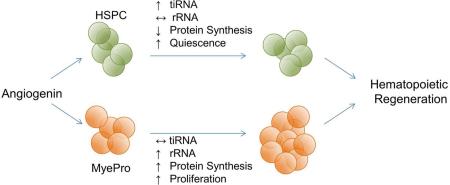
SUMMARY
Regulation of stem and progenitor cell populations is critical in the development, maintenance and regeneration of tissues. Here, we define a novel mechanism by which a niche-secreted ribonuclease, angiogenin (ANG), distinctively alters the functional characteristics of primitive hematopoietic stem/progenitor cells (HSPC) compared with lineage-committed myeloid-restricted progenitors (MyePro). Specifically, ANG reduces the proliferative capacity of HSPC while simultaneously increasing proliferation of MyePro. Mechanistically, ANG induces cell type-specific RNA processing events: tRNA-derived stress-induced small RNA (tiRNA) generation in HSPC and ribosomal RNA (rRNA) induction in MyePro, leading to respective reduction and increase in protein synthesis. Recombinant ANG protein improves survival of irradiated animals and enhances hematopoietic regeneration of mouse and human HSPC in transplantation. Thus, ANG plays a non-cell autonomous role in regulation of hematopoiesis by simultaneously preserving HSPC stemness and promoting MyePro proliferation. These cell type-specific functions of ANG suggest considerable therapeutic potential.
Graphical abstract
INTRODUCTION
A population of quiescent adult stem cells with self-renewal and differentiation capabilities is required for tissue homeostasis and regeneration (Orford and Scadden, 2008). Stem cell quiescence has been shown to protect cells from exhaustion, especially under stress, which is essential for both continuous cell output and prevention of malignant transformation (Nakamura-Ishizu et al., 2014). In the hematopoietic system, this is achieved by both cell-intrinsic and extrinsic factors. Cell cycle and epigenetic regulators as well as pathways involved in growth control, including cyclin dependent kinases and inhibitors, Rb, PI3K, and p53, have been demonstrated as cell-intrinsic regulators of HSPC proliferation (Ito and Suda, 2014; Nakamura-Ishizu et al., 2014). A variety of secreted and cell-surface factors which are produced by bone marrow (BM), including angiopoetin-1, thrombopoietin, SCF, CXCL12, and TGF-β (Ito and Suda, 2014; Mendelson and Frenette, 2014; Morrison and Scadden, 2014), has been shown to extrinsically regulate HSPC.
Recent strides have been made to therapeutically harness growth control properties of hematopoietic stem cells (HSC) in an effort to improve hematopoietic regeneration in the clinic. In the context of hematopoietic stem cell transplantation (SCT), in particular, low numbers of HSPC result in low transplantation efficacy, which can markedly affect the survival of patients undergoing SCT (Smith and Wagner, 2009). Therefore, expanding transplantable cell number has been a longstanding goal (Boitano et al., 2010; Delaney et al., 2010; Fares et al., 2014; Himburg et al., 2010; North et al., 2007). Preserving HSC function can be at odds with expansion strategies, but advances in improved BM homing (Li et al., 2015) and maintained stemness through protection against extraphysiologic oxygen shock (Mantel et al., 2015) are being made. To our knowledge, however, no studies to date have accomplished preserving HSC regenerative capacity through quiescence while enabling progenitor expansion.
Angiogenin (ANG), also known as ribonuclease 5 (RNase5), is a member of the secreted vertebrate-specific ribonuclease superfamily and has angiogenic (Fett et al., 1985), neurogenic (Subramanian and Feng, 2007), neuroprotective (Subramanian et al., 2008), and immune-regulatory functions (Hooper et al., 2003). Under growth conditions, ANG promotes proliferation and enhances survival in a variety of cell types, including endothelial (Kishimoto et al., 2005), neuronal (Kieran et al., 2008), and cancer cells (Yoshioka et al., 2006). The growth stimulatory function of ANG is mediated through ribosomal RNA (rRNA) transcription (Tsuji et al., 2005), and requires nuclear translocation of ANG (Xu et al., 2003). Under stress, ANG is translocated to stress granules (SG) and mediates the production of tRNA-derived stress-induced small RNA (tiRNA); these small RNA species enhance cellular survival by simultaneously suppressing global protein translation, saving anabolic energy, and permitting internal ribosomal entry sequence (IRES)-mediated protein translation of anti-apoptotic genes (Emara et al., 2010; Ivanov et al., 2011; Yamasaki et al., 2009).
In this study, we demonstrate that ANG restricts proliferation of primitive HSPC, but stimulates proliferation of myeloid-restricted progenitors (MyePro). We also demonstrate that ANG mediates tiRNA production in HSPC, but promotes rRNA transcription in MyePro. Importantly, these properties of ANG are reflected by enhanced hematopoietic regeneration and animal survival upon treatment with recombinant ANG protein following radiation-induced BM failure and a dramatic increase in the level of hematopoietic reconstitution by ANG-treated mouse long-term (LT)-HSCs and human CD34+ CB cells. Therefore, ANG is a previously unrecognized regulator of HSPC with unique RNA processing function relevant to radiation-induced BM failure and clinical stem cell transplantation.
RESULTS
ANG plays a non-cell autonomous role in regulation of LT-HSC quiescence and self-renewal
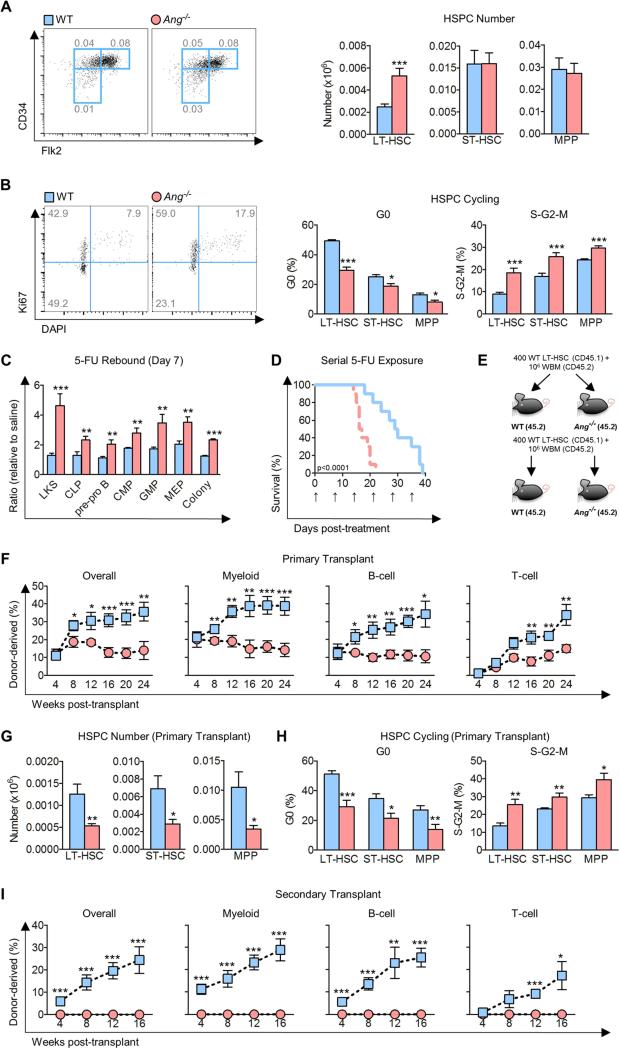
We sought to examine the functional role of ANG in hematopoiesis because it was originally found to be differentially expressed in bone marrow osteolineage cells in close proximity to transplanted HSPC. The presence of ANG mRNA in mesenchymal cells of bone marrow was confirmed by qPCR of sorted subsets of cells (Figure S1A). We then profiled HSPC (Figure S1B) in the BM of Ang knockout (Ang−/−) mice and found a 2-fold increase in the number of LT-HSCs, but not short-term (ST)-HSCs or multi-potent progenitors (MPPs) in Ang−/− BM (Figure 1A). Consistent with this finding, a reduction in G0 phase and a corresponding increase in S/G2/M phases of the cell cycle (Figure 1B), as well as enhanced BrdU incorporation (Figure S1C) was observed in Ang−/− LT-HSCs. Ang−/− ST-HSCs and MPPs also displayed increased cycling (Figure 1B, S1C) but to a less severe degree (for LT-HSC, ST-HSC, and MPP, respectively, the percentage increase in S-G2-M was 107.7±23.7, 52.2±10.0, and 21.9±4.6, and the percent decrease in G0 was 40.1±4.3, 24.3±7.0, and 37.6±10.3), which, combined with elevated apoptosis across hematopoietic lineages in Ang−/− mice (Figure S1D), might partially explain why no difference in cell number was observed for ST-HSC and MPP (Figure 1A). These patterns were also observable by alternative cell surface markers (SLAM/CD48) for HSPC subtypes (Cabezas-Wallscheid et al., 2014) (Figure S1E-F), confirming that Ang−/− LT-HSCs cycle more actively.
