ABSTRACT
The sodium/bicarbonate cotransporter (NBC) transports extracellular Na+ and HCO3− into the cytoplasm upon intracellular acidosis, restoring the acidic pHi to near neutral values. Two different NBC isoforms have been described in the heart, the electroneutral NBCn1 (1Na+:1HCO3−) and the electrogenic NBCe1 (1Na+:2HCO3−). Certain non-genomic effects of aldosterone (Ald) were due to an orphan G protein-couple receptor 30 (GPR30). We have recently demonstrated that Ald activates GPR30 in adult rat ventricular myocytes, which transactivates the epidermal growth factor receptor (EGFR) and in turn triggers a reactive oxygen species (ROS)- and PI3K/AKT-dependent pathway, leading to the stimulation of NBC. The aim of this study was to investigate the NBC isoform involved in the Ald/GPR30-induced NBC activation. Using specific NBCe1 inhibitory antibodies (a-L3) we demonstrated that Ald does not affect NBCn1 activity. Ald was able to increase NBCe1 activity recorded in isolation. Using immunofluorescence and confocal microscopy analysis we showed in this work that both NBCe1 and GPR30 are localized in t-tubules. In conclusion, we have demonstrated that NBCe1 is the NBC isoform activated by Ald in the heart.
KEYWORDS: aldosterone, electrogenic, GPR30, heart, sodium/bicarbonate cotransporter
Introduction
The NBC membrane family proteins are sodium/bicarbonate cotransporters localized in all sarcolemmal membrane zones of the cardiac ventricular myocytes, most notably in the transverse tubules (t-tubules).1 NBC transports extracellular Na+ and HCO3− into the cytoplasm upon intracellular acidosis, restoring the acidic pHi to near neutral values. Two different NBC isoforms, the electroneutral NBCn1 (1Na+:1HCO3−) and the electrogenic NBCe1 (1Na+:2HCO3−)2, have been described in the heart. We have previously demonstrated that these NBC isoforms can be independently regulated by angiotensin II, which stimulates the NBCn1 and inhibits the NBCe1.3,4
It is important to note that NBCe1 mediates the movement of one Na+ and 2 HCO3− in the same direction, resulting in the influx of one negative charge across the plasma membrane in each complete cycle of transport activity. The NBCe1 current (INBC) has been characterized in myocardium as an anionic bicarbonate and sodium-dependent current which reversed at around −85 mV.5,6 Furthermore, the INBC modulates the shape and duration of the cardiac action potential.
The steroid hormone aldosterone (Ald) plays a classic role acting through mineralocorticoids receptors (MR) located in the cytosol, which act as ligand-induced transcription factors. However, activated MR can also elicit additional non-genomic effects. It has been recently proposed that certain non-genomic effects of Ald were due to the activation of an orphan G protein-coupled receptor 30 (GPR30).7,8 Consistently, we have recently demonstrated that Ald activates GPR30 in adult rat ventricular myocytes, which transactivates the epidermal growth factor receptor (EGFR) and in turn triggers a reactive oxygen species (ROS)- and PI3K/AKT-dependent pathway, leading to the stimulation of NBC.9 Nevertheless, the NBC isoform involved in the stimulatory effect of Ald remains unknown. Thus, in this addendum to that previous study,9 we investigated which was the NBC isoform involved in the Ald/GPR30-induced NBC activation.
Methods
All procedures followed during this investigation conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the experimental protocol was approved by the Animal Welfare Committee of La Plata School of Medicine. Male rats (body weight 300–400 g) were anaesthetized by intra-peritoneal injection of sodium pentobarbital (35 mg/kg body weight) and hearts rapidly excised when plane 3 of phase III of anesthesia was reached.
