Abstract
Clinical concepts of mental illness have always been modulated by underlying theoretical considerations. For the past fifty years, schizophrenia has been considered primarily a disease of dopaminergic neurotransmission. Although this conceptualization has helped greatly in explaining the clinical effects of psychostimulants and guiding the clinical use of both typical and atypical antipsychotics, it has nevertheless shaded how we look at the disorder from both a pathophysiological and therapeutic perspective. For example, most explanatory research in schizophrenia has focused on dopamine-rich regions of the brain, with little investigation of regions of the brain that are relatively dopamine poor. Starting approximately twenty years ago, an alternative formulation of schizophrenia was proposed based upon actions of the “dissociative anesthetic” class of psychotomimetic agents, including phencyclidine (PCP), ketamine and various designer drugs. These compounds induce psychosis by blocking neurotransmission at N-methyl-D-aspartate (NMDA)-type glutamate receptors, suggesting an alternative model for pathogenesis in schizophrenia. As opposed to dopamine, the glutamatergic system is widely distributed throughout the brain and plays a prominent role in sensory processing as well as in subsequent stages of cortical analysis. Glutamatergic theories of schizophrenia, thus, predict that cortical dysfunction will be regionally diffuse but process specific. In addition, NMDA receptors incorporate binding sites for specific endogenous brain compounds, including the amino acids glycine and D-serine and the redox modulator glutathione, and interact closely with dopaminergic, cholinergic and γ-aminobutyric acid (GABA)-ergic systems. Glutamatergic theories, thus, open new potential approaches for treatment of schizophrenia, most of which are only now entering clinical evaluation.
Keywords: Schizophrenia, Glutamate, NMDA Receptor, Visual, Auditory, Review
Introduction
Schizophrenia is a severe mental disorder associated with both a specific profile of symptoms and a complex pattern of neurocognitive dysfunction. The first effective treatments for schizophrenia were discovered fortuitously in the mid-1950s (1), and were subsequently shown to mediate their effects at dopamine (DA) D2 receptors in the mid-1970s (2, 3). The DA hypothesis has been the dominant neurochemical model of schizophrenia (4) and has proven heuristically valuable since that time. For example, all current treatments for schizophrenia mediate their effects via blockade of the DA (D2) receptor.
Given the limited distribution of DA neurons in the brain, schizophrenia was traditionally seen as a disease affecting only a few key brain regions. More recent findings, however, implicate widespread cortical and subcortical dysfunction, suggesting more generalized etiology. Over the past twenty years, attention has turned increasingly to dysfunction of the brain glutamate system as a fundamental mechanism underlying brain dysfunction in schizophrenia. Glutamate is a widespread neurotransmitter in both cortex and subcortical systems and accounts for as many as 60% of brain synapses (see [5] for review). Based on observations that the N-methyl-D-aspartate (NMDA)-type glutamate receptors antagonists, such as phencyclidine (PCP) or ketamine, uniquely reproduce the symptomatic, neurocognitive and neurochemical aspects of the disorder, particular emphasis has been placed on NMDA dysfunction as a potential final common pathway leading from pathogenesis to symptoms, as reviewed in (6).
Clinical Phenomenology of Schizophrenia
Clinicians have grown comfortable dividing symptoms of schizophrenia into positive and negative factors, and in considering treatment effects on each factor independently (7, 8). Further, Kraeplin’s original conceptualization (9) of schizophrenia as a dementia has returned greatly to prominence. Positive symptoms, while they obviously remain distressing to a large number of patients and debilitating for some, can often be controlled to the point where they do not of themselves limit recovery.
Over the past twenty years, attention has turned increasingly to dysfunction of the brain glutamate system as a fundamental mechanism underlying brain dysfunction in schizophrenia.
Although subfractionation of the phenomenology of schizophrenia is extremely valuable for assessment and new treatment development, what is frequently lost is the conceptualization of schizophrenia as a syndromal whole. Positive and negative symptoms, while independent factors, fluctuate in parallel (10) (e.g., improve or worsen in parallel) and must be viewed as separate manifestations of the same underlying pathogenic mechanisms, rather than as separate and distinct nosological entities. The treatment of positive symptoms, such as hallucinations and delusions, is clearly important, but may only account for 5% of the variance of quality of life (11). In contrast, most studies have found that the persistent disability of schizophrenia often comes from negative symptoms and cognitive difficulties (12–15). In a recent analysis, negative symptoms affected interpersonal skills independently of other symptoms (16). Moreover, negative symptoms can have an additive effect with neuropsychological deficits on overall functioning and, according to a meta-analysis, patients with primary negative symptoms perform especially poorly on tests of global cognition (Cohen’s d=0.52) (17).
Accordingly, another key component of schizophrenia is neurocognitive dysfunction. When tested on basic IQ tests, such as the Wechsler Adult Intelligence Scale (WAIS), patients with established schizophrenia typically score about 1 standard deviation, or 15 IQ points, below the population mean. While positive symptoms fluctuate in severity throughout the course of the illness (see [18] for a review), cognitive deficits are typically present at first episode and remain relatively constant over the course of the illness (19) and see (20) for review. In studies that have utilized comprehensive neuropsychological batteries, similar levels of deficit have been observed across multiple neurocognitive domains, particularly in learning and declarative memory formation (19, 21–23) and not prefrontal “executive” dysfunction per se. Prevalence of cognitive dysfunction can also be estimated using baseline data from the large scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study in which, despite cognitive dysfunction not being part of the inclusion criteria, the majority of patients had profound cognitive deficits (21). Moreover, the global composite was significantly correlated with Positive and Negative Symptoms Scale (PANSS) negative symptoms (r=0.27, p<0.0001), but unrelated to positive symptoms, suggesting both an interrelationship between these core deficits and a relative distinction from positive symptoms.
While positive symptoms increase dramatically just prior to the first hospitalization (24), prospective, follow-back and cross-sectional data suggest that cognitive functioning may decline during the three to four years immediately preceding the onset of schizophrenia symptoms. For example, in one prospective study, poor educational achievement at age fifteen was a significant predictor of schizophrenia (25). Two follow-back studies have investigated performance on standardized educational testing (Iowa test) during childhood and adolescence in individuals who subsequently developed schizophrenia. Compared with the general population, such individuals showed only modest deficits even when assessed during 4th and 8th grade, but showed a marked decline in performance between 8th and 11th grade (26, 27).
Similarly, individuals with prodromal schizophrenia who have not yet converted to psychosis show cognitive deficits that are intermediate between those of first-episode and control subjects, and such deficits may predict subsequent conversion to psychosis (28). In one study, lower than expected IQ at age seventeen—based upon childhood reading and spelling abilities—was a significant risk factor for schizophrenia but not bipolar disorders, such that individuals showing a 10 point or greater discrepancy between expected and actual IQ showed an approximately twofold elevated risk for developing schizophrenia (29).
It also appears that intellectual performance remains relatively constant between age seventeen and subsequent illness onset in individuals who go on to develop schizophrenia, suggesting that most of the cognitive decline occurs premorbidly, although further deterioration in some domains may be observed (30). While multiple other factors (31, 32), including positive symptoms, also significantly contribute to quality of life and functional impairment, overall, these findings highlight neurocognitive dysfunction as a key manifestation of schizophrenia that precedes onset of symptoms, and must therefore be considered central to etiological hypotheses.
