ABSTRACT
Annexin A2 (AnxA2) is present in multiple cellular compartments and interacts with numerous ligands including calcium, proteins, cholesterol, negatively charged phospholipids and RNA. These interactions are tightly regulated by its post-translational modifications. The levels of AnxA2 and its Tyr23 phosphorylated form (pTyr23AnxA2) are increased in many cancers and the protein is involved in malignant cell transformation, metastasis and angiogenesis. Our previous studies of rat pheochromocytoma (PC12) cells showed that reactive oxygen species (ROS) induce rapid, simultaneous and transient dephosphorylation of nuclear AnxA2, most likely associating with PML bodies, while AnxA2 associated with F-actin at the cell cortex undergoes Tyr23 phosphorylation. The pTyr23AnxA2 in the periphery of the cells is incorporated into intraluminal vesicles of multivesicular endosomes and subsequently released to the extracellular space. We show here that extracellular vesicles (EVs) from cells exposed to ROS prime untreated PC12 cells to better tolerate subsequent oxidative stress, thus enhancing their survival. There is an increase in the levels of pTyr23AnxA2 and AnxA2 in the primed cells, suggesting that AnxA2 is involved in their survival. This increase is due to an upregulation of AnxA2 expression both at the transcriptional and translational levels after relatively short term (2 h) exposure to primed EVs.
Keywords: annexin A2, extracellular vesicles, tyrosine phosphorylation
Background
Annexin A2 (AnxA2), a member of a large family of Ca2+-dependent anionic phospholipid-binding proteins, is a key player in tumor progression, neo-angiogenesis and metastasis,1-3 i.e. processes that all require cytoskeletal rearrangements and thus result in morphological changes. These changes that are related to the ability of AnxA2 to bind certain lipids and both G- and F-actin,4 as well as to interact with different cadherin isoforms,3,5 are regulated by Tyr23 phosphorylation of AnxA2.6 Thus, silencing of AnxA2 expression, inactivation of the Tyr23 site, or treatment with AnxA2 antibodies inhibit cancer metastasis and prolong cell survival.7 Up-regulation of AnxA2 and/or its Tyr23 phosphorylation are not only involved in severe malignant development of cancers and a poor prognosis, but may also play a role in chemotherapy resistance.8,9 Accordingly, AnxA2 has been associated with DNA replication, transcription and translation10 i.e. processes that have been linked to increased resistance of cancer cells to chemotherapy.11
The involvement of AnxA2 in the above-mentioned processes points to a protein capable of responding to one or several signaling pathways in different cellular compartments. Our recent results indicate that different subcellular responses may occur simultaneously.12 Thus, when cells are exposed to oxidative stress, AnxA2 in the nucleus is dephosphorylated on Tyr23, while cortical AnxA2 is phosphorylated on the same residue by the Src kinase.12 AnxA2 itself may in turn act both as a regulator and effector of the Src kinase, at least in the case of v-Src.13 Tyr23 phosphorylation of AnxA2 leads to its binding to the cytoplasmic surface of endosomes,14 which subsequently develop into multivesicular bodies (MVBs),15 due to the invagination of the endosomal membrane. As a consequence of this outside-in conversion in topology,16 Tyr23 phosphorylated AnxA2 ends up residing in the lumen of these intraluminal vesicles. As the MVBs fuse with the plasma membrane, these vesicles are released from the MVB lumen to the extracellular space as exosomes.16 Thus, the luminal Tyr23 phosphorylated AnxA2 may be involved in the exosomal transport of cargo between cells. Indeed, it was recently shown that AnxA2 is involved in the packaging of miRNAs into exosomes derived from human breast and prostate cancer cell lines.17 Interestingly, the presence of AnxA2 did not appear to influence the extent of exosomes released from these cells,17 further suggesting a role in cargo transfer. Extracellular vesicles (EVs) are believed to be involved in genetic exchange between cells in the form of transfer of mRNAs and small RNAs,18 thus emerging as important mediators of intercellular communication. For example, it is thought that extracellular vesicle-mediated signaling is involved in the modulation of the tumor microenvironment.19
