Abstract
AIM
To investigate anti-hypersensitive effects of α2δ-1 ligands in non-inflammatory and inflammation-associated colonic hypersensitivity (CHS) mouse models.
METHODS
To induce an inflammation-associated CHS, 1% dextran sulfate sodium (DSS) was administered to C57Bl/6J male mice, in drinking water, for 14 d. Regarding the non-inflammatory neonatal maternal separation (NMS) -induced CHS model, wild-type C57BI/6J pups were isolated from their mother from day 2 to day 14 (P2 to P14), three hours per day (from 9:00 a.m. to 12:00 p.m.). Colorectal distension was performed by inflating distension probe from 20 μL to 100 μL by 20 μL increment step every 10 s. After a first colorectal distension (CRD), drugs were administered subcutaneously, in a cumulative manner, (Gabapentin at 30 mg/kg and 100 mg/kg; Pregabalin at 10 mg/kg and 30 mg/kg; Carbamazepine at 10 mg/kg and 30 mg/kg) and a second CRD was performed one hour after each injection.
RESULTS
The visceromotor response (VMR) to CRD was increased by our NMS paradigm protocol in comparison to non-handled (NH) mice, considering the highest distension volumes (80 μL: 0.783 ± 0.056 mV/s vs 0.531 ± 0.034 mV/s, P < 0.05 and 100 μL: 1.087 ± 0.056 mV/s vs 0.634 ± 0.038 mV/s, P < 0.05 for NMS and NH mice, respectively). In the inflammation-associated CHS, DSS-treated mice showed a dramatic and significant increase in VMR at 60 and 80 μL distension volumes when compared to control mice (60 μL: 0.920 ± 0.079 mV/s vs 0.426 ± 0.100 mV/s P < 0.05 and 80 μL: 1.193 ± 0.097 mV/s vs 0.681 ± 0.094 mV/s P < 0.05 for DSS- and Water-treated mice, respectively). Carbamazepine failed to significantly reduce CHS in both models. Gabapentin significantly reduced CHS in the DSS-induced model for both subcutaneous injections at 30 or 100 mg/kg. Pregabalin significantly reduced VMR to CRD in the non-inflammatory NMS-induced CHS model for the acute subcutaneous administration of the highest cumulative dose (30 mg/kg) and significantly reduced CHS in low-dose DSS-treated mice in a dose-dependent manner. Finally, the percent decrease of AUC induced by acute GBP or Pregabalin treatment were higher in the inflammatory DSS-induced CHS model in comparison to the non-inflammatory NMS-induced CHS model.
CONCLUSION
This preclinical study demonstrates α2δ-1 ligands efficacy on inflammation-associated CHS, highlighting their potential clinical interest in patients with chronic abdominal pain and moderate intestinal inflammation.
Keywords: Neonatal maternal separation, Dextran sulfate sodium, Colonic hypersensitivity mouse models, Colorectal distension, α2δ-1 ligands
Core tip: Chronic abdominal pain is commonly reported by patients suffering from gastrointestinal disorders. These patients form a very heterogeneous group in regards to their colonic inflammation status. Such heterogeneity may explain the current lack of satisfactory options regarding pain management in these patients. This preclinical study shows that α2δ-1 ligands are effective against colonic hypersensitivity, especially when they are used in the case of moderate intestinal inflammation. Thus, our results pointed out the need for assessing the clinical benefits of α2δ-1 ligands on chronic abdominal pain based on the level of intestinal inflammation observed in patients.
INTRODUCTION
Visceral pain is a diffuse and stabbing sensation, which may result from functional visceral disorders or from inflammation of a visceral organ. Irritable bowel syndrome (IBS), a common functional gastrointestinal disorder characterized by Rome III criteria[1], is characterized by changes in bowel habits and development of colonic hypersensitivity (CHS) in the absence of macroscopic organic lesions. In contrast, inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are characterized by active phases with active mucosal inflammation ultimately leading to gut wall alterations[2]. These active phases are interspersed with remission periods, characterized by low-grade inflammation and IBS-like symptoms[3,4].
Patients suffering from IBS and IBD during active or remission phases often complain about abdominal pain[5,6]. This common symptom is a crucial feature because of his strong impairments on patient’s quality of life, in the absence of efficient therapies[3]. The modulation of intestinal sensory neurotransmission has been considered as a therapeutic approach in IBS patients. For many years, the ability pain of many potential targets expressed in intestinal sensory afferents to modulate abdominal has been tested in several preclinical models and in different clinical conditions[7,8]. Notably, tricyclic antidepressants and selective serotonin reuptake inhibitors have proved to be effective[9], but were associated with strong adverse effects such as sedation, central nervous system dysfunction or anticholinergic symptoms[10]. Inflammatory Bowel Diseases treatments mostly target inflammation either via administration of aminosalicylates compounds, glucocorticoids or anti-tumor necrosis factor-α antibodies strategy[11], or by reducing the immune response using immunosuppressive thiopurines azathioprine or 6-mercaptopurine[12,13]. However, to date, no therapy aiming to relieve CHS associated with IBD is currently available. In this context, development of innovative treatments is needed to alleviate abdominal pain in IBS patients and in IBD patients during the quiescent periods.
Anticonvulsants, initially designed to modulate GABAergic or glutamatergic systems, target neuronal excitability by modulating ion channels, receptors, and intracellular signaling pathways[14,15]. Among them, carbamazepine (CBZ) induces a decrease of sodium (Na+) channel conductance, whereas gabapentin (GBP) and pregabalin (PGB), a new class of anticonvulsants called α2δ-1 ligands, act on voltage-gated calcium (Ca2+) channels (Ca(v)) to inhibit pre-synaptic glutamate release[14,16,17]. Drugs designed as α2δ-1 ligands have provided new options for pain treatment, notably for neuropathic pain management[18,19]. Concerning colonic pain, all studies were focused on IBS pathology, which share some features with neuropathic pain, such as an increase in sensitivity, without clear macroscopic organic dysfunction. Some clinical studies have highlighted the beneficial effect of GBP[20] and PGB[21] on IBS-associated symptoms, and their analgesic activity was confirmed in rat models of non-inflammatory visceral pain[22-24]. Surprisingly, although their antihyperalgesic impact on somatic inflammatory pain has been widely described[25-28], the effects of these compounds have never been evaluated either in specific inflammatory colonic pain models or in IBD patients, among which 35% exhibit IBS-like symptoms[3].
In this context, this preclinical study investigates α2δ-1 ligand (GBP and PGB) beneficial effects on CHS, in different mouse models, associated or not to intestinal inflammation. They will be compared to those receiving another anticonvulsant drug, i.e., CBZ. The main question addressed by this preclinical study will be to describe the effect of α2δ-1 ligand compounds, in a CHS mouse model associated with a colonic inflammation induced by dextran sulfate sodium (DSS) administration compared those obtained in a non-inflammatory IBS-like model, induced by neonatal maternal separation (NMS).
