Abstract
Background
Acute lung injury (ALI) is a complication of hemorrhagic shock (HS). Histone deacetylase inhibitors (HDACI) such as valproic acid (VPA) can improve survival following HS, however, their effects on late organ injury are unknown. Here, we have investigated the effects of HS and VPA treatment on ALI as well as circulating cytokines that may serve as biomarkers for the development of organ injury.
Materials and Methods
Anesthetized Wistar-Kyoto rats (250-300g) underwent 40% blood volume hemorrhage over 10 minutes followed by 30 minutes of un-resuscitated shock and were treated with 1) VPA 300mg/kg or 2) vehicle control. Blood samples were obtained at baseline, following shock, and prior to sacrifice (1h, 4h, and 20h; n=3-4/timepoint/group). Serum samples were screened for possible biomarkers using a multiplex electrochemiluminescence detection assay, and results were confirmed using ELISA. Additionally, lung tissue lysate was examined for chemokine and myeloperoxidase (MPO) levels as a marker for neutrophil infiltration and ALI. Additionally, lung CINC-1 (a chemokine belonging to the IL-8 family that promotes neutrophil chemotaxis) mRNA levels were measured by real-time PCR.
Results
Serum screening revealed that hemorrhage rapidly altered levels of circulating CINC-1. ELISA confirmed that CINC-1 protein was significantly elevated in the serum as early as 4h, and in the lung at 20h following hemorrhage, without any significant changes in the CINC-1 mRNA expression. Lung MPO levels were also elevated 4h and 20h after hemorrhage. VPA treatment attenuated these changes
Conclusions
Hemorrhage resulted in development of ALI, which was prevented with VPA treatment. Circulating CINC-1 levels rose rapidly after hemorrhage, and serum CINC-1 levels correlated with lung CINC-1 and MPO levels. This suggests that circulating CINC-1 could be used as an early marker for the subsequent development of organ inflammation and injury.
Keywords: Hemorrhage, shock, resuscitation, valproic acid, neutrophil, lung, CINC-1
INTRODUCTION
Hemorrhage is a major cause of morbidity and mortality among trauma patients. Despite prompt hemorrhage control and adequate resuscitation, patients may still suffer numerous complications during their hospital course. Common complications include acute lung injury (ALI), multi-system organ failure and sepsis, among others. Development of secondary complications of hemorrhagic shock is thought to be related to aberrant, deregulated activation of the immune system, which may manifest as a relatively immunosuppressed or pro-inflammatory state (1). In the case of ALI, the diagnosis is made when the patient's clinical condition deteriorates (development of bilateral infiltrates on chest x ray and Pa02/FI02<300 in the absence of cardiac failure) (2,3). Accurate prediction of patients who will develop ALI remains a challenge. There is considerable interest in discovering and validating circulating biomarkers that can aid in the early diagnosis and prognostication of ALI (4).
Our lab has explored the concept of pharmacologic resuscitation for traumatic hemorrhagic shock (HS) using histone deacetylase inhibitors (HDACI) such as valproic acid (VPA). These drugs promote acetylation of nuclear histone proteins as well as non-histone proteins located in both the cytoplasm and the nucleus. There are several reasons why HDACI are suitable for pharmacologic resuscitation. It is known that hemorrhage leads to an imbalance in acetylation of proteins, and that HDACI can restore this balance (5,6). Also, acetylation can impact many critical cellular processes such as gene expression, protein signaling cascades, and protein stability (7). HDACI treatment can reduce pro-apoptotic caspase-3 activation in the liver (8) and can acetylate β-catenin and promote transcription of bcl-2, a pro-survival gene in neuronal tissue (9). Treatment with HDACI (without any additional fluid resuscitation) improves early survival in rats subjected to lethal hemorrhagic shock (10,11), as well as swine poly-trauma models (12). In these studies, the survival advantage became apparent immediately following treatment, presumably as the cells became more resistant to the deleterious effect of HS. In addition, HDACI may also have delayed benefits through favorable modulation of the post-injury immune response (13,14). Thus, the protective effects of HDACI extend beyond the acute phase to include an attenuation of delayed organ injury as well.
This study was designed to: (1) discover whether HS altered circulating proteins that could predict subsequent development of organ injury; and (2) determine if these circulating proteins could be prevented by treatment with an HDACI, valproic acid. Recently, new multiplex research tools have become available that can measure multiple compounds simultaneously in biological samples. We utilized one such multiplex assay for the initial screening, followed by focused evaluation of the discovered molecules.
MATERIALS AND METHODS
Animals
This study adhered to the principles stated in The Guide for the Care and Use of Laboratory Animals (7th ed., National Academies Press, 1996), and was approved by the Institutional Animal Care and Use Committee. Male Wistar Kyoto rats (250-300 grams) were purchased from Harlan (Indianapolis, IN). Rats were allowed food and water ad libitum.
