ABSTRACT
Bacterial volatiles protect plants either by directly inhibiting a pathogenic fungus or by improving the defense capabilities of plants. The effect of bacterial volatiles on fungal growth was dose-dependent. A low dosage did not have a noticeable effect on Botrytis cinerea growth and development, but was sufficient to elicit induced resistance in Arabidopsis thaliana. Bacterial volatiles displayed negative effects on biofilm formation on a polystyrene surface and in in planta leaf colonization of B. cinerea. However, bacterial volatile-mediated induced resistance was the major mechanism mediating protection of plants from B. cinerea. It was responsible for more than 90% of plant protection in comparison with direct fungal inhibition. Our results broaden our knowledge of the role of bacterial volatiles in plant protection.
KEYWORDS: bacterial volatile organic compounds, fungal inhibition, induced systemic resistance, leaf surface attachment, plant growth-promoting rhizobacteria
Bacteria emit a vast array of volatile organic compounds belonging to various chemical groups.1 More than 120 individual volatiles have been identified in actinomycetes, a group of Gram-positive bacteria.2 Based on this diversity, bacterial volatiles have different and even opposite biological activities in natural and artificial systems. They increase or inhibit the growth of fungi,2-9 induce resistance to biotic10-16 and abiotic17 stresses, and promote9,15 or suppress plant growth.18
Plant protection by bacterial volatiles is mediated by 2 distinct. First, bacterial volatiles are able to protect plants via inhibition of fungal growth and development. Volatiles from Bacillus spp. decreased pigmentation in Fusarium oxysporum6 and B. cinerea.8 In our previous work, we revealed that the effect of volatiles on B. cinerea was dose-dependent.7 Exposure of fungi to bacterial volatiles from one colony of B. subtilis GB03 did not have a significant effect on fungal growth, spore production, and spore germination (Fig. 1). However, there was a linear relationship between the volatile concentration and fungal inhibition.7 Quintana-Rodriguez and coworkers10 also showed that volatiles emitted from the common bean plant were able to directly inhibit conidia germination in vivo and in vitro in a dose-dependent manner. Furthermore, bacterial volatiles had a negative effect on biofilm formation on a polystyrene surface in a dose-dependent manner.7 It should be noted that some volatiles were able to increase mycelial growth and spore production and germination of different fungi. Volatiles of Klebsiella pneumonia increased growth and spore germination of the mycorrhizal fungus Glomus mosseae.19 Acetoin, 2,3-butanediol, and 3-pentanol improved fungal growth and spore production of B. cinerea at a concentration of 100 µM.8
Figure 1.
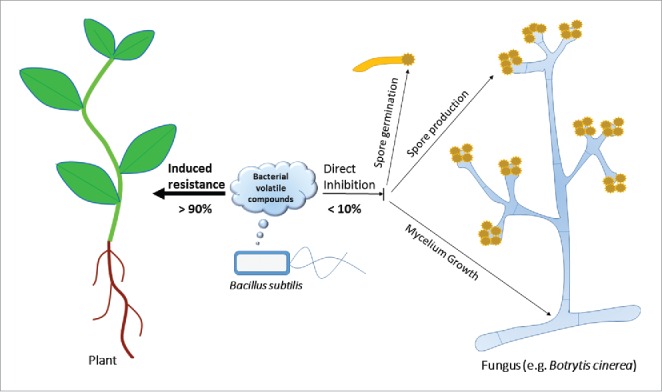
Illustrated model of the role of bacterial volatile compounds (BVCs) in plant protection against pathogenic fungi. The major mechanism for plant protection by BVCs from the soil bacterium Bacillus subtilis is induced resistance (responsible for more than 90% of plant protection). BVCs attenuate mycelial growth, spore production, and spore germination of fungi including Botrytis cinerea when used at high dosages, but this direct effect is responsible for less than 10% of plant protection when the optimum dosage is used.
Secondly, volatiles can protect plants via induced systemic resistance (ISR) against pathogens. The long-chain volatiles tridecane14 and hexadecane15 induced resistance in Arabidopsis against Pectobacterium carotovorum and Pseudomonas syringae, respectively. A low dosage of butanediol suppressed Microdochium nivale in Agrostis stolonifera by up to 90%.12 The same concentration of acetoin induced resistance against P. syringae pv. tomato in Arabidopsis.16 In our previous work, volatiles of B. subtilis GB03 and 100 µM 2-hydroxy-3-pentanone suppressed the growth of B. cinerea on Arabidopsis.8
In our previous work,7 we designed an experiment to determine the contribution of each mechanism, direct fungal inhibition or boosting of plant immunity, to protection of Arabidopsis against B. cinerea. We found that a low concentration of volatiles was sufficient to elicit induced resistance in plants, but was not sufficient to inhibit fungal growth and development.7 ISR and direct fungal inhibition were responsible for more than 90% and less than 10% of plant protection, respectively (Fig. 1).7 Microscopic inspection showed that a low dose of volatiles affected leaf colonization of B. cinerea by increasing epiphytic growth of the fungus, but this effect was unstable.