MATERIALS AND METHODS
Drugs
Gabapentin (2-[1-(aminomethyl)cyclohexyl]acetic acid; Zhejiang Chiral Medicine Chemicals Co, Ltd, Hanghzou, China) and Pregabalin [(S)-(+)-3-(aminomethyl)-5-methylhexanoic acid; Pierre Fabre Laboratories, Castres, France] were dissolved in 0.9% saline. Carbamazepine [5-carbamoyl-5H-dibenzo(b,f)azépine; Sigma-Aldrich, Saint Quentin Fallavier, France] was suspended in 0.9% NaCl and sonicated for 45 min, to allow proper suspension.
Animal models of CHS
Regarding the non-inflammatory NMS-induced CHS model, C57Bl/6J pregnant mice were purchased from Janvier laboratories (Le Genest Saint Isle, France) and single-housed, up to birth of the pups. Briefly, wild-type C57BI/6J pups were isolated from their mother from day 2 to day 14 (P2 to P14), three hours per day (from 9:00 a.m. to 12:00 p.m.) and placed in individual temperature-regulated boxes (37 °C) in a separate room set up with similar environmental conditions, as previously described[29]. These mice, named NMS, were compared to non-handled (NH) mice, used as control (Figure 1A). Pups were then left with their mothers up to weaning (P21). All experiments were then performed on nine-week-old male mice.
Figure 1.
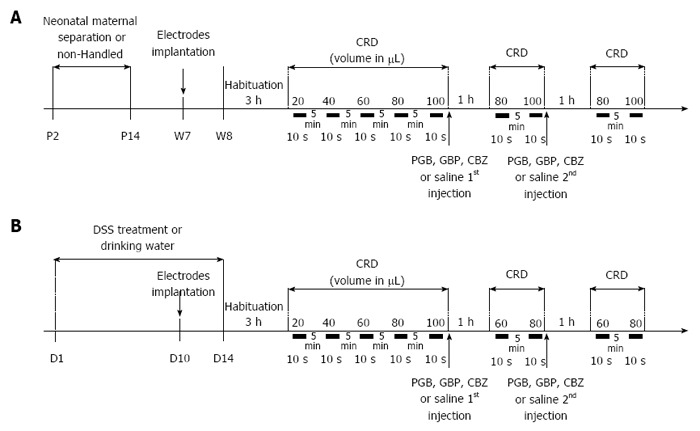
Experimental design of anticonvulsant treatment assessment on colonic hypersensitivity. A: Non-inflammatory hypersensitivity was induced by neonatal maternal separation from day 2 to day 14 (P2 to P14). At week seven, electrodes were surgically implanted in abdominal muscle. On week eight, mice were accustomed to restraint device for 3 h before probe insertion for the colorectal distension (CRD). Balloon was inflated from 20 to 100 μL to assess basal colonic sensitivity. Then mice were subcutaneously injected with a first dose of anticonvulsants [carbamazepine (CBZ) 10 mg/kg, gabapentin (GBP) 30 mg/kg or pregabalin (PGB) 10 mg/kg] or saline and colonic sensitivity was assessed 1 h later for the two distension volumes displaying the highest significant differences in visceromotor response (VMR) between control and sensitized groups (80 and 100 μL). A second subcutaneous injection was performed to reach the second dose of anticonvulsants (CBZ 30 mg/kg, GBP 100 mg/kg or PGB 30 mg/kg) or saline and the same protocol was repeated; B: A moderate intestinal inflammation was induced by replacing drinking water by 1% DSS at D1 for 14 d. At D10, electrodes were surgically implanted in abdominal muscle. On the last day of treatment (D14), mice were accustomed to restraint device for 3h before probe insertion for the CRD. Balloon was inflated from 20 to 100 μL to assess basal colonic sensitivity. Then mice were subcutaneously injected with a first dose of anticonvulsants (CBZ 10 mg/kg, GBP 30 mg/kg or PGB 10 mg/kg) or saline and colonic sensitivity was assessed 1h later for the two distension volumes displaying the highest significant differences in VMR between control and sensitized groups (60 and 80 μL). A second subcutaneous injection was performed to reach the second dose of anticonvulsants (CBZ 30 mg/kg, GBP 100 mg/kg or PGB 30 mg/kg) or saline and the same protocol was repeated. CRD: Colorectal distension.
Regarding the inflammatory DSS-induced CHS model, C57Bl/6J male mice weighing 20-24 g were purchased from Janvier laboratories (Le Genest Saint Isle, France), housed 8 per cage and allowed to acclimatize to the animal care facility for at least one week after their arrival and before any experiment. Briefly, inflammation-associated CHS induction in mice was adapted from Okayasu et al[30] by administering 1% DSS (MP Biomedicals, Illkirch, France) in drinking water for 14 days. Colorectal distension test was performed on the last day of treatment at D14 (Figure 1B).
All animals were housed in a temperature-controlled environment with a 12/12 h light/dark cycle and ad libitum access to food and water. All experiments were performed following the ethical guidelines set out by the International Association for the Study of Pain (IASP)[31], with EU guidelines and the regulations of the French Agriculture and Forestry Ministry (decree 874848) and with approval from local ethical committee (N° CE08-10 and N° CE09-10).
Colorectal distension and CHS measurement
Prior to colonic sensitivity measurement, mice were anaesthetized with an intraperitoneal injection (0.1 mL/10 g) of a Ketamine and Xylazine 2:1 (v/v) mixture diluted in 0.9% NaCl, the left abdominal external musculature was exposed by skin incision and two nickel-chromium electrodes (Nikrothal 80, Kanthal, Sweden) were implanted into the abdominal oblique muscle. Electrodes were secured with 5-0 sutures (Ethicon, Somerville, NJ, United States). The opposite electrode ends were externalized subcutaneously through an incision at the back of the neck and wrapped around a polyethylene catheter attached to the skin with three 3-0 sutures (Ethicon, Somerville, NJ, United States). Mice were allowed to recover at least five days before experiments.
On experimental day, mice were accustomed to the restraint device for 3 h before colorectal distension (CRD). They were then briefly anaesthetized (2% Isoflurane) in order to insert the distension probe (Fogarty Arterial Embolectomy Catheter 4, Edward Lifescience, CA, United States) at 1 cm from the anal margin, and a nickel-chromium reference electrode (Nikrothal 80, Kanthal, Sweden) was inserted subcutaneously in the tail. Animals were placed in home-made restriction cages, tape-maintained on the tail and allowed to recover for 30 min prior CRD. Electrodes were connected to a Bio-Amp® recording system (AD Instruments, Oxford, United Kingdom) linked to an acquisition interface device (Power-Lab®, AD Instruments, Oxford, United Kingdom). Electromyographic signal was amplified, filtered at 100 Hz, integrated and smoothed. Colorectal distension was performed by inflating the distension probe from 20 μL to 100 μL, by 20 μL increment step (Figure 1). A 5 min interval period was observed between stimulations. Recording was performed 10 s before stimulation (baseline) and 10 s during the stimulation period. Visceromotor responses were quantified as the area under the electromyogram activity curve during the 10 s stimulation period minus the area measured during the 10 s baseline. Treated mice displaying VMR values lower than the mean minus 2 SEM for all distension volumes were considered as non-sensitized and excluded from the analysis.
