Abstract
The importance of relationships between handedness, language lateralization and localization, and white matter tracts for language performance is unclear. The goal of the study was to investigate these relationships by examining arcuate fasciculus (AF) structural asymmetry (DTI) and functional asymmetry (fMRI) in language circuits, handedness, and linguistic performance. A large sample of right‐handed (n = 158) and atypical‐handed (n = 82) healthy adults underwent DTI at 3 T to assess number of streamlines and fractional anisotropy (FA) of the AF, and language fMRI. Language functions were assessed using standard tests of vocabulary, naming, verbal fluency, and complex ideation. Laterality indices (LIs) illustrated degree of asymmetry and lateralization patterns for the AF (streamlines and FA) and verb generation fMRI. Both handedness groups showed leftward lateralization bias for streamline and fMRI LIs and symmetry for FA LI. The proportion of subjects with left, right, or symmetric lateralization were similar between groups if based on AF LIs, but differed if based on fMRI LIs (p = 0.0016). Degree of right‐handedness was not associated with AF lateralization, but was associated with fMRI language lateralization (p = 0.0014). FA LI was not associated with performance on language assessments, but streamline LI was associated with better vocabulary and complex ideation performance in atypical‐handed subjects (p = 0.022 and p = 0.0098, respectively), and better semantic fluency in right‐handed subjects (p = 0.047); however, these did not survive multiple comparisons correction. We provide evidence that AF asymmetry is independent of hand preference, and while degree of right‐handedness is associated with hemispheric language lateralization, the majority of atypical‐handed individuals are left‐lateralized for language. Hum Brain Mapp 37:3297–3309, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: handedness, tractography, arcuate fasciculus, fMRI, structural asymmetry, language lateralization, lateralization index
INTRODUCTION
In the majority of humans, language functions are lateralized to the left hemisphere. The left‐hemispheric language lateralization has genetic roots that are further modified by environmental, developmental, pathological, and societal influences [Corballis, 2009; Francks et al., 2002, 2007]. Recent noninvasive imaging studies have shown that about 85–95% of right‐handers have left‐hemispheric dominance for various aspects of language [Knecht et al., 2000a, 2000b; Mazoyer et al., 2014; Mellet et al., 2014; Pujol et al., 1999; Somers et al., 2015; Springer et al., 1999; Szaflarski et al., 2006]. In subjects with atypical handedness (i.e., left‐handed or ambidextrous), the incidence of left‐hemispheric language dominance is approximately 60–80% with the remaining subjects having bilateral or right‐hemispheric language representation [Mazoyer et al., 2014; Mellet et al., 2014; Pujol et al., 1999; Somers et al., 2015; Szaflarski et al., 2002, 2012]. Further, these and other studies suggest that even when left‐lateralized, the strength of the language lateralization in subjects with atypical handedness is lower than in typical right‐handers with many contributing factors including personal and familial handedness and structural brain asymmetries in the gray matter and the underlying white matter tracts [Binder et al., 1996; Shapleske et al., 1999; Somers et al., 2015; Szaflarski et al., 2002; Tzourio‐Mazoyer et al., 2010a, 2010b]. There is great interest in the relationship between handedness and hemispheric language dominance, and interestingly, recent studies show that lateralization of hand preference is only weakly related to or may be even independent of lateralization of language functions [Groen et al., 2013; Mazoyer et al., 2014 ; Somers et al., 2015 ; Willems et al., 2014].
In addition to a number of studies on handedness and hemispheric language lateralization, an increased focus on their relation to the symmetry of language‐related brain structures with particular attention to the arcuate fasciculus (AF) has been noted. The increased focus on the AF is based on the lack of clarity of its specific role in normal language functions (e.g., repetition and naming) and pathological language processing/recovery from injury as well as the recent availability of noninvasive ways of visualizing it in humans [Berthier et al., 2012; Catani and Mesulam, 2008; Dick and Tremblay, 2012; van Hees et al., 2014; Wang et al., 2013]. Anatomically, the AF connects frontal and temporal language areas via several direct and indirect pathways—the direct pathway being the classical AF with the indirect pathways traveling through the inferior parietal lobe contributing to the observed diversity in the presentation of postinjury aphasias [Catani et al., 2005]. In a recent study, left‐lateralization of the reconstructed AF streamlines of the direct pathway was observed in >80% of healthy subjects while the remaining subjects had fairly symmetric AF representation [Catani et al., 2007]. The AF symmetry was associated with better verbal recall of newly learned words while asymmetry of the AF did not provide any advantage in that study. Another small study reported leftward asymmetry of the AF among 18 right‐handers with left > right fractional anisotropy (FA) but no differences in mean diffusivity [Rodrigo et al., 2007]. An overall leftward asymmetry of the AF was also found irrespective of handedness or functional language lateralization in 20 healthy subjects, with the functional and structural asymmetries correlating in right‐ but not in left‐handers [Vernooij et al., 2007]. Thus, although there has been some evidence within these studies, the influence of handedness on structural asymmetry of the AF and the relationship between structural asymmetry and specific language functions remains unclear. This is partly due to paucity of studies that include large numbers of individuals and, in particular, subjects with atypical handedness.
In this study, we have acquired a large sample consisting of 249 healthy individuals with varying degrees of handedness, allowing for the investigation of the relationships between handedness, the structural asymmetry of the AF using diffusion tensor imaging (DTI), functional asymmetry in language processing using verb generation fMRI, and performance on standardized tests of language capabilities. DTI is a noninvasive MRI technique that measures random displacements of water molecules in brain tissue to reconstruct and visualize white matter fiber pathways in vivo [Basser et al., 1994, 2000; Conturo et al., 1999; Le Bihan et al., 2001; Pierpaoli et al., 1996]. Fractional anisotropy (FA) is a DTI measure shaped by features such as myelination and/or axonal density within white matter; it reflects the strength and integrity of anatomical connections such as the AF [Beaulieu, 2002]. By applying deterministic fiber tracking, we can reconstruct streamlines belonging to major white‐matter fiber tracts in the brain. This allows us to specifically examine the lateralization patterns of the direct pathway of the AF in healthy individuals with right and atypical handedness and investigate the relationship between AF asymmetry (i.e., FA and number of streamlines) and performance on a number of language domains including naming and verbal fluency. Recent studies in patients with aphasia following left‐hemispheric stroke have shown that lesion load of the left AF is predictive of naming ability [Marchina et al., 2011] and that increased mean generalized FA of the left AF is associated with retaining improved naming abilities from anomia therapy [van Hees et al., 2014]. Based on the available data, we hypothesized that a greater proportion of the right‐handed subjects would exhibit leftward AF asymmetry in the number of streamlines when compared to participants with atypical handedness and that an increased leftward FA asymmetry for the AF would contribute to better linguistic performance.