pHi measurements
pHi was measured in single myocytes with an epi-fluorescence system (Ion Optix, Milton, MA). Myocytes were incubated at room temperature for 10 min with 10 µM BCECF-AM followed by 30 min washout. Dye-loaded cells were placed in a chamber on the stage of an inverted microscope (Nikon.TE 2000-U) and continuously superfused with a solution containing (mM) 5 KCl, 118 NaCl, 1.2 MgSO4, 0.8 Cl2Mg, 1.35 Cl2Ca, 10 glucose, 20 NaHCO3, pH 7.4 after continuous bubbling with 5% CO2 and 95% O2. The myocytes were stimulated via 2-platinum electrodes on either side of the bath at 0.5 Hz. Dual excitation (440 and 495 nm) was provided by a 75-watt Xenon arc lamp and transmitted to the myocytes. Emitted fluorescence was collected with a photomultiplier tube equipped with a band-pass filter centered at 535 nm. The 495-to-440 nm fluorescence ratio was digitized at 10 kHz (ION WIZARD fluorescence analysis software). At the end of each experiment, the fluorescence ratio was converted to pH by in vivo calibrations using the high K+-nigericin method.10
Ammonium pulse
As described above, the experiments were performed in HCO3− buffered solution. Under these conditions, both alkalizing pHi regulatory systems are operative, the Na+/H+ exchanger (NHE1) and NBC. Thus, all the experiments were performed in the presence of the NHE1 inhibitor HOE642 (10 µM) in order to examine the NBC activity in isolation. The total NBC activity was assessed by evaluating the pHi recovery from a double ammonium pre-pulse (the first was the control of the second pulse). The dpHi/dt at each pHi, obtained from an exponential fit of the recovery phase, was analyzed to calculate the net HCO3− influx (JHCO3-), then JHCO3-=βtot dpHi/dt, where βtot is total intracellular buffering capacity. βtot was calculated by the sum of the intracellular buffering due to CO2 (βCO2) plus the intrinsic buffering capacity (βi). βCO2 was calculated as, βCO2=2.3 [HCO3−]i, where [HCO3−]i = [ HCO3−]o 10 pHi-pH.11,12 βi of the myocytes was measured by exposing cells to varying concentrations of NH4Cl in Na+-free HEPES bathing solution. pHi was allowed to stabilize in Na+-free solution before application of NH4Cl. βi was calculated from the equation βi = Δ[NH4+]i/ΔpHi and referred to the mid-point values of the measured changes in pHi. βi at different levels of pHi were estimated from the least squares regression lines βi vs. pHi plots.
Potassium pulse
To investigate the NBCe1 activity in isolation we performed a potassium pulse as previously described.2 Increasing isotonically extracellular K+ [K+]o from 5 to 45 mM produced a depolarization of approximately 60 mV that enhanced the electrogenic NBC activity and in turn increased pHi. The high K+ was applied for 14 minutes and during this period the pHi was recorded. The data were expressed as increase of pHi units in comparison to the zero time point in high K+ solution. The HCO3−-buffered solution used in the K+-induced depolarization experiments contained (mM): 118 NaCl, 5 KCl, 1 MgSO4, 0.35 NaH2PO4, 10 glucose, 40 choline chloride, 20 NaHCO3, pH 7.4 after continuous bubbling with 5% CO2 and 95% O2. K+-induced depolarization was assessed by replacing 40 mM choline chloride with 40 mM KCl, maintaining ionic strength.
Immunostaining of cardiac myocytes and analysis by confocal microscopy
Isolated adult rat cardiomyocytes plated on laminin-coated coverslips were fixed with 4% paraformaldehyde in phosphate-buffered saline solution, permeabilised with 0.1% Triton X-100 and blocked with 2 % bovine serum albumin. Myocytes were then incubated with 1:50 dilution of the primary antibody followed by incubation with 1:200 dilution of secondary antibodies coupled to Alexa Fluor 488 donkey anti-rabbit (Invitrogen). In control experiments, samples were incubated with primary or secondary antibody alone. Coverslips were mounted on slides with fluorescent mounting medium (ProLong Gold Antifade, Invitrogen). Images were acquired using an inverted laser scanning microscope (Olympus Bx61). Images were collected with an oil immersion ×60 1.4 objective (numerical aperture 0.2, plan Apochromat, Zoom 1.5×). Excitation/emission wavelengths were 488/nm (argon laser)/500–600 nm.
Statistics
Data were expressed as means ± SEM and were compared with Students's t test or One-way ANOVA followed by Student-Newman-Keuls post-hoc test. A value of P < 0.05 was considered statistically significant (2-tailed test).
