Abstract
The genera Microascus and Scopulariopsis comprise species commonly isolated from soil, decaying plant material and indoor environments. A few species are also recognised as opportunistic pathogens of insects and animals, including humans. In the past, the taxonomy of these fungi has been based on morphology only. With the aim to clarify the taxonomy and phylogeny of these fungi, we studied a large set of clinical and environmental isolates, including the available ex-type strains of numerous species, by means of morphological, physiological and molecular analyses. Species delineation was assessed under the Genealogical Phylogenetic Species Recognition (GCPSR) criterion using DNA sequence data of four loci (ITS region, and fragments of rDNA LSU, translation elongation factor 1-α and β-tubulin). The genera Microascus and Scopulariopsis were found to be separated in two distinct lineages. The genus Pithoascus is reinstated and the new genus Pseudoscopulariopsis is erected, typified by P. schumacheri. Seven new species of Microascus and one of Scopulariopsis are described, namely M. alveolaris, M. brunneosporus, M. campaniformis, M. expansus, M. intricatus, M. restrictus, M. verrucosus and Scopulariopsis cordiae. Microascus trigonosporus var. macrosporus is accepted as a species distinct from M. trigonosporus. Nine new combinations are introduced. Microascus cinereus, M. longirostris, P. schumacheri and S. flava are neotypified. A table summarising the morphological features of the species treated and identification keys for each genus are provided.
Keywords: Ascomycota, Microascaceae, Microascales, multigene phylogeny, taxonomy
INTRODUCTION
Scopulariopsis was erected by Bainier (1907) for a group of fungi with asexual propagation, with S. brevicaulis as type species and two additional taxa, S. rubellus and S. rufulus. Scopulariopsis brevicaulis was originally described as Penicillium brevicaule by Saccardo (1882) and included in the Penicillium section Anomala (Biourge 1923). In the current sense, the distinctive features of Scopulariopsis are its annellidic conidiogenesis with mostly thick-walled, basally truncate conidia arranged in long, dry chains and its colony colour varying from white to brown or black, but never in bright green shades like Penicillium (Morton & Smith 1963, Samson et al. 2010). Some asexual genera with morphological features similar to those of Scopulariopsis, such as Acaulium, Masoniella, Phaeoscopulariopsis and Torula were considered to be synonymous (Curzi 1930, Morton & Smith 1963). Scopulariopsis currently comprises species with a worldwide distribution that are commonly isolated from soil, air, plant debris and dung (Domsch et al. 2007). In addition, some species have been described as colonisers or pathogens of mammals, including humans and insects (de Hoog et al. 2011, Iwen et al. 2012, Sandoval-Denis et al. 2013).
Several authors (Curzi 1930, 1931, Abbott et al. 1998, Abbott & Sigler 2001, Issakainen et al. 2003) have demonstrated by culturing, mating studies and molecular methods, that the sexual morphs of Scopulariopsis belong to the ascomycete genus Microascus. Abbott & Sigler (2001) confirmed the existence of both homothallic and heterothallic species. Microascus was included in the family Microascaceae (1951), order Microascales, together with other fungi with annellidic conidiogenesis (Lumbsch & Huhndorf 2007). Microascus is characterised by globose to ampulliform perithecial ascomata with cylindrical or papillate necks, and a dark peridium of textura angularis. The asci are ovate to globose, unitunicate, non-pedicellate, and evanescent, formed in basipetal rows and containing eight 1-celled ascospores. The ascospores are typically asymmetrical, reniform, lunate or triangular, dextrinoid when young, often with an inconspicuous germ-pore, and extruded in a long cirrhus or a gelatinous ball at the top of the ascomata (Barron et al. 1961, Morton & Smith 1963, Guarro et al. 2012).
Von Arx (1973a) erected Pithoascus with three species, i.e. P. intermedius, P. nidicola (type species) and P. schumacheri. These three species were previously included in Microascus and had ascomata with rudimentary or inconspicuous ostioles, navicular to fusiform ascospores without germ pores, while they lacked asexual morphs. Von Arx (1978) added Pithoascus langeronii, which produced an arthroconidial asexual morph. Nevertheless, species of Pithoascus (i.e. P. intermedius, P. schumacherii) were shown to produce a reduced, scopulariopsis-like asexual morph (Roberts 1985, Valmaseda et al. 1986). Valmaseda et al. (1986) erected the new monotypic genus Pithoascina for the arthroconidia-forming species P. langeronii. Based on these features, P. langeronii was later transferred to the genus Eremomyces (Eremomycetaceae, Dothideomycetes) by Malloch & Sigler (1988) and more recently to Arthrographis, being renamed as Arthrographis arxii (Giraldo et al. 2014).
Several authors consider Pithoascus s.str. as a synonym of Microascus (Malloch & Hubart 1987, Abbott et al. 2002, Guarro et al. 2012) since some species show intermediate morphological characteristics. In addition, other asexual genera of the Microascaceae phylogenetically close to Scopulariopsis, i.e. Wardomyces and Wardomycopsis, also produce a Microascus sexual form (Malloch 1970, Udagawa & Furuya 1978); these authors maintained wider generic concepts.
Barron et al. (1961) and later Morton & Smith (1963) published comprehensive monographic reviews on Microascus and Scopulariopsis based on morphological criteria. Morphology seems to be insufficient for establishing species limits in these fungi. Although most species can be identified by detailed morphological study, phenotypic characters appear to overlap in several cases (Sandoval-Denis et al. 2013). DNA sequencing and multilocus phylogenetic analysis have considerably improved our understanding of species concepts in many fungal groups (Lackner & de Hoog 2011, Summerbell et al. 2011, Lackner et al. 2014, Samson et al. 2014), but as yet no such study has been undertaken to revise Microascus, Scopulariopsis and allied genera.
Presently, 77 species are accepted in Scopulariopsis and 32 in Microascus. In addition, many described species are of doubtful identity because their type materials are lost and their protologues are uninterpretable. A further complicating factor is that the new International Code of Nomenclature for Fungi, Algae and Plants no longer allows dual nomenclature for those fungal species that present both sexual and asexual morphs (Hawksworth et al. 2011, Hibbett & Taylor 2013). However, to resolve which name has priority, both at genus and species levels, requires understanding of relationships among species, as well as a stable and well-defined generic circumscription. In the case if Scopulariopsis and Microascus would be congruent, the former name has been recommended (Hawksworth 2012, Sandoval-Denis et al. 2013).
In a recent study on Scopulariopsis and Microascus species associated with human disease, we characterised several isolates that could not be identified (Sandoval-Denis et al. 2013). The present work aims to clarify the taxonomic position of these putative new species using the Genealogical Phylogenetic Species Recognition (GCPSR) criterion (Taylor et al. 2000). We provide a multigene phylogeny of Scopulariopsis, Microascus and related fungi based on a large set of isolates, which includes all available ex-type cultures and well-identified reference strains from international culture collections.
MATERIALS AND METHODS
Isolates
In the present study we evaluate a total of 141 fungal strains, representing 67 fungal species (Table 1). The strains were mainly obtained from different international culture collections, but also from human clinical specimens in the USA.
Table 1.
Strains and sequence accession numbers included in this study.
| Current name | Original name | Strain number1 | Source | Origin | Sequence accession number2 |
|||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | EF-1α | TUB | |||||
| Aspergillus baarnensis | Scopulariopsis halophilica | CBS 380.74 (ex-type) | Undaria pinnatifida | Japan | LM652376 | LM652499 | – | – |
| Doratomyces purpureofuscus | Doratomyces purpureofuscus | CBS 139.42; NBRC 7677 | Manure | The Netherlands: Limburg | 00767701* | 00767701* | – | – |
| Doratomyces stemonitis | Doratomyces stemonitis | CBS 127.22; MUCL 4031 | Seed | The Netherlands: Wageningen | LM652377 | DQ836907 | – | – |
| Gamsia aggregata | Wardomyces aggregatus | CBS 251.69 (ex-isotype) | Dung of carnivore | USA | LM652378 | LM652500 | – | – |
| Gamsia simplex | Wardomyces simplex | CBS 546.69 (ex-isotype) | Milled Oryza sativa | Japan | LM652379 | LM652501 | – | – |
| Graphium penicillioides | Graphium penicillioides | CBS 102632 (ex-epitype) | Wood, Populus nigra | Czech Republic | AB038432 | AF175961 | – | – |
| Hypocrea atroviridis | Hypocrea atroviridis | CBS 110086; NBRC 101776 (ex-type) | Decorticated wood | France | 11776204* | 11776205* | – | – |
| Kernia nitida | Magnusia nitida | CBS 282.52; NBRC 8200 | Chrysolina sanguinolenta | France | 00820001* | 00820001* | – | – |
| Kernia pachypleura | Kernia pachypleura | NBRC 30413; UAMH 8858 | Soil of paddy field | Japan | 03041301* | 03041301* | – | – |
| Lophotrichus macrosporus | Lophotrichus macrosporus | FMR 5571; NBRC 32894 | Sheep dung | Iraq | 03289401* | 03289401* | – | – |
| Lophotrichus plumbescens | Lophotrichus plumbescens | NBRC 30864; UAMH 8710 (ex-type) | Soil | Thailand; Bangkok | 03086401* | 03086401* | – | – |
| Microascus alveolaris sp. nov. | Microascus sp. | UTHSC 04-1534; FMR 12354 | Human BAL | USA | LM652380 | HG380482 | HG380405 | LM652596 |
| Microascus sp. | UTHSC 05-3416; FMR 12350 | Human BAL | USA | LM652381 | HG380483 | HG380406 | LM652597 | |
| Microascus sp. | UTHSC 05-1041; FMR 12351 | Human sputum | USA | LM652382 | HG380488 | HG380411 | LM652598 | |
| Microascus sp. | UTHSC 06-3152; FMR 12346 | Human BAL | USA | LM652383 | HG380487 | HG380410 | LM652599 | |
| Microascus sp. | UTHSC 07-1823; FMR 12342 | Human Sputum | USA | LM652384 | HG380489 | HG380412 | LM652600 | |
| Microascus sp. | CBS 139501; UTHSC 07-3491; FMR 12252 (ex-type) | Human BAL | USA | LM652385 | HG380484 | HG380407 | LM652601 | |
| Microascus sp. | UTHSC 08-886; FMR 12340 | Human BAL | USA | LM652386 | HG380485 | HG380408 | LM652602 | |
| Microascus sp. | UTHSC 10-214; FMR 12336 | Human BAL | USA | LM652387 | HG380486 | HG380409 | LM652603 | |
| Microascus sp. | UTHSC R-4634; FMR 12333 | Human lung Tissue | USA | LM652388 | HG380490 | HG380413 | LM652604 | |
| Microascus albonigrescens† | Microascus albonigrescens | IHEM 18560 | Litter treated with urea | Japan: Nemuro-shi | LM652389 | LM652502 | – | – |
| Microascus brunneosporus sp. nov. | Microascus sp. | CBS 138276; UTHSC 06-4312; FMR 12343 (ex-type) | Human BAL | USA | LM652390 | HG380497 | HG380420 | LM652605 |
| Microascus campaniformis sp. nov. | Microascus sp. | CBS 138126; UTHSC 10-565; FMR 12334 (ex-type) | Human BAL | USA | LM652391 | HG380495 | HG380418 | LM652606 |
| Microascus caviariformis† | Microascus caviariformis | CBS 536.87: UAMH 5592 (ex-type) | Decaying meat | Belgium | LM652392 | LM652503 | – | – |
| Microascus chartarus comb. nov. | Masonia chartarum | CBS 294.52; MUCL 9001 (ex-type) | Mouldy wall-paper in a house | England: London. | LM652393 | HG380463 | HG380386 | LM652607 |
| Microascus cinereus | Microascus cinereus | UTHSC 06-3278; FMR 12345 | BAL | USA | LM652394 | HG380347 | HG380424 | LM652608 |
| Microascus cinereus | UTHSC 08-3181: FMR 12339 | Human sternum tissue | USA | LM652395 | HG380348 | HG380425 | LM652609 | |
| Microascus cinereus | UTHSC 09-573; FMR 12239 | Human BAL | USA | LM652396 | HG380349 | HG380426 | LM652610 | |
| Microascus cinereus | UTHSC 10-2805; FMR 12217 (ex-neotype) | Human BAL | USA | LM652397 | HG380350 | HG380427 | LM652611 | |
| Microascus cinereus | UTHSC 11-383; FMR 12331 | Human BAL | USA | LM652398 | HG380351 | HG380428 | LM652612 | |
| Microascus griseus | CBS 365.65; ATCC 16204 (ex-type) | Soil | India: Maharashtra | LM652399 | HG380346 | HG380423 | LM652613 | |
| Microascus cirrosus | Microascus cirrosus | CBS 217.31 (ex-type) | Leaf of Prunus sp. | Italy | LM652400 | HG380429 | HG380352 | LM652614 |
| Microascus cirrosus | CBS 277.34; MUCL 9050 | Roots of Vitis vinifera | Italy | LM652401 | LM652504 | LM652556 | LM652615 | |
| Microascus desmosporus | CBS 301.61; MUCL 9054 | Unknown | UK | LM652402 | LM652505 | LM652557 | LM652616 | |
| Microascus cirrosus | UTHSC 07-1887: FMR 12256 | Induced human sputum | USA | LM652403 | HG380431 | HG380354 | LM652617 | |
| Microascus cirrosus | UTHSC 11-14; FMR 12332 | Human BAL | USA | LM652404 | HG380432 | HG380355 | LM652618 | |
| Microascus croci comb. nov. | Scopulariopsis chartarum | FMR 3997 | Aquatic sediment, Ebro river | Spain: Tarragona | LM652405 | LM652506 | LM652558 | LM652619 |
| Microascus cirrosus | FMR 4004 | Aquatic sediment, Besós river | Spain: Barcelona | LM652406 | LM652507 | LM652559 | LM652620 | |
| Scopulariopsis croci | CBS 158.44; MUCL 9002 (ex-type) | From Crocus sp. | The Netherlands: Lisse | LM652407 | LM652508 | LM652560 | LM652621 | |
| Masoniella tertia | CBS 296.61; MUCL 9005 (ex-type) | Air | Brazil: Pernambuco | LM652408 | LM652509 | LM652561 | LM652622 | |
| Microascus expansus sp. nov. | Microascus sp. | UTHSC 06-2519; FMR 12267 | Human pleural fluid | USA | LM652409 | HG380491 | HG380414 | LM652623 |
| Microascus sp. | CBS 138127; UTHSC 06-4472; FMR 12266 (ex-type) | Human sputum | USA | LM652410 | HG380492 | HG380415 | LM652624 | |
| Microascus giganteus† | Microascus giganteus | CBS 746.69 (ex-type) | Insect frass in dead log | Canada: Ontario | LM652411 | LM652510 | – | – |
| Microascus gracilis comb. nov. | Paecilomyces fuscatus | CBS 369.70 (ex-isotype) | Food | Japan | LM652412 | HG380467 | HG380390 | LM652625 |
| Scopulariopsis gracilis | UTHSC 09-1351; FMR 12234 | Human joint fluid | USA | LM652413 | HG380476 | HG380399 | LM652626 | |
| Scopulariopsis gracilis | UTHSC 09-1829; FMR 12231 | Human BAL | USA | LM652414 | HG380477 | HG380400 | LM652627 | |
| Scopulariopsis gracilis | UTHSC 10-390; FMR 12335 | Human BAL | USA | LM652415 | LM652511 | LM652562 | LM652628 | |
| Microascus cinereus | CBS 195.61; MUCL 9048 | Soil | England | LM652416 | HG380468 | HG380391 | LM652629 | |
| Microascus cinereus | CBS 300.61; MUCL 9049 | Seed of Zea mays | USA: Iowa | LM652417 | LM652512 | LM652563 | LM652630 | |
| Microascus hyalinus comb. nov. | Kernia hyalina | CBS 766.70 (ex-isotype) | Dung of cow | USA | LM652418 | LM652513 | LM652564 | LM652631 |
| Microascus intricatus sp. nov. | Microascus sp. | CBS 138128; UTHSC 07-156; FMR 12264 (ex-type) | Human BAL | USA | LM652419 | HG380496 | HG380419 | LM652632 |
| Microascus sp. | FMR 12362 | Soil | Argentina: Iguazú | LM652420 | LM652514 | LM652565 | LM652633 | |
| Microascus longirostris | Microascus longirostris | CBS 196.61; MUCL 9058 (ex-neotype) | Wasp’s nest | USA: Maine | LM652421 | LM652515 | LM652566 | LM652634 |
| Microascus longirostris | CBS 415.64 | Soil | Japan | LM652422 | LM652516 | LM652567 | LM652635 | |
| Microascus macrosporus comb & stat. nov. | Microascus trigonosporus var. macrosporus | CBS 662.71 | Soil | USA | LM652423 | LM652517 | LM652568 | LM652636 |
| Microascus murinus comb. nov. | Scopulariopsis murina | CBS 830.70; IHEM 18567 (ex-type) | Composed municipal waste | Germany: Giessen | LM652424 | HG380481 | HG380404 | LM652637 |
| Microascus paisii comb. nov. | Scopulariopsis brumptii | UTHSC 07-639; FMR 12263 | Human BAL | USA | LM652425 | HG380451 | HG380374 | LM652638 |
| Scopulariopsis brumptii | UTHSC 08-1734; FMR 12248 | Human BAL | USA | LM652426 | HG380452 | HG380375 | LM652639 | |
| Scopulariopsis brumptii | UTHSC 09-2391; FMR 12229 | Human sputum | USA | LM652427 | HG380453 | HG380376 | LM652640 | |
| Scopulariopsis brumptii | UTHSC 09-457; FMR 12241 | Human sputum | USA | LM652428 | HG380454 | HG380377 | LM652641 | |
| Scopulariopsis brumptii | UTHSC 09-482; FMR 12240 | Human BAL | USA | LM652429 | HG380455 | HG380378 | LM652642 | |
| Scopulariopsis brumptii | UTHSC 10-2920; FMR 12215 | Human BAL | USA | LM652430 | HG380456 | HG380379 | LM652643 | |
| Scopulariopsis brumptii | UTHSC 11-708; FMR 12210 | Human sputum | USA | LM652431 | HG380457 | HG380380 | LM652644 | |
| Scopulariopsis brumptii | CBS 896.68; MUCL 8989 | Soil on a Triticum sativum field | Germany: Schleswig | LM652432 | HG380449 | HG380372 | LM652645 | |
| Masonia grisea | CBS 295.52; MUCL 9003 (ex-type) | Culture contaminant | England | LM652433 | HG380450 | HG380373 | LM652646 | |
| Torula paisii | CBS 213.27; MUCL 7915 (ex-type) | Man | Italy | LM652434 | LM652518 | LM652569 | LM652647 | |
| Scopulariopsis brumptii | MUCL 8990 | Soil | Germany: Schleswig-Holstein | LM652435 | LM652519 | LM652570 | LM652648 | |
| Scopulariopsis chartarum | CBS 897.68; MUCL 8993 | Soil on a wheat field | Germany | LM652436 | LM652520 | LM652571 | LM652649 | |
| Scopulariopsis melanospora | CBS 272.60; MUCL 9040 (ex-isotype) | Milled Oriza sativa | USA | LM652437 | LM652521 | LM652572 | LM652650 | |
| Scopulariopsis sphaerospora | CBS 402.34; MUCL 9045 (ex-type) | Unknown | Austria | LM652438 | LM652522 | LM652573 | LM652651 | |
| Microascus pyramidus | Microascus pyramidus | CBS 212.65 (ex-isotype) | Desert soil | USA: California | LM652439 | HG380435 | HG380358 | LM652652 |
| Microascus restrictus sp. nov. | Microascus sp. | CBS 138277; UTHSC 09-2704; | Human left hallux | USA | LM652440 | HG380494 | HG380417 | LM652653 |
| FMR 12227 (ex-type) | ||||||||
| Microascus senegalensis | Microascus senegalensis | CBS 277.74; IHEM 18561 (ex-type) | Mangrove soil | Senegal | LM652441 | LM652523 | LM652574 | LM652654 |
| Microascus singularis† | Microascus singularis | CBS 414.64 | Laboratory contaminant | Japan: Tokyo | LM652442 | LM652524 | – | – |
| Microascus trigonosporus | Microascus trigonosporus var. | CBS 218.31 (ex-type) | Unknown | USA | LM652443 | HG380436 | HG380359 | LM652655 |
| trigonosporus | ||||||||
| Microascus trigonosporus | CBS 199.61; MUCL 9061 | Milled rice | Burma, Japan | LM652444 | HG380438 | HG380361 | LM652656 | |
| Scopulariopsis coprophila | CBS 262.35; MUCL 9841 | Mushroom bed | UK | LM652445 | LM652525 | LM652575 | LM652657 | |
| Microascus verrucosus sp. nov. | Microascus sp. | CBS 138278; UTHSC 10-2601; | Human BAL | USA | LM652446 | HG380493 | HG380416 | LM652658 |
| FMR 12219 (ex-type) | ||||||||
| Parascedosporium tectonae | Graphium tectonae | CBS 127.84 (ex-type) | Seed | Jamaica | AY228113 | EF151332 | EF151409 | – |
| Petriella setifera | Petriella setifera | CBS 437.75 | Wood panel in coastal water | Hong Kong | – | DQ470969 | DQ836911 | – |
| Petriella setifera | FMR 7736; NBRC 100025 | Soil | Spain; Canary Islands | 10002501* | 10002501* | – | – | |
| Petriella sordida | Petriella sordida | CBS 124169 | Corner of a bathroom | The Netherlands | GQ426957 | AY281099 | – | – |
| Petriellopsis africana | Petriellidium africanum | CBS 311.72 (ex-type) | Brown sandy soil | Namibia | AJ888425 | EF151331 | – | – |
| Phitoascus ater comb. nov. | Scopulariopsis atra | CBS 400.34; IHEM 18608 (ex-type) | Unknown | Unknown | LM652447 | LM652526 | LM652576 | LM652659 |
| Phitoascus exsertus | Microascus exsertus | CBS 583.75 | From Osmia rufa | Denmark: Sjaelland | LM652448 | LM652527 | LM652577 | LM652660 |
| Microascus exsertus | CBS 819.70 (ex-type) | From Megachile willoughbiella | Denmark: Tastrup | LM652449 | LM652528 | LM652578 | LM652661 | |
| Phitoascus intermedius | Microascus intermedius | CBS 217.32 (ex-type) | Root of Fragaria vesca | USA: North Carolina | LM652450 | LM652529 | LM652579 | LM652662 |
| Phitoascus nidicola | Microascus nidicola | CBS 197.61 (ex-epitype) | From Dipodomys merriami | USA: Utah | LM652451 | LM652530 | LM652580 | LM652663 |
| Pithoascus platysporus† | Pithoascus platysporus | CBS 419.73 (ex-type) | Agricultural soil | The Netherlands | LM652452 | LM652531 | – | – |
| Phitoascus stoveri | Microascus stoveri | CBS 176.71 (ex-type) | Root of Beta vulgaris | USA: Ohio | LM652453 | LM652532 | LM652581 | LM652664 |
| Pseudallescheria ellipsoidea | Petriellidium ellipsoideum | CBS 418.73 (ex-type) | Soil | Tajikistan | EF151323 | EF151323 | – | – |
| Pseudoscopulariopsis hibernica comb. nov. | Scopulariopsis hibernica | UAMH 2643; ATCC 16690 | From soil | Ireland | LM652454 | LM652533 | LM652582 | LM652665 |
| Pseudoscopulariopsis schumacheri comb. nov. | Microascus schumacheri | CBS 435.86 (ex-neotype) | From soil | Spain: Puerto de la Quesera | LM652455 | LM652534 | LM652583 | LM652666 |
| Scedosporium aurantiacum | Scedosporium aurantiacum | CBS 116910 | Ulcer of ankle | Spain | HQ231818 | EF151326 | – | – |
| Scedosporium boydii | Pseudallescheria boydii | CBS 330.93 | Bronchial secretion | The Netherlands | AY863196 | AY882372 | – | – |
| Scopulariopsis acremonium† | Scopulariopsis danica | CBS 290.38; MUCL 9028 (ex-type) | Skin of a horse | Denmark | LM652456 | HG380439 | HG380362 | – |
| Scopulariopsis acremonium | MUCL 8274 | Wheat field soil | Germany: Schleswig-Holstein | LM652457 | LM652535 | – | – | |
| Scopulariopsis acremonium | MUCL 8409 | Soil | Germany: Schleswig-Holstein | LM652458 | LM652536 | – | – | |
| Scopulariopsis asperula | Microascus niger | MUCL 40729; UAMH 7879 | Indoor air | Canada: Alberta | LM652459 | LM652537 | LM652584 | LM652667 |
| Microascus niger | MUCL 40746; UAMH 9029 | Dung of Mephitis mephitis | Canada: Alberta | LM652460 | HG380434 | HG380357 | LM652668 | |
| Scopulariopsis asperula | CBS 853.68 | Compost soil | Germany | LM652461 | JQ434669 | JQ434621 | JQ434558 | |
| Scopulariopsis fusca | UTHSC 10-3405; FMR 12212 | Toenail | USA | LM652462 | HG380461 | HG380384 | LM652669 | |
| Scopulariopsis fusca | CBS 401.34; MUCL 9032 (ex-type) | Carcass of rabbit | Austria | LM652463 | HG380465 | HG380388 | LM652670 | |
| Torula bestae | CBS 289.38; MUCL 9012 (ex-type) | From man | Italy | LM652464 | LM652538 | LM652585 | LM652671 | |
| Scopulariopsis brevicaulis | Microascus brevicaulis | MUCL 40726 (ex-type) | Indoor air | Canada: Alberta | LM652465 | HG380440 | HG380363 | LM652672 |
| Scopulariopsis alboflavescens | CBS 399.34 (ex-type) | Diseased skin | Austria | LM652466 | LM652539 | JQ434600 | JQ434537 | |
| Scopulariopsis brevicaulis | UTHSC 06-277; FMR 12273 | Human hair | USA | LM652467 | HG380441 | HG380364 | LM652674 | |
| Scopulariopsis brevicaulis | UTHSC 06-619; FMR 12271 | Human toenail | USA | LM652468 | HG380442 | HG380365 | LM652675 | |
| Scopulariopsis brevicaulis | UTHSC 06-1072; FMR 12247 | Human BAL | USA | LM652469 | LM652540 | LM652586 | LM652676 | |
| Scopulariopsis brevicaulis | UTHSC 07-1812; FMR 12257 | Human toenail | USA | LM652470 | HG380443 | HG380366 | LM652677 | |
| Scopulariopsis brevicaulis | UTHSC 07-1888; FMR 12255 | Human spine | USA | LM652471 | HG380444 | HG380367 | LM652678 | |
| Scopulariopsis brevicaulis | UTHSC 09-1092; FMR 12236 | Human toe | USA | LM652472 | HG380445 | HG380368 | LM652679 | |
| Scopulariopsis brevicaulis | UTHSC 09-1373; FMR 12233 | Human sputum | USA | LM652473 | LM652541 | LM652587 | LM652680 | |
| Scopulariopsis brevicaulis | UTHSC 11-1240; FMR 12206 | Human lung mass | USA | LM652474 | HG380446 | HG380369 | LM652681 | |
| Scopulariopsis brevicaulis | UTHSC 11-1563; FMR 12204 | Human BAL | USA | LM652475 | HG380447 | HG380370 | LM652682 | |
| Scopulariopsis brevicaulis | UTHSC 11-427; FMR 12211 | Human sputum | USA | LM652476 | HG380448 | HG380371 | LM652683 | |
| Scopulariopsis insectivora | CBS 335.35; MUCL 9035 | Pupa of Pteronus pini | The Netherlands | LM652477 | LM652542 | LM652588 | LM652684 | |
| Scopulariopsis koningii | CBS 208.61 | Elephant | Unknown | LM652478 | LM652543 | LM652589 | LM652685 | |
| Scopulariopsis stercoraria | MUCL 14213 | Soil | Belgium: Heverlee | LM652479 | LM652544 | LM652590 | LM652686 | |
| Scopulariopsis canadensis† | Scopulariopsis canadensis | CBS 204.61 (ex-type) | Seed of Beta vulgaris | Canada | LM652480 | LM652545 | – | – |
| Scopulariopsis candida | Scopulariopsis alboflavescens | MUCL 9007 | Unknown | Unknown | LM652481 | LM652546 | LM652591 | LM652687 |
| Scopulariopsis brevicaulis var. alba | CBS 119.43; MUCL 9016 | Soil | The Netherlands | LM652482 | LM652547 | LM652592 | LM652688 | |
| Scopulariopsis candelabrum | CBS 205.27; MUCL 9026 | Unknown | France | LM652483 | LM652548 | LM652593 | LM652689 | |
| Scopulariopsis candida | MUCL 40743 (ex-epitype) | Indoor air | Canada | LM652484 | HG380458 | HG380381 | LM652690 | |
| Scopulariopsis candida | UTHSC 09-3241; FMR 12226 | Scalp | USA | LM652485 | HG380460 | HG380383 | LM652691 | |
| Scopulariopsis candida | UTHSC 09-2576; FMR 12228 | Sputum | USA | LM652486 | HG380459 | HG380382 | LM652692 | |
| Microascus manginii | MUCL 41467 | Cheese ‘Tome de Savoie’ | France | LM652487 | HG380433 | HG380356 | LM652693 | |
| Nephrospora manginii | CBS 170.27 (ex-type) | Unknown | France | LM652488 | LM652549 | LM652594 | LM652694 | |
| Scopulariopsis carbonaria† | Scopulariopsis carbonaria | MUCL 9027 (ex-type) | Soil | Panama | LM652489 | HG380462 | HG380385 | LM652695 |
| Scopulariopsis coprophila† | Scopulariopsis fimicola | CBS 206.61 | Mushroom bed | UK | LM652490 | LM652550 | – | – |
| Scopulariopsis cordiae sp. nov. | Scopulariopsis sp. | CBS 138129; UTHSC 09-866; | Human finger | USA | LM652491 | HG380499 | HG380422 | LM652673 |
| FMR 12338 (ex-type) | ||||||||
| Scopulariopsis sp. | UTHSC 05-3453; FMR 12349 | Human JP Drain | USA | LM652492 | HG380498 | HG380421 | LM652696 | |
| Scopulariopsis flava | Scopulariopsis flava | CBS 207.61; MUCL 9031 (ex-neotype) | Cheese | UK | LM652493 | HG380464 | HG380387 | LM652697 |
| Scopulariopsis parva | Scopulariopsis parvula | MUCL 9041 (ex-type) | Soil | Canada | LM652494 | LM652551 | – | – |
| Scopulariopsis soppii | Microascus soppii | UAMH 9169 (ex-type) | wood of Populus tremuloides | Canada: Alberta | LM652495 | LM652552 | LM652595 | LM652698 |
| Trichoderma asperellum | Trichoderma asperellum | CBS 433.97; NBRC 101777 (ex-type) | Sclerotia of Sclerotinia minor | USA: Maryland | 11776302* | 11776301* | – | – |
| buried in soil | ||||||||
| Trichurus spiralis | Trichurus spiralis | NBRC 100833 | Mushroom | Japan: Kumamoto-shi | 11058901* | 11058901* | – | – |
| Trichurus terrophilus | Trichurus terrophilus | NBRC 7660; CBS 448.51; UAMH 8848 | Timber of Eucalyptus saligna | South Africa | 00766001* | 00766001* | – | – |
| Wardomyces inflatus | Wardomyces hughesii | CBS 216.61 (ex-isotype) | Wood, Acer sp. | Canada: Québec | LM652496 | LM652553 | – | – |
| Wardomycopsis humicola | Scopulariopsis humicola | CBS 487.66 (ex-type) | Soil | Canada: Ontario | LM652497 | LM652554 | – | – |
| Wardomycopsis inopinata | Wardomycopsis inopinata | FMR 10305 | Soil | Myanmar | LM652498 | LM652555 | – | – |
1 ATCC: American type culture collection, Manassas, VA, USA; CBS: CBS Fungal Biodiversity Centre, Utrecht, The Netherlands; FMR: Facultat de Medicina i Ciències de la Salut, Reus, Spain; IHEM: Biomedical Fungi and Yeasts Collection, Scientific Institute of Public Health, Belgium; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; NBRC: National Biological Resource Centre, Japan; UAMH: University of Alberta Microfungus Collection and Herbarium, Canada; UTHSC: Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center, San Antonio, USA.
