Abstract
Chronic activation of the myocardial renin angiotensin system (RAS) elevates the local level of angiotensin II (Ang II) thereby inducing pathological cardiac hypertrophy, which contributes to heart failure. However, the precise underlying mechanisms have not been fully delineated. Herein we report a novel paracrine mechanism between cardiac fibroblasts (CF)s and cardiomyocytes whereby Ang II induces pathological cardiac hypertrophy. In cultured CFs, Ang II treatment enhanced exosome release via the activation of Ang II receptor types 1 (AT1R) and 2 (AT2R), whereas lipopolysaccharide, insulin, endothelin (ET)-1, transforming growth factor beta (TGFβ)1 or hydrogen peroxide did not. The CF-derived exosomes upregulated the expression of renin, angiotensinogen, AT1R, and AT2R, downregulated angiotensin-converting enzyme 2, and enhanced Ang II production in cultured cardiomyocytes. In addition, the CF exosome-induced cardiomyocyte hypertrophy was blocked by both AT1R and AT2R antagonists. Exosome inhibitors, GW4869 and dimethyl amiloride (DMA), inhibited CF-induced cardiomyocyte hypertrophy with little effect on Ang II-induced cardiomyocyte hypertrophy. Mechanistically, CF exosomes upregulated RAS in cardiomyocytes via the activation of mitogen-activated protein kinases (MAPKs) and Akt. Finally, Ang II-induced exosome release from cardiac fibroblasts and pathological cardiac hypertrophy were dramatically inhibited by GW4869 and DMA in mice. These findings demonstrate that Ang II stimulates CFs to release exosomes, which in turn increase Ang II production and its receptor expression in cardiomyocytes, thereby intensifying Ang II-induced pathological cardiac hypertrophy. Accordingly, specific targeting of Ang II-induced exosome release from CFs may serve as a novel therapeutic approach to treat cardiac pathological hypertrophy and heart failure.
Keywords: Angiotensin II, Exosomes, Cardiac hypertrophy, Heart failure
1. Introduction
The renin-angiotensin system (RAS) mainly consists of angiotensinogen (Agt), renin, angiotensin-converting enzyme (ACE), angiotensin II (Ang II), and Ang II receptors AT1R, and AT2R [1, 2]. Ang II is the primary effector of RAS and is derived from angiotensinogen by the successive enzymatic actions of renin and ACE. Most of the known actions of Ang II are mediated via AT1R and AT2R. Traditionally, RAS is considered as an endocrine system in which circulating Ang II is generated from the cleavage of liver-secreted Agt by kidney-released renin and ACE located on the luminal side of the vascular endothelium, referred to as systemic RAS. Additionally, Ang II is produced locally in tissues that express all of the RAS components, designated as the local RAS. A tightly controlled activity of RAS is required not only for maintaining systemic hemodynamics and blood volume but also for controlling cell proliferation, differentiation, and tissue remodeling in target organs like the heart. However, chronic activation of local RAS in the heart results in elevated levels of myocardial Ang II thereby leading to pathological cardiac hypertrophy and heart failure [1]. Several lines of evidence have demonstrated that Ang II could directly induce cardiomyocyte hypertrophy [3, 4]. Nevertheless, the precise mechanism for elevating myocardial levels of Ang II remains unclear.
Exosomes are small extracellular membrane vesicles of endocytic origin with diameters ranging from 30 to 100 nm and constitutively released by fusion with the cell membrane [5]. Exosomes mediate local and systemic cell communication through the horizontal transfer of bioactive materials [5, 6]. While exosomes have been implicated in cardiovascular diseases [7], a recent study demonstrated that Ang II increased the levels of miRNA (miR)-21* (miR-21-3p) in cardiac fibroblast (CF)-derived exosomes and the CF exosomes delivered miR-21* into cardiomyocytes thereby leading to cellular hypertrophy [8]. These findings suggest a novel paracrine mechanism between CF and cardiomyocyte in Ang II-induced cardiac hypertrophy. However, little is known regarding the regulatory mechanisms responsible for exosome release from cardiac fibroblasts. It is also unclear whether there are other mechanisms by which CF exosomes mediate Ang II-induced cardiac hypertrophy.
In the present study, we identified that Ang II stimulates exosome release from CFs via activation of both AT1R and AT2R. CF exosomes in turn enhances the expression of renin, Agt, AT1R, and AT2R while suppressing the expression of ACE2, and increases Ang II production in cardiomyocytes, thereby amplifying Ang II-inducing cardiac pathological hypertrophy. Our findings demonstrate for the first time that Ang II-induced exosome release from CFs serves as a crucial paracrine mechanism by which Ang II intensifies its own signaling in cardiomyocytes, thus leading to cardiac pathological hypertrophy.
2. Methods
Isolation and culture of rat neonatal cardiac myocytes and fibroblasts, and mouse adult CFs were performed as previously described [9–11] with a minor modification. The purity of cultured CFs is ~100% (Supplementary Fig. 1). Isolation and purification of exosomes from the culture medium of rat neonatal CFs were performed by multi-step centrifugation [12–14]. The CF-derived exosomes were characterized by transmission electron microscopy and Western blot analysis. All methods including the procedures for determining cardiomyocyte hypertrophy, for inducing myocardial hypertrophy via Ang II infusion in vivo, and for the various bioassays are detailed in Online-only Supplement.
3. Results
3.1. Characterization of Cardiac Fibroblast-derived Exosome-induced Cardiomyocyte Hypertrophy
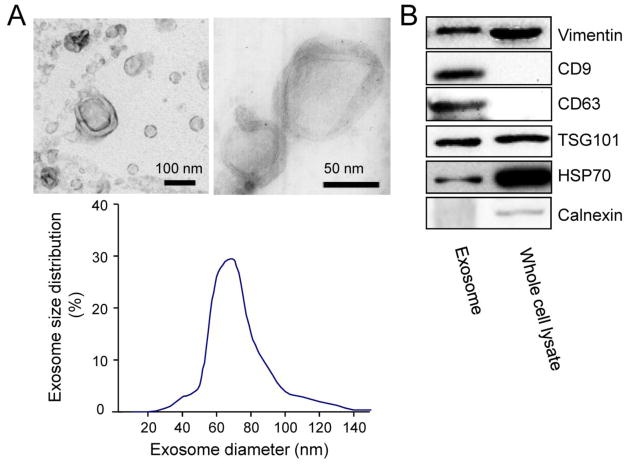
To address a role of cardiac fibroblast (CF)-derived exosomes in the heart, we first isolated and characterized exosomes derived from neonatal rat CFs. Neonatal rat CFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% exosome-depleted FBS for 48 h. Membrane vesicles were isolated from the CF culture media. More than 95% of the vesicles had a cup-shaped morphology with a diameter range of 50 – 100 nm (Fig. 1A), which are characteristic features of most exosomes [15]. In addition, the isolated vesicles were positive for vimentin [16] (Fig. 1B), indicating their fibroblast origin and they also contained the established exosome-associated protein markers such as CD9, CD63, tumor susceptibility gene (TSG)101 and heat-shock protein (HSP)70 [5, 6], but were negative for calnexin, a protein which is not expressed in exosomes [17] (Fig. 1B). These results demonstrated that the isolated vesicles released from neonatal rat CFs are exosomes.
Fig. 1.
Characterization of cardiac fibroblasts-derived exosomes. (A) Upper panel: Transmission electron microscopic images of neonatal rat cardiac fibroblast-derived (CF) exosomes; Lower panel: Distribution of CF exosome sizes. (B) Western blot analysis of several exosome biomarkers in CF exosomes. Immunoblots are representative of six separate experiments.