MATERIALS AND METHODS
Participants
Participants included 249 healthy male and female subjects between the ages of 18 and 76 years, who were recruited from December 2008 to January 2014 through local advertisements and by word of mouth. This research was part of a larger study (NIH R01‐NS048281), which was approved by the University of Cincinnati (UC), Cincinnati Children's Hospital Medical Center (CCHMC), and University of Alabama at Birmingham (UAB) Institutional Review Boards. All study procedures were performed in accordance with the ethics principles of the Declaration of Helsinki and the principles of informed consent. Each subject provided written informed consent prior to participation in the study and had no known contraindications to receiving MRI at 3 T. Health status was confirmed by asking participants a series of questions evaluating for the presence and/or absence of any pre‐existing or ongoing neurological or psychiatric conditions. Handedness was determined by using the Edinburgh Handedness Inventory (EHI) [Oldfield, 1971]. Subjects scoring >50 on the EHI were considered right‐handed, whereas the remaining subjects were categorized as exhibiting atypical handedness, although EHI was also assessed as a continuous variable to characterize relationships with the degree of right‐handedness (see Data Analysis section below). There were 201 participants at UC (137 right‐handers and 64 with atypical handedness) and 48 at UAB (26 right‐handers and 22 with atypical handedness). Of the 249 subjects, 8 were not included in analyses due to claustrophobia/inability to complete the scan (n = 5), presence of psychiatric condition disclosed following study completion (n = 1), or not undergoing the scan protocol (n = 2). An additional subject with atypical handedness was excluded from analysis due to no detectable direct pathway of the arcuate fasciculus in either of the hemispheres during deterministic fiber tracking. Thus, the final sample included 240 healthy subjects (108 male, 132 female), who were either right‐handed (158 subjects; 71 male, 87 female) or had atypical handedness (82 subjects; 37 male, 45 female). Demographic variables are summarized in Table 1.
Table 1.
Demographic and performance characteristics of subjects enrolled in the study
| Right‐handed | Atypical | |||
|---|---|---|---|---|
| N, % females | 158 | 55.1 | 82 | 54.9 |
| Age, yearsa | 43.5 | 15.1 | 35.7 | 12.3 |
| Cerebral volume, ml | 1426.5 | 133.7 | 1441.8 | 168.1 |
| Language assessments | ||||
| Boston Naming Test score | 56.3 | 4.3 | 55.7 | 4.3 |
| Peabody Picture Vocabulary Test scoreb | 213.4 | 12.0 | 211.3 | 10.8 |
| Controlled Oral Word Association Test score | 39.6 | 11.5 | 40.7 | 12.1 |
| Semantic Fluency Test score | 56.6 | 12.7 | 56.3 | 14.1 |
| Complex Ideation score | 11.5 | 0.8 | 11.4 | 1.0 |
| Noun recognition accuracy, % correctc | 92.8 | 7.7 | 93.6 | 8.5 |
Note. Data are reported as mean and S.D. except for number of subjects (N), which are reported as frequency and percentages.
Right‐handed subjects were significantly older than those with atypical handedness (p < 0.001).
Not assessed for one right‐handed female subject.
Assessed for 141 right‐handed subjects and 73 subjects with atypical handedness who completed the verb generation fMRI task.
Language Assessments
Language measures that were administered included the Boston Naming Test, Second Edition (BNT; Kaplan et al. [2001]) to assess naming performance, the Peabody Picture Vocabulary Test, Fourth Edition (PPVT) to examine receptive vocabulary [Dunn and Dunn, 2007], two tests of verbal fluency which were scored based on the number of words generated in one minute for a given letter (Controlled Oral Word Association Test (COWAT); Lezak [1995]) or a given category (Semantic Fluency Test (SFT); Kozora and Cullum [1995]; Lezak [1995]), and the Complex Ideation subset of the Boston Diagnostic Aphasia Examination (CI) to test oral comprehension and recall of information [Goodglass and Kaplan, 1972].
Functional MRI Language Task
In addition to DTI, subjects also completed a well‐established verb generation task [Petersen et al., 1988]. This task involves the auditory presentation of a series of nouns in a 30 s block, one noun every 5 s, during which subjects are instructed to mentally generate as many related verbs for each noun. The subjects were required to generate verbs covertly to reduce the motion artifact related with speech. Five 30 s blocks of the active condition (verb generation) were interleaved with control blocks. During the six 30 s control blocks, subjects were instructed to perform bilateral finger tapping every time they heard a modulated 400 Hz tone. The rate of finger tapping was paced by the target tone, once every 5 s. This task was designed as a control for the auditory stimulation present in the verb generation task, and to distract the subjects from generating verbs during the control period; it has been used to assess functional hemispheric language dominance in many fMRI studies including ours [e.g., Allendorfer et al., 2012b; Szaflarski et al., 2008, 2012]. All subjects learned the verb generation task and performed a trial run to ensure their understanding of the task. Subjects were required to generate at least one verb associated with each of the presented nouns during the trial run before entering the scanner. Immediately after scanning was complete, all subjects were administered a noun recognition test to assess engagement in the verb generation task.
MRI Data Acquisition
Imaging of 201 healthy subjects was performed at the Cincinnati Children's Hospital Imaging Research Center (IRC) using a 3.0 T Philips MR system and 8‐channel head coil. An additional 48 subjects were scanned using a 3.0 T Siemens MR System and a circular polarized head coil at the Civitan Functional Neuroimaging Laboratory (CFNL) at the University of Alabama at Birmingham. All subjects were fitted with an MRI‐compatible headset to communicate with research staff. Subjects also held a response device in their left hand, and were equipped with either video goggles at the IRC or a mirror/screen system at the CFNL for viewing visual stimuli as part of a functional MRI study. After subjects were positioned in the scanner, a three‐plane localizer scan was performed for alignment and brain localization, followed by a shim procedure to generate a homogeneous magnetic field. Next, anatomical scans were acquired for localization of brain activation maps. A high‐resolution T1‐weighted 3D anatomical scan was acquired for brain localization using a magnetization‐prepared rapid acquisition with gradient echo (MP‐RAGE) sequence. At the IRC, MP‐RAGE parameters were as follows: TR/TE = 8.1/3.7 ms, flip angle = 8°, FOV 25.0 × 21.0 × 18.0 cm, matrix 252 × 210, slice thickness = 1 mm. MP‐RAGE parameters at the CFNL were as follows: TR/TE = 2300/2.17 ms, flip angle = 9°, FOV 25.6 × 25.6 × 19.2 cm, matrix 256 × 256, slice thickness = 1 mm. The DTI was performed using an echo planar image sequence with care taken to keep the number of diffusion directions and b‐values consistent between scanners: one image with no diffusion weighting (b = 0 s/mm2) and diffusion‐weighted images (DWIs) in 32 distinct directions (b = 800 s/mm2). This allowed us to acquire the same number of distinct directions for tensor estimation and keep in line with the 30 directions determined to be required for reliable estimation of the tensor orientation [Jones, 2004]. The DTI parameters at the IRC were as follows: TR/TE = 9403/69 ms, FOV 18.0 × 16.1 cm, matrix 76 × 67, slice thickness = 2.37 mm. The DTI parameters at the CFNL were TR/TE = 9400/89 ms, FOV 24.0 × 24.0 cm, matrix 96 × 96, slice thickness = 2.5 mm. T2*‐weighted fMRI scans were acquired using the following parameters at the IRC and the CFNL: TR/TE = 2000/38 ms, FOV 24.0 × 24.0 cm, matrix 64 × 64, flip angle = 90°, slice thickness = 4 mm.
Functional MRI Data Processing
All functional imaging data were preprocessed and modeled using Matlab toolbox SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). For each run, the first 15 volumes were discarded. Then, the spatial discrepancy between slices due to head motion was corrected, and structural scans were spatially registered to functional scans. Following coregistration, an algorithm for unified segmentation was used to spatially normalized functional scans to MNI space [Ashburner and Friston, 2005]. Finally, functional scans were spatially smoothed with an 8‐mm kernel full width half‐maximum. Data were modeled using a general linear model (GLM) in SPM8 (first‐level analysis, by convolution of the block‐design tasks with the canonical hemodynamic response function). Group random effects were computed using a one‐sample t‐test analysis on all individual contrasts obtained after first‐level analysis.