2 ITS: Internal transcribed spacer regions of the rDNA and 5.8S region; LSU: partial large subunit of the rDNA; EF-1α: Partial translation elongation factor gene; TUB: partial beta-tubulin gene.
† Excluded or doubtful species name.
* Sequences obtained from the NBRC database. Sequences newly generated in this study are indicated in bold.
DNA extraction, amplification and phylogenetic analysis
All the strains were cultured on YES agar (20 g yeast extract, 150 g sucrose, 20 g agar, 1 L distilled water) for 5 d at 25 °C. Fresh mycelium was removed by scrapping the agar surface and total genomic DNA extraction was obtained using the PrepmanUltra sample preparation reagent (Applied Biosystems, Foster City, CA, USA), according to manufacturer’s conditions.
Four nuclear DNA regions were amplified and sequenced. These comprised a fragment (490 bp) including the internal transcribed spacer ITS-1 and ITS-2 and the 5.8S rDNA gene (ITS), a fragment (450 bp) including the D1/D2 regions of the LSU rDNA gene, a fragment (820 bp) of the translation elongation factor 1-alpha (EF-1α) and a fragment (470 bp) of the beta-tubulin gene (TUB). The different loci were amplified using the primer pairs ITS5/ITS4 for the ITS region (White et al. 1990), NL1/NL4b for the LSU region (O’Donnell 1993), 983F/2218R for EF-1α (Rehner & Buckley 2005) and BT2a/BT2b for TUB (Glass & Donaldson 1995). PCR amplification reaction had a total volume of 40 μL and consisted in 20 mM Tris-HCl (pH 8.4), 50 mM KCl (10X PCR reaction buffer; Invitrogen, Life Technologies Ltd, Paisley, UK) 1.5 mM MgCl2 (Invitrogen, Life Technologies Ltd, Paisley, UK), 125 μM of each deoxynucleoside triphosphate (GeneAmp® dNTP mix with dTTP, Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, USA), 5 % dimethyl sulfoxyde (DMSO; Panreac Química S.L.U, Barcelona, Spain), 1.2 μM of each primer and 1.25 U of Taq DNA Polymerase (Invitrogen, Life Technologies Ltd, Paisley, UK). The amplification programme consisted of an initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at a suitable temperature for 1 min, extension for 1 min and 20 s at 72 °C, and a final extension for 1 min at 72 °C. Annealing temperatures for each gene were 55 °C for ITS, 51 °C for LSU and 57 °C for EF-1α and TUB. The amplified products were purified with Diffinity Rapid Tip® purification system (Sigma-Aldrich, St. Louis, MO, USA) and stored at -20 °C until sequencing.
Sequencing was conducted in both directions with the same primer pair used for amplification at Macrogen Europe (Macrogen Inc. Amsterdam, The Netherlands). Consensus sequences were obtained using SeqMan v. 7.0.0 (DNASTAR Lasergene, Madison, WI, USA). The newly generated sequences obtained in this study and their GenBank accession numbers are summarised in Table 1. Additionally, 167 relevant sequences, obtained from public databases (GenBank, NITE) and selected on the basis of BLAST homology search results, were incorporated in the phylogenetic analyses (Table 1).
Sequences were aligned individually for each locus using ClustalW (Thompson et al. 1994), under MEGA v. 5.05 (Tamura et al. 2011), refined with MUSCLE (Edgar 2004) under the same platform and manually adjusted if needed. Phylogenetic reconstructions by maximum likelihood (ML) and bayesian inference were carried out using MEGA v. 5.05 and MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001), respectively. The best nucleotide substitution model for each locus and the combined dataset (GTR+G+I) were estimated using MrModeltest v. 2.3 (Nylander 2004). ML phylogeny was first made separately for each locus (data not shown) and assessed for their concordance by comparing the phylogenetic placement and monophyly of the terminal clades and internal nodes with significant bootstrap (bs) support. Since there was no discordance, the loci were combined into two different datasets. A first analysis was carried out using sequences of both ITS and LSU loci in order to establish the boundaries of the genera with all the available ex-type strains of Microascus / Scopulariopsis species complemented with several sequences of related genera of the Microascaceae and Graphiaceae. To establish the species distribution among the genera, a second combined dataset was created including LSU, ITS, EF-1α and TUB sequences made up of a subset of those previously analysed strains and numerous environmental and clinical isolates morphologically identified as Microascus or Scopulariopsis species.
For ML analysis, the trees were inferred using Nearest-Neighbour-Interchange as a heuristic method and gaps were treated as partial deletion with a 95 % site coverage cut-off. The robustness of branches was assessed by a bootstrap analysis of 1 000 replicates (Felsenstein 1985). Bootstrap values over 70 % were considered significant.
The Bayesian analyses consisted of two parallel runs of four incrementally heated Markov Chains starting from a random tree topology. The analyses lasted for five million generations with a sampling frequency of every 100 generations. The 50 % majority rule consensus trees and posterior probabilities (pp) were calculated from 37 500 trees after discarding 12 500 trees for burn-in. Posterior probability values equal or above 0.95 were considered significant. The resulting trees were plotted using FigTree v.1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/). The alignments originated in this study have been deposited in TreeBASE (http://www.treebase.org).
Morphology
All isolates were grown on oatmeal agar (OA; 30 g filtered oat flakes, 20 g agar, 1 L distilled water) and potato-carrot agar (PCA; 20 g each of filtered potatoes and carrots, 20 g agar, 1 L distilled water). They were incubated at different temperatures (5, 15, 25, 30, 35, 37, 40 and 45 °C) and examined at 7 and 14 d to determine colony growth rates. In descriptions, colour notations of the colonies were from Kornerup & Wanscher (1978). Measurements and descriptions of microscopic structures were made using an Olympus CH2 light microscope (Olympus Corporation, Tokyo, Japan) from cultures on PCA or OA at 25 °C for 14 and 21 d to ensure ascomata development. All isolates were examined on slides mounted on 85 % lactic acid. Features of the sexual morph structures were obtained from squash preparations or spore mounts. Photographs of the microscopic structures were made using a Zeiss Axio Imager M1 light microscope (Zeiss, Oberkochen, Germany) with a mounted DeltaPix Infinity X digital camera using Nomarski differential interference contrast and phase contrast optics. Nomenclatural data was deposited in MycoBank (Crous et al. 2004).
RESULTS
Generic circumscription of the Microascaceae
To delineate generic boundaries, we conducted a phylogenetic analysis using the combined LSU and ITS datasets including 54 currently accepted species belonging to 12 genera of Microascaceae and one species of the family Graphiaceae. Trichoderma asperellum and Trichoderma atroviride were selected as outgroup (Fig. 1). The final alignment consisted of 63 taxa and contained 996 characters (LSU 504, ITS 492), of which 618 were conserved and 297 were phylogenetically informative (LSU 98, ITS 199). Fig. 1 shows the ML tree including bs and pp values. The trees obtained from ML and Bayesian analyses of the individual loci and the combined analysis showed congruent topologies.
Fig. 1.
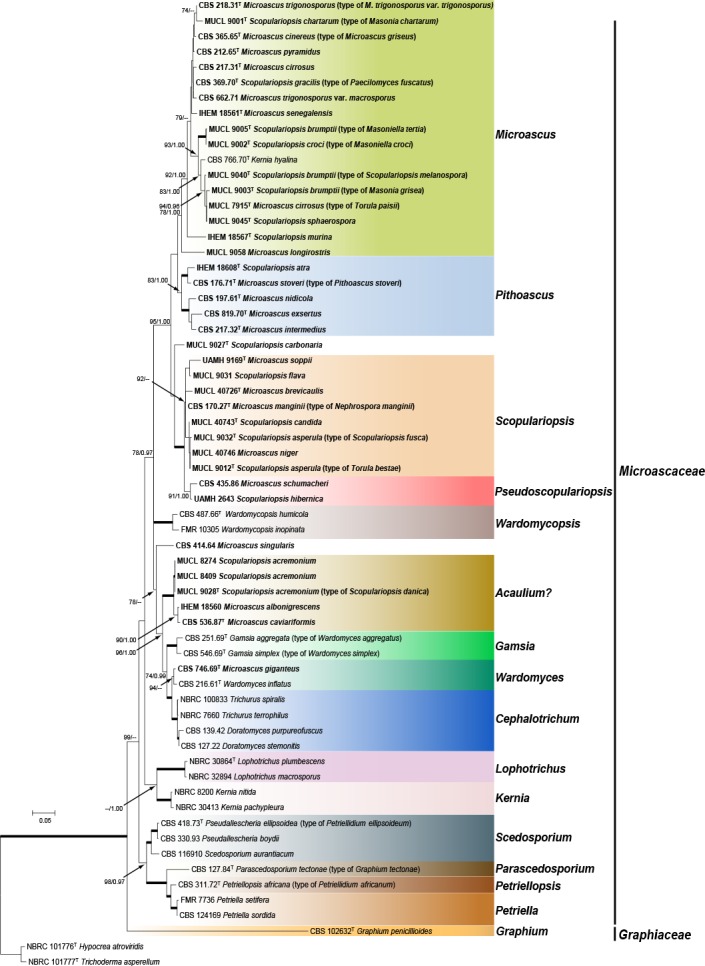
Maximum likelihood (ML) tree obtained from the combined LSU and ITS sequences of 61 representative taxa of Microascaceae and Graphiaceae. Numbers on the branches are ML bootstrap values (bs) above 70 %, followed by Bayesian posterior probabilities (pp) above 0.95. Full supported branches are indicated in bold. Branch lengths are proportional to distance. Strains considered current members of the genera Microascus or Scopulariopsis genera are represented in bold. Ex-type strains are indicated with T. The original name of each strain, when applied, is given between parenthesis. The tree was rooted to Hypocrea atroviridis (NBRC 101776) and Trichoderma asperellum (NBRC 101777).
The phylogenetic inferences showed that Microascus / Scopulariopsis were polyphyletic, with species distributed into several distant lineages. However, most species of Microascus / Scopulariopsis clustered into a single large, well-supported lineage (bs = 95 % / pp = 1.00). This lineage comprised four sublineages, which we interpret as three distinct genera, Microascus, Pithoascus and Scopulariopsis, the fourth one representing a putative undescribed genus.
The members of a sublineage referred to as Microascus were characterised by dark-coloured colonies and mostly brown to green-brown mycelia, conidiogenous apparatus and conidia. The conidiogenous cells (annellides) were born singly on aerial hyphae or on penicillate conidiophores. They were ampulliform or lageniform and usually had a long and narrow cylindrical annellated zone tapering gradually to the conidiogenous locus, and produced smooth to roughened conidia. Sexual morphs were observed in 13 species. Ascomata were ostiolate, rarely nonostiolate, mostly globose to ampulliform, glabrous or hairy, papillate or with long cylindrical necks, and had a dark brown to black peridium of textura angularis with exception of the unidentified strains FMR 12362 and UTHSC 07-156, which showed perithecia with peridia of textura intricata. The ascospores ranged from reniform to ellipsoidal, triangular or quadrangular, were straw-coloured to pale brown and exhibited a single, mostly inconspicuous germ pore (Fig. 2a–h).
Fig. 2.
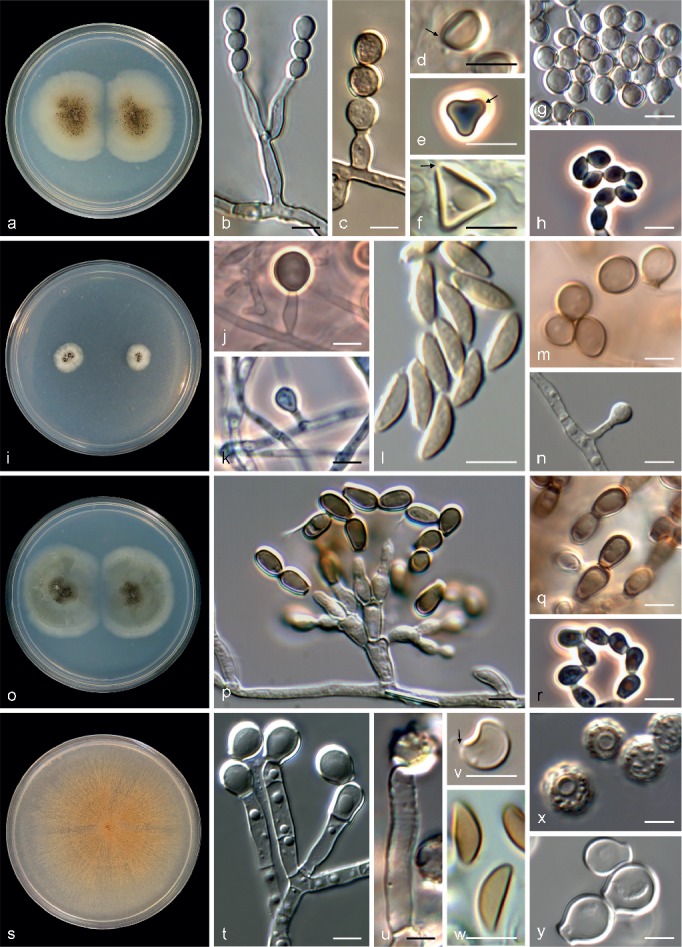
Key morphological features to distinguish Microascus (a–h), Pithoascus (Pi.) (i–n), Pseudoscopulariopsis (Ps.) (o–r) and Scopulariopsis (s–y). a, i, o, s. Colonies on PCA after 21 d at 25 °C; b, c, j, k, p, t, u. conidiogenous cells; d–f, l, v, w. ascospores (germ pores indicated with arrows); g, h, m, n, q, r, x, y. conidia (a, b, d. M. cinereus CBS 365.65; c. M. restrictus CBS 138277; e. M. trigonosporus CBS 218.31; f. M. pyramidus CBS 212.65; g. M. chartarus MUCL 9001; h. M. gracilis CBS 369.70; i, k, l, n. Pi. nidicola CBS 197.61; j, m. Pi. ater IHEM 18608; o–r. Ps. hibernica UAMH 2643; s, u, x. S. brevicaulis MUCL 40726; t, v, y. S. candida MUCL 40743; w. S. soppii UAMH 9169. — Scale bars: 5 μm.
Members of the Pithoascus sublineage showed flat, white to grey colonies without aerial mycelia. The mycelium and the conidiogenous apparatus were subhyaline and the latter consisted of solitary, short, mostly ampulliform annellides with a short-cylindrical neck. With the exception of strain IHEM 18608, all strains of the Pithoascus clade exhibited a sexual morph characterised by black ascomata with an inconspicuous ostiole and navicular to fusiform ascospores without germ pores (Fig. 2i–n).
The Scopulariopsis sublineage included fungi with white, pale grey, tan or brown colonies. The mycelium was mostly hyaline. Annellides were hyaline or pale brown, more or less cylindrical, with a wide, flat conidiogenous opening and mostly formed on densely penicillate conidiophores. Conidia were hyaline or pale brown, smooth or distinctly roughened, often showing a protruding base. A sexual morph was observed in four species and was characterised by dark, globose to subglobose perithecia with a peridium of textura angularis and with a papillate or a long cylindrical ostiolar neck. Ascospores were reniform to broadly lunate, hyaline or pale yellow, with a single, inconspicuous germ pore (Fig. 2s–y).
The reference strains of Microascus schumacheri (CBS 435.86) and Scopulariopsis hibernica (UAMH 2643) formed a well-supported clade (bs 91, pp 1.00), basal to the Scopulariopsis clade. Because the former two taxa shared several morphological features that deviated from those of Scopulariopsis, they were accommodated in a new genus named Pseudoscopulariopsis. Members of this clade were characterised by forming grey or olivaceous colonies and hyaline to subhyaline conidiogenous cells, which usually consisted of annellides arising from swollen basal cells. The annellides were short, more or less ampulliform and with a cylindrical annellated zone. The sexual morph was only observed in P. schumacheri, which produced black perithecia and fusiform or navicular, straw-coloured ascospores without germ pores (Fig. 2o–r).
However, this phylogenetic approach had insufficient resolution to establish the limits among the different species included in each genus. Similarly, the ex-type strain of Scopulariopsis carbonaria (MUCL 9027) was related to the Scopulariopsis clade, but its position was not resolved with this analysis.
The reference strain of Microascus singularis (CBS 414.64) formed a solitary branch in an incertae sedis position. The main morphological distinction of this isolate was the production of conidia showing longitudinal bands. A strongly-supported clade, composed by the ex-type strains of Microascus caviariformis (CBS 536.87) and Scopulariopsis danica (MUCL 9028), two reference strains of Scopulariopsis acremonium (MUCL 8274 and MUCL 8409) and a reference strain of Microascus albonigrescens (IHEM 18560), clustered apart from the genera included in the study. The ex-type strain of Microascus giganteus (CBS 746.69) was placed very far from the Microascus clade. It formed a well-supported clade with the ex-type strain of Wardomyces inflatus (CBS 216.61) and with another fully supported clade, which included several reference strains of the genera Doratomyces and Trichurus. Our phylogenetic analyses were concordant with the observations made by Abbott (2000), who considered Doratomyces and Trichurus as congeneric with Cephalotrichum, all being characterised by the formation of dry-spored synnemata and lacking sexual morphs.
Lophotrichus and Kernia, characterised by hairy ascomata and ellipsoidal ascospores with two germ-pores and graphium- or scopulariopsis-like asexual morphs, respectively, formed well-supported clades related to Scedosporium and allied genera (i.e., Parascedosporium, Petriella and Petriellopsis), characterised by scedosporium-like asexual morphs with slimy conidia.
Some species traditionally included in Pithoascus and Scopulariopsis clustered in orders different from Microascales. The ex-type strain of Pithoascus platysporus (CBS 419.73) and a reference strain of Scopulariopsis coprophila (CBS 206.61) were closely related to the Hypocreales; the ex-type strain of Scopulariopsis canadensis (CBS 204.61) grouped with members of the Xylariales; the ex-type strains of Scopulariopsis parva (MUCL 9041) and Scopulariopsis halophilica (CBS 380.74) clustered outside the Sordariomycetes, being related to members of the Eurotiales (data not shown).
Species distribution in Microascus, Pithoascus, Pseudoscopulariopsis and Scopulariopsis
The final alignment of the combined matrix included 106 strains from Microascus, Pithoascus, Scopulariopsis and Pseudoscopulariopsis species and involved 2 219 characters (LSU 437, ITS 493, EF-1α 816, TUB 473), of which 1 493 were conserved, 673 were variable and 486 were phylogenetically informative (LSU 47, ITS 112, EF-1α 176, TUB 151). Petriella setifera and Parascedosporium tectonae were selected as outgroup taxa. The resulting ML tree is shown in Fig. 3 including bs and pp values. The topology of the trees obtained from ML and Bayesian analyses from each individual locus and the combined analysis were concordant. The multilocus analysis confirmed the results obtained from phylogenetic inferences using the combined LSU and ITS dataset. In total 34 well-supported clades were resolved and were distributed among four main lineages corresponding to Microascus, Pithoascus, Scopulariopsis and the new genus Pseudoscopulariopsis proposed here. The Microascus lineage comprised 20 well-supported subclades, 13 of which included an ex-type strain of a known species or a strain considered to be authentic for a particular species, while seven subclades corresponded to new species, which are described here. Pithoascus comprised five well-supported monophyletic subclades, each of which included an ex-type strain of a known species. Scopulariopsis encompassed six well-supported subclades, of which five included an ex-type strain or a strain considered as authentic, while one subclade corresponded to a new species described here. The new genus Pseudoscopulariopsis encompassed two subclades, each one including a single reference strain of a species previously identified as Microascus or Scopulariopsis, respectively.