Results
NBCe1 is the NBC isoform stimulated by Ald and GPR30 activation
We have previously demonstrated that Ald stimulates NBC in adult rat ventricular myocytes, accelerating pHi recovery during acidosis,9 as also shown herein in Figure 1A. We performed a double ammonium pulse in HCO3− buffered solution in the presence of the blocker of the NHE1 HOE642 (10 µM). Ald (10 nM) was applied to the extracellular solution 10 min before the second pulse. As observed in the representative recordings (Fig. 1A upper panel), the average HCO3− influx (JHCO3-) (Fig. 1A lower panel) or the percentage effect of Ald on JHCO3- corrected by the normal attenuation of the recovery rate of the second pulse9 (Fig. 1C), Ald was able to enhance NBC mediated pHi recovery from acidosis in a clear significant manner. In order to study if the effect of Ald is due to the stimulation of NBCe1, NBCn1 or both isoforms, we next performed a double ammonium pulse in the presence of a specific and inhibitory antibody against NBCe1 isoform, called a-L3.2 Figure 1B–C illustrates that the pre-incubation of the myocytes with a-L3 (dilution 1/500) prevented the stimulatory effect of Ald during the pHi recovery, suggesting that Ald has no effect on NBCn1 and exclusively stimulates the NBCe1 isoform.
Figure 1.

Aldosterone stimulates the electrogenic NBC isoform, NBCe1. (A) upper panel: Representative traces of intracellular pH (pHi) during the application of 2 consecutive ammonium pulses (20 mM NH4Cl), in the absence (first pulse) and presence of 10 nM aldosterone (Ald; second pulse). A, lower panel: Average bicarbonate influx (JHCO3-), carried by the NBC, before and after application of 10 nM Ald. * indicates P < 0.05 vs. control. (B) upper and lower panels: Same as panel A but in the continuous presence of an inhibitory antibody of NBCe1; a-L3 (1/500). a-L3 was applied 10 minutes before the first pulse and maintained throughout the experiment. (C) Average JHCO3- obtained at pHi of 6.8 expressed as the percentage of increase in the JHCO3- during the second pulse in comparison to the first pulse (% of ΔJHCO3-); n values are shown inside bars. * indicates P<0.05 vs. control.
In another set of experiments, isolated myocytes were exposed to an isosmotic high extracellular K+ solution. This hyperkalemic solution induced a depolarization of the membrane potential which selectively stimulated NBCe1 and therefore resulted in cellular alkalization. Figure 2A shows representative traces of continuous pHi recordings of myocytes exposed to a-L3 (1/500), Ald (10 nM), Ald and G15 (1 µM; antagonist of GPR30) or G1 (1 µM; agonist of GRP30). As observed in the average results (Fig. 2B), Ald increased NBCe1 activity, which was abrogated by the addition of G15. Furthermore, G1 significantly stimulates NBCe1 activity. These results confirm that Ald specifically stimulates NBCe1 isoform through GPR30. As expected, a-L3 canceled the alkalization produced by the membrane depolarization, indicating that the increase in pHi was due to the stimulation of NBCe1 and confirming that this strategy is useful to study the function of this NBC isoform in isolation.
Figure 2.
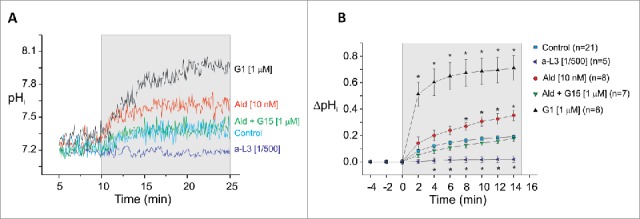
NBCe1 activity is mediated by GPR30. (A) Representative traces of pHi recorded from isolated ventricular myocytes during exposure to the potassium pulse in the presence of GPR30 agonist (G1, 1 μM), Ald (10 nM), Ald with the GPR30 antagonist (G15, 1 μM) or in the presence of the inhibitory antibody of NBCe1; a-L3 (1/500). (B) Average data of the time course of the effect of pHi alkalization induced by the hyperkalemic-induced depolarization of membrane potential in control and in the presence of 10 nM Ald, Ald plus the GPR30 blocker G15 (1 μM), G1 (1 μM) or in the presence of a-L3 (1/500). Data are expressed as an increase of pHi units in comparison to the zero time point in high K+ solution. n values are shown between brackets. * indicates P < 0.05 vs. control.
Co-localization of NBCe1 and GPR30
The spatial distribution of NBCe1 and GPR30 was studied with immunofluorescence and confocal microscopy. Fixed and permeabilised isolated rat ventricular myocytes were incubated with antibodies against the cytoplasmatic domain of NBCe1 (Millipore) or against GPR30 (Abcam) coupled to anti-rabbit Alexa Fluor 488 and confocally imaged. The immunostaining for both proteins showed strong transverse striated pattern (Fig. 3A–B). The normalized longitudinal line-scans (right of panels A-B) displayed oscillatory patterns for NBCe1 and GPR30 with an average in-phase periodicity of ∼1.7 μm (Fig. 3C) (Auto TT analysis program13). These results strongly suggest a co-localization of NBCe1 and GPR30 in the t-tubules. No labeling was detected in control experiments in which primary or secondary antibodies were omitted (not shown). Figure 3D shows the specificity of NBCe1 and GPR30 antibodies by Western blot of homogenates of rat ventricular myocytes. NBCe1 antibody showed a band at approximately 120 kDa as previously described.2 On the other hand, GPR30 antibody recognized a predicted major band at ∼42 kDa.14
Figure 3.
