Abstract
Neonatal encephalopathy due to intrapartum events occurs in approximately 1-2/1000 live births in high-income countries. Outcomes have improved over the past decade due to implementation of therapeutic hypothermia, the only clinically available neuroprotective strategy for hypoxic-ischemic encephalopathy (HIE). Neurocritical care is an emerging subspecialty that combines the expertise of neurology and critical care medicine. Neonatal encephalopathy is the most common condition treated within a neonatal neurocritical care unit. There is strong evidence for improved outcomes among adults treated by a specialized neurocritical care team. Neonates with encephalopathy may benefit from a neurocritical care approach through prevention of secondary brain injury by attention to basic physiology, earlier recognition and treatment of neurological complications such as seizures, consistent management using guidelines and protocols, and use of optimized teams at dedicated referral centers.
Keywords: Neurocritical Care, Infant, Critical care, Therapeutic Hypothermia, Neonatal seizures, Cerebral Palsy, Neonatal Encephalopathy, Hypoxic-Ischemic Encephalopathy
Introduction
Neonatal encephalopathy due to intrapartum events is estimated to occur in 1-2/1000 live births in high-income countries.1 Outcomes following neonatal encephalopathy due to birth asphyxia include death and neurological disabilities such as cerebral palsy, epilepsy and cognitive impairment.
Neonatal neurocritical care has emerged over the last decade as a subspecialty that involves a culture change toward a ‘brain-focused’ approach with all bedside providers (physicians, nurses, respiratory technologists and trainees) maintaining constant awareness of the potential neurological complications of critical illnesses, as well as the impact of management on the developing or injured brain. Several important advances have prompted this culture change, including increased survival from critical illness, as well as the advent of digital monitoring and safe, high-resolution magnetic resonance imaging (MRI). Conditions cared for in a neurocritical care unit include neonatal encephalopathy (and hypoxic-ischemic encephalopathy, HIE), seizures, intracranial hemorrhage, ischemic stroke and intracranial infection, among others. A neurocritical care approach to monitoring, diagnosis, and treatment of neurological conditions has been shown to improve outcomes among adults.2,3 In neonates, a neurocritical care approach may mitigate adverse outcomes among neonates with HIE by preventing secondary brain injury, rapid recognition and treatment of neurological complications like seizures, early identification of HIE mimics like neonatal onset epileptic encephalopathies, consistent management using guidelines and protocols, and use of optimized teams at dedicated referral centers, although long-term outcome studies are needed to show the benefits of this management.
Neonatal encephalopathy due to birth asphyxia, or HIE is the commonest condition treated by a neurocritical care service.4,5 Neonates with HIE require rapid implementation of neuroprotection with hypothermia, have high rates of multi-organ failure, as well as neurological signs and symptoms such as encephalopathy, seizures and brain injury. Therefore, this condition lends itself to the neurocritical care approach. In principle, a neuro-intensive care nursery can lessen adverse outcomes as a result of prevention of secondary brain injury through attention to basic physiology, earlier recognition and treatment of neurological complications such as seizures, consistent management using guidelines and protocols, and use of optimized teams at dedicated referral centers, as discussed below.6 Moreover, the neuro-intensive care nursery can also serve as an ideal platform for research. Early diagnosis will allow interventions during critical neuroplasticity windows7–9, high intensity therapies10, and patient stratification for novel interventions. For example, recent early phase safety studies have evaluated hypothermia combined with administration of potential biological (e.g., erythropoietin11,12), inhaled (e.g., Xenon13) and cell-based (e.g., cord blood stem cells14) therapeutics.
Establishing a Neuro-Intensive Care Nursery
The neurocritical care approach involves a culture shift for the entire Neonatal Intensive Care Unit (NICU) toward brain-focused care, such that providers at every level are continually aware of the potential neurological complications of critical illnesses and the impact of their management strategies on the developing brain. From the time of birth through patient discharge, the neonatal neurocritical care team serves to prevent secondary injury, implement neuroprotective strategies including therapeutic hypothermia, manage neurological complications, optimize developmental care, and establish outpatient developmental services and high-risk follow-up.
