Abstract
Nanomolar intravascular concentrations of drag-reducing polymers (DRP) have been shown to improve hemodynamics and survival in animal models of ischemic myocardium and limb, but the effects of DRP on the cerebral microcirculation have not yet been studied. We recently demonstrated that DRP enhance microvascular flow in normal rat brain and hypothesized that it would restore impaired microvascular perfusion and improve outcomes after focal ischemia and traumatic brain injury (TBI). We studied the effects of DRP (high molecular weight polyethylene oxide, 4,000 kDa, i.v. at 2 µg/mL of blood) on microcirculation of the rat brain: 1) after permanent middle cerebral artery occlusion (pMCAO); and 2) after TBI induced by fluid percussion. Using in-vivo two-photon laser scanning microscopy (2PLSM) over the parietal cortex of anesthetized rats we showed that both pMCAO and TBI resulted in progressive decrease in microvascular circulation, leading to tissue hypoxia (NADH increase) and increased blood brain barrier (BBB) degradation. DRP, injected post insult, increased blood volume flow in arterioles and red blood cell (RBC) flow velocity in capillaries mitigating capillary stasis, tissue hypoxia and BBB degradation, which improved neuronal survival (Fluoro-Jade B, 24 hours) and neurologic outcome (Rotarod, 1 week). Improved microvascular perfusion by DRP may be effective in the treatment of ischemic stroke and TBI.
1 Introduction
Although ischemic stroke and traumatic brain injury (TBI) arise from very different initial insults, the mechanisms involved in the injury process have many similarities [1]. Among them is a reduction in cerebral blood flow (CBF) leading to microvascular circulation impairment and deprivation of oxygen and glucose delivery to tissue.
Currently, there are no approved therapies targeting impaired CBF after TBI and only one targeting impaired CBF after ischemic stroke, but that one is limited by a short treatment window.
Rheological modulation of the blood circulation by minute quantities of drag reducing polymers (DRP) was demonstrated to improve circulation and survival in animal models of hemorrhagic shock, ischemic myocardium and limbs. However, except for one qualitative study [2], the effects of DRP on the impaired cerebral circulation have not yet been studied. We recently showed that DRP enhanced microvascular flow and tissue oxygenation in a healthy rat brain. Here, we examine the acute effects of DRP on impaired cerebral microcirculation and oxygen delivery after TBI induced by fluid percussion, and ischemic stroke induced by permanent middle cerebral artery occlusion (pMCAO), and the translation of these effects into long-term neurologic outcome and function.
2 Methods
2.1 Study Paradigm
Most of the procedures used in these studies were previously described [3, 4]. The fluid percussion was used as a model of TBI and was induced by 1.5 ATA 50 ms pulse from a custom-built Pneumatic Impactor connected to the brain through a pressure transducer filled with artificial cerebrospinal fluid [5]. The suture pMCAO was used as a model of ischemic injury [6].
The acute effects of DRP on microvascular blood flow velocity, tissue oxygenation (NADH) and blood brain barrier (BBB) permeability were measured on laboratory acclimated, male, Sprague-Dawley rats weighing 250–300 grams by two-photon laser scanning microscopy (2PLSM) under 2% isoflurane/69%nitrous oxide/28% oxygen anesthesia via a cranial window over the left parietal cortex. After baseline imaging, TBI or pMCAO was induced and, after post-insult imaging and DRP (2 µg/ml in blood) or saline i.v. injection, the consequent imaging was followed during 4 hours. Doppler flux was measured via a lateral temporal window using a 0.9 mm diameter probe (DRT4, Moor Inst., Axminster, UK) in the same region of the brain studied by 2PLSM. Brain and rectal temperatures were monitored and controlled at 38±0.5°C. Arterial blood gases, electrolytes, hematocrit and pH were measured hourly.
Neurodegeneration was examined by Fluoro-Jade B staining at 24 hours after insult [7]. Coordination and motor deficits were evaluated by Rotarod at one week after insults [8]. Time to dismount with increasing speed of rotation was used as a measure of deficit.