Figure 1. Ang deficiency results in loss of HSPC quiescence and defective transplantation.
(A-B) Quantification of primitive hematopoietic cell number per femur (A, n=12) and cell cycle status (B, n=8) in Ang−/− mice.
(C) Quantification of stem and progenitor cells in Ang−/− mice on day 7 post-exposure to 150 mg/kg 5-FU (n=8).
(D) Survival of Ang−/− mice following weekly 5-FU (150 mg/kg) exposure (n=10). Arrows indicate day of injection.
(E) Experimental schema of serial transplant using WT or Ang−/− hosts.
(F-H) Multi-lineage donor cell chimerism (F), HSPC number per femur (G), and HSPC cell cycle status (H) after competitive primary transplantation of WT LT-HSCs into lethally-irradiated WT or Ang−/− recipients (n=8).
(I) Chimerism after secondary transplantation of sorted LT-HSCs from primary recipients into WT or Ang−/− secondary recipients (n=8).
See also Figures S1 and Tables 1-2.
Despite the significant increase in LT-HSC number in Ang−/− BM (Figure 1A, S1E), only mild lymphocytosis was apparent in 8-12 week old mice at baseline (Table S1). However, under conditions of stress, progenitor response to the genotoxic agent, 5-fluorouracil (5-FU), was markedly exaggerated in Ang−/− mice (Figure 1C). Further, exposure of these animals to serial proliferative stress, such as weekly injections of 5-FU, resulted in excess animal morality (Figure 1D). Consistent with the phenotype of stress-induced exhaustion (Orford and Scadden, 2008), aged 22 month old Ang−/− mice developed leukopenia (Table S2) and showed a marked reduction in the number of primitive hematopoietic cells in the BM (Figure S1G), accompanied by more active HSPC cycling (Figure S1H). Aged Ang−/− mice also displayed reduced functional capabilities by in vitro methylcellulose assays (Figure S1I-J) and in vivo competitive transplantation (Figure S1K-L).
To further characterize the functional significance of elevated cycling in Ang−/− HSPC, we performed transplant experiments by injecting either sorted LT-HSCs (Figure 1E) or total BM (Figure S1M) into lethally-irradiated WT or Ang−/− hosts. In both experiments, impaired long-term multi-lineage reconstitution was observed in Ang−/− hosts (Figure 1F, S1N) with particularly pronounced impairment at later time points. Notably, WT HSPC in the ANG-deficient microenvironment displayed significantly reduced HSPC number, accompanied by more active cycling (Figure 1G-H). To rule out a homing defect as a cause of impaired reconstitution in Ang−/− hosts, CFSE-labeled CD45.1 Lin− cells were injected into irradiated WT or Ang−/− recipients, and no difference in the percentage of LKS cells or MyePro in the BM of these animals was observed 16 hours after transplantation (Figure S1O).
In order to evaluate the effect of niche-derived ANG on HSC self-renewal, we carried out serial transplantation experiments. While competitive transplantation demonstrated no detectable hematopoietic contribution by LT-HSCs that had been passaged through ANG-deficient primary recipients (Figure 1I), non-competitive transplantation of whole BM cells from primary Ang−/− recipients resulted in death of all secondary Ang−/− recipients (Figure S1P). The marked inability to reconstitute in both transplant settings indicates severe loss of HSC self-renewal capacity in ANG-deficient hosts. Taken together, these data demonstrate that ANG plays a non-cell autonomous role in regulation of quiescence and self-renewal of primitive hematopoietic cells, particularly LT-HSC.
ANG enhances MyePro proliferation
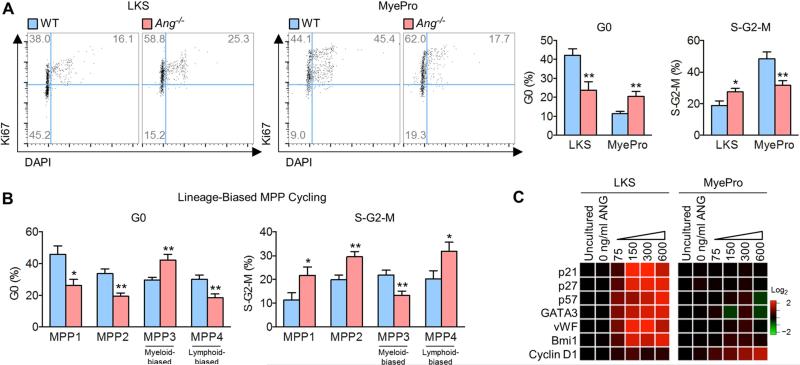
The finding that ANG restricts cell cycling of HSPC is the first known evidence for a suppressive activity of ANG on cell proliferation, as all previous studies revealed that ANG promotes cell proliferation (Li and Hu, 2010). We therefore examined cell type-specific effects of ANG in various cells of the hematopoietic lineage. We observed that while Ang−/− LKS cells cycle more actively, Ang−/− MyePro showed reduced cycling by Ki67 (Figure 2A) and BrdU (Figure S2A) staining.
Figure 2. Dichotomous effect of ANG in LKS and MyePro cycling.
(A-B) Cell cycle status of LKS cells and MyePro (A, n=8) and MPP1-4 cells (B, n=6) in Ang−/− mice.
(C) qRT-PCR analysis of pro-self-renewal transcripts from sorted LKS cells or MyePro treated with mouse ANG protein (0-600 ng/ml, n=6).
See also Figure S2.
The cell type specificity of ANG was further illustrated by analyzing lymphoid-restricted progenitors (LymPro) and MyePro including common lymphoid progenitor (CLP), pre-pro B, common myeloid progenitor (CMP), granulocyte-macrophage progenitor (GMP), and megakaryocyte-erythroid progenitor (MEP) cell types. As with HSPC, we found that Ang−/− CLPs and pre-pro B cells (Figure S2B) cycle more actively (Figure S2C) and incorporate more BrdU (Figure S2D), suggesting ANG restricts LymPro proliferation. In contrast, Ang−/− MyePro, including CMP, GMP, and MEP, displayed less active cycling (Figure S2F) and reduced BrdU incorporation (Figure S2G), accompanied by a reduction of CMP and GMP number (Figure S2E). Importantly, we observed restricted proliferation of myeloid-biased MPP3s and more active cycling of lymphoid-biased MPP4s (Cabezas-Wallscheid et al., 2014) in Ang−/− mice (Figure 2B). Together, these data indicate that the function of ANG is cell type-specific: while ANG restricts cell proliferation in primitive HSPC and LymPro, it promotes proliferation of MyePro. This cell type-specificity is observable within the earliest phenotypically-defined lineage-biased progenitor cell types: MPP3 and MPP4.
