Abstract
Background
This study aimed to explore the therapeutic effect of external application of ligustrazine combined with holistic nursing on pressure sores, as well as the underlying mechanism.
Material/Methods
From February 2014 to March 2015, a total of 32 patients with Phase II and Phase III pressure sores were enrolled and randomly assigned to an experimental group or a control group. The clinical data were comparable between the 2 groups. In addition to holistic nursing, the patients in the experimental group received 4 weeks of continuous external application of ligustrazine, whereas patients in the control group received compound clotrimazole cream. Therapeutic effect and healing time were recorded. HaCaT cells were used as an in vitro model for mechanism analysis of the effect of ligustrazine in treating pressure sores. After culturing with different concentrations of ligustrazine or the inhibitor of AKT (LY294002) for 72 h, cell viability, clone formation numbers, and levels of phosphatidyl inositol 3-kinase (PI3K), p-AKT, and p-mammalian target of rapamycin (mTOR) were determined.
Results
Compared to the control group, the total effective rate in the experimental group was significantly higher, and the healing time was significantly reduced. Cell viability and clone formation numbers were significantly upregulated by ligustrazine in a dose-dependent manner. Both the cell viability and clone formation numbers were significantly inhibited by application of LY294002.
Conclusions
Our results suggest that ligustrazine combined with holistic nursing is an effective treatment of pressure sores. The protective effect may be associated with the promotion of cell growth by activation of the PI3K/AKT pathway.
MeSH Keywords: Holistic Nursing, Pharmacologic Actions, Pressure Ulcer
Background
Pressure sores are a frequent complication among patients hospitalized at acute and long-term care facilities, especially in elderly patients [1,2]. Pressure sores result from both extrinsic (e.g., pressure and friction) and intrinsic (e.g., contractures and malnutrition) etiologies [3,4]. Continuous or chronic pressure on the skin and muscle cause circulation disorder, leading to the destruction and necrosis of tissue. The incidence and prevalence rates of pressure sores are rapidly increasing because of the growth of the elderly population and increasing prevalence of chronic diseases. It has been reported that the prevalence rates vary from 8.8% to 53.2% [5,6] and the incidence rates range from 7% to 71.6% [7,8]. The occurrence of pressure sores considerably increases hospitalization time and medical expenditure. The prevention and care of pressure sores have always been a challenge to the nursing profession because the responsibility for prevention and care usually falls to the nurses [9]. Therefore, early intervention and appropriate treatment is critical to prevent the development of pressure sores.
Currently, the treatment of pressure sores involves a wide range of traditional interventions, including surgical treatment (e.g., debridement and myocutaneous flaps) and nonsurgical treatment (e.g., elemental pastes, antimicrobials, dressing, and solutions). In addition to traditional interventions, Traditional Chinese Medicine (TCM) has been used extensively in treating pressure sores [10]. Ligustrazine, a major active alkaloid extracted from the plant Ligusticum chuanxiong Hort, is a frequent Chinese medicinal herb that has been widely used for many years to treat diseases such as atherosclerosis, hypertension, cancers, and ischemic stroke [11–14]. In addition to the clinical administration, it is now widely used in food preparation as a health-promoting ingredient. Ligustrazine displays numerous bioactivities such as antioxidant, neuron-protection, antifibrosis, anti-nociception, vasorelaxation, anti-inflammation, and anti-proliferation [15]. Although various bioactivities have been substantiated in in vitro and in vivo studies, the molecular mechanisms of action are still unclear, which creates a major obstacle to further clinical application. Little information is available concerning the effect of ligustrazine on pressure sores and the possible underlying mechanism.
Therefore, in the present study, we aimed to explore the effect of ligustrazine on pressure sores and tried to clarify the underlying mechanism. We compared the therapeutic effect of compound clotrimazole cream vs. ligustrazine with holistic nursing. Additionally, we explored the mechanism in vitro by using HaCaT cells.