Neurochemical Models of Schizophrenia
Neurochemical models of schizophrenia based upon DA have had substantial heuristic value in explaining key symptoms of schizophrenia; in particular, positive symptoms, and in guiding treatment considerations. Nevertheless, significant limitations with regard to the DA hypothesis remain. First, few intrinsic deficits have been observed within the DA system to account for the presumed hyperdopaminergia associated with schizophrenia (see [6] and [33] for reviews). Second, reconceptualizations of the DA hypothesis propose that subcortical hyperdopaminergia may coexist with cortical hypodopaminergia, although mechanisms underlying the differential cortical and subcortical abnormalities remain to be determined (see [34] for review). Finally, dopaminergic dysfunction, in general, accounts poorly for both symptom classes in schizophrenia and for the pattern of neurocognitive dysfunction associated with schizophrenia (see [35] for review). Thus, alternative conceptual models of schizophrenia have been proposed.
In initial studies with PCP and ketamine in the early 1960s, it was noted that both agents produced what would now be considered positive, negative and cognitive symptoms of schizophrenia (see [36, 37] for reviews), although no formal rating scales were used. However, recent studies in healthy volunteers using scales such as the PANSS or the Brief Psychosis Rating Scale (BPRS), or the Scale of Assessment of Negative Symptoms (SANS), have documented significant increases not only in positive symptoms, but also in negative and cognitive symptoms after ketamine administration (38–40). Levels of symptoms during acute ketamine challenge, moreover, tend to show a similar pattern across factors as they do in schizophrenia. When patients with schizophrenia are exposed to ketamine, they also show increases in positive symptoms, as well as negative symptoms (41, 42), suggesting that NMDA antagonists affect a brain system that is already vulnerable in schizophrenia. This model has been increasingly adopted and is now considered to be one of the useful models for both etiological conceptualization of schizophrenia and new treatment development (see [43–47] for reviews).
No objective measures have been developed that adequately differentiate primarily positive from primarily negative patients. Because PCP produces negative symptoms as prominently or more than positive symptoms, it has occasionally been argued that PCP psychosis should be seen primarily as a model for negative-symptom schizophrenia, whereas amphetamine or lysergic acid diethylamide (LSD, e.g., serotoninergic) psychosis should be seen as a model for positive symptoms (48). However, in PCP- or ketamine-induced psychosis, the relative proportions of positive and negative symptoms are highly similar to those observed in both acute and chronic schizophrenia, whereas in amphetamine- or LSD-induced psychosis the ratio of positive to negative symptoms is far in excess of the pattern observed even in acute schizophrenia stages (35, 48). Thus, dopaminergic models may be most appropriate to those patients with primarily positive symptoms and rapid response to antipsychotic; NMDA models may be more appropriate to individuals with more balanced positive and negative symptoms and poor antipsychotic response, as reviewed in (6).
Nevertheless, a potentially informative difference between ketamine-induced symptoms and those of schizophrenia is in the production of hallucinations. In established schizophrenia, auditory hallucinations consisting of voices of various types are common, whereas visual hallucinations are rare; but during ketamine-induced psychosis, visual perceptual distortions are common but organized auditory hallucinations are rare. Therefore, the pattern of hallucinations observed during ketamine challenge does not closely resemble the pattern observed in established schizophrenia.
Thus, dopaminergic models may be most appropriate to those patients with primarily positive symptoms and rapid response to antipsychotic; NMDA models may be more appropriate to individuals with more balanced positive and negative symptoms and poor antipsychotic response, as reviewed in (6).
The pattern of ketamine-induced auditory and visual disturbances, however, does resemble the pattern observed early in the course in schizophrenia (49, 50), where both auditory and visual perceptual disturbances are common, and auditory hallucinations have not yet crystallized to the point of being identifiable as speech. Additional support for the similarities between early psychosis and glutamatergic models comes from imaging studies. A significant increase of glutamine was seen in the anterior cingulate cortex following ketamine administration to healthy human volunteers (Rowland et al., 2005), which is similar to the increases seen in studies of first-episode (51) and prodromal patients (52). Thus, acute ketamine challenge may be viewed best as a model of prodromal or acute incipient schizophrenia, rather than later, more chronic, phases.
Pathological and Genetic Evidence
NMDA in Neurodegenerative and Neurodevelopmental Models of Schizophrenia
In addition to producing symptoms and cognitive deficits in acute challenge experiments in humans, preclinically, NMDA antagonists lead to neurodegenerative and neurodevelopmental changes. These changes are seen in specific populations of cortical pyramidal neurons, particularly in frontal, posterior cingulate and retrosplenial brain regions, although delayed spread to larger brain networks (e.g., parietal, temporal, entorhinal cortex, hippocampus and amygdala) (43, 53). Neurotoxicity was observed following even a single large dose of PCP, although it could also be seen following longer duration, lower dose administration (54). NMDA receptors are also expressed as well in oligodendrocytes (which give rise to myelin), and play a critical role both in neurodevelopment and in ischemia-induced damage (55, 56).
NMDA in Excitotoxicity Models
NMDA blockade may lead to rebound hyperglutamatergia, presumably due to reduced excitatory drive within local GABAergic feedback loops. Because glutamatergic transmission is designed to be highly phasic, the elevation of tonic glutamate levels is pathological, and leads to impairments in function over and above those induced by NMDA blockade itself. Based upon this theory, the primary goal of glutamate-based treatments is neither to increase nor decrease NMDA function per se, but rather to restore balance between excitation and inhibition within cortical regions (57, 58). Non-neuronal NMDA receptors have also been studied in excitotoxicity models (59), but would also predict that a reduction in NMDA receptor stimulation would lead to reduced oligodendrocyte growth and proliferation during adulthood (60), consistent with the observations in schizophrenia.