Cell type-specific regulation of ANG was confirmed by the fact that Ang deletion resulted in decreased expression of cycle checkpoint or self-renewal genes including p21, p27, p57, GATA3, vWF and Bmi1 (Cheng et al., 2000; Frelin et al., 2013; Kent et al., 2009; Matsumoto et al., 2011; Park et al., 2003) in LKS cells but not in MyePro (Figure S2H). In contrast, the cell cycle-related gene, cyclin D1, was decreased in MyePro but not in LKS cells upon Ang deletion (Figure S2H). We then examined the effect of recombinant ANG protein on cultured HSPC and MyePro. Remarkably, culture with ANG for 2 hours in PBS led to a dose-dependent increase in the expression of pro-self-renewal genes in LKS cells (Figure 2C). No such change was noted in MyePro. In contrast, cyclin D1 was enhanced by culture with ANG in MyePro but not in LKS cells (Figure 2C). A similar pattern was observed in LT-HSCs cultured with ANG for 2 hours in PBS (Figure S2I) or under longer culture conditions in cytokine-supplemented S-clone media (Figure S2J). Notably, addition of exogenous ANG led to elevated levels of pro-self-renewal genes in Ang−/− LT-HSC, as was seen in WT cells (Figure S2K). Together, these data demonstrate that ANG differentially regulates gene expression in HSPC and MyePro, including genes relevant for proliferation and self-renewal.
ANG dichotomously regulates protein synthesis in LKS and MyePro cells
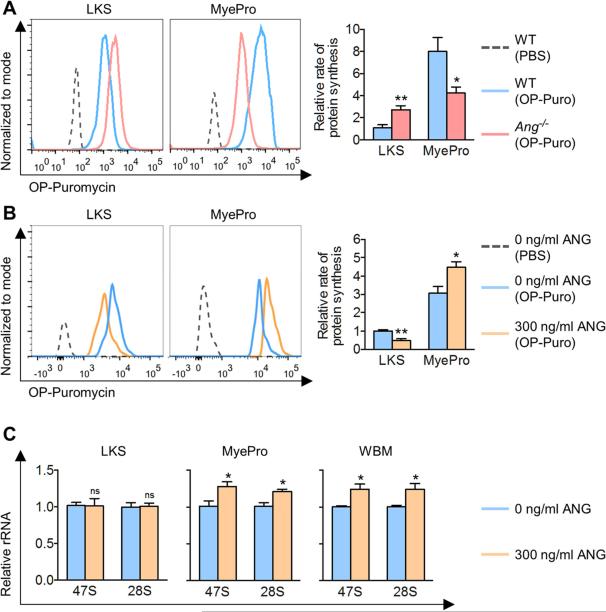
ANG has been shown in other cell types to regulate global protein synthesis, a housekeeping function recently shown to be tightly regulated in primitive HSCs (Signer et al., 2014). To determine whether ANG regulates protein synthesis in HSPC, we assessed in vivo protein synthesis in Ang−/− mice by a fluorogenic assay using O-propargyl-puromycin (OP-Puro) (Signer et al., 2014). Consistent with their cell cycle profile (Figure 2A, S2A), Ang−/− LKS cells showed a higher rate of protein synthesis while Ang−/− MyePro demonstrated reduced protein synthesis (Figure 3A). This cell type-specificity was also evident when BM was analyzed with more specific hematopoietic cell markers (Figure S3A), or when assessing cells in G0/G1 (Figure S3B). In vivo administration of OP-Puro did not alter BM cellularity or LT-HSC frequency (Figures S3C-D). Significantly, in vitro culture of LKS cells with ANG led to reduced protein synthesis, while addition of ANG to MyePro led to enhanced protein synthesis (Figure 3B). Together, these data demonstrate that the effect of ANG on protein synthesis is cell type-specific.
Figure 3. ANG-mediated regulation of protein synthesis is cell type-specific.
(A) In vivo OP-Puro incorporation in WT or Ang−/− LKS cells and MyePro. Cells were analyzed 1 h after OP-Puro administration. Bar graphs are relative values to WT LKS (n=5).
(B) In vitro OP-Puro incorporation following 2 h ANG treatment of LKS cells and MyePro. Bar graphs are relative values to untreated LKS (n=6).
(C) qRT-PCR analysis of rRNA with primers targeting mature and precursor transcripts following 2 h ANG treatment of LKS cells and MyePro, using various primer sets (n=3). Bar graphs are relative values to untreated cells.
See also Figures S3-4.
The restrictive function of ANG in HSPC is mediated by tiRNA
To reveal the biochemical mechanism for this dichotomous effect of ANG on protein synthesis, we first assessed rRNA transcription, which is stimulated by ANG in other cell types (Kishimoto et al., 2005; Tsuji et al., 2005). Addition of ANG led to enhanced rRNA transcription in MyePro and whole BM cells, but not in LKS cells (Figure 3C), while Ang deletion resulted in reduced rRNA transcription in MyePro and whole BM but not in LT-HSC (Figure S3E). These findings are consistent with increased proliferation and protein synthesis observed in MyePro following ANG treatment.
ANG has been shown to reprogram protein synthesis as a stress response to promote survival under adverse conditions. This function of ANG is mediated by tiRNA, a noncoding small RNA that specifically permits translation of anti-apoptosis genes while global protein translation is suppressed so that stressed cells have adequate time and energy to repair damage, collectively promoting cell survival (Emara et al., 2010; Ivanov et al., 2011; Yamasaki et al., 2009). To assess whether ANG-mediated regulation of protein synthesis is tiRNA-dependent, we assessed bulk small RNA production by electrophoresis. LKS cells exhibited dramatically higher small RNA production than MyePro at baseline (Figure 4A). tiRNA level was normally low in Lin+ differentiated cell types although it became detectable when a higher amount (15 μg) of total RNA was assessed (Figure S3F). Importantly, addition of ANG led to markedly elevated tiRNA levels in LKS cells (Figure 4A). Equal loading was affirmed by tRNA levels (indicated by arrows, Figure 4A). Addition of ANG to Lin+ cells did not result in an increase in tiRNA levels, in contrast to significantly elevated tiRNA levels following ANG treatment of HSPC (Figure S3F, compared to Figure 4A). Further, tiRNA levels in Ang−/− LKS cells were substantially reduced, but not completely diminished (Figure S3G). As ANG is the only RNase that has been demonstrated to mediate tiRNA production (Yamasaki et al., 2009), this finding suggests that other unidentified RNases may be responsible for the remaining level (29%) of tiRNA. Moreover, we also observed an increase in tiRNA production in MyePro by sodium arsenite (SA), which was suppressed by exogenous ANG protein (Figure S3H). In contrast, tiRNA production in LKS cells was not increased by SA, but was enhanced by ANG both at steady-state and under SA-induced oxidative stress (Figure S3H). These results demonstrate that ANG differentially regulates tiRNA in LKS cells and MyePro under both homeostatic and stress conditions.
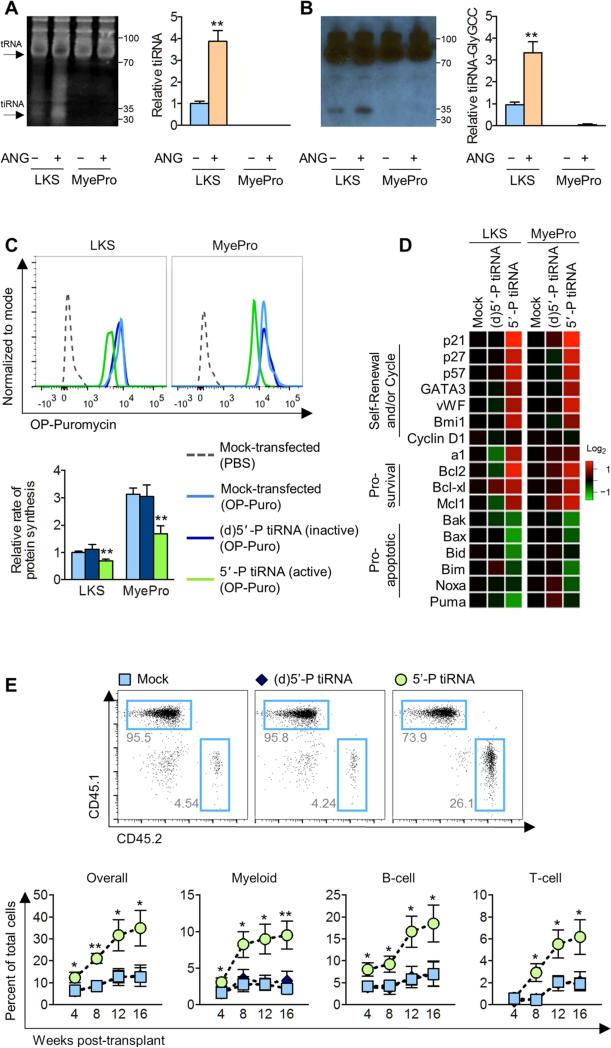
Figure 4. ANG-mediated regulation of protein synthesis is correlated with cell type-specific tiRNA production.