Fig. 3.
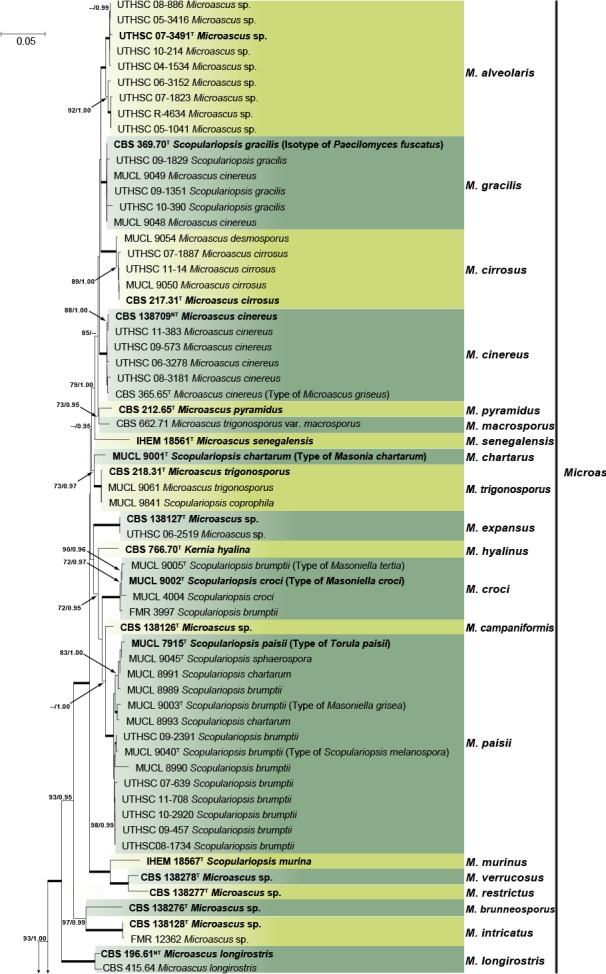
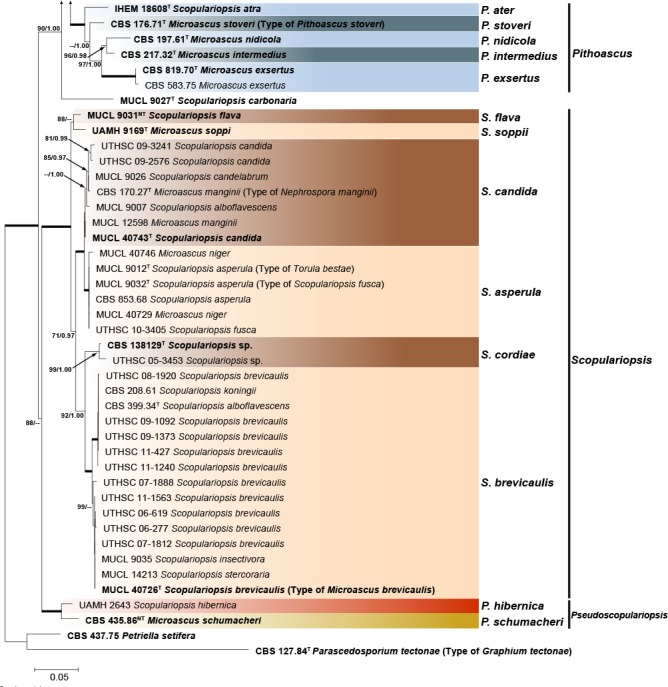
Maximum likelihood (ML) tree obtained from the combined ITS, LSU, EF-1α and TUB sequences of 105 strains from Microascus, Pithoascus, Pseudoscopulariopsis and Scopulariopsis species. Numbers on the branches are ML bootstrap values (bs) above 70 %, followed by Bayesian posterior probabilities (pp) above 0.95. Full supported branches are indicated in bold. Branch lengths are proportional to distance. Ex-type strains are indicated with T. Ex-neotype strains are indicated with NT. The original name of each strain, when applied, is given on parenthesis. The tree was rooted to Petriella setifera (CBS 437.75) and Parascedosporium tectonae (CBS 127.84).
In the combined phylogenetic analysis, the ex-type strain of Scopulariopsis carbonaria (MUCL 9027) was basal to the Microascus and Pithoascus clades. According to the original description (Morton & Smith 1963), this species showed a high similarity in annellidic and conidial morphology with members of the Microascus lineage; however, after several attempts to induce sporulation this strain remained sterile, and thus its taxonomic position could not be resolved.
TAXONOMY
Based on the results of the above multilocus sequence analysis and a morphological analysis, the boundaries of the genera Microascus, Pithoascus and Scopulariopsis have been reassessed accordingly. Their current circumscription is revised and several new taxa and combinations are proposed as follows:
Microascus Zukal, Verh. Zool.-Bot. Ges. Wien 35: 339. 1885
= Peristomium Lechmere, Compt. Rend. Hebd. Séances Acad. Sci. 154: 178. 1912.
= Masonia G. Sm., Trans. Brit. Mycol. Soc. 35: 149. 1952.
≡ Masoniella G. Sm., Trans. Brit. Mycol. Soc. 35: 237. 1952.
Type species. Microascus longirostris Zukal.
Colonies restricted or spreading, pale grey, brown, olivaceous or black, velvety, floccose or fasciculate, granular and often forming concentric rings due to the production of ascomata. Ascomata perithecial, immersed or superficial, scattered or aggregated, globose to ampulliform, glabrous or covered with scattered hairs, ostiolate, usually with a neck of variable length and shape, sometimes with a tuft of ostiolar hairs; peridium dark brown or black, composed of thick-walled, slightly flattened cells, textura angularis or textura intricata. Asci unitunicate, 8-spored, obovate, barrel-shaped or nearly globose, formed in basipetal rows, evanescent. Ascospores 1-celled, asymmetrical, reniform, heart-shaped, triangular or quadrangular, dextrinoid when young, extruded through the ostiole into a gelatinous drop or a long cirrhus. Conidiogenous cells annellidic, borne singly and laterally on the vegetative hyphae, or in groups of 2–5 on short simple or little branched conidiophores, ampulliform or lageniform, subhyaline or darkening with age, smooth- or rough-walled with a distinct cylindrical annellated zone, Conidia 1-celled, pale yellowish to dark brown, globose to subglobose, obovate or clavate, with a truncate base and rounded or pointed at the apex, smooth- and thin-walled or finely rough- and thick-walled, produced singly or in basipetal dry chains. Solitary conidia present in some species, borne sessile or on short stalks from the vegetative hyphae.
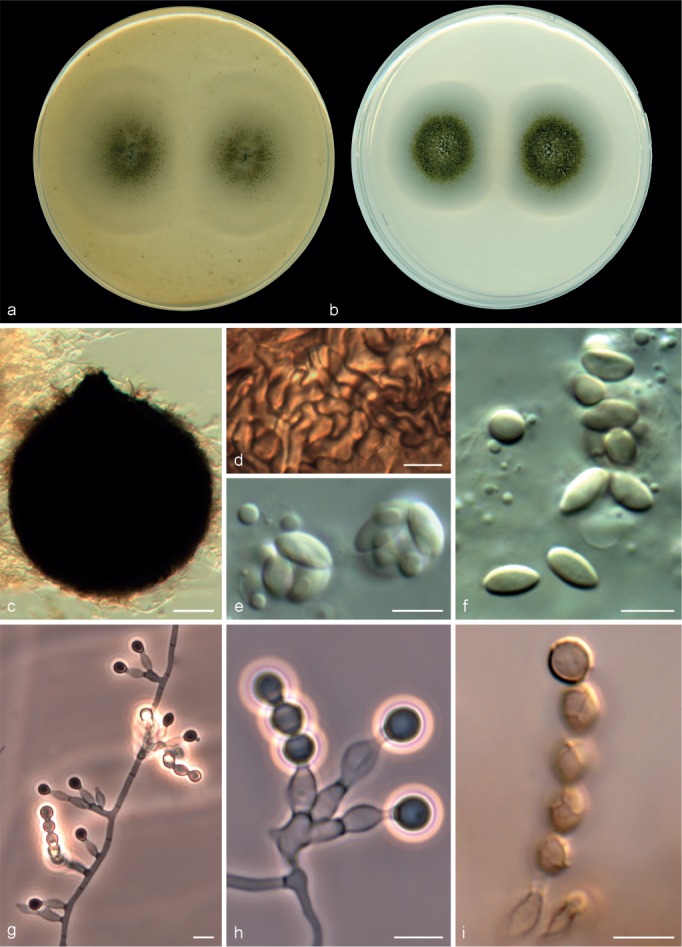
Microascus alveolaris Sandoval-Denis, Gené & Guarro, sp. nov. — MycoBank MB809418, Fig. 4
Fig. 4.
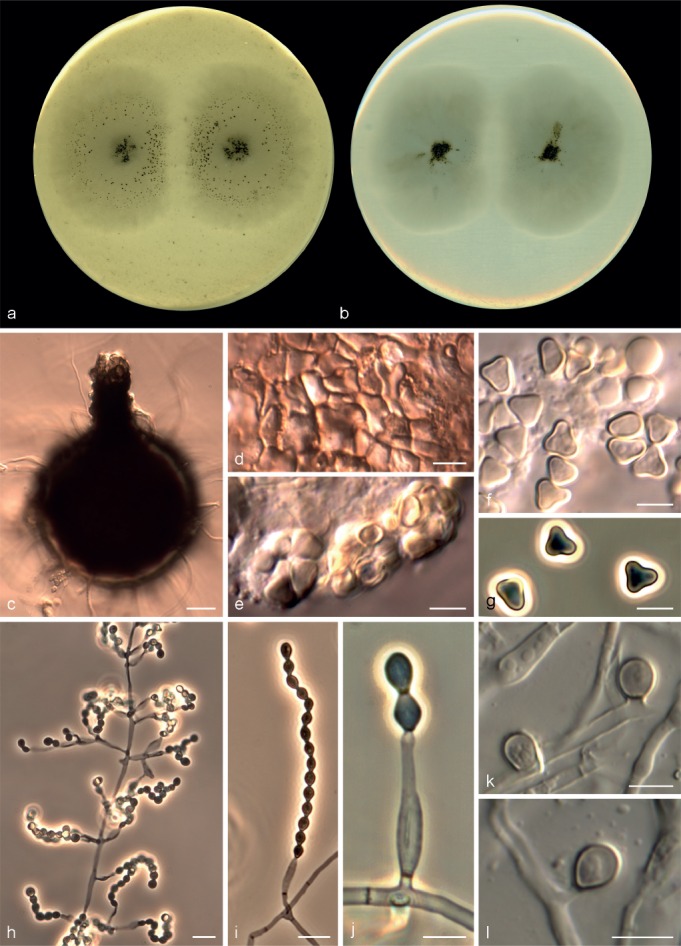
Microascus alveolaris CBS 139501. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c. ascoma; d. peridium; e–g. asci and ascospores; h–j. conidiophores, annellides and conidia; k, l. solitary conidia. — Scale bars: c = 30 μm; h, i = 10 μm; all others = 5 μm.
Etymology. In reference to the isolation source of most isolates.
Colonies on OA and PCA at 25 °C attaining 31–36 and 18–29 mm diam, after 14 d, respectively, flat, slightly velvety, somewhat granular at the centre due to the presence of ascomata, white to grey (4B1), abundant submerged mycelium in the outer zone, with a wide white margin; reverse white to grey (4B1). Vegetative hyphae septate, hyaline to light brown, smooth- and thin-walled, 1.5–3 μm wide. Ascomata superficial or immersed, formed predominantly at the centre of the colony, globose to subglobose, 110–290 μm diam, usually with an ostiolar cylindrical neck up to 100 μm long, black, glabrous, the apice sometimes with a tuft of hyaline, septate and acicular hairs, up to 60 μm long; peridium of textura angularis. Asci irregularly ellipsoidal, 8–12 × 7.5–11 μm. Ascospores broadly triangular, rarely reniform, 4–6 × 3–5 μm, with a single germ pore, straw coloured, bright yellow in mass. Conidiophores absent or as a basal single cell of 5–12 × 2–2.5 μm, bearing groups of 2–3 annellides, rarely slightly branched up to 80 μm long, hyaline to subhyaline, smooth-walled. Annellides mostly sessile, single and lateral on vegetative hyphae, lageniform, 6–17 × 1.5–3.5 μm, tapering slightly towards the annellated zone 1–2 μm wide, hyaline to subhyaline, smooth- and thin-walled. Conidia ellipsoidal, navicular or bullet-shaped, 3–5 × 2–3.5 μm, with truncate base and rounded apex, subhyaline to pale brown, brown in mass, thin- and smooth-walled, arranged in long chains. Solitary conidia sometimes present, borne laterally from vegetative hyphae, sessile or on short stalks, unicellular, subglobose or obovoidal, 3–5 × 2.5–4 μm, subhyaline or pale brown, smooth- and more or less thick-walled.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 40 °C, minimum 15 °C.
Specimens examined. USA, from bronchoalveolar lavage fluid, 2007, D.A. Sutton (holotype CBS H-22111, culture ex-type CBS 139501 = UTHSC 07-3491 = FMR 12252); from sputum, 2005, D.A. Sutton (UTHSC 05-1041 = FMR 12351); from bronchoalveolar lavage fluid, 2005, D.A. Sutton (UTHSC 05-3416 = FMR 12350); from bronchoalveolar lavage fluid, 2006, D.A. Sutton (UTHSC 06-3152 = FMR 12346); from sputum, 2007, D.A. Sutton (UTHSC 07-1823 = FMR 12342); from bronchoalveolar lavage fluid, 2004, D.A. Sutton (UTHSC 04-1534 = FMR 12354); from bronchoalveolar lavage fluid, 2008, D.A. Sutton (UTHSC 08-886 = FMR 12340); from bronchoalveolar lavage fluid, 2010, D.A. Sutton (UTHSC 10-214 = FMR 12336); from lung tissue, D.A. Sutton (UTHSC R-4634 = FMR 12333).
Notes — All the strains included in this species were isolated from the respiratory tract of human patients. Morphologically, M. alveolaris is close to M. campaniformis, M. macrosporus, M. pyramidus and M. trigonosporus, all showing similar triangular-shaped ascospores. Microascus alveolaris can be differentiated by its membranous and white colonies, the smaller size of the ascospores and narrower conidia.
Microascus brunneosporus Sandoval-Denis, Gené & Guarro, sp. nov. — MycoBank MB809419, Fig. 5
Fig. 5.
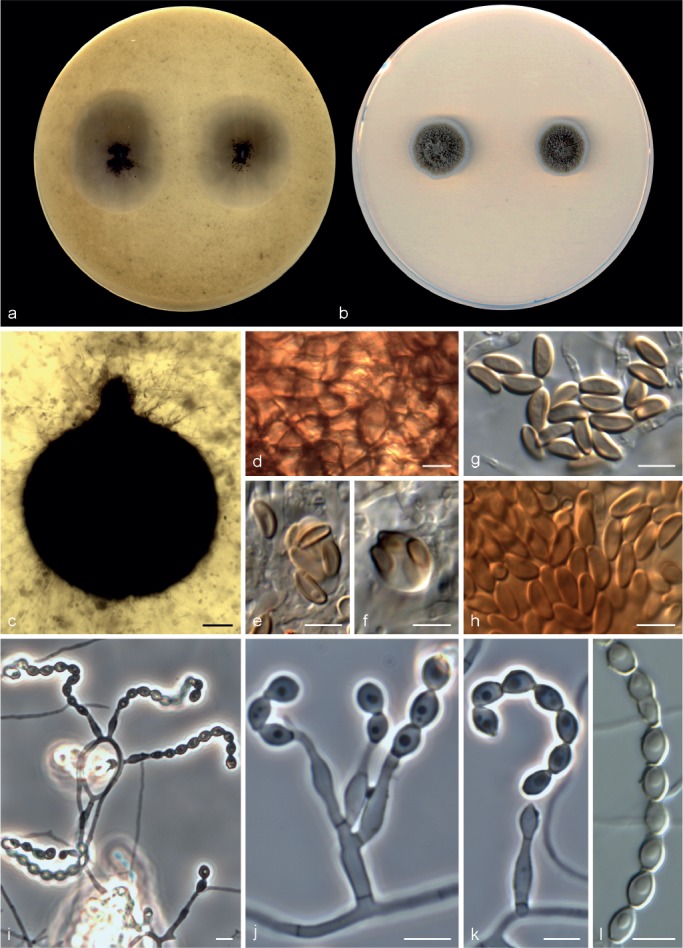
Microascus brunneosporus CBS 138276. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c. ascoma; d. peridium; e–h. asci and ascospores; i–k. conidiophores, annellides and conidia; l. conidial chain. — Scale bars: c = 50 μm; all others = 5 μm.
Etymology. From the Latin brunneus-, brown, referring to the colour of the ascospores.
Colonies on OA at 25 °C attaining 21–25 mm diam in 14 d, flat, velvety, granular at the centre due to the presence of ascomata, dull green (30E3) to olive-brown (4F4), with submerged mycelium towards the outer zone, margin regular; reverse dark green (30F4). On PCA at 25 °C attaining 15–17 mm diam in 14 d, slightly elevated, downy, fasciculate at the centre, dull green (30E3), with a white and regular margin; reverse dull green (30D4). Vegetative hyphae septate, subhyaline to pale brown, smooth- and thin-walled, 1.5–3 μm wide. Ascomata immersed, globose, 110–205 μm diam, with a short cylindrical ostiolar neck up to 40 μm long, black, glabrous; peridium with a textura angularis. Asci irregularly ellipsoidal or ovoidal, 11–14 × 7–8 μm. Ascospores ellipsoidal to allantoid, 5–7 × 2–3 μm, light yellow-brown, brown in mass, with a single and inconspicuous germ pore. Conidiophores absent or as a basal single cell of 5–15 × 1.5–2.5 μm, bearing 1–3 annellides, rarely slightly branched up to 30 μm long, subhyaline, smooth-walled. Annellides mostly sessile, single and lateral on vegetative hyphae, more or less lageniform, 9–14 × 2–2.5 μm, tapering to a cylindrical annellated zone 1–1.5 μm wide, subhyaline, smooth- or rough-walled, thin-walled. Conidia subglobose, ellipsoidal or navicular, 4–5 × 2.5–5 μm, with truncate base, light green-brown, thin- and smooth-walled, arranged in long chains. Solitary conidia not observed.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 35 °C, minimum 15 °C.
Specimen examined. USA, from bronchoalveolar lavage fluid, 2006, D.A. Sutton (holotype CBS H-21783, culture ex-type CBS 138276 = UTHSC 06-4312 = FMR 12343).
Notes — This species is similar to M. cinereus and M. gracilis. However, the latter two species produce reniform or broadly lunate, straw coloured ascospores with an often conspicuous germ pore.
Microascus campaniformis Sandoval-Denis, Cano & Deanna A. Sutton, sp. nov. — MycoBank MB809205, Fig. 6
Fig. 6.
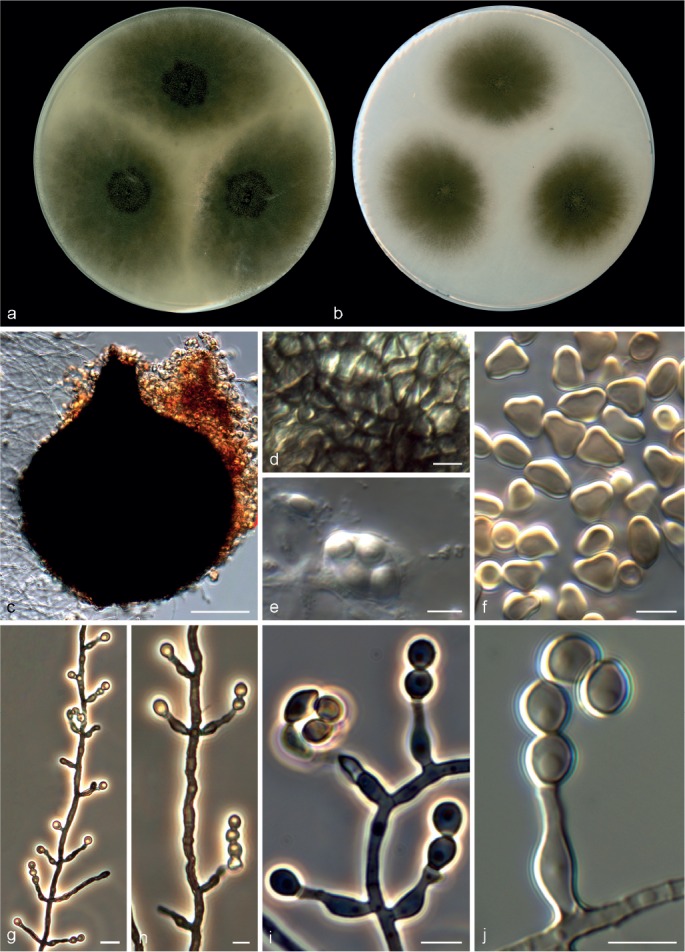
Microascus campaniformis CBS 138126. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c. ascoma; d. peridium; e, f. asci and ascospores; g–j. conidiophores, annellides and conidia. — Scale bars: c = 50 μm; g = 10 μm; all others = 5 μm.
Etymology. From the Latin campanus-, bell, referring to the shape of the ascospores.
Colonies on OA at 25 °C attaining 27–34 mm diam in 14 d, flat, velvety to slightly granular at the centre, dull green (30E4), with an irregular margin; reverse dull green (30E4). On PCA at 25 °C, colonies attaining 14–25 mm diam in 14 d, flat, velvety, fluffy at the centre, dull green (28E4) to dark green (30E4), with a white and regular margin; reverse dark green (28F3). Vegetative hyphae septate, subhyaline, smooth- or rough- and thin-walled, 1.5–2.5 μm wide. Ascomata immersed or superficial, usually formed at the periphery of the colony, globose to subglobose, 150–220 μm diam, with a short cylindrical ostiolar neck up to 80 μm long, widening at the ostiolar opening, rarely with a tuft of hyaline, straight and septate hairs up to 50 μm long, black, glabrous; peridium with a textura angularis. Asci irregularly ellipsoidal or subglobose, 18–21 × 10–15 μm. Ascospores broadly triangular, 6–7 × 4–4.5 μm, often with an elongated side towards a single germ pore, straw coloured, bright yellow-orange in mass. Conidiophores absent or as a basal cell of 5 × 2 μm, bearing groups of 5–8 annellides, or slightly branched up to 60 μm long, hyaline to subhyaline, smooth-walled. Annellides somewhat lageniform, 9–14 × 2–3 μm, with a more or less swollen base and tapering abruptly to a cylindrical annellated zone, 1–1.5 μm wide. Conidia subglobose to broadly ellipsoidal, 4–5 × 2.5–3.5 μm, with a truncate base, light green-brown, dark brown in mass, thick-walled, arranged in long chains. Solitary conidia and chlamydospores not observed.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 40 °C, minimum 15 °C.
Specimen examined. USA, from bronchoalveolar lavage fluid, 2010, D.A. Sutton (holotype CBS H-21784, culture ex-type CBS 138126 = UTHSC 10-565 = FMR 12334).
Notes — Microascus campaniformis is similar to M. alveolaris, M. macrosporus, M. pyramidus and M. trigonosporus in having distinctive triangular shaped ascospores. However, M. campaniformis can be differentiated by its green colonies and inequilateral ascospores that show an elongation at one side towards the germ pore. In contrast, the ascospores of M. alveolaris, M. macrosporus and M. trigonosporus are almost equilateral with rounded ends, while those of M. pyramidus have attenuated ends acquiring a nearly square shape. Microascus campaniformis is phylogenetically close to M. paisii sharing similar annellides. However, a sexual morph has not been observed in M. paisii.
Microascus chartarus (G. Sm.) Sandoval-Denis, Gené & Guarro, comb. nov. — MycoBank MB809206
Basionym. Masonia chartarum G. Sm., Trans. Brit. Mycol. Soc. 35: 150. 1952.
≡ Masoniella chartarum (G. Sm.) G. Sm., Trans. Brit. Mycol. Soc. 35: 237. 1952.
≡ Scopulariopsis chartarum (G. Sm.) F.J. Morton & G. Sm., Mycol. Pap. 86: 64. 1963.
Specimen examined. UK, London, isolated from mouldy wall-paper, 1950, K. Maunsell (Masonia chartarum ex-type culture CBS 294.52 = MUCL 9001).