It has been well documented that CFs produce pro-hypertrophic substances to induce cardiomyocyte hypertrophy [18, 19]. A recent study showed that CF exosomes are the sole carrier of these pro-hypertrophic substances [8]. To reproduce these observations, we examined the effects of neonatal rat CF- and HEK293-conditioned culture medium, as well as different fractions of the cardiac fibroblast-conditioned culture medium including the exosome depleted fraction on hypertrophic growth in neonatal rat cardiomyocytes. As shown in Supplementary Fig. 2, it was the CF-conditioned but not the HEK293-conditioned culture medium that induced increases in cell surface areas, [3H]-Leucine uptake, and early fetal gene expression including an upregulation of atrial natriuretic factor (ANF), B-type natriuretic peptide (BNP), and beta-myosin heavy chain (βMHC), and a downregulation of alpha-myosin heavy chain (αMHC), which are characteristic of pathological cardiomyocyte hypertrophy [9]. The pro-hypertrophic effects of CF-conditioned medium were largely dependent on substances with molecular weights >100 kDa (Supplementary Fig. 3A). However, the pro-hypertrophic effects of fractioned CF-conditioned medium (>100 kDa) were dramatically attenuated by depleting exosomes (Supplementary Fig. 3B–E). These results indicate that exosomes are not the sole transporter of all the pro-hypertrophic substances secreted from CFs but instead act as a major mediator of the CF-induced cardiomyocyte hypertrophy.
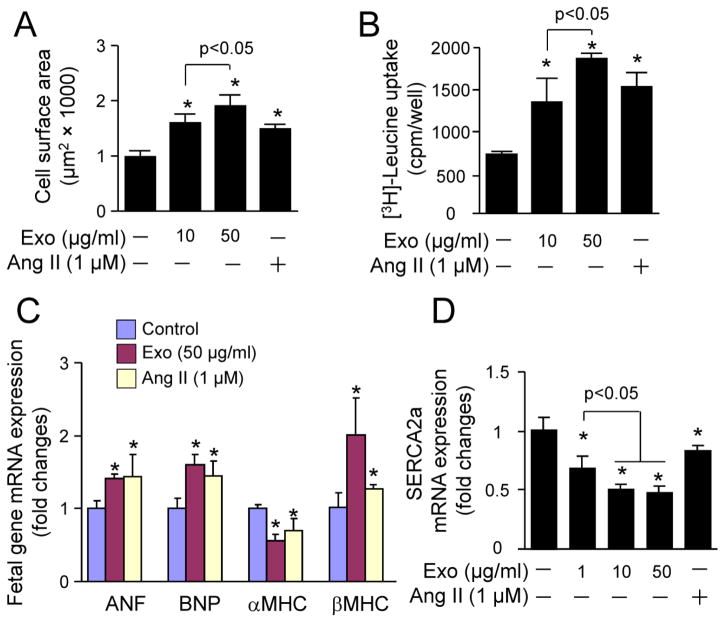
We further determined the pro-hypertrophic effect of CF exosomes in neonatal rat cardiomyocyes using Ang II as a positive control. Indeed, Ang II induced cardiomyocyte hypertrophy (Fig. 2A and B) associated with the activation of fetal hypertrophic gene expression (Fig. 2C) aforementioned and a downregulation of sarco(endo)plasmic reticulum calcium ATPase2a (SERCA2a) (Fig. 2D), a critical regulator of cardiomyocyte function [20]. It has been established that the up-regulation of βMHC expression and down-regulation of αMHC (αMHC/βMHC switch) contribute to the reduction of myofibrillar ATPase activity and cardiac myofiber shortening and velocity of shortening thereby leading to eventual contractile dysfunction [21]. In addition to the αMHC/βMHC switch, a down-regulation of SERCA2a with consequent loss of efficient Ca2+ cycling is involving in pathological myocardial hypertrophy and heart failure [20, 21]. Thus, the Ang II-induced cardiomyocyte hyperytrophy is pathological. Like Ang II, the CF exosomes induced pathological cardiomyocyte hypertrophy (Fig. 2). Interestingly, the magnitude of CF-derived exosomes-induced cardiomyocyte hypertrophy was greater than that of Ang II (Fig. 2). Collectively, these results demonstrate that CF-derived exosomes are capable of inducing pathological cardiomyocyte hypertrophy in vitro.
Fig. 2.
The effect of cardiac fibroblast-derived exosomes on cardiomyocyte hypertrophy. Neonatal rat cardiomyocytes were treated with neonatal rat cardiac fibroblasts-derived exosomes (Exo) or angiotensin II (Ang II) as indicated for 48 h and then subjected to (A) cell surface area measurement, (B) [3H]-Leucine uptake, (C and D) qPCR analysis of gene expression. n=4, *p<0.05 vs. vehicle treated control (−).
3.2. Ang II Stimulates Release of Exosomes From Cardiac Fibroblasts
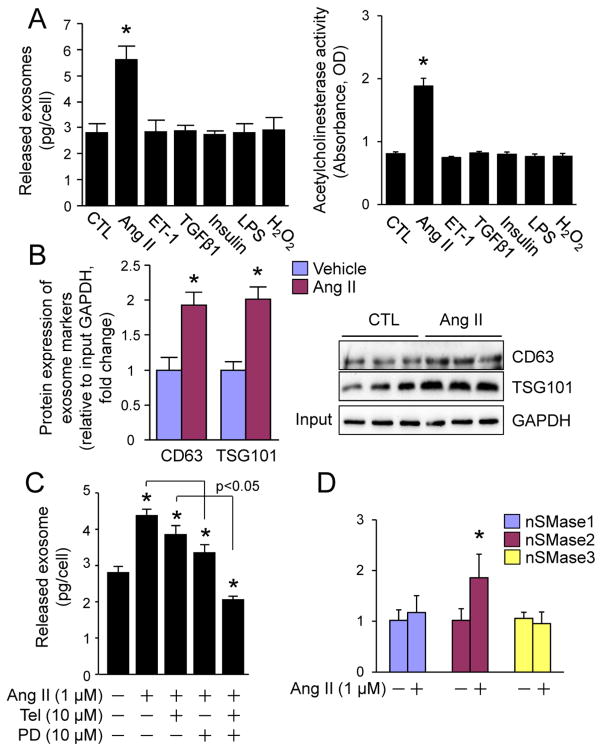
The pathophysiological relevance of CF exosomes remains to be further explored. Therefore, we determined whether CF exosome release is altered in various pathophysiological settings including RAS over-activation, hyperinsulinemia, inflammation, and oxidative stress in cultured neonatal rat CFs. Ang II, insulin, lipopolysaccharide (LPS), and hydrogen peroxide (H2O2) were used to mimic these settings as described elsewhere [10, 22–24]. The effects of pro-hypertrophic factors, ET-1 [25] and TGFβ1 [26] on release of exosomes from neonatal CFs were also studied. We measured the total amount of proteins (concentrations), the activity of acetylcholinesterase (AChE), an exosome specific enzyme [27] and protein expression levels of TSG101 and CD63, two exosome markers [5, 6] in total exosomes released from the same number of CFs after the treatment as mentioned above. Intriguingly, only Ang II enhanced protein quantity, AChE activity, and TSG101 and CD63 protein expression in the released exosomes (Fig. 3A and B). The increases in protein concentrations are well proportional with the increases in TSG101 and CD63 protein levels or AChE activities (Fig. 3A and B). These results indicate that the total protein level of CF exosomes reflects the number of CF exosomes in a setting of Ang II stimulation.
