Abstract
OBJECTIVE
To investigate the comparative efficacy of bevacizumab (Avastin®) and ranibizumab (Lucentis®) for diabetic macular edema (DME) using a crossover study design.
DESIGN
Randomized, double-masked, 36-week, three-period crossover clinical trial.
PARTICIPANTS
56 subjects with DME involving the center of the macula in one or both eyes.
INTERVENTION
Monthly intravitreous injections of bevacizumab (1.25 mg) or ranibizumab (0.3 mg).
MAIN OUTCOME MEASURES
Comparison of mean changes in visual acuity and central retinal thickness, tested using a linear mixed-effect model.
RESULTS
Based on the linear mixed-effect model, the three-month estimated mean improvement in visual acuity was 5.3 letters for bevacizumab and 6.6 letters for ranibizumab (difference of 1.3 letters (p = 0.039)). Estimated change in optical coherence tomography (OCT) central subfield mean thickness (CSMT) was −89 μm for bevacizumab and −137 μm for ranibizumab (difference of 48 μm (p < 0.001)). Incorporating cumulative treatment benefit, the model yielded a predicted 36-week (9-month) average improvement in visual acuity of 7.1 letters (95% CI [5.0, 9.2]) for bevacizumab and 8.4 letters (95% CI [6.3, 10.5]) for ranibizumab, and change in OCT CSMT of −128 μm (95% CI [−155, −100]) for bevacizumab and −176 μm (95% CI [−202, −149]) for ranibizumab.
There was no significant treatment-by-period interaction (i.e., treatment difference was constant in all three periods), nor was there a significant differential carry-over effect from one period to the next.
CONCLUSIONS
This trial demonstrates a statistically significant but small relative clinical benefit of ranibizumab compared with bevacizumab for treatment of DME, using a markedly reduced sample size relative to a full comparative efficacy study. The effects on visual acuity and central retinal thickness for the two drugs are consistent with those reported at one year for the concurrent parallel-group trial by the Diabetic Retinopathy Clinical Research Network (DRCR.net) testing bevacizumab, ranibizumab, and aflibercept for DME. The three-period crossover design allowed for meaningful and efficient comparison, suggesting that this approach might be useful for future comparative efficacy studies of anti-vascular endothelial growth factor (anti-VEGF) drugs for DME.
TRIAL REGISTRATION
Clinicaltrials.gov identifier NCT01610557.
The comparative efficacy of bevacizumab (Avastin®, Genentech), ranibizumab (Lucentis®, Genentech), and aflibercept (Eylea, Regeneron Pharmaceuticals) for treatment of diabetic macular edema (DME) is being investigated in a large, randomized, parallel-group clinical trial carried out by the Diabetic Retinopathy Clinical Research Network (DRCR.net) (ClinicalTrials.gov identifier NCT01627249). Recently reported one-year results for this study demonstrated efficacy for all three drugs.1 Analysis of the primary outcome, mean change in visual acuity at one year, showed that there was an overall relative benefit of aflibercept compared with the other two drugs. However, there was a statistically significant interaction between baseline visual acuity and the treatment effect for aflibercept, warranting stratification of the results by baseline visual acuity. The treatment effect was similar among the three drugs for eyes with baseline visual acuity letter score ≥ 69 (approximately 20/40 or better), and demonstrated superiority of aflibercept for eyes with baseline visual acuity letter score < 69 (worse than 20/40).
Ranibizumab (0.3 mg) and aflibercept (2 mg) are approved by the US Food and Drug Administration (FDA) for the treatment of DME, based on results of several randomized clinical trials.2–5 Bevacizumab has not been tested for this indication in a large clinical trial prior to the DRCR.net study, but has been widely used off-label in recent years on the basis of benefit shown in case series and small trials,6–11 and has shown efficacy equal to that of ranibizumab in large clinical trials for neovascular age-related macular degeneration.12–17
The findings of the DRCR.net trial offer invaluable and definitive guidance about the comparative efficacy of available anti-vascular endothelial growth factor (anti-VEGF) agents for treatment of DME. Such studies are the gold standard for comparative efficacy research, but the investment necessary to execute these projects is large, and the time necessary to organize and carry out these trials is considerable.
We asked whether a crossover study design might offer a meaningful and efficient comparison of two intravitreally-administered anti-VEGF drugs for DME, using a smaller sample size than required for a traditional parallel-group trial. We specifically wanted to compare findings from a small crossover study to those for the large comparative efficacy trial being planned by the DRCR.net. Crossover studies, in which every participant receives both treatments being compared, offer statistical efficiency that permits use of a smaller sample size than would be required for a parallel-group trial, in which each participant receives only one treatment being tested. Some crossover trial designs can be problematic, particularly when carry-over effects (residual effects) of one drug complicate measurement of the effects of a second drug in subjects given one and then the other, making it difficult or impossible to evaluate a treatment difference. Two-period, two-sequence designs susceptible to such problems have been criticized and are infrequently used in biomedical research.18 However, extended crossover designs making use of additional treatment periods and sequences have been developed to overcome these shortcomings under appropriate conditions.19,20
The treatment effect of anti-VEGF drugs on DME, which is rapid, easily measured, and typically reversible in the short-term, combined with the similarities of the drugs, seemed well-suited to this design. We chose to compare bevacizumab and ranibizumab, the two anti-VEGF drugs most widely used for treatment of DME at the time of study initiation, and carried out this trial concurrently with the DRCR.net study, in order to compare findings from the two study designs.
