Abstract
The family Stachybotriaceae was recently introduced to include the genera Myrothecium, Peethambara and Stachybotrys. Members of this family include important plant and human pathogens, as well as several species used in industrial and commercial applications as biodegraders and biocontrol agents. However, the generic boundaries in Stachybotriaceae are still poorly defined, as type material and sequence data are not readily available for taxonomic studies. To address this issue, we performed multi-locus phylogenetic analyses using partial gene sequences of the 28S large subunit (LSU), the internal transcribed spacer regions and intervening 5.8S nrRNA (ITS), the RNA polymerase II second largest subunit (rpb2), calmodulin (cmdA), translation elongation factor 1-alpha (tef1) and β-tubulin (tub2) for all available type and authentic strains. Supported by morphological characters these data resolved 33 genera in the Stachybotriaceae. These included the nine already established genera Albosynnema, Alfaria, Didymostilbe, Myrothecium, Parasarcopodium, Peethambara, Septomyrothecium, Stachybotrys and Xepicula. At the same time the generic names Melanopsamma, Memnoniella and Virgatospora were resurrected. Phylogenetic inference further showed that both the genera Myrothecium and Stachybotrys are polyphyletic resulting in the introduction of 13 new genera with myrothecium-like morphology and eight new genera with stachybotrys-like morphology.
Keywords: biodegraders, generic concept, human and plant pathogens, indoor mycobiota, multi-gene phylogeny, species concept, taxonomy
INTRODUCTION
The family Stachybotriaceae was established by Crous et al. (2014) in the order Hypocreales (Hypocreomycetidae, Sordariomycetes, Pezizomycotina, Ascomycota) to accommodate the genera Myrothecium (Myr.), Peethambara (Pe.) and Stachybotrys (St.). These genera include approximately 210 species (www.mycobank.org; www.indexfungorum.org). The majority of these fungi are saprobic or pathogenic to plants and animals, and some pose a serious risk to human health. They are characterised by asexual morphs with mononematous to sporodochial to synnematous conidiomata, usually with phialidic conidiogenous cells that produce 0–1-septate conidia in dark green to black slimy or dry masses. Some are linked to sexual morphs with perithecial ascomata that are either semi or totally immersed in host tissue, bright to dark yellow to orange or black that do not change colour in KOH.
Past phylogenetic studies by Castlebury et al. (2004) and Summerbell et al. (2011) showed that the genera Myrothecium, Peethambara and Stachybotrys formed a well-supported monophyletic lineage distinct from the other families in the Hypocreales. Arguing that more sexual morphs are required for these genera, Castlebury et al. (2004) refrained from introducing a family for these fungi at that time. As the sexual and asexual morphs are now regarded as equal with the abolishment of dual nomenclature for fungi (Hawksworth et al. 2011, Rossman et al. 2013), Crous et al. (2014) introduced the fungal family Stachybotriaceae to accommodate these genera.
The asexual genus Myrothecium, based on Myr. inundatum, was first introduced by Tode in 1790. He described these fungi as cup-shaped fungi surrounded by a sheath with spores slowly becoming sticky, and included five species in the genus (Preston 1943, Tulloch 1972). Link (1809) emended Tode’s generic concept of Myrothecium and only retained Myr. inundatum in the genus, noting for the first time the conidia of this fungus as globose. Ditmar (1813) also described and illustrated Myr. inundatum with globose conidia, and included Myr. verrucaria (= Peziza verrucaria). Both Link (1809) and Ditmar (1813) referred to the woolly margins surrounding the conidial mass of these fungi. Fries (1829) accepted both Link and Ditmar’s generic concept of Myrothecium and included four species in the genus. Later, Saccardo (1886) introduced the genus Hymenopsis, which he distinguished from Myrothecium by the absence of a well-defined margin and included six species, which he previously included in Myrothecium. Von Höhnel (1905) compared Myrothecium to Volutella and Amerosporium, and noted that the conidia of Myr. inundatum were oblong rod-shaped, and not globose as previously recorded. At the same time, he also noted white setae at the margin of young sporodochia, not previously recognised in Myrothecium. Preston (1943, 1948, 1961) recognised six species in Myrothecium, and also provided a detailed historical survey. Pidoplichko & Kirilenko (1971) were the first to provide a key to species in the genus, which included 11 species. In the first comprehensive monographic study of Myrothecium, Tulloch (1972) recognised 12 species and provided the first link to a sexual morph, Nectria bactridioides. Samuels (1988) linked three other nectria-like species, i.e., N. chlorogloea, N. pityrodes and N. ralfsii, to Myrothecium based on the green conidia produced by the asexual morphs of these species. However, the first phylogenetic study to include Myr. inundatum by Rossman et al. (2001), disproved the link between N. pityrodes and Myrothecium. Nag Raj (1993, 1995) strongly questioned Tulloch’s broad generic concept for Myrothecium and introduced two genera, Xepicula and Xepiculopsis, for Myr. leucotrichum and Myr. gramineum, respectively. Additionally, Nag Raj (1993) also synonymised several Myrothecium species under Hymenopsis. The treatment of Myrothecium by Nag Raj (1993) was reconsidered by Seifert et al. (2003), who regarded both Xepicula and Xepiculopsis as synonyms of Myrothecium based on 28S large subunit rDNA (LSU) sequence data.
Several Myrothecium species are well-known for their cellulolytic activity (Pope 1944, White et al. 1948, Whitaker 1953, Grimes et al. 1957, Halliwell 1961, Bollenbacher & Fulton 1963, Updegraff 1971, Okunowo et al. 2010), resulting in their extensive use as standard test organisms in mould proofing textiles (Tulloch 1972) and biodegradation of waste paper (Updegraff 1971, Okunowo et al. 2010). Additionally, Myrothecium species are also known to produce a cocktail of secondary metabolites that have strong antifungal and antibiotic activity (Brian & McGowan 1946, Brian 1948, Ôkuchi et al. 1968, Gülay & Grossman 1994, Kobayashi et al. 2004, Liu et al. 2006, Xu et al. 2006, Ruma et al. 2015). Some Myrothecium species also produce macrocyclic trichothecenes, biochemical compounds being exploited for their strong cytotoxicity towards human and murine lymphocytic leukaemia and solid tumours (Murakami et al. 2001, Namikoshi et al. 2001, Amagata et al. 2003, Oda et al. 2005, Xu et al. 2006, Liu et al. 2015). Both Myr. roridum and Myr. verrucaria have also been associated with mycotoxicoses of livestock and humans (Mortimer et al. 1971, Martinovich et al. 1972, Trapp et al. 1998, Abbas et al. 2002). Thus far, only Myr. roridum and Myr. verrucaria are considered serious plant pathogens associated with dieback and leaf spots of various plant hosts (Tulloch 1972, Yang & Jong 1995, Han et al. 2014, Li et al. 2014, Ben et al. 2015, Fujinawa et al. 2015) resulting in the exploitation of these fungi as bioherbicides of weeds (Boyette et al. 2014a, b, Piyaboon et al. 2014, Weaver et al. 2016).
There have been a limited number of phylogenetic studies done that included the genus Myrothecium. Rossman et al. (2001) were able to demonstrate the close phylogenetic relationship between Myrothecium and the sexual morph Peethambara based on LSU sequence data. This was further supported by Seifert et al. (2003), Castlebury et al. (2004) and Tang et al. (2007), with the latter two studies also providing evidence of a close relationship with Stachybotrys. Decock et al. (2008) used internal transcribed spacer regions and intervening 5.8S rDNA (ITS) sequence data to distinguish Septomyrothecium from Myrothecium. This study also revealed that the genus Myrothecium is paraphyletic, but the authors did not contemplate this at that time. Surprisingly, all recent taxonomic studies of this genus (Castañeda-Ruiz et al. 2008, Alves et al. 2010, Jiang et al. 2014, Wu et al. 2014) did not include any sequence data. A general search on NCBIs GenBank (www.ncbi.nlm.nih.gov) revealed that there are several ITS and LSU sequences available for Myrothecium, as well as sequences of gene clusters associated with mycotoxin production and other secondary metabolite pathways. However, no phylogenetic study focused on the genus Myrothecium could be located.
The sexual genus Peethambara, based on Pe. sundara, was introduced by Subramanian & Bhat (1978a) linked to the asexual morph Putagraivam sundaram (Subramanian & Bhat 1978b), which was later synonymised under Didymostilbe (Di.) as Di. sundara by Seifert (1985). Rossman et al. (1998, 1999) placed Peethambara in the Bionectriaceae, allied with other asexual genera characterised by synnema and green multiseptate conidia. A second species, Pe. spirostriata (= Nectria spirostriata; Rossman 1983) was later introduced by Rossman et al. (1999) and linked to the asexual morph, Di. echinofibrosa (= Virgatospora echinofibrosa; Finley 1967) based on its phenotypic similarity to Pe. sundara.
Peethambara is characterised by yellow, globose perithecial ascomata having a synnematous asexual morph producing thick-walled, 1-septate conidia in green slimy masses (Seifert 1985, Rossman et al. 1999). Little is known of its ecology and it is presumed to be saprobic based on the substrates it has been isolated from (Subramanian & Bhat 1978a, b, Seifert 1985, Rossman et al. 1999).
The asexual morph Stachybotrys, based on St. atra, was first introduced by Corda (1837), and described as having 2-celled conidia. Bisby (1943) questioned Corda’s generic concept and revised the genus to include species with single-celled conidia containing two guttules, which gave it the 2-celled appearance. At the same time, Bisby (1943) also reduced 19 of the known species to synonymy under St. atra, while retaining St. subsimplex as a second species in the genus. Hughes (1958) studied the type material of St. atra and Stilbospora chartarum (Ehrenberg 1818) and concluded that these fungi were conspecific and provided the combination St. chartarum based on priority.
Bisby (1943) also considered the genus Memnoniella (Mem.), first introduced by Von Höhnel (1924), based on Mem. aterrima, recognising its close relationship to Stachybotrys. Galloway (1933) provided a new combination for Mem. aterrima, as Mem. echinata, based on an earlier description and illustration of a similar fungus by Rivolta (1873). However, Smith (1962), considered both Memnoniella and Stachybotrys as congeneric, arguing that the conidial disposition in dry chains (Memnoniella) or in slimy masses (Stachybotrys) are insufficiently important to distinguish between these genera.
This view was largely ignored by Verona & Mazzucchetti (1968), who recognised 16 Stachybotrys and three Memnoniella species in their monographic study. Jong & Davis (1976) also considered Memnoniella and Stachybotrys as distinct genera and included two Memnoniella and 11 Stachybotrys species in their culture-based study of these fungi. Although Haugland et al. (2001) suggested that Memnoniella should be synonymised under Stachybotrys based on their ITS phylogenetic study of nine Stachybotrys and three Memnoniella species, Pinruan et al. (2004) recognised 55 Stachybotrys and four Memnoniella species in their key to both genera. Wang et al. (2015) formally demoted Memnoniella to synonymy under Stachybotrys, recognising 74 Stachybotrys species and suggested that more species and allied genera need to be studied phylogenetically to determine the relationship between these fungi.
Stachybotrys has been linked to the sexual genera Melanopsamma (Castlebury et al. 2004, Tang et al. 2007, Wang et al. 2015) and Ornatispora (Hyde et al. 1999, Whitton et al. 2012, Wang et al. 2015). Melanopsamma pomiformis (Saccardo 1878), the type species of the genus, is linked to the asexual morph St. albipes, under which it was synonymised by Wang et al. (2015). Whitton et al. (2012) linked Ornatispora novae-zelandiae to St. freycinetiae and showed that O. nepalensis and O. taiwanensis have Stachybotrys asexual morphs based on occurrence of both morphs on the same materials studied. The asexual morph of Ornatispora palmicola, the type of the genus, is also likely a member of Stachybotrys based on the occurrence of sterile conidiophore-like structures on the ascomata illustrated by Hyde et al. (1999). However, another species, O. gamsii, has been linked with another asexual morph Di. aurantiospora (Hyde et al. 1999). Despite the obvious heterogeneity in the asexual morphs linked to Ornatispora, Wang et al. (2015) synonymised Ornatispora under Stachybotrys based on priority.
Several studies in the past have focused on the phylogenetic diversity of St. chartarum (Haugland & Heckman 1998, Haugland et al. 2001, Cruse et al. 2002, Andersen et al. 2003, Koster et al. 2003, 2009, Jie et al. 2013). Haugland & Heckman (1998) were the first to develop species-specific primers of nuclear rDNA for the detection and identification of toxigenic St. chartarum strains. Using only ITS sequence data, Haugland et al. (2001) investigated the sequence variability within and between Memnoniella species and its phylogenetic relationship with Stachybotrys. Cruse et al. (2002) investigated the cryptic nature of St. chartarum using sequences of multiple nuclear protein coding regions and identified two phylogenetic lineages within this species. These two phylogenetic lineages were also recognised by Andersen et al. (2003) and Koster et al. (2003) using multi-gene sequence data, resulting in the introduction of St. chlorohalonata for one of these lineages by Andersen et al. (2003), while retaining the other lineage as St. chartarum. A detailed summary of the history of phylogenetic studies of Stachybotrys and allied genera is provided by Wang et al. (2015).
The importance and impact of Stachybotrys, and in particular St. chartarum, on human and animal health have been well documented in the past. Known as ‘toxic black mould’ to the public and in the media, these fungi are associated with long-term water damage to buildings better known as ‘Sick Building Syndrome’ (Redlick et al. 1997, Crook & Burton 2010) that has been linked to respiratory diseases in humans (Cooley et al. 1998, Mahmoudi & Gershwin 2000, Straus et al. 2003, Brasel et al. 2005, Shoemaker & House 2005, Frazer et al. 2012). These human respiratory diseases include acute infant pulmonary haemorrhage (Etzel et al. 1998, Dearborn et al. 1999, Flappan et al. 1999, Vesper et al. 2000, Thrasher et al. 2014), asthma (Mahmoudi & Gershwin 2000, Viana et al. 2002, Kirjavainen et al. 2015) and nasal and tracheal bleeding (Dearborn et al. 1999, Flappan et al. 1999, Vesper & Vesper 2002). Stachybotryotoxicosis (Drobotko 1945) of animal livestock associated with lip edema, stomatitis, oral necrosis, rhinitis and conjunctivitis (Ozegovic et al. 1971, Schneider et al. 1979, Harrach et al. 1983) have been reported globally (Wang et al. 2015).
Wang et al. (2015) highlighted the need for a more comprehensive phylogenetic study of Stachybotrys and its allied genera. In the present study, the phylogenetic relationships within and between genera in the Stachybotriaceae are evaluated. The goal is to provide a phylogenetic backbone for the family Stachybotriaceae and to resolve the taxonomic irregularities noted in past literature as mentioned above.
MATERIALS AND METHODS
Isolates
Fungal strains were obtained from the culture collections of the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands; the Canadian Collection of Fungal Cultures (DAOMC), Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada; Mycothèque de l’Université Catholique de Louvain (BCCM/MUCL), Belgium, and the working collection of Pedro W. Crous (CPC) housed at the CBS (Table 1).
Table 1.
Details of strains included in phylogenetic analyses. GenBank accession numbers in italics were newly generated in this study.
| Species | Isolate nr.1 | Substrate | Collector/ Depositor | Locality | GenBank Accession No.2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| cmdA | ITS | LSU | rpb2 | tef1 | tub2 | |||||
| Achroiostachys betulicola | CBS 142.97 = INIFAT C96/121 | Bambusa vulgaris | R.F. Castañeda | Spain | KU845770 | KU845790 | KU845810 | KU845830 | KU845846 | KU845751 |
| CBS 399.65 = ATCC 22173 | Zea mays | I. Focke | Germany | KU845771 | KU845791 | KU845811 | DQ676584 | KU845847 | KU845752 | |
| CBS 136397 = MUCL 4167 = DAOMC 87338 | Betula lutea | E.A. Peterson | Canada | KU845772 | KU845792 | KU845812 | KU845831 | KU845848 | KU845753 | |
| CBS 136398 = MUCL 4318 | Triticum aestivum | J. Jooste | USA | KU845773 | KU845793 | KU845813 | – | KU845849 | KU845754 | |
| CBS 136401 = MUCL 4308 | Triticum aestivum | J. Jooste | USA | KU845774 | KU845794 | KU845814 | KU845832 | KU845850 | KU845755 | |
| CBS 136548 = MUCL 4319 | Triticum aestivum | J. Jooste | USA | KU845775 | KU845795 | KU845815 | KU845833 | KU845851 | KU845756 | |
| DAOMC 222969 | Soil | J.A. Traquair | Canada | KU845776 | KU845796 | KU845816 | KU845834 | – | KU845757 | |
| Ac. aurantispora | CBS 187.73 | Italy | KU845783 | KU845803 | KU845823 | KU845839 | KU845858 | KU845764 | ||
| DAOMC 225565 | Straw | G. White | Thailand | KU845784 | KU845804 | KU845824 | KU845840 | KU845859 | – | |
| Ac. humicola | CBS 317.72 | Soil | G. Tichelaar | The Netherlands | KU845777 | KU845797 | KU845817 | KU845835 | KU845852 | KU845758 |
| CBS 598.69 | Soil | G.C. Bhatt | Canada | KU845778 | KU845798 | KU845818 | KU845836 | KU845853 | KU845759 | |
| CBS 868.73 | M. Karman | Turkey | KU845779 | KU845799 | KU845819 | KU845837 | KU845854 | KU845760 | ||
| CBS 136394 = MUCL 15104 | Soil | G.C. Bhatt | Canada | KU845780 | KU845800 | KU845820 | – | KU845855 | KU845761 | |
| CBS 136404 = MUCL 15471 | Soil | G.C. Bhatt | Canada | KU845781 | KU845801 | KU845821 | – | KU845856 | KU845762 | |
| DAOMC 226830 | Soil | D. Overy | Canada | KU845782 | KU845802 | KU845822 | KU845838 | KU845857 | KU845763 | |
| Ac. levigata | CBS 185.79 = ATCC 22172 | Soil | B.P.R. Vittal | Sudan | KU845785 | KU845805 | KU845825 | KU845841 | KU845860 | KU845765 |
| CBS 363.58 | Soil | H.J. Swart | Mozambique | KU845786 | KU845806 | KU845826 | KU845842 | KU845861 | KU845766 | |
| Ac. phyllophila | CBS 136181 = MUCL 53217 = FMR 11019 | Plant debris | M. Hernández & K. Rodriguez | Spain | KU845787 | KU845807 | KU845827 | KU845843 | KU845862 | KU845767 |
| Ac. saccharicola | CBS 268.76 | Saccharum officinarum | T. Watanabe | Taiwan | KU845788 | KU845808 | KU845828 | KU845844 | KU845863 | KU845768 |
| CBS 136393 = MUCL 39119 | Dead twig | C. Decock | Nepal | KU845789 | KU845809 | KU845829 | KU845845 | KU845864 | KU845769 | |
| Albosynnema elegans | GB 3101 | – | – | AF193226 | – | – | – | |||
| Albifimbria lateralis | CBS 117712 | USA | KU845865 | KU845881 | KU845900 | KU845919 | KU845938 | KU845957 | ||
| Al. terrestris | CBS 109378 = NRRL 31066 | Dead hardwood | D.T. Wicklow | USA | KU845866 | KU845882 | KU845901 | KU845920 | KU845939 | KU845958 |
| CBS 126186 | Soil | M. Christensen | Namibia | KU845867 | KU845883 | KU845902 | KU845921 | KU845940 | KU845959 | |
| CBS 127838 | Soil | M. Christensen | Namibia | KU845868 | KU845884 | KU845903 | KU845922 | KU845941 | KU845960 | |
| Al. verrucaria | CPC 30056 | KU845869 | KU845885 | KU845904 | KU845923 | KU845942 | KU845961 | |||
| CBS 176.27 | Soil | E.V. Abbott | USA | KU845870 | KU845886 | KU845905 | KU845924 | KU845943 | KU845962 | |
| CBS 187.46 = IMI 140056 | Old canvas shoe | N.C. Preston | England | – | KU845887 | KU845906 | KU845925 | KU845944 | KU845963 | |
| CBS 188.46 = IMI 140057 | Citrus fruit | N.C. Preston | Zimbabwe | KU845871 | KU845888 | KU845907 | KU845926 | KU845945 | KU845964 | |
| CBS 189.46 = IMI 140060 | Solanum tubersum | N.C. Preston | Cyprus | KU845872 | KU845889 | KU845908 | KU845927 | KU845946 | KU845965 | |
| CBS 207.30 = IMI 140055 | B.B. Kanouse | USA | KU845873 | KU845890 | KU845909 | KU845928 | KU845947 | KU845966 | ||
| CBS 208.72 | Soil | J.H. van Emden | Java | – | KU845891 | KU845910 | KU845929 | KU845948 | KU845967 | |
| CBS 231.56 = IMI 140059 | Kooiman | The Netherlands | KU845874 | KU845892 | KU845911 | KU845930 | KU845949 | KU845968 | ||
| CBS 328.52 = CBS 253.47 = IMI 045541 = MUCL 19018 = NRRL 2003 = ATCC 9095 = QM 460 | Baled cotton | G.A. Greathouse | USA | KU845875 | KU845893 | KU845912 | KU845931 | KU845950 | KU845969 | |
| CBS 390.39 | K. Saito | Japan | KU845876 | KU845894 | KU845913 | KU845932 | KU845951 | KU845970 | ||
| CBS 962.95 | Soil | A. Aptroot | Papua New Guinea | KU845877 | KU845895 | KU845914 | KU845933 | KU845952 | KU845971 | |
| CBS 121142 = NRRL 45892 | Basidioma | D.T. Wicklow | Hawaii | KU845878 | KU845896 | KU845915 | KU845934 | KU845953 | KU845972 | |
| Al. viridis | CBS 244.78 | Air | I.S. Damirdagh | Iraq | – | KU845897 | KU845916 | KU845935 | KU845954 | KU845973 |
| CBS 449.71 = BCC 37540 | M.N. Kamat | India | KU845879 | KU845898 | KU845917 | KU845936 | KU845955 | KU845974 | ||
| CBS 127346 | Soil | M. Christensen | USA | KU845880 | KU845899 | KU845918 | KU845937 | KU845956 | KU845975 | |
| Alfaria caricicola | CBS 113567 | Litter of Carex sp. | W. Gams & R. Zare | Iran | KU845976 | KU845983 | KU845992 | KU846001 | KU846008 | KU846014 |
| Alf. cyperi-esculenti | CPC 23153 | Cyperus esculentus | A.M. Pérez Sierra | Spain | – | KJ869143 | KJ869200 | – | – | – |
| Alf. ossiformis | CBS 324.54 = IMI 055309 = MUCL 11831 = QM 7979 = BCC 38221 | Soil | P.A. Orpurt | USA | KU845977 | KU845984 | KU845993 | KU846002 | KU846009 | KU846015 |
| Alf. putrefolia | CBS 112037 | Rotten leaf | A. Stchigel & J. Guarro | Brazil | – | KU845985 | KU845994 | KU846003 | – | KU846016 |
| Alf. putrefolia | CBS 112038 | Rotten leaf | A. Stchigel & J. Guarro | Brazil | – | KU845986 | KU845995 | KU846004 | – | KU846017 |
| Alf. terrestris | CBS 168.97 | Leaf litter | R.F. Castañeda | Spain | KU845978 | KU845987 | KU845996 | KU846005 | KU846010 | KU846018 |
| CBS 477.91 | Soil | G. Turhan | Turkey | KU845979 | KU845988 | KU845997 | KU846006 | KU846011 | KU846019 | |
| CBS 127305 = RMF 8009 | Soil | M. Christensen | USA | KU845980 | KU845989 | KU845998 | – | KU846012 | KU846020 | |
| Alf. thymi | CBS 447.83 | Thymus serpullum | The Netherlands | KU845981 | KU845990 | KU845999 | – | KU846013 | KU846021 | |
| Alfaria sp. | CPC 22153 | Carex sp. | A. Anon | France | KU845982 | KU845991 | KU846000 | KU846007 | KU846022 | |
| Brevistachys globosa | CBS 397.73 | Musa sp. | W. Gams | Sri Lanka | KU846023 | KU846037 | KU846056 | KU846073 | KU846084 | KU846100 |
| CBS 141056 = CPC 16059 | Musa sp. | M. de Jesus Yarez-Morales | Mexico | KU846024 | KU846038 | KU846057 | – | KU846085 | KU846101 | |
| CPC 15951 | Euphorbia sp. | M. de Jesus Yarez-Morales | Mexico | – | KU846039 | KU846058 | – | KU846086 | KU846102 | |
| CPC 15952 | Euphorbia sp. | M. de Jesus Yarez-Morales | Mexico | KU846025 | KU846040 | KU846059 | – | KU846087 | KU846103 | |
| CPC 15953 | Euphorbia sp. | M. de Jesus Yarez-Morales | Mexico | KU846026 | KU846041 | KU846060 | – | KU846088 | KU846104 | |
| CPC 16060 | Musa sp. | M. de Jesus Yarez-Morales | Mexico | – | KU846042 | KU846061 | – | KU846089 | KU846105 | |
| Br. lateralis | CBS 141058 = CPC 17350 | Musa sp. | P.W. Crous | Australia | KU846027 | KU846043 | KU846062 | KU846074 | KU846090 | KU846106 |
| Br. ossiformis | CBS 696.73 = ATCC 32334 | Zingiber sp. | W. Gams | Sri Lanka | – | KU846044 | KU846063 | – | – | KU846107 |
| CBS 112792 = FMR 7685 | Musa paradisiaca | A. Stchigel & J. Guarro | Brazil | KU846028 | KU846045 | KU846064 | KU846075 | KU846091 | KU846108 | |
| CPC 16031 | Musa sp. | M. de Jesus Yarez-Morales | Mexico | KU846029 | KU846046 | KU846065 | – | KU846092 | KU846109 | |
| Br. subsimplex | ATCC 32888 | Eichhornia crassipes | USA | – | AF205439 | – | – | – | – | |
| Br. variabilis | CBS 141057 = CPC 17349 | Musa sp. | P.W. Crous | Australia | KU846030 | KU846047 | KU846066 | KU846076 | KU846093 | KU846110 |
| Calonectria ilicicola | CBS 190.50 | Solanum tuberosum | K.B. Boedjin & J. Reitsma | Java | – | – | GQ280727 | KM232307 | – | – |
| Capitofimbria compacta | CBS 111739 | Decaying leaf | A. Stchigel & J. Guarro | Brazil | KU846261 | KU846287 | KU846317 | KU846349 | KU846378 | KU846404 |
| MUCL 50238 | Bark | C. Decock | Zimbabwe | – | KU878556 | KU878557 | KU878558 | – | KU878559 | |
| Cymostachys coffeicola | CBS 252.76 | Coffea arabica | W. Gams | Cuba | KU846035 | KU846052 | KU846071 | KU846081 | KU846097 | KU846113 |
| CPC 25009 | Poinsettia sp. | P.W. Crous | Thailand | – | KU846053 | – | – | – | – | |
| Cy. fabispora | CBS 136180 = MUCL 39004 = INIFAT C93/322 | Decaying leaf | R.F. Castañeda | Cuba | KU846036 | KU846054 | KU846072 | KU846082 | KU846098 | KU846114 |
| CPC 24352 | Aloe ferox | M.J. Wingfield | Tanzania | – | KU846055 | – | KU846083 | KU846099 | – | |
| Didymostilbe aurantispora | CBS 616.85 | Arenga tremula var. englerii | K. Tubaki | Japan | – | – | KU846344 | – | – | – |
| Di. matsushimae | CBS 549.84 | Arenga engleri | R.J. Bandoni | Japan | – | – | KU846345 | – | – | – |
| CCFC 54984 | – | – | AY283545 | – | – | – | ||||
| Dimorphiseta terrestris | CBS 127345 = RMF 8243 | Soil | M. Christensen | USA | KU846284 | KU846314 | KU846346 | KU846375 | KU846401 | KU846431 |
| Fusarium sambucinum | CBS 146.95 | Solanum tuberosum | H.I. Nirenberg | UK | – | – | KM231682 | KM232381 | – | – |
| Globobotrys sansevieriicola | CBS 138872 = CPC 24316 | Sansevieria ehrenbergii | M.J. Wingfield | Tanzania | – | KR476717 | KR476752 | – | KR476793 | KR476794 |
| Grandibotrys pseudotheobromae | CBS 136170 = MUCL 39293 | Decaying wood | C. Decock | Nepal | – | KU846135 | KU846161 | KU846188 | KU846215 | KU846241 |
| CBS 136391 = MUCL 39289 | Decaying wood | C. Decock | Nepal | – | KU846136 | KU846162 | KU846189 | KU846216 | KU846242 | |
| Gra. xylophila | CBS 136179 = MUCL 39288 | Decaying wood | C. Decock | Nepal | KU846115 | KU846137 | KU846163 | KU846190 | KU846217 | – |
| Gregatothecium humicola | CBS 205.96 | Soil | A. Aptroot | Papua New Guinea | KU846285 | KU846315 | KU846347 | KU846376 | KU846402 | KU846432 |
| Inaequalispora prestonii | CBS 175.73 = IMI 160372 = ATCC 24427 | Soil | W.H. Tong | Malaysia | KU846286 | KU846316 | KU846348 | KU846377 | KU846403 | KU846433 |
| Kastanostachys aterrima | CBS 101310 | Fagus sylvatica | M. Réblová | Czech Republic | – | – | AF178565 | KU846191 | – | – |
| Melanopsamma pomiformis | CBS 325.90 | Fagus sylvatica | D. Sisto | Italy | KU846031 | KU846048 | KU846067 | KU846077 | KU846094 | KU846111 |
| CBS 101322 | Fagus sylvatica | K. Prásil | Czech Republic | KU846032 | KU846049 | KU846068 | KU846078 | – | – | |
| CBS 114119 = UPSC 2528 | Tilia cordata | K. Holm & L. Holm | Sweden | KU846033 | KU846050 | KU846069 | KU846079 | KU846095 | KU846112 | |
| Mel. xylophila | CBS 100343 | Decaying wood | W. Gams | Japan | KU846034 | KU846051 | KU846070 | KU846080 | KU846096 | – |
| Memnoniella brunneoconidiophora | CBS 109477 | Decayed leaf | R.F. Castañeda-Ruiz | Venezuela | – | KU846138 | KU846165 | KU846192 | KU846218 | KU846243 |
| CBS 136191 = MUCL 43313 | Decayed leaf | R.F. Castañeda-Ruiz | Venezuela | KU846116 | KU846139 | KU846166 | KU846193 | KU846219 | KU846244 | |
| Mem. dichroa | ATCC 18913 = IMI 61337 | Senecio jacobaea | A.H.S. Brown | England | – | AF081472 | – | – | – | – |
| CBS 526.50 = ATCC 18917 = IMI 017506 = MUCL 9482 | Herbaceous stem | M.B. Ellis | England | KU846117 | KU846140 | KU846167 | KU846194 | KU846220 | – | |
| CBS 123800 | Ilex aquifolium | W. Gams | The Netherlands | KU846118 | KU846141 | KU846168 | KU846195 | KU846221 | – | |
| Mem. echinata | CBS 216.32 | Cotton yarn | L.B. Galloway | England | KU846119 | KU846142 | KU846169 | KU846196 | KU846222 | KU846245 |
| CBS 304.54 | P.B. Marsh | USA | KU846120 | KU846143 | KU846170 | KU846197 | KU846223 | – | ||
| CBS 343.50 | Filter paper | K.B. Boedjin & J. Reitsma | Indonesia | KU846121 | KU846144 | KU846171 | KU846198 | KU846224 | KU846246 | |
| CBS 344.39 | Sake lees | K. Saito | Japan | KU846122 | KU846145 | KU846172 | KU846199 | KU846225 | KU846247 | |
| CBS 406.80 | Pulvinula constellatio | H.A. van der Aa | The Netherlands | KU846123 | KU846146 | KU846173 | KU846200 | KU846226 | KU846248 | |
| CBS 627.66 = IMI 045547 = NRRL 2181 | Tent canvas | W.H. Weston | Solomon Islands | KU846124 | KU846147 | KU846174 | KU846201 | KU846227 | KU846249 | |
| DAOMC 173162 | KU846125 | JN942886 | JN938868 | KU846202 | KU846228 | KU846250 | ||||
| DAOMC 235365 | Air | H. McGregor | Canada | KU846126 | KU846149 | KU846176 | KU846203 | KU846229 | KU846251 | |
| Mem. ellipsoidea | CBS 136199 = MUCL 39088 | Dead twig | C. Decock | Nepal | KU846127 | KU846150 | KU846177 | KU846204 | KU846230 | KU846252 |
| CBS 136200 = MUCL 39089 | Dead twig | C. Decock | Nepal | KU846128 | KU846151 | KU846178 | KU846205 | KU846231 | KU846253 | |
| CBS 136201 = MUCL 39090 | Dead twig | C. Decock | Nepal | KU846129 | KU846152 | KU846179 | KU846206 | KU846232 | KU846254 | |
| CBS 136202 = MUCL 41876 | Bromelia sp. | R.F. Castañeda-Ruiz | Brazil | – | KU846153 | KU846180 | KU846207 | KU846233 | KU846255 | |
| Mem. humicola | CBS 463.74 | Soil | J.H. van Emden | Suriname | KU846130 | KU846154 | KU846181 | KU846208 | KU846234 | – |
| Mem. longistipitata | ATCC 22699 | Soil | T. Matsushima | Japan | – | AF081471 | – | – | – | – |
| CBS 136197 = MUCL 33065 | Dead wood | G.L. Hennebert | Malawi | KU846131 | KU846155 | KU846182 | KU846209 | KU846235 | KU846256 | |
| Mem. oenanthes | ATCC 22844 = IMI 016185 | Oenanthe crocata | E.A. Ellis & M.B. Ellis | Channel Islands | – | AF081473 | – | – | – | – |
| CBS 388.73 = ATCC 32255 | Euphobia tirukalli | W. Gams | India | – | KU846156 | KU846183 | KU846210 | KU846236 | – | |
| Mem. pseudonilagirica | CBS 136405 = MUCL 39120 | Ceiba pentandra | C. Decock | Nepal | KU846132 | KU846157 | KU846184 | KU846211 | KU846237 | KU846257 |
| Mem. putrefolia | CBS 101177 | Melastomataceae | W. Gams | Puerto Rico | – | KU846158 | KU846185 | KU846212 | KU846238 | KU846258 |
| CBS 136171 = MUCL 41166 = INIFAT C98/65-2 | Decayed leaf | R.F. Castañeda-Ruiz | Brazil | KU846133 | KU846159 | KU846186 | KU846213 | KU846239 | KU846259 | |
| Memnoniella sp. | MUCL 50191 | KU846134 | KU846160 | KU846187 | KU846214 | KU846240 | KU846260 | |||
| Myrothecium inundatum | CBS 196.74 | Russula sp. | H.A. van der Aa | The Netherlands | KU846434 | KU846451 | KU846473 | – | KU846513 | KU846532 |
| CBS 275.48 = IMI 008983 = QM 7988 | Russula adusta | P.W. Brian | England | KU846435 | KU846452 | KU846474 | – | KU846514 | KU846533 | |
| CBS 616.70 | Leaf litter | G.C. Bhatt | Canada | KU846436 | KU846453 | KU846475 | – | – | KU846534 | |
| CBS 116539 | Agaric | K.A. Seifert | Canada | KU846437 | KU846454 | KU846476 | – | KU846515 | KU846535 | |
| CBS 120646 | Decaying toadstool | M. Gube | Germany | KU846438 | KU846455 | KU846477 | – | KU846516 | KU846536 | |
| Myr. simplex | CBS 582.93 | Decaying agaric | W. Gams | Japan | KU846439 | KU846456 | KU846478 | – | KU846517 | KU846537 |
| CBS 100287 | Russula nigricans | W. Gams et al. | Japan | KU846440 | KU846457 | KU846479 | – | KU846518 | KU846538 | |
| Myxospora aptrootii | CBS 101263 | Leaf litter | A. Aptroot | China | KU846441 | KU846458 | KU846480 | KU846496 | KU846519 | KU846539 |
| Myx. crassiseta | CBS 731.83 | Dead twig | W. Gams | Japan | KU846442 | KU846459 | KU846481 | KU846497 | KU846520 | KU846540 |
| CBS 121141 = NRRL 45891 | Pyrenomycete | D.T. Wicklow | Hawaii | KU846443 | KU846460 | KU846482 | KU846498 | KU846521 | KU846541 | |
| Myx. graminicola | CBS 116538 = AR 3507 | Decaying grass leaf | G. Bills | USA | KU846444 | KU846461 | KU846483 | KU846499 | KU846522 | KU846542 |
| Myx. masonii | CBS 174.73 = IMI 158346 = ATCC 24426 | Glyceria sp. | E.A. Ellis | England | KU846445 | KU846462 | KU846484 | KU846500 | KU846523 | KU846543 |
| Myx. musae | CBS 265.71 = IMI 155922 | Musa sp. | E. Laville | Madagascar | – | KU846463 | KU846485 | KU846501 | KU846524 | KU846544 |
| CPC 25150 | Tarspot lesion | J. Roux | South Africa | KU846446 | KU846464 | – | KU846502 | KU846525 | KU846545 | |
| Myxospora sp. 1 | CBS 100347 | Leaf litter | W. Gams | Japan | KU846447 | KU846465 | KU846486 | KU846503 | KU846526 | KU846546 |
| Myxospora sp. 2 | MUCL 55239 | – | KU846466 | KU846487 | KU846504 | – | KU846547 | |||
| Nectria cinnabarina | CBS 125165 | Aesculus sp. | C. Lechat | France | – | – | HM484562 | KM232402 | – | – |
| Neomyrothecium humicola | CBS 310.96 | Soil | A. Aptroot | Papua New Guinea | KU846448 | KU846467 | KU846488 | KU846505 | KU846527 | – |
| Paramyrothecium acadiense | CBS 123.96 = DAOMC 221473 = UAMH 7653 | Tussilago farfara | G. Sampson | Canada | – | KU846288 | KU846318 | KU846350 | KU846379 | KU846405 |
| Pa. breviseta | CBS 544.75 | A. Subrahmanian | India | KU846262 | KU846289 | KU846319 | KU846351 | KU846380 | KU846406 | |
| Pa. cupuliforme | CBS 126167 | Soil | M. Christensen | Namibia | KU846263 | KU846290 | KU846320 | KU846352 | KU846381 | KU846407 |
| CBS 127789 | Soil | M. Christensen | Namibia | KU846264 | KU846291 | KU846321 | KU846353 | KU846382 | KU846408 | |
| Pa. foeniculicola | CBS 331.51 = IMI 140051 | Foeniculum vulgare | The Netherlands | – | KU846292 | KU846322 | KU846354 | KU846383 | KU846409 | |
| Pa. foliicola | CBS 419.93 = INIFAT C93/60 | Air | R.F. Castañeda | Cuba | KU846265 | KU846293 | KU846323 | KU846355 | KU846384 | KU846410 |
| CBS 113121 = INIFAT C02/104 | Decaying leaf | R.F. Castañeda-Ruiz | Brazil | KU846266 | KU846294 | KU846324 | – | KU846385 | KU846411 | |
| Pa. humicola | CBS 127295 | Soil | M. Christensen | USA | – | KU846295 | KU846325 | KU846356 | KU846386 | KU846412 |
| Pa. nigrum | CBS 116537 = AR 3783 | Soil | G. Bills | Spain | KU846267 | KU846296 | KU846326 | KU846357 | KU846387 | KU846413 |
| Pa. parvum | CBS 142.42 = IMI 155923 = MUCL 7582 | Dune sand | F. Moreau | France | KU846268 | KU846297 | KU846327 | KU846358 | – | KU846414 |
| Pa. parvum | CBS 257.35 = IMI 140049 | Viola sp. | N.C. Preston | UK | – | KU846298 | KU846328 | KU846359 | KU846388 | KU846415 |
| Pa. roridum | CBS 212.95 | Water | E.S. van Reenen-Hoekstra | The Netherlands | KU846269 | KU846299 | KU846329 | KU846360 | KU846389 | KU846416 |
| CBS 357.89 | Gardenia sp. | G. Giunchi | Italy | KU846270 | KU846300 | KU846330 | KU846361 | KU846390 | KU846417 | |
| CBS 372.50 = IMI 140050 | Coffea sp. | O. Urhan | Colombia | KU846271 | KU846301 | KU846331 | KU846362 | KU846391 | KU846418 | |
| Pa. tellicola | CBS 478.91 | Soil | G. Turhan | Turkey | KU846272 | KU846302 | KU846332 | KU846363 | – | KU846419 |
| Pa. terrestris | CBS 564.86 | Soil | G. Turhan | Turkey | KU846273 | KU846303 | KU846333 | KU846364 | – | KU846420 |
| CBS 565.86 | Soil | G. Turhan | Turkey | KU846274 | KU846304 | KU846334 | KU846365 | KU846392 | KU846421 | |
| CBS 566.86 | Soil | G. Turhan | Turkey | KU846275 | KU846305 | KU846335 | KU846366 | KU846393 | KU846422 | |
| CBS 872.85 | Soil | G. Turhan | Turkey | KU846276 | KU846306 | KU846336 | KU846367 | KU846394 | KU846423 | |
| Pa. viridisporum | CBS 563.86 | Soil | G. Turhan | Turkey | KU846277 | KU846307 | KU846337 | KU846368 | KU846395 | KU846424 |
| CBS 873.85 | Soil | G. Turhan | Turkey | KU846278 | KU846308 | KU846338 | KU846369 | KU846396 | KU846425 | |
| CBS 125821 | Soil | M. Christensen | USA | KU846279 | KU846309 | KU846339 | KU846370 | – | KU846426 | |
| CBS 125835 | Soil | M. Christensen | USA | KU846280 | KU846310 | KU846340 | KU846371 | KU846397 | KU846427 | |
| CBS 126942 | Soil | J.S. States | USA | KU846281 | KU846311 | KU846341 | KU846372 | KU846398 | KU846428 | |
| CBS 127785 | Soil | M. Christensen | USA | KU846282 | KU846312 | KU846342 | KU846373 | KU846399 | KU846429 | |
| CBS 127843 | Soil | M. Christensen | USA | KU846283 | KU846313 | KU846343 | KU846374 | KU846400 | KU846430 | |
| Parasarcopodium ceratocaryi | CBS 110664 | Ceratocaryi decipientis | S. Lee | South Africa | – | – | AY425026 | – | – | – |
| Parvothecium terrestre | CBS 198.89 | Soil | L. Pfenning | Brazil | KU846449 | KU846468 | KU846489 | KU846506 | KU846528 | KU846548 |
| CBS 534.88 = INIFAT C87/234 | Leaf litter | R.F. Castañeda | Cuba | KU846450 | KU846469 | KU846490 | KU846507 | KU846529 | KU846549 | |
| Peethambara sundara | CBS 521.96 = MUCL 39093 | Dead twig | C. Decock | Nepal | – | KU846470 | KU846491 | KU846508 | KU846530 | KU846550 |
| CBS 646.77 | Dead twig | C.V. Subramanian | India | – | KU846471 | AF193245 | KU846509 | KU846531 | KU846551 | |
| Septomyrothecium maraitiense | MUCL 47202 | Decaying leaf | C. Decock | French Guyana | – | – | KU846493 | KU846510 | – | – |
| Sept. uniseptatum | CBS 100966 = INIFAT C98/23-1 | Leaf litter | R.F. Castañeda | Venezuela | – | KU846472 | KU846494 | KU846511 | – | KU846552 |
| MUCL 52944 | – | – | KU846495 | KU846512 | – | – | ||||
| Sirastachys castanedae | CBS 164.97 | Decaying leaf | R.F. Castañeda-Ruiz | Spain | KU846553 | KU846658 | KU846771 | KU846885 | KU846990 | KU847094 |
| CBS 531.69 = IMI 144477 | Soil | G.C. Bhatt | Canada | KU846554 | KU846659 | KU846772 | KU846886 | KU846991 | KU847095 | |
| CBS 136403 = MUCL 39994 | Decaying leaf | R.F. Castañeda-Ruiz | Spain | KU846555 | KU846660 | KU846773 | KU846887 | KU846992 | KU847096 | |
| CPC 20373 | Morus sp. | M. Arzanlou | Iran | KU846556 | KU846661 | KU846774 | KU846888 | KU846993 | KU847097 | |
| Si. cylindrospora | CBS 136166 = MUCL 41106 = INIFAT C98/42 | Decaying leaf | R.F. Castañeda-Ruiz | Brazil | KU846557 | KU846662 | KU846775 | KU846889 | – | KU847098 |
| CBS 13654 = MUCL 41088 = INIFAT C98/30 | Decaying leaf | R.F. Castañeda-Ruiz | Brazil | KU846558 | KU846663 | KU846776 | KU846890 | KU846994 | KU847099 | |
| Si. longispora | ATCC 32451 | Ilex latifolia | T. Matsushima | Japan | – | AF081482 | – | – | – | – |
| Si. pandanicola | CBS 136545 = MUCL 49906 | Pandanus sp. | O. Laurence | Singapore | – | KU846664 | KU846777 | – | – | KU847100 |
| Si. phaeospora | CBS 253.75 | Soil | J.A. Stalpers | The Netherlands | KU846559 | KU846665 | KU846778 | – | – | KU847101 |
| CBS 100155 | Decaying leaf | J. Guarro | Cuba | KU846560 | KU846666 | KU846779 | KU846891 | KU846995 | KU847102 | |
| CBS 136167 = MUCL 41195 | Decaying leaf | R.F. Castañeda-Ruiz | Brazil | KU846561 | KU846667 | KU846780 | KU846892 | KU846996 | KU847103 | |
| CBS 136185 = MUCL 41191 | Decaying leaf | R.F. Castañeda-Ruiz | Brazil | KU846562 | KU846668 | KU846781 | KU846893 | KU846997 | KU847104 | |
| CPC 16092 | Cycas sp. | P.W. Crous | South Africa | KU846563 | KU846669 | KU846782 | KU846894 | – | KU847105 | |
| CPC 16093 | Cycas sp. | P.W. Crous | South Africa | KU846564 | KU846670 | KU846783 | KU846895 | – | KU847106 | |
| Si. phyllophila | CBS 173.97 | Decaying leaf | R.F. Castañeda | Spain | KU846565 | KU846671 | KU846784 | KU846896 | KU846998 | KU847107 |
| CBS 136169 = MUCL 39919 | Decaying leaf | R.F. Castañeda | Spain | KU846566 | KU846672 | KU846785 | KU846897 | KU846999 | KU847108 | |
| Si. pseudolongispora | CBS 417.93 = INIFAT C93/213-3 | Leaf litter | R.F. Castañeda | Cuba | KU846567 | KU846673 | KU846786 | KU846898 | KU847000 | KU847109 |
| CBS 100154 | Decaying leaf | J. Guarro | Cuba | KU846568 | KU846674 | KU846787 | KU846899 | – | KU847110 | |
| Sirastachys sp. | CBS 308.56 = ATCC 18877 = IMI 062338 = MUCL 9485 | Soil | J.A. Meyer | Zaire | KU846569 | KU846675 | KU846788 | KU846900 | KU847001 | KU847111 |
| Smaragdiniseta bisetosa | CBS 459.82 | Rotten bark | V. Rao & A.C. Rao | India | KU847206 | KU847229 | KU847255 | KU847281 | KU847303 | KU847319 |
| “Stachybotrys albipes” | ATCC 18873 = IMI 056393 | Ulmus sp. | C. Booth | England | – | AF081478 | – | – | – | – |
| St. aloeticola | CBS 137940 = CPC 19705 | Aloe sp. | P.W. Crous | South Africa | KU846570 | KJ817888 | KJ817890 | KU846901 | – | KJ817886 |
| CBS 137941 = CPC 19706 | Aloe sp. | P.W. Crous | South Africa | KU846571 | KJ817889 | KJ817891 | KU846902 | – | KJ817887 | |
| “St. breviuscula” | HGUP 0106 | Soil | J.J. Xu | China | – | KC305229 | – | – | – | – |
| HGUP 0186 | Soil | J.J. Xu | China | – | KC305272 | – | – | – | – | |
| HGUP 0204 | Soil | J.J. Xu | China | – | KC305320 | – | – | – | – | |
| HGUP 0205 | Soil | J.J. Xu | China | – | KC305342 | – | – | – | – | |
| St. chartarum | CBS 129.13 | H.A. Dale | KM231452 | KM231858 | KM231738 | KM232434 | KM231994 | KM232127 | ||
| CBS 177.42 = ATCC 18848 | Soil | F. Moreau | France | KU846572 | KU846678 | KU846791 | KU846903 | KU847002 | KU847114 | |
| CBS 182.80 | Cheese wrapping | The Netherlands | KU846573 | KU846679 | KU846792 | KU846904 | KU847003 | KU847115 | ||
| CBS 215.92 | Air | A.L. Klodiussen | Norway | KU846574 | KU846680 | KU846793 | KU846905 | KU847004 | KU847116 | |
| CBS 363.49 | Clematis sp. | I. de Boer | The Netherlands | KU846575 | KU846681 | KU846794 | KU846906 | KU847005 | KU847117 | |
| CBS 414.95 | P. Parikka | Finland | KU846576 | KU846682 | KU846795 | KU846907 | KU847006 | KU847118 | ||
| CBS 485.48 | Soil | S. Blumer | Switzerland | KU846577 | KU846683 | KU846796 | KU846908 | KU847007 | KU847119 | |
| CBS 492.96 = INIFAT C96/47 | Leaf litter | R.F. Castañeda | Cuba | KU846578 | KU846684 | KU846797 | KU846909 | KU847008 | KU847120 | |
| CBS 101146 = IMI 082021 | Cotton fabric | R.M. Everett | England | KU846579 | KU846685 | KU846798 | KU846910 | KU847009 | KU847121 | |
| CBS 109287 | Home | B. Sorenson | USA | KU846580 | KU846686 | KU846799 | KU846911 | KU847010 | KU847122 | |
| CBS 109288 | Paint | B. Andersen | Denmark | KU846581 | KU846687 | KU846800 | KU846912 | KU847011 | KU847123 | |
| CBS 109289 | Building material | K.F. Nielsen | Denmark | KU846582 | KU846688 | KU846801 | KU846913 | KU847012 | KU847124 | |
| CBS 109290 | Home | B. Sorenson | USA | KU846583 | KU846689 | KU846802 | KU846914 | KU847013 | KU847125 | |
| CBS 109291 | Home | B. Sorenson | USA | KU846584 | KU846690 | KU846803 | KU846915 | KU847014 | KU847126 | |
| CBS 109292 | Building material | A. Hyvärinen | Finland | KU846585 | KU846691 | KU846804 | KU846916 | KU847015 | KU847127 | |
| CBS 109561 | Gypsum liner board | J. Peltola | Finland | KU846586 | KU846692 | KU846805 | KU846917 | KU847016 | – | |
| CBS 109562 | Gypsum liner board | J. Peltola | Finland | KU846587 | KU846693 | KU846806 | KU846918 | KU847017 | KU847128 | |
| CBS 109563 | Fibre board | J. Peltola | Finland | KU846588 | KU846694 | KU846807 | KU846919 | KU847018 | KU847129 | |
| CBS 112385 | Cardboard | U. Weidner | Germany | KU846589 | KU846695 | KU846808 | KU846920 | KU847019 | KU847130 | |
| CBS 112541 = INIFAT C02/114-1 | Decaying leaf | R.F. Castañeda-Ruiz | Spain | KU846590 | KU846696 | KU846809 | KU846921 | KU847020 | KU847131 | |
| CBS 119366 | Home | J. Houbraken | The Netherlands | KU846591 | KU846697 | KU846810 | KU846922 | KU847021 | KU847132 | |
| CBS 119369 | Home | J. Houbraken | The Netherlands | KU846592 | KU846698 | KU846811 | KU846923 | KU847022 | KU847133 | |
| CBS 119370 | Home | J. Houbraken | The Netherlands | KU846593 | KU846699 | KU846812 | KU846924 | KU847023 | KU847134 | |
| CBS 119371 | Indoor air | J. Houbraken | The Netherlands | KU846594 | KU846700 | KU846813 | KU846925 | KU847024 | KU847135 | |
| CBS 136159 = MUCL 308 | Wall-paper | V. Estienne | Belgium | KU846595 | KU846701 | KU846814 | KU846926 | KU847025 | KU847136 | |
| CBS 136161 = MUCL 18140 | Soil | B. Desai | Belgium | KU846596 | KU846702 | KU846815 | KU846927 | KU847026 | KU847137 | |
| CBS 136163 = MUCL 3820 | Wall-paper | G.L. Hennebert | Belgium | KU846597 | KU846703 | KU846816 | KU846928 | KU847027 | KU847138 | |
| CBS 136172 = MUCL 40562 | Mouldy drywall | J. Scott | Canada | KU846598 | KU846704 | KU846817 | KU846929 | KU847028 | KU847139 | |
| CBS 136175 = MUCL 21588 | Wall-paper | G.L. Hennebert | Belgium | KU846599 | KU846705 | KU846818 | KU846930 | KU847029 | KU847140 | |
| CBS 136176 = MUCL 15918 | G. Ola’h | Canada | KU846600 | KU846706 | KU846819 | KU846931 | KU847030 | KU847141 | ||
| CBS 136178 = MUCL 14285 | Soil | J.A. Meyer | Belgium | KU846601 | KU846707 | KU846820 | KU846932 | KU847031 | KU847142 | |
| CBS 136186 = MUCL 28869 | Gyproc plate | G.L. Hennebert | Belgium | KU846602 | KU846708 | KU846821 | KU846933 | KU847032 | KU847143 | |
| CBS 136188 = MUCL 2443 | Anthacobia sp. | C. Decock | Canada | KU846603 | KU846709 | KU846822 | KU846934 | KU847033 | KU847144 | |
| CBS 136189 = MUCL 30782 | Wall-paper | C. Decock | Belgium | KU846604 | KU846710 | KU846823 | KU846935 | KU847034 | KU847145 | |
| CBS 136400 = MUCL 2538 | Cardboard | G.L. Hennebert | Belgium | KU846605 | KU846711 | KU846824 | KU846936 | KU847035 | KU847146 | |
| DAOMC 18104 | Pisum sativum | J.W. Groves | Canada | KU846606 | KU846712 | KU846825 | KU846937 | KU847036 | KU847147 | |
| DAOMC 75756 | Culture contaminent | W.B. Kendrick | Canada | KU846607 | KU846713 | KU846826 | KU846938 | KU847037 | KU847148 | |
| DAOMC 84862 | Filter paper | B. Cumming | KU846608 | KU846714 | KU846827 | KU846939 | KU847038 | KU847149 | ||
| DAOMC 144338 | Soil | J. Pearn | Canada | KU846609 | KU846715 | KU846828 | KU846940 | KU847039 | KU847150 | |
| DAOMC 155625 | Paper | J. Bissett | Canada | KU846610 | KU846716 | KU846829 | KU846941 | KU847040 | KU847151 | |
| DAOMC 183927 | Fibreglass insulation | J. Bissett | KU846611 | KU846717 | KU846830 | KU846942 | KU847041 | KU847152 | ||
| DAOMC 189444 | Pressed wood fibre | C.J. Shirtliffe | Canada | KU846612 | KU846718 | KU846831 | KU846943 | KU847042 | KU847153 | |
| DAOMC 225489 | Drywall | T. Rand | Hawaii | KU846613 | KU846719 | KU846832 | KU846944 | KU847043 | KU847154 | |
| DAOMC 226913 | Drywall | J. Bissett | Canada | KU846614 | KU846720 | KU846833 | KU846945 | KU847044 | KU847155 | |
| DAOMC 235364 | Air | H. McGregor | Canada | KU846615 | KU846721 | KU846834 | KU846946 | KU847045 | KU847156 | |
| DAOMC 240054 | KU846616 | KU846722 | KU846835 | KU846947 | KU847046 | KU847157 | ||||
| St. chlorohalonata | CBS 113.97 = INIFAT C96/120 | Bambusa vulgaris | R.F. Castañeda | Spain | KU846635 | KU846742 | KU846855 | KU846965 | KU847065 | KU847176 |
| CBS 127.94 | Plastic | A. Hyvärinen | Finland | KU846636 | KU846743 | KU846856 | KU846966 | KU847066 | KU847177 | |
| CBS 222.46 = ATCC 18842 = MUCL 9481 | Flax fibre | R. Bok | The Netherlands | KU846637 | KU846744 | KU846857 | KU846967 | KU847067 | KU847178 | |
| CBS 250.89 | Desert sand | J.C. Krug | Namibia | KU846617 | KU846723 | KU846836 | KU846948 | KU847047 | KU847158 | |
| St. chlorohalonata | CBS 251.89 | Wood, paper and tile | R.S. Khan | Canada | KU846618 | KU846724 | KU846837 | KU846949 | KU847048 | KU847159 |
| CBS 328.37 = ATCC 18844 | Paper | O. Verona | Italy | KU846619 | KU846725 | KU846838 | KU846950 | KU847049 | KU847160 | |
| CBS 330.37 | Paper | O. Verona | Italy | KU846620 | KU846726 | KU846839 | KU846951 | KU847050 | KU847161 | |
| CBS 341.35 = ATCC 18847 = MUCL 9477 | N.F. Conant | USA | KU846638 | KU846745 | KU846858 | KU846968 | KU847068 | KU847179 | ||
| CBS 608.94 | Paper | A. Gargouri | Tunisia | KU846621 | KU846727 | KU846840 | KU846952 | KU847051 | KU847162 | |
| CBS 109281 | Contaminant | B. Andersen | Denmark | KU846639 | KU846746 | KU846859 | – | KU847069 | KU847180 | |
| CBS 109283 | Building material | K.F. Nielsen | Denmark | KU846622 | KU846728 | KU846841 | KU846953 | KU847052 | KU847163 | |
| CBS 109285 | Cardboard | K.F. Nielsen | Denmark | KU846623 | KU846729 | KU846842 | KU846954 | KU847053 | KU847164 | |
| CBS 122763 | Plant debris | J. Capilla, R.F. Castañeda- Ruiz & C. Silvera | Portugal | KU846624 | KU846730 | KU846843 | KU846955 | KU847054 | KU847165 | |
| CBS 125896 | Soil | M. Christensen | USA | KU846640 | KU846747 | KU846860 | – | KU847070 | KU847181 | |
| CBS 129226 | Soil | M. Christensen | USA | KU846625 | KU846731 | KU846844 | – | KU847055 | KU847166 | |
| CBS 136158 = MUCL 49910 | Decayed wood | C. Decock | Singapore | KU846626 | KU846732 | KU846845 | KU846956 | KU847056 | KU847167 | |
| CBS 136160 = MUCL 258 | Soil | J. van Holder | Belgium | KU846641 | KU846748 | KU846861 | KU846969 | KU847071 | KU847182 | |
| CBS 136192 = MUCL 3139 | Grain | G.L. Hennebert | KU846642 | KU846749 | KU846862 | KU846970 | KU847072 | KU847183 | ||
| CBS 136194 = MUCL 4311 | Triticum aestivum | J.W. Jooste | USA | KU846627 | KU846733 | KU846846 | KU846957 | KU847057 | KU847168 | |
| CBS 136196 = MUCL 18020 | Soil | G.L. Hennebert | France | KU846643 | KU846750 | KU846863 | KU846971 | KU847073 | KU847184 | |
| DAOMC 235557 | J.D. Miller | Canada | KU846644 | KU846751 | KU846864 | KU846972 | KU847074 | KU847185 | ||
| St. dolichophialis | DAOMC 227011 | Soil | K.A. Seifert | South Africa | KU846628 | KU846734 | KU846847 | KU846958 | – | KU847169 |
| “St. elegans” | HGUP 0208 | Soil | Y. Wang | China | – | JX978445 | – | – | – | – |
| HGUP 0310 | Soil | Y. Wang | China | – | KC305357 | – | – | – | – | |
| St. limonispora | CBS 128809 | Soil | M. Christensen | USA | KU846629 | KU846735 | KU846848 | KU846959 | KU847058 | KU847170 |
| CBS 136165 = MUCL 18730 | Quisqualis indica | G.L. Hennebert | India | KU846630 | KU846736 | KU846849 | KU846960 | KU847059 | KU847171 | |
| “St. mangiferae” | HGUP 0158 | Soil | J.J. Xu | China | – | KC305253 | – | – | – | – |
| St. microspora | ATCC 18852 = IMI 124902 | Arachis hypogaea | D. McDonald | Nigeria | – | AF081475 | – | – | – | – |
| CBS 186.79 | Soil | B.P.R. Vittal | Sudan | KU846631 | KU846737 | KU846850 | DQ676580 | KU847060 | KU847172 | |
| St. pallescens | HGUP 0146 | Soil | Y.L. Jiang | China | – | KC305345 | KC305345 | – | – | – |
| “St. parvispora” | NRRL 54531 | Decaying wood | D.T. Wicklow | Hawaii | – | JN093263 | – | – | – | – |
| St. phaeophialis | KAS 525 | Seed | G.P. White | China | KU846632 | KU846738 | KU846851 | KU846962 | KU847061 | KU847173 |
| St. reniformis | ATCC 18839 | Japan | – | AF081476 | – | – | – | – | ||
| CBS 976.95 | Soil | A. Aptroot | Papua New Guinea | KU846633 | KU846739 | KU846852 | KU846963 | KU847062 | KU847174 | |
| CBS 136198 = MUCL 39087 | Dead twig | C. Decock | Nepal | – | KU846740 | KU846853 | – | KU847063 | – | |
| “St. sansevieriae” | HGUP 0103 | Soil | J.H. Kong | China | – | JX998165 | – | – | – | – |
| HGUP 0180 | Soil | J.J. Xu | China | – | KC305267 | – | – | – | – | |
| St. subcylindrospora | HGUP 0201 | Soil | Y.L. Zhang | China | – | KC305354 | – | – | – | – |
| St. subreniformis | HGUP 1051 | Soil | Q.R. Li | China | – | KC305344 | – | – | – | – |
| “St. subsimplex” | ATCC 18838 | – | AF205441 | – | – | – | – | |||
| ATCC 22700 | Soil | T. Matsushima | Papua New Guinea | – | AF205440 | – | – | – | – | |
| ATCC 32334 | Zingiber sp. | W. Gams | Sri Lanka | – | AF205442 | – | – | – | – | |
| St. subsylvatica | CBS 126205 | Soil | M. Christensen | Namibia | KU846634 | KU846741 | KU846854 | KU846964 | KU847064 | KU847175 |
| “St. terrestris” | HGUP 0488 | – | KC305289 | – | – | – | – | |||
| “St. theobromae” | ATCC 18905 = IMI 105321 | Theobroma cacao | T.H. Williams | Malaysia | – | AF081479 | – | – | – | – |
| “St. yunnanensis” | HGUP 0142 | Soil | Y.L. Jiang | China | – | KC305246 | – | – | – | – |
| HGUP 0144 | Soil | Y.L. Jiang | China | – | KC305247 | – | – | – | – | |
| HGUP 0745 | Soil | Y.L. Jiang | China | – | KC305322 | – | – | – | – | |
| “St. zeae” | HGUP 0143 | Soil | Y.L. Jiang | China | – | KC305308 | – | – | – | – |
| Stachybotrys sp. | CBS 525.50 = IMI 032542 = MUCL 9475 | Soil | J.H. Warcup | England | KU846645 | KU846752 | KU846865 | – | KU847075 | KU847186 |
| Striatobotrys atypica | CBS 141059 = CPC 18423 | Iris sp. | P.W. Crous | France | KU846646 | KU846753 | KU846866 | KU846973 | KU847076 | KU847187 |
| CPC 18422 | Iris sp. | P.W. Crous | France | KU846647 | KU846754 | KU846867 | KU846974 | KU847077 | KU847188 | |
| Stri. eucylindrospora | CBS 203.61 = ATCC 18851 = IMI 085334 = MUCL 9483 | Soil | G.L. Barron | Canada | KU846648 | KU846755 | KU846868 | KU846975 | KU847078 | KU847189 |
| CBS 949.72 | Turkey | – | KU846756 | KU846869 | KU846976 | KU847079 | KU847190 | |||
| CBS 136399 = MUCL 4251 | Plant debris | G.L. Hennebert | USA | – | KU846757 | KU846870 | KU846977 | KU847080 | KU847191 | |
| CBS 136547 = MUCL 15039 | Soil | G.C. Bhatt | Canada | KU846649 | KU846758 | KU846871 | KU846978 | KU847081 | KU847192 | |
| Stri. humicola | CBS 102408 | Soil | M. Christensen | USA | KU846650 | KU846759 | KU846872 | KU846979 | KU847082 | KU847193 |
| Stri. oleronensis | CBS 137258 | Iris pseudacorus | M. Hairaud | France | – | KF777192 | KU846873 | KU846980 | KU847083 | KU847194 |
| Stri. rhabdospora | CBS 528.80 | Soil | I.J. Kapoor | Germany | KU846651 | KU846760 | KU846874 | KU846981 | KU847084 | KU847195 |
| CBS 878.68 = ATCC 16276 | Soil | W. Gams | Germany | – | KU846761 | KU846875 | KU846982 | KU847085 | KU847196 | |
| CBS 119043 | Soil | W. Gams | Switzerland | – | KU846762 | KU846876 | KU846983 | KU847086 | KU847197 | |
| CBS 121801 | Plant debris | C. Silvera | Spain | KU846652 | KU846763 | KU846877 | KU846984 | KU847087 | KU847198 | |
| CBS 136168 = MUCL 6030 | Soil | K. Domsch | Germany | KU846653 | KU846764 | KU846878 | KU846985 | KU847088 | KU847199 | |
| CBS 136203 = MUCL 17023 | – | KU846765 | KU846879 | – | – | KU847200 | ||||
| CBS 136395 = MUCL 22116 | Asbestos cement tile | G.L. Hennebert | Belgium | KU846654 | KU846766 | KU846880 | KU846986 | KU847089 | KU847201 | |
| CBS 136396 = MUCL 2012 | Caltha palustris | G.L. Hennebert | USA | KU846655 | KU846767 | KU846881 | KU846987 | KU847090 | KU847202 | |
| DAOMC 70309 | Soil | O.A. Olsen | Canada | – | KU846768 | KU846882 | KU846988 | KU847091 | KU847203 | |
| DAOMC 189389 | Wall board | M.L. Florian | Canada | KU846656 | KU846769 | KU846883 | – | KU847092 | KU847204 | |
| Stri. yuccae | CBS 390.68 | Yucca flaccida | W. Gams | The Netherlands | KU846657 | KU846770 | KU846884 | KU846989 | KU847093 | KU847205 |
| Striaticonidium brachysporum | CBS 131.71 = IMI 158441 = ATCC 22270 | Soil | Ukrain | KU847207 | KU847230 | KU847256 | KU847282 | KU847304 | KU847320 | |
| CBS 177.65 | Acacia karroo | M.C. Papendorf | South Africa | KU847208 | KU847231 | KU847257 | KU847283 | – | KU847321 | |
| CBS 513.71 = IMI 115293 | Dune sand | J. Nicot | Iran | KU847209 | KU847232 | KU847258 | KU847284 | KU847305 | KU847322 | |
| CBS 126552 | Soil | P.A. Orpurt & J.T. Curtis | USA | KU847210 | KU847233 | KU847259 | KU847285 | KU847306 | KU847323 | |
| CBS 127287 | Soil | M. Christensen | USA | KU847211 | KU847234 | KU847260 | KU847286 | KU847307 | KU847324 | |
| CBS 128163 | Soil | P.A. Orpurt & J.T. Curtis | USA | KU847212 | KU847235 | KU847261 | KU847287 | KU847308 | KU847325 | |
| Str. cinctum | CBS 277.48 = IMI 001526 | Soil | J.C. Neill | New Zealand | KU847213 | KU847236 | KU847262 | KU847288 | KU847309 | KU847326 |
| CBS 373.50 = IMI 140052 | Soil | J. van Holder | Belgium | KU847214 | KU847237 | KU847263 | KU847289 | – | KU847327 | |
| CBS 528.69 = IMI 140637 = ATCC 18947 | Soil | G.C. Bhatt | Canada | KU847215 | KU847238 | KU847264 | – | KU847310 | KU847328 | |
| CBS 932.69 = IMI 145760 | Soil | J.W. Veenbaas-Rijks | The Netherlands | KU847216 | KU847239 | KU847265 | KU847290 | – | KU847329 | |
| Stri. humicola | CBS 258.76 | Soil | J.A. von Arx | Spain | – | KU847240 | KU847266 | KU847311 | KU847330 | |
| CBS 388.97 | Soil | A. Aptroot | Papua New Guinea | KU847217 | KU847241 | KU847267 | KU847291 | KU847312 | KU847331 | |
| Stri. synnematum | CBS 479.85 | Palm leaf | K.A. Seifert | Japan | KU847218 | KU847242 | KU847268 | KU847292 | – | KU847332 |
| Tangerinosporium thalitricola | CBS 317.61 = IMI 034815 | Thalictrum flavum | M.B. Ellis | UK | KU847219 | KU847243 | KU847269 | – | – | KU847333 |
| Virgatospora echinofibrosa | CBS 110115 | Theobroma cacao | G.J. Samuels | Ecuador | KU847220 | KU847244 | KU847270 | KU847293 | KU847313 | KU847334 |
| MUCL 39092 = ATCC 200437 | Trewia nudiflora | C. Decock | Nepal | – | KU847245 | KU847271 | KU847294 | – | KU847335 | |
| Xenomyrothecium tongaense | CBS 598.80 | Halimeda sp. | B. Kendrick | Tonga | KU847221 | KU847246 | KU847272 | KU847295 | KU847314 | KU847336 |
| Xepicula crassiseta | CBS 392.71 | Soil | Spain | KU847222 | KU847247 | KU847273 | KU847296 | KU847315 | KU847337 | |
| X. jollymannii | CBS 276.48 = MUCL 11830 | Nicotiana tabacum | F.W. Jollymann | Malawi | KU847223 | KU847248 | KU847274 | KU847297 | KU847316 | KU847338 |
| CBS 511.76 | Clerodendron inerme | R.B. Somani | India | – | KU847249 | KU847275 | – | – | KU847339 | |
| CBS 126168 | Soil | M. Christensen | Namibia | KU847224 | KU847250 | KU847276 | KU847298 | KU847317 | KU847340 | |
| X. leucotricha | CBS 131.64 = IMI 103664 = ATCC 16686 | Soil | P. Rama Rao | India | KU847225 | KU847251 | KU847277 | KU847299 | – | KU847341 |
| CBS 256.57 = MUCL 9860 | Soil | O. Verona & P. Joly | Brazil | KU847226 | KU847252 | KU847278 | KU847300 | – | KU847342 | |
| CBS 278.78 | Soil | J. Veerkamp | Colombia | KU847227 | KU847253 | KU847279 | KU847301 | – | KU847343 | |
| CBS 483.78 | Soil | J. Veerkamp | Colombia | KU847228 | KU847254 | KU847280 | KU847302 | KU847318 | KU847344 | |
