Abstract
The aim of this article is to provide an up-to-date review of the state-of-the-art in cerebral autoregulation, particularly as it may relate to the clinician scientist whose expertise is in the area of neuroscience in anesthesia and critical care. Topics covered range from biological mechanisms, methods used for assessment of autoregulation, effects of anesthetics, role in control of cerebral hemodynamics in health and disease such as traumatic brain injury where dysregulation is evident, and emerging areas such as role of age and sex in contribution to dysautoregulation. Emphasis will be placed on bidirectional translational research wherein the clinical informs the study design of basic science studies, which in turn, informs the clinical to result in development of improved therapies for treatment of CNS pathology.
Keywords: cerebral autoregulation, traumatic brain injury, anesthetics, cerebral blood flow
Introduction
Cerebral autoregulation is a homeostatic process that regulates and maintains cerebral blood flow (CBF) constant across a range of blood pressures. The original conceptualization was proposed by Lassen1 as a triphasic curve consisting of the lower limit, the plateau and the upper limit. In healthy adults, the limits are between 50 and 150 mm Hg cerebral perfusion pressure (CPP) or 60 and 160 mm Hg mean arterial pressure (MAP), where CPP = MAP – intracranial pressure (ICP). This homeostatic mechanism ensures that as MAP or CPP increases, resistance increases (vasoconstriction) in the small cerebral arteries. Conversely, this process maintains constant CBF by decreasing cerebrovascular resistance or vasodilation when MAP or CPP decreases. However, given that the lower limit of cerebral autoregulation frequently influences clinical management, it should be noted that this value has been challenged as being too low.2
Mechanism of Autoregulation
At least four mechanisms are proposed for autoregulation:
Myogenic
Neurogenic
Metabolic
Endothelial
The myogenic component concerns the ability of the vascular smooth muscle to constrict or dilate in response to changes in transmural pressure. The neurogenic mechanism occurs through an extensive nerve supply to cerebral vessels. For example, activation of alpha adrenoceptors shifts the limits of autoregulation to higher pressures and the cerebral vessels respond to this with vasoconstriction. The metabolic mechanism probably chiefly contributes to autoregulation in the microvasculature, where changes in the microenvironment such as for pCO2 and H+ will lead to vasodilation. Additionally, endothelial factors, such as nitric oxide, may also contribute to autoregulation. Autoregulation is important in the match of CBF to metabolic demand.
Methods Used For Assessment of Autoregulation
Assessment of cerebral autoregulation:
Static
Dynamic
In the static method, only steady state relationships between CBF and MAP are considered without taking into account the time course of changes in these two parameters. Determination of a steady state relationship can be accomplished through administration of drugs which change MAP but have no effect on metabolism, yielding two values of CBF and their difference in relation to the MAP change being indicative of autoregulation.3 Degree of intactness of autoregulation can be quantified via calculation of the autoregulatory index (ARI), where ARI = % ΔCVR / % ΔCPP and CVR is cerebrovascular resistance. A value of 0 means absent autoregulation while a value of 1 denotes perfect autoregulation.
In the dynamic method, assessment is based on determination of dynamic changes of CBF in response to dynamic changes in MAP. The idea is that after a change in MAP, CBF will first react to such a change and then will return to its original value within a finite amount of time. The faster the return, the better is the degree of autoregulation.
Several approaches are used to assess dynamic cerebral autoregulation via CBF recovery time. In one, which uses the thigh cuff method introduced by Aaslid,4 regulation is defined by the slope of CVR recovery, where CVR = CPP / CBF; the steeper the slope of CVR, the better is the autoregulation.5 In a second approach, the CBF recovery after the cuff release is quantified as an autoregulation index, calculated as a second-order differential equation relating changes in MAP and CBF.5 A third method considers study of CBF response to slow oscillations in MAP induced by paced breathing, head up tilting, or periodic thigh-cuff inflation.6–8 Transfer function analysis is then performed using beat-to-beat MAP measurements as input and CBF as output.9 Here, time delay of phase difference between MAP and CBF as a function of frequency can be used to determine the degree of intactness of autoregulation. This is an important alternative approach since the thigh cuff inflation/deflation technique presents problems in the traumatic brain injury (TBI) patient, where manipulation of blood pressure (secondary to thigh cuff deflation) would occur at a time when the injured brain may be least able to tolerate it. The Mx is one such measure of dynamic auotoregulation. It is a Pearson correlation coefficient between CPP and flow velocity measured by transcranial Doppler (TCD) and can indicate autoregulation if the magnitude of CPP fluctuations is large enough to activate an autoregulatory response (> 5 mm Hg). Positive values for Mx indicate impaired autoregulation, while zero or negative values indicate intact autoregulation. This index correlates significantly with the leg cuff test.10 Another similar measure of dynamic autoregulation is PRx, defined as the correlation coefficient between slow waves in ICP and arterial blood pressure. The PRx is negative for intact autoregulation, and is positive for impaired autoregulation.11
CBF in all of these methods is typically determined non invasively via TCD. TCD, however, has the disadvantage of only being able to measure CBF velocity in larger vessels such as the middle cerebral artery (MCA). Recently, it has been shown that use of near infrared spectroscopy (NIRS) will allow for measurement of CBF in small cerebral arterioles, yielding a quantitative determination of dynamic autoregulation in the cerebral microvasculature of the human.12 A related technique, diffuse correlation spectroscopy (DCS), has been successfully demonstrated to obtain regional CBF non invasively in patients under a variety of conditions, including TBI, orthostatic stress in acute ischemia, and post operatively in neonatal cardiac surgery.13,14 Calibration studies of absolute CBF values obtained via DCS have been recently completed,13 making the future outlook for use of DCS as a quantitative method for determination of autoregulation in the microvasculature more attractive.
