Abstract
Objective
This study aimed to evaluate a co-encapsulated pegylated nano-liposome system based on two herbal anti-tumor drugs, silibinin and glycyrrhizic acid, for delivery to a hepatocellular carcinoma (HCC) cell line (HepG2).
Materials and Methods
In this experimental study, co-encapsulated nano-liposomes by the thin layer film hydration method with HEPES buffer and sonication at 60% amplitude. Liposomes that co-encapsulated silibinin and glycyrrhizic acid were prepared with a specified molar ratio of dipalmitoylphosphatidylcholine (DPPC), cholesterol (CHOL), and methoxy-polyethylene glycol 2000 (PEG2000)–derived distearoyl phosphatidylethanolamine (mPEG2000-DSPE). We used the MTT technique to assess cytotoxicity for various concentrations of co-encapsulated nano-liposomes, free silibinin (25% w/v) and glycyrrhizic acid (75% w/v) on HepG2 and fibroblast cell lines over a 48-hour period.
Results
Formulation of pegylated nano-liposomes showed a narrow size distribution with an average diameter of 46.3 nm. The encapsulation efficiency (EE) for silibinin was 24.37%, whereas for glycyrrhizic acid it was 68.78%. Results of in vitro cytotoxicity showed significantly greater co-encapsulated nano-liposomes on the HepG2 cell line compared to the fibroblast cell line. The half maximal inhibitory concentration (IC50) for co-encapsulated pegylated nanoliposomal herbal drugs was 48.68 µg/ml and free silibinin with glycyrrhizic acid was 485.45 µg/ml on the HepG2 cell line.
Conclusion
This in vitro study showed that nano-liposome encapsulation of silibinin with glycyrrhizic acid increased the biological activity of free drugs, increased the stability of silibinin, and synergized the therapeutic effect of silibinin with glycyrrhizic acid. The IC50 of the co-encapsulated nano-liposomes was lower than the combination of free silibinin and glycyrrhizic acid on the HepG2 cell line.
Keywords: Encapsulation, Silibinin, Glycyrrhizic Acid, Hepatocellular Carcinoma
Introduction
Worldwide, cancer is recognized as a deadly disease; in this regard, hepatocellular carcinoma (HCC) is the sixth most common cancer in the world. However because of poor prognosis, HCC is considered the third cause of cancer related deaths (1). Conventional anticancer drugs cause various side effects that result from non-selective toxicity and distribution of the drug to normal cells (2).
Nano-liposomes are drug carriers with lipoid membranes used to improve drug delivery. Nanophytosomes are nano-phyto-liposome vesicles formed due the interaction of hydrogen bonds between phospholipids of the lipid membrane and phytomolecules for improving the delivery of therapeutic agents. Specifically, the anti-cancer potency of drugs increases with decreasing size of the liposome (3).
Most studies of phyto-liposomes focus on the liver-protectant flavonoid (silymarin mixture) from the milk thistle [Silybum marianum (L.)], a medicinal plant from Iran (4). Silymarin properties include immunomodulation, anti- fibrotic, membrane stabilizing, anti-oxidative, anti-inflammatory, and liver regeneration that play essential roles in experimental models of liver diseases (5). The components of silymarin are used for hepatoprotection, while having poor aqueous solubility and low bioavailability. In vivo studies regarding oral consumption of powdered components of silymarin have shown ng/ml elevated concentrations in the blood plasma (6, 7). Various clinical and pharmacological effects of silymarin have been reported, such as targeting cancer cell metastasis. Unfortunately, silymarin’s poor solubility in water and oil results in permeation through the intestinal epithelial membrane (8). The dried extracts from milk thistle seeds contain approximately 60% silymarin. Silymarin consists of silibinin (~50 to 60%), isosilibinin (~5%), silichristin (~20%), and silidanin (~10%). Silibinin is the most important antioxidant substance and the main biological active compound of silymarin (9). Silibinin is an anti-cancer drug; its antitumor efficacy lies mainly in decreasing N-nitrosodiethylamine in liver cancer cells (10). An advantage of the interaction between hydroxyl groups and the silibinin molecule is the rational identification of suitable sites for silibinin derivation while maintaining the biological activity of the resultant conjugates (11). Chu et al. (12) have shown that silibinin exerted a prohibitive effect on the encroachment and intrusion of advanced metastatic A549 cells, but had little effect on adhesion. Silymarin mediated these effects by decreasing the expressions of matrix metalloproteases (MMP)-2 and u-PA, and enhancing the expression of tissue inhibitor of MMP (TIMP)-2 (12). Oral absorption of silibinin is limited and solubility of silibinin is poor in water and lipid media (13).
Therefore, there is a strong need to develop new drug delivery systems that improve the solubility and bioavailability of silibinin. In previous studies, silymarin has been encapsulated to liposomes with a mixture of lecithin, cholesterol (CHOL), stearyl amine, and Tween 20 at a 9:1:1:0.5 molar ratio as the lipophilic phase with a particle size of 800 nm (14).
In addition, we studied glycyrrhizic acid, an herbal drug obtained from Glycyrrhiza glabra (L.). Glycyrrhizic acid possesses hepatoprotector, anti-viral, anti-tumor, and anti-inflammatory properties. The anti-tumor efficacy of glycyrrhizic acid is due to inhibition of MMPs and protection of DNA in cancer cells. In previous studies such as Japan’s drug of neo-minophagen c based glycyrrhizic acid was used for hepatic disease, Italy’s drug of glycyrrhetinic acid phytosome was prepared based on liposomal glycyrrhizic acid, and Russia’s drug of phosphoglive includes vegetative phospholipids with glycyrrhizic acid (15).
All previous studies and products focused on preparation of liposome based on one herbal drug, either glycyrrhizic acid or silymarin but in this study, we co-entrapped two herbal drugs (silibinin and glycyrrhizic acid) to a pegylated nano-liposome in order to assess the in vitro cytotoxicity of co-encapsulated nano-liposomes on a liver cancer cell line (HepG2).
