ABSTRACT
In animal cells cytokinesis relies on the contraction of an actomyosin ring that pulls the plasma membrane to create a cleavage furrow, whose ingression finally divides the mother cell into two daughter cells. Fungal cells are surrounded by a tough and flexible structure called cell wall, which is considered to be the functional equivalent of the extracellular matrix in animal cells. Therefore, in addition to cleavage furrow ingression, fungal cytokinesis also requires the centripetal formation of a septum wall structure that develops between the dividing cells, whose genesis must be strictly coordinated with both the actomyosin ring closure and plasma membrane ingression. Here we briefly review what is known about the septum structure and composition in the fission yeast Schizosaccharomyces pombe, the recent progress about the relationship between septum biosynthesis and actomyosin ring constriction, and the importance of the septum and ring in the steady progression of the cleavage furrow.
KEYWORDS: cell wall, cleavage furrow, contractile actomyosin ring, cytokinesis, glucan, septum
Introduction
Cytokinesis is the final step of the eukaryotic cell cycle where, after the mitotic exit, the ingression of a cleavage furrow allows the partition of the cell into two new cells. In animal cells furrow formation requires the formation, maintenance, and closure of a contractile actomyosin ring (CAR), tied to the deposition of new plasma membrane material. Fungal cells are enveloped by a cell wall, whose rigidity and resistance are determined by its composition and the mechanical force exerted against the hydraulic turgor pressure inside the cell.1-3 Therefore, fungal cell division requires that CAR contracts in coordination with the centripetal biosynthesis of a special wall structure called division septum.3,4
Animal cells are surrounded by an extracellular matrix, a structure composed of polysaccharides and proteins. Although the extracellular matrix does not provide osmotic support, it is considered to be the functional analog of the fungal cell wall. Similar to fungal cells, some extracellular matrix polymers have been depicted as being important for cytokinesis.5-8
The cell wall and septum are essential structures for cell shape maintenance, and thus extending our knowledge of the morphogenesis processes is significantly important.2,3,9 The fission yeast Schizosaccharomyces pombe has become widely popular for the study of eukaryotic morphogenesis and cell division as it exhibits a rod shape with a simple polarized growth pattern, and because its cell cycle and cytokinesis are remarkably similar to that of animal cells.10 Here we summarize how the septum is constructed in coordination with the CAR and plasma membrane ingression, followed by a debate regarding the impact of septum and ring biogenesis in cleavage furrow ingression in fission yeast.
Cell wall and septum in fission yeast
In fission yeast two glucose polysaccharides are the main structural polymers of the cell wall, β(1,3)-D-glucan with 14% of β(1,6) branches (B-BG) that constitutes 48-54% of the cell wall, and α(1,3)-D-glucan with 7% of α(1,4) bonds located at the reducing end of each chain, representing 28-32% of the cell wall.11-14 The β(1,6)-D-glucan with 75% of β(1,3) branches only represents 5-10%.15,16 Additionally, the galactomannan bound to proteins forms the glycoproteins.11,17,18 Under electron microscopy the cell wall shows two electron dense layers of galactomannan,18 separated by a non-dense layer of B-BG and α(1,3)-D-glucan, with the β(1,6)-D-glucan appearing closer to the outer galactomannan layer (Fig. 1).12,16,19
Figure 1.
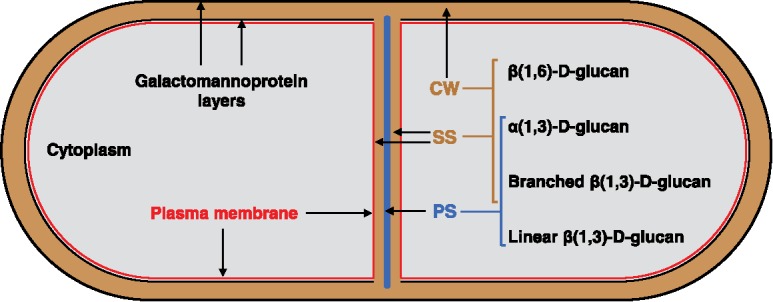
Scheme showing the differential composition of the cell wall and the septum structures. Under transmission electron microscopy, the cell wall (CW) presents two electron dense layers of galactomannoproteins, separated by a non-dense layer composed of B-BG, α(1,3)-D-glucan and β(1,6)-D-glucan. The three-layered septum structure displays a middle primary septum (PS) flanked by two layers of secondary septum (SS). Both septum structures contain B-BG and α(1,3)-D-glucan. The β(1,6)-D-glucan is only detected in the SS; while the L-BG is exclusively found in the PS.
