Abstract
Tetraploid/diploid mosaicism is a rare chromosomal abnormality that is infrequently reported in patients with severe developmental delay, growth retardation, and short life span. Here, we present a 6-year-old patient with severe penoscrotal hypospadias and a coloboma of the left eye but with normal growth, normal psychomotor development, and without dysmorphisms. We considered a local, mosaic sex chromosomal aneuploidy as a possible cause of his genital anomaly and performed karyotyping in cultured fibroblasts from the genital skin, obtained during surgical correction. Tetraploid/diploid (92,XXYY/46,XY) mosaicism was found in 43/57 and 6/26 metaphases in 2 separate cultures, respectively. Buccal smear cells, blood lymphocytes, and cells from urine sediment all showed diploidy. We investigated whether this chromosomal abnormality could be found in other patients with severe hypospadias and karyotyped genital fibroblasts of 6 additional patients but found only low frequencies (<11%) of tetraploid cells, not statistically different from those found in control males with no hypospadias. This is the first time tetraploid mosaicism is found in such a high percentage in a patient without psychomotor retardation, dysmorphisms or growth delay. Although the relationship between this observed mosaicism in cultured cells and the underlying pathogenetic mechanism in penoscrotal hypospadias remains to be determined, our data clearly illustrate the power of cytogenetic techniques in detecting mosaicism compared to next-generation sequencing techniques, in which DNA pooled from multiple cells is used.
Key Words: Cultured fibroblasts, Penoscrotal hypospadias, Tetraploid/diploid mosaicism
Tetraploid mosaicism has very rarely been reported in patients with congenital anomalies including growth retardation and developmental delay [Schinzel, 2001]. Tetraploid mosaicism has also been described as a somatic chromosomal abnormality in a variety of conditions such as Gardner syndrome [Danes, 1976], cancers [Ganem et al., 2007], and hydatidiform moles [Sundvall et al., 2013]. Low levels of tetraploid cells were found in lymphocytes from patients with polycystic ovary syndrome [Scarbrough et al., 1984; Rojanasakul et al., 1985], in the mother of a patient with nonmosaic tetraploidy [Scarbrough et al., 1984], and in gingival cells from patients with generalized aggressive periodontitis [Tözüm et al., 2005; Olgun-Erdemir et al., 2010]. Although polyploid cells including tetraploid cells are present in vivo in a variety of non-neoplastic tissues from normal individuals [Biesterfeld et al., 1994], some of the published cases with low frequencies of tetraploid cells may represent culture artifacts [Schinzel, 2001] as diploid cells fail to divide correctly both in vitro and in vivo [Rooney and Czepulkowski, 1992]. Indeed, it is well known that tetraploidy occurs as an artifact in human fibroblast cultures with frequencies of up to 5% of cells [Mittwoch et al., 1965; Danes, 1976; Annerén, 1982] as well as in amniocyte cultures [Sperling and Salig, 1971].
Tetraploid mosaicism has never been associated with hypospadias. Hypospadias is a common congenital malformation in boys, occurring in ∼0.3–0.5% of live births in Western countries [Baskin, 2004; van der Zanden et al., 2012]. Anatomical studies of mouse embryogenesis suggest that disruption of fusion, remodeling and migration of epithelial cells at the urethral fold leads to severe hypospadias [Baskin et al., 2001]. Both environmental and genetic factors have been implicated in the etiology of hypospadias [Carmichael et al., 2012; van der Zanden et al., 2012; Geller et al., 2014]. Environmental factors include pregnancy complications such as maternal hypertension and pre-eclampsia, whereas evidence for an effect of exposure to endocrine disrupting agents during pregnancy is inconclusive [Carmichael et al., 2012; van der Zanden et al., 2012]. Twin and family studies are in support of a genetic basis of hypospadias [van der Zanden et al., 2012]. Genome-wide association and gene expression studies indicate a contribution of several dozens of genes involved in the formation of the male external genitalia. These include genes encoding transcription factors, growth factors, growth factor receptors, and components of signaling pathways involved in patterning of the genital tubercle as well as genes that function in sex hormone synthesis and metabolism [Li et al., 2006; van der Zanden et al., 2012; Geller et al., 2014].