We recently showed that oxidative stress induced by ROS changes rapidly (within minutes) the subcellular pattern of Tyr23 phosphorylated AnxA2. The nuclear pTyr23AnxA2 is dephosphorylated and while the phosphorylated form partially localizes to nuclear PML bodies, the dephosphorylated form preferentially localizes to SC-35 positive nuclear speckles, possibly involved in transcription. Simultaneously, a pool of cortical AnxA2 becomes Tyr23 phosphorylated by the Src kinase.12 The release of pTyr23AnxA2-containing vesicles to the extracellular space was most pronounced after about 1 h after exposure to ROS, and was significantly reduced after 2 h.12 Since pTyr23AnxA2 has been shown to associate with the endosomal pathway including MVBs, as well as with exosomes released from MVBs,14,15 we investigated whether it would be present in EVs including exosomes released from cells after their exposure to ROS and found this to be the case.12 In fact, AnxA2 has been regarded as a marker of exosomes.20
Results and discussion
Here, we investigated the functional significance of the release of EVs in response to the exposure of PC12 cells to ROS. EVs including exosomes were isolated from the medium of PC12 cells following their treatment for 1 h with 1 mM H2O2 (peak release of EVs after H2O2 exposure). Untreated PC12 cells were then pre-incubated for 2 h in the presence of these EVs (denoted primed EVs). Indeed, we observed that these cells developed a higher tolerance for a subsequent 1 h exposure to 1 mM H2O2, increasing their viability from ∼84% to ∼93% (Fig. 1). Similar results regarding viability after exposure to H2O2 have been obtained with MC/9 cells.21
Figure 1.
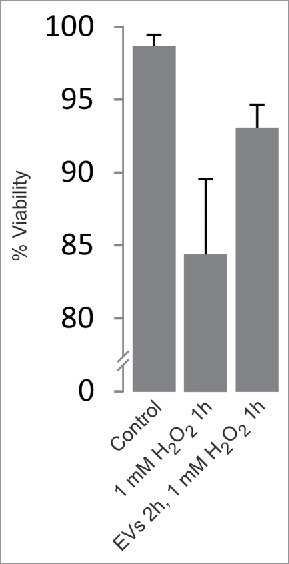
Two hour pre-incubation of PC12 cells with EVs derived from H2O2-treated cells increases their viability upon subsequent 1 h exposure to H2O2. Exosome-depleted medium was used and EVs were resuspended in 1/20 of the original volume of cell medium. Viability was calculated by trypan blue exclusion assay (n = 6). Viability: p < 0.005.
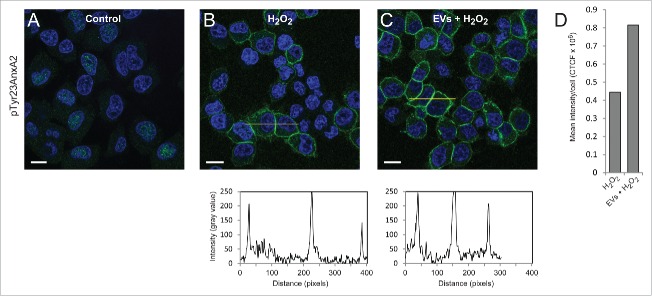
Interestingly, the level of pTyr23AnxA2 at the cell cortex increase significantly in cells pre-incubated with primed EVs and subsequently exposed to H2O2 for 15 min when compared to cells only exposed to H2O2 (Fig. 2, compare Panel B with C). It should be noted that not all cells respond to H2O2 treatment by increasing their cortical pool of pTyr23AnxA2. The poorly responding cells apparently contain low initial levels of AnxA2, both in their cytoplasm and nucleus (Fig. 2B and C), most likely due to cell cycle-dependent expression of AnxA2.12,22 Therefore, we have only given the mean intensity/cell (Fig. 2D). These analyses are not given for the control cells since the localization of pTyr23AnxA2 is predominantly in the nucleus (Fig. 2A). To verify that the increased levels of pTyr23AnxA2 in cells exposed to primed EVs and subsequently to H2O2 are indeed related to their pre-incubation with primed EVs, a Western blot analysis was performed on lysates from control cells, cells treated with H2O2 alone, cells pre-incubated with primed EVs only, or cells pre-incubated with EVs and subsequently exposed to H2O2 (Fig. 3). It is evident that exposure to ROS in the form of H2O2 leads to increased Tyr23 phosphorylation of AnxA2 in general, while 2 h pre-incubation with EVs from cells previously exposed to ROS increases the level of total AnxA2 (Fig. 3). Apparently, pre-exposure to EVs from H2O2-treated cells and subsequent H2O2-exposure lead to a higher increase in the level of pTyrAnxA2 than H2O2 treatment alone (Fig. 3, compare lanes 4 and 2). Together with the imaging data (Fig. 2), these results strongly indicate that the pre-exposure of cells to EVs derived from ROS-stressed PC12 cells increase their level of pTyr23AnxA2 during their subsequent ROS-exposure (Figs. 2 and 3).
