Abstract
We introduce 15 new species of Penicillium isolated from a diverse range of locations, including Canada, Costa Rica, Germany, Italy, New Zealand, Tanzania, USA and the Dry Valleys of Antarctica, from a variety of habitats, including leaf surfaces in tropical rain forests, soil eaten by chimpanzees, infrabuccal pockets of carpenter ants, intestinal contents of caterpillars and soil. The new species are classified in sections Aspergilloides (1), Canescentia (2), Charlesia (1), Exilicaulis (3), Lanata-Divaricata (7) and Stolkia (1). Each is characterised and described using classical morphology, LC-MS based extrolite analyses and multigene phylogenies based on ITS, BenA and CaM. Significant extrolites detected include andrastin, pulvilloric acid, penitrem A and citrinin amongst many others.
Keywords: beta-tubulin, calmodulin, internal transcribed spacer rDNA region, Trichocomaceae
INTRODUCTION
Because of the standardised approach to delineating species of Penicillium now available, with ex-type or representative strains and barcode sequences designated for almost all described species, including those considered synonyms (Visagie et al. 2014b), routine description of new species can now be approached with reasonable confidence. The exact set of genes used for multi-locus sequence typing (MLST) or Genealogical Concordance Phylogenetic Species Recognition (GCPSR) is not standardised among different laboratories, but most tend to use a similar core set of loci. Phenotypes such as irregular conidiophore branching, culture sectoring, and the relative abundance of conidia, sterile mycelium, soluble pigments or exudates can be interpreted as phylogenetically informative characters or artefacts of culture degeneration according to their correlation with robustly analysed DNA sequence data.
The historical focus of Penicillium taxonomy on strains isolated from food, soil and the indoor environment is understandable given the importance of these fungi as spoilage agents, producers of potent mycotoxins, and sources of valuable antibiotics. Much of the expansion of the number of species in Penicillium and its sister genus Talaromyces can be traced to the antibiotic revolution and the ease with which soil could be collected by travellers and used to isolate new strains upon return to the laboratory. This practise, however, obscured how Penicillium species might actually function in nature. It was easy to assume that most species are generalists found wherever we care to make isolations.
In this paper, 15 species are described that originated from several studies in three Canadian laboratories over the past 20 years, which are now recognised as novel taxa because of the more complete reference data. Three species were isolated from soil of termite mounds selectively eaten by chimpanzees (Pan troglodytes) in the Mahale Mountains and Gombe areas of Tanzania (Ketch 1998). Thirty-one genera were identified from these termitarium soils and surrounding control soils. The most commonly isolated genus was Penicillium with over 42 species recovered, the most common of which was Penicillium citrinum. Clark (2002) isolated microfungi from the infrabuccal pockets of carpenter ants, especially Camponotus pennsylvaticus. These sac-like organs in the mouths of ants filter solids from their liquid foods to be expelled later, and thus capture fungal material collected during feeding and grooming. About 60 strains of Penicillium were isolated from extracted infrabuccal masses from ants collected in New Brunswick (Canada), Germany and the Netherlands, representing six morphospecies; three are described as new species here. A US National Science Foundation Microbial Observatory study of caterpillar intestines from Costa Rica previously yielded two undescribed species, P. mallochii and P. guanacastense that clearly were significant components of that niche, along with P. citrinum (Rivera et al. 2012). An additional new species, represented as a single strain among the 88 isolated, is described here. Other new species described in this paper were isolated from leaf surfaces of tropical plants, nuts in temperate hardwood forests, peat bogs and soil remote from agricultural regions.
Extrolite profiling brought a new dimension to Penicillium taxonomy about 25 years ago (Filtenborg et al. 1990), accompanied by a hypothesis that different isolates of a single species should produce a consistent profile of core extrolites. With the availability of a new generation of LC-MS-MS technology, we generated extrolite profiles for all the new species described in this paper. Although reports are unavailable for the metabolites produced by many species related to our new taxa, these observations will be useful for future comparative chemotaxonomic studies.
MATERIALS AND METHODS
Strains
Cultures were isolated either by direct or dilution plating from various substrates or niches as indicated in the species descriptions, usually on 2 % malt extract agar with antibacterial antibiotics. Strains were recovered from the working collections of Keith Seifert (Agriculture and Agri-Food Canada, Ottawa) and David Malloch (formerly University of Toronto), the latter now maintained in the Seifert laboratory. Ex-type and other representative strains were deposited into the Canadian Collection of Fungal Cultures (DAOMC) and the CBS-KNAW Fungal Biodiversity Centre (CBS) in the Netherlands.
Morphology
Colony characters were recorded from various media incubated for 7 d, including Czapek yeast autolysate agar (CYA), Blakeslee’s (1915) malt extract agar (MEAbl), yeast extract sucrose agar (YES), oatmeal agar (OA) and creatine sucrose agar (CREA). BactoTM malt extract was used for MEAbl preparation. Media preparations, inoculations, incubation conditions and microscopic preparations followed the recommendations by Visagie et al. (2014b). Colour names and codes used in descriptions are from Kornerup & Wanscher (1967). Microscopic observations were made on an Olympus SZX12 dissecting microscope and Olympus BX50 compound microscope equipped with Infinity3 and InfinityX cameras driven by Infinity Analyze v. 6.5.1 software (Lumenera Corp., Ottawa, Canada).
DNA extraction, sequencing and phylogenetic analysis
Strains were grown on MEAbl for 7 d and DNA extracted using the UltracleanTM Microbial DNA isolation Kit (MoBio Laboratories Inc., Solana Beach, USA). ITS barcodes (Schoch et al. 2012), partial β-tubulin (BenA) and partial calmodulin (CaM) genes were amplified using the Illustra pureTaq Ready-To-GoTM PCR Beads (GE Healthcare Life Sciences) with conditions and primers as suggested in Visagie et al. (2014b). Sequencing reactions were set up with the same primer pairs and the Big Dye Terminator Cycle Premix Kit with products run on the ABI PRISMTM 310 DNA automated sequencer (Applied Biosystems, Waltham, USA). Contigs were assembled and edited in Geneious v. 8.1.5 (BioMatters Ltd., Auckland, New Zealand). Newly generated sequences were submitted to GenBank under accession numbers KT887760–KT887876.
Gene sequences of the new species were compared with a reference sequence dataset (Visagie et al. 2014b), with phylogenies calculated for the relevant taxonomic sections in the system of Houbraken & Samson (2011). Datasets were aligned using MAFFT v. 7.221 (Katoh & Standley 2013), with the L-INS-i algorithm selected for ITS and G-INS-i for BenA and CaM. Aligned datasets were adjusted and trimmed manually in Geneious. Datasets were subsequently analysed using Maximum Parsimony (MP) and Bayesian tree Inference (BI). MP heuristic searches were performed in PAUP v. 4.0b10 (Swofford 2003). BI analyses were run in MrBayes v. 3.2.5 (Ronquist & Huelsenbeck 2003). Model selections were made using MrModeltest v. 2.3 (Nylander et al. 2004) based on the lowest Akaike information criterion (AIC) value. Aligned datasets with the respective command blocks for MP and BI were uploaded onto TreeBASE (www.treebase.org) with accession no. S18638. Trees were prepared for publication in Adobe Illustrator CS6.
Extrolites
For extrolite analyses, all strains were grown in 9 cm polystyrene Petri dishes on YES (Frisvad 1981, Filtenborg et al. 1990) and CYA (Pitt 1980) incubated at 25 °C for 14 d. Strains were also grown in 200 mL of CYA broth incubated at 25 °C for 14 d. Extrolites were extracted from the agar plates using an established method (Bragulat et al. 2001) with some modifications. Three agar plugs from each fungal isolate were removed with a sterilised 7 mm cork borer and placed in a 7 mL glass scintillation vial containing 1.5 mL of 75 % methanol in water. The vials were vortexed for 30 s and sonicated at room temperature for 30 min. The extrolites in CYA broth media were extracted using 2× 150 mL ethyl acetate, combined, dried and reconstituted in 1 mL of methanol. The solutions were filtered into 1.5 mL amber HPLC glass vials using 0.45 μm syringe filters. All extracts were analysed in both positive and negative ionization modes on a Thermo Q-Exactive Orbitrap coupled to an Agilent 1290 HPLC. Extrolites were identified by comparison with authentic standards when possible. When standards were unavailable, compounds were putatively identified by screening the high-resolution/accurate mass m/z signals with AntiBase2013 and comparing product ions observed with those published in the literature.
RESULTS
Morphology
We introduce 15 new species in the Taxonomy section below. These species belong to sections Aspergilloides, Canescentia, Charlesia, Exilicaulis, Lanata-Divaricata and Stolkia based on the phylogenetic analyses. Strains conformed to the general morphological characters previously observed for species accepted in these sections. All of the new species were compared with respective close relatives, with notes provided on distinguishing characters after each species description in the taxonomy section.
Phylogeny
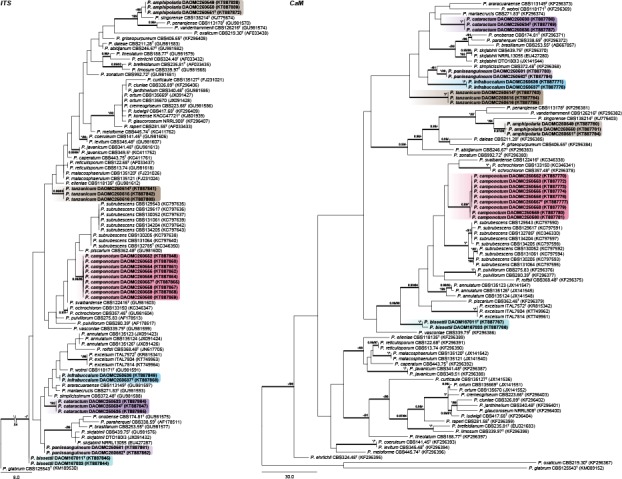
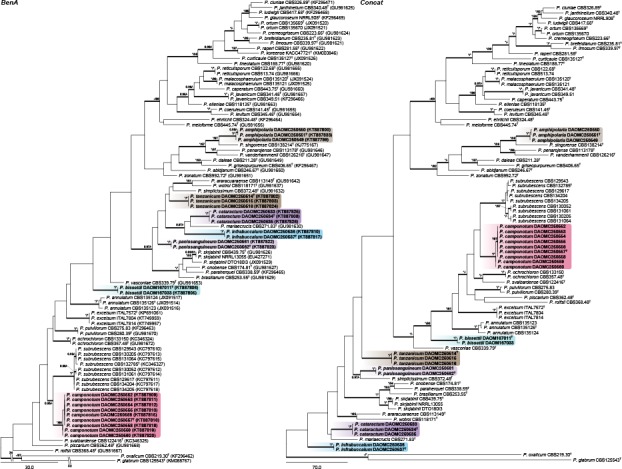
A local BLAST search placed the new species into sections Aspergilloides, Canescentia, Charlesia, Exilicaulis, Lanata-Divaricata and Stolkia. Phylogenies of each section were prepared for the ITS region, BenA and CaM genes, as well as a concatenation of the three. Phylogenies are presented in Fig. 1, 2, 3, 4, 5, 6, 7, 8. The length, number of parsimony informative and uninformative sites, and substitution models (for BI) for each dataset are summarised in Table 1. Tree topologies did not differ between MP and BI analyses and MP trees were thus used for representing results, with BI posterior probability values marked on relevant branches. The exception was the concatenated dataset for section Aspergilloides, where P. longicatenatum was resolved in an unsupported clade with ML (Fig 2, indicated by red branch), whilst in the BI tree the latter two were resolved in a well-supported clade with the P. glabrum and P. thomii clades, with P. longicatenatum a distant relative to this main clade.
Fig. 1.
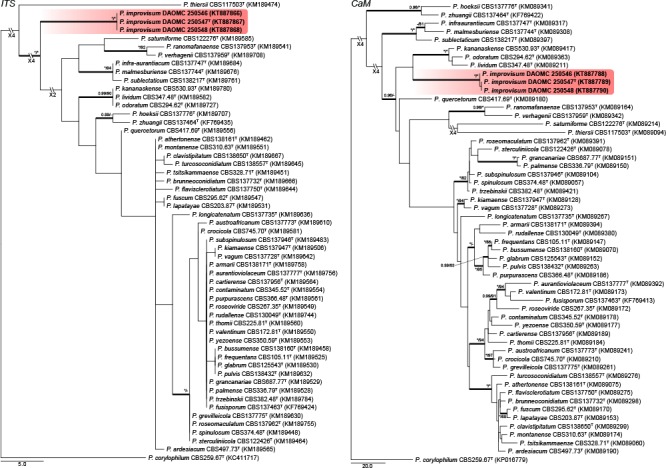
Phylogenetic trees for ITS & CaM datasets of Penicillium sect. Aspergilloides. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets.
Fig. 2.
Phylogenetic trees for BenA & Concatenated (ITS, BenA & CaM) datasets of Penicillium sect. Aspergilloides. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets.
Fig. 3.
Phylogenetic trees for ITS, BenA, CaM & Concatenated (ITS, BenA & CaM) datasets of Penicillium sect. Canescentia. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets.
Fig. 4.
Phylogenetic trees for ITS, BenA, CaM & Concatenated (ITS, BenA & CaM) datasets of Penicillium sections Charlesia and Stolkia. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets.
Fig. 5.
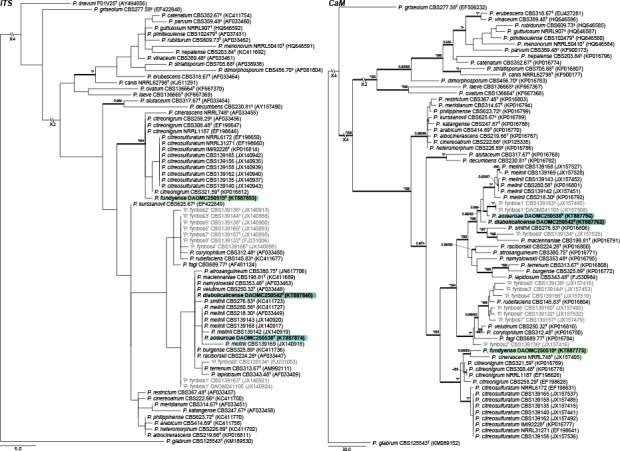
Phylogenetic trees for ITS & CaM datasets of Penicillium sect. Exilicaulis. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets. Taxon labels in grey, indicate unpublished new species from Visagie et al. (2016b).
Fig. 6.
Phylogenetic trees for BenA & Concatenated (ITS, BenA & CaM) datasets of Penicillium sect. Exilicaulis. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets. Taxon labels in grey, indicate unpublished new species from Visagie et al. (2016b).
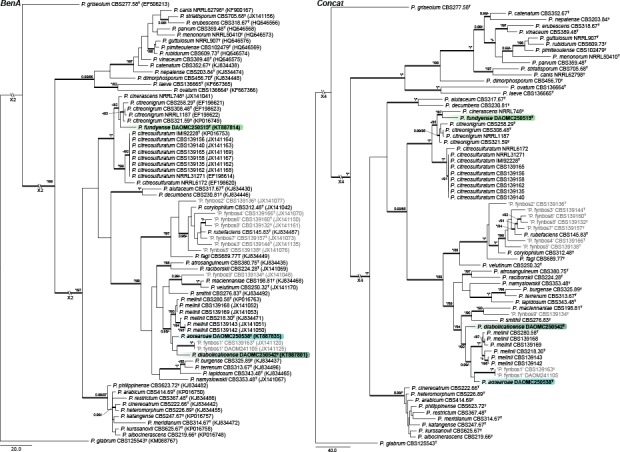
Fig. 7.
Phylogenetic trees for ITS & CaM datasets of Penicillium sect. Lanata-Divaricata. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets.
Fig. 8.
Phylogenetic trees for BenA & Concatenated (ITS, BenA & CaM) datasets of Penicillium sect. Lanata-Divaricata. Branch support in nodes higher than 80 % bs and/or 0.95 pp are indicated above thickened branches (T = ex-type; * = 100 % bs or 1.00 pp; - = support lower than 80 % bs and/or 0.95 pp). New species are indicated by coloured blocks. GenBank accession numbers are provided between brackets.
Table 1.
Overview and details used for phylogenetic analyses.
| Dataset |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| section Aspergilloides | section Canescentia | section Charlesia & Stolkiae | section Exilicaulis | section Lanata-Divaricata | ||||||
| ITS dataset | Length (bp) | 550 | 510 | 532 | 513 | 502 | ||||
| Parsimony uninformative sites | 34 | 31 | 22 | 51 | 41 | |||||
| Parsimony informative sites | 43 | 12 | 100 | 49 | 59 | |||||
| Substitution model (BI) | GTR+G+I | HKY+I | GTR+G+I | GTR+G+I | GTR+G+I | |||||
| BenA dataset | Length (bp) | 450 | 403 | 465 | 489 | 535 | ||||
| Parsimony uninformative sites | 52 | 37 | 17 | 68 | 80 | |||||
| Parsimony informative sites | 168 | 110 | 197 | 197 | 215 | |||||
| Substitution model (BI) | HKY+G+I | HKY+G | SYM+G+I | HKY+G | GTR+G+I | |||||
| CaM dataset | Length (bp) | 521 | 388 | 557 | 527 | 571 | ||||
| Parsimony uninformative sites | 55 | 41 | 25 | 47 | 65 | |||||
| Parsimony informative sites | 207 | 130 | 236 | 241 | 255 | |||||
| Substitution model (BI) | GTR+G+I | K80+G | GTR+I+G | SYM+I+G | GTR+G+I | |||||
| Concatenated dataset | Length (bp) | 1521 | 1301 | 1554 | 1513 | 1608 | ||||
| Parsimony uninformative sites | 141 | 109 | 64 | 164 | 188 | |||||
| Parsimony informative sites | 418 | 252 | 533 | 483 | 522 | |||||
Section Aspergilloides — Fig. 1, 2
Houbraken et al. (2014) reviewed section Aspergilloides and divided it into 12 clades. All of our phylogenies show that P. improvisum strains comprise a well-supported 13th clade. The new species can easily be identified using the ITS barcode, with BenA and CaM also distinguishing the species.
