Abstract
Mitochondrial disease refers to a heterogeneous group of disorders resulting in defective cellular energy production due to abnormal oxidative phosphorylation (oxphos). Primary mitochondrial disease (PMD) is diagnosed clinically and ideally, but not always, confirmed by a known or indisputably pathogenic mitochondrial DNA (mtDNA) or nuclear DNA (nDNA) mutation. The PMD genes either encode oxphos proteins directly or they affect oxphos function by impacting production of the complex machinery needed to run the oxphos process. However, many disorders have the ‘mitochondrial’ phenotype without an identifiable mtDNA or nDNA mutation or they have a variant of unknown clinical significance. Secondary mitochondrial dysfunction (SMD) can be caused by genes encoding neither function nor production of the oxphos proteins and accompanies many hereditary non-mitochondrial diseases. SMD may also be due to nongenetic causes such as environmental factors. In our practice, we see many patients with clinical signs of mitochondrial dysfunction based on phenotype, biomarkers, imaging, muscle biopsy, or negative/equivocal mtDNA or nDNA test results. In these cases, it is often tempting to assign a patient's phenotype to ‘mitochondrial disease’, but SMD is often challenging to distinguish from PMD. Fortunately, rapid advances in molecular testing, made possible by next generation sequencing, have been effective at least in some cases in establishing accurate diagnoses to distinguish between PMD and SMD. This is important, since their treatments and prognoses can be quite different. However, even in the absence of the ability to distinguish between PMD and SMD, treating SMD with standard treatments for PMD can be effective. We review the latest findings regarding mitochondrial disease/dysfunction and give representative examples in which differentiation between PMD and SMD has been crucial for diagnosis and treatment.
Key Words: Mitochondria, Mitochondrial DNA, Non-mitochondrial disorder, Nuclear DNA, Primary mitochondrial disease, Secondary mitochondrial dysfunction
Mitochondria are complex cellular organelles governing many metabolic processes including fatty acid oxidation, Krebs cycle, oxidative phosphorylation (oxphos) in the electron transport chain (ETC), and many others. Mitochondrial disease refers to a group of disorders that primarily affects the ETC and therefore the production of adenosine-triphosphate (ATP), the major cellular energy carrier [Chinnery, 2000]. However, with recent advances in genetic testing, mitochondrial dysfunction is being recognized to be even more complex than originally thought. Therefore, the terms primary mitochondrial disease (PMD) and secondary mitochondrial dysfunction (SMD) have been used to describe mitochondrial pathophysiology.
Distinction between PMD and SMD
PMDs can occur due to germline mutations in mitochondrial DNA (mtDNA) and/or nuclear DNA (nDNA) genes encoding ETC proteins. Point mutations can occur in any of the mtDNA's 37 genes encoding 13 proteins, 22 transfer RNAs (tRNA), and 2 ribosomal RNAs which are essential for optimal ETC function [Chinnery, 2000]. Approximately 250-300 genes are estimated to govern ETC from the nucleus [Powell et al., 2015], and about 1,500 nuclear genes in total are believed to involve mitochondrial processes, including non-ETC functions such as fatty acid oxidation and Krebs cycle. The ETC is organized in 5 complexes including complex I (NADH:ubiquinone oxidoreductase), complex II (succinate dehydrogenase), complex III (CoQ-cytochrome c reductase), complex IV (cytochrome c oxidase), and complex V (ATP synthase). For example, mtDNA genes (such as MT-ND1) encode 7 subunits of the complex I, while nDNA genes (such as NDUFS1) encode additional 38 subunits of complex I [Mimaki et al., 2012]. Complex II is encoded entirely by nDNA genes, while complexes III, IV and V, similar to complex I, have contribution from both mtDNA and nDNA.
PMD occurs not only due to defective genes encoding ETC proteins, but also due to germline mutations in other nDNA genes that affect oxphos function by impacting production of the complex machinery needed for the ETC to perform optimally. Some of the examples include POLG encoding mtDNA polymerase gamma, which replicates mtDNA, and C10ORF2 encoding the twinkle protein, which catalyzes mtDNA unwinding. Even though POLG and C10ORF2 do not encode ETC proteins directly, without these genes ETC function is impaired. When they are mutated they can cause PMD. Deletions in mtDNA may be germline (inherited from the mother) or secondary to nDNA mutations (inherited from the mother and/or father) such as POLG gene mutations, which can cause mtDNA deletions or depletion resulting in PMD. Table 1 gives some examples of mtDNA and nDNA genes implicated in PMD.
Table 1.
Some representative examples of PMDs and causative genes in both mtDNA and nDNA
| PMD name | PMD features | Gene |
|---|---|---|
| Leigh syndrome (mtDNA defect) | complex I deficiency due to mtDNA defect, muscle weakness/hypotonia, cardiomyopathy, grey and white matter abnormalities | MT-ND1; mtDNA gene encoding one of 7 complex I subunits governed by mtDNA |
| Leigh syndrome (nDNA defect) | complex I deficiency due to nDNA defect, cardiomyopathy, encephalomyopathy, renal/hepatic dysfunction | NDUFS1; nDNA gene encoding one of 38 complex I subunits governed by nDNA |
| Kearns-Sayre syndrome (mtDNA defect) | PEO <20 years of age, pigmentary retinopathy, heart block | mtDNA deletion; heteroplasmic, includes several mtDNA genes |
| Alpers-Huttenlocher syndrome (nDNA defect) | hypotonia, seizures, liver failure, mtDNA deletion/depletion (secondary) | POLG; nDNA gene encoding mitochondrial polymerase gamma |
| MELAS (mtDNA defect) | encephalomyopathy, lactic acidosis, stroke-like episodes at age <40 years | MT-TL1; mtDNA gene encoding tRNA with specific point mutations m.3243A>G, m.3271T>C, m.3251A>G |
| Ataxia neuropathy syndrome, (nDNA defect) | SANDO, epilepsy, cerebellar ataxia, myopathy | C10ORF2; nDNA gene encoding twinkle protein |
MELAS = Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes; PEO = progressive external ophthalmoplegia; SANDO = sensory ataxia neuropathy, dysarthria, ophthalmoplegia.
In contrast, SMD can accompany many pathologic processes not involving oxphos, including inherited diseases with germline mutations in non-oxphos genes. SMD can also be acquired secondary to adverse environmental effects which can cause oxidative stress. The latter can result in mtDNA alterations as seen in a variety of other processes adversely impacting mitochondria such as aging, inflammatory response, mitotoxic drugs, etc. [Orrenius et al., 2007; Circu et al., 2009; Rachek et al., 2009]. Thus, SMD can be inherited or acquired which is an important distinction from PMD, which can only be inherited. Distinguishing whether mitochondrial dysfunction is inherited or acquired is extremely challenging, and at present, the best method for making this distinction is still poorly understood. One of the most reliable (but not all-encompassing) tools in this daunting task is comprehensive molecular testing of both mtDNA and nDNA which, at least in some cases, can ultimately distinguish between PMD and SMD.
We examine PMD and SMD in detail below with a caveat that distinction between them is often blurred and contributes to incomplete penetrance and variable expressivity, which make diagnosis and treatment of mitochondrial diseases quite challenging. Table 2 gives some examples of non-PMD disorders that can result in SMD and causative nDNA genes.
Table 2.
Some representative examples of non-PMD disorders that can result in SMD and their causative nDNA genes
| Disorders causing SMD | Features of disorders causing SMD | Gene (nDNA) |
|---|---|---|
| Spinal muscular atrophy | loss of motor neurons, hypotonia, muscle weakness | SMN1 |
| Friedreich's ataxia | ataxia, progressive muscle weakness, hypertrophic cardiomyopathy, diabetes mellitus | FXN |
| Charcot-Marie-Tooth type 2K | weakness/fatigue, peripheral neuropathy, motor regression | GDAP1 |
| Hereditary spastic paraplegia 7 | weakness/spasticity, peripheral neuropathy, ataxia | SPG7 |
| Wilson's disease | liver disease due to copper deposition; neurological features: tremors, ataxia, etc.; psychiatric features: neurosis, depression, etc.; Kayser-Fleischer rings | ATP7B |
Primary Mitochondrial Disease
PMD is caused by germline mutations that are passed through nDNA of both the egg and sperm and through mtDNA of the egg only. Although transmission of mtDNA from sperm to zygote can occur, paternal mtDNA is eliminated [Stewart and Larsson, 2014]. Unless there is reversion or other restoration of the wild-type gene, germline mutations will be present in nDNA of all cells. Mitochondria are unique, being the only cellular organelles carrying their own DNA outside of the nucleus. Thousands of mitochondria may be present in one cell depending on its function (higher numbers in more metabolically active tissues). Each mitochondrion may have roughly 10 copies of mtDNA. Therefore, even within the same cell, some mtDNA copies can have germline mutations, while the other mtDNA copies are wild type. This is one aspect of heteroplasmy. As the zygote divides, germline mutations in mtDNA from the mother may end up in all cells, or only in specific cell lines, with other cell lines having wild-type genetic material [Schmiedel et al., 2003; Saneto and Sedensky, 2013]. Repair of mutations in mtDNA is less efficient than that of nDNA, which results in an mtDNA mutation rate 10-20 times higher than nDNA [Boesch et al., 2011].
Mitochondria in the oocytes can have mutated mtDNA. Heteroplasmy can originate when wild type and mutant mtDNA are both present in one egg; this results in both wild type and mutant mtDNA present at a zygotic level; this then propagates to different embryonic cell lines. Unequal distribution of mutant mitochondria among various organs and tissues can take place during fetal development. The proportion of mutant mtDNA in the egg that ends up distributed in the tissues is apparently a random process [Smeets et al., 2015]. Throughout life there may be selective pressures in cells and tissues leading to changes in the proportion of mutant mitochondria and the worsening or amelioration of symptoms.