2.2 Two-Photon Laser Scanning Microscopy
Fluorescent serum (tetramethylrhodamine isothiocyanate (TAMRA) dextran, 500 kDa in physiological saline, 5% wt/vol) was visualized using an Olympus BX 51WI upright microscope and water-immersion LUMPlan FL/IR 20X/0.50 W objective. Excitation was provided by a PrairieView Ultima multiphoton microscopy laser scan unit powered by a Millennia Prime 10 W diode laser source pumping a Tsunami Ti:Sapphire laser (Spectra-Physics, Mountain View, CA, USA) tuned to 750 nm center wavelength. Band-pass-filtered epifluorescence (560–660 nm for TAMRA and 425–475 nm for NADH) was collected by photomultiplier tubes of the Prairie View Ultima system. Images (512 X 512 pixels, 0.15 um/pixel in the x- and y-axes) or line scans were acquired using Prairie View software. Red blood cell flow velocity was measured in microvessels ranging from 3–50 µm diameter up to 500 µm below the surface of the parietal cortex as previously described [3, 4]. Tissue hypoxia was assessed by measurement of NADH autofluorescence and BBB permeability by TAMRA transcapillary extravasation. In offline analyses using NIH ImageJ software, three-dimensional anatomy of the vasculature in areas of interest were reconstructed from two-dimensional (planar) scans of the fluorescence intensity obtained at successive focal depths in the cortex (XYZ stack).
Statistical analyses were done by Student’s t-test or Kolgomorov-Smirnov test where appropriate. Differences between groups were determined using two-way analysis of variance (ANOVA) for multiple comparisons and post hoc testing using the Mann-Whitney U-test. Statistical significance level was set at P<0.05.
3 Results
3.1 Acute effects on cerebral blood flow and metabolism
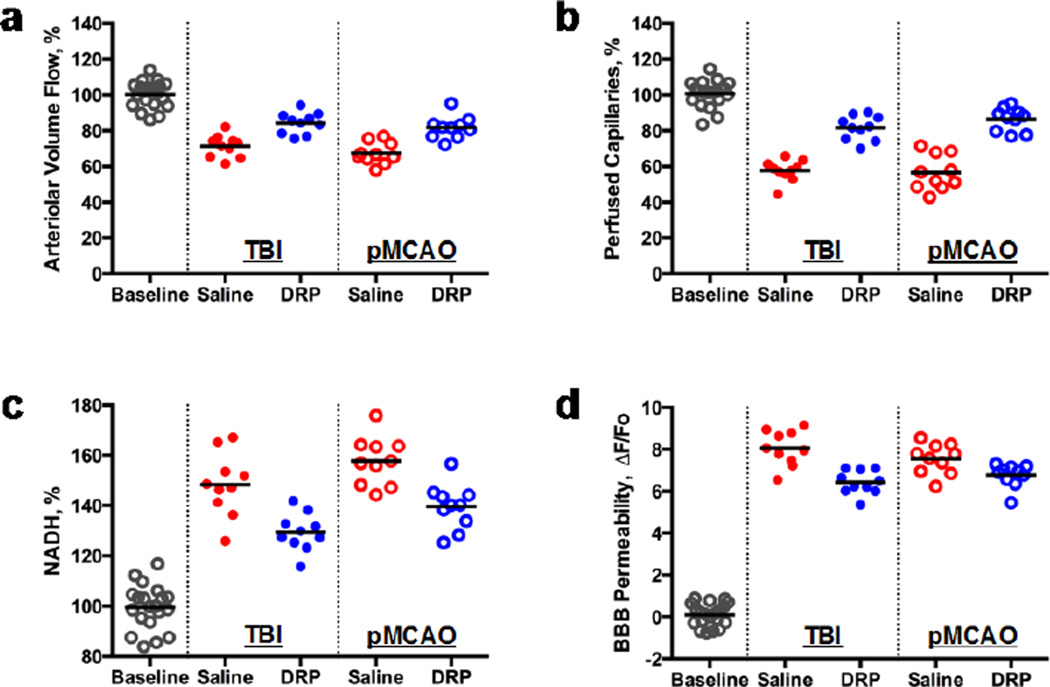
Both TBI and pMCAO progressively decreased microvascular circulation with development of tissue hypoxia and BBB damage in peri-injury or penumbra zones of the rat brain, respectively. DRP injection significantly increased arteriolar blood volume flow in both, TBI (from 71.3 ± 10.5% to 84.3 ± 9.4%) and pMCAO (from 67.3 ± 11.3% to 81.8 ± 10.2%) that was not observed in saline-treated animals (Fig. 1a, p < 0.05). The increase in arteriolar blood volume flow restored circulation in collapsed capillaries by 24 ± 6.7% and 31.0 ± 7.1% compared to saline after TBI and pMCAO, respectively (Fig. 1b, p < 0.05). The improved capillary perfusion increased tissue