METHODS
This randomized, double-masked, 36-week, three-period, two-treatment crossover clinical trial was conducted at two sites, the National Eye Institute (Bethesda, MD) and University Hospitals Bristol National Health Service Foundation Trust (Bristol, UK), with the Emmes Corporation (Rockville, MD) acting as the Data and Statistical Coordinating Center. Institutional review board/independent ethics committee approval was obtained at both sites and all participants gave written informed consent. The study was conducted in accordance with the tenets of the Declaration of Helsinki. No stipend was given for participation. An independent data and safety monitoring committee provided study oversight and approved this manuscript. The study is registered at www.clinicaltrials.gov under identifier NCT01610557. This project has been funded with Federal funds from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN263201200001C. Patient recruitment and clinical research staff costs were also supported in the United Kingdom by the National Institute for Health Research’s Clinical Research Network West of England and Moorfields Biomedical Research Center, as part of the Universities and National Institutes Transatlantic Eye (UNITE) consortium.
STUDY POPULATION
Eligible participants had type 1 or type 2 diabetes mellitus, were at least 18 years old, and could enter one or both eligible eyes in the study. Principal eligibility criteria for a study eye included: 1.) presence of DME involving the center of the macula; 2.) Early Treatment of Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity letter score 78 to 24 (20/32 to 20/400); and 3.) central subfield mean thickness (CSMT) of ≥330 μm on Cirrus (Carl Zeiss Meditec) optical coherence tomography (OCT). Major exclusion criteria for the study eye included presence of factors or other conditions judged to impact the course of edema or preclude possible improvement in vision with treatment; panretinal photocoagulation, focal/grid laser photocoagulation, or depot corticosteroid injection within the previous 3 months; ocular injection with an anti-VEGF agent within the previous 2 months; more than 4 injections with an anti-VEGF agent within the previous year; or prior vitrectomy. Potential participants were excluded for history of renal failure (requiring hemodialysis or renal transplant) and for a measured systolic blood pressure of >180 mmHg or diastolic pressure >110 mmHg.
STUDY DESIGN
This study used a randomized, double-masked, three-period, two-treatment crossover design with four treatment sequence patterns. Each of three 12-week periods consisted of three intravitreous injections of ranibizumab (0.3 mg) or bevacizumab (1.25 mg), given every 4 weeks, with evaluation of the treatment period four weeks following the third dose (i.e., weeks 12, 24, and 36).
Each study eye received nine monthly injections over the course of the trial, according to a pattern of treatments determined by one of four randomly-assigned sequences: R-R-B, R-B-B, B-B-R, or B-R-R, where R indicates a series of three consecutive ranibizumab injections, and B represents a series of three consecutive bevacizumab injections. Participants were assigned to one of the four treatment sequences using a randomization list generated by the Data and Statistical Coordinating Center prior to study initiation, with balance following every 12 enrollments. The list was provided to unmasked pharmacists at each site who confirmed a valid participant identification code prior to dispensing study treatment. Both clinical sites utilized the same randomized list, but selected treatment assignments from opposite ends. For participants entering both eyes in the trial, the right eye was randomly assigned as above to one of the four treatment sequences, and the left eye was automatically assigned to the sequence with the inverse schedule (for example, B-R-R in the right eye and R-B-B in the left eye).
TREATMENT
Participants and investigators were masked to treatment. Site staff collecting study data, including research coordinators, technicians, and photographers, were also masked. Bevacizumab (1.25 mg) or ranibizumab (0.3 mg)179 * was administered every four weeks according to a study eye’s randomly-assigned schedule. Visits were scheduled within a window of ±10 days from the target date, but treatment could not be repeated within 14 days of a previous injection. The injection protocol required use of a lid speculum and application of povidone iodine. Participants with both eyes entered in the study could elect bilateral same-day treatment, or could return on a second day within the visit window for injection of the other eye.
Study eyes meeting pre-defined criteria for significant worsening of DME at week 12 or later could receive focal/grid laser photocoagulation. Fellow eyes in participants only enrolling one eye could receive any necessary ocular treatment.
EXAMINATION PROCEDURES
Best-corrected visual acuity measured using an ETDRS chart with standardized manifest refraction was obtained at baseline (week 0) and at weeks 12, 24, and 36, corresponding to time points four weeks following the third injection of each 12-week period. Testing done at all visits included visual acuity and intraocular pressure measurement, slit lamp biomicroscopy, dilated fundus examination, and OCT scanning obtained on a Cirrus machine. Technical difficulties with a Cirrus machine mandated a protocol amendment during the study, permitting OCT scanning using a Spectralis (Heidelberg Engineering) device for instances in which a Cirrus was unavailable. The amendment stipulated collection of both Cirrus and Spectralis scans at subsequent visits at the affected site, including visits during a pre-specified extension phase of the study through week 52, to allow for development and validation of a function to convert Spectralis values to Cirrus equivalents at all visits for which a Cirrus scan was not performed (please see Analysis for details). OCT scans for all visits were graded by a masked external Reading Center (Duke University, Durham, NC).
OUTCOMES
The analysis of this crossover study tests for a difference in 12-week treatment effect between bevacizumab and ranibizumab. The primary outcome was the mean change in best-corrected visual acuity from baseline, estimated for a three-month dosing period in a linear mixed-effect model. The main pre-specified secondary outcome was the change in central retinal thickness, measured as OCT central subfield mean thickness (CSMT), estimated for a three-month dosing period using the linear mixed-effect model.