Recent findings suggested that some mtDNA heteroplasmic mutations are associated with chronic diseases such as atherosclerosis and cancer [Sobenin et al., 2014]. Undoubtedly, the impact of environmental stressors can contribute to changes of both egg and zygote mtDNA. Epigenetics refers to processes which alter the expression of genes, independent of changes in the genetic code. Epigenetic processes, particularly methylation, can be influenced by environmental factors and the metabolic state of the cell [Wallace and Fan, 2010]. Considerable research shows that epigenetic alterations may contribute to both PMD and SMD with modifier genes and retrograde signaling as some of the few examples [Guha and Avadhani, 2013; Chen et al., 2015].
PMDs due to germline mtDNA mutations are caused by a variety of maternally inherited mtDNA defects in both heteroplasmic and homoplasmic states. Over 250 different mtDNA mutations have been reported with an incidence of pathogenic variants in as high as 1 in 200 live births. Most of individuals with these mutations do not have any clinical manifestations [Schaefer et al., 2008; Spinazzola, 2011]. Indeed, only 1 in 5,000 individuals is estimated to phenotypically express clinical manifestations of these mutations [Skladal et al., 2003; Poulton et al., 2010]. Aberrations in mtDNA include point mutations and deletions and, in rare cases, duplications [Mancuso et al., 2004].
Mitochondrial DNA-Related PMD
These disorders are strictly maternally inherited. Commonly the mother has an mtDNA low-level heteroplasmic mutation which may or may not cause mild symptoms depending on a threshold at which heteroplasmic level results in symptoms. This so-called ‘threshold effect’ reflects the proportion of normal to mutant mtDNA within a cell where mitochondrial dysfunction occurs [Vu et al., 2002]. This threshold can be different depending on the organ involved. Less often, the mother has an mtDNA mutation only in her oocytes (germline de novo), in which case she would be asymptomatic. Some of the representative PMDs caused by mtDNA mutations include diseases involving protein-encoding genes such as Leber hereditary optic neuropathy, and neuropathy, ataxia and retinitis pigmentosa (NARP). Mutations in mitochondrial tRNA encoding genes can result in diseases such as mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) and myoclonic epilepsy with ragged-red fibers (MERRF). mtDNA deletions and rarely duplications are seen in Kearns-Sayre (retinopathy, cardiac conduction defects, and ragged-red fibers) and Pearson (anemia, pancytopenia, and pancreatic insufficiency) syndromes. Aminoglycoside-induced nonsyndromic deafness occurs due to a mutation in the gene encoding ribosomal RNA; this mitochondrial gene is frequently included in genetic panels for hearing loss. The above-mentioned mtDNA-related diseases may occur due to mutated mtDNA in germline before conception (inherited), which would lead to PMDs. These mtDNA mutations can also occur after conception (acquired) secondary to a high level of oxidative stress or environmental insults, thereby causing SMD rather than PMD. In other words, PMD only originates from the germline mtDNA mutated before conception, while wild-type germline mtDNA can be mutated secondary to some process after conception, giving rise to SMD.
Nuclear DNA-Related PMD
These disorders are more complex and often inherited in an autosomal recessive pattern, although dominant and X-linked patterns have also been described [Carrozzo et al., 1998]. Some of the representative PMDs caused by nDNA point mutations include Leigh syndrome which has multigenic causes (NDUFS1, SURF1, etc.). PMDs such as Alpers-Huttenlocher (POLG) and mitochondrial neurogastrointestinal encephalomyopathy or MNGIE (TYMP) are associated with mtDNA deletions and depletion. Again, nDNA mutations that induce mtDNA defects are different from those environmentally induced due to factors such as oxidative stress. The PMD genes either encode oxphos proteins directly or affect oxphos function by impacting production of the complex machinery needed to run the oxphos process.
PMDs have variable clinical and biochemical characteristics. This is due to the fact that their clinical effects rarely occur in isolation. Indeed, in addition to the innate damage to mitochondrial function caused by these mutations before conception, additional factors modulate the clinical severity of these PMDs. Such factors include secondary damage induced by the environment to modifier genes which can either exacerbate or compensate for the primary mutation. Therefore, there is a fine line between PMD and SMD, with a significant overlap which makes accurate diagnosis quite challenging. Just as mtDNA-related PMD, germline nDNA defects causing PMDs occur before conception, while environmentally induced epigenetic alterations after conception or somatic nDNA mutations could result in SMD.
Secondary Mitochondrial Dysfunction
SMD essentially refers to any abnormal mitochondrial function other than PMD, i.e., the process caused by the genes encoding the ETC proteins directly or impacting the production of the machinery needed for ETC. Although the primary mitochondrial function is ATP production, nonbioenergetic capabilities of these complex organelles can be affected by mitochondrial dynamics [Archer, 2013]. SMD does not involve inborn defects of genes controlling oxphos and usually presents after conception. The impact of another pathologic process on mitochondria as seen in many inherited and acquired disorders (non-PMD disorders) can secondarily attenuate mitochondrial ability to generate ATP and alter mitochondrial dynamics, which can impact the non-ATP producing capabilities as well. For instance, mitochondrial fission and fusion, as examples of mitochondrial dynamics, are often implicated in multifactorial disorders such as diabetes [Yoon et al., 2011], heart disease [Ong et al., 2013], cancer [Boland et al., 2013], kidney diseases [Zhan et al., 2013], and neurodegenerative disorders [Jiang et al., 2015]. Therefore, these complex disorders are frequently accompanied by SMD.
Diagnosis of both PMD and SMD often relies on one or several mitochondrial disease criteria (MDC) scoring systems designed to diagnose the energy producing mitochondrial function such as Nijmegen [Wolf and Smeitink, 2002], modified Walker [Bernier et al., 2002; Scaglia et al., 2004], Morava [Morava et al., 2006] criteria, and others. These criteria take into account biochemical, clinical, tissue, and molecular characteristics and assign points based on the importance or specificity of certain clinical or laboratory findings. These criteria typically rate the significance of the clinical and biochemical findings as consistent with a definite, probable, or possible diagnosis of mitochondrial disease, or that mitochondrial disease is unlikely. In some cases, patients meet enough criteria to be diagnosed with a mitochondrial disorder despite absence of molecular confirmation of an mtDNA or nDNA mutation. This raises the question of whether this diagnosed mitochondrial disorder is a PMD or SMD.
MDC systems use some symptoms and signs that are nonspecific and can be part of non-PMD conditions. However, there are currently no consensus guidelines, universally used, for diagnosis of mitochondrial disease (whether primary or secondary) because of the significant variable expressivity and incomplete penetrance. Therefore, no MDC system can be perfect because PMD and SMD can have overlapping symptoms and signs. A recent publication by Parikh et al. [2015] makes an attempt to provide some consensus recommendations based on the literature and the authors' experience, and many diagnostic parameters are discussed. However, since no minimal criteria are given and no single patient would have all the mentioned variables, diagnosis heavily relies on subjective analysis and experience, which vary widely between clinicians. Therefore, in our opinion (in absence of consensus guidelines), the MDC scoring systems, while imperfect, currently represent the best guidance on the minimal diagnostic criteria sufficient to diagnose PMD or SMD. In other words, certain minimal clinical phenotype and/or genotype (combination of symptoms, signs, and tests) should be considered to diagnose a patient with PMD or SMD for clinical purposes, given that a vast majority of patients do not perfectly fit all the MDC components. In a practical sense, no diagnostic criteria for any disorder can capture all patients with that disorder simply due to the variable expressivity and incomplete penetrance characteristic to virtually all diseases. For instance, diagnosis of tuberous sclerosis complex (TSC) is based on the diagnostic criteria similar to MDC. TSC criteria indicate definite, probable and possible TSC, and genetic testing is used to support the clinical diagnosis [Northrup et al., 1999]. However, testing for detection of a definitive mutation in the TSC1 or TSC2 genes is only 80% sensitive. Therefore, TSC remains to be primarily a clinical diagnosis based on the diagnostic criteria which are neither sensitive nor specific for every TSC patient and sometimes without a genetic confirmation.
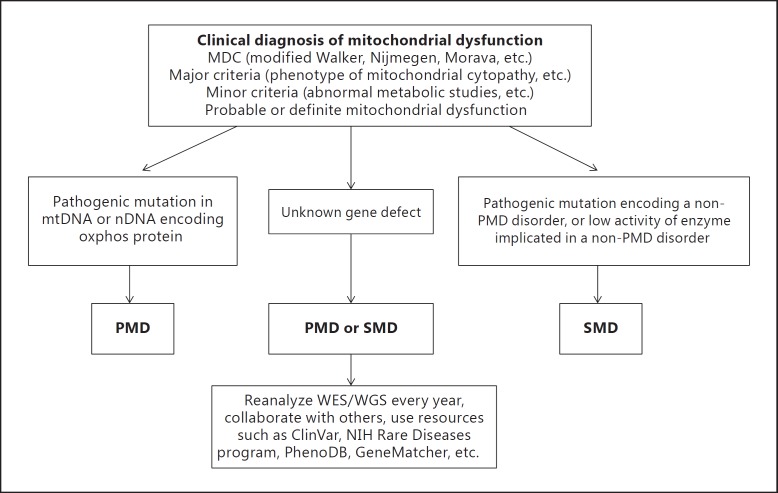
The MDC systems strive to balance diagnostic specificity and sensitivity to achieve a high enough specificity without too many patients being undiagnosed, and a high enough sensitivity without too many patients being overdiagnosed. This is a daunting but essential task to accomplish in the field of mitochondrial medicine, which also applies to any other medical field. In a recent report, the MDC scoring system had a high sensitivity for diagnosis of mitochondrial encephalomyopathy. The authors stated that the simplified MDC may distinguish between mitochondrial and non-mitochondrial disorders and aid in early diagnosis before invasive muscle biopsy is undertaken [Niu et al., 2013]. In some cases, the minimum MDC can be used to decide whether a muscle biopsy is warranted [Morava et al., 2006]. In our practice, we use muscle biopsy as a last resort due to its invasiveness and anesthesia requirement, while we heavily use other noninvasive methods including advanced molecular DNA testing. In figure 1, we propose a pathway to help distinguish between PMD and SMD.