Materials and Methods
Materials
Dipalmitoylphosphatidylcholine (DPPC) and methoxy-polyethylene glycol (PEG2000)-derivatized distearoyl phosphatidylethanolamine (mPEG2000-DSPE) were obtained from Lipoid GmbH (Ludwigshafen, Germany). CHOL, silibinin and HEPES buffer (10 mM, pH=5.5) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glycyrrhizic acid was purchased from Shirin Darou co. (Shiraz, Iran). Deionized water was used throughout the experiments. The in vitro release measurement was carried out at pH=7.4 in phosphate buffer and at pH=5.5 in HEPES buffer at 37˚C. Maltose, etha- nol and the remainder of chemicals used in this study were analytical grade quality. HepG2 and fibroblast cell lines were supplied by the Pasteur Institute of Iran.
Preparation of co-encapsulated nano-liposomes
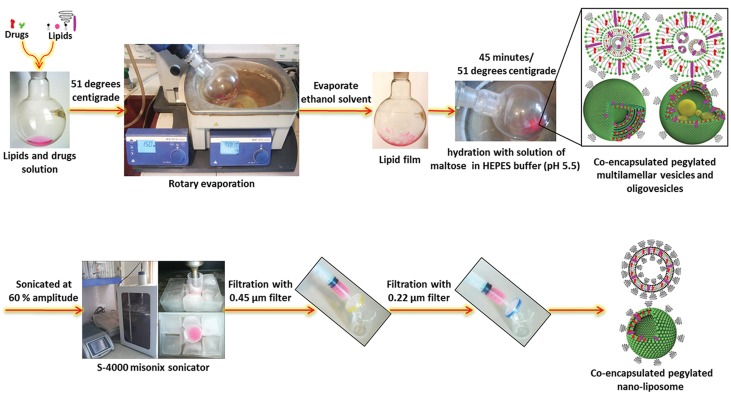
We prepared the co-encapsulated nano-liposomes by the thin layer film hydration method with HEPES buffer and sonication. First, we dissolved a mixture of DPPC, CHOL, and mPEG2000-DSPE at a specified molar ratio with two herbal drugs of silibinin and glycyrrhizic acid at a 1.74:1 molar ratio, as the lipophilic phase in absolute ethanol. The organic solvent was evaporated using a rotary evaporator to produce a thin lipid film. Before hydration, the lipid film was flushed with nitrogen. Liposomes were formed by hydration of the lipid film with a solution that contained maltose in HEPES buffer (10 mM, pH=5.5) as the hydrophilic phase and heated to 51˚C. The mean liposome diameter decreased with sonication. Multilamellar vesicles were sonicated at 60% amplitude for 10 minutes by an S-4000 Misonix sonicator and filtered for synthesis of small unilamellar vesicles (SUV) or nano-liposomes (Fig.1). The suspension was then freeze-dried using an Alpha1-2LD plus Christ Freez Dryer at −48˚C for 48 hours.
Fig.1.
Schematic model for the preparation process of co-encapsulated nano-liposomes.
Morphology of multilamellar vesicles
The morphology (structure and size) of multilamellar vesicles was analyzed by light microscopy.
Size and zeta potential characterization of co-encapsulated nano-liposomes
The particle size and zeta potential of the coencapsulated nano-liposomes were determined by dynamic light scattering (DLS) and a zeta analyzer using a Brookhaven BI-90 particle size and zeta analyzer V7.2 (Brookhaven Instruments, Holtsville, USA).
Measurement of encapsulation efficiency
We used HPLC to determine the encapsulation efficiency of the nano-liposomes that coencapsulated silibinin and glycyrrhizic acid. A reverse phase of the C18 column was used. The mobile phase consisted of a mixture of acetonitrile and phosphoric acid (0.1%), (51:49 v/v) delivered at a flow rate of 1.00 ml/minute with a pump (WellChrom K-1000, Knauer, Germany) (16, 17). First, to remove all free drugs, the coencapsulated nano-liposomes were dialyzed with a Spectra/Pore® Dialysis membrane (12000-14000 Da molecular weight cutoff) against the buffer. We added 200 μl of methanol to 100 μl aliquots of the extruded suspension in tubes and mixed the solution, followed by sonication for 10 minutes. A total of 60 μl of the supernatant was analyzed by the high performance liquid chromatography (HPLC) system. The absorbance of silibinin and glycyrrhizic acid were measured in a UV/V is spectrometer by HPLC in the range of 200-350 nm. Column elute was monitored spectrophotometrically at a 240 nm wavelength with a UV detector (WellChrom K-2500, Knauer, Germany). The calibration curve of silibinin and glycyrrhizic acid were linear over the range of standard concentrations 0.125, 0.25, and 0.5 mg/ ml with a correlation coefficient of R2>0.97 and R2>0.99, respectively.
In order to determine encapsulation efficiency (EE), we used the following equation (1):
In vitro silibinin and glycyrrhizic acid release
Drug release rate was measured by HPLC. The ability to release silibinin and glycyrrhizic acid at pH=7.4 was evaluated. We placed 1 mL of liposomal suspension into a dialysis bag of cellulose that had a 12000-14000 Da molecular weight cutoff (Membrane Filtration Products, Inc.) The dialysis bags were placed in 50 mL of phosphate-buffered saline (PBS), pH=7.4, and the media were stirred with a magnetic bar at 100 rpm at 37˚C. At different time points, we removed 200 μL of the suspension for HPLC analysis in the range of 240 nm.
Stability study
The stability of the liposomal suspension was evaluated after 3 months of storage at 4˚C. The particle size distribution, morphology and drug EE of the samples were determined as a function of the storage time.
Morphology of co-encapsulated pegylated nano-liposomes
The morphology (shape and size) of the nanoliposomes was examined by scanning electron microscope (SEM, KYKY-EM3200-30KV, China) at an acceleration voltage of 25 kV. The size and formation of nano-liposomes membranes with negative-stain was verified through their morphological aspect, as determined by transmission electron microscope (TEM, ZEISS, EM 10, Germany). Samples were then observed in a microscope at the accelerating voltage of 150 kV.