oxygenation reflected by a decrease of NADH autofluorescence by 18 ± 4.6% and 17.0 ± 6.5% comparing saline after TBI and pMCAO, respectively (Fig. 1c, p < 0.05). DRP also reduced BBB damage in microvessels, as quantified by measurement of perivascular tissue fluorescence reflecting the rate of TAMRA dextran extravasation. The fluorescence ratios (ΔF/Fo[pre-injury]) were 8.7 ± 0.8 vs. 7.1 ± 0.9 for TBI saline vs. DRP and 8.3 ± 0.9 vs. 7.2 ± 0.6 for pMCAO saline vs. DRP, respectively (Fig. 1d, p < 0.05).
Fig. 1.
Intravenous injection of DRP in acute period of TBI or pMCAO: (a) increases arteriolar volume flow; (b) increases number of perfused capillaries; (c) improves tissue oxygenation (NADH); and (d) attenuates BBB damage (N=10 rats per group).
3.2 Long-term outcome
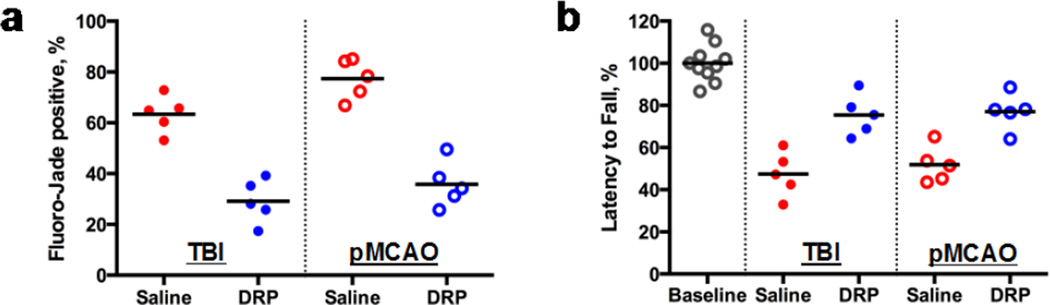
The microvascular flow and tissue oxygenation improved by DRP reduced neurodegeneration by 37.6 ± 11.2% (TBI) and 44.2 ± 12.6% (pMCAO) compared to saline as measured 24 hours post-insult by Fluoro-Jade B staining (Fig. 2a, p<0.05). Evaluation of neurological motor deficits by Rotarod tests at one week after insults revealed that DRP-treated rats performed significantly better than saline-treated animals. Rotarod latency times were 75.6 ± 16.0% vs. 47.3 ± 15.2%, for TBI-DRP vs. TBI-saline rats and 78.2 ± 19.0% vs. 51.4 ± 17.3%, for pMCAO-DRP vs. pMCAO-saline from a baseline, respectively (Fig. 2b, p<0.05).
Fig. 2.
(a) Intravenous injection of DRP reduces neurodegeneration as measured 24 hours post-insult by Fluoro-Jade B staining and (b) improves neurologic outcome as measured by Rotarod test for motor and coordination deficits 1 week after TBI or pMCAO (N=5 rats per group).
4 Discussion
After TBI or ischemic stroke, DRP improve impaired cerebral microvascular perfusion thereby reducing tissue hypoxia and BBB degradation and protecting neurons from death, which translates into improved neurologic outcome. Based on our data and previous in-vitro [9, 10] and in-vivo [11] studies, we could speculate that the mechanisms of increasing the arteriolar blood volume flow include an increase of flow velocity by reduction of flow separations and vortices at vessel bifurcations and increasing a pressure gradient across the arterial vessel network due to the viscoelastic properties of DRP. This leads to a rise in the pre-capillary blood pressure thus enhancing capillary perfusion and countering capillary stasis.