1 Ex-type and ex-epitype cultures indicated in bold.
AR: Collection of A.Y. Rossman; ATCC: American Type Culture Collection, USA; BCC: BIOTEC Culture Collection, National Center for Genetic Engineering and Biotechnology (BIOTEC), Bangkok, Thailand; CBS: CBS-KNAW Fungal Diversity Centre, Utrecht, The Netherlands; CPC: Collection of P.W. Crous; DAOMC: Agriculture and Agri-Food Canada, Canadian Collection of Fungal Cultures, Canada; FMR: Facultad de Medicina, Reus, Tarragona, Spain; HGUP: Herbarium of Guizhou University, Plant Pathology, China; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; INIFAT: INIFAT Fungus Collection, Ministerio de Agricultura Habana; KAS: Collection of K.A. Seifert; MUCL: Mycothèque de l’Université Catholique de Louvian, Belgium; NRRL: Agriculatural Research Service Culture Collection, USA; QM: Quatermaster Research and Development Center, US Army, Natick, MA, USA; RMF: Collection of M. Christensen.
2 cmdA: calmodulin; ITS: internal transcribed spacer regions and intervening 5.8S ribosomal RNA; LSU: 28S ribosomal RNA large subunit; rpb2: RNA polymerase II second largest subunit; tef1: translation elongation factor 1-alpha; tub2: beta-tubulin.
DNA isolation, amplification and analyses
Total genomic DNA was extracted from 7–14-d-old axenic cultures grown on potato dextrose agar (2 % w/w; PDA) using the Wizard® Genomic DNA purification Kit (Promega Corporation, Madison, USA) following the protocol provided by the manufacturer. Partial gene sequences were determined for LSU, using the primers LR0R (Rehner & Samuels 1995) and LR5 (Vilgalys & Hester 1990); ITS, using primers ITS5 and ITS4 (White et al. 1990); RNA polymerase II second largest subunit (rpb2), using primers RPB2-5F2 and RPB2-7cR (O’Donnell et al. 2007); β-tubulin (tub2), using primers Bt2a and Bt2b (Glass & Donaldson 1995); calmodulin (cmdA), using primers CAL-228F (Carbone & Kohn 1999) and CAL2Rd (Groenewald et al. 2013); and translation elongation factor 1-alpha (tef1) using primers EF1-728F (Carbone & Kohn 1999) and EF2 (O’Donnell et al. 1998). Amplicons for each locus were generated following the protocols listed in Lombard et al. (2015a).
Integrity of the sequences was ensured by sequencing the amplicons in both directions using the same primer pairs used for amplification. A consensus sequence for each locus was assembled in MEGA v. 6 (Tamura et al. 2013) and additional sequences were obtained form GenBank (Table 1). Subsequent alignments for each locus were generated in MAFFT v. 7 (Katoh & Standley 2013) and manually corrected where necessary. Congruency of the loci were tested using the 70 % reciprocal bootstrap criterion (Mason-Gamer & Kellogg 1996) following the protocols of Lombard et al. (2015b). All novel sequences generated in this study were deposited in GenBank (Table 1) and alignments and trees in TreeBASE.
Phylogenetic analyses were based on Bayesian inference (BI), Maximum Likelihood (ML) and Maximum parsimony (MP). For both the BI and ML analyses, the evolutionary model for each partition was determined using MrModeltest (Nylander 2004) and incorporated into the analyses. For BI analyses, MrBayes v. 3.2.1 (Ronquist & Huelsenbeck 2003) was used to generate phylogenetic trees under optimal criteria for each locus. A Markov Chain Monte Carlo (MCMC) algorithm of four chains was initiated in parallel from a random tree topology with a heating parameter set at 0.3. The MCMC analyses lasted until the average standard deviation of split frequencies were below 0.01 with trees saved every 1 000 generations. The first 25 % of saved trees were discarded as the ‘burn-in’ phase and posterior probabilities (PP) were determined from the remaining trees.
The ML analyses were performed using RAxML v. 8.0.9 (Stamatakis 2014) through the CIPRES website (http://www.phylo.org). The robustness of the analyses was evaluated by bootstrap support (ML-BS) with the number of bootstrap replicates automatically determined by the software.
The MP analyses were done using PAUP (Phylogenetic Analysis Using Parsimony, v. 4.0b10; Swofford 2003) with phylogenetic relationships estimated by heuristic searches with 1 000 random addition sequences. Tree-bisection-reconnection was used, with branch swapping options set on ‘best trees’ only. All characters were weighted equally and alignment gaps were treated as fifth state. Measures calculated for parsimony included tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC). Bootstrap analyses (MP-BS; Hillis & Bull 1993) were based on 1 000 replicates.
Taxonomy
Axenic cultures were sub-cultured onto cornmeal agar (CMA), oatmeal agar (OA), PDA (recipes in Crous et al. 2009) and synthetic low-nutrient agar (SNA; Nirenberg 1981) without any additional materials and incubated for 7–14 d at room temperature (22–25 °C) under ambient light conditions. Gross morphological characters were examined by mounting fungal structures in clear lactic acid and measurements were made at ×1 000 magnification using a Zeiss Axioscope 2 microscope with differential interference contrast (DIC) illumination. The 95 % confidence levels were determined for the conidial measurements with extremes given in parentheses while only extremes are provided for other structures. At the same time, colony morphology was assessed on CMA, OA and PDA (reverse described only on PDA) using the colour charts of Rayner (1970). All descriptions, illustrations and nomenclatural data were deposited in MycoBank (Crous et al. 2004).
RESULTS
Phylogeny
Approximately 500–650 bases were determined for the cmdA, ITS, tef1 and tub2 gene regions and approximately 800–900 bases for the LSU and rpb2 gene regions. The congruency analyses revealed a large number of conflicts between the cmdA, ITS, tef1 and tub2 gene regions, which could only be resolved by separating the data into two datasets representing the Myrothecium s.l. dataset and Stachybotrys s.l. dataset, respectively. However, the tef1 gene region of the Myrothecium s.l. dataset still provided a conflicting tree topology due to the large number of ambiguous regions and was therefore excluded from the analyses. The LSU and rpb2 gene regions provided similar tree topologies, with the only variation observed in the support values of the deeper branches and were therefore combined to obtain a generic level phylogeny. For the BI and ML analyses, a GTR+I+G model was selected for all six gene regions analysed and incorporated into the analyses. The Bayesian consensus tree for the three datasets confirmed the tree topologies obtained from the ML and MP analyses, and therefore, only the ML trees are presented.
The combined LSU and rpb2 sequence dataset presented in Fig. 1, included representatives of each clade resolved in the larger analyses of both gene regions. This dataset included 66 ingroup taxa, with Calonectria ilicicola (CBS 190.50), Fusarium sambucinum (CBS 146.95) and Nectria cinnabarina (CBS 125165) as outgroup taxa. The sequence dataset consisted of 1 581 characters, including alignment gaps. Of these, 965 were constant, 147 parsimony-uninformative and 469 parsimony-informative. The MP analysis yielded 40 trees (TL = 3 625; CI = 0.275; RI = 0.635; RC = 0.175) and a single best ML tree with –lnL = -16731.731268 which is presented in Fig. 1. The BI lasted for 1 075 M generations, and the consensus tree, with posterior probabilities, was calculated from 1 614 trees left after 538 trees were discarded as the ‘burn-in’ phase.
Fig. 1.
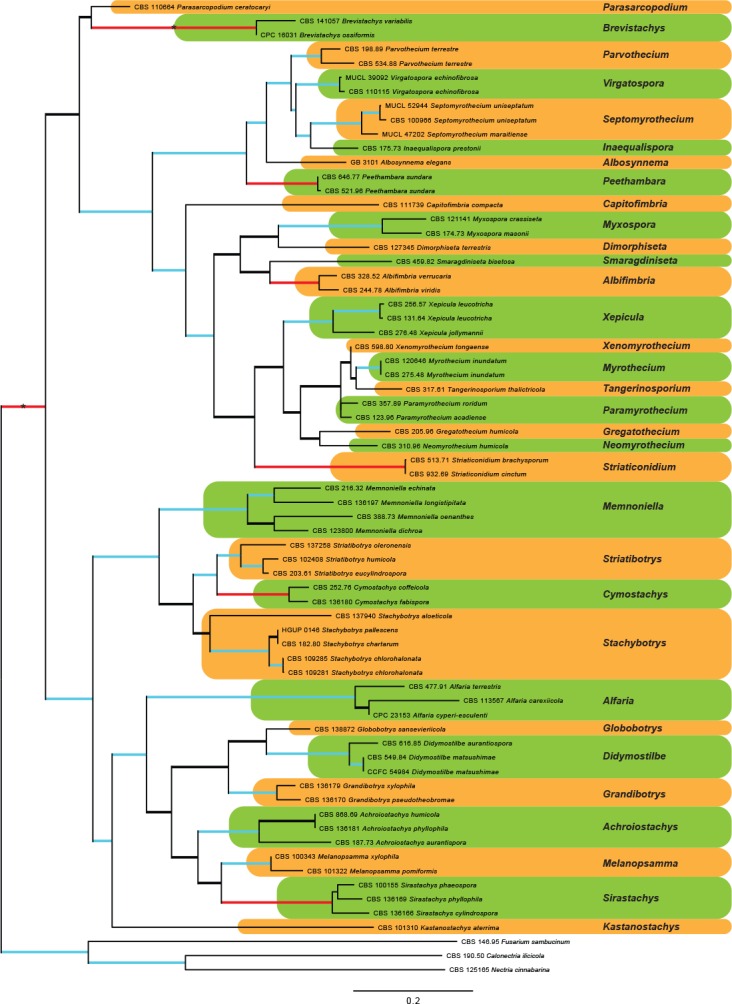
The ML consensus tree inferred from the combined LSU and rpb2 sequence alignments. Thickened branches indicate branches present in the ML, MP and Bayesian consensus trees. Branches with ML-BS & MP-BS = 100 % and PP = 1.0 are in red. Branches with ML-BS & MP-BS ≥ 75 % and PP ≥ 0.95 are in blue. The scale bar indicates 0.2 expected changes per site. Coloured blocks represent the accepted genera. The tree is rooted to Calonectria ilicicola (CBS 190.50), Fusarium sambucinum (CBS 146.95) and Nectria cinnabarina (CBS 125165).
In the phylogenetic tree (Fig. 1), the ingroup taxa resolved into a total of 21 well- to highly-supported clades and 12 single lineages. Of these, six clades and eight single lineages represented species previously considered members of the genus Myrothecium. Several myrothecium-like strains also clustered in a well-supported clade with Alfaria cyperi-esculenti (ex-type CPC 23513; Crous et al. 2014), a monophyletic sexual morph genus. Representatives of the genus Stachybotrys were resolved into nine well- to highly-supported clades and two single lineages. The remaining four well- to highly-supported clades represented established genera that included Didymostilbe, Peethambara, Septomyrothecium and Virgatospora. Of the remaining three single lineages, two represented the monophyletic genera Albosynnema and Parasarcopodium, with the third representing Chaetosphaeria aterrima (Réblová 1998).
The combined cmdA, ITS, rpb2 and tub2 sequence dataset representing Myrothecium s.l. included 108 ingroup taxa and Fusarium sambucinum (CBS 146.95) as outgroup taxon. This dataset consisted of 2 610 characters, of which 1 173 were constant, 236 parsimony-uninformative and 1 201 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 8 863; CI = 0.335; RI = 0.801; RC = 0.269) and a single best ML tree with –lnL = -35437.984026 which is presented in Fig. 2. The BI lasted for 1 245 M generations, and the consensus tree, with posterior probabilities, was calculated from 1 870 trees left after 622 trees were discarded as the ‘burn-in’ phase.
Fig. 2.
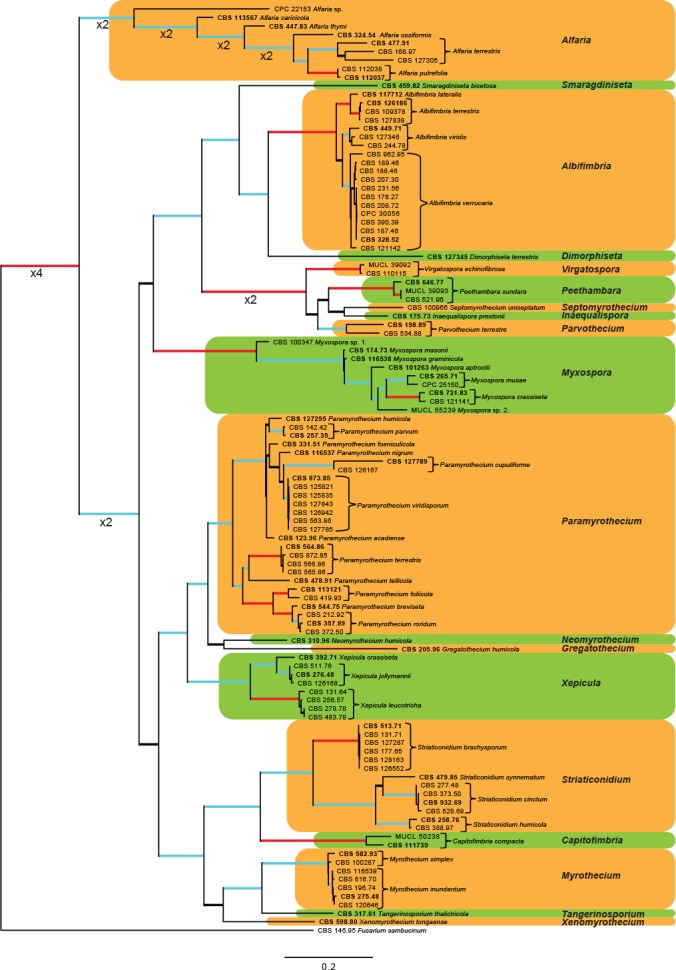
The ML consensus tree inferred from the combined cmdA, ITS, rpb2 and tub2 sequence alignments of the Myrothecium s.l. dataset. Thickened branches indicate branches present in the ML, MP and Bayesian consensus trees. Branches with ML-BS & MP-BS = 100 % and PP = 1.0 are in red. Branches with ML-BS & MP-BS ≥ 75 % and PP ≥ 0.95 are in blue. The scale bar indicates 0.2 expected changes per site. The tree is rooted to Fusarium sambucinum (CBS 146.95). Epi- and ex-type strains are indicated in bold.
The combined cmdA, ITS, rpb2, tef1 and tub2 sequence dataset representing Stachybotrys s.l. included 190 ingroup taxa and Peethambara sundara (CBS 646.77) as outgroup taxon. This dataset consisted of 3 273 characters, of which 1 184 were constant, 262 parsimony-uninformative and 1 827 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 14 474; CI = 0.316; RI = 0.857; RC = 0.271) and a single best ML tree with –lnL = -54412.890264 which is presented in Fig. 3. The BI lasted for 64.86 M generations, and the consensus tree, with posterior probabilities, was calculated from 97 292 trees left after 32 430 trees were discarded as the ‘burn-in’ phase.
Fig. 3.

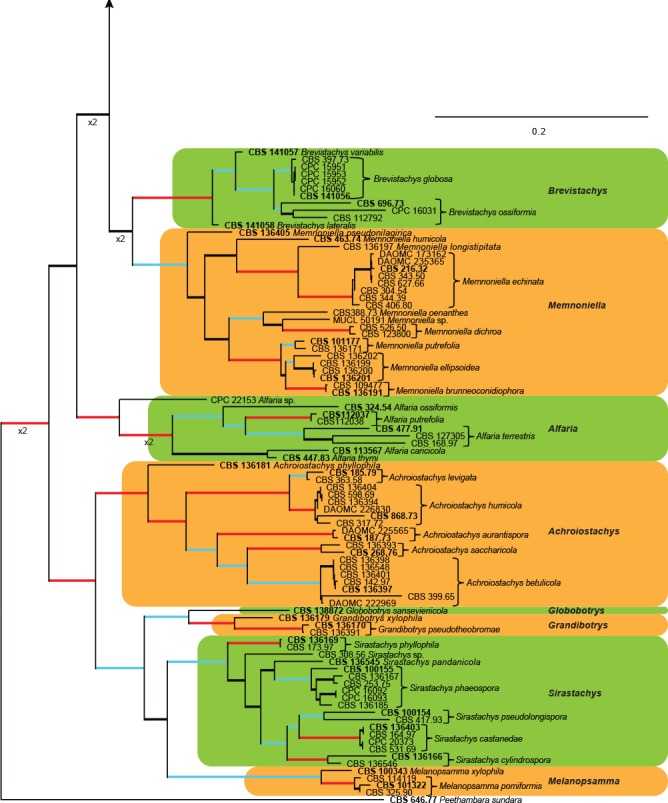
The ML consensus tree inferred from the combined cmdA, ITS, rpb2, tef1 and tub2 sequence alignments of the Stachybotrys s.l. dataset. Thickened branches indicate branches present in the ML, MP and Bayesian consensus trees. Branches with ML-BS & MP-BS = 100 % and PP = 1.0 are in red. Branches with ML-BS & MP-BS ≥ 75 % and PP ≥ 0.95 are in blue. The scale bar indicates 0.2 expected changes per site. The tree is rooted to Peethambara sundara (CBS 646.77). Epi- and ex-type strains are indicated in bold.
In the phylogenetic tree (Fig. 3), the ingroup taxa also resolved into similar well-, to highly-supported clades as was observed for the combined LSU and rpb2 phylogenetic inference. These clades also included several well- to highly-supported subclades and single lineages representing possible new phylogenetic species (see notes in Taxonomy section).
Additionally, an ITS sequence dataset of Stachybotrys s.l. was also analysed and presented in Fig. 4. This dataset included sequences obtained from GenBank for Stachybotrys species known in literature and also included ex- and epitype strains for which only ITS sequence data is available at present (Wang et al. 2015). This dataset included 172 ingroup taxa, with Peethambara sundara (CBS 646.77) as outgroup taxon. This dataset consisted of 536 characters, of which 218 were constant, 69 parsimony-uninformative and 249 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 1 289; CI = 0.458; RI = 0.913; RC = 0.418) and a single best ML tree with –lnL = -4850.513612 which is presented in Fig. 4. The BI lasted for 2 055 M generations, and the consensus tree, with posterior probabilities, was calculated from 3 084 trees left after 1 028 trees were discarded as the ‘burn-in’ phase.
Fig. 4.
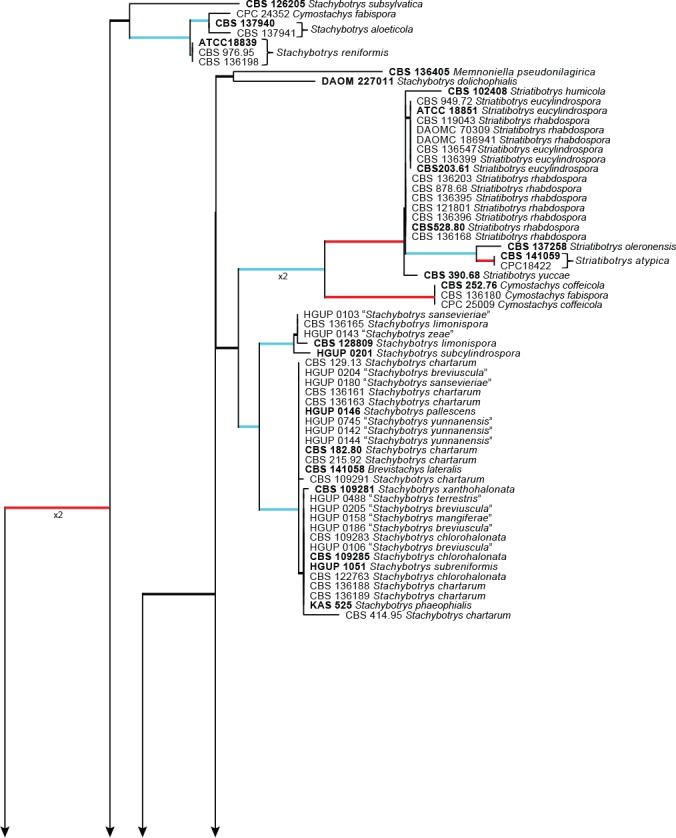
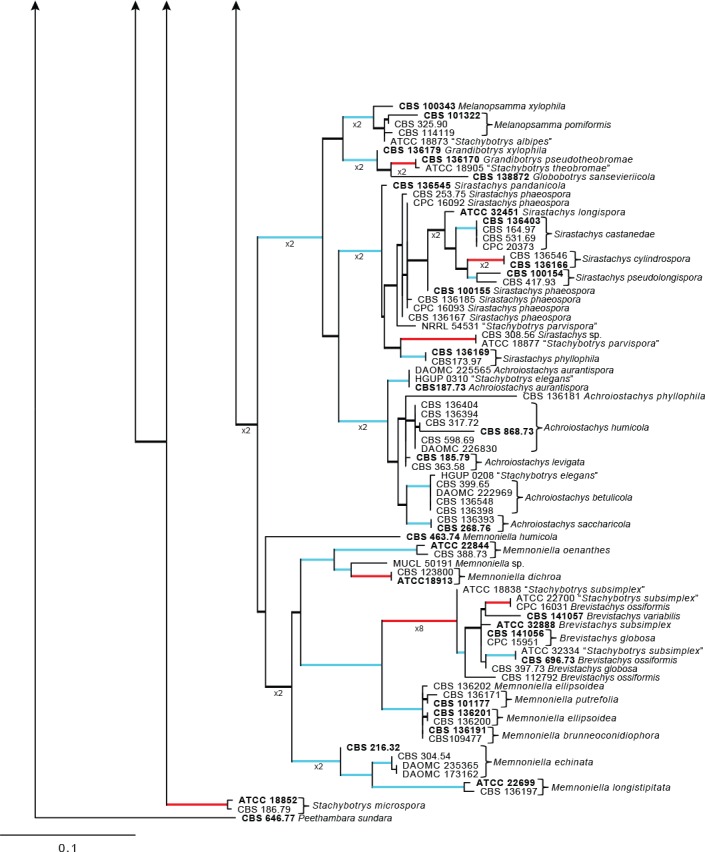
The ML consensus tree inferred from the ITS sequence alignment of the Stachybotrys s.l. dataset. Thickened branches indicate branches present in the ML, MP and Bayesian consensus trees. Branches with ML-BS & MP-BS = 100 % and PP = 1.0 are in red. Branches with ML-BS & MP-BS ≥ 75 % and PP ≥ 0.95 are in blue. The scale bar indicates 0.1 expected changes per site. The tree is rooted to Peethambara sundara (CBS 646.77). Epi- and ex-type strains are indicated in bold.
Taxonomy
Based on phylogenetic inference supported by morphological observations, several novel genera and species, previously treated as members of the genera Myrothecium and Stachybotrys, were identified in this study. Recognised clades representing novel genera and species are described below. Several sterile strains (CBS 308.56, CBS 525.50 and MUCL 50191 in the Stachybotrys s.l. dataset; CBS 100347, CPC 22153 and MUCL 55239 in the Myrothecium s.l. dataset) were not treated here as these represent single lineages and more data is required to confirm their novelty. However, one species in the Alfaria clade is sterile and described here based on DNA sequence data following the approach of Gomes et al. (2013) and Lombard et al. (2014).
Achroiostachys L. Lombard & Crous, gen. nov. — MycoBank MB815916
Etymology. Name reflects the hyaline (Greek = áchroios) conidiophores of this genus.
Type species. Achroiostachys humicola L. Lombard & Crous.
Sexual morph unknown. Conidiophores macronematous, mononematous, erect, solitary or in groups, unbranched or rarely branched, thin-walled, hyaline, smooth, sometimes becoming slightly verrucose at the base, 1–3-septate, with an apical cluster of 2–6 conidiogenous cells. Conidiogenous cells phialidic, elongate ampulliform to ventricose to subcylindrical, smooth to slightly verrucose, hyaline, with a somewhat protruding apical opening. Conidia aseptate, hyaline, smooth, ellipsoidal to limoniform to globose to subglobose, containing 1–2 large or several small guttules, rounded at both ends or with rounded base and acute apex.
Notes — The asexual genus Achroiostachys (Ac.) is established here for a group of stachybotrys-like fungi that formed a highly-supported clade distantly related to the Stachybotrys s.str. clade. Members of this genus are distinguished by their hyaline, mostly smooth, thin-walled conidiophores and hyaline, smooth, ellipsoidal to limoniform conidia. Although morphologically reminiscent of St. elegans (Domsch et al. 1980), the conidiophores of Achroiostachys (up to 140 μm) are shorter than those reported for St. elegans (up to 200 μm; Domsch et al. 1980). The conidiogenous cells of St. elegans are subclavate (Domsch et al. 1980), whereas those of Achroiostachys are ampulliform to ventricose to subcylindrical.
Achroiostachys aurantispora L. Lombard & Crous, sp. nov. — MycoBank MB815917; Fig. 5
Fig. 5.

Achroiostachys aurantispora (DAOMC 225565). a–c. Conidiophores; d. conidiophores on substrate; e. conidia. — Scale bar = 10 μm (apply to b–d).
Etymology. Name reflects the pale orange colour of the conidial mass formed by this fungus.
Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 1–2-septate, slightly thick-walled towards the base, smooth, hyaline and glassy, mostly 70–100 × 2.5–3.5 μm, bearing a whorl of 5–9 conidiogenous cells. Conidiogenous cells terminal, elongate ampulliform to ventricose or clavate, hyaline, smooth, 10–13 × 3.5–4.5 μm, narrowing to a short neck about 1 μm wide. Conidial mass slimy, pale orange. Conidia aseptate, ellipsoidal, sometimes flattened on one side, smooth, hyaline, (7–)7.5–8.5(–10) × 4–5 μm (av. 8 × 4 μm), containing 1–2 large guttules, with an inconspicuous basal hilum and a rounded apex.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium, buff to honey, reverse on PDA buff to honey.
Materials examined. ITALY, Mortara, substrate unknown, Jan. 1973, collector unknown, CBS 187.73. – THAILAND, precise origin uncertain, from straw in a cushion seized at the Vancouver International Airport, 13 Jan. 1998, G. White (holotype DAOM 695772, culture ex-type, DAOMC 225565 = M97-670).
Notes — The conidiophores of Achroiostachys aurantispora (up to 100 μm) are longer than those of Ac. betulicola (up to 85 μm), Ac. humicola (up to 65 μm), Ac. levigata (up to 75 μm) and Ac. phyllophila (up to 70 μm), but shorter than those of Ac. saccharicola (up to 140 μm). Phylogenetic inference in this study (Fig. 3) showed that Ac. aurantispora formed a highly supported clade closely related to Ac. betulicola and Ac. saccharicola.
Achroiostachys betulicola L. Lombard & Crous, sp. nov. — MycoBank MB815919; Fig. 6
Fig. 6.
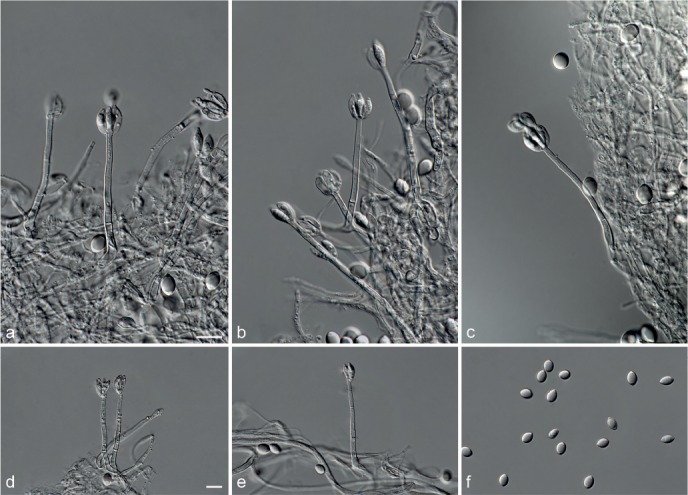
Achroiostachys betulicola (CBS 136397). a–e. Conidiophores; f. conidia. — Scale bars = 10 μm.
Etymology. Name reflects the host genus Betula from which the holotype was isolated.
Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 1–3-septate, thin-walled, smooth, hyaline, 35–85 × 3–5 μm, bearing solitary or a whorl of 2–4 conidiogenous cells. Conidiogenous cells terminal, elongate ampulliform to subcylindrical, hyaline, smooth, 8–11 × 3–5 μm, with a somewhat protruding apical opening. Conidia aseptate, globose to limoniform to ellipsoidal, smooth, hyaline, (7–)9–11(–12) × (5–)5.5–6.5(–7) μm (av. 10 × 6 μm), containing 1–2 large guttules, rounded at both ends or with rounded base and acute apex.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium, buff to rosy buff to salmon, with abundant conidiophores forming on the surface of the medium, bearing slimy hyaline conidial masses, reverse on PDA rosy buff to buff.
Materials examined. CANADA, Ontario, Dorset area, from roots of Betula lutea, 1961, E.A. Peterson (holotype CBS H-22419, culture ex-type CBS 136397 = MUCL 4167 = DAOMC 87338); Ontario, from soil, J.A. Traquair, DAOMC 222969. – GERMANY, Bernburg, Saale, from root of Zea mays, Dec. 1965, I. Focke, CBS 399.65 = ATCC 22173. – SPAIN, from leaf litter of Bambusa vulgaris, July 1996, CBS 142.97 = INIFAT C96/121. – USA, New York, Ithaca, campus of Cornell University, from rhizosphere of Triticum aestivum, May 1962, J. Jooste, CBS 136398 = MUCL 4318, CBS 136401 = MUCL 4308, CBS 136548 = MUCL 4319.
Notes — Phylogenetic inference in this study showed that Ac. betulicola formed a well-supported clade closely related to Ac. saccharicola and Ac. aurantispora (Fig. 3). The conidiophores of Ac. betulicola (up to 85 μm) are longer than those of Ac. humicola (up to 65 μm), Ac. levigata (up to 75 μm) and Ac. phyllophila (up to 70 μm) but shorter than those of Ac. saccharicola (up to 140 μm). Additionally, the conidia of Ac. betulicola are slightly larger than those of the other species in the genus.
Achroiostachys humicola L. Lombard & Crous, sp. nov. — MycoBank MB815920; Fig. 7
Fig. 7.
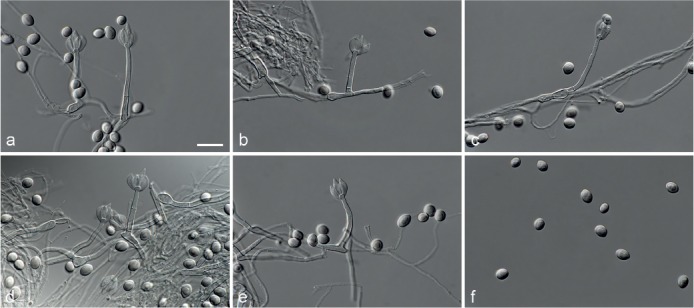
Achroiostachys humicola (CBS 868.73). a–e. Conidiophores; f. conidia. — Scale bar = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 1–2-septate, thin-walled, smooth, hyaline, 30–65 × 3–5 μm, bearing a whorl of 2–6 conidiogenous cells. Conidiogenous cells terminal, elongate ampulliform to ventricose, hyaline, smooth, 7–12 × 3–5 μm, with a somewhat protruding apical opening. Conidia aseptate, globose to limoniform, smooth, hyaline, (7–)7.5–8.5(–10) × (5–)5.5–6.5(–7) μm (av. 8 × 6 μm), containing 1–2 large guttules, rounded at both ends or with rounded base and acute apex.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to pale luteous to rosy buff aerial mycelium, with abundant conidiophores forming on the aerial mycelium and surface of the medium, bearing slimy hyaline conidial masses, reverse on PDA rosy buff to pale luteous.
Materials examined. CANADA, Ontario, South March, from soil under Zea mays, 1969, G.C. Bhatt, CBS 598.69, CBS 136404 = MUCL 15471; Waterloo, from soil in corn field, July 1967, G.C. Bhatt, CBS 136394 = MUCL 15104. – THE NETHERLANDS, Wageningen, from agricultural soil, 1969, G. Tichelaar, CBS 317.72. – TURKEY, Izmir-Bornova, substrate unknown, 1973, M. Karman (holotype CBS H-22420, culture ex-type CBS 868.73).
Notes — Achroiostachys humicola formed a highly supported clade closely related to Ac. levigata (Fig. 3). This species can be distinguished from other species in the genus by their short conidiophores (up to 65 μm).
Achroiostachys levigata L. Lombard & Crous, sp. nov. — MycoBank MB815921; Fig. 8
Fig. 8.
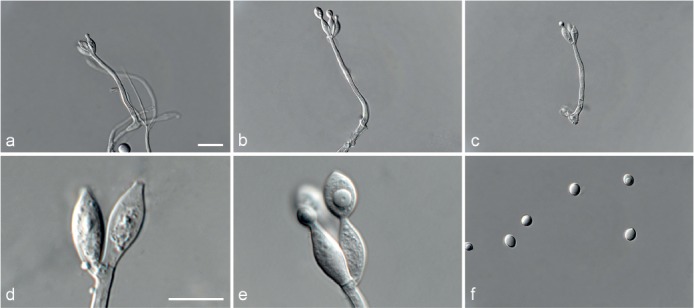
Achroiostachys levigata (CBS 185.79). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 10 μm (apply to b–c, f); d = 10 μm (apply to e).
Etymology. Name reflects the smooth-walled conidiophores and conidia formed by this fungus.
Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 1–2-septate, thin-walled, smooth, hyaline, 30–75 × 3–5 μm, bearing a whorl of 2–3 conidiogenous cells. Conidiogenous cells terminal, elongate ampulliform to ventricose, hyaline, smooth, 9–15 × 3–5 μm, with a somewhat protruding apical opening. Conidia aseptate, globose to limoniform, smooth, hyaline, (7–)8.5–9.5(–10) × (6–)6.5–7.5(–8) μm (av. 9 × 7 μm), containing 1–2 large guttules, with rounded base and acutely rounded apex.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium, buff to honey, with abundant conidiophores forming on the surface of the medium, bearing slimy, hyaline conidial masses, reverse on PDA buff to honey.
Materials examined. MOZAMBIQUE, Inhaca Island, from soil in mangrove swamp, June 1958, H.J. Swart, CBS 363.58. – SUDAN, Elephant White Nile Island, from soil in citrus field, Mar. 1979, B.P.R. Vittal (holotype CBS H-22421, culture ex-type CBS 185.79 = ATCC 22172).
Notes — Phylogenetic inference showed that Ac. levigata formed a well-supported clade closely related to Ac. humicola (Fig. 3). The conidia of Ac. levigata ((7–)8.5–9.5(–10) × (6–)6.5–7.5(–8) μm (av. 9 × 7 μm)) are broader than those of Ac. betulicola ((7–)9–11(–12) × (5–)5.5–6.5(–7) μm (av. 10 × 6 μm)), Ac. humicola ((7–)7.5–8.5(–10) × (5–)5.5–6.5(–7) μm (av. 8 × 6 μm)), Ac. phyllophila ((8–)8.5–9.5(–10) × 3–4 μm (av. 9 × 3 μm)) and Ac. saccharicola ((7–)7.5–8.5(–10) × 3–4 μm (av. 8 × 3 μm)).
Achroiostachys phyllophila L. Lombard & Crous, sp. nov. — MycoBank MB815922; Fig. 9
Fig. 9.
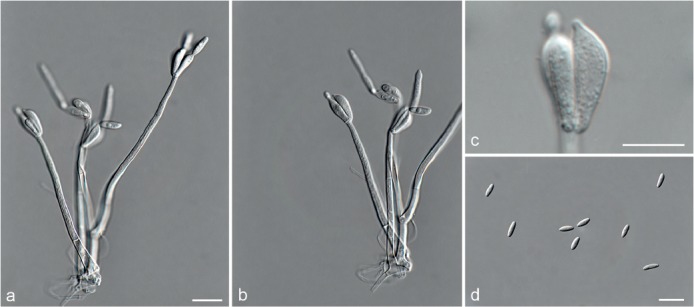
Achroiostachys phyllophila (CBS 136181). a–b. Conidiophores; c. conidiogenous cells; d. conidia. — Scale bars: a = 10 μm (apply to b); c–d = 10 μm.
Etymology. Name reflects the substrate, plant debris (Greek = phyllophilus), from which this fungus was isolated.
Conidiophores macronematous, mononematous, single or in groups, mostly unbranched, erect, straight, 1–3-septate, thin-walled, smooth, hyaline, 40–70 × 3–5 μm, bearing a whorl of 2–6 conidiogenous cells. Conidiogenous cells terminal, elongate ampulliform to ventricose, hyaline, smooth, 6–13 × 3–4 μm, with a somewhat protruding apical opening. Conidia aseptate, ellipsoidal to limoniform, smooth, hyaline, (8–)8.5–9.5(–10) × 3–4 μm (av. 9 × 3 μm), containing several small guttules, with rounded base and acutely rounded apex.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium, buff to honey, with abundant conidiophores forming on the surface of the medium, bearing slimy hyaline conidial masses, reverse on PDA buff to honey.
Material examined. SPAIN, Valencia, Requena, Sot de Chera, from plant debris, June 2010, M. Hernández & K. Rodriguez (holotype CBS H-22422, culture ex-type CBS 136181 = MUCL 53217 = FMR 11019).
Notes — Achroiostachys phyllophila formed a single lineage basal to the other phylogenetic species in the Achroiostachys clade (Fig. 3). The conidiophores of Ac. phyllophila (up to 70 μm) are intermediate in length between Ac. humicola (up to 65 μm) and Ac. levigata (up to 75 μm).
Achroiostachys saccharicola L. Lombard & Crous, sp. nov. — MycoBank MB815923; Fig. 10
Fig. 10.
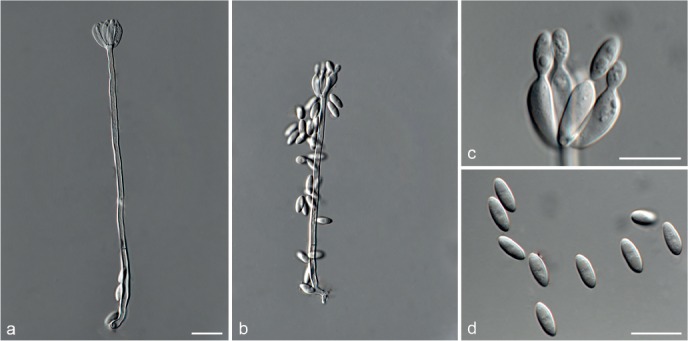
Achroiostachys saccharicola (CBS 268.76). a–b. Conidiophores; c. conidiogenous cells; d. conidia. — Scale bars: a = 10 μm (apply to b); c–d = 10 μm.
Etymology. Name reflects the host genus Saccharum from which this fungus was isolated.
Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 1–3(–4)-septate, thin-walled, smooth, hyaline, 55–140 × 3–5 μm, bearing a whorl of 2–6 conidiogenous cells. Conidiogenous cells terminal, elongate ampulliform to ventricose to subcylindrical, hyaline, smooth, 9–12 × 3–4 μm, with a somewhat protruding apical opening. Conidia acrogenous, aseptate, ellipsoidal, smooth, hyaline, (7–)7.5–8.5(–10) × 3–4 μm (av. 8 × 3 μm), containing several small guttules, rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white to pale luteous aerial mycelium with mostly immersed mycelium, rosy buff, with abundant conidiophores forming on the surface of the medium, bearing slimy hyaline conidial masses, reverse on PDA rosy buff.
Materials examined. NEPAL, Narayani, Royal Chitwan National Park, riparian forest, from dead twig, Dec. 1994, C. Decock, CBS 136393 = MUCL 39119. – TAIWAN, from root of Saccharum officinarum, 1976, T. Watanabe (holotype CBS H-18499, culture ex-type CBS 268.76).
Notes — Achroiostachys saccharicola formed a highly supported clade (Fig. 3) and can be distinguished from other species in the genus by the formation of long conidiophores (up to 140 μm).
Albifimbria L. Lombard & Crous, gen. nov. — MycoBank MB815924
Etymology. Name reflects the characteristic white fringe surrounding the sporodochia.
Type species. Albifimbria verrucaria (Alb. & Schwein.) L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial or absent, reduced to simple conidiophores. Simple conidiophores arising directly from superficial hyphae, doliiform to reniform to allantoid, hyaline, smooth. Sporodochia stromatic, superficial, cupulate to discoid, scattered to gregarious, oval to elongate or irregular in outline, with a white fringe surrounding a pale olivaceous green to dark green slimy mass of conidia. Stroma well-developed, hyaline, of textura globulosa or textura angularis. Setae simple, unbranched, septate, hyaline, verrucose, thin-walled, straight to circinate, arising from the basal stroma between the conidiophores or from the white fringe. Sporodochial conidiophores macronematous, irregularly, verticillately or penicillately branched, hyaline, smooth. Conidiogenous cells phialidic, hyaline, smooth, cylindrical to allantoid, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, ellipsoidal to fusiform to limoniform to subglobose, hyaline, smooth, sometimes bearing a funnel-shaped mucoid apical appendage.
Notes — Phylogenetic inference in this study placed the ex-neotype strain (CBS 328.52 = IMI 45541; Tulloch 1972) of Myrothecium verrucaria (= Peziza verrucaria) in a highly supported clade distant to the Myrothecium s.str. clade (Fig. 1, 2). Therefore, the new generic name, Albifimbria (Al.), is introduced here for this clade and a new combination is provided for M. verrucaria. Members of Albifimbria are characterised by the formation of verrucose setae surrounding the sporodochia and conidia sometimes bearing a funnel-shaped mucoid appendage, not observed in Myrothecium s.str. (see below).