Surgical Procedure
The left femoral artery and vein of rats were cannulated as described previously (11). Briefly, rats were sedated with isoflurane (Abbott Laboratories, North Chicago, IL) delivered through a nose cone scavenging system connected to a veterinary anesthesia vaporizer and delivery system (Kent Scientific Corporation, Torrington, CT). After local anesthesia was achieved by injecting 0.2mL of 0.75% bupivicaine (AstraZeneca, Wilmington, DE), the left femoral vessels were dissected and cannulated with polyethylene 50 (PE50) catheters (Clay Adams, Sparks, MD). The venous cannula was used for hemorrhage and drug administration while the arterial catheter was connected to the Ponemah Physiology Platform (Gould Instrument Systems, Valley View, OH) for continuous hemodynamic monitoring.
Hemorrhagic shock (HS) protocol
The volume of hemorrhage was based on each animal's estimated total blood volume which was calculated as follows: estimated total blood volume (mL) = weight (g) × 0.06 (mL/g) + 0.77 (15). Blood was withdrawn from the animal using infusion and withdrawal syringe pumps (Kent Scientific Corporation). On the day of experimentation, VPA (Calbiochem, San Diego, CA) solution was prepared fresh by dissolving it in filtered distilled water to create a 400 mg/mL solution. To induce sublethal HS, baseline (BL) arterial blood samples were obtained, and 40% of estimated blood volume was withdrawn over 10 minutes through the venous cathether, followed by un-resuscitated shock for 30 minutes. At this time, Post-shock (PS) arterial blood samples were obtained, and animals were treated with either: 1) valproic acid (VPA 300mg/kg IV; volume = 750 μL/kg; given over 5 minutes), or 2) vehicle control (750 μL/kg of 0.9% saline IV; given over 5 minutes), followed by 200 μL of 0.9% saline flush over 15 minutes. The dose of VPA (300 mg/kg) was based upon our previous experience, and on an observation that lower doses do not induce acetylation (5-12). Catheters were removed at this stage and the vessels were ligated. Aesthesia was discontinued and animals were allowed to recover in their cages. They were sacrificed at 1h, 4h, and 20h after completion of treatment. At the time of sacrifice, venous blood samples were collected by puncturing the inferior vena cava and organs were harvested. Lung tissue was rinsed with cold saline, frozen in liquid nitrogen, and stored at −80°C. All blood samples were analyzed using Stat Profile Critical Care Xpress (Nova Biomedical, Waltham, MA) to measure pH, lactate, and hemoglobin.
Meso Scale Discovery Cytokine Immunoassay
A rat multiplex kit (IL-1β, CINC-1, IL-4, IL-5, TNFα, IFNγ, IL-13) including all reagents and standards were purchased from Meso Scale Discovery (MSD; Gaithersburg, Maryland). Serum was prepared by allowing blood samples to clot for 1 hour at room temperature and then centrifuging at 1,500 × g at 4 °C for 10 minutes. Cytokine concentrations were detected in 25μL of serum by following the manufacture's instructions, using the MSD Sector Imager 2400 plate reader. Samples were run in triplicate, and samples with a coefficient of variation greater than 20% were excluded.
Enzyme Linked Immunosorbent Assay (ELISA)
CINC-1 levels in the serum and lung were quantified using the Quantikine rat CINC-1 ELISA kit (R and D, Minneapolis, MN). Rat lung tissue and serum were prepared as described above. CINC-1 concentrations were quantified in 50μL of lung tissue supernatant or serum according to the manufacture's instructions by measuring optical densitometry values at 450 nm in a SpectramaxPlus 384 microplate reader (Molecular Devices, Sunnyvale, CA).
Myeloperoxidase Activity
Pulmonary myeloperoxidase (MPO) levels were quantified using the rat MPO enzyme-linked immunosorbent assay (ELISA) kit (Cell Sciences, Canton, MA). Rat lung tissue (50 mg per sample) was homogenized using a glass hand-held homogenizer in 1 mL of a lysis buffer (200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycine, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL leupeptide, 28 μg/mL aprotinin). The samples were centrifuged twice at 1,500 × g at 4 °C for 15 min, and supernatants were analyzed for MPO levels by following the manufacturer's instructions.
RNA Isolation and Real-Time Polymerase Chain Reaction (real-time PCR)
Total RNA was prepared from lung tissue in RNAlater solution using the RNeasy Mini Kit (Qiagen, Valencia, CA), and mRNA was reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacture's instructions. Equal amounts of cDNA were submitted for PCR in the presence of SYBR Green Master Mix and forward and reverse primers using the ABI PRISM 7300 Real-Time PCR detection machine (Applied Biosystems). The primer for CINC-1 (forward: 5’-CACCCAAACCGAAGTCATAG-3’; reverse: 5’-TTCTTCAATACTTGGGGACA-3’) was synthesized by Real Time Primers (Elkins Park, PA). GAPDH (forward : 5’-CCTGGAGAAACCTGCCAAGTAT-3’; reverse: 5’-CTCGGCCGCCTGCTT-3’) was used as an internal control and was synthesized by Invitrogen (Carlsbad, CA). PCR was performed with 40 cycles of 15 seconds at 95 °C, and 1 minute at 60 °C. Each sample was run in triplicates. Relative quantification of mRNA expression was performed using the ΔΔCt method where ΔCt is the calculated difference in the Ct (threshold cycle) values between CINC-1 and GAPDH, and ΔΔCt is the calculated difference between the ΔCt for a given sample and the ΔCt for sham, such that the relative quantity of mRNA, normalized to GAPDH and sham, is calculated as 2−ΔΔCt.