Effect of anticonvulsants on CHS
In order to reduce the number of animals included in the study, as recommended by ethical guidelines, all drugs were administered subcutaneously in a cumulative manner (Figure 1). Briefly, after basal assessment of visceral sensitivity, mice were subcutaneously injected with a first dose of anticonvulsants (GBP 30 mg/kg, PGB 10 mg/kg and CBZ 10 mg/kg) and their effects on colonic sensitivity were assessed 1h later concerning the two distension volumes displaying the highest significant differences in VMR, between control and sensitized groups (80 and 100 μL for NMS model and 60 and 80 μL for DSS model). A second subcutaneous injection was performed to reach the second dose of anticonvulsants (GBP 100 mg/kg, PGB 30 mg/kg and CBZ 30 mg/kg) and colonic sensitivity was assessed again 1h later with the same distension volume.
Tissue myeloperoxidase assay
A piece of colon (around 50 mg) was thoroughly washed in PBS and homogenized (50 mg/mL) in 0.5% hexadecyltrimethylammonium bromide (Sigma) in 50 mmol/L PBS, (pH 6.0), freeze-thawed 3 times, sonicated and centrifuged. Myeloperoxidase (MPO) was assayed in the supernatant by adding 1mg/mL of dianisidine dihydrochloride (Sigma) and 5 × 10%-5 × 4% H2O2 and the change in optical density measured at 450 nm. Human neutrophil MPO (Sigma) was used as standard. One unit of MPO activity was defined as the amount that degraded 1.0 μmol of peroxide/min at 25 °C[32].
Histology
Colons were fixed for 24 h in 4% buffered formalin at 4 °C and then subjected to hematoxylin and eosin staining on tissue sections of 5 μm thickness. Stained slides were scored, blindly from the study protocol as previously described[33]. Slides were scored for the presence/absence of active inflammation, the intensity of inflammation (average number of neutrophils and the number of fields that were involved), the extent of inflammation (mucosa, submucosa, or serosa), the presence or absence of ulceration, architectural disarray, and the pattern of involvement.
Statistical analysis
All data were expressed as mean ± SE. Statistical analyses were performed with GraphPad Prism software. For VMR analysis in model validation, a two-way (Volume and Treatment) ANOVA followed by Bonferroni post-hoc test for multiple comparisons were used. For the effects of anticonvulsants on VMR, a one-way (Treatment) ANOVA followed by Bonferroni post-hoc test for multiple comparisons were used. A P value less than 0.05 was considered statistically significant.
RESULTS
Effect of acute treatment with α2δ-1 ligands in a non-inflammatory NMS-induced CHS model
As shown in Figure 2A, the VMR to CRD of both mouse groups, NH (n = 17) and NMS (n = 26) mice, gradually increased in response to balloon inflation. Three mice were excluded after our NMS paradigm from the analysis because they were considered as non-sensitized (see material and methods) (Figure 2A, grey circles). Thus, among NMS mice used for model validation, 23 of the 26 mice (88.5%) were considered to develop CHS (sensitized NMS mice), by our NMS paradigm protocol, in comparison to NH mice (Figure 2A). When compared to NH mice, maternal deprivation induced a significant increase in VMR to CRD for the highest distension volumes reflecting CHS (80 μL: 0.783 ± 0.056 mV/s vs 0.531 ± 0.034 mV/s, P < 0.05 and 100 μL: 1.087 ± 0.056 mV/s vs 0.634 ± 0.038 mV/s, P < 0.05 for NMS sensitized and NH mice, respectively) (Figure 2B). The area under the curve (AUC) between 80 and 100 μL exhibited a significant (P < 0.0001) 1.61-fold increase of the colonic sensitivity in sensitized NMS mice in comparison to NH mice (18.72 ± 1.02 and 11.65 ± 0.58, respectively) (Figure 2B, top-right insert).
Figure 2.
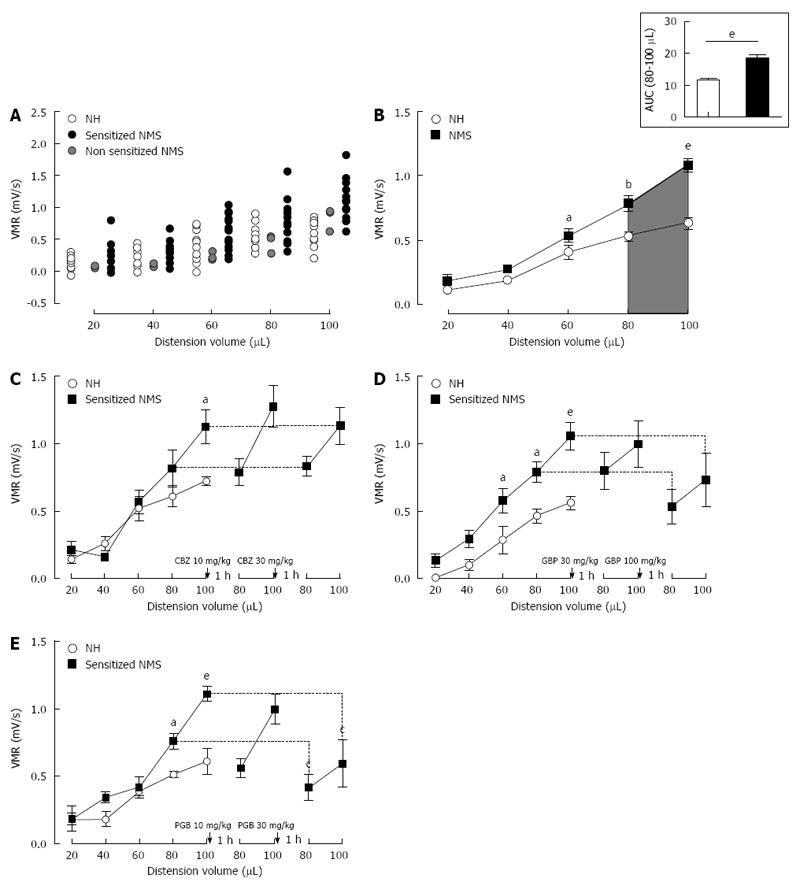
Mouse model of non-inflammatory colonic hypersensitivity and assessment of pregabalin, gabapentin and carbamazepine treatments. A: After colorectal distension (CRD) test, three mother-separated mice are considered non-sensitized (grey circles) as described in material and methods and are excluded from the analysis. Distribution of visceromotor response (VMR) values to CRD test highlights an increased response in sensitized neonatal mother-separated (sensitized NMS) (black circles n = 23) compared to non-handled (NH) (white circles n = 17) mice; B: Assessment of colonic sensitivity in sensitized NMS (black squares n = 23) and non-handled (white circles n = 17) mice displays significant differences between groups for highest distension volumes (60, 80 and 100 μL). Top right insert represents the area under the curve (AUC) between 80 μL and 100 μL; C: Carbamazepine (CBZ) administration (10 and 30 mg/kg s.c.) has no effect on VMR response in neonatal mother-separated mice (NMS : n = 8; NH : n = 6); D: Both doses (30 and 100 mg/kg s.c.) of gabapentin (GBP) have no effect on CHS induced by neonatal maternal separation (NMS: n = 8; NH: n = 6); E: Only the highest cumulative dose (30 mg/kg s.c.) of pregabalin (PGB) induces a VMR reduction in response to CRD in NMS mice (NMS : n = 8; NH : n = 6). aP < 0.05; bP < 0.01, eP < 0.001, Neonatal Mother-separated vs Non-handled ANOVA 2 Way (Animal housing; Volume) followed by Bonferroni post-hoc test for multiple comparisons and cP < 0.05 Pre-injection vs Post-injection; ANOVA 2 Way (Treatment; Volume) followed by Bonferroni post-hoc test for multiple comparisons. NMS: Neonatal maternal separation; NH: Non-handled.