Linkage/Association Studies in Schizophrenia
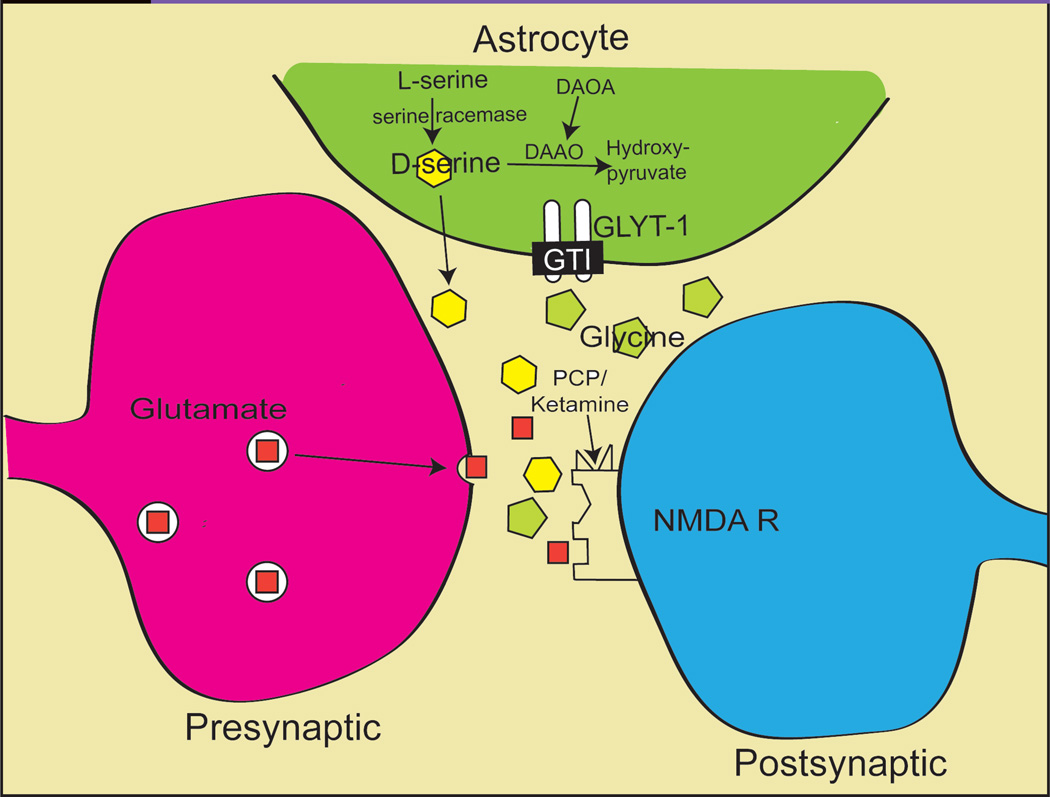
A consistent finding from genetic studies in schizophrenia is that several of the identified genes interact closely with glutamatergic mechanisms in general and NMDA receptors in particular. As such, these studies provide additional support for glutamatergic theories of the disorder. One of the best established candidate genes for schizophrenia is neuregulin (NRG1), which might mediate its risk-enhancing effects based upon interaction with NMDA receptors (see [61] for a review). D-amino acid oxidase (DAAO), the enzyme responsible for D-serine (an NMDA receptor modulator) metabolism, has been linked to schizophrenia (62), as has the DAAO regulator, D-amino oxidase activator (DAOA) (62–66) (see Figure 1).
Figure 1.
Schematic Model of the NMDA Receptor Complex and Synaptic Glutamate  , D-serine
, D-serine  , and Glycine
, and Glycine  , Regulation and Metabolism. (The non-physiologic NMDA antagonist PCP and ketamine site is also shown.)
, Regulation and Metabolism. (The non-physiologic NMDA antagonist PCP and ketamine site is also shown.)
Other potential risk genes for schizophrenia such as dysbindin (DTNBP1) (see [67] for review), Disrupted-in-schizophrenia-1 (DISC-1), and RGS4 may also converge on glutamatergic systems (68–70), although further clarification of the role of these genes in normal brain function is required. Linkages to metabotropic glutamate receptor genes, including GRM3 (71, 72) and GRM7 (73) have also been reported.
Cognitive Deficits Following NMDA Antagonist Treatment
Glutamatergic models provide a framework from which to view the pattern of neuropsychological dysfunction associated with schizophrenia. Although glutamatergic systems are widespread, within each brain region, NMDA receptors participate in only a subset of processes. For example, NMDA receptor activation is required for the initiation, but not maintenance, of long-term potentiation (74). The observation that patients with schizophrenia (as opposed to those with the amnestic syndrome) show deficits in memory formation (19), but not retention, is thus consistent with an NMDA pattern of dysfunction (35, 75–80).
As with symptoms, initial studies conducted with PCP in the early 1960s also showed cognitive deficits that are highly reminiscent of schizophrenia (see [37] for review). Studies conducted with ketamine over the last fifteen years have further confirmed and extended these findings. NMDA antagonists also reproduce core neuropsychological abnormalities of schizophrenia, including executive functioning (38, 81–83), attention/vigilance (35, 81, 82, 84–86), verbal fluency (38, 77, 87), visual and verbal working memory (35, 76, 78, 81, 82, 86–92). Moreover, in monkeys treated with ketamine, characteristic, schizophrenia-like deficits in a task-switching paradigm (93, 94) are reproduced. Ketamine infusion also reproduces both the severity and type of thought disorder seen in schizophrenia with both, for example, being associated with high levels of poverty of speech, circumstantiality and loss of goal, and relatively low levels of distractive or stilted speech or paraphasias (95). Given the importance of neurocognitive dysfunction to the conceptualization of schizophrenia, these findings support the etiological involvement of NMDA dysfunction in the pathophysiology of schizophrenia.
As opposed to ketamine, administration of dopaminergic agonists such as amphetamine does not reproduce the pattern of deficit observed in schizophrenia. For example, in one study that directly compared effects of amphetamine and ketamine in normal volunteers, both ketamine and amphetamine induced positive symptoms and conceptual disorganization. However, only ketamine produced perceptual changes, concrete ideation or negative symptoms. Further, only ketamine induced schizophrenia-like disruptions in delayed recall. Finally, amphetamine did not induce working memory disturbances, and it significantly reversed ketamine-induced disruptions. These findings suggest that augmentation, rather than blockade of frontal dopaminergic systems, may be beneficial in schizophrenia (35).
In schizophrenia, amphetamine treatment does not further impair cognition and may, in fact, lead to cognitive improvement in schizophrenia (96). These findings, therefore, suggest greater involvement of NMDA, rather than DA, receptors in the pathophysiology of cognitive impairment in schizophrenia. Thus, reduction in NMDA functioning within the brain could serve as a single unifying feature to account for the otherwise complex pattern of deficits observed in the disorder.
NMDA Dysfunction and Sensory Processing Impairment
Another key difference between dopaminergic and NMDA models of schizophrenia is the predicted involvement of sensory processing. As opposed to D2 receptors, which are strongly localized to striatum and frontotemporal systems (97), NMDA receptors are diffusely distributed throughout brain, with nearly equal representation in both lower and higher order brain regions. Thus, NMDA receptor models suggest that deficits in sensory processing should co-exist in schizophrenia with deficits in more complex forms of cognition such as memory, attention or working memory. Such deficits were not emphasized in dopaminergic models of schizophrenia. However, over the past decade, such deficits have become increasingly well documented and, in several cases, shown to conform to the pattern induced by NMDA antagonists, such as ketamine, in animal models and normal volunteers. Such deficits thus provide convergent evidence for NMDA receptor dysfunction in schizophrenia.
Behavioral and electrophysiological studies of sensory dysfunction in schizophrenia have been performed primarily in auditory and visual systems, although schizophrenia is known to affect other sensory processes such as weight discrimination (98) and other somatosensory processes (99). Because of its high temporal resolution and noninvasive nature, the electroencephalogram (EEG) and event-related potentials (ERP) are excellent measures of the neurophysiology and early sensory processing of the brain. ERPs are standardized reactions of the brain to a particular stimulus, and because they index underlying neuronal processes, they allow us to look at the basic functioning of the brain and the root cause of neuropsychological dysfunction (see [100] for a review). These deficits are often highly heritable, stable across time and well validated.