DTI Data Processing
The anatomical MP‐RAGE scans were reconstructed using the Analysis of Functional NeuroImages (AFNI) software [Cox, 1996] and DTI scans were reconstructed using the dcm2niigui application in MRIcron [Rorden et al., 2007]. Alignment of the anatomical and DTI data for each subject was performed using tools in AFNI. Gross spatial alignment of the MP‐RAGE to the DTI scans was performed using the “Nudge Dataset” plugin and using the values as input for 3drefit. The DTI image with no diffusion weighting was then aligned with the MP‐RAGE using the local Pearson correlation method [Saad et al., 2009] in the 3dAllineate program, followed by eddy current distortion correction and motion‐correction of the 32 DWIs using 3D affine registration to the first acquired DWI. We then combined the intermediate spatial transformations and applied a single interpolation to the original DTI data to align to the MP‐RAGE and correct for head motion and gradient distortions. To account for the combined alignments of DTI to MP‐RAGE and motion‐correction steps to the 32 diffusion gradients, Octave (http://www.octave.org) was used to perform polar decomposition to obtain the rigid body component of the motion corrections that were applied [Rohde et al., 2004].
Fiber Tracking of the Arcuate Fasciculus
White‐matter deterministic fiber tracking was performed using the Diffusion Toolkit and TrackVis software [Wang et al., 2007]. The aligned and motion‐corrected DTI data and diffusion gradients were used as inputs into Diffusion Toolkit, where deterministic tractography was performed using the Fiber Assignment by Continuous Tracking (FACT) algorithm with an angle threshold of 41° [Wakana et al., 2004, 2007], FA threshold of 0.20, and application of a spline filter. The thresholds and tracking parameters were implemented to minimize the inclusion of voxels that are not part of white matter tracts [Lebel and Beaulieu, 2009]. Diffusion Toolkit was then used to visualize the 3D tract reconstruction and to isolate the streamlines that comprised the direct pathway of the AF using a region of interest (ROI) approach. Diffusion tensor tractography using previously published anatomical landmarks [Lebel and Beaulieu, 2009; Rodrigo et al., 2007] and the two‐ROI approach was used to manually delineate the direct pathway of the AF in each hemisphere for each subject (Fig. 1A). Each ROI was drawn as a large rectangle that does not cross through the plane of the corresponding ROI in the same hemisphere to encompass all possible streamlines belonging to the AF. Using the color‐coded diffusion map (Fig. 1A‐1, left panel), the starting ROIs were drawn in the temporal part of each hemisphere on an axial slice lateral to the ventricular trigone (L1 in the left hemisphere and R1 in the right hemisphere), through which the AF passes in the inferior–superior direction. Initially, all streamlines passing through any part of the two starting ROIs were visualized (Fig. 1A‐1, right panel). Again, using the color‐coded diffusion map (Fig. 1A‐2, left panel), the target ROI in the left hemisphere was selected on a coronal slice at the level of the frontal operculum (L2), through which the AF passes in the anterior–posterior direction [Lebel and Beaulieu, 2009; Rodrigo et al., 2007]. Only streamlines passing through both L1 and L2 were used to define the left AF, but at this step, all streamlines passing through R1 were still visualized (Fig. 1A‐2, right panel). The right‐hemisphere target ROI (R2) was drawn in a similar fashion on the color‐coded diffusion map (Fig. 1A‐3, left panel), and only streamlines passing through both R1 and R2 were used to define the right AF (Fig. 1A‐3, right panel). Thus, the selected landmarks and included streamlines correspond to the AF proper (i.e., the direct pathway or the long segment of the AF) as previously described [Catani et al., 2005], and only streamlines passing through these two ROIs in each hemisphere were then included in the statistical analysis.
Figure 1.
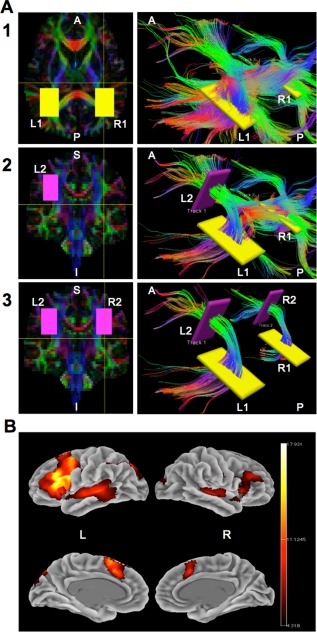
Visualization of the reconstructed streamlines of the direct pathway of the arcuate fasciculus (AF) and the group fMRI activation maps. (A) Diffusion Toolkit was used to visualize the 3D tract reconstruction (right column) and delineate the arcuate fasciculus streamlines in each hemisphere for each subject using the color‐coded diffusion map and a region of interest (ROI) approach (left column). Portions of streamlines along the anterior–posterior direction are represented in green, along the left‐to‐right direction are represented in red/orange, and along the superior–inferior direction are represented in blue/purple. (1) First, two large rectangular ROIs were manually drawn for each hemisphere on an axial slice (left panel) lateral to the ventricular trigone in the temporal region (L1 and R1 in yellow). All streamlines passing through any part of these two ROIs are visualized in the right panel. (2) Next, a large rectangular ROI is drawn on a coronal slice (left panel) at the level of the left frontal operculum (L2 in pink). Only streamlines that pass through both L1 and L2 are counted as part of the left hemisphere AF (Track 1), but all streamlines passing through R1 are still visualized, as shown in the right panel. (3) Then, a second rectangular ROI is drawn on the coronal slice (left panel) at the level of the right frontal operculum (R2 in pink). Only streamlines that pass through both R1 and R2 are counted as part of the right hemisphere AF (Track 2). (B) Statistical parametric map of group activation during the verb generation fMRI task for a cohort of 214 healthy subjects. Clusters of significant activation (FWE p < 0.05) were utilized to create a binary mask for calculating a lateralization index value for each subject. L = left, R = right, A = anterior, P = posterior, S = superior, I = inferior.
Lateralization Index and Laterality
A lateralization index (LI) was adopted from previous fMRI studies [Szaflarski et al., 2006; Tillema et al., 2008], and DTI‐based LI in our study was calculated for each subject based on the structural asymmetry of AF [Lebel and Beaulieu, 2009] to determine whether the sample included in this study is representative of the population as a whole and to allow generalization of the findings. Once the AF streamlines for each subject have been delineated using the two ROI approach, Diffusion Toolkit reports the average number of streamlines in each hemisphere. FA values were also calculated by averaging across all voxels in the streamlines comprising the reconstructed direct pathway of the AF for each hemisphere. DTI‐based LI for the number of streamlines (SLI) and for FA (FALI) were defined as (L − R)/(L + R) − the difference between the number of streamlines or average FA in the left and right hemisphere AF streamlines divided by the sum of streamlines or average FA in the left‐ and right‐hemisphere AF streamlines. This formula yields LIs ranging between −1.0 (strong right‐hemisphere dominance) and +1.0 (strong left‐hemisphere dominance). To further characterize DTI asymmetry, LI values were then classified akin to fMRI studies as left‐lateralized with LI ≥ 0.1, symmetric (−0.1 < LI < +0.1), or right‐lateralized with LI ≤ −0.1 [Szaflarski et al., 2006; Tillema et al., 2008]. These threshold values were originated statistically for fMRI data by determining the mean number of activated voxels across all subjects and testing different hypothetical partitions of this total between hemispheres against a null hypothesis of equal activation in each hemisphere using χ 2 analysis [Binder et al., 1995].