ANALYSIS
Differences in mean change in visual acuity and OCT CSMT were tested using a 2-sided Type 3 F-test of the treatment effect in a linear mixed-effect regression model, where the final model included fixed-effects for treatment (bevacizumab or ranibizumab), period (1, 2, or 3), clinical site (National Eye Institute or Bristol), and baseline visual acuity score; and with random effects for subject and eye nested within subject.19 The model was fit using the GLIMMIX procedure in SAS (Cary, NC). Protocol-defined model building steps included evaluation of first-order carry-over effect (i.e., effect of treatment received in the preceding period, where applicable), period-by-treatment interactions, sequence effects, and sequence-by-period interactions, none of which was found to be significant or have substantive impact on the estimated effect of treatment when included in the model. Twelve- and 36-week changes in visual acuity and OCT CSMT are model estimates based on data from all subjects/eyes and all treatment periods. This four-sequence design has been shown to provide unbiased estimates of treatment and first-order carry-over effects (where carry-over effects that persist for only one period are termed first-order; two periods, second-order; and so-on), and is considered to be the optimal three-period, two-treatment, four-sequence design for estimating treatment differences in the presence of differential or symmetric first-order carry-over effects.20,21
Stratified analysis of eyes with baseline ETDRS visual acuity letter score ≥ 69 letters (approximately 20/40 or better) and eyes with baseline score < 69 letters (worse than 20/40) was not pre-specified in the analytic plan. The DRCR.net trial published one-year results using such stratification, on the basis of a significant interaction between baseline visual acuity and treatment effect for aflibercept, so we have added a similar analysis in order to allow for additional comparison to the DRCR.net results.
In approximately 12% of key visits (i.e., week 12, 24, or 36), participants underwent Spectralis OCT scanning rather than the pre-specified Cirrus OCT testing because of technical difficulties with a Cirrus device. Following repair of the Cirrus, both Cirrus and Spectralis OCT scans were captured at 150 subsequent participant visits, enabling development and validation of a linear conversion function for CSMT from Spectralis to Cirrus, similar to work done previously by the DRCR.net.22 Prediction error of the conversion function was evaluated using a bootstrap cross-validation routine and was estimated to be 8.4 μm (95% CI [8.4, 8.6]). Spectralis values were converted and utilized as Cirrus CSMT values for the 12% of key visits at which the Cirrus scan was not performed. A worst-case sensitivity analysis, adding and subtracting twice the prediction error for imputed CSMT values for observations in the ranibizumab and bevacizumab groups, respectively, did not impact the statistical significance and reduced the estimated effect size by less than 5.6% (i.e., 2.7 μm).
The study sample size was determined through simulation and utilized the exact model and outcomes as described above, but assumed only a single eye per participant. Within- and between-subject standard deviations were each assumed to be 5 ETDRS letters (0.1 logMAR). A differential first-order carry-over effect of 20% (i.e., 20% of effect of previous period would be maintained through a subsequent period) was assumed. Under these conditions, a study of 60 eyes was expected to have 87% power to detect a 2.5 letter (0.05 logMAR) difference between treatments, rising to 89% if no carry-over effect was present.
RESULTS
Fifty-six participants were enrolled in the study between June 2012 and January 2014, including six participants with both eyes enrolled. One participant with a single eye assigned to the R-B-B group withdrew after the week 4 visit following a cerebrovascular accident. All remaining participants completed the study, including week 12, 24, and 36 visits, and were included in this analysis.
Baseline characteristics for all participants are shown in Table 1. The largest imbalances among the four study groups were for participants/eyes assigned to the R-B-B sequence. Compared to the overall mean, age was 2.9 years greater, hemoglobin A1c was 0.2% higher, visual acuity was 3 letters lower, and OCT CSMT was 33 μm less in this group.
Table 1. Baseline Characteristics.
Participants with both eyes enrolled are counted twice (by eye), once for each of the treatment sequences to which an eye was randomly assigned.