Once the CAR is formed and matures throughout anaphase,4 coordinated and simultaneous CAR closure and septum formation only initiate after breakage of the mitotic spindle.20 The three-layered septum structure displays a middle electron-transparent primary septum (PS) flanked by an electron-dense secondary septum (SS) on each side (Fig. 1). After completion, the septum thickness increases through an additional round of SS synthesis.2,7,21 The fission yeast septum comprises different essential glucans. β(1,6)-D-glucan is localized in the SS; a linear β(1,3)-D-glucan (L-BG) is located and abundant in the PS; and B-BG and α(1,3)-D-glucan are located in both PS and SS (Fig. 1).2,19,22 The electron dense glycoprotein layers are not observed in the septum structure, however galatomannoproteins have been detected in the SS by immunoelectron microscopy with a gold particle-labeled lectin specific for terminal residues of galactose.18,23
Synthesis of the fission yeast septum
As stated above, the fission yeast septum is mainly composed of essential α- and β-glucans. Although the β(1,6)-D-glucan must be important to interconnect the wall polysaccharides, our knowledge about how it is synthesized and incorporated into the fission yeast cell wall is still very limited.24
β(1,3)-D-glucan synthases
In fungal cells, the in vitro β(1,3)-D-glucan synthase (GS) activity is responsible for the biosynthesis of short chains of linear β(1,3)-D-glucan. The essential GTPase Rho1 is a regulatory subunit of this activity.25 The GS catalytic subunit is formed by the family Bgs/Fks in fungi, and the callose synthases, CalS, in plants. All of these are large proteins (∼200 KDa) with 15-16 putative transmembranal domains along two hydrophobic regions. Their central hydrophilic region displays a high identity (> 80%) between all Bgs/Fks/CalS proteins. This region is thought to be located on the cytoplasmic face of the plasma membrane and to be essential for the function of the GS.26,27 In fission yeast four GS catalytic subunits have been identified, three of them being essential (Bgs1, 3 and 4) during vegetative growth, and the last one (Bgs2), being only essential for the GS activity required for the synthesis of the spore wall β(1,3)-D-glucan during the sexual phase of the life cycle.22,28-33 Although the absence of bgs3+ causes the death of the cell, the specific function of Bgs3 in the cell wall and septum assembly still remains unknown. Bgs1, 3 and 4 appear in the CAR and septum during cytokinesis and in the cell ends during polar growth. Additionally, they are detected in the sites of wall synthesis during sexual differentiation. Since they are essential for cell survival, the GS catalytic subunits must display differential and vital non-overlapping roles in the biogenesis of β-glucans during cell wall and septum assembly. In the next sections we will describe the known roles of Bgs1 and Bgs4 during the cell cycle, mainly cytokinesis. Despite Bgs3 has to be crucial for septum and/or cell wall assembly, its specific role is unknown (see above), and therefore this subunit will not be additionally discussed.
Bgs1/Cps1
The gene bgs1+ was initially identified by complementation of the cps1-12 mutant hypersensitive to the spindle poison chlorpropham and to Papulacandin, a specific inhibitor of the GS. This mutant displayed a multiseptated and branched phenotype, and thus it was proposed that Bgs1 could be a GS involved in cytokinesis, polarity and cell wall morphogenesis.34 Two other mutants, swl1-N12 (cps1-N12) and drc1-191 (cps1-191), were described as forming a stable CAR, but unable to assemble the division septum, implicating Bgs1 in a septation checkpoint.35,36 The cytokinesis mutant phenotypes described above, together with the findings that Bgs1 was localized at the CAR, and that it was essential for cell survival, allowed to suggest that it could be required for PS formation.29,31 However, the fact that other GSs also localize to the CAR, and Bgs1 localizes not only to the CAR, but also to the growing poles, made more complicated to draw conclusions about the specific role of Bgs1 during septation,29 and therefore, additional experiments were required to undoubtedly demonstrate the function of Bgs1 synthetizing the L-BG of the PS. Depletion or absence of Bgs1 induces a phenotype of multiseptated cells that eventually die. Analysis of the septa formed in bgs1Δ cells from germinating bgs1Δ spores under electron microscopy established that Bgs1 is responsible for the L-BG synthesis and PS formation, and that the fluorochrome calcofluor binds specifically to the L-BG of the chitin-lacking PS of fission yeast.22
Bgs4/Cwg1/Pbr1
Bgs4 is the only subunit that has been shown to form part of the GS enzyme. It is responsible for the synthesis of the cell wall B-BG and the major in vitro GS activity. The B-BG produced by Bgs4 is vital to maintain cell shape and integrity and for SS formation and correct PS completion during cytokinesis.7,28,37-39 Fungal resistance to GS inhibitors is clearly associated with mutations grouped in conserved short regions (hot spots) of the Bgs/Fks proteins,40,41 indicating that this resistance mechanism is well conserved in fungi (Fig. 2). To date, the only identified mutants of fission yeast that display reduced levels of cell wall β-glucan and GS activity,38,39 or resistance to the specific GS inhibitors,42 are due to point mutations in the Bgs4 sequence. The study of these resistant mutants extended the resistance hot spot 1 to 13 aminoacids, and distingued a new resistance hot spot 1-2 (Fig. 2).41 The fact that a simple mutation within Bgs4 conferring resistance to specific GS inhibitors, with Bgs1 and Bgs3 being wild-type, suggests that Bgs1 and Bgs3 are intrinsically natural resistant to known GS inhibitors. Thus, the available GS inhibitors (echinocandins, enfumafungin and papulacandins) only suppress GS activity through Bgs4, and not those derived from Bgs1 or Bgs3.41 In accordance with this, Bgs4 depletion induces the same lytic phenotype as that observed in wild-type cells treated with lethal doses of GS inhibitors,28,41,43 as this is not the case for Bgs1 and Bgs3.
Figure 2.