Notes — Microascus chartarus has been reported from soil, dust and indoor-air (Domsch et al. 2007). It was originally described as a member of Masonia G. Sm. (1952a). However, Masonia is an illegitimate homonym of Masonia Hansford (1944), and thus the new genus Masoniella was erected (Smith 1952b). Most members of Masoniella were later transferred to Scopulariopsis (Morton & Smith 1963); both genera share the same conidiogenesis (annellidic, percurrent) and conidiogenous cells, distinctly narrower at the base, then swollen, and ending in a slender annellidic zone. Our phylogenetic analysis shows that M. chartarus is included in the Microascus sublineage and it is closely related to M. trigonosporus. Microascus trigonosporus can be distinguished by the production of a sexual morph, with triangular ascospores and mostly globose to subglobose and pale brown conidia. No sexual morph is known for M. chartarus and its conidia are ovate, often with a pointed end, green-brown (Morton & Smith 1963). Microascus croci and M. paisii resemble M. chartarus and also lack a sexual morph. However, these two species can be differentiated from M. chartarus by their conidial shape and colour, which are globose and ellipsoidal to short clavate in M. croci and M. paisii, respectively, and pale brown in both species. In addition, M. croci is able to grow from 5–30 °C and M. paisii grows from 15–37 °C, while M. chartarus has a narrower temperature range growing from 15–25 °C.
Microascus cinereus (Émile-Weil & Gaudin) Curzi, Boll. Staz. Patolog. Veget. Roma 11: 60. 1931.
Basionym. Scopulariopsis cinerea Émile-Weil & Gaudin, Arch. Méd. Exp. Anat. Path. 28: 452. 1919.
= Scopulariopsis oidiospora Zach, Oesterr. Bot. Z. 83: 182. 1934.
= Microascus lunasporus P.M. Jones, Mycologia 28: 503. 1936.
≡ Scopulariopsis lunaspora P.M. Jones, Mycologia 28: 504. 1936.
= Microascus pedrosoi C.A. Fuentes & F.A. Wolf, Mycologia 48: 63. 1956.
= Microascus griseus P.N. Matur & Thirum., Sydowia 16: 49. 1962.
= Microascus reniformis Orr, Persoonia 8: 194. 1975.
Specimens examined. INDIA, Maharashtra, Poona, from soil, 1965, M.J. Thirumalachar (M. griseus ex-type culture CBS 365.65 = ATCC 16204). – USA, from bronchoalveolar lavage fluid, 2010, D.A. Sutton (neotype of M. cinereus designated here CBS H-21937, MBT198511) culture ex-neotype CBS 138709 = UTHSC 10-2805 = FMR 12217; from bronchoalveolar lavage fluid, 2006, D.A. Sutton (UTHSC 06-3278 = FMR 12345); from sternum tissue, 2008, D.A. Sutton (UTHSC 08-3181 = FMR 12339); from bronchoalveolar lavage fluid, 2009, D.A. Sutton (UTHSC 09-573 = FMR 12239); from bronchoalveolar lavage fluid, 2009, D.A. Sutton (UTHSC 11-383 = FMR 12331).
Notes — Microascus cinereus has a widespread distribution and a wide range of substrates. It has been isolated mainly from stored cereals, soil and dung (Barron et al. 1961, Udagawa 1962, Guarro et al. 2012), but it has also been described as an opportunistic pathogen of animals and humans (Baddley et al. 2000, de Hoog et al. 2011, Sandoval-Denis et al. 2013). Descriptions of M. cinereus are available in Barron et al. (1961) and Guarro et al. (2012). However, according to our observations, their measurements might have included isolates of Microascus gracilis from which M. cinereus has to be differentiated (Sandoval-Denis et al. 2013). The isolates of M. cinereus studied here showed asci 7–12 × 5–10 μm, ascospores 4–5.5 × 2.5–4 μm and conidia 3–5 × 2–3 μm. In addition, while M. cinereus produces pale to dark or black-grey colonies, at first velvety becoming slightly granular due to the presence of ascomata, M. gracilis produces dull green colonies, becoming olive-grey to olive-brown with ascomata mostly covered by aerial mycelium. Since no ex-type material of M. cinereus is available, the strain CBS 138709 (UTHSC 10-2805) is proposed here as neotype. Despite the existence of an ex-type culture of M. griseus, a synonym of M. cinereus, we consider it important to neotypify M. cinereus in order to conserve the oldest and most widely used epithet of this taxon. The original description of M. cinereus was based on an isolate obtained from a human nail but none of the isolates available share this original substrate. However, we believe that the isolate CBS 138709 (UTHSC 10-2805), obtained from human bronchoalveolar fluid agrees with the original and modern descriptions of this species (Émile-Weil & Gaudin 1919, Barron et al. 1961, Guarro et al. 2012).
Microascus cirrosus Curzi, Boll. Staz. Patol. Veg. Roma 10: 308. 1930
Specimens examined. ITALY, from a leaf of Prunus sp., 1931, M. Curzi (ex-type culture CBS 217.31); from root of Vitis vinifera, 1934, M. Curzi (CBS 277.34 = MUCL 9050). – UK, from unknown substrate, 1961, G. Smith (CBS 301.61 = MUCL 9054). – USA, from sputum, 2007, D.A. Sutton (UTHSC 07-1887 = FMR 12256); from bronchoalveolar lavage fluid, 2011, D.A. Sutton (UTHSC 11-14 = FMR 12332).
Notes — Microascus cirrosus is a saprobic species with a worldwide distribution, commonly isolated from soil and dung (Barron et al. 1961, von Arx et al. 1988, Guarro et al. 2012). It has also been associated to superficial and respiratory human infections (de Hoog et al. 2011, Sandoval-Denis et al. 2013). Morton & Smith (1963) considered the asexual morph of this species to be conspecific with Scopulariopsis paisii (see Microascus paisii). However, according to our results, the ex-type strain of Torula paisii (MUCL 7915) was shown to be phylogenetically distant to the ex-type strain of M. cirrosus (CBS 217.31), and thus should be considered as a distinct species. Microascus cirrosus can be distinguished by having subglobose to obovate conidia measuring 4–6.5 × 4–6 μm, while those of M. paisii are broadly ellipsoidal to short clavate, measuring 4–6 × 2–4.5 μm. Microascus cirrosus is also similar to M. cinereus. However, M. cirrosus produces broadly reniform ascospores measuring 5–6 × 3–4 μm and larger conidia, while M. cinereus produces broadly lunate or almost triangular ascospores measuring 4–5.5 × 2.5–4 μm, and obovate to clavate conidia measuring 3–5 × 2–3 μm.
Microascus croci (J.F.H. Beyma) Sandoval-Denis, Gené & Guarro, comb. nov. — MycoBank MB809207
Basionym. Scopulariopsis croci J.F.H. Beyma, Antonie van Leeuwenhoek 10: 52. 1945.
≡ Masoniella croci (J.F.H. Beyma) G. Sm., Trans. Brit. Mycol. Soc. 37: 166. 1954.
= Masoniella tertia Bat., J.A. Lima & C.T. Vasconc., Publções Inst. Micol. Recife. 263: 14. 1960.
Specimens examined. BRAZIL, Pernambuco, Recife, isolate from air, 1952, A. Batista (Masoniella tertia ex-type culture MUCL 9005 = CBS 296.61). – SPAIN, Tarragona, Riumar, from aquatic sediment of the Ebro River, May 1991, K. Ulfig & J. Gené (FMR 3997); Barcelona, from aquatic sediment of the Besós river, July 1991, J. Gené (FMR 4004). – THE NETHERLANDS, Lisse, from Crocus sp. Queen of the blues, 1943, H. Diddens (Scopulariopsis croci ex-type culture MUCL 9002 = CBS 158.44).
Notes — The clade representing M. croci included isolates from air, aquatic sediments, soil and plants, originating from Europe and South America. Masoniella tertia was considered a synonym of S. melanospora (Udagawa 1959) and a later synonym of S. brumptii (Morton & Smith 1963). However, the current combined analysis showed that the ex-type cultures of M. tertia and S. melanospora are phylogenetically unrelated, which agrees with their morphological features. All the isolates included in this clade have mostly globose conidia and are able to grow from 5–30 °C. Although no sexual morph has been reported for this species, one of the strains tested here (FMR 4004) was able to produce small and sterile peritecial-like ascomata after 8 mo of incubation on OA.
Microascus expansus Sandoval-Denis, Gené & Cano, sp. nov. — MycoBank MB809208, Fig. 7
Fig. 7.
Microascus expansus CBS 138127. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c–g. conidiophores, annellides and conidia; h. conidial chains. — Scale bars: c, d = 10 μm; all others = 5 μm.
Etymology. From the Latin expansio-, expansion, referring to the quick growth of the colonies.
Colonies on OA and PCA at 25 °C growing rapidly, 65–81 and 70–75 mm diam, respectively, in 14 d, flat, velvety to powdery, more or less funiculose at the centre, olive (3F3) to grey-brown (4–5F3), with an irregular margin; reverse olive grey (2F2) or olive (2F4). Vegetative hyphae septate, hyaline to pale brown, smooth- and thin-walled, 1.5–3 μm wide. Conidiophores absent or as a basal single cell of 4–5 × 2–4 μm, bearing groups of 2–5 annellides, or slightly branched up to 20 μm long, hyaline to subhyaline, smooth-walled. Annellides slightly lageniform or somewhat subulate, 5–12 × 1.5–3.5 μm, tapering to a cylindrical annellated zone 1.5–2 μm wide, smooth-walled. Conidia bullet-shaped or broadly clavate, 4–8 × 2.5–3.5 μm, with a distinctive truncate base and rounded or slightly pointed apex, subhyaline to pale brown in mass, smooth- or finely roughened, thick-walled, arranged in long chains. Sexual morph not observed.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 40 °C, minimum 15 °C.
Specimens examined. USA, from sputum, 2006, D.A. Sutton (holotype CBS H-21785, culture ex-type CBS 138127 = UTHSC 06-4472 = FMR 12266); from pleural fluid, 2006, D.A. Sutton (UTHSC 06-2519 = FMR 12267).
Notes — Microascus expansus is known thus far from clinical isolates of human origin. Both isolates are able to grow at 40 °C. Other Microascus species able to grow at this temperature are M. alveolaris, M. campaniformis, M. cinereus, M. cirrosus, M. gracilis, M. intricatus, M. macrosporus, M. pyramidus and M. restrictus. However, except M. restrictus, all these species produce sexual morphs, while M. expansus produces only the asexual morph. Microascus expansus can be differentiated from M. restrictus by a faster growth rate, reaching > 60 mm diam at 25–30 °C in 14 d.
Microascus gracilis (Samson) Sandoval-Denis, Gené & Guarro, comb. nov. — MycoBank MB809209
Basionym. Scopulariopsis gracilis Samson, Arch. Mikrobiol. 85: 179. 1972.
≡ Paecilomyces fuscatus N. Inagaki, Trans. Mycol. Soc. Japan 4: 4. 1962.
Specimens examined. JAPAN, from wheat flour, 1970, N. Inagaki (Paecilomyces fuscatus ex-type culture CBS 369.70). – UK, isolate from soil, 1959, J. Mendy (MUCL 9048 = CBS 195.61). – USA, Iowa, isolate from a seed of Zea mays, 1961, G.L. Barron (MUCL 9049 = CBS 300.61); from synovial fluid, 2009, D.A. Sutton (UTHSC 09-1351 = FMR 12234); from bronchoalveolar lavage fluid, 2009, D.A. Sutton (UTHSC 09-1829 = FMR 12231); from bronchoalveolar lavage fluid, 2010, D.A. Sutton (UTHSC 10-390 = FMR 12335).
Notes — Scopulariopsis gracilis was proposed by Samson & von Klopotek (1972) as a new name for Paecilomyces fuscatus, probably to avoid nomenclatural conflict with Scopulariopsis fusca (Zach 1934).
Microascus gracilis has been isolated mainly from food in Asia, North and South America, and from soil in Europe. Recently, this species was reported from human clinical specimens, but its pathogenicity has not been demonstrated (Sandoval-Denis et al. 2013). Microascus gracilis and M. cinereus are very similar making their identification difficult in the absence of the sexual morph; in fact two reference strains (MUCL 9048 and MUCL 9049) and some clinical isolates were previously identified as M. cinereus. However, sequence comparison revealed that these species only showed 98.1 %, 97.8 % and 97 % sequence similarity for ITS, EF-1α and TUB, respectively. Morphologically M. gracilis can be differ-entiated from M. cinereus by its lunate ascospores, measuring 4.5–6.5 × 2–4 μm (as opposed to reniform to broadly lunate ascospores measuring 4–5.5 × 2.5–4 μm in M. cinereus), asci measuring 8–18 × 6–10 μm (against 7–12 × 5–10 μm in M. cinereus), the formation of complex conidiophores and the morphology and colour of the colony. The asexual-morph of M. gracilis also resembles to that of M. murinus and M. paisii. However, M. gracilis produces annellides 5–20 × 1–2.5 μm, usually formed on well-defined and branched conidiophores, and subglobose to ellipsoidal conidia 3.5–5.5 × 2–3.5 μm; the annellides of M. murinus and M. paisii are shorter (6.5–11 × 1.7–2.5 μm and 10–14 × 2–2.5 μm, respectively) borne mostly from the aerial mycelium and producing cylindrical and broadly ellipsoidal conidia, respectively.
Microascus hyalinus (Malloch & Cain) Sandoval-Denis, Gené & Guarro, comb. nov. — MycoBank MB809210
Basionym. Kernia hyalina Malloch & Cain, Canad. J. Bot. 49: 860. 1971.
Specimen examined. USA, from cow dung, 1964, J.C. Krug (ex-type culture CBS 766.70).
Notes — This species has been isolated from soil and dung in Europe and North America (Malloch & Cain 1971, Guarro et al. 2012). The species was originally described in Kernia by Malloch & Cain (1971), although deviating considerably from the typical features of Kernia such as restricted growth, nonostiolate, hairy ascomata, and ellipsoidal to reniform, orange to copper coloured ascospores with a germ pore at each end (Malloch & Cain 1971, von Arx 1978). Although several species of Kernia have been described with a scopulariopsis-like asexual morph, our phylogenetic analysis based on a combined LSU and ITS sequence dataset (Fig. 1) showed Kernia to be phylogenetically distant to both Scopulariopsis and Microascus. However, K. hyalina is shown to have more affinity with species of Microascus rather than with species of Kernia nested within the Microascus lineage, a relationship previously suggested by Issakainen et al. (2003). The lack of ascomatal appendages, the production of hyaline to yellowish ascospores with a single germ pore, the shape and colour of the annellides and conidia, and the growth rate of the colonies point toward Microascus rather than toward Kernia. Therefore, our phylogenetic and morphological data confirm this taxon as a distinct species in Microascus.
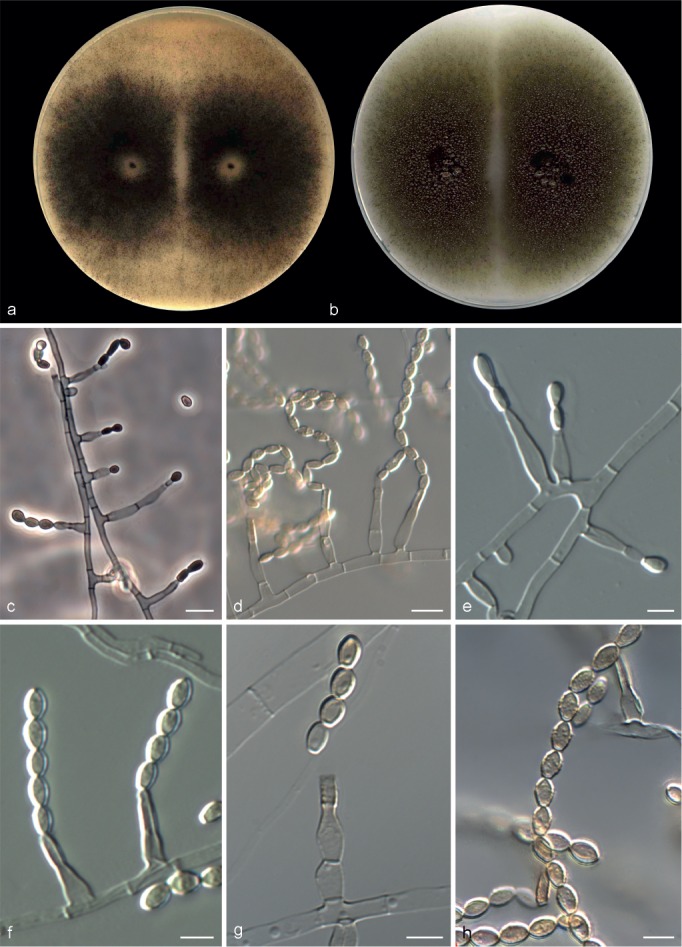
Microascus intricatus Sandoval-Denis, Stchigel & Deanna A. Sutton, sp. nov. — MycoBank MB809211, Fig. 8
Fig. 8.
Microascus intricatus CBS 138128. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c. ascoma; d. peridium; e, f. asci and ascospores; g, h. conidiophores, annellides and conidia; i. conidial chain. — Scale bars: c = 40 μm; all others = 5 μm.
Etymology. Referring to the textura intricata of the peridium.
Colonies on OA at 25 °C growing rather slowly, attaining 28–30 mm diam in 14 d, flat, finely granular, with scarce aerial mycelium, olive grey (2F2), with a white regular margin; reverse white to grey. On PCA at 25 °C colonies attaining 35–38 mm diam in 14 d, flat, velvety to finely granular, with a densely fasciculate centre, olive brown (4F3/4F4), with a white regular margin; reverse olive brown (4F3/4F4). Vegetative hyphae septate, subhyaline to light brown, smooth- and thin-walled, 2–2.5 μm wide. Ascomata immersed or superficial, globose to subglobose, 140–200 μm diam, with a papillate to short cylindrical ostiolar neck up to 40 μm long, black, glabrous; peridium with a textura intricata. Asci irregularly ellipsoidal or subglobose, 7.5–9.5 × 5.5–6.5 μm. Ascospores fusiform, 5–6 × 2.5–3.5 μm, straw coloured, yellow-orange in mass, with one inconspicuous germ pore. Conidiophores absent or as a basal single cell, of 2.5–3 × 3–5 μm, bearing groups of 2–3 annellides, or slightly branched up to 50 μm long, septate, subhyaline, smooth-walled. Annellides mostly sessile, single and lateral on vegetative hyphae, more or less ampulliform, 8–10(–11) × 2–2.5 μm, with a swollen base, tapering abruptly to a cylindrical annellated zone, 1–1.5 μm wide, subhyaline, smooth-walled. Conidia globose to broadly ellipsoidal, 4–5 × 3–3.5 μm, with truncate base, pale brown, dark brown in mass, and smooth- to rough-walled, thin-walled, arranged in long chains.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 40 °C, minimum 15 °C.
Specimens examined. ARGENTINA, Iguazú, from soil, Calduch, Guarro & Stchigel (FMR 12362). – USA, from bronchoalveolar lavage fluid, 2007, D.A. Sutton (holotype CBS H-21786, culture ex-type CBS 138128 = UTHSC 07-156 = FMR 12264).
Notes — Microascus intricatus is described on the basis of two strains, isolated from a clinical (human) sample in the USA and from soil, in Argentina. This species deviates from the other congeneric species in having a perithecial peridium wall with textura intricata and by forming short fusiform ascospores. Nonetheless, the abundant conidiation and ascomata with straw-coloured ascospores bearing a single germ pore match with the circumscription of Microascus, confirming our phylogenetic results.
Microascus longirostris Zukal, Verh. Zool.-Bot. Ges. Wien 35: 33. 1885
= Microascus variabilis Massee & E.S. Salmon, Ann. Bot., Lond. 15: 349. 1901.
Specimens examined. JAPAN, Tokyo, from soil, 1962, S. Udagawa (CBS 415.64 = NBRC 7554). – USA, Maine, Kittery Point, from a wasp’s nest, 1961, R. Thaxter (neotype designated here CBS H-14440, MBT198046) culture ex-neotype CBS 196.61 = MUCL 9058.
Notes — Microascus longirostris has been reported from many sources, mostly from dung of several mammals, soil, wood, seeds, air, as well from clinical samples in South and North America, Europe and Australia (Barron et al. 1961). The protologue of this species was made on the basis of ascomata on the natural substrata only (dog dung and rotten wood) (Zukal 1885, Barron et al. 1961). No ex-type strain or holotype material of this species was available. Microascus longirostris is the type of Microascus and, in order to stabilize the nomenclature, a neotype is here designated. Although none of the cultures studied here have the same geographical origin or host as the original specimen, the morphological characteristics of the two strains studied agree with the fungus described by Zukal in its original publication (Zukal 1885). The neotype culture selected here also corresponds with the modern descriptions of M. longirostris based on cultural characteristics given by Barron et al. (1961), Morton & Smith (1963) and von Arx et al. (1988), being also part of the material revised and considered as authentic by those authors.
Microascus macrosporus (G.F. Orr) Sandoval-Denis, Gené & Guarro, comb. & stat. nov. — MycoBank MB809212
Basionym. Microascus trigonosporus C.W. Emmons & B.O. Dodge var. macrosporus G.F. Orr, Canad. J. Bot. 39: 1617. 1961.
Specimen examined. USA, California, from soil, 1971, G.F. Orr (CBS 662.71 = UAMH 9336).
Notes — This species was originally described from desert soil as a variety of M. trigonosporus. However, while M. macrosporus has ascospores measuring 5–6.5 × 5.5–7.5 μm, those of M. trigonosporus are distinctly smaller (3–5 × 3–4 μm). Microascus pyramidus is another phylogenetically closely related and morphologically similar species. However, its ascospores have distinctly attenuated ends and its conidia are 4.5–5.5 × 3–4 μm. Microascus macrosporus produces ascospores with rounded ends and larger conidia (5–7 × 4–5 μm).
Microascus murinus (Samson & Klopotek) Sandoval-Denis, Gené & Guarro, comb. nov. — MycoBank MB809218
Basionym. Scopulariopsis murina Samson & Klopotek, Arch. Mikrobiol. 85: 175. 1972.
Specimen examined. GERMANY, Giessen, from composed municipal waste, 1970, A. von Klopotek (ex-type culture CBS 830.70 = IHEM 18567).
Notes — This species was originally isolated from domestic waste in Germany. Although M. murinus shares morphological features with M. chartarus, M. croci, M. paisii, M. restrictus and M. verrucosus, it can be differentiated by having smaller cylindrical conidia, measuring 4–6 × 1.5–2 μm and slightly larger annellides, measuring 6.5–11 × 1.5–2.5 μm.
Microascus paisii (Pollacci) Sandoval-Denis, Gené & Guarro, comb. nov. — MycoBank MB809213
Basionym. Torula paisii Pollacci (as ‘pais’), Atti Ist. Bot. Univ. Pavia, ser. 2, 18: 130. 1921.
≡ Phaeoscopulariopsis paisii (Pollacci) M. Ota, Jap. J. Dermatol. Urol. 28: 5. 1928. nom. inval. (Seifert et al. 2011).
≡ Scopulariopsis paisii (Pollacci) Nannf., Repertorio sistematico dei miceti dell’uomo e degli animali 4: 259. 1934.
= Scopulariopsis sphaerospora Zach, Oesterr. Bot. Z. 83: 180. 1934.
= Scopulariopsis brumptii Salv.-Duval, Thèse Fac. Pharm. Paris. 23: 58. 1935.
= Scopulariopsis versicolor Salv.-Duval, Thèse Fac. Pharm. Paris. 23: 63. 1935.
= Masoniella grisea (G. Sm.) G. Sm., Trans. Brit. Mycol. Soc. 35: 237. 1952.
≡ Masonia grisea G. Sm., Trans. Brit. Mycol. Soc. 35: 149. 1952, nom. illeg.
= Scopulariopsis melanospora Udagawa, J. Agric. Sci. (Tokyo) 5: 18. 1959.
Specimens examined. AUSTRIA, from unknown substrate, 1934, F. Zach (S. sphaerospora ex-type culture MUCL 9045 = CBS 402.34). – GERMANY, Schleswig-Holstein, Kiel, Kitzeberg, from soil on a Triticum sativum field, 1966, W. Gams (MUCL 8989 = CBS 896.68); Schleswig-Holstein, Kiel, from soil, 1966, W. Gams (MUCL 8990); from soil on a wheat field, 1966, W. Gams (MUCL 8993 = CBS 897.68). – ITALY, from human, 1927, G. Pollacci (T. paisii ex-type culture MUCL 7915 = CBS 213.27). – UK, isolated as a culture contaminant, 1946, G. Smith (M. grisea ex-type culture MUCL 9003 = CBS 295.52). – USA, from milled Oriza sativa, 1955, S. Udagawa (S. melanospora ex-type culture MUCL 9040 = CBS 272.60); from bronchoalveolar lavage fluid, 2007, D.A. Sutton (UTHSC 07-639 = FMR 12263); from bronchoalveolar lavage fluid, 2008, D.A. Sutton (UTHSC 08-1734 = FMR 12248); from sputum, 2009, D.A. Sutton (UTHSC 09-457 = FMR 12241); from bronchoalveolar lavage fluid, 2009, D.A. Sutton (UTHSC 09-482 = FMR 12240); from sputum, 2009, D.A. Sutton (UTHSC 09-2391 = FMR 12229); from bronchoalveolar lavage fluid, 2010, D.A. Sutton (UTHSC 10-2920 = FMR 12215); from sputum, 2011, D.A. Sutton (UTHSC 11-708 = FMR 12210).
Notes — Microascus paisii has had a confusing nomenclatural history. As Scopulariopsis paisii, it was erroneously considered the asexual morph of M. desmosporus by Morton & Smith (1963). We have also observed discrepancies concerning T. paisii among fungal databases. In MycoBank, T. paisii is considered as the asexual morph of M. cirrosus whereas Index Fungorum lists T. paisii as a synonym of Scytalidium thermophilum. Our phylogenetic analysis showed that the ex-type of T. paisii (MUCL 7915) belongs to the Microascus lineage. Within this lineage, it belongs to a well-supported subclade together with the ex-type strains of Masoniella grisea, S. melanospora and S. sphaerospora and several reference strains of S. brumptii. This subclade might represent the rarely opportunist species S. brumptii. However, since there is no type material of S. brumptii available, and T. paisii being the oldest type strain included in this subclade, the latter name has preference according to the nomenclatural principle that the correct name is the oldest legitimate one (McNeill et al. 2012). Therefore, according to our data the new combination Microascus paisii should be adopted.
