Abstract
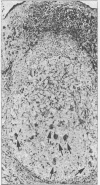
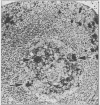
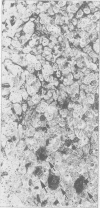
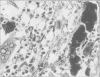
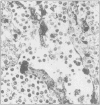
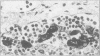
Structures corresponding to histiocytic and dendritic reticulum cells have been shown in human tonsillar tissue, "reactive" lymph nodes and spleens by means of a zinc iodide-osmium technique. These cell types have been shown in various locations in these tissues using paraffin and resin embedded sections produced after fixation/staining of the tissue in zinc iodide-osmium. The quality of morphology attained by this procedure is much improved compared with the demonstration of the two cell types by means of alpha-naphthyl acetate esterase reactions performed on frozen sections. The zonal architecture of the lymphoid follicle is emphasised by this technique. In lymph nodes, sinus lining cells are also shown. Lymphoid cells, polymorphs, fibroblasts, and endothelial cells are negative with the zinc iodide-osmium method. In addition, interdigitating cells are not stained. The results of this procedure are compared with those with those of other methods for the demonstration of histiocytic and dendritic reticulum cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnett D., Crocker J., Vaughan A. T. Synthesis of cathepsin B by cells derived from the HL60 promyelocytic leukaemia cell line. J Cell Physiol. 1983 Jun;115(3):249–254. doi: 10.1002/jcp.1041150306. [DOI] [PubMed] [Google Scholar]
- Crocker J., Burnett D., Jones E. L. Immunohistochemical demonstration of cathepsin B in the macrophages of benign and malignant lymphoid tissues. J Pathol. 1984 Jan;142(1):87–94. doi: 10.1002/path.1711420114. [DOI] [PubMed] [Google Scholar]
- Crocker J., Jones E. L., Curran R. C. The form factor of alpha-naphthyl acetate esterase-positive cells in non-Hodgkin's lymphomas and reactive lymph nodes. J Clin Pathol. 1983 Mar;36(3):303–306. doi: 10.1136/jcp.36.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J. The enzyme histochemistry of lymphoid and non-lymphoid cells of the human palatine tonsil: a basis for the study of lymphomas. J Pathol. 1981 May;134(1):81–95. doi: 10.1002/path.1711340109. [DOI] [PubMed] [Google Scholar]
- Curran R. C., Gregory J., Jones E. L. The distribution of immunoglobulin and other plasma proteins in human reactive lymph nodes. J Pathol. 1982 Apr;136(4):307–332. doi: 10.1002/path.1711360406. [DOI] [PubMed] [Google Scholar]
- Curran R. C., Jones E. L. The lymphoid follicles of the human palatine tonsil. Clin Exp Immunol. 1978 Feb;31(2):251–259. [PMC free article] [PubMed] [Google Scholar]
- Howie A. J. Scanning and transmission electron microscopy on the epithelium of human palatine tonsils. J Pathol. 1980 Feb;130(2):91–98. doi: 10.1002/path.1711300205. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Tjernlund U., Forsum U., Peterson P. A. Epidermal Langerhans cells express Ia antigens. Nature. 1977 Jul 21;268(5617):248–250. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975 Feb;28(2):124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. F., Bhan A. K., Sato S., Mihm M. C., Jr, Harrist T. J. A new immunologic marker for human Langerhans cells. N Engl J Med. 1981 Mar 26;304(13):791–792. doi: 10.1056/NEJM198103263041320. [DOI] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez E. M., Caorsi I. A second look at the ultrastructure of the Langerhans cell of the human epidermis. J Ultrastruct Res. 1978 Dec;65(3):279–295. doi: 10.1016/s0022-5320(78)80065-3. [DOI] [PubMed] [Google Scholar]
- Shamoto M. Langerhans cells increase in the dermal lesions of adult T cell leukaemia in Japan. J Clin Pathol. 1983 Mar;36(3):307–311. doi: 10.1136/jcp.36.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl G., Wolff-Schreiner E. C., Pichler W. J., Gschnait F., Knapp W., Wolff K. Epidermal Langerhans cells bear Fc and C3 receptors. Nature. 1977 Jul 21;268(5617):245–246. doi: 10.1038/268245a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamaguchi H., Ishizeki J., Nakajima T., Nakazato Y. Immunohistochemical and immunoelectron microscopic localization of S-100 protein in the interdigitating reticulum cells of the human lymph node. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(2):125–135. doi: 10.1007/BF02892562. [DOI] [PubMed] [Google Scholar]
- Veerman A. J., van Rooijen N. Lymphocyte capping and lymphocyte migration as associated events in the in vivo antigen trapping process. An electron-microscopic autoradiographic study in the spleen of mice. Cell Tissue Res. 1975 Aug 18;161(2):211–217. doi: 10.1007/BF00220369. [DOI] [PubMed] [Google Scholar]
- Wolff K., Schreiner E. Uptake, intracellular transport and degradation of exogenous protein by Langerhans cells. An electron microscopic-cytochemical study using peroxidase as tracer substance. J Invest Dermatol. 1970 Jan;54(1):37–47. doi: 10.1111/1523-1747.ep12551501. [DOI] [PubMed] [Google Scholar]