Language lateralization indices (VLIs) were computed using LI‐toolbox [Wilke and Lidzba, 2007] on T‐maps obtained after GLM analysis for verb generation fMRI data (Fig. 1B) and similar to the LI calculations used by our group in the past in healthy and diseased populations [Allendorfer et al., 2012a; Szaflarski and Allendorfer, 2012; Szaflarski et al., 2012]. To better reflect the language lateralization of cortical activity of brain regions for each participant, a functional mask was applied to their T‐map that defined the voxels used for VLI calculation. This functional mask was created using the thresholded verb generation fMRI group GLM maps (FWE p < 0.05) that were first binarized (one sample t‐test over all participants), and then voxels above threshold in the left hemisphere were mirrored over the right hemisphere and vice versa to obtain a symmetric mask. Voxels located into a 20 mm midline (axial) were not included into the mask. For each T‐map, the mean intensity of the voxels in the functional mask served as a threshold (i.e., “adaptive threshold” in the LI‐toolbox). In this method of LI calculation, voxels with T‐values above that internal threshold are added to give a global value for each hemisphere. Then, the VLI value is calculated based on the same equation as for the DTI data, in which VLI is defined as the difference in the number of voxels meeting the above‐described threshold between the left and right hemispheres and divided by the sum of the number of voxels meeting threshold between the two hemispheres. Statistical outliers were removed by using data clustering and variance weighting options [Wilke and Lidzba, 2007, eqs 3 and 4 for details].
Data Analysis
Statistical analyses were performed using SAS (Statistical Analysis System version 9.4, Cary, NC). Independent‐samples t‐tests (two‐tailed) were used to assess group differences between right‐handed and atypical‐handed subjects in age, cerebral volume, language assessment scores, SLI, FALI, VLI, and the noun recognition post‐test. The F‐test for equal variances was performed, and the Satterthwaite method was used in cases of unequal variance. Chi‐squared tests were performed to assess differences between handedness groups in their distribution of sex and lateralization patterns (left‐lateralized, symmetric, and right‐lateralized) based on SLI, FALI, and VLI. Regression analysis was used to assess the effect of handedness on the number of AF streamlines and FA in each hemisphere while accounting for cerebral volume and scanner type. Regression analysis was also performed to examine the relationship between the degree of right‐handedness (i.e., analyzing EHI as a continuous variable) and all three types of LIs while accounting for scanner type (associations are significant at p = 0.016 after Bonferroni correction for multiple comparisons).
The relationship between tract‐specific asymmetry of the AF (i.e., SLI and FALI) and language performance were assessed using regression analysis while accounting for scanner type (associations are significant at p = 0.0041 after Bonferroni correction for multiple comparisons). These analyses were performed for the combined group of subjects and for each handedness group. Since sex differences in AF white matter structure have been previously described [e.g., Catani et al., 2007; Madhavan et al., 2014], we also investigated the effect of sex in these measures given the inconsistencies in the literature and the previous findings of similar language performance, verb generation fMRI activation patterns, and language LI scores between males and females [Allendorfer et al., 2012b], in addition to similar FA LI values for the AF [Catani et al., 2007].
RESULTS
Demographic and Performance Variables
Handedness groups did not differ in the distribution of males and females (χ 2 = 0.007; p = 0.98). Right‐handed subjects were significantly older than those with atypical handedness (p < 0.001); therefore, correlations of age with all three types of LIs (SLI, FALI, and VLI) were performed to test potential effects, and none were significant (all p > 0.05). All subjects completed the language assessments, except one subject did not correctly perform the PPVT and was excluded from assessment analyses that involved this measure. There were no significant differences in scores on the BNT (p = 0.27), PPVT (p = 0.19), COWAT (p = 0.50), SFT (p = 0.86), and CI (p = 0.21) between the right‐handed and atypical‐handed subjects. A subset of 214 subjects successfully completed the verb generation fMRI task, and performance on the postscan noun recognition test was similar between handedness groups (p = 0.49). Table 1 summarizes the demographic and performance variables for each handedness group.
DTI Fiber Tracking
Across the entire sample of 240 subjects, the left‐hemisphere AF streamlines were tracked in 236 subjects (98.3%), the right‐hemisphere AF streamlines were tracked in 170 subjects (70.8%), and AF in both hemispheres were tracked in 166 subjects (69.2%). Our inability to track the AF streamlines in both hemispheres for some subjects is consistent with previous studies including Catani et al. [2007] that were not able to identify a right hemisphere AF in 62.5% of their right‐handed adults, and Lebel and Beaulieu [2009] that found 63 individuals to have no right‐hemisphere AF streamlines and 6 to have no left‐hemisphere AF streamlines in their large sample of 207 individuals.
There was a significant effect of handedness on the number of streamlines in the left hemisphere AF after accounting for cerebral volume and scanner type (F = 5.77, p = 0.017) in which right‐handed subjects had a greater number of streamlines (mean ± S.D. = 143.09 ± 92.73) compared to atypical‐handed subjects (mean ± S.D. = 114.05 ± 79.92, p = 0.017). Both groups had a similar number of streamlines in the right hemisphere AF (mean ± S.D. = 52.66 ± 63.64 in right‐handed; mean ± S.D.= 47.06 ± 65.55 in atypical‐handed; F = 0.43, p = 0.51). Similar to the strategy of Lebel and Beaulieu [2009], the asymmetry of FA values was investigated only for individuals with bilateral streamlines (n = 166). FA was similar between groups for both the left hemisphere AF (mean ± S.D. = 0.53 ± 0.024 in right‐handed; mean ± S.D = 0.54 ± 0.028 in atypical‐handed; F = 1.64, p = 0.20) and the right‐hemisphere AF (mean ± S.D. = 0.50 ± 0.030 in right‐handed; mean ± S.D = 0.51 ± 0.038 in atypical‐handed; F = 0.69, p = 0.41). The number of streamlines and FA for each handedness group is summarized in Table 2.
Table 2.
The number of streamlines and fractional anisotropy of the arcuate fasciculus in the left and right hemispheres
| Right‐handed | Atypical | |||||
|---|---|---|---|---|---|---|
| Mean | S.D. | Range | Mean | S.D. | Range | |
| Number of streamlines | ||||||
| Left hemispherea | 143.09 | 92.73 | 0–431 | 114.05 | 79.92 | 0–372 |
| Right hemisphere | 52.66 | 63.64 | 0–328 | 47.06 | 65.55 | 0–323 |
| Fractional anisotropy | ||||||
| Left hemisphere | 0.53 | 0.027 | 0.42–0.60 | 0.54 | 0.024 | 0.44–0.58 |
| Right hemisphere | 0.50 | 0.029 | 0.42–0.57 | 0.51 | 0.037 | 0.41–0.60 |
There was a significant effect of handedness on the number of streamlines in the left hemisphere after accounting for scanner type and cerebral volume (F = 5.77, p = 0.017).
