Abstract
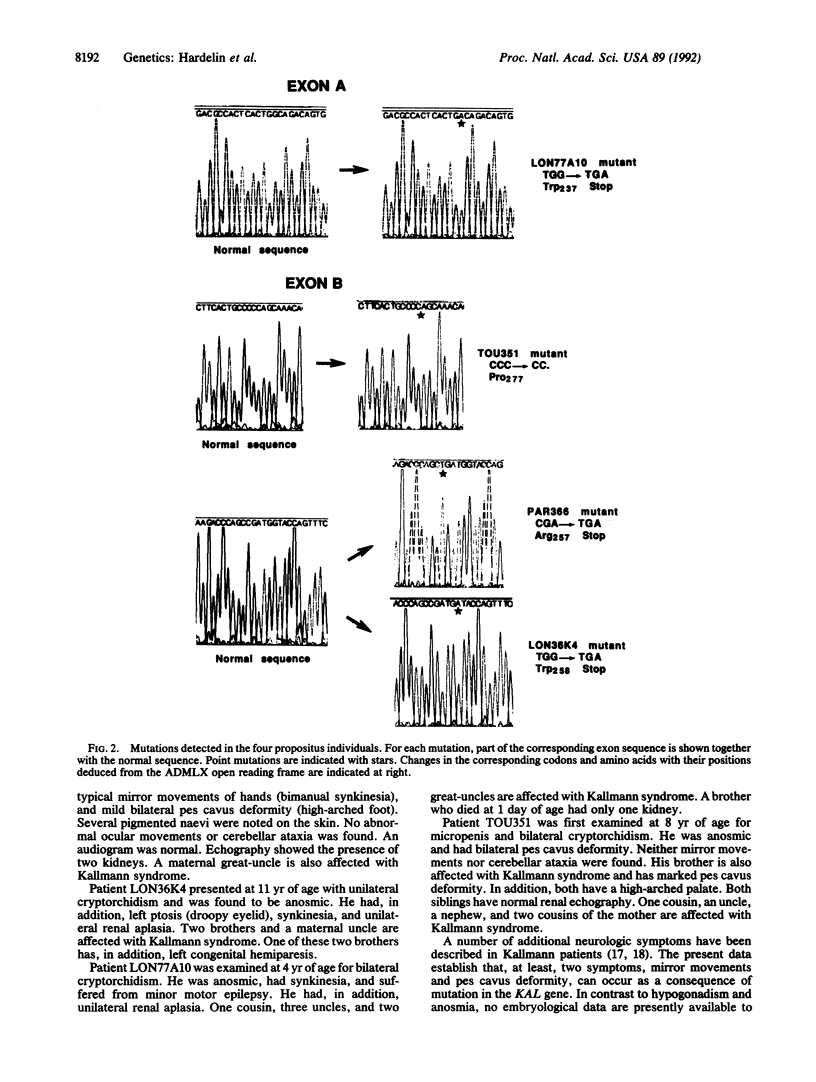
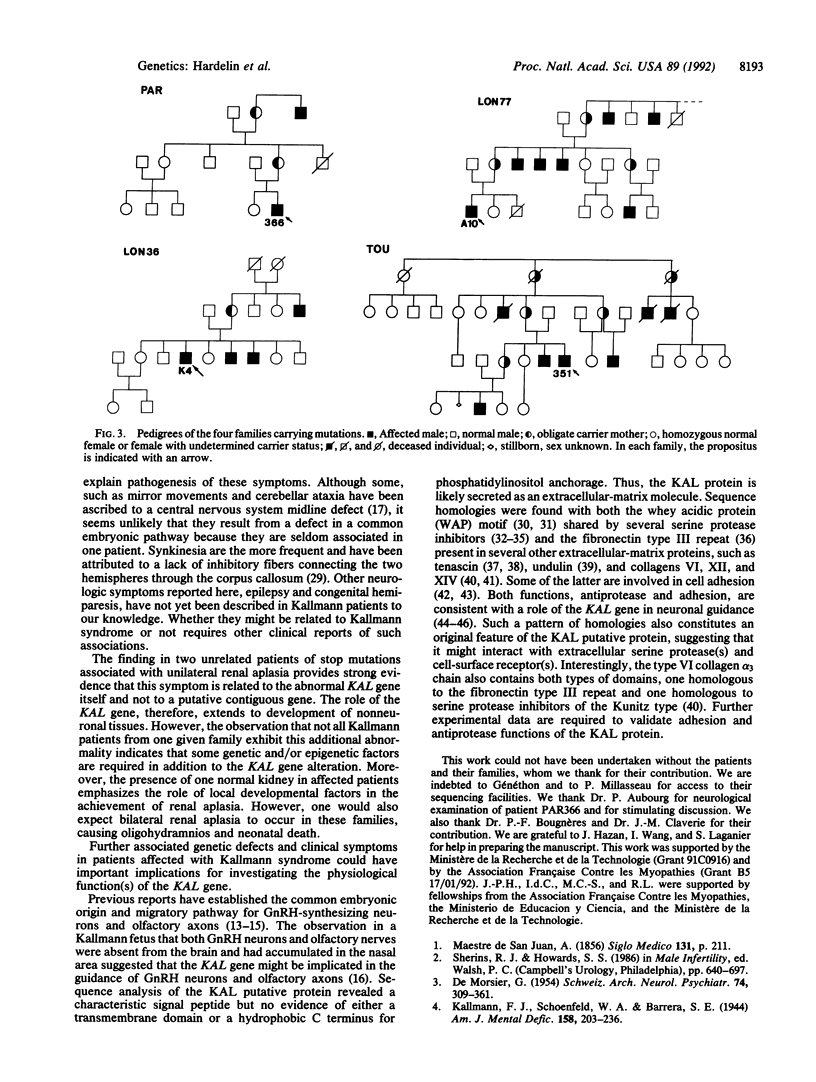
Kallmann syndrome represents the association of hypogonadotropic hypogonadism with anosmia. This syndrome is from a defect in the embryonic migratory pathway of gonadotropin-releasing hormone synthesizing neurons and olfactory axons. A candidate gene for the X chromosome-linked form of the syndrome was recently isolated by using a positional cloning strategy based on deletion mapping in the Xp22.3 region. With the PCR, two exons of this candidate gene were amplified on the genomic DNAs from 18 unrelated patients affected with the X chromosome-linked Kallmann syndrome. Three different base transitions--all leading to a stop codon--and one single-base deletion responsible for a frameshift were identified. We thus conclude that the candidate gene is the actual KAL gene responsible for the X chromosome-linked Kallmann syndrome. Furthermore, unilateral renal aplasia in two unrelated patients carrying a stop mutation indicates that the KAL gene is itself responsible for this Kallmann syndrome-associated anomaly. The gene is, therefore, also involved in kidney organogenesis. Additional neurologic symptoms in Kallmann patients are also discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballabio A., Sebastio G., Carrozzo R., Parenti G., Piccirillo A., Persico M. G., Andria G. Deletions of the steroid sulphatase gene in "classical" X-linked ichthyosis and in X-linked ichthyosis associated with Kallmann syndrome. Hum Genet. 1987 Dec;77(4):338–341. doi: 10.1007/BF00291422. [DOI] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussain J. L., Toublanc J. E., Feingold J., Naud C., Vassal J., Job J. C. Mode of inheritance in familial cases of primary gonadotropic deficiency. Horm Res. 1988;29(5-6):202–206. doi: 10.1159/000181003. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Zhang R. Z., Pan T. C., Stokes D., Conway D., Kuo H. J., Glanville R., Mayer U., Mann K., Deutzmann R. Mosaic structure of globular domains in the human type VI collagen alpha 3 chain: similarity to von Willebrand factor, fibronectin, actin, salivary proteins and aprotinin type protease inhibitors. EMBO J. 1990 Feb;9(2):385–393. doi: 10.1002/j.1460-2075.1990.tb08122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MORSIER G. Etudes sur les dysraphies crânio-encéphaliques. I. Agénésie des lobes olfactifs (télencéphaloschizis latéral) et des commissures calleuse et antérieure (télencéphaloschizis médian); la dysplasie olfacto-génitale. Schweiz Arch Neurol Psychiatr. 1954;74(1-2):309–361. [PubMed] [Google Scholar]