Using the non-inflammatory NMS-induced CHS model, CBZ (n = 8) at 10 and 30 mg/kg failed to significantly reduce VMR in response to CRD (Figure 2C). Gabapentin (n = 8) at the highest cumulative dose of 100 mg/kg slightly reduces the VMR, but this effect is not significant (Figure 2D). Subcutaneous administration of Pregabalin also reduces VMR to CRD and this effect was significant for the highest cumulative dose of 30 mg/kg (1.111 ± 0.056 mV/s vs 0.595 ± 0.174 mV/s) (Figure 2E).
Effect of acute treatment with α2δ-1 ligands in an inflammatory DSS-induced CHS model
Among 1% DSS-treated mice used for model validation, none of them was considered as non-sensitized (see material and methods) (Figure 3A). Control mice (Water, n = 7) and DSS-treated mice (DSS 1%, n = 22) exhibit an increased VMR to balloon inflation. DSS-treated mice showed a dramatic and significant increase in VMR at 60 and 80 μL when compared to control mice (60 μL: 0.920 ± 0.079 mV/s vs 0.426 ± 0.100 mV/s P < 0.05 and 80 μL: 1.193 ± 0.097 mV/s vs 0.681 ± 0.094 mV/s P < 0.05 for DSS- and Water-treated mice, respectively) (Figure 3B). Unlike in NMS model, the most significant increase occurred for 60 and 80 μL of distension volumes (Figure 3B). The AUC between 60 and 80 μL is significantly (P = 0.0053) increased by 110.3% in DSS-treated mice compared to water-drinking mice (23.30 ± 3.10 and 11.08 ± 1.84, respectively) (Figure 3B, top-right insert). In addition, as expected, low-dose 1% DSS treatment induced moderate alterations of colonic mucosa characterized by a few focused epithelium disorganization and inflammatory cell infiltration (Figure 4). Thus, a slight but significant increase of the histological score was observed in the low-dose 1% DSS-treated mice (Table 1). Moreover, MPO activity was not significantly modify in low-dose 1% DSS-treated mice compared to control water-treated mice (Table 1). All together, these results tended to highlight a moderate colonic inflammatory impairment associated to CHS.
Figure 3.
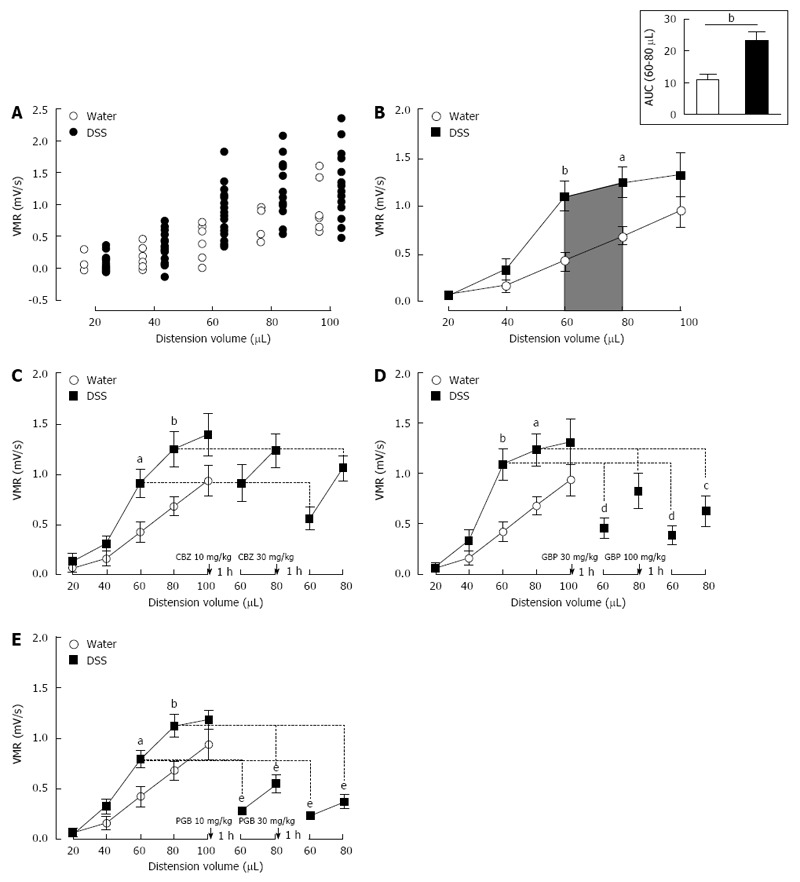
Mouse model of inflammation-associated colonic hypersensitivity and assessment of pregabalin, gabapentin and carbamazepine treatments. A: All animals were considered as sensitized by dextran sulfate sodium (DSS) treatment. Distribution of visceromotor response (VMR) values highlights an increased response in DSS-treated (black circles n = 22) compared to control (white circles n = 7) mice; B: Assessment of colonic sensitivity in DSS-treated (n = 22) and control mice (n = 7) displays significant differences between groups for 60 and 80 μL distension volumes. Top right insert represents the area under the curve (AUC) between 60 μL and 80 μL; C: Carbamazepine (CBZ) administration (10 and 30 mg/kg s.c.) has no significant effect on VMR response in DSS-treated mice (DSS n = 6; Water n = 7); D: Both doses (30 and 100 mg/kg s.c.) of gabapentin (GBP) induce a VMR reduction in DSS-treated mice (DSS n = 7; Water n = 7); E: Pregabalin (PGB) administration (10 and 30 mg/kg s.c.) induces a highly significant reduction of VMR in response to CRD in DSS-treated mice (DSS n = 9; Water n = 7).aP < 0.05; bP < 0.01, DSS-treated vs Water ANOVA 2 Way (Treatment; Volume) followed by Bonferroni post-hoc test for multiple comparisons; cP < 0.05; dP < 0.01, eP < 0.001, Pre-injection vs Post-injection ANOVA 2 Way (Treatment; Volume) followed by Bonferroni post-hoc test for multiple comparisons. CRD: Colorectal distension.
Figure 4.
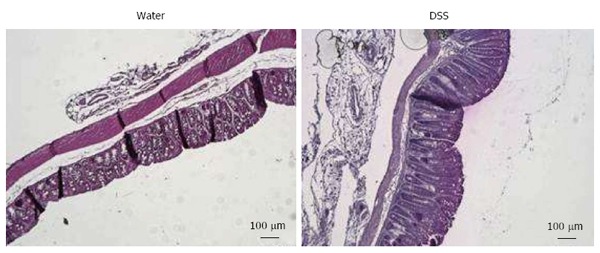
Representative histologic examinations of low dose 1% dextran sulfate sodium-treated mice colons after hematoxylin and eosin-staining (scales bars = 100 μm). DSS: Dextran sulfate sodium.
Table 1.