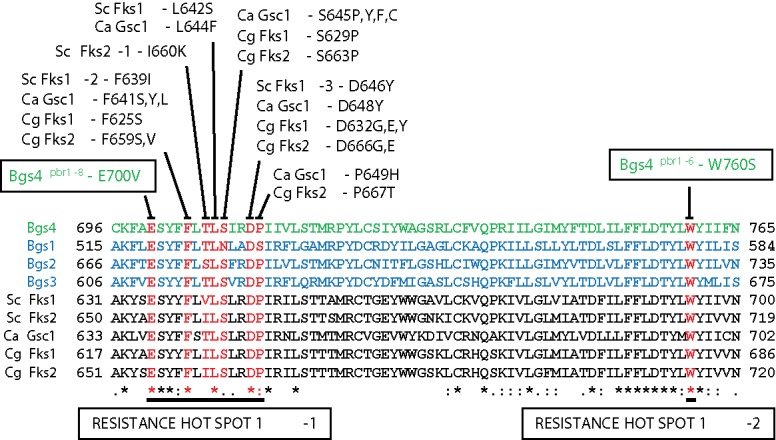
Protein alignments of two conserved regions of Bgs1, Bgs2, Bgs3 (blue), and Bgs4 (green) from S. pombe, and Fks1, Fks2 and Gsc1 (Fks1) from Saccharomyces cerevisiae (Sc), Candida albicans (Ca), and Candida glabrata (Cg). Mutations in the residues depicted in red confer resistance to specific GS antifungals, defining a resistance hot spot 1. The Bgs4pbr1-8 mutation is N-terminal located in a hot spot 1-1 of 13-amino acids conferring resistance to the GS inhibitors. The Bgs4pb1-6 change defines a hot spot 1-2 of resistance located C-terminal from hot spot 1-1. Adapted from ref. 41.
α(1,3)-D-glucan Synthase: Ags1/Mok1
In contrast to GS activity, an in vitro α(1,3)-D-glucan synthase activity has not yet been detected. Ags1 is the putative α(1,3)-D-glucan synthase responsible for the synthesis of the cell wall α(1,3)-D-glucan.44,45 Similar to Bgs proteins, Ags1 is found in the CAR, septum, growing poles, and sites of wall synthesis during sexual differentiation.2 During septation Ags1 is required for the straight progression of the PS, suggesting that Ags1 might collaborate with Bgs1 and the CAR. In addition, together with Bgs4, it is responsible for the assembly of the SS, and the maintenance of cell integrity. Importantly, Ags1 provides the strength needed to PS to counteract the turgor pressure for a gradual cell separation.2 Four additional ags1+/mok1+ homologs, which are only expressed during sporulation, have been identified in fission yeast.46
β-glucans participate in the anchorage of the ring before septation
To create two daughter cells, the CAR must be placed and kept in the cell middle before the onset of septation. In fission yeast the nucleus and the anillin Mid1 mark the site that localizes the CAR in the cell middle.4 However, during and after assembly this ring must be spatially maintained in the same place for proper cell division to occur. Studies using protoplasts, deprived of their cell walls, suggested that new membranes and/or septum ingression might stabilize and maintain the ring in the middle of the cell.47,48 Similarly, in arrested cps1-191 mutant cells lacking either microtubules or Mid1 the CAR can be observed sliding sideways.49,50 Since the CAR begins constriction after mitosis completion,20 it seems probable that the extracellular cell wall in combination with some transmembranal proteins might help to keep the correct position of CAR until septum and cleavage furrow ingression begin at the end of anaphase. The observation of misplaced and unstable rings in cells with reduced Bgs4-dependent B-BG supports this hypothesis, and suggests that the CAR is bound to the extracellular cell wall B-BG through the plasma membrane.7 As described above, and similar to Bgs4, Bgs1 also participates in the stable maintenance of the CAR in the cell middle. The F-BAR protein Cdc15 may contribute to the transport of Bgs1 (and probably the rest of Bgs and Ags1 proteins) from the Golgi apparatus to the plasma membrane.51 Thus, when Bgs1 location in the cell division site is delayed by the presence of a compromised Cdc15, the CAR slides away from the cell middle.51 However a similar delay is also observed in Ags1, suggesting that the reason for the late localization of Bgs1 could be a general delay and/or the formation of a compromised CAR when the essential function of Cdc15 is reduced.2,4,52 Despite these observations, to date it is not known whether Bgs1 itself or the synthetized chains of L-BG are responsible for the stable CAR placement, and how the CAR is attached to the plasma membrane and connected to the cell wall glucan. Recently, it has been reported that the absence of paxillin, Pxl1, a conserved ring protein required for CAR integrity and whose localization depends on the SH3 domains of the homologs Cdc15 and Imp2,53-55 induces simultaneous Bgs1 and CAR sliding from the cell middle until the CAR begins to constrict and the PS is detected.56 This observation, and the fact that Bgs1 mutant cps1-191 also displays CAR sliding,49-51,56 suggest that the mere presence of Bgs1 is not enough to stably maintain the ring location. Interestingly, the combined reduction of function of Pxl1 and Cdc15 induces Bgs1 and CAR sliding even after activation of synthesis of a L-BG material, which is visualized along the longitudinal axis of the cell without cleavage furrow formation. Therefore, suggesting that cooperation between both CAR proteins is needed to coordinate the simultaneous activation of Bgs1 GS synthesis and CAR constriction.56