Statistical Analysis
All continuous variables are expressed as means +/− standard error of the mean (SEM). Data were analyzed using SPSS statistical software (SPSS, Chicago, IL). Differences between 3 or more groups were assessed using one way analysis of variance (ANOVA) followed by Bonferroni post hoc testing for multiple comparisons. The independent samples t-test was used for comparisons between 2 groups. A paired-sample t-test was used to compare variables within a group. In all cases, statistical significance was defined as p<0.05.
RESULTS
Hemorrhagic shock
Animals were hemorrhaged 40% of their total blood volume to induce sub lethal HS, and were sacrificed at 1, 4, and 20 hours following hemorrhage and treatment. This degree of blood loss was selected to allow 100% of animals in all groups to survive until the designated time of sacrifice. Selected laboratory values were measured, and were indicative of moderate HS (mean lactate 0.6 mmol/L at baseline, 4.3 mmol/L after hemorrhage; mean baseline hemoglobin 12.0 g/dL at baseline, 9.6 g/dL after hemorrhage).
Circulating CINC-1
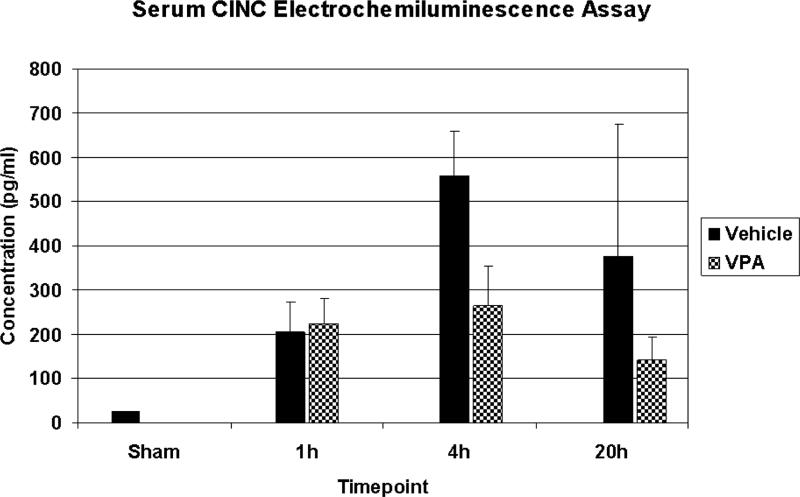
Serum samples (n=3-4/timepoint/group) were screened using a multiplex electrochemiluminescence assay to determine if there were any changes in circulating cytokines and chemokines. Of the 7 cytokines analyzed (IL-1β, CINC-1, IL-4, IL-5, TNFα, IFNγ, IL-13), CINC-1 exhibited a robust rise following HS, which appeared to be attenuated by VPA treatment (Figure 1). Two samples had to be eliminated from the sham group (CINC-1 below the lower limit of detection) and 1 sample had to be eliminated from the vehicle 20h group (CINC-1 coefficient of variation greater than 20%). As this was a screening test, statistical analysis was not performed. Rather, conventional ELISA was used to confirm these data. The other cytokines were either below the lower limit of detection, or did not exhibit obvious differences between groups so no further analysis of these cytokines was undertaken.
Figure 1.
Serum screening using a multiplex electrochemiluminescence assay. Rats were hemorrhaged 40% of their blood volume, and were treated with VPA 300mg/kg IV or normal saline vehicle (VEH). Serum was collected at the time of sacrifice (1h, 4h, and 20h) as well from sham animals, and analyzed. Data shown as mean CINC-1 concentration ± SEM.
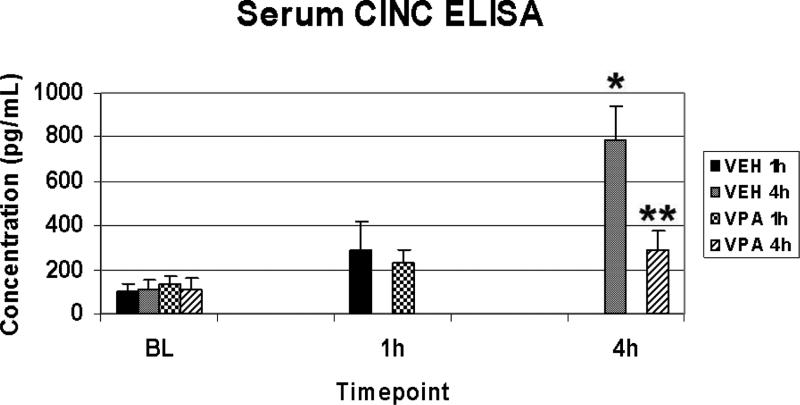
Conventional ELISA was used to confirm the data obtained using the multiplex electrochemiluminescence assay. At BL, all groups exhibited similar low levels of circulating CINC-1. HS increased the amount of circulating CINC-1, and VPA treatment significantly attenuated this effect at 4 hours (Figure 2).