(A and B) Small RNA production (A, n=3) and Northern blot analysis of tiRNA-Gly-GCC (B, n=3) following 2 h treatment of LKS cells and MyePro with ANG. Bar graphs are relative values to untreated LKS.
(C-D) OP-Puro incorporation (C, n=5), and qRT-PCR analysis of pro-self-renewal, pro-survival, and pro-apoptotic transcripts (D, n=5) in LKS cells and MyePro transfected with inactive (d)5’-P tiRNA or active 5’-P tiRNA.
(E) Post-transplant reconstitution of LKS cells transfected with inactive (d)5’-P tiRNA or active 5’-P tiRNA (n=7).
See also Figures S3-4.
To ensure that bulk small RNA reflect tiRNA, we analyzed the levels of a representative tiRNA, tiRNA-Gly-CCC, by Northern blotting in ANG-treated LKS cells and MyePro. tiRNA-Gly-GCC was previously shown to be expressed in hematopoietic tissues, including BM and spleen, but was neither examined in primitive hematopoietic cells nor functionally-validated (Dhahbi et al., 2013). Figure 4B shows that tiRNA-Gly-GCC was significantly elevated in LKS cells, relative to MyePro, and was further enhanced by exogenous ANG. Together, these data identify tiRNA as a distinct RNA species that is abundantly expressed in HSPC and that is regulated by ANG.
To determine whether tiRNA is responsible for restricted protein synthesis in HSPC, we transfected synthetic tiRNA-Gly-GCC in LKS cells and MyePro, and assessed protein synthesis in vitro using OP-Puro. As tiRNA requires its 5’-phosphate to suppress protein synthesis (Ivanov et al., 2011), we used an inactive, dephosphorylated synthetic tiRNA-Gly-GCC, termed (d)5’-P-tiRNA, as a negative control. Expectedly, transfection of active 5’-P-tiRNA, but not inactive (d)5’-P-tiRNA, led to a significant reduction in the rate of protein synthesis in both LKS cells and MyePro (Figure 4C). Thus, tiRNA transfection phenocopies exogenous ANG on restriction of protein synthesis in LKS cells, which has been shown in Figure 3B. We also found that myeloid and lymphoid progenitor colony formation was restricted upon transfection of whole BM with active 5’-P tiRNA but not inactive (d)5’-P-tiRNA (Figure S3I). Moreover, transfection of active 5’-P-tiRNA led to upregulation of pro-self-renewal and pro-survival genes, and downregulation of pro-apoptotic genes, in both LKS cells and MyePro by lipofection (Figure 4D) and electroporation (Figure S3J).
The exact subcellular compartment where tiRNA is produced by ANG is currently unknown (Saikia et al., 2014), but the growth and survival function of ANG has been correlated to its SG localization in stressed cells (Pizzo et al., 2013). The finding that ANG produces tiRNA and restricts protein synthesis only in LKS cells prompted us to examine differential localization of ANG in SGs between LKS and MyePro. ANG is known to be internalized through receptor-mediated endocytosis and translocated to either the nucleus or SG, depending on cell state, to mediate rRNA or tiRNA production, respectively (Pizzo et al., 2013). We found that ANG was colocalized with poly-(A) binding protein (PABP), a SG marker, in LKS cells, but not in MyePro (Figure S4A). Further, we found that RNase/ANG inhibitor 1 (RNH1), an endogenous ANG inhibitor that has been shown to regulate subcellular localization of ANG (Pizzo et al., 2013), and is expressed in BM cell subsets under steady-state conditions (Figure S4B), is localized in SGs in MyePro, but not in LKS cells (Figure S4C). This opposing localization pattern of RNH1 and ANG was further examined by double immunofluorescence (Figure S4D) and fluorescence resonance energy transfer (FRET, Figure S4E), which showed that ANG and RNH1 colocalize and interact in the nucleus, but not in the cytoplasm of LKS cells, and in the cytoplasm, but not in the nucleus, of MyePro. Thus, RNH1, which is known to stoichiometrically inhibit ANG with a femto-molar Kd (Lee et al., 1989), likely inhibits nuclear ANG but not cytoplasmic ANG in LKS cells, suppressing rRNA production but permitting tiRNA production, whereas it inhibits cytoplasmic ANG but not nuclear ANG in MyePro to allow rRNA transcription but not tiRNA production.
To assess whether tiRNA-mediated regulation of protein synthesis affects HSPC function, we transfected LKS cells with synthetic tiRNA and competitively transplanted those cells into WT hosts. We observed significantly enhanced long-term multi-lineage post-transplant reconstitution of cells transfected with active 5’-P-tiRNA, relative to untreated LKS cells or cells transfected with inactive (d)5’-P-tiRNA (Figure 4E). As ANG stimulates tiRNA production in LKS cells, these data strongly suggest that ANG may enhance the regenerative potential of HSPC by tiRNA-mediated alterations of protein synthesis.
ANG is a pro-regenerative factor after radio-damage
To assess the pro-regenerative role of ANG, we first examined the function of ANG in the context of radiation-induced cell damage. We found that Ang mRNA levels were elevated in mixed niche cells one day post-irradiation (Figure S5A), but not in whole BM cells (Figure S5B) or sorted LKS and MyePro cells (Figure S5C). Consistent with this finding, ANG serum levels were also significantly elevated (Figure S5D), suggesting that niche cells increase ANG synthesis as a response to irradiation. Ang−/− mice displayed reduced survival following exposure to various doses of γ-radiation (Figure S5E), accompanied by reduced blood leukocyte recovery (Figure S5F). When animals were analyzed at day 7 post-irradiation (Figure S5G), we observed reduced total BM cellularity (Figure S5H, Table S3), reduced HSPC and LymPro number (Figure S5I, S5K), and more active cycling (Figures S5J, S5L). These data are consistent with the quiescence-inducing effect of ANG on HSPC and LymPro. In contrast, Ang−/− MyePro showed reduced cell number (Figure S5M), but restricted proliferation (Figure S5N) following γ-irradiation. Importantly, this dichotomous regulation of proliferation was observed in lineage-biased MPP3 and MPP4 cells under radiation stress (Figure S5O-P), as observed at steady-state (Figure 2B). Ang−/− mice also demonstrated increased apoptosis in all primitive hematopoietic cell types examined (Figure S5Q), as well as reduced lymphoid and myeloid colony formation in response to γ-irradiation (Figure S5R).