| Variable | Category | RRB | RBB | BBR | BRR | Total |
|---|---|---|---|---|---|---|
| Group | Total Eyes | 17 | 15* | 16 | 14 | 62 |
| Age (years) | Min / Median (Mean) / Max | 39 / 61 (62.4) /85 | 39 / 66 (65.9) /87 | 39 / 63 (62.3) /83 | 51 / 61.5 (61.8) /82 | 39 / 62 (63) /87 |
| Diabetes Type | Type 1 | 2 (11.8%) | 2 (13.3%) | 1 (6.3%) | 2 (14.3%) | 7 (11.3%) |
| Type 2 | 15 (88.2%) | 13 (86.7%) | 15 (93.8%) | 12 (85.7%) | 55 (88.7%) | |
| Hemoglobin | Min / Median (Mean) / Max | 6.2 / 7.4 (8.1) / 11.5 | 5.8 / 7.8 (8.4) / 12.2 | 6.2 / 7.9 (7.9) / 10.6 | 5.8 / 7.6 (7.8) / 10.3 | 5.8 / 7.6 (8.1) / 12.2 |
| A1C (%) | ≥ 8.0% | 6 (35%) | 6 (40%) | 7 (43%) | 5 (35%) | 24 (38%) |
| Gender | Female | 4 (23.5%) | 8 (53.3%) | 7 (43.8%) | 5 (35.7%) | 24 (38.7%) |
| Male | 13 (76.5%) | 7 (46.7%) | 9 (56.3%) | 9 (64.3%) | 38 (61.3%) | |
| Race | Asian | 1 (5.9%) | 0 (0%) | 1 (6.3%) | 1 (7.1%) | 3 (4.8%) |
| (Participant-reported) | Black | 2 (11.8%) | 2 (13.3%) | 2 (12.5%) | 2 (14.3%) | 8 (12.9%) |
| Multiple race | 1 (5.9%) | 0 (0%) | 2 (12.5%) | 0 (0%) | 3 (4.8%) | |
| Unknown | 1 (5.9%) | 0 (0%) | 0 (0%) | 1 (7.1%) | 2 (3.2%) | |
| White | 12 (70.6%) | 13 (86.7%) | 11 (68.8%) | 10 (71.4%) | 46 (74.2%) | |
| Ethnicity | Unknown | 0 (0%) | 0 (0%) | 1 (6.3%) | 0 (0%) | 1 (1.6%) |
| (Participant-reported) | Hispanic or Latino | 2 (11.8%) | 0 (0%) | 1 (6.3%) | 0 (0%) | 3 (4.8%) |
| Not Hispanic or Latino | 15 (88.2%) | 15 (100%) | 14 (87.5%) | 14 (100%) | 58 (93.5%) | |
| Best-Corrected Visual Acuity (letters) | Min / Median (Mean) / Max | 38 / 69 (65) / 78 | 32 / 64 (61) / 73 | 44 / 69 (65) / 75 | 50 / 64 (65) / 78 | 32 / 66 (64) / 78 |
| OCT Central Subfield Mean Thickness (μm) | Min / Median (Mean) / Max | 334 / 496 (484) / 720 | 366 / 435 (444) / 602 | 362 / 432 (471) / 606 | 358 / 508 (508) / 653 | 334 / 453 (477) / 720 |
One participant with a single eye assigned to the RBB group withdrew after the week 4 visit following a cerebrovascular accident and was not included in the analysis; all other participants/eyes completed the study and were included in the analysis.
All participants received study medication according to their randomly-assigned schedule, 92% (449/487 injections) given within the protocol-specified window of ±10 days. No study eye experienced significant worsening of DME or received supplemental application of focal/grid laser photocoagulation or other adjuvant treatment for DME.
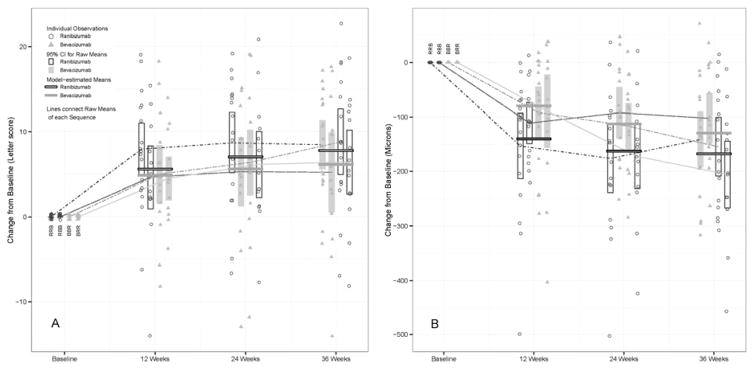
Based on the linear mixed-effect model, the three-month estimated mean improvement in visual acuity was 5.3 letters (95% CI [3.2, 7.4]) for bevacizumab and 6.6 letters (95% CI [4.5, 8.7]) for ranibizumab, with an estimated difference of 1.3 letters (95% CI [0.07, 2.5]; p = 0.039) (Table 2). Model-based estimates of mean change in central retinal thickness measured by OCT CSMT were −89 μm (95% CI [−116, −62]) for bevacizumab and −137 μm (95% CI [−164, −110]) for ranibizumab, with an estimated difference of −48 μm (95% CI [−65, −31], p<0.001) (Table 2). Figure 1 (A and B, respectively) presents change in visual acuity and OCT CSMT from baseline for periods 1, 2, and 3 by treatment group, illustrated as individual measurements, raw means, and model-based estimates from the mixed-effect analysis for each drug.
Table 2. Results of Crossover (Mixed-Effect Model) Analysis. Bevacizumab.
and Ranibizumab columns represent the estimated effect of the drug for a 3-month period, adjusted for period and baseline value. The Difference column represents the estimated difference between the two drugs.
| Bevacizumab [95% CI] | Ranibizumab [95% CI] | Difference [95% CI] | P-Value | |
|---|---|---|---|---|
| Best-Corrected Visual Acuity, Change from Baseline (letters) | 5.3 [3.2, 7.4] | 6.6 [4.5, 8.7] | 1.3 [0.07, 2.5] | 0.039 |
| Best-Corrected Visual Acuity (letters) | 69.5 [67.4, 71.6] | 70.8 [68.7, 72.9] | ||
|
| ||||
| OCT Central Subfield Mean Thickness, Change from Baseline (μm) | −89 [−116, −62] | −137 [−164, −110] | −48 [−65, −31] | <0.001 |
| OCT Central Subfield Mean Thickness (μm) | 388 [361, 415] | 340 [313, 367] | ||
Figure 1.
Change in (A) Visual Acuity and (B) OCT Central Subfield Mean Thickness from baseline for crossover periods 1, 2, and 3 by treatment group.
A significant period effect was identified, indicating a cumulative benefit over time with either drug. For every three-month period, improvement in visual acuity attributable to the period effect was estimated to be 0.9 letters (95% CI [0.2, 1.6]) and decrease in OCT CSMT attributable to the period effect was estimated to be 19 μm (95% CI [9, 29]), whether receiving bevacizumab or ranibizumab.