Volatiles of B. subtilis GB03 primed the expression of PR1 and PDF1.2, but not of ChiB, indicating activation of a salicylic acid (SA)- and jasmonic acid (JA)-dependent signaling pathways. However, the ISR signaling pathways could differ based on the profile of volatiles released by different bacteria. For example, 3-pentanol induced the SA and JA pathways against Xanthomonas axonopodis pv vesicatoria11 and P. syringae pv. tomato.20 Resistance induced by the volatile hexadecane was dependent on SA but not on JA.15 Acetoin treatment invoked the SA, JA, and ethylene signaling pathways.16
In conclusion, we suggest that BVCs may more related ISR as plant protection mechanism of action. Pavlica and coauthors21 declared that only a small number of soil volatiles such as formaldehyde and ammonia could reach a threshold concentration to reduce conidia germination of pathogenic fungi. There is a report that BVCs emission could be 30–200 ng/g depending on the soil type. However, bacteria are able to produce more than 30 g/L acetoin22 and 2,3-butanediol23 in synthetic media, while only 2–200 ng of these compound can be adequate to activate effective systemic resistance against Erwinia carotovora.24 Up to 90% of conidia of Cochliobolus victoria germinated when they were exposed to BVCs in a soil sample in an open vial system.25 In our previous work,8 the BVCs acetoin, 2,3-butanediol, 3-pentanol, 1-pentanol, 2-hydroxy-3-pentanone, methyl jasmonate, and methyl SA did not affect the growth and spore formation of B. cinerea at a concentration of 100 µM, while this concentration significantly suppressed disease. Eventually, volatiles produced by bacteria normally act as infochemicals to communicate to other organisms in their niche and they can be toxic in specific conditions in which they are produced at high concentrations.
Abbreviations
- BVC
bacterial volatile compound
- ISR
induced systemic resistance
- JA
jasmonic acid
- SA
salicylic acid
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We are grateful for financial support from the Industrial Source Technology Development Program of the Ministry of Knowledge Economy (10044909), the Next-Generation BioGreen 21 Program (SSAC grant #PJ01111803), RDA, and the KRIBB initiative program of South Korea.
References
- [1].Schulz S, Dickschat JS. Bacterial volatiles: the smell of small organisms. Natl Prod Rep 2007; 24:814-42; PMID:17653361; http://dx.doi.org/ 10.1039/b507392h [DOI] [PubMed] [Google Scholar]
- [2].Kai M. Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 2009; 81:1001-12; PMID:19020812; http://dx.doi.org/ 10.1007/s00253-008-1760-3 [DOI] [PubMed] [Google Scholar]
- [3].Chen H, Xiao X, Wang J, Wu L, Zheng Z, Yu Z. Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol Lett 2008; 30:919-23; PMID:18165869; http://dx.doi.org/ 10.1007/s10529-007-9626-9 [DOI] [PubMed] [Google Scholar]
- [4].Li Q, Ning P, Zheng L, Huang J, Li G, Hsiang T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol Technol 2010; 58:157-65; http://dx.doi.org/ 10.1016/j.postharvbio.2010.06.003 [DOI] [Google Scholar]
- [5].Li Q, Ning P, Zheng L, Huang J, Li G, Hsiang T. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol Control 2012; 61:113-20; http://dx.doi.org/ 10.1016/j.biocontrol.2011.10.014 [DOI] [Google Scholar]
- [6].Liu W-w, Mu W, Zhu B-y, Du Y-c, Liu F. Antagonistic activities of volatiles from four strains of Bacillus spp. and Paenibacillus spp. against soil-borne plant pathogens. Agr Sci China. 2008; 7(9):1104-14; http://dx.doi.org/ 10.1016/S1671-2927(08)60153-4 [DOI] [Google Scholar]
- [7].Sharifi R, Ryu C-M. Are bacterial volatile compounds poisonous odors to a fungal pathogen Botrytis cinerea, alarm signals to Arabidopsis seedlings for eliciting induced resistance, or both? Front Microbiol 2016; 7:196; PMID:26941721; http://dx.doi.org/ 10.3389/fmicb.2016.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharifi R, Ahmadzade M, Behboudi K, Ryu C- M. Role of Bacillus subtilis volatiles in induction of systemic resistance in Arabidopsis. Iranian J Plant Prot Sci 2013; 44:91-101 [Google Scholar]