Clinical Applications of Autoregulation Testing
A benefit to autoregulation testing relates to management of patients with TBI in the neuro intensive care unit, where sophisticated equipment for determination of dynamic indices of autoregulation is available. However, there are tangible benefits for management of neurologic patients in the operating room through use of simpler determination of static autoregulation. These benefits include:
Perioperative management of patients undergoing carotid endarterectomy.
Blood pressure management in uncontrolled hypertensive patients.
Blood pressure support in patients with carotid artery stenosis.
Effect of Anesthetics on Autoregulation
In general, inhaled anesthetics such as isoflurane and desflurane have a depressive effect on autoregulation,15,16 the one exception being sevoflurane.17,18 In contrast, propofol preserves autoregulation15 and is viewed as the anesthetic of choice in patients with high ICP,19 although one study observed that high dose propofol impaired autoregulation in head injured patients.20 Others have observed that the combination of remifentanil with propofol results in preservation of cerebral autoregulation.19,21 Current neurocritical care of TBI is largely based on TIVA, or total intravenous anesthesia, often involving fentanyl.
Pathophysiology of Cerebral Autoregulation in TBI: Outcome and Treatment
Multiple studies have demonstrated that cerebral autoregulation is absent or impaired in significant numbers of patients after TBI, even when values of CPP and CBF were normal.22 Cerebral hypoperfusion that occurs post TBI may result from the decreased metabolic demand due to coma (with appropriately matched CBF) or may alternatively indicate ischemia. Under normal physiologic conditions, decreases in CPP result in vasodilation and increased cerebral blood volume (CBV), leading to increases in ICP in the context of abnormal intracranial compliance. When autoregulation is impaired, decreases in CPP result in decreases in CBF; in moderate/severe TBI such decreases in CBF may reach ischemic levels, further exacerbating secondary injury. A number of retrospective studies have observed that impaired cerebral autoregulation is associated with worsened outcome (Glasgow Outcome Scale).23–25 Cerebral autoregulation also has been observed to correlate with brain biochemistry via microdialysis in patients with severe head injury.26 Critical autoregulatory thresholds for survival and improvement of outcome may vary as a function of age and sex.23,24
One treatment modality has been derived with specific consideration of cerebral autoregulation. This treatment relies on administration of vasoactive agents that increase MAP to normalize reduced CPP after TBI and the identification of the optimal CPP that maximizes the intactness of autoregulation. Those patients with CPP values below that of the one considered optimal have been shown to be at increased risk of fatal outcome, while too high a value of CPP is equally thought to be bad since it is often associated with increased rate of severe disability.27 Optimal CPP, as determined by PRx in TBI patients, has been shown to be narrowed from a normal range and vary from 65 to 95 mm Hg in 300 patients (with total mean value of 75 mm Hg).25 Similar results were obtained using other technologies to determine degree of intactness of autoregulation, such as direct brain tissue monitoring (ORx) and near-infrared spectroscopy (COx).28,29 While such results would seem to indicate that randomized prospective trials involving CPP (optimal) are needed, to date no such trial has been conducted.
