ABSTRACT
Epicatechin (Epi), a flavanol found in foods such as dark chocolate has previously been shown to enhance memory formation in our model system, operant conditioning of aerial respiration in Lymnaea. In those experiments snails were trained in Epi. Here we ask whether snails exposed to Epi before training, during the consolidation period immediately following training, or 1 h after training would enhance memory formation. We report here that Epi is only able to enhance memory if snails are placed in Epi-containing pond water immediately after training. That is, Epi enhances memory formation if it is applied during the memory consolidation period as well as if snails are trained in Epi-containing pond water.
KEYWORDS: consolidation period, Epicatechin, long-term memory, Lymnaea
In Lymnaea, (−) epicatechin (Epi) a naturally occurring flavanol found in dark chocolate confers on snails an enhanced ability to form memory following operant conditioning of aerial respiratory behavior.1 More recently2 it was found that Epi application quickly overcame a stress-induced state in which neither learning nor memory occur, and restored memory enhancement. Thus, Epi is a powerful bio-active substance that alters cognitive ability. In humans flavanols in dark chocolate, including Epi, also positively affect cognitive ability. Both epidemiological and clinical studies show that the intake of these flavanols is correlated with a lower incidence of cognitive impairment3 and cause significantly better cognitive performance in older people.4-6
Learning and memory are 2 separate but related processes. We define learning as a change in behavior as a result of experience, while memory is the ability to recall the change in behavior. Here we are concerned with long-term memory (LTM) which persists for at least 24 h following training in Lymnaea. LTM formation is dependent on altered gene activity and new protein synthesis.7 It has been known for well over a 100 y that following learning there is a period of time, the consolidation period, in which the learning is encoded into memory. In Lymnaea the consolidation period persists for about 1 h. It is possible to alter memory formation during the consolidation period by either enhancing or suppressing it using different stressors. Here we hypothesize that Epi possesses the ability to enhance memory formation if Epi is applied to the snail either 1 h before training or immediately following conditioning (i.e., during the consolidation process13).
In all the previous studies of Epi in Lymnaea, snails were trained in Epi-containing pond water (10 min acclimatization time + 30 min of training) and then tested for memory in pond water (i.e., Epi not present). That is, the enhanced memory was not dependent on Epi being present in the memory test session. We did not previously study how long Epi’s enhancing effect persisted if applied before training or whether Epi could be applied post-training and still have an enhancing effect on memory formation. In Lymnaea it was recently shown8 that a thermal stressor’s enhancing effect on memory formation could persist for days before training or if applied immediately following training.9 Here we directly test whether: 1) exposure of snails to Epi 1 h before training, 2) exposure immediately after training, or 3) exposure to Epi 1 h after training results in enhanced memory formation. In all cases snails were exposed to the same concentration of Epi previously used1 (15 mg l−1) and for the same period of time (40 min).
To operantly condition aerial respiratory behavior snails were placed in hypoxic pond water and every time they attempted to open their pneumostome to perform aerial respiration they received a tactile stimulus to the pneumostome.10 This tactile stimulus caused the pneumostome to close. Here snails received a single 0.5 h training session (TS) and then a 0.5 h memory test (MT) 24 h later. We plotted and compared the number of attempted pneumostome openings in both the TS and MT sessions. LTM is operationally defined as there being a significant decrease in the number of attempted pneumostome openings in MT compared to the number of attempted openings in TS.
We first exposed snails to Epi-containing pond water for 40 min 1 h before training (Fig. 1A). Following exposure to Epi snails were returned to their home aquarium for 1 h and then received the training session (TS) and a memory test (MT) 24 h later. As can be seen in Figure 1A, LTM was not present. That is, the number of attempted openings in MT was not significantly less than in TS. Next (Fig. 1B), snails were first trained in pond water (TS) and then immediately placed into Epi-containing pond water for 40 minutes. They were then returned to their home aquarium and tested for LTM 24 h later. As can be seen the number of attempted pneumostome openings in MT was significantly less than in TS. Thus, LTM formed as a result of this procedure. Finally (Fig. 1C) we first trained snails in pond water and then waited 1 h before exposing them to Epi-containing pond water for 40 min. When we tested memory 24 h later, we found that the number of attempted openings in MT was not statistically less than in TS. Thus, LTM was not present.
Figure 1.