In the auditory system, one of the strongest indices of sensory dysfunction is impaired generation of an ERP component termed mismatch negativity (MMN). MMN is elicited by a paradigm in which a sequence of repetitive standard stimuli is infrequently interrupted by stimuli that differ in a physical stimulus dimension such as pitch, duration, intensity or location. MMN reflects response of the auditory cortex to infrequent changes in a repetitive pattern of auditory stimulation and MMN deficits are well-established in schizophrenia, with a mean effect size of ∼1 d across studies (101). Similar deficits in MMN can be induced in animals by infusion of PCP (102, 103) and in normal volunteers by infusion of ketamine (40). MMN deficits are associated with impaired ability to match tones following brief delay (104). Deficits in tone matching contribute significantly to impairments in higher order functions that rely on tone matching ability (105), such as the ability to determine emotion based upon vocal modulation (prosody), which are thought to be rate limiting in terms of functional outcome (106).
These findings suggest that augmentation, rather than blockade of frontal dopaminergic systems, may be beneficial in schizophrenia (35).
Similarly, NMDA receptors are located at multiple levels of the early visual system, and studies have also investigated the consequences of NMDA dysfunction in the early visual system. The early visual system consists of two main components: a magnocellular and parvocellular pathway. The magnocellular pathway, which conducts low-resolution visual information rapidly to cortex, is involved in attentional capture and processing of overall stimulus organization (107). In contrast, the parvocellular pathway, which is primarily involved in processing fine-grained stimuli and object recognition, is relatively intact. NMDA receptors may be particularly important in the magnocellular pathway (108).
Early visual processing deficits include Visual P1 (109–111), a measure that reflects responses within the mangocellular pathway and has been shown to predict community outcome (112, 113). Further, deficits in early visual processing produce subsequent impairments on higher order processes such as object identification (114), motion processing (115), reading (116) and illusion sensitivity (117). Change in the physical properties of stimuli can lead to significant improvement in performance in such high-level tasks such as the Wisconsin Card Sorting Test (118). Thus, as evidenced by associations between these NMDA-dependent early auditory and visual processes and higher order functioning, deficits in NMDA activity within sensory regions may lead to impairment in more complex processes (see [119] for review).
Glutamate-DA and Glutamate-GABA Interactions
NMDA dysfunction may also account for both the impaired dopaminergic regulation and the impaired GABAergic neurotransmission that has been documented in schizophrenia. Positron emission (PET) and single photon emission (SPECT) tomographic studies provide insights into patterns of neurochemical receptor dysfunction in schizophrenia. Patients with schizophrenia, as a group, show enhanced DA release to amphetamine challenge, consistent with endogenous dopaminergic hyperactivity/dysregulation (see [120] for review). The enhanced release, however, is observed specifically during acute decompensation, and not during a remission phase, and is associated specifically with increased severity of positive symptoms. Based upon these findings, it has been suggested that dopaminergic instability may account only for the increased severity of symptoms associated with acute decompensation (33). In normal volunteers, pretreatment with ketamine leads to dopaminergic dysregulation similar to that observed in schizophrenia (121), even under conditions where no effect on basal DA release is observed. Similar augmentation of amphetamine-induced DA release is observed in rodents treated with NMDA antagonists (122–124). A primary site of NMDA appears to be local inhibitory interneurons within key brain regions (125). These serve as a “brake” on glutamate-stimulated DA release (126). Thus, failure of this brake may lead to dysregulated DA release of the type observed in schizophrenia.
Changes in GABAergic neurotransmission have also been increasingly well documented over recent years (127–131). GABAergic dysfunction may be directly linked to well-documented deficits in working memory function, and may, therefore, represent an appropriate target of pharmacological intervention (132). There is also evidence for NMDA receptors modulating their regulation of DA in part through presynaptic GABAB and on DA terminals (133).
NMDA-Based Treatments
A critical issue is whether treatment approaches based upon glutamatergic and NMDA models can lead to new treatment approaches. Over the past decade, several new treatment strategies have been proposed. First, direct and indirect approaches have targeted the glycine modulatory site of the NMDA receptor complex (see Figure 1). Direct agonists have included treatment with the naturally occurring amino acids glycine and D-serine, which serve as endogenous modulators of NMDA receptors in vivo, as well the antituberculosis drug D-cycloserine, which fortuitously cross-reacts with the NMDA/glycine site (see [134] for a review). These agents have proven effective in several preclinical models, including reversal of PCP effects in both rodents (135, 137) and primates (137).
A potential indirect approach is to use glycine type I (GlyT1) transport inhibitors (GTIs). Rather than serving as direct glycine precursors, these compounds function similarly to selective serotonin reuptake inhibitors (SSRIs) and increase glycine levels in the brain by preventing glycine removal from the synaptic cleft, leading to endogenous increases in CSF glycine levels (138) (see Figure 1). An initial study with glycyldodeclamide, a relatively low-affinity agent, demonstrated significant reversal of PCP-induced hyperactivity in rodents (139, 140). Since then, the naturally occurring sarcosine has shown preliminary effectiveness, and high affinity GTIs have been synthesized by several pharmaceutical companies, and have shown to be effective in multiple animal models. Several of these compounds are currently in early-stage clinical trials, with results expected over the next several years.
Other treatment strategies have been proposed utililizing nonglycine modulatory sites of the NMDA receptor. One involves examination of the redoxsensitive site on the NMDA receptor. This site is modulated by the oxidized form of glutathione (GSH) (141, 142). Schizophrenia has also been shown to be associated with reduced levels of GSH (143–145), leading to potential dysfunction of NMDA receptors (146). Early studies testing this mechanism have utilized N-acetylcysteine, a glutathione precursor, as a potential psychopharmacological agent.
Another treatment possibility, based upon the observation that NMDA blockade leads to rebound increases in glutamate release that may themselves be pathological (147), proposes that compounds that inhibit presynaptic glutamate release may also be therapeutic (148). Examples of such compounds include the antiepilepsy drug lamotrigine (88) and agonists of metabotropic glutamate type 2/3 (mGluR2/3) receptors (57, 149), which have been shown to be effective in reversing behavioral effects of NMDA antagonists in human and rodent models supporting the potential efficacy of these compounds as novel antipsychotic agents. Other metabotropic ligands, including mGluR5 (150, 151) and mGluR8 (152) agonists, have also been proposed as potential treatments for schizophrenia based upon their ability to modulate NMDA receptor-mediated neurotransmission (153). N-acetylaspartylglutamate (NAAG) may be an endogenous ligand for mGlu2/3 receptors in CNS. NAAG has been tested preclinically and shown to inhibit NMDA antagonist-induced behaviors in animals, consistent with an effect on NMDA receptor-mediated neurotransmission (154, 155).
Results of Clinical Studies
The most studies to date have been performed with NMDA agonists, primarily because several of the agents used have been natural compounds, and so it has not been necessary to wait for structure activity optimization or preclinical toxicity testing (see Figure 2). As permeability of these agents may be limited, delivering optimal doses, therefore, may be impossible (135). Nevertheless, positive studies with these compounds have provided proof-of-concept for development of compounds with higher affinity and specificity.