Given that dysregulation appears to correlate quite well with clinical outcome, one could hypothesize that autoregulation should be therapeutically manipulated to improve recovery.25 While this hypothesis has never been formally prospectively tested, a few observations have hinted that there may indeed be support for it. For example, Mx value was observed to improve with induction of moderate hypocapnia in TBI patients,30 but autoregulation was observed to be impaired when hyperventilation was more intense, resulting in a robust decrease in pCO2.31 Additionally, indomethacin has been shown to decrease ICP and CBFV, thereby increasing CPP, resulting in improved dynamic cerebral autoregulation in still other TBI patients.32 Other clinical work has shown that other therapeutic maneuvers equally change cerebral autoregulation status, such as hypothermia, use of mannitol or hypertonic saline, barbiturates, and various anesthetics.25,33
Role of age and sex in cerebral autoregulation, dysautoregulation, and its treatment after TBI
a. Clinical Studies
Only a few studies of cerebral autoregulation in healthy children have been published and clinicians often assume that there are no age or sex related differences in this process.34 In one study, healthy children anesthetized with low-dose sevoflurane revealed no age-related differences in autoregulatory capacity.35 However, counter to the assumption that the lower limit of cerebral autoregulation in younger children is lower, these investigators also concluded that there were no age related differences in lower limit since that value in children aged 6 months to 2 years receiving sevoflurane was observed to be 60 ± 9 mm Hg.36 Alternatively, there may be age related differences in the latency of cerebral autoregulation. For example, compared to adults, adolescents may have a delayed return of CBF in response to brief hypotension.37 Additionally, there may be sex differences in pediatric cerebral autoregulation.34,37,38 Despite these clinical studies, the mechanisms of cerebral autoregulation in healthy children are not understood completely.39
A number of conditions can disrupt autoregulation in children, including hypoxic conditions, prematurity, congenital heart defects, intracranial hemorrhage, and TBI. Risk of dysautoregulation in preterm infants increases with severity of illness.40 TBI is the leading cause of injury-related death in children and young adults41 and thus will be the focus here, with boys over a larger age range and all children under 4 years having particularly poor outcomes.42–44 Cerebral autoregulation is often impaired after TBI,23 and with concomitant high ICP, lead to poor outcome. In children with impaired autoregulation, lower blood pressure may result in diminished CPP and CBF. Decrease in MAP causes cerebral vasodilation, increase in cerebral blood volume, and thus an increase in ICP. In the context of plateau wave generation, increased ICP will further decrease CPP, leading to more cerebral vasodilation, resulting in a vicious cycle, the vasodilation cascade.45 Critical closing pressure (CCP) has been defined as an arterial pressure threshold below which arterial vessels collapse. Dewey et al (1974)46 identified in their model of CBF regulation a relationship between CCP and cerebral autoregulation indicating that cerebral autoregulation mediated increases in vasomotor tone as predicted by CCP. This concept has recently been re-visited47 but while the difference in CPP-ICP significantly correlated with cerebral autoregulation, it lacked the power to predict outcome after head injury. Nonetheless, by stabilizing CPP at higher levels, ICP might be better controlled without cerebral ischemia.45 Because augmenting MAP in the hyperemic brain could theoretically result in worse edema or cerebral hemorrhage,44,48 it is uncertain if empirically increasing MAP to prevent cerebral ischemia in the presence of impaired cerebral autoregulation and cerebral hyperemia may potentially be harmful.35 Moreover, a fixed blood pressure does not improve nor allow for cerebrovascular adaptation to changing CBF-metabolism requirements. Since cerebroautoregulation changes with time after TBI, it is suggested that blood pressure management strategies can neither be empiric nor fixed but rather should be based on the status of cerebral autoregulation. In this context, vasodilator mechanisms of cerebral autoregulation may be important in improving outcome after TBI. The functional significance of this idea rests on several clinical observations. For example, cognitive outcome (GOS) and risk of death are correlated with degree of cerebral autoregulatory impairment in children after TBI.23,34 Additionally, severity of impaired cerebral autoregulation may be greater in those children who are victims of intentional or abusive TBI compared to children with noninflicted TBI.49
Current 2012 Pediatric Guidelines recommend maintaining CPP above 40 mm Hg, noting that an age-related continuum for the optimal CPP is between 40 and 65 mm Hg.50,51 Despite these current therapeutic targets, guidelines are lacking regarding how this should be achieved. For example, maintaining CPP within these levels is often managed by use of vasopressors to increase CPP and optimize CBF. However, vasoactive agents clinically used to elevate MAP after TBI, such as phenylephrine (Phe), dopamine (DA), epinephrine (EPI) and norepinephrine (NE)52,53 have not sufficiently been compared regarding effect on CPP, CBF, autoregulation, and survival after TBI, and clinically, current vasopressor use is variable. A recent single center study found that: 1). Phe was used nearly twice as often as any other vasopressor, 2). Young children tended to receive DA and EPI whereas older children received Phe and NE, 3). Blood pressure was managed with 1 drug during the first 3 hours, and 4). NE was associated with a higher CPP and lower ICP at 3h post injury.54 Nonetheless, CPP-directed therapy has remained somewhat controversial because it has been observed to either have no effect or in fact worsen outcome.55 Further complicating this issue is that most pressors are neurotransmitters, which normally have no effect on the brain when given peripherally. However, with a disrupted blood brain barrier (BBB), these drugs may cross the BBB and impact on cerebral metabolism. Additionally, CPP has been considered to be a poor surrogate for CBF,56 since regional or local CBF may be markedly reduced even if CPP is normal.55 Therefore, there remains an unmet need to have available appropriate noninvasive bedside monitors which may enable identification of the optimal vasopressor and whether pressor choice determines outcome as a function of age and sex in pediatric TBI.
b. Basic Science Studies
Although several rodent models of TBI have been described,57 all have the disadvantage of not permitting repeated measurements of systemic physiological variables and regional CBF because of the small size of the subjects. Additionally, rodents have a lissencephalic brain containing more grey than white matter. In contrast, pigs have a gyrencephalic brain similar to humans that contains substantial white matter, which is more sensitive to ischemic damage than grey matter.