Figure 2.
Pre-incubation of PC12 cells with EVs derived from H2O2-treated cells results in ∼2-fold higher levels of pTyr23AnxA2 upon subsequent treatment with H2O2, as compared to cells exposed to H2O2 only. PC12 cells were untreated (A), treated for 15 min with 1 mM H2O2 only (B), or treated for 15 min with 1 mM H2O2 after their pre-incubation for 2 h with EVs released from cells exposed for 1 h to H2O2 (C). pTyr23AnxA2 (green, A-C) was detected using monoclonal antibodies. The DAPI-stained nuclei are shown in blue. Scale bars: 10 μm. The diagrams below each panel show the corresponding intensity profiles along the lines indicated in the respective panels. Panel 2D: Corrected total cell fluorescence (CTCF) was measured from cells (20–30) in the samples shown (panels A–C); CTCF were then divided by the number of cells measured.
Figure 3.
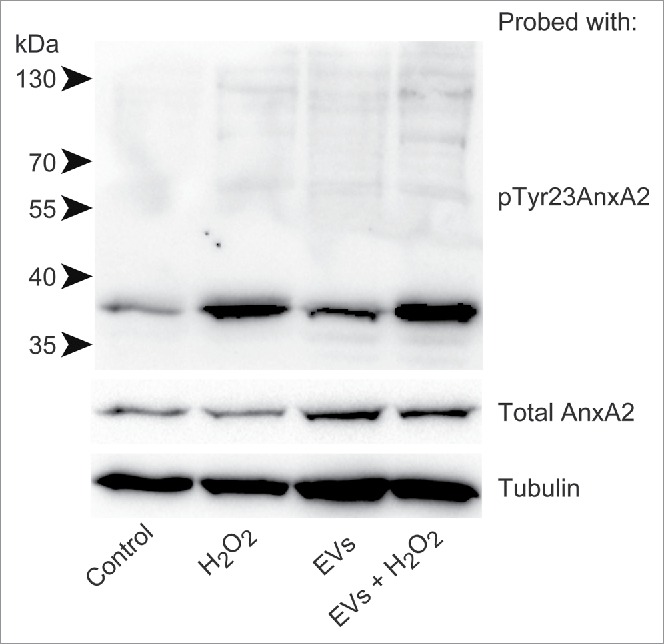
Pre-incubation of PC12 cells with primed EVs increases the levels of pTyr23AnxA2 upon subsequent exposure to H2O2, as compared to cells exposed to H2O2 alone. 100 µg of protein from total lysates derived from PC12 cells grown in the presence of H2O2 for 0 min (control), 15 min (H2O2), or cells that were first pre-incubated for 2 h with primed EVs before exposure to H2O2 for 0 min (EVs) or 15 min (EVs + H2O2) were separated by 10% SDS-PAGE, transferred to nitro-cellulose membranes and probed with antibodies against pTyr23AnxA2, total AnxA2, or tubulin as indicated. Following incubation with HRP-conjugated secondary antibodies and the ECL-reagent, the reactive protein bands were detected using the ChemiDocTM XRS+ molecular imager. Molecular mass standards are indicated to the left of the upper blot.