DRP also reduce the near-wall cell-free layer increasing wall shear stress and decreasing plasma skimming at vessel bifurcations (i.e., lowering hematocrit in daughter branches of microvessels), described in vitro [12, 13] and in vivo [14] which could explain protection of the BBB. Recent studies reported that increased wall shear stress restrains expression of proinflammatory cytokines that initiate a signaling cascade leading to activation of BBB damage [15, 16]
Due to the absence of effective therapies for impaired cerebral microcirculation leading to tissue hypoxia, despite the numerous pharmacological treatments successfully tested in animals that failed clinically, the use of DRP is a promising approach for the treatment of TBI, ischemic stroke and other low CBF pathologic conditions.
Acknowledgments
This work was supported by AHA 14GRNT20380496 and NIH 8P30GM103400 and R21NS091600
References
- 1.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24(2):133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 2.Gannushkina IV, Grigorian SS, Kameneva MV, et al. Possibility of restoring the cerebral blood flow in cerebral ischemia by injecting special polymers into the blood. Pat. Fiz. Eksp Ter. 1982;(3):58–59. [PubMed] [Google Scholar]
- 3.Bragin DE, Bush RC, Muller WS, et al. High intracranial pressure effects on cerebral cortical microvascular flow in rats. J Neurotrauma. 2011;28(5):775–785. doi: 10.1089/neu.2010.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bragin DE, Bush RC, Nemoto EM. Effect of cerebral perfusion pressure on cerebral cortical microvascular shunting at high intracranial pressure in rats. Stroke. 2013;44(1):177–181. doi: 10.1161/STROKEAHA.112.668293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67(1):110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 6.Foley LM, Hitchens TK, et al. Quantitative Temporal Profiles of Penumbra and Infarction During Permanent Middle Cerebral Artery Occlusion in Rats. Transl Stroke Res. 2010;1(3):220–229. doi: 10.1007/s12975-010-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain research. 2000;874(2):123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 8.Hamm RJ, Pike BR, O'Dell DM, et al. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11(2):187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 9.Kameneva MV, Polyakova MS, Fedoseeva EV. Effect of drag-reducing polymers on the structure of the stagnant zones and eddies in models of constricted and branching blood vessels. Fluid Dynamics. 1990;25:956–959. [Google Scholar]
- 10.Kameneva MV, Poliakova MS, Gvozdkova IA. The nature of the effect of polymers reducing hydrodynamic resistance on blood circulation. Doklady Akademii nauk SSSR. 1988;298(5):1253–1256. [PubMed] [Google Scholar]
- 11.Pacella JJ, Kameneva MV, Brands J, et al. Modulation of pre-capillary arteriolar pressure with drag-reducing polymers: a novel method for enhancing microvascular perfusion. Microcirculation. 2012;19(7):580–585. doi: 10.1111/j.1549-8719.2012.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kameneva MV, Wu ZJ, Uraysh A, et al. Blood soluble drag-reducing polymers prevent lethality from hemorrhagic shock in acute animal experiments. Biorheology. 2004;41(1):53–64. [PubMed] [Google Scholar]
- 13.Zhao R, Marhefka JN, Antaki JF, et al. Drag-reducing polymers diminish near-wall concentration of platelets in microchannel blood flow. Biorheology. 2010;47(3–4):193–203. doi: 10.3233/BIR-2010-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brands J, Kliner D, Lipowsky HH, Kameneva MV, et al. New insights into the microvascular mechanisms of drag reducing polymers: effect on the cell-free layer. PloS one. 2013;8(10):e77252. doi: 10.1371/journal.pone.0077252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh TG, Murphy RP, Fitzpatrick P, et al. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. Journal of cellular physiology. 2011;226(11):3053–3063. doi: 10.1002/jcp.22655. [DOI] [PubMed] [Google Scholar]
- 16.Rochfort KD, Collins LE, McLoughlin A, et al. Shear-dependent attenuation of cellular ROS levels can suppress proinflammatory cytokine injury to human brain microvascular endothelial barrier properties. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]