Material and Methods
Research subjects
A total of 32 patients with Phase II and Phase III pressure sores, who were admitted at our hospital from February 2014 to March 2015, were enrolled in this study. Among the 32 patients, 19 were male and 13 were female, with an age range of 65–83 years. The stage of pressure sores was categorized according to the standard pressure ulcer classification revised by the National Pressure Ulcer Advisory Panel (NPUAP) [16]. There were a total of 45 sites of ulcers, including 33 sites of Phase II and 12 sites of Phase III. The locations of pressure sores were: ischial tuberosity (21 sites), sacrum (12 sites), trochanter (5 sites), heel (4 sites), and malleolus (3 sites).
Patients were randomly assigned to the experimental group or the control group. In the experimental group there were 9 males and 7 females, with an average age of 63.33±2.78 years, and the average duration of pressure sores was 1.36±0.68 months. In the control group there were 8 males and 8 females with an average age of 64.21±3.02 years, and the average duration of pressure sores was 1.22±0.37 months. There were no significant differences in sex, age, or average duration of pressure sores between the patients in the 2 groups and the results were comparable. Informed written consent was obtained from all the patients and our study was approved by the local Ethics Committee.
Holistic nursing and external application of ligustrazine vs. compound clotrimazole cream
Basic nursing was provided to all patients, including regular observation of the pressure site and establishment of prevention measures for pressure sores. If there was incontinence, measures were taken and the bedding was replaced in a timely fashion. Patients who had difficulty in turning over or who could not turn over used an air cushion bed or a bedsore mat. A high-protein diet was given to patients to strengthen nutrition. Nutritional support was administered when necessary. Health education was provided to the families. In addition to the basic nursing, patients in the control group received compound clotrimazole cream with an outer layer of wet dressing held in place by a medical-quality rubberized fabric. Dressing change was carried out 1–2 times a day, depending on local exudation. Patients in the experimental group were given ligustrazine prepared according to a previously described method [17]. After disinfection with iodine and rinsing with normal saline (NS), the wound was dried by a high-velocity flow of oxygen for 5 min, and then covered with a transdermal ligustrazine patch. The patch was changed once a week for 4 weeks.
Therapeutic effect
The therapeutic effect of pressure sores was evaluated according to diagnostic efficacy of standard TCM syndrome [18]: healing, markedly effective, effective, and ineffective. Healing means crusting and scaling; markedly effective shows the reduction of wound area, no secretions, with good growth of granulation tissue; effective means that the wound area was not enlarged and the leakage was decreased; and ineffective means the expansion of the wound area and exudate.
Preparation of ligustrazine
For the cell experiment, ligustrazine, purchased from Sigma (St Louis, MO, USA), was extracted according to described protocols [14,19]. Briefly, ligustrazine was pulverized, sieved, weighed, added to aqueous ethanol, immersed for 40 min, boiled, and filtered. After centrifugation, the supernatant was dissolved in dimethyl sulfoxide (DMSO). The final concentration of ligustrazine extracts solution was adjusted to 0.5 mmol/L, 1.0 mmol/L, and 1.5 mmol/L.
Cell culture and treatment
Human keratinocytes cell line HaCaT cells were purchased from the China Center for Type Culture Collection (CCTCC, China). All the cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS (Invitrogen), 100 U/mL penicillin, and 100 U/mL streptomycin (Invitrogen) and maintained in a humidified incubator at 37°C and 5% CO2. Cells were then seeded into a 96-well plate and incubated at 37°C for 24 h. Various concentrations of ligustrazine (0.5 mmol/L, 1.0 mmol/L, and 1.5 mmol/L) or LY294002 (10 μM) (Cell signaling Technology, Beverly, MA, USA) were added to the cells. The cells in the control group only had the culture medium added.
Cell viability
The cell viability was measured by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) colorimetric assay. Briefly, the cells (5×104/mL) were seeded in a 96-well plate and washed with phosphate-buffered saline (PBS). After incubation with various concentrations of ligustrazine or LY294002 for 72 h, 20 μL of 0.5 mg/mL MTT was added to each well and incubated for another 4 h at 37°C. Viable cells were then detected by measuring the absorbance at 490 nm optical density (OD) using a microplate reader (FLUOstar Omega, BMG LABTECH, Offenburg, Germany). The experiment was repeated 3 times.