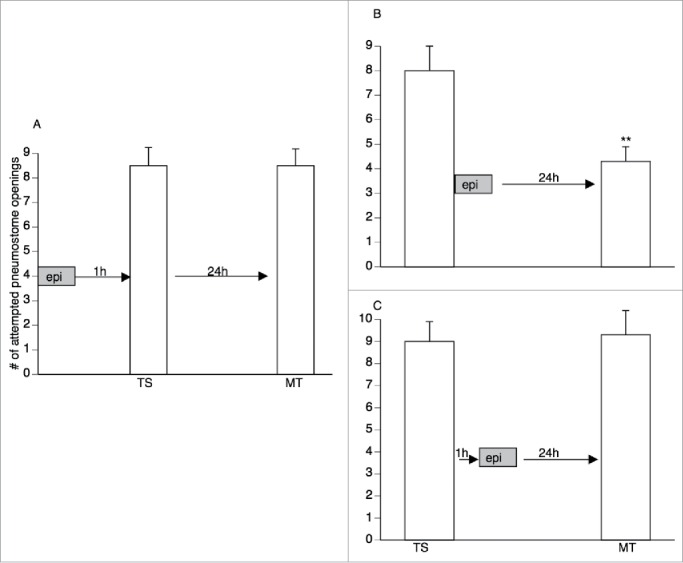
Epicatechin enhances memory formation if applied during the memory consolidation period. (A) Pre-exposure of snails to Epi for 40 min 1 h before training does not result in LTM formation. That is, MT was not significantly less than TS (paired 2 tail t-test; p > 0.05). (B) Snails exposed to Epi for 40 min immediately after operant conditioning training (TS) exhibit LTM. That is, the number of attempted pneumostome openings in MT was significantly less than in TS (2 tailed paired t-test t-value=5.38, p-value <0.001). (C) Snails were exposed to Epi for 40 min 1 h after training. LTM was not observed (paired 2 tail t-test; p > 0.05).
Together, these data show that Epi can enhance memory formation if snails are either trained in Epi1,2,11 or exposed to Epi-containing pond water immediately after training (Fig. 1B). Pretreating snails with Epi 1 h before or delaying exposure to Epi 1 h after training did not result in an Epi-induced enhancement of memory formation. Previously,12-17 it was shown in Lymnaea that it was only possible to block memory formation by applying a cold block (10 min in 4°C pond water) immediately after conditioning training. If applied 1 h after training the cold block was not effective in preventing memory formation. These previous data suggest that the consolidation period begins immediately after the training session and persists for up to 1 h. Thus, applying a cold block during this time is sufficient to block memory formation. Here we found that the application of Epi was sufficient to cause LTM formation, but only during the consolidation process when the molecular processes that underlie memory formation are active.
Previously it was shown that predator detection in Lymnaea also caused memory enhancement, but this enhancement only occurred if the snail detected the presence of the predator during the training session.18,19 However, predator detection occurring during the consolidation period (i.e., immediately after training) did not cause memory enhancement. In studies of how a thermal stressor enhanced memory formation8,9 it was shown that pre-exposing snails to the stressor from 3 d to 1 h beforehand was sufficient to enhance memory formation. Finally, it was shown8 that heat-shock proteins (HSPs), which are elicited by the thermal stimulus20 together with DNA-methylation play a necessary role in the memory enhancement process.2 Whether HSPs and DNA methylation are necessary for memory enhancement caused by Epi remain to be determined.
We had hypothesized, based on the results from the thermal stressor, that Epi pre-treatment 1 h beforehand would cause memory enhancement. This was not the case. Thus, it appears that there are a number of different mechanisms that underlie the enhancement of memory formation. These mechanisms include the elaboration of HSPs and DNA methylation. However, while DNA methylation appears to be involved with predator detection and the thermal stressor,2,21,22 the DNA methylation process involved with predator detection only appears to be activated when the predator is detected during the training procedure. That is, it appears to be an activity dependent process. On the other hand, the thermal stressor-induced memory enhancement persists for many days. Together these data show that while the behavioral phenotype of memory enhancement looks similar there are probably many different mechanisms underlying it.