Figure 2.
Serum CINC-1 levels. Data obtained in Figure 1 were confirmed by ELISA. Rats were hemorrhaged 40% of their blood volume, and were treated with VPA 300mg/kg IV or normal saline vehicle (VEH). Serum was collected for analysis at baseline (BL) and at the time of sacrifice (1h and 4h). Data shown as mean CINC-1 concentration ± SEM. Hemorrhage resulted in a significant increase in serum CINC-1 levels at 4h (*p<0.05 vs. BL), whereas VPA treatment significantly attenuated this effect (**p<0.05 vs. VEH 4h).
Lung CINC-1
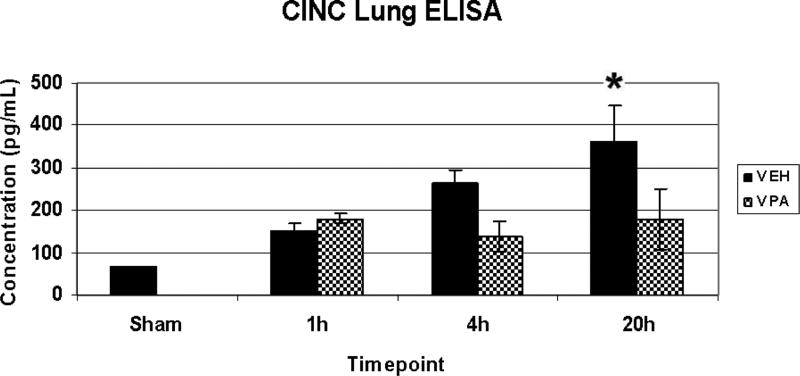
ELISA was used to measure CINC-1 levels in lung whole tissue extract (n=3-4/timepoint/group). Sham animals exhibited a low level of CINC-1 in the lung. HS resulted in a gradual increase of CINC-1 in lung tissue, which became significantly different from sham by 20 hours (Figure 3).
Figure 3.
Lung CINC-1 levels. Rats were hemorrhaged 40% of their blood volume, and were treated with VPA 300mg/kg IV or normal saline vehicle (VEH). Lung tissue was collected for analysis at the time of sacrifice (1h, 4h, 20) as well as from sham animals. Data shown as mean CINC-1 concentration ± SEM. Hemorrhage resulted in a significant increase in lung CINC-1 levels at 20h (*p<0.05 vs. sham). The effects of VPA on lung CINC-1 levels were not statistically significant (p=0.17; comparing VEH vs. VPA at the 20h time point).
Lung MPO
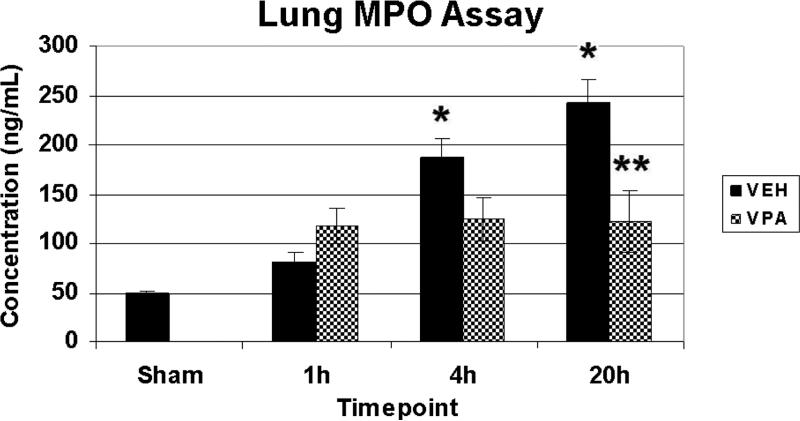
MPO levels were measured in lung whole tissue extract as a marker for neutrophil infiltration (n=3-4/timepoint/group). Sham animals exhibited low levels of MPO in the lung. HS resulted in a gradual increase of lung MPO levels which was highest at 20 h (nearly 5-fold that of sham), and significantly different from sham at 4h, and 20h. VPA treatment significantly attenuated this effect at the 20h time point (Figure 4).
Figure 4.
Lung MPO levels. Rats were hemorrhaged 40% of their blood volume, and were treated with VPA 300mg/kg IV or normal saline vehicle (VEH). Lung tissue was collected for analysis at the time of sacrifice (1h, 4h, 20) as well as from sham animals. Data shown as mean MPO concentration ± SEM. Hemorrhage resulted in a significant increase in lung MPO levels at 4h and 20h (*p<0.05 vs. sham), whereas VPA attenuated this effect (**p<0.05 vs VEH 20h).