Role of β-glucans coupling septum synthesis with cleavage furrow ingression
Recent studies have revealed that both septum synthesis and CAR constriction are required for the correct ingression of the cleavage furrow.7,57 The intimate correlation between CAR closure and septum genesis has traditionally complicated the deciphering of, as in animal cells, weather the CAR produces the mechanical force that invaginates the plasma membrane covering the septum.58 The analysis of the cell division region of wild-type cells under transmission electron microscopy showed growing septa that in some cases appeared bent and misdirected. Furthermore, mutants affected in CAR assembly are able to form septa, suggesting that the sole synthesis of the growing septum is able to push the plasma membrane.58 In support of this, it has been reported that largely advanced septa are slowly completed in the presence of the actin depolymerizing drug latrunculin A. It is important to note that small septa are unable to advance, indicating that the CAR is still essential for general septum ingression. In any case, all these observations together with the reduced rates of septum ingression in cps1-191 mutant cells, lead to propose that Bgs1-dependent L-BG provides the mechanical force needed for plasma membrane ingression.3,57 However, this hypothesis opposes the fact that the absence of the major B-BG, synthesized by Bgs4 and present in both PS and SS, causes misdirected septum formation, indicative of a weak, labile and larger CAR and a faster ring and membrane ingression separated away from the PS synthesis, which is delayed (Fig. 3). This observation led to suggest that cleavage furrow ingression could progress just by the fusion of membrane vesicles to the tip of the advanced septum membrane, without the need of the mechanical force of the newly synthetized glucans or the pulling force of CAR contraction.7 Moreover, the close relationship between CAR and the septum makes drawing conclusions difficult as regards the real influence of both CAR constriction and PS synthesis to the force required for septum membrane ingression. With this in mind, it has been shown that the septum L-BG or Bgs1 seems to contribute to the maintenance of the ring structure during septation, based on the fact that a reduction of Bgs1 function in cps1-191 cells triggers the disorganization of the constricting ring.56 Bgs1 also cooperates with Pxl1 keeping the CAR and allowing septum ingression. Thus, when Bgs1 is depleted in cells deprived of Pxl1 function, the CAR disassembles prematurely and septum formation is abolished.56 Importantly, Pxl1 cooperates with Bgs1 to restrict the region of septum synthesis by delimiting the location of the synthases Ags1, Bgs4, and probably Bgs3 (Fig. 4). Therefore, septum and cleavage furrow formation in cells depleted of Bgs1 depend exclusively on the presence of Pxl1 in the CAR.56
Figure 3.
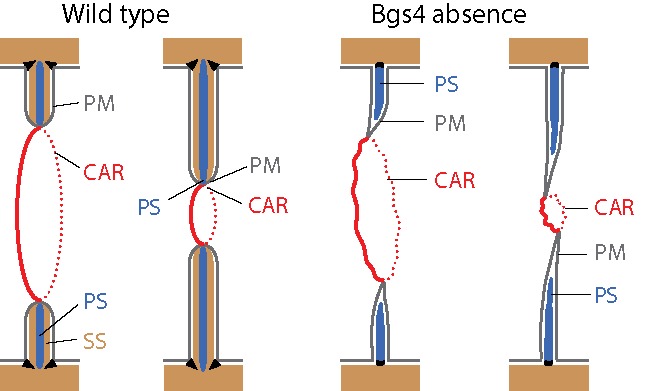
Model of advanced fast CAR and septum membrane ingressions uncoupled from delayed PS synthesis in the absence of the branched β(1,3)-D-glucan synthesized by Bgs4. A loose CAR devoid of tensile force promotes the synthesis of misdirected septum. The septum membrane progresses without CAR constriction and septum synthesis forces. CAR, actomyosin ring; PM, plasma membrane; PS, primary septum; SS, secondary septum. Adapted from ref. 7.
Figure 4.