In order to establish a neuro-intensive care nursery (NICN), a leadership team (with representatives from neonatology, neurology and nursing) must work together to establish a program for the following core functions of the unit:
Training and education for all providers, including physicians, nurses, nurse practitioners and respiratory therapists
Local guidelines for management of neonatal encephalopathy (including resuscitation, implementation and maintenance of hypothermia, and use of extra corporeal membrane oxygenation), as well as neurological monitoring and treatment of complications, including use of electroencephalogram (EEG) amplitude–integrated EEG (aEEG), seizure treatment, and brain imaging using magnetic resonance (MRI)
Ensuring adequate resources, equipment and training for brain monitoring, imaging and application of hypothermia
Community outreach and education to foster timely referrals
Current neuro-intensive care nurseries are closed units with the neonatologist acting as the physician of record and the neurologist acting as a consultant with an active role in decision-making and communication with the family. The NICN itself may have dedicated or a specific area within the NICU, or else operate ‘virtually’ with a team that can operate at any bedside.
Role of the Neonatologist
The neonatologist typically acts as the physician of record and identifies neonates who are eligible for hypothermia and consultation by the NICN team. Neonatologists will perform the initial resuscitation and manage the patient with close attention to physiological homeostasis with a focus on cardiopulmonary support, maintaining normal electrolyte and glucose levels, as well as temperature control to minimize secondary brain injury (Table 1).
Table 1.
Preventing secondary brain injury
| Parameter | Approach |
|---|---|
| Temperature |
|
| Ventilation |
|
| Blood pressure |
|
| Glucose |
Role of the Neurologist
The neurologist takes an early active role from the time of the initial presentation of neurological signs or symptoms. For neonates with encephalopathy due to birth asphyxia, the neurologist is notified at the time of referral or admission. At most centers, the neonatologist makes the decision whether or not to initiate cooling therapy, conferring with the neurologist as needed. The neurologist then serves to document a detailed neurological examination, as well as guide the initial investigation and management decisions, including rapid implementation of hypothermia (if not initiated at the referral center or during transport). The neurologist will often consider other causes of neonatal encephalopathy such as congenital brain anomalies, intracranial infection or hemorrhage, inborn errors of metabolism, neonatal onset epilepsy and other genetic conditions, and plan additional investigations accordingly.
At the time of admission, the neurologist serves to coordinate with the neurophysiology service for application and interpretation of EEG, and urgent cranial imaging if needed (e.g., suspicion for hemorrhage). Along with the neonatologist, the neurologist manages the patient and communicates with the family during the period of critical illness. The neurologist is key in providing guidance for seizure therapy and coordinating with the neuroradiologist to ensure appropriate imaging protocols. Finally, the neurologist perspective is especially important when discussing prognosis and neurological follow-up with the family, and the neurologist assists with planning outpatient services such as physical and occupational therapy or Early Start program, especially if the child is expected to have a long-term disabling neurological condition.
Role of the Specialized Neuro-Intensive Bedside NICU Nurse
The bedside nurse has a vital role in the neuro-intensive care nursery program.15 Didactic and hands on education to care for neonates with neurological conditions distinguishes the specialized neurological nurse from the general NICU nursing pool. S/he learns to recognize neurological signs and symptoms, as well as interpret the aEEG so that the physician can be alerted at the first sign of clinical or electrographic seizure, or worsening of encephalopathy. The bedside nurse can help to optimize care by quickly setting up the cooling blanket and EEG/aEEG machine, which allows for faster treatment. In addition, nurses learn to adhere to management guidelines and anticipate next steps in care, safely transport critically ill neonates to the MR scanner, and communicate effectively with families.
Preventing secondary injury
Perinatal asphyxia puts the neonate at risk for end organ failure, which can lead to cardiopulmonary instability, inadequate brain perfusion and hypoglycemia. Hypotension, hypoxemia, hypocarbia, hyperthermia, and hypo/hyperglycemia can exacerbate brain injury and so these parameters must be carefully monitored and actively managed by all members of the neurocritical care team from the time of birth (Table 1).