Fig. 1.
Schematic diagram proposed to distinguish between PMD and SMD. ClinVar, PhenoDB, and GeneMatcher are online databases of genetic variants and clinical phenotypes.
With the advent of next generation sequencing, powerful genetic tests, such as whole mitochondrial DNA sequencing, panels of multiple nDNA genes, and whole exome/genome sequencing (WES/WGS) for the entire coding and noncoding DNA, can be performed in a time- and cost-effective manner compared to the standard Sanger sequencing [Goh and Choi, 2012]. In a recent study assessing the yield of WES in mitochondrial disease, a molecular diagnosis was achieved in 39% of the patients suspected for mitochondrial disease, and the yield was even higher (57%) in the subgroup of patients highly suspected for mitochondrial disease [Wortmann et al., 2015]. The authors used the MDC scoring system [Morava et al., 2006] to assign their patients into the different severity groups. The WES yield was the highest in those meeting more criteria, thus strengthening support for the use of the MDC (Morava). In several patients in our practices, molecular confirmation was found after a long ‘diagnostic odyssey’ which either confirmed PMD or diagnosed a non-PMD disorder which caused SMD, with significant therapeutic implications (see examples below). Even though genetic testing can be quite useful, it is important to perform it in conjunction with thorough genetic counseling before and after the test. Medical geneticists or genetic counselors are most qualified in selecting the appropriate test, counsel on possible variants of unknown significance and provide post-test counseling including result interpretation and further steps affected by genetic results.
Disorders with SMD
The list of non-PMD disorders and pathologic processes involving SMD is constantly growing as scientists increasingly recognize that many diseases impact mitochondria in ways that have been underappreciated. With the advent and progress of next generation sequencing, it may become increasingly evident that SMD commonly accompanies many non-PMD disorders, supporting evidence that SMD is likely much more common than PMD. This knowledge is crucial for therapeutic management including investigation of potential new therapeutic agents and assignment into potentially life-saving clinical trials. Discussion of all diseases involving SMD is beyond the scope of this review, but we highlight some of the most representative examples commonly seen in patients in our practice.
Neuromuscular Disorders
A wide variety of myopathies and muscular dystrophies have solid evidence of SMD. Examples include spinal muscular atrophy (SMA), limb-girdle muscular dystrophy, Bethlem myopathy, inflammatory myopathies, Charcot-Marie-Tooth (CMT) neuropathy, drug-induced peripheral neuropathies, etc. [Katsetos et al., 2013]. Some proposed mechanisms include dysregulation of the mitochondrial permeability and defective autophagy [Bernardi and Bonaldo, 2013]. Recent molecular studies of muscle samples in various neuromuscular diseases showed mtDNA depletion [Komulainen et al., 2015].
SMA is one of the representative disorders associated with SMD. The SMN1 gene mutation in SMA results in defective spinal neurons leading to denervation and muscular atrophy, which in turn brings about decreased mitochondrial function in myocytes. The carnitine level in muscle is decreased and fatty acids are elevated due to an abnormal fatty acid metabolism [Zolkipli et al., 2012]. SMA patients also have decreased complex I-IV activities in muscle tissue [Berger et al., 2003]. In our practice, we routinely offer our patients SMA ubiquinol and levocarnitine, which improve strength as evidenced by the reports of physical therapists who are blinded to the administration of these agents. Previous investigators indicated that coenzyme Q10 (CoQ10) can be beneficial for physical performance of SMA patients [Harpey et al., 1990; Folkers and Simonsen, 1995].
Chromosomal Defects
Chromosomal rearrangements can result in SMD by disrupting nuclear genes governing mitochondria. Similarly, abnormal copy number variation can disrupt a particular gene or group of genes involved in mitochondrial function [Castro-Gago et al., 2011]. Down syndrome (DS) or trisomy 21 is one of the representative chromosomal disorders associated with mitochondrial dysfunction. Chromosome 21 contains the SOD1 gene encoding Cu, Zn-superoxide dismutase. Gain of an extra SOD1 copy results in a redox imbalance due to an overexpression of Cu, Zn-superoxide dismutase resulting in an excessive oxidative stress in DS due to the overproduction of reactive oxygen species by mitochondria which can contribute to SMD [Valenti et al., 2011; Pagano and Costello, 2012]. Interestingly, deleterious SOD1 gene mutations, which have a dominant negative effect in the pathogenesis of amyotrophic lateral sclerosis, can also cause SMD [Rosen et al., 1993]. Altered mitochondrial function and oxidative stress have been established to play a central role in the pathogenesis of neurodevelopmental impairment in DS [Arbuzova et al., 2002; Perluigi and Butterfield, 2012] and amyotrophic lateral sclerosis. Furthermore, there is a potential role of CoQ10 in modulating DNA repair mechanisms in DS [Tiano et al., 2012]. In our practice, we routinely offer CoQ10 to our patients with trisomy 21. By optimizing the dosing based on CoQ10 level in leukocytes and clinical response, we achieve a faster progress primarily in gross motor skills as evidenced by physical therapy reports while therapists are blinded to the CoQ10 administration. Large scale studies are needed to assess the role of CoQ10 or other antioxidants in motor function of DS patients.
Chromosomal imbalances that cause contiguous gene syndromes can affect mitochondrial genes and, therefore, secondarily affect mitochondrial function. Examples include 22q11.2 deletion or DiGeorge syndrome [Meechan et al., 2011], 22q13 duplication [Frye, 2012] and deletion [Frye et al., 2016] syndromes, and 15q11q13 duplication syndrome [Frye, 2009]. Chromosomal rearrangements can cause autosomal dominant PMDs or unmask recessive PMDs (sporadic mutation on one chromosome and point mutation on the other inherited from a parent). We recently described a female with a de novo 8q21.11 deletion and intellectual disability, failure to thrive, short stature, multiple congenital anomalies, and dysmorphic features and evidence of mitochondrial dysfunction (see Case Report below) [Niyazov and Africk, 2015].
Immunity and Autoimmunity
Mitochondria are considered to be critical components of the innate immune response [Lane et al., 2015]. The immune system requires significant energy, which varies depending on the specific activity. ATP is needed for neutrophil and T-cell function as well as antigen presentation and processing [Ledderose et al., 2014]. During systemic infections, the immune system expends a large amount of ATP to mount an inflammatory response [Harijith et al., 2014]. Inflammatory responses result in catabolism and high rates of ATP consumption from massive release of proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6, which may cause fatigue and malaise [Morris et al., 2015].
At a certain point, if ATP consumption is high enough, SMD can ensue, especially if infection is severe and immune response is strong. However, if an individual already has preexisting SMD, the mitochondria many not be able to support the extra demand required by immune response, resulting in an inability to provide adequate ATP for basic cellular function, giving rise to neurologic regression. Neuroregression in the context of infection is a hallmark of mitochondrial diseases (both PMD and SMD), and it is not uncommon to have severe and even life-threating events with intercurrent infection [Edmonds et al., 2002].
Autoimmunity consumes ATP to mount an immune response to the body's own tissues. SMD has been documented in a variety of autoimmune processes including multiple sclerosis and lupus [Morris et al., 2014, Morris et al., 2015]. Autoimmune diseases of the skin such as pemphigus vulgaris and lupus are associated with increased levels of antimitochondrial antibodies which can result in alterations of mitochondrial energy metabolism [Feichtinger et al., 2014]. Moreover, defective mitochondria can result in autoimmune activation from generated neopeptides [Duvvuri et al., 2014]. This results in an unnecessary ATP consumption and expenditure of immune response which can weaken the immune system and increase susceptibility to infections [Booth et al., 2012]. The latter consume more ATP by triggering an immune response, and this vicious cycle can result in ATP depletion and neurodegeneration.
Fever is a product of immune response that consumes ATP. Immune responses can cause oxidative stress [Phillips et al., 2010; Slifka and Amanna, 2014]. If ATP is already deficient due to PMD or SMD prior to immune response and fever, neurologic regression could ensue after an immune response is triggered, when a certain ‘threshold effect’ is achieved. This threshold is higher in normal cells and lower in cells with PMD or SMD. A variety of cumulative factors including the proportion of mitochondrial heteroplasmy in different organs and tissues can contribute to the level of this threshold.