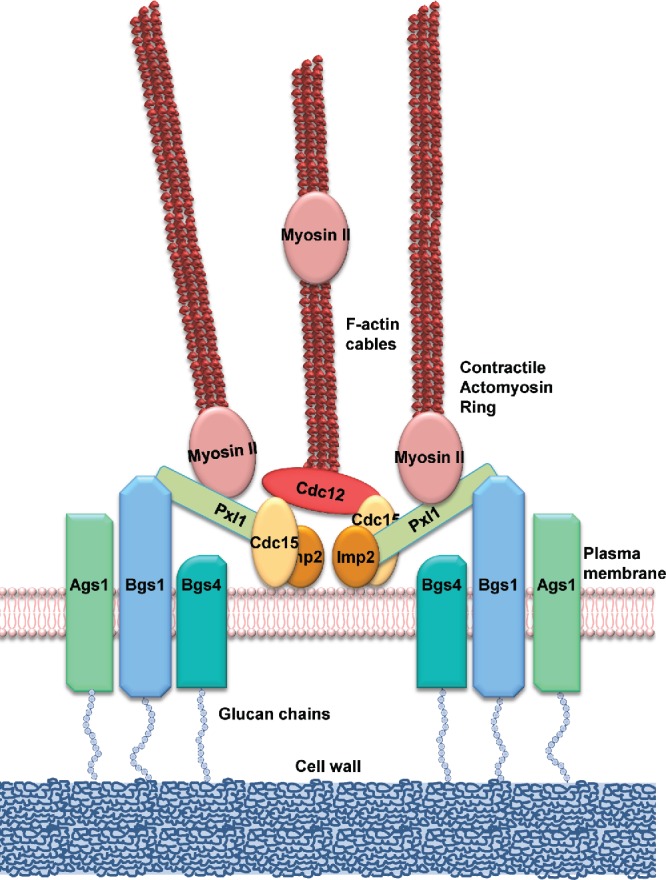
Scheme of the proteins connecting the CAR with the biosynthesis of the septum wall. Type II myosin Myo2, Cdc15 and other essential regulators locate early in the CAR assembly site.20 Cdc15 recruits the formin Cdc12 through its F-BAR domain.60 Later, Cdc15 and Imp2 61 collaborate through their SH3 domains to stabilize the CAR by recruiting the paxillin Pxl1 and many other proteins.52,62 Pxl1 helps to stabilize the CAR through Myo2,53,54 while Cdc15 function is required for the translocation of glucan synthase Bgs1 from the Golgi apparatus to the plasma membrane in the division site.63 Pxl1 and Bgs1 cooperation results essential for septum biogenesis and cleavage furrow ingression, probably because of their role maintaining the stable location of the CAR and the other septum glucan synthases, Ags1, Bgs4 and probably Bgs3.56
Concluding remarks
Bgs1 is responsible for the L-BG synthesis because L-BG and the corresponding PS are absent in bgs1Δ cells.22 However, although the available Bgs1 mutants have proved to be useful in the study of the functions of this GS subunit,34-36,51,56,57 their morphological phenotypes are different from those observed in bgs1Δ cells, and it is unknown how these point mutations compromise biochemically the L-BG synthesis or any additional function of Bgs1. Future studies are required that delve into the GS activity and the cell wall ultrastructure and composition of these mutants.
Our recent study indicates that cooperation of Bgs1 and Pxl1 is required to maintain the other GS subunits in the division site.56 Although, how this is accomplished is still unknown. In focal adhesions, paxillin connects and reinforces the linkages between the extracellular matrix and cytoskeleton through the transmembranal α-integrins.59 Therefore, a noteworthy hypothesis is that Pxl1 might act as a mechanosensor to transmit the CAR tension to activate the Bgs1 function in the plasma membrane, which somehow would help to concentrate Ags1, Bgs3 and Bgs4 in the cell equator (Fig. 4). In the absence of Bgs1 and Pxl1 there is no PS synthesis and this could trigger the breakage of linkages between the plasma membrane and CAR, which would ultimately promote Ags1, Bgs3 and Bgs4 delocalization,56 leading to a widespread SS synthesis and absence of cleavage furrow ingression.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Professor Frantisek Baluska for inviting us to submit this review, and Emma Keck for language revision. Because of space limitations, we apologize to those authors whose work could not be cited.
Funding
This work was supported by the Spanish Ministry of Science and Innovation (BFU2010-15641 and BFU2013-39394-P) to PP. JCR was financed by the Spanish Ministry of Science and Innovation (BIO2012-35372 and BIO2015-69958-P), and the Junta de Castilla y León, Spain (CSI037U14). JCGC was supported by a Juan de la Cierva postdoctoral contract from the Spanish Ministry of Science and Innovation.
References
- [1].Howland JL. The surprising archaea : discovering another domain of life. New York: Oxford University, 2000. [Google Scholar]
- [2].Cortés JCG, Sato M, Muñoz J, Moreno MB, Clemente-Ramos JA, Ramos M, Okada H, Osumi M, Durán A, Ribas JC. Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J Cell Biol 2012; 198:637-56; http://dx.doi.org/ 10.1083/jcb.201202015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Davi V, Minc N. Mechanics and morphogenesis of fission yeast cells. Curr Opin Microbiol 2015; 28:36-45; PMID:26291501; http://dx.doi.org/ 10.1016/j.mib.2015.07.010 [DOI] [PubMed] [Google Scholar]
- [4].Willet AH, McDonald NA, Gould KL. Regulation of contractile ring formation and septation in Schizosaccharomyces pombe. Curr Opin Microbiol 2015; 28:46-52; PMID:26340438; http://dx.doi.org/ 10.1016/j.mib.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hwang HY, Olson SK, Esko JD, Horvitz HR. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature 2003; 423:439-43; PMID:12761549; http://dx.doi.org/ 10.1038/nature01634 [DOI] [PubMed] [Google Scholar]
- [6].Mizuguchi S, Uyama T, Kitagawa H, Nomura KH, Dejima K, Gengyo-Ando K, Mitani S, Sugahara K, Nomura K. Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature 2003; 423:443-8; PMID:12761550; http://dx.doi.org/ 10.1038/nature01635 [DOI] [PubMed] [Google Scholar]
- [7].Muñoz J, Cortés JCG, Sipiczki M, Ramos M, Clemente-Ramos JA, Moreno MB, Martins IM, Pérez P, Ribas JC. Extracellular cell wall β(1,3)glucan is required to couple septation to actomyosin ring contraction. J Cell Biol 2013; 203:265-82; http://dx.doi.org/ 10.1083/jcb.201304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu X, Vogel BE. A secreted protein promotes cleavage furrow maturation during cytokinesis. Curr Biol 2011; 21:114-9; PMID:21215633; http://dx.doi.org/ 10.1016/j.cub.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cabib E, Roh DH, Schmidt M, Crotti LB, Varma A. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J Biol Chem 2001; 276:19679-82; PMID:11309404; http://dx.doi.org/ 10.1074/jbc.R000031200 [DOI] [PubMed] [Google Scholar]