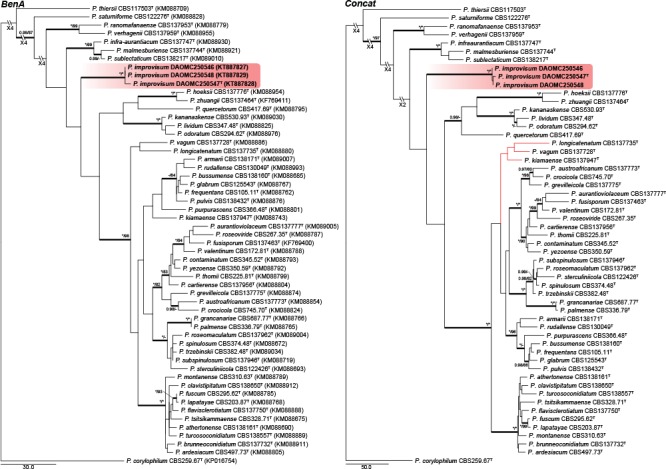
Section Canescentia — Fig. 3
Two new species belong to section Canescentia. Penicillium corvianum is resolved in a distinct clade closely related to species of the P. canescens and P. janczewskii clades, while P. nucicola is distinct from all other species. The ITS barcode has poor discriminatory power in the section. BenA easily distinguishes the new species, but cannot reliably distinguish among P. radiatolobatum and P. murcianum strains. In the strict sense, current data assessed with the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) concept, suggests that these two represent synonyms and P. radiatolobatum (Publ. Soc. Nat. Rom. Pent. Stiinta Sol. 10B: 435, 1971) an older name than P. murcianum (Mycopathologia 74: 37, 1981).
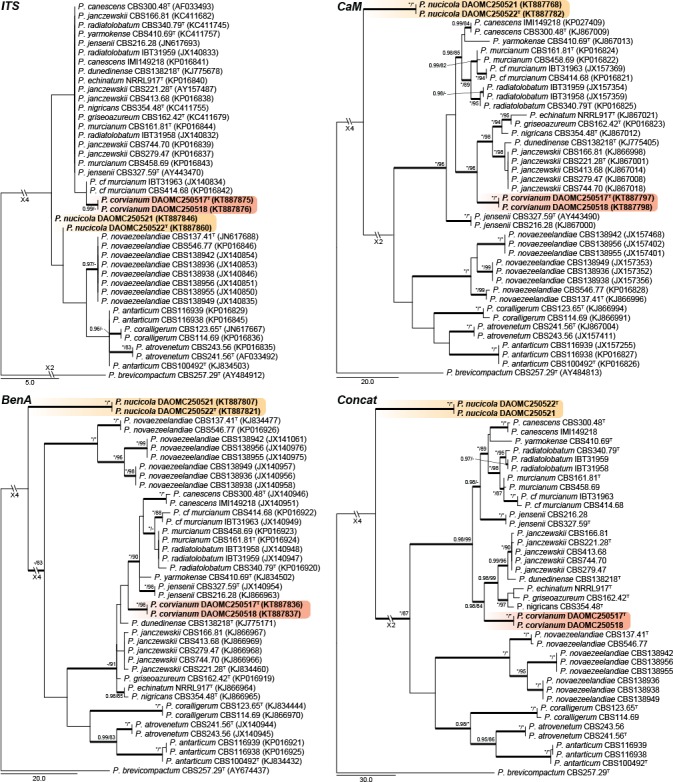
Sections Charlesia and Stolkia — Fig. 4
A new species is introduced in section Charlesia, where species are phylogenetically diverse enough for ITS to result in a positive identification. Phylogenies show the distinct identity of P. charlesii and P. fellutanum, with P. multicolor (CBS 501.73) and P. ebenbitarianum (CBS 415.69) confirmed as synonyms of the latter. The single strain of P. costaricense forms a branch distantly related to all other species of section Charlesia.
Section Stolkia is a small section of rather rare species. As with section Charlesia, the ITS barcode can distinguish between the six species and P. alogum described here. The concatenated phylogeny resolves P. alogum as a close relative of P. stolkiae and P. subarcticum.
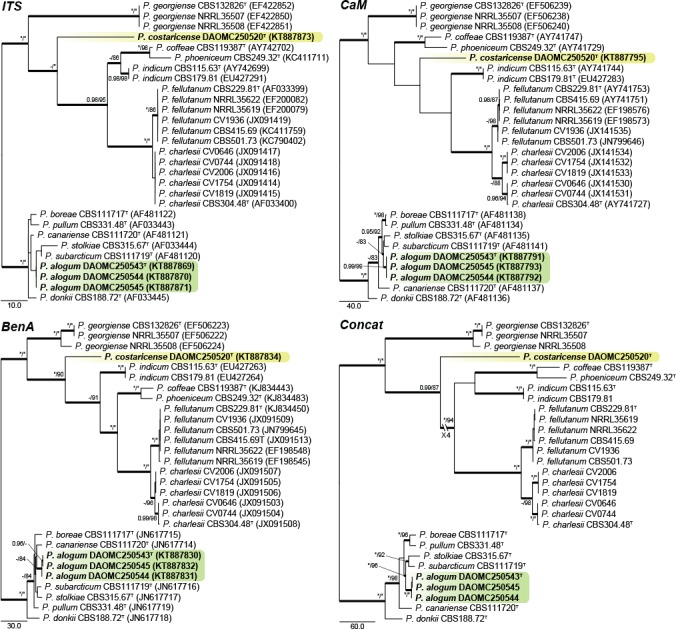
Section Exilicaulis — Fig. 5, 6
Three new species are introduced in section Exilicaulis. Sequence datasets contain some undescribed species from the fynbos, South Africa (in grey, Visagie et al. 2016b). ITS generally has poor resolution in this section. Of the new species, P. diabolicalicense and P. aotearoae share identical sequences with previously known species, whereas P. fundyense has a unique ITS sequence. The BenA and CaM genes distinguish the new species from its close relatives. Penicillium fundyense sits in a clade with P. cinerascens, P. citreonigrum and P. citreosulfuratum (= P. toxicarium fide Serra et al. 2008), recently reviewed in Visagie et al. (2016b). The single strain DAOMC 250519 was sufficiently distinct from other strains in the section to propose a new species. Penicillium aotearoae and P. diabolicalicense is resolved in the P. melinii clade closely related to P. melinii and P. xanthomelinii.
Section Lanata-Divaricata — Fig. 7, 8
ITS performs remarkably well for identification of the seven new species we introduce in section Lanata-Divaricata, with all having unique sequences. BenA and CaM also easily distinguish all species of the section. The concatenated phylogeny resolved P. amphipolaria as a close relative of P. singorense; P. camponotum as closely related to P. subrubescens; while P. tanzanicum, P. bissettii, P. cataractum, P. infrabuccalum and P. panissanguineum resolved in several clades on a poorly supported back bone typically having species with rough walled conidiophores such as P. simplicissimum.
Extrolites
An overview of the extrolites detected during this study and their product ions are summarised in Table 2, while Table 3 summarises the extrolites produced by each species analysed. We also list extrolites produced by each species with their respective descriptions in the taxonomy section.
Table 2.
Overview of the major extrolites detected and product ions.
| Code | m/z | Formula | Product ion m/z | ||||
|---|---|---|---|---|---|---|---|
| andrastin A | 487.2687 | C28H38O7 | 243.1743 | 395.2229 | 427.1995 | 349.2160 | 225.1637 |
| andrastin B* | 487.2702 | C28H40O7 | 427.2486 | 455.2455 | 457.2603 | 419.0410 | |
| andrastin C | 473.2898 | C28H40O6 | 203.1431 | 179.0702 | 441.2272 | 381.2057 | 335.2004 |
| andrastin D | 429.2636 | C26H36O5 | 397.2374 | 369.2425 | 379.2267 | 245.1899 | 351.2318 |
| aurantioclavine | 227.1543 | C15H18N2 | 210.1277 | 171.0916 | 58.0660 | 130.0651 | 154.0651 |
| austinb | 501.2481 | C27H32O9 | 381.2056 | 363.1950 | 409.2004 | 391.1901 | 335.2000 |
| bisdechlorogeodinb | 331.0812 | C17H14O7 | 287.0911 | 299.0549 | 272.0677 | 255.0649 | 317.0653 |
| citrinin | 251.0915 | C13H14O5 | 233.0808 | 205.0860 | |||
| dehydrohistidyltryptophanyldiketopiperazine | 322.1299 | C17H15N5O2 | 193.0720 | 130.0652 | 162.0295 | ||
| fiscalin A | 474.2137 | C26H27N5O4 | 258.1237 | 199.0865 | 171.0916 | ||
| fiscalin C | 488.2290 | C27H29N5O4 | 213.1021 | 258.1236 | 185.1072 | 201.1018 | |
| fumitremorgin B | 480.2523 | C27H33N3O5 | 383.1967 | 355.2018 | 406.1764 | 424.1865 | 462.2385 |
| fusaperazine E | 361.1580 | C19H24N2O3S | 245.0920 | 217.0970 | 153.0367 | 293.0953 | 69.0707 |
| fusaperazine related | 406.1795 | C20H27N3O4S | 107.0495 | 216.1382 | 359.1836 | 290.1133 | 291.1210 |
| glandicoline B | 420.1667 | C22H21N5O4 | 289.0716 | 335.1018 | 261.0767 | 334.0935 | 307.1086 |
| marcfortine A | 478.2699 | C28H35N3O4 | 450.2748 | 419.2314 | 348.1232 | ||
| marcfortine B | 464.2544 | C27H33N3O4 | 436.2593 | 419.2327 | 405.2178 | ||
| meleagrin | 434.1820 | C23H23N5O4 | 334.0931 | 403.1639 | 318.0741 | 289.0718 | 306.0985 |
| mevastatinb | 391.2477 | C23H34O5 | 185.1324 | 159.1167 | 211.1480 | 229.1585 | 235.1482 |
| N,N-dimethylrestricticinb | 366.2639 | C21H35NO4 | 115.0757 | 149.1326 | 231.1743 | 263.2004 | 83.0499 |
| N6-formyl-roquefortine C | 418.1874 | C23H23O3N5 | 333.0977 | 349.1164 | 332.0900 | 350.1243 | |
| neoxaline | 436.1978 | C23H25N5O4 | 336.1087 | 405.1794 | 280.1192 | 263.1187 | 145.0760 |
| oxalicine A | 504.2379 | C30H33NO6 | 148.0392 | 216.0291 | 122.0602 | 234.0396 | 363.1601 |
| oxalicine B | 520.2332 | C30H33NO7 | 148.0394 | 216.0292 | 122.0603 | 234.0398 | 218.0449 |
| oxaline | 448.1981 | C24H25N5O4 | 348.1090 | 417.1794 | 332.0894 | 349.1168 | 386.1612 |
| penicillic acid | 171.0652 | C8H10O4 | 125.0598 | 112.0522 | 97.0652 | 111.0444 | 83.0498 |
| penitrem A | 634.2930 | C37H44ClNO6 | Confirmed by RT and isotope pattern, CYA media only | ||||
| pulvilloric acid* | 295.1186 | C15H20O6 | 195.0293 | 277.1083 | 177.0185 | 151.0389 | 251.1286 |
| restricticinb | 338.2324 | C19H31NO4 | 231.1742 | 170.0811 | 161.1324 | 263.2004 | 306.2063 |
| isofumigaclavine A | 299.1753 | C18H22N2O2 | 239.1542 | 196.1122 | 197.1077 | 144.0808 | 168.0806 |
| isofumigaclavine B | 257.1647 | C16H20N2O | 239.1542 | ||||
| roquefortine C | 390.1925 | C22H23N5O2 | 322.1296 | 193.0719 | 198.1280 | 334.1296 | 358.1661 |
| roquefortine M | 422.1821 | C22H23O4N5 | 192.0642 | 205.0722 | 353.1120 | ||
| roquefortine N | 440.1928 | C22H25O5N5 | 192.0642 | 154.0611 | 210.0747 | 422.1823 | |
| trichodermamide A (penicillazine)b | 433.1242 | C20H20N2O9 | 248.0552 | 220.0603 | 192.0653 | 176.0343 | |
| trichodermamide Cb | 447.1397 | C21H22N2O9 | 262.0709 | 234.0761 | 206.0812 | 219.0764 | 176.0342 |
* Analyzed as [M-H]-1 in negative mode. All other ions are [M+H]+.
b Putative assignments based interpretation of MS2 fragmentation pathways.
Table 3.
Overview of the extrolites produced by the strains of this study.
| P. alogum | P. amphipolaria | P. aotearoae | P. bissettii | P. camponatum | P. cataractum | P. costaricense | P. corvianum | P. diabolicalicense | P. fundyense | P. improvisum | P. infrabuccalum | P. nucicola | P. panissanguineum | P. tanzanicum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| andrastin A | – | – | – | – | + | + | + | – | – | – | – | + | + | + | – |
| andrastin B | – | – | – | – | + | + | – | – | – | – | – | + | + | + | – |
| andrastin C | – | – | – | – | + | + | + | – | – | – | – | + | + | + | – |
| andrastin D | – | – | – | – | + | + | – | – | – | – | – | + | + | + | – |
| aurantioclavine | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – |
| austin | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – |
| bisdechlorogeodin | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + |
| citrininc | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – |
| dehydrohistidyl-tryptophanyl-diketopiperazine | – | – | – | + | – | – | – | – | – | + | – | – | – | – | – |
| fiscalin A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + |
| fiscalin C | – | – | – | – | – | – | – | + | – | – | – | – | – | – | + |
| fumitremorgin B | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| fusaperazine E | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| fusaperazine related | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| glandicoline B | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – |
| marcfortine A | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – |
| marcfortine B | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – |
| meleagrinc | – | – | – | + | – | – | – | – | – | + | – | – | – | – | – |
| mevastatin | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| N,N-dimethylrestricticin | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – |
| N6-formyl-roquefortine C | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – |
| neoxaline | – | – | – | + | – | – | – | – | – | + | – | – | – | – | – |
| oxalicine A | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – |
| oxalicine B | – | – | – | + | – | – | – | – | – | – | – | + | – | – | – |
| oxaline | – | + | – | – | – | – | – | – | – | + | – | – | – | – | – |
| penicillic acid | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – |
| penitrem Ac | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – |
| pulvilloric acid | – | – | – | – | – | + | – | – | – | – | – | + | – | + | + |
| restricticin | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – |
| isofumigaclavine Ac | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – |
| isofumigaclavine B | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – |
| roquefortine Cc | – | – | – | + | – | – | – | – | – | + | – | – | – | – | – |
| roquefortine M | – | – | – | + | – | – | – | – | – | + | – | – | – | – | – |
| roquefortine N | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – |
| trichodermamide C | – | – | – | – | – | + | – | + | – | – | – | – | – | – | – |
| trichodermamide A (penicillazine) | – | – | – | – | – | + | – | – | – | – | – | – | – | – | – |
| C11H15O3N | – | – | – | – | – | – | + | + | – | – | – | – | – | – | – |
| C18H22O5 | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| C29H20O7S2 | – | – | – | – | – | + | – | + | – | – | – | – | – | – | – |
| C20H30O6N2 | – | – | – | – | – | + | – | – | – | – | – | – | – | – | – |
| C22H23O4N3 | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| C27H31O7N3 | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – |
| C14H19O4N | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – |
| C21H28O8 | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| C19H24O8 | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – |
| C15H22O2 | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – |
| C20H20O6 | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – |
c Confirmed by comparison to authentic standard.
Six of the Penicillium species studied here produced andrastin metabolites in varying amounts. Penicillium cataractum and P. infrabuccalum were both major producers of andrastin A–D. Penicillium nucicola, P. panissanguineum and two strains of P. camponotum also produced andrastin, while only lower levels of andrastin A and C were produced by P. costaricense. There was a correlation between the production of andrastins and a pulvilloric acid related compound (Fig. 9). As explained in the Discussion, this compound is either an unreported analogue of pulvilloric acid or the structure of pulvilloric acid has been mischaracterised in the literature. Of all the andrastin producers, only P. camponotum, P. costaricense and P. nucicola did not produce this pulvilloric acid related compound. Penicillium tanzanicum also produced this compound.
Fig. 9.