The increase in the level of total AnxA2 after preincubation with primed EVs (2 h) alone, or combined with exposure to ROS (15 min), could be due to a) cargo delivery of AnxA2 by EVs, or b) increased transcription and/or translation of AnxA2 in these cells. To distinguish between these possibilities, the amount of total cortical AnxA2 was investigated after exposure to ROS alone (Fig. 4B), or in combination with primed EVs (Fig. 4D), and compared to exposure to primed EVs alone (Fig. 4C). While the total cellular level of pTyr23AnxA2 increases after 15 min of oxidative stress without pre-incubation with primed EVs (Fig. 2), there is no equivalent increase in total AnxA2 (Fig. 4G). However, after the priming of cells with EVs for 2 h, there is an almost 2-fold increase in total AnxA2 (Fig. 4G). By contrast, when transcription or translation in the cells incubated with primed EVs was inhibited by Actinomycin D (AcD) and cycloheximide (CHX), respectively, the increase in the level of total AnxA2 was suppressed. This finding suggests that the pre-incubation of PC12 cells with EVs derived from cells exposed to ROS increases the expression of AnxA2, possibly representing an adaptation to oxidative stress.23 Furthermore, the data also suggests that AnxA2 is involved in the EV/exosome-mediated response to ROS, in line with results showing the involvement of ROS in metastasis,24 angiogenesis and tumor growth.25 ROS is known to modulate the activity of several transcription factors, including hypoxia-inducible factor-1 (HIF-1).26 Other examples are the transcription factors SP1 and SP3, which are activated by ROS, leading to activated GC-boxes in the vascular endothelial growth factor A (VEGF-A) promoter, which in turn leads to increased expression of VEGF-A.27 HIF-1 plays a critical role in inducing chemoresistance and metastasis,28 and may also increase the expression of AnxA2.29 Upregulated expression of AnxA2 is associated with metastasis in several cancer types, including gastric adenocarcinoma3 and colon cancers.30 It has been observed that the levels of pTyr23AnxA2 and total AnxA2, in particular associated with the plasma membrane, are considerably increased in cells derived from a metastatic tumor, as compared to the primary tumor derived from the same patient.31 Thus, in addition to its role in intracellular signaling, AnxA2 also appears to be involved in mediating intercellular signaling either directly (for example by binding to small regulatory RNAs in EVs) or indirectly (by being a “responder” in the EV-recipient cell)
Figure 4.
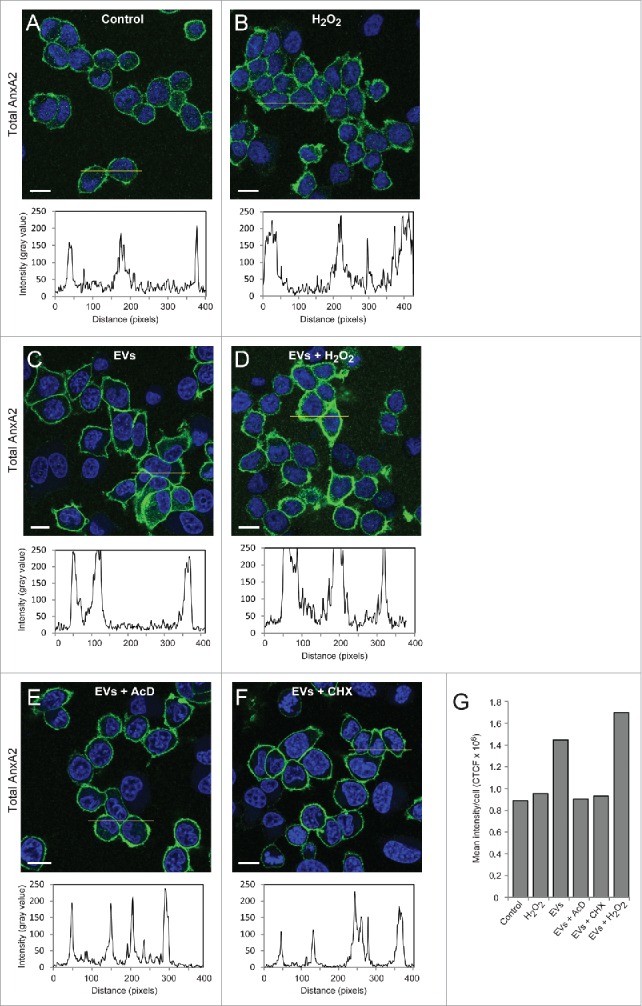
Pre-incubation of PC12 cells with EVs derived from H2O2-treated cells increases the expression of total AnxA2 both at the transcriptional and translational levels. PC12 cells were untreated (A), treated for 15 min with 1 mM H2O2 (B), pre-incubated for 2 h with EVs released from H2O2-treated cells (C-F) either with no additional treatment (C), or with a subsequent 15 min treatment with 1 mM H2O2 (D), in the presence of 3 µg/ml AcD (E) or 10 µg/ml CHX (F). Total AnxA2 (green) was detected using monoclonal polyclonal antibodies. The DAPI-stained nuclei are shown in blue. Scale bars: 10 μm. The diagrams below each panel show the corresponding intensity profiles along the lines indicated in the respective panels. Panel 4G: Corrected total cell fluorescence (CTCF) was measured from cells in the images shown in Panels A-F; CTCF were then divided by the number of cells (20–30) measured.