Inflammatory parameters in low dose 1% dextran sulfate sodium-induced colonic hypersensitivity model
| Water | DSS | |
| Histological score | 0.1 ± 0.1 | 4.0 ± 0.6b |
| MPO activity | 2.4 ± 0.3 | 4.0 ± 0.6 |
On the same mouse, intestinal inflammation has been assessed by measuring the colonic myeloperoxidase (MPO) activity and histological score.
P < 0.01 Water vs dextran sulfate sodium (DSS) treated ANOVA 1 Way followed by Tuckey’s post-hoc test for multiple comparisons.
In the CHS model associated with intestinal low-grade inflammation (1% DSS in drinking water), CBZ (n = 6) administration exhibited any significant antihypersensitive effect in mice sensitized with DSS (Figure 3C). In contrast, both α2δ-1 ligands, GBP (n = 7) and PGB (n = 9), showed a strong antihypersensitive effect. For a 60 μL-distension volume, s.c. administration of GBP and PGB induced a dose-dependent VMR decrease in DSS-treated mice (1.092 ± 0.160 mV/s vs 0.460 ± 0.099 mV/s and vs 0.390 ± 0.090 mV/s for GBP 30 and 100 mg/kg, respectively; 0.793 ± 0.082 mV/s vs 0.283 ± 0.033 mV/s and vs 0.236 ± 0.046 mV/s for PGB 10 and 30 mg/kg, respectively). For a 80 μL-distension volume, the highest cumulative dose of GBP (100 mg/kg) and both doses of PGB (10 and 30 mg/kg) induced a significant reduction of VMR in DSS-treated mice (1.238 ± 0.157 mV/s vs 0.630 ± 0.153 mV/s for GBP 100 mg/kg; 1.123 ± 0.110 mV/s vs 0.550 ± 0.087 mV/s and vs 0.373 ± 0.068 mV/s for PGB 10 and 30 mg/kg, respectively) (Figure 3D and E).
Comparative effect of acute treatment with α2δ-1 ligands on CHS
To compare the effects of anticonvulsant drug (GBP, PGB and CBZ) treatment, the AUC corresponding to the two distension volumes displaying the highest significant differences in VMR between control and sensitized groups (80 and 100 μL for NMS model and 60 and 80 μL for DSS model) were calculated. Carbamazepine failed to significantly reduce CHS in both models (Figure 5A and B). Gabapentin also failed to reduce CHS in NMS-induced model, but significantly reduced it in the DSS-induced model for both subcutaneous injections of 30 or 100 mg/kg (Figure 5C and D). Subcutaneous acute administration of the highest cumulative dose of PGB (30 mg/kg) significantly reduced VMR to CRD (Figure 5E), and significantly reduced CHS in low-dose DSS-treated mice, since s.c. acute administration of PGB induced a dose-dependent inhibition of abdominal contractile responses to CRD (Figure 5F). In addition, none of these drugs (CBZ, GBP and PGB) did modify the colonic sensitivity in control (NH or water-treated) animals.
Figure 5.
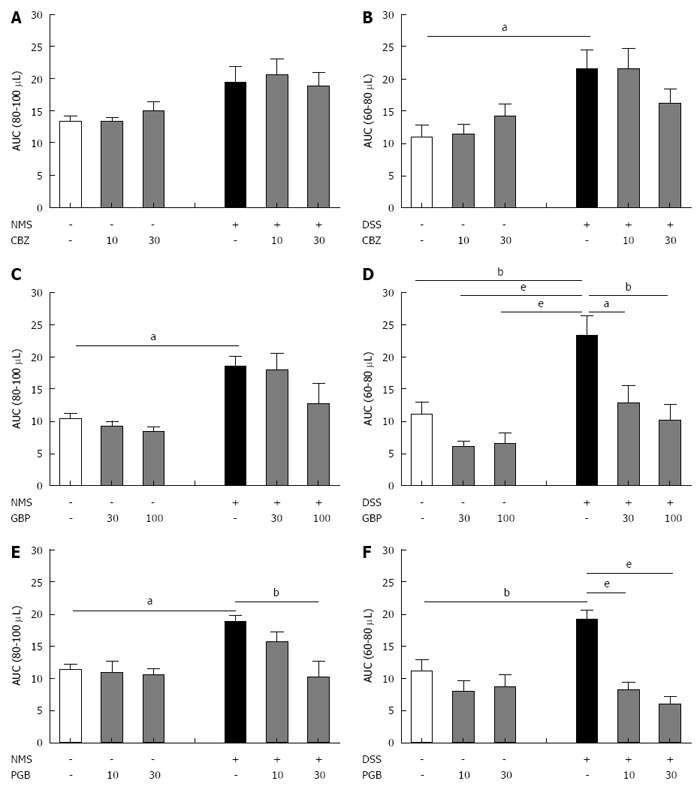
Comparative effect of carbamazepine, gabapentin and pregabalin on visceral hypersensitivity in non-inflammatory and inflammation-associated colonic hypersensitivity mouse models. The area under the curve (AUC) (A, C and E) between 80 μL and 100 μL for neonatal maternal separation (NMS) model and (B, D and F) between 60 μL and 80 μL for Dextran Sulfate Sodium (DSS) model has been calculated for both doses of (A and B) carbamazepine (CBZ), (C and D) gabapentin (GBP) and (E and F) pregabalin (PGB). aP < 0.05; bP < 0.01, eP < 0.001, ANOVA 2 Way (Treatment; Dose) followed by Bonferroni post-hoc test for multiple comparisons for each treatment in one CHS model. CHS: Colonic hypersensitivity; DSS: Dextran sulfate sodium.
Finally, the percent decrease of AUC induced by acute GBP or PGB treatment were higher in the inflammatory DSS-induced CHS model in comparison to the non-inflammatory NMS-induced CHS model (Table 2). This decrease was significant for both doses of PGB.
Table 2.
Comparison of the carbamazepine, gabapentin or pregabalin efficacy on neonatal mother-separated and dextran sulfate sodium induced colonic hypersensitivity models
| NMS | DSS | ||
| CBZ | 10 mg/kg | 9 ± 8 | 6 ± 18 |
| 30 mg/kg | 5 ± 13 | -17 ± 15 | |
| GBP | 30 mg/kg | 15 ± 32 | -45 ± 9 |
| 100 mg/kg | -14 ± 37 | -56 ± 9 | |
| PGB | 10 mg/kg | -16 ± 8 | -54 ± 7a |
| 30 mg/kg | -47 ± 13 | -66 ± 7b |
On the same mouse, the visceromotor response to gradual colorectal distensions (CRD) has been evaluated before and after the first and the second injection of CBZ, GBP or PGB. For each gradual CRD, the area under the curve (AUC) has been calculated between 80 μL and 100 μL in NMS model or between 60 μL and 80 μL for DSS model. Data represented the mean percent variation of the AUC calculated after the first or the second injection in comparison to the AUC calculated before any injection.
P < 0.05,
P < 0.01 NMS I DSS treated ANOVA 1 Way followed by Tuckey’s post-hoc test for multiple comparisons. CBZ: Carbamazepine; GBP: Gabapentin; PGB: Pregabalin; NMS: Neonatal maternal separation; DSS: Dextran sulfate sodium.