Colony formation assay
HaCaT cells were plated in a 6-well plate and then incubated with various concentrations of ligustrazine or LY294002 for 72 h. After washing 3 times with PBS and culturing in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen), cells were supplemented with 10% FBS for another 6 days. Finally, the cells were stained with 0.5% crystal violet at room temperature.
Quantitative real-time RCR (qRT-PCR)
Total RNA was extracted from HaCaT cells with TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. The quantity of total RNA was determined by using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
First-strand complementary DNA (cDNA) was synthesized using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA). Amplification reactions were carried out on an ABI PRISM 7900 HT system using SYBR Green PCR Master Mix (Applied Biosystems). The mRNA expression levels of phosphatidyl inositol 3-kinase (PI3K), p-AKT, and p-mammalian target of rapamycin (mTOR) were normalized on GAPDH with the comparative 2−δδCT method. All samples were processed in triplicate.
Western blotting
HaCaT cells were treated with various concentrations of ligustrazine for 48 h, and then were harvested, washed with cold PBS, and lysed with ice-cold cell lysis (RIPA) buffer. The concentration of protein was assessed by using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). The protein (30 μg) was resolved on sodium dodecyl sulfonate (SDS) – polyacrylamide gel electrophoresis (PAGE), electro-blotted onto nitrocellulose membranes, and then incubated with anti-PI3K antibody (1:1000; Santa Cruz Biotechnology, Inc.) or anti-p-AKT antibody (1:1000; Cell Signaling Technology, Inc., Beverly, MA, USA) at 4oC overnight. Subsequently, the membranes were washed with Tris-Buffered Saline Tween (TBST), incubated with corresponding horseradish peroxidase-conjugated secondary antibodies (1:1000; Cell Signaling Technology) for 2 h at room temperature, and detected by enhanced chemiluminescence and densitometric analysis. GAPDH was used as a loading control.
Statistical analysis
Quantitative data are shown as the mean ± standard deviation (SD). Statistical analyses were conducted using SAS (version 8.1; SAS Institute Inc., Cary, NC). The statistical comparison between groups was performed using Student’s t-test (for 2 groups) or one-way analysis of variance (ANOVA) (for 3 or more groups). Differences were regarded as statistically significant at P<0.05.
Results
Comparisons of clinical data between the 2 groups
The clinical data are shown in Table 1, which shows that there were no significant differences between the 2 groups in sex, average age, average duration of pressure sores, and stages. After a continuous 4 weeks of treatment, the therapeutic effects between the 2 groups were compared. In the experimental group there were 11 healing cases, 9 markedly effective cases, 2 effective cases, and 1 ineffective case. In the control group there were 8 healing cases, 8 markedly effective cases, 2 effective cases, and 4 ineffective cases. The total effective rate was 95% in the experimental group and 81% in the control group. These differences between the 2 groups were significant (P<0.05). The healing time in the experimental group was 9.33±2.08 days (Phase II) and 21.16±3.68 days (Phase III), whereas the healing time in the control group was 11.01±3.22 days (Phase II) and 24.26±2.37 days (Phase III). The healing time in both Phase II and Phase III in the experimental group was significantly reduced compared to the control group (P<0.05).
Table 1.
Clinical data of the 2 groups.
| Group | Sex | Ages (years) | Average duration (months) | Stages | ||
|---|---|---|---|---|---|---|
| Male | Female | II | III | |||
| Experimental | 9 | 7 | 63.33±2.78 | 1.36±0.68 | 17 | 6 |
| Control | 8 | 8 | 64.21±3.02 | 1.22±0.37 | 16 | 6 |
Effect of ligustrazine on cell growth
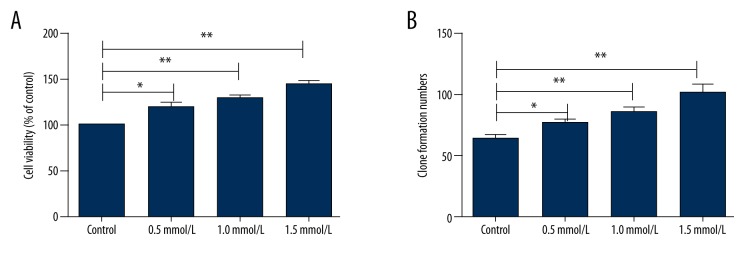
We then explored the underlying mechanism of ligustrazine treatment on pressure sores in vitro. Human HaCaT cells were cultured with various concentrations of ligustrazine (0.5 mmol/L, 1.0 mmol/L, and 1.5 mmol/L), and the cell growth was then evaluated by MTT and colony formation assay after 72 h of culture. As shown in Figure 1A, 1B, the cell viability and clone formation numbers were significantly increased in a dose-dependent manner by culture with ligustrazine (P<0.05 or P<0.01), indicating that ligustrazine promotes cell growth.
