |
| N3 | 0 (0) | 2 (5) | |
| Treatment | |||
| Surgery | 80 (36) | 7 (16) | |
| Surgery + RT | 100 (44) | 25 (57) | |
| Surgery + CRT | 46 (20) | 12 (27) | |
| Extent of neck dissection | < .001 | ||
| I–III | 158 (70) | 10 (23) | |
| I–V/II–V | 65 (29) | 23 (52) | |
| RND/MRND | 3 (1) | 11 (25) | |
| Contralateral I–III | 18 (8) | 10 (23) | |
| Margin status | .45 | ||
| Positive | 37 (16) | 4 (9) | |
| Close (<5 mm) | 61 (28) | 13 (30) | |
| Negative | 127 (56) | 26 (60) | |
| Follow-up of all patients, mo | .78 | ||
| Mean | 63 ± 42 | 60 ± 47 | |
| Median | 52 | 39 | |
| Range | 7–258 | 8–252 |
Abbreviations: RT, radiotherapy; CRT, chemoradiotherapy; RND, radical neck dissection; MRND, modified radical neck dissection.
Overall rate of neck metastases in adenoid cystic carcinoma
Histologically confirmed lymph node metastases were detected in 91 neck level samples from 79 patients (29%). Compared to patients who underwent elective neck dissection, for patients who underwent therapeutic neck dissections, the oral cavity was more often the primary site, advanced T and N classifications were more common, and treatment more often included radiotherapy in addition to surgery (Table 1). Table 2 shows the incidence of neck metastases according to the primary site. Regional metastases presented in 55 of the 148 patients (37%) with oral cavity tumors, compared to 18 of the 95 patients (19%) with major salivary gland ACC (p = .001). Of the 55 patients with oral cavity lymph nodes metastases, 47 (85%) had level I to III metastases, compared to 10 of 18 (55%) of those with major salivary gland tumors (p = .02). Contralateral neck involvement was observed in only 2 patients with oral cavity tumors (both in levels I–III) and concurrent ipsilateral nodal metastasis.
TABLE 2.
Incidence of neck metastases according to the primary site.
| Variables | Major salivary glands (n = 95) |
Oral cavity (n = 148) |
Sinonasal (n = 25) |
Larynx (n = 2) |
p value |
|---|---|---|---|---|---|
| Ipsilateral | .02 | ||||
| I–III | 10 (10%) | 47 (31%) | 4 (16%) | ||
| IV–V | 8 (8.5%) | 8 (5%) | 1 (4%) | 1 (50%) | |
| Contralateral | |||||
| I–III | 2 (1%) |
Rate of occult neck metastases in adenoid cystic carcinoma
The overall rate of occult nodal metastases among patients who underwent elective neck dissection was 17% (39 of 226 patients). Subgroup analysis revealed that the highest incidence rates of occult nodal metastases were in patients with oral cavity tumors (22%; 25 of 116), and in those with cancer of the paranasal sinuses (16%; 4/24). The lowest incidence of occult neck metastases was in patients with major salivary gland tumors (12%; 10/85); p = .2.
Association between incidence rates of lymph node metastases and patient outcomes
Figures 2 and 3 show the Kaplan–Meier curves of patient outcomes after neck dissection, according to the neck dissection type and pathological nodal status. The 5-year OS was 44% in patients undergoing therapeutic neck dissections, compared to 65% and 73% in patients undergoing elective neck dissections, with (cN−/pN+) and without (cN-/pN−) occult metastases, respectively (p = .017). Among patients undergoing therapeutic neck dissection and elective neck dissections with nodal metastases, the 5-year disease-free survival rates were 35% and 32%, respectively, compared to 47% in those without nodal disease (p = .011). Interestingly, although the 5-year regional control rate was significantly lower in patients with neck metastases, there was no statistically significant difference in the 5-year local control rate between patients undergoing therapeutic or elective neck dissection (Figure 3). In accordance with the incidence of regional metastasis, the 5-year distant metastasis rate was significantly higher among patients with nodal metastases than among those without nodal metastases (40% and 27%, respectively; p = .029; Figure 4).
FIGURE 2.
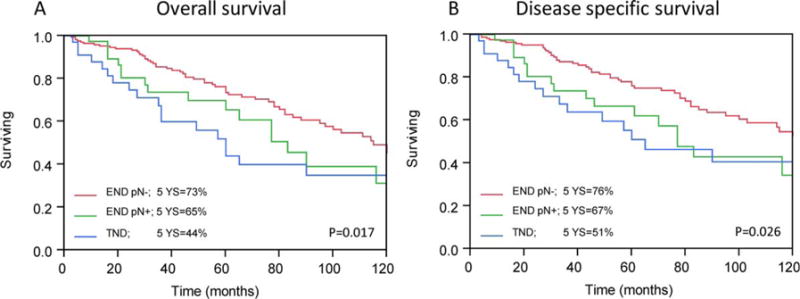
Kaplan–Meier analysis. Five-year (A) overall survival (OS) and (B) disease-specific survival (DSS) rates calculated by the Kaplan–Meier method according to neck management and nodal status (p < .05). pN, pathological nodal status; END, elective neck dissection; TND, therapeutic neck dissection. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 3.