Combining the period and treatment effects in the mixed-effect model yields a predicted nine-month (36-week) average improvement in visual acuity of 7.1 letters (95% CI [5.0, 9.2]) for bevacizumab and 8.4 letters (95% CI [6.3, 10.5]) for ranibizumab, and a predicted nine-month average decrease in OCT CSMT of 128 μm (95% CI [100, 155]) for bevacizumab and 176 μm (95% CI [149, 202]) for ranibizumab.
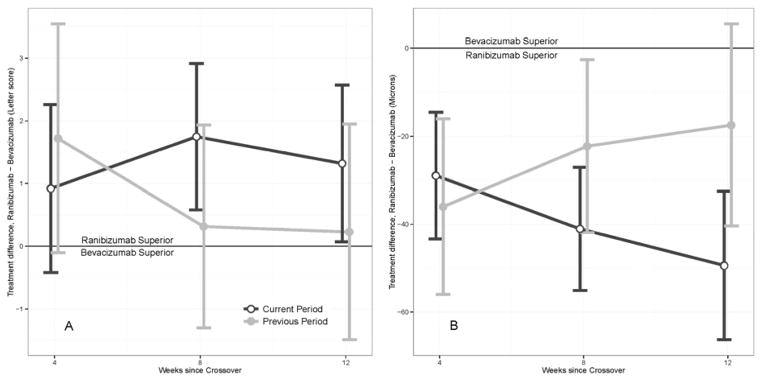
There was no significant treatment-by-period interaction, implying that differences between ranibizumab and bevacizumab were similar in all periods. There was no significant differential first-order carry-over effect at outcome measurement at weeks 12, 24, and 36. Figure 2 (A and B, respectively) shows the estimated differential first-order carry-over effect on change in visual acuity and OCT CSMT at 4, 8, and 12 weeks after crossover, illustrated as a difference between the effect of ranibizumab and bevacizumab from the previous period, with the corresponding treatment effect for the present period shown for comparison. At 4 weeks, this figure shows that the treatment received in the preceding period has a greater impact on outcome than the treatment received in the current period. This is reversed by 8 weeks and further decreases by 12 weeks, when the treatment received in the current period dominates the non-significant impact of treatment received in the preceding period.
Figure 2.
Differential first-order carry-over effect (residual effect of the drug from the preceding period), shown as a treatment effect difference between ranibizumab and bevacizumab given in the previous period for (A) change in visual acuity and (B) central subfield mean thickness on optical coherence tomography at 4, 8, and 12 weeks after crossover. The treatment difference attributable to the first-order carry-over effect is shown in gray. The treatment difference attributable to drugs in the current period (i.e., the differential effect of the two drugs as estimated in the primary analysis of the study) is shown in black for comparison. Note that the treatment difference between ranibizumab and bevacizumab for the current period (in black) at 12 weeks after crossover (at outcome assessment) is the result estimated for 3 and 9 months in the primary analysis (1.3 letters (p=0.039) and −48 microns (p<0.001), both favoring ranibizumab).
We evaluated how many eyes achieved a normal or near-normal central retinal thickness within the first 24 weeks and maintained this level of improvement through 36 weeks. Six (19%) of 31 eyes receiving ranibizumab and 2 (7%) of 30 eyes receiving bevacizumab achieved an OCT CSMT of less than 275 μm in the first period (12 weeks) and maintained a CSMT less than 275 μm through 36 weeks. Considering eyes that did not achieve a CSMT of less than 275 μm during the first period, but did so in the second period, and maintained this improvement through 36 weeks, there were 2 (13%) of 15 assigned to bevacizumab in both periods (B-B); 2 (15%) of 13 assigned to bevacizumab in the first and ranibizumab in the second period (B-R); 2 (14%) of 14 assigned to ranibizumab in both periods (R-R); and none (0%) of 11 assigned to ranibizumab in the first and bevacizumab in the second period (R-B). Through Week 12, the group rate appears higher among those receiving ranibizumab, but this difference could have been achieved by chance (p = 0.25, Fisher’s Exact test). Through Week 24, the rate for those receiving bevacizumab for two consecutive periods is similar to those receiving ranibizumab.
An exploratory analysis, performed to allow additional comparison to one-year results of the DRCR.net study, showed a statistically significant interaction between baseline visual acuity and the difference in treatment effect for bevacizumab and ranibizumab. Baseline characteristics for all participants, stratified by baseline visual acuity for all study eyes, are shown in Table S1 (Available at http://www.X). The linear mixed-effect model was used for each of two strata to estimate three-month mean improvement in visual acuity and mean change in OCT CSMT for bevacizumab and ranibizumab, with analysis stratified for eyes with baseline visual acuity letter score ≥ 69 (20/40 or better) and for eyes with visual acuity letter score < 69 (worse than 20/40). Three-month estimates, stratified by baseline visual acuity, are shown in Table 3, and include the non-stratified values for all eyes for comparison. As in the primary analysis, combination of treatment and period effects allowed estimation of 36-week (9-month) changes in visual acuity and retinal thickness. For better-seeing eyes, average improvement in visual acuity was 4.9 letters (95% CI [2.0, 7.8]) for bevacizumab and 5.3 letters (95% CI [2.4, 8.2]) for ranibizumab, and change in OCT CSMT was −144 μm (95% CI [−182, −106]) for bevacizumab and −184 μm (95% CI [−221, −147]) for ranibizumab at 36 weeks. For worse-seeing eyes, average improvement in visual acuity was 8.6 letters (95% CI [6.0, 11.2]) for bevacizumab and 10.5 letters (95% CI [7.9, 13.1]) for ranibizumab, and change in OCT CSMT was −117 μm (95% CI [−150, −84]) for bevacizumab and −170 μm (95% CI [−203, −137]) for ranibizumab at 36 weeks. Note that, as for the primary analysis, nine-month differences between the two drugs are equivalent to the three-month differences shown in Table 3.