In the vast majority of the published reports on the genetics of hypospadias, genomic DNA isolated from blood has been used. We considered that local, mosaic (sex) chromosomal abnormalities present in genital tissues may be associated with hypospadias in some cases. Therefore, we karyotyped fibroblasts from biopsies of genital skin, obtained during surgical correction of a patient with severe penoscrotal (posterior) hypospadias. In 2 independent cultures, we found tetraploid cells at much higher frequencies than attributable to chance culture artifacts. We studied genital skin fibroblasts of 6 additional patients to discriminate between an exceptional, sporadic finding in our first patient and a more general, and perhaps causal, association between tetraploid mosaicism and hypospadias.
Case Report
Patient
The patient is a 6-year-old boy of black African ethnicity, who was adopted by his Dutch parents at the age of 1.5 years. Nothing is known about his ancestors. He was referred at the age of 6 years for surgical correction of his severe penoscrotal (posterior) hypospadias. He had a micropenis and scrotal testes (fig. 1), and a coloboma of the iris of the left eye (fig. 2). His height was −0.7 SD, weight +0.2 SD, and his OFC was 0 SD. There were no asymmetries and no facial dysmorphisms. Otherwise, he was healthy with a normal psychomotor and intellectual development.
Fig. 1.
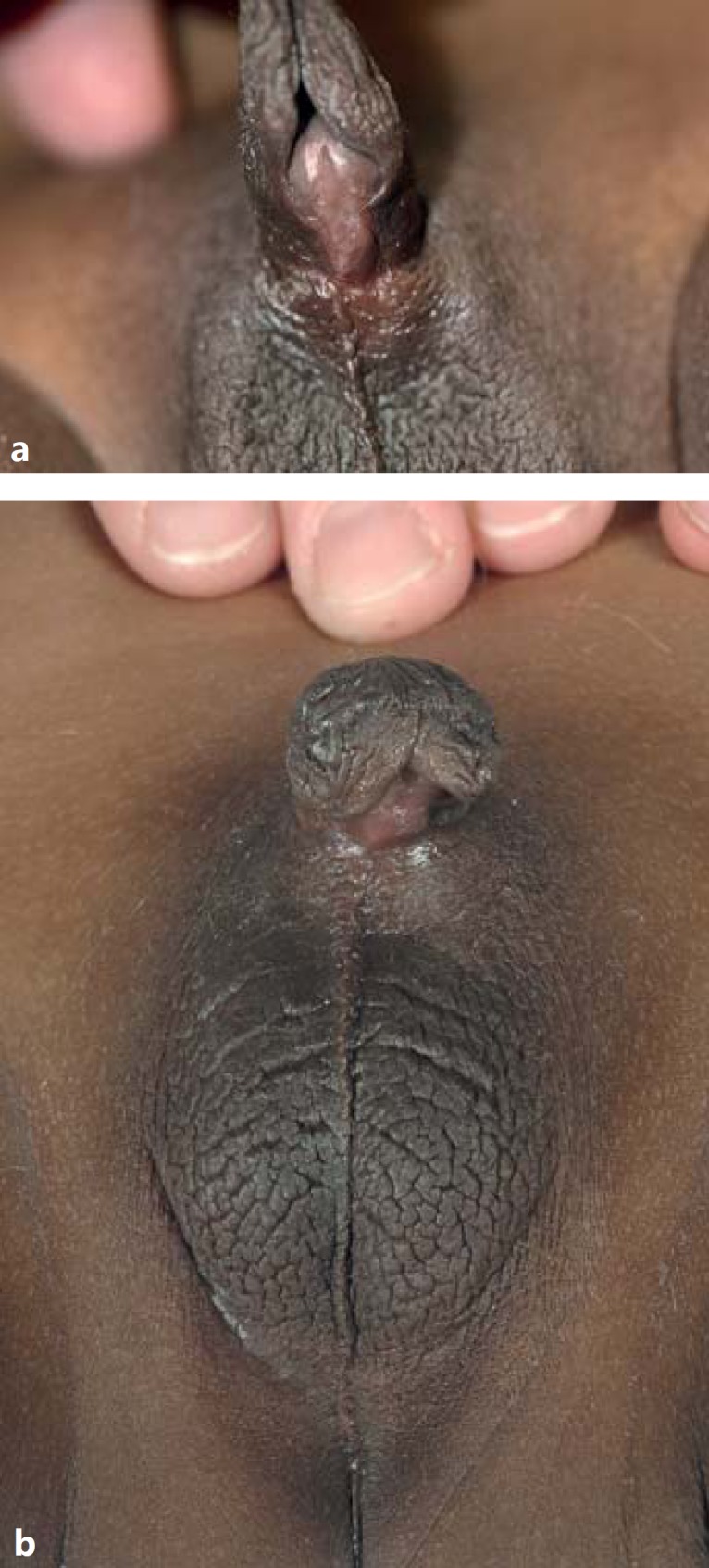
Genitalia of the patient before (a) and after surgical correction (b).