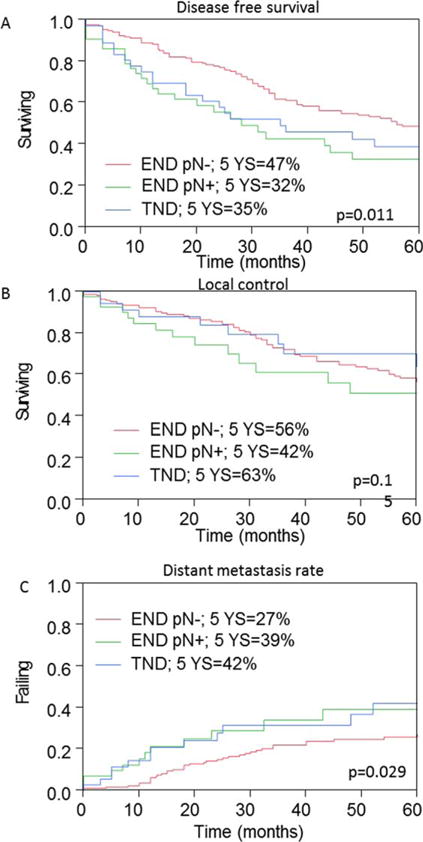
Analysis of outcome measures in patients with adenoid cystic carcinoma (ACC). Five-year (A) disease-free survival, (B) local control, and (C) distant metastases rates calculated by the Kaplan–Meier method according to neck management and nodal status. pN, pathological nodal status; END, elective neck dissection; TND, therapeutic neck dissection. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 4.
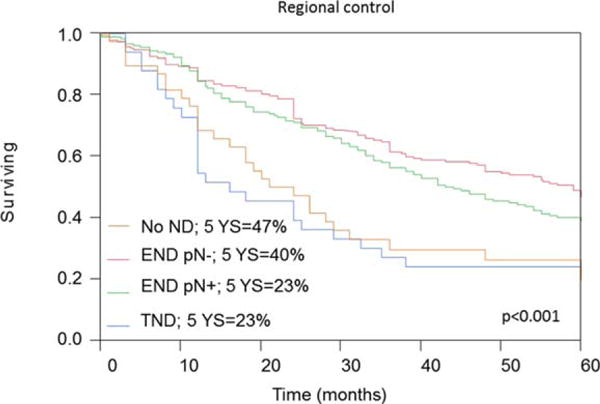
Analysis of regional metastasis measures in patients with adenoid cystic carcinoma (ACC). Five-year regional recurrence rates calculated by the Kaplan–Meier method according to neck management and nodal status. ND, neck dissection; pN, pathological nodal status; END, elective neck dissection; TND, therapeutic neck dissection. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Independent predictors of outcome
Multivariate analysis was used to evaluate the impact of various clinicopathological variables on outcome. The variables that were found to be significant on univariate analysis were introduced into a multivariate model: primary site, margin status, T classification, N classification, tumor grade, extracapsular spread, treatment group, and extent of neck dissection. As shown in Table 3, the variables that were independently associated with both OS and DSS were primary site, nodal classification, and margin status.
TABLE 3.
Multivariate analysis of prognostic factors for overall and disease specific survival.