- Dandekar A. M., Robinson E. A., Appella E., Qasba P. K. Complete sequence analysis of cDNA clones encoding rat whey phosphoprotein: homology to a protease inhibitor. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3987–3991. doi: 10.1073/pnas.79.13.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B., Guioli S., Pragliola A., Incerti B., Bardoni B., Tonlorenzi R., Carrozzo R., Maestrini E., Pieretti M., Taillon-Miller P. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991 Oct 10;353(6344):529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. Mouse whey acidic protein is a novel member of the family of 'four-disulfide core' proteins. Nucleic Acids Res. 1982 Apr 24;10(8):2677–2684. doi: 10.1093/nar/10.8.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Lander A. D. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992 Jan 24;68(2):303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. R., Kemmann E. Olfacto-genital dysplasia in the female. Obstet Gynecol Annu. 1976;5:443–466. [PubMed] [Google Scholar]
- Just M., Herbst H., Hummel M., Dürkop H., Tripier D., Stein H., Schuppan D. Undulin is a novel member of the fibronectin-tenascin family of extracellular matrix glycoproteins. J Biol Chem. 1991 Sep 15;266(26):17326–17332. [PubMed] [Google Scholar]
- Kertzman C., Robinson D. L., Sherins R. J., Schwankhaus J. D., McClurkin J. W. Abnormalities in visual spatial attention in men with mirror movements associated with isolated hypogonadotropic hypogonadism. Neurology. 1990 Jul;40(7):1057–1063. doi: 10.1212/wnl.40.7.1057. [DOI] [PubMed] [Google Scholar]
- Legouis R., Hardelin J. P., Levilliers J., Claverie J. M., Compain S., Wunderle V., Millasseau P., Le Paslier D., Cohen D., Caterina D. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991 Oct 18;67(2):423–435. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- Monard D. Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends Neurosci. 1988 Dec;11(12):541–544. doi: 10.1016/0166-2236(88)90182-8. [DOI] [PubMed] [Google Scholar]
- Nass R. Mirror movement asymmetries in congenital hemiparesis: the inhibition hypothesis revisited. Neurology. 1985 Jul;35(7):1059–1062. doi: 10.1212/wnl.35.7.1059. [DOI] [PubMed] [Google Scholar]
- Pearson C. A., Pearson D., Shibahara S., Hofsteenge J., Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta. EMBO J. 1988 Oct;7(10):2977–2982. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Weissenbach J. Long-range restriction map of the terminal part of the short arm of the human X chromosome. Proc Natl Acad Sci U S A. 1990 May;87(10):3680–3684. doi: 10.1073/pnas.87.10.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Weissenbach J. Physical mapping of the human pseudo-autosomal region; comparison with genetic linkage map. EMBO J. 1988 Aug;7(8):2369–2376. doi: 10.1002/j.1460-2075.1988.tb03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F., Tomaselli K. J. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv O. K., Resko J. A. Ontogeny of gonadotropin-releasing hormone-containing neurons in early fetal development of rhesus macaques. Endocrinology. 1990 Jan;126(1):498–511. doi: 10.1210/endo-126-1-498. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen R. J., Paulsen C. A. Hypogonadotropic eunuchoidism. I. Clinical study of the mode of inheritance. J Clin Endocrinol Metab. 1973 Jan;36(1):47–54. doi: 10.1210/jcem-36-1-47. [DOI] [PubMed] [Google Scholar]