Regarding data pertaining to S. brumptii, M. paisii has a worldwide distribution, being isolated from multiple substrates, including air, decaying wood or soil and is an opportunistic pathogen of human and warm-blooded animals (Morton & Smith 1963, de Hoog et al. 2011, Sandoval-Denis et al. 2013). This species morphologically resembles M. chartarus and M. croci, but it can be differentiated by its dark grey or black colonies and its ability to grow and sporulate well at 37 °C.
Microascus pyramidus G.L. Barron & J.C. Gilman, Canad. J. Bot. 39: 1618. 1961
Specimen examined. USA, from desert soil, 1957, G.L. Barron (ex-type culture CBS 212.65).
Notes — This species was originally isolated from desert soil in North America (Barron et al. 1961). Morphologically, it is similar to other Microascus species producing triangular ascospores as M. alveolaris, M. campaniformis, M. macrosporus and M. trigonosporus. However, ascospores of M. pyramidus are wider (5–6.5 × 5.5–7 μm), have attenuated ends and often acquire a nearly square shape (von Arx et al. 1988). The asexual morph of M. pyramidus is morphologically similar to those of M. macrosporus and M. campaniformis. Microascus macrosporus produces globose to ovoid conidia measuring 5–7 × 4–5 μm, while those of M. pyramidus are markedly narrower measuring 4.5–5.5 × 3–4 μm, and those of M. campaniformis are subglobose to broadly ellipsoidal measuring 4–5 × 2.5–3.5 μm.
Microascus restrictus Sandoval-Denis, Gené & Deanna A. Sutton, sp. nov. — MycoBank MB809420, Fig. 9
Fig. 9.
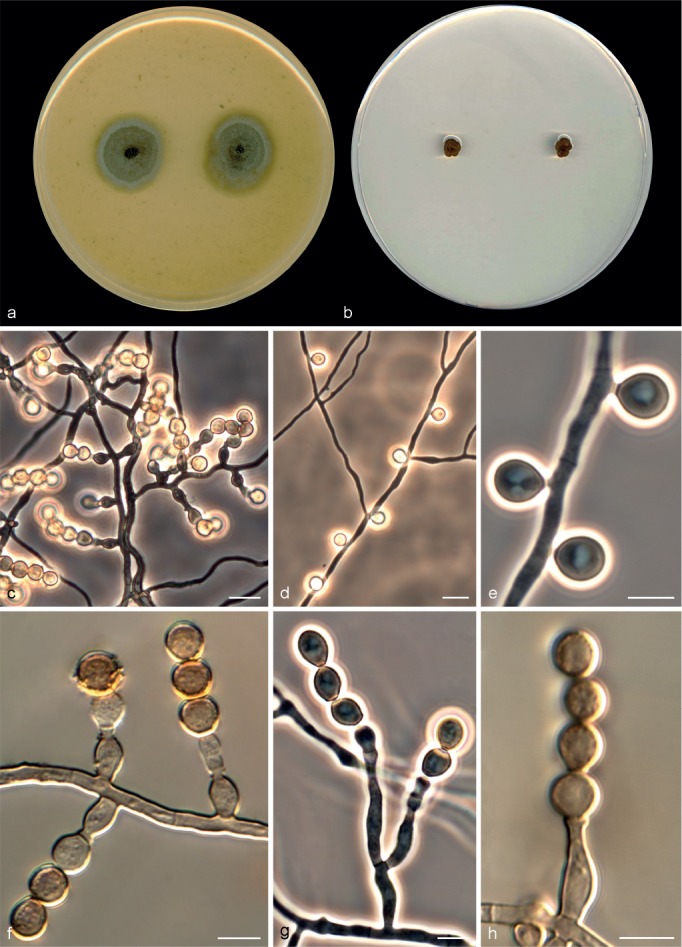
Microascus restrictus CBS 138277. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c, f–h. conidiophores, annellides and conidia; d, e. solitary conidia. — Scale bars: c, d = 10 μm; all others = 5 μm.
Etymology. From the Latin restringere-, restrict, referring to the restricted growth of the colony.
Colonies on OA at 25 °C growing rather slowly, attaining 23–25 mm diam in 14 d, flat, downy, olive grey (3F2) to brown-grey (5F2), with an irregular margin; reverse brown-grey (5F2). On PCA at 25 °C growing restrictedly, attaining 3–5 mm diam after 14 d, membranous, lobulate, with an irregular undulate margin, olive brown (4E5) to brown (5E5); reverse brown-grey (5E2). Vegetative hyphae septate, subhyaline becoming dark brown with age, smooth- and thin-walled, 1.5–3 μm wide. Conidiophores absent or as a basal single cell of 4–6 × 3–5 μm, bearing groups of 2–3 annellides, or slightly branched up to 20 μm long, subhyaline, smooth-walled. Annellides mostly sessile borne single and laterally on vegetative hyphae, ampulliform, 7–19 × 2–4.5 μm, with a swollen base, tapering abruptly to a cylindrical and darker annellated zone 1.5–2 μm wide, subhyaline, becoming darker with age, smooth-walled. Conidia globose to obovoidal, 4.5–6 × 4–5.5 μm, with truncate base, dark brown, smooth or finely roughened, thick-walled, arranged in short chains. Solitary conidia sometimes present, borne laterally from vegetative hyphae, sessile or on short stalks, globose or obovate, 5–5.5 × 4.5–5 μm, dark brown, smooth- and thick-walled. Sexual morph not observed.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 40 °C, minimum 15 °C.
Specimen examined. USA, from human left hallux, 2009, D.A. Sutton (holotype CBS H-21787, culture ex-type CBS 138277= UTHSC 09-2704 = FMR 12227).
Notes — Microascus restrictus is morphologically very similar to M. verrucosus. However, while M. restrictus shows larger smooth-walled annellides measuring 7–19 × 2–4.5 μm, smaller conidia (4.5–6 × 4–5.5 μm) and is able to grow at 40 °C, M. verrucosus has annellides measuring 8–10 × 1–3 μm, typically warted when mature, larger conidia (5–7 × 4.5–6 μm) and is unable to growth at 40 °C.
Microascus senegalensis Arx, Persoonia 8: 194. 1975
Specimen examined. SENEGAL, Joel, from mangrove soil, J.A. von Arx (ex-type culture IHEM 18561 = CBS 277.74).
Notes — This species has been reported from soil, seeds and plant debris as well as from human skin, in Africa, India and North America (von Arx et al. 1988). The most remarkable features of M. senegalensis are the presence of large reniform ascospores (7–9 × 2–3 μm) with a single and often protuberant germ pore (von Arx et al. 1988, Guarro et al. 2012).
Microascus trigonosporus C.W. Emmons & B.O. Dodge, Mycologia 23: 317. 1931
≡ Scopulariopsis trigonospora C.W. Emmons & B.O. Dodge, Mycologia 23: 317. 1931.
?= Microascus trigonosporus C.W. Emmons & B.O. Dodge var. terreus Kamyschko, Novosti Sist. Nizsh. Rast. 76: 175. 1966.
?= Microascus trigonosporus C.W. Emmons & B.O. Dodge var. macroperithecia Sage, Steiman, Seigle-Mur. & Guiraud, Mycotaxon 55: 194. 1995, nom inval. Art. 40.5 (Melbourne).
Specimens examined. JAPAN, Burma, from milled rice, 1961, S. Udagawa (MUCL 9061 = CBS 199.61). – UK, from mushroom bed, 1935, W.M. Ware (MUCL 9841 = CBS 262.35). – USA, from unknown substrate, 1931, C.W. Emmons (M. trigonosporus var. trigonosporus ex-type culture CBS 218.31).
Notes — This is a cosmopolitan species, commonly reported from soil, seeds and dung (Barron et al. 1961). It is also considered as a human pathogen that has been associated with pneumonia in an immunocompromised patient (Mohammedi et al. 2004) and endocarditis (Wang et al. 2011). Among the species producing triangular-shaped ascospores (M. alveolaris, M. campaniformis, M. macrosporus and M. pyramidus), M. trigonosporus produces the smaller ones, measuring 3–5 × 3–4 μm.
Microascus verrucosus Sandoval-Denis, Gené & Cano, sp. nov. — MycoBank MB809421; Fig. 10
Fig. 10.
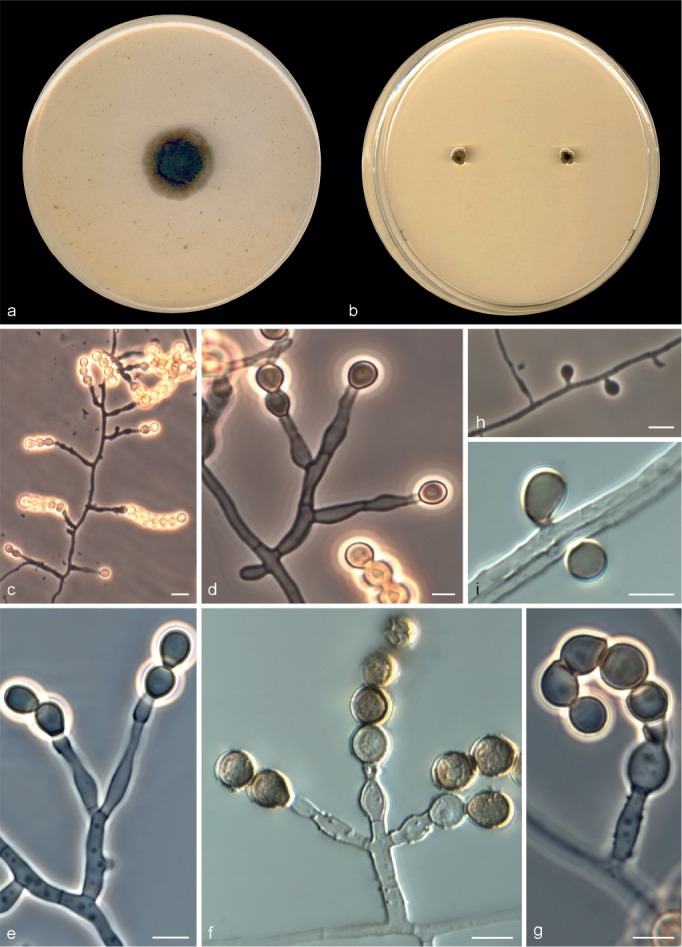
Microascus verrucosus CBS 138278. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c–g. conidiophores, annellides and conidia; h, i. solitary conidia. — Scale bars: c, h = 10 μm; all others = 5 μm.
Etymology. From the Latin verruca-, wart, referring to the warted ornamentation of the annellides.
Colonies on OA at 25 °C growing slowly, attaining 19–22 mm diam in 14 d, flat, finely granular, olive grey (2–3F2), with an immersed and slightly undulated margin; reverse olive grey (1–3F2). On PCA at 25 °C growing restrictedly, attaining 1–5 mm diam in 14 d, adherent, membranous or slightly downy, hemispherical, cerebriform, with lobulated margin, olive (2E3/2E4); reverse olive (2E2) to olive grey (2E3). Vegetative hyphae septate, subhyaline becoming dark brown with age, smooth- to rough-walled, thin-walled, 1.5–2.5 μm wide. Conidiophores absent or as a basal single cell of 4–5 × 2.5–4 μm, bearing groups of 2–3 annellides, rarely slightly branched up to 25 μm long. Annellides mostly sessile and borne directly on vegetative hyphae, lageniform, 8–10 × 1–3 μm, constricted at the basal septum, followed by a slightly swollen portion and tapering to a more or less cylindrical annellated zone 1–1.5 μm wide, usually sparsely warted. Conidia globose to subglobose, 5–7 × 4.5–6 μm, often with an inconspicuous truncate base, dark brown, smooth or finely roughened, thick-walled, arranged in short chains. Solitary conidia sometimes present, borne laterally from vegetative hyphae, sessile or on short stalks, globose or broadly ellipsoidal, 5–6 × 4–5 μm, dark brown, smooth- and thick-walled. Chlamydospores not observed. Sexual morph not observed.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 35 °C, minimum 15 °C.
Specimen examined. USA, from bronchoalveolar lavage fluid, 2010, D.A. Sutton (holotype CBS H-21788, culture ex-type CBS 138278 = UTHSC 10-2601 = FMR 12219).
Notes — Microascus verrucosus can be differentiated from M. restrictus, its closest morphological relative, by its sparsely warted annellides and its inability to grow at 40 °C. Microascus verrucosus is phylogenetically close to M. murinus; however, this latter species has cylindrical conidia with pointed apices.
Pithoascus Arx, Proc. Kon. Ned. Akad. Wetensch. 76: 295. 1973
Type species. Pithoascus nidicola (Massee & E.S. Salmon) Arx.
Colonies restricted, white, becoming grey or darkening due to the production of ascomata; usually with scarce aerial mycelium. Ascomata perithecial, immersed or somewhat superficial, gregarious, often grouped on dense crusts, globose, glabrous, often with an inconspicuous ostiolar opening or with a short cylindrical ostiolar neck; peridium black, composed of thick-walled, slightly flattened cells, textura angularis. Asci unitunicate, 8-spored, broadly clavate or barrel-shaped, evanescent. Ascospores 1-celled, asymmetrical, navicular, fusiform or falcate, yellow, straw- or honey-coloured, dextrinoid when young, without germ pores. Asexual morph present in some species. Conidiogenous cells annellidic, borne singly and laterally on the vegetative hyphae, short, ampulliform, hyaline, smooth-walled. Conidia 1-celled, globose to pyriform, with a truncate base, smooth- and thin-walled, solitary.
Pithoascus ater (Zach) Sandoval-Denis, Cano & Guarro, comb. nov. — MycoBank MB809214
Basionym. Scopulariopsis atra Zach, Oesterr. Bot. Z. 83: 184. 1934.
Specimen examined. From human nail, 1934, F. Zach (ex-type culture IHEM 18608 = CBS 400.34).
Notes — A single strain of this species is available. It was isolated from a human nail, but its pathogenic role was not clearly established (Zach 1934). Pithoascus ater is the only species of the genus for which a sexual morph is unknown and by contrast shows abundant conidial production. However, the ex-type strain of P. ater shows similar morphological characteristics to the known asexual morphs of Pithoascus species, such as P. stoveri and P. intermedius. The main distinctive feature of P. ater is the abundant production of solitary, globose and smooth-walled pale brown conidia measuring 4–9 × 4.5–8.5 μm. In contrast, conidia of P. stoveri and P. intermedius are rarely seen in culture and, when present, are hyaline, obovate to pyriform (5–8 × 3–4 μm) or globose to subglobose (4–8 × 4.5–7.5 μm), respectively. Other species, i.e. P. nidicola and P. exsertus produce only sexual morphs in culture.
Pithoascus exsertus (Skou) Arx, Persoonia 7: 373. 1973
Basionym. Microascus exsertus Skou, Antonie van Leeuwenhoek 39: 533. 1973.
Specimens examined. DENMARK, Sjaelland, Bjerge Strand, from Osmia rufa, 1975, J.P. Skou (CBS 583.75); Tastrup, Hojbakkegard, Experimental Station, from Megachile willoughbiella, 1970, J.P. Skou (ex-type culture CBS 819.70).
Notes — This fungus is considered as an entomogenous species, which has been isolated from a leaf-cutting bee (Megachile willughbiella) and from a Red Mason-bee (Osmia rufa), both in northern Europe. Morphologically, P. exsertus can be differentiated from the other species of the genus by its larger ascomata (210–450 μm diam) and its long, falcate to nearly cylindrical and yellow ascospores, 6.5–12 × 1–2.5 μm.
Pithoascus intermedius (C.W. Emmons & B.O. Dodge) Arx, Proc. Kon. Ned. Akad. Wetensch. 76: 292. 1973
Basionym. Microascus intermedius C.W. Emmons & B.O. Dodge, Mycologia 23: 324. 1931.
Specimen examined. USA, North Carolina, Chadbourn, from decaying root of Fragaria vesca, 1932, C.W. Emmons & B.O. Dodge (ex-type culture CBS 217.32).
Notes — This species has been reported mainly from soil in North America, Europe and Asia, and also as a potential pathogenic species isolated from human hair and nails (von Arx et al. 1988, Guarro et al. 2012). Pithoascus intermedius is morphologically similar to P. nidicola and P. stoveri. However, P. intermedius can be identified by its small, fusiform ascospores, 5–6 × 2–2.5 μm.
Pithoascus nidicola (Massee & E.S. Salmon) Arx, Proc. Kon. Ned. Akad. Wetensch. 76: 292. 1973
Basionym. Microascus nidicola Massee & E.S. Salmon, Ann. Bot., Lond. 15: 313. 1901.
Specimen examined. USA, Utah, from Dipodomys merriami, C.W. Emmons (ex-epitype culture CBS 197.61).
Notes — Pithoascus nidicola was originally isolated from a wasp’s nest in England and later from soil samples in North America (Massee & Salmon 1901, Barron et al. 1961). The ex-epitype culture, however, was isolated from a kangaroo rat in the USA (von Arx 1973b, Abbott et al. 2002). This species is similar to P. stoveri; however, P. nidicola can be differentiated by having larger ascomata (90–160 μm diam) with thicker walls (6–10 μm) and navicular to nearly lunate straw coloured ascospores. In contrast, P. stoveri produces ascomata measuring 50–110 μm diam, with a wall 4–7 μm thick, and navicular, golden to brown coloured ascospores (von Arx 1973b, Abbot et al. 2002, Guarro et al. 2012). Although conidia had never been reported for P. nidicola, we observed the development of a reduced asexual morph on PCA forming globose to ampulliform hyaline conidia, 4–5 × 2.5–3.5 μm, borne on short conidiogenous cells (Fig. 2). These asexual structures resemble those of P. intermedius, but the conidia of the latter are globose to subglobose and larger (4–8 × 4–7.5 μm).
Pithoascus stoveri Arx, Persoonia 7: 373. 1973
≡ Microascus stoveri (Arx) S.P. Abbott, Mycologia 94: 368. 2002.
Specimen examined. USA, Ohio, from root of Beta vulgaris, W.L. White (ex-type culture CBS 176.71).
Notes — This species was originally isolated from a root of sugar beet in the USA. Pithoascus stoveri is morphologically similar to P. nidicola; however, the former species forms an asexual morph in culture, has a smaller ascomata (50–110 μm diam) and navicular golden yellow to brown ascospores. Pithoascus nidicola produces ascomata 90–160 μm diam, and navicular to nearly lunate straw-coloured ascospores.
Pseudoscopulariopsis Sandoval-Denis, Gené & Guarro, gen. nov. — MycoBank MB809215
Type species. Pseudoscopulariopsis schumacheri (Curzi) Sandoval-Denis, Gené & Guarro.
Colonies restricted, greyish, dark olive grey to olivaceous black; floccose with abundant submerged mycelium, often becoming crustose and dark. Ascomata black, globose or ovate, glabrous, with a short cylindrical ostiolar neck and a peridium of textura epidermoidea. Asci unitunicate, 8-spored, ovoid, evanescent. Ascospores 1-celled, asymmetrical, navicular to fusiform, sub-hyaline, pale yellow or brown, without germ pores. Conidiogenous cells short, annellidic, often with a swollen base, mostly borne on a short and swollen supporting cell forming short swollen conidiophores, rarely borne singly on aerial hyphae. Conidia 1-celled, subglobose, obovate to short clavate, with truncate base and rounded apex, smooth- and thin-walled, pale brown to brown-grey, arranged in short chains.
Pseudoscopulariopsis hibernica (A. Mangan) Sandoval-Denis, Gené & Cano, comb. nov. — MycoBank MB809216
Basionym. Scopulariopsis hibernica A. Mangan, Trans. Brit. Mycol. Soc. 48: 617. 1965.
Specimen examined. IRELAND, from soil, A. Mangan (UAMH 2643 = ATCC 16690).
Notes — This is a species described from soil and only a few isolates are available, all of them derived from the same isolation source (Mangan 1965). The isolate studied here, although not the ex-type culture, was isolated and considered authentic by the same authors (Mangan 1965) and matched in all aspects with the protologue (Mangan 1965). Phylogenetically, P. hibernica clustered close to P. schumacheri, both species being characterised by short cylindrical annellides mostly formed in small groups on short and swollen supporting cells, commonly darkening with age. Pseudoscopulariopsis hibernica can be differentiated mainly by its lack of a sexual morph, the presence of shorter (9–15 × 3–5 μm) and darker annellides, and its larger (5–7 × 5–6 μm) subglobose conidia.
Pseudoscopulariopsis schumacheri (E.C. Hansen) Sandoval-Denis, Gené & Guarro, comb. nov. — MycoBank MB809549
Basionym. Sphaerella schumacheri E.C. Hansen, Vidensk. Meddel. Dansk Naturhist. Foren. Kjøbenhavn: 16. 1876.
≡ Rosellinia schumacheri (E.C. Hansen) Sacc., Syll. Fung. 1: 276. 1882.
≡ Microascus schumacheri (E.C. Hansen) Curzi, Boll. Staz. Patol. Veg. Roma, N.S. 23: 8. 1931.
≡ Pithoascus schumacheri (E.C. Hansen) Arx, Proc. Kon. Ned. Akad. Wetensch. 76: 292. 1973.
= Melanospora stysanophora Mattir., Nuovo Giorn. Bot. Ital. 18: 121. 1886.
≡ Microascus stysanophorus (Mattir.) Curzi, Boll. Staz. Patol. Veg. Roma, N.S. 10: 391. 1930.
≡ Microascus stysanophorus (Mattir.) G.L. Barron, Cain & J.C. Gilman, Canad. J. Bot. 39: 1621. 1961.
≡ Pithoascus stysanophorus (Mattir.) Valmaseda, A.T. Martínez & Barrasa, Canad. J. Bot. 65: 1805. 1987.
Specimen examined. SPAIN, Puerto de la Quesera, from soil, 1986, A.T. Martínez (neotype designated here MA-Fungi 16319, MBT178643), culture ex-neotype CBS 435.86.
Notes — This species was originally described from dung of rodents, in Denmark, but no ex-type strain was preserved nor herbarium material listed in the protologue. The modern descriptions of the species by Barron et al. (1961), Valmaseda et al. (1986), von Arx et al. (1988) and Guarro et al. (2012) are based on the same isolate studied here; however, a type specimen has never been designated. We agree with the observations of all these authors in that morphological features of CBS 435.86 match with those of the protologue of the species (Hansen 1876). Therefore, due to the scant live material available and the inexistence of ex-type cultures, we have selected this strain as neotype in order to fix the application of the name. Although neither the substrate nor the geographic origin correspond to that indicated in the protologue of the species, this strain clearly represents P. schumacheri according to concepts maintained by subsequent authors (Valmaseda et al. 1986, von Arx 1988, Guarro et al. 2012).
Morphologically, P. schumacheri resembles Pithoascus species by its navicular to fusiform ascospores lacking germ pores, thus being included in that genus by von Arx (1973a). Nevertheless, P. schumacheri can be differentiated by its restricted growth and the textura epidermoidea of the ascoma wall. Phylogenetically, P. schumacheri is related to P. hibernica; however, the former can be differentiated by the presence of ascomata and the production of obovate to short-clavate conidia, measuring 4.5–6 × 2.5–4 μm, on mostly hyaline annellides which measure 3.5–22.5 × 1.5–2.5 μm.
Scopulariopsis Bainier, Bull. Soc. Mycol. France 23: 98. 1907
?= Acaulium Sopp, Skr. Vidensk.-Selsk. Christiana, Math.-Naturvidensk. Kl. I, 11: 42. 1912.
= Phaeoscopulariopsis M. Ota, Jap. J. Dermatol. Urol. 28: 405. 1928, nom. inval. Art 34 (Seifert et al. 2011).
Type species. Scopulariopsis brevicaulis (Sacc.) Bainier.
Colonies spreading fast, velvety, funiculose or granular, varying from white and grey-white to several shades of buff, brown or dark brown, but never in shades of green or black. Ascomata perithecial, immersed or superficial, developing slowly, scattered; globose to subglobose or pyriform, glabrous, ostiolate, papillate or with a cylindrical neck; peridium black, composed of thick-walled, slightly flattened cells of textura angularis. Asci unitunicate, 8-spored, subglobose, irregularly ovoidal or ellipsoidal, evanescent. Ascospores 1-celled, asymmetrical, short, broadly reniform or lunate, dextrinoid when young, with or without an inconspicuous germ pore. Conidiogenous cells annellidic, borne on branched penicillate conidiophores, occasionally singly on vegetative hyphae or in groups of 2–3 on short stalks, cylindrical, often with a slightly swollen base followed by a cylindrical annellated portion that ends in a flat and wide conidiogenous opening, hyaline, smooth- or rough-walled. Conidia 1-celled, hyaline, avellaneous or brown, globose to ovate, with a rounded or pointed apex and a conspicuously protuberant and truncate base, smooth- or rough- and thick-walled, arranged in long basipetal dry chains.
Scopulariopsis asperula (Sacc.) S. Hughes, Canad. J. Bot. 36: 803. 1958
Basionym. Torula asperula Sacc., Michelia 2: 560. 1882.
= Acaulium nigrum Sopp, Skr. Vidensk.-Selsk. Christiana, Math.-Naturvidensk. Kl. I 11: 47. 1912.
≡ Penicillium nigrum (Sopp) Biourge, Cellule 33: 1043. 1923.