| Variable | OS
|
DSS
|
||||
|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | |
| Primary site | ||||||
| Parotid | .001 | 1 | .002 | 1 | ||
| Major glands | 1.006 | 0.9–1.11 | 1.002 | 1.001–3.1 | ||
| Oral cavity | 1.2 | 0.68–3.22 | 1.1 | 2.3–9.1 | ||
| Sinonasal | 3.16 | 1.47–5.76 | 5.26 | 0.9–1.01 | ||
| Margins | ||||||
| Negative | .012 | 1 | .035 | 1 | ||
| Close (<5 mm) | 2.6 | 1.1–17.2 | 2.33 | 1.2–5 | ||
| Positive | 3 | 1.2–4.1 | 4.36 | 1.5–7.9 | ||
| Pathologic T classification | ||||||
| T1 | .3 | NA | NA | .1 | NA | NA |
| T2 | ||||||
| T3 | ||||||
| T4 | ||||||
| Pathologic N classification | ||||||
| N0 | .01 | 1 | .006 | 1 | ||
| N1 | 2.7 | 1.6–6.1 | 3 | 1.4–5.5 | ||
| N2a | 2.7 | 1.05–6.7 | 3.1 | 1.3–6 | ||
| N2b | 2.05 | 1.15–3.5 | 3.1 | 1.36–3.6 | ||
| N2c | 3.8 | 1.6–7.3 | 4.2 | 2.3–7 | ||
| N3 | 6.8 | 2.3–9 | 22.3 | 10–37.5 | ||
| Extracapsular spread | ||||||
| No | .3 | NA | NA | .39 | NA | NA |
| Yes | ||||||
| Treatment | ||||||
| Surgery | .43 | NA | NA | .39 | NA | NA |
| Surgery + RT | ||||||
| Surgery + CRT | ||||||
| Neck dissection extent | ||||||
| I–III/II–III | .2 | NA | NA | .007 | 1 | |
| I–IV/I–V | 3.1 | 1.1–4.8 | ||||
| RND/MRND | 4 | 2.1–6 | ||||
| Contralateral | 4 | 1.9–7.3 | ||||
Abbreviations: OS, overall survival; DSS, disease-specific survival; HR, hazard ratio; 95% CI, 95% confidence interval; RT, radiation therapy; CRT, chemoradiation therapy; RND, radical neck dissection; MRND, modified radical neck dissection.
DISCUSSION
ACC of the head and neck is an uncommon tumor; treatment includes surgery with or without adjuvant radiotherapy. Because of its rarity, data on the incidence and impact of ACC nodal metastases on outcome is insufficient to determine whether an elective neck treatment is advisable in the management of these patients. Nodal metastasis has previously been shown to be a common cause of treatment failure in this population.18 On the other hand, the reported incidence of neck metastasis in ACC was 6% to 10%, considerably lower than in patients with squamous cell carcinoma of the head and neck.10,19 To overcome the limitation of data collection because of the low incidence of this disease, we performed an international collaborative study involving 9 cancer centers. We collected detailed data on 270 patients who had undergone neck dissection as part of their treatment.
The overall nodal metastases rate of 29% observed in this study is higher than was previously reported. The pattern of spread is similar to squamous cell carcinoma and reflects the lymphatic drainage echelons of these sites.3,5 The incidence rate of occult nodal metastases (17%) was high among patients undergoing elective neck dissection, and particularly among those with ACC of the minor salivary glands (oral cavity and sinonasal). Nodal status was associated with survival outcomes, both among patients who underwent therapeutic neck dissection and among those who underwent elective neck dissections.
Although therapeutic neck dissection is performed as a matter of course in all patients with clinical evidence of nodal metastases, the decision to perform an elective neck dissection is complicated by the limited ability of imaging techniques to assess cervical metastases.20 In head and neck squamous cell carcinoma, an elective neck dissection is indicated if the probability of occult cervical metastases is above 15% to 20%.21 Our finding of a 17% rate of occult neck metastases in patients with minor salivary gland ACC raises the possible need of an elective neck treatment. Although pN status was not associated with the local control rate, evidence of lymphatic spread increased the rate of distant metastases and neck failure.
Multivariate analysis identified margin status, N classification, and primary site as independent predictors of patient outcomes. Data regarding an association between adjuvant radiotherapy and the outcome of patients with ACC are contradictory. Although some authors showed that adjuvant radiotherapy can improve the local control of patients, others found no advantage for such treatment.22–24 The current study did not have the power to determine an association between adjuvant radiotherapy and the outcomes of patients with ACC. In accordance to previous publications, we did not find a correlation between tumor grade and survival. This might be related to the fact that grading was poorly reproducible collecting data from 9 centers.9,25
Because of the retrospective design of this study, data could not be retrieved on the specific levels of the neck metastases. Twenty-eight patients underwent deep lobe parotidectomy. Because of the retrospective design of this study, data regarding intraparotid lymph node involvement was not available. Nevertheless, our data suggest that the most common region of nodal metastases is levels I to III. In the 7% of patients (18 of 27) who had level IV to V lymph node metastases, preoperative positive findings prompted therapeutic neck dissection. Furthermore, the very low rate of contralateral neck metastases (4%) suggest that elective neck treatment should include levels I to III in the ipsilateral side. Also, consequent to the retrospective design, despite the use of uniform data extraction sheets, the heterogeneity of professionals involved (eg, pathologists, radiologists, and radiation oncologists) may have led to inconsistency in reporting.
We did not evaluate whether an elective neck dissection with or without adjuvant radiotherapy can improve patient outcomes. Until such data are available, the tailoring of treatment of ACC should be based on risk stratification of each patient and on decisions of the multidisciplinary teams that manage the treatment of these patients.
CONCLUSION
The incidence of neck metastases in patients with ACC is higher than was previously suspected. In a multivariate analysis, margin status, N classification, and primary site were significant predictors of outcome. The relatively high rate of occult nodal metastases in patients with ACC of the oral cavity suggests that elective neck treatment should be considered for this population.
