Abstract
Objective
To test the hypothesis that different forms of neurodegeneration are differentially related to longitudinal cognitive trajectories in old age.
Methods
Participants are 420 older persons from two clinical-pathologic studies without cognitive impairment at study onset. They completed a battery of 17 cognitive tests annually for a minimum of 5 years, died, and underwent a neuropathologic examination to quantify neuronal neurofibrillary tangles and transactive response DNA-binding protein 43 (TDP-43) pathology and to identify Lewy bodies and hippocampal sclerosis. We used sigmoid mixed models based on the 4-parameter logistic distribution to decompose nonlinear global cognitive trajectories into components and assess the relation of each neuropathologic marker to each trajectory component.
Results
Cognitive function was assessed for a mean of 10.5 years before death. In the absence of pathology, global cognition was relatively stable before declining moderately in the last 3 to 4 years of life. Tangles were related to all trajectory components except initial level. TDP-43 pathology was the only marker related to initial level of function. It was also associated with an earlier midpoint of decline but not to slope of decline. Hippocampal sclerosis was related to an earlier midpoint of decline and more rapid slope of decline. Lewy bodies were associated with faster slope of decline and lower level of function proximate to death.
Conclusion
Neurodegenerative processes are differentially related to cognitive trajectories, with TDP-43 pathology most potently impacting incipient cognitive decline, AD pathology and hippocampal sclerosis affecting the progression of cognitive decline, and Lewy bodies impacting terminal decline.
Keywords: cognitive decline, longitudinal study, neurofibrillary tangles, TDP-43 pathology, hippocampal sclerosis
Introduction
Much of late-life cognitive decline and dementia is thought to be driven by age-related neurodegenerative lesions. On postmortem examination of the brains of older adults, several forms of neurodegeneration are commonly observed. At least trace levels of beta-amyloid-immunoreactive plaques and tau-immunoreactive neurofibrillary tangles, the pathologic hallmarks of Alzheimer’s disease, are present in most older individuals (Boyle et al., 2013). Although these lesions are traditionally associated with dementia, they are also found in the brains of old persons who died with mild (Petersen et al., 2006; Markesbery, 2010) or no (Bennett et al., 2012) cognitive impairment. Transactive response DNA-binding protein 43 (TDP-43) pathology, the primary protein aggregate in amyotrophic lateral sclerosis and frontotemporal lobar dementia (Neumann et al., 2006; Arai et al, 2006), is seen in about half of the brains of older people (Geser et al., 2010; Wilson et al., 2013), particularly in the medial temporal lobe (Higashi et al., 2007; Hu et al., 2008; Arai et al., 2009; Wilson et al., 2013). The presence of TDP-43 pathology has been associated with dementia and cognitive impairment (Josephs et al., 2008; Nelson et al., 2010; Wilson et al., 2013). However, because TDP-43 pathology often co-occurs with other neurodegenerative conditions (Amador-Ortiz et al., 2007; Arai et al., 2009; Robinson et al., 2011), its specific association with late-life cognition has been difficult to establish (Robinson et al., 2011; Wilson et al., 2013). TDP-43 pathology has a particularly strong association with hippocampal sclerosis (Nelson et al., 2011; Nelson et al., 2013), which refers to cell loss and gliosis in the hippocampus. Hippocampal sclerosis is reported in approximately 10% of older persons (Nelson et al., 2011; Nelson et al., 2013; Nag et al., 2015). It has been associated with cognitive impairment and dementia (Nelson et al., 2011; Nelson et al., 2013) though at least some of this association may be due to the correlation between hippocampal sclerosis and TDP-43 pathology (Robinson et al., 2011; Wilson et al., 2013; Nag et al., 2015). Alpha-synuclein-immunoreactive Lewy boies are found in approximately 20% of the brains of old people (Wakisaka et al., 2003; Zaccai et al., 2008; Schneider et al., 2012). Although Lewy bodies are considered a leading cause of dementia (Perry et al., 1990), recent research has suggested that the association is relatively weak (Zaccai, Brayne, Matthews, Ince, & MRC Cognitive Function and Ageing Neuropathology Study, 2015) or mainly due to individuals with more advanced disease (Schneider et al., 2012; Wilson et al., 2015).
Although the association of these neurodegenerative lesions with loss of cognitive function is established, the temporal course of these effects is not well understood. In particular, because these conditions often co-occur and are difficult to quantify during life, little is known about how specific neurodegenerative effects on cognition unfold over time and whether this varies across different forms of neurodegeneration. One approach has been to assess the relation of postmortem pathologic markers to MCI, widely regarded as a precursor to dementia, but this focuses on only one segment of cognitive aging. A more comprehensive approach has been to estimate the relation of neurodegenerative markers to trajectories of cognitive decline. In this approach, one (Hall et al, 2001) or two (Wilson et al., 2012) person-specific change points have been incorporated to accommodate nonlinear change in cognitive function, but these models may not provide a good fit for individuals with minimal or mostly linear decline.