Albifimbria lateralis L. Lombard & Crous, sp. nov. — MycoBank MB815925; Fig. 11
Fig. 11.
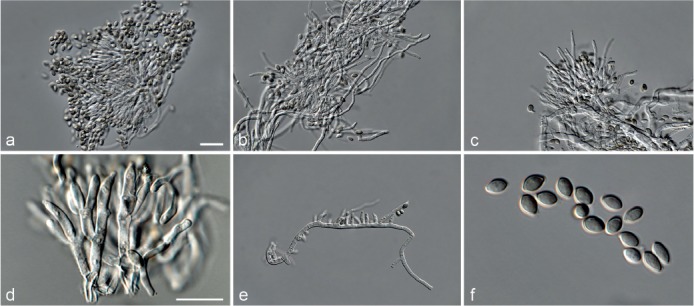
Albifimbria lateralis (CBS 117712). a–c. Sporodochial conidiomata; d. conidiogenous cells; e. simple conidiomata; f. conidia. — Scale bars: a = 20 μm (apply to b–c, e–f); d = 10 μm.
Etymology. Name reflects the characteristic lateral phialides formed on the superficial hyphae produced by this fungus.
Conidiomata sporodochial or absent, reduced to simple conidiophores. Simple conidiophores arising from superficial hyphae, borne singly or gregarious, doliiform to cylindrical, hyaline, smooth, 5–10 × 2–3 μm, sometimes borne on a short hyaline, smooth, aseptate stipe, 3–5 × 2–3 μm. Sporodochia stromatic, superficial, cupulate to discoid, scattered, rarely gregarious, oval to irregular in outline, 45–100 μm diam, 20–80 μm deep, with a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma well-developed, hyaline, of textura globulosa or textura angularis. Setae arising from the basal stroma thin-walled, hyaline, smooth to lightly verrucose, septate, unbranched, straight to flexuous to circinate, with an obtuse apices, 30–100 μm long, 2–3 μm wide. Sporodochial conidiophores arising from the basal stroma, branched, hyaline, smooth, up to 95 μm long. Conidiogenous cells phialidic, cylindrical to elongate doliiform, hyaline, smooth, 5–18 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline, fusiform to subglobose, 4–6 × 2–4 μm (av. 5 × 3 μm).
Culture characteristics — Colonies on PDA, OA and CMA with white to buff aerial mycelium with sporodochia scattered on the surface covered by slimy olivaceous green to mouse grey conidial masses, reverse on PDA honey to buff.
Material examined. USA, other collection details unknown (holotype CBS H-22423, culture ex-type CBS 117712).
Notes — Albifimbria lateralis formed a single lineage basal to the Al. terrestris clade (Fig. 2). This is the only species in this genus for which simple conidiophores were observed on the superficial hyphae. The conidia of Al. lateralis are smaller and also lack the funnel-shaped mucoid apical appendages observed for the other species in this genus.
Albifimbria terrestris L. Lombard & Crous, sp. nov. — MycoBank MB815926; Fig. 12
Fig. 12.
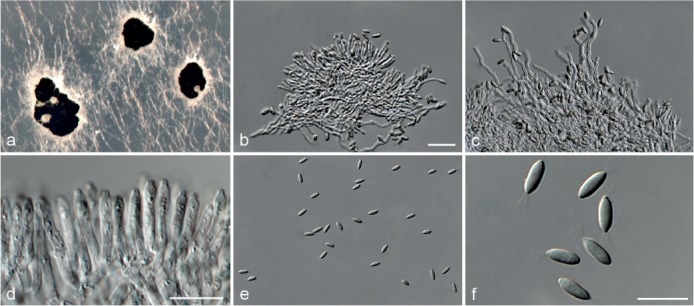
Albifimbria terrestris (CBS 126186). a. Sporodochial conidiomata on SNA; b. sporodochial conidiomata; c. setae; d. conidiogenous cells; e–f. conidia. — Scale bars: b = 20 μm (apply to c, e); d, f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, cupulate to discoid, scattered or gregarious, oval to elongate or irregular in outline, 90–300 μm diam, 35–120 μm deep, with a white setose fringe surrounding a dark olivaceous green agglutinated slimy mass of conidia. Stroma well-developed, hyaline, of textura globulosa or textura angularis. Setae arising from the basal stroma thin-walled, hyaline, verrucose, septate, unbranched, flexuous to circinate, with an obtuse apices, 50–80 μm long, 2–3 μm wide. Conidiophores arising from the basal stroma, branched, hyaline, smooth, up to 65 μm long. Conidiogenous cells phialidic, cylindrical to allantoid, hyaline, smooth to lightly verrucose, 8–15 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline, fusiform, 6–8 × 2–3 μm (av. 7 × 3 μm), bearing a funnel-shaped mucoid apical appendage.
Culture characteristics — Colonies on PDA, OA and CMA with white aerial mycelium with sporodochia scattered on the surface covered by slimy olivaceous green conidial masses, reverse on PDA buff.
Materials examined. NAMIBIA, Etosha National Park, Halali Rest Camp, south of Dolomite Hill, from soil in mopane woodlands, Apr. 2001, M. Christensen (holotype CBS H-22424, culture ex-type CBS 126186), CBS 127838. – USA, Florida, Sabal Palm swamp, Hickory Mounds impoundment, near Ecofina river, Highway 98, from unknown dead hardwood, 20 June 2000, D.T. Wicklow, CBS 109378 = NRRL 31066.
Notes — Albifimbria terrestris formed a highly supported clade (Fig. 2) and is morphologically similar to Al. verrucaria (Tulloch 1972) and Al. viridis. The conidia of Al. terrestris (6–8 × 2–3 μm) are slightly smaller than those of Al. verrucaria (6.5–8 × 2–3.5 μm; Tulloch 1972) and Al. viridis (7–8 × 2–3 μm). However, phylogenetic inference is required to accurately distinguish these species.
Albifimbria verrucaria (Alb. & Schwein.) L. Lombard & Crous, comb. nov. — MycoBank MB815927; Fig. 13
Fig. 13.
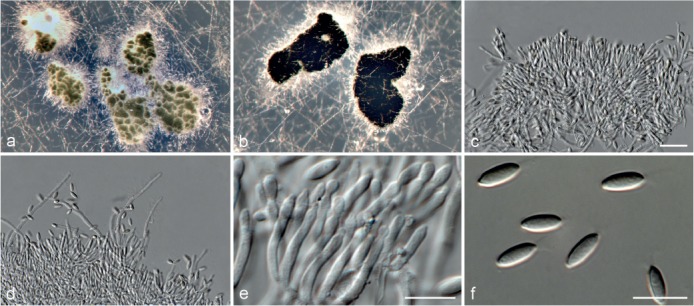
Albifimbria verrucaria (CBS 328.52). a–b. Sporodochial conidiomata on SNA; c–d. sporodochial conidiomata; e. conidiogenous cells; f. conidia. — Scale bars: c = 20 μm (apply to d); e–f = 10 μm.
Basionym. Peziza verrucaria Alb. & Schwein., Consp. Fungorum Lusat.: 340. 1805.
≡ Myrothecium verrucaria (Alb. & Schwein.) Ditmar, Deutschl. Fl., Abt. 3, Pilze Deutschl. 1-1: 7, t. 4. 1813.
= Gliocladium fimbriatum J.C. Gilman & E.V. Abbott, Iowa State Coll. J. Sci. 1: 304. 1927.
= Metarhizium glutinosum S.A. Pope, Mycologia 36: 343. 1944.
Description — See Tulloch (1972).
Materials examined. CYPRUS, from leaf of Solanum tubersum, Mar. 1946, N.C. Preston, CBS 189.46 = IMI 140060. – ENGLAND, from old canvas shoe, 1946, N.C. Preston, CBS 187.46 = IMI 140056. – JAPAN, unknown substrate, Feb. 1939, K. Saito, CBS 390.39. – JAVA, West Java, from soil under Camellia sinensis, 1968, J.H. van Emden, CBS 208.72 – PAPUA NEW GUINEA, Madang Province, Braham, from soil in tropical forest, Nov. 1995, A. Aptroot, CBS 962.95. – USA, Washington DC, from deteriorated baled cotton, 1940, G.A. Greathouse, CBS 328.52 = CBS 253.47 = IMI 045541 = MUCL 19018 = NRRL 2003 = ATCC 9095 = QM 460 (neotype of Peziza verrucaria); Louisiana, from soil, 1927, E.V. Abbott, CBS 176.27 = IMI 140054, NRRL 13972 = QM 7989 (ex-type of Gliocladium fimbriatum); locality and substrate unknown, dep. Aug. 1930, B.B. Kanouse, CBS 207.30 = IMI 140055; Hawaii, Onoma Bay, Alien Wet Forest, Scenic Route 19 at milepost 7, from basidioma of a resupinate polypore on a dead branch, 6 Nov. 2002, D.T. Wicklow, CBS 121142 = NRRL 45892. – ZIMBABWE, from Citrus fruit, 1946, N.C. Preston, CBS 188.46 = IMI140057.
Notes — Phylogenetic inference in this study placed the ex-neotype (CBS 328.85; Tulloch 1972) of Al. verrucaria in a well-supported clade, closely related but distinct to the Al. viridis clade (Fig. 2).
Albifimbria viridis L. Lombard & Crous, sp. nov. — MycoBank MB815928; Fig. 14
Fig. 14.
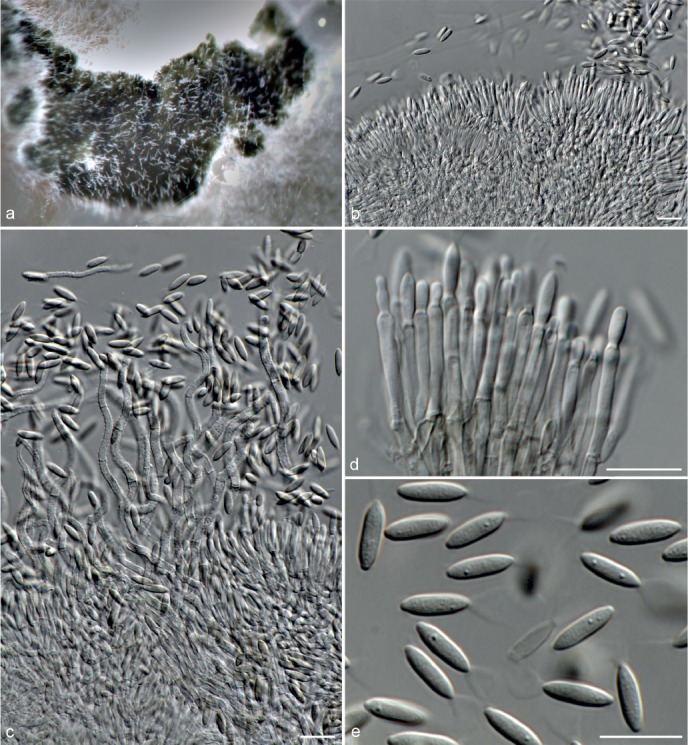
Albifimbria viridis (CBS 449.71). a. Sporodochial conidiomata on SNA; b. sporodochial conidiomata; c. setae; d. conidiogenous cells; e. conidia. — Scale bars = 10 μm.
Etymology. Name reflects the green conidial masses formed on the sporodochia by this fungus.
Conidiomata sporodochial, stromatic, superficial, cupulate to discoid, gregarious, oval to elongate or irregular in outline, 120–650 μm diam, 55–165 μm deep, with a white setose fringe surrounding a dark olivaceous green agglutinated slimy mass of conidia. Stroma well developed, hyaline, of textura globulosa or textura angularis. Setae arising from the basal stroma thin-walled, hyaline, lightly verrucose, septate, unbranched, flexuous to circinate, with an obtuse apices, 60–90 μm long, 3–4 μm wide. Conidiophores arising from the basal stroma, branched, hyaline, smooth, up to 65 μm long. Conidiogenous cells phialidic, cylindrical, hyaline, smooth, 8–15 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline, fusiform, 7–8 × 2–3 μm (av. 8 × 2 μm) bearing a funnel-shaped mucoid apical appendage.
Culture characteristics — Colonies on PDA, OA and CMA with white aerial mycelium becoming pale luteous towards the margins with sporodochia scattered on the surface covered by slimy olivaceous green conidial masses, and pale luteous exudate diffusing into the media, reverse on PDA pale luteous.
Materials examined. INDIA, Poona, from unknown substrate, June 1971, M.N. Kamat (holotype CBS H-22425, culture ex-type CBS 449.71 = BCC 37540). – IRAQ, Kurdistan, Sulaymaniyah, University of Sulaymaniyah, from air, 19 Apr. 1978, I.S. Damirdagh, CBS 244.78. – USA, Kansas, near Manhattan, Konza Prairie Research Natural Area, long term ecological research site, from soil collected in tallgrass prairie, 1986, M. Christensen, CBS 127346 = RMF 8240.
Notes — Albifimbria viridis formed a well-supported clade closely related to Al. verrucaria (Fig. 2) and is the only species that produces a pale luteous exudate that diffuses into the growth medium, which was not observed for the Al. terrestris and Al. verrucaria strains studied here. However, as mentioned, phylogenetic inference is required to accurately distinguish between these three species.
Albosynnema E.F. Morris, Mycopathol. Mycol. Appl. 33, 2: 179. 1967.
Type species. Albosynnema elegans E.F. Morris, Mycopathol. Mycol. Appl. 33, 2: 179. 1967.
Description and illustration — See Morris (1967) and Bills et al. (1994).
Notes — Morris (1967) established the synnematous asexual genus Albosynnema, based on A. elegans, and considered this genus as a member of the Stilbellaceae. A second species, A. filicicola (as A. filicola), was later introduced by Sherwood (1974), characterised by larger (26–32 × 10–11 μm), thick-walled conidia distinguishing it from the smaller (15–22 × 5–8 μm), thin-walled conidia of A. elegans (Morris 1967). However, no cultures are available at this time to determine the phylogenetic position of A. filicicola. Bills et al. (1994), with the neotypification of A. elegans, and Rossman et al. (1999) considered Albosynnema to be closely related to several other synnematous asexual genera, such as Didymostilbe (= Peethambara), Solheimia and Virgatospora. Phylogenetic inference in this study supports this, with the exception of the genus Solheimia (Fig. 1). Bills et al. (1994) suggested that S. costispora, the type species of Solheimia (Morris 1967) could be accommodated in Myrothecium based on the close morphological resemblance of the conidiomata and conidia. However, preliminary analysis of the LSU gene sequence of the neotype strain (GB 3165 = CBS 102798; Bills et al. 1994) of S. costispora was inconclusive, placing this genus with members of the Bionectriaceae and Nectriaceae. Therefore, S. costispora was not included in the phylogenetic inference in this study and requires further investigation to confirm its classification.
Alfaria Crous et al., Persoonia 32: 239. 2014. — MycoBank MB808923
Type species. Alfaria cyperi-esculenti Crous et al., Persoonia 32: 239. 2014.
Ascomata perithecial, black, hypophyllous on leaves, globose, with central ostiole; wall of 6–10 layers of thin-walled brown textura angularis, upper region of perithecium somewhat darker brown than base and sides; ostiolar region contain additional layers. Paraphyses intermingled among asci, hyaline, smooth, subcylindrical, hypha-like, with obtuse apices, septate, at times constricted at septa. Asci fasciculate, hyaline, short stipitate, subcylindrical with obtuse apices, unitunicate, with apical mechanism, containing 2–8 ascospores that are bi- to tri-seriate. Ascospores hyaline, smooth, granular, fusoid-ellipsoid, widest in middle with obtuse ends, 0–3-septate, at times with mucoid sheath or mucoid caps. Conidiomata simple or myrothecium-like, sporodochial or solitary, cupulate to discoid, superficial. Setae, when present, septate, thick-walled or thin-walled, unbranched, smooth to lightly verrucose, straight to flexuous, becoming darkly pigmented towards the base or hyaline, tapering to a sharp or obtuse point at the apice. Conidiophores macronematous, verticillately or penicillately branched, hyaline, smooth to verrucose. Conidiogenous cells phialidic, smooth and hyaline to verrucose and pigmented, allantoid to cylindrical to elongate doliiform. Conidia aggregate in dry or slimy olivaceous green to mouse grey to black masses, cylindrical to ellipsoidal to ossiform, hyaline to lightly pigmented, aseptate.
Notes — The sexual genus Alfaria (Alf.), based on A. cyperi-esculenti, was introduced by Crous et al. (2014), associated with leaf apical necrosis of Cyperus esculentus (tiger nut). In their treatment of this fungus, no mention of the asexual morph was made. Based on phylogenetic inference in this study, several isolates, tentatively identified as Myrothecium species, clustered with the ex-type (CPC 23153) of A. cyperi-esculenti in a well-supported clade (Fig. 1) and are therefore newly described in this genus. Isolate CPC 22153 was sterile and, therefore, not provided with a name here. Pending on the collection of fresh isolates, it will be a new species in Alfaria.
Alfaria caricicola L. Lombard & Crous, sp. nov. — MycoBank MB815929; Fig. 15
Fig. 15.
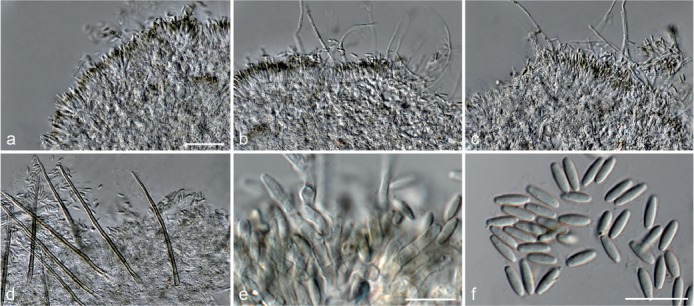
Alfaria caricicola (CBS 113567). a. Sporodochial conidiomata; b–c. sporodochia with Type I setae; d. Type II setae; e. conidiogenous cells; f. conidia; Scale bars: a = 20 μm (apply to b–d); e–f = 10 μm.
Etymology. Name reflects the host genus Carex, from which the species was isolated.
Ascomata not observed. Conidiomata sporodochial, stromatic, superficial, cupulate to discoid, scattered to gregarious, oval to elongate or irregular in outline, 180–550 μm diam, 50–100 μm deep, with a setose fringe surrounding a green agglutinated mass of conidia. Stroma well developed, hyaline, of textura globulosa or textura angularis. Setae arising from the basal stroma of two kinds: Type I scattered among the conidiogenous cells, hypha-like, thin-walled, hyaline, septate, unbranched, smooth, flexuous, with obtuse or rounded apices, 65–95 μm long, 2–5 μm wide; and Type II originating from the fringe, thick-walled, pigmented towards the bottom third, smooth, 1–3-septate, unbranched, straight, narrowing to sharp apices, 80–105 μm long, 3–5 μm wide at the broadest part. Conidiophores arising from the basal stroma, unbranched or branched, hyaline, smooth, up to 45 μm long. Conidiogenous cells phialidic, cylindrical to elongate doliiform, hyaline, smooth, 4–11 × 1–2 μm, with conspicuous collarettes and periclinal thickenings, covered by an olivaceous green mucoid layer. Conidia aseptate, smooth, hyaline, ellipsoidal, 4–6 × 1–2 μm (av. 5 × 2 μm).
Culture characteristics — Colonies on PDA with white aerial mycelium and luteous to pale luteous mycelium on the surface interspersed with sporodochia covered by slimy green conidial masses, reverse sienna in the centre becoming pale luteous towards the margins; on OA and CMA with white to pale luteous aerial mycelium, with pale luteous surface mycelium forming concentric rings interspersed with sporodochia covered by slimy green conidial masses.
Material examined. IRAN, Chirabad waterfall, on litter of Carex sp., June 2003, W. Gams & R. Zare (holotype CBS H-22426, culture ex-type CBS 113567).
Notes — Alfaria caricicola formed a single lineage (Fig. 2, 3) and can be distinguished from other members of this genus by the two types of setae arising from the sporodochia.
Alfaria ossiformis L. Lombard & Crous, sp. nov. — MycoBank MB815930; Fig. 16
Fig. 16.
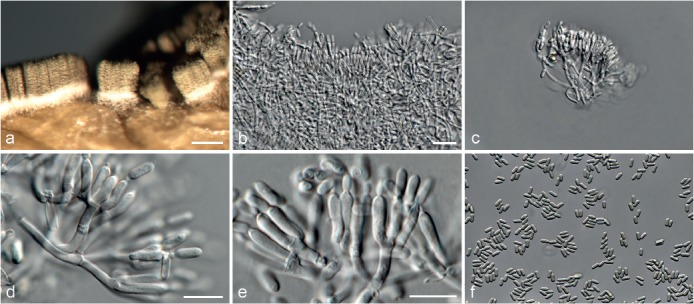
Alfaria ossiformis (CBS 324.54). a. Sporodochia with dry conidial pillars; b–c. sporodochial conidiomata; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 100 μm; b = 20 μm (apply to c, f); d–e = 10 μm.
Etymology. Name reflects the characteristically bone-shaped (ossiform) conidia produced by this fungus.
Ascomata not observed. Conidiomata sporodochial, stromatic, superficial, cupulate to discoid, scattered to gregarious, oval to elongate or irregular in outline, 25–200 μm diam, 50–350 μm deep due to dry conidial pillars on top of sporodochia, without a setose fringe. Stroma well-developed, hyaline, of textura globulosa or textura angularis. Conidiophores arising from the basal stroma, unbranched or branched, hyaline, smooth, up to 65 μm long. Conidiogenous cells phialidic, clavate to cylindrical, hyaline, smooth, 5–10 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline, ossiform to ellipsoidal, straight, (5–)6–7 × 2–3 μm (av. 6 × 2 μm).
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium giving rise to white sporodochia scattered or gregarious on the surface, covered by olivaceous green to mouse grey pillars of conidia, reverse on PDA sienna in the centre becoming pale luteous towards the margins.
Material examined. USA, Wisconsin, from prairie soil, Apr. 1954, P.A. Orpurt (holotype CBS H-22427, culture ex-type CBS 324.54 = IMI 055309 = MUCL 11831 = QM 7979 = BCC 38221).
Notes — Alfaria ossiformis represents another single lineage resolved in the Alfaria clade (Fig. 2. 3) and characteristically produces ossiform conidia not observed in other species of the genus. Furthermore, Alf. ossiformis lacks setae surrounding the sporodochia, distinguishing it from Alf. caricicola and Alf. thymi.
Alfaria putrefolia L. Lombard & Crous, sp. nov. — MycoBank MB815931
Etymology. Name reflects the substrate, rotten leaves, from which this fungus was isolated.
Cultures sterile. Alfaria putrefolia differs from its closest phylogenetic neighbours, Alf. ossiformis and Alf. terrestris, by unique fixed alleles in three loci based on alignments of the separate loci deposited in TreeBASE as study S18962: ITS positions: 75(indel), 78(C), 173(indel), 174(T), 266(indel), 500(T), 501(indel) and 521(indel); rpb2 positions: 23(T), 24(T), 53(T), 128(T), 194(A), 239(C), 254(T), 260(A), 341(T), 368(T), 380(A), 383(C), 389(G), 401(T), 407(C), 444(C), 446(A), 455(A), 549(T), 558(T), 570(T), 573(C), 591(C), 609(A), 621(G), 729(C), 756(G) and 812(G); tub2 positions: 183(A), 185(C), 188(T), 191(C), 214(T) and 335(C).
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium, buff to honey, reverse on PDA buff to honey.
Materials examined. BRAZIL, Corcovado, from rotten leaf, 12 Oct. 2002, A. Stchigel & J. Guarro (holotype CBS 112037, preserved as metabolically inactive culture, culture ex-type CBS 112037), CBS 112038.
Notes — Phylogenetic inference in this study placed Alfaria putrefolia in a highly supported clade closely related to Alf. ossiformis and Alf. terrestris (Fig. 2, 3). All attempts to induce sporulation of both strains of Alf. putrefolia on the defined media failed in this study.
Alfaria terrestris L. Lombard & Crous, sp. nov. — MycoBank MB815932; Fig. 17
Fig. 17.
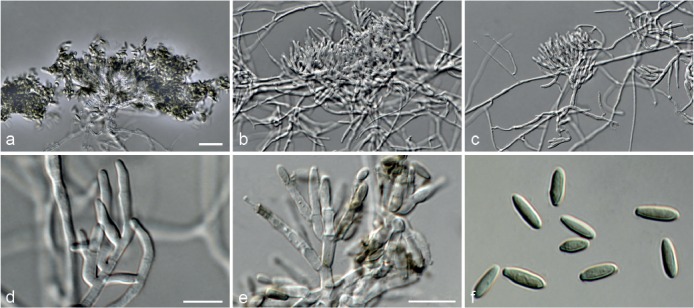
Alfaria terrestris (CBS 477.91). a–c. Sporodochial conidiomata; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c, f); d–e = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Ascomata not observed. Conidiomata sporodochial or absent, reduced to simple conidiophores. Simple conidiophores arising from hyphae, verticillately or penicillately branched, hyaline, smooth, up to 20 μm long. Sporodochia stromatic, superficial, cupulate to discoid, scattered or gregarious, oval to elongate or irregular in outline, 125–350 μm diam, 20–100 μm deep, without a setose fringe, covered by a green to black agglutinated slimy mass of conidia. Stroma well developed, hyaline, of textura globulosa or textura angularis. Sporodochial conidiophores arising from the basal stroma, unbranched or branched, initially hyaline and smooth becoming pigmented and verrucose with age covered by an olivaceous green mucoid layer, up to 50 μm long. Conidiogenous cells phialidic, cylindrical to allantoid, initially hyaline and smooth becoming pigmented and verrucose with age, 5–11 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline, ellipsoidal to limoniform, straight, (4–)5–7× 2–3 μm (av. 6 × 2 μm), with distinct basal hilum.
Culture characteristics — Colonies on PDA, OA and CMA with white to mouse grey aerial mycelium and sporodochia forming throughout the colony covered by slimy olivaceous green to black conidial masses, reverse on PDA olivaceous green to mouse grey.
Materials examined. SPAIN, from leaf litter, July 1996, R.F. Castañeda, CBS 168.97. – TURKEY, from soil, Aug. 1991, G. Turhan (holotype CBS H-22428, culture ex-type CBS 477.91). – USA, Kansas, near Manhattan, Konza Prairie Research Natural Area, long term ecological research site, from soil collected in tallgrass prairie, 1986, M. Christensen, CBS 127305 = RMF 8009.
Notes — Alfaria terrestris formed a well-supported subclade in the Alfaria clade (Fig. 2, 3). Similar to Alf. ossiformis, Alf. terrestris do not produce setae surrounding the sporodochia, distinguishing it from Alf. caricicola and Alf. thymi. Alfaria terrestris can be distinguished from Alf. ossiformis by its ellipsoidal to limoniform conidia and the formation of simple conidiophores.
Alfaria thymi L. Lombard & Crous, sp. nov. — MycoBank MB815933; Fig. 18
Fig. 18.
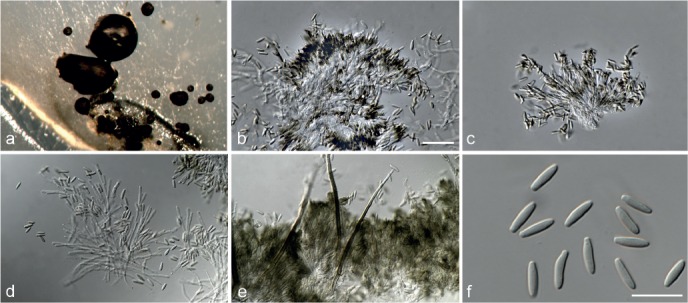
Alfaria thymi (CBS 447.83). a. Sporodochial conidiomata on SNA; b–c. sporodochia; d. conidiogenous cells; e. setae; f. conidia. — Scale bars: b = 20 μm (apply to c–e); f = 10 μm.
Etymology. Name reflects the host genus Thymus, from which this species was isolated.
Ascomata not observed. Conidiomata sporodochial, stromatic, superficial, cupulate to discoid, scattered, rarely gregarious, oval to elongate or irregular in outline, 20–120 μm diam, 80–250 μm deep, without a setose fringe surrounding a green to black agglutinated slimy mass of conidia. Stroma well-developed, hyaline, of textura globulosa or textura angularis. Setae arising from the basal stroma thick-walled, pigmented towards the bottom third, smooth to lightly verrucose, 1–3-septate, unbranched, straight to slightly curved, narrowing to a sharp apices, 70–100 μm long, 2–4 μm wide at the broadest part. Conidiophores arising from the basal stroma, unbranched or branched, initially hyaline and smooth, becoming pigmented and verrucose with age, up to 70 μm long. Conidiogenous cells phialidic, cylindrical to allantoid, initially hyaline and smooth becoming pigmented and verrucose with age, 7–18 × 1–2 μm, with conspicuous collarettes and periclinal thickenings, covered by an olivaceous green mucoid layer. Conidia aseptate, smooth, hyaline, ellipsoidal, straight to slightly curved, 5–7(–8) × 1–2 μm (av. 6 × 2 μm).
Culture characteristics — Colonies on PDA, OA and CMA with white aerial mycelium and sporodochia forming at the margins of the colony covered by slimy black conidial masses, reverse on PDA sienna to buff.
Material examined. THE NETHERLANDS, Limburg, Schin op Geul, on Thymus serpyllum, June 1983, collector unknown (holotype CBS H-22429, culture ex-type CBS 447.83).
Notes — Alfaria thymi also formed a single lineage in the Alfaria clade (Fig. 2, 3). Furthermore, it produces one type of seta surrounding the sporodochia, which distinguishes it from Alf. caricicola.
Brevistachys L. Lombard & Crous, gen. nov. — MycoBank MB815934
Etymology. Name reflects the short stachybotrys-like conidiophores characteristic of these fungi.
Type species. Brevistachys variabilis L. Lombard & Crous.
Sexual morph unknown. Conidiophores macronematous, mononematous, short, erect, solitary or in groups, unbranched or rarely branched, thin- or thick-walled, hyaline or subhyaline, smooth or verrucose, 1-septate towards the bottom third, sometimes with bulbous apice from which a whorl of 3–8 conidiogenous cells radiate. Conidiogenous cells born on the apice or stipe of the conidiophores or directly on vegetative hyphae, phialidic, ellipsoidal to subcylindrical to elongate doliiform, smooth to verrucose, hyaline to subhyaline, with conspicuous collarettes. Conidia aseptate, hyaline to dark brown, smooth to verrucose, obovoid to globose to ossiform to ellipsoidal, aggregating in slimy masses.
Notes — The genus Brevistachys (Br.) is established here to accommodate stachybotrys-like species having distinctly short conidiophores and conidiogenous cells born on conidiophores or directly from vegetative hyphae, not known for Stachybotrys s.str. (Hughes 1958, McKenzie 1991, Wang et al. 2015). Phylogenetic inference in this study showed that representatives of this group of fungi formed a distinct highly supported clade distant to the Stachybotrys s.str. Clade (Fig. 1, 3). ITS sequence data placed the ex-epitype strain (ATCC 32888; Haugland et al. 2001) of St. subsimplex (= Memnoniella subsimplex; Deighton 1960) within the Brevistachys clade (Fig. 4), and therefore a new combination is provided for this species in the genus Brevistachys. Based on the description provided by Rao (1962) for Memnoniella zingiberis (now St. zingiberis; Wang et al. 2015) this species might also belong to this genus. However, due to a lack of DNA sequence data for St. zingiberis to confirm this, we refrain from providing a new combination at this time.
Brevistachys globosa L. Lombard & Crous, sp. nov. — MycoBank MB815935; Fig. 19
Fig. 19.
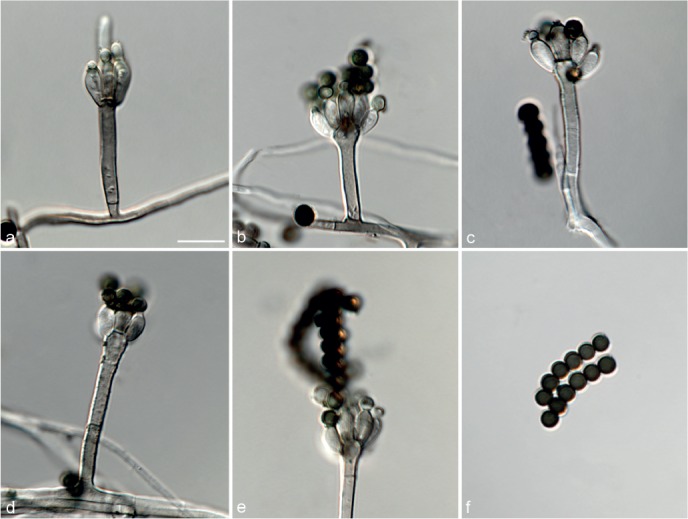
Brevistachys globosa (CBS 141056). a–e. Conidiophores; f. conidia in chains. — Scale bar = 10 μm (apply to all).
Etymology. Name reflects the globose conidia produced by this fungus.
Conidiophores simple, macronematous, mononematous, single or in groups, mostly unbranched, erect, straight to slightly flexuous, 1-septate, thin-walled, initially smooth and hyaline becoming subhyaline and lightly verrucose, 20–40 × 2–4 μm, sometimes with a slightly bulbous apice, 4–5 μm diam, bearing a whorl of 3–8 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to subcylindrical, hyaline, smooth, 5–8 × 2–3 μm, with conspicuous collarettes. Conidia acrogenous, aggregating in slimy masses, aseptate, globose, initially smooth and hyaline becoming darkly pigmented and verrucose, 3–5 × 3–5 μm (av. 4 × 3 μm), borne in chains.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium and conidiophores forming on the surface of the medium, with slimy black conidial masses, reverse on PDA mouse grey.
Materials examined. MEXICO, from Musa sp., 30 Oct. 2008, M. de Jesus Yarez-Morales (holotype CBS H-22473, culture ex-type CBS 141056 = CPC 16059), CPC 16060; Tamaulipas, from Euphorbia sp., 31 Oct. 2008, M. de Jesus Yarez-Morales, CPC 15951–3. – SRI LANKA, Anuradhapura, from dead leave of Musa sp., Mar. 1973, W. Gams, CBS 397.73.
Notes — Brevistachys globosa formed a well-supported clade (Fig. 3) and only produces globose conidia distinguishing it from other species in this genus.
Brevistachys lateralis L. Lombard & Crous, sp. nov. — MycoBank MB815936; Fig. 20
Fig. 20.
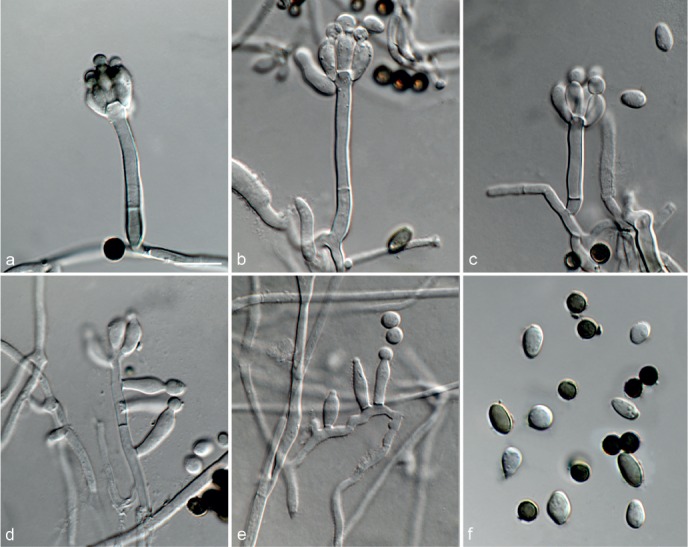
Brevistachys lateralis (CBS 141058). a–c. Conidiophores; d. conidiogenous cells carried laterally on the stipe of a conidiophore; e. conidiogenous cells carried laterally on a hyphae; f. conidia. — Scale bar = 10 μm (apply to all).
Etymology. Name reflects the characteristic lateral phialides formed on the superficial hyphae produced by this fungus.
Conidiophores simple, macronematous, mononematous, single or in groups, mostly unbranched, erect, straight to slightly flexuous, thin-walled, hyaline, 1-septate, smooth, 20–40 × 2–5 μm, sometimes with a slightly bulbous apice, 4–5 μm diam, bearing a whorl of 3–6 conidiogenous cells. Conidiogenous cells terminal or born laterally on vegetative hyphae and stipe of the conidiophores, elongate doliiform to subcylindrical, hyaline, smooth, 5–9 × 3–5 μm, with conspicuous collarettes. Conidia acrogenous, aggregating in slimy masses, aseptate, globose to ellipsoidal, initially smooth and hyaline becoming darkly pigmented and verrucose, 3–7(–8) × 3–4 μm (av. 5 × 4 μm), borne in chains.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium and conidiophores forming on the surface of the medium, with slimy black conidial masses, reverse on PDA mouse grey.
Material examined. AUSTRALIA, Queensland, Cape Tribulation, from Musa sp., 9 Aug. 2009, P.W. Crous (holotype CBS H-22430, culture ex-type CBS 141058 = CPC 17350).
Notes — Brevistachys lateralis formed a basal single lineage in the Brevistachys clade (Fig. 3) and is morphologically similar to Br. variabilis, but can be distinguished by producing globose to ellipsoidal conidia and having lateral phialides forming on the stipes of the conidiophores.
Brevistachys ossiformis L. Lombard & Crous, sp. nov. — MycoBank MB815937; Fig. 21
Fig. 21.
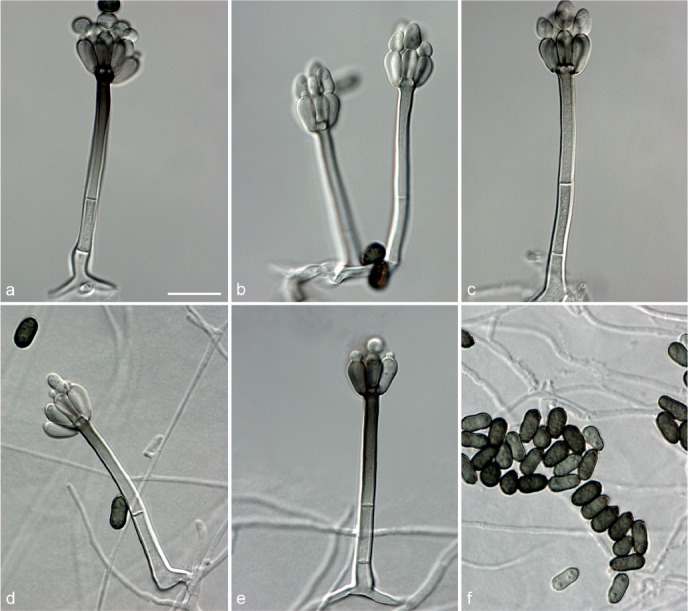
Brevistachys ossiformis (CBS 696.73). a–e. Conidiophores; f. conidia in chains. — Scale bar = 10 μm.
Etymology. Name reflects the characteristically bone-shaped (ossiform) conidia produced by this fungus.
Conidiophores simple, macronematous, mononematous, single or in groups, unbranched, erect, straight to slightly flexuous, 1-septate, thick-walled, smooth, subhyaline becoming darker towards the apice, 15–45 × 2–5 μm, sometimes with a slightly bulbous apice, 4–5 μm diam, bearing a whorl of 3–8 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to subcylindrical, subhyaline, smooth, 4–6 × 2–3 μm, with conspicuous collarettes. Conidia acrogenous, aggregating in slimy masses, aseptate, ossiform to ellipsoidal, initially smooth and hyaline becoming darkly pigmented and verrucose, 3.5–5.5 × 2–3 μm (av. 4.5 × 3 μm), borne in chains.
Culture characteristics — Colonies on PDA with mostly immersed aerial mycelium and conidiophores forming on the surface of the medium, rosy vinaceous to salmon with mouse grey to iron-grey spots of sporulation on the surface, reverse on PDA pale luteous to pale salmon; on OA with mostly immersed white aerial mycelium with mouse grey to iron grey spots of sporulation on the surface; on CMA with mostly immersed aerial mycelium, white to pale luteous, with olivaceous green spots of sporulation on the surface.
Materials examined. BRAZIL, Ceará State, Baturité Mountains, Guaramiranga, from dying leaves and branches of Musa paradisiaca, 3 July 2001, A.M. Stchigel & J. Guarro, CBS 112792 = FMR 7685. – MEXICO, Colima, from Musa sp., 17 Nov. 2008, M. de Jesus Yarez-Morales, CPC 16031. – SRI LANKA, Hakgala Gardens, from dead leaf of Zingiber sp., Aug. 1973, W. Gams (holotype CBS H-14401, culture ex-type CBS 696.73 = ATCC 32334).
Notes — Brevistachys ossiformis is characterised by having the longest conidiophores (up to 45 μm) and producing ossiform conidia, not observed for the other species in this genus. Phylogenetic inference showed that Br. ossiformis formed a well-supported clade closely related to Br. globosa (Fig. 3).
Brevistachys subsimplex (Cooke) L. Lombard & Crous, comb. nov. — MycoBank MB815938
Basionym. Stachybotrys subsimplex Cooke, Grevillea 12: 33. 1883.
≡ Memnoniella subsimplex (Cooke) Deighton, Mycol. Pap. 78: 5. 1960.
= Haplographium musae Sawada, Natn. Taiwan Univ., Coll. Agric., Spec. Publ. 8: 193. 1959.
Description and illustration — See Deighton (1960) and Wang et al. (2015).
Brevistachys variabilis L. Lombard & Crous, sp. nov. — MycoBank MB815939; Fig. 22
Fig. 22.
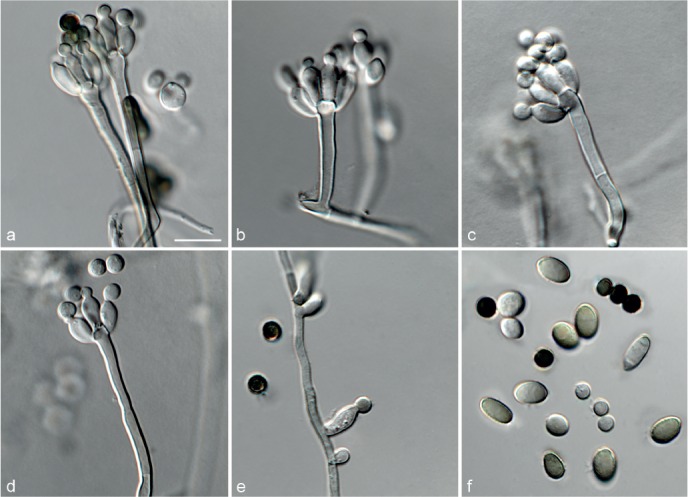
Brevistachys variabilis (CBS 141057). a–d. Conidiophores; e. conidiogenous cells carried laterally on a hyphae; f. conidia. — Scale bar = 10 μm.
Etymology. Name reflects the various conidial shapes produced by this fungus.
Conidiophores simple, macronematous, mononematous, single or in groups, mostly unbranched, erect, straight to slightly flexuous, hyaline, 1-septate, smooth, 20–40 × 2–4 μm, sometimes with a slightly bulbous apice, 4–6 μm diam, bearing a whorl of 3–8 conidiogenous cells. Conidiogenous cells terminal or born laterally on vegetative hyphae, elongate doliiform to subcylindrical, hyaline, smooth, 5–10 × 3–4 μm, with conspicuous collarettes. Conidia acrogenous, aggregating in slimy masses, aseptate, globose to obovoid to ellipsoidal, initially smooth and hyaline becoming darkly pigmented and verrucose, (3–)4–8 × 3–4 μm (av. 6 × 3 μm), borne in chains.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium and conidiophores forming on the surface of the medium, carrying slimy black conidial masses, reverse on PDA mouse grey.
Material examined. AUSTRALIA, Queensland, Cape Tribulation, from Musa sp., 9 Aug. 2009, P.W. Crous (holotype CBS H-22431, culture ex-type CBS 141057 = CPC 17349).
Notes — Brevistachys variabilis formed a single lineage, basal to the Br. globosa and Br. ossiformis clades (Fig. 3). The various conidial shapes produced by Br. variabilis distinguishes this species from other members of this genus.
Capitofimbria L. Lombard & Crous, gen. nov. — MycoBank MB815940
Etymology. Name reflects the capitate apex of the marginal hyphae surrounding the sporodochia of this fungus.
Type species. Capitofimbria compacta (R.F. Castañeda et al.) L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, scattered or rarely gregarious, oval to irregular in outline, amphigenous, pulvinate, with olivaceous green to dark green slimy mass of conidia, lacking a white setose fringe surrounding an olivaceous green to dark green slimy mass of conidia. Stroma well developed, hyaline to subhyaline, of textura globulosa and textura angularis. Marginal hyphae branched or unbranched, septate, terminating in a capitate to clavate, thick-walled cell, coarsely rugose or tuberculate, compactly grouped, pale brown-green becoming dark brown-green at the apex, encircling the sporodochia. Conidiophores macronematous, septate, tightly aggregated, subhyaline to pale olivaceous brown at the apex, smooth. Conidiogenous cells phialidic, aseptate, smooth, cylindrical to slightly subulate, with a conspicuous collarettes and periclinal thickenings. Conidia aseptate, cylindrical, olivaceous brown, smooth, rounded at both ends (adapted from Castañeda-Ruíz et al. 2008).
Notes — Phylogenetic inference in this study placed the ex-type strain (CBS 111739) of Myrothecium compactum (Castañeda-Ruíz et al. 2008) in a highly supported clade distant to the Myrothecium s.str. clade (Fig. 2). Therefore, the new generic name, Capitofimbria, is introduced here for this clade and a new combination is provided for Myr. compactum. Capitofimbria is characterised by the marginal hyphae terminating in a capitate to clavate thick-walled cell surrounding the sporodochia.
Capitofimbria compacta (R.F. Castañeda et al.) L. Lombard & Crous, comb. nov. — MycoBank MB815941; Fig. 23
Fig. 23.
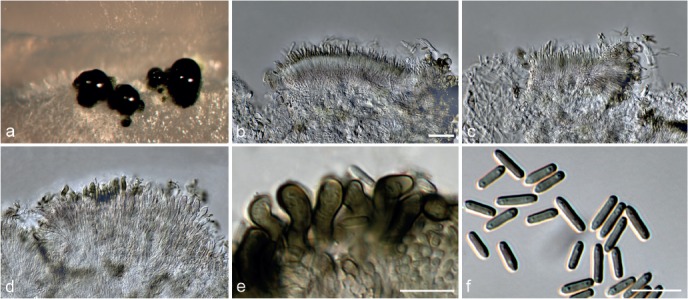
Capitofimbria compacta (CBS 111739). a. Sporodochial conidiomata on SNA; b–c. sporodochia; d–e. marginal hyphae of the sporodochia; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Basionym. Myrothecium compactum R.F. Castañeda et al., Mycotaxon 103: 5. 2008.
Description — See Castañeda-Ruíz et al. (2008).
Materials examined. BRAZIL, Rio de Janeiro, Rio de Janeiro, “Pista Cláudio Coutinho, near Pão de Açúcar, on decaying leaves of unknown plant, 12 Oct. 2002, A.M. Stchigel & J. Guarro (holotype CBS-H6584a, culture ex-type CBS 111739 = IMI 390539 = INIFAT C02/95). – ZIMBABWE, Manicaland, Chipinge Forest Botanical Reserve, mountainous rainforest, on bark of dead branch, 22 Jan. 1996, C. Decock, ZW-96-346 = MUCL 50238 (as Myrothecium flavovirens).
Cymostachys L. Lombard & Crous, gen. nov. — MycoBank MB815942
Etymology. Name reflects the characteristic cymosely branched conidiophores of this genus.
Type species. Cymostachys fabispora L. Lombard & Crous.
Sexual morph unknown. Conidiophores macronematous, mononematous, erect, mostly in groups, irregularly cymosely branched, thin-walled, hyaline to subhyaline, smooth to slightly verrucose, septate, with 3–6 conidiogenous cells radiating from the apex. Conidiogenous cells phialidic, clavate, smooth to slightly verrucose, olivaceous brown to dark brown at the apex becoming hyaline to subhyaline towards the base, with conspicuous collarettes. Conidia aseptate, olivaceous brown to dark brown, smooth to verrucose, fabiform to globose, rounded at both ends, aggregating in dark slimy masses.
Notes — The asexual genus Cymostachys (Cy.) is established here for stachybotrys-like fungi characterised by their irregularly cymosely branched conidiophores and olivaceous brown to dark brown, fabiform conidia. Phylogenetic inference in this study placed these fungi in a well-supported clade distantly related to the Stachybotrys s.str. clade (Fig. 3).
Cymostachys coffeicola L. Lombard & Crous, sp. nov. — MycoBank MB815943; Fig. 24
Fig. 24.
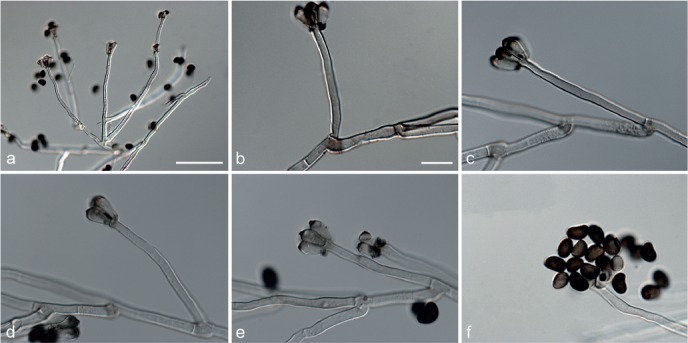
Cymostachys coffeicola (CBS 252.76). a–e. Conidiophores; f. conidia. — Scale bars: a = 50 μm; b = 10 μm (apply to c–f).
Etymology. Name reflects the host genus Coffea, from which this fungus was isolated.
Conidiophores macronematous, mononematous, mostly in groups, thin-walled, cymosely branched, erect, straight to slightly flexuous, hyaline to subhyaline, 1–2-septate, smooth to slightly verrucose, 50–135 × 4–6 μm, bearing a whorl of 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate, olivaceous brown to dark brown at the apex becoming hyaline to subhyaline towards the base, smooth to slightly verrucose, 8–12 × 4–6 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, fabiform, olivaceous brown to dark brown, smooth to verrucose, (7–)7.5–8.5(–10) × (5–)5.5–6.5(–7) μm (av. 8 × 5 μm), rounded at both ends, aggregating in dark slimy masses.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white aerial mycelium, consisting of mostly immersed mycelium, mouse grey becoming dark mouse grey towards the margins; conidiophores forming on the surface of the medium, with slimy black conidial masses, reverse on PDA pale purplish grey to dark mouse grey.
Materials examined. CUBA, La Habana, Cacabual, from Coffea arabica, Apr. 1976, W. Gams (holotype CBS-H18497, culture ex-type CBS 252.76). – THAILAND, Bangkok, Chatuchak district, Queen Sirikit Park, from Poinsettia sp., Aug. 2014, P.W. Crous, CPC 25009.
Notes — Cymostachys coffeicola can be distinguished by their longer conidiophores (up to 135 μm) compared to those of Cy. fabispora (up to 100 μm). The conidia of Cy. coffeicola ((7–)7.5–8.5(–10) × (5–)5.5–6.5(–7) μm (av. 8 × 5 μm)) are also slightly larger than those of Cy. fabispora ((6–)6.5–7.5(–8) × 4–5 μm (av. 7 × 4 μm)). Phylogenetic inference in this study distinguished these two species, placing Cy. fabispora in a well-supported clade, distinct from Cy. coffeicola (Fig. 3).
Cymostachys fabispora L. Lombard & Crous, sp. nov. — MycoBank MB815944; Fig. 25
Fig. 25.

Cymostachys fabispora (CBS 136180). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the fabiform conidia produced by this fungus.
Conidiophores macronematous, mononematous, mostly in groups, thin-walled, cymosely branched, erect, straight to slightly flexuous, hyaline to subhyaline, 1–2-septate, smooth to slightly verrucose, 40–100 × 3–8 μm, bearing a whorl of 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate, olivaceous brown to dark brown at the apex becoming hyaline to subhyaline towards the base, smooth to slightly verrucose, 6–19 × 3–5 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, fabiform, olivaceous brown to dark brown, smooth to verrucose, (6–)6.5–7.5(–8) × 4–5 μm (av. 7 × 4 μm), rounded at both ends, aggregating in dark slimy masses.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium, honey to amber, and conidiophores forming on the surface of the medium, carrying slimy black conidial masses, reverse on PDA sienna to honey.
Materials examined. CUBA, Valencia, Requena, Sot de Chera, from decaying leaf, Oct. 1993, R.F. Castañeda (holotype CBS H-22433, culture ex-type CBS 136180 = MUCL 39004 = INIFAT C93/322). – TANZANIA, Serengeti, from Aloe ferox, Feb. 2014, M.J. Wingfield, CPC 24352.
Notes — See notes under Cym. coffeicola.
Didymostilbe Henn., Hedwigia 41: 148. 1902 (non Didymostilbe Bres. & Sacc. 1902)
Type species. Didymostilbe coffeae Henn., Hedwigia 41: 148. 1902.
Description and illustration — See Seifert (1985).
Notes — Species of Didymostilbe are characterised by large, thick-walled conidia, having prominent apical and/or basal mammiform protuberances (Seifert 1985). This genus is presently classified as a member of the Bionectriaceae (Hypocreales, Hypocreomycetidae) according to MycoBank and Index Fungorum. However, phylogenetic inference in this study showed that representatives of this genus belong to the Stachybotriaceae (Fig. 1). No living cultures of Di. coffeae, the type species of this genus, is presently available for phylogenetic study. Hyde et al. (1999) linked Ornatispora gamsii to the asexual morph Di. aurantiospora based on the close proximity of both morphs in the holotype and several other collections, while at the same time reported differently looking setae or conidiophore-like structures occurring on the ascomatal wall. However, this link still needs to be proven experimentally.
Dimorphiseta L. Lombard & Crous, gen. nov. — MycoBank MB815956
Etymology. Name reflects the two types of setae surrounding the sporodochia of this fungus.
Type species. Dimorphiseta terrestris L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, cupulate, scattered to gregarious, oval to elongate or irregular in outline, surrounded by two types of setae inclosing an olivaceous green to dark green slimy mass of conidia. Stroma well developed, hyaline, of textura globulosa or textura angularis. Type I setae thin-walled, flexuous to circinate, verrucose, hyaline, tapering to an obtuse apice. Type II setae hyaline, septate, thick-walled, smooth, tapering to a sharp apice. Conidiophores macronematous, irregularly, verticillately or penicillately branched, hyaline, smooth or verrucose, sometimes covered by an olivaceous green mucoid layer. Conidiogenous cells phialidic, hyaline, smooth, cylindrical, becoming narrowed at the tip, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, fusiform, hyaline, smooth, with a funnel-shaped mucoid apical appendage.
Notes — The monotypic genus, Dimorphiseta, is introduced here for a strain (CBS 127345) that formed a single lineage basal to the Albifimbria clade and sister to Smaragdiniseta (Fig. 1, 2). Although morphologically similar to Smaragdiniseta, the Type I setae of Dimorphiseta are hyaline, and not emerald green as recorded for Smaragdiniseta (Rao & De Hoog 1983). The Type II setae of Dimorphiseta taper to sharp apices, whereas those of Smaragdiniseta narrow to an obtuse apice (Rao & De Hoog 1983). Additionally, the conidia of Dimorphiseta bear a funnel-shaped mucoid apical appendage, not observed for Smaragdiniseta (Rao & De Hoog 1983).
Dimorphiseta terrestris L. Lombard & Crous, sp. nov. — MycoBank MB815957; Fig. 26
Fig. 26.