ATR-Fourier transform infrared spectroscopy study of co-encapsulated nano-liposomes
The co-encapsulated pegylated nano-liposomes were analyzed by ATR-Fourier transform infrared spectroscopy (ATR-FTIR) and FTIR (Tensor 27 FTIR spectrophotometer, Bruker Corp., Germany) to determine the pegylating surface of nanoliposomes.
Cytotoxicity assay
We decanted 100 µl of suspension that contained 10000 cells from the cultured HepG2 and fibroblast cells into 96-well plates, which were incubated (5% CO2 and 37˚C) separately. The superT - natant was removed after 24 hours, after which we poured different concentrations of pegylated nanoliposomal silibinin (25% w/v) and glycyrrhizic acid (75% w/v), free silibinin (25% w/v) with glycyrrhizic acid (75% w/v), and their controls on the cells, which were then incubated for 48 hours. Subsequently, the supernatant was removed and 100 µl of MTT solution (0.5 mg/ml) was added. After a 3-hour incubation period, we observed a purple color which indicated the formation of formazan. The mixture was then dissolved in viable cells in 100 µl of isopropanol. Light absorbance was measured at 540 nm by a Power wave XS spectrophotometer (BioTek Instruments, USA) and the IC50 was calculated using the Pharmacologic Calculation System (Pharm PCS) statistical package (Springer Verlag, USA).
Statistical analysis
One-way analysis of variance (ANOVA) was performed on the data to assess the impact of the formulation variables on the results. P<0.05 were considered significant. All calculations were performed using the statistical software program SAS 9.1 (SAS Institute, Cary, NC, USA).
Results
Morphologies of silibinin and glycyrrhizic acid multilamellar vesicles
We analyzed the silibinin and glycyrrhizic acid multilamellar vesicles images via light microscopy. There were multi membranes of liposomal herbal drugs that had micrometric sizes before sonication and synthesis of SUV (Fig.2).
Fig.2.
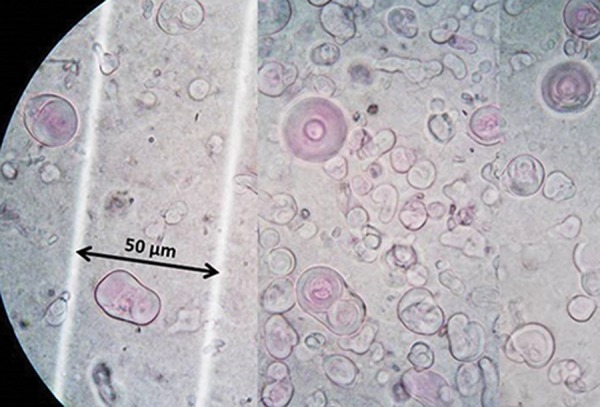
Light microscopic images of co-encapsulated multilamellar vesicles from the same samples before decreasing the size of the liposomes.
Characterization of co-encapsulated nanoliposome size and zeta potential
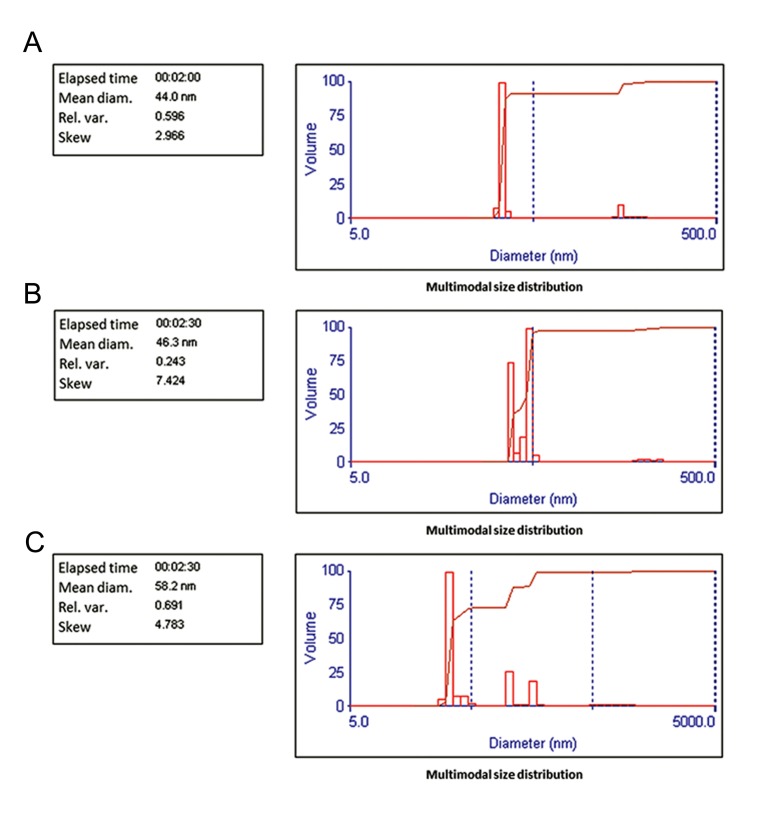
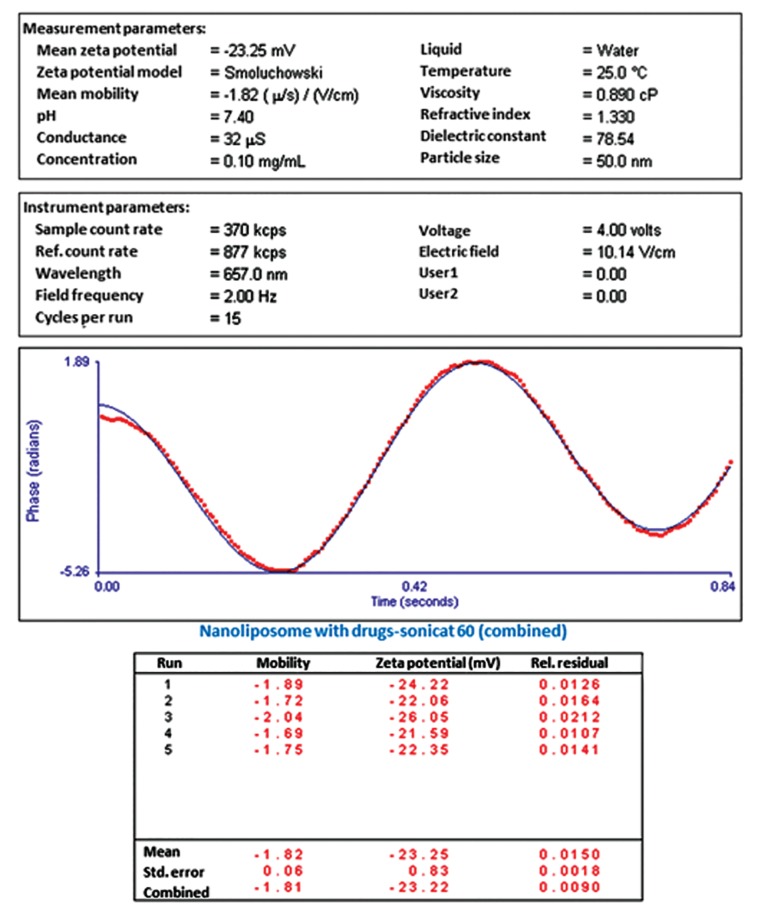
The formulation of pegylated nano-liposomes sonicated at 60% amplitude showed a narrow size distribution with an average diameter of 46.3 nm (Fig.3). The zeta potential of the co-encapsulated nano-phytosome was -23.25 mV (Fig.4).