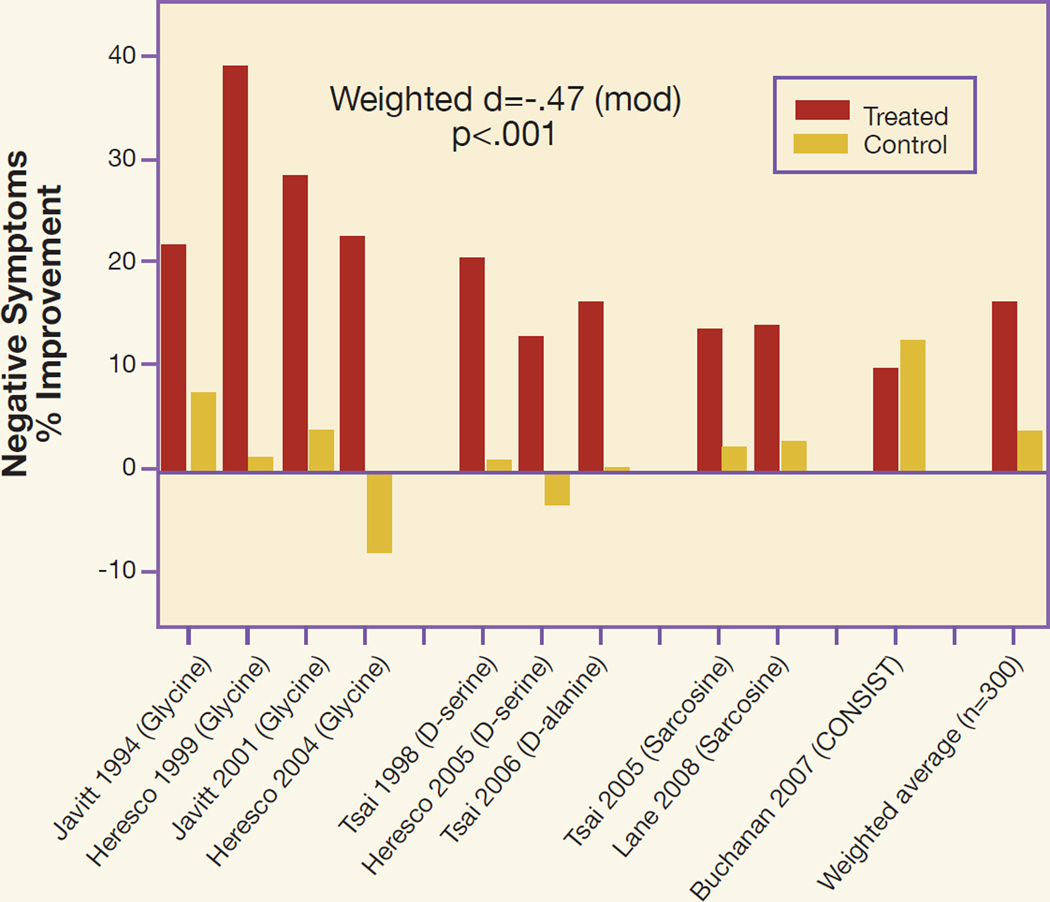
Figure 2.
Summary of Clinical Trials Performed to Date with Full NMDA Agonists Combined with Antipsychotics other than Clozapine
Further details about individual studies are provided in reference #134. CONSIST refers to the Cognitive and Negative Symptoms in Schizophrenia Trial (169). Statistics were calculated as weighted average of % change scores for negative symptoms, across trials.
Studies with naturally occurring compounds to date have primarily used glycine, administered at a dose of up to 800 mg/kg (approx. 60 g/d) (156–159); D-serine, administered at a dose of 30 mg/kg (approx. 2.1 g/d) (160, 161) or D-alanine, administered at a dose of 100 mg/kg (162); and, sarcosine, administered at a dose of 30 mg/kg (approx. 2.1 g/d) (163, 164). For glycine, this represents the highest practical dose because of the quantity of amino acid needed to significantly increase brain glycine levels. Most recently, high dose D-serine (>60 mg/kg) was shown to be more efficacious than lower doses in treating neurocognitive deficits (165). For other compounds, formal dose findings studies have not been performed, and maximum tolerated doses are presently unknown.
Overall, NMDA-based treatments appear to be efficacious in schizophrenia. A meta-analysis conducted in 2005 (166) concluded that in the first 132 patients studied with NMDA allosteric modulators, a highly significant (p=0.0004), moderate effect size improvement in negative symptoms was observed across studies. A more recent meta-analysis (167), which included 312 patients, continues to suggest a moderate effect size, highly significant improvement in negative symptoms (p<0.001). Variability in findings across studies was related primarily to degree of placebo effect within individual trial, with all studies showing a consistent, 15 to 20% improvement in negative symptoms within the experimental group.
One study has evaluated effects of open-label glycine in individuals showing prodromal symptoms of schizophrenia. In that study, large effect-size improvement was observed, including early remission in 3 of 10 subjects (168). These data, if confirmed by double-blind trials, would indicate that NMDA agonists might have potential utility in the schizophrenia prodrome.
Summary
Glutamatergic models of schizophrenia were first proposed over two decades ago, based upon the effects of the agents PCP and ketamine, which were shown to induce their unique psychotomimetic effects by blocking neurotransmission at NMDA-type glutamate receptors. Since that time, glutamatergic models have been strongly supported by NMDA antagonist studies in animals, as well as ketamine challenge studies in humans. Over that time, potential molecular contributors to NMDA dysfunction have been increasingly documented. New treatment approaches based upon glutamatergic approaches are only now reaching the clinical trial stage, and will serve to further elucidate and refine these models over upcoming years. Glutamatergic approaches offer particular hope for treatment of negative symptoms and cognitive deficits in schizophrenia and, thus, for improvement of the clinical situation of millions of patients worldwide.
Acknowledgments
Preparation of this manuscript was supported in part by R01 DA03383, and R37 MH49334.
Footnotes
Confict of Interest
Dr. Javitt holds intellectual property rights for use of glycine, D-serine and glycine transport inhibitors in treatment of schizophrenia and related disorders.