Figure 1.
Effect of ligustrazine on cell growth. Human HaCaT cells were cultured with various concentrations of ligustrazine (0.5 mmol/L, 1.0 mmol/L, and 1.5 mmol/L), and the cell growth was then evaluated by MTT and colony formation assay after 72 h of culture. (A) Cell viability was significantly increased by culture with ligustrazine in a dose-dependent manner. (B) Clone formation numbers were significantly increased in a dose-dependent manner by culture with ligustrazine. MTT – 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide. * P<0.05 compared with the control group; ** P<0.01 compared with the control group.
Effect of ligustrazine on the PI3K/AKT pathway
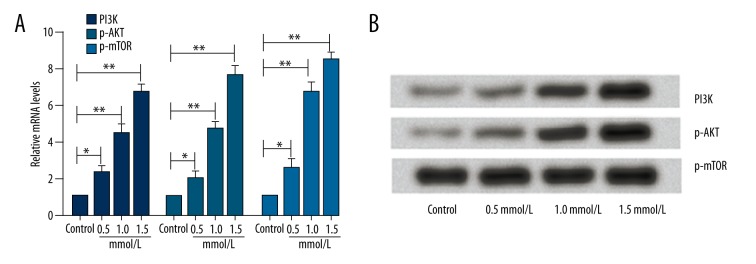
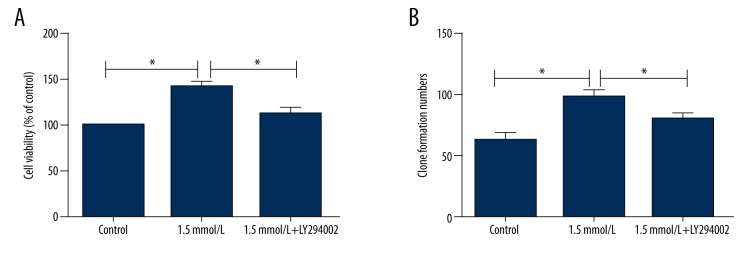
We also investigated the associated pathway involved in the effect of ligustrazine on cell growth. The mRNA expression levels of PI3K, AKT, and p-mTOR were examined. As indicated in Figure 2A, the mRNA expression levels of PI3K, AKT, and p-mTOR were all significantly increased by culture with ligustrazine in a dose-dependent manner compared to the control group (P<0.05 or P<0.01). In line with PCR, the protein levels of PI3K and AKT were also significantly up-regulated by the various concentrations of ligustrazine (Figure 2B). To further confirm the results, we treated cells with the inhibitor of AKT (LY294002). The cell viability and clone formation numbers were then analyzed. As demonstrated in Figure 3A, 3B, the MTT and clone formation experiment showed that both the cell viability and clone formation numbers were significantly inhibited by simultaneous application of 1.5 mmol/L ligustrazine and LY294002. The results suggested that the higher cell growth induced by ligustrazine might be associated with activation of the PI3K/AKT pathway.
Figure 2.
Effect of ligustrazine on the PI3K/AKT pathway. Expression levels of PI3K, AKT, and p-mTOR were all significantly increased in a dose-dependent manner by culture with ligustrazine compared to the control group. (A) Relative mRNA levels of PI3K, AKT, and p-mTOR. (B) Representative images of Western blotting. PI3K – phosphatidyl inositol 3-kinase; mTOR – mammalian target of rapamycin. * P<0.05 compared with the control group; ** P<0.01 compared with the control group.