Fig.3.
Size of: A. Nano-liposome without herbal drugs, B. Co-encapsulated nano-liposomes after 3 days, and C. Co-encapsulated nanoliposomes after 3 months.
Fig.4.
Zeta potential of co-encapsulated nano-liposomes after 3 days.
We observed that the zeta potential of the co-encapsulated nano-phytosome had sufficient charge to inhibit aggregation of the liposomes. Loading of silibinin and glycyrrhizic acid increased the mean diameter and decreased zeta potential negative charge of the nano-liposome, respectively. The results of the mean diameter and zeta potential were obtained from approximately 150 individual liposomes.
Encapsulation efficiency for silibinin and glycyrrhizic acid in pegylated nano-liposomes
We used HPLC to measure EE. Silibinin and glycyrrhizic acid absorbance peaks were found at a wave length of 240 nm. The EE for silibinin was approximately 24.37%, whereas for glycyrrhizic acid it was approximately 68.78%. Figure 5 shows the HPLC graph of silibinin and glycyrrhizic acid loaded in the nano-phytosomes.
Fig.5.
HPLC graph of silibinin and glycyrrhizic acid loaded in the nano-phytosomes. HPLC; High performance liquid chromatography.
In vitro silibinin and glycyrrhizic acid release
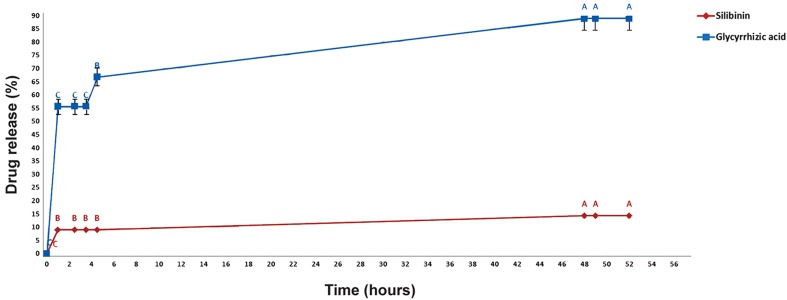
According to HPLC analyses, 14% (W/W) of silibinin and 88% (W/W) of glycyrrhizic acid were released after 48 hours. Release rate decreased over time (Fig.6). As seen in Figure 6, data with different letters show significant differences (P<0.05) from each other.
Fig.6.
In vitro silibinin and glycyrrhizic acid release. Values with different letters demonstrate significant variance (P<0.05) from each other (n=3).
Stability study
Results of the zetasizer tests confirmed the stability of the liposomal suspension. There were negligible changes in size distribution and zeta potential of the liposomal suspension after storage at 4˚C for 3 months (Table 1). Results of SEM analysis of the liposomal suspension after storage for 3 months showed that the co-encapsulated nano-liposomes had an approximate mean diameter of 55 nm (Fig.7). The EE for liposomal silibinin and glycyrrhizic acid after storage in suspension form at 4˚C for 3 months were approximately 19.14% for silibinin and 26.75% for glycrrhizic acid.
Table 1.
Size and zeta potential stability of liposomal suspension (about 150 individual liposomes) after storage for 3 months (mean ± SE)
| Liposomal suspension | Mean zeta potential (mV) | Mean particle size (nm) |
|---|---|---|
| Nano-liposome without herbal drugs after 3 days | 44 ± 2.2 | -31.66 ± 0.39 |
| Co-encapsulated nano-liposomes after 3 days | 46.3 ± 0.4 | -23.25 ± 0.83 |
| Co-encapsulated nano-liposomes after 3 months | -25.52 ± 0.97 | 58.2 ± 3.8 |
Fig.7.
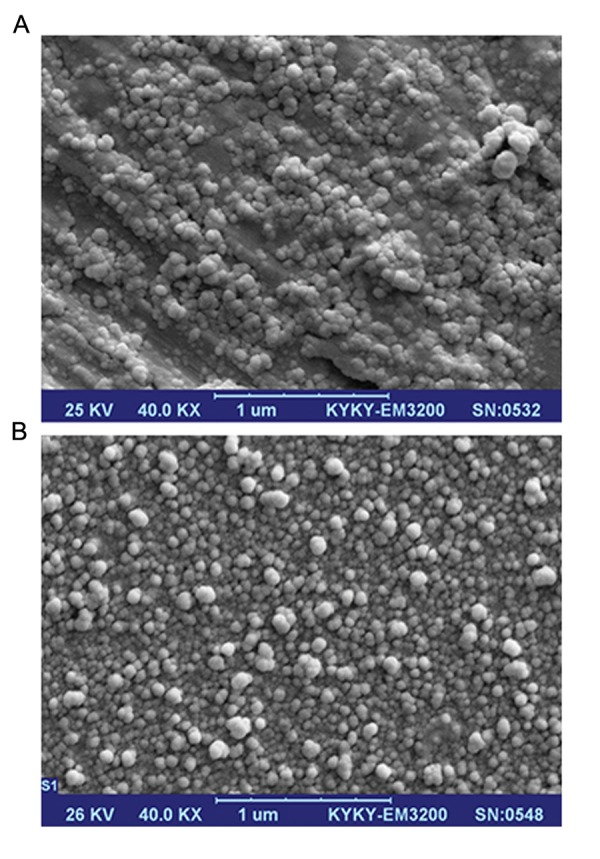
A. Scanning electron microscope (SEM) photograph of co-encapsulated nano-liposomes and B. SEM photograph of co-encapsulated nano-liposomes after 3 months.