Relating Handedness with Hemispheric Lateralization
Based on all three types of LIs (SLI, FALI, and VLI), subjects were divided according to their hemispheric lateralization pattern as showing left, symmetric, or right lateralization (Table 3). SLI was calculated for all subjects, ranging from −1 (streamlines only in the right‐hemisphere AF) and +1 (streamlines only in the left‐hemisphere AF), and were similar between handedness groups (p = 0.85). Left‐hemisphere lateralization for SLI occurred in 128 right‐handed subjects (81.0%) and 67 subjects with atypical‐handedness (81.7%), symmetric in 15 right‐handed (9.5%) and 4 subjects with atypical handedness (4.9%), and right hemisphere lateralization in the remaining 15 subjects who are right‐handed (9.5%) and 11 subjects who had atypical handedness (13.4%). The distribution of laterality based on SLI was not significantly different between groups (χ 2 = 2.22; p = 0.33). SLI was not associated with EHI scores (F = 0.57; p = 0.45).
Table 3.
Lateralization indices (LIs) and hemispheric lateralization for the enrolled subjects
| Arcuate fasciculus number of streamlines (SLI) | Arcuate fasciculus fractional anisotropy (FALI) | Verb generation fMRIa (VLI) | ||||
|---|---|---|---|---|---|---|
| Right‐handed (N = 158) | Atypical (N = 82) | Right‐handed (N = 113) | Atypical (N = 53) | Right‐handed (N = 141) | Atypical (N = 73) | |
| LIs | 0.54 (0.45) | 0.52 (0.52) | 0.032 (0.027) | 0.030 (0.039) | 0.44 (0.30) | 0.35 (0.44) |
| Lateralizationa | ||||||
| Left | 128 (81.0) | 67 (81.7) | 2 (1.8) | 1 (1.9) | 125 (88.7) | 55 (75.34) |
| Symmetric | 15 (9.5) | 4 (4.9) | 111 (98.2) | 52 (98.1) | 12 (8.5) | 6 (8.22) |
| Right | 15 (9.5) | 11 (13.4) | 0 (0) | 0 (0) | 4 (2.8) | 12 (16.44) |
Lateralization indices for fractional anisotropy (FALI) were determined only for the portion of subjects, in which streamlines for the direct pathway of the arcuate fasciculus were reconstructed in both hemispheres. Also, LIs based on the verb generation fMRI task (VLI) were determined only for the portion of subjects who underwent both fMRI and DTI procedures
Note. Data are reported as mean (S.D.) for lateralization indices (LIs) and as frequency (percentages) for the distribution of lateralization (left, symmetric, and right). Lateralization indices for arcuate fasciculus (SLI and FALI) and verb generation fMRI (VLI) were determined with LI ≥ 0.1 as left‐lateralized, LI ≤ −0.1 as right‐lateralized, and −0.1 < LI < 0.1 as symmetric.
Verb generation fMRI lateralization patterns were significantly different between right‐handed subjects and atypical handedness (χ 2 = 12.92; p = 0.0016).
Consistent with the investigation of asymmetry of FA values above [Lebel and Beaulieu, 2009], FALI and corresponding categorization of laterality were performed only for the 166 subjects with bilateral AF streamlines. FALIs were close to zero and did not differ between handedness groups (p = 0.75). The majority of subjects (about 98% for both right‐handed and atypical‐handed subjects) showed symmetric lateralization based on FALI, while few displayed left lateralization (<2% for both groups), and no subjects from either group were lateralized to the right hemisphere. Overall, the distribution of laterality based on FALIs was not significantly different between groups (χ 2 = 0.0028; p = 0.96). FALI was also not associated with EHI (F = 0.17; p = 0.68).
For the verb generation fMRI task, VLIs were not significantly different between right‐handed subjects and subjects with atypical handedness (p = 0.092). We observed leftward predominance of language lateralization based on VLI for both right‐handed subjects (88.7%) and subjects with atypical handedness (75.34%), with similar proportions of symmetric lateralization (8.5% right‐handed and 8.22% atypical‐handed, respectively), and a greater proportion of right‐hemisphere lateralization in subjects with atypical handedness (16.44%) compared to the right‐handed (2.8%) subjects. The overall distribution of laterality based on language fMRI activation was significantly different between handedness groups (χ 2 = 12.92; p = 0.0016). Further, VLI was significantly associated with EHI (F = 10.51; p = 0.0014; association was significant at p = 0.016 after Bonferroni correction).
Relating Arcuate Fasciculus Asymmetry to Language Performance
Associations between asymmetry of the AF and language performance are summarized in Table 4. FALI was not significantly associated with performance on language assessments in the combined sample or in the separate handedness groups. SLI was positively associated with SFT performance in the right‐handed subjects (F = 4.00, p = 0.047), while in subjects with atypical handedness, we observed a positive association between SLI and performance on both the PPVT (F = 5.44, p = 0.022) and the CI (F = 7.00, p = 0.0098), although these associations were not significant after correction for multiple comparisons (statistically significant at p = 0.0041 after Bonferroni correction).
Table 4.
Results of regression analyses investigating how arcuate fasciculus (AF) asymmetry, based on either fractional anisotropy (FALI) or the number of streamlines (SLI), affects performance on language assessments while accounting for scanner type
| BNT | PPVT | COWAT | SFT | CI | VG Post | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| FALI | ||||||||||||
| All subjects | 1.33 | 0.25 | 0.73 | 0.39 | 0.04 | 0.84 | 1.98 | 0.16 | 0.61 | 0.44 | 0.21 | 0.65 |
| Right‐handed | 1.28 | 0.26 | 1.43 | 0.23 | 0.34 | 0.56 | 1.96 | 0.16 | 1.31 | 0.25 | 0.10 | 0.75 |
| Atypical | 1.82 | 0.18 | 0.00 | 0.98 | 0.00 | 0.94 | 0.37 | 0.54 | 0.11 | 0.74 | 1.00 | 0.32 |
| SLI | ||||||||||||
| All subjects | 1.01 | 0.32 | 0.72 | 0.40 | 0.52 | 0.47 | 2.54 | 0.11 | 3.39 | 0.067 | 0.97 | 0.33 |
| Right‐handed | 0.30 | 0.58 | 0.91 | 0.34 | 1.25 | 0.27 | 4.00 | 0.047 | 0.03 | 0.86 | 0.55 | 0.46 |
| Atypical | 3.56 | 0.063 | 5.44 | 0.022 | 0.02 | 0.89 | 0.03 | 0.87 | 7.00 | 0.0098 | 0.34 | 0.56 |
Note. F‐value (F) and corresponding p‐value (p) reported for results in all subjects combined, in right‐handed subjects, and in subjects with atypical handedness. BNT = Boston Naming Test; PPVT = Peabody Picture Vocabulary Test; COWAT = Controlled Oral Word Association Test; SFT = Semantic Fluency Test; CI = Complex Ideation; VG Post = verb generation postscan noun recognition test.
Effects of Sex on Language‐Related Performance and Asymmetry
Preliminary analyses were performed to assess sex differences in language performance and the patterns of lateralization for all subjects combined (132 females; 108 males), in right‐handed subjects (87 females; 71 males) and in those with atypical handedness (45 females; 37 males). Age for males and females were similar in all groups. Performance on all language assessments was not significantly different between males and females in the combined sample or in the handedness groups (all p > 0.05). SLI, FALI, and VLI as well as the corresponding distributions of left, right, and symmetric lateralization were similar between males and females for the entire sample and for each of the handedness groups (all p > 0.05).