To determine whether treatment with ANG enhances survival, we pretreated WT or Ang−/− mice with ANG daily for three successive days and irradiated mice with 8.0 Gy 24 hours following the final ANG treatment (Figure S6A). Significantly, the 30-day survival rate increased from 20% to 90% after ANG treatment, indicating that ANG is radioprotective (Figure 5A). Importantly, 80% of Ang−/− mice also survived following ANG pretreatment whereas 100% of untreated Ang−/− mice died. These results indicate that both endogenous and exogenous ANG are radioprotective. Pre-treatment with ANG protected against radiation-induced loss of total BM number (Figure S6B) and various BM cell subsets (Table S4) and restricted HSPC and LymPro cycling (Figure S6C-D). In contrast, ANG pre-treatment not only prevented radiation-induced loss of MyePro number (Table S4) but also further promoted their proliferation over and above those enhanced by γ-irradiation (Figure S6E). Moreover, ANG protected against irradiation-induced apoptosis in all primitive hematopoietic cell types examined (Figure S6F), and led to enhanced colony formation (Figure S6G) and post-transplant reconstitution (Figure S6H). Together, these data demonstrate the protective function of ANG against radiation-induced BM damage, likely through induction of HSPC quiescence and promotion of MyePro proliferation.
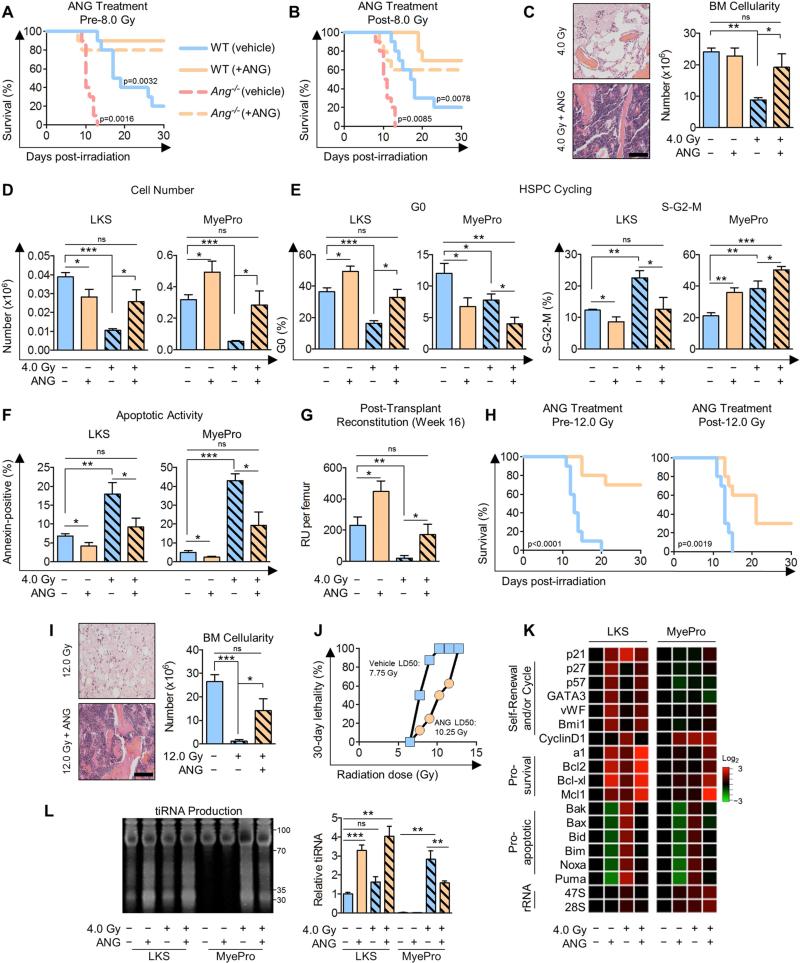
Figure 5. ANG enhances radioprotection and radioresistance.
(A) Survival of WT or Ang−/− mice treated with ANG daily for three successive days 24 h pre-8.0 Gy (n=10).
(B) Survival of WT or Ang−/− mice treated with ANG daily for three successive days 24 h post-8.0 Gy (n=10).
(C-G) H&E and BM cellularity of femurs (C), LKS and MyePro cell number per femur (D), cell cycling (E), apoptotic activity (F), and post-transplant reconstitution (G) of WT mice treated with ANG daily for three successive days 24 h post-4.0 Gy (n=6). Scale bar = 100 μm.
(H) Survival of WT mice treated with ANG daily for three successive days 24 h prior or post-12.0 Gy.
(I) H&E and BM cellularity of femurs of WT mice treated with ANG daily for three successive days 24 h post-12.0 Gy TBI (n=6). Scale bar = 100 μm.
(J) LD50 of mice treated with ANG daily for three successive days beginning 24 h post-TBI (n=8).
(K-L) qRT-PCR analysis of pro-self-renewal, pro-survival, pro-apoptotic, and rRNA transcripts (K, n=6), and tiRNA production (L, n=3) in LKS or MyePro sorted from irradiated mice (4.0 Gy) and treated with 300 ng/ml ANG.
See also Figures S5-6 and Tables S3-5.
To assess a potential therapeutic use of ANG as a radio-mitigating agent, we irradiated mice with 8.0 Gy and immediately began ANG treatment (Figure S6I), and observed enhanced survival in ANG-treated mice (Figure S6J). We also irradiated mice with 8.0 Gy and began ANG treatment 24 hours later (Figure S6K). Significantly, the majority of ANG-treated mice survived, including ANG-treated Ang−/− mice, suggesting that ANG has radio-mitigating capabilities (Figure 5B). Importantly, treatment with ANG 24 hours post-irradiation prevented radiation-induced reduction of overall BM cellularity (Figure 5C), as well as of LKS cells, MyePro (Figure 5C-D), and other BM cells (Table S4). Consistent with its dichotomous role in cell cycle kinetics, ANG restricted proliferation of LKS cells, and simultaneously enhanced proliferation of MyePro under radiation stress (Figure 5E). Further, ANG prevented radiation-induced apoptosis in both cell types (Figure 5F). These effects on cell number, cycling, and apoptosis were also apparent in further defined stem and progenitor cell populations (Figure S6L-O, Table S4). Significantly, defects in colony formation (Figure S6P) and post-transplant reconstitution (Figure 5G) could be rescued by ANG treatment.
We also assessed the protective and mitigative effect of ANG in lethally-irradiated (12.0 Gy) animals and found that ANG treatment either before or after lethal irradiation improved survival (Figure 5H), and enhanced BM cellularity (Figure 5I), as well as peripheral blood content (Table S5). Consistent with these findings, ANG significantly increased the LD50 when treatment was begun 24 hours post-irradiation (Figure 5J). Further, treatment with ANG upregulated pro-self-renewal genes in LKS cells and led to enhanced pro-survival transcript levels and reduced pro-apoptotic transcripts in both LKS cells and MyePro under radiation stress (Figure 5K). ANG treatment enhanced rRNA transcription only in MyePro both before and after irradiation (Figure 5K). In LKS cells, ANG did not promote rRNA transcription (Figure 5K) but enhanced tiRNA production under radiation stress (Figure 5L), as has been observed under steady state (Figures 4A) and under oxidative stress (Figure S3H). These results indicate that the dichotomous role of ANG in regulating proliferation of HSPC and MyePro is preserved under stress conditions. Together, these results establish a model by which ANG simultaneously stimulates proliferation of rapidly responding MyePro and preserves HPSC stemness, in association with enhanced hematopoietic regeneration and improved survival.
Ex vivo treatment of LT-HSCs with recombinant ANG enhances post-transplant reconstitution
The in vitro (Figure 2C, S2H-K) and in vivo (Figure 5, S5, S6) activity of ANG in preserving HSPC stemness and in enhancing regeneration prompted us to assess its capacity in improving SCT and its potential for clinical development. Treatment of LT-HSCs with ANG in culture for 7 days led to a dose-dependent decrease of cell proliferation in WT and Ang−/− cells (Figure 6A), consistent with its ability to restrict HSPC proliferation. Notably, LKS cells cultured in the absence of ANG resulted in a reduction of tiRNA expression relative to uncultured cells (Figure 6B). In contrast, cells cultured in the presence of ANG not only maintained baseline tiRNA levels, but also their responsiveness to further ANG treatment (Figure 6B).