Morphology of co-encapsulated pegylated nano-liposomes
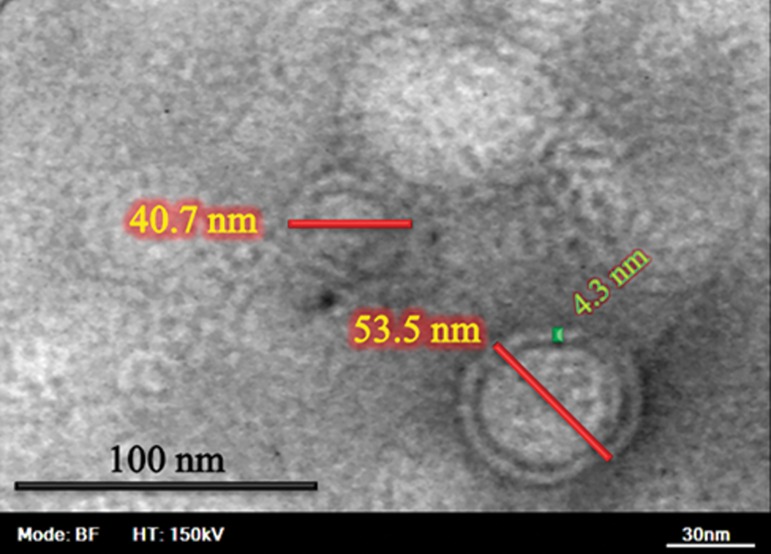
SEM analyses of the surface morphology of the nano-liposomes showed that the co-encapsulated nano-liposomes had a mean diameter of 43 nm (Fig.7). The morphology of the nanoliposomes was determined by TEM. Results of TEM showed that the range of diameter of coencapsulated nano-liposomes was about 40 to 60 nm (Fig.8).
Fig.8.
Transmission electron microscope (TEM) photograph of co-encapsulated nano-liposomes.
Fig.9.
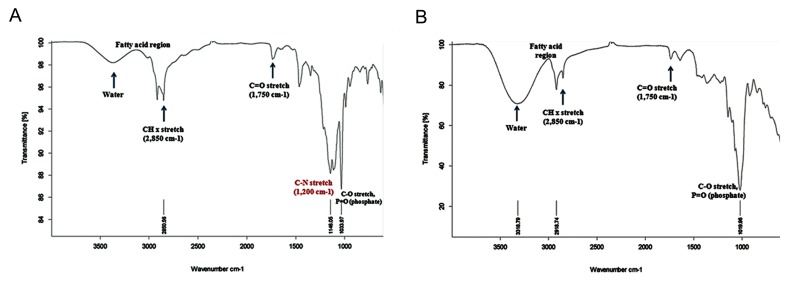
A. ATR-Fourier transform infrared spectroscopy (ATR-FTIR) of methoxy-polyethylene glycol 2000 (PEG2000)–derived distearoyl phosphatidylethanolamine mPEG-DSPE and B. ATR-FTIR of co-encapsulated nano-liposomes.
ATR-Fourier transform infrared spectroscopy study on co-encapsulated nano-liposomes
The co-encapsulated nano-liposomes were analyzed by ATR-FTIR. FTIR results demonstrated a pegylating surface of the co-encapsulated nano-liposomes. Absorption bonds of MPEG-DSPE emerged at wave- numbers 1033 cm-1, 1750 cm-1, and 2850 cm-1 (the distance covered by light in one second). According to Figure 9, there were negligible alterations in chemical bonds of the co-encapsulated nano-liposomes which showed that the surfaces of the co-encapsulated nano- liposomes were pegylated.