References
- 1.Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104–112. [PubMed] [Google Scholar]
- 2.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188(4194):1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 3.Wong DF, Wagner HN, Jr, Tune LE, Dannals RF, Pearlson GD, Links JM, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drugnaive schizophrenics. Science. 1986;234(4783):1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1(3):179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 5.Dingledine R, McBain CJ. Glutamate and aspartate. In: Siegel GJ, American Society for Neurochemistry; Albers RW, Brady S, Price DL, editors. Basic neurochemistry: molecular, cellular and medical aspects. 6th. Burlington (MA): Academic Press; 1999. [Google Scholar]
- 6.Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, editors. Handbook of neurochemistry and molecular neurobiology. 3rd. New York: Springer; 2009. [Google Scholar]
- 7.Carpenter WT, Bartko JJ, Strauss JS, Hawk AB. Signs and symptoms as predictors of outcome: a report from the International Pilot Study of Schizophrenia. Am J Psychiatry. 1978;135(8):940–944. doi: 10.1176/ajp.135.8.940. [DOI] [PubMed] [Google Scholar]
- 8.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39(7):784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 9.Kraepelin E. Dementia praecox and paraphrenia. Edinburgh: Livingston; 1919. [Google Scholar]
- 10.Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P. A longitudinal study of symptom dimensions in schizophrenia. Prediction and patterns of change. Arch Gen Psychiatry. 1995;52(5):352–360. doi: 10.1001/archpsyc.1995.03950170026004. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman JA, Keefe RS. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am J Psychiatry. 2008;165(8):978–987. doi: 10.1176/appi.ajp.2008.07111713. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz MM, Moberg PJ, Ragland JD, Gur RC, Gur RE. Symptoms versus neurocognitive test performance as predictors of psychosocial status in schizophrenia: a 1- and 4-year prospective study. Schizophr Bull. 2005;31(1):167–174. doi: 10.1093/schbul/sbi004. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013–1022. doi: 10.1093/schbul/sbl057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351–356. doi: 10.1176/ajp.151.3.351. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 16.Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- 17.Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT, Jr, Buchanan RW. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr Bull. 2007;33(5):1201–1212. doi: 10.1093/schbul/sbl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman JA. Neurobiology and the natural history of schizophrenia. J Clin Psychiatry. 2006;67(10):e14. [PubMed] [Google Scholar]
- 19.Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157(4):549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 20.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165(5):579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 21.Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- 22.Dickinson D, Ragland JD, Calkins ME, Gold JM, Gur RC. A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophr Res. 2006;85(1–3):20–29. doi: 10.1016/j.schres.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 24.Hafner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Konnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases-a controlled study of schizophrenia, depression and healthy controls. Schizophr Res. 2005;77(1):11–24. doi: 10.1016/j.schres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Jones P, Rodgers B, Murray R, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344(8934):1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 26.Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159(7):1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- 27.Ho BC, Andreasen NC, Nopoulos P, Fuller R, Arndt S, Cadoret RJ. Secondary prevention of schizophrenia: utility of standardized scholastic tests in early identification. Ann Clin Psychiatry. 2005;17(1):11–18. doi: 10.1080/10401230590905272. [DOI] [PubMed] [Google Scholar]
- 28.Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, et al. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62(12):1297–1304. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- 30.Caspi A, Reichenberg A, Weiser M, Rabinowitz J, Kaplan Z, Knobler H, et al. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr Res. 2003;65(2–3):87–94. doi: 10.1016/s0920-9964(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 31.Yamauchi K, Aki H, Tomotake M, Iga J, Numata S, Motoki I, et al. Predictors of subjective and objective quality of life in outpatients with schizophrenia. Psychiatry Clin Neurosci. 2008;62(4):404–411. doi: 10.1111/j.1440-1819.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 32.Savilla K, Kettler L, Galletly C. Relationships between cognitive deficits, symptoms and quality of life in schizophrenia. Aust N Z J Psychiatry. 2008;42(6):496–504. doi: 10.1080/00048670802050512. [DOI] [PubMed] [Google Scholar]
- 33.Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- 34.Abi-Dargham A. Cortical dopamine in schizophrenia. In: Javitt DC, Kantrowitz JT, editors. Handbook of neurochemistry and molecular neurobiology. 3rd. New York: Springer; 2009. [Google Scholar]
- 35.Krystal JH, Perry EB, Jr, Gueorgulieva R, Belger A, Madonick SH, Abi-Dargham A, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62(9):985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- 36.Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF. Model psychoses and schizophrenia. Am J Psychiatry. 1962;119:61–67. doi: 10.1176/ajp.119.1.61. [DOI] [PubMed] [Google Scholar]
- 37.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 38.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 39.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 40.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57(12):1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 41.Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17(3):141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 43.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 44.Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 2004;174(1):32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- 45.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40(5):881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 46.Heresco-Levy U. Glutamatergic neurotransmission modulators as emerging new drugs for schizophrenia. Expert Opin Emerg Drugs. 2005;10(4):827–844. doi: 10.1517/14728214.10.4.827. [DOI] [PubMed] [Google Scholar]
- 47.Javitt DC. Phenomenology, aetiology and treatment of schizophrenia. Novartis Found Symp. 2008;289:4–16. doi: 10.1002/9780470751251.ch2. discussion 17–22, 87–93. [DOI] [PubMed] [Google Scholar]
- 48.Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, et al. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38(6):301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- 49.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 50.Bowers MB., Jr Central dopamine turnover in schizophrenic syndromes. Arch Gen Psychiatry. 1974;31(1):50–54. doi: 10.1001/archpsyc.1974.01760130034005. [DOI] [PubMed] [Google Scholar]
- 51.Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159(11):1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 52.Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66(6):533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 54.Ellison G, Keys A, Noguchi K. Long-term changes in brain following continuous phencyclidine administration: an autoradiographic study using flunitrazepam, ketanserin, mazindol, quinuclidinyl benzilate, piperidyl-3,4-3H(N)-TCP, and AMPA receptor ligands. Pharmacol Toxicol. 1999;84(1):9–17. doi: 10.1111/j.1600-0773.1999.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 55.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 57.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 58.Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16(1):93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- 59.Verkhratsky A, Kirchhoff F. NMDA Receptors in glia. Neuroscientist. 2007;13(1):28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 60.Lindahl JS, Kjellsen BR, Tigert J, Miskimins R. In utero PCP exposure alters oligodendrocyte differentiation and myelination in developing rat frontal cortex. Brain Res. 2008;1234:137–147. doi: 10.1016/j.brainres.2008.06.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen YJJ, Role LW, Talmage DA. Neuregulin 1 and schizophrenia. In: Javitt DC, Kantrowitz J, Lajtha A, editors. Handbook of neurochemistry and molecular neurobiology: schizophrenia. 3rd. New York: Springer; 2009. [Google Scholar]
- 62.Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci U S A. 2002;99(21):13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shinkai T, De Luca V, Hwang R, Muller DJ, Lanktree M, Zai G, et al. Association analyses of the DAOA/G30 and D-amino-acid oxidase genes in schizophrenia: further evidence for a role in schizophrenia. Neuromolecular Med. 2007;9(2):169–177. doi: 10.1007/BF02685890. [DOI] [PubMed] [Google Scholar]