In the present study, we assess the longitudinal cognitive profiles associated with postmortem measures of 4 common forms of neurodegeneration and test the hypothesis that these longitudinal cognitive profiles differ. Participants are 420 older persons from 2 longitudinal clinical-pathologic cohort studies without cognitive impairment at enrollment. After a mean of 10.5 years of annual cognitive assessment, they died and underwent a uniform neuropathologic examination to quantify neuronal neurofibrillary tangles, TDP-43 pathology, hippocampal sclerosis, and Lewy bodies, lesions that are currently difficult to securely identify without a postmortem neuropathologic examination. There are wide individual differences in patterns of cognitive decline including both linear and nonlinear trajectories. Therefore, we used longitudinal sigmoid mixed models based on a 4-parameter logistic distribution to accommodate the heterogeneity among longitudinal cognitive trajectories and to decompose the trajectories into dynamic components. These models, by assessing the relation of each marker to each component, allowed us to test for the hypothesized differences in the longitudinal cognitive footprints of each marker.
Methods
Participants
All data are based on participants in two ongoing clinical-pathologic studies. The Religious Orders Study involves older Catholic priests, nuns, and monks recruited from multiple sites across the United States (Wilson, Bienias, Evans, & Bennett, 2004; Bennett, Schneider, Arvanitakis, & Wilson, 2012). The Rush Memory and Aging Project involves older lay persons recruited from the Chicago area (Bennett et al., 2005; Bennett, Schneider, Buchman et al., 2012). Eligibility for each of these studies was determined at the time of enrollment and required age ≥50 years, absence of a prior clinical diagnosis of dementia, and agreement to annual clinical evaluations and brain autopsy at death. Participants in each study signed an informed consent and anatomical gift act, and the studies were approved by the institutional review board of Rush University Medical Center.
Eligibility for the current analyses required the absence of cognitive impairment at baseline, to allow us to characterize incipient cognitive changes; death with a brain autopsy and completed neuropathologic examination, to provide markers of the 4 neurodegenerative conditions of interest; and completion of at least 5 annual clinical evaluations, to enhance our ability to capture nonlinear change in cognitive function. At the time of these analyses, 3,934 individuals had enrolled in the parent studies and completed their baseline evaluation. We excluded 223 persons with dementia and 945 with mild cognitive impairment. Of the remaining 2,766 individuals without cognitive impairment at baseline, 897 died during follow-up. A brain autopsy was done on 842 (93.9%) and the neuropathological examination had been completed on the first consecutive 706 individuals. Of these, 420 had completed at least 5 clinical evaluations and had valid scores on all four of the postmortem neurodegenerative measures of interest. Analyses are based on this group. They had a mean age at baseline of 78.7 years (SD=6.8; range: 64–96), they had a mean of 16.4 years of education range (SD=3.7; range: 5–28), 128 were men (30.4 %), and 406 were White and not Latino (96.7%). They died at a mean age of 89.2 (SD=6.4; range: 71–104) after completing a mean of 10.5 years of follow-up (SD=3.6; range: 3.7–20.0). The analytic group included 203 persons from the Rush Memory and Aging Project and 217 from the Religious Orders Study. As shown in Table 1, those from the Religious Orders Study had more follow-up, were younger and more educated, and at baseline had slightly better cognitive function and fewer chronic conditions than those from the Rush Memory and Aging Project.
Table 1.
Comparison of participants from the Rush Memory and Aging Project (RMAP) and the Religious Orders Study (ROS) included in the analytic group
| Characteristic | RMAP (n=203) | ROS (n=217) | p-Value | Cohen’s d |
|---|---|---|---|---|
| Age at baseline | 82.0 (5.3) | 75.7 (6.6) | <0.001 | 0.919 |
| Age at death | 90.3 (5.6) | 88.1 (6.9) | <0.001 | 0.340 |
| Study years | 8.4 (2.5) | 12.4 (3.8) | <0.001 | −0.613 |
| Years of education | 14.5 (2.9) | 18.2 (3.5) | <0.001 | −1.000 |
| Women,% | 71.4 | 67.7 | 0.412 | |
| Baseline global cognition | 0.191 (0.363) | 0.262 (0.382) | 0.054 | −0.188 |
| Final global cognition | −0.637 (0.980) | −0.702 (1.184) | 0.537 | 0.060 |
| Baseline MMSE | 28.3 (1.8) | 28.7 (1.4) | 0.008 | −0.141 |
| Final MMSE | 22.7 (7.9) | 21.7 (8.9) | 0.222 | 0.022 |
| Baseline heart disease, % | 11.3 | 11.1 | 0.930 | |
| Baseline cancer, % | 30.1 | 32.7 | 0.556 | |
| Baseline hypertension, % | 57.6 | 44.2 | 0.006 | |
| Baseline thyroid, % | 22.2 | 14.3 | 0.036 | |
| Baseline head injury, % | 6.4 | 11.1 | 0.093 | |
| Baseline diabetes, % | 14.3 | 11.5 | 0.398 | |
| Baseline stroke, % | 11.3 | 3.7 | 0.003 | |
| Incident dementia, % | 30.4 | 37.8 | 0.109 | |
| Postmortem interval, hours | 8.6 (7.8) | 8.7(6.0) | 0.861 | 0.059 |
Note. Values are mean (standard deviation) unless otherwise indicated. MMSE, Mini-Mental State Examination.