Cytotoxicity
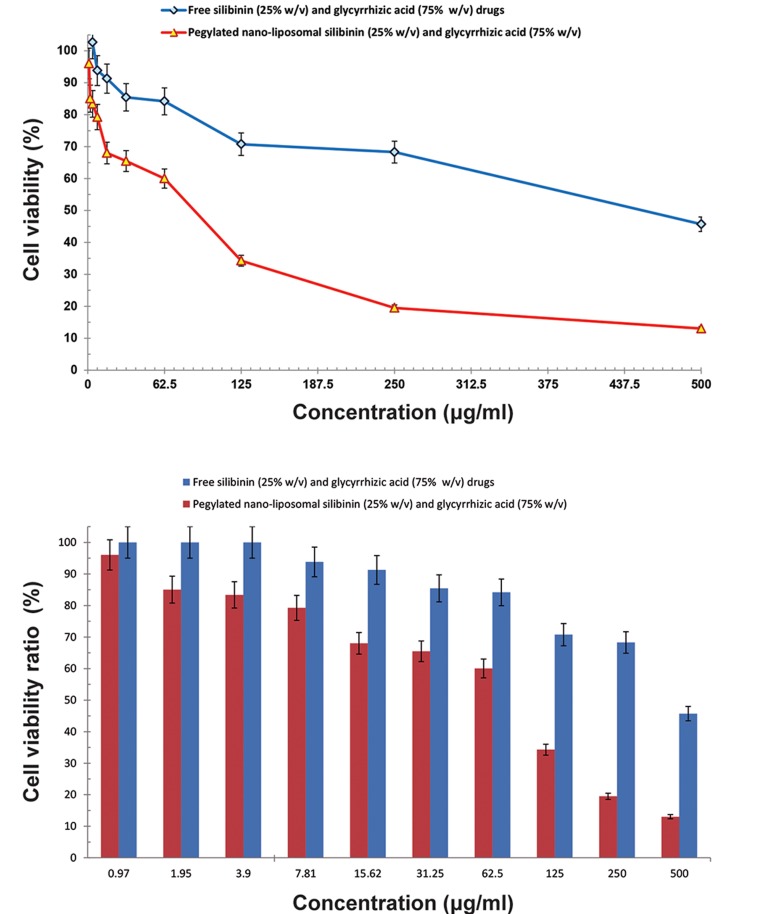
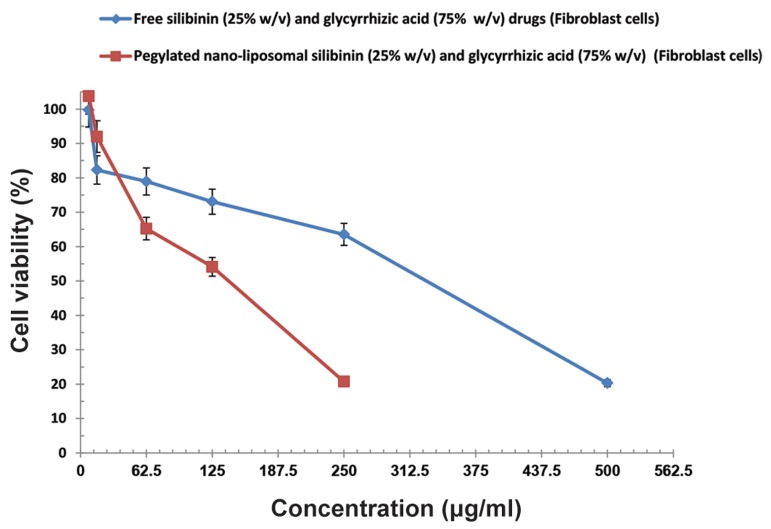
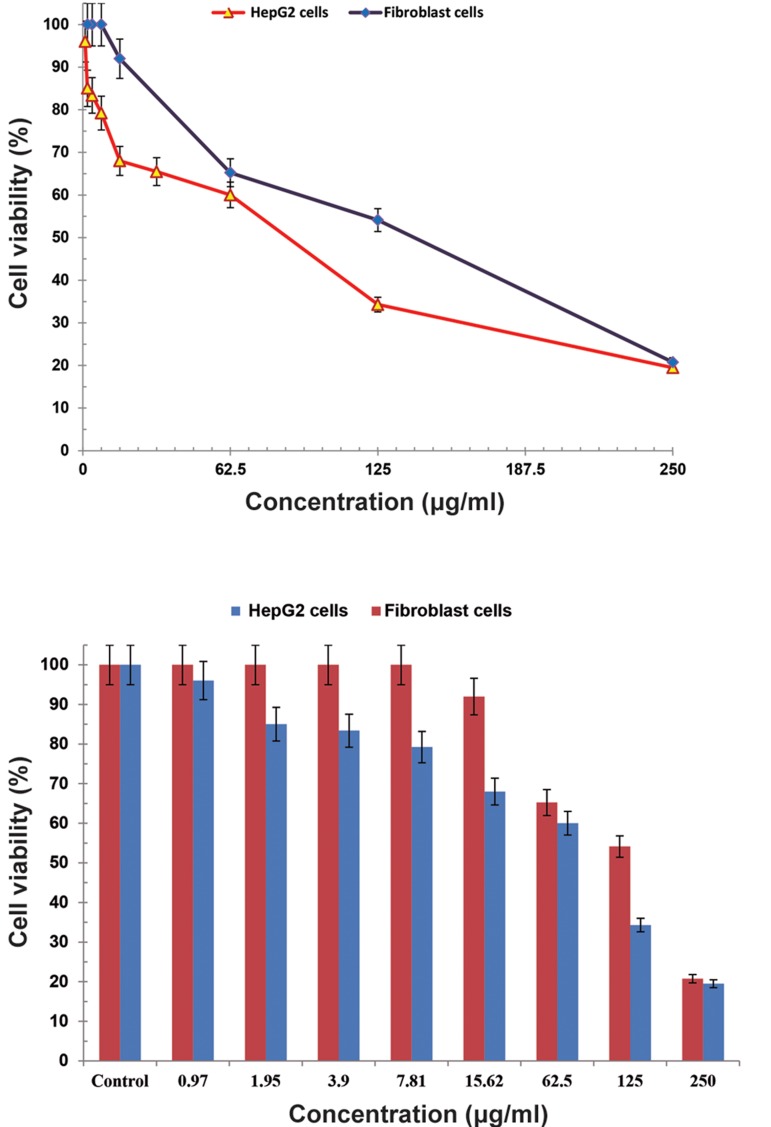
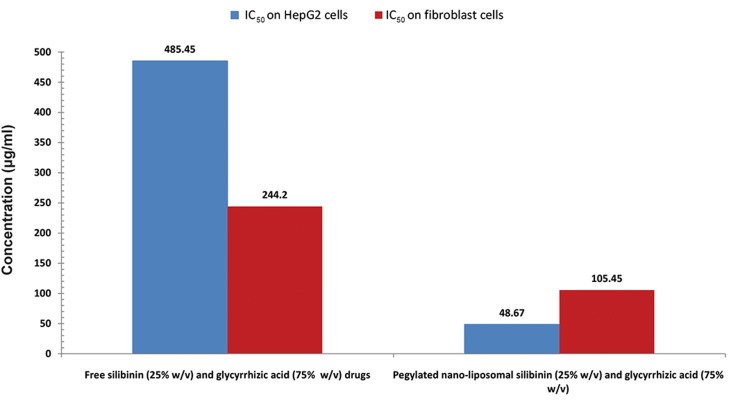
We sought to examine the presence of any drug toxicity at different concentrations based on the MTT technique. We observed the highest cytotoxicity at the time of maximum release which occurred in the first 48 hours. Cell viability of co-encapsulated nanoliposomes and free silibinin (25% w/v) and glycyrrhizic acid (75% w/v) drugs on HepG2 cell line and fibroblast cell line are shown in Figures 10 and 11, respectively. Cell viability comparison of co-encapsulated nano-liposomes on HepG2 cell line and fibroblast is shown in Figure 12. The IC50 for pegylated nanoliposomal silibinin and glycyrrhizic acid was 48.67 µg/ml; for free silibinin and glycyrrhizic acid, this value was 485.45 µg/ml in the HepG2 cell line. In the fibroblast cell line, the IC50 for pegylated nanoliposomal silibinin and glycyrrhizic acid was 105.45 µg/ml, whereas the free silibinin and glycyrrhizic acid IC50 was 244.2 µg/ml (Fig.13). The results showed that the cytotoxicity of pegylated nanoliposomal silibinin and glycyrrhizic acid was about ten times greater than the cytotoxicity of free herbal drugs on the HepG2 cell line. The cytotoxicity of pegylated nanoliposomal silibinin and glycyrrhizic acid on the HepG2 cell line was approximately two times greater than the cytotoxicity on the fibroblast cell line.