- 64.Li D, He L. G72/G30 genes and schizophrenia: a systematic meta-analysis of association studies. Genetics. 2007;175(2):917–922. doi: 10.1534/genetics.106.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi J, Badner JA, Gershon ES, Liu C. Allelic association of G72/G30 with schizophrenia and bipolar disorder: a comprehensive meta-analysis. Schizophr Res. 2008;98(1–3):89–97. doi: 10.1016/j.schres.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maier W. Common risk genes for affective and schizophrenic psychoses. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):37–40. doi: 10.1007/s00406-008-2008-z. [DOI] [PubMed] [Google Scholar]
- 67.Talbot K, et al. Dysbindin-1 and its protein family. In: Javitt DC, Kantrowitz J, Lajtha A, editors. Handbook of neurochemistry and molecular neurobiology: schizophrenia. 3rd. New York: Springer; 2009. [Google Scholar]
- 68.Moghaddam B, Jackson ME. Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci. 2003;1003:131–137. doi: 10.1196/annals.1300.065. [DOI] [PubMed] [Google Scholar]
- 69.Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther. 2005;27(Suppl A):S8–S15. doi: 10.1016/j.clinthera.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 70.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- 71.Mossner R, Schuhmacher A, Schulze-Rauschenbach S, Kuhn KU, Rujescu D, Rietschel M, et al. Further evidence for a functional role of the glutamate receptor gene GRM3 in schizophrenia. Eur Neuropsychopharmacol. 2008;18(10):768–772. doi: 10.1016/j.euroneuro.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Harrison PJ, Lyon L, Sartorius LJ, Buret PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22(3):308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- 73.Ohtsuki T, Koga M, Ishiguro H, Horiuchi Y, Arai M, Niizato K, et al. A polymorphism of the metabotropic glutamate receptor mGluR7 (GRM7) gene is associated with schizophrenia. Schizophr Res. 2008;101(1–3):9–16. doi: 10.1016/j.schres.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 74.Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100(5):433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- 75.Parwani A, Weiler MA, Blaxton TA, Warfel D, Hardin M, Frey K, et al. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacology (Berl) 2005;183(3):265–274. doi: 10.1007/s00213-005-0177-2. [DOI] [PubMed] [Google Scholar]
- 76.Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004;29(1):208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- 77.Radant AD, Bowdle TA, Cowley DS, Kharasch ED, Roy-Byrne PP. Does ketamine-mediated N-methyl-D-aspartate receptor antagonism cause schizophrenia-like oculomotor abnormalities? Neuropsychopharmacology. 1998;19(5):434–444. doi: 10.1016/S0893-133X(98)00030-X. [DOI] [PubMed] [Google Scholar]
- 78.Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20(2):106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 79.Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30(3):633–639. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- 80.Hartvig P, Valtysson J, Lindner KJ, Kristensen J, Karlsten R, Gustafsson LL, et al. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther. 1995;58(2):165–173. doi: 10.1016/0009-9236(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 81.Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145(2):193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- 82.Krystal JH, Karper LP, Bennett A, D’Souza DC, Abi-Dargham A, Morrissey K, et al. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacology (Berl) 1998;135(3):213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- 83.Krystal JH, Bennett A, Abi-Saab D, Belger A, Karper LP, D’Souza DC, et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry. 2000;47(2):137–143. doi: 10.1016/s0006-3223(99)00097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22(3):293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 85.Passie T, Karst M, Wiese B, Emrich HM, Schneider U. Effects of different subanesthetic doses of (S)-ketamine on neuropsychology, psychopathology, and state of consciousness in man. Neuropsychobiology. 2005;51(4):226–233. doi: 10.1159/000085724. [DOI] [PubMed] [Google Scholar]
- 86.Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14(5):301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 87.Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry. 1998;43(11):811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- 88.Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- 89.Ahn KH, Youn T, Cho SS, Ha TH, Ha KS, Kim MS, et al. N-methyl-D-aspartate receptor in working memory impairments in schizophrenia: event-related potential study of late stage of working memory process. Prog Neuropsychopharmacology Biol Psychiatry. 2003;27(6):993–999. doi: 10.1016/S0278-5846(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 90.Harborne GC, Watson FL, Healy DT, Groves L. The effects of sub-anaesthetic doses of ketamine on memory, cognitive performance and subjective experience in healthy volunteers. Journal of Psychopharmacology. 1996;10(2):134–140. doi: 10.1177/026988119601000208. [DOI] [PubMed] [Google Scholar]
- 91.Hetem LA, Danion JM, Diemunsch P, Brandt C. Effect of a subanesthetic dose of ketamine on memory and conscious awareness in healthy volunteers. Psychopharmacology (Berl) 2000;152(3):283–288. doi: 10.1007/s002130000511. [DOI] [PubMed] [Google Scholar]
- 92.Honey RA, Turner DC, Honey GD, Sharar SR, Kumaran D, Pomarol-Clotet E, et al. Subdissociative dose ketamine produces a deficit in manipulation but not maintenance of the contents of working memory. Neuropsychopharmacology. 2003;28(11):2037–2044. doi: 10.1038/sj.npp.1300272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kieffaber PD, Kappenman ES, Bodkins M, Shekhar A, O’Donnell BF, Hetrick WP. Switch and maintenance of task set in schizophrenia. Schizophr Res. 2006;84(2–3):345–358. doi: 10.1016/j.schres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 94.Wylie GR, Clark EA, Butler PD, Javitt DC. Schizophrenia patients show task switching deficits consistent with N-methyl-d-aspartate system dysfunction but not global executive deficits: implications for pathophysiology of executive dysfuntion in schizophrneia. Schizophr Bull. 2010;36(3):585–594. doi: 10.1093/schbul/sbn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, et al. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156(10):1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- 96.Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77(1):43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 97.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14(1):88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Javitt DC, Liedeman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophr Bull. 1999;25(4):763–775. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- 99.Chang BP, Lenzenweger MF. Somatosensory processing and schizophrenia liability: proprioception, exteroceptive sensitivity, and graphesthesia performance in the biological relatives of schizophrenia patients. J Abnorm Psychol. 2005;114(1):85–95. doi: 10.1037/0021-843X.114.1.85. [DOI] [PubMed] [Google Scholar]
- 100.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76(1):1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5(3–4):207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 103.Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, et al. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158(2):705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 104.Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111(10):1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- 105.Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995;152(10):1517–1519. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- 106.Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. 2005;80(2–3):213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 107.Vidyasagar TR. A neuronal model of attentional spotlight: parietal guiding the temporal. Brain Res Brain Res Rev. 1999;30(1):66–76. doi: 10.1016/s0165-0173(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 108.Kwon YH, Nelson SB, Toth LJ, Sur M. Effect of stimulus contrast and size on NMDA receptor activity in cat lateral geniculate nucleus. J Neurophysiol. 1992;68(1):182–196. doi: 10.1152/jn.1992.68.1.182. [DOI] [PubMed] [Google Scholar]
- 109.Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116(9):2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12(7):3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- 111.Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, et al. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63(11):1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- 112.Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59(2–3):233–241. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- 113.Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87(1–3):238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59(11):1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 115.Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82(1):1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87(1–3):238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kantrowitz JT, Butler PD, Schecter I, Silipo G, Javitt DC. Seeing the world dimly: the impact of early visual deficits on visual experience in schizophrenia. Schizophr Bull. 2009;35(6):1085–1094. doi: 10.1093/schbul/sbp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kantrowitz JT, Rebheim N, Pasternak R, Silipo G, Javitt DC. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198–204. doi: 10.1016/j.psychres.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42(3):211–221. [PubMed] [Google Scholar]