Autism
Autism spectrum disorder (ASD) is a multifactorial neurodevelopmental disorder in which genetic and environmental factors appear to contribute equally [Hallmayer et al., 2011; Sandin et al., 2014]. Genetic etiology may account for 30-40% of all cases [Schaefer and Mendelsohn, 2013] with advanced testing techniques such as WES/WGS, suggesting that a genetic component may be present in up to 50% of all cases [Freitag, 2007; Yuen et al., 2015]. A meta-analysis suggests that classic PMD affects 5% of children with ASD, with over 30% exhibiting biomarkers consistent with PMD [Rossignol and Frye, 2012]. Several studies using immune cells demonstrate higher rates of functional mitochondrial abnormalities in ASD [Giulivi et al., 2010; Napoli et al., 2012; Rose et al., 2014]. The much higher rates of abnormal biomarker levels is probably an indication of many types of SMD in ASD. Indeed, mitochondrial dysfunction has been implicated in immune dysregulation in ASD [Rossignol and Frye, 2012; Rose et al., 2014]. Autoimmune diseases are diagnosed significantly more often among children with ASD than among controls [Zerbo et al., 2015] and among their family members [Comi et al., 1999].
Environment
There is evidence to suggest that mitochondria serve as potent epigenetic regulators of the cellular processes in part due to antero- and retrograde signaling pathways [Guha and Avadhani, 2013; Chen et al., 2015]. Environmental influences of diet and exercise can have a significant impact on mitochondrial function. Diet is known to affect the epigenetic regulation of human mitochondrial superoxide dismutase (the Cu, Zn-superoxide dismutase discussed above) [Thaler et al., 2009]. Both overexertion and lack of physical activity can strain mitochondria, but exercise is one of the standard effective treatment modalities for PMD and SMD [Tarnopolsky and Raha, 2005; Safdar et al., 2011; Tarnopolsky, 2014]. The catabolism associated with psychological stress causes a substantial change in mitochondrial function [Batandier et al., 2014]. In addition, acute and chronic infections are among the most potent environmental forces exerting their effects on mitochondria via inflammatory processes [Cloonan and Choi, 2012].
Pharmacologic Agents
Multiple studies over more than 50 years have demonstrated that many medications can induce mitochondrial damage by adversely impacting mitochondrial metabolic pathways and components [Neustadt and Pieczenik, 2008; Olszewska and Szewczyk, 2013]. Mitotoxicity has been shown for several drugs including propofol, statins, doxoribucin, risperidone, acetaminophen, etc. The most metabolically active organs, including the liver [Begriche et al., 2011], heart [Apostolova et al., 2011], and skeletal muscle [Pereira et al., 2009] are usually affected. Statin medications used for hypercholesterolemia are well known to induce a specific (‘statin-induced’) myopathy by depleting CoQ10 in the tissues [Joy and Hegele, 2009]. CoQ10 supplementation reduces severity of myalgia and muscle weakness in statin-induced myopathy [Littarru and Langsjoen, 2007]. As more drugs come to market, research will need to be done to explore their impact on mitochondria.
Aging
Aging negatively affects mitochondrial function [Lane et al., 2015; Payne and Chinnery, 2015; Yin et al., 2016]. Aging contributes to oxidative stress in virtually all organs and tissues in the body and increases the risk for SMD. That is one reason an athlete's endurance is not the same at 20 and 65 years of age [Crane et al., 2013] since mitochondria provide energy to virtually all processes in the body. Their changes are one of the most important factors that contribute to aging. With time, aging and dysfunctional mitochondria accumulate under the plasma membrane in skeletal and smooth muscle cells. This gives an appearance of red-ragged fibers on Gomori trichrome stain of histopathologic muscle sections. RRFs are present in healthy individuals and their numbers increase with age [Cormio et al., 2005]. Point mutations and deletions in mtDNA also increase with age owing to a diminished precision of mitochondrial machinery responsible for mtDNA replication [Simon et al., 2001] and a number of other factors including environmental influences such as diet, exercise, infections, drugs, and radiation. Neurodegenerative diseases such as Alzheimer's and Parkinson's are among the best examples of deterioration of mitochondrial function with aging [Keogh and Chinnery, 2015]. In our practice, we routinely see multiple low-level mtDNA deletions in muscle specimens from adults, much more so than in children. This trend of accumulation of mtDNA deletions and ETC abnormalities in muscle with aging has been described in the literature [Cheema et al., 2015].
Neurodegenerative Disorders
Altered mitochondrial fusion/fission dynamics have been found to be a recurring theme in neurodegeneration [Archer, 2013]. There is evidence of mitochondrial dysfunction in neurodegenerative diseases such as Alzheimer's and Parkinson's, but it is unclear whether it is secondary to the disease process or whether it is due to oxidative stress increased by aging and whether or not it is contributing to the pathogenesis of these diseases. For instance, mutations in the PINK1 gene in Parkinson's disease cause accumulation of the fission protein DRP1. DRP1, being a positive regulator of fission, results in excessive fission which increases oxidative stress and reduces ATP production [Dagda et al., 2009]. Reduced ATP production contributes to SMD as documented in the mouse model of Rett syndrome [Grosser et al., 2012] and in a patient with an MECP2 mutation [Condie et al., 2010].
SMD is well described in some CMT types. Some representative examples include mitofusin-2 protein (encoded by the MFN2 gene) which is implicated in CMT type 2A (CMT2A) [Züchner et al., 2004] and ganglioside-induced differentiation-associated protein 1 (encoded by the GDAP1 gene) implicated in CMT2A (see Case Report below) [Niyazov and Africk, 2015].
At 12 years of age, one of our patients presented with progressive fatigue, weakness, abnormal gait, speech disturbance, drooling, and foot eversion. She had a high lactate acid level and lactate/pyruvate ratio and a low carnitine level on 2 occasions, which along with her clinical phenotype and MDC provided evidence for SMD. Her whole mtDNA testing was negative, but a nuclear mitochondrial panel showed a variant likely disease causing in the MFN2 gene responsible for CMT2A, which is known to cause SMD. However, her asymptomatic father and paternal grandmother had this variant as well which made it less likely to be a culprit, since CMT is almost completely penetrant. WES was ordered next and revealed a de novo mutation in the THAP1 gene implicated in torsion dystonia type 6 (DYT6). Both CMT2A and DYT6 can involve SMD with important differences, especially for therapy. Therefore, the more advanced genetic testing (WES vs. a limited nuclear mitochondrial panel) can provide a more accurate diagnosis with potential implications in prognosis and treatment, such as enrollment into clinical trials utilizing deep brain stimulation and transcranial electrical polarization.
The FXN gene in Friedreich's ataxia encodes frataxin, a mitochondrial protein which plays a role in mitochondrial iron homeostasis [Calabrese et al., 2005]. An expansion of trinucleotide repeats in FXN causes a deficiency of the respiratory chain due to free radical-mediated oxidative damage [González-Cabo and Palau, 2013]. Idebenone was reported to achieve a dose-related neurologic benefit as measured by the International Cooperative Ataxia Rating Scale (ICARS) [Di Prospero et al., 2007]. Due to study duration less than 12 months, this study was excluded from the review by Kearney et al. [2012], who concluded that no randomized trials with idebenone have shown significant benefit on neurological symptoms associated with Friedreich's ataxia. In our experience, one of our Friedreich's ataxia patients was started on idebenone at the age of 10, and now at 17 years of age, her ICARS score has decreased by 4 points.
Cancer
Mutations in genes encoding mitochondrial proteins have been linked to cancer [Singleterry et al., 2014]. For example, defects in succinate dehydrogenase (SDH), also known as complex II of the ETC, may lead to increased oxygen production and increased sensitivity to oxidative stress [Slane et al., 2006]. SDH mutations have been linked to paraganglioma and pheochromocytoma [Astuti et al., 2003]. Mitochondrial dysfunction may result in growth advantage for cancer cells during migration and invasion. Rapidly dividing cancer cells have extreme metabolic requirements and therefore utilize various metabolic pathways to support the high rate of proliferation. Increased requirement for ATP production is a basis of novel therapeutic interventions such as the use of the enzyme L-asparaginase to treat acute lymphoblastic leukemia [Müller and Boos, 1998; Luo et al., 2009].
Inborn Errors of Metabolism
A significant number of metabolic disorders include SMD as a part of their phenotypes. Representative examples include methylmalonic and propionic acidurias [de Keyzer et al., 2009; Baruteau et al., 2014], fatty acid oxidation disorders [Nsiah-Sefaa and McKenzie, 2016], disorders of purine/pyrimidine synthesis [Duley et al., 2011], urea cycle disorders such as argininosuccinic aciduria due to lyase deficiency [Monné et al., 2015], and many others. The importance of distinguishing between PMD and the primary cause of the clinical phenotype with or without SMD is emphasized by a clinical example of the following patient in our practice.
Clinical Example
A boy presented to our practice for the first time at the age of 9 years with developmental delay, seizures, autism, periods of developmental regression, and self-injurious behavior (finger biting). At 3 years of age, he was diagnosed with PMD due to complex III deficiency which was demonstrated on a fresh muscle sample. His lactic acid level and lactate/pyruvate ratio were elevated. His treating physicians thought that most of his clinical phenotype could be explained by the PMD diagnosis, and his self-injurious behavior was attributed to autism. He was treated with CoQ10 and levocarnitine, which did result in improvement of his muscle tone (per physical therapy reports). However, his propensity to harm himself went beyond a typical autistic child. Even though his original plasma uric acid was normal, we tested his hypoxanthine phosphoribosyl-transferase which showed a very low activity (4% of control), consistent with a newly diagnosed Lesch-Nyhan syndrome patient. His HPRT1 gene sequencing confirmed a hemizygous mutation (IVS1 + 1G>T) which was also present in his healthy mother. His renal ultrasound demonstrated bilateral renal calculi (determined to be uric acid calculi) in the renal pelvis close to obstructing the ureters. Prompt therapy with allopurinol and lithotripsy avoided extreme pain of the stone obstruction. Lesch-Nyhan syndrome is an X-linked inborn error of metabolism involving hypoxanthine phosphoribosyl-transferase deficiency which results in abnormal purine synthesis. Defective nucleotide availability affects mtDNA production and causes SMD with ATP depletion [Duley et al., 2011]. To date, the child appears to benefit from his mitochondrial vitamin cocktail as well as allopurinol. The molecular diagnosis of the HPRT1 mutation in the patient and his mother also provided important information on the recurrence risk and reproductive options for the parents.