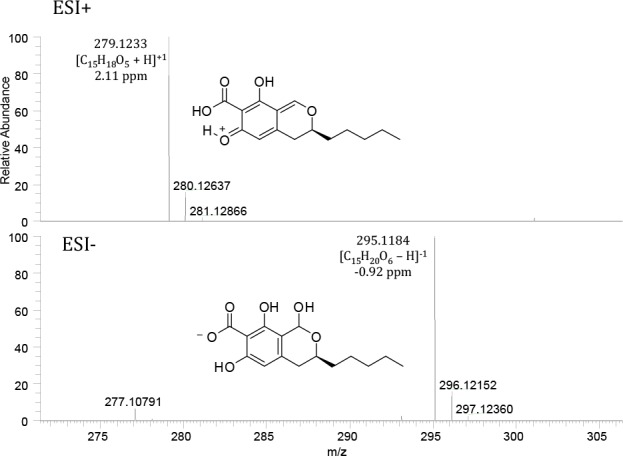
Mass spectra of pulvilloric acid analyzed in ESI+ and ESI-. The intact [M-H]- ion is observed in the negative electrospray ionization mode. The intact [M+H]+ ion is absent in positive electrospray ionization mode and the major ion species is [M-H2O+H]+.
Although all P. camponotum strains produced the indole alkaloid marcfortine A and B, their metabolite profiles could be separated into three groups that partly correlated with geographical origin. Penicillium amphipolaria was the only species that produced fumitremorgin compounds. The compounds produced on YES and CYA agar were fumitremorgin A, fumitremorgin C, dihydroxyfumitremorgin C and oxofumitremorgin B. When grown in CYA broth, other uncharacterised fumitremorgins were the extrolites produced. Very few extrolites were detected in cultures of P. diabolicalicense, but the fungal neurotoxin penitrem A was detected.
The two strains of P. bissettii produced significantly different extrolites. DAOMC 167033 produced the mycotoxin penicillic acid. DAOMC 167011 produced meleagrin, neoxaline and aurantioclavine along with other uncharacterised metabolites.
TAXONOMY
Penicillium section Aspergilloides — Fig. 1, 2
Penicillium improvisum Visagie, Malloch & Seifert, sp. nov. — MycoBank MB815769; Fig. 10
Fig. 10.
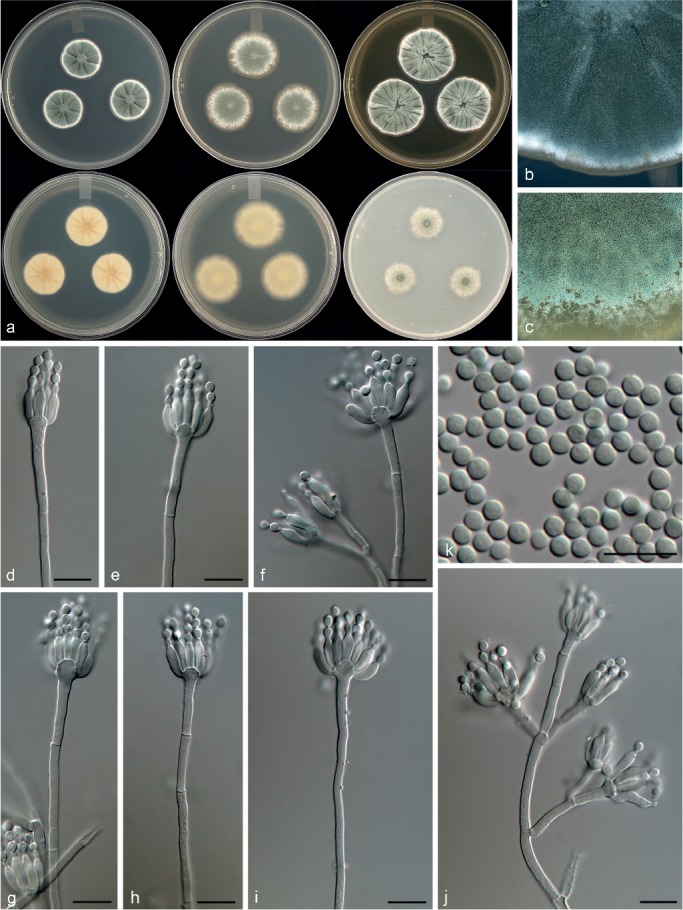
Morphological characters of Penicillium improvisum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Aspergilloides, section Aspergilloides.
ITS barcode: KT887867. Alternative identification markers: BenA = KT887828, CaM = KT887789.
Etymology. Latin, improvisum, meaning unexpected/unforeseen, named for the conidiophores, which have randomly arranged branches.
Type specimen. CANADA, Ontario, Niagara falls, soil from oak/maple/ash forest, 28 Sept. 1996, D. Malloch (holotype DAOM 695768, culture ex-type DAOMC 250547 = CBS 140994 = KAS 2386 = W 156).
Colony morphology — Colony diam, 7 d, in mm: CYA 21–24; CYA 37 °C no growth; MEAbl 24–30; YES 28–31; OA 18–22; CREA 14–15.
CYA 25 °C, 7 d: Colonies moderately deep, raised at centre, radially sulcate; margins low, narrow, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse greyish turquoise (24D5–E5); soluble pigments absent; exudates absent; reverse greyish yellow (3B4), pale to yellowish grey (2A2–B2). MEA 25 °C, 7 d: Colonies low, plane; margins low, wide, irregular; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse greyish turquoise to greyish green (24D4–E5–25E5); soluble pigments absent; exudates absent; reverse greyish yellow (2B3), greenish grey to dull green (1B2–29D4). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, crateriform; margins low, narrow, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse greyish turquoise (24D5–E5); soluble pigments absent; exudates absent; reverse greyish yellow (3B6), pale yellow (3A3). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse greyish turquoise (24D4–E6); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Weak growth, acid weakly produced.
Conidiophores monoverticillate, sometimes divaricate; stipes smooth, some roughened hyphae present, 40–200(–330) × 2.5–3.5 μm; vesicle 4.5–6.5 μm; branches 13–32.5 μm; phialides ampulliform, (3–)5–12 per stipe, 7.5–10.5 × 2.5–3.5 μm (8.9 ± 0.8 × 3.0 ± 0.2); conidia smooth walled, globose to subglobose, 2.5–3 × 2.5–3 μm (2.8 ± 0.1 × 2.8 ± 0.1), average width/length = 0.96, n = 43.
Extrolites — citrinin, isofumigaclavine A, isofumigaclavine B.
Additional material examined. CANADA, Niagara falls, soil from oak/maple/ash forest, 28 Sept. 1996, D. Malloch, culture DAOMC 250546 (= CBS 140993 = KAS 2381 = W 155), DAOMC 250548 (= CBS 140995 = KAS 2393 = W 154).
Notes — Phylogenetic analyses resolved P. improvisum, classified in section Aspergilloides, in a unique clade distinct from the 12 clades recognised by Houbraken et al. (2014) (Fig. 1, 2). Section Aspergilloides species are typically monoverticillate, but some do produce extra subterminal branches as observed in some conidiophores of P. glabrum, P. armarii, P. athertonense and P. bussumense. In addition, more complex patterns occur in P. saturniforme (biverticillate), P. verhagenii (biverticillate and divaricate) and P. ranomafanaense (divaricate). In common with the latter two, P. improvisum produces divaricate conidiophores. However, P. ranomafanaense and P. verhagenii produce rough-walled conidia in contrast to the smooth-walled conidia of P. improvisum. Additionally, P. verhagenii produces larger conidia (3.5–4 μm) than P. improvisum, while P. ranomafanaense has more ellipsoidal conidia in comparison to the globose conidia of the new species.
Penicillium section Canescentia— Fig. 3
Penicillium corvianum Visagie & Seifert, sp. nov. — MycoBank MB815770; Fig. 11
Fig. 11.
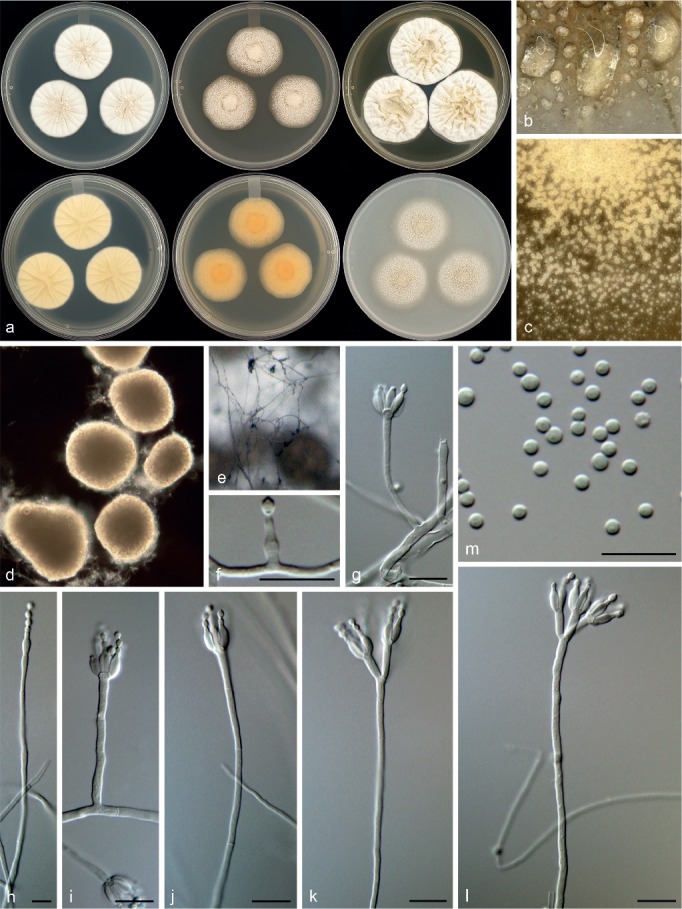
Morphological characters of Penicillium corvianum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d. sclerotia; e–l. conidiophores; m. conidia. — Scale bar: e–k = 10 μm.
In: subgenus Penicillium, section Canescentia.
ITS barcode: KT887875. Alternative identification markers: BenA = KT887836, CaM = KT887797.
Etymology. Latin, corvianum, named after Corviano, Italy, where the isolates originate.
Type specimen. ITALY, Tuscany, Natural Monument Corviano, soil, 27 Aug. 2008, K.A. Seifert (holotype DAOM 695764, culture ex-type DAOMC 250517 = CBS 141000 = KAS 3618 = IT-2008-4-D).
Colony morphology — Colony diam, 7 d, in mm: CYA 30–35; CYA 37 °C no growth; MEAbl (17–)28–32; YES 36–40; OA 30–32; CREA 21–23.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate, brown to brownish yellow sclerotia present; margins moderately deep, wide, entire; mycelia white; texture floccose; sporulation absent to sparse, conidia en masse dull green (26D4); soluble pigments absent; exudates clear; reverse yellowish white to pale yellow (2A2–3), orange (5A5). MEA 25 °C, 7 d: Colonies low, plane, creamish to brown sclerotia; margins low, narrow, entire; mycelia white; texture floccose; sporulation absent, sparse in some colonies, conidia en masse dull green (26D4); soluble pigments absent; exudates absent; reverse pale yellow to light orange (4A3–5A4), orange (6B7) in some colony areas. YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, crateriform, brownish sclerotia sparsely produced; margins low, narrow, entire; mycelia white; texture floccose; sporulation absent to sparse, conidia en masse greenish grey (26B2); soluble pigments absent; exudates clear; reverse brownish orange to light brown (5C4–7C8–D8), pale yellow to light yellow (4A3–4). OA 25 °C, 7 d: Colonies low, plane, cream to brown sclerotia produced; margins low, wide, entire; mycelia white; texture floccose; sporulation absent to sparse, conidia en masse greenish grey (26B2); soluble pigments absent; exudates clear. CREA 25 °C, 7 d: Weak growth, acid not produced.
Conidiophores on MEA very sparse, monoverticillate, minor proportion biverticillate and also phialides born directly on hyphae, on CYA biverticilllate, sometimes monoverticillate; stipes smooth, (20–)100–400 × 1.5–2.5 μm; metulae divergent, 2–4 per stipe, (7.5–)9.5–15 × 2–3(–3.5) μm; phialides ampulliform, when not monophialidic 3–8 per metula/stipe, 6–9 × 2–3 μm (7.3 ± 0.7 × 2.7 ± 0.3); average length metula/phialide 1.6; conidia smooth walled, globose to subglobose, 2–3 × 2–3 μm (2.1 ± 0.2 × 2.1 ± 0.2), average width/length = 0.96, n = 41. Sclerotia 130–265 × 110–230 μm.
Extrolites — fiscalin C, trichodermamide C, mevastatin, uncharacterised C18H22O5, C29H20O7S2, C11H15O3N.
Additional material examined. ITALY, Tuscany, Natural Monument Corviano, soil, 27 Aug. 2008, K.A. Seifert, culture DAOMC 250518 (= CBS 141001 = KAS 3622 = IT-2008-4-A).
Notes — Penicillium corvianum is classified in section Canescentia. Phylogenies (Fig. 3) resolve it in a larger clade with P. canescens, P. jensenii and P. janczewskii. Colonies of the new species are dominated by the presence of abundant cream to brown sclerotia. Sclerotia in the section are not a common feature and are only known from P. coralligerum and P. novaezeelandiae. However, sclerotia generally do not dominate colony appearance in the latter two species. Also, their conidiophores are consistently symmetrically biverticillate compared to the more diverse range of conidiophore branching observed in P. corvianum.
Penicillium nucicola Visagie, Malloch & Seifert, sp. nov. — MycoBank MB815771; Fig. 12
Fig. 12.
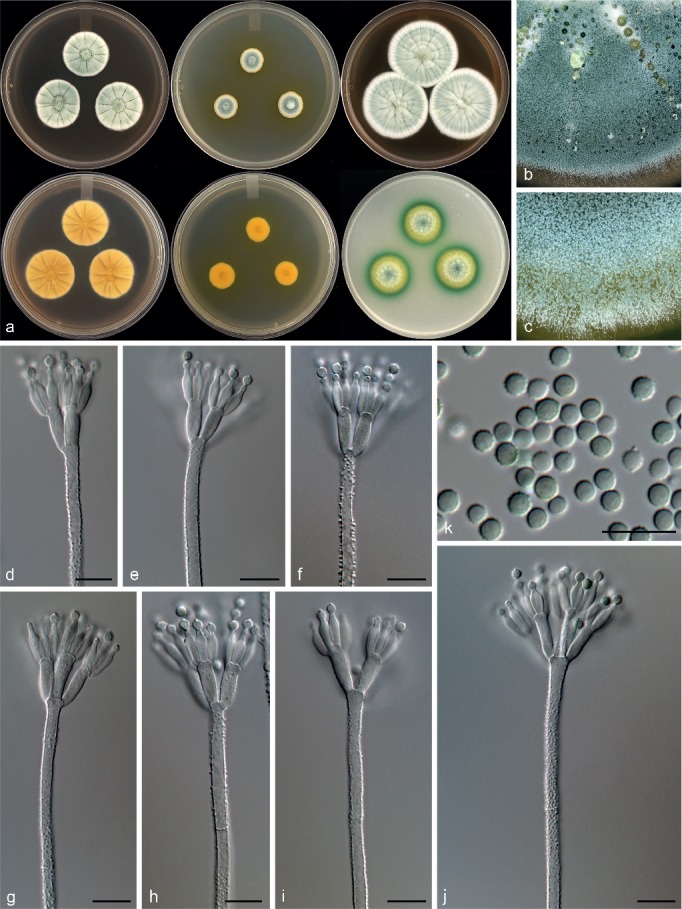
Morphological characters of Penicillium nucicola. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Penicillium, section Canescentia.
ITS barcode: KT887860. Alternative identification markers: BenA = KT887821, CaM = KT887782.
Etymology. Latin, nucicola (from nux), meaning living on nuts.
Type specimen. CANADA, Ontario, Niagara falls, fallen nuts of Carya ovata, May 1996, D. Malloch (holotype DAOM 695770, culture ex-type DAOMC 250522 = CBS 140987 = W 59 = KAS 2203).
Colony morphology — Colony diam, 7 d, in mm: CYA 20–23; CYA 37 °C no growth; MEAbl 12–14; YES 37–40; OA 17–20; CREA 13–15.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins moderately deep, wide, entire; mycelia white, inconspicuously yellow; texture floccose; sporulation moderately dense, conidia en masse greyish green (26D5–E5–E6); soluble pigments reddish pink, yellow halo close to colonies, sometimes absent; exudates clear; reverse greyish yellow to olive (4B4–D7), pale yellow (4A3), sometimes deep green (27E8) at centre. MEA 25 °C, 7 d: Colonies low, plane; margins low, narrow, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse greyish green (26D5–E5–E6); soluble pigments deep yellow, inconspicuous sometimes; exudates absent; reverse olive brown (4D6–E6), light yellow to greyish yellow (4A4–B6). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate; margins low, narrow, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse greyish green (26D5–E5–E6); soluble pigments reddish brown, yellow halo close to colonies, absent sometimes; exudates absent; reverse light yellow (3A5), greyish yellow (4C4). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white, yellow; texture floccose; sporulation moderately dense, conidia en masse greyish green (26D5–E5–E6); soluble pigments yellow close to colonies, deep green surrounding yellow pigment; exudates absent. CREA 25 °C, 7 d: Weak growth, acid moderately produced.