Dimorphiseta terrestris (CBS 127345). a. Sporodochial conidiomata; b. sporodochia with Type I setae; c. sporodochia with Type II setae; d. conidiogenous cells; e. conidia. — Scale bars: a = 20 μm (apply to b); c–e = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, cupulate to discoid, scattered, rarely gregarious, oval to elongate or irregular in outline, 50–150 μm diam, 50–250 μm deep, with a setose fringe surrounding a green to black agglutinated slimy mass of conidia. Stroma well developed, hyaline, of a textura globulosa or textura angularis. Setae arising from the basal stroma, of two types: Type I setae thin-walled, hyaline, verrucose, flexuous to circinate, septate, 35–50 μm long, 2–4 μm wide narrowing to a sharp apices. Type II setae thick-walled, hyaline, smooth, septate, unbranched, straight to slightly curved, narrowing to a sharp apices, 70–95 μm long, 3–4 μm wide at the broadest part. Conidiophores arising from the basal stroma, branched, hyaline, smooth becoming verrucose, up to 45 μm long, sometimes covered by an olivaceous green mucoid layer. Conidiogenous cells phialidic, cylindrical, hyaline, smooth, 11–23 × 1–3 μm, with conspicuous collarettes and periclinal thickenings, sometimes covered by an olivaceous green mucoid layer. Conidia aseptate, smooth, hyaline, fusiform, straight, (7–)8–10 × 2–3 μm (av. 9 × 3 μm), bearing a funnel-shaped mucoid apical appendage.
Culture characteristics — Colonies on PDA, OA and CMA with white aerial mycelium and sporodochia forming on the surface of the medium and on the aerial mycelium covered by slimy olivaceous green to black conidial masses, producing a pale luteous exudate that diffuses into the medium, reverse on PDA pale luteous.
Material examined. USA, Kansas, near Manhattan, Konza Prairie Research Natural Area, long term ecological research site, from soil collected in tallgrass prairie, 1986, M. Christensen (holotype CBS H-22442, culture ex-type CBS 127345 = RMF 8243).
Globobotrys L. Lombard & Crous, gen. nov. — MycoBank MB815990
Etymology. Name reflects the characteristic smooth-walled globose conidia produced by this genus.
Type species. Globobotrys sansevieriicola (Crous & M.J. Wingf.) L. Lombard & Crous.
Sexual morph unknown. Conidiophores macronematous, mononematous, erect, solitary or in groups, mostly unbranched, thin-walled, hyaline, smooth, 1(–2)-septate, with a whorl of 3–8 conidiogenous cells radiating from the apex. Conidiogenous cells phialidic, subcylindrical to clavate to broadly reniform, smooth, thick-walled, hyaline becoming pale brown, with conspicuous collarettes. Conidia aseptate, hyaline to olivaceous brown, smooth, thick-walled, globose to broadly ellipsoidal, with a truncate hilum, containing one or two large guttules.
Notes — Phylogenetic inferences in this study placed the ex-type strain (CBS 138872) of St. sansevieriicola as a single lineage basal to the Didymostilbe and Grandibotrys clades, distinct from the Stachybotrys s.str. clade (Fig. 1, 3). Therefore, the asexual morph genus Globobotrys is established here, characterised by thick and smooth-walled conidia that distinguish it from Stachybotrys s.str. and other Stachybotrys-like genera. Hence, a new combination is provided for St. sansevieriicola in Globobotrys.
Globobotrys sansevieriicola (Crous & M.J. Wingf.) L. Lombard & Crous, comb. nov. — MycoBank MB815991
Basionym. Stachybotrys sansevieriicola Crous & M.J. Wingf., Persoonia 34: 175. 2015.
Description and illustration — See Crous et al. (2015).
Material examined. TANZANIA, Olduvai Gorge, on leaves of Sansevieria ehrenbergii, Feb. 2014, M.J. Wingfield (holotype CBS H-22220, culture ex-type CBS 138872).
Grandibotrys L. Lombard & Crous, gen. nov. — MycoBank MB815992
Etymology. Name reflects the characteristic large conidia produced by this genus.
Type species. Grandibotrys pseudotheobromae L. Lombard & Crous.
Sexual morph unknown. Conidiophores macronematous, mononematous, erect, solitary or in groups, unbranched or branched, thin-walled, hyaline, smooth, septate, with a whorl of 2–4 conidiogenous cells radiating from the apex. Conidiogenous cells phialidic, subcylindrical to clavate to fusiform, smooth, hyaline, with conspicuous collarettes. Conidia aseptate, olivaceous green to dark brown, smooth, thick-walled, limoniform to ellipsoidal, with a mammiform apex and rounded base.
Notes — The asexual genus Grandibotrys (Gra.) is established here for a group of stachybotrys-like fungi characterised by large, olivaceous green to dark brown conidia, having a mammiform apical and/or basal protrudance. Phylogenetic inference in this study showed that these fungi formed a well-supported clade distinct from the Stachybotrys s.str. clade (Fig. 1, 3). Wang et al. (2015) designated the ITS sequence AF081479 (GenBank accession number of ATCC 18905) as epitype of St. theobromae, erroneously linking it to the strain ATCC 18877 (= St. parvispora). Furthermore, Wang et al. (2015) failed to indicate the holotype of St. theobromae, rendering the epitypification invalid (Art. 9.8; McNeill et al. 2012). Phylogenetic inference of the ITS sequence data placed ATCC 18905 within the Grandibotrys clade (Fig. 4), but we refrain from providing a new combination for this species pending valid epitypification.
Grandibotrys pseudotheobromae L. Lombard & Crous, sp. nov. — MycoBank MB815993; Fig. 27
Fig. 27.
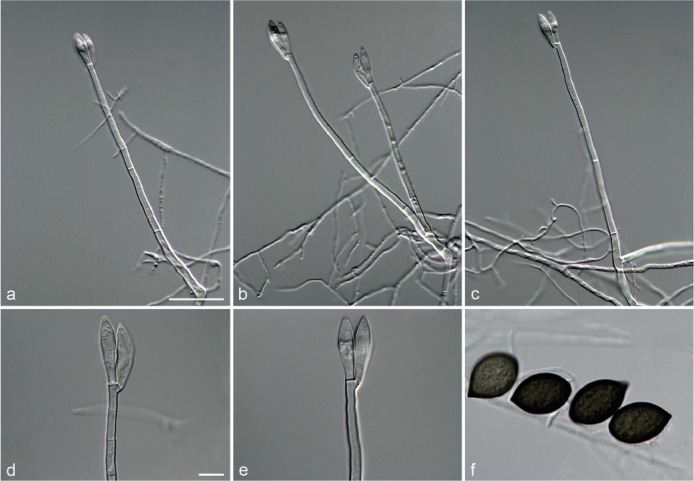
Grandibotrys pseudotheobromae (CBS 136170). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d = 10 μm (apply to e–f).
Etymology. Name reflects the morphological similarity to Stachybotrys theobromae.
Conidiophores macronematous, mononematous, single, thin-walled, unbranched or branched once, erect, straight to slightly flexuous, hyaline, septate, smooth, 95–210 × 4–8 μm, bearing a whorl of 2–4 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical to fusiform, hyaline, smooth, 20–28 × 5–9 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, limoniform, olivaceous brown to dark brown, smooth, thick-walled, (20–)21–25(–27) × (14–)14.5–15.5(–17) μm (av. 23 × 15 μm), with a mammiform apex and rounded base.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to pale luteous aerial mycelium with conidiophores forming on the aerial mycelium, carrying slimy olivaceous green to black conidial masses, reverse on PDA pale luteous to buff.
Materials examined. NEPAL, Narayani, Royal Chitwan National Park, Sauraha area, from decaying wood, Dec. 1994, C. Decock (holotype CBS H-22443, culture ex-type CBS 136170 = MUCL 39293), CBS 136391 = MUCL 39289.
Notes — Grandibotrys pseudotheobromae is morphologically reminiscent of St. theobromae but can be distinguished by having longer conidiophores (up to 210 μm) compared to those of St. theobromae (up to 200 μm; Hansford 1943). The conidia of Gra. pseudotheobromae ((20–)21–25(–27) × (14–)14.5–15.5(–17) μm (av. 23 × 15 μm)) are also slightly smaller than those of St. theobromae (20–28 × 15–18 μm; Hansford 1943).
Grandibotrys xylophila L. Lombard & Crous, sp. nov. — MycoBank MB815994; Fig. 28
Fig. 28.

Grandibotrys xylophila (CBS 136179). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d = 10 μm (apply to e–f).
Etymology. Name reflects the substrate, decaying wood, from which this fungus was isolated.
Conidiophores macronematous, mononematous, single, thin-walled, unbranched or branched once, erect, straight to slightly flexuous, hyaline, septate, smooth, 95–200 × 4–9 μm, bearing a whorl of 2–4 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical to fusiform, hyaline, smooth, 22–30 × 6–9 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, limoniform to ellipsoidal, olivaceous brown to dark brown, smooth, thick-walled, (23–)27–35(–39) × (9–)12–14 μm (av. 31 × 13 μm), with a mammiform apex and rounded base.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to pale luteous aerial mycelium with conidiophores forming on the aerial mycelium, carrying slimy olivaceous green to black conidial masses and producing a pale luteous exudate that diffuse into the medium, reverse on PDA pale luteous to buff.
Material examined. NEPAL, Narayani, Royal Chitwan National Park, Sauraha area, from decaying wood, Dec. 1994, C. Decock (holotype CBS H-22444, culture ex-type CBS 136179 = MUCL 39288).
Notes — Grandibotrys xylophila formed a single lineage basal to the highly supported clade representing Gra. pseudotheobromae (Fig. 3). The conidia of Gra. xylophila ((23–)27–35(–39) × (9–)12–14 μm (av. 31 × 13 μm)) are larger than those of Gra. pseudotheobromae ((20–)21–25(–27) × (14–)14.5–15.5(–17) μm (av. 23 × 15 μm)) and St. theobromae (20–28 × 15–18 μm; Hansford 1943).
Gregatothecium L. Lombard & Crous, gen. nov. — MycoBank MB815995
Etymology. Name reflects the gregarious conidiomata produced by this fungus.
Type species. Gregatothecium humicola L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, consisting of numerous aggregated penicillate conidiophores, or reduced to separate penicillate or subverticillate conidiophores. Sporodochia stromatic, superficial, scattered or gregarious, orbicular or irregular in outline, without a setose fringe forming an olivaceous green slimy mass of conidia from which emerge hyphal-like setae. Stroma poorly developed, hyaline to subhyaline, of textura globulosa and textura angularis. Setae arising from the basal stroma, adjacent to cells that give rise to the conidiophore stipe, unbranched, straight to flexuous, hyaline, thin-walled, septate, tapering to an acutely to subobtusely rounded apex. Conidiophores consist of a stipe, penicillate arrangement of fertile branches and an extension of the stipe. Stipe hyaline to subhyaline, septate becoming constricted at the septum, smooth. Conidiogenous apparatus with several series of aseptate branches (up to 6), hyaline, smooth, with terminal branches producing 3–6 conidiogenous cells. Conidiogenous cells phialidic, hyaline, aseptate, cylindrical to subcylindrical, straight to slightly curved, apex with minute periclinal thickening and an inconspicuous collarette. Conidia aseptate, cylindrical to subcylindrical, rounded at both ends, held in fascicles in olivaceous green slime.
Notes — The monophyletic asexual genus Gregatothecium (Gre.) is established here for a single phylogenetic lineage (Fig. 1, 2), morphologically reminiscent of the nectriaceous genera Calonectria (Lombard et al. 2010, 2015a, b), Cylindrocladiella (Lombard et al. 2012, 2015b) and Dematiocladiella (Crous et al. 2005, Lombard et al. 2015b). This genus can be distinguished from these three nectriaceous genera by the slimy olivaceous green conidial masses produced on the conidiophores and sporodochia, and its phylogenetic placement in the Stachybotriaceae. This fungus is also morphologically reminiscent of Pa. roridum, but lacks the straight to curling, repeatedly branched marginal hyphae as recorded by Tulloch (1972).
Gregatothecium humicola L. Lombard & Crous, sp. nov. — MycoBank MB815996; Fig. 29
Fig. 29.
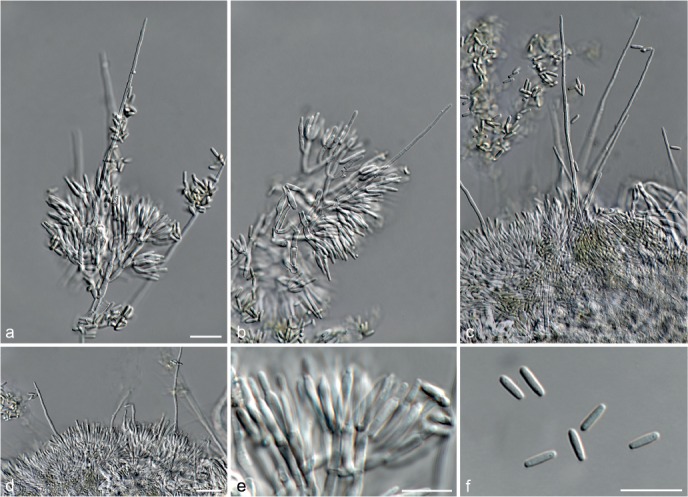
Gregatothecium humicola (CBS 205.96). a–b. Penicillate conidiophores; c–d. sporodochial conidiophores; e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d = 20 μm; e–f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, consisting of numerous aggregated penicillate conidiophores, or reduced to separate penicillate or subverticillate conidiophores. Sporodochia stromatic, superficial, scattered or gregarious, orbicular or irregular in outline, 150–600 μm diam, 65–100 μm deep, without a setose fringe, with a green to black agglutinated slimy mass of conidia. Stroma poorly developed, hyaline to subhyaline, smooth, of textura globulosa and textura angularis. Setae arising from the basal stroma, adjacent to cells that give rise to the conidiophore stipe, unbranched, straight to flexuous, hyaline, thin-walled, septate, 120–140 μm long, 2–3 μm wide tapering to an acutely to subobtusely rounded apex. Conidiophores consist of a stipe, penicillate arrangement of fertile branches and an extension of the stipe. Stipes unbranched, hyaline to subhyaline, septate becoming constricted at the septum, smooth, 10–30 × 2–4 μm; stipe extension septate, straight to flexuous, unbranched, hyaline, thin-walled, 65–100 μm long, 2–3 μm wide at the basal septum, tapering to an acutely to subobtusely rounded apex. Conidiogenous apparatus with several series of aseptate, hyaline branches; primary branches aseptate, unbranched, smooth, 6–10 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 5–7 × 2–3 μm; tertiary branches and additional branches (–6) aseptate, unbranched, smooth, 4–6 × 1–3 μm, each terminal branch producing 3–6 conidiogenous cells. Conidiogenous cells phialidic, hyaline, aseptate, cylindrical to subcylindrical, straight to slightly curved, 6–12 × 1–2 μm, apex with minute periclinal thickening and inconspicuous collarette. Conidia aseptate, smooth, hyaline, cylindrical to subcylindrical, rounded at both ends, 5–6 × 1–2 μm (av. 5 × 1 μm), lacking funnel-shaped mucoid appendages, held in fascicles in olivaceous green slime.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white aerial mycelium that becomes mostly immersed turning buff to sienna. Sporodochia forming on the surface of the medium and on the aerial mycelium covered by slimy olivaceous green to black conidial masses, producing a buff to luteous exudate that diffuses into the medium, reverse on PDA buff to sienna.
Material examined. PAPUA NEW GUINEA, Madang, Jais Aben, from soil along coral reef coast, Nov. 1995, A. Aptroot (holotype CBS H-22445, culture ex-type CBS 205.96).
Inaequalispora L. Lombard & Crous, gen. nov. — MycoBank MB815997
Etymology. Name reflects the asymmetrically ellipsoidal conidia produced by this fungus.
Type species. Inaequalispora prestonii (M.C. Tulloch) L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, scattered or gregarious, orbicular or irregular in outline, with a white setose fringe surrounding an olivaceous green to dark green slimy mass of conidia. Stroma poorly developed, hyaline, composed of textura angularis. Setae thick-walled, septate, flexuous, hyaline, tapering to an obtuse apice that becomes lightly verrucose. Conidiophores macronematous, penicillately branched, hyaline, smooth to lightly verrucose at the base. Conidiogenous cells phialidic, hyaline, smooth, subcylindrical, becoming narrowed at the tip, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, fusiform to ellipsoidal to asymmetrically ellipsoidal, hyaline, smooth, sometimes with a slightly curved acute apex and a narrow truncate base, with a funnel-shaped mucoid apical appendage (adapted from Tulloch 1972 and Nag Raj 1995).
Notes — Nag Raj (1995) questioned Tulloch’s (1972) treatment of Myr. prestonii and provided a narrower concept for this species, while retaining the holotype as IMI 160372 (= isotype strain CBS 175.73). However, phylogenetic inference in this study showed that the isotype of Myr. prestonii formed a unique single lineage, sister to the Septomyrothecium s.str. clade and distant to the Myrothecium s.str. clade (Fig. 1). Therefore, the genus Inaequalispora is introduced here and a new combination is provided for Myr. prestonii.
Inaequalispora prestonii (M.C. Tulloch) L. Lombard & Crous, comb. nov. — MycoBank MB815998; Fig. 30
Fig. 30.
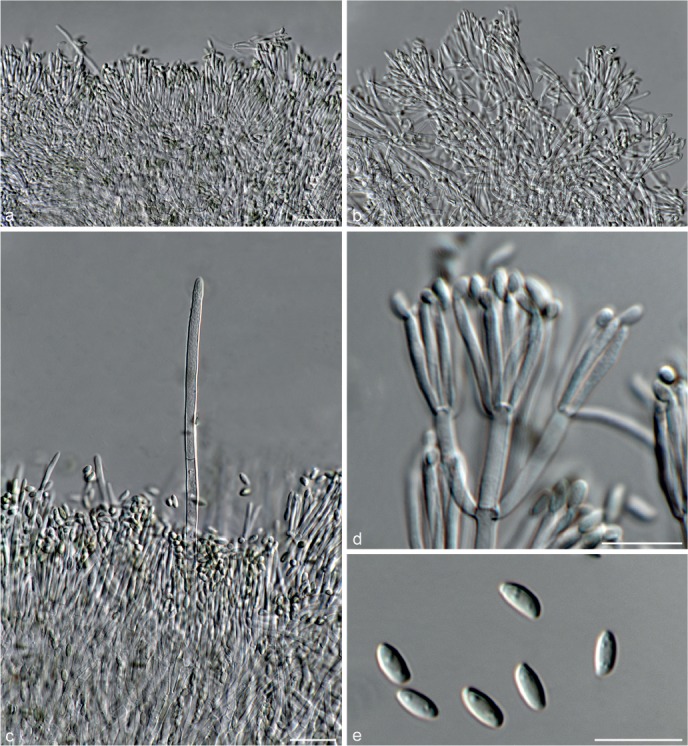
Inaequalispora prestonii (CBS 175.73). a–b. Sporodochial conidiomata; c. seta; d. conidiogenous cells; e. conidia. — Scale bars: a = 20 μm (apply to b); c = 20 μm; d, e = 10 μm.
Basionym. Myrothecium prestonii M.C. Tulloch, Mycol. Pap. 130: 12. 1972.
Description — See Tulloch (1972) and Nag Raj (1995).
Material examined. MALAYSIA, from forest soil, July-Aug. 1971, W.H. Tong (holotype CBS H-7392, culture isotype CBS 175.73 = IMI 160372 = ATCC 24427).
Kastanostachys L. Lombard & Crous, gen. nov. — MycoBank MB815999
Etymology. Name reflects the brown (Greek = kastanós) stachybotrys-like conidiophores in this genus.
Type species. Kastanostachys aterrima (Fuckel) L. Lombard & Crous.
Ascomata perithecial, superficial with base slightly immersed, solitary or in groups of 2–4, globose to subglobose, ostilate, papillate, black, glabrous, bearing conidiophores identical with those arising from the substrate. Ascomatal wall textura prismatica, consisting of two layers: outer layer of thick-walled, melanised cells; inner layer of hyaline, compressed, elongated cells. Asci unitunicate, 8-spored, cylindrical, shortly stipitate, truncate to broadly rounded at the apex, with a visible apical annulus. Paraphyses abundant among asci, branching, anastomosing, septate, hyaline. Ascospores fusiform, 1-septate, not or slightly constricted at the septum, hyaline, smooth, enclosed in a hyaline sheath that disintegrates with age, each cell containing 1–2 oil drops. Conidiophores macronematous, unbranched, thick-walled, septate, erect, with one to occasionally three percurrent proliferations, dark brown, paler towards the apex and enlarging to form a more or less distinct vesicle. Vesicle pale brown to subhyaline, clavate, smooth, bearing phialides in the upper part. Phialides aseptate, hyaline, cylindrical to clavate, straight or curved with indistinct collarettes. Conidia hyaline, smooth, ellipsoidal, slightly truncate at the base, forming a slimy head on the conidiophores (adopted from Réblová 1998).
Notes — Réblová (1998) compared the type material of Melanomma aterrima (Fuckel 1872) to a similar fungus (M.R. 871/96 = CBS 101310) isolated from a decayed stump of Fagus sylvatica collected in Czech Republic and concluded that both were conspecific. Based on the ascomatal morphology, Réblová (1998) then transferred this species to the sexual genus Chaetosphaeria (Chaetosphaeriaceae, Chaetosphaeriales; Réblová et al. 1999, Huhndorf et al. 2004) and linked it to an undescribed Custingophora sp. (Gondwanamycetaceae, Microascales; Réblová et al. 2011). Our phylogenetic inference in this study showed that the Czech isolate (CBS 101310) belongs to the Stachybotriaceae (Fig. 1), and therefore, a new combination is provided for this species in the newly established genus Kastanostachys. As no living type material is available for C. aterrima, we designate CBS 101310 as epitype.
Kastanostachys aterrima (Fuckel) L. Lombard & Crous, comb. nov. — MycoBank MB816000; Fig. 31
Fig. 31.

Kastanostachys aterrima (CBS 101310). a–b. Conidiophores; c. conidia; d–e. conidiogenous cells. — Scale bars: a–b = 50 μm; c–e = 10 μm.
Basionym. Melanomma aterrima Fuckel, Jahrb. Nassauischen Vereins Naturk. 25–26, Nachtr. 1: 304. 1872.
≡ Zignoella aterrima (Fuckel) Sacc., Michelia 1: 346. 1878.
≡ Chaetosphaeria aterrima (Fuckel) Réblová, Czech Mycol. 50: 165. 1998.
Typification. CZECH REPUBLIC, Southern Bohemia, Šumava Mts, glacial cirque of the lake Černé jezero near Železná Ruda, on a decayed stump of Fagus sylvatica, 23 Oct. 1996, M. Réblová (epitype of Kastanostachys aterrima designated here, PRM 934970, MBT204281, culture ex-epitype CBS 101310). – GERMANY, Aepfelbach, on a branch of Fagus sylvatica, autumn, leg. Fuckel (G – holotype of Melanomma aterrima).
Description and illustration — See Réblová (1998).
Additional material examined. F. Petrak, Flora Bohemiae et Moraviae exsiccate Lfg. 1, no. 2: CZECH REPUBLIC, Moravia, Hranice na Moravě (Mährisch Weisskirchen), military school park, on decayed wood of a stump, 3 Oct. 1912, J. Petrak (as Eriosphaeria vermicularioides, PRM 777947).
Melanopsamma Niessl, Verh. Naturf. Vereins Brünn 14: 200. 1876
Type species. Melanopsamma pomiformis (Pers.) Sacc.
Ascomata perithecial, superficial or borne on a poorly developed stroma, solitary or in groups, ostilate, papillate, globose below and flattened above with the upper surface tending to collapse inward forming a shallow cup, black, bearing conidiophores identical with those arising from the substrate. Ascomatal wall usually covered with a wax-like secretion, consisting of two layers: outer layer of thick-walled, melanised cells; inner layer of hyaline, thin-walled, elongated cells. Asci unitunicate, 8-spored, cylindrical to clavate, truncate or occasionally rounded at the thickened apex. Paraphyses abundant among asci, hyaline, anastomosing. Ascospores ellipsoidal, 1-septate, slightly constricted at the septum, hyaline, smooth (adopted from Booth 1957). Conidiophores macronematous, mononematous, erect, solitary or in groups, unbranched, thick-walled, hyaline, smooth or slightly verrucose, 1–5-septate, with bulbous apice from which 4–10 conidiogenous cells radiate. Conidiogenous cells phialidic, subcylindrical to clavate to elongate doliiform, smooth, hyaline, with conspicuous collarettes. Conidia aseptate, hyaline to olivaceous brown to dark brown, smooth to verrucose, limoniform to obovoid to globose to ellipsoidal, containing 1–2 small guttules, with rounded ends or with acute apex and rounded base.
Notes — The sexual genus Melanopsamma (Mel.) is re-instated here after Wang et al. (2015) synonymised this genus under Stachybotrys based on ITS sequence analysis and its link to St. albipes (Jong & Davies 1976, Castlebury et al. 2004, Wang et al. 2015). Phylogenetic inference in this study showed that these fungi formed a well-supported clade, distant to the Stachybotrys s.str. clade (Fig. 1, 3). Booth (1957) studied the type and other authentic material of Mel. pomiformis, the type species of the genus, and designated Persoon’s No. 910 (in Herb. L) as lectotype. As there is no living type material presently available for Mel. pomiformis, we select to designate CBS 101322 as ex-epitype for this species.
Melanopsamma pomiformis (Pers.) Sacc., Michelia 1: 347. 1878 — Fig. 32
Fig. 32.
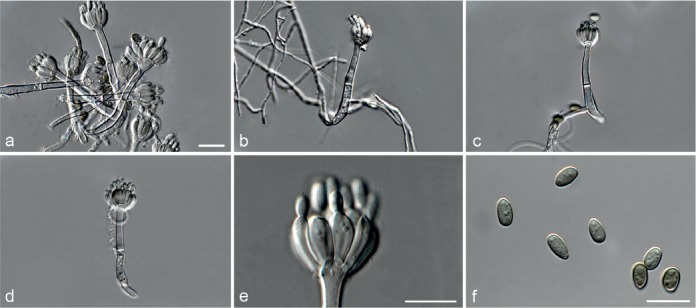
Melanopsamma pomiformis (CBS 101322). a–d. Conidiophores; e. conidiogenous cells; f. conidia. — Scale bars: a = 10 μm (apply to b); e–f = 10 μm.
≡ Sphaeria pomiformis Pers., Syn. Meth. Fung.: 65. 1801.
≡ Melanomma pomiformis (Pers.) Nitschke ex Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 159. 1870.
≡ Eriosphaeria pomiformis (Pers.) Sacc., Michelia 1: 33. 1877.
≡ Psilosphaeria pomiformis (Pers.) Cooke, Grevillea 16: 50. 1887.
≡ Nectria pomiformis (Pers.) Höhn., Sitzungsber. Akad. Wiss. Wien, Math.-Naturwiss. Kl. Abt. I 128: 568. 1919.
≡ Chaetosphaeria pomiformis (Pers.) E. Müll., Beitr. Kryptogamenfl. Schweiz 11: 588. 1962.
= Sporocybe albipes Berk. & Broome, Ann. Mag. Nat. Hist., Ser. II, 8: 19. 1851.
≡ Fuckelina albipes (Berk. & Broome) Höhn., Zentralbl. Bakt. ParasitKde, Abt II 60: 14. 1923.
≡ Stachybotrys albipes (Berk. & Broome) S.C. Jong & E.E. Davis, Mycotaxon 3: 425. 1976.
Typification. Herb. L on dry wood of unknown host, Persoon’s No. 910: 264–737 (lectotype fide Booth). – CZECH REPUBLIC, Southern Bohemia, Šumava Mts, glacial cirque of the lake Černé jezero near Železná Ruda, on a decayed stump of Fagus sylvatica, 7 Nov. 1997, K. Prášil (epitype of Mel. pomiformis designated here, PRM 934971, MBT204282, culture ex-epitype CBS 101322).
Ascomata not examined. Conidiophores simple, macronematous, mononematous, single or in groups, thick-walled, unbranched, erect, straight, hyaline, 1–2-septate, smooth, 30–60 × 5–7 μm, with a bulbous apice, 10–25 μm diam, bearing a whorl of 4–10 conidiogenous cells. Conidiogenous cells phialidic, clavate to elongate doliiform, hyaline, smooth, 7–11 × 2–5 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, obovoid to globose to ellipsoidal, hyaline to olivaceous brown, smooth to slightly verrucose, (5–)6.5–7.5(–9) × (3–)3.5–4.5(–5) μm (av. 7 × 4 μm), rounded at both ends or with an acute apex and rounded base.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium with conidiophores forming on the surface of the medium, carrying slimy olivaceous green conidial masses, reverse on PDA pale olivaceous green.
Additional materials examined. ITALY, Bari, on decaying bark of Fagus sylvatica, 1990, D. Sisto, CBS 325.90. – SWEDEN, Uppland, Dalby, Ekbacken, from Tilia cordata, 5 Mar. 1988, K. Holm & L. Holm, CBS 114119 = UPSC 2528.
Notes — CBS 101322 is designated as ex-epitype of Mel. pomiformis here based on its close morphological resemblance (Booth 1957, Jong & Davies 1976). Phylogenetic inference of the ITS sequence data placed an authentic strain (ATCC 18873; Booth 1957, Jong & Davies 1976) in a well-supported clade with CBS 101322 (Fig 4). The multi-locus phylogenetic inference showed that Mel. pomiformis formed a highly supported clade (Fig. 1, 3) and can be distinguished from Mel. xylophila by its much shorter conidiophores (up to 60 μm vs up to 200 μm) and smaller, smooth-walled conidia.
Melanopsamma xylophila L. Lombard & Crous, sp. nov. — MycoBank MB816001; Fig. 33
Fig. 33.
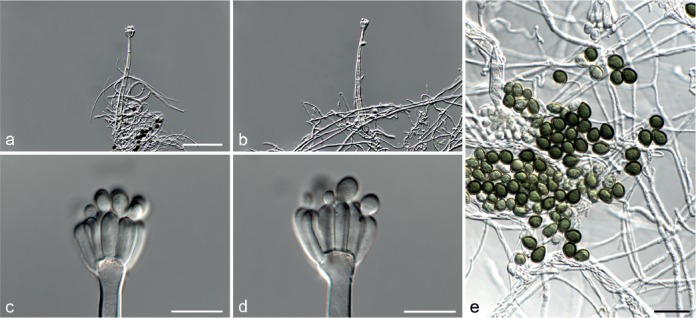
Melanopsamma xylophila (CBS 100343). a–b. Conidiophores; c–d. conidiogenous cells; e. conidia. — Scale bars: a = 50 μm (apply to b); c–e = 10 μm.
Etymology. Name reflects the substrate, decaying wood, from which this fungus was isolated.
Ascomata not observed. Conidiophores simple, macronematous, mononematous, single or in groups, thick-walled, unbranched, erect, straight to slightly flexuous, hyaline, 1–5-septate, smooth, 105–200 × 8–15 μm, with a bulbous apice, 10–20 μm diam, bearing a whorl of 4–10 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical, hyaline, smooth, 8–13 × 3–4 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, limoniform to obovoid, olivaceous brown to dark brown, verrucose, (7–)7.5–8.5(–9) × (5–)5.5–6.5(–7) μm (av. 8 × 6 μm), with an acute apex and rounded base.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium and conidiophores forming on the aerial mycelium and surface of the medium, carrying slimy black conidial masses, reverse on PDA white to rosy buff.
Material examined. JAPAN, Nagano Prefecture, Kakuma Valley, from decaying wood, 13 Aug. 1997, W. Gams (holotype CBS 100343, preserved as metabolically inactive culture, culture ex-type CBS 100343).
Notes — Melanopsamma xylophila formed a single lineage in the Melanopsamma clade, distinct from Mel. pomiformis (Fig. 3).
Memnoniella Höhn., Zentralbl. Bakt. ParasitKde, Abt. II, 60: 16. 1923
= Spinomyces Saito, J. Ferment. Technol. 17: 2. 1939 (non Spinomyces Bat. & Peres).
Type species. Memnoniella echinata (Riv.) Galloway.
Sexual morph unknown. Conidiophores macronematous, mononematous, erect, solitary or in groups, unbranched, thick-walled, hyaline to pale olivaceous brown, smooth to slightly verrucose at the apex, 1–4-septate, with 3–12 conidiogenous cells radiating from the apex. Conidiogenous cells phialidic, subcylindrical to clavate, smooth, hyaline to pale olivaceous brown, with conspicuous collarettes. Conidia aseptate, initially hyaline becoming olivaceous brown to dark brown, smooth to verrucose, thick-walled, ellipsoidal to globose to reniform, rounded at both ends, sometimes carried in dry or slimy chains (adapted from Galloway 1933 and Jong & Davis 1976).
Notes — Wang et al. (2015) demoted Memnoniella to synonymy under Stachybotrys based on priority of the two names following the argument by Smith (1962) that conidial disposition in dry or slimy chains is not sufficient evidence to segregate these two genera. This was further corroborated by ITS sequence data of a limited number of taxa that included nine species of Stachybotrys and three of Memnoniella (Haugland et al. 2001). Phylogenetic inference in this study, which included a larger sampling of taxa and more loci, clearly showed that the isolate treated by Galloway (1933) as Mem. echinata (CBS 216.32) grouped in a well-supported clade distinct from the Stachybotrys s.str. clade (Fig. 1, 3). Therefore, we select to resurrect the genus Memnoniella and epitypify the type species of the genus, Mem. echinata, using Galloway’s strain (see Galloway 1933 for specimen comparisons). Memnoniella can be distinguished from Stachybotrys by the formation of mostly smooth, thick-walled and unbranched conidiophores giving rise to conidia sometimes carried in dry chains.
Memnoniella brunneoconidiophora L. Lombard & Crous, sp. nov. — MycoBank MB816002; Fig. 34
Fig. 34.
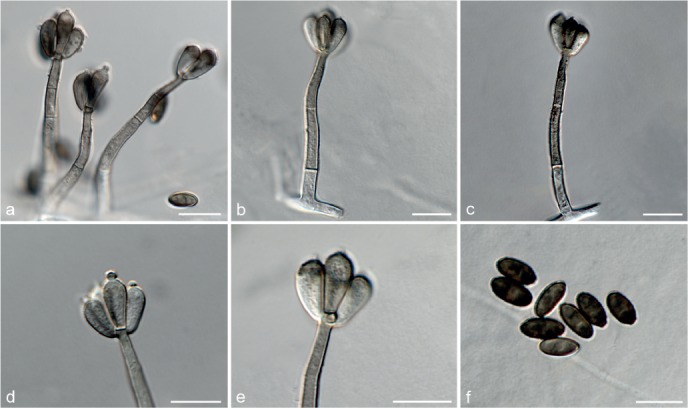
Memnoniella brunneoconidiophora (CBS 136191). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars = 10 μm.
Etymology. Name reflects the olivaceous brown conidiophores of this fungus.
Conidiophores simple, macronematous, mononematous, single, thick-walled, unbranched, erect, straight to slightly flexuous, hyaline at the base becoming olivaceous brown towards the apex, 1–2-septate, smooth to slightly verrucose, 30–45 × 3–5 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical, olivaceous brown, smooth, 7–11 × 3–5 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal, olivaceous brown to dark brown, verrucose, thick-walled, (6–)7.5–8.5(–9) × (3–)3.5–4.5(–5) μm (av. 8 × 4 μm), rounded at both ends or sometimes bearing an apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to aerial mycelium, producing a luteous extracellular pigment that diffuse into the media, with abundant conidiophores forming on the aerial mycelium and surface of the media, carrying dry to slimy mouse grey to black conidial masses, reverse on PDA pale luteous.
Materials examined. VENEZUELA, Colonia Tovar, from decayed leaf, 25 Nov. 2000, R.F. Castañeda-Ruiz (holotype CBS H-22446, culture ex-type CBS 136191 = MUCL 43313), CBS 109477.
Notes — Memnoniella brunneoconidiophora formed a highly supported clade closely related to Mem. ellipsoidea and Mem. putrefolia (Fig. 3). This species can be distinguished from both latter species by its short conidiophores (up to 45 μm) compared to those of Mem. ellipsoidea (up to 140 μm) and those of Mem. putrefolia (up to 90 μm). The conidia of Mem. brunneoconidiophora ((6–)7.5–8.5(–9) × (3–)3.5–4.5(–5) μm (av. 8 × 4 μm)) are slightly smaller than those of Mem. ellipsoidea ((8–)8.5–9.5(–10) × (4–)4.5–5.5(–6) μm (av. 9 × 5 μm)) and Mem. putrefolia ((8–)9–11 × (4–)4.5–5.5(–6) μm (av. 10 × 5 μm)).
Memnoniella dichroa (Grove) L. Lombard & Crous, comb. nov. — MycoBank MB816003; Fig. 35
Fig. 35.
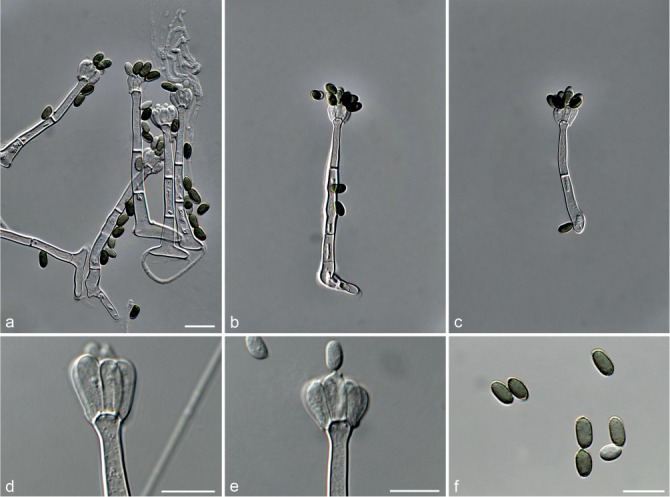
Memnoniella dichroa (CBS 526.50). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d–f = 10 μm.
Basionym. Stachybotrys dichroa Grove, J. Bot., Lond. 24: 201. 1886.
Description and illustrations — See Grove (1886) and Wang et al. (2015).
Materials examined. ENGLAND, Exeter, Devon, from herbaceous stem, 15 Sept. 1947, M.B. Ellis, CBS 526.50 = ATCC 18917 = IMI 017506 = MUCL 9482. – THE NETHERLANDS, Baarn, garden Molenweg, from leaf litter of Ilex aquifolium, Sept. 2008, W. Gams, CBS 123800.
Notes — Phylogenetic inference of the ITS sequence data showed that CBS 123800 grouped in a well-supported clade that included the ex-epitype of St. dichroa (Fig 4; ATCC 18913; Wang et al. 2015). Phylogenetic inference of the combined loci also placed CBS 123800 in the Memnoniella clade (Fig. 3), and therefore a new combination is provided here for St. dichroa.
Memnoniella echinata (Riv.) Galloway, Trans. Brit. Mycol. Soc. 18: 165. 1933 — Fig. 36
Fig. 36.
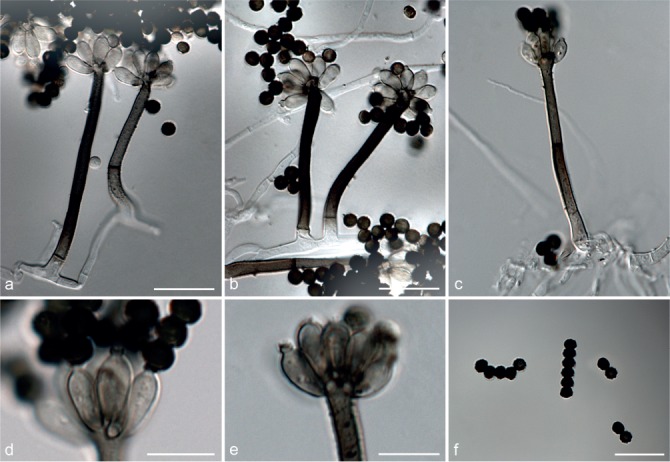
Memnoniella echinata (CBS 216.32). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia in chains. — Scale bars = 10 μm.
≡ Penicillium echinatum Riv., Dei Parassiti Vegetali: 451. 1873.
≡ Haplographium echinatum (Riv.) Sacc., Syll. Fung. 4: 307. 1886.
≡ Stachybotrys echinata (Riv.) G. Sm., Trans. Brit. Mycol. Soc. 45: 392. 1962.
= Periconia papyrogena Sacc., Michelia 1: 273. 1878.
≡ Stachybotrys papyrogena (Sacc.) Sacc., Fungi Ital.: tab. 900. 1881.
≡ Sterigmatobotrys papyrogena (Sacc.) Oud., Ned. Kruidk. Arch., ser. 2, 4: 548. 1886.
= Memnoniella aterrima Höhn., Zentralbl. Bakt. ParasitKde, Abt. 2 60: 16. 1923.
= Spinomyces japonica Saito, J. Ferment. Technol. 17: 2. 1939 (nom. inval. Art. 36).
Typification. ITALY, from clumps of Triticum saltivum, 1873, S. Rivolta (Galloway 1933, f. 1, lectotype of Penicillium echinatum designated here (as Rivolta’s Dei Parassiti Vegetali p. 451, f. 150–151)), MBT204286. – ENGLAND, Manchester, Didsbury, The Towers, Shirely Institute, British Cotton Industry Research Association, from cotton yarn, Feb. 1932, L.B. Galloway (epitype of Penicillium echinatum designated here, CBS H-22447, MBT204287, culture ex-epitype CBS 216.32).
Conidiophores simple, macronematous, mononematous, single, thick-walled, unbranched, erect, straight to slightly flexuous, hyaline becoming olivaceous brown, septate, smooth to slightly verrucose, 40–100 × 4–6 μm, bearing 6–10 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical, olivaceous brown, smooth, 7–10 × 2–5 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, globose, olivaceous brown to dark brown, verrucose, thick-walled, 3–6 × 3–5 μm (av. 5 × 4 μm), formed in long dry chains.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium, amber to sienna, with abundant conidiophores forming on the surface of the media, carrying slimy olivaceous brown to black conidial masses, reverse on PDA amber.
Additional materials examined. CANADA, Ontario, Ottawa, from air using a RCS sampler, date unknown, H. McGregor no. 229.3, DAOM 235365; ibid, from endotracheal tubes in a hospital, Oct. 1979, L. Gour no. 79M-153, DAOM 173162. – INDONESIA, from filter paper, 1 Oct. 1949, K.B. Boedjin & J. Reitsma, CBS 343.50. – JAPAN, from contaminated sake lees, Feb. 1939, K. Saito, CBS 344.39 (isotype of Spinomyces japonica). – SOLOMON ISLANDS, Bougainville Island, from tent canvas, 1944, W.H. Weston, CBS 627.66 = IMI 045547 = NRRL 2181. – THE NETHERLANDS, Eastern Flevoland, Houtribbos, from Pulvinula constellation, 26 June 1980, H.A. van der Aa, CBS 406.80. – USA, substrate unknown, P.B. Marsh, CBS 304.54 = ATCC 9597.
Notes — The newly designated ex-epitype strain (CBS 216.32) of Mem. echinata clustered in a highly supported clade, closely related to the single lineage representing Mem. longistipitata (Fig. 3).
Memnoniella ellipsoidea L. Lombard & Crous, sp. nov. — MycoBank MB816005; Fig. 37
Fig. 37.

Memnoniella ellipsoidea (CBS 136201). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the ellipsoidal conidia produced by this fungus.
Conidiophores simple, macronematous, mononematous, single, thick-walled, unbranched, erect, straight to slightly flexuous, hyaline becoming olivaceous brown towards the apex, 1–2-septate, smooth, 55–140 × 4–8 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical, hyaline, smooth, 8–12 × 4–6 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal, olivaceous brown to dark brown, verrucose, gluttulate, thick-walled, (8–)8.5–9.5(–10) × (4–)4.5–5.5(–6) μm (av. 9 × 5 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to pale luteous aerial mycelium, with abundant conidiophores forming on the aerial mycelium and surface of the media, carrying dry to slimy mouse grey to black conidial masses, reverse on PDA pale luteous.
Materials examined. BRAZIL, from Bromelia sp., R.F. Castañeda-Ruiz, CBS 136202 = MUCL 41876. – NEPAL, Narayani, Royal Chitwan National Park, from a dead twig, Dec. 1994, C. Decock (holotype CBS H-22448, culture ex-type CBS 136201 = MUCL 39090), CBS 136199 = MUCL 39088, CBS 136200 = MUCL 39089.
Notes — Memnoniella ellipsoidea formed a well-supported clade closely related to Mem. brunneoconidiophora and Mem. putrefolia (Fig. 3). For morphological comparisons, see notes under Mem. brunneoconidiophora.
Memnoniella humicola L. Lombard & Crous, sp. nov. — MycoBank MB816006; Fig. 38
Fig. 38.
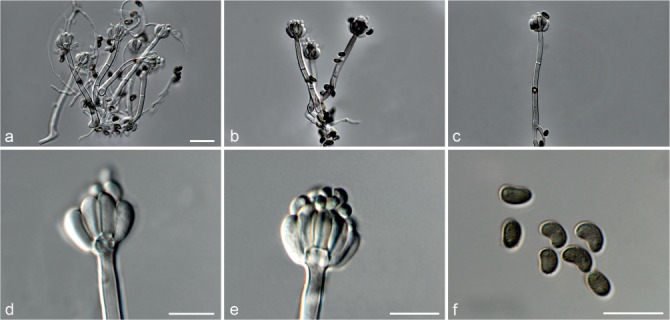
Memnoniella humicola (CBS 463.74). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bar: a = 20 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiophores simple, macronematous, mononematous, single, thick-walled, unbranched, erect, straight to slightly flexuous, hyaline becoming olivaceous brown towards the apex, septate, smooth to slightly verrucose at the apex, 35–70 × 4–6 μm, bearing 6–12 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical, hyaline to olivaceous brown, smooth, 6–9 × 2–4 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to reniform, olivaceous brown to dark brown, verrucose, thick-walled, (5–)5.5–6.5(–7) × 2–3 μm (av. 6 × 3 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium producing a luteous extracellular pigment that diffuse into the media, with abundant conidiophores forming on the surface of the media, carrying slimy olivaceous brown to black conidial masses, reverse on PDA luteous.
Material examined. SURINAME, from soil under Elaeis guineensis, 1974, J.H. van Emden (holotype CBS H-22449, culture ex-type CBS 463.74).
Notes — Memnoniella humicola formed a single lineage in the Memnoniella clade (Fig. 3). This species can be distinguished from Mem. echinata, its closest phylogenetic neighbour, by ellipsoidal to reniform conidia and shorter conidiophores (up to 70 μm) compared to globose conidia and longer conidiophores (up to 100 μm) of Mem. echinata.
Memnoniella longistipitata D.W. Li et al., Mycotaxon 85: 254. 2003
≡ Stachybotrys longistipitata (D.W. Li et al.) D.W. Li et al., Fung. Diversity 71: 57. 2015.
Description and illustrations — See Li et al. (2003) and Wang et al. (2015).
Material examined. MALAWI, Mulungusi Valley, Zomba, Zomba Botanical Gardens, from dead wood, G.L. Hennebert & C. Decock, CBS 136197 = MUCL 33065.
Notes — Phylogenetic inference of the ITS sequence data (Fig. 4) showed that CBS 136197 grouped in a well-supported clade that included the ex-type of Mem. longistipitata (ATCC 22699; Li et al. 2003). Phylogenetic inference of the combined loci also placed CBS 136197 in the Memnoniella clade (Fig. 3) and therefore the species Mem. longistipitata is resurrected here.
Memnoniella oenanthes (M.B. Ellis) L. Lombard & Crous, comb. nov. — MycoBank MB816007
Basionym. Stachybotrys oenanthes M.B. Ellis, Mycol. Pap. 125: 29. 1971.
Description and illustrations — See Ellis (1971) and Wang et al. (2015).
Material examined. INDIA, Tamil Nadu, west of Madras, from old stem of Euphorbia tirukalli, Mar. 1973, W. Gams, CBS 388.73 = ATCC 32255.
Notes — Phylogenetic inference of the ITS sequence data (Fig. 4) showed that isolate CBS 388.73 grouped in a well-supported clade that included the ex-type of St. oenanthes (ATCC 22844; Ellis 1971, Wang et al. 2015). Phylogenetic inference of the combined loci also placed this isolate in the Memnoniella clade (Fig. 3) and therefore a new combination is provided here for St. oenanthes.
Memnoniella pseudonilagirica L. Lombard & Crous, sp. nov. — MycoBank MB816008; Fig. 39
Fig. 39.
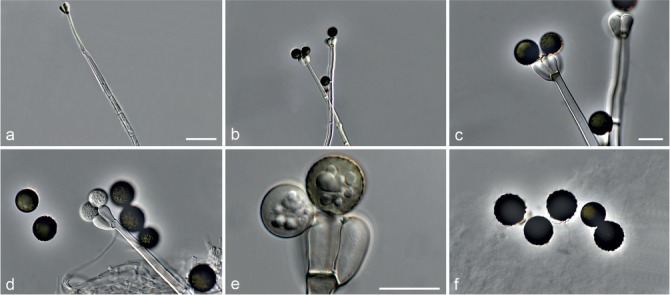
Memnoniella pseudonilagirica (CBS 136405). a–d. Conidiophores; e. conidiogenous cells; f. conidia. — Scale bars: a = 50 μm (apply to b); c = 20 μm (apply to d, f); e = 10 μm.
Etymology. Name reflects the morphological similarity to Stachybotrys nilagirica.
Conidiophores simple, macronematous, mononematous, single, thick-walled, unbranched, erect, straight to slightly flexuous, hyaline becoming pale olivaceous brown at the apex, septate, smooth, 150–300 × 9–15 μm, bearing 2–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical, hyaline, smooth, 13–20 × 6–11 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, globose, olivaceous brown to dark brown, verrucose, thick-walled, (14–)18–22(–23) × (12–)17–21(–22) μm (av. 20 × 19 μm).
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to aerial mycelium becoming pale luteous to light salmon at the margins, forming concentric rings, with conidiophores forming on the aerial mycelium, carrying slimy olivaceous brown to black conidial masses, reverse on PDA luteous.
Material examined. NEPAL, Royal Chitwan National Park, on dead leaf of Ceiba pentandra, Dec. 1994, C. Decock (holotype CBS H-22450, culture ex-type CBS 136405 = MUCL 39120).
Notes — Memnoniella pseudonilagirica formed a single lineage in the Memnoniella clade (Fig. 3) and is morphologically reminiscent of St. nilagirica (Subramanian 1957). It can be distinguished by its longer conidiophores (up to 300 μm) compared to those of St. nilagirica (up to 224 μm; Subramanian 1957). The conidia of Mem. pseudonilagirica ((14–)18–22(–23) × (12–)17–21(–22) μm (av. 20 × 19 μm)) are also smaller than those of St. nilagirica (15.4–28.0 μm; Subramanian 1957).
Memnoniella putrefolia L. Lombard & Crous, sp. nov. — MycoBank MB816009; Fig. 40
Fig. 40.
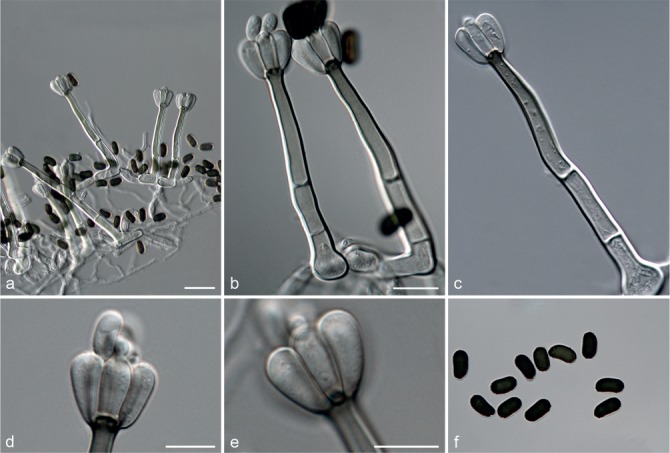
Memnoniella putrefolia (CBS 101177). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm; b = 10 μm (apply to c, f); d–e = 10 μm.
Etymology. Name reflects the substrate, decayed leaves, from which this fungus was isolated.
Conidiophores simple, macronematous, mononematous, single, thick-walled, unbranched, erect, straight to slightly flexuous, hyaline becoming pale olivaceous brown, 1–2-septate towards the base, smooth, 50–90 × 5–9 μm, bearing 3–8 conidiogenous cells. Conidiogenous cells phialidic, clavate to subcylindrical, hyaline, smooth, 10–14 × 3–7 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to rarely reniform, olivaceous brown to dark brown, verrucose, thick-walled, (8–)9–11 × (4–)4.5–5.5(–6) μm (av. 10 × 5 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium, pale luteous, with concentric rings of conidiophores on the surface of the media, carrying dry to slimy olivaceous brown to black conidial masses, reverse on PDA luteous with light olivaceous green rings.
Materials examined. BRAZIL, Mata Avenca-Santa Rita, from decayed leaf, Sept. 1997, R.F. Castañeda-Ruiz, CBS 136171 = MUCL 41166 = INIFAT C98/65-2. – PUERTO RICO, Calmitillo, on decayed leaf of Melastomataceae, 19 June 1998, W. Gams (holotype CBS H-22451, culture ex-type CBS 101177).
Notes — Memnoniella putrefolia formed a well-supported clade closely related to Mem. brunneoconidiophora and Mem. ellipsoidea (Fig. 3). For morphological comparisons, see notes under Mem. brunneoconidiophora.
Myrothecium Tode, Fungi Mecklenburgenses Selecti 1: 25. 1790
= Myxormia Berk. & Broome, Ann. Mag. Nat. Hist., ser. II, 5: 457. 1850.
= Godroniella P. Karst., Hedwigia 23: 88. 1884.
= Myrotheciella Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 20: 460. 1910.
= Exotrichum Syd., Ann. Mycol. 12: 571. 1914.
= Starkeyomyces Agnihothr., J. Indian Bot. Soc. 35: 40. 1956.
Type species. Myrothecium inundatum Tode.
Sexual morph unknown. Conidiomata reduced to solitary conidiophores or sporodochial. Sporodochia stromatic or non-stromatic, superficial, cupulate, scattered or gregarious, oval or irregular in outline, sometimes with a white setose fringe surrounding an olivaceous green to dark green slimy mass of conidia. Stroma well developed, hyaline, of textura globulosa and textura angularis. Marginal hyphae arising from the stroma, straight or curved, thin-walled, irregularly branched. Setae arising from the stroma, thin-walled, septate, hyaline, smooth, tapering to an acute apex. Simple conidiomata consisting of a conidiogenous cell, sometimes carried on a short aseptate stipe, arising directly from vegetative hyphae, monophialidic or rarely polyphialidic. Conidiophores macronematous, subverticillately or penicillately branched, hyaline, smooth. Conidiogenous cells phialidic, hyaline, smooth, cylindrical to subcylindrical, becoming narrowed at the tip, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, ellipsoidal to obovoid, hyaline, smooth, < 5 μm in length, lacking a funnel-shaped mucoid apical appendage.
Notes — The broad generic concept of Myrothecium sensu Tulloch (1972) was contested by Nag Raj (1993, 1995), who introduced the asexual genera Xepicula and Xepiculopsis for Myr. leucotrichum and Myr. gramineum, respectively. Myrothecium sensu Tulloch included species unified by sporodochial and synnematous conidiomata bearing green conidial masses, and conidia with or without mucoid appendages (Nag Raj 1995). This concept has resulted in the description of several Myrothecium spp. solely based on the green conidial masses produced on the sporodochial and/or synnematous conidiomata (Agarwal 1980, DiCosmo et al. 1980, Rao & De Hoog 1983, Sullia & Padma 1985, Matsushima 1989, 1995, Castañeda & Kendrick 1991, Bohn 1993, Escalona 1997, Seifert et al. 2003, Watanabe et al. 2003, Alves et al. 2010, Jiang et al. 2014, Wu et al. 2014). Phylogenetic inference in this study clearly showed that Myrothecium sensu Tulloch includes several genera, and therefore, the generic concept for Myrothecium s.str. is narrowed here to only include species with sporodochia or mononematous conidiophores producing conidia smaller than 5 μm in green slimy masses without mucoid appendages.