Treatment
Suspicion for mitochondrial dysfunction (whether PMD or SMD) is usually based on clinical/family history and laboratory findings, including metabolic and molecular tests, which are frequently aided by the MDC scoring systems (fig. 1). However, failure to demonstrate a mitochondrial diagnosis does not rule it out; further testing must be pursued until it is either confirmed by a different testing method or disproven by finding a non-PMD diagnosis. In various clinical trials, including pharmacological and gene therapy, it is crucial to have a molecular diagnosis. Nevertheless, even if a particular non-PMD disorder is not known to have a mitochondrial component, exclusion of SMD may be rather challenging if the clinical phenotype and biomarkers are present as illustrated in the cases presented in this review. Although it is tempting to treat only SMD, an undiscovered non-PMD etiology may require treatment different from that used in SMD alone. In our opinion, due to the relatively benign nature of treatment with antioxidants, regular caloric intake and exercise, we believe that potential SMD should be addressed in both PMD and non-PMD disorders. SMD should be treated as long as there are sufficient indicators of a probable or definite mitochondrial dysfunction as determined by the MDC.
Table 3 lists some ofthe most common agents used to treat PMD and SMD. These agents are frequently utilized due to their well-documented safety [Tarnopolsky, 2008]. While discussion of each agent's detailed mechanism of action in PMD/SMD is beyond the scope of this review, we have highlighted the most commonly used ubiquinone (CoQ10) and provided examples of some combination therapies. Even though Pfeffer et al. [2012] concluded that there is no clear evidence supporting any interventions in mitochondrial disorders, more promising trials have been conducted recently and are currently in process [Kerr, 2013; Avula et al., 2014; Enns, 2014; Tischner and Wenz, 2015].
Table 3.
Agents commonly used to treat PMD and SMD
| Vitamin | Dose | Adverse effects | Function |
|---|---|---|---|
| Electron transport chain support | |||
| CoQ10 (reduced): ubiquinol | 5–30 mg/kg/day, 1–2×/day | appetite loss, nausea, diarrhea | energy carrier between complex I and III, |
| CoQ10 (oxidized): ubiquinone | 10–30 mg/kg/day, 1–2×/day | at high doses | and complex II and III |
| Electron carrier support | |||
| Niacin (B3) | 50–100 mg given daily | flushing reaction | nicotinamide adenine dinucleotide (NAD) precursor |
| Riboflavin (B2) | 100–400 mg given daily | nausea at high doses | flavin adenine dinucleotide (FAD) precursor |
| Energy Storage | |||
| Creatine monohydrate | 100 mg/kg/day; 1–2×/day | increased urination | high-energy phosphate buffer precursor to phosphocreatine |
| Fatty acid oxidation support | |||
| L-carnitine or acetyl-L-carnitine | 30–120 mg/kg/day, 1–2×/day | stool loose/fishy smell | carrier of long-chain fatty acids |
| Biotin (B7) | 5–10 mg/day given daily | none | cofactor for carboxylase enzymes |
| Mitochondrial enzyme cofactors | |||
| Thiamine (B1) | 50–100 mg given daily | none | cofactor for citric acid cycle enzymes |
| Pantothenic acid (B5) | 5–1,200 mg/day, 1–3×/day | diarrhea at high doses | precursor to coenzyme A |
| Pyridoxine (B6) | 200 mg given daily | headache, paresthesia, nausea, headache at high doses | cofactor for over 100 enzymes |
| Biotin (B7) | as above | none | cofactor for carboxylase enzymes |
| Alpha-lipoic acid | 50–200 mg given daily | headache, paresthesia, rash, muscle cramps | cofactor for citric acid cycle enzymes |
| Antioxidants | |||
| CoQ10 | as above | as above | targets ETC oxidative stress |
| L-carnitine | as above | as above | scavenger of organic acids |
| Vitamin E | 200–400 IU given daily | bleeding at high doses | protects cell membranes |
| Vitamin C | 100–500 mg given daily | diarrhea at high doses | protects iron and copper |
| Redox metabolism support | |||
| Methylcobalamin (B12) | 5–2,000 μg every 1–3 days | hyperactivity, sleep disruption | supports methylation and folate cycles, and glutathione production |
| Reduced folate (B9) | folinic acid 400–800 μg/day | none | supports methylation and folate cycles |
| N-acetyl-L-cysteine (NAC) | 10–70 mg/kg/day, 1–3×/day | diarrhea at high doses | precursor to glutathione |
| Zinc | 10–40 mg daily | suppresses iron and copper absorption | supports superoxide dismutase |
| Central folate support | |||
| Folinic acid/leucovorin calcium (B9) | 0.5–4 mg/kg/day, 1–3×/day | hyperactivity | supports adequate folate levels in the brain |
Ubiquinone (CoQ10), or ubiquinol (a reduced and more bioavailable form of CoQ10) is generally recommended, since it is an essential component of the ETC. It is an obligatory inner mitochondrial membrane cofactor and an antioxidant that reduces excess reactive species at the inner mitochondrial membrane. When mitochondrial dysfunction occurs, whether PMD or SMD, the ETC complexes can produce high levels of oxidative stress. Such oxidative stress can, in turn, cause dysfunction of the ETC. CoQ10, being a part of the ETC, becomes the major antioxidant of the ETC, and if it becomes depleted by high levels of oxidative stress, the ETC will further dysfunction. CoQ10 is naturally produced as part of the cholesterol pathway, and its exogenous form can be given as ubiquinone (the oxidized form) or ubiquinol (the reduced form). Many physicians prefer the ubiquinol form, and studies have suggested that ubiquinol is more bioavailable than ubiquinone [Failla et al., 2014]. Several trials showed beneficial effects of CoQ10 on fatigue and muscle weakness [Folkers and Simonsen, 1995; Caso et al., 2007, Montini et al., 2008]. However, other studies reported no significant improvement [Glover et al., 2010]. Ongoing large-scale studies would further assess the efficacy of CoQ10 in improving mitochondrial function.
There have been few trials examining the effectiveness of mitochondrial supplements in PMD and many of these have used combination therapy. A combination of creatine, CoQ10, and lipoic acid decreased resting plasma lactate, and urinary 8-isoprostanes and decreased a decline in peak ankle dorsiflexion strength and increased fat-free mass in several patients with PMDs [Rodriguez et al., 2007]. The efficacy of L-arginine has been demonstrated in mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes patients [Koga et al., 2005, 2010]. Mitoquinone is a ubiquinone analogue and a potent antioxidant which specifically targets mitochondria and has demonstrated to provide neuroprotection against lipid peroxidation [Kumar and Singh, 2015]. Besides antioxidants, these trials include gene therapy, allotopic expression of wild-type mitochondrial genes, mitochondria-targeted peptides, and alteration of mitochondrial dynamics [Leitão-Rocha et al., 2015].
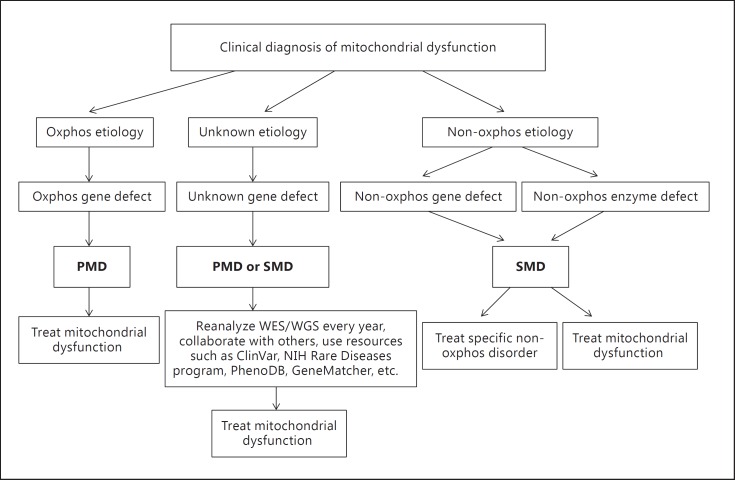
In figure 2, we propose a pathway to diagnose and treat PMD and SMD. In our experience, the treatment is beneficial and safe. However, since the efficacy of some therapeutic agents may not be high in a particular patient, each individual vitamin composition has to be regularly modified based on its clinical effects, safety, and cost. The following case report illustrates our approach to a patient with SMD [Niyazov and Africk, 2015].
Fig. 2.
Schematic diagram of the proposed diagnosis and treatment of PMD and SMD. ClinVar, PhenoDB, and GeneMatcher are online databases of genetic variants and clinical phenotypes.
Case Report
The female proband was seen in our practice for the first time at the age of 12 with a history of developmental delay, intellectual disability, failure to thrive, short stature, cleft palate, congenital heart disease, and dysmorphic features. At the age of 7, she was found to have a de novo 8q21.11 deletion of 11.8 Mb, which explained most of her symptoms until 11 years of age, when she experienced a regression in language, worsening gait, and fatigue which required intermittent use of a wheelchair.
Electromyography showed a mild axonal neuropathy. Her brain MRI showed a global cerebral atrophy which was more pronounced than 5 years prior. Additional new symptoms included tremor and a hoarse voice.