- [10].Chang F, Martin SG. Shaping fission yeast with microtubules. Cold Spring Harb Perspect Biol 2009; 1:a001347; PMID:20066076; http://dx.doi.org/ 10.1101/cshperspect.a001347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bush DA, Horisberger M, Horman I, Wursch P. The wall structure of Schizosaccharomyces pombe. J Gen Microbiol 1974; 81:199-206; PMID:4822119; http://dx.doi.org/ 10.1099/00221287-81-1-199 [DOI] [PubMed] [Google Scholar]
- [12].Kopecka M, Fleet GH, Phaff HJ. Ultrastructure of the cell wall of Schizosaccharomyces pombe following treatment with various glucanases. J Struct Biol 1995; 114:140-52; PMID:7612397; http://dx.doi.org/ 10.1006/jsbi.1995.1013 [DOI] [PubMed] [Google Scholar]
- [13].Grun CH, Hochstenbach F, Humbel BM, Verkleij AJ, Sietsma JH, Klis FM, Kamerling JP, Vliegenthart JF. The structure of cell wall α-glucan from fission yeast. Glycobiol 2005; 15:245-57; http://dx.doi.org/ 10.1093/glycob/cwi002 [DOI] [PubMed] [Google Scholar]
- [14].Manners DJ, Meyer MT. The molecular structures of some glucans from the cell walls of Schizosaccharomyces pombe. Carbohydr Res 1977; 57:189-203; http://dx.doi.org/ 10.1016/S0008-6215(00)81930-8 [DOI] [Google Scholar]
- [15].Magnelli PE, Cipollo JF, Robbins PW. A glucanase-driven fractionation allows redefinition of Schizosaccharomyces pombe cell wall composition and structure: assignment of diglucan. Anal Bio Chem 2005; 336:202-12 [DOI] [PubMed] [Google Scholar]
- [16].Sugawara T, Takahashi S, Osumi M, Ohno N. Refinement of the structures of cell-wall glucans of Schizosaccharomyces pombe by chemical modification and NMR spectroscopy. Carbo Hydr Res 2004; 339:2255-65; http://dx.doi.org/ 10.1016/j.carres.2004.05.033 [DOI] [PubMed] [Google Scholar]
- [17].Ballou CE, Ballou L, Ball G. Schizosaccharomyces pombe glycosylation mutant with altered cell surface properties. Proc Natl Acad Sci U S A 1994; 91:9327-31; PMID:7937765; http://dx.doi.org/ 10.1073/pnas.91.20.9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Horisberger M, Vonlanthen M, Rosset J. Localization of α-galactomannan and of wheat germ agglutinin receptors in Schizosaccharomyces pombe. Arch Microbiol 1978; 119:107-11; PMID:727851; http://dx.doi.org/ 10.1007/BF00964260 [DOI] [PubMed] [Google Scholar]
- [19].Humbel BM, Konomi M, Takagi T, Kamasawa N, Ishijima SA, Osumi M. In situ localization of β-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 2001; 18:433-44; PMID:11255251; http://dx.doi.org/ 10.1002/yea.694 [DOI] [PubMed] [Google Scholar]
- [20].Wu JQ, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell 2003; 5:723-34; PMID:14602073; http://dx.doi.org/ 10.1016/S1534-5807(03)00324-1 [DOI] [PubMed] [Google Scholar]
- [21].Johnson BF, Yoo BY, Calleja GB. Cell division in yeasts: movement of organelles associated with cell plate growth of Schizosaccharomyces pombe. J Bacteriol 1973; 115:358-66; PMID:4717522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cortés JCG, Konomi M, Martins IM, Muñoz J, Moreno MB, Osumi M, Durán A, Ribas JC. The (1,3)β-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol Microbiol 2007; 65:201-17; PMID:17581129; http://dx.doi.org/ 10.1111/j.1365-2958.2007.05784.x [DOI] [PubMed] [Google Scholar]
- [23].Horisberger M, Rouvet-Vauthey M. Cell wall architecture of the fission yeast Schizosaccharomyces pombe. Experientia 1985; 41:748-50; http://dx.doi.org/ 10.1007/BF02012578 [DOI] [Google Scholar]
- [24].Durán A, Pérez P. Cell Wall Synthesis In: Egel R, ed. The Molecular Biology of Schizosaccharomycespombe: Springer-Verlag; Berlin Heidelberg, 2004:269-79 [Google Scholar]
- [25].Arellano M, Durán A, Pérez P. Rho 1 GTPase activates the (1-3)β-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J 1996; 15:4584-91; PMID:8887550 [PMC free article] [PubMed] [Google Scholar]
- [26].Latge JP. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol 2007; 66:279-90; PMID:17854405; http://dx.doi.org/ 10.1111/j.1365-2958.2007.05872.x [DOI] [PubMed] [Google Scholar]
- [27].Lesage G, Bussey H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 2006; 70:317-43; PMID:16760306; http://dx.doi.org/ 10.1128/MMBR.00038-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cortés JCG, Carnero E, Ishiguro J, Sanchez Y, Durán A, Ribas JC. The novel fission yeast (1,3)β-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J Cell Sci 2005; 118:157-74; PMID:15615781; http://dx.doi.org/ 10.1242/jcs.01585 [DOI] [PubMed] [Google Scholar]