Assessment of Cognitive Function
Each annual clinical evaluation included a battery of 19 cognitive tests administered by a trained research assistant in an approximately 60 minute session. Two tests were only used in clinical classification: the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) and a short form (Wilson et al., 2002) of Complex Ideational Material, a measure of auditory verbal comprehension from the Boston Diagnostic Aphasia Examination (Goodglass & Kaplan, 1983). The remaining 17 tests included 7 measure of episodic memory: immediate and delayed recall of the East Boston story (Albert, Scherr, Taylor, Evans, & Funkenstein, 1991; Wilson et al., 2002) and Logical Memory Story A (Wechsler, 1987) plus Word List Memory, Word List Recall, and Word List Recognition (Welsh et al., 1994). Semantic memory was assessed with a 15-item word reading test (Wilson et al., 2002), a measure of verbal fluency involving naming category exemplars in 1 minute trials (Welsh et al., 1994; Wilson et al., 2002), and a 15-item version (Welsh et al., 1994) of the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983). Digit Span Forward and Backward (Wechsler, 1987) plus Digit Ordering (Wilson et al., 2002) were used to assess working memory. Perceptual speed was assessed with the oral form of the Symbol Digit Modalities Test (Smith, 1982) and a modified form (Wilson et al., 2002) of Number Comparison (Ekstrom, French, Harman, & Kermen, 1976). A 15-item version of Judgment of Line Orientation (Benton, Sivan, Hamsher, Varney, & Spreen, 1994) and a 12-item version of Standard Progressive Matrices (Raven, Court, & Raven, 1992) were used to measure visuospatial ability.
The cognitive assessment has 2 aims. First, it supports clinical diagnoses of mild cognitive impairment, dementia, and Alzheimer’s disease. As previously described (Wilson, Boyle, Yang, James, & Bennett, 2015), an algorithm using educationally adjusted cutoff scores on 11 tests provided ratings of impairment in 5 cognitive domains (orientation, attention, memory, language, perception). After review of all test results and information about education, occupation, effort on testing, and potential sensory or motor problems, a neuropsychologist reviewed each rating and either agreed with it or supplied a new rating in the event of disagreement. Following each evaluation, an experienced clinician (i.e., physician or nurse practitioner) diagnosed dementia following the criteria of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984). These criteria require a history of cognitive decline and impairment in 2 or more cognitive domains, one of which must be memory to meet criteria for Alzheimer’s disease. Individuals who had cognitive impairment but did not meet criteria for dementia were diagnosed with mild cognitive impairment. In previous research, these mild cognitive impairment criteria have been associated with subsequent rates of mortality (Bennett et al., 2002) and cognitive decline (Bennett et al., 2002; Boyle, Wilson, Aggarwal, Tang, & Bennett, 2006) and postmortem levels of dementia related pathologies (Bennett, Schneider, Wilson, Bienias, & Arnold, 2005) intermediate between no cognitive impairment and dementia subgroups.
The second aim of the cognitive assessment is to characterize change in cognitive function over time. To minimize floor and ceiling artifacts in longitudinal analyses, we used a composite measure of global cognition based on all 17 tests. Raw scores on the 17 individual tests were transformed to z scores, using the baseline mean and standard deviation of all participants in both parent studies, and the z scores were averaged to get the composite score. Valid scores on a minimum of 6 individual tests were required to compute the composite measure of global cognition. In the current data set, the 420 participants had a total of 4,229 global cognitive scores during the observation period, with 102 (2.4%) global cognitive scores missing for reasons other than death. Further information on the composite measure of global cognition and the individual tests is published elsewhere (Wilson et al., 2002; Wilson, Barnes, & Bennett, 2003; Wilson et al., 2005).
Neuropathologic Examination
A standard protocol was used for brain removal, which occurred a median of 6.3 hours after death (interquartile range: 4.8–9.8), tissue sectioning, and quantifying pathologic findings (Bennett et al., 2004; Schneider et al., 2006), with examiners who were kept unaware of all clinical information. An anti-paired helical filament-tau antibody clone AT8 (ThermoScientific, Rockford, IL;1:2,000) and computer assisted sampling were used to assess density/mm2 of tau-immunoreactive tangles in 8 brain regions (hippocampus [subfield 1 and subiculum], entorhinal cortex, inferior temporal cortex, angular/supramarginal gyrus, superior frontal cortex, dorsal lateral prefrontal cortex, anterior cingulate cortex, primary visual cortex). Density scores in each region were standardized and the standardized regional scores were averaged to yield a composite index of tangle density (Bennett et al., 2004). We assessed TDP-43 pathology in 6 regions (amygdala, hippocampus [subfield 1 and subiculum], dentate gyrus, entorhinal cortex, middle temporal cortex, midfrontal cortex) with monoclonal antibodies to phosphyorylated TDP-43 (pS409/410;1:100) (Neumann et al., 2009) which stains the pathologically phosphorylated TDP-43 proteins but not normal nuclear TDP-43. The TDP-43 cytoplasmic inclusions in each region were rated on a 6-point scale and the mean of the regional scores was used in analyses (Wilson et al., 2013). Hippocampal sclerosis was defined as severe loss of neurons in the pyramidal cell layer of the subiculum or any hippocampal subfield (Wilson et al., 2013; Nag et al., 2015). A monoclonal phosphorylated antibody to alpha-synuclein (Zymed LB 509;1:50) was used to identify Lewy bodies in the substantia nigra, 2 limbic sites (entorhinal cortex, anterior cingulate cortex), and 3 neocortical areas (superior or middle temporal cortex, inferior parietal cortex, midfrontal cortex). Lewy bodies were subdivided into 3 mutually exclusive stages (nigral, limbic, and neocortical) based on McKeith et al. (1996), as previously described (Wilson et al., 2011) but were treated as present or absent in primary analyses.