It is still unclear how Epi brings about memory enhancement. Experiments now in progress show that Epi alters the activity of neuron RPeD1, a neuron known to be necessary for memory formation, reconsolidation, extinction and forgetting.23-26 It is possible that Epi also alters the activity of other neurons involved in mediating aerial respiratory behaviors or neurons up-stream of the central pattern generator that drives aerial respiration.
In conclusion we found that Epi is able to cause an enhancement of memory formation if the snail experienced Epi immediately after training (i.e., during the consolidation period). Epi exposure 1 h before training and 1 h after training was not sufficient to cause memory enhancement. Epi is a very powerful memory enhancer in Lymnaea. However, it must be experienced either during training or immediately after training to effectively enhance memory.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Fruson L, Dalesman S, Lukowiak K. A flavonol present in cocoa [(−)epicatechin] enhances snail memory. J Exp Biol 2012; 215:3566-3576; PMID:23014569; http://dx.doi.org/ 10.1242/jeb.070300 [DOI] [PubMed] [Google Scholar]
- [2].Knezevic B, de Freitas E, Komatsuzaki Y, Lukowiak K. A flavonoid component of chocolate quickly reverses an imposed memory deficit. J Exp Biol 2016; 219:816-23; PMID:26823103; http://dx.doi.org/ 10.1242/jeb.130765 [DOI] [PubMed] [Google Scholar]
- [3].Kuriyama S, Hozaka A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project. Am J Clin Nutr 2006; 83:355-361; PMID:16469995 [DOI] [PubMed] [Google Scholar]
- [4].Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol 2007; 165:1364-1371; PMID:17369607; http://dx.doi.org/ 10.1093/aje/kwm036 [DOI] [PubMed] [Google Scholar]
- [5].Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr 2009; 139:120-127; PMID:19056649; http://dx.doi.org/ 10.3945/jn.108.095182 [DOI] [PubMed] [Google Scholar]
- [6].Mastroiacovo D, Kwik-Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, Righetti R, Bocale R, Lechiara MC, Marini C. et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study—a randomized controlled trial. Am J Clin Nutr 2015; 101:538-548; PMID:25733639; http://dx.doi.org/ 10.3945/ajcn.114.092189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sangha S, Scheibenstock A, McComb C, Lukowiak K. Intermediate and long-term memories of associative learning are differentially affected by transcription versus translation blockers in Lymnaea. J Exp Biol 2003; 206:1605-1613; PMID:12682092; http://dx.doi.org/ 10.1242/jeb.00301 [DOI] [PubMed] [Google Scholar]
- [8].Sunada H, Riaz H, de Freitas E, Lukowiak KS, Swinton C, Swinton E, Protheroe A, Shymansky T, Komatsuzaki Y, Lukowiak K. Thermal stress enhances LTM formation: Role of HSPs and DNA methylation. J Exp Biol 2016; 219:1 337-1345; http://dx.doi.org/ 10.1242/jeb.134296 [DOI] [PubMed] [Google Scholar]
- [9].Teskey M.L, Lukowiak KS, Riaz H, Dalesman S, Lukowiak K. ‘What’s Hot?’: The enhancing effects of thermal stress on long-term memory formation in Lymnaea. J Exp Biol 2012; 215:4322-4329; PMID:22972889; http://dx.doi.org/ 10.1242/jeb.075960 [DOI] [PubMed] [Google Scholar]
- [10].Lukowiak K, Ringseis E, Spencer G, Wildering W, Syed N. Operant conditioning of aerial respiratory behavior in Lymanea stagnalis. J Exp Biol 1996; 199:683-691; PMID:9318425 [DOI] [PubMed] [Google Scholar]
- [11].Knezevic B, Lukowiak K. The flavonol epicatechin reverses the suppressive effects of a stressor on long-term memory formation. J Exp Biol 2014; 217:4004-4009; PMID:25267853; http://dx.doi.org/ 10.1242/jeb.110726 [DOI] [PubMed] [Google Scholar]
- [12].Sangha S, McComb C, Lukowiak K. Forgetting and the Extension of Memory in Lymnaea. J Exp Biol 2003; 206:71-77; PMID:12456698; http://dx.doi.org/ 10.1242/jeb.00061 [DOI] [PubMed] [Google Scholar]