Fig. 3.
Ang II-induced release of exosomes from cardiac fibroblasts. (A, B) The effect of pathological stress on the release of exosomes from cardiac fibroblasts. (A) The amount of proteins and the activity of acetylcholinesterase in total released exosomes from the same number of neonatal rat cardiac fibroblasts that were stimulated with vehicle, Ang II (1 μM), ET-1 (0.1 μM), TGFβ1 (10 ng/ml), insulin (0.1 μM), LPS (0.1 μg/ml), H2O2 (500 μM) as indicated for 48 h. n=3, *p<0.05 vs. vehicle-treated control (CTL). (B) Western blot analysis of CD63 and TSG101 in total exosomes from the same number of neonatal rat cardiac fibroblasts that were stimulated with vehicle or Ang II for 48 h. Left panel shows semi-quantified results. n=3, *p<0.05 vs. vehicle-treated control (CTL). Right panel is the immunoblots (n=3). Input: 10 μl of the neonatal rat cardiac fibroblast lysates. (C) The effect of Telmisartan and PD123319 on Ang II-induced secretion of exosomes from cardiac fibroblasts. Neonatal rat cardiac fibroblasts were treated with Ang II (1 μM), Telmisartan (Tel, 10 μM), PD123319 (PD, 10 μM) as indicated for 48 h. The total proteins of exosomes secreted were measured by BCA assay and normalized by cell number. n=3, *p<0.05 vs. vehicle treated control (−). (D) The effect of Ang II on mRNA expression of neutral sphingomyelinase (nSMase) in cardiac fibroblasts. Neonatal rat cardiac fibroblasts were treated with vehicle of Ang II (1 M) for 48 h and subjected to qPCR analysis of nSMase1, 2, and 3 expression. n=4, *p<0.05 vs. vehicle-treated control (−).
In addition, we found that Ang II stimulated exosome release from neonatal rat CFs via the activation of both AT1R and AT2R (Fig. 3C). On the other hand, Ang II did not affect the expression of neutral sphingomyelinase (nSMase)1 or nSMase3 but significantly upregulated the expression of nSMase2 (Fig. 3D), a critical regulator of exosome release [28], in neonatal rat CFs. These results indicate that Ang II enhances CF exosome release most likely by enhancement of CF nSMase2 activity via activating both AT1R and AT2R. CF exosomes may play a unique role in the Ang II-induced cardiac hypertrophy.
3.3. Cardiac Fibroblast-derived Exosomes Induce Cardiomyocyte Hypertrophy via Upregulation of RAS
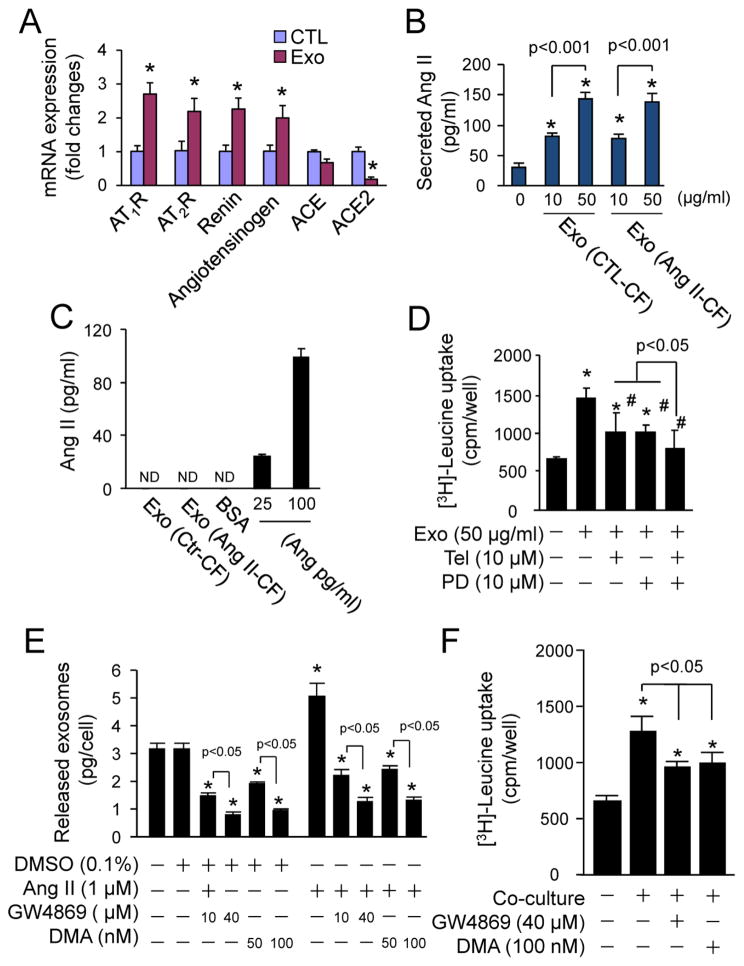
We then investigated whether the Ang II-increased production of CF exosomes functions as a yet unappreciated aspect of the Ang II-induced cardiomyocyte hypertrophy feedback loop. Accordingly, we determined whether CF exosomes are capable of regulating RAS in cultured neonatal cardiomyocytes. As shown in Fig. 4A, CF exosomes upregulated the expression of renin, Agt, AT1R, and AT2R and downregulated the expression of ACE2. In addition, CF exosomes stimulated a dramatic increase in Ang II secretion (Fig. 4B), whereas CF exosomes per se did not contain Ang II (Fig. 4C). These results reveal that CF exosomes activate the cardiomyocyte RAS and cumulatively increase Ang II production and secretion. To investigate a functional link between CF exosome-induced activation of RAS and hypertrophy in cardiomyocytes, we determined the effects of AT1R blocker Telmisartan and AT2R antagonist PD123319 on CF exosomes-induced hypertrophic growth in neonatal rat cardiomyocytes. As shown in Fig. 4D, CF exosome-induced [3H]-Leucine uptake was similarly attenuated by Telmisartan and PD1233319, and completely blocked following co-treatment with Telmisartan and PD123319. These results demonstrate CF exosomes upregulate the expression of AT1R and AT2R and enhance Ang II production and secretion from cardiomyocytes. The increased Ang II in turn activates the upregulated AT1R and AT2R resulting in an exaggerated phenotype of cardiomyocyte hypertrophy.
Fig. 4.