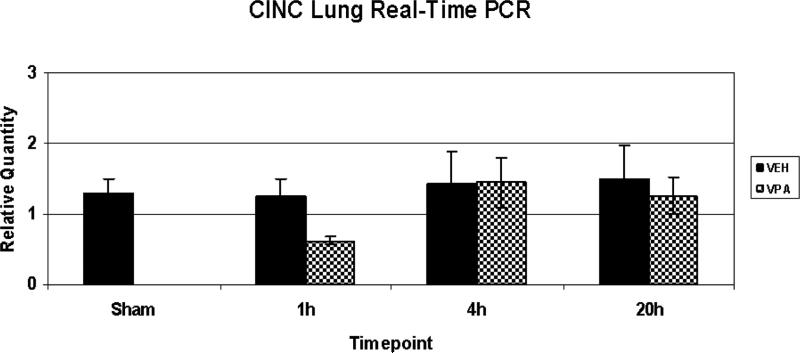
CINC-1 mRNA levels
Lung tissue was assessed for CINC-1 mRNA levels (n=3-4/timepoint/group). Hemorrhage had no effect on CINC-1 mRNA levels over time. There were no significant changes between the groups.
DISCUSSION
There are two main findings of this study. First, circulating CINC-1 levels increase rapidly following HS, along with a rise in CINC-1 and MPO levels in the lung. CINC-1 is a chemokine that promotes neutrophil chemotaxis (16), and MPO is a marker for neutrophil infiltration that is known to occur in ALI. Thus, these findings suggest that organ injury may be associated with a measurable change in circulating proteins such as CINC-1, and these proteins may have some utility as biomarkers for early detection of organ injury. Second, treatment with VPA attenuates these effects. Previously, we have shown that post-shock administration of VPA, without conventional fluid resuscitation, prevents death from lethal blood loss (10,11). The current study suggests that approach may have some delayed benefit as well by decreasing pulmonary neutrophil infiltration.
ALI is a known complication of trauma that is multi-factorial in etiology (2). In ALII, accumulation of neutrophils in the lung is commonly observed, and neutrophil-mediated damage is considered to be a major mechanism (2). Distinguishing those patients who will develop ALI and those who will not prior to clinical deterioration remains a challenge. ALI is a clinical diagnosis made on the basis of simple clinical criteria: new bilateral infiltrates on chest radiograph and Pa02/FI02≤300 in the absence of heart failure (3). By definition, these diagnostic criteria of ALI cannot be used to predict which patients may be at risk for developing ALI. Some authors have suggested that circulating biomarkers could be used to help make the diagnosis of early ALI, or predict those patients at risk (17,4). Many of the proposed biomarkers thus far have established roles in the inflammatory and coagulation cascades (17,4). Theoretically, early detection of inflammatory mediators that contribute to the development of ALI would allow for early diagnosis and better triage of at risk patients.
One goal of this study was to identify changes in circulating proteins that may be associated with HS. We utilized a multiplex assay, based on ELISA and electrochemiluminescence detection principles, to evaluate multiple circulating cytokines simultaneously. The main advantage of utilizing a multiplex platform is that multiple proteins can be simultaneously evaluated within a single sample, which increase efficiency and decreases the amount of biological material required. Admittedly, these newer multiplex platforms also have several disadvantages. For example, the assay cannot be titrated to individual proteins, and while experimental conditions such as sample volume, incubation time, etc. may be idea for detection of one protein, they may be suboptimal for detection of another proteins. Thus, it is possible that a biologically important protein may be missed using this method. Secondly, some proteins included as part of the multiplex panel may not be of particular interest. For example, the panel we utilized included IL-4, IL-5 and IL-13. These cytokines are prominent mediators of eosinophilic reactions and allergy (18), but are likely to be less relevant in HS. Additionally, these multiplex platforms are relatively new, and their accuracy and sensitivity are only beginning to be validated in the literature (19). For these reasons, we designed our study to use the multiplex assay only as a screening tool, and relied on the traditional ELISA to confirm the findings.
Our multiplex screening study suggested that circulating CINC-1 levels were elevated rapidly following HS. Circulating levels of 6 other proteins included in the assay (IL-1β, IL-4, IL-5, TNFα, IFNγ, IL-13) did not appear to be significantly modulated in our model. This was somewhat of an unexpected finding because other authors have shown that circulating TNFα and IL-1β are elevated following hemorrhagic shock in both humans and animal models (20,21,4). There are several possible explanations for this discrepancy between studies. First, in other studies, subjects were resuscitated with crystalloid solution such as lactated Ringer's, and this therapy is well-known to be pro-inflammatory (22). On the other hand, we utilized only pharmacologic resuscitation without additional fluids. Thus, the elevated cytokines may be attributed to the combination of shock and resuscitation. Second, because we only looked at three discrete time points, it is possible that we failed to capture the peak in the circulating levels of these proteins. Third, other variations in experimental design such as variations in the levels and duration of shock may also account for these differences.