Implementing Therapeutic Hypothermia
Neonatal encephalopathy is the most common condition managed by a neurocritical care service, and therapeutic hypothermia is among the most common treatments.5,21 Several randomized controlled trials have shown that treatment with hypothermia leads to lower rates of death or disability at 18–24 months of age (RR 0.75, 95%CI 0.68–0.83), and the benefit appears to be sustained through school age.22–24
Treatment of neonatal encephalopathy in the setting of a specialized neuro-intensive care nursery can offer the following benefits:
Quicker onset of cooling by an experienced team
Rapid, around the clock detection and treatment of seizures
High quality brain imaging
Counseling for parents by experienced physicians and nurses
Timely and accurate diagnosis of conditions that can mimic HIE, such as neonatal onset epilepsies, inborn errors of metabolism and congenital central and peripheral nervous system disorders
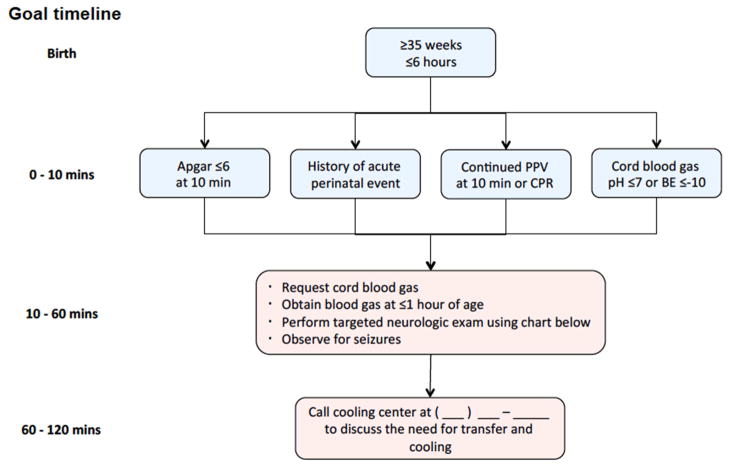
Screening tools, such as the hypothermia toolkit by the California Perinatal Quality Care Collaborative (CPQCC) can help outlying centers to quickly identify neonates who may benefit from hypothermia (Figure 1).25 Both animal and human studies show that early initiation of therapy is associated with improved outcomes, and so rapid implementation of hypothermia is critical.26–28 Implementation of hypothermia at the referral center or by the transport team is safe. Use of a portable servo-controlled cooling device on transport provides more stable temperature management with a higher percentage of temperatures within the target range as compared to neonates who are passively cooled.29
Figure 1.
Screening Criteria for Evaluation of Risk for Neonatal Encephalopathy (NE)
Guidelines and protocols that are site specific and endorsed by neonatology, neurology and nursing can help to standardize the approach to implementation of therapeutic hypothermia (Table 2).
Table 2.
Guidelines for therapeutic hypothermia
| Guideline | Examples of Guideline Contents |
|---|---|
| Therapeutic hypothermia |
|
| Seizures |
|
| Imaging |
|
Managing Neurological Complications
Although recent reports suggest that the burden of seizures among neonates undergoing hypothermia is lower than for neonates who are not cooled, the risk remains approximately 50%.30–32 Neonates with encephalopathy due to perinatal asphyxia should receive neurophysiology monitoring using continuous, video EEG and/or a simplified montage amplitude-integrated EEG (aEEG) monitoring for bedside use. Continuous neurophysiology monitoring is important to evaluate dynamic change in background brain activity and degree of encephalopathy, as well as seizures. Clinical indicators such as resuscitation parameters and degree of encephalopathy do not appear to be associated with risk of seizures. An abnormal initial EEG background (i.e., excessively discontinuous, burst suppression, depressed and undifferentiated or extremely low voltage) is associated with >60% seizure risk. Neonates with a normal initial EEG background have the lowest risk of seizures (~10%).30 The EEG and aEEG recordings also provide important prognostic information that can be used to start counseling parents regarding risk of disability and goals of care. Early normal or mildly abnormal EEG/aEEG is reassuring for a good prognosis, whereas an early severely abnormal EEG/aEEG (e.g., burst suppression, depressed and undifferentiated, extremely low voltage or status epilepticus at the onset of recording) is associated with a poor prognosis and brain injury if it persists beyond 24–36 hours of life.33,34
aEEG and full montage EEG can be recorded using the same system. The aEEG can be displayed at the bedside for the neurocritical care team and the full montage EEG sent to remote servers for access in the neurophysiology lab or personal device. The limited montage of the aEEG can be easily applied at the time of admission so that the bedside nurse and neonatology team can quickly assess the degree of encephalopathy and for the presence of seizures. The full montage EEG is applied as soon as a technician is available. The aEEG is then available as a screening tool for the bedside neurocritical care team and yet the EEG is available to the neurophysiologist as the gold standard to confirm presence or absence of seizures and detect seizures that are not visible on the aEEG recording.35
Neonates undergoing hypothermia are at high risk for brain injury. Magnetic resonance imaging (MRI) is an important tool to assess the location and severity of injury, and to rule out other causes of encephalopathy (e.g., dysgenesis).36,37 Furthermore, moderate-severe injury on MRI is associated with a high risk of death or disability among neonates with moderate to severe injury.38 The neurocritical care team should be prepared to safely take a critically ill neonate to the MRI scanner. Resources for safe transport include MRI compatible incubators, ventilators and cardiopulmonary monitoring equipment, as well as skilled staff who have completed training and mock codes in the MRI suite. The optimal timing of MRI may depend on the resources of the neurocritical care team. Since the appearance of the injury evolves over time, neonates at a given center should be imaged within a standard time frame. Imaging neonates just after cooling has ended (day 4–6) offers several advantages:
Lower need for sedation as the neonate often remains encephalopathic
Serves as a good turning point between the neurocritical care phase of the admission and convalescence
MRI can be performed prior to discharge home
At some centers, the second week of life is the preferred timing for imaging as there are rare reports that the brain injury can evolve over this time period. In order to mitigate issues related to timing of imaging, it is our practice to repeat imaging in a neonate whose early scan is normal but who remains encephalopathic after the first 5–7 days after birth or if the results of ancillary testing are discordant (i.e., very abnormal neurological examination or EEG results and/or difficulty establishing feeding and with a normal MRI).