Since ethical considerations constrain mechanistic studies in children with TBI, we have used an established porcine model of fluid percussion injury (FPI) that mimics TBI to corroborate clinical observations regarding cerebral autoregulation after TBI.58 Newborn and juvenile pigs may approximate the human neonate (6 months- 2yrs old) and child (8–10 yrs old).59
Armstead and Vavilala have recently taken a bidirectional translational approach to investigate the role of vasoactive agent choice in outcome after TBI. Clinical observations were used to inform study design of procedures conducted in pigs after fluid percussion injury (FPI), with intent to use information so derived to improve outcome in brain injured children; e.g. a bedside to bench and back again arrangement. Early studies focused on Phe, given that it is the predominant vasoactive agent administered clinically in young children after TBI.54 We were surprised to observe that while Phe is protective of cerebral hemodynamics, particularly for autoregulation, in newborn female piglets, it aggravates cerebrovascular dysregulation in male newborn piglets post injury.60 Because of this perplexing observation, we speculated that pressor choice may influence outcome. Indeed, another pressor, DA, produced equivalent cerebrohemodynamic protection in both male and female piglets after TBI.61
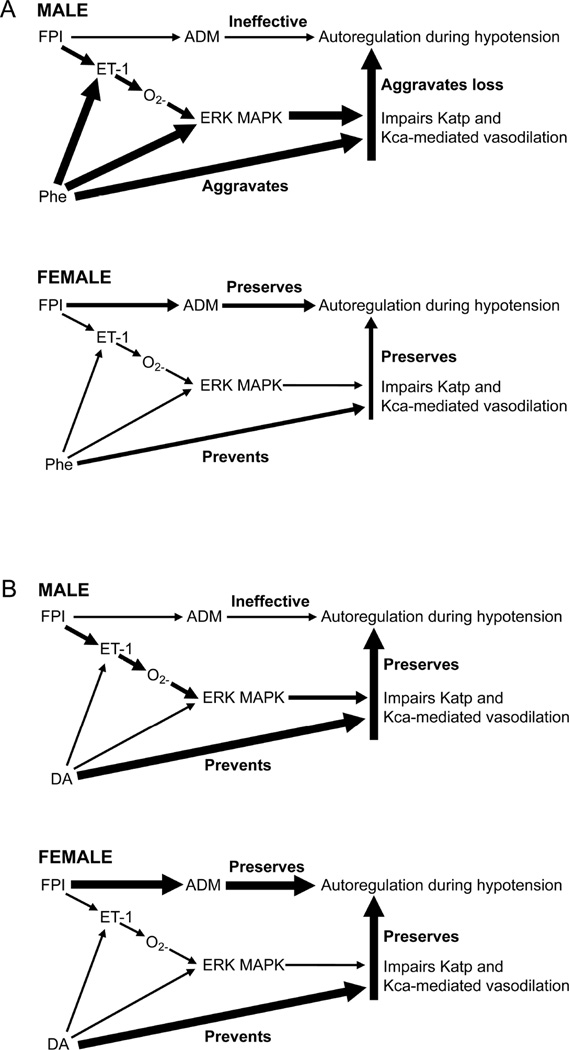
Mechanistic studies were designed to further explore these observations. First, it was found that autoregulation was impaired more in the male compared to the female piglet after FPI, resulting from a shift in the constrictor to dilator ratio, with greater brain concentration of the spasmogen endothelin-1 (ET-1) and little to no increase in the endogenous vasodilator peptide adrenomedullin (ADM), a K channel opener (Fig 1).62,63 Autoregulation had been prior observed to be dependent on opening of K channels (particularly Katp and Kca channels), while ET-1 is known to impair opening of K channels via release of activated oxygen which then activates (phosphorylates) the Extracellular signal-regulated kinase (ERK) isoform of mitogen activated protein kinase (MAPK), a distal signaling system important in control of cerebral hemodynamics (Fig 1).58 Because more ERK MAPK is released after FPI in the male compared to the female,62,64 there is correspondingly greater impairment of autoregulation and Katp and Kca channel agonist-mediated cerebrovasodilation post injury in males compared to females.65 While Phe blunted ET-1 and ERK MAPK upregulation in female piglets after FPI, there was an unanticipated and unwanted Phe-mediated aggravation of ET-1 and ERK MAPK upregulation in male piglets post injury (Fig 1).60 The latter compounded the already greater release of ET-1 and ERK MAPK in males compared to females after FPI and appeared to contribute to the sex dependent impairment of autoregulation.60 The ET-1 antagonist BQ 123 blocked elevation of CSF ERK MAPK and the aggravation of such elevation by Phe after FPI.60 Co-administered BQ 123 with Phe also prevented impairment of autoregulation after FPI, supportive of the intermediary role for ET-1 in sex dependent Phe-mediated hemodynamic dysregulation. Alternatively, Phe also promoted the increase in brain levels of ADM in females but not males after FPI.60 In the context of ADM being an opener of K channels, Phe can be viewed as upregulating an endogenous neuroprotectant in females after FPI. Therefore, Phe augments impairment of autoregulation in males after TBI both because it promotes release of a spasmogen and subsequent block of Katp and Kca channels as well because it simultaneously limits release of an endogenous neuroprotectant (Fig 1).66 In contrast, DA completely blocked ET-1 and ERK MAPK upregulation in both newborn male and female piglets after FPI (Fig 1),61 serving as an explanation for equivalent protection of autoregulation post injury in male and female piglets.
Figure 1.
Comparison of proposed mechanisms for Phe (Upper Panel) and DA (Lower Panel) in control of cerebral hemodynamics after FPI in newborn piglets. Arrow thickness in proportion to probability of action.