Fig. 2.
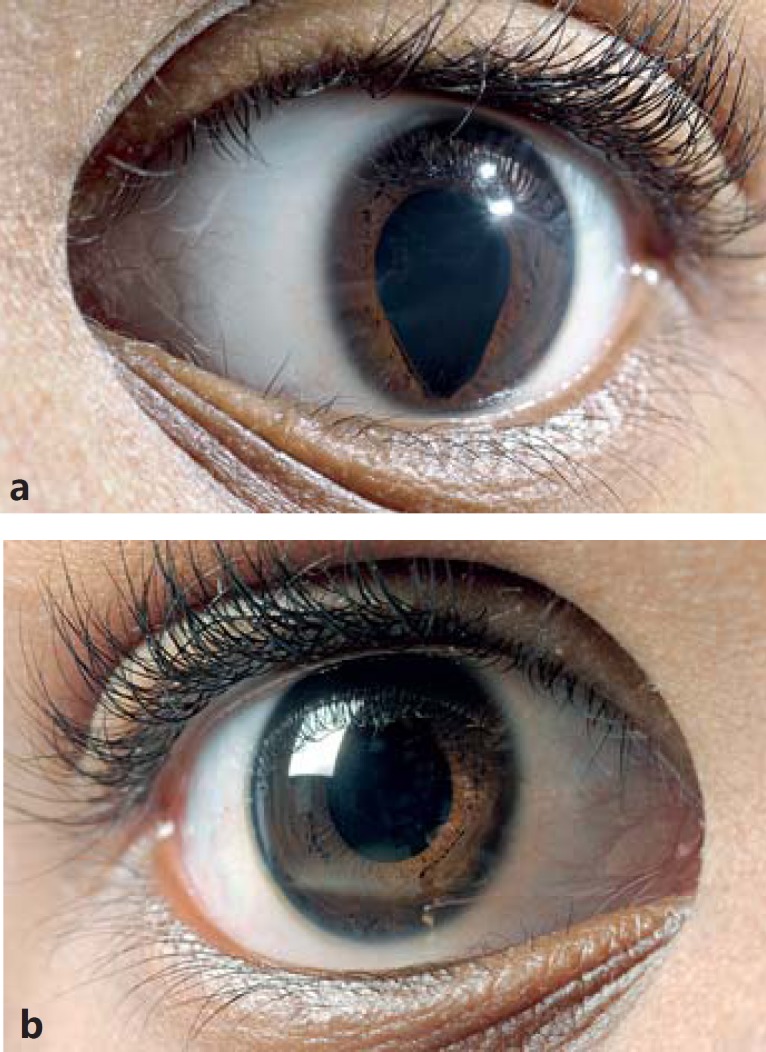
Coloboma of the left eye (a) and the normal right eye (b).
Additional Patients and Control Individuals
Six other patients with penoscrotal hypospadias were included, with ages between 8 months and 9.5 years. Genital skin fibroblasts from 3 patients with other genital anomalies, but without hypospadias, were used as controls. Fibroblasts cultured from arm or leg biopsies in 4 patients without genital anomalies but with suspicion of a metabolic disorder served as another set of controls.