≡ Microascus niger (Sopp) Curzi, Boll. Staz. Patol. Veg. Roma, N.S. 11: 8. 1931.
= Scopulariopsis repens Bainier, Bull. Soc. Mycol. France 23: 125. 1907.
≡ Penicillium repens (Bainier) Biourge, Cellule 33: 225. 1923.
= Monilia arnoldii L. Mangin & Pat., Bull. Soc. Mycol. France 24: 164. 1908.
≡ Scopulariopsis arnoldii (L. Mangin & Pat.) Vuill., Bull. Soc. Mycol. France 27: 148. 1911.
= Scopulariopsis ivorensis H. Boucher, Bull. Soc. Pathol. Exot. 11: 312. 1918.
= Torula bestae Pollacci, Rivista Biol. 4: 317. 1922.
≡ Phaeoscopulariopsis bestae (Pollacci) M. Ota, Jap. J. Dermatol. Urol. 28: 405. 1928, nom. inval. (Seifert et al. 2011).
≡ Scopulariopsis bestae (Pollacci) Nannf., Repertorio sistematico dei miceti dell’uomo e degli animale 4: 254. 1934.
= Scopulariopsis fusca Zach, Oesterr. Bot. Z. 83: 174. 1934.
= Acaulium nigrum Sopp var. glabrum Salv.-Duval, Thèse Fac. Pharm. Paris. 23: 55. 1935.
= Scopulariopsis roseola N. Inagaki, Trans. Mycol. Soc. Japan 4: 1. 1962.
Specimens examined. AUSTRIA, from a carcass of rabbit, 1934, F. Zach (Scopulariopsis fusca ex-type culture MUCL 9032 = CBS 401.34). – CANADA, Alberta, Girouxville, from indoor air ex RCS strip, Apis mellifera overwintering facility, Jan. 1994, S.P. Abbott (MUCL 40729 = UAMH 7879); Alberta, 10 km south of Leduc, from dung of Mephitis mephitis, June 1997, S.P. Abbott (MUCL 40746 = UAMH 9029). – GERMANY, from compost soil, 1958, K.H. Domsch (CBS 853.68). – ITALY, from human, 1938, G. Pollacci (Torula bestae ex-type culture MUCL 9012 = CBS 289.38). – USA, from toenail, 2010, D.A. Sutton (UTHSC 10-3405 = FMR 12212).
Notes — This species has been mostly recovered from environmental samples such as soil, air, mouldy indoor environments, food such as cheese and butter, as well as from human clinical specimens, mainly skin and nails (Ropars et al. 2012, Sandoval-Denis et al. 2013).
Torula bestae was considered by Morton & Smith (1963) to be conspecific with S. koningii. However, although both species show smooth conidia, T. bestae typically exhibits darker fuscous-black colonies and conidia. Later, Abbott & Sigler (2001), using mating experiments, established that S. arnoldii, S. asperula, S. bestae, S. fusca and S. roseola were all synonyms of the heterothallic species Microascus niger. The same authors also selected neotype and epitype cultures for M. niger (UAMH 9489) and S. asperula (UAMH 9037), respectively, which unfortunately were not available for our study. However, all the reference strains studied here were genetically related with the type cultures of S. fusca and S. bestae, both species regarded as synonyms of S. asperula. Recently, Ropars et al. (2012) confirmed the synonymy of these species using a multilocus analysis based on D1/D2, TUB and EF-1α sequences which results are confirmed by our phylogenetic analysis. Morphologically, S. asperula is close to S. brevicaulis and S. flava; however, S. asperula can be differentiated by having dark brown to fuscous or violaceus colonies, and globose to ovate, coarsely verrucose or smooth walled, fuscous to sepia coloured conidia, mostly with a pointed apex and measuring 5–8 × 4–6.5 μm. In contrast, S. brevicaulis shows tan colonies, globose and verrucose pale brown conidia, 6–9 × 5.5–9 μm, while S. flava exhibits white colonies and obovoidal, verrucose and hyaline conidia, 6–9.5 × 5–8.5 μm.
Scopulariopsis brevicaulis (Sacc.) Bainier, Bull. Soc. Mycol. France 23: 99. 1907
Basionym. Penicillium brevicaule Sacc., Fungi Ital. 893: 1881.
= Monilia penicillioides Delacr., Bull. Soc. Mycol. France 13: 114. 1897.
≡ Penicillium penicillioides (Delacr.) Vuill., Bull. Soc. Mycol. France 27: 75. 1911.
≡ Scopulariopsis penicillioides (Delacr.) Smith & Ramsb., Trans. Brit. Mycol. Soc. 5: 164. 1915.
= Monilia koningii Oudem., Arch. Neerl. Sci., sér. 2: 287. 1902.
≡ Scopulariopsis koningii (Oudem.) Vuill., Bull. Soc. Mycol. France 27: 143. 1911.
= Penicillium coccophilum Sacc., Ann. Mycol. Berl. 5: 178. 1907.
= Scopulariopsis rufulus Bainier, Bull. Soc. Mycol. France 23: 105. 1907.
≡ Penicillium rufulum (Bainier) Sacc., Syll. Fung. 22: 1275. 1913.
= Penicillium brevicaule Sacc. var. hominis Brumpt & Langeron in Brumpt, E. Précis de parasitologie, Ed. 1: 838. 1910.
≡ Scopulariopsis brevicaulis (Sacc.) Bainier var. hominis (Brumpt & Langeron) Brumpt & Langeron in Brumpt, E. Précis de parasitologie, Ed 2: 902. 1913.
= Scopulariopsis hominis (Brumpt & Langeron) Sartory, Champ. Parasit. Fasc. 8: 612. 1922.
= Acaulium insectivorum Sopp, Skr. Vidensk.-Selsk. Christiana, Math.-Naturvidensk. Kl. I, 11: 60. 1912.
≡ Penicillium insectivorum (Sopp) Biourge, Cellule 33: 103. 1923.
≡ Scopulariopsis insectivora (Sopp) Thom, The Penicillia: 532. 1930.
= Acaulium anomalum Sopp, Skr. Vidensk.-Selsk. Christiana, Math.-Naturvidensk. Kl. I, 11: 65. 1912.
= Penicillium brevicaule Sacc.var. intermedium Cagnetto, Sperimentale 67, Suppl. to Fasc. 4: 210. 1913.
= Sporotrichum stercorarium Ehrenb., Jahrb. Gewächsk. 1: 178. 1818.
≡ Scopulariopsis stercoraria (Ehrenb.) S. Hughes, Canad. J. Bot. 36: 803. 1958.
= Scopulariopsis alboflavescens Zach, Oesterr. Bot. Z. 83: 177. 1934.
= Microascus brevicaulis S.P. Abbott. Mycologia 90: 298. 1998.
Specimens examined. AUSTRIA, from human diseased skin, 1934, F. Zach (S. alboflavescens ex-type culture CBS 339.34). – BELGIUM, Heverlee, from soil, J. Meyer (as S. stercoraria MUCL 14213). – CANADA, Alberta, from indoor air, March 1994, S.P. Abbott (M. brevicaulis ex-type culture UAMH 7770 = MUCL 40726). – THE NETHERLANDS, Wageningen, from pupa of Pteronus pini, 1935, J. Rozsypal (as S. insectivora CBS 335.35 = MUCL 9035). – Unknown geographical origin, Elephant, 1951, I.M. Scott (as S. koningii CBS 208.61). – USA, from hair, 2006, D.A. Sutton (UTHSC 06-277 = FMR 12273); from toenail, 2006, D.A. Sutton (UTHSC 06-619 = FMR 12271); from toenail, 2007, D.A. Sutton (UTHSC 07-1812 = FMR 12257); from human spine, 2007, D.A. Sutton (UTHSC 07-1888 = FMR 12255); from maxillary sinus, 2008, D.A. Sutton (UTHSC 08-1920 = FMR 12247); from toe, 2009, D.A. Sutton (UTHSC 09-1092 = FMR 12236); from sputum, 2009, D.A. Sutton (UTHSC 09-1373 = FMR 12233); from sputum, 2011, D.A. Sutton (UTHSC 11-427 = FMR 12211); from lung mass, 2011, D.A. Sutton (UTHSC 11-1240 = FMR 12206); from bronchoalveolar lavage fluid, 2011, D.A. Sutton (UTHSC 11-1563 = FMR 12204).
Notes — This is a species with a worldwide distribution. It has been isolated from a wide range of substrates and locations and also recognised as an important human opportunistic pathogen (de Hoog et al. 2011, Sandoval-Denis et al. 2013). The history of this taxon was reviewed by Morton & Smith (1963). These authors synonymised S. insectivora and S. brevicaulis, but regarded S. stercoraria and S. koningii as different species since the latter two taxa exhibited smooth conidia. Later, Abbott & Sigler (2001) based on mating studies demonstrated that S. brevicaulis and S. koningii were conspecific. The present data confirmed the synonymy of the four mentioned species. In contrast, our results showed that the ex-type strain of S. alboflavescens (CBS 339.34), a species currently considered a synonym of S. candida, is conspecific with S. brevicaulis. Scopulariopsis alboflavescens was described as having smooth conidia and colonies at first white becoming pale yellowish, features that distinguished it from S. brevicaulis (Zach 1934). However, our morphological study of the ex-type strain of S. alboflavescens revealed that despite the fact that it forms whitish to pale yellow colonies, it also produces some finely roughened conidia. Ropars et al. (2012) already suggested a relationship between the two species using a phylogenetic analysis based in LSU, TUB and EF-1α sequences, showing that the ex-type strain of S. alboflavescens nested in a clade closely related to S. brevicaulis and far from the S. candida clade. However, those authors concluded that S. candida was a polyphyletic species. Abbott & Sigler (2001) reported the formation of fertile ascomata when crossing the ex-type strain of S. alboflavescens (CBS 339.34) with several strains of S. candida and the ex-types of S. candida (MUCL 40743) and Nephrospora manginii (basionym of M. manginii CBS 170.27), thus supporting the synonymy of S. alboflavescens and S. candida already proposed by Morton & Smith (1963). However, considering that strains of M. manginii (CBS 170.27 and MUCL 12598), the ex-epitype of S. candida (MUCL 40743) and S. alboflavescens (CBS 399.34) were all self-fertile in our study, the phylogenetic results and the placement of S. alboflavescens as a synonym of S. brevicaulis, including strains showing smooth conidia and whitish to pale yellow colonies, is supported.
Scopulariopsis candida (Guég.) Vuill., Bull. Soc. Mycol. France 27: 143. 1911
Basionym. Monilia candida Guég., Bull. Soc. Mycol. France 15: 271. 1899.
= Nephrospora manginii Loubière, Compt. Rend. Hebd. Séances Acad. Sci. 177: 209. 1923.
≡ Microascus manginii (Loubière) Curzi, Boll. Staz. Patol. Veg. Roma 2: 60. 1931.
= Monilia candida auct. non Pers.: Loubière in Compt. Rend. Hebd. Séances Acad. Sci. 177: 209. 1923.
= Scopulariopsis brevicaulis (Sacc.) Bainier var. glabra (Thom) Thom sensu Raper & Thom, Manual of the Penicillia: 699. 1949.
= Chrysosporium keratinophilum (Frey) Carmich. var. denticola C. Moreau, Mycopathol. Mycol. Appl. 37: 37. 1969.
≡ Basipetospora denticola (C. Moreau) C. Moreau, Bull. Soc. Mycol. France 87: 43, 1971.
Specimens examined. CANADA, British Columbia, Chilliwak, from indoor air, March 1997, S.P. Abbott (S. candida ex-type culture MUCL 40743 = UAMH 9004). – FRANCE, from unknown origin, 1927, L. Mangin (N. manginii ex-type culture CBS 170.27); from unknown substrate, 1927, L. Mangin (as S. candelabrum MUCL 9026 = CBS 205.27); from cheese ‘tome de Savoie’, Aug. 1998, C. Decock (as M. manginii MUCL 41467). – Unknown origin, 1966 (as S. alboflavescens MUCL 9007). – USA, from sputum, 2009, D.A. Sutton (UTHSC 09-2576 = FMR 12228); from scalp, 2009, D.A. Sutton (UTHSC 09-3241 = FMR 12226).
Notes — This species has been reported from environmental samples (air, dust and soil) generally from the Northern Hemisphere, especially in Europe and North America; and also from clinical samples, mainly from superficial tissue of humans and animals (de Hoog et al. 2011). It is morphologically close to S. brevicaulis, S. asperula and S. flava. However, S. candida has subglobose to broadly ovate, hyaline, smooth-walled conidia and white colonies. In contrast, S. brevicaulis and S. asperula produce tan or fuscous brown colonies, respectively, while S. flava produces white colonies and obovoidal rough-walled conidia. When the sexual morph is present, it is characterised by globose perithecia, 100–170 μm diam, and reniform to heart-shaped ascospores which are somewhat wider (4–6 × 5–6 μm) than those of its closest relatives such as S. brevicaulis (5–6 × 3.5–4.5 μm), S. cordiae (4.5–5.5 × 3.5–4 μm) and S. soppii (6–7 × 2.5–3 μm).
Scopulariopsis cordiae Sandoval-Denis, Gené & Cano, sp. nov. — MycoBank MB809217, Fig. 11
Fig. 11.

Scopulariopsis cordiae CBS 138129. a, b. Colonies on OA and PCA, respectively, after 21 d at 25 °C; c. ascoma; d. peridium; e, f. asci and ascospores; g–i. conidiophores, annellides and conidia; j, k. conidial chains. — Scale bars: c = 50 μm; all others = 5 μm.
Etymology. From the Latin cordiae-, heart, referring to the heart-shaped ascospores.
Colonies on OA and PCA at 25 °C attaining 35–36 and 48–50 mm diam, respectively, after 14 d, flat, with scarce aerial mycelium, white to light grey (4B1) and granular due to the abundant production of ascomata, regular margin with abundant submerged mycelium; reverse whitish. Vegetative hyphae septate, hyaline, smooth- and thin-walled, 1.5–3.5 μm wide. Ascomata abundant, superficial or immersed, globose or subglobose, 100–150 μm diam, with a long cylindrical ostiolar neck up to 390 μm, black, glabrous; peridium with a textura angularis. Asci irregularly ellipsoidal, 9–15.5 × 7.5–10 μm. Ascospores broadly lunate to reniform, 4.5–5.5 × 3.5–4 μm, straw coloured, bright yellow in mass, with a single and inconspicuous germ pore. Conidiophores absent. Annellides sessile, borne single and laterally on vegetative hyphae, hyaline, smooth and thin-walled, cylindrical, 8–15 × 1.5–3.5 μm, tapering gradually to a cylindrical annellated zone 1–1.5 μm wide. Conidia broadly ellipsoidal to obovoidal, 2.5–6 × 2–5 μm, with truncate base, hyaline, white in mass, smooth- and thick-walled, arranged in chains. Chlamydospores and solitary conidia not observed.
Cardinal temperature for growth — Optimum 25–30 °C, maximum 40 °C, minimum 15 °C.
Specimens examined. USA, from a human J.P Drain, 2005, D.A. Sutton (UTHSC 05-3453 = FMR 12349); from human finger, 2009, D.A. Sutton (holotype CBS H-21789, culture ex-type CBS 138129 = UTHSC 09-866 = FMR 12338).
Notes — Scopulariopsis cordiae morphologically resembles the sexual morph of S. candida in the shape and size of the ascomata, asci and ascospores. Scopulariopsis cordiae can be differentiated by its faster growth rate, the sparkled appearance of the colonies, the presence of long cylindrical necks in numerous submerged ascomata, and the slightly reduced size and shape of its ascospores and conidia.
Scopulariopsis flava (Sopp) F.J. Morton & G. Sm., Mycol. Pap. 86: 43. 1963
Basionym. Acaulium flavum Sopp, Skr. Vidensk.-Selsk. Christiana, Math.-Naturvidensk. Kl. I 11: 53. 1912.
= Penicillium brevicaule Sacc. var. album Thom, Bull. U.S. Bur. Anim. Ind. 118: 47. 1910.
≡ Scopulariopsis brevicaulis (Sacc.) Bainier var. alba (Thom) Thom, The Penicillia: 520. 1930.
= Scopulariopsis aurea Sartory, Champ. Parasit. Fasc. 9: 650. 1922.
= Scopulariopsis casei Loubière, Thèse Fac. Sci. Paris, Sér. 4: 62. 1924.
= Scopulariopsis grylli Sartory, Ann. Mycol., Berl., 30: 469. 1932.
Specimen examined. UK, from cheese, 1948, G. Smith (neotype designated here CBS H-21939, MBT198047) culture ex-neotype CBS 207.61 = MUCL 9031).
Notes — This species is commonly isolated from cheese and soil in Europe and North America (Ropars et al. 2012). However, Sopp’s original description of A. flavum is based on an isolate obtained from an insect larva. There are also reports of this species as human opportunistic pathogen (de Hoog et al. 2011). Scopulariopsis flava is morphologically close to S. brevicaulis. However, while S. brevicaulis produces tan, powdery to granular colonies, and globose to ovoid conidia with rounded or pointed apices, becoming verrucose and pale brown when mature, S. flava produces white floccose to fasciculate colonies, and hyaline globose to obovoidal conidia, with rounded apices and a coarsely roughened wall.
We only could study a single strain of S. flava (MUCL 9031), which is morphologically identical to the asexual morph of A. flavum and fits with the modern concept of S. flava by Morton & Smith (1963). Abbott et al. (2002) considered this strain a probable poorly pigmented variant of S. brevicaulis. However, our analyses show that MUCL 9031 is phylogenetically and morphologically distant from S. brevicaulis. Considering that Morton & Smith (1963) regarded MUCL 9031 as an authentic strain of S. brevicaulis var. alba, the epithet alba would take priority over the younger one flava, however the former epithet was already used in Scopulariopsis (S. alba, currently Doratomyces albus according to Dominik & Majchrowicz (1970)). Thus to avoid nomenclatural confusion we prefer to maintain the epithet flava for the present species. Phylogenetically S. flava is related to S. soppii, but the latter differs morphologically in producing larger conidia (5.5–9 × 5–8 μm vs 6.5–7 × 5.5–6.5 μm in S. flava), and falcate to lunate ascospores measuring 6–7 × 2.5–3 μm. It is noteworthy that a short description of a sexual morph was included in the protologue of A. flavum having ‘oval-round’ ascospores measuring 6–7 μm (Sopp 1912). However, we were not able to induce the production of ascomata in the above-mentioned strain and according to Abbott et al. (2002) no sexual morph has been reported since the original description of the species (Sopp 1912).
Scopulariopsis soppii S.P. Abbott, Mycologia 94: 364. 2002
≡ Microascus soppii S.P. Abbott, Mycologia 94: 364. 2002.
Specimen examined. CANADA, Alberta, Elk Island National Park, from dry, rotten wood of Populus tremuloides, T. Lumley (ex-type culture UAMH 9169).
Notes — This species has been isolated from decayed wood and sandy loam (Abbott et al. 2002). It is phylogenetically and morphologically close to S. flava from which it can be differentiated based on the size of the conidia and the size and shape of its ascospores (see S. flava).
IDENTIFICATION KEYS
According to the morphological features, identification keys were constructed for the different genera including all the phylogenetic species recognised in this study.
Key to Microascus, Scopulariopsis and allied genera
1. Colonies white, tan or brown coloured; conidiogenous cells cylindrical, hyaline or pale brown; conidia thick-walled with a protruding flat base . . . . . . . . . Scopulariopsis
1. Colonies grey-white, olive-green or black; conidiogenous cells ampulliform or lageniform, subhyaline or brown-green; conidia otherwise . . . . . . . . . . . . . . 2
2. Asexual morph absent; if present conidiophores simple, short; ascospores without germ pores . . . . . . . . 3
2. Asexual morph always present, conidiophores often branched up to 80 μm long; ascospores with a germ pore . . . . . . . . . . . . . . . . . . . Microascus
3. Ascomata peridium of textura angularis; asexual morph when present forming short, hyaline and single annellides . . . . . . . . . . . . . . . . Pithoascus
3. Ascomata peridium of textura epidermoidea; asexual morph usually abundant, forming long annellides from short swollen conidiophores, darkening with time . . . . . . . . . . . . . . . . . . . . . Pseudoscopulariopsis
Key to Microascus species
1. Ascomata present in culture . . . . . . . . . . . . . . . . 2
1. Ascomata absent in culture . . . . . . . . 14
2. Growth at 40 °C . . . 3
2. No growth at 40 °C . . . . . . . . . . . . 10
3. Peridium with textura angularis . . . . . . . . . . . . . . . . . . . 4
3. Peridium with textura intricata . . . . . . . . . . . M. intricatus
4. Ascospores always triangular or quadrangular . . . . . 5
4. Ascospores reniform to broadly lunate, rarely triangular . 8
5. Ascospores with rounded ends . . . . . . . . .6
5. Ascospores with attenuated (pointed) ends M. pyramidus
6. Ascospores 5–6.5 × 5.5–7.5 μm . . . . . . M. macrosporus
6. Ascospores narrower . . . . . . . . . . . . . .7
7. Ascospores 4–6 × 3–5 μm, yellow in mass . M. alveolaris
7. Ascospores 6–7 × 4–4.5 μm elongated to one side, yellow to orange in mass . . . . . . . . . . . . . . . M. campaniformis
8. Colonies dull to olive-green; ascospores lunate 4.5–6.5 × 2–4 μm; conidiophores irregularly branched . . M. gracilis
8. Colonies light to brown-grey; ascospores reniform to broadly lunate 4–6 × 2.5–4 μm; conidiophores usually simple . . . . . . . . . . . . . . . . . . . . . . . . 9
9. Ascomata up to 300 μm diam; ascospores pale red-brown in mass; conidia 2–3 μm wide . . . . M. cinereus
9. Ascomata less than 230 μm diam; ascospores straw coloured; conidia 4–6 μm wide . . . . . . . . . M. cirrosus
10. Ascospores triangular 4–5 × 3–4 μm . . M. trigonosporus
10. Ascospores otherwise . . . . . . . . . . . . . . . . . . . . . . . . . 11
11. Ascospores reniform . . . . . . . . . . . . . . . . . . . . . . 12
11. Ascospores broadly ovoid to ellipsoidal . . . . . . . . . . 13
12. Ascospores 7–9 × 2–3 μm, straw coloured, yellow in mass, with a protuberant germ pore . . . . . . . . M. senegalensis
12. Ascospores 3–4 × 2–3.5 μm, hyaline to subhyaline with an indistinct germ pore . . . . . . . . . . . . . . . M. longirostris
13. Ascospores ellipsoidal 5–7 × 2–3 μm, light brown to brown in mass . . . . . . . . . . M. brunneosporus
13. Ascospores broadly ovoid 3.5–5 × 2–3.5 μm, pale yellow in mass . . . . . . . . . . . . . . . . . . . . . . . . . . . . . M. hyalinus
14. Growth at 35 °C . . . . . . . . . . . . . . . . . 15
14. No growth at 35 °C . . . . . . . . . . . . . . 19
15. Colonies fast growing (> 60 mm in 14 d); conidia bulletshaped to broadly clavate, finely roughened 4–8 × 2.5–3.5 μm . . . . . . . M. expansus
15. Colonies growing restrictedly; conidia otherwise . . . . . 16
16. Colonies finely granular, olive-grey; conidiophores sparsely warted . . . . . . . . . . . . . . . M. verrucosus
16. Colonies downy to velvety, dark brown-grey; conidiophores smooth-walled . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
17. Growth at 40 °C; conidia globose to obovoidal 4.5–6 × 4–5.5 μm, thick- and rough-walled . . . . . . M. restrictus
17. No growth at 40 °C; conidia smooth-walled . . . . 18
18. Conidia broadly ellipsoidal to short clavate 4–6 × 2–4.5 μm with rounded apex . . . . . . . . . . . M. paisii
18. Conidia cylindrical, 4–6 × 1.5–2 μm, usually with a pointed apex . . . . . . . . . . . . . . . M. murinus
19. Conidia globose, 3.5–5 × 3–4 μm, brown coloured; slow growth at 5 °C . . . . . . . . . . . . . . . . M. croci
19. Conidia ovate, usually with a pointed apex, 4–5.5 × 3–4 μm, green-brown; no growth at 5 °C . . . . . M. chartarus
Key to Pithoascus species
1. Colonies black, velvety or powdery; sexual morph not formed in culture; asexual morph abundant showing conidia globose to pyriform, 4–9 × 4.5–8.5 μm . . . . . P. ater
1. Colonies light grey to black, becoming crustose by the formation of ascomata; asexual morph scarce or absent . . . . . . . . . . . . . . . .2
2. Ascomata 210–450 μm diam; ascospores falcate with attenuated ends, up to 12 μm long . . . . . . . . . P. exsertus
2. Ascomata less than 170 μm diam; ascospores otherwise . . . . . . . . . . . . . . . . . . . . . . . 3
3. Ascomata 50–110 μm diam; ascospores navicular, 6–7.5 × 2–3 μm, golden yellow to brown coloured . . P. stoveri
3. Ascomata 90–160 μm diam; ascospores fusiform . . .4
4. Ascospores fusiform 5–6 μm long, honey coloured; asexual morph when present forming globose to subglobose hyaline conidia 4–8 × 4–7.5 μm . . . . . . . . P. intermedius
4. Ascospores fusiform, nearly lunate 6–8 μm long, subhyaline to straw coloured; asexual morph when present forming globose to ampulliform hyaline conidia 4–5 × 2.5–3.5 μm on short stalks . . . . . . .P. nidicola
Key to Pseudoscopulariopsis species
1. Colonies grey-white; conidia obovate or short clavate, 4.5–6 × 2.5–4 μm; sexual morph present in culture, with pale brown fusiform or navicular ascospores, measuring 8–13 × 2.5–4 μm . . . . . . . . . . .P. schumacheri
1. Colonies olive-grey; conidia subglobose 5–7 × 5–6 μm; sexual morph absent . . . . . . . . . . P. hibernica
Key to Scopulariopsis species
1. Colonies white, tending to light grey when ascomata are present . . . . . . . . . . . . . . . 2
1. Colonies tan, pale brown to fuscous brown . . . . . . 5
2. Conidia smooth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2. Conidia rough at maturity . . . . . . . . . . . . . 4
3. Conidiophores abundant; conidia 6–9.5 × 5–8.5 μm; ascospores when present hyaline, heart shaped . . S. candida
3. Conidiophores scarce; conidia 2.5–6 × 2–5 μm; ascospores straw coloured, reniform to broadly lunate . . . . . . . . . . . . . . . . . . . S. cordiae
4. Conidia pale yellow in mass, globose to subglobose, 5.5–9 × 5–8 μm; sexual morph present; ascospores falcate to lunate . . . . . . . . . S. soppii
4. Conidia white in mass, globose to obovoidal, 6.5–7 × 5.5–6.5 μm; sexual morph not observed . . . . . . S. flava
5. Conidia brown in mass, verrucose at maturity, globose to ovoid; 6–9 × 5.5–9 μm . . . . . . . . . S. brevicaulis
5. Conidia dark brown to fuscous, smooth or verrucose, globose to ovate usually with a pointed apex, 5–8 × 4–6.5 μm . . . . . . . . . . . . . . . . .S. asperula
EXCLUDED OR DOUBTFUL SPECIES
Microascus albonigrescens (Sopp) Curzi, Boll. Staz. Patol. Veg. Roma 11: 60. 1931
Specimen examined. JAPAN, Hokkaido, from litter, treated with urea, 1967, S. Udagawa (IHEM 18560 = CBS 109.69).