FPI = fluid percussion brain injury, ET-1 = endothelin-1, O2− = oxygen free radical, ERK MAPK = Extracellular signal-regulated kinase (ERK) isoform of mitogen activated protein kinase (MAPK), Phe = phenylephrine, DA = dopamine, ADM = adrenomedullin, Katp and Kca = atp sensitive and calcium sensitive K channels.
From Armstead WM, Vavilala, MS. Age and Sex Differences In Hemodynamics In a Large Animal Model of Brain Trauma. In: Lo EH, Lok J, Ning M, Whalen MJ, editors. Vascular Mechanisms in CNS Trauma. New York: Springer; 2014. p. 269–284; with permission.
A third vasoactive drug, NE, has recently been investigated. In the first study, we observed that NE preferentially protected cerebral autoregulation and prevented neuronal cell necrosis in hippocampal areas CA1 and CA3 in female newborn piglets after FPI.67 However, NE had no protective effect on cerebral autoregulation and potentiated neuronal cell necrosis in male newborn pigs, despite achievement of a similar CPP.67 In the second study, NE protected cerebral autoregulation and limited hippocampal neuronal cell necrosis after FPI in both male and female juvenile pigs,68 indicating that the same vasoactive drug elicits both age and sex dependent differences in outcome under equivalent injury intensity conditions.
Mechanistically, two mediators were investigated in the above studies: ERK MAPK and IL-6. Clinically, IL-6 was observed to be increased in the CSF of children after severe TBI.69 Its role in CNS pathology, however, is less well understood. For example, IL-6 may mediate motor coordination deficits after TBI,70 appears associated with poor neurological outcome following hemorrhagic stroke,71 yet may also be involved in regenerative and repair processes.69 In our first study, NE increased ERK MAPK upregulation in young males, but blocked upregulation in young females after FPI.67 In contrast, in the second study using older pigs, NE blunted ERK MAPK and IL-6 upregulation in both males and females after FPI.68 Co-administration of the ERK MAPK antagonist U 0126 preserved autoregulation and maintained CBF after FPI in newborns, regardless of sex.67 U 0126 also prevented hippocampal neuronal cell necrosis associated with FPI.64 IL-6 was similarly increased after FPI more in young males compared to females and NE aggravated such upregulation in an ERK MAPK dependent manner in males; the ERK MAPK antagonist U 0126 blocked IL-6 release.64 In contrast, NE blocked IL-6 upregulation in young females after FPI.64 These data indicate that ERK MAPK mediates elevation of IL-6, which is associated with greater hemodynamic impairment and hippocampal cell necrosis after FPI in young males.
In the context of the neurovascular unit (NVU), we hypothesize that CBF contributes to neuronal cell integrity; eg normalization of CBF via administration of vasoactive drugs that elevate MAP should limit neuronal cell necrosis associated with TBI. Administration of NE to increase CPP after TBI in the piglet prevented impairment of cerebral autoregulation and neuronal cell necrosis in the CA1 and CA3 hippocampal areas of the brain.67 Clinically, degree of impairment of autoregulation is significantly correlated with GCS.23,34 Although cognition depends on more than the hippocampus and formal cognitive testing was not performed in these studies, these data nonetheless support the idea that targeting CPP and thereby protecting cerebral autoregulatory capacity may improve cognitive outcome. The NVU concept and its potential role in cognition may be somewhat more universal than prior appreciated. For example, administration of tPA-S481A, a catalytically inactive tPA variant that competes with wild type tPA for binding, cleavage and activation of NMDA receptors, after TBI in the pig similarly improved cerebral hemodynamics and prevented impairment of cerebral autoregulation and CA1 and CA3 hippocampal cell necrosis.72 We speculate that choice of pressor may influence cognitive outcome as a function of age and sex in the setting of TBI.
In summary, vasoactive agents clearly have complex sex-dependent effects on CBF and may potentiate impairment (Phe), prevent impairment (DA), or have no effect (NE) on impairment of cerebral autoregulation in newborn males after TBI. Additionally, when considering age, NE may have no effect in the newborn, or prevent impairment in the juvenile male after TBI. These studies strengthen the idea that choice of vasoactive agent is important in determining outcome after pediatric TBI as a function of sex and age. To our knowledge similar studies have not been conducted in the adult.
Concluding Remarks
This article has emphasized a bidirectional translational research approach in summarizing state of the art aspects of cerebral autoregulation that range from the traditional to the newly emerging. Enhanced cross talk between basic science and clinical disciplines will speed the transfer of knowledge resulting in better therapeutic approaches towards improving outcome in patients with CNS pathology such as TBI where there is cerebral dysautoregulation.
Key Points.
Cerebral autoregulation can be assessed by static and dynamic techniques.
Inhaled anesthetics generally depress autoregulation, except for sevoflurane. TIVA is most widely used in neurocritical care of TBI patients.
Autoregulation impaired after TBI. CPP directed therapy often used to improve outcome.
Identification of optimal CPP in health and disease an active area of investigation.
Role of age and sex in cerebral autoregulation in physiology and pathology of TBI is an emerging area of clinical significance.