The effects of cardiac fibroblast-derived exosomes on activation of renin angiotensin system (RAS) in cardiomyocytes. (A) Cardiac fibroblast-derived exosomes (Exo)-induced expression of RAS components. Neonatal rat cardiomyocytes were treated with or without Exo (50 μg/ml) for 48 h and then subjected to qPCR analysis of mRNA expression of AT1R, AT2R, ACE, ACE2, and angiotensinogen. n=4, *p<0.05 vs. vehicle-treated control (CTL). (B) Measurement of Ang II in culture medium of cardiomyocytes treated with Exo. Neonatal cardiomyocytes were treated with or without exosomes derived from neonatal rat cardiac fibroblasts treated with (Ang II-CF) or without Ang II (CTL-CF) for 48 h and then the culture medium were subjected to enzyme immunoassay (EIA) of Ang II. n=4, *p<0.05 vs. vehicle treated control (0). (C) Measurement of Ang II in cardiac fibroblast-derived exosomes (Exo). Lysates (50 μg) of exosomes derived from neonatal rat cardiac fibroblasts treated with (Ang II-CF) or without Ang II (CTL-CF) were subjected to EIA analysis of Ang II as indicated, and 25–100 pg/ml Ang II was used as a positive control. Bovine serum albumin (BSA, 50 μg) was used as a negative control; ND - non-detectable. n=4. (D) The effects of Telmisartan (Tel) and PD123319 (PD) on cardiac fibroblast-derived exosomes (Exo)-induced [3H]-Leucine uptake in cardiomyocytes. Neonatal rat cardiomyocytes were treated with Exo (50 μg/ml), Tel (10 μM), and PD (10 μM) as indicated for 48 h. n=4, *p<0.05 vs. control (−); #p<0.05 vs. Exo (+). (E) The effect of GW4869 and DMA on exosome release from cardiac fibroblasts. Neonatal rat cardiac fibroblasts were treated with GW4869 and DMA as indicated for 48 h and the culture medium was subjected to exosome isolation and quantification. n=4, *p<0.05 vs. vehicle-treated control (−). (F) The effect of GW4869 and DMA on cardiac fibroblast-induced [3H]-Leucine uptake in cardiomyocytes. Neonatal rat cardiac myocytes and fibroblasts were co-cultured with GW4869 and DMA as indicated for 48 h. n=4, *p<0.05 vs. vehicle-treated control (−).
To determine the functional relevance of CF exosomes in cellular communication between cardiac fibroblasts and cardiomyocytes, we examined the effect of exosome inhibitors GW4869 and dimethyl amiloride (DMA) [28, 29] on hypertrophic growth in neonatal rat cardiomyocytes co-cultured with neonatal rat CFs. The effective doses of GW4869 (40 μM) and DMA (100 nM) suppressed both basal and Ang II-induced exosome release from neonatal rat CFs (Fig. 4E) and strongly inhibited [3H]-Leucine uptake in the cardiomyocytes (Fig. 4F). However, Ang II-induced [3H]-Leucine uptake was not affected by GW4869 (40 μM) and DMA (100 nM) (Supplementary Fig. 4). These findings suggest that CF exosomes act as critical paracrine mediator of CF-induced cardiomyocyte hypertrophy.
Taken together, these results indicate that CF exosomes are capable of upregulating the RAS of cardiomyocytes, which results in the pro-hypertrophic autocrine activation of the Ang II-Ang II receptor axis in cardiomyocytes.
3.4. Cardiac Fibroblast-derived Exosomes Upregulate RAS in Cardiomyocytes via the Activation of Mitogen-activated Protein Kinases (MAPKs) and Akt
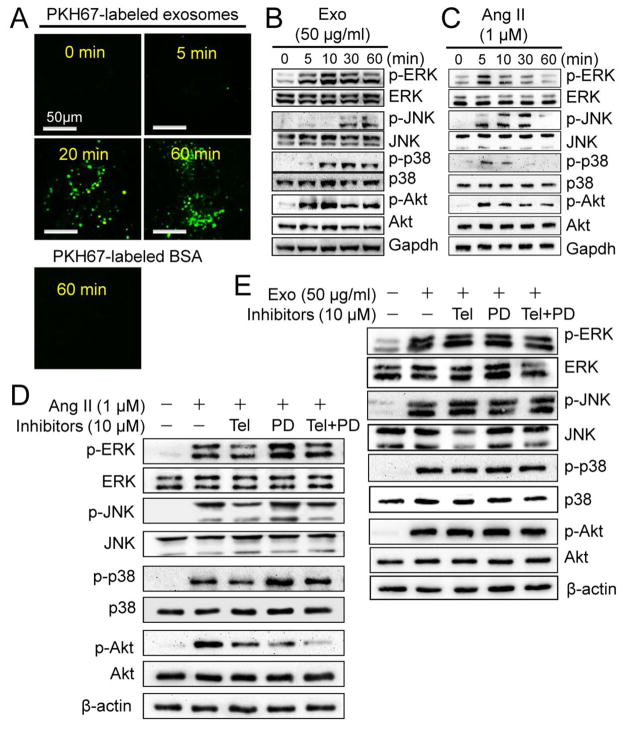
To explore the molecular mechanism by which CF exosomes upregulate RAS in cardiomyocytes, we tracked CF exosome traffic and determined the effect of CF exosomes on the activation of MAPK and Akt, which are capable of regulating RAS [30, 31], in neonatal rat cardiomyocytes. As shown in Fig. 5A, CF exosomes entered the cytoplasm within 5 min, further accumulated at 20 min, and the accumulation was maintained for at least 60 min. In addition, CF exosomes activated MAPKs including ERK and p38, as well as Akt, and their activation was apparent at 5 min, peaked at 10 min and was maintained for at least 60 min (Fig. 5B and Supplementary 5A). CF exosomes also activated MAPK JNK, which appeared at 30 min and was maintained up to 60 min (Fig. 5B and Supplementary 5A). While Ang II also activated these kinases peaking at 5 min, it contrastingly declined to the basal level by 60 min (Fig. 5C and Supplementary 5B). The Ang II-induced activation of MAPKs was inhibited by co-treatment with Telmisartan and enhanced by co-treatment with PD123319 (Fig. 5D and Supplementary 5C). The Ang II-induced Akt activation was inhibited by both Telmisartan and PD123319 (Fig. 5D and Supplementary Fig. 5C). However, the CF exosome-induced activation of these kinases was not affected by co-treatment with either Telmisartan or PD123319 (Fig. 5E and Supplementary Fig. 5D). Taken together, these results demonstrate that CF exosomes activate MAPK and Akt in cardiomyocytes via a mechanism independent of the rapid Ang II release.
Fig. 5.
The effects of cardiac fibroblast-derived exosomes on pro-hypertrophic signaling in cardiomyocytes. (A) Exosomes uptake assay. Cardiac fibroblast-derived exosomes were labeled with green membrane dye PKH67 and incubated with neonatal rat cardiomyocytes as indicated and visualized using a confocal microscopy. The results are representatives of 3 separated experiments. Scale bar = 50 μm. (B) Exosome (Exo)-induced activation of mitogen-activated protein kinases (MAPKs) and Akt. Neonatal rat cardiomyocytes were treated with (5, 10, 30 and 60 min) and without (0 min) Exo (50 μg/ml) derived from neonatal rat cardiac fibroblasts as indicated and then subjected to Western blot analysis of phosphorylated and total ERK1/2, JNK, p38, and Akt. The results are representatives of 4 separated experiments. (C) Ang II-induced activation of MAPKs and Akt. Neonatal rat cardiomyocytes were treated with (5, 10, 30 and 60 min) or without (0 min) Ang II (1 μM) as indicated and then subjected to Western blot analysis of phosphorylated and total ERK1/2, JNK, p38, and Akt. The results are representatives of 4 separated experiments. (D) The effects of Telmisartan (Tel) and PD123319 (PD) on Exo-induced activation of MAPKs and Akt. Neonatal rat cardiomyocytes were treated with Exo, Tel (10 μM), and PD (10 μM) as indicated for 20 min and subjected to Western blot analysis. The immunoblots are representatives of 4 separated experiments. (E) The effects of Tel and PD on Ang II-induced activation of MAPKs and Akt. Neonatal rat cardiomyocytes were treated with Ang II, Tel (10 μM), and PD (10 μM) as indicated for 5 min and subjected to Western blot analysis. The immunoblots are representatives of 4 separate experiments.