Materials and Methods
Surgical Procedure
At the start, a meatoplasty was performed by incision in the midline of the distal urethral plate and transverse closure (Heineke-Mikulicz procedure). Subsequently, a standard meatal advancement and glanduloplasty procedure was performed with mobilization of glans tissue and tubularization around a stent sized 12 French. A biopsy of the penile skin was taken at the tip of the non-closed foreskin. Subsequently, the opened foreskin was reconstructed. The inner and outer parts of the foreskin were separated surgically. The inner layer and the tunica dartos were closed over the first layer. Finally, the outer layer of the foreskin reconstruction was closed, together with the penile skin.
Cell Culture, Karyotyping, FISH, and SNP-Array Analyses
Culturing of fibroblasts and blood lymphocytes as well as metaphase karyotyping was performed according to standard procedures. Skin biopsies were sliced into small pieces which were used for 2 independent simultaneous fibroblast cultures (A and B). Microscope slides for G-banding were made from the first passage of both cultures. We used FISH on interphases to investigate the presence of tetraploid cells in fibroblasts and cells from buccal mucosa, and urine sediment, using 3-color FISH with Vysis/Abbott DNA-probes for loci DXZ1, DYZ3, and D18Z1 [Liehr and Claussen, 2002]. SNP-array copy number profiling and analysis of regions of homozygosity were performed according to standard methods using the Infinium Human Omni Express Exome BeadChip (Illumina, San Diego, Calif., USA). Subsequently, visualizations of SNP-array results and data analysis were performed using Nexus 7.5 software (BioDiscovery, Los Angeles, Calif., USA). This platform is able to identify mosaic gains, losses, or chimerism when present in at least 5% of the cells (= 2.5% of the alleles). The results were classified with Cartagenia BENCH software (Cartagenia, Leuven, Belgium).
Results
Cytogenetic Studies in Our Patient
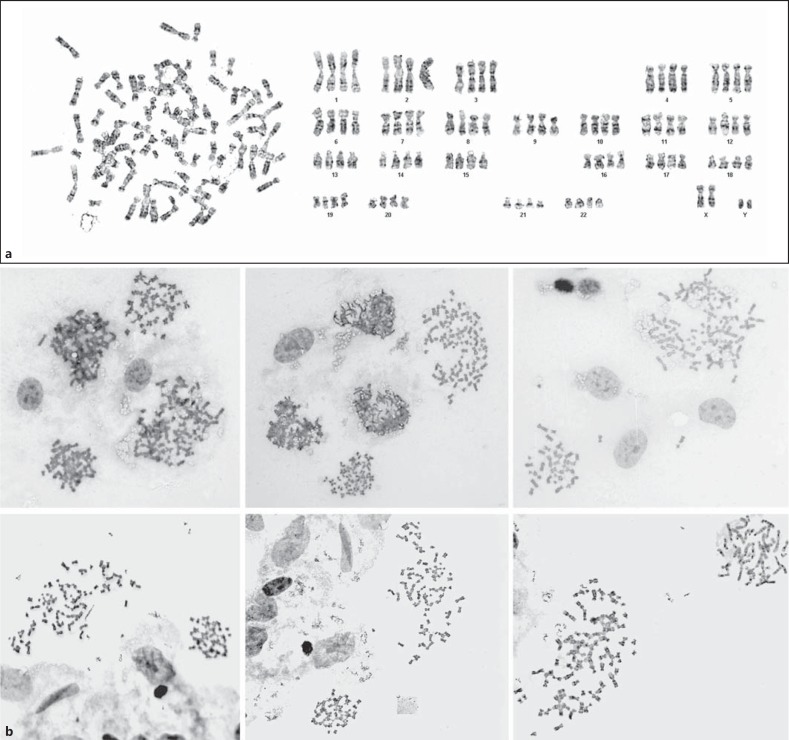
The cytogenetic data are summarized in table 1. Karyotyping revealed 57% tetraploid metaphases in culture A (100 metaphases investigated) and 19% in culture B (32 metaphases). After analysis of a standard number of 32 cells, tetraploid mosaicism was concluded in both cultures; however, the number of metaphases analyzed was extended to 100 only in culture A, whereas in retrospect, it would have been conceivable to do this in both cultures. The other metaphases had a normal male 46,XY karyotype. Representative metaphase spreads of both cultures, showing intermingling of 92,XXYY and 46,XY cells, are shown in figure 3. Because the original biopsy was entirely used for initiating the primary cultures, it was not possible to investigate uncultured cells using interphase FISH. So in future studies, it may be useful to store an uncultured skin fragment for possible interphase analysis. Karyotyping of blood lymphocytes (n = 400), interphase FISH of buccal mucosa cells (n = 100), and urine sediment cells (n = 100) was consistent with 46,XY in all cells. To exclude chimerism as the cause of the tetraploidy, SNP-array analysis of DNA isolated from cells in culture A was performed. No evidence was obtained for the presence of additional alleles, which would have been indicative for chimerism (detection limit ∼5% tetraploid cells).