Statistical Analysis
To characterize nonlinear trajectories of cognitive decline, we implemented a sigmoidal model based on the 4-parameter logistic function (Davidian & Giltinan, 1995) in SAS using PROC NLMIXED. The cognitive function (Y) was assumed to follow the equation
| (1) |
for person i and observation j. The time tij denotes the negative time before death. For example, an observation occurring one year before death would have a time of −1. There are 4 parameters, and changes in these parameters are assumed to occur as linear functions of the covariates. The parameter θ1i represents the final level of cognitive function (proximate to death), and the parameter θ4i represents the initial (starting) level of cognitive function. Random effects associated with the parameters for initial and final levels of cognitive functions are assumed to be normally distributed. The model handles missing observations within a person-specific trajectory through the asymptotic estimation of initial and final levels as well as the strength borrowed from the trajectories of other participants.
The parameter θ2 represents the midpoint of cognitive decline; that is, the point in time at which half of the total decline from study baseline until death has occurred. The parameter θ3 represents the nonlinear trajectory between the initial and final cognitive levels, or the rate of decline. Thus, in combination, the four parameters (θ1– θ4 ) characterize nonlinear trajectories of change in cognitive function over time.
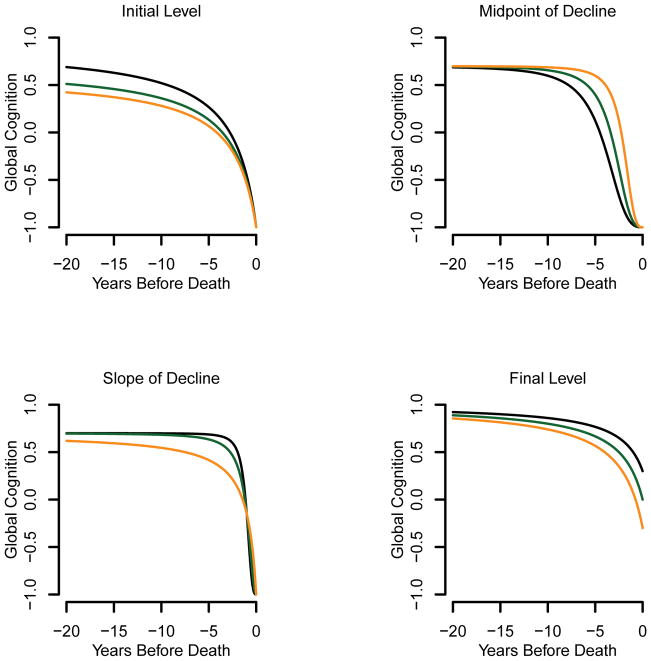
Figure 1 illustrates how changes in one parameter may impact the trajectory of change in the composite measure of global ognition. The parameter θ1i represents the final level as shown in the lower right panel of figure 1. The parameter θ2 represents the midpoint of decline as shown in the upper right panel. The parameter θ3 is represents the rate of decline/nonlinearity, shown in the lower left panel. The parameter θ4i represents the initial level, shown in the upper left panel.
Figure 1.
Illustration of how a shift in one parameter impacts the trajectory of change in the standardized composite measure of global cognition estimated in the sigmoid mixed model: initial level (upper left, θ1=−1, θ2=2.5, θ3=1, θ4= 0.6, 0.7 and 0.9), midpoint of decline (upper right, θ1=−1, θ2=2, 3 and 4, θ3=3, θ4= 0.7), slope (lower left, θ1=−1, θ2=1, θ3=1, 2 and 3, θ4= 0.7), final level (lower right, θ1=−0.3, 0, 0.3, θ2=2.5, θ3=1, θ4=1).
Results
Postmortem Markers of Neurodegeneration
The composite measure of the density of tau positive neurofibrillary tangles had a positively skewed distribution (skewness = 2.0), with scores ranging from 0.001 to 30.5 (mean = 5.1, SD = 5.5). The composite measure of TDP-43 pathology ranged from 0 (in 53.3 % of persons) to 4.5 (mean=0.55, SD=0.90, skewness=1.9). Hippocampal sclerosis was present in 39 persons (9.3 %). Lewy bodies were present in 98 persons (23.3 %), with the stage rated as nigral in 10 (2.4%), limbic in 34 (8.1%), and neocortical in 54 (12.9%). TDP-43 pathology was related to tangles (r=0.31, p<0.001), hippocampal sclerosis (χ2 [1] = 71.7, p<0.001), and Lewy bodies (χ2 [1] = 5.5, p=0.019), but tangles, hippocampal sclerosis, and Lewy bodies were unrelated to one another.