Conidiophores biverticillate; stipes rough, some warted, 180–890 × 2.5–4 μm; metulae divergent, 2–6 per stipe, 10.5–15 × 3–4 μm; phialides ampulliform, 4–10 per metula, 7.5–10.5 × 2.5–3.5 μm (8.9 ± 0.8 × 2.9 ± 0.2); average length metula/phialide 1.4; conidia rough walled, some finely rough, globose to subglobose, 2.5–3 × 2.5–3 μm (2.8 ± 0.2 × 2.7 ± 0.2), average width/length = 0.97, n = 56.
Extrolites — andrastin A, andrastin B, andrastin C, andrastin D, austin, uncharacterised C20H20O6.
Additional material examined. CANADA, Ontario, 5 km NW of Milton, fallen nuts of Fagus grandifolia, 24 Oct. 1995, D. Malloch, DAOMC 250521 (= CBS 140973 = KAS 2101 = W 109).
Notes — Classified in section Canescentia, P. nucicola sits on a well-supported branch distinct from all other species (Fig. 3). Morphologically it resembles P. atrovenetum, P. antarcticum, P. coralligerum, P. novaezeelandiae and P. herquei (the latter classified in section Sclerotiora). These species commonly produce broad, symmetrical biverticillate conidiophores with bright yellow and green colours often evident in colonies, as observed for P. nucicola on OA (Fig. 12). However, the restricted growth on MEA (12–14 mm diam) easily distinguishes P. nucicola from all of these morphologically similar species.
Penicillium section Charlesia & Stolkia — Fig. 4
Penicillium alogum Visagie, Frisvad & Seifert, sp. nov. — MycoBank MB815772; Fig. 13
Fig. 13.
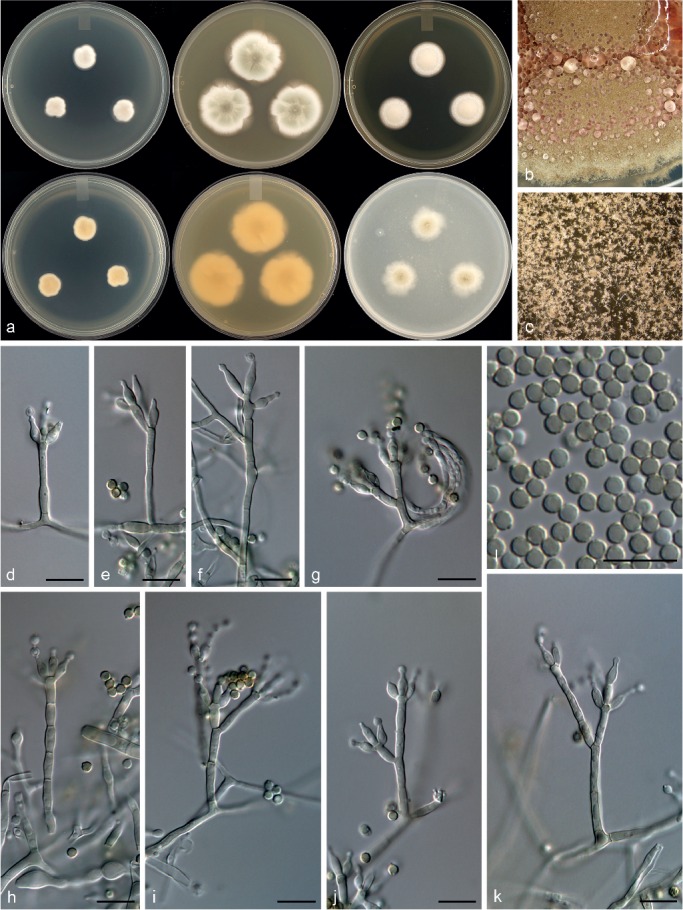
Morphological characters of Penicillium alogum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–k. conidiophores; l. conidia. — Scale bar: d–l = 10 μm.
In: subgenus Aspergilloides, section Stolkia.
ITS barcode: KT887869. Alternative identification markers: BenA = KT887830, CaM = KT887791.
Etymology. Latin, alogum, meaning irrational, irregular, nonsensical. Named for the irregular conidiophore branching observed in the species.
Type specimen. USA, Wyoming, Middle Mountain, Wind River Range, Tundra wetland soil, June 2001, M. Christensen (holotype DAOM 695759, culture ex-type DAOMC 250543 = CBS 140996 = IBT 23947= KAS 2475).
Colony morphology — Colony diam, 7 d, in mm: CYA (15–)19–22; CYA 37 °C no growth; MEAbl 31–35; YES 21–22(–29); OA 22–25; CREA 12–14.
CYA 25 °C, 7 d: Colonies deep, radially and concentrically sulcate, crateriform in some isolates; margins low, narrow, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, conidia en masse greenish grey to dull green (29D2–3); soluble pigments absent; exudates absent, after two weeks inconspicuously red present; reverse dull to greyish yellow (3B4–C4), yellowish white (3A2). MEA 25 °C, 7 d: Colonies low, plane, sometimes slightly raised centrally; margins low, wide, entire; mycelia white; texture velutinous; sporulation moderately dense to sparse, conidia en masse greyish green to dark green (29E5–F5), in some a lighter greyish green (29D4); soluble pigments absent; exudates absent, after two weeks inconspicuously red present; reverse greyish green (29E5), pale yellow (2A3), yellowish grey (2B2). YES 25 °C, 7 d: Colonies deep, plane; margins low, narrow, entire; mycelia white, orange white (5A2–6A2); texture floccose; sporulation absent, conidia en masse not determined; soluble pigments absent; exudates absent; reverse greyish orange (5B5), yellowish white (4A2). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous; sporulation moderately dense near centre, conidia en masse greyish green (30E6); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Weak growth, acid not produced.
Conidiophores monoverticillate and divaricate, sometimes solitary phialides present; stipes smooth, inconspicuous greenish brown pigment, 10–43 × 2–3 μm; metulae divergent, 2 when present, of unequal length on the same conidiophore, 7–12(–20) × 2–3 μm; phialides ampulliform, sometimes solitary and elongated to almost acerose, mostly 3–5 per metula, 5.5–7(–8) × 2.5–3 μm (6.4 ± 0.5 × 2.7 ± 0.2); average length metula/phialide 1.7; conidia thick walled, finely rough walled, globose, 2.5–3 × 2.5–3 μm (2.7 ± 0.1 × 2.7 ± 0.1), average width/length = 0.96, n = 64.
Extrolite — uncharacterised C21H28O8.
Additional material examined. USA, Wind River Mountain, Wyoming, Tundra wetland soil, 1998, M. Christensen, culture DAOMC 250544 (= CBS 126173 = IBT 23948 = KAS 2476); Middle Mountain, Wind River Mountain, Wyoming, Tundra wetland soil, 1998, M. Christensen, culture DAOMC 250545 (= CBS 126150 = IBT 23950 = KAS 2477).
Notes — Penicillium alogum belong to section Stolkia and is closely related to P. stolkiae, P. subarcticum, P. boreae, P. pullum and P. canariense (Fig. 4). These species all produce conidiophores with some brown pigmentation. Penicillium boreae and P. canariense typically produce biverticillate conidiophores, while the others mainly have monoverticillate/simple conidiophore branching patterns. Penicillium pullum produces large, conspicuously rough walled conidia (3.5–4.5 μm) that turn dark with age, which easily distinguish it from P. alogum. On the other hand, P. subarcticum produces bigger conidia than the new species (3–4 μm) (Peterson & Sigler 2002). From a morphological perspective, P. alogum is most similar to P. stolkiae, having similar colony colours, growth rates and conidiophore branching patterns. However, P. stolkiae produces longer phialides (7–10 μm) and slightly larger conidia (2.8–3.5 μm) fide Pitt (1980).
Penicillium costaricense Visagie, M. Urb & Seifert, sp. nov. — MycoBank MB815773; Fig. 14
Fig. 14.
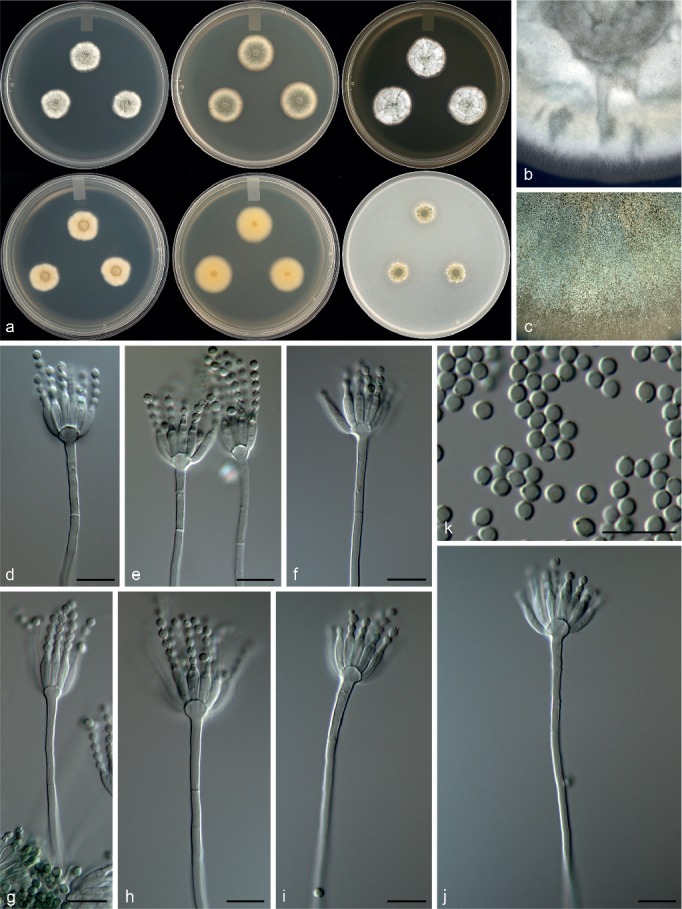
Morphological characters of Penicillium costaricense. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Aspergilloides, section Charlesia.
ITS barcode: KT887873. Alternative identification markers: BenA = KT887834, CaM = KT887795.
Etymology. Latin, costaricense, named after Costa Rica, the origin of the isolate.
Type specimen. COSTA RICA, Area de Conservación Guanacaste, Santa Rosa, intestines of Rothschildia lebeau (01-CACG-589) feeding on Spondias mombin, 19 Oct. 2001, J. Diaz (holotype DAOM 695765, culture ex-type DAOMC 250520 = CBS 140998 = KAS 2597 = 01-RGTHC-294).
Colony morphology — Colony diam, 7 d, in mm: CYA 15–17; CYA 37 °C no growth; MEAbl 19–21; YES 21–22; OA 11–12; CREA 6–7.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate, crateriform; margins low, narrow, entire; mycelia white; texture velutinous; sporulation sparse to moderately dense, conidia en masse greyish turquoise to dull green (25C4–D3); soluble pigments absent; exudates absent; reverse greyish green to olive (1D4–2D4), yellowish white to pale yellow (3A2–3). MEA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse greyish green to dark green (25E5–F5); soluble pigments absent; exudates absent; reverse greyish yellow (2B3–C4–3C5). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, crateriform; margins low, narrow, entire; mycelia white; texture velutinous; sporulation sparse to moderately dense, conidia en masse greyish green to dull green (25C4–D4); soluble pigments absent; exudates absent; reverse olive (2D3–E3), light yellow to dull yellow (3A4–B3). OA 25 °C, 7 d: Colonies low, plane; margins low, narrow, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse greyish green to dark green (25E5–F5); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Weak growth, acid not produced.
Conidiophores monoverticillate; stipes smooth, 50–280 × 2–3 μm; vesicle 4–6.5 μm; phialides ampulliform, 10–20 per stipe, 8.5–11.5 × 2–3.5 μm (9.7 ± 0.7 × 2.7 ± 0.3); conidia smooth walled, subglobose, 2.5–3 × 2–3 μm (2.6 ± 0.1 × 2.4 ± 0.1), average width/length = 0.94, n = 72.
Extrolites — andrastin A, andrastin C, uncharacterised C11H15O3N.
Notes — Penicillium costaricense is known from only the one isolate and forms a subbasal branch distinct from the main body of section Charlesia and P. georgiense, the basal species in the section (Fig. 4). The new species produces monoverticillate conidiophores, readily distinguished from the biverticillate to divaricate conidiophores of P. georgiense. The same character also distinguishes it from P. charlesii and P. fellutanum. The remaining species, P. coffeae, P. phoeniceum and P. indicum all produce monoverticillate conidiophores. However, P. indicum (20–30 mm diam) and P. phoeniceum (5–8 mm diam) are able to grow on CYA at 37 °C, in contrast to P. costaricense, which does not grow at 37 °C. Microscopically, P. costaricense most closely resembles P. coffeae, but the latter grows much slower on CYA and MEA (11–17 mm, 11–13 mm diam) than the new species.
Penicillium section Exilicaulis — Fig. 5, 6
Penicillium aotearoae Visagie & Seifert, sp. nov. — MycoBank MB815774; Fig. 15
Fig. 15.
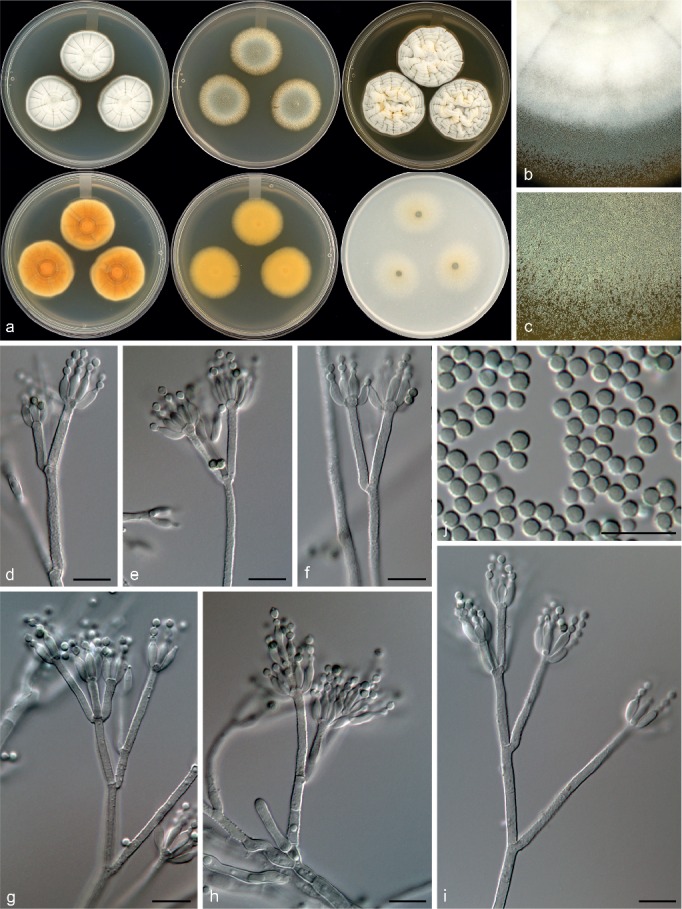
Morphological characters of Penicillium aotearoae. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–i. conidiophores; j. conidia. — Scale bar: d–j = 10 μm.
In: subgenus Aspergilloides, section Exilicaulis.
ITS barcode: KT887874. Alternative identification markers: BenA = KT887835, CaM = KT887796.
Etymology. Latin, aotearoae, from the Maori name for New Zealand, the origin of the isolate.
Type specimen. NEW ZEALAND, South Island, Arthurs Pass National Park, Devil’s Punchbowl, isol. ex clay, light yellow brown, beneath moss and Nothofagus, 18 Feb. 2003, K.A. Seifert (holotype PDD 107543, culture ex-type DAOMC 250538 = CBS 140999 = KAS 3088).
Colony morphology — Colony diam, 7 d, in mm: CYA 30–32; CYA 37 °C no growth; MEAbl 29–30; YES 35–37; OA 33–35; CREA 18–19.
CYA 25 °C, 7 d: Colonies low to moderately deep, radially and concentrically sulcate; margins low, narrow, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, conidia en masse greyish turquoise to greyish green (24D4–25D4); soluble pigments absent; exudates clear to absent; reverse brown (6D7), greyish orange (5B6), light yellow (4A5), greyish green (28C4). MEA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse greyish turquoise to greyish green (24D4–25D4); soluble pigments inconspicuously yellow; exudates absent; reverse dull yellow to olive yellow (3B4–D6). YES 25 °C, 7 d: Colonies deep, radially and concentrically sulcate, crateriform; margins low, narrow, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, conidia en masse greyish green (25B3–C4); soluble pigments inconspicuously yellow; exudates absent; reverse brown (7E7), orange to brownish orange (6B8–C8), yellowish orange (4A6). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous; sporulation sparse to moderately dense, conidia en masse dull green (26E4); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Strong growth, acid weakly produced.
Conidiophores biverticillate, divaricate, some monoverticillate; stipes rough to finely rough, 115–180 × 2–3 μm; branches 16.5–46 μm; metulae divergent, 2–5 per stipe, 9.5–24 × 2–3 μm; phialides ampulliform, 6–20 per metula, 6.5–9 × 2–3 μm (7.5 ± 0.6 × 2.4 ± 0.2); average length metula/phialide 2.1; conidia finely rough walled, globose, 2–3 × 2–3 μm (2.5 ± 0.1 × 2.4 ± 0.1), average width/length = 0.96, n = 67.