DISCUSSION
The present study demonstrates the acute anti-hypersensitive effect of α2δ-1 ligands gabapentin and pregabalin, compared to the non-α2δ-1 ligand anticonvulsant carbamazepine, on CHS models, in mice. A more effective effect of α2δ-1 ligands was observed in CHS associated with colonic inflammation compared to the non-inflammatory model.
The inflammatory animal model of CHS used in this work involved the administration of 1% DSS in drinking water during 14 consecutive days. Dextran sulfate sodium is widely used in rodents to induce colitis and generates quite similar symptoms to those of patients displaying IBD, such as inflammatory cell infiltration, cytokines production and fecal blood[34], showing the relevancy of this model to study intestinal inflammation. A few studies investigated the ability of DSS to induce CHS and displayed discrepant results with either a hypersensitive effect[35,36] or no influence of DSS administration on colonic sensitivity[37,38]. Comparing the data of those works is challenging since different protocols of visceral sensitivity measurement and treatment time course were used. However, it appears that high doses of DSS (> 3%), despite inducing a severe colitis, fail to enhance colonic sensitivity[37,38]. A possible explanation of this phenomenon might be the impairment of nerve terminals due to tissue lesion that leads to primary afferents desensitization. A recent paper has shown that low-dose of DSS (0.5% to 1% DSS) in drinking water of mice did not induce marked inflammation but only signs of low-grade inflammation[39]. Thus, in our study, low-doses of DSS probably do not induce severe tissue lesion and subsequent desensitization, but are sufficient to enhance colonic primary afferents response to colorectal distension.
Studying IBS-like non-inflammatory CHS is more challenging as mechanisms involved in IBS pathophysiology are poorly known. However, several studies have reported a close link between IBS development and early life traumatic events such as neglect or abuse[40]. Accordingly, Coutinho et al[41] pointed out an altered viscero-somatic response of rats separated from their mother during neonatal life. These sensitive disturbances seem to be correlated with hypothalamic-pituitary-adrenal axis dysfunctions[42] and are associated with plastic changes at spinal cord level[43,44] but not with organic injuries of colonic mucosa[45]. It was recently demonstrated that the same separation paradigm led to the increase of VMR to CRD in mice as well[29]. Our results corroborate this observation with a colonic sensitization of the vast majority of mice separated from their mother during postnatal period.
In DSS-induced model, we showed that α2δ-1 ligands were able to decrease CHS at the two used experimental doses, while only PGB significantly reduced the CHS in NMS model. In contrast, CBZ failed to modify VMR scores in both models. Chosen doses of α2δ-1 ligands have been shown to display an analgesic effect up to 2 h on mechanical hypersensitivity developed after spinal cord injury in mice[46]. Carbamazepine was administered at 10 and 30 mg/kg corresponding to doses reducing inflammatory somatic pain related behaviors[47]. Antihyperalgesic properties of α2δ-1 ligands are widely used in the management of neuropathic pain and fibromyalgia[48,49]. Regarding visceral pain, their ability to decrease colonic sensitivity has been demonstrated in two clinical trials in IBS patients[20,21]. At preclinical level, although GBP and PGB efficiency is documented in IBS models, only few studies reported their potential beneficial impact in acute inflammatory models. Indeed, PGB has been shown to decrease visceral hypersensitivity induced by LPS[50]. Our study confirms the ability of an α2δ-1 ligand (PGB) to relieve CHS in a non-inflammatory model. Interestingly, the two α2δ-1 ligands (GBP and PGB) more potently reduce CHS in our inflammatory model. In addition, not all classes of anticonvulsants are suitable to alleviate CHS, as highlighted by CBZ treatment failure, suggesting a specific involvement of α2δ-1 in the mechanism of action of GBP and PGB.
The first evoked action of GBP and PGB on GABAergic system does not seem to occur as conclude Taylor in his review[48]. Instead, their high affinity for α2δ-1 subunits of Ca(v) channels[51,52] could explain their effect on chronic pain. Indeed, it is well documented that α2δ-1 subunit stimulation increase both current amplitude of high voltage activated Ca(v) channels and their trafficking to the plasmatic membrane[53]. Consistent with this observation, mutation in the α2δ-1 Ca2+ channel subunit preventing the binding of α2δ-1 ligands, led to a decrease in their analgesic effects in mouse pain models[54,55]. Regarding visceral sensitivity, Liao et al[56] demonstrated the spinal upregulation of α2δ-1 subunit and the efficacy of GBP in a pancreatitis model in rats. Among Ca(v) channels, several data demonstrate that all subtypes can be involved in chronic pain development including visceral pain[57-59]. Their preponderant role is the increase of spinal synaptic transmission by promoting neurotransmitter-containing vesicle release that may lead to long-term potentiation phenomenon underlying chronic pain state[60]. Involvement of α2δ-1 subunits was also demonstrated in visceral pain conditions. Hence, in a rat model of CHS induced by uterine cross-sensitization a spinal overexpression of α2δ-1 subunit was described[61]. It is, therefore, possible that CHS in NMS mice is related to the up-regulation of Ca(v) channels and associated α2δ-1 subunits at presynaptic terminals of spinal sensory neurons and, in this model, antihyperalgesic effect of PGB is mainly due to spinal neurotransmission inhibition.
Besides spinal mechanisms of α2δ-1 ligands evoked in NMS-induced model and that probably occur in inflammatory model as well, an effect on peripheral sensitization cannot be ruled out explaining a higher efficacy in DSS-induced CHS. In fact, α2δ-1 subunit are retrieved in intestine primary afferent neurons associated with N-type calcium channels[62] and could be overexpressed in an inflammatory context, as observed in somatic inflammatory pain models. In fact, α2δ-1 and Ca(v)2.2 proteins are overexpressed in the nerves arising from L4 and L5 ganglia ipsilateral to the site of CFA administration[63]. In a rat model of osteoarthritic pain an increase in α2δ-1 mRNA levels was observed in ipsilateral L3 and L4 ganglia. In this model, pregabalin inhibit dorsal horn neuronal responses[64]. Other studies demonstrated antinociceptive efficacy of α2δ-1 ligands on behavioral and neuronal responses in cutaneous models of acute inflammatory pain[65,66]. Thereby, with inhibition of spinal synaptic transmission, α2δ-1 ligands could also target inflammation in DSS-induced CHS model, explaining their potent effect in this model.
To conclude, our results, combined with available data of literature, confirmed the beneficial effect of α2δ-1 ligands in the management of visceral hypersensitivity. Moreover, our findings highlight their efficacy in inflammation-induced CHS. Further studies are needed to investigate the mechanism of action of α2δ-1 ligands on peripheral sensitization in an inflammatory context. Finally, this work pointed out the interest to study the clinical relevance of α2δ-1 ligands in IBD patients in remission to alleviate IBS-like symptoms, particularly abdominal pain.
COMMENTS
Background
Colonic hypersensitivity is often reported in patients with abdominal pain as irritable bowel syndrome (IBS) patients or inflammatory bowel diseases (IBD) patients during remission phases. Despite many potential tested targets expressed in intestinal sensory afferents to modulate abdominal pain in several preclinical models or under different clinical conditions, colonic hypersensitivity (CHS) is not targeted by any therapy as of today. In this context, the development of new treatments is needed to provide IBS patients and IBD patients during the quiescent periods with a therapeutic option in alleviating abdominal pain.