Tulloch (1972) designated IMI 158855 as neotype of Myr. inundatum, although the prologue does not agree with the illustration provided. The setae illustrated in f. 1 of Tulloch (1972) are clearly thick-walled, whereas the prologue indicate thin-walled setae. A similar problem was raised by Nag Raj (1995) for Myr. prestonii (now I. prestonii). Therefore, to avoid confusion, we select to epitypify Myr. inundatum here using CBS 275.48, which morphologically agrees with the prologue of Tulloch (1972).
Myrothecium inundatum Tode, Fungi Mecklenburgenses Selecti 1: 25, t. 5:39. 1790 — Fig. 41
Fig. 41.
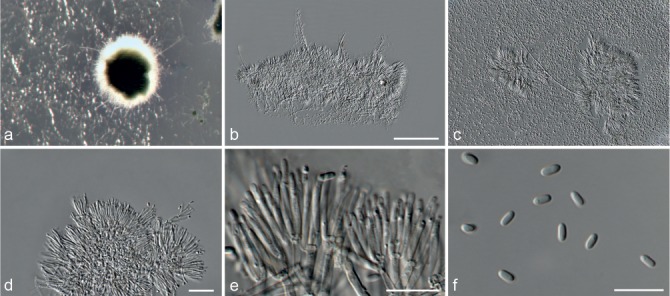
Myrothecium inundatum (CBS 275.48). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 100 μm (apply to c); d = 20 μm; e–f = 10 μm.
= Myrothecium fungicola Peck, Rep. St. Mus. N.Y. 26: 29. 1872.
Typification. ENGLAND, Norfolk, Wheatfern, on decaying pileus of Russula nigricans, 29 July 1971, E.A. Ellis, IMI 158855 (neotype of Myr. inundatum fide Tulloch). – ENGLAND, on Russula adusta, Nov. 1948, P.W. Brian (epitype of Myr. inundatum designated here, CBS H-14898, MBT204294, culture ex-epitype CBS 275.48 = IMI 008983 = QM 7988).
Ascomatal morph unknown. Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or gregarious, oval to elongate or irregular in outline, 150–850 μm diam, 45–130 μm deep, with a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma well developed, hyaline, of textura globulosa and textura angularis. Marginal hyphae arising from the stroma, straight to curved thin-walled, hyaline, smooth, irregularly branched. Setae arising from the stroma, thin-walled, smooth, unbranched, hyaline, septate, 80–200 μm long, 2–4 μm wide, terminating in a blunt apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched or rarely branched, hyaline, septate, smooth, 12–25 × 1–3 μm; primary branches aseptate, unbranched, smooth, 6–11 × 1–2 μm; secondary branches aseptate, unbranched, smooth, 5–7 × 1–2 μm; terminating in a whorl of 2–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 7–25 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, obovoid to ellipsoidal, 3–4 × 1–2 μm (av. 3 × 1 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the surface of the medium, covered by slimy herbage to olivaceous green conidial masses, reverse on PDA sienna to buff.
Additional materials examined. CANADA, Ontario, Petawawa, from leaf litter under Populus tremuloides, Aug. 1969, G.C. Bhat, CBS 616.70; Quebec, Gatineau Park, Lusk Cave Trail, on an old agaric, 17 Sept. 1993, K.A. Seifert, CBS 116539 = AR 2738. – GERMANY, Schwäbische Alb, Sternberg bei Gomadingen, on an old decaying toadstool, 5 Oct. 2006, M. Gube, CBS 120646. – THE NETHERLANDS, Baarn, woods along road to Hilversum, near Hoge Vuursche, on Russula sp., 29 July 1973, H.A. van der Aa, CBS 194.74.
Notes — The newly designated epitype (CBS 275.48) of Myr. inundatum clustered in a well-supported clade, sister to Myr. simplex (Fig. 2). This clade represents the Myrothecium s.str. clade.
Myrothecium simplex L. Lombard & Crous, sp. nov. — MycoBank MB816010; Fig. 42
Fig. 42.
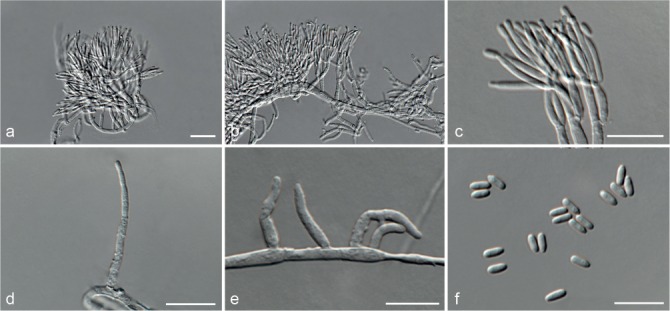
Myrothecium simplex (CBS 582.93). a–b. Sporodochial conidiomata; c. conidiogenous cells; d–e. simple conidiomata; f. conidia. — Scale bar: a = 20 μm (apply to b); c–f = 10 μm.
Etymology. Name reflects the simple conidiophores produced by this fungus.
Conidiomata sporodochial or simple. Sporodochia non-stromatic, superficial, scattered, oval or irregular in outline, 30–65 μm diam, 35–100 μm deep, consisting of closely interwoven conidiophores arising from the vegetative hyphae, bearing an olivaceous green to dark green agglutinated slimy mass of conidia. Simple conidiomata monophialidic or rarely polyphialidic, consisting of conidiogenous cells arising directly from the vegetative hypha or carried on an aseptate, smooth, hyaline stipe, 5–15 × 2–3 μm. Conidiophores consisting of a stipe and a penicillately to subverticillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 12–26 × 2–3 μm; primary branches aseptate, unbranched, smooth, 8–15 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 6–10 × 1–3 μm; terminating in a whorl of 2–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 6–15 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, obovoid to ellipsoidal, 3–4 × 1–2 μm (av. 4 × 1 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with a floccose to felt appearance with extensive sporulation on the aerial mycelium, covered by slimy herbage to olivaceous green conidial masses, reverse on PDA buff to olivaceous green.
Materials examined. JAPAN, Sugadaira, from decaying agaric, 2 Sept. 1993, W. Gams (holotype CBS H-22452, culture ex-type CBS 582.93); University of Tsukuba, Sugadaira Research Center, Daimyojin waterfall, from a rotten basidioma of Russula nigricans, 11 Aug. 1997, H.-J. Schroers, W. Gams, T. Gräfenhan & M. Klamer, CBS 100287.
Notes — Myrothecium simplex can be distinguished from Myr. inundatum by the simple conidiomata formed on the vegetative hyphae and lack of setae.
Myxospora L. Lombard & Crous, gen. nov. — MycoBank MB816011
Etymology. Name reflects the slimy conidial masses produced on the conidiomata of these fungi.
Type species. Myxospora masonii (M.C. Tulloch) L. Lombard & Crous.
Sexual morph unknown. Conidiomata synnematous or sporodochial. Synnemata cylindrical to pyriform, unbranched, broadening towards the apex, consisting of bundles of parallel, longitudinal, closely compacted hyphae, terminating in whorls of 2–4 conidiogenous cells, covered by an olivaceous green to black slimy mass of conidia with marginal hyphae terminating in hyaline bulbous, verrucose cells and carried on a poorly developed stroma. Sporodochia stromatic, superficial, scattered or rarely gregarious, oval or irregular in outline, without a white setose fringe surrounding an olivaceous green to dark green slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae rare, thick-walled, septate, flexuous, subhyaline, tapering to an obtuse apice protruding through the conidial masses. Conidiophores macronematous, verticillately or penicillately branched, hyaline, smooth to lightly verrucose. Conidiogenous cells phialidic, hyaline, smooth, subcylindrical, becoming narrowed at the tip, with a conspicuous collarettes and periclinal thickenings. Conidia aseptate, fusiform, hyaline sometimes becoming pigmented with age, smooth, with an apical hilum lacking a funnel-shaped mucoid apical appendage.
Notes — Nag Raj (1993), following the approach of Rao & Sutton (1975), selected to retain Myr. masonii and suggested that Myrothecium only accommodates sporodochial and synnematal species. Based on phylogenetic inference in this study, the ex-type (CBS 174.73) of Myr. masonii clustered in a well-supported clade distant to the Myrothecium s.str. clade. Therefore, the new generic name, Myxospora (Myx.), is introduced here for this clade and a new combination is provided for Myr. masonii. Members of Myxospora are characterised by the formation of mostly fusiform conidia, bearing an apical hilum without funnel-shaped mucoid appendages and some species forming synnematal conidiomata.
Myxospora aptrootii L. Lombard & Crous, sp. nov. — MycoBank MB816012; Fig. 43
Fig. 43.
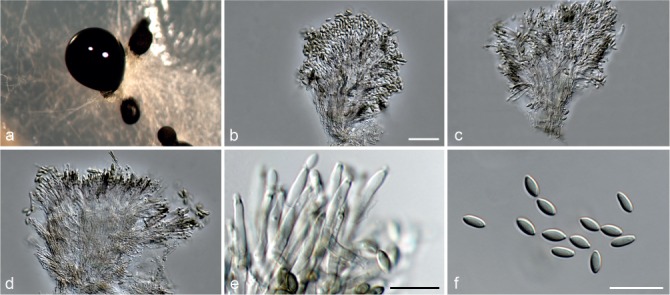
Myxospora aptrootii (CBS 101263). a. Conidiomata on SNA; b–d. synnematous conidiomata; e. conidiogenous cells; f. conidia. — Scale bars: b = 50 μm (apply to c–d); e–f = 10 μm.
Etymology. Named after Dr. André Aptroot, who collected this fungus.
Conidiomata synnematous, solitary, 125–200 μm high, 20–45 μm wide at the base, 80–130 μm at the apex, pyriform, unbranched, broadening towards the apex, consisting of bundles of parallel, longitudinal, closely compacted hyphae, terminating in whorls of 2–4 conidiogenous cells, covered by an olivaceous green to black slimy mass of conidia without marginal hyphae terminating in hyaline bulbous, verrucose cells. Stroma poorly developed, hyaline, of a textura angularis. Conidiogenous cells phialidic, subcylindrical, hyaline, smooth, 7–14 × 2–3 μm, with conspicuous collarettes and periclinal thickenings becoming pigmented at the apex. Conidia aseptate, smooth, hyaline becoming pigmented with age, fusiform, 4–5 × 2–3 μm (av. 5 × 2 μm), with an apical hilum, borne in chains.
Culture characteristics — Colonies on PDA, OA and CMA with white to buff aerial mycelium with synnemata forming on the aerial mycelium and surface of the medium covered by slimy olivaceous green conidial masses, reverse on PDA buff to pale luteous.
Material examined. CHINA, Hong Kong, from leaf litter, June 1998, A. Aptroot (holotype CBS H-22453, culture ex-type CBS 101263).
Notes — Myxospora aptrootii formed a single lineage closely related to Myx. crassiseta and Myx. musae (Fig. 2). This species produces synnematous conidiomata, not observed for both Myx. crassiseta and Myx. musae. The synnemata of Myx. aptrootii are shorter than those of Myx. masonii (150–900 μm; Tulloch 1972) but show some overlap with those of Myx. graminicola (80–220 μm). The conidia of Myx. aptrootii (4–5 × 2–3 μm (av. 5 × 2 μm)) are slightly smaller that those of Myx. graminicola ((5–)6(–7) × 2–3 μm (av. 6 × 2 μm)) and carried in chains, not observed for Myx. graminicola.
Myxospora crassiseta L. Lombard & Crous, sp. nov. — MycoBank MB816013; Fig. 44
Fig. 44.
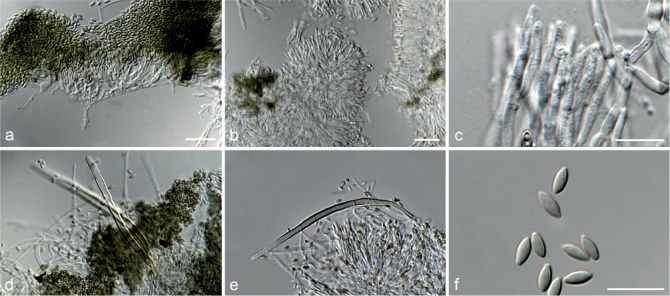
Myxospora crassiseta (CBS 731.83). a–b. Sporodochial conidiomata; c. conidiogenous cells; d–e. setae; f. conidia. — Scale bars: a = 50 μm; b = 20 μm (apply to d–e); c, f = 10 μm.
Etymology. Name reflects the thick-walled setae formed by this fungus.
Conidiomata sporodochial, stromatic, superficial, scattered, rarely gregarious, oval to elongate or irregular in outline, 25–95 μm diam, 25–50 μm deep, without a setose fringe surrounding a green to black agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, smooth, of a textura angularis. Setae rare, extending through the conidial mass, thick-walled, subhyaline, smooth, septate, 100–185 μm long, 4–6 μm wide, with a blunt obtuse apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 10–30 × 2–4 μm; conidiogenous apparatus consists of primary and secondary branches that terminate in 2–4 conidiogenous cells; primary branches aseptate, unbranched, smooth, 6–11 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 6–12 × 2–3 μm. Conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, 8–21 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline becoming pigmented with age, fusiform, 4–6 × 2–3 μm (av. 4 × 2 μm), with an apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium with sporodochia forming on the surface of the medium covered by slimy olivaceous green to mouse grey conidial masses, reverse on PDA pale olivaceous green.
Materials examined. JAPAN, Kyoto, Daitokuij Temple, from dead twig, 28 Aug. 1983, W. Gams (holotype CBS H-22454, culture ex-type CBS 731.83). – USA, Hawaii, Puna District, Mackenzie State Park, coastal forest, from a black stoma of a Pyrenomycete on a dead hardwood branch, 9 June 2003, D.T. Wicklow, CBS 121141 = NRRL 45891.
Notes — Myxospora crassiseta formed a highly supported clade closely related to Myx. aptrootii and Myx. musea (Fig. 2). This species can be distinguished from Myx. musae by the formation of thick-walled setae that are projected through the conidial masses. Myxospora crassiseta is further distinguished from Myx. aptrootii by the formation of sporodochial conidiomata.
Myxospora graminicola L. Lombard & Crous, sp. nov. — MycoBank MB816014; Fig. 45
Fig. 45.

Myxospora graminicola (CBS 116538). a. Conidiomata on SNA; b–d. synnematous conidiomata; e. conidiogenous cells; f. conidia. — Scale bars: b = 50 μm (apply to c); d = 20 μm; e–f = 10 μm.
Etymology. Name reflects the substrate, decaying grass leaf, from which this fungus was isolated.
Conidiomata synnematous, solitary or gregarious, 80–220 μm high, 25–55 μm wide at the base, 45–75 μm at the apex, cylindrical, unbranched, broadening towards the apex, consisting of bundles of parallel, longitudinal, closely compacted hyphae, terminating in whorls of 2–4 conidiogenous cells, covered by an olivaceous green to black slimy mass of conidia with marginal hyphae terminating in hyaline bulbous, verrucose cells, 5–12 × 2–5 μm. Stroma poorly developed, hyaline, of a textura angularis. Conidiogenous cells phialidic, subcylindrical, hyaline, smooth, 6–16 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline becoming pigmented with age, fusiform, (5–)6(–7) × 2–3 μm (av. 6 × 2 μm), with an apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium pale luteous to buff with synnemata forming on the surface of the medium covered by slimy olivaceous to herbage green conidial masses, reverse on PDA olivaceous green where synnemata form.
Material examined. USA, New Jersey, Union County, Scotch Plains and Edison, Clark, Ash Brook Reservation, from decaying grass leaf, 2 Oct. 2000, G. Bills (holotype CBS H-22455, culture ex-type CBS 116538 = A.R. 3507).
Notes — Myxospora graminicola formed a single lineage closely related to Myx. masonii (Fig. 2). The synnemata of Myx. graminicola are shorter than those reported for Myx. masonii (150–900 μm; Tulloch 1972) and the conidia of Myx. masonii are slightly larger (4.5–9 × 1.5–2.5 μm; Tulloch 1972) than those of Myx. graminicola.
Myxospora masonii (M.C. Tulloch) L. Lombard & Crous, comb. nov. — MycoBank MB816015
Basionym. Myrothecium masonii M.C. Tulloch, Mycol. Pap. 130: 21. 1972.
Description and illustration — See Tulloch (1972).
Material examined. ENGLAND, Norfolk, Wheatfern Board, on leaves of Glyceria sp., 5 June 1971, E.A. Ellis, culture ex-type CBS 174.73 = IMI 158346 = ATCC 24426.
Myxospora musae L. Lombard & Crous, sp. nov. — MycoBank MB816016; Fig. 46
Fig. 46.
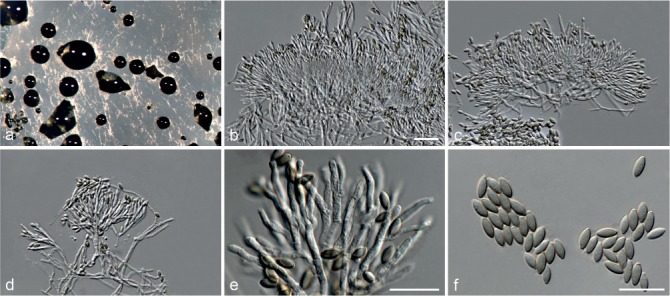
Myxospora musae (CBS 265.71). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the plant host, Musa sp., from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, scattered, rarely gregarious, oval to elongate or irregular in outline, 35–100 μm diam, 25–65 μm deep, without a setose fringe surrounding a green to black agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, smooth, of a textura angularis. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 17–30 × 2–3 μm; conidiogenous apparatus consists of primary and secondary branches that terminate in 2–4 conidiogenous cells; primary branches aseptate, unbranched, smooth, 7–14 × 1–2 μm; secondary branches aseptate, unbranched, smooth, 6–10 × 1–2 μm. Conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, 9–18 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline becoming pigmented with age, fusiform, (4–)5(–6) × 2–3 μm (av. 5 × 2 μm), with an apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the aerial mycelium and surface of the medium covered by slimy olivaceous green conidial masses, reverse on PDA sienna to buff.
Material examined. MADAGASCAR, from Musa sp., Mar. 1971, E. Laville (holotype CBS H-14937, culture ex-type CBS 265.71 = IMI 155922). – SOUTH AFRICA, Limpopo Province, Agatha, from tarspot lesion on unknown host, 9 Sept. 2014, J. Roux, CPC 25150.
Notes — Myxospora musae formed a well-supported clade closely related to Myx. aptrootii and Myx. crassiseta (Fig. 2). As the only other sporodochial species in this genus thus far, Myx. musae can be distinguished from Myx. crassiseta by the lack of setae formed in the sporodochia.
Neomyrothecium L. Lombard & Crous, gen. nov. — MycoBank MB816017
Etymology. Name reflects its morphological similarity to the genus Myrothecium.
Type species. Neomyrothecium humicola L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, pulvinate, scattered or gregarious, oval or irregular in outline, covered by an olivaceous green to dark green slimy mass of conidia, lacking a white setose fringe. Stroma poorly developed, hyaline, of a textura angularis. Setae rarely seen, thin-walled, septate, unbranched, straight to flexuous, hyaline, tapering to an acute apice. Conidiophores macronematous, penicillately branched, hyaline, smooth. Conidiogenous cells phialidic, hyaline, smooth, cylindrical to subcylindrical, straight to slightly curved, with a conspicuous collarettes and periclinal thickenings. Conidia aseptate, cylindrical, hyaline, rounded at both ends.
Notes — The monophyletic asexual genus Neomyrothecium is established here for a fungal isolate from soil that is morphologically similar to members of Paramyrothecium but phylogenetically distinct (Fig. 2). Neomyrothecium produces only a few setae in culture, which are multiseptate and unbranched, whereas those of Paramyrothecium are 1–3(–4)-septate, and sometimes branched beneath the most apical septum. The pulvinate sporodochia of Neomyrothecium lack a white setose fringe compared to the cupulate sporodochia of Paramyrothecium with a distinct white setose fringe.
Neomyrothecium humicola L. Lombard & Crous, sp. nov. — MycoBank MB816018; Fig. 47
Fig. 47.
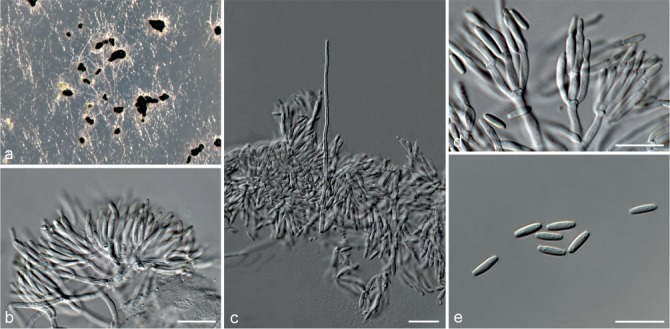
Neomyrothecium humicola (CBS 310.96). a. Sporodochial conidiomata on SNA; b. sporodochia; c. setae; d. conidiogenous cells; e. conidia. — Scale bars: b, d–e = 10 μm; c = 20 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, scattered or gregarious, oval to elongate or irregular in outline, 60–450 μm diam, 50–100 μm deep, without a setose fringe surrounding an olivaceous green to black agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, smooth, of a textura angularis. Setae rarely seen, arising from the basal stroma, thin-walled, hyaline, smooth, straight to flexuous, septate, 85–140 μm long, 2–3 μm wide, tapering towards an acute apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 15–25 × 2–3 μm; conidiogenous apparatus consists of a whorl of primary and secondary branches with the terminal branches producing 3–6 conidiogenous cells; primary branches aseptate, hyaline, smooth, 7–15 × 2–3 μm; secondary branches aseptate, hyaline, smooth, 5–10 × 2–3 μm; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, 7–14 × 2 μm, with conspicuous collarettes and periclinal thickenings, sometimes covered by an olivaceous green mucoid layer. Conidia aseptate, smooth, hyaline, cylindrical, 5–7 × 1–2 μm (av. 6 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the surface of the medium covered by slimy olivaceous green conidial masses, reverse on PDA buff.
Material examined. PAPUA NEW GUINEA, Madang, Jais Aben, from soil along coral reef coast, Nov. 1995, A. Aptroot (holotype CBS H-22456, culture ex-type CBS 310.96).
Paramyrothecium L. Lombard & Crous, gen. nov. — MycoBank MB815988
Etymology. Name reflects its morphological similarity to the genus Myrothecium.
Type species. Paramyrothecium roridum (Tode) L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or gregarious, oval or irregular in outline, with or without a white setose fringe surrounding an olivaceous green to dark green slimy mass of conidia. Stroma poorly or well-developed, hyaline to subhyaline, composed of hyphae disposed in a textura globulosa and/or textura angularis. Setae thin-walled, 1–3(–4)-septate, straight to flexuous, sometimes becoming sinuous above the apical septum, hyaline, tapering to an acute apice. Conidiophores macronematous, penicillately branched, hyaline, smooth. Conidiogenous cells phialidic or percurrent, hyaline sometimes becoming darker at the apex, smooth to lightly verrucose, cylindrical to subcylindrical, becoming narrowed at the tip, with a conspicuous collarettes and periclinal thickenings. Conidia aseptate to 1-septate, cylindrical to ellipsoidal to obovoid, straight to bent, hyaline to pale green, smooth.
Notes — Phylogenetic inference in this study showed that members of this group of fungi formed a highly supported clade distant to the Myrothecium s.str. clade (Fig. 2). Therefore, the new generic name, Paramyrothecium, is introduced here for this clade. Tulloch (1972) designated Fuckel’s Fungi Rhenani no. 166 as neotype of Myr. roridum. As Pa. roridum (= Myr. roridum) is the type species of the genus, and no living material is available for this neotype, we consider it important to designate an epitype for this species. The morphological features of the strain CBS 357.89 best fit the description of Myr. roridum provided by Tulloch (1972), and hence is designated as epitype here. Members of Paramyrothecium can be distinguished from Myrothecium s.str. and the other myrothecium-like genera by their 1–3-septate, thin-walled setae surrounding the sporodochia.
Paramyrothecium acadiense (Seifert & G. Sampson) L. Lombard & Crous, comb. nov. — MycoBank MB815945
Basionym. Myrothecium acadiense Seifert & G. Sampson, Mycotaxon 87: 320. 2003.
Description and illustration — See Seifert et al. (2003).
Material examined. CANADA, Nova Scotia, Shubenacadie, from leaves of Tussilago farfara, 2 Oct. 1989, G. Sampson, CBS 123.96 = DAOMC 221473 = UAMH 7653 (ex-type strain of Myr. acadiense).
Notes — The ex-type strain (CBS 123.96; Seifert et al. 2003) of Myr. acadiense clustered in the Paramyrothecium clade (Fig. 2), and therefore a new combination is provided for this species in the asexual genus Paramyrothecium. Paramyrothecium acadiense can be distinguished from other species in this genus by the formation of 0–1-septate conidia that are straight or bent, and percurrently extending rather than phialidic conidiogenous cells (Seifert et al. 2003).
Paramyrothecium breviseta L. Lombard & Crous, sp. nov. — MycoBank MB815946; Fig. 48
Fig. 48.
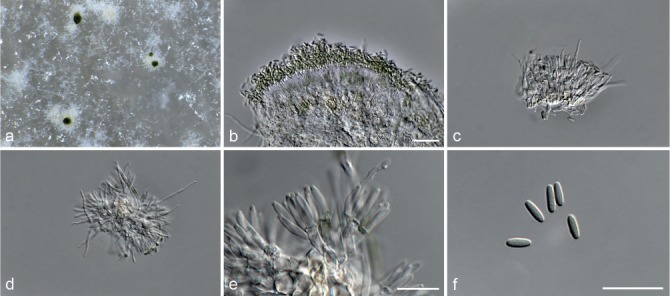
Paramyrothecium breviseta (CBS 544.75). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the short setae produced by this fungus.
Conidiomata sporodochial, stromatic, cupulate, superficial, scattered or rarely gregarious, oval or irregular in outline, 45–100 μm diam, 45–70 μm deep, with a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma well developed, hyaline to subhyaline, of a textura globulosa and textura angularis. Setae arising from the stroma thin-walled, hyaline, 1–3-septate, straight to flexuous, 25–40 μm long, 2–3 μm wide, tapering to an acutely rounded apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 6–9 × 2–4 μm; primary branches aseptate, unbranched, smooth, 5–8 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 4–7 × 2–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 6–11 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 4–5 × 1–2 μm (av. 5 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to rosy buff aerial mycelium with sporodochia forming on the surface of the medium, covered by slimy olivaceous green conidial masses, reverse on PDA rosy buff.
Material examined. INDIA, substrate unknown, Feb. 1975, A. Subrahmanian (holotype CBS H-14915, culture ex-type CBS 544.75).
Notes — Paramyrothecium breviseta formed a single lineage basal to the Pa. roridum clade (Fig. 2) and can be distinguished from other species in this genus by the formation of characteristic short setae surrounding the sporodochia.
Paramyrothecium cupuliforme L. Lombard & Crous, sp. nov. — MycoBank MB815947; Fig. 49
Fig. 49.
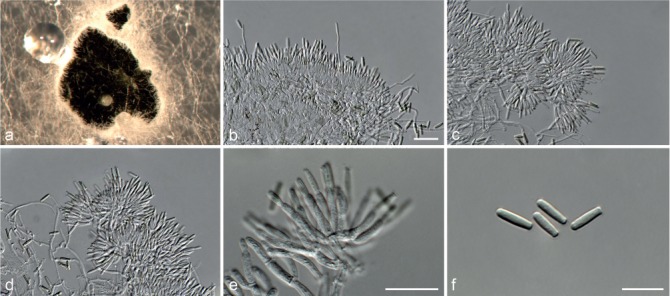
Paramyrothecium cupuliforme (CBS 127789). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the cupulate sporodochia produced by this fungus.
Conidiomata sporodochial, stromatic, cupulate, superficial, scattered or gregarious, oval or irregular in outline, 75–900 μm diam, 45–135 μm deep, with a white setose fringe surrounding an olivaceous green to black agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma, thin-walled, hyaline, 1–3-septate, straight becoming sinuous above the apical septum, 45–90 μm long, 2–3 μm wide, tapering to an acutely rounded apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 15–25 × 2–4 μm; primary branches aseptate, unbranched, smooth, 8–15 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 4–10 × 2–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 4–11 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 6–8 × 1–2 μm (av. 7 × 2 μm), with a flattened apex and rounded at the base.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to buff aerial mycelium with sporodochia forming on the surface of the medium, covered by slimy olivaceous green to black conidial masses, reverse on PDA buff to pale luteous.
Materials examined. NAMIBIA, 30 km west of Maltahole on Highway C19, from surface soil in desert, Apr. 2001, M. Christensen (holotype CBS H-22434, culture ex-type CBS 127789), CBS 126167.
Notes — Paramyrothecium cupuliforme formed a well-supported clade closely related to Pa. nigrum and Pa. viridisporum (Fig. 2). This species is morphologically similar to Pa. humicola, Pa. tellicola and Pa. terrestris. The setae of Pa. cupuliforme (up to 90 μm) are longer than those of Pa. humicola (up to 65 μm), Pa. tellicola (up to 80 μm) and Pa. terrestris (up to 70 μm). Additionally, the conidia of Pa. cupuliforme (6–8 × 1–2 μm (av. 7 × 2 μm)) are slightly larger than those of Pa. humicola (6–7 × 1–2 μm (av. 6 × 1 μm)), but slightly smaller than those of Pa. tellicola ((7–)7.5–8.5(–9) × 1–3 μm (av. 8 × 2 μm)) and Pa. terrestris ((7–)7.5–8.5(–10) × 1–3 μm (av. 8 × 2 μm)).
Paramyrothecium foeniculicola L. Lombard & Crous, sp. nov. — MycoBank MB815948; Fig. 50
Fig. 50.
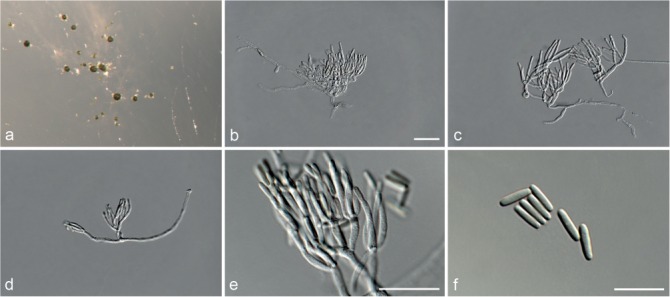
Paramyrothecium foeniculicola (CBS 331.51). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the host genus Foeniculum, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, scattered, oval in outline, 25–45 μm diam, 30–50 μm deep, lacking a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 7–17 × 2–3 μm; primary branches aseptate, unbranched, smooth, 5–11 × 1–2 μm; secondary branches aseptate, unbranched, smooth, 5–8 × 1–2 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth to lightly verrucose, straight to slightly curved, 6–16 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 5–7 × 1–2 μm (av. 6 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA rosy buff, with mostly immersed mycelium with sporodochia forming on the surface of the medium, covered by slimy herbage to olivaceous green conidial masses, reverse on PDA rosy buff.
Material examined. THE NETHERLANDS, from leaf sheath of Foeniculum vulgare, 1951, collector unknown (holotype CBS H-14914, culture ex-type CBS 331.51 = IMI 140051).
Notes — Paramyrothecium foeniculicola formed a single lineage in the Paramyrothecium clade (Fig. 2). The sporodochia of Pa. foeniculicola lack a white setose fringe as was observed for Pa. parvum and no setae were observed for either species. The conidia of Pa. foeniculicola (5–7 × 1–2 μm (av. 6 × 2 μm)) are slightly larger than those of Pa. parvum (4–5 × 1–2 μm (av. 5 × 2 μm)).
Paramyrothecium foliicola L. Lombard & Crous, sp. nov. — MycoBank MB815949; Fig. 51
Fig. 51.
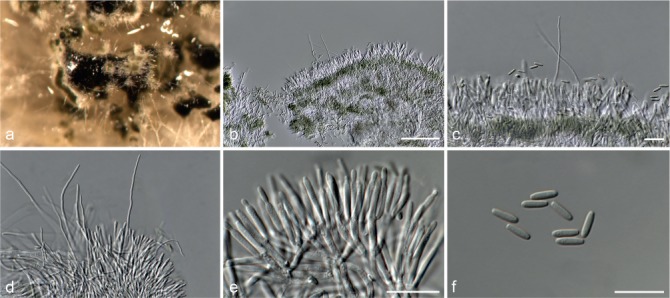
Paramyrothecium foliicola (CBS 113121). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the substrate, a rotten leaf, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or gregarious, oval or irregular in outline, 50–850 μm diam, 90–165 μm deep, with a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma thin-walled, hyaline, 1–3-septate, straight becoming sinuous above the apical septum, 60–100 μm long, 2–3 μm wide, tapering to an acutely rounded apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline sometimes covered by a green mucoid layer, septate, smooth, 15–25 × 2–3 μm; primary branches aseptate, unbranched, smooth, 8–15 × 1–2 μm; secondary branches aseptate, unbranched, smooth, 5–10 × 2 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline becoming darker at the apex, smooth, straight to slightly curved, 8–14 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 5–6 × 1–2 μm (av. 6 × 1 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the aerial mycelium and surface of the medium, covered by slimy olivaceous green to mouse grey conidial masses, producing a rosy buff exudate into the medium, reverse on PDA luteous to rosy buff.
Materials examined. BRAZIL, Pista Claudio Coutiño near Pao de Açucar, from rotten leaf of unknown host, 12 Oct. 2002, R.F. Castañeda-Ruiz (holotype CBS H-22435, culture ex-type CBS 113121 = INIFAT C02/104). – CUBA, La Habana, from air, July 1993, R.F. Castañeda, CBS 419.93 = INIFAT C93/60.
Notes — Paramyrothecium foliicola formed a highly supported clade closely related but distinct to Pa. breviseta and Pa. roridum (Fig. 2). This species share several morphological features with Pa. nigrum and Pa. roridum. The conidiophore stipes of Pa. foliicola (up to 25 μm long) are shorter than those of Pa. nigrum (up to 45 μm long) and Pa. roridum (up to 40 μm long). Furthermore, Pa. foliicola produces a rosy buff exudate that diffuses into the growth medium, which was not seen for both Pa. nigrum and Pa. roridum.
Paramyrothecium humicola L. Lombard & Crous, sp. nov. — MycoBank MB815950; Fig. 52
Fig. 52.

Paramyrothecium humicola (CBS 127295). a–c. Sporodochial conidiomata; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d, f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or gregarious, oval or irregular in outline, 85–550 μm diam, 40–120 μm deep, lacking a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma, thin-walled, smooth, unbranched, straight to flexuous, hyaline, 1–2-septate, 55–65 μm long, 2–3 μm wide, terminating in an acute apice. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline sometimes covered by a pale green mucoid layer, septate, smooth, 12–22 × 2–3 μm; primary branches aseptate, unbranched, smooth, 7–17 × 1–2 μm; secondary branches aseptate, unbranched, smooth, 8–11 × 1–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline becoming darker at the apex, smooth, straight to slightly curved, 8–13 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 6–7 × 1–2 μm (av. 6 × 1 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium with sporodochia forming on the surface of the medium, covered by slimy herbage to olivaceous green conidial masses, reverse on PDA buff.
Material examined. USA, Kansas, near Manhattan, Konza Prairie Research Natural Area, long term ecological research site, from soil collected in tallgrass prairie, 1986, M. Christensen (holotype CBS H-22436, culture ex-type CBS 127295).
Notes — Paramyrothecium humicola formed a single lineage basal to the Pa. parvum clade (Fig. 2). This species is easily distinguished from Pa. parvum by the lack of setae formed surrounding the sporodochia of Pa. parvum. The conidia of Pa. humicola (6–7 × 1–2 μm (av. 6 × 1 μm)) are also slightly larger than those of Pa. parvum (4–5 × 1–2 μm (av. 5 × 2 μm)).
Paramyrothecium nigrum L. Lombard & Crous, sp. nov. — MycoBank MB815951; Fig. 53
Fig. 53.
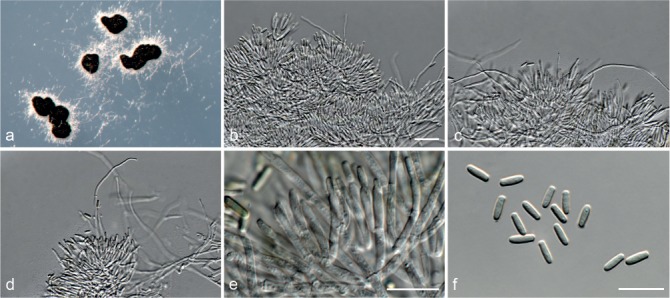
Paramyrothecium nigrum (CBS 116537). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the black conidial masses produced on the sporodochia by this fungus.
Conidiomata sporodochial, stromatic, superficial, scattered or gregarious, oval or irregular in outline, 50–850 μm diam, 55–165 μm deep, with a white setose fringe surrounding black, rarely olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma thin-walled, hyaline, 1–3-septate, straight becoming sinuous above the apical septum, 60–100 μm long, 2–3 μm wide, tapering to an acutely rounded apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 25–45 × 2–4 μm; primary branches aseptate, unbranched, smooth, 9–16 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 7–13 × 1–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth to slightly verrucose, straight to slightly curved, 8–13 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 5–6 × 1–2 μm (av. 6 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the surface of the medium in concentric rings, covered by slimy black, rarely olivaceous green, conidial masses, reverse on PDA pale luteous.
Material examined. SPAIN, Mallorca, Es Verger, from soil, 2001, G. Bills (holotype CBS H-22437, culture ex-type CBS 116537 = A.R. 3783).
Notes — Paramyrothecium nigrum formed a single lineage closely related to Pa. cupuliforme, Pa. foeniculicola and Pa. viridisporum (Fig. 2). Morphologically this species can be distinguished from the latter three species by their setae (up to 100 μm), which are longer than those of Pa. cupuliforme (up to 90 μm) and shorter than those of Pa. viridisporum (up to 140 μm), whereas Pa. foeniculicola did not produce any setae surrounding its sporodochia.
Paramyrothecium parvum L. Lombard & Crous, sp. nov. — MycoBank MB815952; Fig. 54
Fig. 54.
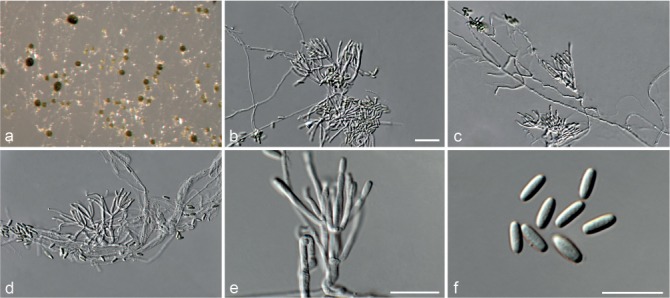
Paramyrothecium parvum (CBS 257.35). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the small conidia produced by this fungus.
Conidiomata sporodochial, stromatic, superficial, scattered, oval in outline, 25–50 μm diam, 35–65 μm deep, lacking a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline sometimes covered by a pale green mucoid layer, septate, smooth, 12–26 × 2–4 μm; primary branches aseptate, unbranched, smooth, 5–12 × 1–3 μm; secondary branches aseptate, unbranched, smooth, 5–11 × 1–2 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 7–23 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 4–5 × 1–2 μm (av. 5 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA buff to pale luteous, with mostly immersed mycelium with sporodochia forming on the surface of the medium, covered by slimy olivaceous green conidial masses, reverse on PDA buff.
Materials examined. FRANCE, from dune sand, Mar. 1942, F. Moreau, CBS 142.42 = IMI 155923 = MUCL 7582. – UK, Shropshire, Wellington, from Viola sp., Mar. 1935, N.C. Preston (holotype CBS H-14907, culture ex-type CBS 257.35 = IMI 140049).
Notes — Phylogenetic inference in this study showed that Pa. parvum formed a well-supported clade closely related to Pa. humicola (Fig. 2). See note under Pa. humicola for morphological differences.
Paramyrothecium roridum (Tode) L. Lombard & Crous, comb. nov. — MycoBank MB815989; Fig. 55
Fig. 55.
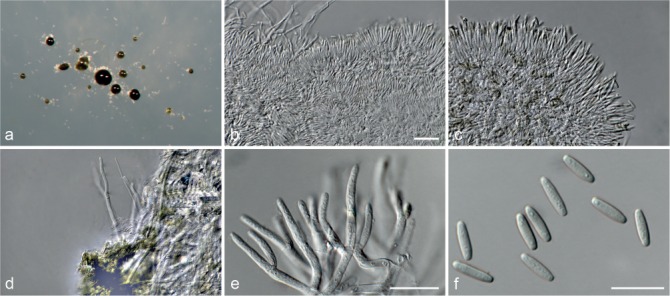
Paramyrothecium roridum (CBS 357.89). a. Sporodochial conidiomata on SNA; b–c. sporodochia; d. setae; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Basionym. Myrothecium roridum Tode, Fungi Mecklenburgenses Selecti 1: 25, t. 5: 38. 1790.
= Myrothecium advena Sacc., Ann. Mycol. 6: 560. 1908.
= Myrotheciella catenuligera Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 20: 460. 1910.
= Exothecium leucomelas Syd., Ann. Mycol. 12: 571. 1914.
≡ Myrothecium leucomelas (Syd.) Höhn., Mitt. Bot. Inst. Tech. Hochsch. Wien 2: 95. 1925.
= Myrothecium fragosianum Sacc., Not. Mycol. 22: 162. 1917.
?= Hymenospsis tenuis Petch, Ann. Roy. Bot. Gard. (Peradeniya) 10: 178. 1927.
= Myrothecium roridum Tode var. eichhorniae Ponnappa, Hyacinth Control Journal 8: 18. 1970.
Typification. Herb. K on Allium sativum, Fuckel’s Fungi Rhenani no. 166 (neotype fide Tulloch). – ITALY, Bologna, on Gardenia sp., Sept. 1989, G. Giunchi (epitype of D. roridum designated here, CBS H-22438, MBT204246, culture ex-epitype CBS 357.89).
Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or rarely gregarious, oval in outline, 20–100 μm diam, 100–210 μm deep, with a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma thin-walled, hyaline, 1–3(–4)-septate, smooth, straight to flexuous becoming sinuous above the apical septum, 60–100 μm long, 2–6 μm wide, tapering to an acutely rounded apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 15–40 × 2–4 μm; primary branches aseptate, unbranched, smooth, 10–20 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 6–20 × 2–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline becoming darkly pigmented at the collarettes, smooth, straight to slightly curved, 7–33 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, (5–)6.5–7.5(–8) × 2 μm (av. 7 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to pale luteous aerial mycelium with sporodochia forming on the surface of the medium, covered by slimy olivaceous green conidial masses, reverse on PDA pale luteous.
Additional materials examined. COLOMBIA, Chinchina, Caldas, from twig of Coffea sp., Aug. 1950, O. Urhan, CBS 372.50 = IMI 140050. – THE NETHERLANDS, from water from nursery, Apr. 1992, E.S. van Reenen-Hoekstra, CBS 212.92.
Notes — The ex-epitype strain (CBS 357.89) designated here for Pa. roridum clustered in a well-supported clade closely related to Pa. breviseta and Pa. foliicola (Fig. 2). For morphological differences see notes under Pa. breviseta and Pa. foliicola.
Paramyrothecium tellicola L. Lombard & Crous, sp. nov. — MycoBank MB815953; Fig. 56
Fig. 56.
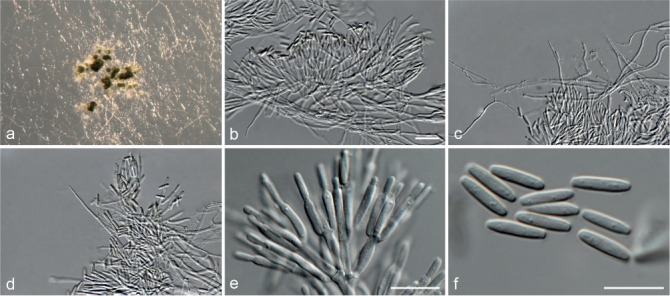
Paramyrothecium tellicola (CBS 478.91). a. Sporodochial conidiomata on SNA; b–c. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or gregarious, oval or irregular in outline, 45–750 μm diam, 80–165 μm deep, with a white setose fringe surrounding an olivaceous green to mouse grey agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma, sometimes branching at the basal septum, thin-walled, hyaline, 1–3-septate, smooth, straight becoming sinuous above the apical septum, tapering to an acutely rounded apex, 45–80 μm long, 2–3 μm wide. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 15–30 × 2–4 μm; primary branches aseptate, unbranched, smooth, 10–17 × 1–3 μm; secondary branches aseptate, unbranched, smooth, 7–11 × 1–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 7–17 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, (7–)7.5–8.5(–9) × 1–3 μm (av. 8 × 2 μm), with a flattened apex and rounded at the base.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to rosy buff aerial mycelium with sporodochia forming on the aerial mycelium and on the surface of the medium, covered by slimy olivaceous green to mouse grey conidial masses, reverse on PDA pale luteous to buff.
Material examined. TURKEY, Canakkale-Ezine, from soil, 1991, G. Turhan (holotype CBS H-22439, culture ex-type CBS 478.91).
Notes — Paramyrothecium tellicola formed a distinct single lineage basal to the Pa. terrestris clade (Fig. 2). The setae of Pa. tellicola (up to 80 μm) are longer than those of Pa. terrestris (up to 70 μm).
Paramyrothecium terrestris L. Lombard & Crous, sp. nov. — MycoBank MB815954; Fig. 57
Fig. 57.
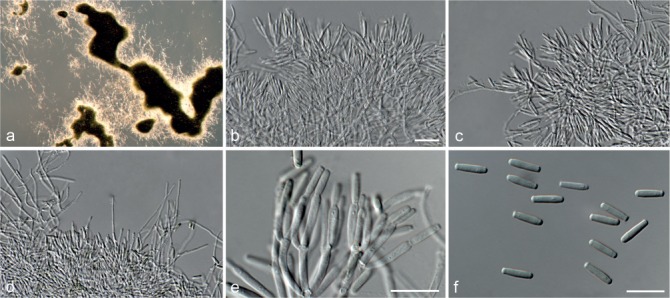
Paramyrothecium terrestris (CBS 564.86). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or gregarious, oval or irregular in outline, 20–750 μm diam, 70–145 μm deep, with a white setose fringe surrounding an olivaceous green to black agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma thin-walled, hyaline, 1–3-septate, smooth to slightly verrucose, straight becoming sinuous above the apical septum, tapering to an acutely rounded apex, 35–70 μm long, 2–3 μm wide. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 15–30 × 2–3 μm; primary branches aseptate, unbranched, smooth, 10–25 × 1–3 μm; secondary branches aseptate, unbranched, smooth, 5–12 × 1–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 7–12 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, (7–)7.5–8.5(–10) × 1–3 μm (av. 8 × 2 μm), with a flattened apex and rounded at the base.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white to buff aerial mycelium with sporodochia forming on the surface of the medium, covered by slimy olivaceous green to black conidial masses, reverse on PDA pale luteous to buff.
Materials examined. TURKEY, Canakkale-Ezine, from soil under Lycopersicon esculentum, 1986, G. Turhan (holotype CBS H-22440, culture ex-type CBS 564.86), CBS 566.86; Baslikesir-Aksabal, from soil beneath Helianthus annuus, 1986, G. Turhan, CBS 565.86; from soil, 1985, G. Turhan, CBS 872.85.
Notes — Phylogenetic inference showed that Pa. terrestris formed a highly supported clade, closely related to the single lineage representing Pa. tellicola (Fig. 2). For morphological differences, see notes under Pa. tellicola.
Paramyrothecium viridisporum L. Lombard & Crous, sp. nov. — MycoBank MB815955. Fig. 58
Fig. 58.
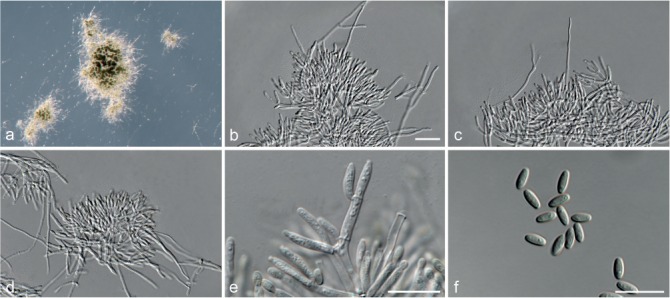
Paramyrothecium viridisporum (CBS 873.85). a. Sporodochial conidiomata on SNA; b–d. sporodochia; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the pale green conidia produced by this fungus.
Conidiomata sporodochial, cupulate, stromatic, superficial, scattered or gregarious, oval or irregular in outline, 100–900 μm diam, 30–85 μm deep, with a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae arising from the stroma thin-walled, unbranched, hyaline, 1–3-septate, straight becoming sinuous above the apical septum, tapering to an acutely rounded apex 60–140 μm long, 2–3 μm wide. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 15–35 × 2–3 μm; primary branches aseptate, unbranched, smooth, 8–17 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 5–9 × 1–2 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to elongate doliiform, hyaline, smooth, straight to slightly curved, 6–12 × 3–5 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, obovoid to ellipsoidal, 3–5 × 2 μm (av. 5 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the surface of the medium in concentric rings, covered by slimy mouse grey to olivaceous green conidial masses, reverse on PDA white to pale luteous.
Materials examined. TURKEY, from soil, 1985, G. Turhan (holotype CBS H-22441, culture ex-type CBS 873.85); Usak-Sükraniye, from soil under Cicer arietinum, 1986, G. Turhan, CBS 563.86. – USA, Wyoming, Rock Springs, DOE site, 11 km west of Rock Springs, from soil in sagebrush grassland, 1978, M. Christensen, CBS 125821; from soil in bunchgrass rhizosphere, 1978, M. Christensen, CBS 125835; Grand Teton National Park, from soil in sagebrush grasslands, 1966, J.S. States, CBS 126942; near Dubois, from soil in desert grassland, 1997, M. Christensen, CBS 127843; 6 miles north and west of Hanna, from soil in strip mine area, 1976, M. Christensen, CBS 127785.
Notes — Paramyrothecium viridisporum produced the longest setae (up to 140 μm) distinguishing it from other species in the genus. Additionally, this species is also characterised by obovoid to ellipsoidal pale green conidia, not seen for the other species. Phylogenetic inference in this study showed that Pa. viridisporum formed a well-supported clade closely related to Pa. cupuliforme and Pa. nigrum (Fig. 2).
Parasarcopodium Mel’nik et al., Mycol. Progr. 3: 22. 2004
Description and illustration — See Mel’nik et al. (2004).
Type species. Parasarcopodium ceratocaryi Mel’nik et al., Mycol. Progr. 3: 24. 2004.
Notes — This monotypic genus, based on Parasarcopodium ceratocaryi, was initially classified as a member of the Bionectriaceae (Mel’nik et al. 2004) based on sequences data of the 18S small subunit rDNA gene (SSU) and LSU gene regions. However, phylogenetic inference in this study placed the ex-type strain (CBS 110664) in the Stachybotriaceae (Fig. 1), which include a much larger sampling of taxa. Therefore, Parasarcopodium should be considered as a member of the Stachybotriaceae.
Parvothecium L. Lombard & Crous, gen. nov. — MycoBank MB816019
Etymology. Name reflects the characteristic small sporodochia formed in culture by this fungus.
Type species. Parvothecium terrestre L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, scattered or rarely gregarious, oval or irregular in outline, covered by an olivaceous green to dark green slimy mass of conidia. Stroma well developed, hyaline, of a textura angularis. Setae rarely seen, thin-walled, septate, flexuous, hyaline, tapering to an obtuse apice. Conidiophores macronematous, penicillately branched, hyaline, smooth to verrucose. Conidiogenous cells phialidic, hyaline, smooth to verrucose, cylindrical, becoming narrowed at the tip, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, ellipsoidal to asymmetrically ellipsoidal, hyaline becoming pigmented with age, smooth, sometimes with a slightly curved acute apex and a narrow truncate base.
Notes — The monotypic genus, Parvothecium, is introduced here for a well-supported clade, closely related but distinct from the Virgatospora and Septomyrothecium s.str. clades and the single lineage representing Inaequalispora prestonii (Fig. 2). Morphologically, Parvothecium is similar to Inaequalispora, but can be distinguished by thin-walled setae and a verrucose conidiogenous apparatus compared to the thick-walled setae and smooth conidiogenous apparatus of Inaequalispora (Tulloch 1972, Nag Raj 1995).
Parvothecium terrestre L. Lombard & Crous, sp. nov. — MycoBank MB816020; Fig. 59
Fig. 59.
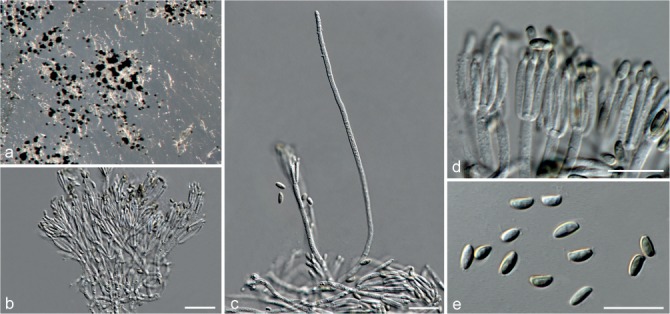
Parvothecium terrestre (CBS 198.89). a. Sporodochial conidiomata on SNA; b. sporodochia; c. setae; d. conidiogenous cells; e. conidia. — Scale bars: b = 20 μm; c–e = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, scattered, rarely gregarious, oval to elongate or irregular in outline, 45–85 μm diam, 50–100 μm deep, without a setose fringe surrounding a green to black agglutinated slimy mass of conidia. Stroma well developed, hyaline, smooth to verrucose, of a textura angularis. Setae rare, arising from the basal stroma, thin-walled, hyaline, smooth, flexuous, septate, 100–135 μm long, 2–3 μm wide, narrowing to an obtuse apice. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth to verrucose, 25–50 × 2–4 μm; conidiogenous apparatus consists of a whorl of 3–5 primary branches terminating in 3–6 conidiogenous cells; primary branches aseptate, unbranched, smooth to verrucose, 10–18 × 2–3 μm; conidiogenous cells phialidic, cylindrical, hyaline, smooth to verrucose, 8–15 × 1–2 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, smooth, hyaline becoming pigmented with age, ellipsoidal to asymmetrically ellipsoidal, 4–5 × 2–3 μm (av. 4 × 2 μm), sometimes with a slightly curved acute apex and a narrow truncate base.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed aerial mycelium and sporodochia forming on the surface of the medium, covered by slimy olivaceous green to black conidial masses, reverse on PDA pale olivaceous green.
Materials examined. BRAZIL, Pará, Capitão Poço, c. 200 km east of Belém, Experimental Station Embrapa, from soil in virgin forest, Feb. 1989, L. Pfenning (holotype CBS H-4380, culture ex-type CBS 198.89). – CUBA, Habana, Santiago de las Vegas, from leaf litter of Andira inermis, 9 July 1987, R.F. Castañeda, CBS 534.88 = INIFAT C87/234.
Peethambara Subram. & Bhat, Rev. Mycol. (Paris) 42: 52. 1978 — Fig. 60
Fig. 60.
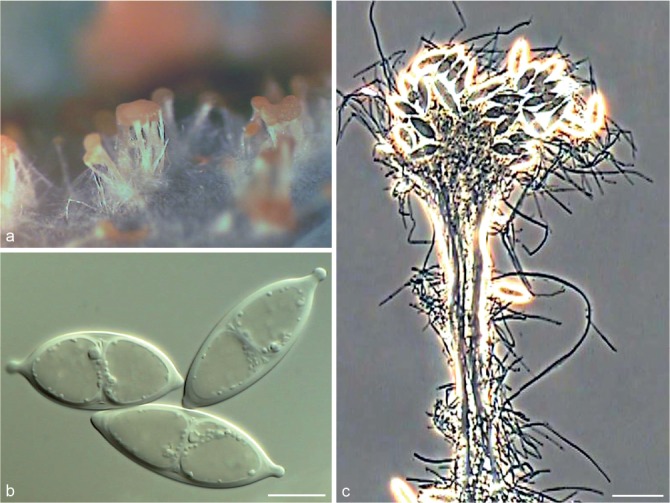
Peethambara sundara (CBS 521.96). a. Synnemata on substrate; b. conidia; c. synnemata. — Scale bars: b = 10 μm; c = 50 μm.