Figure 3.
Effect of LY294002 on cell growth. The cell viability and clone formation numbers were analyzed after administration of the inhibitor of AKT (LY294002). (A) Cell viability was significantly inhibited by simultaneous application of 1.5 mmol/L ligustrazine and LY294002. (B) Clone formation numbers were significantly inhibited by simultaneous application of 1.5 mmol/L ligustrazine and LY294002.* P<0.05 compared with the control group.
Discussion
In the present study, the therapeutic effect on pressure sores of external application of ligustrazine combined with holistic nursing was investigated. The underlying mechanism was also explored in vitro by using HaCaT cells. The results showed that compared to the patients who received compound clotrimazole cream, the patients who received external application of ligustrazine had higher total effective rate and shorter healing time. The in vitro study suggested that the protective role of ligustrazine in pressure sores might be associated with activation of the PI3K/AKT pathway.
Pressure sores, also known as pressure ulcers or bedsores, are a source of pain, major discomfort and disruption, and infection, limiting ability to perform daily activities. This disease not only represents a major burden to individuals and society, but also reduces the quality of life for patients and their caregivers. Although tremendous progress has been made in recent years in basic science research and clinical guidelines on the treatment of pressure sores, prevention and treatment of pressure sores is still an issue that needs to be addressed by doctors and nurses. TCM, one of the most important complementary and alternative medicines, has been well demonstrated to have significant treatment effects on pressure sores [10]. Ligustrazine is an effective TCM herb isolated from Ligusticum chuanxiong Hort, which was first recorded in the Divine Husbandman’s Classic of the Materia Medica (Shen Nong Ben Cao Jing). This plant is widely used when treating various diseases in vivo and in vitro. For example, it has been reported that ligustrazine can protect against accelerated nephritis due to its antioxidant characteristics and inhibition of reactive oxygen species (ROS) [20]. Ligustrazine may attenuate atherosclerosis and hepatic accumulation by alleviating oxidative stress and dyslipidemia [12,21]. In addition, ligustrazine has been reported to play a protective role in ischemia-reperfusion injury [22,23]. However, the therapeutic effect of ligustrazine on pressure sores has rarely been studied. Therefore, we explored the therapeutic effect of ligustrazine in combination with holistic nursing on pressure sores.
Patients with Phase II and Phase III pressure sores were enrolled in our study. Ligustrazine was externally administrated to the experimental group patients by using a dermal patch, whereas compound clotrimazole cream was administered to the patients in the control group. Compared with conventional oral administration, the medication delivered by a dermal patch reduces adverse effects and improves compliance with medication and dosage control by avoidance of first-pass hepatic metabolism and destruction in the gastrointestinal tract [24]. Therefore, in our study we administrated ligustrazine using dermal patches. In addition to the pharmacotherapy, nursing intervention is also extremely important. Effective and good nursing care with targeted interventions is essential for prevention of pressure sores and is generally able to reduce the incidence and development of pressure sores. In addition, exemplary skin care is now considered as one of the nurse-sensitive outcome measures [25] and was reported in the National Database of Nursing Quality Indicators [26]. The total effective rate and healing time were recorded after 4 continuous weeks of treatment. Our results showed that external application of ligustrazine significantly improved the total effective rate and decreased healing time. Since ligustrazine can promote the recovery of pressure sores, we speculated that ligustrazine might promote cell regeneration and enhance cell proliferation ability. To further clarify the underlying mechanism of the protective role of ligustrazine in pressure sores, the phosphorylation and expression of PI3K, AKT, and p-mTOR were examined through qRT-PCR and Western blotting analysis. AKT is an important anti-apoptotic protein, which is activated via the PI3K pathway. The PI3K/AKT pathway has been demonstrated to play a key role in cell proliferation and survival [27–29], and activation of the PI3K/AKT pathway inhibits cell death and enhances cells survival [30]. Ligustrazine and its analogue, CXC195, can both protect against ischemia reperfusion injury in rats by activation of the PI3K/AKT pathway [23,31,32]. Chen et al. suggested that combination treatment of Adriamycin and (E)-2-(2, 4-dimethoxystyryl)-3, 5, 6-trimethylpyrazine (DLJ14) (a ligustrazine derivate) suppressed resistant breast cancer progression, partly by inhibition of the EGFR/PI3K/AKT survival pathway [33]. In our study, the expression levels of PI3K, phosphorylated AKT, and mTOR were significantly up-regulated in a dose-dependent manner by application of ligustrazine. However, this effect was abolished by co-treatment with the inhibitor of AKT (LY294002), suggesting that ligustrazine activates the PI3K/Akt pathway. Our results were similar to those of the above studies.