Research frontiers
Clinical studies highlighted the antinociceptive effect of alpha-2-delta-1 (α2δ-1) ligands, initially commercialized as anticonvulsant, in patients suffering from IBS. However, no investigation was done based on the intestinal inflammation state.
Innovations and breakthroughs
The goal of this study was to determine if the α2δ-1 ligands (gabapentin and pregabalin) have beneficial effects on CHS, using different mouse models of IBS and IBD. They will be compared to those of another anticonvulsant drug, carbamazepine. The main question addressed by this preclinical study will be the effect of α2δ-1 ligand compounds in a CHS mouse model associated with a colonic inflammation induced by dextran sulfate sodium administration and compare these effects with those obtained in a non-inflammatory IBS-like model, induced by neonatal maternal separation.
Applications
The present study suggests that α2δ-1 ligands may be beneficial for the management of visceral hypersensitivity. In particular, our findings show the efficacy of α2δ-1 ligands on inflammation-induced CHS and further studies on the mechanisms of action of α2δ-1 ligands on peripheral sensitization in an inflammatory context will pave the way for new CHS treatments for IBD patients. Finally, this work shows the clinical relevance of using α2δ-1 ligands also in IBD patients who are in remission in order to alleviate IBS-like symptoms, such as abdominal pain.
Terminology
Mouse CHS is evaluated in our different animal models using a colorectal distension procedure and by evaluating the animal discomfort reflected by changes in the visceromotor response, a pseudoaffective reflex response widely used as index of visceral pain in rodents, to colorectal distension.
Peer-review
This preclinical study demonstrates α2δ-1 ligands efficacy on inflammation-associated CHS, showing their potential clinical interest for patients with chronic abdominal pain and moderate intestinal inflammation.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Auvergne University Institutional review board.
Institutional animal care and use committee statement: All animal experiments were performed following the ethical guidelines set out by the International Association for the Study of Pain (IASP), with EU guidelines and the regulations of the French Agriculture and Forestry Ministry (decree 874848) and with approval from local ethical committee (N° CE08-10 and N° CE09-10).
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Data sharing statement: There are no additional data available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 21, 2016
First decision: May 27, 2016
Article in press: July 6, 2016
P- Reviewer: Elpek GO S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 2.Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–1728. doi: 10.1053/j.gastro.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 3.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 4.Piche T, Ducrotté P, Sabate JM, Coffin B, Zerbib F, Dapoigny M, Hua M, Marine-Barjoan E, Dainese R, Hébuterne X. Impact of functional bowel symptoms on quality of life and fatigue in quiescent Crohn disease and irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:626–e174. doi: 10.1111/j.1365-2982.2010.01502.x. [DOI] [PubMed] [Google Scholar]
- 5.Stacher G, Christensen J. Visceral hypersensitivity in irritable bowel syndrome: a summary review. Dig Dis Sci. 2006;51:440–445. doi: 10.1007/s10620-006-3152-9. [DOI] [PubMed] [Google Scholar]
- 6.Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Crohn’s disease in the elderly: a comparison with young adults. J Clin Gastroenterol. 1998;27:129–133. doi: 10.1097/00004836-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hicks GA. TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterol Motil. 2006;18:590–594. doi: 10.1111/j.1365-2982.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- 9.Bradesi S, Herman J, Mayer EA. Visceral analgesics: drugs with a great potential in functional disorders? Curr Opin Pharmacol. 2008;8:697–703. doi: 10.1016/j.coph.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clouse RE. Antidepressants for irritable bowel syndrome. Gut. 2003;52:598–599. doi: 10.1136/gut.52.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutgeerts P, Van Assche G, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593–1610. doi: 10.1053/j.gastro.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 12.Beattie RM, Croft NM, Fell JM, Afzal NA, Heuschkel RB. Inflammatory bowel disease. Arch Dis Child. 2006;91:426–432. doi: 10.1136/adc.2005.080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:157–165. doi: 10.1016/j.bpg.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Johannessen Landmark C. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22:27–47. doi: 10.2165/00023210-200822010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Landmark CJ. Targets for antiepileptic drugs in the synapse. Med Sci Monit. 2007;13:RA1–RA7. [PubMed] [Google Scholar]
- 16.Lynch JJ, Honore P, Anderson DJ, Bunnelle WH, Mortell KH, Zhong C, Wade CL, Zhu CZ, Xu H, Marsh KC, et al. (L)-Phenylglycine, but not necessarily other alpha2delta subunit voltage-gated calcium channel ligands, attenuates neuropathic pain in rats. Pain. 2006;125:136–142. doi: 10.1016/j.pain.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald RL, Kelly KM. Mechanisms of action of currently prescribed and newly developed antiepileptic drugs. Epilepsia. 1994;35 Suppl 4:S41–S50. doi: 10.1111/j.1528-1157.1994.tb05955.x. [DOI] [PubMed] [Google Scholar]
- 18.Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10:685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- 19.Waszkielewicz AM, Gunia A, Słoczyńska K, Marona H. Evaluation of anticonvulsants for possible use in neuropathic pain. Curr Med Chem. 2011;18:4344–4358. doi: 10.2174/092986711797200408. [DOI] [PubMed] [Google Scholar]
- 20.Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:981–988. doi: 10.1111/j.1365-2036.2005.02685.x. [DOI] [PubMed] [Google Scholar]
- 21.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56:1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diop L, Raymond F, Fargeau H, Petoux F, Chovet M, Doherty AM. Pregabalin (CI-1008) inhibits the trinitrobenzene sulfonic acid-induced chronic colonic allodynia in the rat. J Pharmacol Exp Ther. 2002;302:1013–1022. doi: 10.1124/jpet.302.3.1013. [DOI] [PubMed] [Google Scholar]
- 23.Million M, Wang L, Adelson DW, Roman F, Diop L, Taché Y. Pregabalin decreases visceral pain and prevents spinal neuronal activation in rats. Gut. 2007;56:1482–1484. doi: 10.1136/gut.2007.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravnefjord A, Brusberg M, Larsson H, Lindström E, Martínez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br J Pharmacol. 2008;155:407–416. doi: 10.1038/bjp.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepanović-Petrović RM, Tomić MA, Vucković SM, Kocev N, Ugresić ND, Prostran MS, Bosković B. GABAergic mechanisms are involved in the antihyperalgesic effects of carbamazepine and oxcarbazepine in a rat model of inflammatory hyperalgesia. Pharmacology. 2008;82:53–58. doi: 10.1159/000127841. [DOI] [PubMed] [Google Scholar]