Notes — Our phylogenetic study demonstrates that this taxon does not belong to Microascus s.str. It nested in a clade related to the genera Cephalotrichum, Gamsia and Wardomyces, and might represent the former genus Acaulium, which was typified by Sopp (1912) with Scopulariopsis albonigrescens (sexual morph M. albonigrescens). Given the absence of type material, however, we prefer not to introduce any taxonomic changes until a more accurate study of the available reference material can be carried out.
Microascus caviariformis Malloch & Hubart, Canad. J. Bot. 65: 2384. 1987
Specimen examined. BELGIUM, Prov. de Liège, Flemalle, Cave de Ramioul, from decaying meat, 1985, J.M. Hubart (ex-type culture CBS 536.87).
Notes — As in the case of M. albonigrescens, our phylogenetic data revealed that this taxon shows affinity with members of Cephalotrichum, Gamsia and Wardomyces rather than Microascus. It could represent another species of the former genus Acaulium.
Microascus decorticatus C. Ram, Nova Hedwigia 21: 226. 1972
Notes — The original description stated a close morphological relationship of this species with M. cinereus and M. gracilis. Microascus decorticatus was described having slightly larger ascomata, while the asci and ascospores are nearly identical in size and shape to those of M. cinereus. Because the ex-type culture (IMUFPe 2194) was not available for study, the taxonomy of this species remains unclear.
Microascus desmosporus (Lechmere) Curzi, Boll. Staz. Patol. Veg. Roma 11: 60. 1931
≡ Peristomium desmosporum Lechmere, Compt. Rend. Hebd. Séances Acad. Sci. 155: 178. 1912.
?= Peristomium desmosporum Lechmere var. verticillium Lechmere, Bull. Trimestriel Soc. Mycol. France: 178. 1913.
= Microascus desmosporus (Lechmere) Curzi var. macroperithecia Sage, Steiman, Seigle-Mur. & Guiraud, Mycotaxon 55: 191. 1995, nom inval. Art. 40.5 (Melbourne).
Notes — This species has a controversial taxonomy. Barron et al. (1961) recognize M. desmosporus as a species distinct from M. cirrosus while Morton & Smith (1963) regarded both species as conspecific. However, the ex-type strain of M. cirrosus was not examined by the latter authors. Von Arx (1975) and von Arx et al. (1988) regarded M. desmosporus as a doubtful species considering that Peristomium desmosporum and M. desmosporus are based on two different fungi. In the absence of type cultures the taxonomy of these fungi remains unknown.
Microascus dimonatus Sage, Steiman, Seigle-Mur. & Guiraud, Mycotaxon 55: 195. 1995
Notes — This name is currently considered as invalid in fungal databases (Index Fungorum, MycoBank) following the Art. 40.5 of the International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). As stated in the original description, an ex-type culture of this fungus exists (CMPG 1274); however, it was not available for examination. Therefore, according to Guarro et al. (2012) further studies are necessary to clarify the taxonomy of this species.
Microascus giganteus Malloch, Mycologia 62: 731. 1970
Specimen examined. CANADA, Ontario, from insect frass in dead log, 1968, D.W. Malloch (ex-type culture CBS 746.69 = UAMH 9425).
Notes — This is a coprophilous species, originally isolated from insect dung (Malloch 1970), which resembles Microascus species in many morphological characteristics, particularly in those of the sexual state. However, it shows large (up to 750 μm) and hairy ascomata, the asci are formed irregularly at the centre of the perithecia and it has a Wardomyces asexual morph. The genus Wardomyces, typified by W. anomalus, is characterised by having swollen conidiogenous cells forming conidia in lateral or basipetal succession, the conidia are subglobose, ovoid or ellipsoid, brown to blackish and present a single longitudinal germ-slit (Brooks & Hansford 1923, Hennerbert 1962). According to our combined LSU and ITS sequence analysis (Fig. 1), M. giganteus is closely related to W. inflatus, thus the morphological features and phylogenetic evidence seem to demonstrate that M. giganteus is a Wardomyces species. However, sequence comparison with the type species of Wardomyces is required for taxonomical changes.
Microascus inopinatus Udagawa & Furuya, Mycotaxon 7: 91. 1978
≡ Wardomycopsis inopinata Udagawa & Furuya, Mycotaxon 7: 92. 1978.
Specimen examined. MYANMAR, from soil, 2008, C. Hartung (FMR 10305).
Notes — This taxon was described as unusual among the genus Microascus since its asexual morph exhibits annellated conidiogenous cells producing short catenate and globose conidia with a prominent longitudinal germ-slit. Thus, the genus Wardomycopsis was established to accommodate its asexual state. Although the ex-type culture was not available, the reference strain included in our study fits clearly with the protologue of the species. The phylogenetic analysis of the LSU and ITS sequences showed that this taxon was located far from the Microascus clade forming a highly supported clade with the ex-type strain of Wardomycopsis humicola (basionym Scopulariopsis humicola CBS 487.66).
Microascus microcordiformis Matsush., Matsush. Mycol. Mem. 9: 16. 1996
Notes — The original description correlates with a Microascus species; being morphologically close to M. longirostris. However, in absence of live type material, the status of this name remains unknown.
Microascus pilosus Valldos. & Guarro, Nova Hedwigia 57: 123. 1993
Specimen examined. SPAIN, Burgos, from rabbit dung, 1986, M. Hernandez (ex-isotype FMR 2604).
Notes — No live ex-type material is available for phylogenetic analyses. An isotype specimen (FMR 2604) is preserved at the Universitat Rovira i Virgili. The morphological examination of this specimen corresponded with the original description showing that this species clearly belongs to Microascus (Guarro et al. 2012).
Microascus singularis (Sacc.) Malloch & Cain, Canad. J. Bot. 49: 859. 1971
Basionym. Fairmania singularis Sacc., Ann. Mycol. 4: 276. 1906.
= Microascus doguetii Moreau, Rev. Mycol. 18: 177.1953.
Specimen examined. JAPAN, Tokyo, laboratory contaminant, 1962, S. Udagawa (CBS 414.64).
Notes — The morphological features of the asexual morph, with conidia showing longitudinal bands, and the analysis of the LSU and ITS sequences of a reference isolate (CBS 414.64) showed that this taxon formed a phylogenetic lineage related to Wardomyces and Wardomycopsis. However, given the absence of an ex-type strain, a deeper phylogenetic analysis is required.
Microascus tardifaciens Y. Horie & Udagawa, Mycotaxon 17: 331. 1983
≡ Scopulariopsis tardifaciens Y. Horie & Udagawa, Mycotaxon 17: 331. 1983.
Notes — According to the protologue, this species is similar morphologically in colony features and its asexual morph to M. albonigrescens and S. acremonium. In this sense, our phylogenetic analysis (Fig. 1) showed that these species could probably correspond to a species of the former genus Acaulium. However, the ex-type culture (NHL 2912) of M. tardifaciens was not available for study.
Pithoascus platysporus Arx & Veenb.-Rijks, Persoonia 7: 374. 1973
Specimen examined. THE NETHERLANDS, Wageningen, from agricultural soil, date unknown, J.W. Veenbaas-Rijks (ex-type culture CBS 419.73).
Notes — This species was described showing reddish brown, broadly cylindrical ascospores that differs from the main characteristics of the genus Pithoascus. According to Abbott et al. (2002), the ascospore morphology suggests a closer affinity to Kernia or Lophotrichus. Although we were unable to obtain sporulation from the ex-type strain (CBS 419.73), the analysis of the LSU and ITS sequences showed that this taxon was phylogenetically far from the Microascales and was closely related with the Hypocreales.
Scopulariopsis acremonium (Delacr.) Vuill., Bull. Soc. Mycol. France 27: 148. 1911
Basionym. Monilia acremonium Delacr., Soc. Mycol. Fr. 13: 114. 1897.
= Scopulariopsis communis Bainier, Bull. Soc. Mycol. France 23: 125.1907.
= Penicillium scopulariopsis Sacc., Syll. Fung. 22: 1275. 1913.
= Oospora glabra Hanzawa, J. Coll. Agric. Tohuko Imper. Univ. 4: 1912.
= Scopulariopsis candelabrum Loubière, Rech. Struct. Mucor. (Thesis), Paris: 63. 1924.
= Penicillium brevicaule Sacc. var. glabrum Thom, Bull. U.S. Bur. Anim. Ind. 118: 48. 1910.
≡ Scopulariopsis brevicaulis (Sacc.) Bainier var. glabra (Thom) Thom in The Penicillia: 250. 1930.
= Scopulariopsis danica J.F.H. Beyma, Zentralbl. Bakteriol., 2 Abt. 99: 390. 1939.
Specimens examined. DENMARK, from horse skin infected with Trichophyton sp., 1938, C. Werdelin (Scopulariopsis danica ex-type culture MUCL 9028). – GERMANY, from wheat field soil, 1963, W. Gams (MUCL 8274); from soil, collector unknown (MUCL 8409).
Notes — This species was transferred to Scopulariopsis from Monilia acremonium by Vuillemin (1911). No ex-type strain of M. acremonium exists. This taxon was later considered conspecific with Scopulariopsis danica by Morton & Smith (1963), from which an ex-type culture (MUCL 9028) was available. Our phylogenetic and morphological studies seem to confirm this synonymy; however, this species is phylogenetically distant from Scopulariopsis and related to the genera Cephalotrichum, Gamsia, Trichurus and Wardomyces, clustering with a reference strain of S. albonigrescens and probably corresponding to a species of the former genus Acaulium (see M. albonigrescens).
Scopulariopsis argentea Szilvinyi, Zentralbl. Bakteriol., 2 Abt. 103: 173. 1941
Notes — Type material was studied by Morton & Smith (1963) and considered as ‘unidentifiable’. The protologue suggest a species of Paecilomyces. No living ex-type material is available.
Scopulariopsis bertaccini Redaelli, Giorn. Ital. Derm. Syph. 75: 825. 1934
Notes — Type material was studied by Morton & Smith (1963), and considered not to be a Scopulariopsis species. No living type material is available.
Scopulariopsis canadensis F.J. Morton & G. Sm., Mycol. Pap. 86: 55. 1963
Specimen examined. CANADA, British Columbia, from seed of Beta vulgaris, 1958, S.J. Hughes (ex-type culture CBS 204.61).
Notes — Our phylogenetic data showed that the ex-type culture (CBS 204.61) is related to the Xylariales.
Scopulariopsis carbonaria F.J. Morton & G. Sm., Mycol. Pap. 86: 59. 1963
Specimen examined. PANAMA, from soil, 1961, R. Coghill (ex-type culture MUCL 9027 = CBS 205.61).
Notes — Our phylogenetic data on LSU, ITS, EF-1α and TUB showed that the ex-type strain (MUCL 9027) formed an isolated lineage basal to the Microascus and Pithoascus clades. However, the ex-type culture was sterile, impeding further comparisons.
Scopulariopsis castellanii M. Ota & Komaya, Dermatol. Wochenschrift 78: 163. 1924
Notes — Morton & Smith (1963) considered its original description as ‘unidentifiable’. Since no living type material exists, the identity of this taxon remains unknown.
Scopulariopsis coprophila (Cooke & Massee) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 207. 1971
Basionym. Monosporium coprophilum Cooke & Massee, Grevillea 16: 10. 1887.
= Monilia fimicola Costantin & Matr., Rev. Gén. Bot. 6: 292. 1894.
≡ Oospora fimicola (Costantin & Matr.) Cub. & Megliola, C. R. Accad. Lincei: 440. 1903.
≡ Scopulariopsis fimicola (Costantin & Matr.) Vuill., Bull. Soc. Mycol. France 27: 143. 1911.
Specimen examined. UK, from mushroom bed, 1946, C.J. La Touche (as Scopulariopsis fimicola MUCL 9030 = CBS 206.61).
Notes — Originally described as Monosporium coprophilum, this species was transferred to Scopulariopsis by Gams (1971) who also considered it to be conspecific with Monilia fimicola. The latter taxon had been previously transferred to Scopulariopsis by Vuillemin (1911) as Scopulariopsis fimicola, a species that was regarded as valid by Morton & Smith (1963); however, these authors did not consider M. coprophilum, whose oldest epithet has priority over fimicola. According to their observations, S. fimicola shares colonial and micromorphological characteristics with Scopulariopsis baarnensis, both species being included in different series of Scopulariopsis (Morton & Smith 1963). The latter species was transferred to the genus Gliomastix, as G. murorum var. polychroma by Dickinson (1968), currently named Gliomastix polychromum (Bionectriaceae, Hypo-creales) (Summerbell et al. 2011).
Although no ex-type culture of S. coprophila is available, two reference strains were included in this study, one of them (MUCL 9641) was reidentified here as M. trigonosporus. Interestingly, the second strain (CBS 206.61), according to its LSU and ITS sequences it is phylogenetically related with members of Bionectriaceae.
Scopulariopsis finkii Sartory & R. Sartory ex Vuill., Encycl. Mycol. 2: 65. 1931
Notes — Original description of this fungus is too vague and inadequate for recognition of the species (Morton & Smith 1963). Type material does not exist.
Scopulariopsis halophilica Tubaki, Trans. Mycol. Soc. Japan 14: 367. 1973
Specimen examined. JAPAN, Osaka, from Undaria pinnatifida, 1974, K. Tubaki (ex-type culture CBS 380.74).
Notes — This name was considered by Pitt & Hocking (1985) to be a synonym of Basipetospora halophila, a fungus formerly described as Oospora halophila by van Beyma (1933). Recently, Samson et al. (2014) transferred this species to the genus Aspergillus under the new name A. baarnensis. Our phylogenetic data on LSU and ITS confirmed those results, showing its relationships with the Eurotiales.
Scopulariopsis hanii Moustafa & Abdul-Wahid, Nova Hedwigia 51: 476. 1990
Notes — According to the protologue, this fungus is morphologically compatible with the asexual morph of Microascus and should be considered as member of this genus. In many aspects, this species resembles M. restrictus and M. verrucosus from which it can be differentiated by having annellides, conidia and solitary sessile conidia at least two times larger. The protologue indicates that holotype material was deposited in the Herbarium of the Royal Botanic Gardens (IMI 326933). However, this accession number corresponds to an isolate of Scopulariopsis croci. No living culture of S. hanii was available for study.
Scopulariopsis lanosa J.F.H. Beyma, Zentralbl. Bakteriol., 2 Abt. 99: 423. 1937
Notes — This species was excluded from the genus by Morton & Smith (1963). No ex-type strain is available.
Scopulariopsis lilacea Szilvinyi, Zentralbl. Bakteriol., 2 Abt. 103: 174. 1941
Notes — Morton & Smith (1963) regarded the species as ‘unidentifiable’. The illustrations included in the protologue seem to represent a conidial apparatus similar to Fusarium, while none of the described structures fits with those of Scopulariopsis. No live cultures were available.
Scopulariopsis lingualis Neto bis & C. Martins, Compt. Rend. Seanc. Soc. Biol. 106: 1179. 1931
Notes — Morton & Smith (1963) regarded the species as ‘unidentifiable’. No living type material was available.
Scopulariopsis longipes H.Q. Pan & T.Y. Zhang, Mycosystema 33: 2. 2014
Notes — This species was recently described based only on morphological features but no phylogenetic study was carried out. Unfortunately, the type material listed in the protologue (holotype HMAS 196252, dried culture HSAUP II 074334) is not available for comparison. According to the authors, S. longipes morphologically resembles S. fusca (syn. S. asperula), but differs in having smaller conidia (3.5–5 μm wide vs 5–8 μm in S. fusca).
Scopulariopsis maduramycosis Q.T. Chen, Chin. Med. J. 99: 378. 1986
Notes — This name has been invalidated since it was published without an adequate description or holotype information.
Scopulariopsis mencieri C.W. Dodge, Medical Mycology. Fungous diseases of men and other mammals: 648. 1935
Notes — According to Morton & Smith (1963) the description is inadequate. No living type material is available.
Scopulariopsis minima Sartory, Hufschm. & J. Mey., Bull. Acad. Méd. Paris, sér. 3, 103: 606. 1930
Notes — This species is not a Scopulariopsis according to Morton & Smith (1963). No living material is available.
Scopulariopsis mottai Vuill., Encycl. Mycol. 2: 62. 1931
Notes — Considered as a doubtful species by Dodge (1935), and probably not a Scopulariopsis according to Morton & Smith (1963). No living material is available.
Scopulariopsis musae Matsush., Matsush. Mycol. Mem. 5: 27. 1987
Notes — The protologue of this species has morphological features that could correspond with an asexual state of Microascus. However, it shows asymmetrical, curved and extremely large conidia, features that do not match with the typical characteristics of the genus. No living culture is available for study.
Scopulariopsis nicotianae J.F.H. Beyma, Zentralbl. Bakteriol., 2 Abt. 91: 354. 1933
Notes — According to Morton & Smith (1963) the fungus probably belongs to a fungal genus different from Scopulariopsis. No living material is available.
Scopulariopsis nivea Demelius, Verh. Zool.-Bot. Ges. Wien 66: 490. 1916
Notes — According to the original description the fungus might represent a Scopulariopsis species; however, the description is too vague. No living specimen is available.
Scopulariopsis olivacea Szilvinyi, Zentralbl. Bakteriol., 2 Abt. 103: 174. 1941
Notes — Description and illustrations of the protologue seem to indicate that this is a Penicillium species. No living cultures are available.
Scopulariopsis parva (A.H.S. Br. & G. Sm.) Samson, Stud. Mycol. 6: 102. 1974
Basionym. Paecilomyces parvus A.H.S. Br. & G. Sm., Trans. Brit. Mycol. Soc. 40: 58. 1957.
Specimen examined. CANADA, Alberta, from soil, 1961, J.W. Carmichael (Scopulariopsis parvula ex-type culture MUCL 9041 = CBS 209.61).
Notes — This species was originally described as Scopulariopsis parvula by Morton & Smith (1963). Samson (1974) con-sidered this taxon as synonym of the older species Paecilomyces parvus, transferred to Scopulariopsis since it produces conidia with a truncate base, thus the new combination S. parva was established. However, the analysis of the LSU and ITS sequences of the ex-type culture (basionym Scopulariopsis parvula MUCL 9041) showed that this fungus is related with the Eurotiomycetes.
Scopulariopsis penicillioides H.Q. Pan, Y.L. Jiang, H.F. Wang & T.Y. Zhang, Mycosystema 33: 3. 2014
Notes — This name is an illegitimate later homonym of Scopulariopsis penicillioides (Delacroix) Smith & Ramsbottom (1915), a species considered a synonym of S. brevicaulis in Morton & Smith (1963), but not listed in the repositories for fungal names such as Index Fungorum or MycoBank. Comparing the original descriptions of these two fungi we conclude that they are different species. While S. penicillioides (Delacroix) Smith & Ramsbottom, a species based on Monilia penicillioides Delacroix (1897), shows pale-yellow, oval and echinulate conidia, the fungus described by Pan et al. (2014) has pale-brown, ellipsoidal to broadly obovoid and smooth-walled conidia. The latter fungus resembles Pseudoscopulariopsis hibernica but mainly differs in having narrower conidia (3–4.5 μm vs 5–6 μm in P. hibernica). The molecular study of these fungi has not been carried out because the type material listed in the protologue of S. penicillioides (holotype HMAS 196253, dried culture HSAUP II 074299) is not available for comparison.
Scopulariopsis polychromica Szilvinyi, Zentralbl. Bakteriol., 2 Abt. 103: 175. 1941
Notes — According to Morton & Smith (1963) the fungus is unrecognisable from the original description. The original illustrations seem to represent a ‘degenerated’ strain. A type does not exist.
Scopulariopsis rosacea Szilvinyi, Zentralbl. Bakteriol., 2 Abt. 103: 175. 1941
Notes — ‘Unidentifiable’ according to Morton & Smith (1963). No living material is available.
Scopulariopsis rubellus Bainier, Bull. Soc. Mycol. France 23: 104. 1907
Notes — Inadequately described according to Morton & Smith (1963). An ex-type strain does not exist.
Scopulariopsis sehnsuchta Mello, Bull. Soc. Pathol. Exot. 25: 296. 1932
Notes — This fungus was poorly described according to Morton & Smith (1963). No living material is available.
Scopulariopsis silvatica (Oudem.) Apinis, Nova Hedwigia 5: 73. 1963
Basionym. Spicaria silvatica Oudem., Arch. Néerl. Sci., sér. 2, 7: 291. 1902.
Notes — The description of S. silvatica (Apinis 1962) and the illustrations of the basionym Spicaria silvatica, seem to represent a fungus not belonging to Scopulariopsis, showing a phialidic conidiogenesis with abundant intercalary phialides. No living material is available for examination.
Scopulariopsis spinosa E. Müll. & Pacha-Aue, Nova Hedwigia 18: 161. 1970
Notes — The original illustrations of the fungus suggest the conidial apparatus typical of a Penicillium species. No living culture is available.
Scopulariopsis sputicola (Galippe) C.W. Dodge, Medical Mycology. Fungous diseases of men and other mammals: 648. 1935
Basionym. Monilia sputicola Galippe, J. Anatomie 21: 538. 1885.
Notes — ‘Unidentifiable’ according to Morton & Smith (1963). No living material is available.
Scopulariopsis tritici K.B. Deshp. & K.S. Deshp., Curr. Sci. 34: 222. 1965
Notes — The original description and illustration seem to belong to a species of Stachybotrys or related taxa. According to the authors of the species, the holotype and an ex-type culture were deposited in the Herbarium Cryptogamae India Orientalia (New Delhi) and in the Herbarium of the Botany Department, Marathwada University (Aurangabad) from India. However, no records of this species were found in the respective catalogues.
Scopulariopsis venerei Greco, Origine des Tumeurs (Etiologie du Cancer, etc.) et Observations de Mycoses (Blastomycoses, etc.) Argentines (Buenos Aires): 716. 1916
Notes — Morton & Smith (1963) considered this fungus as a possible species of Botrytis. No living material is available.
Scopulariopsis verticillioides Kamyschko, Notul. Syst. Sect. Cryptog. Inst. Bot. Acad. Sci. U.S.S.R. 14: 225. 1961
Notes — Living material of this species is not available.
Scopulariopsis verrucaria (‘verrucifera’) H.F. Wang & T.Y. Zhang, Mycosystema 33: 4. 2014
Notes — This species was recently described based only on morphological features. This has not been included in our phylogenetic study because the type material listed in the protologue (holotype HMAS 196254, dried culture HSAUP II 064334) is not available for comparison. According to the original description and illustration of the species, it is similar to Microascus verrucosus. However, S. verrucaria differs in having dark brown colonies, and conidia of 3–5 μm wide covered by a gelatinous membrane, while M. verrucosus has olive grey colonies and rough, somewhat wider conidia (5–7 μm).
Scopulariopsis vignololutatii (Matr.) C.W. Dodge, Medical Mycology. Fungous diseases of men and other mammals: 650. 1935
Basionym. Acaulium vignololutatii Matr. (as Vignoli-Lutatii), in Vignolo-Lutati, Arch. Derm. Syph., Berlin 118: 690. 1913.
Notes — Dodge (1935), although with no strong conviction due to its inadequate description, transferred Acaulium vignolilutatii to Scopulariopsis. Morton & Smith (1963) considered this species as a possible member of the Scopulariopsis brumptii series, but with an inadequate description for recognition of the fungus. The original description and illustration seem to refer to a fungus morphologically close to M. paisii. No living cultures are available for study.
Scopulariopsis yunnanensis Q.T. Chen & C.L. Jiang, Acta Mycol. Sin. 4: 167. 1985
Notes — The original description seems to refer to a Scopulariopsis species. However, it was described forming bifurcate chains of conidia. In addition, the conidia are smaller than those of the currently known Scopulariopsis species. According to the authors (Jiang et al. 1985), S. yunnanensis is similar to S. parva, a species that according to our results is phylogenetically related with the Eurotiomycetes. The ex-type strain of this species is not available.