Table 3. Results of Crossover (Mixed-Effect Model) Analysis, Stratified by Visual Acuity at Baseline (Post hoc Analysis). Bevacizumab.
and Ranibizumab columns represent the estimated effect of the drug for a 3-month period, adjusted for period and baseline value. The Difference column represents the estimated difference between the two drugs.
| Baseline visual acuity letter score | Bevacizumab [95% CI] | Ranibizumab [95% CI] | Difference [95% CI] | P-Value | |
|---|---|---|---|---|---|
| Best-Corrected Visual Acuity, Change from Baseline (letters) | Any (all eyes) | 5.3 [3.2, 7.4] | 6.6 [4.5, 8.7] | 1.3 [0.07, 2.5] | 0.039 |
| ≥ 69 letters | 3.1 [0.2, 6.0] | 3.6 [0.7, 6.5] | 0.45 [−1.4, 2.3] | 0.64 | |
| < 69 letters | 6.9 [4.3, 9.4] | 8.7 [6.2, 11.3] | 1.9 [0.3, 3.5] | 0.022 | |
| OCT Central Subfield Mean Thickness, Change from Baseline (μm) | Any (all eyes) | −89 [−116, −62] | −137 [−164, −110] | −48 [−65, −31] | <0.001 |
| ≥ 69 letters | −106 [−144, −68] | −145 [−183, −108] | −40 [−66, −14] | 0.0032 | |
| < 69 letters | −78 [−111, −45] | −132 [−165, −99] | −54 [−76, −31] | < 0.001 |
ADVERSE EVENTS
There were no cases of endophthalmitis, retinal detachment, traumatic cataract, or vision loss ≥ 15 letters. A single instance of hemorrhagic cerebrovascular accident occurred in a participant who received ranibizumab at baseline (week 0) and week 4, eighteen days following the second injection.
DISCUSSION
This randomized crossover clinical trial demonstrates a statistically significant, but small (1.3 letter difference in visual acuity, 48 μm difference in central retinal thickness) relative estimated benefit of ranibizumab compared with bevacizumab for treatment of DME. By comparison, the large, ongoing parallel-group trial performed by the DRCR.net found a one-year benefit of ranibizumab of 1.4 letters (not statistically significant, p = 0.12) and 51μm (statistically significant, p < 0.001) relative to bevacizumab,1 results essentially identical to those obtained in our study. Although caution is warranted in comparing results of an estimated three-month period in our study to twelve-month findings in the DRCR.net trial, our analysis allows estimation of the treatment difference at 36 weeks (9 months). The rapid development of a large treatment benefit during the first several months of serial injections, with maintenance of the effect thereafter, is characteristic of the available ophthalmic anti-VEGF drugs across a number of indications,1–5,10,12–17,23 making nine and twelve month results very similar in these studies, including the present DRCR.net trial.
Compared to parallel-group trials, crossover trials achieve similar statistical power with fewer participants, by utilizing each subject as his or her own control. The increase in power comes at the cost of additional assumptions that are not necessary in a randomized parallel-group trial. The principal and primary assumption of all crossover studies is that the condition to be treated, whether stable or progressive over the course of the trial, would revert to the untreated state (or close approximation) if an effective intervention were ceased. In other words, it is assumed that the interventions tested are not curative during the period of treatment. This assumption seems justified based on clinical experience with treatment of DME with anti-VEGF agents during the first year of treatment, and is corroborated by the results of the DRCR.net trial, in which study eyes receiving bevacizumab or ranibizumab required a median of 10 injections (of a possible 13) in the first year, in the context of a complex re-treatment algorithm.1 We evaluated the rate of potential “cure” in our study by considering eyes that achieved an OCT CSMT of less than 275 μm during the first or second period of treatment and maintained this resolution of central edema through 36 weeks. Even conservatively defining all such eyes as “cured” (and not simply dependent on continued monthly treatment to maintain improvement), we found that the rate of such “cure” is low and occurs slightly more often with ranibizumab. If this difference is real, the analysis is biased toward a reduced effect of ranibizumab, implying that the result presented here is a conservative estimate of the superiority of ranibizumab. However, the statistical significance of our results, and their similarity to those of DRCR.net trial, suggest that our analysis was not meaningfully compromised or influenced by “cure” of eyes in the study.