- 121.Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48(7):627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 122.Balla A, Hashim A, Burch S, Javitt DC, Lajtha A, Sershen H. Phencyclidine-in-duced dysregulation of dopamine response to amphetamine in prefrontal cortex and striatum. Neurochem Res. 2001;26(8–9):1001–1006. doi: 10.1023/a:1012396820510. [DOI] [PubMed] [Google Scholar]
- 123.Balla A, Koneru R, Smiley J, Sershen H, Javitt DC. Continuous phencyclidine treatment induces schizophrenia-like hyperreactivity of striatal dopamine release. Neuropsychopharmacology. 2001;25(2):157–164. doi: 10.1016/S0893-133X(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 124.Balla A, Sershen H, Serra M, Koneru R, Javit DC. Subchronic continuous phencyclidine administration potentiates amphetamine-induced frontal cortex dopamine release. Neuropsychopharmacology. 2003;28(1):34–44. doi: 10.1038/sj.npp.1300019. [DOI] [PubMed] [Google Scholar]
- 125.Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30(4):649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- 126.Carlsson ML. Are the disparate pharmacological profiles of competitive and uncompetitive NMDA antagonists due to different baseline activities of distinct glutamatergic pathways? (Hypothesis) J Neural Transm Gen Sect. 1993;94(1):1–10. doi: 10.1007/BF01244978. [DOI] [PubMed] [Google Scholar]
- 127.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24(3):349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 128.Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154(7):1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 129.Reynolds GP, Beasley CL. GABAergic neuronal subtypes in the human frontal cortex--development and deficits in schizophrenia. J Chem Neuroanat. 2001;22(1–2):95–100. doi: 10.1016/s0891-0618(01)00113-2. [DOI] [PubMed] [Google Scholar]
- 130.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 131.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 132.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174(1):143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 133.Balla A, Nattini ME, Sershen H, Lajtha A, Dunlop DS, Javitt DC. GABA(B)/NMDA receptor interaction in the regulation of extracellular dopamine levels in rodent prefrontal cortex and striatum. Neuropharmacology. 2009;56(5):915–921. doi: 10.1016/j.neuropharm.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry. 2006;19(2):151–157. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- 135.Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycinesite agonists. Neuropsychopharmacology. 2004;29(2):300–307. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- 136.Contreras PC. D-serine antagonized phencyclidine- and MK-801-induced stereotyped behavior and ataxia. Neuropharmacology. 1990;29(3):291–293. doi: 10.1016/0028-3908(90)90015-j. [DOI] [PubMed] [Google Scholar]
- 137.Linn GS, O’Keeffe RT, Lifshitz K, Schroeder C, Javitt DC. Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine. Psychopharmacology (Berl) 2007;192(1):27–38. doi: 10.1007/s00213-007-0771-6. [DOI] [PubMed] [Google Scholar]
- 138.Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, et al. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol. 2001;60(6):1414–1420. doi: 10.1124/mol.60.6.1414. [DOI] [PubMed] [Google Scholar]
- 139.Javitt DC, Sershen H, Hashim A, Lajtha A. Reversal of phencyclidine-induced hyperactivity by glycine and the glycine uptake inhibitor glycyldodecylamide. Neuropsychopharmacology. 1997;17(3):202–204. doi: 10.1016/S0893-133X(97)00047-X. [DOI] [PubMed] [Google Scholar]
- 140.Javitt DC, Frusciante M. Glycyldodecylamide, a phencyclidine behavioral antagonist, blocks cortical glycine uptake: implications for schizophrenia and substance abuse. Psychopharmacology (Berl) 1997;129(1):96–98. doi: 10.1007/s002130050168. [DOI] [PubMed] [Google Scholar]
- 141.Sucher NJ, Lipton SA. Redox modulatory site of the NMDA receptor-channel complex: regulation by oxidized glutathione. J Neurosci Res. 1991;30(3):582–591. doi: 10.1002/jnr.490300316. [DOI] [PubMed] [Google Scholar]
- 142.Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, et al. Cysteine regulation of protein function--as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002;25(9):474–480. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- 143.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12(10):3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 144.Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS ONE. 2008;3(4):e1944. doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22(1–2):83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Steullet P, Neijt HC, Cuenod M, Do KQ. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience. 2006;137(3):807–819. doi: 10.1016/j.neuroscience.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 147.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004;174(1):39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- 149.Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 2005;179(1):303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 150.Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry. 2007;62(7):739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodriguez AL, Williams R. Recent progress in the development of allosteric modulators of mGluR5. Curr Opin Drug Discov Devel. 2007;10(6):715–722. [PubMed] [Google Scholar]
- 152.Robbins MJ, Starr KR, Honey A, Soffin EM, Rourke C, Jones GA, et al. Evaluation of the mGlu8 receptor as a putative therapeutic target in schizophrenia. Brain Res. 2007;1152:215–227. doi: 10.1016/j.brainres.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 153.Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9(11):984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- 154.Olszewski RT, Wegorzewska MM, Monteiro AC, Krolikowski KA, Zhou J, Kozikowski AP, et al. Phencyclidine and dizocilpine induced behaviors reduced by N-acetylaspartylglutamate peptidase inhibition via metabotropic glutamate receptors. Biol Psychiatry. 2008;63(1):86–91. doi: 10.1016/j.biopsych.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Olszewski RT, Bukhari N, Zhou J, Kozikowski AP, Wroblewski JT, Shamimi-Noori S, et al. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89(4):876–885. doi: 10.1111/j.1471-4159.2004.02358.x. [DOI] [PubMed] [Google Scholar]
- 156.Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP. Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry. 1994;151(8):1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- 157.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56(1):29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 158.Javitt DC, Silipo G, Cienfuegos A, Shelley AM, Bark N, Park M, et al. Adjunctive high-dose glycine in the treatment of schizophrenia. Int J Neuropsychopharmacol. 2001;4(4):385–391. doi: 10.1017/S1461145701002590. [DOI] [PubMed] [Google Scholar]
- 159.Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 2004;55(2):165–171. doi: 10.1016/s0006-3223(03)00707-8. [DOI] [PubMed] [Google Scholar]
- 160.Tsai G, Yang P, Chung LC, Lange N, Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998;44(11):1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 161.Heresco-Levy U, Javitt DC, Ebstein R, Bass A, Lichtenberg P, Bar G, et al. D-serine efcacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57(6):577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 162.Tsai GE, Yang P, Chang YC, Chong MY. D-alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2005;59(3):230–234. doi: 10.1016/j.biopsych.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 163.Tsai G, Lane HY, Yang P, Chong MY, Lange N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2004;55(5):452–456. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 164.Lane HY, Chang YC, Liu YC, Chiu CC, Tsai Ge. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebocontrolled study. Arch Gen Psychiatry. 2005;62(11):1196–1204. doi: 10.1001/archpsyc.62.11.1196. [DOI] [PubMed] [Google Scholar]
- 165.Kantrowitz JT, Malhotra AK, Cornblatt BA, Silipo G, Balla A, Suckow RF, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1–3):125–130. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;72(2–3):225–234. doi: 10.1016/j.schres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 167.Javitt DC. Glycine transport inhibitors and the treatment of schizophrenia. Biol Psychiatry. 2008;63(1):6–8. doi: 10.1016/j.biopsych.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 168.Woods SW, et al. Effects of oral glycine in the schizophrenia prodrome. Schizophr Res. 2004;70(Suppl 1):79. [Google Scholar]
- 169.Buchanan RW, Javitt DC, Marder ST, Schooler NR, Gold JM, McMahon RP, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164(10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]