Haploinsufficiency of more than 35 genes in the deleted region explained many of her symptoms. However, unbalanced chromosomal rearrangements in general do not result in developmental regression and neurologic deterioration. This prompted us to look for a gene, among the deleted ones, that could be implicated in these symptoms. Analysis of the deleted genes revealed the GDAP1 gene encoding ganglioside-induced differentiation-associated protein 1. Haploinsufficiency of the GDAP1 is implicated in an autosomal dominant CMT2K, which likely contributed to her regression based on the GDAP1 involvement in mitochondrial function and a signal transduction pathway in neuronal development [Palomares et al., 2011]. In fact, we used one of the MDC scoring systems such as Modified Walker criteria [Bernier et al., 2002] to show a probable or definite mitochondrial dysfunction or SMD. According to this MDC, she met 1 major criterion (pathogenic nDNA abnormality) and 3 minor criteria (incomplete mitochondrial clinical phenotype, antibody-based demonstration of a widespread defective complex I respiratory chain subunit expression, and a decreased I+III complex activity in the muscle, and abnormal metabolic studies such as a high lactate acid level and lactate/pyruvate ratio, low plasma and urine carnitine, high alanine and glutamine levels). This is a good example of SMD caused by a primary disorder, which, in this case, was a sporadic chromosomal deletion. Treatment of the patient's SMD included improved nutrition via a gastrostomy tube, using a high-fat regular caloric intake with no more than 3 h fasting including at night. She also had a regular exercise program and physical therapy. Her vitamin cocktail included ubiquinol, creatine, and lipoic acid and needed to be regularly modified based on efficacy, safety, and cost. The girl experienced a stabilization of her neurological status and an improvement in several developmental parameters including motor and language skills as evidenced by reports of physical, occupational and speech therapists, while they were blinded to the nutritional changes and antioxidant administration.
Conclusions
The many manifestations of mitochondrial dysfunction are extremely complex and, at this time, still poorly understood. Nevertheless, some of these complexities are being unraveled with newly created diagnostic tools and therapeutic interventions. Mitochondrial dysfunction can be inherited and/or acquired as well as continuously modified by environment factors through several mechanisms including epigenetics. There is an important distinction between PMD and SMD, since at times they require different therapeutic approaches. While we advocate treating both PMD and SMD using diet, exercise, and specific vitamin supplements it is crucial to identify a possible non-mitochondrial etiology, since it may require separate treatment to decrease morbidity and mortality. Even though there are no consensus guidelines on the diagnosis of mitochondrial disorders, the MDC scoring systems are available to aid the diagnosis and can be quite beneficial. While effective treatment remains to be elucidated in the current and upcoming trials, patients with mitochondrial disease and dysfunction desperately seek intervention. The standard interventions for PMD should be strongly considered, while new, more effective treatments such as gene therapy are being investigated. Successful clinical trials will eventually lead to FDA approval. In our opinion, standard treatment used in PMD can be effective for some individuals with non-PMD disorder and SMD, and given their safety and availability, systematically starting such treatments, even without a firm diagnosis, is reasonable.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Apostolova N, Blas-García A, Esplugues JV. Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-γ inhibition. Trends Pharmacol Sci. 2011;32:715–725. doi: 10.1016/j.tips.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Arbuzova S, Hutchin T, Cuckle H. Mitochondrial dysfunction and Down's syndrome. Bioessays. 2002;24:681–684. doi: 10.1002/bies.10138. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL. Mitochondrial dynamics - mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 4.Astuti D, Hart-Holden N, Latif F, Lalloo F, Black GC, et al. Genetic analysis of mitochondrial complex II subunits SDHD, SDHB and SDHC in paraganglioma and phaeochromocytoma susceptibility. Clin Endocrinol (Oxf) 2003;59:728–733. doi: 10.1046/j.1365-2265.2003.01914.x. [DOI] [PubMed] [Google Scholar]
- 5.Avula S, Parikh S, Demarest S, Kurz J, Gropman A. Treatment of mitochondrial disorders. Curr Treat Options Neurol. 2014;16:292. doi: 10.1007/s11940-014-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baruteau J, Hargreaves I, Krywawych S, Chalasani A, Land JM, et al. Successful reversal of propionic acidaemia associated cardiomyopathy: evidence for low myocardial coenzyme Q10 status and secondary mitochondrial dysfunction as an underlying pathophysiological mechanism. Mitochondrion. 2014;17:150–156. doi: 10.1016/j.mito.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Batandier C, Poulet L, Hininger I, Couturier K, Fontaine E, et al. Acute stress delays brain mitochondrial permeability transition pore opening. J Neurochem. 2014;131:314–322. doi: 10.1111/jnc.12811. [DOI] [PubMed] [Google Scholar]
- 8.Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol. 2011;54:773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Berger A, Mayr JA, Meierhofer D, Fötschl U, Bittner R, et al. Severe depletion of mitochondrial DNA in spinal muscular atrophy. Acta Neuropathol. 2003;105:245–251. doi: 10.1007/s00401-002-0638-1. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi P, Bonaldo P. Mitochondrial dysfunction and defective autophagy in the pathogenesis of collagen VI muscular dystrophies. Cold Spring Harb Perspect Biol. 2013;5:a011387. doi: 10.1101/cshperspect.a011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59:1406–1411. doi: 10.1212/01.wnl.0000033795.17156.00. [DOI] [PubMed] [Google Scholar]
- 12.Boesch P, Weber-Lotfi F, Ibrahim N, Tarasenko V, Cosset A, et al. DNA repair in organelles: pathways, organization, regulation, relevance in disease and aging. Biochim Biophys Acta. 2011;1813:186–200. doi: 10.1016/j.bbamcr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth E, Myhill S, McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Int J Clin Exp Med. 2012;5:208–220. [PMC free article] [PubMed] [Google Scholar]
- 15.Calabrese V, Lodi R, Tonon C, D'Agata V, Sapienza M, et al. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. J Neurol Sci. 2005;233:145–162. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Carrozzo R, Hirano M, Fromenty B, Casali C, Santorelli FM, et al. Multiple mitochondrial DNA deletions features in autosomal dominant and recessive diseases suggest distinct pathogeneses. Neurology. 1998;50:99–106. doi: 10.1212/wnl.50.1.99. [DOI] [PubMed] [Google Scholar]
- 17.Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–1412. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 18.Castro-Gago M, Blanco-Barca MO, Pérez-Gay L, Eirís-Puñal J. Chromosomopathy manifesting as mitochondrial disease. J Child Neurol. 2011;26:659–660. doi: 10.1177/0883073811404068. [DOI] [PubMed] [Google Scholar]
- 19.Cheema N, Herbst A, McKenzie D, Aiken JM. Apoptosis and necrosis mediate skeletal muscle fiber loss in age-induced mitochondrial enzymatic abnormalities. Aging Cell. 2015;14:1085–1093. doi: 10.1111/acel.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Chen Y, Guan MX. A peep into mitochondrial disorder: multifaceted from mitochondrial DNA mutations to nuclear gene modulation. Protein Cell. 2015;6:862–870. doi: 10.1007/s13238-015-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinnery PF. Mitochondrial disorders overview. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] Seattle: University of Washington; 2000. http://www.ncbi.nlm.nih.gov/books/NBK1224/?report=classic. [Google Scholar]
- 22.Circu ML, Moyer MP, Harrison L, Aw TY. Contribution of glutathione status to oxidant-induced mitochondrial DNA damage in colonic epithelial cells. Free Radic Biol Med. 2009;47:1190–1198. doi: 10.1016/j.freeradbiomed.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cloonan SM, Choi AM. Mitochondria: commanders of innate immunity and disease? Curr Opin Immunol. 2012;24:32–40. doi: 10.1016/j.coi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- 25.Condie J, Goldstein J, Wainwright MS. Acquired microcephaly, regression of milestones, mitochondrial dysfunction, and episodic rigidity in a 46,XY male with a de novo MECP2 gene mutation. J Child Neurol. 2010;25:633–636. doi: 10.1177/0883073809342004. [DOI] [PubMed] [Google Scholar]
- 26.Cormio A, Milella F, Vecchiet J, Felzani G, Gadaleta MN, Cantatore P. Mitochondrial DNA mutations in RRF of healthy subjects of different age. Neurobiol Aging. 2005;26:655–664. doi: 10.1016/j.neurobiolaging.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Crane JD, Macneil LG, Tarnopolsky MA. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J Gerontol A Biol Sci Med Sci. 2013;68:631–638. doi: 10.1093/gerona/gls237. [DOI] [PubMed] [Google Scholar]
- 28.Dagda RK, Cherra SJ, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Keyzer Y, Valayannopoulos V, Benoist JF, Batteux F, Lacaille F, et al. Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res. 2009;66:91–95. doi: 10.1203/PDR.0b013e3181a7c270. [DOI] [PubMed] [Google Scholar]
- 30.Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich's ataxia: randomised, placebo-controlled trial. Lancet Neurol. 2007;6:878–886. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- 31.Duley JA, Christodoulou J, de Brouwer AP. The PRPP synthetase spectrum: what does it demonstrate about nucleotide syndromes? Nucleosides Nucleotides Nucleic Acids. 2011;30:1129–1139. doi: 10.1080/15257770.2011.591747. [DOI] [PubMed] [Google Scholar]