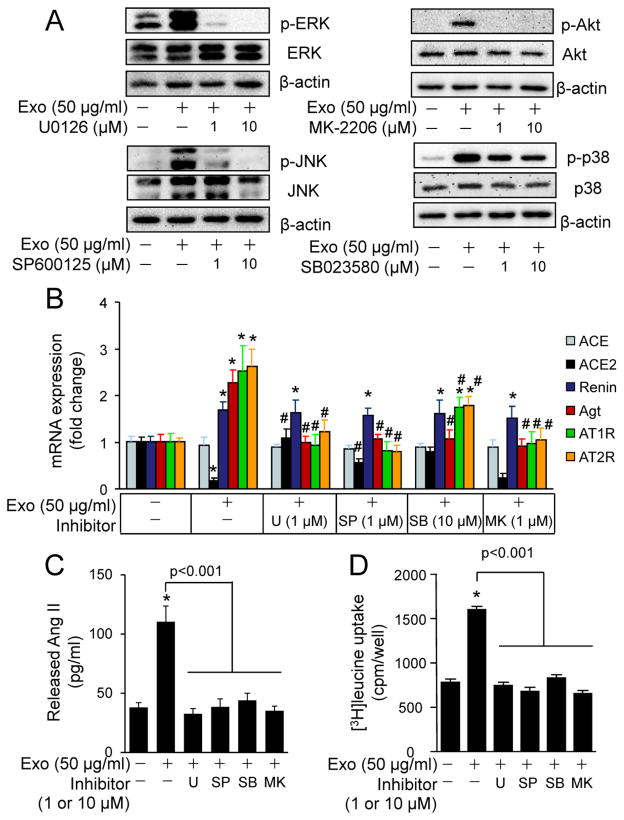
To establish a mediator role of MAPKs and Akt in CF exosome-induced upregulation of RAS in cardiomyocytes, we utilized kinase inhibitors. The effective doses of ERK (U0126; 1 μM), p38 (SB023580; 10 μM), JNK (SP600125; 1 μM), and Akt (MK-2206; 1 μM) inhibited CF exosome-induced upregulation of RAS in neonatal rat cardiomyocytes (Fig. 6A and B). In addition, CF exosome-induced upregulation of renin, Atg, AT1R, and AT2R was blocked by the Akt inhibitor (Fig. 6B) while ACE and ACE2 were unaffected. Moreover, CF exosome-induced Ang II release and [3H]-Leucine uptake was blocked by these inhibitors individually (Fig. 6C and D). Collectively, these results indicate that CF exosomes are capable of increasing Ang II production and release from cardiomyocytes via the activation of MAPKs and Akt.
Fig. 6.
The effects of MAPK and Akt inhibitors on cardiac fibroblast-derived exosomes-induced activation of RAS in cardiomyocytes. (A) Dose response of MAPK and Akt inhibitors on cardiac fibroblast-derived exosomes (Exo)-induced activation of MAPKs and Akt. Neonatal rat cardiomyocytes were treated with or without Exo (50 μg/ml), U0126, SP600125, MK-2206, and SB023580 for 20 min and subjected to Western blot analysis. The results are from 4 separate experiments. (B) The effects of MAPK and Akt inhibitors on Exo-induced mRNA expression of RAS components. Neonatal rat cardiomyocytes were treated with or without Exo (50 μg/ml), U0126 (U, 1 μM), SP600125 (SP, 1 μM), SB023580 (SB, 10 μM), and MK-2206 (MK, 1 μM) for 48 h and then subjected to qPCR analysis of mRNA expression of ACE, ACE2, renin, angiotensinogen (Agt), AT1R, and AT2R. n=4, *p<0.05 vs. Control (−). #p<0.05 vs. Exo (+) group. (C) The effects of MAPK and Akt inhibitors on Exo-induced Ang II release. Neonatal rat cardiomyocytes were treated with or without Exo (50 μg/ml), U0126 (U, 1 μM), SP600125 (SP, 1 μM), SB023580 (SB, 10 μM), and MK-2206 (MK, 1 μM) for 48 h. Ang II was measured in the culture medium. n=4, *p<0.05 vs. Control (−). (D) The effects of MAPK and Akt inhibitors on Exo-induced [3H]-Leucine uptake. Neonatal rat cardiomyocytes were treated with or without Exo (50 μg/ml), U0126 (U, 1 μM), SP600125 (SP, 1 μM), SB023580 (SB, 10 μM), and MK-2206 (MK, 1 μM) for 48 h. n=4, *p<0.05 vs. Control (−).
3.5. Proteomics Analysis of Cardiac Fibroblast-derived Exosomes
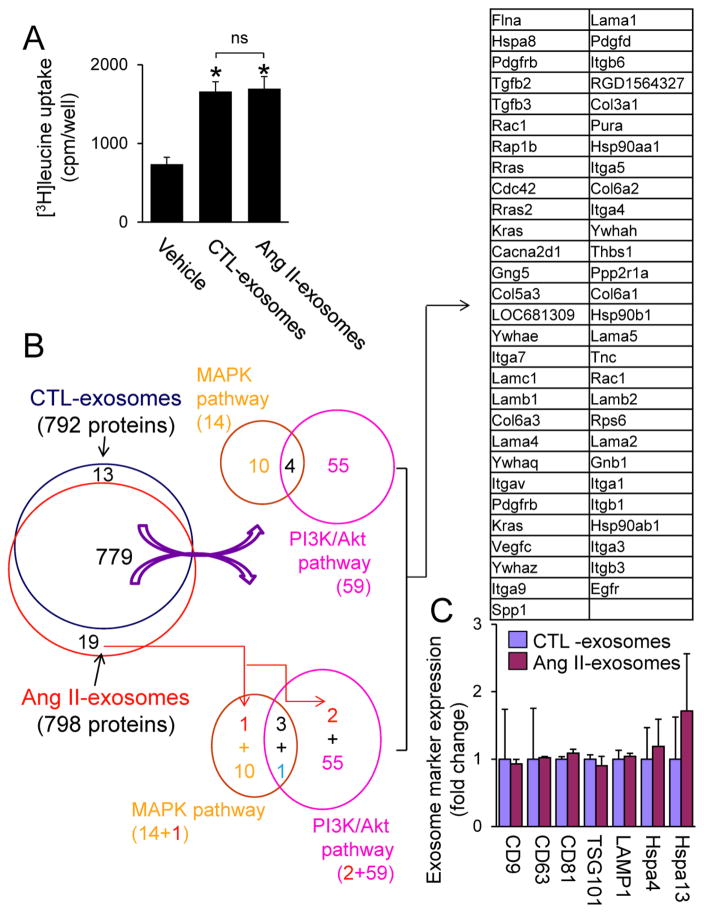
Given that Ang II increases the levels of several miRNAs (miRs), such as miR-132 which has potential to regulate Ang II signaling [32], in cardiac fibroblasts [8], it is likely that the enriched miRs in CF exosomes are responsible for the autocrine enhancement of Ang II-induced cardiomyocyte hypertrophy. However, surprisingly, we found that the magnitude of cardiomyocyte hypertrophy induced by exosomes derived from CFs in the presence or absence of Ang II stimulation is similar (Fig. 7A). The precise reason for these discrepancies is unclear. One possibility is that the phenotype of cultured CFs may be different due to the different experimental conditions. Alternatively, the amount of exosomes derived from vehicle control-treated neonatal rat CFs (CTL-exosomes) may be more than that derived from Ang II-treated neonatal CFs (Ang II-exosomes) due to the limitation of measuring methods in our study. In addition, except the well-characterized miRs [8], other substances such as proteins secreted by CF exosomes mediate the paracrine intensification of Ang II-induced cardiomyocyte hypertrophy. Therefore, we performed quantitative proteomics analysis (n=3) using equal amount of exosomes (20 μg) released from neonatal rat CFs with or without Ang II stimulation. We found that there are 792 proteins in CTL-exosomes (Supplementary table 1). KEGG analysis indicates that CTL exosome proteins are linked to 254 pathway maps (Supplementary table 2) including PI3K/Akt and MAPK pathways, hypertrophic cardiomyopathy, and dilated cardiomyopathy (Supplementary Fig. 6 and 7). Given that CF exosomes upregulate RAS via activation of PI3K/Akt and MAPK pathways (Fig. 6), we analyzed the CTL exosome proteins which are capable of regulating both pathways. Among the 59 PI3K/Akt (Supplementary table 3) and 14 MAPK pathway (Supplementary table 4) related CF exosome proteins, we found four such proteins including platelet derived growth factor receptor beta (Pdgfrb), Ras-related C3 botulinum toxin substrate 1 (Rac1), Guanine nucleotide-binding protein subunit gamma (Gng12), and GTPase Kras (Kras) (Fig. 7A). On the other hand, there are 798 proteins in Ang II-exosomes (Fig. 7B and Supplementary table 5). We found that 98% of the proteins in Ang II-exosomes are identical with that in CTL-exosomes, suggesting that Ang II has minor impact on the protein expression of CF exosomes (Fig. 7B, Supplementary tables 1 and 5). Indeed, there is no difference in the expression of exosome marker proteins including CD9, CD63, CD81, TSG101, lysosomal-associated membrane protein (LAMP)1, and HSP70 [5] between the CTL- and Ang II-exosomes (Fig. 7C). These results suggest that Ang II may have minor impact on the protein compositions of CF exosomes, instead increase exosome release from CFs aforementioned (Fig. 3A and B).