Fig.10.
Cell viability of co-encapsulated nano-liposomes, free silibinin (25% w/v) and glycyrrhizic acid (75% w/v) on the HepG2 cell line. Values are expressed as mean ± SD. Error bars with 5% value.
Fig.11.
Cell viability of co-encapsulated nano-liposomes, free silibinin (25% w/v) and glycyrrhizic acid (75% w/v) on a fibroblast cell line. Values are expressed as mean ± SD.
Fig.12.
Cell viability of co-encapsulated nano-liposomes on HepG2 and fibroblast cell lines. Values are expressed as mean ± SD. Error bars with 5% value.
Fig.13.
The half maximal inhibitory concentration (IC50) of co-encapsulated nano-liposomes, free silibinin (25% w/v) and glycyrrhizic acid (75% w/v) on HepG2 and fibroblast cells. Values are expressed as mean ± SD.
Discussion
In this study, there was significantly greater in vitro cytotoxicity in the co-encapsulated nanoliposome on HepG2 cells compared to the fibroblast cell line. The cytotoxicity of co-encapsulated pegylated nano-liposome was ten times greater than free silibinin (25% w/v) and glycyrrhizic acid (75% w/v) on the liver cancer cells (HepG2 cell line).
Nano-phyto liposomes (nano-phytosome) are innovative formulations that improve bioavailability of hydrophilic flavonoids. It has been demonstrated that polar phyto constituents such as flavonoids when mixed with phospholipids (e.g., phosphatidylcholine) generate a new drug delivery system called nano-phyto liposome with a better absorption profile and more suitable lipid solubility which make them competent to cross biological membranes (4). Bombardelli et al. (18) have reported that phyto liposomes increased the specific activity of silymarin and had greater durability and stability than the free constituents. With respect to the antioxidant percentage and free radical clean- ing properties, in humans liposomal silibinin effectively is absorbed into the target tissue of liver. The results have suggested that the absorbency of liposomal silibinin is approximately 7 times greater than the absorbency of free silibinin (6). The anti-hepatotoxic activity of liposomal silymarin is better than silymarin alone and can protect broiler chicks against the toxic effects of aflatoxin B1 (19). Yanyu et al. (20) have prepared a silibinin-phospholipid complex. Due to an impressive improvement of the lipophilic, oral administration of phyto liposomal silibinin increased its bioavailability in rats and had an improved biological effect. Different clinical studies have shown the bioavailability and pharmacological safety of silymarin. Experiments are now in progress to demonstrate the clinical influence of silymarin on different cancers (21). Silibinin is the most important anti-tumor substance of the silymarin compound and largely responsible for its antihepatotoxic activity (8-10). Components of silymarin (such as silibinin) have poor solubility in aqueous and lipid and low bioavailability (6, 7, 13).
Therefore, there is a strong need to develop new drug delivery systems such as phyto-liposomes to improve both the solubility and synergize the therapeutic effect and bioavailability of silibinin. In this study, we have co-encapsulated two herbal anti-tumor drugs, silibinin and glycyrrhizic acid, to a pegylated nano-liposome. The EE for silibinin was greater than 24% and glycyrrhizic acid was over 68%. In this study, the two herbal drugs were co-entrapped to nano-phyto liposome by hydrogen bond interactions between the phospholipids and the herbal drugs. These nano-phyto liposomes could improve the delivery of phytomolecules.
El-Samaligy et al. (14) prepared liposome based on one herbal drug, silymarin, that had a mean particle size of 800 nm. Archakov et al. prepared phosphoglive using vegetative phospholipids with glycyrrhizic acid and reported a mean particle size less than 100 nm (15). All previous studies and products dealt with preparation of liposome based on one herbal drug, either glycyrrhizic acid or silymarin. However, the present study investigated co-entrapping two herbal drugs and the formulation of pegylated nano-liposomes. We observed a narrow size distribution with diameters of approximately 40 to 50 nm.
In this study, SEM photographs showed that the mean diameter of the co-encapsulated nanoliposomes was 43 nm. We have observed that the zeta potential for the herbal drug loaded liposomes was -23.25 mV. A zeta potential greater than 30 mV is necessary for effective stability and to inhibit aggregation. In the current study, we have observed that the zeta potential of the co-encapsulated nano-phytosomes had sufficient charge to inhibit liposome aggregation.
The size stability of the co-encapsulated nanoliposomes in buffer solution (4˚C) was suitable; the mean diameter of co-encapsulated nano-liposomes after 3 months was 58.2 nm. A stability study showed that the EE of liposomal suspension changed dramatically after 3 months. For increased stability of herbal drug entrapment, it was suggested that the nano-liposomal suspension should be freeze-dried after which the co-encapsulated nano-liposome stability would be analyzed after lyophilizing.
In this study, ATR-FTIR results of co-encapsulated nano-liposomes demonstrated that the surface of the co-encapsulated nano-liposomes was pegylated. Nano-liposomes could be coated with hydrophilic polymers like PEG, which gives them long-circulating properties (22, 23). The most significant finding of this study was that co-encapsulated nano-liposomes with good silibinin and glycyrrhizic acid content and suitable stability could be successfully developed.