CINC-1 is a rat chemokine known to be involved in neutrophil chemotaxis, and binds to CXCR2 receptor (16,23). CINC-1 belongs to the IL-8 family for chemokines, and is homologous to the mouse keratinocyte-derived chemokine (KC), and to the human growth related oncogene alpha (GROα) protein (16,23). We discovered that CINC-1 becomes detectable in the serum rapidly after HS. Because of the association of HS and ALI and the central role of neutrophils, we also analyzed the lung, and found that pulmonary CINC-1 expression was elevated following HS. The rise in lung CINC-1 appeared to parallel a rise in lung MPO, a marker for pulmonary neutrophil infiltration. We also assessed CINC-1 mRNA production in the lung. Our results were inconclusive in this regard because there was no change in CINC-1 mRNA between the sham and HS animals. It is possible that CINC-1 is produced by only a subpopulation of cells within the lung, for example, alveolar macrophages, and that our whole tissue extraction technique was not sufficiently sensitive to detect these changes in RNA levels. Also, it is possible that small changes in RNA, below the level of detection in our assays, could produce larger changes in protein levels due to amplification. Other groups have shown that lung CINC-1 is produced mostly by alveolar macrophages, and depletion of these cells result in less CINC-1 and less lung injury following infectious challenge (24). Alternatively, lung epithelial, endothelial, and fibroblast cells in culture also produce CINC-1 (25,26). Further studies are required to determine the source of CINC-1 in our model.
The fact that CINC-1 can be detected in the serum following HS is significant because this may used as a circulating biomarker for the subsequent development of organ injury. A recent study looking at a mouse model of 30% blood volume hemorrhage also found that lung and plasma KC was elevated 4 hours after hemorrhage (27). In a retrospective study looking at a panel of biomarkers to distinguish patients with and without ALI, IL-8 was one of the biomarkers found to be important (4). Others have also suggested that KC could be a circulating biomarker for kidney injury following ischemia and reperfusion (28). In our model, we did not find any evidence of kidney injury (data not shown), however, this is probably because moderate HS (40%) in our model was not severe enough to result in kidney injury. In lethal models of HS, however, we have shown that renal injury can be prevented by VPA treatment (29). Detection of circulating CINC-1 is likely an early marker for organ injury, and not specific for a particular organ. Its utility as a biomarker would be its ability to suggest that in a distant organ inflammation and neutrophil recruitment is ongoing, and that clinical manifestation of this organ injury may be expected. In our study, CINC-1 levels in serum peaked well in advance (Fig 2) of the tissue levels (Fig 3), which also supports the hypothesis that circulating CINC-1 levels may be used as a marker for the prediction of ALI. Another goal of this project was to determine if VPA treatment would have any impact on ALI, as well as on circulating and tissue CINC-1 levels. We studied lung CINC-1 levels as well as MPO levels as a marker for neutrophil infiltration and found that treatment with VPA attenuated hemorrhage-induced elevation of circulating CINC-1, lung CINC-1, and lung MPO levels. Thus, VPA appeared to inhibit several inflammatory changes associated with HS.
Previously, we have studied phamarcologic resuscitation using HDACI such as VPA, and found that these compounds promote survival following otherwise lethal HS. In both rat and pig experiments, untreated control animals tend to die within 1-2 hours of hemorrhage, while VPA treated animals continued to survive despite ongoing hypotension, shock, and acidosis (10,11,12). Because the benefits are immediate (within 1-2 hours), the likely mechanism involves modulating cellular signaling cascades (through restoration of cellular acetylation), protein stability, or similar mechanisms, rather than new gene expression. Indeed, prior studies have shown activation (phosphorylation) of MAP kinases 1h, and 4h after HS, but not at 20h (11,30). ALI, however, is a later inflammatory complication of HS, typically manifesting hours or days after resuscitation when the patient is no longer in shock. Thus, this study suggests that the beneficial effects of VPA may last beyond the initial period of shock, and that VPA may modulate the post-trauma inflammatory response.
It is not known how VPA decreases CINC-1 and pulmonary neutrophil infiltration, but several hypotheses can be generated. One possibility is that VPA stabilized the intestinal barrier which prevents systemic inflammation. It is known that HS induces tight junction disruption and gut permeability, allowing toxic substances to translocate from the lumen into the intestinal lymphatics, ultimately resulting in pulmonary injury as well as systemic inflammation (31,32,33). In particular, the tight junction protein claudin-3 is shed from the intestine during HS, but VPA prevents claudin-3 loss (34). A second possibility is that VPA directly exerts an anti-inflammatory effect. Several lines of evidence support this possibility. HDACI are known to be anti-inflammatory, and have been proposed as therapeutic options for inflammatory diseases such as rheumatoid arthritis (35). Data from our lab also indicate that SAHA, another HDACI, can modulate the inflammatory response of splenic leukocytes from trauma patients exposed to a second insult (ex-vivo) of hypoxia or LPS (13,14). In addition, it was recently reported that the activation of the MAP kinases JNK and ERK1/2 promote CINC-1 release from rat astrocytes (36). Previously, we have shown that VPA attenuates HS-induced activation of both JNK and ERK (11,30). Thus, it is possible that CINC-1 attenuation observed in this study is related to a direct anti-inflammatory effect of VPA, or an effect mediated by regulation of MAP kinase signaling. Further studies would be required to test these hypotheses.