Table 1.
Summary of cytogenetic findings in patients with hypospadias and controls
| Cases | Age at corrective surgery | Fibroblasts from penile, non-closed foreskin biopsy |
Peripheral blood lymphocyte metaphases | Buccal mucosa cell metaphases | Urine sediment cell interphases | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| metaphase culture A |
metaphase culture B |
||||||||||||
| cells | tetraploidy | p | cells | tetraploidy | p | cells | tetraploidy | cells | tetraploidy | cells | tetraploidy | ||
| 1 | 6 ys | 100 | 57% | <0.00001 | 32 | 19% | 0.024 | 400 | 0% | 100 | 0% | 100 | 0% |
| 2 | 12 m | 100 | 4% | 0.751 | 100 | 1% | 0.212 | 200 | 0% | n.d. | n.d. | 100 | 0% |
| 3 | 13 m | 132 | 10% | 0.219 | 25 | 4% | 0.746 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4 | 2 ys 7 m | 100 | 4% | 0.751 | 100 | 1% | 0.212 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 5 | 9 ys 6 m | 100 | 11% | 0.096 | 100 | 5% | 1 | 32 | 0% | n.d. | n.d. | n.d. | n.d. |
| 6 | 8 m | 100 | 1% | 0.212 | 100 | 1% | 0.212 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 7 | 12 m | 100 | 7% | 0.384 | 62 | 1.6% | 0.948 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
p values were determined by the Fisher's exact test under the assumption that 5 out of 100 metaphases in cultured fibroblasts from any skin biopsy would be tetraploid. Values in bold are significantly aberrant. m = Months; n.d. = not determined; ys = years.
Fig. 3.
a 92,XXYY metaphase from genital skin biopsy. b Representative fields from 2 fibroblast cultures (upper and lower row, respectively) that were established using the hypospadias biopsy of our first patient, showing mosaicism of diploid and tetraploid metaphases in both cultures.
Cytogenetic Studies in Additional Patients with Hypospadias and in Controls
To replicate the finding of tetraploid mosaicism in our patient, we studied genital fibroblasts of 6 additional patients with penoscrotal hypospadias. Indeed, tetraploid cells were observed, although at much lower levels (1-11%). In 3 of those patients (case 2, 6, and 7), we also studied uncultured interphase fibroblasts and found no tetraploid cells (data not shown). We also studied 2 types of controls. First, we karyotyped cultured fibroblasts from genital skin biopsies from 3 male patients with no hypospadias but other genital anomalies (table 2). At least 100 metaphases were studied, and in these patients, low levels of tetraploid cells were also found (1.5, 4, and 5%, respectively). Second, we took skin biopsies from the arm (n = 3) or leg (n = 1) from male controls with no hypospadias or any other genital anomalies and also found tetraploid fibroblast metaphases at frequencies varying from 1 to 5% (table 3). Compared to the highest level of 5% tetraploid metaphases in controls, comparable to earlier reports by others [Mittwoch et al., 1965; Danes, 1976; Annerén, 1982], the frequencies of tetraploid metaphases found in our patient were significantly higher (57%, p < 0.00001 and 19%, p = 0.024, respectively). The maximum frequency of 11% tetraploidy found in other patients with hypospadias did not reach statistical significance (p = 0.096).
Table 2.
Genital skin biopsies from males with no hypopadias
| Cases | Cells | Tetraploidy |
|---|---|---|
| 1 | 150 | 4% |
| 2 | 100 | 5% |
| 3 | 132 | 1.5% |
Table 3.