Extrolites — uncharacterised C19H24O8.
Notes — Penicillium aotearoae is classified in section Exilicaulis and is closely related to P. melinii, P. ‘fynbos1’ (an undescribed species from the fynbos in South Africa, Visagie et al. 2016b) and P. diabolicalicense described below (Fig. 5, 6). The new species can be distinguished from the others based on colony growth rates. It grows more restrictedly than the fynbos species on MEA, but faster on MEA compared to P. melinii, and faster on CYA but more restricted on MEA compared to P. diabolicalicense.
Penicillium diabolicalicense Visagie & Seifert, sp. nov. — MycoBank MB815775; Fig. 16
Fig. 16.
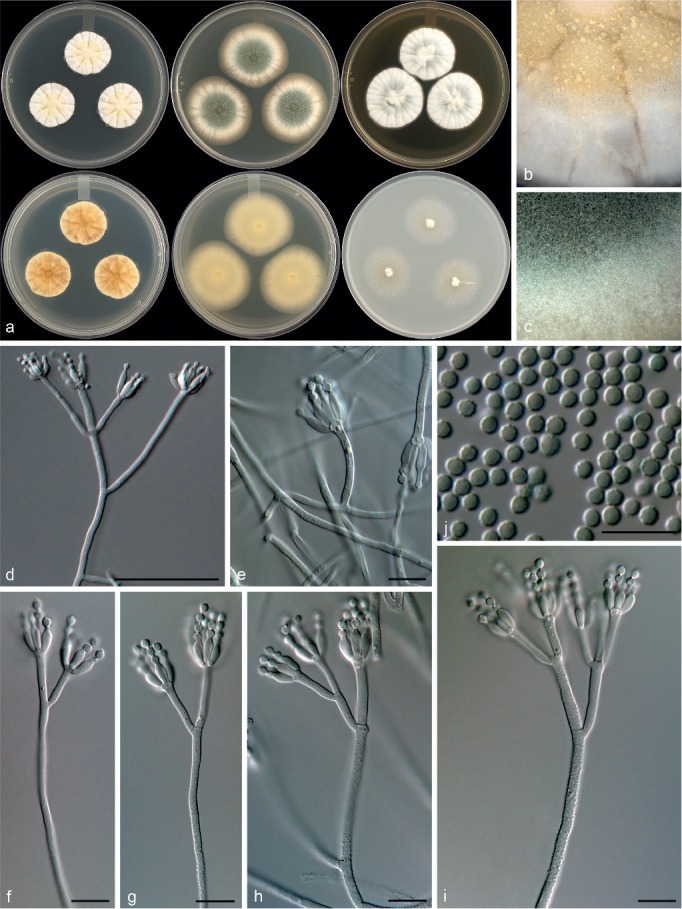
Morphological characters of Penicillium diabolicalicense. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–i. conidiophores; j. conidia. — Scale bar: d = 50 μm, e–j = 10 μm.
In: subgenus Aspergilloides, section Exilicaulis.
ITS barcode: KT887840. Alternative identification markers: BenA = KT887801, CaM = KT887762.
Etymology. Latin, diabolicalicense, meaning Devil’s Punchbowl, the origin of type strain – diabolus, diaboli = devil; calix, calicis = drinking bowl.
Type specimen. NEW ZEALAND, Arthurs Pass National Park, Devil’s Punchbowl, light yellow brown, beneath moss and Nothofagus, 18 Feb. 2003, Keith Seifert (holotype PDD 107542, culture ex-type DAOMC 250542 = CBS 140967 = KAS 1726).
Colony morphology — Colony diam, 7 d, in mm: CYA 20–25; CYA 37 °C no growth; MEAbl 36–40; YES 31–34; OA 30–31; CREA 20–21.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins low, narrow, entire; mycelia white, deep yellow; texture floccose; sporulation absent to sparse, conidia en masse not determined; soluble pigments absent; exudates absent; reverse brown (5F6), light brown (5D3), pale yellow to greyish yellow (4A3–B3). MEA 25 °C, 7 d: Colonies moderately deep, plane; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse dark green (25F6–26F6); soluble pigments absent; exudates absent; reverse dull yellow to greyish green (3B3–29B3–C3), yellowish white (2A2–3A2). YES 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins moderately deep, narrow, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, conidia en masse greenish grey to greyish green (25B2–D4); soluble pigments absent; exudates absent; reverse greyish orange (5B6), pale yellow to light yellow to yellowish orange (4A3–6). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous; sporulation sparse to moderately dense, conidia en masse greyish green (25E5); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Strong growth, acid strongly produced.
Conidiophores biverticillate, often divaricate; stipes rough, 200–800 × 2–3 μm; branches 20–68 μm; metulae divergent, 2–4 per stipe, unequal length on same conidiophore, 12–26 × 2–3 μm; phialides ampulliform, 4–8 per metula, (5.5–)6.5–8(–9) × 2.5–3 μm (7.2 ± 0.7 × 2.7 ± 0.2); average length metula/phialide 2.1; conidia rough walled, subglobose, minor proportion globose, 2–3 × 2–2.5 μm (2.6 ± 0.2 × 2.5 ± 0.0), average width/length = 0.96, n = 41.
Extrolites — N,N-dimethylrestricticin (CYA), penitrem A (CYA), restricticin.
Notes — Penicillium diabolicalicense is classified in section Exilicaulis and is closely related to P. melinii, P. ‘fynbos1’ (Visagie et al. 2016b) and P. aotearoae described above (Fig. 5, 6). The new species can easily be distinguished from its close relatives based on its more restricted growth on CYA at 25 °C compared to the fynbos species, faster growth on MEA compared to P. melinii, and growth slower on CYA but faster growth on MEA compared to P. aotearoae.
Penicillium fundyense Visagie, David Clark & Seifert, sp. nov. — MycoBank MB815776; Fig. 17
Fig. 17.
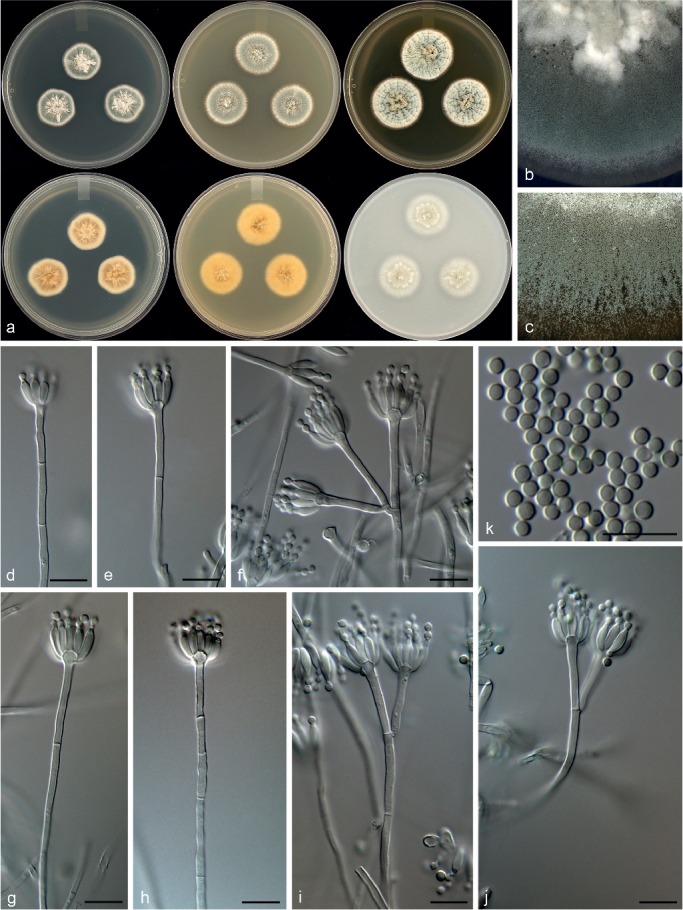
Morphological characters of Penicillium fundyense. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Aspergilloides, section Exilicaulis.
ITS barcode: KT887853. Alternative identification markers: BenA = KT887814, CaM = KT887775.
Etymology. Latin, fundyense, named after the Bay of Fundy, origin of the strain.
Type specimen. CANADA, New Brunswick, St. Andrews, Carpenter ants (Camponotus pennsylvanicus), Aug. 2000, D. Clark (holotype DAOM 695767, culture ex-type DAOMC 250519 = CBS 140980 = NBBR-2-3 = W466 = KAS 2174).
Colony morphology — Colony diam, 7 d, in mm: CYA 20–21; CYA 37 °C no growth; MEAbl 22–24; YES 28–30; OA 25–26; CREA 14–15.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate, crateriform; margins low, narrow, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse dull green to greyish green (25E4–5); soluble pigments absent; exudates absent; reverse olive (3D4), yellowish white (2A2). MEA 25 °C, 7 d: Colonies low, lightly sulcate centrally; margins low, narrow, entire; mycelia white; texture velutinous; sporulation moderately dense, conidia en masse dull green to greyish green (25E4–5); soluble pigments absent; exudates absent; reverse olive (3E4), greyish yellow (3B4–C4). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, crateriform; margins low, narrow, entire; mycelia white; texture velutinous; sporulation sparse to moderately dense, conidia en masse greyish green (25B3–C4); soluble pigments absent; exudates deep yellow, inconspicuous; reverse brownish orange (5C6), light yellow to greyish yellow (4A4–B5). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous; sporulation sparse to moderately dense, conidia en masse greyish green to dull green (26C3–D3); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Strong growth, acid not produced.
Conidiophores monoverticillate, biverticillate and divaricate both common, often difficult to distinguish between branches and metulae; stipes smooth, 15–200(–400) × 2–2.5 μm; branches 16–30(–47) μm; metulae divergent, 2–4 per stipe, 16–30 × 2–2.5 μm; phialides ampulliform, 8–16 per metula, 6–8.5 × 2–2.5 μm (7.4 ± 0.7 × 2.4 ± 0.2); average length metula/phialide 3.3; conidia smooth walled, globose, 2–3 × 2–3 μm (2.4 ± 0.2 × 2.4 ± 0.2), average width/length = 0.96, n = 46.
Extrolites — dehydrohistidyltryptophanyldiketopiperazine, meleagrin, N6-formyl-roquefortine C, neoxaline, oxaline, roquefortine C, roquefortine M.
Notes — Penicillium fundyense is classified in section Exilicaulis, with phylogenies resolving it in a clade with P. cinerascens, P. citreonigrum and P. citreosulfuratum. The species of this complex are almost impossible to distinguish morphologically, but chemically they represent distinct species, which resulted in Visagie et al. (2016b) suggesting the use of ITS and BenA sequences for identifications of strains in this clade. This approach is followed here, because P. fundyense shows only minor morphological differences from its close relatives, but is phylogenetically distinct from the other three.
Penicillium section Lanata-Divaricata — Fig. 7, 8
Penicillium amphipolaria Visagie, Malloch & Seifert, sp. nov. — MycoBank MB815777; Fig. 18
Fig. 18.
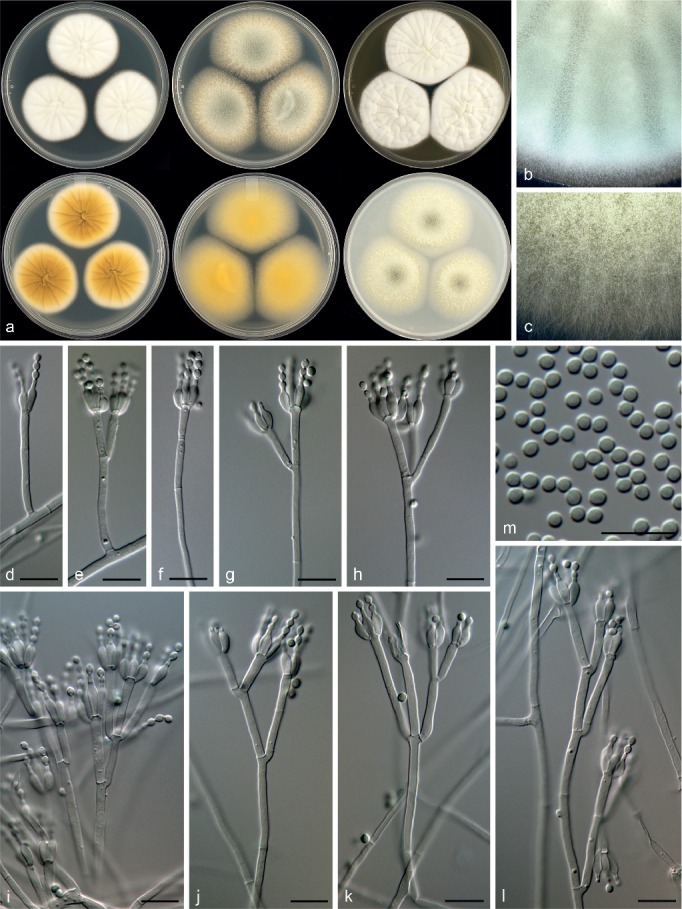
Morphological characters of Penicillium amphipolaria. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–l. conidiophores; m. conidia. — Scale bar: d–m = 10 μm.
In: subgenus Aspergilloides, section Lanata-Divaricata.
ITS barcode: KT887872. Alternative identification markers: BenA = KT887833, CaM = KT887794.
Etymology. Latin, amphipolaria, meaning both poles, named to indicate the origin of the two strains on opposite sides of the Earth, close to the north and south poles.
Type specimen. ANTARCTICA, Quartermain Mountains of Antarctic Dry Valleys (-77.842741° 161.122229°), soil, Jan. 1998, W.C. Mahaney (holotype DAOM 695760, culture ex-type DAOMC 250551 = CBS 140997 = W 284 = KAS 2555).
Colony morphology — Colony diam, 7 d, in mm: CYA (30–)36–39; CYA 37 °C 5–7; MEAbl 50–52; YES 48–51; OA 45–50; CREA 24–29.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white, inconspicuously yellow; texture floccose; sporulation very sparse, moderate in the ex-type, conidia en masse dull green (26D4) in the ex-type; soluble pigments absent; exudates clear to absent; reverse greyish yellow to olive brown (4C6–D6), light yellow (2A4), pale yellow (3A3) in DAOMC 250550. MEA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture floccose and velutinous; sporulation moderately dense, conidia en masse dull green (26D3–4); soluble pigments deep yellow, absent in DAOMC 250550; exudates absent; reverse yellow to olive yellow (3B6–C6), greenish grey (1B2) in KAS1396. YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, raised at centre; margins low, wide, entire; mycelia white, inconspicuously yellow; texture floccose; sporulation very sparse, moderately dense in the ex-type, conidia en masse greenish grey to dull green (26B3–D4); soluble pigments absent; exudates absent; reverse greyish yellow to olive brown (4C6–D6), light yellow (2A4), pale yellow (3A3) in DAOMC 250550. OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture floccose and velutinous; sporulation moderately dense, conidia en masse greenish white to dull green (26B2–D3–E3); soluble pigments yellow, absent in DAOMC 250550; exudates absent. CREA 25 °C, 7 d: Strong growth, acid moderately produced.
Conidiophores biverticillate and divaricate, minor proportion monoverticillate; stipes smooth, 240–460 × 2–2.5 μm; branches 12–42 μm; metulae divergent, 2–4 per stipe, 9.5–21 × 2–3 μm; phialides ampulliform, 3–6 per metula, 6.5–10 × 2–3 μm (7.4 ± 0.8 × 2.5 ± 0.8); average length metula/phialide 2.1; conidia smooth walled, mainly broadly ellipsoidal, some globose, 2–3 × 2–2.5 μm (2.4 ± 0.1 × 2.2 ± 0.1), average width/length = 0.9, n = 78.
Extrolites — fumitremorgin B, fusaperazine E, fusaperazine related (C20H27N3O4S), oxaline, uncharacterised C22H23O4N3, C27H31O7N3.
Additional material examined. CANADA, Quebec, peat moss factory, 2001, C. Duchaine, cultures DAOMC 250549 (= CBS 140965 = KAS 1392 = R-273), DAOMC 250550 (= CBS 140966 = KAS 1396 = R-293).
Notes — Penicillium amphipolaria is classified in section Lanata-Divaricata and is a close relative of P. singorense (Fig. 7, 8). The new species can easily be distinguished from its close relatives by its poor growth on CYA at 37 °C, in contrast to the strong growth of P. singorense (40–43 mm diam) (Visagie et al. 2014a). Also, P. amphipolaria has a more complex conidiophore branching pattern compared to the generally simple conidiophores of P. singorense (Visagie et al. 2014a).
Penicillium bissettii Visagie & Seifert, sp. nov. — MycoBank MB815778; Fig. 19
Fig. 19.