In summary, HS results in early elevation of serum CINC-1 levels as well as elevation of lung CINC-1 and MPO levels, indicating neutrophil infiltration and inflammation –hallmarks of ALI. Pharmacologic resuscitation with VPA attenuates these effects. CINC-1 may be an early marker for HS-induced organ injury, and pharmacologic resuscitation with HDACI such as VPA may offer anti-inflammatory benefits following HS.
Figure 5.
Lung CINC-1 RNA relative quantity. Rats were hemorrhaged 40% of their blood volume, and were treated with VPA 300mg/kg IV or normal saline vehicle (VEH). Lung tissue was collected for analysis at the time of sacrifice (1h, 4h, 20) as well as from sham animals. Data shown as CINC-1 relative quantity ± SEM. There were no significant differences between groups.
Acknowledgements
Supported by the NIH grant RO1 GM084127 (HBA). Data presented at the 6th Annual Academic Surgical Congress, Huntington Beach, CA (February, 2011)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alam HB, Rhee P. New developments in fluid resuscitation. Surg Clin North Am. 2007;87:55–72. doi: 10.1016/j.suc.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 4.Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR, Matthay MA, May AK, Ware LB. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010;68:1121–7. doi: 10.1097/TA.0b013e3181c40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin T, Alam HB, Chen H, Britten-Webb J, Rhee P, Kirkpatrick J, Koustova E. Cardiac histones are substrates of histone deacetylase activity in hemorrhagic shock and resuscitation. Surgery. 2006;139:365–76. doi: 10.1016/j.surg.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Gonzales E, Chen H, Munuve R, Mehrani T, Britten-Webb J, Nadel A, Alam HB, Wherry D, Burris D, Koustova E. Valproic acid prevents hemorrhage-associated lethality and affects the acetylation pattern of cardiac histones. Shock. 2006;25:395–401. doi: 10.1097/01.shk.0000209522.28120.c8. [DOI] [PubMed] [Google Scholar]
- 7.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–9. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt MU, Sailhamer EA, Li Y, Liu B, Shuja F, Velmahos GC, DeMoya M, King DR, Alam HB. Pharmacologic resuscitation: cell protective mechanisms of histone deacetylase inhibition in lethal hemorrhagic shock. J Surg Res. 2009;156:290–6. doi: 10.1016/j.jss.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Liu B, Sailhamer EA, Yuan Z, Shults C, Velmahos GC, deMoya M, Shuja F, Butt MU, Alam HB. Cell protective mechanism of valproic acid in lethal hemorrhagic shock. Surgery. 2008;144:217–24. doi: 10.1016/j.surg.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Shults C, Sailhamer EA, Li Y, Liu B, Tabbara M, Butt MU, Shuja F, Demoya M, Velmahos G, Alam HB. Surviving blood loss without fluid resuscitation. J Trauma. 2008;64:629–38. doi: 10.1097/TA.0b013e3181650ff3. [DOI] [PubMed] [Google Scholar]
- 11.Fukudome EY, Kochanek AR, Li Y, Smith EJ, Liu B, Kheirbek T, Lu Jennifer, Kim K, Hamwi K, Velmahos GC, Alam HB. Pharmacologic resuscitation promotes survival and attenuates hemorrhage-induced activation of extracellular signal-regulated kinase 1/2. J Surg Res. 2010;163:118–26. doi: 10.1016/j.jss.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam HB, Shuja F, Butt MU, Duggan M, Li Y, Zacharias N, Fukudome EY, Liu B, Demoya M, Velmahos GC. Surviving blood loss without blood transfusion in a swine poly-trauma model. Surgery. 2009;146:325–33. doi: 10.1016/j.surg.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Sailhamer EA, Li Y, Smith EJ, Liu B, Shuja F, Soupir CP, DeMoya MA, Velmahos GC, Alam HB. Hypoxic “second hit” in leukocytes from trauma patients: Modulation of the immune response by histone deacetylase inhibition. Cytokine. 2010;49:303–11. doi: 10.1016/j.cyto.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Sailhamer EA, Li Y, Smith EJ, Shuja F, Shults C, Liu B, Soupir C, deMoya M, Velmahos G, Alam HB. Acetylation: a novel method for modulation of the immune response following trauma/hemorrhage and inflammatory second hit in animals and humans. Surgery. 2008;144:204–16. doi: 10.1016/j.surg.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–6. [PubMed] [Google Scholar]
- 16.Watanabe K, Koizumi F, Kurashige Y, Tsurufuji S, Nakagawa H. Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo. Exp Mol Pathol. 1991;55:30–7. doi: 10.1016/0014-4800(91)90016-q. [DOI] [PubMed] [Google Scholar]