Skin biopsies from arm or leg from males with no hypopadias
| Cases | Cells | Tetraploidy |
|---|---|---|
| 1 | 200 | 1% |
| 2 | 200 | 5% |
| 3 | 50 | 4% |
| 4 | 200 | 1% |
Discussion
In this study, we present a patient with penoscrotal hypospadias and an iris coloboma of the left eye. Two fibroblast cultures from a biopsy of the penile skin taken at the tip of the non-closed foreskin revealed 57 and 19% tetraploid cells, respectively. Apparently, the percentages of tetraploid cells can be quite different between one spot and another. Because the entire biopsy was used for initiating fibroblast cultures, it was not possible to directly demonstrate the presence of tetraploid cells in tissue sections or in uncultured material. However, the percentages of tetraploid cells are significantly higher than the maximum of 5% tetraploid cells in cultured fibroblasts from control subjects with no hypospadias, either from genital skin or from skin of arms or legs. Our patient may be an exceptional case because in other patients with hypospadias, we found frequencies varying from 1 to 11% tetraploid metaphases in biopsies taken during surgical correction from non-closed penile skin. Under the assumption that 5% tetraploid cells can be found in every fibroblast culture, the frequency of 11% tetraploid cells is below the significance level (p = 0.096).
Given the considerable variation of tetraploid cells between the different biopsies and given the absence of tetraploid cells in interphases of some other tissues, we considered an artifact as the cause of the tetraploidy. However, there are several arguments against this hypothesis. First, in more than 30 years in which over 1,500 fibroblast cultures were performed, we never observed such a high level of tetraploid mosaicism. Culture conditions including temperature and carbon dioxide are always kept at constant levels. At least one parallel culture from another patient did not show any tetraploid metaphases.
Tetraploidy usually is the result of aberrant cell division (mitotic slippage or cytokinesis failure) [Storchova and Kufer, 2008]. SNP-array analysis in our patient's fibroblasts gave no evidence for additional sets of alleles. This excludes chimerism and indicates that a defect in cell division also was the cause of the mosaicism in this case. This defect in cell division at its turn may be due to a mosaic single gene mutation. For example, Yan et al. [2014] demonstrated the presence of tetraploid cells in cells with defects in one of the genes involved in the 3M complex which controls microtubule integrity.
Thus, although the presence of only low percentages of tetraploid cells in penile skin biopsies from 6 other patients with hypospadias suggests that our first patient represents a sporadic, exceptional case, we consider a role of tetraploid mosaicism in the etiology of hypospadias not as totally impossible. First, Stefanova et al. [2010] reported a renal/urinary tract anomaly and/or genital ambiguity in 1 out of 14 patients with tetraploid mosaicism and in 8 of 10 patients with complete tetraploidy. Second, biological pathway analysis of genes associated with hypospadias points to fibroblasts as the cell type of particular relevance, since fibroblasts play a key role in the closing of the urethral groove [Geller et al., 2014]. We speculate that the mixture of diploid and tetraploid fibroblasts in the fusing epithelial edges of the urethral folds disturbs the normal fusion, remodeling, and migration of epithelial cells during closure of the urethral groove [cf. Baskin et al., 2001], thereby leading to hypospadias. This may be due to the reduced proliferative capabilities of tetraploid cells that arise from normal diploid cells [Storchova and Kuffer, 2008; Ito et al., 2013; Ganem et al. 2014] and possibly also due to a disturbed interaction with neighboring cells, similar to tetraploid aortic endothelial cells which show both a reduced growth rate and upregulation of genes involved in extracellular matrix remodeling [Borradaile and Pickering, 2010]. Additional studies in other patients are required to further substantiate these hypotheses.
Recent studies in mice show that disrupting the androgen receptor of the genital tubercle at different developmental stages can produce hypospadias, confirming that hypospadias may be caused by a dysfunction of molecules at tissue (mosaic) level [Zheng et al., 2015].
Alternatively, it is conceivable that one or more unknown factors predispose both to hypospadias and tetraploidy in vitro. If so, tetraploidy would rather be an epiphenomenon.