Neurodegeneration and Global Cognition
The outcome for analyses was a composite measure of global cognition which was constructed by averaging z scores on 17 individual tests. At baseline, it had a mean of 0.274, which is greater than zero because persons with mild cognitive impairment and dementia were excluded; it had a standard deviation of 0.370, which is less than one because persons with cognitive impairment were excluded and the z scores of individual tests were correlated; and scores ranged from −0.845 to 1.419. Proximate to death, scores ranged from −4.145 to 1.658 (mean = −0.671, SD = 1.089). A lower level of global cognition proximate to death was associated with higher levels of tangles (r = −0.40, p <0.001) and TDP-43 pathology (r = −0.30, p < 0.001) and the presence of hippocampal sclerosis (t[42.7] = 4.3, p < 0.001) and Lewy bodies (t[132.6] = 4.1, p<0.001).
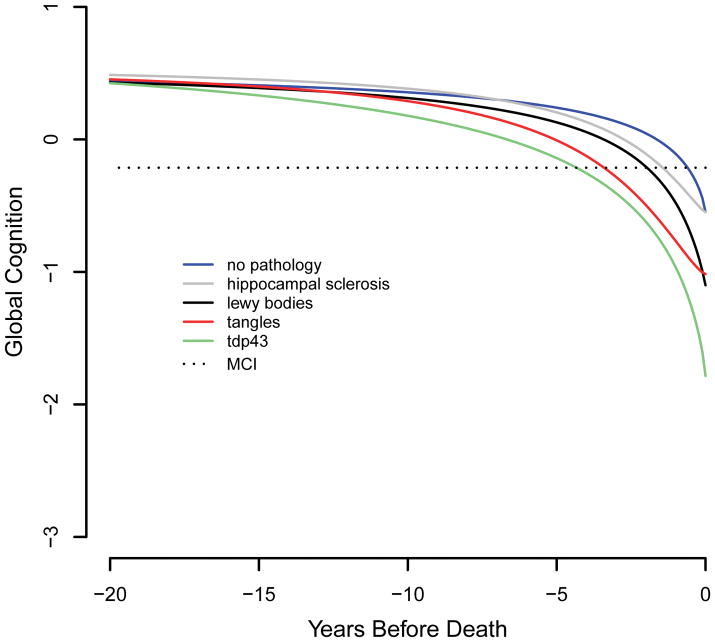
We estimated the relation of the pathologic markers to trajectories of global cognitive decline during a mean of 10.5 years of observation (SD=3.8) using a sigmoid mixed model adjusted for age at death, sex, and education (Table 2). The model results are portrayed in Figure 2. To provide a clinical context, the figure includes the mean global cognitive score associated with incident mild cognitive impairment for the entire cohort (mean estimate = −0.214, SD = 0.270). The figure shows that in the absence of these 4 forms of neurodegeneration (blue line), cognition is relatively stable until about 3–4 years prior to death, when gradual cognitive decline becomes evident.
Table 2.
Relation of Postmortem Neurodegenerative Markers to Trajectories of Global Cognitive Decline
| Model Parameter | Estimate | SE | p |
|---|---|---|---|
| Initial level | 0.577 | 0.042 | <0.001 |
| Midpoint of decline | 1.745 | 0.185 | <0.001 |
| Slope of decline | 0.804 | 0.080 | <0.001 |
| Final level | −0.549 | 0.117 | <0.001 |
| Tangles x decline | 0.136 | 0.016 | <0.001 |
| Tangles x post midpoint slope | 0.049 | 0.006 | <0.001 |
| Tangles x final level | −0.039 | 0.014 | 0.006 |
| TDP-43 pathology x initial level | 0.146 | 0.034 | <0.001 |
| TDP-43 pathology x midpoint of decline | 0.493 | 0.116 | <0.001 |
| TDP-43 pathology x final level | −0.618 | 0.122 | <0.001 |
| Hippocampal sclerosis x midpoint of decline | 1.114 | 0.315 | <0.001 |
| Hippocampal sclerosis x slope | 0.446 | 0.105 | <0.001 |
| Lewy bodies x slope | 0.152 | 0.069 | 0.027 |
| Lewy bodies x final level | −0.551 | 0.160 | <0.001 |
Note. Estimated from a sigmoidal mixed model adjusted for age at death, sex, and education. SE, standard error; TDP-43, transactive DNA-binding protein 43.
Figure 2.
Trajectories of change in the standardized composite measure of global cognition associated with no pathology (blue line), tangles only (orange line), TDP-43 pathology only (green line), hippocampal sclerosis only (gray line), or Lewy bodies only (black line), from a sigmoid mixed model adjusted for age at death, sex, and education.