= Putagraivam Subram. & Bhat, Proc. Indian Acad. Sci., B, 87: 103. 1978.
Description and illustration — See Subramanian & Bhat (1978a, b), Seifert 1985 and Rossman et al. (1999).
Type species. Peethambara sundara Subram. & Bhat, Rev. Mycol. (Paris) 42: 52. 1978.
= Putagraivam sundarum Subram. & Bhat, Proc. Indian Acad. Sci., B, 87: 103. 1978.
≡ Didymostilbe sundara (Subram. & Bhat) Seifert, Stud. Mycol. 27: 140. 1985.
Notes — Phylogenetic inference in this study placed the ex-type (CBS 646.77) of P. sundara in a well-supported clade, distantly related to the Didymostilbe clade and Virgatospora clade containing the second species, P. spirostriata as V. echinofibrosa (Fig. 2). Therefore, the sexual genus Peethambara is considered monotypic here. Peethambara and its asexual morph, Putagraivam, both contain a single species, however, the latter generic name is less commonly used in literature. Therefore, we choose to retain Peethambara and regard Putagraivam as synonym.
Septomyrothecium Matsush., Bull. Natl. Sci. Mus. Tokyo 14: 469. 1971
?= Nectria septomyrothecii Samuels, Brittonia 40: 326. 1988.
Type species. Septomyrothecium uniseptatum Matsush., Bull. Natl. Sci. Mus. Tokyo 14: 470. 1971.
Description and illustration — See Matsushima (1971a, b), Samuels (1988) and Decock et al. (2008).
Notes — The genus Septomyrothecium, based on S. uniseptatum (Matsushima 1971a, b), is distinguished from Myrothecium s.str. by long dichotomously branched hyphoid extensions extending beyond the conidial masses, and some species producing 1-septate conidia (Matsushima 1971a, b, Decock et al. 2008). This genus includes two additional species, i.e. S. maraitense and S. setiramosum (Decock et al. 2008) of which the latter along with M. dimorphum (Watanabe et al. 2003) probably represents another new genus. Both these fungi produce mostly unbranched and thick-walled hypoid extensions that terminates in a grown of 3–6 short protuberances (Castañeda 1986, Watanabe et al. 2003). However, no sequences or strains for both species were available during this study and their classification remains unresolved. Samuels (1988) was able to link the sexual morph Nectria septomyrothecii to S. uniseptatum based on morphology. However, the ex-type strain of this sexual morph is no longer viable (Samuels 1988) to confirm this connection.
Sirastachys L. Lombard & Crous, gen. nov. — MycoBank MB816021
Etymology. Name reflects the characteristic rope-like (Greek = sira) synnematous hyphae formed in culture by these fungi.
Type species. Sirastachys phaeospora L. Lombard & Crous.
Sexual morph unknown. Synnemata cylindrical, hyaline, slender to robust, straight to curved, consisting of bundles of parallel, longitudinal, closely compacted hyphae. Conidiophores arising laterally from synnemata, macronematous, mononematous, erect, solitary or in groups, unbranched or branched, thin-walled or thick-walled, hyaline to pale olivaceous, smooth to verrucose, 1–3-septate, with an apical cluster of 6–12 conidiogenous cells. Conidiogenous cells phialidic, elongate doliiform to clavate to subclavate, smooth to slightly verrucose, hyaline to pale olivaceous, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline to pale olivaceous brown to dark brown, smooth to verrucose, ellipsoidal to obovoid to cylindrical, rounded at both ends.
Notes — The new asexual genus Sirastachys (Si.) is established here for a group of stachybotrys-like fungi characterised by the formation of synnemata in culture from which the conidiophores arise laterally. Phylogenetic inference in this study showed that members of this genus formed a well-supported clade distantly related to the Stachybotrys s.str. clade (Fig. 3). ITS sequence data placed the ex-type strain (ATCC 32451; Matsushima 1975, Wang et al. 2015) of St. longispora within the Sirastachys clade (Fig. 4), and therefore a new combination is provided for this species here.
Sirastachys castanedae L. Lombard & Crous, sp. nov. — MycoBank MB816022; Fig. 61
Fig. 61.

Sirastachys castanedae (CBS 136403). a–b. Conidiophores; c. conidiogenous cells; d. conidia. — Scale bars: a = 20 μm (apply to b); c–d = 10 μm.
Etymology. Name honours eminent Cuban mycologist Dr. R.F. Castañeda, who collected the type of this fungus.
Synnemata cylindrical, hyaline, slender, straight to curved, consisting of bundles of parallel, longitudinal, closely compacted hyphae, 10–15 μm diam. Conidiophores macronematous, mononematous, single or in groups, unbranched or rarely branched once, erect, straight, 2–3(–4)-septate, thin-walled, smooth to verrucose, hyaline to olivaceous brown towards the apex, 45–90 × 3–5 μm, bearing a whorl of 4–8 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to clavate, hyaline to subhyaline, smooth to verrucose, 7–12 × 3–4 μm, with conspicuous collarettes and periclinal thickenings. Conidia acrogenous, aseptate, obovoid, verrucose, darkly olivaceous to dark brown, (4–)4.5–5.5(–6) × 2–3 μm (av. 5 × 3 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white to pale luteous aerial mycelium forming synnemata and immersed mycelium, buff to pale luteous, with abundant conidiophores forming on the synnemata, bearing slimy olivaceous green to mouse grey conidial masses, reverse on PDA pale luteous to sienna.
Materials examined. CANADA, Ontario, Aberfoyle, from soil under Thuja occidentalis, July 1964, G.C. Bhatt, CBS 531.69 = IMI 144477. – IRAN, West Azerbaijan Province, Urmia, from Morus sp., 2010, M. Arzanlou, CPC 20737. – SPAIN, from decaying leaf, July 1996, R.F. Castañeda-Ruiz (holotype CBS H-22457, culture ex-type CBS 136403 = MUCL 39994); from decaying leaf, July 1996, R.F. Castañeda-Ruiz, CBS 164.97.
Notes — Sirastachys castanedae formed a highly supported clade closely related to Si. cylindrospora and Si. pseudolongispora (Fig. 3). The conidiophores of Si. castanedae (up to 90 μm) are longer than those of Si. cylindrospora (up to 50 μm) and Si. pseudolongispora (up to 55 μm). The conidia of Si. castanedae ((4–)4.5–5.5(–6) × 2–3 μm (av. 5 × 3 μm)) are smaller than those of Si. cylindrospora ((7–)8.5–9.5(–10) × 2–3 μm (av. 9 × 2 μm)) and Si. pseudolongispora ((8–)9.5–10.5(–11) × 2 μm (av. 10 × 2 μm)).
Sirastachys cylindrospora L. Lombard & Crous, sp. nov. — MycoBank MB816023; Fig. 62
Fig. 62.
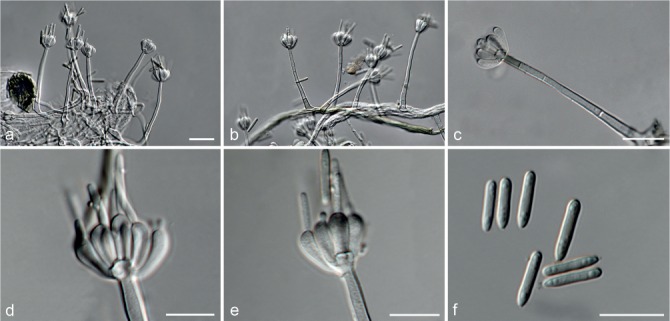
Sirastachys cylindrospora (CBS 136166). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 10 μm (apply to b); c–f = 10 μm.
Etymology. Name reflects the cylindrical conidia produced by this fungus.
Synnemata cylindrical, hyaline, slender, straight to curved, consisting of bundles of parallel, longitudinal, closely compacted hyphae, 10–15 μm diam. Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 2–3-septate, thin-walled, smooth, hyaline, 35–50 × 3–5 μm, bearing a whorl of 4–8 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to clavate, hyaline, smooth, 6–8 × 3–4 μm, with conspicuous collarettes and periclinal thickenings. Conidia acrogenous, aseptate, cylindrical, straight, hyaline, (7–)8.5–9.5(–10) × 2–3 μm (av. 9 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium forming synnemata and immersed mycelium, buff to pale luteous, with abundant conidiophores forming on the synnemata, bearing slimy olivaceous green conidial masses, reverse on PDA pale luteous to sienna.
Materials examined. BRAZIL, from decaying leaf, Sept. 1997, R.F. Castañeda-Ruiz (holotype CBS H-22458, culture ex-type CBS 136166 = MUCL 41106 = INIFAT C98/42), CBS 136546 = MUCL 41088 = INIFAT C98/30.
Notes — Sirastachys cylindrospora formed a highly supported clade closely related to Si. castanedae and Si. pseudolongispora (Fig. 3). However, this species shares several morphological features with Si. pseudolongispora, and can be distinguished by the conidiophores that remain hyaline, where as those of Si. pseudolongispora become olivaceous brown towards the apex.
Sirastachys longispora (Matsush.) L. Lombard & Crous, comb. nov. — MycoBank MB816024
Basionym. Stachybotrys longispora Matsush., Icon. Microfung. Matsush. Lect.: 145. 1975.
Description and illustration — See Matsushima (1975) and Wang et al. (2015).
Notes — The conidiophores of Si. longispora (up to 75 μm; Matsushima 1975) are longer than those of Si. cylindrospora and Si. pseudolongispora (both up to 50 μm). Furthermore, the conidia of Si. longispora are longer (8.8–12 × 2–2.4 μm; Matsushima 1975) than those of Si. cylindrospora and Si. pseudolongispora.
Sirastachys pandanicola L. Lombard & Crous, sp. nov. — MycoBank MB816025; Fig. 63
Fig. 63.
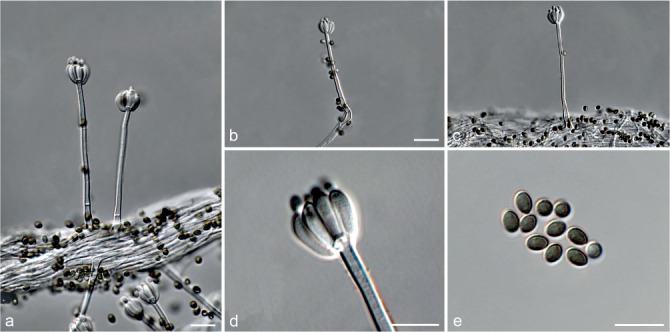
Sirastachys pandanicola (CBS 136545). a–c. Conidiophores; d. conidiogenous cells; e. conidia. — Scale bar: a, d, e = 10 μm; b = 20 μm (apply to c).
Etymology. Name reflects the host genus Pandanus from which this fungus was isolated.
Synnemata cylindrical, hyaline, robust, straight to curved, consisting of bundles of parallel, longitudinal, closely compacted hyphae, 15–45 μm diam. Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 1–3-septate, thin-walled, smooth, hyaline, 55–75 × 3–5 μm, bearing a whorl of 4–8 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to clavate, hyaline, smooth, 6–9 × 2–4 μm, with conspicuous collarettes and periclinal thickenings. Conidia acrogenous, aseptate, obovoid to ellipsoidal, verrucose, darkly olivaceous, 3–4 × 2–3 μm (av. 4 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium forming synnemata, with abundant conidiophores forming on the synnemata, bearing slimy olivaceous green conidial masses, reverse on PDA buff to pale luteous.
Material examined. SINGAPORE, MacRitchie Nature Reserve, from decaying leaf of Pandanus sp., 2008, O. Laurence (holotype CBS H-22459, culture ex-type CBS 136545 = MUCL 49906).
Notes — Sirastachys pandanicola formed a single lineage basal to the Si. phaeospora clade (Fig. 3). This species can be distinguished from Si. phaeospora by its shorter (up to 45 μm) conidiophores compared to those of Si. phaeospora (up to 65 μm).
Sirastachys phaeospora L. Lombard & Crous, sp. nov. — MycoBank MB816026; Fig. 64
Fig. 64.

Sirastachys phaeospora (CBS 100155). a–c. Conidiophores; d. conidiogenous cells; e. conidia. — Scale bars: a, d–e = 10 μm; b = 20 μm.
Etymology. Name reflects the dark brown conidia produced by this fungus.
Synnemata cylindrical, hyaline, slender to robust, straight to curved, consisting of bundles of parallel, longitudinal, closely compacted hyphae, 10–65 μm diam. Conidiophores macronematous, mononematous, single or in groups, unbranched or branched once, erect, straight, 1–2(–3)-septate, thin-walled, smooth to verrucose, hyaline, 40–65 × 3–5 μm, bearing a whorl of 4–8 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to clavate, hyaline, smooth to slightly verrucose, 7–9 × 2–4 μm, with conspicuous collarettes and periclinal thickenings. Conidia acrogenous, aseptate, obovoid to ellipsoidal, verrucose, darkly olivaceous to dark brown, 4–5 × 2–3 μm (av. 4 × 3 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant aerial mycelium forming synnemata and immersed mycelium, ochreous to amber, with abundant conidiophores forming on the synnemata, bearing slimy mouse grey conidial masses, reverse on PDA ochreous to sienna.
Materials examined. BRAZIL, Mata Atlântica, João Pessoa, from decaying leaf of unknown host, Sept. 1997, R.F. Castañeda-Ruiz, CBS 136167 = MUCL 41195; Rio Tinto, from decaying leaf of unknown host, Sept. 1997, R.F. Castañeda-Ruiz, CBS 136185 = MUCL 41191. – CUBA, Trinidad, from decaying leaves in rain forest, Jan. 1996, J. Guarro (holotype CBS H-22460, culture ex-type CBS 100155). – SOUTH AFRICA, Western Cape Province, from Cycas sp., 1 Jan. 2008, P.W. Crous, CPC 16092, 16093. – THE NETHERLANDS, Wageningen, from soil, Apr. 1975, J.A. Stalpers, CBS 253.75.
Notes — Sirastachys phaeospora formed a well-supported clade closely related to Si. pandanicola (Fig. 3). For morphological differences see notes under Si. pandanicola.
Sirastachys phyllophila L. Lombard & Crous, sp. nov. — MycoBank MB816027; Fig. 65
Fig. 65.

Sirastachys phyllophila (CBS 136169). a–b. Conidiophores; c. conidiogenous cells; d. conidia. — Scale bars = 10 μm.
Etymology. Name reflects the substrate, plant debris (Greek = phyllophilus), from which this fungus was isolated.
Synnemata not observed. Conidiophores macronematous, mononematous, single or in groups, unbranched, erect, straight, 1–3-septate, thick-walled, smooth to slightly verrucose, hyaline, 80–150 × 3–5 μm, bearing a whorl of 6–12 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to clavate, hyaline, smooth to slightly verrucose, 7–11 × 2–4 μm, with conspicuous collarettes and periclinal thickenings. Conidia acrogenous, aseptate, ellipsoidal, verrucose, darkly olivaceous, 4–5 × 2–3 μm (av. 5 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium, pale luteous, with abundant conidiophores forming on the surface of the medium, bearing slimy mouse grey conidial masses, reverse on PDA sienna to luteous.
Materials examined. SPAIN, from decaying leaves, July 1996, R.F. Castañeda (holotype CBS H-22461, culture ex-type CBS 136169 = MUCL 39919), CBS 173.97.
Notes — Sirastachys phyllophila formed a highly supported clade (Fig. 3) and is the only species for which no synnemata were observed in culture. Additionally, Si. phyllophila produced the longest conidiophores (up to 150 μm) compared to the other species in this genus.
Sirastachys pseudolongispora L. Lombard & Crous, sp. nov. — MycoBank MB816028; Fig. 66
Fig. 66.
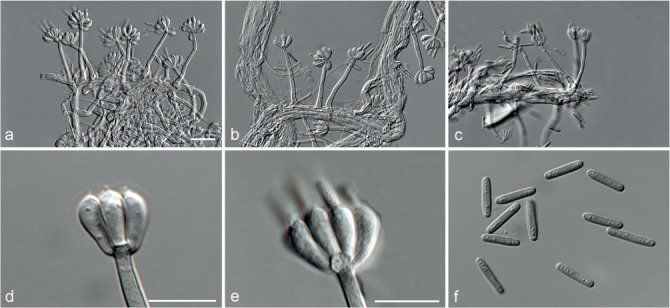
Sirastachys pseudolongispora (CBS 100154). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 10 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the morphological similarity to Sirastachys longispora.
Synnemata cylindrical, hyaline, slender to robust, straight to curved, consisting of bundles of parallel, longitudinal, closely compacted hyphae, 10–55 μm diam. Conidiophores macronematous, mononematous, single or in groups, unbranched or branched, erect, straight, 1–2-septate, thin-walled, smooth to slightly verrucose, hyaline or olivaceous brown becoming paler towards the base, 35–50 × 3–5 μm, bearing a whorl of 4–8 conidiogenous cells. Conidiogenous cells terminal, elongate doliiform to clavate, hyaline to olivaceous brown, smooth to verrucose, 6–8 × 3–4 μm, with conspicuous collarettes and periclinal thickenings. Conidia acrogenous, aseptate, cylindrical, smooth, hyaline to olivaceous brown, (8–)9.5–10.5(–11) × 2 μm (av. 10 × 2 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant aerial mycelium forming synnemata and immersed mycelium, ochreous to sienna, centre brown vinaceous, with abundant conidiophores forming on the synnemata, bearing slimy olivaceous green to mouse grey conidial masses, producing a pale luteous exudate diffusing into the media, reverse on PDA ochreous to sienna.
Materials examined. CUBA, Trinidad, from decaying leaves in rain forest, Jan. 1996, J. Guarro (holotype CBS H-22462, culture ex-type CBS 100154); La Habana, from leaf litter, 6 May 1993, R.F. Castañeda, CBS 417.93 = INIFAT C93/213-3.
Notes — Sirastachys pseudolongispora formed a well-supported clade (Fig. 3) and is the only species that produced a pale luteous extracellular pigment into the media.
Smaragdiniseta L. Lombard & Crous, gen. nov. — MycoBank MB816029
Etymology. Name reflects the characteristic emerald green setae surrounding the sporodochia.
Type species. Smaragdiniseta bisetosa (V.G. Rao & de Hoog) L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, cupulate, scattered to gregarious, oval to elongate or irregular in outline, surrounded by two types of setae inclosing an olivaceous green to dark green slimy mass of conidia. Stroma well developed, hyaline, of textura globulosa or textura angularis. Type I setae compacted, thick-walled, verrucose, emerald green. Type II setae originating from the marginal hyphae, hyaline, septate, thick-walled, smooth to lightly verrucose, tapering to an obtuse apice, soon becoming overgrown by setae of the first type. Conidiophores macronematous, irregularly, verticillately or penicillately branched, hyaline, smooth. Conidiogenous cells phialidic, hyaline, smooth, cylindrical, becoming narrowed at the tip, without conspicuous collarettes and periclinal thickenings. Conidia aseptate, obclavate to narrowly ellipsoidal, hyaline, smooth (adopted from Rao & De Hoog 1983).
Notes — Based on phylogenetic inference in this study, the ex-type strain (CBS 459.82) of Myr. bisetosum formed a single lineage sister to the Albifimbria clade, distant to the Myrothecium s.str. clade (Fig. 1, 2). Therefore, the monotypic genus Smaragdiniseta is introduced here and Myr. bisetosum is provided with a new combination below. Smaragdiniseta can be distinguished from members of Albifimbria by the formation of two types of setae and conidia lacking a funnel-shaped mucoid apical appendage.
Smaragdiniseta bisetosa (V.G. Rao & de Hoog) L. Lombard & Crous, comb. nov. — MycoBank MB816030
Basionym. Myrothecium bisetosum V.G. Rao & de Hoog, Persoonia 12: 99. 1983.
Description and illustration — See Rao & De Hoog (1983).
Material examined. INDIA, Adilabad, Pranheeta Valley, on inner side of rotten bark, 16 Jan. 1981, V. Rao & A.C. Rao, ex-type CBS 459.82 (sterile).
Stachybotrys Corda, Icon. Fungorum 1: 21. 1837
= Gliobotrys Höhn., Sber. Akad. Wiss. Wien Math.-naturw. Kl., Abt I, 111: 1048. 1902.
= Hyalobotrys Pidopl., Mykrobiol. Zh. Kiev 9, 2–3: 55. 1948.
= Hyalostachybotrys Sriniv., J. Indian Bot. Soc. 37: 340. 1958.
= Ornatispora K.D. Hyde, Goh, J.E. Taylor & J. Fröhl., Mycol. Res. 103: 1432. 1999.
Type species. Stachybotrys chartarum (Ehrenb.) S. Hughes.
Ascomata perithecial, superficial except at the base, stromatic, solitary or occasionally in pairs, subglobose to obpyriform, black, surface glabrous, papillate ostiolar region without periphyses, with setae irregularly arranged over the surface. Ascomatal wall consisting of a single layer of textura angularis. Setae erect, irregularly flexuous, brown fading towards the apex, thick-walled, septate, smooth or verrucose, with a rounded apex, infrequently branching towards the apex, sometimes developing into fertile conidiophores. Asci clavate, 8-spored, apex rounded to nearly truncate with a refractive apical ring. Ascospores cylindrical, 1-septate, subhyaline, verrucose, with slightly tapering apices, surrounded by a thick mucoid sheath (adapted from Hyde et al. 1999, Whitton et al. 2012). Conidiophores macronematous, mononematous, erect, solitary or in groups, unbranched or branched, thin-walled, hyaline to olivaceous brown, smooth or verrucose, 1–2(–4)-septate, with 3–12 conidiogenous cells radiating from the apex. Conidiogenous cells phialidic, subcylindrical to clavate to fusiform to elongate doliiform, smooth to verrucose, hyaline to olivaceous brown, with conspicuous collarettes. Conidia aseptate, initially hyaline becoming olivaceous brown to dark brown, smooth to verrucose, thick-walled, ellipsoidal to globose to fusiform to limoniform, rounded at both ends or with an apical hilum.
Notes — Phylogenetic inference in this study clearly illustrates the polyphyletic nature of Stachybotrys s.l., resulting in the segregation of this genus into 10 genera, which is also supported by morphological observations. Therefore, the generic concept for Stachybotrys s.str. is restricted to include only species characterised by conidiophores branching at the basal septum and the formation of thick-walled conidia sometimes bearing ornamentations. Stachybotrys chartarum is the type species of the genus, for which no living type material presently exists. Hughes (1958) was able to study the holotype of Stilbospora chartarum (≡ St. chartarum; Ehrenberg 1818) lodged at UPS, which can no longer be located at UPS (I.O. Ibarguren, pers. comm.). However, a slide made by Hughes, of Ehrenberg’s holotype material, was located at DAOM and preserved as DAOM 51026. This slide was studied and compared with CBS 182.80, an isolate obtained from cheese wrapping in the Netherlands. The structures observed in the type slide were identical to those observed in CBS 182.80. Hence, we designate CBS 182.82 as epitype for St. chartarum.
Stachybotrys aloeticola L. Lombard & Crous, Persoonia 32: 283. 2014
Description and illustrations — See Crous et al. (2014).
Materials examined. SOUTH AFRICA, Eastern Cape Province, Grahamstown, on Aloe sp., 26 July 2011, P.W. Crous, CBS 137940 = CPC 19705 (ex-type of Stachybotrys aloeticola), CBS 137941 = CPC 19706.
Notes — Stachybotrys aloeticola formed a highly supported clade closely related to St. reniformis (Fig. 3).
Stachybotrys chartarum (Ehrenb.) S. Hughes, Canad. J. Bot. 36: 812. 1958 — Fig. 67
Fig. 67.

Stachybotrys chartarum. a–c. DAOM 51026. a. Conidiophore; b. conidia; c. conidiogenous cells. — d–i. CBS 182.80; d–h. Conidiophores; i. conidia. — Scale bars: a–c = 10 μm (apply to g–h); d = 20 μm (apply to e–f); i = 10 μm.
≡ Stilbospora chartarum Ehrenb., Sylv. Mycol. Berol.: 9, 21. 1818.
≡ Oidium chartarum (Ehrenb.) Link, Linn. Spec. Plant. IV, 6: 124. 1824.
≡ Oospora chartarum (Ehrenb.) Wallr., Fl. Crypt. Germ. 2: 184. 1833.
= Stachybotrys atra Corda, Icon. Fungorum 1: 21. 1837.
= Synsporium biguttatum Preuss, Klotzsch. Herb. Vivum. Mycol. No. 1285. 1849.
= Memnonium sphaerospermum Fuckel, Symb. Mycol.: 358. 1870. (for more synonymies see Wang et al. 2015)
Typification. GERMANY, Leipzig, C.G. Ehrenberg, in charta emporetica putrida; domi, 1818 (UPS, holotype of Stilbospora chartarum – missing; slide of holotype ex-UPS = DAOM 51026). – THE NETHERLANDS, from cheese wrapping, 1980, collector unknown (epitype of Stilbospora chartarum designated here, CBS H-18496, MBT204311, culture ex-epitype CBS 182.80).
Ascomata not observed. Conidiophores macronematous, mononematous, single or in groups, thin-walled, unbranched or branched, erect, straight to slightly flexuous, subhyaline to olivaceous brown, 1–3(–4)-septate, smooth, 40–110 × 3–6 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subclavate, subhyaline to olivaceous brown, smooth, 9–14 × 3–6 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to subcylindrical, thick-walled, olivaceous brown to dark brown, verrucose, (8–)8.5–9.5(–11) × (3–)3.5–4.5(–5) μm (av. 9 × 4 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium, buff to pale luteous, with conidiophores forming on the surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA rosy vinaceous to salmon.
Additional materials examined. BELGIUM, Fagnolles, from wall-paper, 1990, G.L. Hennebert, CBS 136189 = MUCL 30782; Hacquegnies, from mouldy wall-paper in moist house, Aug. 1963, G.L. Hennebert, CBS 136174 = MUCL 21588, CBS 136163 = MUCL 3820; Heverlee, from humid soil in greenhouse, June 1971, B. Desai, CBS 136161 = MUCL 18140; Leuven, from cardboard in garden, Aug. 1961, V. Estienne, CBS 136400 = MUCL 2538; from mouldy wall-paper in house, Sept. 1956, V. Estienne, CBS 136159 = MUCL 308; Louvian-la-Neuve, from gyproc plate, May 1986, G.L. Hennebert, CBS 136186 = MUCL 28869. – CANADA, Ontario, Toronto, Manulife Centre, from mouldy drywall, Nov. 1996, J. Scott, CBS 136172 = MUCL 40562; Ottawa, from Anthacobia sp., date and collector unknown, CBS 136188 = MUCL 2443; Québec, substrate unknown, Feb. 1970, G. Ola’h, CBS 136176 = MUCL 15918. – CUBA, from leaf litter, 1996, R.F. Castañeda-Ruiz, CBS 492.96. – DENMARK, Copenhagen, from paint, May 1992, collector unknown, CBS 109288; from building material, 20 Jan. 1997, K.F. Nielsen, CBS 109289. – ENGLAND, Birmingham, from cotton fabric, 1960, R.M. Everett, CBS 101146. – FINLAND, Helsinki, from water-damaged fibre board, 2001, J. Peltola, CBS 109563; from water-damaged gypsum liner board, CBS 109561, CBS 109562; Kuopio, from building material, 20 Mar. 2000, A. Hyvärinen, CBS 109292. – FRANCE, from sand dune, 1942, F. Moreau, CBS 177.42. – NORWAY, Tromsø, from air, 1992, A.-L. Klodiussen, CBS 215.92. – SPAIN, Poblet, Tarragona, from submerged rotten leaf, 3 Nov. 2002, R.F. Castañeda-Ruiz, CBS 112541. – SWITZERLAND, from soil, 1948, S. Blumer, CBS 485.48. – THE NETHERLANDS, from wilting Clematis sp., Mar. 1949, I. de Boer, CBS 363.49; from house, June 2005, J. Houbraken, CBS 119370. – USA, Ohio, Cleveland, from a house, 29 Oct. 1999, J. Simpson, CBS 109287, CBS 109290.
Notes — Stachybotrys chartarum formed a highly supported clade closely related to St. chlorohalonata (Fig. 3).
Stachybotrys chlorohalonata B. Andersen & Thrane, Mycologia 95: 1228. 2003
Description and illustrations — See Andersen et al. (2003).
Materials examined. BELGIUM, Gent, from soil, J. van Holder, CBS 136160 = MUCL 258. – CANADA, Ontario, Scarborough, Hildenboro Square, North of Finch, west of Warden Ave., from wood, paper and tile, 9 Dec. 1988, R.S. Khan, CBS 251.89. – DENMARK, Copenhagen, from building material, 20 Jan. 1997, K.F. Nielsen, CBS 109283; Lyngby, Technical University of Denmark, contamination on Petri dish, June 2000, B. Andersen, CBS 109281; Sjælland, from cardboard on gypsum board, Oct. 1997, K.F. Nielsen, CBS 109285 = IBT 9467 (ex-type culture of Stachybotrys chlorohalonata). – FINLAND, Jokioinen, Masku, from plastic of insulation wall in play room of day-care-centre, 1994, A. Hyvarinen, CBS 127.94. – ITALY, from paper, July 1937, O. Verona, CBS 330.37, CBS 328.37 = ATCC 18844. – NAMIBIA, South east of Swakopmund, near Gobabeb, from desert sand, 7 Jan. 1986, J.C. Krug, CBS 250.89. – PORTUGAL, Vilarinho, from plant debris, Nov. 2007, J. Capilla, R.F. Castañeda-Ruiz & C. Silvera, CBS 122763. – SINGAPORE, Bukit Timah Nature Reserve, from decayed wood, 2008, O. Laurence, CBS 136158 = MUCL 49910. – THE NETHERLANDS, from raw flax fibre, June 1946, R. Bok, CBS 222.46 = ATCC 18842. – TUNISIA, Sfax, Centre de Biotechnologie de Sfax, from contaminated paper in laboratory, Jan. 1995, A. Gargouri, CBS 608.94. – USA, substrate unknown, June 1935, N.F. Conant, CBS 341.35 = ATCC 18847 = MUCL 9477; New York, Ithaca, Campus of Cornell University, from rhizosphere of Triticum aestivum, 1962, J.W. Jooste, CBS 136194 = MUCL 4311; Wisconsin, from soil in willow-cottonwood forest, 1962, M. Christensen, CBS 129226.
Notes — Phylogenetic inference in this study agreed with the results obtained by Andersen et al. (2003), recognising St. chlorohalonata as a distinct species from St. chartarum (Fig. 3).
Stachybotrys dolichophialis L. Lombard & Crous, sp. nov. — MycoBank MB816031; Fig. 68
Fig. 68.
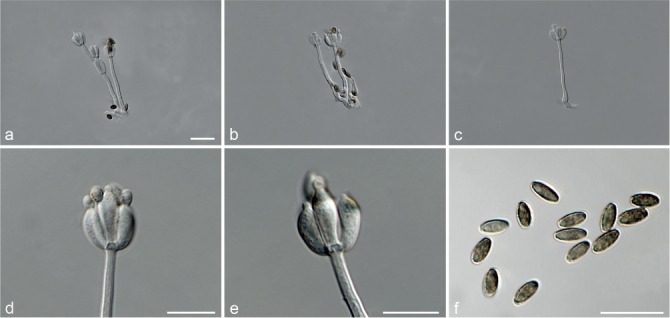
Stachybotrys dolichophialis (DAOM 227011). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the elongated central conidiogenous cell characteristic of this fungus.
Ascomata not observed. Conidiophores macronematous, mononematous, single or in groups, thin-walled, unbranched, erect, straight to slightly flexuous, hyaline, 1–3-septate, smooth, 30–55 × 2–5 μm, bearing 4–12 conidiogenous cells with the central conidiogenous cell extending above the rest. Conidiogenous cells phialidic, clavate to subclavate, hyaline, smooth, 7–11 × 3–4 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to fusiform, thick-walled, olivaceous brown to dark brown, verrucose, (5–)5.5–6.5(–7) × 2–3 μm (av. 6 × 3 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium forming concentric rings, with conidiophores forming on the aerial mycelium and surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA amber in the centre becoming pale luteous towards the margins.
Material examined. SOUTH AFRICA, KwaZulu-Natal, near KwamboNambi, from soil, 26 Jan. 1996, K.A. Seifert (holotype CBS H-22463, culture ex-type DOAMC 227011).
Notes — Stachybotrys dolichophialis formed a well-supported clade closely related to St. chartarum and St. chlorohalonata (Fig. 3). This species is characterised by the elongated central conidiogenous cell that extend above the rest, distinguishing it from the other species in the genus (McKenzie 1991, Pinruan et al. 2004, Wang et al. 2015).
Stachybotrys limonispora L. Lombard & Crous, sp. nov. — MycoBank MB816032; Fig. 69
Fig. 69.
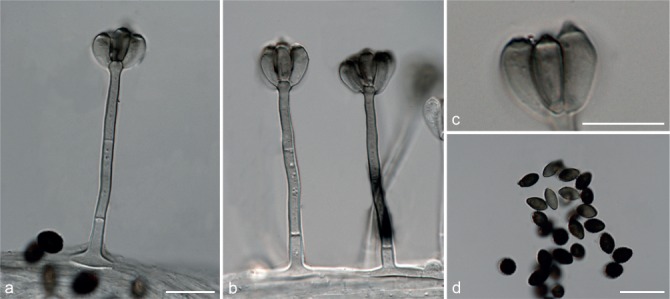
Stachybotrys limonispora (CBS 128809). a–b. Conidiophores; c. conidiogenous cells; d. conidia. — Scale bars: a = 10 μm (apply to b); c–d = 10 μm.
Etymology. Name reflects the limoniform conidia produced by this fungus.
Ascomata not observed. Conidiophores macronematous, mononematous, single or in groups, thin-walled, unbranched, erect, straight to slightly flexuous, hyaline to subhyaline, 1–3-septate, smooth, 40–75 × 3–7 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subclavate, hyaline to subhyaline, smooth, 8–14 × 4–7 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to limoniform, thick-walled, olivaceous brown to dark brown, verrucose, (6–)6.5–7.5(–9) × 3–4 μm (av. 7 × 4 μm), rounded at the base with an apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium becoming immersed at the margins, with conidiophores forming on the aerial mycelium and surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA sienna to amber.
Materials examined. INDIA, Jaipur, Rajasthan, from twig of Quisqualis indica, Dec. 1971, G.L. Hennebert, CBS 136165 = MUCL 18730. – USA, Kansas, near Manhattan, Konza Prairie Research Area, Long-term Ecological Research site, from soil in tallgrass prairie, 1986, M. Christensen (holotype CBS H-22464, culture ex-type CBS 128809).
Notes — Stachybotrys limonispora is closely related to St. subcylindrospora (Jie et al. 2013) based on phylogenetic inference of the ITS sequence data (Fig. 4) but can be distinguished by the formation of characteristic limoniform conidia ((6–)6.5–7.5(–9) × 3–4 μm (av. 7 × 4 μm)) that are smaller than the cylindrical to subcylindrical conidia of St. subcylindrospora ((9.7–)11.6–13.8(–14.7) × (2.9–)3.8–4.6(–5) μm; Jie et al. 2013).
Stachybotrys microspora (B.L. Mathur & Sankhla) S.C. Jong & E.E. Davis, Mycotaxon 3: 448. 1976
≡ Stachybotrys atra Corda var. microspora B.L. Mathur & Sankhla, Sci. & Cult. 32: 93. 1966.
Description and illustrations — See Jong & Davis (1976) and Wang et al. (2015).
Material examined. SUDAN, White Nile Island, from soil in Mangifera field, Mar. 1979, B.P.R. Vittal, CBS 186.79.
Notes — Phylogenetic inference of the ITS sequence data placed CBS 186.79 in a well-supported clade that included the epitype strain (ATCC 18852; Jong & Davis 1976) of St. microspora (Fig. 4), which was confirmed by morphological observations.
Stachybotrys pallescens Y.L. Jiang & T.Y. Zhang, Mycosystema 28: 646. 2009
Description and illustrations — See Jiang & Zhang (2009).
Notes — Phylogenetic inference placed the ex-type strain (HGUP 0146; Jiang & Zhang 2009) of St. pallescens in the Stachybotrys s.str. clade (Fig. 1).
Stachybotrys phaeophialis L. Lombard & Crous, sp. nov. — MycoBank MB816033; Fig. 70
Fig. 70.
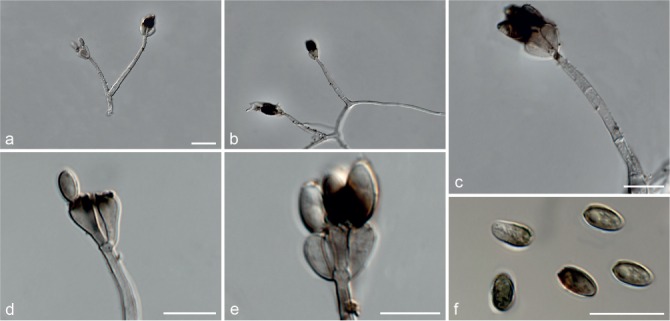
Stachybotrys phaeophialis (KAS 525). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 10 μm (apply to b); c–f = 10 μm.
Etymology. Name reflects the darkly pigmented conidiogenous cells characteristic of this fungus.
Ascomata not observed. Conidiophores macronematous, mononematous, single or in groups, thin-walled, unbranched or branched once, erect, straight to slightly flexuous, hyaline to subhyaline, 1–3-septate, smooth to slightly verrucose, 20–40 × 3–4 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subclavate, subhyaline to pale olivaceous brown, smooth, 6–9 × 3–4 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to fusiform, thick-walled, olivaceous brown to dark brown, verrucose, (6–)6.5–7.5(–9) × 3–4 μm (av. 7 × 4 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white aerial mycelium, consisting mostly of immersed mycelium, buff to pale luteous, with conidiophores forming on the aerial mycelium and surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA sienna in the centre becoming pale luteous towards the margins.
Material examined. CHINA, specific location uncertain, from seed of unidentified herb imported into Canada, 1998, G.P. White no. M98-063a (CBS H-22465, culture ex-type = KAS 525).
Notes — Stachybotrys phaeophialis is phylogenetically closely related to St. dolichophialis (Fig. 3) and can be distinguished by their shorter conidiophores (up to 40 μm) terminating in subhyaline to pale olivaceous brown conidiogenous cells. The conidiophores of St. dolichophialis (up to 55 μm) terminate in hyaline conidiogenous cells.
Stachybotrys reniformis Tubaki, Trans. Mycol. Soc. Japan 4: 86. 1963
?= Stachybotrys nephrospora Hansf., Proc. Linn. Soc. London 155: 44. 1943.
?= Stachybotrys sinuatophora Matsush., Bull. Natl. Sci. Mus., Tokyo 14: 476. 1971.
Description and illustrations — See Jong & Davis (1976) and Wang et al. (2015).
Materials examined. NEPAL, Narayani, Royal Chitwan National Park, from dead twig, C. Decock, CBS 136198 = MUCL 39087. – PAPUA NEW GUINEA, Central Province, Varirata National Park near Port Moresby, from soil in dry forest remnants, Oct. 1995, A. Aptroot, CBS 976.95.
Notes — Phylogenetic inference of the ITS sequence data placed both CBS 976.95 and CBS 136198 in a well-supported clade which included the ex-type strain (ATCC 18839) of St. reniformis (Fig. 4; Jong & Davis 1976). Jong & Davis (1976) synonymised both St. nephrospora and St. sinuatophora under St. reniformis. However, Wang et al. (2015) concluded, after examination of the various type materials, that these three species are not conspecific. Presently, there is no living type material available for St. nephrospora and no sequence data for the type (ATCC 22706; Jong & Davis 1976) of St. sinuatophora to determine whether these three species are truly conspecific or not.
Stachybotrys subcylindrospora C.Y. Jie et al., Mycol. Progr. 12: 695. 2013
Description and illustration — See Jie et al. (2013).
Notes — Phylogenetic inference of the ITS sequence data (Fig. 4) placed the ex-type strain (HGUP 0201; Jie et al. 2013) of St. subcylindrospora in the Stachybotrys s.str. clade.
Stachybotrys subreniformis Q.R. Li & Y.L. Jiang, Mycotaxon 115: 171. 2011
Description and illustrations — See Li & Jiang (2011).
Notes — Phylogenetic inference of the ITS sequence data showed that the ex-type strain (HGUP 1051; Li & Jiang 2011) of St. subreniformis is closely related to St. chartarum and St. chlorohalonata (Fig. 4).
Stachybotrys subsylvatica L. Lombard & Crous, sp. nov. — MycoBank MB816034; Fig. 71
Fig. 71.
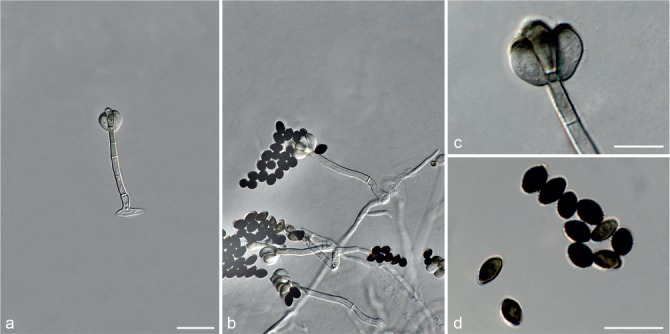
Stachybotrys subsylvatica (CBS 126205). a–b. Conidiophores; c. conidiogenous cells; d. conidia. — Scale bars: a = 10 μm (apply to b); c–d = 10 μm.
Etymology. Name reflects the environment, soil in a woodland, from which this fungus was collected.
Ascomata not observed. Conidiophores macronematous, mononematous, single or in groups, thin-walled, unbranched, erect, straight to slightly flexuous, hyaline to subhyaline, 1–4-septate, smooth, 30–65 × 3–5 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subclavate, subhyaline to pale olivaceous brown at the apex, smooth, 6–11 × 4–6 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to limoniform, thick-walled, olivaceous brown to dark brown, verrucose, (5–)5.5–6.5(–7) × 3–4 μm (av. 6 × 4 μm), rounded at both ends.
Culture characteristics — Colonies on PDA, OA and CMA with mostly immersed mycelium, buff to pale luteous, with conidiophores forming on the surface of the medium, carrying slimy olivaceous green to mouse grey conidial masses, reverse on PDA pale luteous.
Material examined. NAMIBIA, Halali Rest Camp, south of Dolomite Hill, S19°02.189’ E16°28.378’, from soil in Colophospermum mopane woodland, Apr. 2001, M. Christensen (holotype CBS H-22466, culture ex-type CBS 126205).
Notes — Stachybotrys subsylvatica formed a single lineage closely related to St. microspora (Fig. 3) from which it can be distinguished by their slightly smaller conidia ((5–)5.5–6.5(–7) × 3–4 μm (av. 6 × 4 μm)) compared to those of St. microspora (6–8 × 5–5 μm; Jong & Davies 1976). The conidiophores of St. subsylvatica (up to 65 μm) are also longer than those reported for St. microspora (up to 55 μm; Jong & Davies 1976).
Striatibotrys L. Lombard & Crous, gen. nov. — MycoBank MB816035
Etymology. Name reflects the characteristic striate conidia produced by these fungi.
Type species. Striatibotrys eucylindrospora (D.W. Li) L. Lombard & Crous.
Ascomata perithecial, scattered, subglobose to obpyriform, non-stromatic, totally immersed in host tissue, with only rounded papillate apex with setae protruding at surface of periderm, orange, not changing in KOH, completely covered by thick-walled intertwined hyphae, except at ostiolar region. Setae erect, hyaline, cylindrical, thick-walled, 1–2-septate, with rounded apex. Ascomatal wall consisting of a single layer of textura globulosa. Asci clavate, 8-spored, apex rounded to nearly truncate with a refractive apical ring. Ascospores ellipsoidal to fusiform, rounded at both ends, hyaline becoming orange en mass, verrucose. Paraphyses branched containing numerous oily droplets (adapted from Crous et al. 2013). Conidiophores macronematous, mononematous, erect, solitary or in groups, unbranched or branched once, thin-walled, hyaline to subhyaline with apex becoming olivaceous brown, smooth with the apex becoming verrucose, 1–5-septate, with an apical cluster of 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate to subclavate, smooth to slightly verrucose, hyaline to pale olivaceous brown, with conspicuous collarettes. Conidia aseptate, pale olivaceous to dark brown, smooth, longitudinally striate, ellipsoidal to subcylindrical to fusiform, rounded at the base with an apical hilum.
Notes — Phylogenetic inference in this study placed several stachybotrys-like fungi characterised by the formation of longitudinally striate conidia in a highly supported clade, distinct from the Stachybotrys s.str. clade (Fig. 1, 3). This clade also included the ex-type strains of St. eucylindrospora (CBS 203.61 = ATCC 18851; Li 2007) and St. oleronensis (CBS 137258; Crous et al. 2013), for which new combinations are provided in the newly established genus Striatibotrys (Stri.).
Striatibotrys atypica L. Lombard & Crous, sp. nov. — MycoBank MB816036; Fig. 72
Fig. 72.
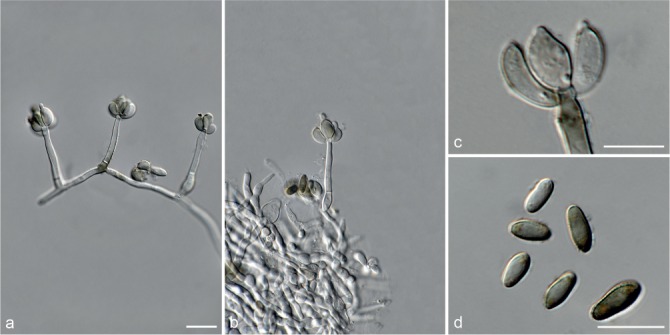
Striatibotrys atypica (CBS 141059). a–b. Conidiophores; c. conidiogenous cells; d. conidia. — Scale bars: a = 20 μm (apply to b); c–d = 10 μm.
Etymology. Name reflects the atypical morphology of this fungus.
Ascomata not observed. Conidiophores simple, macronematous, mononematous, single or in groups, thin-walled, unbranched, erect, straight, hyaline to subhyaline, 1-septate near the base, smooth, 20–40 × 3–4 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, subclavate with central cell becoming swollen and subglobose, hyaline to subhyaline, smooth, 6–10 × 3–6 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to subcylindrical, olivaceous brown to dark brown, smooth, rarely longitudinally striate, (6–)7–9(–10) × 3–4 μm (av. 8 × 3 μm), with a rounded base and apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with immersed mycelium, buff to pale luteous, with conidiophores forming on the surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA buff to pale luteous.
Material examined. FRANCE, Domain Le Fraysse, from Iris sp., 16 July 2010, P.W. Crous (holotype CBS H-22468, culture ex-type CBS 141059 = CPC 18423).
Notes — Striatibotrys atypica formed a highly supported clade closely related to Stri. oleronensis (Fig 3). This species is morphologically an atypical species in this genus, rarely producing longitudinally striate conidia and having a central conidiogenous cell that becomes swollen.
Striatibotrys eucylindrospora (D.W. Li) L. Lombard & Crous, comb. nov. — MycoBank MB816037
Basionym. Stachybotrys eucylindrospora D.W. Li, Mycologia 99: 333. 2007.
Description and illustrations — See Li (2007).
Materials examined. CANADA, Ontario, Guelph, from soil under Thuja occidentalis, Nov. 1960, G.L. Barron, CBS 203.61 = ATCC 18851 = IMI 085334 = MUCL 9483 (ex-type of Stachybotrys eucylindrospora); Aberfoyle area, from soil in a cedar forest, July 1964, G.C. Bhatt, CBS 136547 = MUCL 15039. – TURKEY, Izmir, Bornova, substrate unknown, 1972, collector unknown, CBS 949.72. – USA, New York, Ithaca, from plant debris, May 1962, G.L. Hennebert, CBS 136399 = MUCL 4251.
Striatibotrys humicola L. Lombard & Crous, sp. nov. — MycoBank MB816038; Fig. 73
Fig. 73.
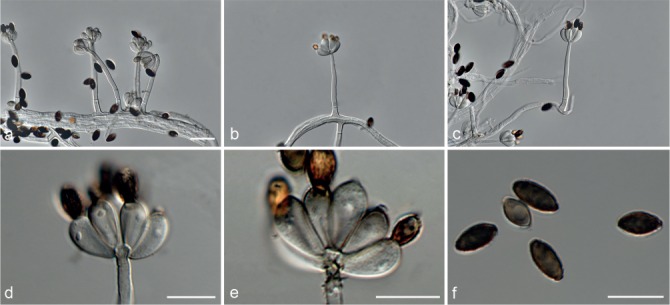
Striatibotrys humicola (CBS 102408). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 10 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Ascomata not observed. Conidiophores simple, macronematous, mononematous, single or in groups, thin-walled, unbranched, erect, straight to slightly flexuous, hyaline to subhyaline, 1-septate towards the base, smooth, 35–55 × 4–5 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate, hyaline to subhyaline, smooth, 7–12 × 3–5 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, fusiform, olivaceous brown to dark brown, smooth, longitudinally striate, (7–)7.5–8.5(–10) × (3–)3.5–4.5(–5) μm (av. 8 × 4 μm), with a rounded base and apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white to pale luteous aerial mycelium, consisting mostly of immersed mycelium producing luteous exudates diffusing into the medium and luteous droplets forming on the aerial mycelium; conidiophores forming on the aerial mycelium and surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA sienna in the centre becoming luteous towards the margins.
Material examined. USA, Wyoming, Laramie, from soil, 1999, M. Christensen (holotype CBS H-22469, culture ex-type CBS 102408).
Notes — Striatibotrys humicola formed a single lineage in the Striatibotrys clade (Fig. 3). The conidiophores of Stri. humicola (up to 55 μm) are shorter than those of Stri. eucylindrospora (up to 200 μm; Li 2007), Stri. rhabdospora and Stri. yuccae (both up to 70 μm).
Striatibotrys oleronensis (Lechat et al.) L. Lombard & Crous, comb. nov. — MycoBank MB816039
Basionym. Stachybotrys oleronensis Lechat et al., Persoonia 31: 283. 2013.
Description and illustrations — See Crous et al. (2013).
Material examined. FRANCE, Charente Maritime, Île d’Oléron, Saint Trojan, on leaf of Iris pseudacorus, 16 Apr. 2012, M. Hairaud, CBS 137258 = CIRM BRFM MH 160412 (ex-type of Stachybotrys oleronensis).
Notes — All attempts to induce the formation of the asexual morph of this fungus on the media defined in this study failed.
Striatibotrys rhabdospora L. Lombard & Crous, sp. nov. — MycoBank MB816040; Fig. 74
Fig. 74.
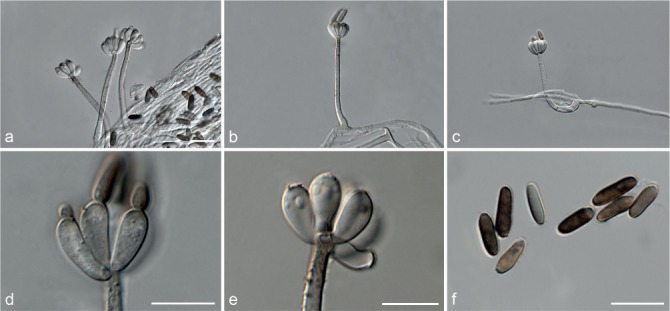
Striatibotrys rhabdospora (CBS 528.80). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 20 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the longitudinally striate conidia produced by this fungus.
Ascomata not observed. Conidiophores simple, macronematous, mononematous, single or in groups, thin-walled, unbranched or branched once, erect, straight to slightly flexuous, smooth and hyaline at the base becoming subhyaline to pale olivaceous brown and verrucose at the apex, 1–2-septate, 50–70 × 4–6 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate, hyaline to subhyaline, smooth, 8–10 × 4–6 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to subcylindrical, olivaceous brown to dark brown, smooth, longitudinally striate, (9–)9.5–10.5(–11) × 3–4 μm (av. 10 × 3 μm), with a rounded base and apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white aerial mycelium, consisting of mostly immersed mycelium producing luteous exudates diffusing into the medium with conidiophores forming on the surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA luteous to pale luteous.
Materials examined. BELGIUM, Heverlee, from asbestos cement tile on building roof, Mar. 1969, G.L. Hennebert, CBS 136395 = MUCL 22116. – GERMANY, Kiel-Kitzeberg, Schleswig-Holstein, from soil under Triticum sp., K.H. Domsch, CBS 136168 = MUCL 6030; from soil, Oct. 1980, I.J. Kapoor (holotype CBS H-18492, culture ex-type CBS 528.80). – SPAIN, Asturias, Picos de Europa, Covadonga, from plant debris, Oct. 2006, A. Mercado & C. Silvera, CBS 121801 = FMR 9485. – SWITZERLAND, Flüeli, Schüpberg, from soil with Trichophaea woolhopeia, 14 Oct. 2005, H. Meier, CBS 119043. – USA, New York, Labrador Lake, from petiole of Caltha palustris, Apr. 1961, G.L. Hennebert, CBS 136396 = MUCL 2012.
Notes — Striatibotrys rhabdospora formed a well-supported clade (Fig. 3) and is morphologically similar to Stri. yuccae, but can be distinguished by the abundant luteous exudates produced in culture by Stri. rhabdospora which was not seen for Stri. yuccae.
Striatibotrys yuccae L. Lombard & Crous, sp. nov. — MycoBank MB816041; Fig. 75
Fig. 75.
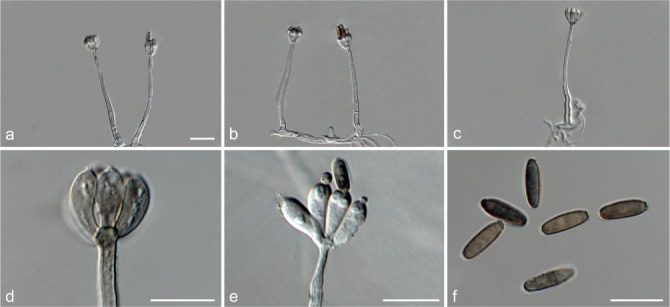
Striatibotrys yuccae (CBS 390.68). a–c. Conidiophores; d–e. conidiogenous cells; f. conidia. — Scale bars: a = 10 μm (apply to b–c); d–f = 10 μm.
Etymology. Name reflects the host genus Yucca, from which this fungus was isolated.
Ascomata not observed. Conidiophores simple, macronematous, mononematous, single or in groups, thin-walled, unbranched, erect, straight to slightly flexuous, hyaline to subhyaline, 1–2-septate, smooth to slightly verrucose at the apex, 40–70 × 3–6 μm, bearing 3–6 conidiogenous cells. Conidiogenous cells phialidic, clavate, hyaline to subhyaline, smooth, 7–11 × 3–5 μm, with conspicuous collarettes. Conidia acrogenous, aseptate, ellipsoidal to subcylindrical, olivaceous brown to dark brown, smooth, longitudinally striate, 9–9.5(–11) × 3–4 μm (av. 9 × 3 μm), with a rounded base and apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white aerial mycelium, consisting mostly of immersed mycelium with conidiophores forming on the surface of the medium, carrying slimy mouse grey to black conidial masses, reverse on PDA mouse grey.
Material examined. THE NETHERLANDS, Baarn, Cantonspark, from old leaf of Yucca flaccida, Apr. 1968, W. Gams (holotype CBS H-22470, culture ex-type CBS 390.68).
Notes — Striatibotrys yuccae formed a single lineage in the Striatibotrys clade with Stri. humicola as its closest phylogenetic neighbour (Fig. 3). The conidiophores of Str. yuccae (up to 70 μm) are longer than those of Stri. humicola (up to 55 μm). Additionally, the conidia of Stri. yuccae (9–9.5(–11) × 3–4 μm (av. 9 × 3 μm)) are slightly longer than those of Stri. humicola ((7–)7.5–8.5(–10) × (3–)3.5–4.5(–5) μm (av. 8 × 4 μm)).