In vitro cytotoxicity showed that the IC50 of coencapsulated nano-liposomes was lower than free silibinin (25% w/v) and glycyrrhizic acid (75% w/v). Toxicity tests indicated that cytotoxicity of the drug nano-carrier was approximately three times greater than the cytotoxicity of free drug (24). Silibinin was shown to seriously synergize the remedial efficacy of doxorubicin in advanced prostate cancer cells (25). An in vitro study showed that nano-liposome encapsulation of silibinin with glycyrrhizic acid increased the biological activity of free drugs, increased the stability of silibinin, and synergized the therapeutic effect of silibinin with glycyrrhizic acid on an HCC cell line.
Conclusion
The results showed significant improvement in the co-entrapment of silibinin and glycyrrhizic acid to pegylated nano-liposome which had suitable EE, stability, controlled release rate of the drugs, good surface zeta potential and suitable mean diameter (less than 50 nm). The results have shown that the least IC50 belonged to co-encapsulated pegylated nanoliposomal silibinin and glycyrrhizic acid was less than that of free silibinin (25% w/v) and glycyrrhizic acid (75% w/v) on the HepG2 cancer cell line. Its usage is suggested for in vivo experiments.
Acknowledgments
This study is part of a Ph.D. thesis and financially supported by the University of Tehran. The authors would like to thank the Research Center for New Technologies in Life Science Engineering, University of Tehran, Islamic Republic of Iran for their laboratory service. There is no con- flict of interest.
References
- 1.Jin C, Yang W, Bai L, Wang J, Dou K. Preparation and characterization of targeted DOX-PLGA-PEG micelles decorated with bivalent fragment HAb18 F (ab′) 2 for treatment of hepatocellular carcinoma. J Control Release. 2011;152(Suppl 1):e14–15. doi: 10.1016/j.jconrel.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 2.Cheng WW, Allen TM. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab′ fragments and single chain Fv. J Control Release. 2008;126(1):50–58. doi: 10.1016/j.jconrel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathore P, Swami G. Planterosomes: a potential phytophospholipid carriers for the bioavailability enhancement of herbal extracts. Int J Pharm Sci Res. 2012;3(3):737–755. [Google Scholar]
- 5.Post-White J, Ladas EJ, Kelly KM. Advances in the use of milk thistle (Silybum marianum) Integr Cancer Ther. 2007;6(2):104–109. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 6.Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur J Drug Metab Pharmacokinet. 1990;15(4):333–338. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- 7.Schandalik R, Gatti G, Perucca E. Pharmacokinetics of silybin in bile following administration of silipide and silymarin in cholecystectomy patients. Arzneimittelforschung. 1992;42(7):964–968. [PubMed] [Google Scholar]
- 8.Chen MW, Tan W, Wang ShP, Zhong ZhF, Wang YT. Advances in the nanoparticle drug delivery systems of silymarin. J Chin Pharm Sci. 2011;20(5):442–446. [Google Scholar]
- 9.Radjabian T, Fallah Huseini H. Anti-hyperlipidemic and anti-atherosclerotic activities of silymarins from cultivated and wild plants of silybum marianum L.with different content of flavonolignans. Iranian J pharmacol Ther. 2010;9(2):63–67. [Google Scholar]
- 10.Ramakrishnan G, Raghavendran HR, Vinodhkumar R, Devaki T. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact. 2006;161(2):104–114. doi: 10.1016/j.cbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Gažák R, Marhol P, Purchartová K, Monti D, Biedermann D, Riva S, et al. Large-scale separation of silybin diastereoisomers using lipases. Process Biochem. 2010;45(10):1657–1663. [Google Scholar]
- 12.Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40(3):143–149. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Zhuang J, Guo J, Xiao Y, Ping Q. Preparation and properties of a silybin-phospholipid complex. Pharmazie. 2008;63(1):35–42. [PubMed] [Google Scholar]
- 14.El-Samaligy MS, Afifi NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308(1-2):140–148. doi: 10.1016/j.ijpharm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Archakov AI, Guseva MK, Uchaikin VF, Tikhonova EG, Ipatova OM, inventors. Medicinal forms of phospholipid preparations and methods for their preparation.United States Patent (USPTO) Publication type of patent: grant. 2014 Available from: http://www.google.com/patents/US8680061. (5 Apr 2016).
- 16.Park JH, Park JH, Hur HJ, Woo JS, Lee HJ. Effects of silymarin and formulation on the oral bioavailability of paclitaxel in rats. Eur J Pharm Sci. 2012;45(3):296–301. doi: 10.1016/j.ejps.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Ren P, Sun G. HPLC determination of glycyrrhizic acid and glycyrrhetinic acid in fuzilizhong pills. Asian J Trad Med. 2008;3(3):110–116. [Google Scholar]
- 18.Bombardelli E, Cristoni A, Morazzoni P. Phytosomes in functional cosmetics. Fitoterapia. 1994;65(5):387–401. [Google Scholar]
- 19.Tedesco D, Steidler S, Galletti S, Tameni M, Sonzogni O, Ravarotto L. Efficacy of silymarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poult Sci. 2004;83(11):1839–1843. doi: 10.1093/ps/83.11.1839. [DOI] [PubMed] [Google Scholar]
- 20.Yanyu X, Yunmei S, Zhipeng C, Qineng P. The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm. 2006;307(1):77–82. doi: 10.1016/j.ijpharm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26(6B):4457–4498. [PubMed] [Google Scholar]
- 22.Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113(2):171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 23.Nunes SS, Barros ALB. The use of coating agents to enhance liposomes blood circulation time. J Mol Pharm Org Process Res. 2015;3(1):1–2. [Google Scholar]
- 24.Bagherpour Doun SK, Alavi SE, Koohi Moftakhari Esfahani M, Ebrahimi Shahmabadi H, Alavi F, Hamzei S. Efficacy of Cisplatin-loaded poly butyl cyanoacrylate nanoparticles on the ovarian cancer: an in vitro study. Tumor Biol. 2014;35(8):7491–7497. doi: 10.1007/s13277-014-1996-8. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin Cancer Res. 2002;8(11):3512–3519. [PubMed] [Google Scholar]