There are many different crossover study designs, and each relies on different assumptions about carry-over effects. A differential carry-over effect (or residual effect) occurs when an intervention from a preceding period influences the assessment of treatment differences in the current period. Carry-over effects that persist for only one period are termed first-order; two periods, second-order; and so-on. All two-treatment, two-period crossover designs (e.g., AB/BA or AA/AB/BB/BA designs) assume no carryover effect; violation of this assumption is a common critique of many such studies.24 Extended, or higher-order, crossover designs can provide unbiased estimates of treatment effects when first-order carry-over effects are present. For example, a two-treatment, three-period, three-sequence AAB/BBA design is optimal in the presence of a first-order carry-over effect, but is invalid in the presence of a second-order carry-over effect, or a treatment-by-period interaction.20 The two-treatment, three-period, four-sequence AAB/ABB/BBA/BAA design used in this study has been shown to be unbiased and near-optimal in the presence of a simple first-order carry-over effect and robust even when small second-order carry-over effects or more complex treatment-by-period interactions exist.25
In crossover studies, a ‘washout’ interval between periods is included to mitigate the possibility of carry-over effects and reduce the possibility that treatments from prior periods influence outcome measures of the current period. In a typical ‘washout’ interval, participants receive no doses of investigational product for a period of time, often designated as five times the half-life of the drug.26,27 Both bevacizumab and ranibizumab have an intraocular half-life of less than 10 days.28,29 In DME, with effective therapy available, and the possibility of permanent damage to vision without treatment, a pure ‘washout’ period has potential to compromise care of participants. This study utilized an ‘active washout,’ where patients were treated every month, but the intervals between primary outcome assessments were 12 weeks (84 days) apart. Further, since the last dose in each series of three injections was 4 weeks prior to each outcome assessment, each outcome assessment occurred 16 weeks (112 days) following the last dose of the previous period. Although clinical benefit likely exceeds bioavailability of either drug in the vitreous, Figure 2 shows the magnitude and diminution of the carry-over effect and demonstrates that the 12-week ‘active washout’ period effectively limits the impact of treatments in prior periods on outcome assessment of the current period.
Although rare in ophthalmology, crossover trials are common in other areas of medical research.30–36 Regulatory guidance documents for clinical evaluation of drugs for proarrhythmic potential and, more generally, for support of a New Drug Application to the FDA fully integrate crossover studies as valid for evaluation of drug effects and treatment differences under appropriate circumstances.26,37 Specifically, the FDA Guideline for the Format and Content of the Clinical and Statistical Sections of an Application recommends consideration of “the likelihood of spontaneous change in disease during the study, and need (or lack of need) for re-establishment of baseline between treatment periods, or a plan to estimate residual effects to show that they are inconsequential” when evaluating suitability of a crossover study design.37 The International Conference on Harmonisation Statistical Principles for Clinical Trials discusses appropriate use of crossover designs, indicating that “the disease under study be chronic and stable” and that the problem of unequal carryover will bias direct treatment comparisons in a two-period, two-sequence design, but that this problem is “less acute in higher order designs.”38 These considerations were critical in designing and analyzing this study. In particular, the first-order differential carry-over (residual) effect was shown to be inconsequential, and the carry-over effect common to both drugs did not bias estimates of treatment effect difference.
The route for approval of novel anti-VEGF agents and of existing drugs evaluated for new indications typically involves comparison to a single already-approved medication, not comparison to off-label drugs like bevacizumab (which is a drug of interest because of cost considerations), and not comparison to multiple agents in the class. Parallel-group trials remain the gold standard for comparison of these drugs, but the expense of these trials limits comparative efficacy research. In the setting of an expanding number of anti-VEGF drugs for an increasing array of ophthalmic indications, it may not be practical to execute a large traditional trial to provide guidance in every instance, particularly for less common diseases. Given the similarity between our findings and those of the large DRCR.net trial, we believe that the crossover design used in this study merits further evaluation as a potentially rapid and economic means of obtaining data on the comparative efficacy of anti-VEGF drugs for diseases amenable to such analysis. The appropriate circumstances for a crossover study need to be assessed carefully. If a course of anti-VEGF therapy for a given condition results in a high frequency of disease-modifying effects shortly after treatment initiation, a crossover approach is not appropriate. For example, choroidal neovascularization secondary to myopic degeneration frequently exhibits a durable response to treatment with anti-VEGF drugs within a few injections (with resolution of exudation visualized by OCT and angiography that often does not recur after cessation of treatment),39,40 precluding use of a crossover design in this setting. In addition to consideration of factors related to the disease in question and the nature of the treatment effects to be compared, it is important to bear in mind that this design has very low statistical power to assess adverse experiences. A study of this kind seems most appropriate when there is experience with the drugs from previous large trials.
Supplementary Material
Acknowledgments
Financial support: This project has been funded with Federal funds from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN263201200001C. Patient recruitment and clinical research staff costs were also supported in the United Kingdom by the National Institute for Health Research’s Clinical Research Network West of England and Moorfields Biomedical Research Center as part of the Universities and National Institutes Transatlantic Eye (UNITE) consortium.
Abbreviations/Acronyms
- Anti-VEGF
Anti-Vascular Endothelial Growth Factor
- CI
Confidence Interval
- CSMT
Central Subfield Mean Thickness
- DME
Diabetic Macular Edema
- DRCR.net
Diabetic Retinopathy Clinical Research Network
- ETDRS
Early Treatment of Diabetic Retinopathy Study
- FDA
US Food and Drug Administration
- OCT
Optical Coherence Tomography
Footnotes
Eleven doses of ranibizumab 0.5 mg were given to participants at the start of the study. After publication of two large trials reporting no difference in efficacy between ranibizumab 0.3 mg and 0.5 mg for DME4 and subsequent FDA approval of the 0.3 mg dose for DME, the protocol was amended and ranibizumab 0.3 mg was used for the remainder of the study (98% of all ranibizumab injections).
Meeting presentation:
Paper Presentation, Association for Research in Vision and Ophthalmology 2015 Annual Meeting, May 2015, Denver, CO
Author conflict of interest disclosures: No conflicting relationship exists for any author.