DISCUSSION
In this study we have reviewed the taxonomic circumscription of Microascus and Scopulariopsis, traditionally referred to as sexual and asexual morphs, respectively, and related genera using a polyphasic approach based on the evaluation of molecular, physiological and morphological data. These results show that Microascus and Scopulariopsis constitute two phylogenetically distant lineages, which are clearly different from Pithoascus, a genus revalidated in the present work, and from the lineage proposed here as the novel genus Pseudoscopulariopsis. Furthermore, combining the results of a multilocus sequence analysis and phenotypic data, we were able to delineate the accepted species of the four genera, proposing several new ones.
One of the first attempts to clarify phylogenetically the relationships among the different genera of the Microascales by the use of partial LSU sequences was that of Issakainen et al. (2003). That study demonstrated polyphyly of several genera of Microascaceae and raised questions concerning correct positions of several members of the family and their generic circumscriptions, suggesting a possible subdivision of Microascus and Scopulariopsis into several smaller genera. However, the LSU fragments used were too small and poorly informative, thus no final conclusions were made. Nevertheless, our phylogenetic analysis based on combined, longer LSU and ITS sequences proved to be useful to resolve topology of the different genera in the Microascacae. Confirming the findings of Issakainen et al. (2003), we demonstrated that Microascus and Scopulariopsis are clearly polyphyletic and that their currently accepted species are grouped in at least seven different lineages. In addition, the combined LSU and ITS phylogeny showed that the dry-spored synnematous genera Cephalotrichum, Doratomyces, Stysanus, and Trichurus are conspecific, which agrees with Abbott (2000) who synonymised and integrated these four genera under the name Cephalotrichum. However, given the lack of ex-type strains for most of the species, no formal decision is made at the moment. The genus Cephalotrichum, as well as several other genera of the Microascaceae, such as Kernia, Wardomyces and Wardomycopsis, have been considered in the past as probably congeneric with Scopulariopsis or Microascus (Morton & Smith 1963, Abbott 2000). Our phylogeny demonstrates that, although these genera share similar morphological and ecological traits, they are in fact genetically distant. The phylogenetic data is supported by relevant morphological differences, such as the presence of germ slits, synnemata or conspicuously hairy ascomata (Abbott 2000, Issakainen et al. 2003).
The recognition of Pithoascus as a valid genus has always been a matter of discussion. Probably one of the strongest reasons to synonymise the genus with Microascus was the publication of the species M. caviariformis by Malloch & Hubart (1987), a fungus exhibiting intermediate characteristics between Microascus and Pithoascus. Microascus caviariformis showed the typical ascospores of Pithoascus, although with an inconspicuous germ pore, and produced abundant conidia. Our study demonstrated that both genera were clearly separated, thus confirming the original observations made by Skou (1973) and von Arx (1973a, b). Moreover, our data showed that M. caviariformis was located outside the main clades, which represent the genera Microascus, Pithoascus, Scopulariopsis and Pseudoscopulariopsis, forming a distant and strongly supported clade with S. acremonium and a reference strain of M. albonigrescens. The latter fungus was described as the sexual morph of the type species of Acaulium (Sopp 1912), and this genus was considered as congeneric with Microascus (Curzi 1930, Barron et al. 1961, Morton & Smith 1963). However, our phylogenetic analysis suggests that the old genus Acaulium might be revalidated. Considering that neither original cultures of Sopp, nor an ex-type strain of M. albonigrescens were available for analysis, we think it is best to not propose further taxonomic changes at the moment. Furthermore, M. singularis proved to be related to the putative Acaulium clade, which also constituted a new lineage among the Microascaceae. More study is needed on members of these clades and their closest phylogenetic relatives.
Although the morphological differences distinguishing the genera treated here are subtle, they correlate with the phylogenetic data, shape, size and colour of annellides and conidia, shape of ascospores and presence of germ pores being the most informative morphological characters (Table 2). Other features, such as shape and size of ascomata and number and shape of ostiolar necks, are frequently associated with environmental changes related to incubation conditions (Barron et al. 1961). On the basis of our data, we recommend using PCA or OA culture media to achieve the best growth and sporulation ratio. On PCA these fungi produce fast and abundant sporulation but some species, particularly those with little growth of their asexual morphs, might sporulate better on OA. It is most likely that variable morphological differences observed in the past might have led to incorrect identification of strains due to overlapping characteristics between closely related species. For example, two strains (MUCL 9048 and MUCL 9049) that were received as M. cinereus were finally reidentified as belonging to the undescribed sexual morph of M. gracilis, a species morphologically close to M. cinereus but differentiated by size and complexity of conidiophores and shape of ascospores (Table 2). Barron et al. (1961) and Udagawa (1962) mentioned a wide variation in size and shape of ascospores of isolates identified as M. trigonosporus, i.e. from triangular to nearly quadrangular, with some isolates showing spores distinctly longer at one side, but judging from our data this ascospore shape variation might correspond to different phylogenetic species. Most of the species of Microascus newly described here showed triangular ascospores of variable size. The new species M. campaniformis produced inequilateral spores with the longer side towards the germ pore, although the measurements do not coincide with those previously described by the authors mentioned above.
Table 2.
Relevant phenotypic features of members of Microascus, Pithoascus, Pseudoscopulariopsis and Scopulariopsis.
| Species | Colony | Sexual morph | Asexual morph | Ascomata diam (μm) | Ascospore size (μm) | Ascospore shape | Germ pore | Conidial size (μm) | Conidial shape | Growth at (° C) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 25 | 30 | 35 | 40 | ||||||||||
| Microascus | ||||||||||||||
| M. alveolaris | white to grey | + | + | 110–290 | 4–6 × 3–5 | broadly triangular | + | 3–5 × 2–3.5 | ellipsoidal to bullet shaped | – | + | + | + | + |
| M. brunneosporus | dull green to olive brown | + | + | 110–205 | 5–7 × 2–3 | ellipsoidal | + | 4–5 × 2.5–5 | subglobose to navicular | – | + | + | + | – |
| M. campaniformis | dull green | + | + | 150–220 | 6–7 × 4–4.5 | triangular with an elongated side | + | 4–5 × 2.5–3.5 | subglobose to broadly ellipsoidal | – | + | + | + | + |
| M. chartarus | grey to smoke grey | – | + | 4–5.5 × 3–4 | ovate | – | + | – | – | – | ||||
| M. cinereus | light to dark grey | + | + | 95–300 | 4–5.5 × 2.5–4 | reniform, broadly lunate, rarely triangular | + | 3–5 × 2–3 | obovate or clavate | – | + | + | + | + |
| M. cirrosus | brown-grey | + | + | 140–230 | 5–6 × 3–4 | broadly reniform | + | 4–6.5 × 4–6 | subglobose to obovate | – | + | + | + | + |
| M. croci | grey to mouse grey | – | + | 3.5–5 × 3–4 | globose | + | + | + | – | – | ||||
| M. expansus | olive to grey-brown | – | + | 4–8 × 2.5–3.5 | bullet shaped to broadly clavate | + | + | + | + | + | ||||
| M. gracilis | dull to olive green | + | + | 130–300 | 4.5–6.5 × 2–4 | lunate | + | 3.5–5.5 × 2–3.5 | subglobose or ellipsoidal | – | + | + | + | + |
| M. hyalinus | dark grey | + | + | 50–180 | 3.5–5 × 2–3.5 | broadly ovoid | + | 3.5–5 × 2–3.5 | ovoid | – | + | + | + | – |
| M. intricatus | white to olive grey | + | + | 140–200 | 5–6 × 2.5–3.5 | fusiform | + | 4–5 × 3–3.5 | globose to broadly ellipsoidal | – | + | + | + | + |
| M. longirostris | grey-black to brown-black | + | + | 180–300 | 3–4 × 2–3.5 | reniform | + | 4–6 × 3–4 | obovate | – | + | + | – | – |
| M. macrosporus | grey opaque | + | + | 130–250 | 5–6.5 × 5.5–7.5 | triangular | + | 5–7 × 4–5 | globose to ovoid | – | + | + | + | + |
| M. murinus | pale to dark grey | – | + | 4–6 × 1.5–2 | cylindrical | – | + | + | + | – | ||||
| M. paisii | grey-brown grey-black | – | + | 4–6 × 2–4.5 | broadly ellipsoidal to short clavate | – | + | + | + | – | ||||
| M. pyramidus | grey to violaceus grey | + | + | 125–250 | 5–6.5 × 5.5–7 | triangular or quadrangular with attenuated ends | + | 4.5–5.5 × 3–4 | obovate | – | + | + | + | + |
| M. restrictus | olive to brown-grey | – | + | 4.5–6 × 4–5.5 | globose to obovoidal | – | + | + | + | + | ||||
| M. senegalensis | pale brown | + | + | 180–250 | 7–9 × 2–3 | reniform | + | 4.5–5.5 × 3.5–4 | obovate | – | + | + | + | – |
| M. trigonosporus | grey | + | + | 130–250 | 3–5 × 3–4 | triangular | + | 2.5–3.5 × 3.5–5.5 | globose to ovoid | – | + | + | + | – |
| M. verrucosus | olive grey | – | + | 5–7 × 4.5–6 | globose to subglobose | – | + | + | + | – | ||||
| Pithoascus | ||||||||||||||
| P. ater | brown-black | – | + | 4–9 × 4.5–8.5 | globose to pyriform | + | + | + | + | – | ||||
| P. exsertus | white | + | – | 210–450 | 6.5–12 × 1–2.5 | falcate | – | – | + | + | + | + | ||
| P. intermedius1 | white to light grey | + | + | 95–150 | 5–6 × 2–2.5 | fusiform | – | 4–8 × 4–7.5 | globose to subglobose | – | + | + | + | – |
| P. nidicola | white to light grey | + | + | 90–160 | 6–8 × 2–2.5 | fusiform, nearly lunate | – | 4–5 × 2.5–3.5 | globose to ampulliform | – | + | + | + | + |
| P. stoveri2 | light to dark grey | + | + | 50–110 | 6–7.5 × 2–3 | navicular | – | 5–8 × 3–4 | obovate to pyriform | – | + | + | – | – |
| Pseudoscopulariopsis | ||||||||||||||
| P. hibernica | olive grey | – | + | 5–7 × 5–6 | subglobose | – | + | – | – | – | ||||
| P. schumacheri3 | grey-white | + | + | 140–190 | 8–13 × 2.5–4 | fusiform or navicular | – | 4.5–6 × 2.5–4 | obovate, short clavate | – | + | – | – | – |
| Scopulariopsis | ||||||||||||||
| S. asperula4 | dark brown | + | + | 130–190 | 4.5–6.5 × 3.5–4 | broadly reniform | 5–8 × 4–6.5 | globose to ovate | – | + | + | + | – | |
| S. brevicaulis | tan | + | + | 70–150 | 5–6 × 3.5–4.5 | broadly reniform | + | 6–9 × 5.5–9 | globose to ovate | – | + | + | + | + |
| S. candida | white | + | + | 100–170 | 4–6 × 5–6 | reniform, heart shaped | + | 6–9.5 × 5–8.5 | subglobose to broadly ovate | – | + | + | + | – |
| S. cordiae | white to light grey | + | + | 100–150 | 4.5–5.5 × 3.5–4 | reniform | + | 2.5–6 × 2–5 | broadly ellipsoidal to obovoidal | – | + | + | + | + |
| S. flava | white | – | + | 6.5–7 × 5.5–6.5 | globose to obovoidal | – | + | + | + | – | ||||
| S. soppii | white | + | + | 130–200 | 6–7 × 2.5–3 | lunate | + | 5.5–9 × 5–8 | globose to subglobose | – | + | + | + | – |
1 Asexual morph data obtained from Roberts (1985).
2 Asexual morph data obtained from von Arx et al. (1988).
3 Sexual morph data obtained from von Arx et al. (1988).
4 Sexual morph data obtained from Abbott & Sigler (2001).
One of the main objectives of the present work was to assess the phylogenetic relationships among members of Microascus and Scopulariopsis in order to comply with the requirements of the new International Code of Nomenclature for fungi, algae and plants (Hawksworth et al. 2011). As has been discussed extensively by Hawksworth (2012), for this particular dual-name combination an option might be to retain the most ‘widely-used’ name. Accordingly, Scopulariopsis should have priority over Microascus, primarily because of the abundant medical literature on this genus (Hawksworth 2012, Sandoval-Denis et al. 2013). Our proposal to separate both genera is an alternative approach that maintains the names of the most relevant species of each genus, including those of the species that are significant in medicine as well as some important plant pathogens.
Acknowledgments
We thank Chantal Planard and Marijke Hendrickx (Belgian Coordinated Collections of Microorganisms, BCCM/IHEM), Lynne Sigler and Connie F.C. Gibas (University of Alberta Microfungus Collection and Herbarium, UAMH) for providing cultures. This study was in part supported by the Spanish Ministerio de Economía y Competitividad, grant CGL 2011-27185.
REFERENCES
- Abbott SP. 2000. Holomorph studies of the Microascaceae (PhD dissertation). Edmonton, Alberta, University Alberta. [Google Scholar]
- Abbott SP, Lumley TC, Sigler L. 2002. Use of holomorph characters to delimit Microascus nidicola and M. soppii sp. nov., with notes on the genus Pithoascus. Mycologia 94: 362–369. [PubMed] [Google Scholar]
- Abbott SP, Sigler L. 2001. Heterothallism in the Microascaceae demonstrated by three species in the Scopulariopsis brevicaulis series. Mycologia 93: 1211–1220. [Google Scholar]
- Abbott SP, Sigler L, Currah RS. 1998. Microascus brevicaulis sp. nov., the teleomorph of Scopulariopsis brevicaulis, supports placement of Scopulariopsis with the Microascaceae. Mycologia 90: 297–302. [Google Scholar]
- Apinis AE. 1962. Occurrence of thermophilous microfungi in certain alluvial soils near Nottingham. Nova Hedwigia 5: 57–78. [Google Scholar]
- Arx JA von. 1973a. Ostiolate and nonostiolate pyrenomycetes. Koninklijke Nederlandse Akademie van Wetenschappen, Amsterdam. [Google Scholar]
- Arx JA von. 1973b. The genera Petriellidium and Pithoascus (Microascaceae). Persoonia 7: 367–375. [Google Scholar]
- Arx JA von. 1975. Revision of Microascus with the description of a new species. Persoonia 8: 191–197. [Google Scholar]
- Arx JA von. 1978. Notes on Microascaceae with the description of two new species. Persoonia 10: 23–31. [Google Scholar]
- Arx JA von, Figueras MJ, Guarro J. 1988. Sordariaceous ascomycetes with-out ascospore ejaculation. Beihefte zur Nova Hedwigia 94: 1–104. [Google Scholar]
- Baddley JW, Moser SA, Sutton DA, et al. 2000. Microascus cinereus (anamorph Scopulariopsis) brain abscess in a bone marrow transplant recipient. Journal of Clinical Microbiology 38: 395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainier G. 1907. Mycothèque de l’École de Pharmacie, XIV. Scopulariopsis (Penicillium pro parte) genre nouveau de mucédinées. Bulletin Trimestriel de la Société Mycologique de France 23: 98–105. [Google Scholar]
- Barron GL, Cain RF, Gilman JC. 1961. The genus Microascus. Canadian Journal of Botany 39: 1609–1631. [Google Scholar]
- Beyma FH van. 1933. Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures - Baarn (Holland. Zentralblatt für Bakteriologie und Parasitenkunde Abteilung 2, 88: 132–141. [Google Scholar]
- Biourge P. 1923. Les moisissures du groupe Penicillium Link: etude monographique. La Cellule 33: 7–331. [Google Scholar]
- Brooks FT, Hansford CG. 1923. Mould growths upon cold-store meat. Transactions of the British Mycological Society 8: 113–142. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Curzi M. 1930. Una nuova specie di Microascus. Bolletino della Stazione di Patologia Vegetale di Roma 10: 302–309. [Google Scholar]
- Curzi M. 1931. Rapporti fra i generi Microascus Zukal e Scopulariopsis Bainier. Bolletino della Stazione di Patologia Vegetale di Roma 11: 55–60. [Google Scholar]
- Delacroix EG. 1897. Quelques espéces nouvelles. Bulletin de la Société Mycologique de France 13: 114–127. [Google Scholar]
- Dickinson CH. 1968. Gliomastix Guéguen. Mycological Papers 115: 1–24. [Google Scholar]
- Dodge CW. 1935. Medical mycology; fungous diseases of men and other mammals. St. Louis, The C.V. Mosby Company. [Google Scholar]
- Dominik T, Majchrowicz I. 1970. Further contribution to the knowledge of keratinolytic and keratinophilic fungi of the region of Szczecin. Keratinolytic and keratinophilic fungi in the excrements of farm animals. Ekologia Polska 18: 571–611. [Google Scholar]
- Domsch KH, Gams W, Anderson TH. 2007. Compendium of soil fungi ed 2. Eching, IHW Verlag. [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Émile-Weil P, Gaudin L. 1919. Contribution a l’étude des onychomycoses. Archives de Médecine Expérimentale et d’Anatomie Pathologique 28: 452–467. [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Gams W. 1971. Cephalosporium-artige Schimmelpilze (Hyphomycetes). Fischer Verlag, Stuttgart. [Google Scholar]
- Giraldo A, Gené J, Sutton DA, et al. 2014. Phylogenetic circumscription of Arthrographis (Eremomycetaceae, Dothideomycetes). Persoonia 32: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarro J, Gené J, Stchigel AM, et al. 2012. Atlas of soil ascomycetes. CBS Biodiversity Series. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Hansen EC. 1876. De Danske Gjøningssvampe (Fungi fimicoli Danici). Videns-kabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjøbenhavn. 38: 207–354. [Google Scholar]
- Hansford CG. 1944. Contribution towards the fungus flora of Uganda. VI. New records. Proceedings of the Linnean Society London 156: 102–124. [Google Scholar]
- Hawksworth DL. 2012. Managing and coping with names of pleomorphic fungi in a period of transition. IMA Fungus 3: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, et al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebert GL. 1962. Wardomyces and Asteromyces. Canadian Journal of Botany 40: 1203–1216. [Google Scholar]
- Hibbett DS, Taylor JW. 2013. Fungal systematics: is a new age of enlightenment at hand? Nature Reviews Microbiology 11: 129–133. [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, et al. 2011. Atlas of clinical fungi. CD-ROM version 3.1. CBS-KNAW Fungal Biodiversity Centre, Utrecht. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Issakainen J, Jalava J, Hyvönen J, et al. 2003. Relationships of Scopulariopsis based on LSU rDNA sequences. Medical Mycology 41: 31–42. [DOI] [PubMed] [Google Scholar]
- Iwen P, Schutte SD, Florescu DF, et al. 2012. Invasive Scopulariopsis brevicaulis infection in an immunocompromised patient and review of prior cases caused by Scopulariopsis and Microascus species. Medical Mycology 50: 561–569. [DOI] [PubMed] [Google Scholar]
- Jiang CI, Xu LH, Chen QT. 1985. A new species of Scopulariopsis Bainier. Acta Mycologica Sinica 4: 167–170. [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour, 3rd edition London, Methuen. [Google Scholar]
- Lackner M, Hoog GS de. 2011. Parascedosporium and its relatives: phylogeny and ecological trends. IMA Fungus 21: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner M, Hoog GS de, Yang L, et al. 2014. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Diversity 67: 1–10. [Google Scholar]
- Lumbsch HT, Huhndorf SM. 2007. Outline of Ascomycota – 2007. Myconet 13: 1–58. [Google Scholar]
- Malloch D. 1970. New concepts in the Microascaceae illustrated by two new species. Mycologia 62: 727–740. [Google Scholar]
- Malloch D, Cain RF. 1971. The genus Kernia. Canadian Journal of Botany 49: 855–867. [Google Scholar]
- Malloch D, Hubart JM. 1987. An undescribed species of Microascus from the Cave of Ramioul. Canadian Journal of Botany 65: 2384–2388. [Google Scholar]
- Malloch D, Sigler L. 1988. The Eremomycetaceae (Ascomycotina). Canadian Journal of Botany 66: 1929–1932. [Google Scholar]
- Mangan A. 1965. Scopulariopsis hibernica sp. nov. Transactions of the British Mycological Society 48: 617–620. [Google Scholar]
- Massee GE, Salmon ES. 1901. Researches on coprophilous fungi. Annals of Botany 15: 313–357. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. (eds). 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Vegetabile 146. Gantner Verlag, Ruggell. [Google Scholar]
- Mohammedi I, Piens M, Audigier-Valette C, et al. 2004. Fatal Microascus trigonosporus (anamorph Scopulariopsis) pneumonia in a bone marrow transplant recipient. European Journal of Clinical Microbiology & Infectious Diseases 23: 215–217. [DOI] [PubMed] [Google Scholar]
- Morton FJ, Smith G. 1963. The genera Scopulariopsis Bainier, Microascus Zukal, and Doratomyces Corda. Mycological Papers 86: 1–96. [Google Scholar]
- Nylander JA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- O’Donnell K. 1993. Fusarium and its near relatives. In: Reynolds DR, Taylor JW. (eds), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics: 225–233. CAB International, Wallingford. [Google Scholar]
- Pan HQ, Wang HF, Jiang YL, et al. 2014. Scopulariopsis: three new species and a key to species from soils in China. Mycosystema 33: 1–6. [Google Scholar]
- Pitt JI, Hocking AD. 1985. New species of fungi from Indonesian dried fish. Mycotaxon 22: 197–208. [Google Scholar]
- Rehner SA, Buckley E. 2005. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Roberts RG. 1985. The anamorph of Microascus intermedius Emmons & Dodge (abstract). Mycological Society of America Newsletter 36: 37. [Google Scholar]
- Ropars J, Cruaud C, Lacoste S, et al. 2012. A taxonomic and ecological overview of cheese fungi. International Journal of Food Microbiology 155: 199–210. [DOI] [PubMed] [Google Scholar]
- Saccardo PA. 1882. Fungi veneti novi vel critici vel mycologiae venetae addend. Series 13. Michelia 2: 528–563. [Google Scholar]
- Samson RA. 1974. Paecilomyces and some allied Hyphomycetes. Studies in Mycology 6: 1–119. [Google Scholar]
- Samson RA, Houbraken J, Thrane U, et al. 2010. Food and indoor fungi. CBS Laboratory Manual Series 2. CBS-Fungal Biodiversity Centre, Utrecht. [Google Scholar]
- Samson RA, Klopotek A von. 1972. Scopulariopsis murina, a new fungus from self-heated compost. Archiv für Mikrobiologie 85: 175–180. [Google Scholar]
- Samson RA, Visagie CM, Houbraken J, et al. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78: 141–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Denis M, Sutton DA, Fothergill AW, et al. 2013. Scopulariopsis, a poorly known opportunistic fungus: spectrum of species in clinical samples and in vitro responses to antifungal drugs. Journal of Clinical Microbiology 51: 3937–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA, Morgan-Jones GA, Gams W, et al. (eds). 2011. The genera of Hyphomycetes. CBS Biodiversity Series 9, CBS Fungal Diversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Skou JP. 1973. Microascus exsertus sp. nov. associated with a leaf-cutting bee, with considerations on relationships of species in the genus Microascus Zukal. Antonie van Leeuwenhoek 39: 529–538. [DOI] [PubMed] [Google Scholar]
- Smith AL, Ramsbottom J. 1915. New or rare microfungi. Transactions of the British Mycological Society 5: 156–168. [Google Scholar]
- Smith G. 1952a. Masonia, a new genus of Hyphomycetes. Transactions of the British Mycological Society 35: 149–151. [Google Scholar]
- Smith G. 1952b. Masoniella nom. nov. Transactions of the British Mycological Society 35: 237. [Google Scholar]
- Sopp OJ. 1912. Monographie der Pilzgruppe Penicillium mit besonderer Berücksichtigung der in Norwegen gefunden Arte. Videnskaps Selskapets Skrifter 1. Matematisk-Naturvidenskabelig Klasse 11: 1–207. [Google Scholar]
- Summerbell RC, Gueidan C, Schroers HJ, et al. 2011. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Studies in Mycology 68: 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa S. 1959. Taxonomic studies of fungi on stored rice grain. III. Pe-nicillium group (penicillia and related genera) 2. Journal of Agricultural Science Tokyo Nogyo Daigaku 5: 5–21. [Google Scholar]
- Udagawa S. 1962. Microascus species new to mycoflora of Japan. Journal of General and Applied Microbiology 8: 39–51. [Google Scholar]
- Udagawa S, Furuya K. 1978. A new species of Microascus and its peculiar conidial state. Mycotaxon 7: 91–96. [Google Scholar]
- Valmaseda M, Martínez T, Barrasa JM. 1986. Annellidic conidiogenesis in Pithoascus schumacheri and redefinition of Pithoascus and related fungi. Canadian Journal of Botany 65: 1802–1805. [Google Scholar]
- Vuillemin P. 1911. Différence fondamentale entre le genre Monilia et les genres Scopulariopsis, Acmosporium et Catenularia. Bulletin de la Société Mycologique de France 27: 137–152. [Google Scholar]
- Wang P, Wang H, Zhao Y, et al. 2011. A case of endocarditis caused by Micro-ascus trigonosporus. Chinese Journal of Mycology 6: 162–165. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, New York. [Google Scholar]
- Zach F. 1934. Untersuchungen über einige neue Arten der Gattung Scopulariopsis Bainier. Österreichische Botanische Zeitschrift 83: 173–186. [Google Scholar]
- Zukal H. 1885. Ueber einige neue Pilze, Myxomyceten und Bakterien. Verhandlungen der Zoologisch-Botanischen Gesellschaft Wien 35: 333–342. [Google Scholar]