- [29].Cortés JCG, Ishiguro J, Durán A, Ribas JC. Localization of the (1,3)β-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J Cell Sci 2002; 115:4081-96; http://dx.doi.org/ 10.1242/jcs.00085 [DOI] [PubMed] [Google Scholar]
- [30].Liu J, Tang X, Wang H, Balasubramanian M. Bgs2p, a 1,3-β-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe. FEBS Lett 2000; 478:105-8; PMID:10922478; http://dx.doi.org/ 10.1016/S0014-5793(00)01828-7 [DOI] [PubMed] [Google Scholar]
- [31].Liu J, Tang X, Wang H, Oliferenko S, Balasubramanian MK. The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol Biol Cell 2002; 13:989-1000; PMID:11907277; http://dx.doi.org/ 10.1091/mbc.01-12-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Martín V, Garcia B, Carnero E, Durán A, Sánchez Y. Bgs3p, a putative 1,3-β-glucan synthase subunit, is required for cell wall assembly in Schizosaccharomyces pombe. Eukaryot Cell 2003; 2:159-69; http://dx.doi.org/ 10.1128/EC.2.1.159-169.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Martin V, Ribas JC, Carnero E, Duran A, Sanchez Y. bgs2+, a sporulation-specific glucan synthase homologue is required for proper ascospore wall maturation in fission yeast. Mol Microbiol 2000; 38:308-21; PMID:11069657; http://dx.doi.org/ 10.1046/j.1365-2958.2000.02118.x [DOI] [PubMed] [Google Scholar]
- [34].Ishiguro J, Saitou A, Duran A, Ribas JC. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J Bacteriol 1997; 179:7653-62; PMID:9401022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Le Goff X, Woollard A, Simanis V. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol Gen Genet 1999; 262:163-72; PMID:10503548; http://dx.doi.org/ 10.1007/s004380051071 [DOI] [PubMed] [Google Scholar]
- [36].Liu J, Wang H, Balasubramanian MK. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J Cell Sci 2000; 113:1223-30; PMID:10704373 [DOI] [PubMed] [Google Scholar]
- [37].Verde F, Mata J, Nurse P. Fission yeast cell morphogenesis: identification of new genes and analysis of their role during the cell cycle. J Cell Biol 1995; 131:1529-38; PMID:8522609; http://dx.doi.org/ 10.1083/jcb.131.6.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ribas JC, Díaz M, Durán A, Perez P. Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1-3)β-D-glucan. J Bacteriol 1991; 173:3456-62; PMID:1828464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ribas JC, Roncero C, Rico H, Durán A. Characterization of a Schizosaccharomyces pombe morphological mutant altered in the galactomannan content. FEMS Microbiology Letters 1991; 79:263-7; http://dx.doi.org/ 10.1111/j.1574-6968.1991.tb04539.x [DOI] [PubMed] [Google Scholar]
- [40].Pfaller MA, Messer SA, Jones RN, Castanheira M. Antifungal susceptibilities of Candida, Cryptococcus neoformans and Aspergillus fumigatus from the Asia and Western Pacific region: data from the SENTRY antifungal surveillance program (2010–2012). J Antibiot (Tokyo) 2015; 68:556-61; PMID:25899126; http://dx.doi.org/ 10.1038/ja.2015.29 [DOI] [PubMed] [Google Scholar]
- [41].Martins IM, Cortes JCG, Muñoz J, Moreno MB, Ramos M, Clemente-Ramos JA, Durán A, Ribas JC. Differential activities of three families of specific β(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J Biol Chem 2011; 286:3484-96; PMID:21115488; http://dx.doi.org/ 10.1074/jbc.M110.174300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Castro C, Ribas JC, Valdivieso MH, Varona R, del Rey F, Durán A. Papulacandin B resistance in budding and fission yeasts: isolation and characterization of a gene involved in (1,3)β-D-glucan synthesis in Saccharomyces cerevisiae. J Bacteriol 1995; 177:5732-9; PMID:7592316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Miyata M, Kitamura J, Miyata H. Lysis of growing fission-yeast cells induced by aculeacin A, a new antifungal antibiotic. Arch Microbiol 1980; 127:11-6; PMID:7425782; http://dx.doi.org/ 10.1007/BF00414349 [DOI] [PubMed] [Google Scholar]
- [44].Hochstenbach F, Klis FM, van den Ende H, van Donselaar E, Peters PJ, Klausner RD. Identification of a putative α-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc Natl Acad Sci U S A 1998; 95:9161-6; PMID:9689051; http://dx.doi.org/ 10.1073/pnas.95.16.9161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Katayama S, Hirata D, Arellano M, Pérez P, Toda T. Fission yeast a-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J Cell Biol 1999; 144:1173-86; PMID:10087262; http://dx.doi.org/ 10.1083/jcb.144.6.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Garcia I, Tajadura V, Martin V, Toda T, Sanchez Y. Synthesis of α-glucans in fission yeast spores is carried out by three α-glucan synthase paralogues, Mok12p, Mok13p and Mok14p. Mol Microbiol 2006; 59:836-53; PMID:16420355; http://dx.doi.org/ 10.1111/j.1365-2958.2005.04995.x [DOI] [PubMed] [Google Scholar]