Fig. 7.
Protein array analysis of cardiac fibroblast (CF)-derived exosomes. A, The effect of released exosomes of control vehicle-treated (CTL-exosomes) and Ang II-treated neonatal cardiac fibroblasts (CFs) (Ang II-exosomes) on cardiomyocyte hypertrophy. Neonatal rat cardiomyocytes were treated with or without CTL-exosomes (50 μg/ml) and Ang II-exosomes (50 μg/ml) for 48 h and then subjected to [3H]leucine uptake assay. n=4, *p<0.05 vs. vehicle control. B, C, Exosome proteins extracted from CTL-exosomes (20 μg) and Ang II-exosomes (20 μg) (n=3) were subjected to proteomics analysis as described in Online Supplement. B, The effect of Ang II on overall distribution of CF exosome proteins which potentially regulate PI3K/Akt and MAPK pathways. Ang II downregulates one protein (blue) and upregulates 2 proteins (red) in CF exosomes (Supplementary tables 6–8). C, The effect of Ang II on CF exosome marker expression in CTL- and Ang II-exosomes. There is no difference in the expression of exosome marker proteins in CTL- and Ang II-exosomes. Hspa4; Heat shock 70 kDa protein 4, Hspa13; Heat shock 70 kDa protein 13.
Ang II affected the expression of a few CF exosome proteins which are capable of regulating PI3K/Akt and MAPK pathways; whereas none of them have been individually linked to Ang II-mediated cardiac hypertrophy except Osteopontin (Spp1) [33] and epidermal growth factor receptor (Egfr) [34] (Supplementary tables 6 and 7). A comparison of the differential protein expression between CTL- and Ang II-exosomes which are involved in regulating PI3K/Akt and MAPK pathways, we narrowed down 57 CF exosome proteins (Fig. 7A and Supplementary table 8). It is likely that a net effect of these CF exosome proteins on PI3K/Akt and MAPK cascades is responsible for CF exosome-mediated upregulation of RAS leading to cardiomyocyte hypertrophy. However, we noticed that the expression of Spp1 and Egfr was not detected in CTL-exosomes (Supplementary tables 6 and 7). Given that in the experiment of CF exosome-induced cardiomyocyte hypertrophy, the used amount of Ang II-exosomes may be less than that of CTL-exosomes aforementioned, and Ang II-exosomes are more potent in inducing cardiomyocyte hypertrophy compared with CTL-eoxosomes [8], it is possible that the observed activation of PI3K/Akt and MAPK-RAS upregulation-cardiomyocyte hypertrophy is predominantly caused by Egfr and Spp1 in Ang II-exosomes. Moreover, considering 98% of CF exosome proteins in Ang II-exosomes are identical with that in CTL-exosomes, it is likely that other molecules in the exosomes such as DNA and ncRNAs are also responsible for the observed phenotype.
3.6. Exosome Inhibitors Attenuate Ang II-induced Cardiac Hypertrophy in vivo
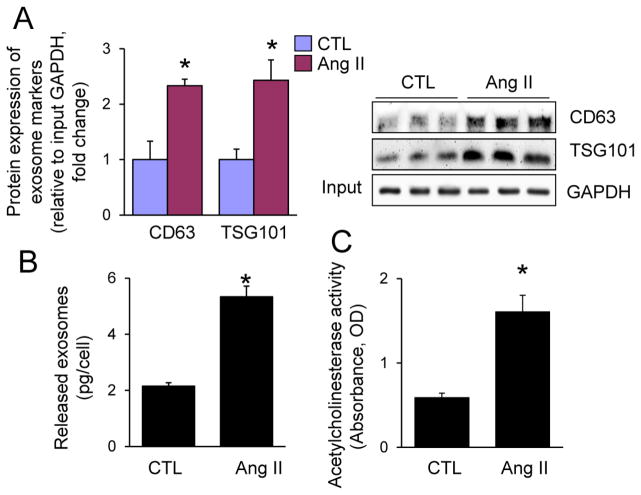
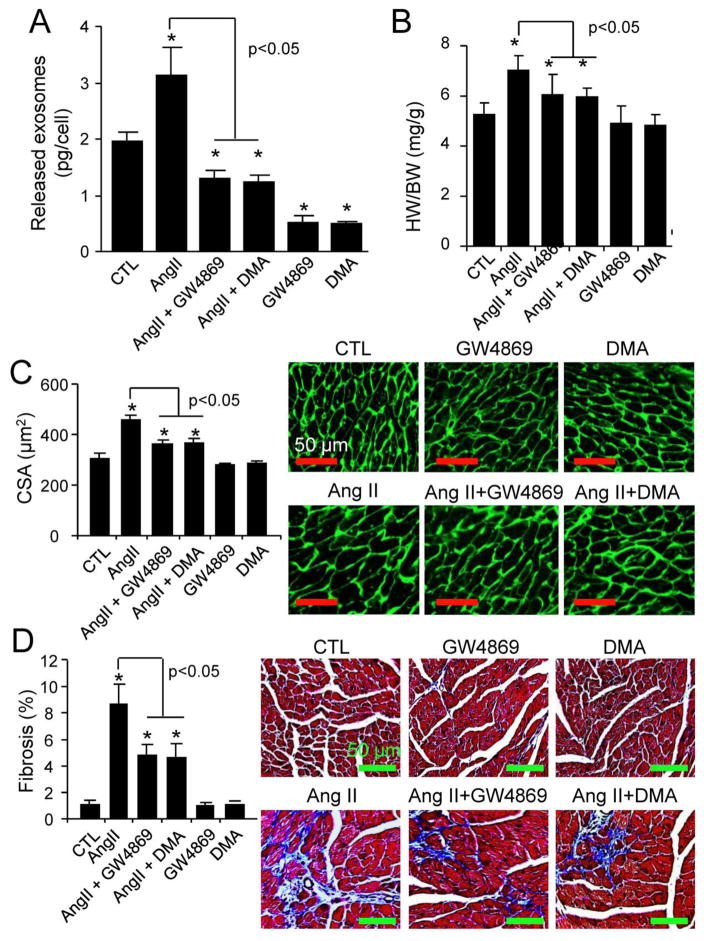
Since these findings in cultured neonatal CFs and cardiomyocytes may not be used as a sole surrogate for the cellular communication in adult myocardium, we explored the pathophysiological relevance of CF exosomes in Ang II-induced cardiac hypertrophy in vivo. We found that like in neonatal rat CFs (Fig. 1A–C), Ang II stimulated exosome release from cultured mouse adult CFs (Fig. 8). We further determined the effect of GW4869 and DMA on Ang II-induced CF exosome release and cardiac hypertrophy in adult mice. Cardiac hypertrophy was induced by sustained Ang II infusion as described elsewhere [10, 35]. GW4869 and DMA were administrated by previously established protocols in mice [29, 36]. Nevertheless, we verified the efficacy of GW4869 and DMA treatments in suppressing CF exosome release in the heart. As shown in Fig. 9A and Supplementary Fig. 8, CFs isolated from Ang II-treated mice released much more exosomes, compared to the vehicle-treated control. The Ang II-induced enhancement of exosome release was blocked by GW4869 or DMA treatment, whilst the basal exosome release was also inhibited by GW4869 or DMA (Fig. 9A, Supplementary Fig. 8). Ang II-induced myocardial hypertrophy and cardiac fibrosis were markedly prevented by the treatment of GW4869 and DMA (Fig. 9B–D). In addition, the systemic delivery of GW4869 and DMA at the doses used did not cause any cellular damage in the kidney and liver (Supplementary Fig. 9). Taken together, these results indicate that Ang II induces myocardial hypertrophy at least partly via increasing CF exosome release in the heart.