- [13].Sangha S, Morrow R, Smyth K, Cooke R, Lukowiak K. Cooling Blocks ITM and LTM Formation and Preserves Memory. Neurobiol Learning & Memory 2003; 80:130-139; http://dx.doi.org/ 10.1016/S1074-7427(03)00065-0 [DOI] [PubMed] [Google Scholar]
- [14].Parvez K, Moisseev V, Lukowiak K. A context-specific single contingent-reinforcing stimulus boosts intermediate-term memory into long-term memory. Eur J Neurosci 2006; 24:606-616; PMID:16903862; http://dx.doi.org/ 10.1111/j.1460-9568.2006.04952.x [DOI] [PubMed] [Google Scholar]
- [15].Martens K, Amarell M, Parvez K, Hittel K, De Caigny P, Ito E, Lukowiak K. One-trial conditioning of aerial respiratory behaviour in Lymnaea stagnalis. Neurobiol Learn Mem 2007; 88:232-242; PMID:17540582; http://dx.doi.org/ 10.1016/j.nlm.2007.04.009 [DOI] [PubMed] [Google Scholar]
- [16].Fulton D, Kemenes I, Andrew R, Benjamin P. Time-window for sensitivity to cooling distinguishes the effects of hypothermia and protein synthesis inhibition on the consolidation of long-term memory. Neurobiol Learn Mem 2008; 90:65 1-4; PMID:18342551; http://dx.doi.org/ 10.1016/j.nlm.2008.08.006 [DOI] [PubMed] [Google Scholar]
- [17].Takahashi T, Takigami S, Sunada H, Lukowiak K, Sakakibara M. Critical period of memory enhancement during taste avoidance conditioning in Lymnaea stagnalis. PLoS One Oct 2013; 3;8(10):e75276; http://dx.doi.org/ 10.1371/journal.pone.0075276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Orr M, El-Bekai M, Lui M, Watson K, Lukowiak K. Predator detection in Lymnaea stagnalis. J Exp Biol 2007; 210:4150-4158; PMID:18025014; http://dx.doi.org/ 10.1242/jeb.010173 [DOI] [PubMed] [Google Scholar]
- [19].Orr M, Lukowiak K. Electrophysiological and behavioral evidence demonstrating that predator detection alters adaptive behaviors in the snail Lymnaea. J Neurosci 2008; 28:2726-34; PMID:18337402; http://dx.doi.org/ 10.1523/JNEUROSCI.5132-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Foster NL, Lukowiak K, Henry TB. Time related expression profiles for heat shock protein gene transcripts (HSP40, HSP70) in the central nervous system of Lymnaea stagnalis exposed to thermal stress. Communicative & Integrative Biology 2015; 8:3 e1040954; http://dx.doi.org/ 10.1080/19420889.2015.1040954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han J-I, Janes T, Lukowiak K. The role of serotonin in the enhancement of Long-term memory resulting from predator detection in Lymnaea. J Exp Biol 2010; 213:3603-3614; PMID:20952608; http://dx.doi.org/ 10.1242/jeb.048256 [DOI] [PubMed] [Google Scholar]
- [22].Lukowiak K, Heckler B, Bennett T, Schriner E, Wyrick K, Jewett C, Todd R, Sorg B. Enhanced memory persistence is blocked by DNA methyltransferase inhibitor in the snail Lymnaea stagnalis. J Exp Biol 2014; 217:2920-2929; PMID:24902747; http://dx.doi.org/ 10.1242/jeb.106765 [DOI] [PubMed] [Google Scholar]
- [23].Scheibenstock A, Krygier D, Haque Z, Syed S, Lukowiak K. The soma of RPeD1 must be present for LTM formation of associative learning in Lymnaea. J Neurophysiol 2002; 88:1584-1591; PMID:12364489 [DOI] [PubMed] [Google Scholar]
- [24].Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of RPeD1. J Neurosci 2003a; 23:8034-8040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sangha S, Scheibenstock A, Morrow R, Lukowiak K. Extinction requires new RNA and protein synthesis and the soma of the cell RPeD1 in Lymnaea stagnalis. J Neurosci 2003b; 23:9842-9851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sangha S, Scheibenstock A, Martens K, Varshney N, Cooke R, Lukowiak K. Impairing forgetting by preventing new learning and memory. Behav Neurosci 2005; 119:787-796; PMID:15998200; http://dx.doi.org/ 10.1037/0735-7044.119.3.787 [DOI] [PubMed] [Google Scholar]