- 27.Tomić MA, Vucković SM, Stepanović-Petrović RM, Ugresić N, Prostran MS, Bosković B. The anti-hyperalgesic effects of carbamazepine and oxcarbazepine are attenuated by treatment with adenosine receptor antagonists. Pain. 2004;111:253–260. doi: 10.1016/j.pain.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Vucković SM, Tomić MA, Stepanović-Petrović RM, Ugresić N, Prostran MS, Bosković B. The effects of alpha2-adrenoceptor agents on anti-hyperalgesic effects of carbamazepine and oxcarbazepine in a rat model of inflammatory pain. Pain. 2006;125:10–19. doi: 10.1016/j.pain.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Miquel S, Martín R, Lashermes A, Gillet M, Meleine M, Gelot A, Eschalier A, Ardid D, Bermúdez-Humarán LG, Sokol H, et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci Rep. 2016;6:19399. doi: 10.1038/srep19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho FA, Barnich N, Sauvanet P, Darcha C, Gelot A, Darfeuille-Michaud A. Crohn’s disease-associated Escherichia coli LF82 aggravates colitis in injured mouse colon via signaling by flagellin. Inflamm Bowel Dis. 2008;14:1051–1060. doi: 10.1002/ibd.20423. [DOI] [PubMed] [Google Scholar]
- 33.Onyeagocha C, Hossain MS, Kumar A, Jones RM, Roback J, Gewirtz AT. Latent cytomegalovirus infection exacerbates experimental colitis. Am J Pathol. 2009;175:2034–2042. doi: 10.2353/ajpath.2009.090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sha T, Igaki K, Yamasaki M, Watanabe T, Tsuchimori N. Establishment and validation of a new semi-chronic dextran sulfate sodium-induced model of colitis in mice. Int Immunopharmacol. 2013;15:23–29. doi: 10.1016/j.intimp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Boué J, Basso L, Cenac N, Blanpied C, Rolli-Derkinderen M, Neunlist M, Vergnolle N, Dietrich G. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4(+) T cells in mice. Gastroenterology. 2014;146:166–175. doi: 10.1053/j.gastro.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Jain P, Hassan AM, Koyani CN, Mayerhofer R, Reichmann F, Farzi A, Schuligoi R, Malle E, Holzer P. Behavioral and molecular processing of visceral pain in the brain of mice: impact of colitis and psychological stress. Front Behav Neurosci. 2015;9:177. doi: 10.3389/fnbeh.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson MH, Miketa A, Martinez V. Lack of interaction between psychological stress and DSS-induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress. 2009;12:434–444. doi: 10.1080/10253890802626603. [DOI] [PubMed] [Google Scholar]
- 38.Larsson MH, Rapp L, Lindström E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol Motil. 2006;18:144–152. doi: 10.1111/j.1365-2982.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 39.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heitkemper MM, Cain KC, Burr RL, Jun SE, Jarrett ME. Is childhood abuse or neglect associated with symptom reports and physiological measures in women with irritable bowel syndrome? Biol Res Nurs. 2011;13:399–408. doi: 10.1177/1099800410393274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 42.Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol. 2011;14:666–683. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- 43.Chung EK, Zhang XJ, Xu HX, Sung JJ, Bian ZX. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neuronal plasticity in rat spinal cord. Neuroscience. 2007;149:685–695. doi: 10.1016/j.neuroscience.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 44.Tsang SW, Zhao M, Wu J, Sung JJ, Bian ZX. Nerve growth factor-mediated neuronal plasticity in spinal cord contributes to neonatal maternal separation-induced visceral hypersensitivity in rats. Eur J Pain. 2012;16:463–472. doi: 10.1016/j.ejpain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 45.O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Tanabe M, Ono K, Honda M, Ono H. Gabapentin and pregabalin ameliorate mechanical hypersensitivity after spinal cord injury in mice. Eur J Pharmacol. 2009;609:65–68. doi: 10.1016/j.ejphar.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Sahebgharani M, Hossein-Abad AA, Zarrindast MR. On the mechanism of carbamazepine-induced antinociception in the formalin test. Int J Neurosci. 2006;116:1097–1113. doi: 10.1080/00207450600808669. [DOI] [PubMed] [Google Scholar]
- 48.Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin--calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain. 2009;142:13–16. doi: 10.1016/j.pain.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, Kouvelas D. Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta-analysis. J Clin Pharm Ther. 2010;35:639–656. doi: 10.1111/j.1365-2710.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- 50.Eutamene H, Coelho AM, Theodorou V, Toulouse M, Chovet M, Doherty A, Fioramonti J, Bueno L. Antinociceptive effect of pregabalin in septic shock-induced rectal hypersensitivity in rats. J Pharmacol Exp Ther. 2000;295:162–167. [PubMed] [Google Scholar]
- 51.Cheng JK, Chiou LC. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci. 2006;100:471–486. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- 52.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 53.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 54.Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, Walker LC. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006;1075:68–80. doi: 10.1016/j.brainres.2005.12.084. [DOI] [PubMed] [Google Scholar]
- 55.Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao XZ, Zhou MT, Mao YF, Xu H, Chen H, Sun JH, Xiong YC. Analgesic effects of gabapentin on mechanical hypersensitivity in a rat model of chronic pancreatitis. Brain Res. 2010;1337:104–112. doi: 10.1016/j.brainres.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 57.Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrère C, Pizzoccaro A, Muller E, Nargeot J, Snutch TP, et al. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci USA. 2011;108:11268–11273. doi: 10.1073/pnas.1100869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGivern JG. Targeting N-type and T-type calcium channels for the treatment of pain. Drug Discov Today. 2006;11:245–253. doi: 10.1016/S1359-6446(05)03662-7. [DOI] [PubMed] [Google Scholar]
- 59.Swayne LA, Bourinet E. Voltage-gated calcium channels in chronic pain: emerging role of alternative splicing. Pflugers Arch. 2008;456:459–466. doi: 10.1007/s00424-007-0390-4. [DOI] [PubMed] [Google Scholar]
- 60.Youn DH, Gerber G, Sather WA. Ionotropic glutamate receptors and voltage-gated Ca2+ channels in long-term potentiation of spinal dorsal horn synapses and pain hypersensitivity. Neural Plast. 2013;2013:654257. doi: 10.1155/2013/654257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Zhang M, Xie F, Li X, Bao M, Yang N, Shi R, Wang Z, Wu A, Guan Y, et al. Upregulation of α2δ-1 Calcium Channel Subunit in the Spinal Cord Contributes to Pelvic Organ Cross-Sensitization in a Rat Model of Experimentally-Induced Endometriosis. Neurochem Res. 2015;40:1267–1273. doi: 10.1007/s11064-015-1592-3. [DOI] [PubMed] [Google Scholar]
- 62.Needham K, Bron R, Hunne B, Nguyen TV, Turner K, Nash M, Furness JB. Identification of subunits of voltage-gated calcium channels and actions of pregabalin on intrinsic primary afferent neurons in the guinea-pig ileum. Neurogastroenterol Motil. 2010;22:e301–e308. doi: 10.1111/j.1365-2982.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 63.Lu SG, Zhang XL, Luo ZD, Gold MS. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain. 2010;151:633–643. doi: 10.1016/j.pain.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain. 2009;5:45. doi: 10.1186/1744-8069-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanfa LC, Singh L, Williams RG, Dickenson AH. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997;8:587–590. doi: 10.1097/00001756-199702100-00002. [DOI] [PubMed] [Google Scholar]
- 66.Yoon MH, Yaksh TL. Evaluation of interaction between gabapentin and ibuprofen on the formalin test in rats. Anesthesiology. 1999;91:1006–1013. doi: 10.1097/00000542-199910000-00021. [DOI] [PubMed] [Google Scholar]