Fig. 8.
Ang II-induced exosome release from mouse adult cardiac fibroblasts. Mouse adult cardiac fibroblasts were treated with vehicle control (CTL) or Ang II (1 μM) for 48 h and then subjected to (A) Western blot analysis of CD63 and TSG101, (B) protein quantity measurement, and (C) acetylcholinesterase activity assay. n=3, *p<0.05 vs. CTL.
Fig. 9.
The effects of GW4869 and DMA on Ang II-induced cardiac hypertrophy. A, Adult male C57BL/6N mice (n=10) at 8 weeks of age were treated with Ang II, GW4869, and DMA for 5 days as described in “Methods”, and then cardiac fibroblasts were isolated for measuring exosome release. *p<0.05 vs. vehicle-treated control (CTL). B–D, Adult male C57BL/6N mice (n=6~10) at 8 weeks of age were treated with Ang II, GW4869, and DMA for 14 days as described in “Methods”, and then (B) heart weight (HW)/body weight (BW) ratio, (C) cardiomyocyte cross sectional area (CSA), and (D) cardiac fibrosis were quantified. n=6~10. *p<0.05 vs. vehicle-treated control (CTL).
4. Discussion
Over the last century, it has been firmly established that the abnormal and chronic activation of AT1R by excessive Ang II plays a causative role in the pathogenesis of cardiovascular diseases, such as hypertension and heart failure [1, 2]. However, the precise mechanism of Ang II-mediated adverse effects in the heart is still lacking a comprehensive understanding. To this end, an emerging role of exosomes carrying miRs in pro-hypertrophic communication between cardiac fibroblasts and cardiomyocytes has been highlighted by a recent report that exosomes released from cardiac fibroblasts are major components contributing to cardiomyocyte hypertrophy [8]. In the present study, we identified that Ang II stimulates cardiac fibroblasts to release exosomes, which act as a paracrine mediator to upregulate renin, Agt, AT1R, AT2R while downregulating ACE2 in cardiomyocytes, thereby intensifying the autocrine activation of the Ang II-AT1R axis that leads to pathological cardiomyocyte hypertrophy (Fig. 10). In addition, it is likely that a group of proteins in cardiac fibroblast-derived exosomes are responsible for activating PI3K/Akt- and MAPK-mediated upregulation of RAS leading to cardiomyocyte hypertrophy. Thus, our findings indicate that the increase in exosome secretion from cardiac fibroblasts serves as a novel paracrine mechanism of Ang II-induced myocardial hypertrophy.
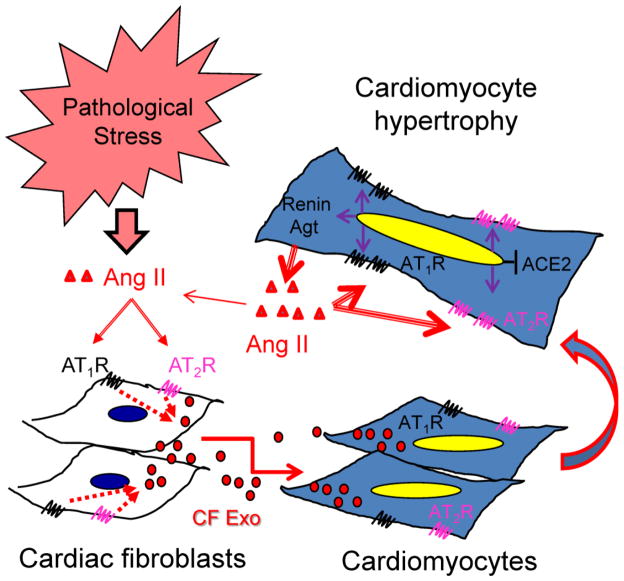
Fig. 10.
Schematic working hypothesis. Under pathological stress, myocardial Ang II stimulates exosome release from cardiac fibroblasts via activation of both AT1R and AT2R and then the cardiac fibroblast-derived exosomes upregulate expression of renin, angiotensinogen, AT1R, and AT2R while downregulating ACE2 in cardiomyocytes in a paracrine manner. Thus, cardiomyocytes produce and release more Ang II, which in turn activates the upregulated AT1R and AT2R to induce hypertrophic growth in an autocrine fashion. As a result, Ang II intensifies its own pro-hypertrophic signaling in cardiomyocytes by upregulation of RAS via increasing paracrine release of exosomes from cardiac fibroblasts.
Since therapy that inhibits the engagement of Ang II to AT1R, such as ACE inhibitors (ACEI) or AT1R blockers (ARBs), clearly retard the progression of hypertension and reduce the risk of adverse cardiovascular events, they are widely used as the first line of treatment in hypertension and heart failure [37]. However, a substantial portion of patients receiving ACEI or ARB therapy develop side effects, such as first-dose hypotension, hyperkalemia and renal failure, cough, and angioedema [37, 38]. These adverse effects may be caused by the suppression or blockade of normal Ang II-mediated physiological functions in target organ systems [2, 38, 39] thereby creating a need for an alternative approach to selective targeting of the Ang II-mediated detrimental effects in the heart. In this regard, our results indicate that the suppression of Ang II-induced exosome release from cardiac fibroblasts represents a potential alternate therapeutical approach for the prevention of pathological cardiac hypertrophy secondary to sustained Ang II stimulation.