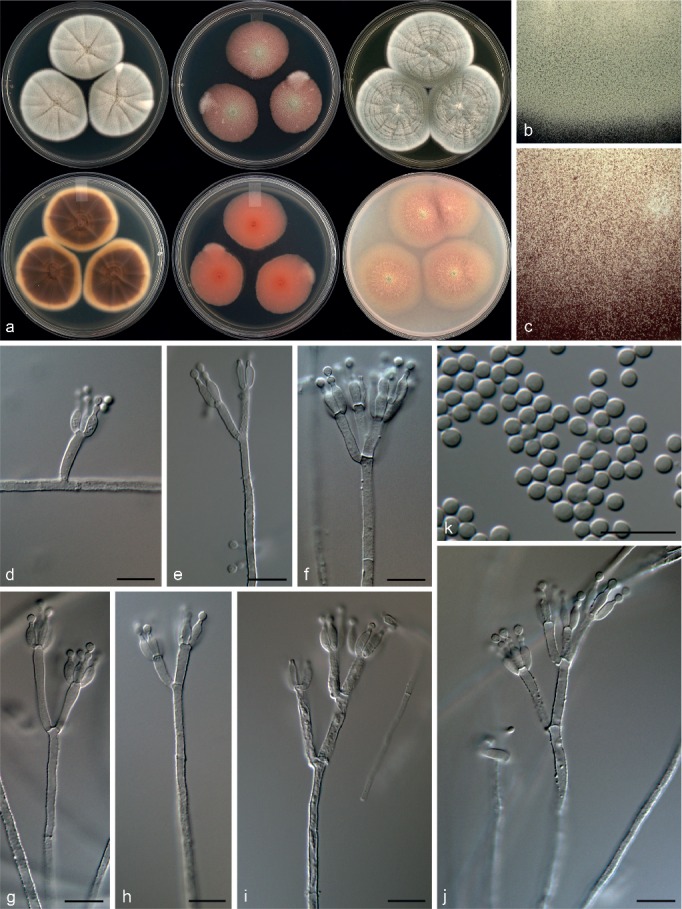
Morphological characters of Penicillium bissettii. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Aspergilloides, section Lanata-Divaricata.
ITS barcode: KT887845. Alternative identification markers: BenA = KT887806, CaM = KT887767.
Etymology. Latin, bissettii, named after John Bissett, recently retired mycologist, AAFC Ottawa, who collected and isolated the strains, in honour of his contributions to soil fungus ecology.
Type specimen. CANADA, Quebec, near Lacolle, soil from Spruce forest, 5 Apr. 1977, J. Bissett (holotype DAOM 695761, culture ex-type DAOMC 167011 = CBS 140972 = KAS 1951).
Colony morphology — Colony diam, 7 d, in mm: CYA 42–43; CYA 37 °C no growth; MEAbl 35–43; YES 47–52; OA 45–48; CREA 23–26.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense, sparse in DAOMC 167033, conidia en masse greyish green to dull green (26C3–D4), greenish grey (26B2); soluble pigments absent; exudates absent; reverse brown to dark brown (7E6–F6), yellowish white to greyish yellow (4A2–B4). MEA 25 °C, 7 d: Colonies low, plane, pinkish red underneath mycelial and sporulating areas; margins low, wide, entire; mycelia white, red submerged in media; texture floccose and velutinous; sporulation absent, sparse in some colonies, conidia en masse greyish green to dull green (26D4–E5), greenish grey (26B2); soluble pigments absent; exudates absent; reverse greyish red to reddish brown (8B6–D6). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, some strains sporulating in concentrical rings; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense, absent in some strains, conidia en masse dull green (26D4–E4), greenish white (26B2); soluble pigments absent; exudates absent; reverse brownish orange (6C5), light yellow to greyish yellow (4A3–B5). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture velutinous and floccose; sporulation sparse, conidia en masse greenish grey to greyish green (26B2–3); soluble pigments pink, absent in DAOMC 167033; exudates absent. CREA 25 °C, 7 d: Weak growth, acid not produced.
Conidiophores biverticillate, terverticillate, minor proportion divaricate; stipes rough, 190–670 × 2.5–3.5 μm; branches 14–25 μm; metulae divergent, 3–4 per stipe or branch, 11–15.5 × 2.5–3.5 μm; phialides ampulliform, 3–6 per metula, 6.5–9 × 2.5–3 μm (7.5 ± 0.7 × 2.6 ± 0.2); average length metula/phialide 1.8; conidia smooth walled, globose to subglobose, 2–3 × 2–3 μm (2.6 ± 0.2 × 2.4 ± 0.1), average width/length = 0.92, n = 72.
Extrolites — DAOMC 167033: penicillic acid, uncharacterised C14H19O4N. DAOMC 167011: aurantioclavine, dehydrohistidyltryptophanyldiketopiperazine, glandicoline B, meleagrin, oxalicine B, roquefortine C, roquefortine M, roquefortine N.
Additional material examined. CANADA, Quebec near Lacolle, soil from Spruce forest, 5 Apr. 1977, J. Bissett, culture DAOMC 167033 (= CBS 140971 = KAS 1949).
Notes — Penicillium bissettii is classified in section Lanata-Divaricata in a clade distantly related to all other species in the section. The multigene phylogeny places it closest to P. vasconiae and P. annulatum (Fig. 7, 8), but on a poorly supported backbone. Penicillium bissettii and P. vasconiae share the production of red mycelia submerged in the media (Ramírez & Martinez 1980). However, P. vasconiae has smooth walled conidiophores, in comparison with the roughened conidiophores of the new species. Penicillium annulatum grows well on CYA at 37 °C in contrast to P. bissettii, which does not grow at this temperature.
Penicillium camponotum Visagie, David Clark & Seifert, sp. nov. — MycoBank MB815779; Fig. 20
Fig. 20.
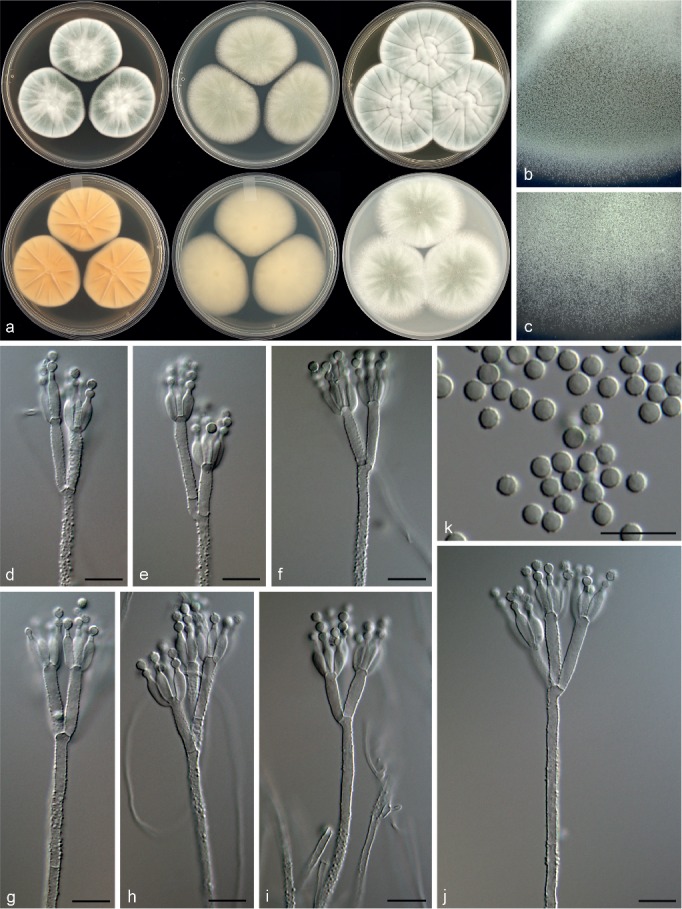
Morphological characters of Penicillium camponotum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Aspergilloides, section Lanata-Divaricata.
ITS barcode: KT887855. Alternative identification markers: BenA = KT887816, CaM = KT887777.
Etymology. Latin, camponotum , named in relation to genus of Carpenter ants that the species was isolated from.
Type specimen. CANADA, New Brunswick, St. Andrews, Carpenter ants (Camponotus pennsylvanicus), Aug. 2000, D. Clark (holotype DAOM 695762, culture ex-type DAOMC 250557 = CBS 140982 = NBBR-2-1 = W 471 = KAS 2177).
Colony morphology — Colony diam, 7 d, in mm: CYA (23–)37–45; CYA 37 °C no growth to microcolonies; MEAbl 48–55(–60); YES 53–60; OA 49–52; CREA 20–25.
CYA 25 °C, 7 d: Colonies moderately deep, lightly radially sulcate; margins low, narrow, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, absent in some strains, conidia en masse greyish green to dull green (25C4–E4–26E4); soluble pigments reddish brown, absent in some isolates; exudates clear; reverse greyish yellow (4B4–5), yellowish white to pale yellow (3A2–3), brownish orange (5C5–6C5) in isolates with soluble pigments. MEA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse dull green (26D3–4–E4); soluble pigments absent; exudates absent; reverse greenish grey to greyish green (30B2–B3), yellowish white (2A2). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate; margins low, wide, entire; mycelia white, inconspicuously pinkish grey; texture floccose; sporulation moderately dense, conidia en masse dull green to greyish green (25D4–26D4–E5); soluble pigments absent; exudates absent; reverse brownish orange (5C3), greyish orange (5B4–5), pale yellow to light yellow (4B3–5). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture floccose; sporulation absent to sparse to moderately dense, conidia en masse greyish green to dull green (26C3–D3–4); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Weak growth, acid moderately produced.
Conidiophores biverticillate, minor proportion divaricate; stipes rough, 220–620 × 2.5–3.5 μm; branches 13–44 μm; metulae divergent, 2–4 per stipe, sometimes of unequal length on same conidiophore, 12–26 × 2.5–4 μm; phialides ampulliform, 4–8 per metula, 8–11.5 × 2.5–3.5 μm (9.5 ± 0.8 × 2.9 ± 0.2); average length metula/phialide 1.7; conidia rough to finely rough walled, globose, 2.5–3.5 × 2.5–3.5 μm (2.9 ± 0.1 × 2.9 ± 0.1), average width/length = 0.97, n = 72.
Extrolites — andrastin B, andrastin C, andrastin D, uncharacterised C15H22O2 (only New Brunswick strains), andrastin A (only German strains), marcfortine A, marcfortine B (all strains).
Additional material examined. CANADA, New Brunswick, St. Andrews, Carpenter ants (Camponotus pennsylvanicus), Aug. 2000, D. Clark, culture DAOMC 250552 (= CBS 140975 = NBCP-17-4 = W 447 = KAS 2166), DAOMC 250553 (= CBS 140977 = NBCP-9-1 = W 451 = KAS 2169), DAOMC 250554 (= CBS 140978 = NBCP-1-1 = W 452 = KAS 2170), DAOMC 250555 (= CBS 140979 = NBCP-17-4B = W 463 = KAS 2172), DAOMC 250556 (= CBS 140981 = NBCP-23-1 = W 469 = KAS 2176), DAOMC 250558 (= CBS 140984 = NBCP-5-1 = W 477 = KAS 2182). – GERMANY, Grosse-Pfahl, Bayern, ant nest in Picea abies, Aug. 2000, D. Clark, DAOMC 250559 (= CBS 140985 = TP-4-1= W 498 = KAS 2193); Pfahl Viechtach, Bayern, Carpenter ants (Camponotus herculeanus), Aug. 2000, D. Malloch, culture DAOMC 250560 (= CBS 140986 = TPF-8-3 = W 512 = KAS 2199).
Notes — Penicillium camponotum is classified in section Lanata-Divaricata as a close relative to P. subrubescens (Fig. 7, 8). However, the new species produces weak acid on CREA, which is absent in P. subrubescens (Mansouri et al. 2013). Also, P. camponotum has larger globose conidia compared to the smaller subglobose to broadly ellipsoidal conidia of P. subrubescens.
Penicillium cataractum Visagie, Malloch & Seifert, sp. nov. — MycoBank MB815780; Fig. 21
Fig. 21.
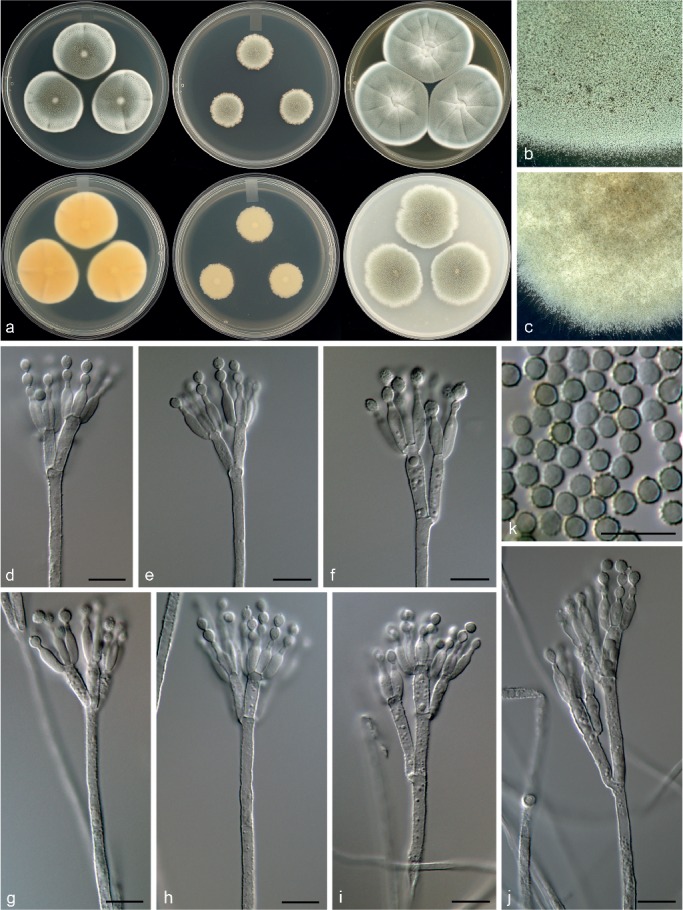
Morphological characters of Penicillium cataractum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Aspergilloides, section Lanata-Divaricata.
ITS barcode: KT887847. Alternative identification markers: BenA = KT887808, CaM = KT887769.
Etymology. Latin, cataractum, meaning waterfall, reflecting the origin of the strains, Niagara Falls, Ontario.
Type specimen. CANADA, Niagara falls, on fallen nuts of Carya cordiformis, May 1996, D. Malloch (holotype DAOM 695763, culture ex-type DAOMC 250534 = CBS 140974 = W 4 = KAS 2145).
Colony morphology — Colony diam, 7 d, in mm: CYA 34–42; CYA 37 °C no growth; MEAbl 15–25; YES 45–60; OA 28–37; CREA 13–15.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, conidia en masse dull green (26E4), greyish green (25C3); soluble pigments absent; exudates clear; reverse greyish yellow to greyish orange (4B4–5B4), pale yellow (4A2). MEA 25 °C, 7 d: Colonies low to moderately deep, plane; margins low, wide, irregular; mycelia white; texture floccose; sporulation moderately dense, conidia en masse dull green (25D4–E4); soluble pigments absent; exudates absent; reverse greyish yellow to greyish orange (4B4–5B4), pale yellow (4A2), yellowish grey (4B2). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, raised at centre; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse greyish green to dull green (25C4–E5); soluble pigments absent; exudates absent; reverse greyish yellow (4B5), pale yellow to light yellow (4A3–4). OA 25 °C, 7 d: Colonies low, plane, orange colour in some isolates underneath sporulation; margins low, wide, entire; mycelia white; texture floccose and velutinous; sporulation sparse to moderately dense, conidia en masse dull green (26D4–E4); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Moderate growth, acid strongly produced.
Conidiophores biverticillate, terverticillate, minor proportion divaricate; stipes rough, (120–)200–650 × 3–4 μm; branches 13–30 μm; metulae divergent, 2–5 per stipe or branch, 11–20 × 3–4 μm; phialides ampulliform, 5–10 per metula, 8–10.5 × 3–3.5 μm (9.5 ± 0.7 × 3.1 ± 0.2); average length metula/phialide 1.4; conidia rough walled, globose to minor proportion broadly ellipsoidal, 3–3.5 × 3–3.5 μm (3.3 ± 0.1 × 3.3 ± 0.1), average width/length = 0.95, n = 60.
Extrolites — andrastin A, andrastin B, andrastin C, andrastin D, pulvilloric acid, trichodermamide A, trichodermamide C, uncharacterised C29H20O7S2, C20H30O6N2.
Additional material examined. CANADA, Niagara falls, on fallen nuts of Carya ovata, May 1996, D. Malloch, culture DAOMC 250533 (= CBS 140991 = W 6 = KAS 2270); Niagara falls, on fallen nuts of Carya cordiformis, May 1996, D. Malloch, culture DAOMC 250535 (= CBS 140992 = W 7 = KAS 2271).
Notes — Penicillium cataractum belongs to section Lanata-Divaricata and is a close relative of P. mariae-crucis and the new species P. infrabuccalum (Fig. 7, 8). Penicillium mariae-crucis produces sclerotia and reddish brown colonies, which are absent in both of the closely related new species. Penicillium cataractum has more restricted growth on MEA and has larger conidia than P. infrabuccalum.
Penicillium infrabuccalum Visagie, David Clark & Seifert, sp. nov. — MycoBank MB815782; Fig. 22
Fig. 22.