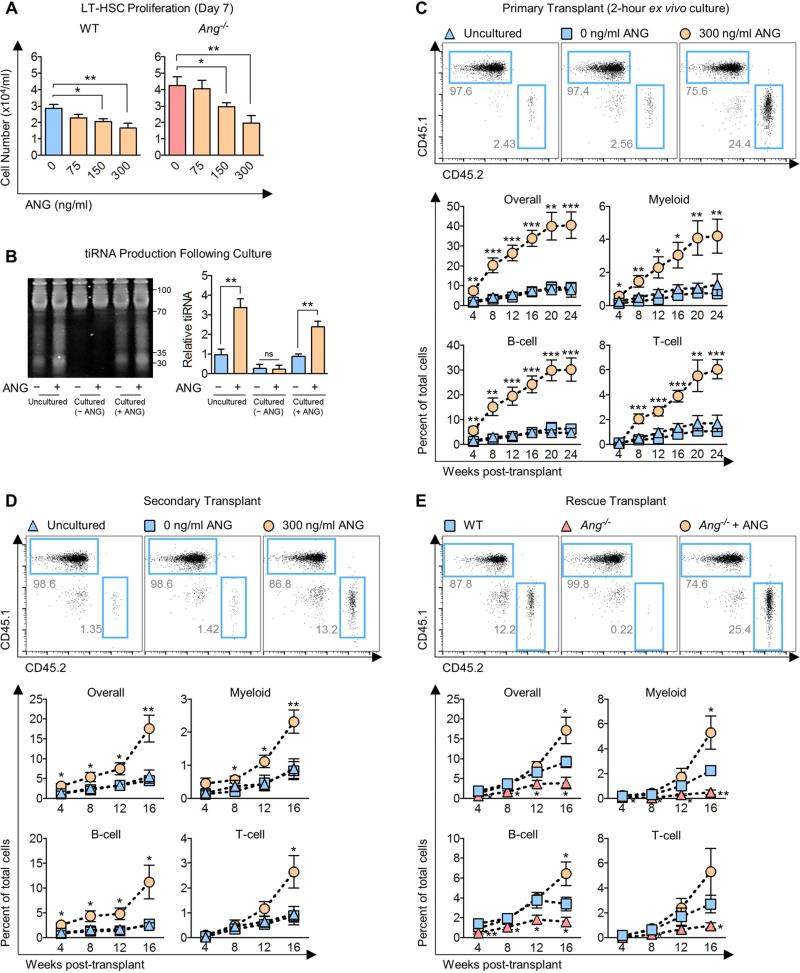
Figure 6. ANG enhances post-transplant reconstitution.
(A) Cell density on day 7 from sorted WT or Ang−/− LT-HSCs (1,875 cells/ml) cultured in the presence of various doses of ANG (n=6).
(B) tiRNA levels following 7 day culture with 0 or 300 ng/ml ANG. After culture, cells were harvested and again treated with 0 or 300 ng/ml ANG (indicated by + or −) for 2 h prior to analysis by electrophoresis (n=3).
(C) Post-transplant reconstitution of WT LT-HSCs (CD45.2) after 2 h ex vivo treatment with ANG (n=8-9).
(D) Secondary transplant of WT LT-HSCs (CD45.2) without further ex vivo ANG treatment (n=7-8).
(E) Post-transplant reconstitution of WT or Ang−/− LT-HSCs which were cultured in the presence or absence of 300 ng/ml ANG for 2 h and competitively transplanted in WT hosts (n=7).
To test whether restriction of proliferation would enhance transplantation efficiency, we competitively transplanted LT-HSCs that were either freshly isolated or had been cultured for 2 hours with or without 300 ng/ml ANG (Figure S7A), the physiological concentration of both mouse (Figure S5D) and human (Yoshioka et al., 2006) ANG. Significantly, we observed that a 2 hour treatment with ANG led to a dramatic increase in multi-lineage post-transplant reconstitution over 24 weeks (Figure 6C). We also found that improved transplant efficiency was observed with LT-HSCs cultured with ANG for 7 days (Figure S7B). Enhanced regeneration was observed over 16 weeks upon secondary transplant without further ANG treatment (Figure 6D), and elevated peripheral blood counts were observed one year-post transplant without any indication of leukemia development (Table S6). Significantly, removal of ANG from the media after 7 days in culture did not induce proliferation (Figure S7C) and enhanced levels of pro-self-renewal transcripts were retained (Figure S7D). Treatment with ANG had no effect on homing (Figure S7E). Importantly, treatment of Ang−/− LT-HSCs with exogenous ANG ameliorated post-transplant reconstitution defect of Ang−/− cells, and led to enhanced reconstitution over WT cells by week 16 (Figure 6E). Together, these data demonstrate that treatment of LT-HSCs with exogenous ANG significantly enhances their regenerative capabilities upon relatively short exposure, and this effect is long-lasting.
ANG improves regeneration of human cells
Given that ANG significantly improved transplantation efficiency of mouse LT-HSCs, we examined whether human ANG has similar pro-regenerative capabilities in human CD34+ CB cells. Consistent with the anti-proliferative effect of ANG on mouse LT-HSCs, treatment with human ANG led to a dose-dependent reduction of CD34+ CB cell proliferation over 7 days (Figure 7A) and elevated level of pro-self-renewal transcripts (Figure S7F), whereas ANG variants that are defective in its ribonucleolytic activity (K40Q) or in receptor binding (R70A) (Hallahan et al., 1991) were inactive (Figure 7A, S7F). Interestingly, R33A ANG, despite having a defective nuclear localization sequence (Moroianu and Riordan, 1994), recapitulated the effect of WT ANG in restricting proliferation (Figure 7A) and enhancing self-renewal signature (Figure 7B, S7F). It is significant to note that a 2 hour exposure to human ANG is adequate for CD34+ human CB cells to up-regulate pro-self-renewal genes (Figure 7B), which greatly enhances the translational capability of ANG in improving SCT. The fact that R33A ANG variant is as active as WT ANG points to the dispensable role of nuclear ANG in HSPC, and reinforces the finding that cytoplasmic localization of ANG is important in preservation of HSPC stemness. Further, ANG treatment of CB cells led to slightly elevated numbers of primitive colonies (Figure S7G). Together, these data indicate that in vitro properties of mouse ANG faithfully translate in a human setting, and suggest that the cellular mechanisms underlying mouse HSC regeneration may also translate into human cells.
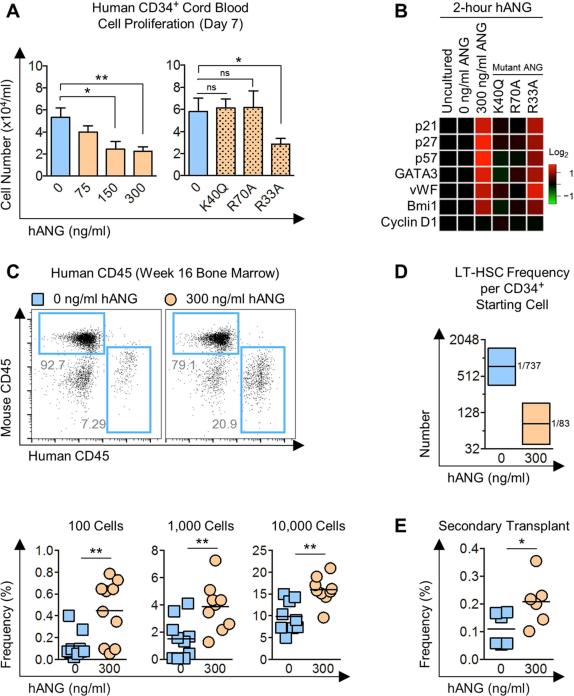
Figure 7. ANG enhances post-transplant reconstitution of human CD34+ CB cells.
(A) Cell number on day 7 from human CD34+ CB cells (2,500 cells/ml) cultured in the presence of various doses of ANG or ANG variants: K40Q (enzymatic variant), R70A (receptor-binding variant), or R33A (nuclear localization variant) at 300 ng/ml (n=6).