Acknowledgments
Sources of Financial Support: NIH RO1 NS090998
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lassen LA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- 2.Drummond JC. The lower limit of autoregulation: time to revise our thinking? Anesthesiology. 1997;86:1431–1433. doi: 10.1097/00000542-199706000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Lassen LA. Control of cerebral circulation in health and disease. Circ Res. 1974;34:749–760. doi: 10.1161/01.res.34.6.749. [DOI] [PubMed] [Google Scholar]
- 4.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 5.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 6.Reinhard M, Wehrle-Wieland E, Grabiak D, et al. Oscillatory cerebral hemodynamics-the macro vs microvascular level. J Neurol Sci. 2006;250:103–109. doi: 10.1016/j.jns.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke. 1995;26:1801–1804. doi: 10.1161/01.str.26.10.1801. [DOI] [PubMed] [Google Scholar]
- 8.Aaslid R, Blaha M, Sviri G, Douville CM, Newell DW. Asymmetric dynamic cerebral autoregulatory response to cyclic stimuli. Stroke. 2007;38:1465–1469. doi: 10.1161/STROKEAHA.106.473462. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 10.Czosnyka M, Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral autoregulation following head injury. J Neurosurg. 1974;95:756–763. doi: 10.3171/jns.2001.95.5.0756. [DOI] [PubMed] [Google Scholar]
- 11.Steiner LA, Coles JP, Czosnyka M, et al. Cerebrovascular pressure reactivity is related to global cerebral oxygen metabolism after head injury. J Neurol Neurosurg Psychiatry. 2003;74:765–770. doi: 10.1136/jnnp.74.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kainerstorfer JM, Sassaroli A, Tgavalekos KT, Fantini S. Cerebral autoregulation in the microvasculature measured with near-infrared spectroscopy. J Cereb Blood Flow Metab. 2015;35:959–966. doi: 10.1038/jcbfm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durduran T, Yodh AG. Diffuse correlation spectroscopy for non invasive, microvascular cerebral blood flow measurement. NeuroImage. 2014;85:51–63. doi: 10.1016/j.neuroimage.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MN, Durduran T, Frangos S, et al. Noninvasive measurement of cerebral blood flow and blood oxygenation using near infrared and diffuse correlation spectroscopies in critically brain injured adults. Neurocrit Care. 2010;12:173–180. doi: 10.1007/s12028-009-9305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strebel S, Lam AM, Batta B, Mayberg TS, Aaslid R, Newell DW. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83:66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Dagal A, Lam AM. Cerebral autoregulation and anesthesia. Current Opinion in Anesthesiol. 2009;22:547–552. doi: 10.1097/ACO.0b013e32833020be. [DOI] [PubMed] [Google Scholar]
- 17.Summors AC, Gupta AK, Mat BF. Dynamic cerebral autoregulation during sevoflurane anesthesia: a comparison with isoflurane. Anesth Analg. 1999;88:341–345. doi: 10.1097/00000539-199902000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Heath K, Matta BF. Effect of incremental does of sevoflurane on cerebral pressure autoregulation in humans. Br J Anaesth. 1997;79:469–472. doi: 10.1093/bja/79.4.469. [DOI] [PubMed] [Google Scholar]
- 19.Cole CD, Gottfried ON, Gupta DK, Couldwell WT. Total intravenous anesthesia: advantages for intracranial surgery. Neurosurgery. 2007;61(Suppl 2):369–377. doi: 10.1227/01.neu.0000303996.74526.30. [DOI] [PubMed] [Google Scholar]
- 20.Grathwohl KW, Black IH, Spinella PC, et al. Total intravenous anesthesia including ketamine versus volatile gas anesthesia for combat-related operative traumatic brain injury. Anesthesiology. 2008;109:44–53. doi: 10.1097/ALN.0b013e31817c02e3. [DOI] [PubMed] [Google Scholar]
- 21.Engelhard K, Werner C, Mollenberg O, et al. Effects of remifentanil/propofol in comparison with isoflurane on dynamic cerebrovascular autoregulation in humans. Acta Anaesthesiol Scand. 2001;45:9971–9976. doi: 10.1034/j.1399-6576.2001.450809.x. [DOI] [PubMed] [Google Scholar]
- 22.Rangel-Castilla L, Gasco J, Nauta HJW, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus. 2008;25:1–8. doi: 10.3171/FOC.2008.25.10.E7. [DOI] [PubMed] [Google Scholar]
- 23.Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology. 2008;108:588–595. doi: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- 24.Sorrentino E, Diedler J, Kasprowicz M, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16:258–266. doi: 10.1007/s12028-011-9630-8. [DOI] [PubMed] [Google Scholar]
- 25.Czonsyka M, Miller C. Monitoring of cerebral autoregulation. Neurocrit Care. 2014;21:S95–S102. doi: 10.1007/s12028-014-0046-0. [DOI] [PubMed] [Google Scholar]
- 26.Chan TV, Ng SC, Lam JM, Poon WS, Gin T. Monitoring of autoregulation using intracerebral microdialysis in patients with severe head injury. Act Neurochir Suppl. 2005;95:113–116. doi: 10.1007/3-211-32318-x_24. [DOI] [PubMed] [Google Scholar]
- 27.Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 28.Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006;34:1783–1788. doi: 10.1097/01.CCM.0000218413.51546.9E. [DOI] [PubMed] [Google Scholar]