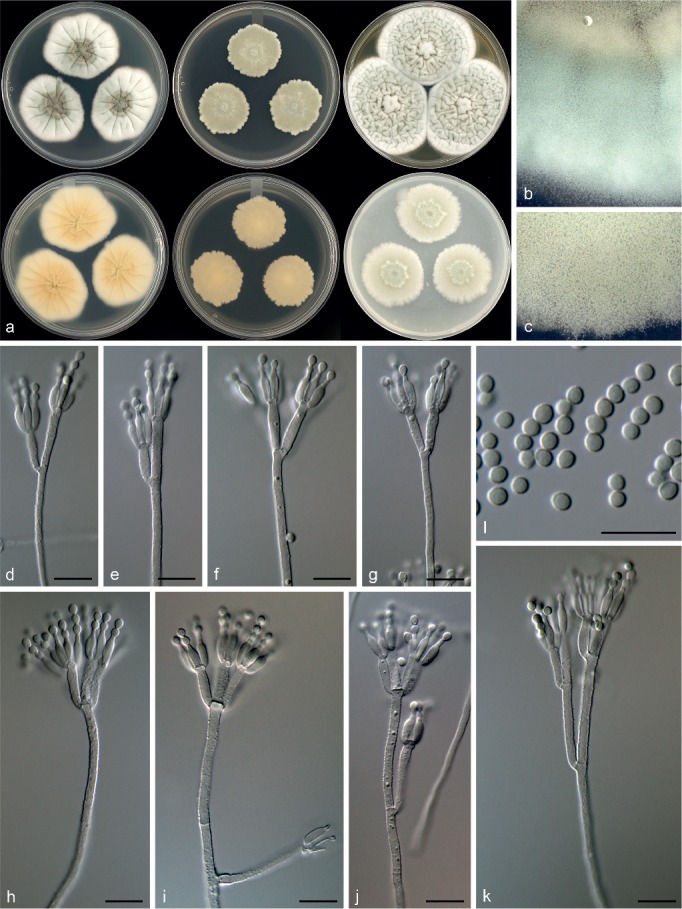
Morphological characters of Penicillium infrabuccalum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–k. conidiophores; l. conidia. — Scale bar: d–l = 10 μm.
In: subgenus Aspergilloides, section Lanata-Divaricata.
ITS barcode: KT887856. Alternative identification markers: BenA = KT887817, CaM = KT887778.
Etymology. Latin, infrabuccalum, meaning from infrabuccal pockets of the Carpenter ant Camponotus pennsylvanicus, the niche of this fungus.
Type specimen. CANADA, New Brunswick, St. Andrews, Carpenter ants (Camponotus pennsylvanicus), Aug. 2000, D. Clark (holotype DAOM 695769, culture ex-type DAOMC 250537 = CBS 140983 = NBSM-6-2 = W 475 = KAS 2181).
Colony morphology — Colony diam, 7 d, in mm: CYA 37–45; CYA 37 °C no growth; MEAbl 29–31; YES 54–58; OA 35–37; CREA 28–30.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, conidia en masse dull green (26E4–27E3–4), greenish white (25A2); soluble pigments absent; exudates clear; reverse greyish orange (5B3), greyish yellow (4B3), pale yellow (3A2). MEA 25 °C, 7 d: Colonies low to moderately deep, plane; margins low, narrow, irregular; mycelia white; texture floccose; sporulation moderately dense to dense, conidia en masse dull green (26D3–27D3–4); soluble pigments absent; exudates absent; reverse greenish white (30A2), greenish grey to greyish green (30B2–3). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate; margins low, wide, entire; mycelia white; texture floccose; sporulation sparse to moderately dense, conidia en masse greyish green (25B3–4–26B3–4); soluble pigments absent; exudates absent; reverse brownish orange (5C6), greyish yellow to greyish orange (4C3–5C3). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, irregular; mycelia white; texture floccose; sporulation moderately dense, conidia en masse greyish green to dull green (26C3–D4); soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Weak growth, acid not produced.
Conidiophores biverticillate, terverticillate, minor proportion divaricate; stipes rough, 210–640 × 2–3 μm; branches 20–30(–48) μm; metulae divergent, 2–5 per stipe or branch, 10–19 × 2.5–3.5 μm; phialides ampulliform, 5–10 per metula, 7.5–9.5 × 2–3 μm (8.3 ± 0.5 × 2.4 ± 0.2); average length metula/phialide 1.5; conidia finely rough walled, subglobose to broadly ellipsoidal, 2.5–3 × 2–3 μm (2.7 ± 0.2 × 2.4 ± 0.1), average width/length = 0.89, n = 49.
Extrolites — andrastin A, andrastin B, andrastin C, andrastin D, oxalicine A, oxalicine B, pulvilloric acid.
Additional material examined. CANADA, New Brunswick, St. Andrews, Carpenter ants (Camponotus pennsylvanicus), Aug. 2000, D. Clark, culture DAOMC 250536 (= CBS 140976 = NBSM-4-4 = W 449 = KAS 2168).
Notes — Penicillium infrabuccalum is classified in section Lanata-Divaricata. The single gene phylogenies resolved it in a clade with P. mariae-crucis and the new species P. cataractum (Fig. 7, 8). However, P. mariae-crucis produces sclerotia and reddish brown colonies, which are absent in both of the closely related new species. Penicillium infrabuccalum grows faster on MEA and has smaller conidia than P. cataractum.
Penicillium panissanguineum Visagie, Ketch & Seifert, sp. nov. — MycoBank MB815783; Fig. 23
Fig. 23.
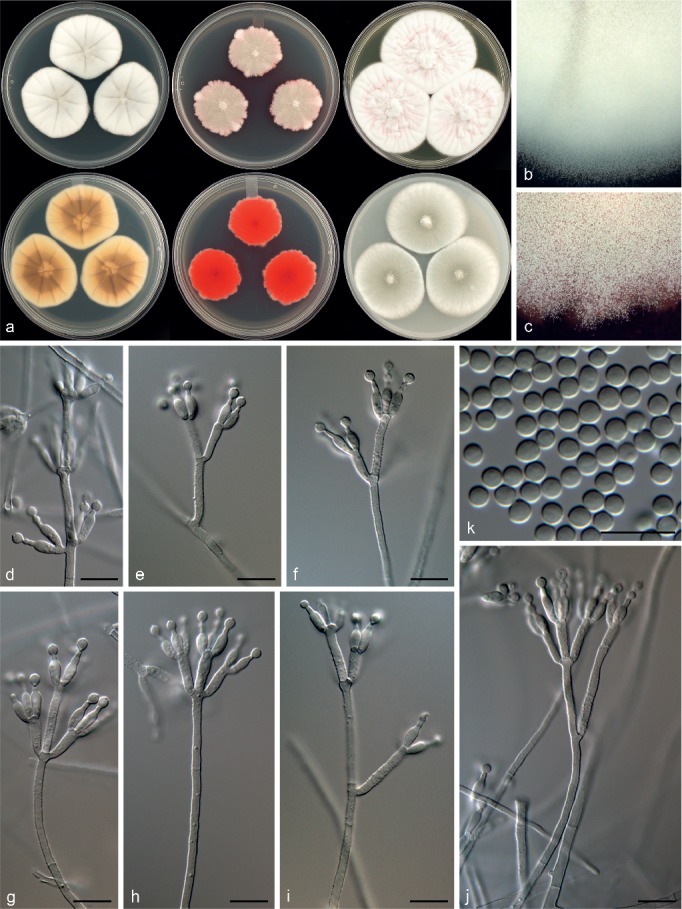
Morphological characters of Penicillium panissanguineum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d–j. conidiophores; k. conidia. — Scale bar: d–k = 10 μm.
In: subgenus Aspergilloides, section Lanata-Divaricata.
ITS barcode: KT887862. Alternative identification markers: BenA = KT887823, CaM = KT887784.
Etymology. Latin, panissanguineum, meaning blood of a chimpanzee, named in relation to chimpanzees that ate the soil from where strains were isolated and the red colony reverse on MEA.
Type specimen. TANZANIA, Kigoma region, soil related with termite mounds, Oct. 1996, coll. M.A. Huffman, isol. L. Ketch (holotype DAOM 695771, culture ex-type DAOMC 250562 = CBS 140989 = W 93 = KAS 2209).
Colony morphology — Colony diam, 7 d, in mm: CYA 41–44; CYA 37 °C no growth; MEAbl 30–35; YES 48–55; OA 40–45; CREA 20–21.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse greyish green to dull green (26C3–D4), greenish grey (26B2); soluble pigments absent; exudates absent; reverse brown to dark brown (7E6–F6), yellowish white to greyish yellow (4A2–B4). MEA 25 °C, 7 d: Colonies low, plane, pinkish red underneath mycelial and sporulating areas; margins low, wide, irregular; mycelia white; texture floccose and velutinous; sporulation moderately dense, conidia en masse greyish green to dull green (26D4–E5), greenish grey (26B2); soluble pigments pink; exudates absent; reverse greyish red to deep red (10B5–C8). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, raised at centre; margins low, wide, entire; mycelia white, pinkish areas present; texture floccose; sporulation very sparse, conidia en masse light grey; soluble pigments absent; exudates absent; reverse greyish red to red (10B5–7), pale yellow (4A3). OA 25 °C, 7 d: Colonies low, plane; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse greenish white to dull green (26B2–D3–E3); soluble pigments pink and absent; exudates absent. CREA 25 °C, 7 d: Weak growth, acid not produced.
Conidiophores biverticillate, minor proportion terverticillate and divaricate, solitary phialides sometimes present; stipes rough, 200–730 × 2–2.5 μm; branches 19.5–34 μm; metulae divergent, 2–4 per stipe or branch,10–16.5(–21) × 2.5–3 μm; phialides ampulliform, 3–7 per metula, 6.5–10 × 2.5–3.5 μm (8.2 ± 0.8 × 2.8 ± 0.2); average length metula/phialide 1.6; conidia smooth walled, globose to broadly ellipsoidal, 2.5–3.5 × 2.5–3.5 μm (3.1 ± 0.2 × 3 ± 0.1), average width/length = 0.95, n = 76.
Extrolites — andrastin A, andrastin B, andrastin C, andrastin D, pulvilloric acid.
Additional material examined. TANZANIA, Kigoma region, soil related with termite mounds, Oct. 1996, coll. M.A. Huffman, isol. L. Ketch, culture DAOMC 250561 (= CBS 140988 = W 85 = KAS 2208).
Notes — Penicillium panissanguineum is classified in section Lanata-Divaricata and is in a clade of species that typically produce rough-walled conidiophores, such as P. simplicissimum. However, the inability of the new species to grow on CYA at 37 °C and its bright red colony reverse colour on MEA distinguish it from all other species in this clade.
Penicillium tanzanicum Visagie, Ketch & Seifert, sp. nov. — MycoBank MB815781; Fig. 24
Fig. 24.
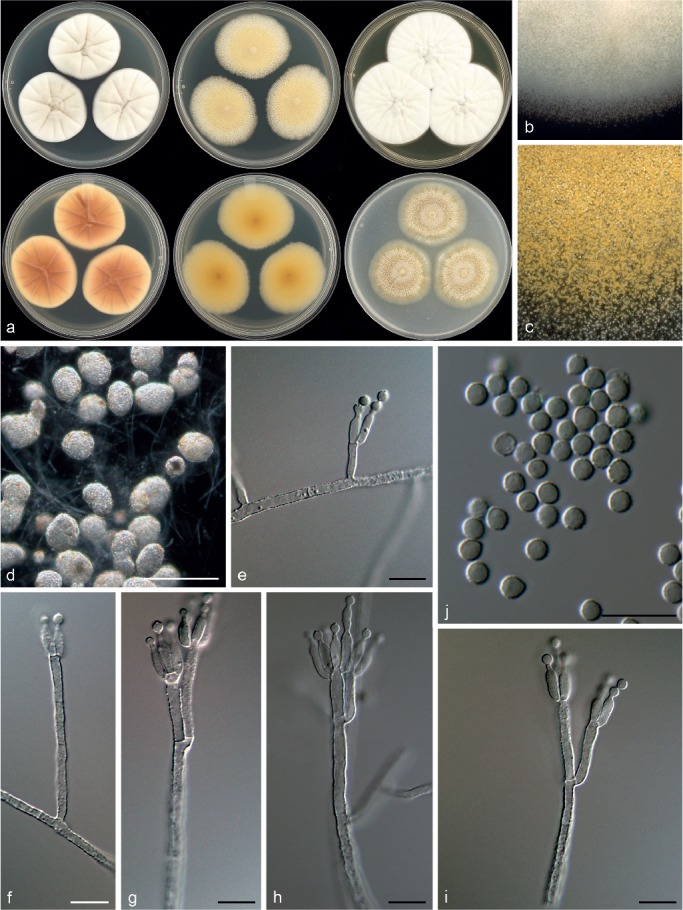
Morphological characters of Penicillium tanzanicum. a. Colonies from left to right (top row) CYA, MEA, YES; (bottom row) CYA reverse, MEA reverse, YES reverse; b. colony texture on CYA; c. colony texture on MEA; d. sclerotia; e–i. conidiophores; j. conidia. — Scale bar: d = 50 μm, e–j = 10 μm.
In: subgenus Aspergilloides, section Lanata-Divaricata.
ITS barcode: KT887841. Alternative identification markers: BenA = KT887802, CaM = KT887763.
Etymology. Latin, tanzanicum, named after Tanzania, origin of the strains.
Type specimen. TANZANIA, Kigoma region, soil related with termite mounds, Oct. 1996, coll. M.A. Huffman, isol. L. Ketch (holotype DAOM 695766, culture ex-type DAOMC 250514 = CBS 140968 = 50.118 = KAS 1946).
Colony morphology — Colony diam, 7 d, in mm: CYA 36–40; CYA 37 °C no growth; MEAbl 41–43; YES 47–50; OA 39–40; CREA 28–31.
CYA 25 °C, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white, inconspicuously yellowish grey; texture floccose; sporulation sparse, conidia en masse greenish grey (27B2–D2); soluble pigments absent; exudates absent; reverse brownish orange (6C4–7C4), yellowish white to orange white (4A2–5A2). MEA 25 °C, 7 d: Colonies low, plane, yellow to greyish yellow colour due to abundant sclerotia dominating colony appearance; margins low, wide, entire; mycelia white, inconspicuously yellow; texture floccose; sporulation absent to very sparse, conidia en masse indeterminable; soluble pigments absent; exudates absent; reverse yellowish brown (5D5), pale yellow to greyish yellow (4A2–B3–5). YES 25 °C, 7 d: Colonies moderately deep, radially and concentrically sulcate, raised at centre; margins low, wide, entire; mycelia white; texture floccose; sporulation very sparse, conidia en masse not determined; soluble pigments absent; exudates absent; reverse yellowish brown (5D5), pale yellow to greyish yellow (4A2–B3–5). OA 25 °C, 7 d: Colonies low, plane, yellow to greyish yellow colour due to abundant sclerotia; margins low, wide, entire; mycelia white, inconspicuously yellow; texture floccose; sporulation very sparse, conidia en masse not determined; soluble pigments absent; exudates absent. CREA 25 °C, 7 d: Weak growth, acid not produced.
Conidiophores biverticillate, divaricate; stipes rough, (80–)200–875 × 2.5–3.5 μm; branches 12.5–13.5; metulae divergent, 2–4 per stipe, unequal length on same conidiophore, 11.5–26 × 2.5–3.5 μm; phialides ampulliform, 4–8 per metula, 8–11 × 2.5–3.5 μm (9.5 ± 0.0.7 × 3.0 ± 0.3); average length metula/phialide 1.9; conidia rough walled, some finely rough, globose to subglobose, often pointed on one side with connective still visible, 2.5–3.5 × 2.5–3.5 μm (3.1 ± 0.2 × 3.0 ± 0.1), average width/length = 0.96, n = 55; sclerotia 33–105 × 32–96 μm.
Extrolites — bisdechlorogeodin, fiscalin A, fiscalin C, pulvilloric acid.
Additional material examined. TANZANIA, Kigoma region, soil related with termite mounds, Oct. 1996, coll. M.A. Huffman, isol. L. Ketch, cultures DAOMC 250515 (= CBS 140969 = 51.92 = KAS 1947), DAOMC 250516 (= CBS 140990 = 50.11 = KAS 2256).
Notes — Penicillium tanzanicum is classified in section Lanata-Divaricata. The phylogeny assigns it to a clade of species typically having roughened conidiophores, i.e. P. simplicissimum, P. araracuaraense and P. wotroi (Fig. 7, 8). Penicillium tanzanicum does not grow on CYA at 37 °C, in contrast with P. wotroi and P. simplicissimum, which grow at 37 °C. It should be noted that this character has been considered unstable for delineating species in the P. simplicissimum complex (Pitt 1980, Houbraken et al. 2011b). Additionally, P. tanzanicum also grows more restrictedly than P. simplicissimum. Penicillium araracuaraense produces a weak acid on CREA, whereas acid production is absent in P. tanzanicum. The main character distinguishing the new species from all three of its close relatives, is the abundant yellowish sclerotia on MEA, which are absent in close relatives.