Conclusions
Our results demonstrate that external application of ligustrazine combined with holistic nursing protects against pressure sores. The protective effect may be associated with the promotion of cell growth by activation of the PI3K/AKT pathway.
Footnotes
Conflict of interests
No conflict of interests exists.
Source of support: Departmental sources
References
- 1.Berlowitz DR, Wilking SV. Risk factors for pressure sores. A comparison of cross-sectional and cohort-derived data. J Am Geriatr Soc. 1989;37:1043–50. doi: 10.1111/j.1532-5415.1989.tb06918.x. [DOI] [PubMed] [Google Scholar]
- 2.Woolsey RM, McGarry JD. The cause, prevention, and treatment of pressure sores. Neurol Clin. 1991;9:797–808. [PubMed] [Google Scholar]
- 3.Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg. 2007;44:101–43. doi: 10.1067/j.cpsurg.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Staas WE, Jr, Cioschi HM. Pressure sores – a multifaceted approach to prevention and treatment. West J Med. 1991;154:539–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Davis CM, Caseby NG. Prevalence and incidence studies of pressure ulcers in two long-term care facilities in Canada. Ostomy Wound Manage. 2001;47:28–34. [PubMed] [Google Scholar]
- 6.Tannen A, Bours G, Halfens R, Dassen T. A comparison of pressure ulcer prevalence rates in nursing homes in the Netherlands and Germany, adjusted for population characteristics. Res Nurs Health. 2006;29:588–96. doi: 10.1002/nur.20160. [DOI] [PubMed] [Google Scholar]
- 7.Scott JR, Gibran NS, Engrav LH, et al. Incidence and characteristics of hospitalized patients with pressure ulcers: State of Washington, 1987 to 2000. Plast Reconstr Surg. 2006;117:630–34. doi: 10.1097/01.prs.0000197210.94131.39. [DOI] [PubMed] [Google Scholar]
- 8.Whittington KT, Briones R. National Prevalence and Incidence Study: 6-year sequential acute care data. Adv Skin Wound Care. 2004;17:490–94. doi: 10.1097/00129334-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Wang X-R, Han B-R. Logistic regression analysis and nursing interventions for high-risk factors for pressure sores in patients in a surgical intensive care unit. Chinese Nursing Research. 2015;2:51–54. [Google Scholar]
- 10.Zhang QH, Sun ZR, Yue JH, et al. Traditional Chinese medicine for pressure ulcer: A meta-analysis. Int Wound J. 2013;10:221–31. doi: 10.1111/j.1742-481X.2012.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen KJ, Chen K. Ischemic stroke treated with Ligusticum chuanxiong. Chin Med J. 1992;105:870–73. [PubMed] [Google Scholar]
- 12.Jiang F, Qian J, Chen S, et al. Ligustrazine improves atherosclerosis in rat via attenuation of oxidative stress. Pharm Biol. 2011;49:856–63. doi: 10.3109/13880209.2010.551776. [DOI] [PubMed] [Google Scholar]
- 13.Hou YZ, Zhao GR, Yang J, et al. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004;75:1775–86. doi: 10.1016/j.lfs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Xie X, Tian Y, Yin S, et al. Anticancer effects of Ligusticum chuanxiong Hort alcohol extracts on HS766T cell. Afr J Tradit Complement Altern Med. 2013;10(6):542–46. doi: 10.4314/ajtcam.v10i6.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran X, Ma L, Peng C, et al. Ligusticum chuanxiong Hort: A review of chemistry and pharmacology. Pharm Biol. 2011;49:1180–89. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 16.Black J, Baharestani M, Cuddigan J, et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Dermatology nursing/Dermatology Nurses’ Association. 2007;19:343–49. quiz 350. [PubMed] [Google Scholar]