Palliative Care
Unfortunately therapeutic hypothermia does not prevent death or developmental disabilities in all patients with neonatal encephalopathy due to birth asphyxia; approximately 50% have adverse outcome.39 When a neonate has multi-organ failure that is not compatible with life, and/or is expected to develop severe and permanent developmental disabilities, the neurocritical care team may wish to discuss the option of transition to a palliative approach. Using information from the neurological examination, EEG and aEEG, and MRI, an experienced team can predict those children that are likely to suffer severe disabilities, and counsel the family accordingly.40 The entire neurocritical care team, including the neonatologist, neurologist and bedside nurse must work together to provide a consistent message to the family and provide compassionate supportive care.
Compassion fatigue and burn out are common among bedside providers who frequently care for children with adverse outcomes. All members of the team should be given the option to request a different patient assignment in case of ethical concerns or compassion fatigue. An important aspect of the neuro-intensive care nursery is to provide specialized neurological nurses with adequate breaks, psychological support and a safe space to debrief difficult cases, as well as updates on children with good outcomes.
Optimizing Developmental Care
Once the neonate with encephalopathy has recovered from the critical illness, the focus of the neurocritical care team should turn toward achieving oral feeds and optimizing developmental outcomes. Inpatient services include consultation with physical and occupational therapists, as well as lactation consultants. Neonates with neurological disorders may need assistance with state regulation, positioning, and oral feeding readiness and preparation, as well as optimizing tone, strength and ability to take in external stimuli. The family should also learn about developmentally appropriate exercises (e.g., upright positioning, tummy time, language exposure, and early exposure to fine motor tasks). Enriched environments can provide the intensive, repetitive, task-specific interventions that are needed for improved outcomes.7,8,41,42
Outpatient Developmental Services and Neurological Care
Survivors of neonatal encephalopathy due to perinatal asphyxia are at high risk for long-term disabilities, including cerebral palsy, epilepsy and intellectual or learning disabilities. A neonatal neuro-intensive care program should make provisions for outpatient care by a neurologist and/or high-risk infant program. The American Academy of Pediatrics recommends that longitudinal neurodevelopmental outcome be monitored in all neonates that undergo hypothermia.43 While practically speaking, this means follow up until 18–24 months of age, this is inadequate to capture major learning milestones. Consideration of follow up through age 6 is encouraged to better evaluate the ultimate impact of neonatal neuro-intensive care interventions.
Summary/Discussion
Neonates with encephalopathy are often critically ill with multi-organ failure. They are at high risk for brain injury and seizures, which can lead to death or long-term disabilities. A neonatal neuro-intensive care nursery can optimally support neonates by providing brain-focused care from the time of resuscitation through discharge home. Members of the neurocritical care team include a neonatologist, neurologist and specialized bedside nurse. Guidelines and protocols can help to standardize care and optimize therapies. Early recognition and treatment of neurological complications such as seizures, as well as prevention of secondary brain injury through attention to basic physiology can minimize brain injury. Finally, experienced teams at dedicated referral centers provide the specialized care that children and parents need, which includes close follow up to address late-emerging issues.
Synopsis.
Neonatal encephalopathy due to intrapartum events is estimated at 1-2/1000 live births in high-income countries. Outcomes have improved over the past decade due to implementation of therapeutic hypothermia, the only clinically available neuroprotective strategy for hypoxic-ischemic encephalopathy (HIE). Neonatal encephalopathy is the most common condition treated within a neonatal neurocritical care unit. There is strong evidence for improved outcomes among adults treated by a specialized neurocritical care team. Neonates with encephalopathy benefit from a neurocritical care approach due to prevention of secondary brain injury through attention to basic physiology, earlier recognition and treatment of neurological complications such as seizures, consistent management using guidelines and protocols, and use of optimized teams at dedicated referral centers.