Materials and methods
EVs were isolated as previously described.12 The kit precipitates both EVs that bud off from the plasma membrane and EVs of endosomal origin (termed exosomes) as well as other secreted membrane-enclosed vesicles. Thus, the term EVs will be used.32 Briefly, EVs were derived from PC12 cells after treatment with H2O2 for 1 h. 1.4 ml ExoQuick-TC was mixed with 7 ml media from PC12 cells exposed to H2O2 for 1 h and grown in exosome-depleted medium (System Biosciences). This was left ON at 4°C and centrifuged at 1500 × g for 30 min before a wash of the exosomal pellet in exosome-depleted medium and another centrifugation was performed. The resulting pellet was resuspended in 1 ml exosome-depleted medium before further dilution when added to cells. Fresh PC12 cells were treated with these EVs for 2 h, and subsequently exposed to H2O2 for 15 min or 1 h (viability assays).
Cells, fixed and permeabilised as described earlier,33 were stained for immunofluorescence with primary antibodies against pTyr23AnxA2 (sc-135753, Santa Cruz Biotechnologies, 1:20) or AnxA2 (ab41803, Abcam, 1:250), with one modification; before staining with the polyclonal antibodies against AnxA2, antigenic sites were exposed by incubation for 5 min with 6 M guanidine-HCl in 50 mM Tris, pH 7.4.34 This treatment is not necessary to detect pTyrAnxA2. When the fixed cells were treated with guanidine-HCl and stained with polyclonal antibodies against AnxA2 (recognizes aa residues 150–250), AnxA2 was found predominantly associated with the cortical region under the plasma membrane, with an additional faint signal in the rest of the cytoplasm (Fig. 4). However, in non-guanidine-HCl-treated cells the protein was mainly detected in the nucleus (see also ref. 35, in addition to being detected in the cytoplasm (See Fig. 1, Panels I–II in ref. 12).
Fluorescence intensity graphs/profiles were measured in ImageJ 1.48o. Total cellular fluorescence was calculated using ImageJ 1.48o. An outline was drawn around the cells, and integrated density, mean gray value (fluorescence) and area were measured. Background measurements were also carried out. The corrected total cell fluorescence (CTCF) was calculated as: integrated density – (selected area x mean gray value of background).36
Total cell lysates were prepared from PC12 cells that had been first extensively washed in PBS. Subsequently, the cells were lysed in RIPA buffer (25 mM Tris; pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing 2 mM EGTA. The resultant supernatant after centrifugation at 12000 g for 20 min was the total cell lysate. SDS-PAGE and western blot analysis were performed as described.12 Total AnxA2 and its Tyr23 phosphorylated form were detected using monoclonal antibodies directed against AnxA2 (610069; BD Biosciences; 1:1000) and pTyr23AnxA2 (sc-135753, Santa Cruz Biotechnologies, 1:200 dilution), respectively. Monoclonal antibodies against tubulin (A01410–40, Genscript, 1:8000) were used as loading control.
Abbreviations
- AcD
Actinomycin D
- AnxA2
Annexin A2
- CHX
cycloheximide
- CTCF
corrected total cell fluorescence
- EV
extracellular vesicle
- MVBs
multivesicular bodies
- PC12
rat pheochromocytoma cell
- pTyr23AnxA2
Tyr23 phosphorylated Annexin A2
- ROS
reactive oxygen species
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We greatly appreciate Prof. Jaakko Saraste for valuable comments.
Funding
This study was funded by the University of Bergen (A.K.G. and A.V.), Helse Vest (grant no 911499 to A.V.) and The Research Council of Norway (NFR)(grant no 240400/F20 to A.V.).