DISCUSSION
Penicillium species are reported often in ecological surveys. If identified to the species rank, they are usually determined using micromorphological and colony characters. Although there are some recent comprehensive monographs of some subgenera or sections of Penicillium (Frisvad & Samson 2004, Houbraken et al. 2011a, b, 2014, Visagie et al. 2013, 2016a, b, Peterson et al. 2015), the available generic monographs are now 35 years out of date and the species concepts employed were biased heavily towards isolates from food or other niches of particular interest to humans. Species with apparently diverse ecologies, such as e.g. P. citrinum (according to Pitt 1980 one of the most common eukaryotes on Earth), P. glabrum, P. janthinellum and P. simplicissimum, incorporate great phenotypic diversity. Variability in growth, form and ecology made reliable identification of new isolates very difficult. The consequence is that before the development of a standardised molecular approach and extrolite profiling, species with more restricted ecological niches that do not intrude on the human economy tended to be lumped into other species, their unique genetic or biochemical status unrecognised. Many of the strains described as new in this paper were identified originally as known species using micromorphology before molecular data indicated the significance of some of the deviating or inconspicuous characters.
There is increasing evidence of ecological specialisation in Penicillium. Several of the new species described in this paper come from studies of esoteric ecological niches, such as soil eaten by chimpanzees, infrabuccal cavities of carpenter ants, caterpillar intestines, nuts in temperate forests, or leaf surfaces in tropical rain forests. It seems likely that several of our new species are actual functional components and not incidental contaminants of the ecosystem samples. There was no particular effort to target Penicillium specifically in most of these studies, so the strains were isolated among other fungi at differing frequencies. The Tanzanian soil eaten by chimpanzees, for example, yielded a broad diversity of hyphomycetes, including many Penicillium species that were a significant part of the mycota (Ketch 1998). Even though it is likely that some penicillia are actually true soil fungi or associates of roots, we speculate that many species have other primary habitats where they may perform various functions, similar to the close association that a number of subgenus Penicillium species have with food related products (Frisvad & Samson 2004) such as grains, dairy products or fruits. This may explain why it is often difficult to obtain numerous strains of one species when making isolations from soil.
Previous studies hint at possible interactions between Penicillium and insect hosts or habitats. Penicillium brocae has a very close association with coffee-berry borers (Peterson et al. 2003) while P. mallochii and P. guanacastense are known only from the intestines of Costa Rican caterpillars (Rivera et al. 2012). Penicillium herquei and the leave-rolling weevil (Euops chinensis) have a close association (Kobayashi et al. 2008, Li et al. 2012), and mites are suspected dispersal vectors for some Penicillium species in Protea repens infructescences (Visagie 2012). The dominant fungi in buccal cavities of carpenter ants were species of Mortierella (Clark 2002), but our new species P. camponotum seems to be a consistent inhabitant of this niche, and occurred in both Canada and Germany. The other two species, P. infrabuccalum with two isolates and P. fundyense with one, are known only from this niche but with so few strains available, it is difficult to interpret the significance of the association.
Whatever the actual ecological roles of the species described in this paper, it seems likely that the extrolites they produce will have crucial ecological functions. From the 15 species described here, 36 known extrolites were identified and 11 uncharacterised compounds detected (Table 3). As shown repeatedly in Penicillium, extrolite profiling is a valuable addition to morphological characterisation and gene comparisons for species delimitation (e.g. Frisvad & Filtenborg 1990, Sonjak et al. 2007, Tuthill et al. 2001), although chemotaxonomic comparisons with close relatives were not possible for most of our new species because of the lack of data for sister species. Among the species we studied, P. diabolicalicense, P. improvisum, P. alogum and P. aotearoae produced unique, previously unknown extrolites. Isofumigaclavine A and citrinin were extrolites of P. improvisum. Restricticins are broad spectrum antifungal polyenes previously only identified in cultures of P. restrictum (Hensens et al. 1991, Schwartz et al. 1991) and Penicillium sp. NR6564 (Matsukuma et al. 1992). Penicillium diabolicalicense also produces these compounds, but the less active N,N-dimethylrestricticin occurred in greater concentrations. In this study, P. diabolicalicense (section Exilicaulis) was the only producer of the neurotoxin penitrem A (on CYA), which is otherwise well-known among species of subgenus Penicillium. Penicillium alogum and P. aotearoae produced uncharacterised extrolites of formula C21H28O8 and C19H24O8, respectively. Penicillium amphipolaria was the only producer of fusaperazine E, fumitremorgin B and several related, uncharacterised metabolites. Andrastins were the extrolites of P. nucicola, P. cataractum, P. infrabuccalum, P. panissanguineum and some P. camponotum. Despite these similarities, all species produced unique combinations of additional extrolites (Table 3).
Infraspecific variation of extrolite production was observed for P. camponotum, P. cataractum and P. bissettii. All strains of P. camponotum produced marcfortine A and marcfortine B, yet the predominant extrolites produced by Canadian and German strains differed significantly. The seven Canadian strains isolated in New Brunswick from carpenter ants (Camponotus pennsylvanicus) produced an uncharacterised compound of formula C15H22O2 that was absent in the German strains isolated from Camponotus herculeanus, which produced andrastin A. The greatest infraspecific variation of extrolites occurred within strains of P. bissettii, both of which were isolated from soil samples collected from a spruce forest in Quebec, Canada on the same date. DAOMC 167011 produced high amounts of meleagrin and aurantioclavine in all three growth media tested whereas these extrolites were absent in DAOMC 167033, which produced penicillic acid.
Pulvilloric acid (C15H18O5) was first identified in cultures of P. pulvillorum (Brian et al. 1957), and its structure was elucidated (Barber et al. 1986, Barret et al. 1969, McOmie et al. 1966) following isolation under acidic conditions (pH = 1). Recently, pulvilloric acid was identified in the related P. wotroi and P. araracuaraense (Houbraken et al. 2011b). Using LC-high resolution mass spectrometry (LC-HRMS) in negative ionization mode, we’ve identified a similar compound of chemical formula C15H20O6 (m/z 295.1186) as an extrolite of P. panissanguineum, P. cataractum, P. infrabuccalum and P. tanzanicum. An isochroman similar to pulvilloric acid, with an identical chemical formula to that reported in this study, was characterised in cultures of the phylogenetically related species P. simplicissimum and patented as an amyloid aggregation inhibitor (Hirai et al. 2001). Interestingly, when these strains were screened by positive mode LC-HRMS, the m/z of the major peak was 279.1226, which corresponds to the chemical formula of pulvilloric acid (C15H18O5) (Fig. 9). The LC conditions were adjusted to ensure that the two contradictory signals observed in positive and negative ionization modes were indeed from the same compound. Non-aqueous extraction solutions were also used to determine if the extraction method was causing an undesired chemical reaction. We determined that during ionization in positive mode, protonation of this compound causes it to immediately dissociate by neutral loss of water, producing a [C15H18O5+H]+ product ion that can be mistaken as the molecular ion. This is a charge-directed fragmentation phenomenon known to occur when the protonated, gas-phase ion is highly unstable (Renaud et al. 2013). In negative mode ionization, the molecular charge is obtained through the deprotonation of the carboxylic acid and the compound does not undergo extensive in-source fragmentation, exemplifying the benefits of LC-MS screening with both polarities. Our results suggest that ‘pulvilloric acid’ which was previously identified in cultures of P. wotroi and P. araracuaraense and quite possibly P. pulvillorum itself, is not the structure as it exists on artificial solid and liquid growth media; it is the patented isochroman isolated from cultures of P. simplicissimum. The originally reported pulvilloric acid structure is known to be unstable and require careful extraction and work-up methodology, to avoid rearrangement and decomposition reactions from occurring during isolation (Tanenbaum & Nakajima 1969). Therefore, it is reasonable to believe that the two structures could be inter-convertible due to an increased stability of the molecule provided by the aromatisation of the originally proposed structure of pulvilloric acid to its isochroman. However, the true form of pulvilloric acid within the natural environments of its producers remains unknown. In solid and liquid growth media, the formula of this extrolite is C15H20O6 and we will simply maintain the name of pulvilloric acid.
Acknowledgments
This paper is warmly dedicated to Dr Robert A. Samson on the occasion of his 70th birthday. Rob is a teacher, mentor and colleague to the senior authors of this paper and we celebrate the remarkable achievements of his career, in particular his contributions to Penicillium taxonomy and phylogeny, with this paper. KAS thanks Peter Johnston, Mycology & Bacteriology, Landcare Research, Auckland, New Zealand, for his assistance in arranging a collecting permit for Arthur’s Pass National Park, where two of the new species in this paper were collected in 2003. Walter Gams assisted with collection of soil samples in Italy in 2008. Walter Gams and Shaun Pennycook assisted with the derivation of appropriate epithets for some of our species. KAS, RA, GL-S, JR and MS received operating support from several competitive grants offered by Agriculture & Agri-Food Canada Science and Technology Branch. DWM acknowledges nearly three decades of support from the Natural Sciences and Engineering Research Council of Canada (grants OGP0000145 and STR0149534). LK was supported by the Natural Science and Engineering Research Council of Canada (NSERC) postgraduate scholarship program. MU acknowledges support from NSERC and the US National Science Foundation (NSF) to R. Greg Thorn, from NSF to Daniel H. Janzen and from CR-USA to Costa Rican parataxonomists at the Area de Conservación Guanacaste who collected and reared the caterpillar host of P. costaricense and isolated and sent the fungi to the Thorn lab in London, Ontario. We are grateful to the Molecular Microbiology Technology Laboratory group at ORDC for their support with DNA sequencing.
REFERENCES
- Barber J, Bradley SM, Pope SM. 1986. Isolation, purification and nuclear magnetic resonance spectra of pulvilloric acid. Mycotoxin Research 2: 25–32. [DOI] [PubMed] [Google Scholar]
- Barret GC, McOmie JWF, Nakajima S, et al. 1969. Absolute configuration of pulvilloric acid 1969: 1068–1069. [Google Scholar]
- Blakeslee AF. 1915. Lindner’s roll tube method of separation cultures. Phytopathology 5: 68–69. [Google Scholar]
- Bragulat MR, Abarca ML, Cabanes FJ. 2001. An easy screening method for fungi producing ochratoxin A in pure culture. International Journal of Food Microbiology 71: 139–144. [DOI] [PubMed] [Google Scholar]
- Brian PW, Curtis PJ, Hemming HG, et al. 1957. Pulvilloric acid, an antibiotic obtained from cultures of Penicillium pulvillorum. Transactions British Mycological Society 40: 369–374. [Google Scholar]
- Clark DW. 2002. Fungi associated with the infrabuccal pockets of Camponotus pennsylvanticus and other formicine ants. MSc thesis, University of Toronto, Department of Botany, Canada. [Google Scholar]
- Filtenborg O, Frisvad JC, Thrane U. 1990. The significance of yeast extract composition on metabolite production in Penicillium. Modern concepts in Penicillium and Aspergillus classification: 433–441. [Google Scholar]
- Frisvad JC. 1981. Physiological criteria and mycotoxin production as aids in identification of common asymmetric Penicillia. Applied and Environmental Microbiology 41: 568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad JC, Filtenborg O. 1990. Revision of Penicillium subgenus Furcatum based on secondary metabolites and conventional characters. In: Samson RA, Pitt JI. (eds), Modern concepts in Penicillium and Aspergillus classification: 159–172. Plenum Press, New York. [Google Scholar]
- Frisvad JC, Samson RA. 2004. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Studies in Mycology 49: 1–174. [Google Scholar]
- Hensens OD, Wichmann CF, Liesch JM, et al. 1991. Structure elucidation of restricticin, a novel antifungal agent from Penicillium restrictum. Tetrahedron 47: 3915–3924. [Google Scholar]
- Hirai H, Ichiba T, Tonai H. 2001. Isochroman compounds and their production process. Pfizer Inc, New York: (patent CA2291931). [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. 2011a. Taxonomy of Penicillium section Citrina. Studies in Mycology 70: 53–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Lopez-Quintero CA, Frisvad JC, et al. 2011b. Penicillium araracuarense sp. nov., Penicillium elleniae sp. nov., Penicillium penarojense sp. nov., Penicillium vanderhammenii sp. nov. and Penicillium wotroi sp. nov., isolated from leaf litter. International Journal of Systematic and Evolutionary Microbiology 61: 1462–1475. [DOI] [PubMed] [Google Scholar]
- Houbraken J, Samson RA. 2011. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology 70: 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Visagie CM, Meijer M, et al. 2014. A taxonomic and phylogenetic revision of Penicillium section Aspergilloides. Studies in Mycology 78: 373–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketch LA. 1998. Microbiological investigations of geophage in chimpanzees. MSc thesis, Univeristy of Toronto, Canada. [Google Scholar]
- Kobayashi C, Fukasawa Y, Hirose D, et al. 2008. Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evolutionary Ecology 22: 711–722. [Google Scholar]
- Kornerup A, Wanscher JH. 1967. Methuen handbook of colour. Methuen & Co Ltd, Copenhagen. [Google Scholar]
- Li X, Guo W, Ding J. 2012. Mycangial fungus benefits the development of a leaf-rolling weevil, Euops chinesis. Journal of Insect Physiology 58: 867–873. [DOI] [PubMed] [Google Scholar]
- Mansouri S, Houbraken J, Samson RA, et al. 2013. Penicillium subrubescens, a new species efficiently producing inulinase. Antonie van Leeuwenhoek 103: 1343–1357. [DOI] [PubMed] [Google Scholar]
- Matsukuma S, Ohtsuka T, Kotaki H, et al. 1992. A new series of natural antifungals that inhibit P450 lanosterol C-14 demethylase. I. Taxonomy, fermentation, isolation and structural elucidation. The Journal of Antibiotics 45: 151–159. [DOI] [PubMed] [Google Scholar]
- McOmie JWF, Turner AB, Tute MS. 1966. The structure of pulvilloric acid. Journal of the Chemical Society C 1966: 1608–1613. [Google Scholar]
- Nylander AJJ, Ronquist F, Huelsenbeck JP, et al. 2004. Bayesian phylogenetic analysis of combined data. Systematic Biology 53: 47–67. [DOI] [PubMed] [Google Scholar]
- Peterson SW, Jurjevič Ž, Frisvad JC. 2015. Expanding the species and chemical diversity of Penicillium section Cinnamopurpurea. PLoSOne 10: e0121987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SW, Pérez J, Vega F, et al. 2003. Penicillium brocae, a new species associated with the coffee berry borer in Chiapas, Mexico. Mycologia 95: 141–147. [PubMed] [Google Scholar]
- Peterson SW, Sigler L. 2002. Four new Penicillium species having Thysanophora-like melanized conidiophores. Mycological Research 106: 1109–1118. [Google Scholar]
- Pitt JI. 1980. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London. [Google Scholar]
- Ramírez C, Martinez C. 1980. Some new species of Penicillium recovered from the atmosphere in Madrid and from other substrata. Mycopathologia 72: 181–191. [DOI] [PubMed] [Google Scholar]
- Renaud JB, Overton S, Mayer PM. 2013. Energy and entropy at play in competitive dissociations: The case of uneven positional dissociation of ionized triacylglycerides. International Journal of Mass Spectrometry 352: 77–86. [Google Scholar]
- Rivera KG, Díaz J, Chavarría-Díaz F, et al. 2012. Penicillium mallochii and P. guanacastense, two new species isolated from Costa Rican caterpillars. Mycotaxon 119: 315–328. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the United States of America 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Dufresne C, Flor JE, et al. 1991. Restricticin, a novel glycine-containing antifungal agent. The Journal of Antibiotics 44: 463–471. [DOI] [PubMed] [Google Scholar]
- Serra R, Peterson S, CTCOR , et al. 2008. Multilocus sequence identification of Penicillium species in cork bark during plank preparation for the manufacture of stoppers. Research in Microbiology 159: 178–186. [DOI] [PubMed] [Google Scholar]
- Sonjak S, Uršič V, Frisvad JC, et al. 2007. Penicillium svalbardense, a new species from Arctic glacial ice. Antonie van Leeuwenhoek 92: 43–51. [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Massachusetts. [Google Scholar]
- Tanenbaum SW, Nakajima S. 1969. The biosynthesis of pulvilloric acid. I. Production, isolation, and degradation. Biochemistry 8: 4622–4626. [DOI] [PubMed] [Google Scholar]
- Tuthill D, Frisvad JC, Christensen M. 2001. Systematics of Penicillium sim-plicissimum based on rDNA sequences, morphology and secondary metabolites. Mycologia 93: 298–308. [Google Scholar]
- Visagie CM. 2012. The polyphasic taxonomy of Penicillium and Talaromyces spp. isolated from the diverse fynbos biome. PhD thesis, Stellenbosch University, Department of Microbiology, South Africa. [Google Scholar]
- Visagie CM, Hirooka Y, Tanney JB, et al. 2014a. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Studies in Mycology 78: 63–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Dijksterhuis J, et al. 2016a. A taxonomic review of Penicillium species producing conidiophores with solitary phialides, classified in section Torulomyces. Persoonia 36: 134–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Frisvad JC, et al. 2014b. Identification and nomenclature of the genus Penicillium. Studies in Mycology 78: 343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Rodriques C, et al. 2013. Five new Penicillium species in section Sclerotiora: a tribute to the Dutch Royal family. Persoonia 31: 42–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Seifert KA, Houbraken J, et al. 2016b. A phylogenetic revision of Penicillium sect. Exilicaulis, including nine new species from fynbos in South Africa. IMA fungus: accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]