- Schwankhaus J. D., Currie J., Jaffe M. J., Rose S. R., Sherins R. J. Neurologic findings in men with isolated hypogonadotropic hypogonadism. Neurology. 1989 Feb;39(2 Pt 1):223–226. doi: 10.1212/wnl.39.2.223. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Bick D., Pfaff D. W. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989 Dec;6(4):311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Pfaff D. W. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989 Mar 9;338(6211):161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Seemüller U., Arnhold M., Fritz H., Wiedenmann K., Machleidt W., Heinzel R., Appelhans H., Gassen H. G., Lottspeich F. The acid-stable proteinase inhibitor of human mucous secretions (HUSI-I, antileukoprotease). Complete amino acid sequence as revealed by protein and cDNA sequencing and structural homology to whey proteins and Red Sea turtle proteinase inhibitor. FEBS Lett. 1986 Apr 7;199(1):43–48. doi: 10.1016/0014-5793(86)81220-0. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Shapiro M. B., Harris N. L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Simpson R. W., Paulsen C. A. Familial hypogonadotropic hypogonadism with anosmia. Arch Intern Med. 1968 Jun;121(6):534–538. [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Stetler G., Brewer M. T., Thompson R. C. Isolation and sequence of a human gene encoding a potent inhibitor of leukocyte proteases. Nucleic Acids Res. 1986 Oct 24;14(20):7883–7896. doi: 10.1093/nar/14.20.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegenke J. D., Uehling D. T., Wear J. B., Jr, Gordon E. S., Bargman J. G., Deacon J. S., Herrmann J. P., Opitz J. M. Familial Kallmann syndrome with unilateral renal aplasia. Clin Genet. 1975 May-Jun;7(5):368–381. doi: 10.1111/j.1399-0004.1975.tb00344.x. [DOI] [PubMed] [Google Scholar]
- White B. J., Rogol A. D., Brown K. S., Lieblich J. M., Rosen S. W. The syndrome of anosmia with hypogonadotropic hypogonadism: a genetic study of 18 new families and a review. Am J Med Genet. 1983 Jul;15(3):417–435. doi: 10.1002/ajmg.1320150307. [DOI] [PubMed] [Google Scholar]
- Wiedow O., Schröder J. M., Gregory H., Young J. A., Christophers E. Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1990 Sep 5;265(25):14791–14795. [PubMed] [Google Scholar]
- Wray S., Grant P., Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M., Yamada K. M., Yamada S. S., Shinomura T., Tanaka H., Nishida Y., Obara M., Kimata K. The complete primary structure of type XII collagen shows a chimeric molecule with reiterated fibronectin type III motifs, von Willebrand factor A motifs, a domain homologous to a noncollagenous region of type IX collagen, and short collagenous domains with an Arg-Gly-Asp site. J Cell Biol. 1991 Oct;115(1):209–221. doi: 10.1083/jcb.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]