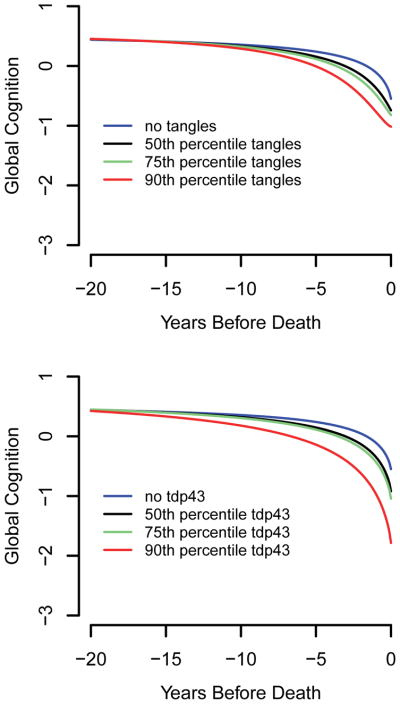
Tangles were related to each trajectory component except initial level of cognitive function. As shown in Figure 2 (red line, 90th percentile), the association of tangles with global cognition was not evident at the beginning of the observation period, which occurred a mean of 10.5 years before death, but emerged a few years later. The upper panel of Figure 3 shows that the degree of divergence of the tangle profile (black, green, and orange lines) from healthy cognitive aging (blue line) is dose dependent.
Figure 3.
Trajectories of change in the standardized composite measure of global cognition associated with different levels of tangles (upper panel) and TDP-43 pathology (lower panel) from sigmoid mixed models adjusted for age at death, sex, and education.
TDP-43 pathology had a unique cognitive footprint. As shown in Table 2, it was the only neuropathologic marker related to initial cognitive level and the only marker not related to the slope of cognitive decline. It was also associated with an earlier midpoint of decline. Figure 2 (green line, 90th percentile) suggests that the association of TDP-43 pathology with cognition emerges before the effects of the other neurodegenerative processes and several years before the mean start of the observation period in this study, with gradual acceleration thereafter. The lower panel of Figure 3 suggests that this effect is dose dependent.
Hippocampal sclerosis was related to 2 trajectory components, the midpoint and slope (Table 2). That is, individuals with hippocampal sclerosis reached the midpoint of their decline trajectory earlier and declined more rapidly than individuals without the condition, as shown in Figure 2 (gray line).
Lewy bodies were associated with two trajectory components, the slope of decline and the final level of cognition. As shown in Figure 2, the Lewy body cognitive profile (black line) did not diverge from healthy aging until a few years before death but had a strong negative association with cognition as death approached.
Multiple Forms of Neurodegeneration
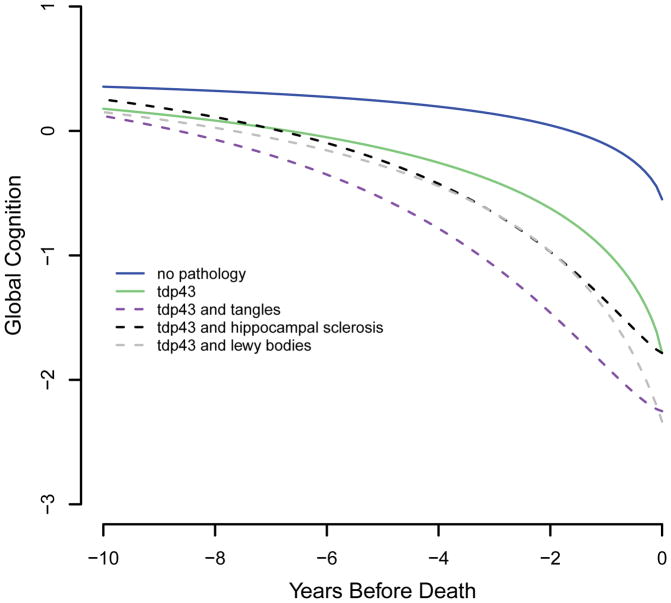
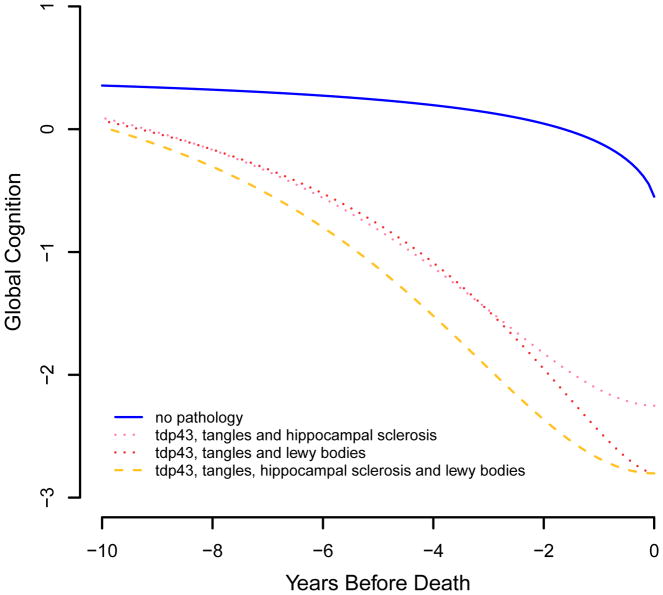
Although it is important to identify the longitudinal cognitive profiles associated with specific neurodegenerative processes, most late-life cognitive impairment reflects multiple pathologies, (Jicha et al., 2006; Schneider, Arvanitakis, Bang, & Bennett, 2007; Jellinger & Attems, 2010; Kovacs et al., 2013; Kawas et al., 2015). Therefore, we used the core model (Table 2) to estimate the cognitive trajectories of individuals with multiple forms of neurodegeneration, focusing on TDP-43 pathology because it is correlated with the other postmortem markers. As shown in Figure 4, cognitive decline was substantially more rapid in those with TDP-43 pathology plus a second neurodegenerative condition, especially tangles (dashed purple line), compared to those with only TDP-43 (solid green line) or no (solid blue line) pathology. Figure 5 shows that the addition of two (dotted pink and red lines) or three (dashed yellow lines) other neurodegenerative conditions is associated with even more precipitous cognitive decline.