DISCUSSION
This study utilized DTI to primarily examine the relationships between handedness and hemispheric asymmetry of the direct pathway of the AF and whether or not asymmetry in the strength/integrity of the AF fronto‐temporal connections correlates with language abilities in a large sample of 240 healthy individuals. We hypothesized that a greater proportion of right‐handed subjects would exhibit leftward AF asymmetry in the number of streamlines when compared to subjects with atypical handedness and that an increased leftward asymmetry of FA for the AF would contribute to better language performance. Our main findings are that (1) there is a left‐greater‐than‐right hemispheric asymmetry of the AF with regard to SLI and symmetry with regard to FALI regardless of handedness; (2) handedness groups exhibit similar distribution patterns of lateralization (i.e., left, right, and symmetric) based on SLI and FALI (i.e., handedness has no effect on AF symmetry) but significantly different distribution patterns of language lateralization based on VLI, and while the degree of right‐handedness (EHI) was not associated AF lateralization, it was associated with fMRI language lateralization; and (3) leftward asymmetry in the number of streamlines is significantly associated with SFT performance in right‐handed subjects, PPVT and CI performance in atypical‐handed subjects, as well as CI performance when handedness groups are combined. We discuss these findings in greater detail.
Our results indicate that right‐handed subjects and subjects with atypical handedness are similar not only in language performance, but also in that the proportion of the number of AF streamlines favors the left hemisphere when compared to the right hemisphere; this is in line with previous DTI studies that focused on right‐handed subjects [Catani et al., 2007; Lebel and Beaulieu, 2009; Nucifora et al., 2005; Powell et al., 2006]. Further, our findings provide evidence that subjects with atypical handedness also exhibit leftward asymmetry in the number of AF streamlines, confirming reports from smaller studies that both handedness groups demonstrate a high number of AF streamlines in the left relative to the right hemisphere irrespective of the direction of hand preference [Propper et al., 2010; Vernooij et al., 2007]. Finally, these results parallel recent findings in large studies showing that the planum temporale, a small section of the superior temporal gyrus that partially overlaps with Wernicke's area and that is often the focus in the discussion of left hemisphere language laterality, is also leftward asymmetric regardless of handedness [Dos Santos Sequeira et al., 2006; Eckert et al., 2006; Tzourio‐Mazoyer et al., 2010b ; Willems et al., 2014], although the relationship between the AF and planum temporale asymmetries is not straightforward. While it should be noted that right‐handed subjects demonstrated a greater number of streamlines in the left‐hemisphere AF compared to atypical‐handed subjects, this did not translate into significant differences in SLI, FALI, or the corresponding AF lateralization patterns between the handedness groups.
With our classification of lateralization, FALIs for both handedness groups are considered symmetric. However, the positive mean values for FALIs indicate a bias towards left‐greater‐than‐right asymmetry of FA for the direct pathway of the AF. Our findings agree with previous research in right‐handers that found mean FA values were significantly greater in the left hemisphere AF than in the right [Buchel et al., 2004; Cao et al., 2003; Floel et al., 2009; Lebel and Beaulieu, 2009; Powell et al., 2006; Rodrigo et al., 2007]. Upadhyay et al. [2008] reported a trend of greater FA in left‐hemisphere AF in their small sample of four right‐handed males, and when restricting FA measurements to intra‐axonal space of the AF using diffusion tensor spectroscopy of N‐acetyl aspartate (NAA), they observed a significant leftward asymmetry [Upadhyay et al., 2008]. They also showed significant leftward asymmetry of axial diffusivity and rightward asymmetry of radial diffusivity for NAA of the AF, which was not found using conventional DTI; these results suggest that the leftward FA asymmetry for the AF observed in numerous studies including ours may be rooted in the intra‐axonal differences of the AF in the left and right hemispheres (e.g., axonal diameter or conduction velocities).
Additionally, when examining SLI, FALI, and VLI averaged across the groups of subjects (Table 3), all were positive values indicating a leftward bias. Similarly, a small study by Propper et al. [2010] did not find differences between handedness groups when examining LIs from language fMRI and AF volume/length [Propper et al., 2010]. Other small studies have also found positive values for LI/asymmetry index for leftward asymmetry of FA and/or the number of AF streamlines (or relative fiber density/tract volume) both in subjects who are right‐handed [Catani et al., 2007; Lebel and Beaulieu, 2009; Nucifora et al., 2005] and those who are atypical in handedness [Propper et al., 2010; Vernooij et al., 2007]. Furthermore, none of the LIs were significantly different between sexes regardless of handedness, in line with previous studies indicating a similar degree of asymmetry between sexes for FA of the AF [Catani et al., 2007] and verb generation fMRI [Allendorfer et al., 2012b]. However, unlike Catani et al. [2007], we did not find sex differences in SLI‐based lateralization patterns in right‐handed subjects, which is likely due to our much larger sample size (i.e., more than double the number of male subjects and more than four times the number of female subjects). The lack of sex differences in all LIs and corresponding lateralization patterns holds true in our subjects with atypical handedness and in the combined sample of subjects questioning the results and conclusions of the previous smaller studies.
When categorizing asymmetry patterns based on LIs as left‐lateralized, symmetric, and right‐lateralized, the distribution of SLI‐based lateralization were predominantly leftward for both handedness groups and consistent with a previous study in right‐handed children and adults [Lebel and Beaulieu, 2009]. The distribution of FALI‐based lateralization patterns were also similar between handedness groups and revealed that the majority (98%) of both right‐handed and atypical‐handed individuals exhibited symmetric lateralization, which was somewhat expected given how we defined the lateralization categories and that the mean FALI is 0.03 for both handedness groups. In contrast, the distribution patterns for VLI‐based lateralization in our study showed a significantly greater proportion of right‐handed subjects to be left‐lateralized for language compared to those with atypical handedness. These results coincide with former language lateralization studies that include typical and atypical language dominance in subjects who are right‐handed and atypical in handedness [Knecht et al., 2000b; Mazoyer et al., 2014; Perlaki et al., 2013; Propper et al., 2010; Pujol et al., 1999; Springer et al., 1999; Szaflarski et al., 2002, 2012]. While we showed that right‐handed subjects had a greater number of streamlines in the left hemisphere AF compared to atypical‐handed subjects, the distribution patterns for SLI‐based and FALI‐based lateralization were similar between handedness groups and do not parallel the VLI‐based lateralization differences between groups. Sex was also not a factor in the distribution of lateralization patterns, whether based on SLI, FALI, or VLI. Further, while we found no relationship between AF structural asymmetry (i.e., SLI and FALI) and the degree or right‐handedness based on the EHI, we did observe a significant association between VLI and EHI, consistent with previous results showing that the degree of right‐handedness is positively associated with left‐hemisphere language processing [Groen et al., 2013; Knecht et al., 2000b], although more recent evidence shows that handedness is only weakly associated with or may even be independent of hemispheric lateralization of language functions [Groen et al., 2013 ; Mazoyer et al., 2014 ; Somers et al., 2015 ; Willems et al., 2014]. We posit that our inclusion of a large sample of individuals with atypical handedness provided the necessary range and variability in the data to detect the relationship between VLI and degree of right‐handedness. Despite such a dataset, our tractography findings strongly suggest lack of a relationship between hemispheric language dominance and DTI‐based structural asymmetry of the AF.