It is unclear whether the iris coloboma of our patient is associated with tetraploid mosaicism, since such an abnormality was reported only once in another patient with tetraploid mosaicism [Witwer and Witwer, 1985] and in 2 out of 10 patients with complete tetraploidy [Stefanova et al., 2010].
In patients with hypospadias, studies using urogenital biopsies have only rarely been performed. Therefore, tetraploid mosaicism may be an underestimated feature of hypospadias and perhaps other congenital anomalies of the urogenital tract. In recent years, classical karyotyping has increasingly been replaced by array technology (array-CGH, SNP-array) and next-generation sequencing (i.e., whole exome sequencing and whole genome sequencing) as diagnostic tools in patients with multiple congenital anomalies and/or mental retardation [Kloosterman and Hochstenbach, 2014]. Despite their diagnostic power, these approaches are not suited for identifying non-chimeric tetraploid mosaicism. Moreover, it is evident that applying these techniques to DNA derived from lymphocytes does not allow detecting abnormalities only present in other tissues. Hence, our data illustrate that it may be useful to perform genetic investigations in other tissues than blood and that non-chimeric (mosaic) tetraploidy can be revealed by karyotyping, but not by array-based techniques. Also, with other state-of-the-art molecular techniques without demonstration of the total amount of DNA per cell, such as next-generation sequencing, non-chimeric (mosaic) tetraploidy cannot be identified.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank Dr. Martin Poot for his constructive criticism, the technicians of the cytogenetics laboratory for their expert assistance, and Dr. Wigard Kloosterman and Dr. Zusanna Storchova for discussion.
References
- 1.Annerén G. Increased frequency of tetraploidy in cultured skin fibroblasts from extremities with reduction of malformations. Hereditas. 1982;96:255–259. doi: 10.1111/j.1601-5223.1982.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 2.Baskin LS. Hypospadias. Adv Exp Med Biol. 2004;545:3–22. doi: 10.1007/978-1-4419-8995-6_1. [DOI] [PubMed] [Google Scholar]
- 3.Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR. Urethral seam formation and hypospadias. Cell Tissue Res. 2001;305:379–387. doi: 10.1007/s004410000345. [DOI] [PubMed] [Google Scholar]
- 4.Biesterfeld S, Gerres K, Fischer-Wein G, Böcking A. Polyploidy in non-neoplastic tissues. J Clin Pathol. 1994;47:38–42. doi: 10.1136/jcp.47.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borradaile NM, Pickering JG. Polyploidy impairs human aortic endothelial cell function and is prevented by nicotinamide phosphoribosyltransferase. Am J Physiol Cell Physiol. 2010;298:C66–C74. doi: 10.1152/ajpcell.00357.2009. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael SL, Shaw GM, Lammer EJ. Environmental and genetic contributors to hypospadias: a review of the epidemiologic evidence. Birth Defects Res A Clin Mol Teratol. 2012;94:499–510. doi: 10.1002/bdra.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danes BS. The Gardner syndrome: increased tetraploidy in cultured skin fibroblast. J Med Genet. 1976;13:52–56. doi: 10.1136/jmg.13.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Ganem NJ, Cornils H, Chiu SY, O'Rourke KP, Arnaud J, et al. Cytokinesis failure triggers Hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geller F, Feenstra B, Carstensen L, Pers TH, van Rooij IALM, et al. Genome-wide association analyses identify variants in developmental genes associated with hypospadias. Nat Genet. 2014;46:957–963. doi: 10.1038/ng.3063. [DOI] [PubMed] [Google Scholar]