Supplementary Material
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Bhayani MK, Yener M, El-Naggar A, et al. Prognosis and risk factors for early-stage adenoid cystic carcinoma of the major salivary glands. Cancer. 2012;118:2872–2878. doi: 10.1002/cncr.26549. [DOI] [PubMed] [Google Scholar]
- 2.Spiro RH, Alfonso AE, Farr HW, Strong EW. Cervical node metastasis from epidermoid carcinoma of the oral cavity and oropharynx. A critical assessment of current staging. Am J Surg. 1974;128:562–567. doi: 10.1016/0002-9610(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 3.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–409. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 4.Snow GB, van den Brekel MW, Leemans CR, Patel P. Surgical management of cervical lymph nodes in patients with oral and oropharyngeal cancer. Recent Results Cancer Res. 1994;134:43–55. doi: 10.1007/978-3-642-84971-8_6. [DOI] [PubMed] [Google Scholar]
- 5.Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer. 1990;66:109–113. doi: 10.1002/1097-0142(19900701)66:1<109::aid-cncr2820660120>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446–1449. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Spiro JD, Spiro RH, Shah JP, Sessions RB, Strong EW. Critical assessment of supraomohyoid neck dissection. Am J Surg. 1988;156:286–289. doi: 10.1016/s0002-9610(88)80293-9. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd S, Yu JB, Ross DA, Wilson LD, Decker RH. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Int J Radiat Oncol Biol Phys. 2010;76:169–175. doi: 10.1016/j.ijrobp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Vander Poorten V, Hunt J, Bradley PJ, et al. Recent trends in the management of minor salivary gland carcinoma. Head Neck. 2014;36:444–455. doi: 10.1002/hed.23249. [DOI] [PubMed] [Google Scholar]
- 10.Min R, Siyi L, Wenjun Y, et al. Salivary gland adenoid cystic carcinoma with cervical lymph node metastasis: a preliminary study of 62 cases. Int J Oral Maxillofac Surg. 2012;41:952–957. doi: 10.1016/j.ijom.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Schramm VL, Jr, Imola MJ. Management of nasopharyngeal salivary gland malignancy. Laryngoscope. 2001;111:1533–1544. doi: 10.1097/00005537-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134:536–538. doi: 10.1001/archotol.134.5.536. [DOI] [PubMed] [Google Scholar]
- 13.Pathology Group. Guidelines for the examination and reporting of head and neck cancer specimens. Vol. 2007. Leeds, UK: Yorkshire Cancer Network; 2007. pp. 1–12. [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 17.Gil Z, Patel SG, Singh B, et al. Analysis of prognostic factors in 146 patients with anterior skull base sarcoma: an international collaborative study. Cancer. 2007;110:1033–1041. doi: 10.1002/cncr.22882. [DOI] [PubMed] [Google Scholar]
- 18.Terhaard CH, Lubsen H, Van der Tweel I, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck. 2004;26:681–692. doi: 10.1002/hed.10400. discussion 692–693. [DOI] [PubMed] [Google Scholar]
- 19.Balamucki CJ, Amdur RJ, Werning JW, et al. Adenoid cystic carcinoma of the head and neck. Am J Otolaryngol. 2012;33:510–518. doi: 10.1016/j.amjoto.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Wei WI, Ferlito A, Rinaldo A, et al. Management of the N0 neck–reference or preference. Oral Oncol. 2006;42:115–122. doi: 10.1016/j.oraloncology.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Pitman KT. Rationale for elective neck dissection. Am J Otolaryngol. 2000;21:31–37. doi: 10.1016/s0196-0709(00)80121-0. [DOI] [PubMed] [Google Scholar]
- 22.Adachi M, Mitsudo K, Yamamoto N, et al. Chemoradiotherapy for maxillary sinus adenoid cystic carcinoma using superselective intra-arterial infusion via a superficial temporal artery. Head Neck. 2013;35:E89–E93. doi: 10.1002/hed.21925. [DOI] [PubMed] [Google Scholar]
- 23.Verweij J, de Mulder PH, de Graeff A, et al. Phase II study on mitoxantrone in adenoid cystic carcinomas of the head and neck. EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1996;7:867–869. doi: 10.1093/oxfordjournals.annonc.a010770. [DOI] [PubMed] [Google Scholar]
- 24.Budd GT, Groppe CW. Adenoid cystic carcinoma of the salivary gland. Sustained complete response to chemotherapy. Cancer. 1983;51:589–590. doi: 10.1002/1097-0142(19830215)51:4<589::aid-cncr2820510405>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992;164:623–628. doi: 10.1016/s0002-9610(05)80721-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