- 32.Duvvuri B, Duvvuri VR, Wang C, Chen L, Wagar LE, et al. The human immune system recognizes neopeptides derived from mitochondrial DNA deletions. J Immunol. 2014;192:4581–4591. doi: 10.4049/jimmunol.1300774. [DOI] [PubMed] [Google Scholar]
- 33.Edmonds JL, Kirse DJ, Kearns D, Deutsch R, Spruijt L, Naviaux RK. The otolaryngological manifestations of mitochondrial disease and the risk of neurodegeneration with infection. Arch Otolaryngol Head Neck Surg. 2002;128:355–362. doi: 10.1001/archotol.128.4.355. [DOI] [PubMed] [Google Scholar]
- 34.Enns G. Treatment of mitochondrial disorders: antioxidants and beyond. J Child Neurol. 2014;29:1235–1240. doi: 10.1177/0883073814538509. [DOI] [PubMed] [Google Scholar]
- 35.Failla M, Chitchumroonchokchai C, Aoki F. Increased bioavailability of ubiquinol compared to that of ubiquinone is due to more efficient micellarization during digestion and greater GSH-dependent uptake and basolateral secretion by Caco-2 cells. J Agric Food Chem. 2014;62:7174–7182. doi: 10.1021/jf5017829. [DOI] [PubMed] [Google Scholar]
- 36.Feichtinger RG, Sperl W, Bauer JW, Kofler B. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607–614. doi: 10.1111/exd.12484. [DOI] [PubMed] [Google Scholar]
- 37.Folkers K, Simonsen R. Two successful double-blind trials with coenzyme Q10 (vitamin Q10) on muscular dystrophies and neurogenic atrophies. Biochim Biophys Acta. 1995;1271:281–286. doi: 10.1016/0925-4439(95)00040-b. [DOI] [PubMed] [Google Scholar]
- 38.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 39.Frye R. 15q11.2-13 duplication, mitochondrial dysfunction, and developmental disorders. J Child Neurol. 2009;24:1316–1320. doi: 10.1177/0883073809333531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frye R. Mitochondrial disease in 22q13 duplication syndrome. J Child Neurol. 2012;27:942–949. doi: 10.1177/0883073811429858. [DOI] [PubMed] [Google Scholar]
- 41.Frye R, Cox D, Slattery J, Tippett M, Kahler S, et al. Mitochondrial dysfunction may explain symptom variation in Phelan-McDermid syndrome. Sci Rep. 2016;6:19544. doi: 10.1038/srep19544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glover E, Martin J, Maher A, Thornhill R, Moran G, Tarnopolsky M. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve. 2010;42:739–748. doi: 10.1002/mus.21758. [DOI] [PubMed] [Google Scholar]
- 44.Goh G, Choi M. Application of whole exome sequencing to identify disease-causing variants in inherited human diseases. Genomics Inform. 2012;10:214–219. doi: 10.5808/GI.2012.10.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.González-Cabo P, Palau F. Mitochondrial pathophysiology in Friedreich's ataxia. J Neurochem 126 Suppl. 2013;1:53–64. doi: 10.1111/jnc.12303. [DOI] [PubMed] [Google Scholar]
- 46.Grosser E, Hirt U, Janc OA, Menzfeld C, Fischer M, et al. Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiol Dis. 2012;48:102–114. doi: 10.1016/j.nbd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Guha M, Avadhani NG. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion. 2013;13:577–591. doi: 10.1016/j.mito.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harpey JP, Charpentier C, Paturneau-Jouas M, Renault F, Romero N, Fardeau M. Secondary metabolic defects in spinal muscular atrophy type II. Lancet. 1990;336:629–630. doi: 10.1016/0140-6736(90)93426-p. [DOI] [PubMed] [Google Scholar]
- 51.Jiang Z, Wang W, Perry G, Zhu X, Wang X. Mitochondrial dynamic abnormalities in amyotrophic lateral sclerosis. Transl Neurodegener. 2015;4:14. doi: 10.1186/s40035-015-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150:858–868. doi: 10.7326/0003-4819-150-12-200906160-00009. [DOI] [PubMed] [Google Scholar]
- 53.Katsetos CD, Koutzaki S, Melvin JJ. Mitochondrial dysfunction in neuromuscular disorders. Semin Pediatr Neurol. 2013;20:202–215. doi: 10.1016/j.spen.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Kearney M, Orrell RW, Fahey M, Pandolfo M. Antioxidants and other pharmacological treatments for Friedreich ataxia. Cochrane Database Syst Rev. 2012;4:CD007791. doi: 10.1002/14651858.CD007791.pub3. [DOI] [PubMed] [Google Scholar]
- 55.Keogh M, Chinnery P. Mitochondrial DNA mutations in neurodegeneration. Biochim Biophys Acta. 2015;1847:1401–1411. doi: 10.1016/j.bbabio.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Kerr DS. Review of clinical trials for mitochondrial disorders: 1997-2012. Neurotherapeutics. 2013;10:307–319. doi: 10.1007/s13311-013-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koga Y, Akita Y, Nishioka J, Yatsuga S, Povalko N, et al. L-arginine improves the symptoms of strokelike episodes in MELAS. Neurology. 2005;64:710–712. doi: 10.1212/01.WNL.0000151976.60624.01. [DOI] [PubMed] [Google Scholar]
- 58.Koga Y, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T. MELAS and L-arginine therapy: pathophysiology of stroke-like episodes. Ann NY Acad Sci. 2010;1201:104–110. doi: 10.1111/j.1749-6632.2010.05624.x. [DOI] [PubMed] [Google Scholar]
- 59.Komulainen T, Hautakangas MR, Hinttala R, Pakanen S, Vähäsarja V, et al. Mitochondrial DNA Depletion and Deletions in Paediatric Patients with Neuromuscular Diseases: Novel Phenotypes. JIMD Rep. 2015;23:91–100. doi: 10.1007/8904_2015_438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar A, Singh A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer's disease and other neurological conditions. Front Pharmacol. 2015;6:206. doi: 10.3389/fphar.2015.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lane RK, Hilsabeck T, Rea SL. The role of mitochondrial dysfunction in age-related diseases. Biochim Biophys Acta. 2015;1847:1387–1400. doi: 10.1016/j.bbabio.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ledderose C, Bao Y, Lidicky M, Zipperle J, Li L, et al. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J Biol Chem. 2014;289:25936–25945. doi: 10.1074/jbc.M114.575308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leitão-Rocha A, Guedes-Dias P, Pinho BR, Oliveira JM. Trends in mitochondrial therapeutics for neurological disease. Curr Med Chem. 2015;22:2458–2467. doi: 10.2174/0929867322666150209160317. [DOI] [PubMed] [Google Scholar]
- 64.Littarru GP, Langsjoen P. Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion. 2007;7(Suppl):S168–S174. doi: 10.1016/j.mito.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Luo J, Solimini N, Elledge SJ. Principles of cancer therapy: oncogene and nononcogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mancuso M, Vives-Bauza C, Filosto M, Marti R, Solano A, et al. A mitochondrial DNA duplication as a marker of skeletal muscle specific mutations in the mitochondrial genome. J Med Genet. 2004;41:e73. doi: 10.1136/jmg.2003.012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meechan DW, Maynard TM, Tucker ES, LaMantia AS. Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes. Int J Dev Neurosci. 2011;29:283–294. doi: 10.1016/j.ijdevneu.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta. 2012;1817:851–862. doi: 10.1016/j.bbabio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Monné M, Miniero DV, Daddabbo L, Palmieri L, Porcelli V, Palmieri F. Mitochondrial transporters for ornithine and related amino acids: a review. Amino Acids. 2015;47:1763–1777. doi: 10.1007/s00726-015-1990-5. [DOI] [PubMed] [Google Scholar]
- 70.Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med. 2008;358:2849–2850. doi: 10.1056/NEJMc0800582. [DOI] [PubMed] [Google Scholar]
- 71.Morava E, van den Heuvel L, Hol F, De Vries MC, Hogeveen M. Mitochondrial disease criteria: diagnostic applications in children. Neurology. 2006;67:1823–1826. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- 72.Morris G, Berk M, Galecki P, Maes M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs) Mol Neurobiol. 2014;49:741–756. doi: 10.1007/s12035-013-8553-0. [DOI] [PubMed] [Google Scholar]
- 73.Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. doi: 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28:97–113. doi: 10.1016/s1040-8428(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 75.Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, et al. Mitochondrial dysfunction in Pten haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PLoS One. 2012;7:e42504. doi: 10.1371/journal.pone.0042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neustadt J, Pieczenik S. Medication-induced mitochondrial damage and disease. Mol Nutr Food Res. 2008;52:780–788. doi: 10.1002/mnfr.200700075. [DOI] [PubMed] [Google Scholar]
- 77.Niu F, Chang L, Meng F, Zhang P, Niu Q, et al. Evaluation of a mitochondrial disease criteria scoring system on mitochondrial encephalomyopathy in Chinese patients. Int J Neurosci. 2013;123:93–98. doi: 10.3109/00207454.2012.732975. [DOI] [PubMed] [Google Scholar]
- 78.Niyazov D, Africk D. Mitochondrial dysfunction in a patient with 8q21.11 deletion and Charcot-Marie-Tooth disease type 2K due to GDAP1 haploinsufficiency. Mol Syndromol. 2015;6:204–206. doi: 10.1159/000440660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Northrup H, Koenig MK, Pearson DA, Au KS. Tuberous Sclerosis Complex. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] Seattle: University of Washington; 1999. http://www.ncbi.nlm.nih.gov/books/NBK1220/ [PubMed] [Google Scholar]
- 80.Nsiah-Sefaa A, McKenzie M. Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci Rep. 2016;36:e00313. doi: 10.1042/BSR20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olszewska A, Szewczyk A. Mitochondria as a pharmacological target: magnum overview. IUBMB Life. 2013;65:273–281. doi: 10.1002/iub.1147. [DOI] [PubMed] [Google Scholar]