We also hypothesized that leftward FA asymmetry of AF would contribute to better linguistic performance. Our results do not support this hypothesis, which may be due to a small effect of FA asymmetry on linguistic performance. However, in light of two recent studies investigating naming abilities in individuals with aphasia following left hemisphere stroke [Marchina et al., 2011; van Hees et al., 2014], it could be that the leftward bias of the strength/integrity of the AF plays a functional role in naming abilities and may be of greater importance in the context of language function recovery. One can suppose that in the event of a left‐hemisphere lesion, an individual with a large number of left‐hemisphere AF fibers may have a greater chance of retaining some of those fibers, but it may be the increased strength/integrity of those remaining fibers that would be a factor in better naming performance. The fact of the matter is that the question of whether the AF is necessary or sufficient for naming or other language abilities is difficult to answer. In the study of human language function, it would be ideal, as in molecular biology, to have techniques that can achieve a partial or full knockout of a particular white matter tract or brain region to assess function. While brain stimulation studies have been performed to create “virtual lesions,” e.g., Bruckner et al. [2013], this is still being developed, and the current bulk of our understanding of human language pulls together knowledge from normative and lesion studies.
As part of our analyses, we also evaluated SLI and its relationship to language performance, and showed weak associations. We found that SLI was associated with SFT performance in right‐handed but not atypical‐handed subjects, suggesting that the increased number of AF streamlines in right‐handed subjects may confer some benefit with respect to verbal fluency. We also found that PPVT performance was positively associated with SLI in atypical‐handed subjects, consistent with Lebel and Beaulieu [2009] who showed that increasing LI for AF streamlines was related to better PPVT performance in right‐handed children [Lebel and Beaulieu, 2009]. It is unclear why we do not observe this relationship in our right‐handed subjects. The Lebel and Beaulieu [2009] study did not find AF lateralization to change with age, but they also did not perform cognitive assessments on subjects older than 13 years of age. Thus, we can only speculate that the involvement of leftward asymmetry of AF streamlines in processing of receptive vocabulary may be more similar between children who are right‐handed and adults with atypical handedness vs right‐handed adults. Given that Lebel and Beaulieu [2009] only investigated right‐handed individuals, it would be interesting to test if the relationship between LI for AF streamlines and PPVT performance also holds true for children with atypical handedness. It should be noted that our right‐handed subjects were older than our atypically handed subjects, although age was not associated with AF asymmetry in any of the groups and, thus, was not included as a covariate in examining relationships between AF asymmetry and language performance. The observed age differences in the presence of atypical handedness are consistent with the population data showing decreases of the incidence of atypical handedness with age [Gilbert and Wysocki, 1992] and were not altogether unexpected. Finally, we showed that SLI was associated with CI performance in atypical‐handed subjects but not in right‐handed subjects. This association in atypical‐handed subjects is unique in that no other studies to date have described this effect. The CI assessment tests comprehension and recall of complex information, and it is unclear why the association between performance on this assessment and leftward asymmetry in the number of the AF streamlines would only be observed in subjects with atypical handedness. More studies investigating AF asymmetry and language performance in large samples of subjects with atypical handedness are necessary to clarify these discrepancies.
CONCLUSIONS
In summary, we found leftward asymmetry and lateralization patterns of the AF to be similar between right‐handed subjects and subjects with atypical handedness. Interestingly, these similar patterns of AF structural asymmetry in the handedness groups do not parallel the differential functional language lateralization patterns that we, and others, have shown. Our sample of individuals that ranged widely in handedness enabled us to detect an association between the degree of right‐handedness and hemispheric language function, albeit contrary to some recent studies; we did not observe a similar association between handedness and AF asymmetry. We argue that the inclusion of individuals with atypical handedness in language studies, and in cognitive studies in general, is necessary to glean greater understanding of such cognitive processes (see Willems et al. [2014] for review). We also showed associations between leftward asymmetry of the AF and performance on some of the language assessments including the PPVT, SFT, and CI; although these were weak effects that did not survive correction for multiple comparisons, they are still relevant to the discussion of language functions and anatomical asymmetries. Given the small effect sizes, it is not surprising that studies with smaller samples did not find significant relationships between AF asymmetry and language performance, and this should also be kept in mind when reviewing small studies that find significant associations. The lack of associations between AF asymmetry and many of the language assessments may also be attributed to the types of measures and language processes we are investigating. Catani et al. [2007] found a strong positive relationship between FA LI for the direct pathway of the AF connecting the frontal and temporal regions and verbal recall on the California Verbal Learning Test [Catani et al., 2007]. A recent study by the same group also showed that word learning performance was positively correlated with both the radial diffusivity of the AF direct segment pathway in the left hemisphere as well as the functional connectivity strength of these fronto‐temporal connections [Lopez‐Barroso et al., 2013]. These studies support a greater role for AF asymmetry in verbal learning abilities. Therefore, it would be informative for future studies integrating language fMRI, DTI, and language assessment to focus on the verbal learning aspects of language in both right‐handed subjects and subjects with atypical handedness. And, while DTI tractography enabled for the reconstruction of the AF white‐matter streamlines and assessment of its structural properties in relation to language function, investigations of a more expanded language network beyond the AF are necessary to advance our understanding of structure–function relationships since multiple brain regions are involved in various aspects of language processing.
ACKNOWLEDGMENT
The authors would like to thank Christi Banks and Amber N. Martin for their contribution in data collection.
REFERENCES
- Allendorfer JB, Kissela BM, Holland SK, Szaflarski JP (2012a): Different patterns of language activation in post‐stroke aphasia are detected by overt and covert versions of the verb generation fMRI task. Med Sci Monit 18:CR135–CR147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorfer JB, Lindsell CJ, Siegel M, Banks CL, Vannest J, Holland SK, Szaflarski JP (2012b): Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex 48:1218–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000): In vivo fiber tractography using DT‐MRI data. Magn Reson Med 44:625–632. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system ‐ a technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Lambon Ralph MA, Pujol J, Green C (2012): Arcuate fasciculus variability and repetition: The left sometimes can be right. Cortex 48:133–143. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, Jesmanowicz A, Hyde JS (1995): Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol 52:593–601. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW (1996): Function of the left planum temporale in auditory and linguistic processing. Brain 119:1239–1247. [DOI] [PubMed] [Google Scholar]
- Bruckner S, Kiefer M, Kammer T (2013): Comparing the after‐effects of continuous theta burst stimulation and conventional 1 Hz rTMS on semantic processing. Neuroscience 233:64–71. [DOI] [PubMed] [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA (2004): White matter asymmetry in the human brain: A diffusion tensor MRI study. Cereb Cortex 14:945–951. [DOI] [PubMed] [Google Scholar]
- Cao Y, Whalen S, Huang J, Berger KL, DeLano MC (2003): Asymmetry of subinsular anisotropy by in vivo diffusion tensor imaging. Hum Brain Mapp 20:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK (2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A 104:17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam M (2008): The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME (1999): Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A 96:10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis MC (2009): The evolution and genetics of cerebral asymmetry. Philos Trans R Soc Lond B Biol Sci 364:867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Dick AS, Tremblay P (2012): Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain 135:3529–3550. [DOI] [PubMed] [Google Scholar]