- 17.Liu X, Liu H, Liu J, et al. Preparation of a ligustrazine ethosome patch and its evaluation in vitro and in vivo. Int J Nanomedicine. 2011;6:241–47. doi: 10.2147/IJN.S16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Committee TSDeose. Criteria of Diagnosis and therapeutic effect of diseases and syndromes in Traditional Chinese Medicine (the People’s Republic of China TCM Industry Standard ZY/T001. 1∼001.9-94) China: State Administration of Traditional Chinese Medicine of the People’s Republic of China; 1994. [Google Scholar]
- 19.Xiao Y, Wang YC, Li LL, et al. Lactones from Ligusticum chuanxiong Hort. reduces atherosclerotic lesions in apoE-deficient mice via inhibiting over expression of NF-κB-dependent adhesion molecules. Fitoterapia. 2014;95:240–46. doi: 10.1016/j.fitote.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Fu H, Li J, Li QX, et al. Protective effect of ligustrazine on accelerated anti-glomerular basement membrane antibody nephritis in rats is based on its antioxidant properties. Eur J Pharmacol. 2007;563:197–202. doi: 10.1016/j.ejphar.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Luo Z-y, Song D, Mao C-m, Wang W-t. Effect of ligustrazine injection on the change of oxidative stress system during renal ischemia reperfusion injury in rabbits. Global Journal of Integrated Chinese Medicine and Western Medicine. 2015;3:1. [Google Scholar]
- 22.Feng L, Ke N, Cheng F, et al. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. The Journal of Surgical Research. 2011;166:298–305. doi: 10.1016/j.jss.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Lv L, Meng Q, Xu J, et al. Ligustrazine attenuates myocardial ischemia reperfusion injury in rats by activating the phosphatidylinositol 3-kinase/Akt pathway. Ann Clin Lab Sci. 2012;42:198–202. [PubMed] [Google Scholar]
- 24.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–24. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 25.Association AN, Association AN. Nursing report card for acute care. American Nurses Publishing. 1995 [Google Scholar]
- 26.Association AN. National database of nursing quality indicators. Retrieved June. 2012;10:2012. [Google Scholar]
- 27.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 28.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Wang M, Chen H, et al. Hypothermia protects the brain from transient global ischemia/reperfusion by attenuating endoplasmic reticulum response-induced apoptosis through CHOP. PloS One. 2013;8:e53431. doi: 10.1371/journal.pone.0053431. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Shibata M, Yamawaki T, Sasaki T, et al. Upregulation of Akt phosphorylation at the early stage of middle cerebral artery occlusion in mice. Brain Res. 2002;942:1–10. doi: 10.1016/s0006-8993(02)02474-5. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Wei X, Chen L, et al. Tetramethylpyrazine analogue CXC195 protects against cerebral ischemia/reperfusion injury in the rat by an antioxidant action via inhibition of NADPH oxidase and iNOS expression. Pharmacology. 2013;92:198–206. doi: 10.1159/000354722. [DOI] [PubMed] [Google Scholar]
- 32.Lv L, Jiang SS, Xu J, et al. Protective effect of ligustrazine against myocardial ischaemia reperfusion in rats: The role of endothelial nitric oxide synthase. Clin Exp Pharmacol Physiol. 2012;39:20–27. doi: 10.1111/j.1440-1681.2011.05628.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Wang W, Wang H, et al. Combination treatment of ligustrazine piperazine derivate DLJ14 and adriamycin inhibits progression of resistant breast cancer through inhibition of the EGFR/PI3K/Akt survival pathway and induction of apoptosis. Drug Discov Ther. 2014;8:33–41. doi: 10.5582/ddt.8.33. [DOI] [PubMed] [Google Scholar]