(B) qRT-PCR analysis of pro-self-renewal transcripts in human CD34+ CB cells following 2 h culture with 300 ng/ml human ANG protein (n=6).
(C) Human CD45 cells in BM of NSG mice transplanted with human CD34+ CB cells treated with or without human ANG (300 ng/ml) for 2 h. BM was harvested 16 weeks post-transplant (n=9-10).
(D) LT-HSC frequencies (black line) and 95% confidence intervals (shaded boxes) for each transplant condition from Figure 7C (p=8.28×10−5).
(E) Secondary transplant of ANG-treated human CD34+ CB cells from primary recipient. BM was harvested 4 weeks post-transplant (n=6)
See also Figure S7.
To assess whether ANG improves transplantation efficiency of human cells, we transplanted CD34+ CB cells that had been cultured for 2 hours in the presence or absence of ANG into NSG mice at limit dilution and found that treatment with ANG led to elevated frequencies of human CD45+ cells across all doses examined in BM at 16 weeks post-transplant (Figure 7C). Importantly, enhanced regeneration was multi-lineage, as confirmed by the presence of both CD19 B-lymphoid cells and CD33 myeloid cells in BM (Figure S7H-I). Remarkably, calculated LT-HSC frequency was 8.9-fold higher in ANG-treated human CD34+ CB cells relative to untreated cells (Figure 7D). Further, enhanced reconstitution was observed upon secondary transplant without further ANG treatment (Figure 7E). These data highlight the translational capacity of ANG in improved transplantation efficacy of clinically-relevant human cells.
DISCUSSION
Our study highlights several important findings. First, ANG has a cell type-specific role in regulating proliferation of HSPC versus MyePro cells: while promoting quiescence in the former, ANG stimulates proliferation in the latter. Second, we identify a novel RNA-based mechanism by which hematopoiesis is regulated. Importantly, ANG promotes tiRNA production in LKS cells, in association with enhanced stemness in vitro and in vivo. We also show that increased tiRNA production results in reduced levels of global protein synthesis in HSPC. In contrast, ANG stimulates rRNA transcription in MyePro, but not in HSPC, leading to increased protein synthesis and proliferation. To our knowledge, this is the first report of cell type specificity in RNA processing that leads to or originates from a different cellular state. How cell differentiation state results in the very distinctive effects of ANG is intriguing and of practical consequence as demonstrated by our studies. Defining the basis for this cell type specificity is beyond the scope of this report, but will be of particular interest for how exogenous signals and intrinsic properties may induce distinctive RNA processing in specific cell types in response to ANG.
Our findings are of importance given recent reports demonstrating tight regulation of protein synthesis in HSPC (Signer et al., 2014). Further, a number of mutations or defects in ribosome function or protein synthesis have been shown to either promote or resist malignant hematopoiesis (Narla and Ebert, 2010). To our knowledge, however, no factors have been shown to link the regulation of HSPC quiescence at the level of protein synthesis. Moreover, a potential therapeutic benefit of using these properties to promote hematopoietic regeneration has not been explored. Modulating tiRNA to alter protein synthesis and cell fate is unique among prior reports of regulatory mechanisms and is of particular interest because of its ability to be affected by a cell exogenous source. The notion that tiRNA can be cell state-specific in regulating hematopoiesis offers the possibility that similar distinct mechanisms may apply to other tissue types. Discerning whether this is the case and how they may induce altered cell characteristics will help define whether tiRNA represent a common regulatory lever in mammalian biology.
Third, we demonstrate two potential therapeutic uses for ANG. We found that recombinant ANG recapitulates the growth suppressive properties in vitro, and can remarkably improve post-transplant reconstitution of mouse LT-HSCs and human CD34+ CB cells in vivo. Previous studies have identified numerous factors that expand stem cell number in vitro and in vivo (Boitano et al., 2010; Delaney et al., 2010; Fares et al., 2014; Himburg et al., 2010; North et al., 2007); however, it has been noted that cycling HSPC engraft less well upon transplantation and undergo faster exhaustion (Nakamura-Ishizu et al., 2014; Passegue et al., 2005), likely as a consequence of differentiation and loss of stemness. Our finding that ANG improves HSPC stemness warrants further testing of ANG as a means of improving transplantation outcomes in the setting of limiting HSPC cell numbers. Further, the ability of ANG to serve as a radio-mitigant is also of considerable interest, particularly given its ability to rescue animals when administered 24 hours post-irradiation injury. Translation of this ability to humans is obviously complex, but the potential to reduce mortality following radiation exposure is of considerable significance. Functionally, we demonstrate that ANG simultaneously preserves stemness and promotes progenitor cell proliferation following radiation damage, in contrast to other reported approaches of HSPC regeneration or protection from genotoxic injury. The success of hematopoietic regeneration depends upon rapid reconstitution of mature blood cell pools to avoid infections and bleeding complications and long-term generation of mature cells from a durable cell source (Doulatov et al., 2012; Smith and Wagner, 2009). These two functions are provided by progenitor and stem cell populations, respectively.
Currently, there are no FDA-approved drugs to treat severely irradiated individuals (Singh et al., 2015). A number of hematopoietic growth factors have been shown to mitigate hematopoietic syndrome of acute radiation syndrome, however only a few candidates have been demonstrated to improve survival when administered 24 hours post-irradiation (Himburg et al., 2014), an efficacy requirement mandated by The Radiation and Nuclear Countermeasures Program at the National Institute of Allergy and Infectious Diseases. Moreover, current standard-of-care approaches, including granulocyte colony-stimulating factor (G-CSF) and its derivatives, target a limited progenitor cell pool and require repeated doses to combat radiation-induced neutropenia (Singh et al., 2015). In this regard, ANG is a promising candidate as a medical countermeasure for radiation exposure, as only three ANG treatments are needed for improved animal survival, even if started 24 hours after a lethal (12.0 Gy) dose. The long-term effect of ANG treatment in post-irradiated mice, however, is not clear and is the subject of future studies. It will therefore be important to assess whether ANG promotes survival of genetically aberrant HSPC and/or leads to development of leukemia.
Overall, we demonstrate that the unique growth and survival properties of ANG in primitive hematopoietic cells can be therapeutically harnessed for improvement of tissue regeneration.
EXPERIMENTAL PROCEDURES
Animal Studies
Ang−/− mice were generated in-house. B6.SJL and NSG mice were purchased from The Jackson Laboratory. For aged animal experiments, 22-month old WT (NIH/NIA) and Ang−/− mice were used. For all other studies, age-matched 7-12 week old mice were used. Littermates and gender-matched animals were used whenever possible. All procedures were performed in accordance with protocols approved by Institutional Animal Care and Use Committee of Tufts University/Tufts Medical Center.
Cell Surface Markers for Stem and Progenitor Subtypes
The following cell surface markers were used: LKS (Lin−c-Kit+Sca1+), MyePro (Lin−c-Kit+Sca1−), LT-HSC (Flk2−CD34− LKS or CD150+C48− LKS), ST-HSC (Flk2−CD34+ LKS or CD150+C48+ LKS), MPP (Flk2+CD34+ LKS or CD150−C48+ LKS), MPP1 (CD150+CD48−CD135−CD34+ LKS), MPP2 (CD150+CD48+CD135−CD34+ LKS), MPP3 (CD150−CD48+CD135−CD34+ LKS), MPP4 (CD150−CD48+CD135+CD34+ LKS), CLP (Lin−c-KitmedSca1medIL7R+ Flk2+ B220−), pre-pro B (Lin−c-KitmedSca1medIL7R+ Flk2+ B220+), CMP (Lin−c-Kit+Sca1−CD34+CD16/32−), GMP (Lin−c-Kit+Sca1−CD34+CD16/32+), and MEP (Lin−c-Kit+Sca1−CD34−CD16/32−).