This article contains additional online-only material. The following should appear online only: Table S1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. The New England Journal of Medicine. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77. e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–50. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Scott IU, Edwards AR, Beck RW, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kook D, Wolf A, Kreutzer T, et al. Long-term effect of intravitreal bevacizumab (Avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2008;28:1053–60. doi: 10.1097/IAE.0b013e318176de48. [DOI] [PubMed] [Google Scholar]
- 9.Soheilian M, Ramezani A, Obudi A, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116:1142–50. doi: 10.1016/j.ophtha.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078–86. e2. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Gillies MC, Lim LL, Campain A, et al. A Randomized Clinical Trial of Intravitreal Bevacizumab versus Intravitreal Dexamethasone for Diabetic Macular Edema: The BEVORDEX Study. Ophthalmology. 2014;121:2473–81. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Subramanian ML, Abedi G, Ness S, et al. Bevacizumab versus ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked, randomised clinical trial. Eye. 2010;24:1708–15. doi: 10.1038/eye.2010.147. [DOI] [PubMed] [Google Scholar]
- 13.Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. The New England Journal of Medicine. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswas P, Sengupta S, Choudhary R, et al. Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration. Indian Journal of Ophthalmology. 2011;59:191–96. doi: 10.4103/0301-4738.81023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Kodjikian L, Souied EH, Mimoun G, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: Results from the GEFAL noninferiority randomized trial. Ophthalmology. 2013;120:2300–09. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Krebs I, Schmetterer L, Boltz A, et al. A randomized, double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. British Journal of Ophthalmology. 2013;97:266–71. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 18.Woods JR, Williams JG, Tavel M. The two-period crossover design in medical research. Annals of Internal Medicine. 1989;110:560–6. doi: 10.7326/0003-4819-110-7-560. [DOI] [PubMed] [Google Scholar]
- 19.Ebbutt AF. Three-period crossover designs for two treatments. Biometrics. 1984;40:219–24. [PubMed] [Google Scholar]
- 20.Carriere KC. Crossover designs for clinical trials. Statistics in Medicine. 1994;13:1063–9. doi: 10.1002/sim.4780131008. [DOI] [PubMed] [Google Scholar]
- 21.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials. 2. Chapman and Hall / CRC; 2003. [Google Scholar]
- 22.Bressler SB, Edwards AR, Chalam KV, et al. Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmology. 2014;132:1113–22. doi: 10.1001/jamaophthalmol.2014.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thach AB, Yau L, Hoang C, et al. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. Ophthalmology. 2014;121:1059–66. doi: 10.1016/j.ophtha.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Deutsches Ärzteblatt International. 2012;109:276–81. doi: 10.3238/arztebl.2012.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carriere KC, Huang R. Crossover designs for two-treatment clinical trials. Journal of Statistical Planning and Inference. 2000;87:125–34. [Google Scholar]
- 26.The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use [Internet]; Geneva, Switzerland. 2005. [Accessed July 17, 2015]. p. E14. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. [Google Scholar]
- 27.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) [Internet]. Silver Spring, Maryland. [Accessed July 17, 2015];Guidance for Industry: Statistical Approaches to Establishing Bioequivalence. 2001 Available at http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf.
- 28.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. American Journal of Ophthalmology. 2008;146:508–12. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 29.Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. American Journal of Ophthalmology. 2012;154:682–6. e2. doi: 10.1016/j.ajo.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Gilron I, Bailey JM, Tu D, et al. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374:1252–61. doi: 10.1016/S0140-6736(09)61081-3. [DOI] [PubMed] [Google Scholar]
- 31.Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. The New England Journal of Medicine. 2010;363:513–22. doi: 10.1056/NEJMoa0805538. [DOI] [PubMed] [Google Scholar]
- 32.Leissinger C, Gringeri A, Antmen B, et al. Anti-inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. The New England Journal of Medicine. 2011;365:1684–92. doi: 10.1056/NEJMoa1104435. [DOI] [PubMed] [Google Scholar]
- 33.Vanpouille C, Lisco A, Grivel JC, et al. Valacyclovir Decreases Plasma HIV-1 RNA in HSV-2 Seronegative Individuals: A Randomized Placebo-Controlled Crossover Trial. Clinical Infectious Diseases. 2015;60:1708–14. doi: 10.1093/cid/civ172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthon BS, Gibson PG, McElduff P, et al. Effects of short-term oral corticosteroid intake on dietary intake, body weight and body composition in adults with asthma - a randomized controlled trial. Clinical and Experimental Allergy. 2015;45:908–19. doi: 10.1111/cea.12505. [DOI] [PubMed] [Google Scholar]
- 35.Lebovitz HE, Ludvik B, Kozakowski J, et al. Gastric electrical stimulation treatment of type 2 diabetes: effects of implantation versus meal-mediated stimulation. A randomized blinded cross-over trial. Physiological Reports. 2015;3:e12456. doi: 10.14814/phy2.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thabit H, Tauschmann M, Allen JM, et al. Home Use of an Artificial Beta Cell in Type 1 Diabetes. The New England Journal of Medicine. doi: 10.1056/NEJMoa1509351. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) [Internet]. Silver Spring, Maryland. [Accessed October 22, 2015];Guideline for the Format and Content of the Clinical and Statistical Sections of an Application. 1988 Available at http://www.fda.gov/downloads/Drugs/.../Guidances/UCM071665.pdf.
- 38.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use [Internet]. Geneva, Switzerland. [Accessed October 22, 2015];Statistical Principles for Clinical Trials. 1998 :E9. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf.
- 39.Iacono P, Parodi MB, Papayannis A, et al. Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina. 2012;32:1539–46. doi: 10.1097/IAE.0b013e31826956b7. [DOI] [PubMed] [Google Scholar]
- 40.Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121:682–92. doi: 10.1016/j.ophtha.2013.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.