Figure 4.
Trajectories of change in the standardized composite measure of global cognition associated with no pathology (blue solid line), TDP-43 pathology only (green solid line), TDP-43 pathology and tangles (purple dashed line), TDP-43 pathology and hippocampal sclerosis (black dashed line), and TDP-43 pathology and Lewy bodies (gray dashed line), from a sigmoid mixed model adjusted for age at death, sex, and education.
Figure 5.
Trajectories of change in the standardized composite measure of global cognition associated with no pathology (blue solid line); TDP-43 pathology, tangles, and hippocampal sclerosis (pink dotted line); TDP-43 pathology, tangles, and Lewy bodies (red dotted line); and TDP-43 pathology, tangles, hippocampal sclerosis, and Lewy bodies (yellow dashed line), from a sigmoid mixed model adjusted for age at death, sex, and education.
Discussion
Cognitive function was assessed annually for a mean of about one decade in a group of more than 400 older persons who were cognitively healthy at enrollment. At death, common neurodegenerative lesions were quantified in a uniform neuropathologic examination. Analyses linked postmortem neurodegenerative markers with components of longitudinal cognitive trajectories. The results support the hypothesis that specific forms of neurodegeneration have distinctive longitudinal cognitive profiles.
Knowledge about the cognitive trajectories associated with different neurodegenerative lesions is limited. To date, neuroradiologic methods have had limited success in assessing intracellular pathology though there have been advances in tau imaging (Villemagne, Fodero-Tavoletti, Masters, & Rowe, 2015). In addition, most clinical-pathologic studies have focused on cross-sectional cognitive outcomes or linear change in cognitive function, although much of cognitive decline in old age is nonlinear (Wilson et al., 2010; Proust-Lima, Amieva, & Jacqmin-Gadda, 2013). In previous mixed-effects analyses with a change point to accommodate nonlinear trajectories of cognitive decline, tangles (Wilson et al., 2010) and Lewy bodies (Wilson, Segawa, Buchman, et al., 2012) have been related to decline before and after the change point. However, the present analyses using data from the same parent studies found clear differences in how tangles, TDP-43 pathology, Lewy bodies, and hippocampal sclerosis were related to different components of late-life cognitive trajectories. Of particular note, the association of TDP-43 pathology with cognitive decline was evident substantially earlier than the effects of the other neurodegenerative conditions. To our knowledge, this has not been previously reported. It remains uncertain whether the impairment associated with TDP-43 pathology reflects a toxic effect or the loss of normal TDP-43 functions (Lee, Lee, & Trojanowski, 2012; Sloan & Barres, 2013). Though hardly definitive, the observation that the negative association of TDP-43 pathology with cognition appears to emerge early and progress slowly is arguably more consistent with a gradual loss of normal cellular function than a cytotoxic process. Here, as in previous research (Amador-Oritz et al., 2007; Arai et al., 2009; Robinson et al., 2011), TDP-43 pathology was related to other common neurodegenerative conditions raising the possibility that it somehow exacerbates them or marks a more virulent form of neurodegeneration associated with each pathology. Overall, the finding that different neurodegenerative lesions vary in their associations with components of late-life cognitive trajectories suggests a level of complexity in the timing of pathologic effects. This issue has received little focus in aging studies but may have important clinical implications. Effective interventions to prevent cognitive decline in old age will likely need to be multifaceted and varied in terms of the timing of their administration.
These data further underscore that multiple biological processes underlie late-life dementia. It is noteworthy that except at high levels a single neurodegenerative condition was not sufficient to cause severe cognitive impairment (Figure 2). In contrast, the presence of two (Figure 4) or more (Figure 5) neurodegenerative conditions was associated with precipitous decline in global cognition. These observations are consistent with previous reports that most dementia (Jicha et al., 2006; Schneider, Arvanitakis, Bang, & Bennett, 2007; Jellinger & Attems, 2010; Kovacs et al., 2013; Kawas et al., 2015) and mild cognitive impairment (Peterson et al., 2006; Markesbery, 2010) reflects multiple neuropathological conditions.
After statistical adjustment for the deleterious effects of these pathologies, there was little evidence of cognitive decline until about 3–4 years prior to death when the rate of decline accelerated, resulting in about 1 year of mild cognitive impairment. This supports the idea that loss of cognition in old age is mainly due to mortality related and neurodegenerative processes rather than to age related developmental processes.