References
- [1].Mussunoor S, Murray GI. The role of annexins in tumour development and progression. J Pathol 2008; 216:131-40; PMID:18698663; http://dx.doi.org/ 10.1002/path.2400 [DOI] [PubMed] [Google Scholar]
- [2].Hedhli N, Falcone DJ, Huang B, Cesarman-Maus G, Kraemer R, Zhai H, Tsirka SE, Santambrogio L, Hajjar KA. The annexin A2/S100A10 system in health and disease: emerging paradigms. J Biomed Biotechnol 2012; 2012:406273; PMID:23193360; http://dx.doi.org/ 10.1155/2012/406273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Han Y, Ye J, Dong Y, Xu Z, Du Q. Expression and significance of annexin A2 in patients with gastric adenocarcinoma and the association with E-cadherin. Exp Ther Med 2015; 10:549-54; PMID:26622352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J 2006; 25:1816-26; PMID:16601677; http://dx.doi.org/ 10.1038/sj.emboj.7601078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hitchcock JK, Katz AA, Schafer G. Dynamic reciprocity: the role of annexin A2 in tissue integrity. J Cell Commun Signal 2014; 8:125-33; PMID:24838661; http://dx.doi.org/ 10.1007/s12079-014-0231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V. Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci 2008; 121:2177-85; PMID:18565825; http://dx.doi.org/ 10.1242/jcs.028415 [DOI] [PubMed] [Google Scholar]
- [7].Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT, et al.. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One 2011; 6:e19390; PMID:21572519; http://dx.doi.org/ 10.1371/journal.pone.0019390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Spijkers-Hagelstein JAP, Pinhancos SM, Schneider P, Pieters R, Stam RW. Src kinase-induced phosphorylation of annexin A2 mediates glucocorticoid resistance in MLL-rearranged infant acute lymphoblastic leukemia. Leukemia 2013; 27:1063-71; PMID:23334362; http://dx.doi.org/ 10.1038/leu.2012.372 [DOI] [PubMed] [Google Scholar]
- [9].Chen CY, Lin YS, Chen CL, Chao PZ, Chiou JF, Kuo CC, Lee FP, Lin YF, Sung YH, Lin YT, et al.. Targeting annexin A2 reduces tumorigenesis and therapeutic resistance of nasopharyngeal carcinoma. Oncotarget 2015; 6:26946-59; PMID:26196246; http://dx.doi.org/ 10.18632/oncotarget.4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vedeler A, Hollas H, Grindheim AK, Raddum AM. Multiple roles of annexin A2 in post-transcriptional regulation of gene expression. Curr Protein Pept Sci 2012; 13:401-12; PMID:22708494; http://dx.doi.org/ 10.2174/138920312801619402 [DOI] [PubMed] [Google Scholar]
- [11].Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013; 13:714-26; PMID:24060863; http://dx.doi.org/ 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- [12].Grindheim AK, Hollas H, Raddum AM, Saraste J, Vedeler A. Reactive oxygen species exert opposite effects on Tyr23 phosphorylation of the nuclear and cortical pools of annexin A2. J Cell Sci 2016; 129:314-28; PMID:26644180; http://dx.doi.org/ 10.1242/jcs.173195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hayes MJ, Moss SE. Annexin 2 has a dual role as regulator and effector of v-Src in cell transformation. J Biol Chem 2009; 284:10202-10; PMID:19193640; http://dx.doi.org/ 10.1074/jbc.M807043200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morel E, Gruenberg J. Annexin A2 binding to endosomes and functions in endosomal transport are regulated by tyrosine 23 phosphorylation. J Biol Chem 2009; 284:1604-11; PMID:18990701; http://dx.doi.org/ 10.1074/jbc.M806499200 [DOI] [PubMed] [Google Scholar]
- [15].Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem 2011; 286:30911-25; PMID:21737841; http://dx.doi.org/ 10.1074/jbc.M111.271155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000; 113 Pt 19:3365-74; PMID:10984428. [DOI] [PubMed] [Google Scholar]
- [17].Hagiwara K, Katsuda T, Gailhouste L, Kosaka N, Ochiya T. Commitment of Annexin A2 in recruitment of microRNAs into extracellular vesicles. FEBS Lett 2015; 589:4071-8; PMID:26632510; http://dx.doi.org/ 10.1016/j.febslet.2015.11.036 [DOI] [PubMed] [Google Scholar]