Striaticonidium L. Lombard & Crous, gen. nov. — MycoBank MB816042
Etymology. Name reflects the characteristic striate conidia produced by these fungi.
Type species. Striaticonidium cinctum (Corda) L. Lombard & Crous.
Sexual morph unknown. Conidiomata synnematous or sporodochial or reduced to simple conidiophores. Synnemata cylindrical to pyriform, unbranched, broadening towards the apex, consisting of bundles of parallel, longitudinal, closely compacted hyphae, terminating in whorls of 2–4 conidiogenous cells, covered by an olivaceous green to black slimy mass of conidia. Marginal hyphae of synnemata olivaceous green, verrucose, sinuous, terminating in a blunt apex. Sporodochia stromatic, superficial, scattered or gregarious, oval or irregular in outline, with a white to grey setose fringe surrounding an olivaceous green to dark green slimy mass of conidia. Stroma poorly- or well-developed, hyaline, of textura globulosa or textura angularis. Simple conidiophores consisting of conidiogenous cells arising directly from vegetative hypha, solitary or rarely in groups. Setae short, thin-walled, septate, sinuous, subhyaline to olivaceous green, verrucose, with an obtuse apice. Conidiophores macronematous, verticillately or penicillately branched, hyaline, smooth to verrucose. Conidiogenous cells phialidic, hyaline, smooth, cylindrical to subcylindrical, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, fusiform to ellipsoidal, olivaceous green to brown, longitudinally striate, with an apical hilum lacking a funnel-shaped mucoid apical appendage.
Notes — The new asexual genus Striaticonidium (Str.) is introduced here for a group of myrothecium-like fungi characterised by striate conidia, which is further supported by phylogenetic inference in this study (Fig. 1, 2). Tulloch (1972) synonymised all Myrothecium species with striate conidia under Myr. cinctum based on the similarity in conidial dimensions and marginal ornamentation of the conidiomata. Nag Raj (1993) later synonymised Myr. cinctum under Hymenopsis ellipsospora, a species in a genus that requires taxonomic revision. We select to retain the epithet ‘cinctum’ (1842) for the type species of Striaticonidium as it predates ‘ellipsospora’ (1886) (Hawksworth 2012, McNeill et al. 2012) and designate an epitype for this species. Furthermore, a new combination is provided in Striaticonidium for this species.
Striaticonidium brachysporum (Nicot) L. Lombard & Crous, comb. nov. — MycoBank MB816043; Fig. 76
Fig. 76.
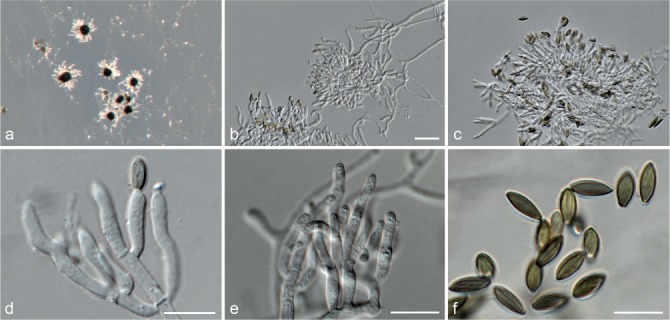
Striaticonidium brachysporum (CBS 513.71). a. Sporodochial conidiomata on SNA; b, c. sporodochia; d–e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c); d–f = 10 μm.
Basionym. Myrothecium brachysporum Nicot, Rev. Gén. Bot. 68: 684. 1961.
= Myrothecium ucrainicum Pidopl., Mykrobiol. Zh. Kiev 31: 161. 1969.
Conidiomata sporodochial or absent, reduced to simple conidiophores. Sporodochia stromatic, superficial, scattered or gregarious, oval to elongate or irregular in outline, 55–220 μm diam, 25–85 μm deep, with a white to grey setose fringe surrounding an olivaceous green to mouse grey agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Simple conidiomata consisting of conidiogenous cells arising directly from the vegetative hypha. Setae sinuous, rarely branched, hyaline to subhyaline, verrucose, 10–25 μm long, 3–5 μm wide, terminating in a blunt apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched or rarely branched, hyaline, septate becoming constricted at the septum, smooth, 18–33 × 2–4 μm; conidiogenous apparatus consists of a whorl of 3–5 primary branches terminating in 3–6 conidiogenous cells; primary branches aseptate, unbranched, smooth, 12–18 × 2–3 μm; conidiogenous cells phialidic, cylindrical to clavate to doliiform, hyaline, smooth to lightly verrucose, straight to flexuous or bent at the upper third, 5–22 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, longitudinally striate, olivaceous green to brown, fusiform to ellipsoidal, (6–)6.5–7.5(–9.5) × (2–)2.5–3.5(–5) μm (av. 7 × 3 μm), with a distinct apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium, olivaceous green to mouse grey in the centre becoming white towards the margins; sporodochia forming on the aerial mycelium and the surface of the medium, covered by slimy olivaceous green to mouse grey conidial masses, reverse on PDA pale luteous to white.
Materials examined. IRAN, bank of Caspian sea near Nochahr, from dune sand, 1961, J. Nicot (culture ex-type CBS 513.71 = IMI 115293 (ex-type of Myr. brachysporum)). – SOUTH AFRICA, North West Province, Potchefstroom, from leaf litter of Acacia karroo, 1965, M.C. Papendorf, CBS 177.65 = IMI 140053. – UKRAIN, Kiev, from soil, 1965, collector unknown, CBS 131.71 = IMI 158441 = ATCC 22270 (ex-type of Myr. ucrainicum). – USA, Kansas, near Manhattan, Konza Prairie Research Natural Area, long-term Ecological Research site, from soil in tallgrass prairie, 1986, M. Christensen, CBS 127287; Wisconsin, from soil in grassland prairie, 1950, P.A. Orpurt & J.T. Curtis, CBS 126552, CBS 128163.
Notes — Phylogenetic inference in this study placed the ex-type strain (CBS 513.71; Nicot & Olivry 1961) of Str. brachysporum in a highly supported subclade in the Striaticonidium clade, which also included the ex-type strain of Myr. ucrainicum (Fig. 2, CBS 131.71; Pidoplichko & Kirilenko 1969). As the epithet ‘brachysporum’ (1961) predates ‘urainicum’ (1969) we select to apply the former epithet to this clade, based on ICN (Hawksworth 2012, McNeill et al. 2012). Pidoplichko & Kirilenko (1969) distinguished Myr. ucrainicum from Myr. brachysporum by six phenotypic characters (see Tulloch 1972) although the conidial dimensions overlapped (Nicot & Olivry 1961, Pidoplichko & Kirilenko 1969, Tulloch 1972). Morphological comparisons of both ex-type strains under similar culturing conditions in this study revealed no phenotypic differences, indicating that the culturing conditions applied by both Nicot & Olivry (1961) and Pidoplichko & Kirilenko (1969) could have contributed to the phenotypic differences observed in the past.
Striaticonidium cinctum (Corda) L. Lombard & Crous, comb. nov. — MycoBank MB816044; Fig. 77
Fig. 77.
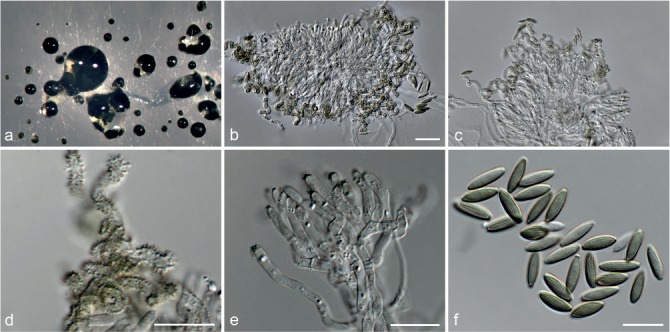
Striaticonidium cinctum (CBS 932.69). a. Sporodochial conidiomata on SNA; b–c. sporodochia; d. setae; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c); d–f = 10 μm.
Basionym. Fusarium cinctum Corda, Icon. Fungorum 5: 80. 1842.
≡ Myrothecium cinctum (Corda) Sacc., Syll. Fung. 4: 751. 1886.
?= Myrothecium ellipsosporum Fuckel, Symb. Mycol.: 364. 1870.
= Hymenopsis ellipsospora (as ellipsosporum) (Fuckel) Sacc., Syll. Fung. 4: 745. 1886.
= Myrothecium striatisporum N.C. Preston, Trans. Brit. Mycol. Soc. 31: 275. 1948.
= Myrothecium longistriatisporum Matsush., Microfungi Solomon Isl. Papua-New Guinea: 39. 1971.
Typification. GERMANY, on Trifolium repens, in herb. PR ex herb. A.C.I. Corda (labelled Fusarium cinctum Corda), no. 155489, Mus. Hof. 1840 (holotype of Myr. cinctum). – THE NETHERLANDS, Eastern Flevoland, from agricultural soil, 7 Oct. 1969, J.W. Veenbaas-Rijks (epitype of Myr. cinctum designated here CBS H-22471, MBT204324, ex-epitype culture CBS 932.69 = IMI 145760).
Conidiomata sporodochial, stromatic, superficial, scattered or gregarious, oval to elongate or irregular in outline, 250–600 μm diam, 45–85 μm deep, with a white to grey setose fringe surrounding an olivaceous green to mouse grey agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae sinuous, rarely branched, hyaline to subhyaline to olivaceous green, verrucose, 45–120 μm long, 3–5 μm wide, terminating in a blunt apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched or rarely branched, hyaline, septate, smooth, 6–15 × 2–3 μm; primary branches aseptate, unbranched, smooth, 7–10 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 5–10 × 2–3 μm; terminating in a whorl of 2–4 conidiogenous cells; conidiogenous cells phialidic, clavate to cylindrical to subcylindrical, hyaline, smooth to lightly verrucose, straight to slightly curved or bent at the upper third, 7–25 × 1–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, longitudinally striate, olivaceous green to brown, fusiform to ellipsoidal, (6–)7–9 × 2–3 μm (av. 8 × 3 μm), with a distinct apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the aerial mycelium and the surface of the medium, covered by slimy olivaceous green to mouse grey conidial masses, reverse on PDA pale luteous to buff.
Additional materials examined. BELGIUM, West Vlaanderen, from clay soil, 1947, J. van Holder, CBS 373.50 = IMI 140052. – CANADA, Ontario, Acton, from soil under Thuja occidentalis, July 1964, G.C. Bhat, CBS 528.69 = IMI 140637 = ATCC 18947. – NEW ZEALAND, from clay soil, collection date unknown, J.C. Neill, CBS 277.48 = IMI 001526 (ex-type of Myr. striatisporum).
Notes — Striaticonidium cinctum is the type species of the genus and as no living type material exists for this species, we consider it important to designate an epitype. The holotype (as F. cinctum = Myr. cinctum) was studied by Tulloch (1972) and based on the similarities to the protologue and illustrations provided by Tulloch, as well as the close proximity to the holotype location, we designate CBS 932.69 as epitype. Although Str. cinctum is morphologically similar to Str. brachysporum, it can be distinguished by its longer setae (45–120 μm) compared to those of Str. brachysporum (10–25 μm).
Striaticonidium humicola L. Lombard & Crous, sp. nov. — MycoBank MB816046; Fig. 78
Fig. 78.
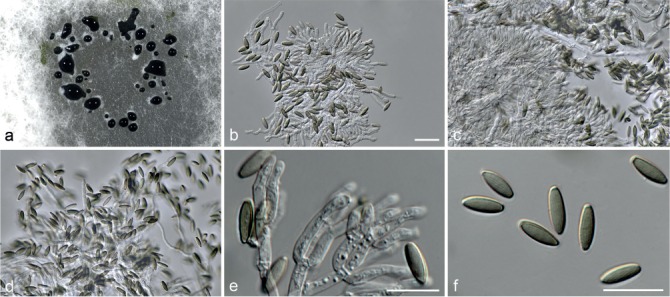
Striaticonidium humicola (CBS 258.76). a. Sporodochial conidiomata on SNA; b–c. sporodochia; d. setae; e. conidiogenous cells; f. conidia. — Scale bars: b = 20 μm (apply to c–d); e–f = 10 μm.
Etymology. Name reflects the substrate, soil, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, scattered or gregarious, oval to elongate or irregular in outline, 120–650 μm diam, 65–125 μm deep, with a white setose-like fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae rare, sinuous, unbranched, hyaline to subhyaline, verrucose, 35–75 μm long, 2–3 μm wide, terminating in a blunt apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 7–16 × 2–5 μm; primary branches aseptate, unbranched, smooth, 8–12 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 5–11 × 2–3 μm; terminating in a whorl of 2–4 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth to lightly verrucose, straight to slightly curved, 7–17 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, longitudinally striate, olivaceous green to brown, fusiform to ellipsoidal, (6–)6.5–7.5(–9) × 2–3 μm (av. 7 × 3 μm), with a distinct apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with sporodochia forming on the aerial mycelium and the surface of the medium, covered by slimy olivaceous green conidial masses, reverse on PDA pale luteous to olivaceous green.
Materials examined. PAPUA NEW GUINEA, Madang Province, Braham, from soil in tropical forest, Nov. 1995, A. Aptroot, CBS 388.97. – SPAIN, Gran Canaria, from soil, Apr. 1976, J.A. von Arx (holotype CBS H-14894, culture ex-type CBS 258.76).
Notes — Striaticonidium humicola formed a well-supported clade closely related to Str. cinctum and Str. synnematum (Fig. 2). This species can be distinguished from the other species in this genus by the sparsely formed setae, that are not as darkly pigmented as those observed for the other species in this genus.
Striaticonidium synnematum L. Lombard & Crous, sp. nov. — MycoBank MB816047; Fig. 79
Fig. 79.
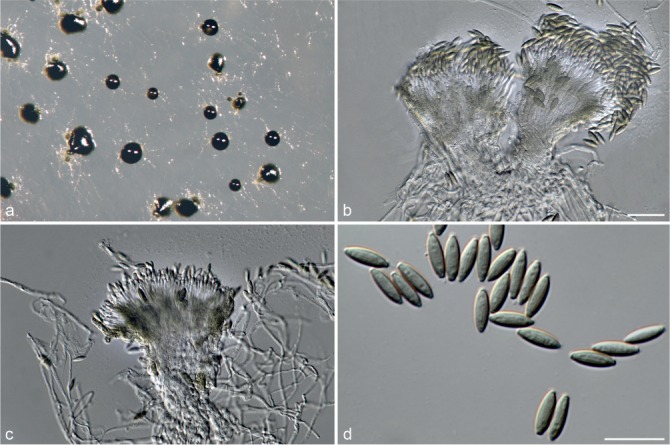
Striaticonidium synnematum (CBS 479.85). a. Conidiomata on SNA; b–c. synnematous conidiomata; d. conidia. — Scale bars: b = 10 μm (apply to c); d = 10 μm.
Etymology. Name reflects the synnematous conidiomata formed by this fungus.
Conidiomata synnematous, solitary, 50–85 μm high, 35–70 μm wide at the base, 45–85 μm at the apex, cylindrical, unbranched, broadening towards the apex, consisting of bundles of parallel, longitudinal, closely compacted hyphae, terminating in whorls of 2–4 conidiogenous cells, enclosed by marginal hyphae arising from the stroma and covered by an olivaceous green to mouse grey slimy mass of conidia. Marginal hyphae 2–3 μm wide, sinuous, unbranched to rarely branched, hyaline to olivaceous green, verrucose, with a blunt apex. Stroma well developed, hyaline, of textura globulosa and textura angularis. Conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, 18–30 × 1–2 μm in vivo, 5–10 × 1–2 μm in vitro, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, longitudinally striate, hyaline to olivaceous green, fusiform to ellipsoidal, 7–8 × 2–3 μm (av. 7 × 3 μm), with a distinct apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with abundant white aerial mycelium with synnemata forming on the surface of the medium, covered by slimy herbage to olivaceous green conidial masses, reverse on PDA buff to olivaceous green.
Material examined. JAPAN, Kamakura, from leaf of unknown palm, 29 Aug. 1983, K.A. Seifert no. 312 (holotype CBS H-14895, culture ex-type CBS 479.85).
Notes — Striaticonidium synnematum formed a single lineage basal to the Str. cinctum clade (Fig. 2) and is the only species in this genus that only produces synnematous conidiomata in culture.
Tangerinosporium L. Lombard & Crous, gen. nov. — MycoBank MB816048
Etymology. Name reflects the characteristic orange conidial masses covering the sporodochia produced by these fungi.
Type species. Tangerinosporium thalitricola L. Lombard & Crous.
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, pulvinate, scattered or gregarious, oval or irregular in outline, covered by an apricot to orange slimy or dry mass of conidia, surrounded by a white fringe. Stroma poorly developed, hyaline, of a textura angularis. Setae rarely seen, thin-walled, septate, unbranched, straight to flexuous, hyaline, tapering to an acute apice. Conidiophores macronematous, penicillately branched, hyaline, smooth. Conidiogenous cells phialidic, hyaline, smooth, cylindrical to subcylindrical, straight to slightly curved, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, ellipsoidal to ossiform, hyaline, with a rounded basal end and apical hilum.
Notes — The monophyletic asexual morph genus Tangerinosporium is established here for a fungus that characteristically produces orange conidial masses on its sporodochia, which distinguish it from the other myrothecium-like genera. Phylogenetic inference in this study showed that Tangerinosporium forms a basal sister lineage to the Myrothecium s.str. clade (Fig. 2).
Tangerinosporium thalictricola L. Lombard & Crous, sp. nov. — MycoBank MB816049; Fig. 80
Fig. 80.

Tangerinosporium thalitricola (CBS 317.61). a. Sporodochia with orange conidial masses on colony surface; b–c. sporodochial conidiomata; d. conidiogenous cells; e. seta; f. conidia. — Scale bars: b = 20 μm (apply to c, e); d, f = 10 μm.
Etymology. Name reflects the genus Thalictrum, from which this fungus was isolated.
Conidiomata sporodochial, stromatic, superficial, scattered or gregarious, oval to elongate or irregular in outline, 300–750 μm diam, 50–250 μm deep, with a white setose-like fringe surrounding an apricot to orange agglutinated slimy or dry mass of conidia. Stroma poorly developed, hyaline, of a textura angularis. Setae rare, flexuous, unbranched, hyaline, smooth, up to 120 μm long, 2–3 μm wide, terminating in an acute apice. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes unbranched, hyaline, septate, smooth, 7–20 × 2–3 μm; primary branches aseptate, unbranched, smooth, 7–14 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 4–9 × 2–3 μm; terminating in a whorl of 3–6 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, 6–11 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, ellipsoidal to ossiform, (5–)5.5–6.5(–7) × 2–3 μm (av. 6 × 2 μm), rounded at the base with a distinct apical hilum.
Culture characteristics — Colonies on PDA, OA and CMA with sparse white aerial mycelium with sporodochia forming on the surface of the medium, covered by slimy to dry apricot to orange conidial masses, reverse on PDA luteous to honey.
Material examined. UK, from Thalictrum flavum, 1949, M.B. Ellis (holotype CBS H-22472, culture ex-type CBS 317.61 = IMI 034815).
Virgatospora Finley, Mycologia 59: 538. 1967
Type species. Virgatospora echinofibrosa Finley, Mycologia 59: 538. 1967.
≡ Didymostilbe echinofibrosa (Finley) Rossman, Stud. Mycol. 42: 56. 1999.
= Nectria spirostriata Rossman, Mycol. Pap. 150: 61. 1983.
≡ Peethambara spirostriata (Rossman) Rossman, Stud. Mycol. 42: 56. 1999.
Description and illustration — See Finley (1967), Rossman (1983) and Rossman et al. (1999).
Notes — The monotypic asexual genus, Virgatospora, was established by Finley (1967), based on V. echinofibrosa, described from a dead twig collected on the Barro Colorado Island. Rossman (1983) was able to experimentally link V. echinofibrosa to the sexual morph Nectria spirostriata. Later, Rossman et al. (1999) transferred N. spirostriata to the sexual genus Peethambara (as P. spirostriata) and at the same time provided a new combination for V. echinofibrosa as Didymostilbe echinofibrosa, based on morphological similarities for both generic morphs. Based on phylogenetic inference in this study, authentic strains of P. echinofibrosa (see Rossman 1983 and Decock et al. 2008) formed a well-supported clade, distant to the Peethambara and Didymostilbe clades (Fig. 1). Therefore, we select to apply the asexual generic name Virgatospora to this clade, pending recollection from the type locality and epitypification as no living ex-type strain is available for phylogenetic analyses at present.
Xenomyrothecium L. Lombard & Crous, gen. nov. — MycoBank MB816050
Etymology. Name reflects its morphological similarity to the genus Myrothecium.
Type species. Xenomyrothecium tongaense (W.B. Kendr., et al.) L. Lombard & Crous
Sexual morph unknown. Conidiomata sporodochial, stromatic, superficial, scattered or gregarious, irregular in outline, covered by an olivaceous green to dark green slimy mass of conidia. Margin well developed, slightly involute, of textura intricata, composed of branched, septate, hyaline, verrucose, loosely coiled hyphae. Stroma poorly developed, hyaline, of a textura angularis. Conidiophores arising from the stroma branched, septate, hyaline, smooth. Conidiogenous cells phialidic, pale green, verrucose, subcylindrical, with conspicuous collarettes. Conidia aseptate, oblong-ellipsoidal, pale green, smooth, apex truncate, base constricted and truncate (adapted from DiCosmo et al. 1980).
Notes — Based on phylogenetic inference in this study, the ex-type strain (CBS 598.80) of Myr. tongaense formed a single lineage sister to the Myrothecium s.str. clade and Tangerinosporium lineage (Fig. 1, 2). Therefore, the monotypic genus, Xenomyrothecium, is introduced here and Myr. tongaense is provided with a new combination below. Xenomyrothecium can be distinguished from Myrothecium s.str. and Tangerinosporium by its oblong-ellipsoidal conidia and lack of setae formed in the sporodochia. Xenomyrothecium is also morphologically reminiscent of Paramyrothecium, but can also be distinguished by conidial morphology and lack of setae formation.
Xenomyrothecium tongaense (W.B. Kendr. et al.) L. Lombard & Crous, comb. nov. — MycoBank MB816051
Basionym. Myrothecium tongaense W.B. Kendr. et al., Mycotaxon 12: 220. 1980.
Description and illustration — See DiCosmo et al. (1980).
Material examined. TONGA, Pangai, Lifuka, Haápai Group, from calcified portions of a dead thallus of Halimeda sp., 1 May 1980, B. Kendrick (holotype DAOM 176764 (not seen), culture isotype CBS 598.80).
Xepicula Nag Raj, Coelemycetous anamorphs with appendage-bearing conidia: 979. 1993
Type species. Xepicula leucotricha (Peck) Nag Raj.
Description and illustration — See Nag Raj (1993).
Notes — Nag Raj (1993) introduced the asexual genus Xepicula, based on X. leucotricha (= Myr. leucotechium), to resolve the taxonomic problems associated with the broad generic concept for Myrothecium as proposed by Tulloch (1972). At the same time, Nag Raj (1993) synonymised Myr. indicum (Rama Roa 1963) and Myr. jollymannii (Preston 1948) under X. leucotricha. Based on phylogenetic inference in this study, the ex-type strain of Myr. jollymannii (CBS 276.48) clustered in a well-supported subclade, distinct from the well-supported subclade that includes the ex-type of Myr. indicum (CBS 131.64) (Fig. 2). We select to retain the name X. leucotricha for the latter subclade and therefore resurrect Myr. jollymannii and provide a new combination in the genus Xepicula.
Xepicula crassiseta L. Lombard & Crous, sp. nov. — MycoBank MB816052; Fig. 81
Fig. 81.
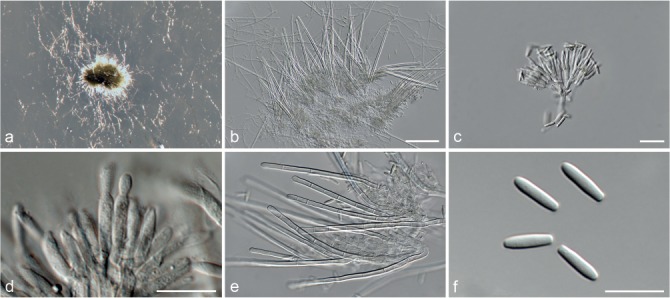
Xepicula crassiseta (CBS 392.71). a. Sporodochial conidiomata on SNA; b–c. sporodochia; d. conidiogenous cells; e. setae; f. conidia. — Scale bars: b = 100 μm; c = 20 μm (apply to e); d, f = 10 μm.
Etymology. Name reflects the thick-walled setae produced by this fungus.
Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or rarely gregarious, oval to elongate or irregular in outline, 175–450 μm diam, 55–220 μm deep, with a white setose fringe surrounding an olivaceous green agglutinated slimy mass of conidia. Stroma well-developed, hyaline, of textura globulosa or textura angularis. Setae arising from the stroma, unbranched, straight to flexuous, thick-walled, septate, hyaline to subhyaline, 85–170 μm long, 3–6 μm wide, terminating in a blunt apex. Conidiophores arising from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus; stipes branched or unbranched, hyaline, septate, smooth to slightly verrucose, 15–25 × 3–4 μm; primary branches aseptate, unbranched, smooth to slightly verrucose, 8–14 × 2–3 μm; secondary branches aseptate, unbranched, smooth to slightly verrucose, 6–11 × 2–3 μm; terminating in a whorl of 2–4 conidiogenous cells; conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth to lightly verrucose, straight to slightly curved, 7–15 × 2–3 μm, with conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, fusiform to ellipsoidal, (7–)8–10 × 2 μm (av. 9 × 2 μm), with an obtuse apex and truncate base, lacking a funnel-shaped mucoid apical appendage.
Culture characteristics — Colonies on PDA, OA and CMA with abundant luteous aerial mycelium with sporodochia forming on the aerial mycelium and the surface of the medium, covered by slimy olivaceous to herbage green conidial masses, with luteous to buff exudates diffusing into the medium; reverse on PDA luteous.
Material examined. SPAIN, Gran Canaria, from forest soil under Pinus canariensis, collector and date unknown (holotype CBS H-14902, culture ex-type CBS 392.71).
Notes — Xepicula crassiseta formed a single lineage basal to the X. jollymannii clade (Fig. 2). The setae of X. crassiseta are shorter than those reported for X. leucotrichia (up to 200 μm; Nag Raj 1993), X. leucotrichoides (up to 300 μm; Nag Raj 1993) and X. jollymannii (up to 260 μm; Preston 1948). Furthermore, the conidia of X. crassiseta, similar to X. jollymannii (Preston 1948) do not bear any funnel-shaped mucoid apical appendages as recorded for the former two species, but the conidia are slightly smaller ((7–)8–10 × 2 μm (av. 9 × 2 μm)) than those reported for X. jollymannii (8–12 × 2.5 μm; Preston 1948).
Xepicula jollymannii (N.C. Preston) L. Lombard & Crous, comb. nov. — MycoBank MB816053; Fig. 82
Fig. 82.
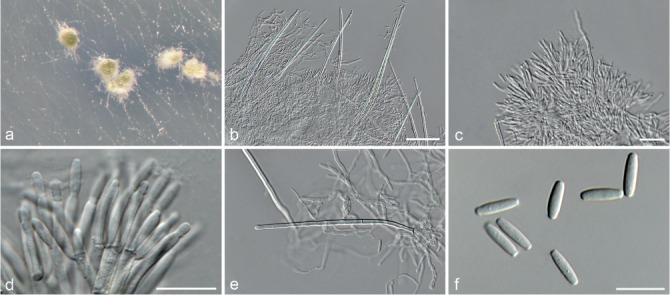
Xepicula jollymannii (CBS 276.48). a. Sporodochial conidiomata on SNA; b–c. sporodochia; d. conidiogenous cells; e. setae; f. conidia. — Scale bars: b = 100 μm; c = 20 μm (apply to e); d, f = 10 μm.
Basionym. Myrothecium jollymannii N.C. Preston, Trans. Brit. Mycol. Soc. 31: 272. 1948.
Description and illustration — See Preston (1948).
Materials examined. INDIA, in garden of Agricultural College Akola, from branch of Clerodendron inerme, Sept. 1975, R.B. Somani, CBS 511.76. – MALAWI, Nyasaland, from dried leaf of Nicotiana tabacum, 1936, F.W. Jollymann (holotype IMI 001495, culture ex-type CBS 276.48 = MUCL 11830 = QM 1229). – NAMIBIA, 30 km west of Maltahohe on Highway C19, from surface soil in desert, Apr. 2001, M. Christensen, CBS 126168.
Xepicula leucotricha (Peck) Nag Raj, Coelemycetous anamorphs with appendage-bearing conidia: 980. 1993
≡ Excipula leucotricha Peck, Rep. St. Mus. N.Y. 29: 48. 1878.
≡ Amerosporium leucotrichum (Peck) Sacc., Syll. Fung. 3: 682. 1884.
≡ Myrothecium leucotrichum (Peck) M.C. Tulloch, Mycol. Pap. 130: 12. 1972.
= Volutella piracicabana Verona & S. Joly, Rc. Accad. Naz. Lincei 21 (1–2): 121. 1956.
= Myrothecium indicum P. Rama Rao, Antonie van Leeuwenhoek 29: 180. 1963.
Materials examined. BRAZIL, Piracicaba, from soil, 1956, O. Verona & P. Joly, CBS 256.57 = MUCL 9860 (ex-type culture of Volutella piracicabana). – COLOMBIA, Dep. del Meta, Municipio de Villavicencio, 25 km from Villavicencio to Acacías, from maize-field soil, May 1978, J. Veerkamp, CBS 278.78, CBS 483.78. – INDIA, Hyderabad, Andhra Pradesh, from uncultivated soil, 1963, P. Rama Rao, CBS 131.64 = IMI 103664 = ATCC 16686 (isotype of Myrothecium indicum).
EXCLUDED GENERA AND SPECIES
Chaetosphaeria aspergilloides M.E. Barr & J.L. Crane, Canad. J. Bot. 57: 835. 1979
Description and illustration — See Barr & Crane (1979).
Notes — Réblová (1998) compared Chaetosphaeria aterrima (now Kastanostachys aterrima) to Chaetosphaeria aspergilloides (Barr & Crane 1979) and found that both these species share morphological similarities in their sexual and asexual morphs. However, the type material of C. aspergilloides, lodged at NY (México, Universite Nacional Autonomia de México, Distrito Federal, on decayed woody material, tropical greenhouse, 7 Sept. 1972, J.M. Trappe 3642, NY), could not be located for comparative studies (Réblová 1998). The author also examined isotype (ILLS 37868), which contained several microscopic preparations; all observed morphological structures matched the description of sexual and asexual morphs of C. aspergilloides (Barr & Crane 1979). No living culture could be located for this species in this study. This species probably also belongs to the genus Kastanostachys, but formal placement in this genus requires recollection and epitypification of this species.
Melanconis groenlandica (M. Bohn) L. Lombard & Crous, comb. nov. — MycoBank MB816054
Basionym. Myrothecium groenlandicum M. Bohn, Mycotaxon 46: 336. 1993.
Description and illustration — See Bohn (1993).
Notes — Analyses of the ITS (GenBank accession KU878552), LSU (KU878553), tef1 (KU878554) and tub2 (KU878555) sequence data revealed that the ex-type strain (UPSC 3407 = CBS 116540; Bohn 1993) of Myr. groenlandicum belongs to the sexual genus Melanconis (Voglmayr et al. 2012, Rossman et al. 2015; data not shown here). Therefore, this new combination is introduced here.
Memnoniella indica Kesh. Prasad et al., Mycotaxon 85: 341. 2003
≡ Stachybotrys indicoides Yong Wang et al., Fung. Diversity 70: 54. 2015.
Description and illustration — See Keshava Prasad et al. (2003) and Wang et al. (2015).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Based on the thick-walled, verrucose conidia formed in chains, this species probably belongs to the genus Memnoniella.
Memnoniella leprosa R.F. Castañeda, Fungi Cubenses: 10. 1986
≡ Stachybotrys leprosa (R.F. Castañeda) R.F. Castañeda, Fung. Diversity 70: 57. 2015.
Description — See Castañeda (1986).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Castañeda (1986) characterised this species with conidia borne in dry chains, placing it in the genus Memnoniella.
Memnoniella levispora Subram., J. Indian Bot. Soc. 33: 40. 1954
≡ Stachybotrys levispora (Subram.) Yong Wang et al., Fung. Diversity 70: 57. 2015.
Description and illustration — See Subramanian (1954).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species produces globose, smooth-walled conidia in dry chains (Subramanian 1954) and therefore belongs to the genus Memnoniella.
Memnoniella mohanramii Manohar. et al., Indian Phytopathol. 59: 489. 2006
≡ Stachybotrys mohanramii (Manohar. et al.) Yong Wang et al., Fung. Diversity 70: 58. 2015.
Description and illustration — See Manoharachary et al. (2006).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species produces ellipsoidal, smooth, thick-walled conidia in chains (Manoharachary et al. 2006) and therefore belongs to the genus Memnoniella.
Memnoniella stilboidae (Munjal & J.N. Kapoor) M.B. Ellis, More dematiaceous Hyphomycetes: 464. 1976
≡ Stachybotrys stilboidea Munjal & J.N. Kapoor, Mycopathol. Mycol. Appl. 39: 121. 1969.
Description and illustration — See Munjal & Kapoor (1969) and Ellis (1979).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species forms characteristic synnematous conidiomata (Munjal & Kapoor 1969, Ellis 1979) not known for Memnoniella and therefore might represent a novel genus allied to Memnoniella.
Myrothecium advena Sacc. var. terricola H.Q. Pan & T.Y. Zhang, Mycosystema 33: 8. 2014
Description and illustration — See Jiang et al. (2014).
Notes — Although Tulloch (1972) synonymised Myr. advena under Myr. roridum (now Paramyrothecium roridum), Jiang et al. (2014) introduced this new variety of Myr. advena based on colony colour and conidial dimensions. The broad species description and illustration by Jiang et al. (2014) and lack of sequence data makes it difficult to place this variety. It probably represents Pa. roridum, pending re-examination of the type material and phylogenetic inference.
Myrothecium baciliforme Y.L. Jiang & T.Y. Zhang, Mycosystema 33: 9. 2014
Description and illustration — See Jiang et al. (2014).
Notes — Jiang et al. (2014) distinguished Myr. baciliforme from Pa. roridum based on conidial morphology and dimensions. Based on the description provided by Jiang et al. (2014), Myr. baciliforme is morphologically similar to Pa. parvum. Unfortunately, the ex-type strain (HMAS 196271) or associated DNA sequence data were unavailable to us to confirm its link to Pa. parvum. This species probably belongs to the genus Paramyrothecium.
Myrothecium biforme Y.L. Jiang & T.Y. Zhang, Mycosystema 33: 10. 2014
Description and illustration — See Jiang et al. (2014).
Notes — Jiang et al. (2014) distinguished this species from all other Myrothecium species based on the production of two kinds of conidia. Based on the conidial dimension indicated by Jiang et al. (2014), this species does not belong to the genus Myrothecium, but might represent another new allied genus. However, neither the ex-type strain (HMAS 196272) nor associated DNA sequence data were available to us to determine its relationship to the genus Myrothecium.
Myrothecium macrosporum H.F. Wang & T.Y. Zhang, Mycosystema 33: 10. 2014
Description and illustration — See Jiang et al. (2014).
Notes — Jiang et al. (2014) compared this species to Myr. verrucaria (now Albifimbria verrucaria) and Myr. flavovirens (≡ Hymenopsis flavovirens; Nag Raj 1993), distinguishing it based on conidial dimensions, pigmentation and gluttulae. However, the conidial dimensions exclude this species from the genus Myrothecium. Based on the illustration of the unique conidiogenous cells and conidia (Jiang et al. 2014), this myrothecium-like species probably represents a new allied genus. Neither the ex-type strain (HMAS 196273) nor associated DNA sequence data were available to us to determine its relationship to the genus Myrothecium.
Myrothecium miconiae J.L. Alves et al., Mycologia 102: 73. 2010
Description and illustration — See Alves et al. (2010).
Notes — This synnematous myrothecium-like species was introduced by Alves et al. (2010) based on an isolate obtained from a leaf lesion on Miconia calvescens. Based on its 1-septate, subconical conidia, this species does not belong to the genus Myrothecium, and probably represent another new allied genus. No living type material could be located for this study to determine its phylogenetic relationship to the genus Myrothecium.
Myrothecium mori Sullia & Padma, Curr. Sci. 54: 757. 1985
Description — See Sullia & Padma (1985).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Sullia & Padma (1985) distinguished Myr. mori from Al. verrucaria, Pa. roridum and Myr. advena based on conidial shape and dimensions. However, the broad species concept provided by Sullia & Padma (1985) makes it difficult to identify in which myrothecium-like genus this species belongs.
Myrothecium mucunae R.F. Castañeda & W.B. Kendr., Univ. Waterloo, Biol. Ser. 35: 73. 1991
Description and illustration — See Castañeda-Ruiz & Kendrick (1991).
Notes — No living material could be located for this species to determine its phylogenetic placement in this study. Castañeda-Ruiz & Kendrick (1991) distinguished this species from other Myrothecium species based on the thick-walled, verrucose setae and conidial shape and dimensions. This species clearly does not belong to the genus Myrothecium based on the formation of a synnematous conidiomata and thick-walled setae, characteristics also not observed for the other myrothecium-like genera treated here.
Myrothecium nipponicum Matsush., Matsushima Mycol. Mem. 8: 27. 1995
Description and illustration — See Matsushima (1995).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Based on the description and illustrations provided by Matsushima (1995) this species could belong to the genus Myxospora.
Myrothecium renaudii Escalona, Mycotaxon 61: 82. 1997
Description and illustration — See Escalona (1997).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This fungus is characterised by percurrent vegetative growth of the synnematous conidiomata, which was not observed for any of the synnematous myrothecium-like genera treated here. Therefore, this fungus might represent another new myrothecium-like genus.
Myrothecium variabile Y.M. Wu & T.Y. Zhang, Mycotaxon 129: 404. 2014
Description and illustration — See Wu et al. (2014).
Notes — The ex-type strain (HMAS 196284) of this species was not available to determine its phylogenetic placement in this study. Based on the description and illustrations provided by Wu et al. (2014), this species belongs to the genus Striaticonidium. However, as no sequence data is presently available to confirm this, we refrain from providing a new combination for this species at this time.
Myrothecium viride S.C. Agarwal, Curr. Sci. 49: 281. 1980
Description and illustration — See Agarwal (1980).
Notes — The ex-type strain (IMI 132173) of this species could not be located to determine its phylogenetic placement in this study. The overly broad description and poor illustration provided by Agarwal (1980) makes it difficult to determine to which myrothecium-like genus this species belongs. However, based on conidial shape and dimensions, it clearly does not belong to Myrothecium s.str.
Myrothecium xigazense Y.M. Wu & T.Y. Zhang, Mycotaxon 129: 405. 2014
Description and illustration — See Wu et al. (2014).
Notes — The ex-type strain (HMAS 196285) of this species was not available to determine its phylogenetic placement in this study. Based on the description and illustrations provided by Wu et al. (2014), this species belongs to the genus Xepicula. However, as no sequence data are presently available to confirm this, we refrain from providing a new combination for this species at this time.
Stachybotrys bambusicola Rifai, Trans. Brit. Mycol. Soc. 47: 270. 1964
Description and illustration — See Rifai (1964).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This fungus is characterised by percurrent vegetative growth of the conidiophore, which produces pink conidia in mass (Rifai 1964). These characteristics were not observed for any of the studied stachybotrys-like genera introduced here.
Stachybotrys breviuscula McKenzie, Mycotaxon 41: 180. 1991
Description and illustration — See McKenzie (1991).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is morphologically very similar to St. chartarum (McKenzie 1991, Wang et al. 2015) and its placement in the genus Stachybotrys is probably correct but needs to be confirmed experimentally.
Stachybotrys cordylines McKenzie, Fung. Diversity 17: 146. 2004
Description and illustration — See Pinruan et al. (2004).
Notes — The ex-type strain (ICMP 15219; Pinruan et al. 2004) of this species was not available to determine its phylogenetic placement in this study.
Stachybotrys crassa Marchal, Bull. Acad. Roy. Sci. Belgique, Cl. Sci., sér. 5, 34: 140. 1895
Description — See Wang et al. (2015).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species appears to produce conidia borne in chains, which could place it in the genus Memnoniella.
Stachybotrys elegans (Pidopl.) W. Gams, Compendium of Soil Fungi: 746. 1980
≡ Hyalobotrys elegans Pidopl., Fungus flora of coarse fodder: 186. 1948.
= Stachybotrys pallida Orpurt, Studies on the soil microfungi of Wisconsin prairies, Diss. Univ. Wisconsin: 95. 1954 [nom. invalid., Art. 30.5, 36.1].
= Hyalostachybotrys bisby Sriniv., J. Indian Bot. Soc. 37: 340. 1964.
For additional synonyms see Wang et al. 2015.
Description and illustration — See Domsch et al. (1980).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is characterised by hyaline, smooth, limoniform to fusiform conidia, which would place it in the stachybotrys-like genus Achroiostachys.
Stachybotrys freycinetiae McKenzie, Mycotaxon 41: 183. 1991
Description and illustration — See McKenzie (1991).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is morphologically very similar to St. chartarum (McKenzie 1991, Wang et al. 2015), and its placement in the genus Stachybotrys is probably correct but needs to be confirmed experimentally.
Stachybotrys frondicola (K.D. Hyde et al.) Yong Wang et al., Fung. Diversity 70: 52. 2015
≡ Ornatispora frondicola K.D. Hyde et al., Mycol. Res. 103: 1438. 1999.
Description and illustration — See Hyde et al. (1999).
Notes — No ex-type strain of this species is available to determine its phylogenetic placement. Hyde et al. (1999) did not include a description of the asexual morph of O. frondicola in their treatment of this fungus.
Stachybotrys globose P.C. Misra & S.K. Srivast., Trans. Brit. Mycol. Soc. 78: 556. 1982
Description and illustration — See Misra & Srivastava (1982).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is morphologically very similar to St. microspora (Wang et al. 2015) and its placement in the genus Stachybotrys is probably correct but needs to be confirmed experimentally.
Stachybotrys guttulispora Muhsin & Al-Helfi, Sydowia 34: 133. 1981
Description and illustration — See Muhsin & Al-Helfi (1981).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Muhsin & Al-Helfi (1981) and Wang et al. (2015) considered it morphologically similar to St. albipes (= Melanopsamma pomiformis) but distinguished it based on the biguttulate, ellipsoidal, smooth conidia formed on thin-walled, verrucose, hyaline conidiophores. These are distinct characters not observed for any of the stachybotrys-like fungi studied here, which exclude this species from Stachybotrys s.str., pending availability of sequence data to confirm this.
Stachybotrys havanensis Mercado & J. Mena, Acta Bot. Cub. 55: 2. 1988
Description — See Mercado Sierra et al. (1997).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys jiangziensis Y.M. Wu & T.Y. Zhang, Mycotaxon 114: 459. 2010
Description and illustration — See Wu & Zhang (2010).
Notes — The ex-type strain (HMAS 196256; Wu & Zhang 2010) of this species was not available to determine its phylogenetic placement in this study.
Stachybotrys kampalensis Hansf., Proc. Linn. Soc. London 155: 45. 1943
Description and illustration — See Hansford (1943).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys kapiti Whitton et al., New Zealand J. Bot. 39: 493. 2001
Description and illustration — See Whitton et al. (2001).
Notes — No ex-type strain of this species is available to determine its phylogenetic placement.
Stachybotrys mangiferae P.C. Misra & S.K. Srivast., Trans. Brit. Mycol. Soc. 78: 556. 1982
Description and illustration — See Misra & Srivastava (1982).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys mexicanus J. Mena & Heredia, Boln Soc. Micol. Madrid 33: 12. 2009
Description — See Wang et al. (2015).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Based on the hyaline, smooth, ellipsoidal to limoniform conidia (Wang et al. 2015) produced by this fungus, it probably belongs to the genus Achroiostachys.
Stachybotrys nepalensis (Whitton et al.) Whitton et al., Fung. Diversity 70: 59. 2015
≡ Ornatispora nepalensis Whitton et al., Fungi associated with Pandanaceae. Fung. Divers. Res. Ser. 21: 86. 2012.
Description and illustration — See Whitton et al. (2012) and Wang et al. (2015).
Notes — No ex-type strain of this species is available to determine its phylogenetic placement.
Stachybotrys nephrodes McKenzie, Mycotaxon 41: 185. 1991
Description and illustration — See McKenzie (1991).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is morphologically very distinct, having tightly curled or tightly reniform conidia (McKenzie 1991, Wang et al. 2015) and its placement in the genus Stachybotrys is probably incorrect but needs to be confirmed experimentally.
Stachybotrys nephrospora Hansf., Proc. Linn. Soc. London 155: 45. 1943
Description and illustration — See Hansford (1943).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. See notes under St. reniformis for more comments.
Stachybotrys nielamuensis Y.M. Wu & T.Y. Zhang, Mycotaxon 109: 461. 2009
Description and illustration — See Wu & Zhang (2009).
Notes — The ex-type strain (HMAS 196254; Wu & Zhang 2009) of this species was not available to determine its phylogenetic placement in this study. The smooth, thick-walled conidiophores with a slightly bulbous apex as illustrated by Wu & Zhang (2009) indicates that this species might belong to the genus Melanopsamma.
Stachybotrys nilagirica Subram., Proc. Indian Acad. Sci., Pl. Sci. 46: 331. 1957
Description and illustration — See Subramanian (1957) and Wang et al. (2015).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. See notes under Mem. pseudonilagiria for more comments.
Stachybotrys palmae Pinruan, Fung. Diversity 17: 146. 2004
Description and illustration — See Pinruan et al. (2004).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is distinct in producing hyaline, verrucose conidia on conidiophores that are darkly pigmented for the bottom two-thirds of its length (Pinruan et al. 2004) making its placement in Stachybotrys s.str. highly unlikely. This species probably represents a new stachybotrys-like genus, but requires sequence data to confirm this.
Stachybotrys palmicola (K.D. Hyde et al.) Yong Wang et al., Fung. Diversity 70: 61. 2015
≡ Ornatispora palmicola K.D. Hyde et al., Mycol. Res. 103: 1438. 1999.
Description and illustration — See Hyde et al. (1999).
Notes — No ex-type strain of this species is available to determine its phylogenetic placement. Hyde et al. (1999) was unable to include a description of the asexual morph of O. palmicola in their treatment of this fungus, as only scattered remnants of conidiophore-like structures on the ascomata were found and illustrated.
Stachybotrys palmijunci Rifai, Reinwardtia 8: 537. 1974
Description and illustration — See Rifai (1974).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This fungus is characterised by percurrent vegetative growth of the conidiophore, which produces pink conidia in mass (Rifai 1974), similar to St. bambusicola (Rifai 1964). These characteristics were not observed for any of the studied stachybotrys-like genera introduced here.
Stachybotrys parvispora S. Hughes, Mycol. Pap. 48: 74. 1952
Description and illustration — See Hughes (1952).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is morphologically similar to St. chartarum, but is distinguished by smaller, smooth-walled conidia (Wang et al. 2015).
Stachybotrys proliferata K.G. Karand., S.M. Kulk. & Patw., Biovigyanam 18: 79. 1992
Description — See Wang et al. (2015).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is characterised by reniform conidia and indeterminate conidiophores, which proliferate through the apex in place of the phialides (Wang et al. 2015). These characteristics excludes this species from Stachybotrys s.str. and its phylogenetic relationship needs to be determined.
Stachybotrys punctata (Dulym. et al.) Yong Wang et al., Fung. Diversity 70: 61. 2015
≡ Ornatispora punctata Dulym. et al., Fung. Diversity 8: 95. 2001.
Description and illustration — See Dulymamode et al. (2001).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Dulymamode et al. (2001) did not include a description of the asexual morph in their treatment of this fungus.
Stachybotrys queenslandica Matsush., Matsushima Mycol. Mem. 6: 40. 1989
Description and illustration — See Matsushima (1989).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Based on the description and illustrations provided by Matsushima (1989), this species might belong to the new genus Melanopsamma.
Stachybotrys ramosa Dorai & Vittal, Trans. Brit. Mycol. Soc. 87: 642. 1987
Description and illustration — See Dorai & Vittal (1987).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is characterised by sympodially branched conidiophores that are loosely intertwined, which excludes this species from Stachybotrys s.str. and its phylogenetic relationship needs to be determined.
Stachybotrys renispora P.C. Misra, Mycotaxon 4: 161. 1976
Description and illustration — See Misra (1976).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is morphologically similar to St. reniformis and St. renisporoides, which Wang et al. (2015) considers the latter as a possible synonym of St. renispora, pending further study.
Stachybotrys renisporoides K.G. Karand., S.M. Kulk. & Patw., Biovigyanam 19: 79
Description — See Wang et al. (2015).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species is probably a synonym of St. renispora, which requires further investigation.
Stachybotrys reniverrucosa Whitton et al., New Zealand J. Bot. 39: 496. 2001
Description and illustration — See Whitton et al. (2001).
Notes — No ex-type strain of this species is available to determine its phylogenetic placement.
Stachybotrys ruwenzoriensis Matsush., Matsushima Mycol. Mem. 4: 17. 1985
Description and illustration — See Matsushima (1985).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys sansevieriae G.P. Agarwal & N.D. Sharma, J. Indian Bot. Soc. 53: 78. 1974
= Stachybotrys indica P.C. Misra, Mycotaxon 2: 107. 1975.
Description and illustration — See Sharma & Agarwal (1974).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys sinuatophora Matsush., Bull. Natl. Sci. Mus., Tokyo 14: 476. 1971
Description and illustration — See Kobayashi (1971).
Notes — The ex-type strain (ATCC 22706; Jong & Davis 1976) of this species was not available to determine its phylogenetic placement in this study. This species was synonymised under St. nephrospora by Jong & Davis (1976) based on conidial morphology. However, Wang et al. (2015) considered it distinct from St. nephrospora after examination of type material. Stachybotrys sinuatophora is characterised by repeatedly, alternately branched, undulating to coiling conidiophores (Kobayashi 1971) not known for St. nephrospora (Hansford 1943) or other species of Stachybotrys s.str.
Stachybotrys sphaerospora Morgan-Jones & R.C. Sinclair, Mycotaxon 10: 372. 1980
Description and illustration — See Morgan-Jones & Sinclair (1980).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys suthepensis Photita et al., Cryptog. Mycol. 24: 149. 2003
Description and illustration — See Photita et al. (2003).
Notes — The ex-type strain (BCC 9776; Photita et al. 2003) of this species was not available to determine its phylogenetic placement in this study.
Stachybotrys taiwanensis (Sivan. & W.H. Hsieh) Yong Wang et al., Fung. Diversity 70: 70. 2015
≡ Niesslia taiwanensis Sivan. & W.H. Hsieh, Mycol. Res. 93: 342. 1989.
Description and illustration — See Sivanesan & Hsieh (1989).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Wang et al. (2015) provided a new combination for Niesslia taiwanensis in Stachybotrys without explanation. Sivanesan & Hsieh (1989) considered the conidiophores formed in culture could represent Monocillium but could not confirm this due to a lack of conidia. Placement of this species in Stachybotrys is doubtful pending further investigation.
Stachybotrys terrestris J.H. Kong & T.Y. Zhang, Mycosystema 26: 200. 2007
Description and illustration — See Kong et al. (2007).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys thaxteri D.W. Li, Mycotaxon 115: 240. 2011
Description and illustration — See Li (2011).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. The smooth-walled, striate conidia produced by this fungus (Li 2011) indicate that this species probably belongs to the new genus Striatibotrys.
Stachybotrys theobromae Hansf., Proc. Linn. Soc. London 155: 45. 1943
Description and illustration — See Hansford (1943).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. See notes under Grandibotrys.
Stachybotrys thermotolerans McKenzie, Fung. Diversity 17: 149. 2004
Description — See Pinruan et al. (2004).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys variabilis H.F. Wang & T.Y. Zhang, Mycosystema 28: 23. 2009
Description and illustration — See Wang & Zhang (2009).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys verrucispora Matsush., Matsushima Mycol. Mem. 4: 18. 1985
Description and illustration — See Matsushima (1985).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys virgata Krzemien. & Badura, Acta Soc. Bot. Poloniae 23: 759. 1954
Description and illustration — See Wang et al. (2015).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys waitakere Whitton, McKenzie & K.D. Hyde, New Zealand J. Bot. 39: 497. 2001
Description and illustration — See Whitton et al. (2001).
Notes — No ex-type strain of this species is available to determine its phylogenetic placement.
Stachybotrys xanthosomatis Mercado & J. Mena, Acta Bot. Cub. 55: 4. 1988
Description and illustration — See Mercado Sierra et al. (1997).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys xigazenensis Y.M. Wu & T.Y. Zhang, Mycotaxon 114: 461. 2010
Description and illustration — See Wu & Zhang (2010).
Notes — The ex-type strain (HMAS 196257; Wu & Zhang 2010) of this species was not available to determine its phylogenetic placement in this study.
Stachybotrys yunnanensis H.Z. Kong, Mycotaxon 62: 427. 1997
Description and illustration — See Kong (1997).
Notes — The ex-type strain (HMAS 71158; Kong 1997) of this species was not available to determine its phylogenetic placement in this study.
Stachybotrys zeae Morgan-Jones & Karr, Mycotaxon 4: 510. 1976
Description and illustration — See Morgan-Jones & Karr (1976).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Stachybotrys zhangmuensis Y.M. Wu & T.Y. Zhang, Mycotaxon 109: 463. 2009
Description and illustration — See Wu & Zhang (2009).
Notes — The ex-type strain (HMAS 196255; Wu & Zhang 2009) of this species was not available to determine its phylogenetic placement in this study.
Stachybotrys zingiberis (V. Rao) Yong Wang et al., Fung. Diversity 70: 70. 2015
≡ Memnoniella zingiberis V. Rao, Sydowia 16: 43. 1962.
Description and illustration — See Rao (1962).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. See notes under Brevistachys.
Stachybotrys zuckii K. Matsush. & Matsush., Matsushima Mycol. Mem. 8: 53. 1995
Description and illustration — See Matsushima & Matsushima (1995).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. This species produces conidia in both slimy masses and in dry chains (Matsushima & Matsushima 1995), which indicates that this species might belong to the genus Memnoniella.
Xepicula leucotrichoides Nag Raj, Coelomycetous anamorphs with appendage-bearing conidia: 980. 1993
Description and illustration — See Nag Raj (1993).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study. Nag Raj (1993) distinguished X. leucotrichoides from X. leucotricha based on the longer setae of X. leucotrichoides (up to 300 μm) compared to those of X. leucotricha (up to 200 μm). See notes under Xepicula.
Xepiculopsis Nag Raj, Coelomycetous anamorphs with appendage-bearing conidia: 983. 1993
Type species. Xepiculopsis graminea (Lib.) Nag Raj.
Description and illustration — See Nag Raj (1993).
Notes — Nag Raj (1993) introduced the asexual genus Xepiculopsis (Xe.), based on X. graminea (≡ Myr. gramineum), distinguishing it from Xepicula by the aseptate, thick-walled setae and two kinds of sterile excipular elements. Unfortunately, no living cultures could be located representing this genus.
Xepiculopsis graminea (Lib.) Nag Raj, Coelomycetous anamorphs with appendage-bearing conidia: 983. 1993
≡ Myrothecium gramineum Lib., Pl. Crypt. Arduenna: 380. 1837.
Description and illustration — See Nag Raj (1993).
Notes — No living type material could be located for this species to determine its phylogenetic placement in this study.
Xepiculopsis perpulchra Nag Raj, Coelomycetous anamorphs with appendage-bearing conidia: 983. 1993
Description and illustration — See Nag Raj (1993).
Notes — Nag Raj (1993) distinguished Xe. graminea from Xe. perpulchra based on conidial dimensions. No living type material could be located for this species to determine its phylogenetic placement in this study.