- 29.Zweifel C, Castellani G, Czosnyka M, et al. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J Neurotrauma. 2010;27:1951–1958. doi: 10.1089/neu.2010.1388. [DOI] [PubMed] [Google Scholar]
- 30.Haubrich C, Steiner L, Kim DJ, Kasprowicz M, Smielewski P, Diehl RR, Pickard JD, Czosnyka M. How does moderate hypocapnia affect cerebral autoregulation in response to changes in perfusion pressure in TBI patients? Acta Neurochirurgia Suppl. 2012;114:153–156. doi: 10.1007/978-3-7091-0956-4_28. [DOI] [PubMed] [Google Scholar]
- 31.Newell DW, Weber JP, Watson R, AAslid R, Winn HR. Effect of transient moderate hyperventilation on dynamic cerebral autoregulation after severe head injury. Neurosurgery. 1996;39:35–43. doi: 10.1097/00006123-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Puppo C, Lopez L, Caragna E, Moraes L, Iturralde A, Biestro A. Indomethacin and cerebral autoregulation in severe head injured patients: a transcranial Doppler study. Act Neurochir (Wien) 2007;149:139–149. doi: 10.1007/s00701-006-1074-0. [DOI] [PubMed] [Google Scholar]
- 33.Steiner LA, Johnston AJ, Chatfield DA, et al. The effects of large dose propofol on cerebrovascular pressure autoregulation in head injured patients. Anesth Analg. 2003;97:572–576. doi: 10.1213/01.ANE.0000070234.17226.B0. [DOI] [PubMed] [Google Scholar]
- 34.Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225–234. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vavilala MS, Lee LA, Lee M, Graham A, Visco E, Lam AM. Cerebral autoregulation in children during sevoflurane anesthesia. Br J. Anaesh. 2003;90:636–641. doi: 10.1093/bja/aeg119. [DOI] [PubMed] [Google Scholar]
- 36.Vavilala MS, Lee LA, Lam AM. The lower limit of cerebral autoregulation in children during sevoflurane anesthesia. J Neurosurg Anesthesiol. 2003;15:307–312. doi: 10.1097/00008506-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Vavilala MS, Newell DW, Junger E, et al. Dynamic cerebral autoregulation in healthy adolescents. Acta Anaesthesiol Scand. 2002;46:393–397. doi: 10.1034/j.1399-6576.2002.460411.x. [DOI] [PubMed] [Google Scholar]
- 38.Vavilala MS, Kincaid MS, Muangman SL, Suz P, Rozet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in health children. Pediatr Res. 2005;58:574–578. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philip S, Udomphorn Y, Kirkham FJ, Vavilala MS. Cerebrovascular pathophysiology in pediatric traumatic brain injury. J Trauma. 2009;67:S128–S134. doi: 10.1097/TA.0b013e3181ad32c7. [DOI] [PubMed] [Google Scholar]
- 40.Williams M, Lee JK. Intraoperative blood pressure and cerebral perfusion: strategies to clarify hemodynamics. Ped Anesth. 2014;24:657–667. doi: 10.1111/pan.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au AK, Carcillo JA, Clark RSB, Bell MJ. Brain injuries and neurological system failure are the most common proximate causes of death in children admitted to a pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:566–571. doi: 10.1097/PCC.0b013e3181fe3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Newacheck PW, Inkelas M, Kim SE. Heath services use and health care expenditures for children with disabilities. Pediatrics. 2004;114:79–85. doi: 10.1542/peds.114.1.79. [DOI] [PubMed] [Google Scholar]
- 44.Mandera M, Larysz D, Wojtacha M. Changes in cerebral hemodynamics assess by transcranial Doppler Ultrasonography in children after head injury. Childs Nerv Syst. 2002;18:124–128. doi: 10.1007/s00381-002-0572-5. [DOI] [PubMed] [Google Scholar]
- 45.Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949–962. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 46.Dewey RC, Pieper HP, Hunt WE. Experimental cerebral hemodynamics. Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg. 1974;41:597–606. doi: 10.3171/jns.1974.41.5.0597. [DOI] [PubMed] [Google Scholar]
- 47.Czosnyka M, Smielewski P, Piechnik S, Al-Rawi PG, Kirkpatrick PJ, Matta PJ, et al. Critical closing pressure in cerebrovascular circulation. J of Neurol, Neurosurg, and Psychiatry. 1999;66:606–611. doi: 10.1136/jnnp.66.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aldrich EF, Eisenberg HM, Saydjari C, et al. Diffuse brain swelling in severely head-injured children: a report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1992;76:45–454. doi: 10.3171/jns.1992.76.3.0450. [DOI] [PubMed] [Google Scholar]
- 49.Vavilala MS, Muangman S, Waitayawinu P, et al. Neurointensive care; impaired cerebral autoregulation in infants and young children early after inflicted traumatic brain injury: a preliminary report. J Neurotrauma. 2007;24:87–96. doi: 10.1089/neu.2006.0058. [DOI] [PubMed] [Google Scholar]
- 50.Carter BG, Butt W, Taylor A. ICP and CPP. Excellent predictors of long term outcome in severely brain injured children. Childs Nervous System. 2008;24:245–251. doi: 10.1007/s00381-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 51.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-Second Edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S24–S29. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 52.Biestro A, Barrios E, Baraibar J, et al. Use of vasopressors to raise cerebral perfusion pressure in head injured patients. Acta Neurochir. 1998;71:5–9. doi: 10.1007/978-3-7091-6475-4_2. [DOI] [PubMed] [Google Scholar]