- [18].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654-9; PMID:17486113; http://dx.doi.org/ 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- [19].Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ, Martin CK, Li F, et al.. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol 2012; 14:159-67; PMID:NOT_FOUND; http://dx.doi.org/ 10.1038/ncb2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009; 9:4997-5000; PMID:19810033; http://dx.doi.org/ 10.1002/pmic.200900351 [DOI] [PubMed] [Google Scholar]
- [21].Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One 2010; 5:e15353; PMID:21179422; http://dx.doi.org/ 10.1371/journal.pone.0015353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chiang Y, Schneiderman MH, Vishwanatha JK. Annexin II expression is regulated during mammalian cell cycle. Cancer Res 1993; 53:6017-21; PMID:8261416 [PubMed] [Google Scholar]
- [23].Madureira PA, Hill R, Miller VA, Giacomantonio C, Lee PW, Waisman DM. Annexin A2 is a novel cellular redox regulatory protein involved in tumorigenesis. Oncotarget 2011; 2:1075-93; PMID:22185818; http://dx.doi.org/ 10.18632/oncotarget.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee DJ, Kang SW. Reactive oxygen species and tumor metastasis. Mol Cells 2013; 35:93-8; PMID:23456330; http://dx.doi.org/ 10.1007/s10059-013-0034-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [25].Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 2007; 67:10823-30; PMID:18006827; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0783 [DOI] [PubMed] [Google Scholar]
- [26].Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2014; 2:535-62; PMID:24634836; http://dx.doi.org/ 10.1016/j.redox.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schafer G, Cramer T, Suske G, Kemmner W, Wiedenmann B, Hocker M. Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J Biol Chem 2003; 278:8190-8; PMID:12509426; http://dx.doi.org/ 10.1074/jbc.M211999200 [DOI] [PubMed] [Google Scholar]
- [28].Nagaraju GP, Bramhachari PV, Raghu G, El-Rayes BF. Hypoxia inducible factor-1alpha: Its role in colorectal carcinogenesis and metastasis. Cancer Lett 2015; 366:11-8; PMID:26116902; http://dx.doi.org/ 10.1016/j.canlet.2015.06.005 [DOI] [PubMed] [Google Scholar]
- [29].Huang B, Deora AB, He KL, Chen K, Sui G, Jacovina AT, Almeida D, Hong P, Burgman P, Hajjar KA. Hypoxia-inducible factor-1 drives annexin A2 system-mediated perivascular fibrin clearance in oxygen-induced retinopathy in mice. Blood 2011; 118:2918-29; PMID:21788340; http://dx.doi.org/ 10.1182/blood-2011-03-341214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Duncan R, Carpenter B, Main LC, Telfer C, Murray GI. Characterisation and protein expression profiling of annexins in colorectal cancer. Br J Cancer 2008; 98:426-33; PMID:18071363; http://dx.doi.org/ 10.1038/sj.bjc.6604128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tristante E, Martinez CM, Jimenez S, Mora L, Carballo F, Martinez-Lacaci I, de Torre-Minguela C. Association of a characteristic membrane pattern of annexin A2 with high invasiveness and nodal status in colon adenocarcinoma. Transl Res 2015; 166:196-206; PMID:25795236; http://dx.doi.org/ 10.1016/j.trsl.2015.02.006 [DOI] [PubMed] [Google Scholar]
- [32].Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al.. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014; 3:26913; PMID:25536934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grindheim AK, Hollas H, Ramirez J, Saraste J, Trave G, Vedeler A. Effect of serine phosphorylation and Ser25 phospho-mimicking mutations on nuclear localisation and ligand interactions of annexin A2. J Mol Biol 2014; 426:2486-99; PMID:24780253; http://dx.doi.org/ 10.1016/j.jmb.2014.04.019 [DOI] [PubMed] [Google Scholar]
- [34].Peranen J, Rikkonen M, Kaariainen L. A method for exposing hidden antigenic sites in paraformaldehyde-fixed cultured cells, applied to initially unreactive antibodies. J Histochem Cytochem 1993; 41:447-54; PMID:8429208; http://dx.doi.org/ 10.1177/41.3.8429208 [DOI] [PubMed] [Google Scholar]
- [35].Madureira PA, Hill R, Lee PW, Waisman DM. Genotoxic agents promote the nuclear accumulation of annexin A2: role of annexin A2 in mitigating DNA damage. PLoS One 2012; 7:e50591; PMID:23226323; http://dx.doi.org/ 10.1371/journal.pone.0050591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014; 13:1400-12; PMID:24626186; http://dx.doi.org/ 10.4161/cc.28401 [DOI] [PMC free article] [PubMed] [Google Scholar]