Key Points.
In neonatal neurocritical ‘brain-focused’ care units, all bedside providers maintain constant awareness of the neurological complications of critical illnesses, and the impact of management on the developing brain.
Neonatal encephalopathy due to birth asphyxia, or hypoxic-ischemic encephalopathy (HIE) is the commonest condition treated by a neonatal neurocritical care service.
A neurocritical care approach may mitigate adverse outcomes among neonates with HIE by preventing secondary brain injury, rapid recognition and treatment of neurological complications, consistent management using guidelines and protocols, and use of optimized teams at dedicated referral centers.
Best Practices Box.
What is the current practice?
Therapeutic hypothermia is standard of care for neonates with encephalopathy due to perinatal asphyxia who would have fulfilled inclusion and exclusion criteria for the clinical trials.
Best Practice/Guideline/Care Path Objective(s)
Neonates with encephalopathy should be quickly identified and transferred to a center with experience in management of multi-organ failure, neurological complications, and therapeutic hypothermia.
What changes in current practice are likely to improve outcomes?
Careful attention to basic physiology, including temperature regulation, glucose homeostasis, oxygenation, and blood pressure support to prevent secondary injury;
Use of protocols and/or guidelines;
Early recognition and treatment of neurological complications;
Management by an experienced, multidisciplinary neurocritical care team in a dedicated referral unit.
Is there a Clinical Algorithm?
Major Recommendations
Establish an experienced team of neonatologists, neurologists and bedside nurses to manage neonates with encephalopathy due to perinatal asphyxia
Establish guidelines to manage implementation of therapeutic hypothermia, brain monitoring, seizure treatment and brain imaging.
Clinical Algorithm(s)
Rating for the Strength of the Evidence
Bibliographic Source(s)
Summary Statement
A neonatal neuro-intensive care nursery can optimally support neonates with encephalopathy due to birth asphyxia by providing brain focused care from the time of resuscitation through discharge home.
Footnotes
Disclosures
The authors have no financial conflicts of interest to disclose. HCG is supported by the NINDS K23NS066137 and the Neonatal Brain Research Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(Suppl 1):50–72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egawa S, Hifumi T, Kawakita K, et al. Impact of neurointensivist-managed intensive care unit implementation on patient outcomes after aneurysmal subarachnoid hemorrhage. Journal of critical care. 2015 doi: 10.1016/j.jcrc.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Josephson SA, Douglas VC, Lawton MT, English JD, Smith WS, Ko NU. Improvement in intensive care unit outcomes in patients with subarachnoid hemorrhage after initiation of neurointensivist co-management. J Neurosurg. 2010;112:626–30. doi: 10.3171/2009.8.JNS09441. [DOI] [PubMed] [Google Scholar]
- 4.Glass HC, Bonifacio SL, Shimotake T, Ferriero DM. Neurocritical care for neonates. Curr Treat Options Neurol. 2011;13:574–89. doi: 10.1007/s11940-011-0144-7. [DOI] [PubMed] [Google Scholar]
- 5.Mulkey SB, Swearingen CJ. Advancing neurologic care in the neonatal intensive care unit with a neonatal neurologist. J Child Neurol. 2014;29:31–5. doi: 10.1177/0883073812469051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon F, Mayer SA. Neurocritical care: a distinct discipline? Current opinion in critical care. 2007;13:115–21. doi: 10.1097/MCC.0b013e32808255c6. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd RB. Cerebral Palsy in Infancy. New York: Churchill Livingstone Elsevier; 2014. [Google Scholar]
- 8.Morgan C, Novak I, Badawi N. Enriched environments and motor outcomes in cerebral palsy: systematic review and meta-analysis. Pediatrics. 2013;132:e735–46. doi: 10.1542/peds.2012-3985. [DOI] [PubMed] [Google Scholar]
- 9.Novak I. Evidence-Based Diagnosis, Health Care, and Rehabilitation for Children With Cerebral Palsy. J Child Neurol. 2014;29:1141–56. doi: 10.1177/0883073814535503. [DOI] [PubMed] [Google Scholar]