This study has strengths and limitations. Participation in follow-up clinical evaluations and brain autopsy was high, reducing the likelihood that selective attrition biased results. Cognition was assessed with a psychometrically sound measure at regular intervals for a mean of a decade, enhancing our ability to reliably characterize nonlinear trajectories of change. The sigmoid mixed model provided a novel way to decompose nonlinear cognitive trajectories into component parts. A limitation is that participants were selected and predominantly White so that it will be important to replicate these findings in other groups. Despite apparent cognitive health at baseline and a decade of observation, it is likely that some cognitive decline predated the study, underscoring the need for longer term longitudinal studies.
Acknowledgments
This research was supported by NIH grants R01AG17917, P30AG10161, RF1AG15819, R01AG042210, R01AG33678, and R01AG34374, and by the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors thank the many Illinois residents for participating in the Rush Memory and Aging Project and the many Catholic nuns, priests, and monks for participating in the Religious Orders Study; Traci Colvin, MPH, and Karen Skish, MS, for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; and Alysha Kett, MS, for statistical programming.
References
- Albert M, Scherr P, Taylor J, Evans D, Funkensten H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. International Journal of Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Annals of Neurology. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Nizato K, Tsuchiya K, … Akiyama H. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathologica. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religous orders study. Current Alzheimer Research. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer Research. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias LL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer’s disease and level of cognitive function. Archives of Neurology. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Mild cognitive impairment is related to Alzheimer’s disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Annals of Neurology. 2012;72:599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, de Hamsher KS, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2. New York: Oxford University Press; 1994. [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer's disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Annals of Neurology. 2013;74:478–489. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidian M, Giltinan DM. Nonlinear models for repeated measurement data. London: Chapman & Hall; 1995. [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Kermen D. Manual for kit of factor- referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, … Trojanowski JQ. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Archives of Neurology. 2010;67:1238–1250. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, … Arai H. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Research. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathologica. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathologica. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, … Peterson RC. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Archives of Neurology. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, … Dickson DW. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, … Budka H. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathologica. 2013;126:365–384. doi: 10.1007/s00401-013-1157-y. [DOI] [PubMed] [Google Scholar]
- Lee EB, Lee VMY, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nature Reviews Neuroscience. 2012;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR. Neuropathologic alterations in mild cognitive impairment: a review. Journal of Alzheimer’s Disease. 2010;19:221–228. [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, … Perry RH. Consensus guidelines for clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of consortium of DLB International Workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Annals of Neurology. 2015;77:942–952. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, … Markesbery WR. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathology. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, … Kryscio RJ. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, Neltner JH, … Schmitt FA. Hippocampal sclerosis of aging, a prevalent and high morbidity brain disease. Acta Neuropathologica. 2013;126:161–177. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, … Lee VM. Phosphorylation of S409/410 in TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathologica. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RH, Irving D, Tomlinson BE. Lewy body prevalence in the aging brain: relationship to neuropsychiatric disorders, Alzheimer-type pathology and catecholarminergic nuclei. Journal of the Neurological Sciences. 1990;100:223–233. doi: 10.1016/0022-510x(90)90037-n. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, … Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Archives of Neurology. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Proust-Lima C, Amieva H, Jacqmin-Gadda H. Analysis of multivariate mixed longitudinal data: a flexible latent process approach. British Journal of Mathematical and Statistical Psychology. 2013;66:470–487. doi: 10.1111/bmsp.12000. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary: Standard Progressive Matrices. Oxford, England: Oxford Psychologists Press; 1992. [Google Scholar]
- Robinson JL, Geser F, Corrada MM, Berlau DJ, Arnold SE, Lee VM, … Trojanowski JQ. Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain. 2011;134:3708–3715. doi: 10.1093/brain/awr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135:3005–3014. doi: 10.1093/brain/aws234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Annals of Neurology. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- Sloan SA, Barres BA. Glia as primary drivers of neuropathology in TDP-43 proteinopathies. Proceedings of the National Academy of Science USA. 2012;110:4439–4440. doi: 10.1073/pnas.1301608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test manual-revised. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurology. 2015;14:114–124. doi: 10.1016/S1474-4422(14)70252-2. [DOI] [PubMed] [Google Scholar]
- Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T. Age-associated prevalence and risk factors of Lewy body pathology in a general population; the Hisayama study. Acta Neuropathologica. 2003;106:374–382. doi: 10.1007/s00401-003-0750-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part V: a normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society. 2005;11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging, Neuropsychology and Cognition. 2004;11:280–303. [Google Scholar]
- Wilson RS, Boyle PA, Yang J, James BD, Bennett DA. Early life instruction in foreign language and music and incidence of mild cognitive impairment. Neuropsychology. 2015;29:292–302. doi: 10.1037/neu0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chemical Senses. 2011;36:367–373. doi: 10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Buchman AS, Boyle PA, Hizel LP, Bennett DA. Terminal decline in motor function. Psychology and Aging. 2012;27:998–1007. doi: 10.1037/a0028182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA. TDP-43 pathology, cognitive decline, and dementia in old age. Journal of the American Medical Association Neurology. 2013;70:1418–1424. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai J, Brayne C, Matthews FE, Ince PG MRC Cognitive Function and Ageing Neuropathology Study. Alpha-synucleinopathy and neuropsychological symptoms in a population-based cohort of the elderly. Alzheimer’s Research & Therapy. 2015;7:19. doi: 10.1186/s13195-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]