KEY TO GENERA IN STACHYBOTRIACEAE
1. Genera with sporodochial, myrothecium-like conidiomata . 2
1. Genera with stachybotrys-like conidiophores . . 18
1. Genera with synnematous conidiomata or not as above . 28
2. Sporodochia surrounded by white to grey fringe . . . . 3
2. Sporodochia lacking a fringe . . . . . . . . . . . . . . . . . . . . 13
3. One type of setae present . . 4
3. Two types of setae present . . . . 12
3. Sterile hyphoid extensions protruding through the olivaceous green to black conidial masses, hyaline, septate, smooth, simple or apically dichotomously branched; conidia 0–1-septate, cylindrical, straight to curved . . . . . . . . .Septomyrothecium
4. Setae thick-walled . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
4. Setae thin-walled . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
5. Setae septate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
5. Setae aseptate, smooth-walled; marginal hyphae of sporodochia of two kinds; conidiophores branched or unbranched, smooth, hyaline; conidiogenous cells phialidic, subcylindrical to lagenifom to ampulliform, smooth, hyaline; conidia aseptate, fusiform to ellipsoidal, smooth, with apical, funnel-shaped mucoid appendage . . . . Xepiculopsis
6. Conidia bearing an apical funnel-shaped mucoid appendage . . . . 7
6. Conidia lacking an apical funnel-shaped mucoid appendage, fusiform, initially hyaline becoming darker with age; conidiophores hyaline, smooth to lightly verrucose . . . . . . . . . . Myxospora
7. Conidia hyaline, smooth-walled, fusiform to ellipsoidal to asymmetrically ellipsoidal; setae hyaline, smooth to lightly verrucose . . . . Inaequalispora
7. Conidia pale green, smooth-walled, fusiform to ellipsoidal; setae hyaline, smooth-walled . . . . . . . . Xepicula
8. Conidial mass olivaceous green to black . . . . . . . . . . . 9
8. Conidial mass orange; conidia smooth-walled, ellipsoidal to ossiform . . . . . . . . . . . Tangerinosporium
9. Conidia lacking ornamentations . . . . . . . . . . . . . . . . . 10
9. Conidia with longitudinal striations, olivaceous green to brown, fusiform to ellipsoidal . . . . . . . . Striaticonidium
10. Conidia > 5 μm in length . . . . . . . . . . . . . 11
10. Conidia < 5 μm in length, hyaline, smooth, ellipsoidal to obovoid; conidiogenous cells sometimes arising directly from vegetative hyphae directly or borne on a short stipe . . . . . . . . . . . . . . Myrothecium
11. Conidia aseptate, ellipsoidal to fusiform to limoniform to subglobose, hyaline, sometimes bearing an apical funnel-shaped mucoid appendage; setae hyaline, verrucose, straight to circinate . . . . . . . . . . . . . . . . . . . . Albifimbria
11. Conidia 0–1-septate, cylindrical to ellipsoidal to obovoid, straight to slightly bent, hyaline to pale green; setae hyaline, smooth-walled, becoming sinuous above the apical septum . . . . . . Paramyrothecium
12. Type I setae thin-walled, flexuous to circinate, verrucose, hyaline; Type II setae hyaline, septate, thick-walled, smooth; conidia fusiform, bearing an apical funnel-shaped mucoid appendage . . . . . . . . . . Dimorphiseta
12. Type I setae compacted, thick-walled, verrucose, emerald green; Type II setae hyaline, septate, thick-walled, smooth to lightly verrucose; conidia obclavate to narrowly ellipsoidal . . . . . . . . . . . . . . . Smaragdiniseta
13. Setae present . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
13. Setae absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
14. Setae hyaline . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
14. Setae darkening towards the base; conidia cylindrical to ellipsoidal to ossiform, hyaline to subhyaline . . . . Alfaria
15. Setae with acute apices . . . . . . . . . . . . . . . . . . . 16
15. Setae with obtuse apices, conidia ellipsoidal, smooth-walled, hyaline . . . . . . . . . . . . . Parvothecium
16. Conidiophore stipes hyaline to subhyaline, septate, becoming constricted at the septa, smooth; conidiogenous cells hyaline, cylindrical to subcylindrical, straight to slightly curved; conidia cylindrical to subcylindrical . . . . . . . . . . . . . . . . . . . . . . . . . . Gregatothecium
16. Conidiophore stipes hyaline, septate without constrictions; conidiogenous cells hyaline cylindrical to subcylindrical, straight to slightly curved, covered by olivaceous green mucoid layer; conidia cylindrical . . . Neomyrothecium
17. Marginal hyphae terminating in a capitate to clavate thickwalled cell, coarsely rugose or tuberculate; conidiophores subhyaline to pale olivaceous brown; conidiogenous cells hyaline, smooth; conidia cylindrical, smooth, olivaceous brown . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Capitofimbria
17. Marginal hyphae well-developed, slightly involute, verrucose, loosely coiled; conidiophores hyaline; conidiogenous cells olivaceous green, verrucose; conidia oblong-ellipsoidal, smooth pale olivaceous green . . . Xenomyrothecium
18. Conidiophores thin-walled . . . . . . . 19
18. Conidiophores thick-walled . . . . . . . . . . . . 25
19. Conidiophores hyaline . . . . . . . . . . . . 20
19. Conidiophores hyaline to subhyaline to pale green . . . 22
20. Conidia initially hyaline becoming pigmented with age . 21
20. Conidia remaining hyaline, smooth, ellipsoidal to limoniform to globose to subglobose, containing 1–2 large guttules; conidiophores smooth with verrucose base, 1–3-septate; phialides elongate ampulliform to ventricose to subcylindrical, smooth to verrucose . . . . . . . . . . . . . Achroiostachys
21. Conidia globose to broadly ellipsoidal, thick-walled, containing 1–2 guttules, with truncate hilum . . . . Globobotrys
21. Conidia limoniform to ellipsoidal, thick-walled, with mammiform apice and rounded base . . . Grandibotrys
22. Conidiophores unbranched or branched once . . . . . . 23
22. Conidiophores irregularly cymosely branched, smooth to slightly verrucose; conidia olivaceous to dark brown, smooth to verrucose, fabiform to globose . . . . . Cymostachys
23. Conidiophores carried on vegetative hyphae . . . . . 24
23. Conidiophores carried on synnematous hyphae, smooth with verrucose apex; conidia hyaline to olivaceous brown to dark brown, smooth to verrucose, ellipsoidal to obovoid to cylindrical . . . . . . . . . . . . . Sirastachys
24. Conidiophores 1–3-septate, smooth to verrucose; conidia olivaceous brown to dark brown, smooth to verrucose, ellipsoidal to globose to fusiform to limoniform . . . . . . . . . . . . . . . Stachybotrys
24. Conidiophores 1–5-septate, smooth with verrucose apex, conidia pale olivaceous brown to dark brown, smooth with longitudinal striations, ellipsoidal to fusiform to subcylindrical . . . . . . . . . . . . . . Striatibotrys
25. Conidiophores hyaline to subhyaline to pale brown . . . 26
25. Conidiophores dark brown, with 1–3 percurrent proliferations at the apex; conidia smooth, hyaline, ellipsoidal . . . . . . . . . . . . . . . . . . . . Kastanostachys
26. Conidiophores with bulbous apex . . . . . . 27
26. Conidiophores without bulbous apex, unbranched, smooth to lightly verrucose; conidia initially hyaline becoming olivaceous brown to dark brown, smooth to verrucose, thickwalled, ellipsoidal to globose to reniform, sometimes borne in chains . . . . . . . . . . . . . . . . . . . . . . . . . . . Memnoniella
27. Conidiophores 1-septate, terminating in 3–8 conidiogenous cells; conidiogenous cells carried on the apice of the conidiophores or on the stipe; conidia hyaline to dark brown, smooth to verrucose, obovoid to globose to ossiform to ellipsoidal . . . . . . . . . . . . . . . Brevistachys
27. Conidiophores 1–5-septate, terminating in 4–10 conidiogenous cells; conidia hyaline to dark brown, smooth to verrucose, limoniform to obovoid to globose to ellipsoidal, containing 1–2 guttules . . . . . . Melanopsamma
28. Conidiomata synnematous . . . . . . . . . . . . 29
28. Conidiomata not synnematous, straight to flexuous, verrucose becoming warty with age, hyaline to subhyaline, branched in the upper regions with spiralling to twisted ends, conidiogenous cells carried in the middle or on branches in rows or whorls; conidia cylindrical, straight to curved with mucoid appendages at both ends . Parasarcopodium
29. Synnemata white to yellow . . . . . . . . . . . . . . . . . . . . 30
29. Synnemata white to dark brown or black to olivaceous grey . . . . . . . . . . . . 31
30. Conidia ellipsoidal to oblong, thick-walled, dark brown to black, 3-septate . . . . . . . . . . . . Albosynnema
30. Conidia ellipsoidal to limoniform, thick-walled, hyaline to subhyaline, 1-septate, with mammiform basal and/or apical protrudance . . . . . . . . . . . . . . . . . . . . . . . . Peethambara
31. Conidia 0–1-septate, ellipsoidal to fusiform, thick-walled, with mammiform basal and/or apical protrudance . . . . . . . Didymostilbe
31. Conidia 3-septate, fusiform with papillate and truncate ends, olivaceous grey, coarsely striate . . . . . . . Virgatospora
DISCUSSION
This study represents the first multi-locus sequence analysis of the family Stachybotriaceae, and provides a broad phylogenetic backbone and framework for future studies of this fungal family. In this study, we were able to resolve 33 genera in the Stachybotriaceae, of which 21 are newly introduced here. The remaining 12 genera include established genera (i.e., Myrothecium, Peethambara and Stachybotrys), of which some (i.e., Albosynnema, Alfaria, Didymostilbe, Parasarcopodium, Septomyrothecium and Xepicula) were previously treated as either incertae sedis or members of the Bionectriaceae (Nag Raj 1993, Rossman et al. 1999, Seifert et al. 2003, Mel’nik et al. 2004, Decock et al. 2008, Crous et al. 2014). Several older generic names were also resurrected here (i.e., Melanopsamma, Memnoniella and Virgatospora) based on phylogenetic inference.
This study supports the view of Nag Raj (1993, 1995) that the overly broad generic concept for the genus Myrothecium (Tulloch 1972) has resulted in the misplacement of several fungal species in this genus based solely on the formation of green, slimy, aseptate conidia (Matsushima 1995, Escalona 1997, Seifert et al. 2003, Watanabe et al. 2003, Castañeda-Ruiz et al. 2008, Decock et al. 2008, Alves et al. 2010, Jiang et al. 2014, Wu et al. 2014). Multi-locus phylogenetic inference, supported by phenotypic characters in this study revealed that Myrothecium fide Tulloch is generically diverse with the introduction of 13 new genera (Albifimbria, Capitofimbria, Dimorphiseta, Gregatothecium, Inaequalispora, Myxospora, Neomyrothecium, Paramyrothecium, Parvothecium, Smaragdiniseta, Striaticonidium, Tangerinosporium and Xenomyrothecium). Septomyrothecium (Matsushima 1971a) and Xepicula (Nag Raj 1993) are also recognised as distinct genera in this study. Additionally, several strains deposited in the CBS collection as Myrothecium species surprisingly belonged to the presumed monotypic genus Alfaria (Crous et al. 2014) based on phylogenetic inference.
Of the 13 new myrothecium-like genera introduced here, nine are monotypic, i.e., Capitofimbria compacta (= Myr. compactum; Castañeda-Ruíz et al. 2008), Inaequalispora prestonii (= Myr. prestonii; Tulloch 1972, Nag Raj 1995), Smaragdiniseta bisetosa (= Myr. bisetosum; Rao & De Hoog 1983) and Xenomyrothecium tongaense (= Myr. tongaense; DiCosmo et al. 1980) were initially treated as members of Myrothecium. The remaining five monotypic genera include Dimorphiseta terrestris, Gregatothecium humicola, Neomyrothecium humicola, Parvothecium terrestre and Tangerinosporium thalicicola. Albifimbria, based on Al. verrucaria (= Myr. verrucaria; Tulloch 1972), Myxospora, based on Myx. masonii (Tulloch 1972), Paramyrothecium, based on Pa. roridum (= Myr. roridum; Tulloch 1972) and Striaticonidium, based on Stri. cinctum (= Myr. cinctum; Tulloch 1972) also include well-known species, previously treated as members of Myrothecium.
The present morphological emendment of Myrothecium to include only species producing conidia that are less than 5 μm in length, borne in olivaceous to dark green slimy masses surrounded by a setose fringe, reduces this genus to only two species. Although the remaining myrothecium-like genera share several phenotypic characters, suitable morphological features could be identified to distinguish these fungal genera. For example, the genera Albifimbria, Alfaria, Dimorphiseta, Inaequalispora, Myxospora, Paramyrothecium, Smaragdiniseta, Xepicula and Xepiculopsis each produce setae that have distinct morphologies (Tulloch 1972, Rao & De Hoog 1983, Nag Raj 1993); Capitofimbria is characterised by marginal hyphae terminating in thick-walled cells (Castañeda-Ruíz et al. 2008); Gregatothecium is characterised by sporodochial and penicillately branched conidiomata; Neomyrothecium is characterised by the absence of a setose fringe, but producing thin-walled setae; Parvothecium is also characterised by the absence of a setose fringe but producing verrucose conidiogenous cells; Septomyrothecium is characterised by long hyphoid extensions (Decock et al. 2008); Striaticonidium is characterised by striate conidia not seen for other genera in this group; Tangerinosporium is the only genus in this group to produce orange conidial masses; Xenomyrothecium is characterised by the lack of setae formation.
Comparisons of the ecological characters of the myrothecium-like genera included in this study revealed that the majority of the genera are soil-borne fungi displaying a saprobic lifestyle. Only a small number of species were originally obtained from symptomatic plant material, but their relevance as plant pathogens require further investigation. Based on the narrowed generic concept for Myrothecium applied in this study, this genus appears to be fungicolous as the majority of the strains included were originally isolated from dry or decaying agarics.
This study represents the largest collection of Myrothecium s.l. species subjected to DNA sequence analyses thus far. DNA sequence data for the cmdA, ITS, LSU, rpb2, tef1 and tub2 gene regions have been extensively used to explore the phylogenetic relationships within and between genera in the Hypocreales (Chaverri et al. 2011, Gräfenhan et al. 2011, Lombard et al. 2015b). In this regard, analyses of the individual gene regions (results not shown) showed that partial sequences of the rpb2 gene region followed by cmdA and tub2 gene regions provided the best statistical support to resolve genera and underlying species for this group of fungi. The LSU sequence data also provided significant support at the generic level, but failed to resolve most of the species within the myrothecium-like genera. The ITS gene region, the standard fungal barcode region (Schoch et al. 2012), provided the least resolution at the generic and species level, although most were resolved with low to moderate statistical support. Sequences of the tef1 gene region for the Myrothecium s.l. species included numerous ambiguous regions, complicating alignment of the sequences within and between the myrothecium-like genera. Phylogenetic inference of this gene region produced conflicting results compared to the other five gene regions used in this study. Therefore, this gene region was excluded in the multi-locus analyses.
Wang et al. (2015) questioned whether Stachybotrys represents a monophyletic or polyphyletic genus. Phylogenetic inference in this study clearly illustrated that this genus harbours several phylogenetic genera of which seven were provided with names in this study. At the same time, the genera Melanopsamma and Memnoniella are resurrected to genus level based on the well-supported monophyletic clades resolved in this study. Wang et al. (2015) synonymised both Melanopsamma and Memnoniella under Stachybotrys based on ITS sequence data and following the arguments of Haugland et al. (2001) and Castlebury et al. (2004). The greatest problem facing taxonomic studies of the genus Stachybotrys, is the availability of living type material for molecular study. Of the 74 Stachybotrys species recognised by Wang et al. (2015), sequence data of type material were available for only 15 species (Haugland et al. 2001, Andersen et al. 2003, Li 2007, Jie et al. 2013, Wang et al. 2015).
Of the eight new stachybotrys-like genera introduced in this study, only Globobotrys sansevieriicola (= St. sansevieriicola; Crous et al. 2015) and Kastanostachys aterrima (= Chaetosphaeria aterrima; Réblová 1998) formed single lineages distant to the Stachybotrys s.str. clade. The remaining six genera introduced here (Achroiostachys, Brevistachys, Cymostachys, Grandibotrys, Sirastachys and Striatibotrys) each formed well to highly supported monophyletic clades, distant or closely related to the Melanopsamma, Memnoniella and Stachybotrys s.str. clades, which was further supported by subtle but suitable phenotypic characters (see notes in Taxonomy section). Of these genera with stachybotrys-like morphology, only Brevistachys did not group in the complex of genera centred on Stachybotrys, but formed together with Parasarcopodium a statistically unsupported sister lineage to the clade containing myrothecium-like genera.
Sequence comparisons of the individual loci defined in this study (results not shown) for Stachybotrys s.l., showed that the cmdA and rpb2 gene regions provided similar and the highest statistical support to resolve genera and underlying species for this group of fungi. Both the tef1 and tub2 gene regions were also able to provide resolution, although with lower statistical support, at the generic and species level, with LSU sequence data only providing significant support at the generic level. The ITS sequence data did not resolve the same clades as was observed for the Stachybotrys s.l. dataset based on five loci. Where similar clades were resolved, the support values were lower or equal, with less phylogenetic species resolved. This lack of consistency between the results of the ITS phylogenetic inference and that of the combined five-locus phylogenetic inference highlights the limitations of using ITS sequences to identify Stachybotrys species and species in allied genera (Stielow et al. 2015).
The ecological niche of Stachybotrys s.str. has been well documented in the past (Ellis 1971, 1979, Jong & Davis 1976, Mckenzie 1991, Redlick et al. 1997, Whitton et al. 2001, Andersen et al. 2003, Tang et al. 2007, Pinruan et al. 2004, Li 2007, Crook & Burton 2010, Jie et al. 2013, Wang et al. 2015) and comparison with the remaining 10 genera showed some overlap. Species in the genera Achroiostachys, Grandibotrys, Kastanostachys, Memnoniella, Sirastachys and Striatibotrys were mostly isolated from soil and decaying plant material, indicating a possible saprobic lifestyle. Species of Brevistachys, Cymostachys, Globobotrys and Melanopsamma were mostly isolated from living plant material, although it is not clear whether these fungi were isolated from symptomatic plant material. Therefore, the relevance of these fungi as plant pathogens requires further investigation.
The inclusion of Parasarcopodium ceratocaryi within the Stachybotriaceae was surprising, as this fungus was initially classified as a member of the Bionectriaceae based on LSU sequence data (Mel’nik et al. 2004). This fungus displays a distinct morphology unlike any observed for the other genera studied here. Additionally, the phylogenetic placement of Alfaria, with its myrothecium-like asexual morph morphology, and Didymostilbe, with its synnematous conidiomata (Seifert 1985), in close relationship with the stachybotrys-like genera Achroiostachys, Globobotrys, Grandibotrys, Melanopsamma and Sirastachys was unexpected. This pose interesting questions pertaining to the morphological evolution of these fungi, which requires further investigation.
In conclusion, this study should serve as a backbone for future taxonomic studies of the Stachybotriaceae. A large number of species, especially those with a stachybotrys-like morphology, still need to be included in a phylogenetic study to clarify the taxonomy of this apparently heterogenous group of fungi. This also highlights the importance of maintaining living type cultures in public culture collections, as molecular analysis techniques are becoming commonplace in modern taxonomic studies of fungi. At present only one genome sequence is available for Myr. inundatum (http://genome.jgi.doe.gov.) and for Stachybotrys only genome sequences of St. chartarum (Semeiks et al. 2014, Betancourt et al. 2015) and St. chlorohalonata (Semeiks et al. 2014) are presently available. This under-representation of these fungi in genomic studies is unexpected, especially since they play such an important role in human health. Further genomic studies are therefore urgently required to address this issue.
Acknowledgments
This study was financially supported by the NWO Joint Scientific Thematic Research Programme – Joint Research Projects 2012 ALW file number 833.13.2005 titled ‘Building the fungal quarantine & quality barcode of life database to ensure plant health’. The first author thanks the technical staff, A. van Iperen, Y. Vlug and D. Vos for their valuable assistance with cultures. We are grateful to Keith Seifert for providing some cultures used in this study, microphotographs of some species, and for providing the description of Achroiostachys aurantispora. Martina Réblová was supported by a long-term research development project of the Institute of Botany, Academy of Sciences (RVO 67985939). Cony Decock also acknowledges the financial support received from the Belgian Federal Science Policy (BELSPO). The strain Septomyrothecium uniseptatum MUCL 52944 = 2P-085 was kindly sent to MUCL by Shionogi & Co., Ltd., Pharmaceutical Research Division (Dr. Shuhei Koshida, Strategic Research Planning) to whom we are thankful.
REFERENCES
- Abbas HK, Johnson BB, Shier WT, et al. 2002. Phytotoxicity and mammalian cytotoxicity of macrocyclic trichothecene mycotoxins from Myrothecium verrucaria. Phytochemistry 59: 309–313. [DOI] [PubMed] [Google Scholar]
- Agarwal SC. 1980. A new species of Myrothecium from Indian alkaline soils. Current Science 49: 281–282. [Google Scholar]
- Alves JL, Barreto RW, Pereira OL. 2010. Additions to the mycobiota of the invasive weed Miconia calvescens (Melastomataceae). Mycologia 102: 69–82. [DOI] [PubMed] [Google Scholar]
- Amagata T, Rath C, Rigot JF, et al. 2003. Structures and cytotoxic properties of trichoverroids and their macrolide analogues produced by saltwater culture of Myrothecium verrucaria. Journal of Medical Chemistry 46: 4342–4350. [DOI] [PubMed] [Google Scholar]
- Andersen B, Nielsen KF, Thrane U, et al. 2003. Molecular and phenotypic descriptions of Stachybotrys chlorohalonata sp. nov. and two chemotypes of Stachybotrys found in water-damaged buildings. Mycologia 95: 1227–1238. [DOI] [PubMed] [Google Scholar]
- Barr ME, Crane JL. 1979. Another conidial state for a species of Chaetosphaeria. Canadian Journal of Botany 57: 835–837. [Google Scholar]
- Ben HY, Gao W, Qu HY, et al. 2015. New host record of Myrothecium roridum causing leaf spot on Abutilon megapotamicum from China. Journal of Phytopathology doi: 10.1111/jph.12439. [Google Scholar]
- Betancourt DA, Dean TR, Kim J, et al. 2015. Genome sequence of Stachybotrys chartarum strain 51-11. Genome Announcements 3: e01114–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills FB, Rossman AY, Polishook JD. 1994. Rediscovery of Albosynnema elegans and Solheimia costaspora. Sydowia 46: 1–10. [Google Scholar]
- Bisby G. 1943. Stachybotrys. Transactions of the British Mycological Society 26: 133–143. [Google Scholar]
- Bohn M. 1993. Myrothecium groenlandicum sp. nov., presumed endophytic fungus of Betula nana (Greenland). Mycotaxon 46: 335–341. [Google Scholar]
- Bollenbacher K, Fulton ND. 1963. Myrothecium striatisporum: Its occurrence in Arkansas soil and its cellulolytic activity. Mycologia 55: 786–789. [Google Scholar]
- Booth C. 1957. Studies of Pyrenomycetes II: Melanopsamma pomiformis and its Stachybotrys conidia. Mycological Papers 68: 16–27. [Google Scholar]
- Boyette CD, Hoagland RE, Stetina KC. 2014a. Biological control of the weed hemp sesbania (Sesbania exaltata) in rice (Oryza sativa) by the fungus Myrothecium verrucaria. Agronomy 4: 74–89. [Google Scholar]
- Boyette CD, Hoagland RE, Weaver MA, et al. 2014b. Interaction of the bioherbicide Myrothecium verrucaria and glyphosate for kudzu control. American Journal of Plant Sciences 5: 3943–3956. [Google Scholar]
- Brasel TL, Martin JM, Carriker CG, et al. 2005. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Applied Environmental Microbiology 71: 7376–7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian PW. 1948. Production of antibiotics by species of Myrothecium. Mycologia 40: 363–368. [PubMed] [Google Scholar]
- Brian PW, McGowan JC. 1946. Biologically active metabolic products of the mould Metarrhizium glutinosum Pope. Nature 157: 334. [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castañeda RF. 1986. Deuteromycotina de Cuba, Hyphomycetes IV. Instituto de Investigaciones Fundamentales en Agriculture Tropical “Alejandro de Humboldt” (La Habana): 1–17; f. 1–19. [Google Scholar]
- Castañeda-Ruíz RF, Gusmão LFP, Guarro J, et al. 2008. Two new anamorphic fungi from Brazil: Dictyochaetopsis polysetosa and Myrothecium compactum. Mycotaxon 103: 1–8. [Google Scholar]
- Castañeda-Ruíz RF, Kendrick B. 1991. Ninety-nine conidial fungi from Cuba and three from Canada. University of Waterloo Biology series 35: 1–132. [Google Scholar]
- Castlebury LA, Rossman AY, Sung GH, et al. 2004. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108: 864–872. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Salgado C, Hirooka Y, et al. 2011. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Studies in Mycology 68: 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley JD, Wong WC, Jumper CA, et al. 1998. Correlation between the prevalence of certain fungi and Sick Building Syndrome. Occupation and Environmental Medicine 55: 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda ACJ. 1837. Icones fungorum hucusque cognitorum 1: 1–32. [Google Scholar]
- Crook B, Burton NC. 2010. Indoor moulds, Sick Building Syndrome and building related to illness. Fungal Biology Reviews 24: 106–113. [Google Scholar]
- Crous PW, Allegrucci N, Arambarri AM, et al. 2005. Dematiocladium celtidis gen. sp. nov. (Nectriaceae, Hypocreales), a new genus from Celtis leaf litter in Argentina. Mycological Research 109: 833–840. [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014. Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. (eds). 2009. Fungal Biodiversity. CBS Laboratory Manual Series No. 1. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2013. Fungal Planet description sheets: 154–213. Persoonia 31: 188–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015. Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse M, Telerant R, Gallagher T, et al. 2002. Cryptic species in Stachybotrys chartarum. Mycologia 94: 814–822. [PubMed] [Google Scholar]
- Dearborn DG, Yike I, Sorenson WG, et al. 1999. Overview of investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environmental Health Perspectives 107: 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decock C, Huret S, Bivort C. 2008. Anamorphic fungi from French Guyana. Septomyrothecium sp. nov. and S. setiramosum comb. nov. (anamorphic Hypocreales, Ascomycota). Cryptogamie Mycologie 29: 321–331. [Google Scholar]
- Deighton FC. 1960. African Fungi. I. Mycological Papers 78: 1–43. [Google Scholar]
- DiCosmo F, Michaelides J, Kendrick B. 1980. Myrothecium tongaense anam.-sp. nov. Mycotaxon 12: 219–224. [Google Scholar]
- Ditmar LPF. 1813. Deutschlands Flora, Abt. III. Die Pilze Deutschlands; Strum, Nürnberg, Germany. [Google Scholar]
- Domsch KH, Gams W, Anderson T-H. 1980. Compendium of soil fungi. IHW-Verlag, Eching, Germany. [Google Scholar]
- Dorai M, Vittal BPR. 1987. A new Stachybotrys from Eucalyptus litter. Transactions of the British Mycological Society 87: 642–644. [Google Scholar]
- Drobotko VG. 1945. Stachybotryotoxicosis. A new disease of horses and humans. American Review of Soviet Medicine 2: 238–242. [Google Scholar]
- Dulymamode R, Cannon PF, Hyde KD, et al. 2001. Four new ascomycetes species from endemic Pandanus of Mauritius. Fungal Diversity 8: 87–96. [Google Scholar]
- Ehrenberg CG. 1818. Sylvae Mycologicae Berolinensis: 1–32. [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England. [Google Scholar]
- Ellis MB. 1979. More dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England. [Google Scholar]
- Escalona F. 1997. Myrothecium renaudii sp. nov. on Heliconia psittacorum H. spathocircinata. Mycotaxon 61: 81–86. [Google Scholar]
- Etzel RA, Montaña E, Sorenson WG, et al. 1998. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Archives of Pediatrics and Adolescent Medicine Journal 158: 757–762. [DOI] [PubMed] [Google Scholar]
- Finley DE. 1967. Virgatospora: a new genus of Stilbellaceae. Mycologia 59: 538–541. [Google Scholar]
- Flappan SM, Portnoy J, Jones P, et al. 1999. Infant pulmonary hemorrhage in a suburban home with water damage and mould (Stachybotrys atra). Environmental Health Perspectives 107: 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer S, Pestka J, Kim JK, et al. 2012. Impact of environmental factors on growth and satratoxin G production by strains of Stachybotrys chartarum. World Mycotoxin Journal 5: 37–43. [Google Scholar]
- Fries EM. 1829. Systema Mycologicum 3: 1–260. Moritz, Greifswald, Germany. [Google Scholar]
- Fuckel L. 1872. Symbolae mycologicae. Beiträge zur Kenntniss der rheinischen Pilze. Erster Nachtrag. Jahrbücher des Nassauischen Vereins für Naturkunde 25–26: 287–346. [Google Scholar]
- Fujinawa MF, De Carvalho Pontes N, Martins do Vale HM, et al. 2015. First report of Myrothecium roridum causing Myrothecium leaf spot on Begonia in Brazil. Plant disease notes http://dx.doi.org/10.1094/PDIS-09-15-1097-PDN. [Google Scholar]
- Galloway LD. 1933. Note on an unusual mould fungus. Transactions of the British Mycological Society 18: 163–166. [Google Scholar]
- Glass NL, Donaldson G. 1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RR, Glienke C, Videira SIR, et al. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräfenhan T, Schroers H-J, Nirenberg HI, et al. 2011. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68: 79–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes RM, Duncan CW, Hoppert CA. 1957. Multiplicity of cellulolytic enzymes of Myrothecium verrucaria. Archives of Biochemistry and Biophysics 68: 412–424. [DOI] [PubMed] [Google Scholar]
- Groenewald JZ, Nakashima C, Nishikawa J, et al. 2013. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75: 115–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove WB. 1886. New or noteworthy fungi. Part III. Journal of Botany British and Foreign 24: 197–207. [Google Scholar]
- Gülay T, Grossman F. 1994. Antagonistic activity of five Myrothecium species against fungi and bacteria in vitro. Journal of Phytopathology 140: 97–113. [Google Scholar]
- Halliwell G. 1961. The action of cellulolytic enzymes from Myrothecium verrucaria. Biochemical Journal 79: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KS, Choi SK, Kim HH, et al. 2014. First report of Myrothecium roridum causing leaf and stem rot disease of Peperomia quadragularis in Korea. Mycobiology 42: 203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford GC. 1943. Contributions towards the fungus flora of Uganda, V. Fungi Imperfecti. Proceedings of the Linnean Society London 155: 34–67. [Google Scholar]
- Harrach B, Bata A, Bajmócy E, et al. 1983. Isolation of satratoxins from the bedding straw of a sheep flock with fatal stachybotryotoxicosis. Applied and Environmental Microbiology 45: 1419–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland RA, Heckman JL. 1998. Identification of putative sequence specific PCR primers for detection of the toxigenic fungal species Stachybotrys chartarum. Molecular and Cellular Probes 12: 387–396. [DOI] [PubMed] [Google Scholar]
- Haugland RA, Vesper SJ, Harmon SM. 2001. Phylogenetic relationships of Memnoniella and Stachybotrys species and evaluation of morphological features for Memnoniella species identification. Mycologia 93: 54–65. [Google Scholar]
- Hawksworth DL. 2012. Managing and coping with names of pleomorphic fungi in a period of transition. IMA Fungus 3: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, et al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192. [Google Scholar]
- Hughes SJ. 1952. Fungi from the Gold Coast. I. Mycological Papers 48: 1–91. [Google Scholar]
- Hughes SJ. 1958. Revisiones hyphomycetum aliquot cum appendice de nomi-nibus rejiciendis. Canadian Journal of Botany 36: 727–836. [Google Scholar]
- Huhndorf SM, Miller AN, Fernández FA. 2004. Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia 96: 368–387. [PubMed] [Google Scholar]
- Hyde KD, Goh TK, Taylor JE, et al. 1999. Byssosphaeria, Chaetosphaeria, Niesslia and Ornatispora gen. nov. from palms. Mycological Research 103: 1423–1439. [Google Scholar]
- Jiang YL, Wang HF, Pan HQ, et al. 2014. Myrothecium (Hyphomycetes): Three new species, one variety and a key to species and varieties of the genus known from soil in China. Mycosystema 33: 7–14. [Google Scholar]
- Jiang YL, Zhang T. 2009. Notes on soil dematiaceous hyphomycetes from Sichuan Province, China. Mycosystema 28: 644–647. [Google Scholar]
- Jie CY, Geng K, Jiang YL, et al. 2013. Stachybotrys from soil in China, identified by morphology and molecular phylogeny. Mycological Progress 12: 693–698. [Google Scholar]
- Jong SC, Davis EE. 1976. Contributions to the knowledge of Stachybotrys and Memnoniella in culture. Mycotaxon 3: 409–485. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshava Prasad T, Asha L, Bhat D. 2003. A new species of Memnoniella from India. Mycotaxon 85: 341–344. [Google Scholar]
- Kirjavainen PV, Täubel M, Karvonen AM, et al. 2015. Microbial secondary metabolites in homes in association with moisture damage and asthma. Indoor Air doi:10.1111/ina.12213. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kanasaki R, Ezaki M, et al. 2004. FR227244, a novel antifungal antibiotic from Myrothecium cinctum No. 002 I. Taxonomy, fermentation, isolation and physio-chemical properties. Journal of Antibiotics 57: 780–787. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. 1971. Mycological reports from New Guinea and the Solomon Island (1–11). Bulletin of the National Science Museum Tokyo 14: 367–551. [Google Scholar]
- Kong HZ. 1997. Stachybotrys yunnanensis sp. nov. and Neosartorya delicate sp. nov. isolated from Yunnan, China. Mycotaxon 62: 427–433. [Google Scholar]
- Kong JH, Zhang TY, Zhang W. 2007. Notes on soil dematiaceous hyphomycetes from Hexi Corridor, Gansu Province. Mycosystema 26: 196–201. [Google Scholar]
- Koster B, Scott J, Wong B, et al. 2003. A geographically diverse set of isolates indicates two phylogenetic lineages within Stachybotrys chartarum. Canadian Journal of Botany 81: 633–643. [Google Scholar]
- Koster B, Wong B, Straus N, et al. 2009. A multi-gene phylogeny for Stachybotrys evidences lack of trichodiene synthase (tri5) gene for isolates of one of three intrageneric lineages. Mycological Research 113: 877–886. [DOI] [PubMed] [Google Scholar]
- Li BJ, Ben HY, Shi YX, et al. 2014. First report of Myrothecium roridum causing leaf spot on Zantedeschia aethiopica in China. Plant Disease 98: 854. [DOI] [PubMed] [Google Scholar]
- Li DW. 2007. Stachybotrys eucylindrospora sp. nov. resulting from re-examination of Stachybotrys cylindrosporia. Mycologia 99: 332–339. [DOI] [PubMed] [Google Scholar]
- Li DW. 2011. Stachybotrys thaxteri sp. nov. and the nomenclatural status of three Stachybotrys species. Mycotaxon 115: 239–250. [Google Scholar]
- Li DW, Yang CS, Haugland R, et al. 2003. A new species of Memnoniella. Mycotaxon 85: 253–257. [Google Scholar]
- Li QR, Jiang YL. 2011. Stachybotrys subreniformis, new from soil in China. Mycotaxon 115: 171–173. [Google Scholar]
- Link HF. 1809. Observationes in ordines plantarum naturales. Dissertatio I. Magazin der Gesellschaft Naturforschenden Freunde Berlin 3: 3–42. [Google Scholar]
- Liu JY, Huang LL, Ye YH, et al. 2006. Antifungal and new metabolites of Myrothecium sp. Z16, a fungus associated with white croaker Argyromosum argentatus. Journal of Applied Microbiology 100: 195–202. [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang S, Zhu J, et al. 2015. Two new amides from a halotolerant fungus, Myrothecium sp. GS-17. Journal of Antibiotics 68: 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Chen SF, Mou X, et al. 2015a. New species, hyper-diversity and potential importance of Calonectria spp. from Eucalyptus in South China. Studies in Mycology 80: 151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, et al. 2010. Phylogeny and systematics of the genus Calonectria. Studies in Mycology 66: 31–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Shivas RG, To-Anun C, et al. 2012. Phylogeny and taxonomy of the genus Cylindrocladiella. Mycological Progress 11: 835–868. [Google Scholar]
- Lombard L, Van der Merwe NA, Groenewald JZ, et al. 2014. Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathologia Mediterranea 53: 515–532. [Google Scholar]
- Lombard L, Van der Merwe NA, Groenewald JZ, et al. 2015b. Generic concepts in Nectriaceae. Studies in Mycology 80: 189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, Gershwin ME. 2000. Sick Building Syndrome. III. Stachybotrys chartarum. Journal of Asthma 37: 191–198. [DOI] [PubMed] [Google Scholar]
- Manoharachary C, Agarwal DK, Sureshkumar G, et al. 2006. Memnoniella mohanramii sp. nov. and Zygosporium anupamvarmae sp. nov. from India. Indian Phytopathology 59: 489–491. [Google Scholar]
- Martinovich D, Mortimer PH, Di Menna ME. 1972. Similarities between so-called kikuyu poisoning of cattle and two experimental mycotoxicoses. New Zealand Veterinary Journal 20: 57–58. [DOI] [PubMed] [Google Scholar]
- Mason-Gamer R, Kellogg E. 1996. Testing for phylogenetic conflict among molecular datasets in the tribe Triticeae (Graminae). Systematic Biology 45: 524–545. [Google Scholar]
- Matsushima T. 1971a. Some interesting fungi imperfecti. Bulletin Natural Science Museum Tokyo 14: 460–480. [Google Scholar]
- Matsushima T. 1971b. Microfungi of the Solomon Islands and Papua-New Guinea. Matsushima ed.: 1–78. [Google Scholar]
- Matsushima T. 1975. Icones Microfungorum: a Matsushima lectorum. [Google Scholar]
- Matsushima T. 1985. Matsushima Mycological Memoirs No. 4: 1–68. [Google Scholar]
- Matsushima T. 1989. Matsushima Mycological Memoirs No. 6: 1–100. [Google Scholar]
- Matsushima T. 1995. Matsushima Mycological Memoirs No. 8: 1–53. [Google Scholar]
- Matsushima K, Matsushima T. 1995. Fragmenta Mycologica – 1. Matsushima Mycological Memoirs 8: 45–54. [Google Scholar]
- McKenzie EHC. 1991. Dematiaceous hyphomycetes on Freycinetia (Pandanaceae). 1. Stachybotrys. Mycotaxon 41: 179–188. [Google Scholar]
- McNeill J, Barrie FF, Buck WR, et al. 2012. International Code of Nomenclature for algae, fungi and plants (Melbourne Code). Gantner Verlag KG; [Regum Vegetabile no. 154]. [Google Scholar]
- Mel’nik V, Lee S, Groenewald JZ, et al. 2004. New hyphomycetes from Restionaceae in fynbos: Parasarcopodium ceratocaryi gen. et sp. nov., and Rhexodenticula elegiae sp. nov. Mycological Progress 3: 19–28. [Google Scholar]
- Mercado Sierra A, Holubová-Jechová V, Mena Portales J. 1997. Hifomicetes demaciáceos de Cuba Enteroblásticos. Museo Regionale di Scienze Naturali, Monografie XXIII, Torino, Cuba. [Google Scholar]
- Misra PC. 1976. Stachybotrys renispora sp. nov. Mycotaxon 4: 161–162. [Google Scholar]
- Misra PC, Srivastava SK. 1982. Two undescribed Stachybotrys species from India. Transactions of the British Mycological Society 78: 556–559. [Google Scholar]
- Morgan-Jones G, Karr GW., Jr 1976. Notes on Hyphomycetes. XVI. A new species of Stachybotrys. Mycotaxon 4: 510–512. [Google Scholar]
- Morgan-Jones G, Sinclair RC. 1980. Notes on Hyphomycetes. XXXIII. Stachybotrys sphaerospora sp. nov. from South Africa. Mycotaxon 10: 372–374. [Google Scholar]
- Morris EF. 1967. Studies on the synnematous Fungi Imperfecti. Mycopathologia et Mycologia Applicata 33: 179–185. [DOI] [PubMed] [Google Scholar]
- Mortimer PH, Campbell J, Di Menna ME, et al. 1971. Experimental myrotheciotoxicosis and poisoning in ruminants by verrucarin A and roridin A. Research in Veterinary Science 12: 508–515. [PubMed] [Google Scholar]
- Muhsin TM, Al-Helfi MA. 1981. Hyphomycetes from Iran – The genus Stachybotrys. Sydowia 34: 130–134. [Google Scholar]
- Munjal RL, Kapoor JN. 1969. Some Hyphomycetes from the Himalayas. Mycopathologia et Mycologia Applicata 39: 121–128. [Google Scholar]
- Murakami Y, Okuda T, Shindo K. 2001. Roridin L, M and Verrucarin M, new macrocyclic trichtheciene group antitumor antibiotics, from Myrothecium verrucaria. Journal of Antibiotics 54: 980–983. [DOI] [PubMed] [Google Scholar]
- Nag Raj TR. 1993. Coelomyceous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario, Canada. [Google Scholar]
- Nag Raj TR. 1995. What is Myrothecium prestonii? Mycotaxon 53: 295–310. [Google Scholar]
- Namikoshi M, Akano K, Meguro S, et al. 2001. A new macrocyclic trichothecene, 12,13-deoxyroridin E, produced by the marine derived fungus Myrothecium roridum collected in Palau. Journal of Natural Products 64: 396–398. [DOI] [PubMed] [Google Scholar]
- Nicot J, Olivry C. 1961. Contribution à l’étude du genre Myrothecium Tode. 1. Les espèces à spores striées. Revue Générale de Botanique 68: 672–685. [Google Scholar]
- Nirenberg HI. 1981. A simplified method for identifying Fusarium spp. occurring on wheat. Canadian Journal of Botany 59: 1599–1609. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v. 2. Programme distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, et al. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Science of the United States of America 95: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Sarver BA, Brandt M, et al. 2007. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. Journal of Clinical Microbiology 45: 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Namikoshi M, Akano K, et al. 2005. Verrucarin A inhibition of MAP Kinase activation in a PMA-simulated promyelocytic leukemia cell line. Marine Drugs 3: 64–73. [Google Scholar]
- Ôkuchi M, Itoh M, Kaneko Y, et al. 1968. A new antifungal substance produced by Myrothecium. Agricultural and Biological Chemistry 32: 394–395. [Google Scholar]
- Okunowo WO, Gbenle GO, Osuntoki AA, et al. 2010. Production of cellulolytic and xylanolytic enzymes by a phytopathogenic Myrothecium roridum and some avirulent fungal isolates from water hyacinth. African Journal of Biotechnology 9: 1074–1078. [Google Scholar]
- Ozegovic L, Pavlovic R, Milosev B. 1971. Toxic dermatitis, conjunctivitis, rhinitis, pharyngitis and laryngitis in fattening cattle and farm workers caused by moulds from contaminated straw (stachybotryotoxicosis?). Veterinaria 20: 263–267. [Google Scholar]
- Photita W, Lumyong P, McKenzie EHC, et al. 2003. Memnoniella and Stachybotrys species from Musa acuminate. Cryptogamie Mycologie 24: 147–152. [Google Scholar]
- Pidoplichko NM, Kirilenko TS. 1969. New species of the genus Myrothecium Tode. Mykrobiologichnyi Zhurnal Kiev 31: 158–163. [PubMed] [Google Scholar]
- Pidoplichko NM, Kirilenko TS. 1971. On the taxonomy of the genus Myrothecium. In: Pidoplichko NM. (ed), Metabolites of soil micromycetes. Dumka, Naukova, Kiev, Ukrain: 157–171. [Google Scholar]
- Pinruan U, McKenzie EHC, Gareth Jones EB, et al. 2004. Two new species of Stachybotrys, and a key to the genus. Fungal Diversity 17: 145–157. [Google Scholar]
- Piyaboon O, Unartngam A, Unartngam J. 2014. Effectiveness of Myrothecium roridum for controlling water hyacinth and species identification based on molecular data. African Journal of Microbiology Research 8: 1444–1452. [Google Scholar]
- Pope S. 1944. A new species in Metarrhizium active in decomposing cellulose. Mycologia 36: 343–350. [Google Scholar]
- Preston NC. 1943. Observations on the genus Myrothecium Tode. I. Transactions of the British Mycological Society 26: 158–168. [Google Scholar]
- Preston NC. 1948. Observations on the genus Myrothecium Tode. II. Myrothecium gramineum Lib. and two new species. Transactions of the British Mycological Society 31: 271–276. [Google Scholar]
- Preston NC. 1961. Observations on the genus Myrothecium Tode. III. The cylindrical spored species of Myrothecium known in Britain. Transactions of the British Mycological Society 44: 31–41. [Google Scholar]
- Rama Rao P. 1963. A new species of Myrothecium from soil. Antonie van Leeuwenhoek 29: 180–182. [Google Scholar]
- Rao V, Sutton BC. 1975. Synnematous Fungi. I. Kavaka 3: 21–28. [Google Scholar]
- Rao VG. 1962. Some new records of Fungi-imperfecti from India. Sydowia 16: 41–45. [Google Scholar]
- Rao VG, De Hoog GS. 1983. A new species of Myrothecium. Persoonia 12: 99–101. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, Kew, Surrey. British Mycological Society. [Google Scholar]
- Réblová M. 1998. Revision of three Melanomma species described by L. Fuckel. Czech Mycology 50: 161–179. [Google Scholar]
- Réblová M, Barr ME, Samuels GJ. 1999. Chaetosphaeriaceae, a new family for Chaetosphaeria and its relatives. Sydowia 51: 49–70. [Google Scholar]
- Réblová M, Gams W, Seifert KA. 2011. Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Studies in Mycology 68: 163–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlick CA, Sparer J, Cullen MR. 1997. Sick-building syndrome. The Lancet 349: 1013–1016. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. 1995. Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Canadian Journal of Botany 73: S816–S823. [Google Scholar]
- Rifai MA. 1964. Stachybotrys bambusicola sp. nov. Transactions of the British Mycological Society 47: 269–272. [Google Scholar]
- Rifai MA. 1974. Another pink-spored and brown-stalked species of Stachybotrys. Reinwardtia 8: 537–540. [Google Scholar]
- Rivolta S. 1873. Dei Parassiti Vegetali: 1–592. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rossman AY. 1983. The phragmosporous species of Nectria and related genera. Mycological Papers 150: 1–164. [Google Scholar]
- Rossman AY, Adams GC, Cannon PF, et al. 2015. Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, McKemy JM, Pardo-Schultheiss RA, et al. 2001. Molecular studies of the Bionectriaceae using large subunit rDNA sequences. Mycologia 93: 100–110. [Google Scholar]
- Rossman AY, Newell S, Schultheiss RA. 1998. Bionectria erubescens on smooth cordgrass along the coast of eastern North America with comments on a new family, Bionectriaceae, Hypocreales. Inoculum 49: 45. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, et al. 1999. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Rossman AY, Seifert KA, Samuels GJ, et al. 2013. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales) proposed for acceptance and rejection. IMA Fungus 4: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruma K, Sunil K, Kini KR, et al. 2015. Genetic diversity and antimicrobial activity of endophytic Myrothecium spp. isolated from Calophyllum apelatum and Garcinia Morella. Molecular Biology Reports 42: 1533–1543. [DOI] [PubMed] [Google Scholar]
- Saccardo PA. 1878. Fungi Italici autographice delineati. Michelia 1: 326–350. [Google Scholar]
- Saccardo PA. 1886. Sylloge Fungorum 4: 744. [Google Scholar]
- Samuels GJ. 1988. Species of Nectria (Ascomycetes, Hypocreales) having orange perithecia and colorless, striate ascospores. Brittonia 40: 306–331. [Google Scholar]
- Schneider DJ, Marasas WF, Kuys DJC, et al. 1979. A field outbreak of suspected stachybotryotoxicosis in sheep. Journal of South African Veterinary Association 50: 73–81. [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences 109: 6241–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA. 1985. A monograph of Stilbella and some allied Hyphomycetes. Studies in Mycology 27: 1–235. [Google Scholar]
- Seifert KA, Louis-Seize G, Sampson G. 2003. Myrothecium acadiense, a new hyphomycete isolated from the weed Tussilago farfara. Mycotaxon 87: 317–327. [Google Scholar]
- Semeiks J, Borek D, Otwinowski Z, et al. 2014. Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genomics 15: 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma ND, Agarwal GP. 1974. Fungi causing plant diseases at Jabalpur (M.P.) – XVI. Journal of the Indian Botanical Society 53: 76–82. [Google Scholar]
- Sherwood M. 1974. New Hyphomycetes from Guadeloupe, F.W.I. Albosynnema filicola, Tetracrium musicola, and Thozetellopsis calicioides. Mycotaxon 1: 117–120. [Google Scholar]
- Shoemaker RC, House DE. 2005. A time-series study of Sick Building Syndrome: chronic, biotoxin-associated illness from exposure to water-damaged buildings. Neurotoxicology and Teratology 27: 29–46. [DOI] [PubMed] [Google Scholar]
- Sivanesan A, Hsieh WH. 1989. New species and new records of ascomycetes from Taiwan. Mycological Research 93: 340–351. [Google Scholar]
- Smith G. 1962. Some new and interesting species of micro-fungi. III. Transactions of the British Mycological Society 45: 387–394. [Google Scholar]
- Stamatakis A. 2014. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stielow JB, Lévesque CA, Seifert KA, et al. 2015. One fungus, which gene? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35: 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DC, Cooley D, Wong WC, et al. 2003. Studies on the role of fungi in Sick Building Syndrome. Archives of Environmental Health 58: 475–478. [DOI] [PubMed] [Google Scholar]
- Subramanian CV. 1954. Fungi Imperfecti from Madras – VI. Journal of the Indian Botanical Society 33: 36–42. [Google Scholar]
- Subramanian CV. 1957. Hyphomycetes – IV. Proceedings of the Indian Academy of Science Section B 46: 324–335. [Google Scholar]
- Subramanian CV, Bhat DJ. 1978a. Peethambara, a new genus of the Hypocreales. Revue de Mycologie 42: 49–55. [Google Scholar]
- Subaramanian CV, Bhat DJ. 1978b. Putagraivam, a new genus of the Hyphomycetes. Proceedings of the Indian National Science Academy, Part B. Biological Sciences 87: 99–104. [Google Scholar]
- Sullia SB, Padma SD. 1985. Myrothecium mori sp. nov. – A new leaf spot pathogen of mulberry. Current Science 54: 757–758. [Google Scholar]
- Summerbell RC, Gueidan C, Schroers H-J, et al. 2011. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Studies in Mycology 68: 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods), v. 4.0b10. Computer programme. Sinauer Associates, Sunderland, Massachusetts, USA. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AMC, Jeewon R, Hyde KD. 2007. Phylogenetic utility of protein (RPB2, β-tubulin) and ribosomal (LSU, SSU) gene sequences in the systematics of Sordariomycetes (Ascomycota, Fungi). Antonie van Leeuwenhoek 91: 327–349. [DOI] [PubMed] [Google Scholar]
- Thrasher JD, Hooper DH, Taber J. 2014. Family of six, their health and the death of a 16 month old male from pulmonary hemorrhage: Identification of mycotoxins and mold in the home and lungs, liver and brain of deceased infant. Global Journal of Medical Research 14: 1–11. [Google Scholar]
- Trapp SC, Hohn TM, McCormick S, et al. 1998. Characterization of the gene cluster for biosynthesis of macrocyclic trichothecens in Myrothecium roridum. Molecular and General Genetics 257: 421–432. [DOI] [PubMed] [Google Scholar]
- Tulloch M. 1972. The genus Myrothecium Tode ex Fr. Mycological Papers 130: 1–42. [Google Scholar]
- Updegraff DM. 1971. Utilization of cellulose from waste paper by Myrothecium verrucaria. Biotechnology and Bioengineering 13: 77–97. [DOI] [PubMed] [Google Scholar]
- Verona O, Mazzucchetti G. 1968. I Generi “Stachybotrys” e “Memnoniella”. Publicazioni Dell’Ente Nazionale, per la Cellulosa e per la Carta, Laboratorio di Cartotecnica Speciale, Roma. [Google Scholar]
- Vesper S, Dearborn DG, Yike I, et al. 2000. Evaluation of Stachybotrys chartarum in the house of an infant with pulmonary hemorrhage: Quantitative assessment before, during, and after remediation. Journal of Urban Health 77: 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper SJ, Vesper MJ. 2002. Stachylysin may be a cause of hemorrhaging in humans exposed to Stachybotrys chartarum. Infection and Immunity 70: 2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana ME, Coates NH, Gavett SH, et al. 2002. An extract of Stachybotrys chartarum causes allergic asthma-like responses in a BALB/c mouse model. Toxicological Sciences 70: 98–109. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, et al. 2012. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. [Google Scholar]
- Von Höhnel FV. 1905. Über Myrothecium und Formverwandte Gattungen. Annales Mycologici 3: 559–560. [Google Scholar]
- Von Höhnel FV. 1924. Studien über Hyphomyzete. Zentralblatt für Bakteriologie und Parasitenkunde Abteilung 2, 60: 1–26. [Google Scholar]
- Wang HF, Zhang TY. 2009. Notes on soil dematiaceous hyphomycetes from the Qaidam Basin, Qinghai province, China. Mycosystema 28: 20–24. [Google Scholar]
- Wang Y, Hyde KD, McKenzie EHC, et al. 2015. Overview of Stachybotrys (Memnoniella) and current species status. Fungal Diversity 70: 17–83. [Google Scholar]
- Watanabe T, Watanabe Y, Nakamura K. 2003. Myrothecium dimorphum, sp. nov., a soil fungus from beach sand in the Bonin (Ogasawara) Islands, Japan. Mycoscience 44: 283–286. [Google Scholar]
- Weaver MA, Boyette CD, Hoagland RE, et al. 2016. Management of kudzu by the bioherbicide, Myrothecium verrucaria, herbicides and integrated control programmes. Biocontrol Science and Technology 26: 136–140. [Google Scholar]
- Whitaker DR. 1953. Purification of Myrothecium verrucaria cellulase. Archives of Biochemistry and Biophysics 43: 253–268. [DOI] [PubMed] [Google Scholar]
- White TJ, Burns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 282–287. Academic Press, USA. [Google Scholar]
- White WL, Darby RT, Stechart GM, et al. 1948. Assays of cellulolytic activity of molds isolated from fabrics and related items exposed in the tropics. Mycologia 40: 34–84. [PubMed] [Google Scholar]
- Whitton SR, McKenzie EHC, Hyde KD. 2001. Microfungi on the Pandanaceae: Stachybotrys with three new species. New Zealand Journal of Botany 39: 489–499. [Google Scholar]
- Whitton SR, McKenzie EHC, Hyde KD. 2012. Fungi associated with Pandanaceae. Fungal Diversity Research Series 21: 1–457. [Google Scholar]
- Wu YM, Wang HF, Zhang TY. 2014. Two new species of Myrothecium from Qinghai-Tibet Plateau Area, China. Mycotaxon 129: 403–406. [Google Scholar]
- Wu YM, Zhang TY. 2009. Two new species of Stachybotrys from soil. Mycotaxon 109: 461–464. [Google Scholar]
- Wu YM, Zhang TY. 2010. Two new species of Stachybotrys from soil. Mycotaxon 114: 459–462. [Google Scholar]
- Xu J, Takasaki A, Kobayashi H, et al. 2006. Four new macrocyclic trichothecens from two strains of marine-derived fungi of the genus Myrothecium. Journal of Antibiotics 59: 451–455. [DOI] [PubMed] [Google Scholar]
- Yang SM, Jong SC. 1995. Factors influencing pathogenicity of Myrothecium verrucaria isolated from Euphorbia esula and species of Euphorbia. Plant Disease 79: 998–1002. [Google Scholar]