- 82.Ong SB, Hall AH, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 84.Pagano G, Castello G. Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol. 2012;724:291–299. doi: 10.1007/978-1-4614-0653-2_22. [DOI] [PubMed] [Google Scholar]
- 85.Palomares M, Delicado A, Mansilla E, De Torres ML, Vallespín E, et al. Characterization of an 8q21.11 microdeletion syndrome associated with intellectual disability and a recognizable phenotype. Am J Hum Genet. 2011;89:295–301. doi: 10.1016/j.ajhg.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parikh S, Goldstein A, Koenig M, Scaglia F, Enns G, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med. 2015;17:689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Payne BA, Chinnery PF. Mitochondrial dysfunction in aging: much progress but many unresolved questions. Biochim Biophys Acta. 2015;1847:1347–1353. doi: 10.1016/j.bbabio.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pereira CV, Moreira AC, Pereira SP, Machado NG, Carvalho FS, et al. Investigating drug-induced mitochondrial toxicity: a biosensor to increase drug safety? Curr Drug Saf. 2009;4:34–54. doi: 10.2174/157488609787354440. [DOI] [PubMed] [Google Scholar]
- 89.Perluigi M, Butterfield DA. Oxidative stress and Down syndrome: a route toward Alzheimer-like dementia. Curr Gerontol Geriatr Res. 2012;2012:724904. doi: 10.1155/2012/724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery P. Treatment for mitochondrial disorders. Cochrane Database Syst Rev. 2012;4:CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phillips M, Cataneo RN, Chaturvedi A, Danaher PJ, Devadiga A, et al. Effect of influenza vaccination on oxidative stress products in breath. J Breath Res. 2010;4:026001. doi: 10.1088/1752-7155/4/2/026001. [DOI] [PubMed] [Google Scholar]
- 92.Poulton J, Chiaratti MR, Meirelles FV, Kennedy S, Wells D, Holt IJ. Transmission of mitochondrial DNA diseases and ways to prevent them. PLoS Genet. 2010;6:e1001066. doi: 10.1371/journal.pgen.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Powell C, Nicholls T, Minczuk M. Nuclear-encoded factors involved in post-transcriptional processing and modification of mitochondrial tRNAs in human disease. Front Genet. 2015;6:79. doi: 10.3389/fgene.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rachek LI, Yuzefovych LV, Ledoux SP, Julie NL, Wilson GL. Troglitazone, but not rosiglitazone, damages mitochondrial DNA and induces mitochondrial dysfunction and cell death in human hepatocytes. Toxicol Appl Pharmacol. 2009;240:348–354. doi: 10.1016/j.taap.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 96.Rose S, Frye RE, Slattery J, Wynne R, Tippett M, et al. Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS One. 2014;9:e85436. doi: 10.1371/journal.pone.0085436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 98.Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Safdar A, Bourgeois J, Ogborn D, Little J, Hettinga B, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci USA. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman C, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saneto RP, Sedensky MM. Mitochondrial disease in childhood: mtDNA encoded. Neurotherapeutics. 2013;10:199–211. doi: 10.1007/s13311-012-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scaglia F, Towbin JA, Craigen WJ, Belmont JW, Smith EO, et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004;114:925–931. doi: 10.1542/peds.2004-0718. [DOI] [PubMed] [Google Scholar]
- 103.Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, et al. Prevalence of mitochondrial DNA disease in adults. Ann Neurol. 2008;63:35–39. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- 104.Schaefer BG, Mendelsohn NJ, Professional Practice and Guidelines Committee Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med. 2013;15:399–407. doi: 10.1038/gim.2013.32. [DOI] [PubMed] [Google Scholar]
- 105.Schmiedel J, Jackson S, Schäfer J, Reichmann H. Mitochondrial cytopathies. J Neurol. 2003;250:267–277. doi: 10.1007/s00415-003-0978-3. [DOI] [PubMed] [Google Scholar]
- 106.Simon D, Lin M, Ahn C, Liu G, Gibson G, et al. D: Low mutational burden of individual acquired mitochondrial DNA mutations in brain. Genomics. 2001;73:113–116. doi: 10.1006/geno.2001.6515. [DOI] [PubMed] [Google Scholar]
- 107.Singleterry J, Sreedhar A, Zhao Y. Components of cancer metabolism and therapeutic interventions. Mitochondrion. 2014;17:50–55. doi: 10.1016/j.mito.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skladal D, Halliday J, Thorburn D. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126:1905–1912. doi: 10.1093/brain/awg170. [DOI] [PubMed] [Google Scholar]
- 109.Slane B, Aykin-Burns N, Smith B, Kalen A, Goswami P, et al. Mutation of succinate dehydrogenase subunit C results in increased O2-, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- 110.Slifka M, Amanna I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine. 2014;32:2948–2957. doi: 10.1016/j.vaccine.2014.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smeets H, Sallevelt S, Dreesen J, De Die-Smulders C, de Coo IF. Preventing the transmission of mitochondrial DNA disorders using prenatal or preimplantation genetic diagnosis. Ann NY Acad Sci. 2015;1350:29–36. doi: 10.1111/nyas.12866. [DOI] [PubMed] [Google Scholar]
- 112.Sobenin I, Mitrofanov K, Zhelankin A, Sazonova M, Postnov Y, et al. Quantitative assessment of heteroplasmy of mitochondrial genome: perspectives in diagnostics and methodological pitfalls. Biomed Res Int. 2014;2014:292017. doi: 10.1155/2014/292017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spinazzola A. Mitochondrial DNA mutations and depletion in pediatric medicine. Semin Fetal Neonatal Med. 2011;16:190–196. doi: 10.1016/j.siny.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Stewart JB, Larsson NG. Keeping mtDNA in shape between generations. PLoS Genet. 2014;10:e1004670. doi: 10.1371/journal.pgen.1004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tarnopolsky MA. The mitochondrial cocktail: rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv Drug Deliv Rev. 2008;60:1561–1567. doi: 10.1016/j.addr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 116.Tarnopolsky MA. Exercise as a therapeutic strategy for primary mitochondrial cytopathies. J Child Neurol. 2014;29:1225–1234. doi: 10.1177/0883073814538512. [DOI] [PubMed] [Google Scholar]
- 117.Tarnopolsky M, Raha S. Mitochondrial myopathies: diagnosis, exercise intolerance, and treatment options. Med Sci Sports Exerc. 2005;37:2086–2093. doi: 10.1249/01.mss.0000177341.89478.06. [DOI] [PubMed] [Google Scholar]
- 118.Thaler R, Karlic H, Rust P, Haslberger AG. Epigenetic regulation of human buccal mucosa mitochondrial superoxide dismutase gene expression by diet. Br J Nutr. 2009;101:743–749. doi: 10.1017/S0007114508047685. [DOI] [PubMed] [Google Scholar]
- 119.Tiano L, Padella L, Santoro L, Carnevali P, Principi F, et al. Prolonged coenzyme Q10 treatment in Down syndrome patients, effect on DNA oxidation. Neurobiol Aging. 2012;33:626.e1–8. doi: 10.1016/j.neurobiolaging.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 120.Tischner C, Wenz T. Keep the fire burning: current avenues in the quest of treating mitochondrial disorders. Mitochondrion. 2015;24:32–49. doi: 10.1016/j.mito.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 121.Valenti D, Manente GA, Moro L, Marra E, Vacca RA. Deficit of complex Iactivity in human skin fibroblasts with chromosome 21 trisomy and overproduction of reactive oxygen species by mitochondria: involvement of the cAMP/PKA signalling pathway. Biochem J. 2011;435:679–688. doi: 10.1042/BJ20101908. [DOI] [PubMed] [Google Scholar]
- 122.Vu T, Hirano M, DiMauro S. Mitochondrial diseases. Neurol Clin N Am. 2002;20:809–839. doi: 10.1016/s0733-8619(01)00017-2. [DOI] [PubMed] [Google Scholar]
- 123.Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2010;10:12–31. doi: 10.1016/j.mito.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolf N, Smeitink J. Mitochondrial disorders: a proposal for consensus diagnostic criteria in infants and children. Neurology. 2002;59:1402–1405. doi: 10.1212/01.wnl.0000031795.91814.d8. [DOI] [PubMed] [Google Scholar]
- 125.Wortmann S, Koolen D, Smeitink J, van den Heuvel L, Rodenburg R. Whole exome sequencing of suspected mitochondrial patients in clinical practice. J Inherit Metab Dis. 2015;38:437–443. doi: 10.1007/s10545-015-9823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin F, Sancheti H, Liu Z, Cadenas E. Mitochondrial function in ageing: coordination with signalling and transcriptional pathways. J Physiol. 2016;594:2025–2042. doi: 10.1113/JP270541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoon Y, Galloway C, Jhun B, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuen R, Thiruvahindrapuram B, Merico D, Walker S, Tammimies K, et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat Med. 2015;21:185–191. doi: 10.1038/nm.3792. [DOI] [PubMed] [Google Scholar]
- 129.Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, Croen LA. Immune mediated conditions in autism spectrum disorders. Brain Behav Immun. 2015;46:232–236. doi: 10.1016/j.bbi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhan M, Brooks C, Liu F, Sun L, Dong Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013;83:568–581. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zolkipli Z, Sherlock M, Biggar WD, Taylor G, Hutchison JS, et al. Abnormal fatty acid metabolism in spinal muscular atrophy may predispose to perioperative risks. Eur J Paediatr Neurol. 2012;16:549–553. doi: 10.1016/j.ejpn.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Züchner S, Mersiyanova I, Muglia M, Bissar-Tadmouri N, Rochelle J, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]