- 10.Kolb B, Muhammad A. Harnessing the power of neuroplasticity for intervention. Frontiers in human neuroscience. 2014;8:377. doi: 10.3389/fnhum.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130:683–91. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers EE, Bonifacio SL, Glass HC, et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol. 2014;51:657–62. doi: 10.1016/j.pediatrneurol.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azzopardi D, Robertson NJ, Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)00347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotten CM, Murtha AP, Goldberg RN, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164:973–9. e1. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass HC, Rogers EE, Peloquin S, Bonifacio SL. Interdisciplinary approach to neurocritical care in the intensive care nursery. Semin Pediatr Neurol. 2014;21:241–7. doi: 10.1016/j.spen.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 17.Kasdorf E, Perlman JM. Strategies to prevent reperfusion injury to the brain following intrapartum hypoxia-ischemia. Semin Fetal Neonatal Med. 2013;18:379–84. doi: 10.1016/j.siny.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Wong DS, Poskitt KJ, Chau V, et al. Brain injury patterns in hypoglycemia in neonatal encephalopathy. AJNR Am J Neuroradiol. 2013;34:1456–61. doi: 10.3174/ajnr.A3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam EW, Haeusslein LA, Bonifacio SL, et al. Hypoglycemia is associated with increased risk for brain injury and adverse neurodevelopmental outcome in neonates at risk for encephalopathy. J Pediatr. 2012;161:88–93. doi: 10.1016/j.jpeds.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filan PM, Inder TE, Cameron FJ, Kean MJ, Hunt RW. Neonatal hypoglycemia and occipital cerebral injury. J Pediatr. 2006;148:552–5. doi: 10.1016/j.jpeds.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–9. doi: 10.1007/s12028-009-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–9. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 24.Shankaran S. Outcomes of hypoxic-ischemic encephalopathy in neonates treated with hypothermia. Clin Perinatol. 2014;41:149–59. doi: 10.1016/j.clp.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Early Screening and Identification of Candidates for Neonatal Therapeutic Hypothermia Toolkit. California Perinatal Quality Care Collaborative, 2015. 2015 at https://www.cpqcc.org/qi-tool-kits/early-screening-and-identification-candidates-neonatal-therapeutic-hypothermia-toolkit.
- 26.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 27.Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology. 2013;104:228–33. doi: 10.1159/000353948. [DOI] [PubMed] [Google Scholar]
- 28.Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2006;3:154–69. doi: 10.1016/j.nurx.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akula VP, Joe P, Thusu K, et al. A Randomized Clinical Trial of Therapeutic Hypothermia Mode during Transport for Neonatal Encephalopathy. J Pediatr. 2015 doi: 10.1016/j.jpeds.2014.12.061. [DOI] [PubMed] [Google Scholar]
- 30.Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: A multicenter cohort study. Neurology. 2014;82:1239–44. doi: 10.1212/WNL.0000000000000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Low E, Boylan GB, Mathieson SR, et al. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F267–72. doi: 10.1136/archdischild-2011-300716. [DOI] [PubMed] [Google Scholar]
- 32.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8. doi: 10.1177/0883073810390036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–62. doi: 10.1212/WNL.0b013e31820af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–9. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 35.Glass HC, Wusthoff CJ, Shellhaas RA. Amplitude-integrated electro-encephalography: the child neurologist’s perspective. J Child Neurol. 2013;28:1342–50. doi: 10.1177/0883073813488663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mrelashvili A, Bonifacio SL, Rogers EE, Shimotake TK, Glass HC. Outcome After Therapeutic Hypothermia in Term Neonates With Encephalopathy and a Syndromic Diagnosis. J Child Neurol. 2015;30:1453–8. doi: 10.1177/0883073815569307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felix JF, Badawi N, Kurinczuk JJ, Bower C, Keogh JM, Pemberton PJ. Birth defects in children with newborn encephalopathy. Dev Med Child Neurol. 2000;42:803–8. doi: 10.1017/s0012162200001493. [DOI] [PubMed] [Google Scholar]
- 38.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 40.Bonifacio SL, deVries LS, Groenendaal F. Impact of hypothermia on predictors of poor outcome: How do we decide to redirect care? Semin Fetal Neonatal Med. 2015 doi: 10.1016/j.siny.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Physical therapy. 2006;86:1534–40. doi: 10.2522/ptj.20050397. [DOI] [PubMed] [Google Scholar]
- 42.Novak I, McIntyre S, Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. 2013;55:885–910. doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- 43.Newborn ACoFa. Hypothermia and Encephalopathy. Pediatrics. 133:1146–50. doi: 10.1542/peds.2014-0899. [DOI] [PubMed] [Google Scholar]