- [47].Flor-Parra I, Bernal M, Zhurinsky J, Daga RR. Cell migration and division in amoeboid-like fission yeast. Biol Open 2014; 3:108-15; PMID:24357230; http://dx.doi.org/ 10.1242/bio.20136783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mishra M, Huang Y, Srivastava P, Srinivasan R, Sevugan M, Shlomovitz R, Gov N, Rao M, Balasubramanian M. Cylindrical cellular geometry ensures fidelity of division site placement in fission yeast. J Cell Sci 2012; 125:3850-7; PMID:22505610; http://dx.doi.org/ 10.1242/jcs.103788 [DOI] [PubMed] [Google Scholar]
- [49].Pardo M, Nurse P. Equatorial retention of the contractile actin ring by microtubules during cytokinesis. Science 2003; 300:1569-74; PMID:12791993; http://dx.doi.org/ 10.1126/science.1084671 [DOI] [PubMed] [Google Scholar]
- [50].Huang Y, Yan H, Balasubramanian MK. Assembly of normal actomyosin rings in the absence of Mid1p and cortical nodes in fission yeast. J Cell Biol 2008; 183:979-88; PMID:19075108; http://dx.doi.org/ 10.1083/jcb.200806151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Arasada R, Pollard TD. Contractile ring stability in S. pombe depends on F-BAR Protein Cdc15p and Bgs1p Transport from the golgi complex. Cell Rep 2014; 8:1533-44; PMID:25159149; http://dx.doi.org/ 10.1016/j.celrep.2014.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roberts-Galbraith RH, Chen JS, Wang J, Gould KL. The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring. J Cell Biol 2009; 184:113-27; PMID:19139265; http://dx.doi.org/ 10.1083/jcb.200806044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ge W, Balasubramanian MK. Pxl1p, a paxillin-related protein, stabilizes the actomyosin ring during cytokinesis in fission yeast. Mol Biol Cell 2008; 19:1680-92; PMID:18272786; http://dx.doi.org/ 10.1091/mbc.E07-07-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pinar M, Coll PM, Rincón SA, Pérez P. Schizosaccharomyces pombe Pxl1 is a paxillin homologue that modulates Rho1 activity and participates in cytokinesis. Mol Biol Cell 2008; 19:1727-38; PMID:18256290; http://dx.doi.org/ 10.1091/mbc.E07-07-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Roberts-Galbraith RH, Ohi MD, Ballif BA, Chen JS, McLeod I, McDonald WH, Gygi SP, Yates JR 3rd, Gould KL. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol Cell 2010; 39:86-99; PMID:20603077; http://dx.doi.org/ 10.1016/j.molcel.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cortés JCG, Pujol N, Sato M, Pinar M, Ramos M, Moreno B, Osumi M, Ribas JC, Pérez P. Cooperation between Paxillin-like protein Pxl1 and glucan synthase Bgs1 is essential for actomyosin ring stability and septum formation in fission yeast. PLoS Genet 2015; 11:e1005358; http://dx.doi.org/ 10.1371/journal.pgen.1005358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Proctor SA, Minc N, Boudaoud A, Chang F. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr Biol 2012; 22:1601-8; PMID:22840513; http://dx.doi.org/ 10.1016/j.cub.2012.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Johnson BF, Yoo BY, Calleja GB, Kozela CP. Second thoughts on septation by the fission yeast, Schizosaccharomyces pombe: pull vs. push mechanisms with an appendix–dimensional modelling of the flat and variable septa. Antonie van Leeuwenhoek 2005; 88:1-12; PMID:15928972; http://dx.doi.org/ 10.1007/s10482-004-7074-2 [DOI] [PubMed] [Google Scholar]
- [59].Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 2010; 188:877-90; PMID:20308429; http://dx.doi.org/ 10.1083/jcb.200906012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Carnahan RH, Gould KL. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J Cell Biol 2003; 162:851-62; PMID:12939254; http://dx.doi.org/ 10.1083/jcb.200305012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Demeter J, Sazer S. imp2, a new component of the actin ring in the fission yeast Schizosaccharomyces pombe. J Cell Biol 1998; 143:415-27; PMID:9786952; http://dx.doi.org/ 10.1083/jcb.143.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ren L, Willet AH, Roberts-Galbraith RH, McDonald NA, Feoktistova A, Chen JS, Huang H, Guillen R, Boone C, Sidhu SS, et al.. The Cdc15 and Imp2 SH3 domains cooperatively scaffold a network of proteins that redundantly ensure efficient cell division in fission yeast. Mol Biol Cell 2015; 26:256-69; PMID:25428987; http://dx.doi.org/ 10.1091/mbc.E14-10-1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Arasada R, Pollard TD. Distinct roles for F-BAR proteins Cdc15p and Bzz1p in actin polymerization at sites of endocytosis in fission yeast. Curr Biol 2011; 21:1450-9; PMID:21885283; http://dx.doi.org/ 10.1016/j.cub.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]