It should be noted that the precise mechanism by which cardiac fibroblast-derived exosomes upregulate and activate RAS in cardiomyocytes have not been fully dissected in the present study. Although our results indicate that except miRs [], a group of proteins in CF exosomes may contribute to the paracrine intensification of Ang II-induced cardiomyocyte hypertrophy; miRs as paracrine mediators may mediate Ang II-induced cardiac hypertrophy via other potential mechanisms such as cardiac fibroblast-derived extracellular complex of miRs and RNA-binding proteins [40]. In addition, except Ang II over stimulation, the impact of other pathological settings on exosome release from cardiac fibroblasts has not been fully characterized. Whether time frames of the exosome under hyperinsulinemia, inflammation, and oxidative stress are different with the Ang II’s is warranted. Another limitation of our study is the systemic delivery of Ang II and exosome inhibitors. Such systemic suppression of exosome release could not exclude a potential involvement of extra-myocardial Ang II-induced exosome release in cardiac hypertrophy. This issue may be clarified by cardiac specific suppression of exosome release in a setting of cardiac restricted overstimulation of Ang II.
Further investigations of cardiac specific targeting the axis of Ang II-cardiac fibroblast exosome-cardiomyocyte hypertrophy as well as the putative substances in cardiac fibroblast-derived exosomes or from cardiac fibroblasts are needed. The outcome will provide not only novel insights into Ang II-operated pro-hypertrophic signaling in the heart but also new perspectives for the development of novel therapeutic approaches for the treatment of cardiac disease associated with chronic activation of Ang II signaling.”
Supplementary Material
Highlights.
Ang II stimulates the release of exosomes from cardiac fibroblasts (CF exosomes).
CF exosomes upregulate renin angiotensin system in cardiomyocytes.
CF exosomes induces cardiomyocyte hypertrophy via activating AT1R and AT2R.
Ang II-induced cardiac hypertrophy is inhibited by suppression of exosome production.
Acknowledgments
We acknowledge the technique supports by Dr. Xue Liu at Key Laboratory of Cardiovascular Remodeling and Function Research, Qilu Hospital of Shandong University, China.
Sources of Fundings
This work was supported by NIH NCCAM PO20 GM103641 and 2PO1AT003961-06A1, Shandong University National Qianren Scholar Fund, Shandong University Taishan Scholar fund, and the National Natural Science Foundation of China (No. 81370267).
Nonstandard Abbreviations and Acronyms
- Ang II
Angiotensin II
- RAS
Renin angiotensin system
- AT1R
Angiotensin II type 1 receptor
- AT2R
Angiotensin II type 2 receptor
- Agt
Angiotensinogen
- ACE
Angiotensin-converting enzyme
- ROS
Reactive oxygen species
- MAPK
Mitogen-activate protein kinase
- ET-1
Endothelin-1
- TGFβ1
Transforming growth factor 1
- ANF
Atrial natriuretic factor
- BNP
B-type natriuretic peptide/brain natriuretic factor
- αMHC
alpha-myosin heavy chain
- βMHC
beta-myosin heavy chain
- SERCA2a
Sarcoplasmic reticulum calcium ATPase2a
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wollert KC, Drexler H. The renin-angiotensin system and experimental heart failure. Cardiovascular research. 1999;43:838–49. doi: 10.1016/s0008-6363(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 2.Balakumar P, Jagadeesh G. A century old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal. 2014;26:2147–60. doi: 10.1016/j.cellsig.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Aoki H, Richmond M, Izumo S, Sadoshima J. Specific role of the extracellular signal-regulated kinase pathway in angiotensin II-induced cardiac hypertrophy in vitro. Biochem J. 2000;347(Pt 1):275–84. [PMC free article] [PubMed] [Google Scholar]
- 4.Gul R, Shawl AI, Kim SH, Kim UH. Cooperative interaction between reactive oxygen species and Ca2+ signals contributes to angiotensin II-induced hypertrophy in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2012;302:H901–9. doi: 10.1152/ajpheart.00250.2011. [DOI] [PubMed] [Google Scholar]
- 5.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90:1549–57. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 8.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. The Journal of clinical investigation. 2014;124:2136–46. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–50. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, et al. Up-regulation of p27(kip1) contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy. Cardiovascular research. 2011;90:315–24. doi: 10.1093/cvr/cvr010. [DOI] [PubMed] [Google Scholar]
- 11.Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, et al. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. Journal of molecular and cellular cardiology. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 13.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. Journal of cellular physiology. 2010;225:631–7. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. Journal of cellular physiology. 2005;202:891–9. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 19.LaFramboise WA, Scalise D, Stoodley P, Graner SR, Guthrie RD, Magovern JA, et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol. 2007;292:C1799–808. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- 20.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovascular research. 2008;77:265–73. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 21.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. The Journal of clinical investigation. 2005;115:538–46. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–33. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Abdalrahman A, Lai Y, Janicki JS, Ward KW, Meyer CJ, et al. Dihydro-CDDO-trifluoroethyl amide suppresses inflammatory responses in macrophages via activation of Nrf2. Biochem Biophys Res Commun. 2014;444:555–61. doi: 10.1016/j.bbrc.2014.01.101. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Ichikawa T, Jin Y, Hofseth LJ, Nagarkatti P, Nagarkatti M, et al. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J Ethnopharmacol. 2010;130:222–30. doi: 10.1016/j.jep.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drawnel FM, Archer CR, Roderick HL. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. British journal of pharmacology. 2013;168:296–317. doi: 10.1111/j.1476-5381.2012.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovascular research. 2004;63:423–32. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. Journal of cell science. 2002;115:2505–15. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 28.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. The Journal of biological chemistry. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. The Journal of clinical investigation. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172:1174–83. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshmanan AP, Watanabe K, Thandavarayan RA, Sari FR, Harima M, Giridharan VV, et al. Telmisartan attenuates oxidative stress and renal fibrosis in streptozotocin induced diabetic mice with the alteration of angiotensin-(1-7) mas receptor expression associated with its PPAR-gamma agonist action. Free Radic Res. 2011;45:575–84. doi: 10.3109/10715762.2011.560149. [DOI] [PubMed] [Google Scholar]
- 32.Eskildsen TV, Schneider M, Sandberg MB, Skov V, Bronnum H, Thomassen M, et al. The microRNA-132/212 family fine-tunes multiple targets in Angiotensin II signalling in cardiac fibroblasts. J Renin Angiotensin Aldosterone Syst. 2014 doi: 10.1177/1470320314539367. [DOI] [PubMed] [Google Scholar]
- 33.Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, et al. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension. 2004;43:1195–201. doi: 10.1161/01.HYP.0000128621.68160.dd. [DOI] [PubMed] [Google Scholar]
- 34.Messaoudi S, Zhang AD, Griol-Charhbili V, Escoubet B, Sadoshima J, Farman N, et al. The epidermal growth factor receptor is involved in angiotensin II but not aldosterone/salt-induced cardiac remodelling. PloS one. 2012;7:e30156. doi: 10.1371/journal.pone.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, Suzaki Y, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. The Journal of biological chemistry. 2005;280:29661–6. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- 36.Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiology of aging. 2014;35:1792–800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robles NR, Cerezo I, Hernandez-Gallego R. Renin-angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther. 2014;19:14–33. doi: 10.1177/1074248413501018. [DOI] [PubMed] [Google Scholar]
- 38.Steckelings UM, Artuc M, Wollschlager T, Wiehstutz S, Henz BM. Angiotensin-converting enzyme inhibitors as inducers of adverse cutaneous reactions. Acta Derm Venereol. 2001;81:321–5. doi: 10.1080/000155501317140007. [DOI] [PubMed] [Google Scholar]
- 39.Steckelings UM, Wollschlager T, Peters J, Henz BM, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13:148–54. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic acids research. 2010;38:7248–59. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.