- 53.Steiner LA, Johnston AJ, Czosnyka M, et al. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head injured patients. Crit Care Med. 2004;32:1049–1054. doi: 10.1097/01.ccm.0000120054.32845.a6. [DOI] [PubMed] [Google Scholar]
- 54.DiGennaro JL, Mack CD, Malkouti A, Zimmerman JJ, Armstead W, Vavilala MS. Use and effect of vasopressors after pediatric brain injury. Dev Neurosci. 2010;32:420–430. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coles JP, Steiner A, Johnston AJ, et al. Does induced hypertension reduce cerebral ischemia within traumatized human brain? Brain. 2004;127:2479–2490. doi: 10.1093/brain/awh268. [DOI] [PubMed] [Google Scholar]
- 56.Cremer OL, van Dijk GW, van Wensen E, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med. 2005;33(10):2207–2213. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- 57.Robertson CL, Scafidi S, McKenna MC, Fiskum G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp Neurol. 2009;218:371–380. doi: 10.1016/j.expneurol.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armstead WM. Age dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation. 2000;7:225–235. [PubMed] [Google Scholar]
- 59.Dobbing J. The later development of the brain and its vulnerability. In: Davis JA, Dobbing J, editors. Scientific Foundations of Pediatrics. London: Heineman Medical; 1981. pp. 744–759. [Google Scholar]
- 60.Armstead WM, Kiessling JW, Kofke WA, Vavilala MS. Impaired cerebral blood flow autoregulation during post traumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by ERK MAPK upregulation. Crit Care Med. 2010;38:1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armstead WM, Riley J, Vavilala MS. Dopamine prevents impairment of autoregulation after TBI in the newborn pig through inhibition of upregulation of ET-1 and ERK MAPK. Ped Crit Care Med. 2013;14:e103–e111. doi: 10.1097/PCC.0b013e3182712b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstead WM, Kiessling JW, Bdeir K, Kofke WA, Vavilala MS. Adrenomedullin prevents sex dependent impairment of autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J Neurotrauma. 2010;27:391–402. doi: 10.1089/neu.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armstead WM, Vavilala MS. Adrenomedullin reduces gender dependent loss of hypotensive cerebrovasodilation after newborn brain injury through activation of ATP-dependent K channels. J Cereb Blood Flow Metab. 2007;27:1702–1709. doi: 10.1038/sj.jcbfm.9600473. [DOI] [PubMed] [Google Scholar]
- 64.Armstead WM, Riley J, Vavilala MS. TBI sex dependently upregulates ET-1 to impair autoregulation which is aggravated by phenylephrine in males but is abrogated in females. J Neurotrauma. 2012;29:1483–1490. doi: 10.1089/neu.2011.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armstead WM, Vavilala MS. Age and Sex Differences In Hemodynamics In a Large Animal Model of Brain Trauma. 2014:269–284. Book chapter in "Vascular Mechanisms in CNS Trauma" Eng Lo editor. [Google Scholar]
- 66.Armstead WM, Kiessling JW, Riley J, Kofke WA, Vavilala MS. Phenylephrine infusion prevents impairment of ATP and Calcium sensitive K channel mediated cerebrovasodilation after brain injury in female but aggravates impairment in male piglets through modulation of ERK MAPK upregulation. J Neurotrauma. 2011;28:105–111. doi: 10.1089/neu.2010.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstead WM, Riley J, Vavilala MS. Preferential protection of cerebral autoregulation and reduction of hippocampal necrosis with norepinephrine after traumatic brain injury in female piglets. Pediatr Crit Care Med. doi: 10.1097/PCC.0000000000000603. in press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Armstead WM, Riley J, Vavilala MS. Norepinephrine protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury via block of ERK MAPK and IL-6 in juvenile pigs. J Neurotrauma. doi: 10.1089/neu.2015.4290. in press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Bell MJ, Kochanek PM, Doughty LA, et al. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma. 1997;14:451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- 70.Yang SH, Gangidine M, Pritts TA, et al. Interleuking 6 mediates neuroinflammation and motor coordination deficits after mild traumatic brain injury and brief hypoxia in mice. Shock. 2013;40:471–475. doi: 10.1097/SHK.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oto J, Suzue A, Inui D, et al. Plasma proinflammatory and anti-inflammatory cytokine and catecholamine concentrations as predictors of neurological outcome in acute stroke patients. J Anesth. 2008;22:207–212. doi: 10.1007/s00540-008-0639-x. [DOI] [PubMed] [Google Scholar]
- 72.Armstead WM, Riley J, Yarovoi S, Cines CB, Smith DH, Higazi AAR. tPA S-481A prevents neurotoxicity of endogenous tPA in traumatic brain injury. J Neurotrauma. 2012;29:1794–1802. doi: 10.1089/neu.2012.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]