- [9].Vespermann A, Kai M, Piechulla B. Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 2007; 73:5639-41; PMID:17601806; http://dx.doi.org/ 10.1128/AEM.01078-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Quintana-Rodriguez E, Morales-Vargas AT, Molina-Torres J, Ádame-Alvarez RM, Acosta-Gallegos JA, Heil M. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J Ecol 2015; 103:250-60; http://dx.doi.org/ 10.1111/1365-2745.12340 [DOI] [Google Scholar]
- [11].Choi HK, Song GC, Yi H-S, Ryu C-M. Field evaluation of the bacterial volatile derivative 3-pentanol in priming for induced resistance in pepper. J Chem Ecol 2014; 40:882-92; PMID:25149655; http://dx.doi.org/ 10.1007/s10886-014-0488-z [DOI] [PubMed] [Google Scholar]
- [12].Cortes-Barco A, Hsiang T, Goodwin P. Induced systemic resistance against three foliar diseases of Agrostis stolonifera by (2R,3R)-butanediol or an isoparaffin mixture. Ann Appl Biol 2010; 157:179-89; http://dx.doi.org/ 10.1111/j.1744-7348.2010.00417.x [DOI] [Google Scholar]
- [13].Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J Gen Plant Pathol 2007; 73:35-7 [Google Scholar]
- [14].Lee B, Farag MA, Park HB, Kloepper JW, Lee SH, Ryu CM. Induced resistance by a long-chain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS One 2012; 7(11):e48744; PMID:23209558; http://dx.doi.org/ 10.1371/journal.pone.0048744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park HB, Lee B, Kloepper JW, Ryu C-M. One shot-two pathogens blocked: Exposure of Arabidopsis to hexadecane, a long chain volatile organic compound, confers induced resistance against both Pectobacterium carotovorum and Pseudomonas syringae. Plant Signal Behav 2013; 8:e24619; PMID:23603940; http://dx.doi.org/ 10.4161/psb.24619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rudrappa T, Biedrzycki ML, Kunjeti SG, Donofrio NM, Czymmek KJ, Pare PW, Bais H.P. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun Integr Biol 2010; 3:130-8; PMID:20585504; http://dx.doi.org/ 10.4161/cib.3.2.10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cho SM, Kang BR, Han SH, Anderson AJ, Park JY, Lee YH, Cho BH, Yang KY, Ryu CM, Kim YC. 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol Plant Microbe Interact 2008; 21:1067-75; PMID:18616403; http://dx.doi.org/ 10.1094/MPMI-21-8-1067 [DOI] [PubMed] [Google Scholar]
- [18].Blom D, Fabbri C, Eberl L, Weisskopf L. Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol 2011; 77:1000-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Effmert U, Kalderás J, Warnke R, Piechulla B. Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 2012; 38: 665-703; PMID:22653567; http://dx.doi.org/ 10.1007/s10886-012-0135-5 [DOI] [PubMed] [Google Scholar]
- [20].Song GC, Choi HK, Ryu C-M. Gaseous 3-pentanol primes plant immunity against a bacterial speck pathogen, Pseudomonas syringae pv. tomato via salicylic acid and jasmonic acid-dependent signaling pathways in Arabidopsis. Front Plant Sci 2015; 6:821; PMID:26500665; http://dx.doi.org/ 10.3389/fpls.2015.00821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pavlica DA, Hora TS, Bradshaw JJ, Skogerboe RK, Baker R. Volatiles from soil influencing activities of soil fungi. Phytopathology 1978; 68, 758-65; http://dx.doi.org/ 10.1094/Phyto-68-758 [DOI] [Google Scholar]
- [22].Xiao ZJ, Liu PH, Qin JY, Xu P. Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl Microbiol Biotechnol 2007; 74:61-8; PMID:17043817; http://dx.doi.org/ 10.1007/s00253-006-0646-5 [DOI] [PubMed] [Google Scholar]
- [23].Ma C, Wang A, Qin J, Li L, Ai X, Jiang T, Tang H, Xu P. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl Microbiol Biotechnol 2009; 82:49-57; PMID:18949476; http://dx.doi.org/ 10.1007/s00253-008-1732-7 [DOI] [PubMed] [Google Scholar]
- [24].Ryu C-M, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 2004; 134:1017-26; PMID:14976231; http://dx.doi.org/ 10.1104/pp.103.026583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liebman JA, Epstein L. Partial characterization of volatile fungistatic compound(s) from soil. Phytopathology 1994; 84:442-6; http://dx.doi.org/ 10.1094/Phyto-84-442 [DOI] [Google Scholar]