- 11.Ito H, Oga A, Furuya T, Ikemoto K, Amakawa G, et al. Elucidation of proliferative capability of mononuclear tetraploid cells, emerging spontaneously from diploid cells, using image cytometry and fluorescence in situ hybridization. Cel Prolif. 2013;46:356–363. doi: 10.1111/cpr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloosterman WP, Hochstenbach R. Deciphering the pathogenic consequences of chromosomal aberrations in human genetic disease. Mol Cytogenet. 2014;7:100. doi: 10.1186/s13039-014-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Willingham E, Baskin LS. Gene expression profiles in mouse urethral development. BJU Int. 2006;98:880–885. doi: 10.1111/j.1464-410X.2006.06435.x. [DOI] [PubMed] [Google Scholar]
- 14.Liehr T, Claussen U. FISH on chromosome preparations of peripheral blood. In: Rautenstrauss B, Liehr T, editors. FISH Technology. Berlin: Springer; 2002. pp. 73–81. [Google Scholar]
- 15.Mittwoch U, Lele KP, Webster WS. Deoxyribonuceic acid synthesis in cultured human cells and its bearing on the concepts of endoreduplication and polyploidy. Nature. 1965;208:242–244. doi: 10.1038/208242a0. [DOI] [PubMed] [Google Scholar]
- 16.Olgun-Erdemir E, Yildirim RS, Karşiyaka M. Generalized aggressive peridontitis in a child with 92,XXYY/46,XY mosaicism: report of a second case. Turk J Pediatr. 2010;52:94–96. [PubMed] [Google Scholar]
- 17.Rojanasakul AK, Gustavson H, Lithell H, Nilius SJ. Tetraploidy in two sister with polycystic ovary syndrome. Clin Genet. 1985;27:167–174. doi: 10.1111/j.1399-0004.1985.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 18.Rooney DE, Czepulkowski BH, editors. Constitutional Analysis. I. Oxford: IRL Press; 1992. Human Cytogenetics: A Practical Approach. [Google Scholar]
- 19.Scarbrough PR, Hersch J, Kukolich MK, Carrol AJ, Finley SC, et al. Tetraploidy: a report of three live-born infants. Am J Med Genet. 1984;19:29–37. doi: 10.1002/ajmg.1320190106. [DOI] [PubMed] [Google Scholar]
- 20.Schinzel A. Catalogue of Unbalanced Chromosome Aberrations in Man. ed 2. Berlin: De Gruyter; 2001. [Google Scholar]
- 21.Sperling K, Saling E. Prenatal chromosome analysis with mosaic statement 46,XX-92,XXXX. Possibility of an erroneous diagnosis [in German]. Humangenetik. 1971;11:139–145. doi: 10.1007/BF00393794. [DOI] [PubMed] [Google Scholar]
- 22.Stefanova I, Jenderny J, Kaminsky E, Mannhardt A, Meinecke P, et al. Mosaic and complete tetraploidy in live-born infants: two new patients and review of the literature. Clin Dysmorphol. 2010;19:123–127. doi: 10.1097/MCD.0b013e3283353877. [DOI] [PubMed] [Google Scholar]
- 23.Storchova Z, Kuffer C. The consequences of tetraploidy and aneuploidy. J Cell Sci. 2008;121:3859–3866. doi: 10.1242/jcs.039537. [DOI] [PubMed] [Google Scholar]
- 24.Sundvall L, Lund H, Niemann I, Jensen U, Bolund L, Sunde L. Tetraploidy in hydatidiform moles. Hum Reprod. 2013;28:2010–2020. doi: 10.1093/humrep/det132. [DOI] [PubMed] [Google Scholar]
- 25.Tözüm TF, Berker E, Akincibay H, Ozer O, Aktaş O, et al. Tetraploid/diploid mosaicism with generalized aggressive peridontitis. J Peridontol. 2005;76:1567–1571. doi: 10.1902/jop.2005.76.9.1567. [DOI] [PubMed] [Google Scholar]
- 26.van der Zanden LF, van Rooij IALM, Feitz WFJ, Franke B, Knoers NVAM, Roeleveld N. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update. 2012;18:260–283. doi: 10.1093/humupd/dms002. [DOI] [PubMed] [Google Scholar]
- 27.Witwer BB, Witwer HB. Informations about diploid-tetraploid mosaicism in a six-year-old male. Clin Genet. 1985;28:567–568. doi: 10.1111/j.1399-0004.1985.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 28.Yan J, Yan F, Li Z, Sinnot B, Cappell KM, et al. The 3M complex maintains microtubule and genome integrity. Mol Cell. 2014;54:791–804. doi: 10.1016/j.molcel.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Z, Armfield BA, Cohn MJ. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc Natl Acad Sci USA. 2015;112:E7194–E7203. doi: 10.1073/pnas.1515981112. [DOI] [PMC free article] [PubMed] [Google Scholar]