- Dos Santos Sequeira S, Woerner W, Walter C, Kreuder F, Lueken U, Westerhausen R, Wittling RA, Schweiger E, Wittling W (2006): Handedness, dichotic‐listening ear advantage, and gender effects on planum temporale asymmetry–a volumetric investigation using structural magnetic resonance imaging. Neuropsychologia 44:622–636. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn M (2007): Peabody Picture Vocabulary Test. Minneapolis, MN: NCS Pearson, Inc. [Google Scholar]
- Eckert MA, Leonard CM, Possing ET, Binder JR (2006): Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain Lang 98:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, de Vries MH, Scholz J, Breitenstein C, Johansen‐Berg H (2009): White matter integrity in the vicinity of Broca's area predicts grammar learning success. Neuroimage 47:1974–1981. [DOI] [PubMed] [Google Scholar]
- Francks C, Fisher SE, MacPhie IL, Richardson AJ, Marlow AJ, Stein JF, Monaco AP (2002): A genomewide linkage screen for relative hand skill in sibling pairs. Am J Hum Genet 70:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francks C, Maegawa S, Lauren J, Abrahams BS, Velayos‐Baeza A, Medland SE, Colella S, Groszer M, McAuley EZ, Caffrey TM, Timmusk T, Pruunsild P, Koppel I, Lind PA, Matsumoto‐Itaba N, Nicod J, Xiong L, Joober R, Enard W, Krinsky B, Nanba E, Richardson AJ, Riley BP, Martin NG, Strittmatter SM, Moller HJ, Rujescu D, St Clair D, Muglia P, Roos JL, Fisher SE, Wade‐Martins R, Rouleau GA, Stein JF, Karayiorgou M, Geschwind DH, Ragoussis J, Kendler KS, Airaksinen MS, Oshimura M, DeLisi LE, Monaco AP (2007): LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol Psychiatry 12:1129–1139. 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AN, Wysocki CJ (1992): Hand preference and age in the United States. Neuropsychologia 30:601–608. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E (1972): The Assessment of Aphasia and Related Disorders. Philadelphia: Lea & Febiger. [Google Scholar]
- Groen MA, Whitehouse AJ, Badcock NA, Bishop DV (2013): Associations between handedness and cerebral lateralisation for language: A comparison of three measures in children. PLoS One 8:e64876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK (2004): The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn Reson Med 51:807–815. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S (2001): Boston Naming Test. Baltimore, MD: Lippincott Williams & Wilkins. [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H (2000a): Language lateralization in healthy right‐handers. Brain 123:74–81. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H (2000b): Handedness and hemispheric language dominance in healthy humans. Brain 123:2512–2518. [DOI] [PubMed] [Google Scholar]
- Kozora E, Cullum CM (1995): Generative naming in normal aging ‐ Total output and qualitative changes using phonemic and semantic constraints. Clinical Neuropsychologist 9:313–320. [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001): Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging 13:534–546. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C (2009): Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30:3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M (1995): Neuropsychological Assessment. New York: Oxford University Press. [Google Scholar]
- Lopez‐Barroso D, Catani M, Ripolles P, Dell'Acqua F, Rodriguez‐Fornells A, de Diego‐Balaguer R (2013): Word learning is mediated by the left arcuate fasciculus. Proc Natl Acad Sci U S A 110:13168–13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan KM, McQueeny T, Howe SR, Shear P, Szaflarski J (2014): Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res 1562:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G (2011): Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 42:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Jobard G, Crivello F, Joliot M, Perchey G, Mellet E, Petit L, Tzourio‐Mazoyer N (2014): Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. PLoS One 9:e101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellet E, Zago L, Jobard G, Crivello F, Petit L, Joliot M, Mazoyer B, Tzourio‐Mazoyer N (2014): Weak language lateralization affects both verbal and spatial skills: An fMRI study in 297 subjects. Neuropsychologia 65:56–62. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC (2005): Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport 16:791–794. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Perlaki G, Horvath R, Orsi G, Aradi M, Auer T, Varga E, Kantor G, Altbacker A, John F, Doczi T, Komoly S, Kovacs N, Schwarcz A, Janszky J (2013): White‐matter microstructure and language lateralization in left‐handers: A whole‐brain MRI analysis. Brain Cogn 82:319–328. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331:585–589. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996): Diffusion tensor MR imaging of the human brain. Radiology 201:637–648. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler‐Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS (2006): Hemispheric asymmetries in language‐related pathways: A combined functional MRI and tractography study. Neuroimage 32:388–399. [DOI] [PubMed] [Google Scholar]
- Propper RE, O'Donnell LJ, Whalen S, Tie Y, Norton IH, Suarez RO, Zollei L, Radmanesh A, Golby AJ (2010): A combined fMRI and DTI examination of functional language lateralization and arcuate fasciculus structure: Effects of degree versus direction of hand preference. Brain Cogn 73:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A (1999): Cerebral lateralization of language in normal left‐handed people studied by functional MRI. Neurology 52:1038–1043. [DOI] [PubMed] [Google Scholar]
- Rodrigo S, Naggara O, Oppenheim C, Golestani N, Poupon C, Cointepas Y, Mangin JF, Le Bihan D, Meder JF (2007): Human subinsular asymmetry studied by diffusion tensor imaging and fiber tracking. AJNR Am J Neuroradiol 28:1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C (2004): Comprehensive approach for correction of motion and distortion in diffusion‐weighted MRI. Magn Reson Med 51:103–114. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L (2007): Improving lesion‐symptom mapping. J Cogn Neurosci 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW (2009): A new method for improving functional‐to‐structural MRI alignment using local Pearson correlation. Neuroimage 44:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS (1999): The planum temporale: A systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev 29:26–49. [DOI] [PubMed] [Google Scholar]
- Somers M, Aukes MF, Ophoff RA, Boks MP, Fleer W, de Visser KC, Kahn RS, Sommer IE (2015): On the relationship between degree of hand‐preference and degree of language lateralization. Brain Lang 144:10–15. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM (1999): Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 122:2033–2046. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA (2002): Language lateralization in left‐handed and ambidextrous people: fMRI data. Neurology 59:238–244. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW (2006): fMRI study of language lateralization in children and adults. Hum Brain Mapp 27:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M (2008): Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav 12:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Allendorfer JB (2012): Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav 24:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, Schapiro MB, Plante E, Holland SK (2012): Left‐handedness and language lateralization in children. Brain Res 1433:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillema JM, Byars AW, Jacola LM, Schapiro MB, Schmithorst VJ, Szaflarski JP, Holland SK (2008): Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang 105:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Petit L, Razafimandimby A, Crivello F, Zago L, Jobard G, Joliot M, Mellet E, Mazoyer B (2010a): Left hemisphere lateralization for language in right‐handers is controlled in part by familial sinistrality, manual preference strength, and head size. J Neurosci 30:13314–13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Simon G, Crivello F, Jobard G, Zago L, Perchey G, Herve PY, Joliot M, Petit L, Mellet E, Mazoyer B (2010b): Effect of familial sinistrality on planum temporale surface and brain tissue asymmetries. Cereb Cortex 20:1476–1485. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Hallock K, Ducros M, Kim DS, Ronen I (2008): Diffusion tensor spectroscopy and imaging of the arcuate fasciculus. Neuroimage 39:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA (2014): Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil Neural Repair 28:325–334. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A (2007): Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right‐ and left‐handed healthy subjects: A combined fMRI and DTI study. Neuroimage 35:1064–1076. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Marchina S, Norton AC, Wan CY, Schlaug G (2013): Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci 7:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Weeden VJ (2007): Diffusion Toolkit: A software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med 15:3720. [Google Scholar]
- Wilke M, Lidzba K (2007): LI‐tool: A new toolbox to assess lateralization in functional MR‐data. J Neurosci Methods 163:128–136. [DOI] [PubMed] [Google Scholar]
- Willems RM, Van der Haegen L, Fisher SE, Francks C (2014): On the other hand: Including left‐handers in cognitive neuroscience and neurogenetics. Nat Rev Neurosci 15:193–201. [DOI] [PubMed] [Google Scholar]