Stem Cell Cultures
Mouse LT-HSCs were cultured per manufacturer's instructions in PBS or in S-clone media (Sanko Junyaku) for 2 hour or 2-14 days, respectively. Human CD34+ CB cells were cultured per manufacturer's instructions in PBS or in StemSpan SFEM for 2 hours or 7 days, respectively.
Transplantation
Transplantation of conditional knockout donor cells (1:1 competitive), B6.SJL donor BM into conditional knockout recipients, ex vivo reconstitution assays, serial and rescue cells, tiRNA-transfected donor cells, irradiated or 5-FU-treated donor cells, and treated human CD34+ CB cells was done as described in Supplemental Experimental Procedures.
Statistical Analyses
All bar graphs represent mean ± SEM and all heatmaps represent mean. All data are derived from 2-4 independent experiments. For comparisons of two experimental groups, an unpaired two-tailed Student's t-test was used (Excel). Kaplan-Meier survival curves were analyzed by log rank tests (Prism 6). Heatmaps were generated with RStudio. LDA was assessed by ELDA (http://bioinf.wehi.edu.au/software/elda). For all analyses, *p<0.05, **p<0.01, ***p<0.001, and ns=not significant.
Supplementary Material
Highlights.
- HSPC quiescence and progenitor cell proliferation is simultaneously enhanced by ANG
- The dichotomous effect of ANG is related to differential RNA processing
- ANG prevents and mitigates radiation-induced bone marrow failure
- ANG dramatically improves transplantation efficiency of mouse and human HSPC
Acknowledgements
We thank Kathryn Folz-Donahue, Steven Kwok, Allen Parmalee, Laura Prickett-Rice, and Meredith Weglarz for assistance with cell sorting, Dr. Alenka Lovy for assistance with microscopy. Aged WT mice were kindly provided by NIH/NIA, and Osx and Ocn cDNA were kind gifts from Dr. Deepak Balani/Dr. Henry Kronenberg. This work was supported by NIH fellowship F31HL128127 (KAG), Sackler Dean's Fellow Award (KAG), Sackler Families Collaborative Cancer Biology Award (KAG/GFH), Leukemia & Lymphoma Research UK/Leukemia & Lymphoma Society Fellowships (LS), NSFC Grant #81272674 (SL), NIH grants R01DK050234 and R01HL097794 (DTS), and R01CA105241 and R01NS065237 (GFH). DTS is a consultant and stockholder for Magenta Therapeutics, Fate Therapeutics and a consultant for Bone Therapeutics, Teva, Dr. Reddy's and Apotex. GFH is a consultant and stockholder of Fuyuan Biopharmaceuticals and Karma Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
KAG, LS, DTS and GFH conceived the project, designed experiments, and analyzed data. KAG, LS, SL, NS, MGH and HY performed experiments. KAG, DTS and GFH wrote the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, seven figures, and six tables.
REFERENCES
- Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas-Wallscheid N, Klimmeck D, Hansson J, Lipka DB, Reyes A, Wang Q, Weichenhan D, Lier A, von Paleske L, Renders S, et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat. Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Boffelli D, Mote P, Martin DI. 5' tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics. 2013;14:298–312. doi: 10.1186/1471-2164-14-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10:120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J. Biol. Chem. 2010;285:10959–10968. doi: 10.1074/jbc.M109.077560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, Csaszar E, Knapp DJ, Miller P, Ngom M, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345:1509–1512. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochem. 1985;24:5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Frelin C, Herrington R, Janmohamed S, Barbara M, Tran G, Paige CJ, Benveniste P, Zuniga-Pflucker JC, Souabni A, Busslinger M, et al. GATA-3 regulates the self-renewal of long-term hematopoietic stem cells. Nat. Immunol. 2013;14:1037–1044. doi: 10.1038/ni.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan TW, Shapiro R, Vallee BL. Dual site model for the organogenic activity of angiogenin. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2222–2226. doi: 10.1073/pnas.88.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, Chi JT, Salter AB, Lento WE, Reya T, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat. Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg HA, Yan X, Doan PL, Quarmyne M, Micewicz E, McBride W, Chao NJ, Slamon DJ, Chute JP. Pleiotrophin mediates hematopoietic regeneration via activation of RAS. J. Clin. Invest. 2014;124:4753–4758. doi: 10.1172/JCI76838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-Induced tRNA Fragments Inhibit Translation Initiation. Mol. Cell. 2011;43:613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent DG, Copley MR, Benz C, Wohrer S, Dykstra BJ, Ma E, Cheyne J, Zhao Y, Bowie MB, Zhao Y, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113:6342–6350. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- Kieran D, Sebastia J, Greenway MJ, King MA, Connaughton D, Concannon CG, Fenner B, Hardiman O, Prehn JH. Control of motoneuron survival by angiogenin. J. Neurosci. 2008;28:14056–14061. doi: 10.1523/JNEUROSCI.3399-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–456. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- Lee F, Shapiro R, Vallee B. Tight-Binding Inhibition of Angiogenin and Ribonuclease A by Placental Ribonuclease Inhibitor. Biochem. 1989;1989:225–230. doi: 10.1021/bi00427a031. [DOI] [PubMed] [Google Scholar]
- Li P, Lahvic JL, Binder V, Pugach EK, Riley EB, Tamplin OJ, Panigrahy D, Bowman TV, Barrett FG, Heffner GC, et al. Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature. 2015;523:468–471. doi: 10.1038/nature14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hu GF. Angiogenin-mediated rRNA transcription in cancer and neurodegeneration. Int. J. Biochem. Mol. Biol. 2010;1:26–35. [PMC free article] [PubMed] [Google Scholar]
- Mantel CR, O'Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, Brustovetsky N, Srour EF, Lee MR, Messina-Graham S, et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell. 2015;161:1553–1565. doi: 10.1016/j.cell.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y, Nakayama K, Nakayama KI. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2011;9:262–271. doi: 10.1016/j.stem.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Identification of the nucleolar targeting signal of human angiogenin. Biochem. Biophys. Res. Commun. 1994;203:1765–1772. doi: 10.1006/bbrc.1994.2391. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Ishizu A, Takizawa H, Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141:4656–4666. doi: 10.1242/dev.106575. [DOI] [PubMed] [Google Scholar]
- Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Park I, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J. Exp. Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo E, Sarcinelli C, Sheng J, Fusco S, Formiggini F, Netti P, Yu W, D'Alessio G, Hu GF. Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J. Cell Sci. 2013;126:4308–4319. doi: 10.1242/jcs.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia M, Jobava R, Parisien M, Putnam A, Krokowski D, Gao XH, Guan BJ, Yuan Y, Jankowsky E, Feng Z, et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol. Cell. Biol. 2014;34:2450–2463. doi: 10.1128/MCB.00136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer RA, Magee JA, Salic A, Morrison SJ. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature. 2014;509:49–54. doi: 10.1038/nature13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. 2015;71:22–37. doi: 10.1016/j.cyto.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: place of umbilical cord blood. Br. J. Haematol. 2009;147:246–261. doi: 10.1111/j.1365-2141.2009.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Crabtree B, Acharya KR. Human angiogenin is a neuroprotective factor and amyotrophic lateral sclerosis associated angiogenin variants affect neurite extension/pathfinding and survival of motor neurons. Hum. Mol. Genet. 2008;17:130–149. doi: 10.1093/hmg/ddm290. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Feng Y. A new role for angiogenin in neurite growth and pathfinding: implications for amyotrophic lateral sclerosis. Hum. Mol. Genet. 2007;16:1445–1453. doi: 10.1093/hmg/ddm095. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, Hirukawa S, Hu GF. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 2005;65:1352–1360. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- Xu ZP, Tsuji T, Riordan JF, Hu GF. Identification and characterization of an angiogenin-binding DNA sequence that stimulates luciferase reporter gene expression. Biochem. 2003;42:121–128. doi: 10.1021/bi020465x. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14519–14524. doi: 10.1073/pnas.0606708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.