- 17.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, Korpak A, Matthay MA. Acute Respiratory Distress Syndrome Network, National Heart, Lung, and Blood Institute. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–50. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–10. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods. 2009;340:55–64. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Frink M, Hsieh YC, Hsieh CH, Pape HC, Choudhry MA, Schwacha MG, Chaudry IH. Keratinocyte-derived chemokine plays a critical role in the induction of systemic inflammation and tissue damage after trauma-hemorrhage. Shock. 2007;28:576–81. doi: 10.1097/shk.0b013e31814b8e0d. [DOI] [PubMed] [Google Scholar]
- 21.Douzinas EE, Andrianakis I, Livaditi O, Paneris P, Tasoulis M, Pelekanou A, Betrosian A, Giamarellos-Bourboulis EJ. The level of hypotension during hemorrhagic shock is a major determinant of the post-resuscitation systemic inflammatory response: an experimental study. BMC Physiol. 2008;8:15. doi: 10.1186/1472-6793-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee P, Burris D, Kaufmann C, Pikoulis M, Austin B, Ling G, Harviel D, Waxman K. Lactated Ringer's solution resuscitation causes neutrophil activation after hemorrhagic shock. J Trauma. 1998;44:313–9. doi: 10.1097/00005373-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Shibata F, Konishi K, Nakagawa H. Identification of a common receptor for three types of rat cytokine-induced neutrophil chemoattractants (CINCs). Cytokine. 2000;12:1368–73. doi: 10.1006/cyto.2000.0739. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto S, Pittet JF, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanabe K, Tsurufuji S, Wiener-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:L819–28. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 25.Lukaszewicz GC, Souba WW, Abcouwer SF. Induction of cytokine-induced neutrophil chemoattractant (CINC) mRNA in the lungs of septic rats. J Trauma. 1996;41:222–8. doi: 10.1097/00005373-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, Paulauskis JD, Kobzik L. Rat KC cDNA cloning and mRNA expression in lung macrophages and fibroblasts. Biochem Biophys Res Commun. 1992;184:922. doi: 10.1016/0006-291x(92)90679-f. [DOI] [PubMed] [Google Scholar]
- 27.Lee BH, Lee TJ, Jung JW, Oh DJ, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. The role of keratinocyte-derived chemokine in hemorrhage-induced acute lung injury in mice. J Korean Med Sci. 2009;24:775–81. doi: 10.3346/jkms.2009.24.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molls RR, Savransky V, Liu M, Bevans S, Mehta T, Tuder RM, King LS, Rabb H. Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2006;290:F1187–93. doi: 10.1152/ajprenal.00342.2005. [DOI] [PubMed] [Google Scholar]
- 29.Zacharias N, Sailhamer EA, Li Y, Liu B, Butt MU, Shuja F, Velmahos GC, de Moya M, Alam HB. Histone deacetylase inhibitors prevent apoptosis following lethal hemorrhagic shock in rodent kidney cells. Resuscitation. 2010 Oct 29; doi: 10.1016/j.resuscitation.2010.09.469. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochanek AR, Fukudome EY, Li Y, Smith EJ, Liu B, Velmahos GC, deMoya M, King D, Alam HB. Histone deacetylase inhibitor treatment attenuates MAP kinase pathway activation and pulmonary inflammation following hemorrhagic shock in a rodent model. J Surg Res. 2011 doi: 10.1016/j.jss.2011.06.007. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164–9. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- 32.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–27. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deitch EA, Forsythe R, Anjaria D, Livingston DH, Lu Q, Xu DZ, Redl H. The role of lymph factors in lung injury, bone marrow suppression, and endothelial cell dysfunction in a primate model of trauma-hemorrhagic shock. Shock. 2004;22:221–8. doi: 10.1097/01.shk.0000133592.55400.83. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Liu B, Dillon ST, Fukudome EY, Kheirbek T, Sailhamer EA, Velmahos G, Demoya M, Libermann TA, Alam HB. Identification of a Novel Potential Biomarker in a Model of Hemorrhagic Shock and Valproic Acid Treatment. J Surg Res. 2010;159:474–81. doi: 10.1016/j.jss.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Grabiec AM, Tak PP, Reedquist KA. Targeting histone deacetylase activity in rheumatoid arthritis and asthma as prototypes of inflammatory disease: should we keep our HATs on? Arthritis Res Ther. 2008;10:226. doi: 10.1186/ar2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Luo W, Stricker R, Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J Neurochem. 2006;98:1046–60. doi: 10.1111/j.1471-4159.2006.03950.x. [DOI] [PubMed] [Google Scholar]