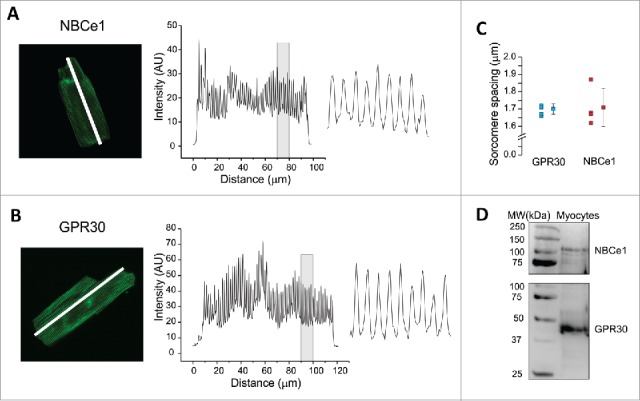
NBCe1 and GPR30 are co-localized in t-tubules. Confocal images of rat ventricular myocytes stained with the antibody against NBCe1 (A) or the antibody against GPR30 (B). Fluorescence intensity profiles were normalized to the peak (arbitrary units, AU) along the longitudinal white line depicted in the images. A fraction of each graph has been expanded on the right for better visualization. (C) Individual and average values of the fluorescent peak spacing, showing no differences in NBCe1 and GPR30 pattern. (D) Typical immunoblots of homogenates of rat ventricular cardiomyocytes showing bands of 120 kDa and 42 kDa, corresponding to NBCe1 and GPR30, respectively.
Discussion
In our previous study we found that Ald induced stimulation of cardiac NBC via activation of the novel receptor GPR30.9 Herein, we have identified that the NBC isoform implicated in such effect is NBCe1. Using specific NBCe1 inhibitory antibodies we demonstrated that Ald does not affect NBCn1 activity. Moreover, Ald was able to increase NBCe1 activity recorded in isolation with the potassium pulse. It was recently reported that NBC is localized in t-tubules while NHE1 is only confined to the intercalated disks.1 Using immunofluorescence and confocal microscopy analysis we show in this work that both NBCe1 and GPR30 are localized in t-tubules.
It has been reported that the activation of GPR30 is cardioprotective. The administration of G1 reduced the infarct size in ischemia-reperfusion,15,16 diminished left ventricular wall thickness and myocyte hypertrophy,17 attenuated heart failure18 and induced a decrease in perivascular fibrosis.19 Moreover, the activation of GPR30 inhibited angiotensin II-induced hypertrophy in H9c2 cardiomyocytes.20
The NBC is responsible for 30 % of Na+ influx into the cells during the recovery from acidosis, being the other 70 % due to the NHE1. However, both transporters are equally operative at pH close to basal.4,21-23 The increase in [Na+]i is crucial for cardiac pathophysiology because, as it is well-known, it decreases the driving force of the forward mode (extruding Ca2+ mode) of the Na+/Ca2+ exchanger (NCXf) or even favors the reverse mode of this transporter (NCXr), leading to an increase in [Ca2+]i.24-26 Due to its stoichiometry, the NBCe1 acts as a Na+-sparing transporter, because it needs half amount of Na+ to mediate the influx of the same amount of HCO3− than the NBCn1. Thus, it could be possible to speculate that the activation of NBCe1 through GPR30 results in a decreased Na+ uptake upon defending the cell against intracellular acidosis, explaining at least in part the cardioprotective properties of G1 commented above. Additional experiments are needed to elucidate this interesting possibility.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by grants of the Agencia Nacional de Promoción Científica y Tecnológica de Argentina, ANPCyT (PICT2014 2594 to EAA) and the National Council of Research, CONICET (PIP 00664 to EAA).
References
- [1].Garciarena CD, Ma YL, Swietach P, Huc L, & Vaughan-Jones RD. Sarcolemmal localisation of Na+/H+ exchange and Na+-HCO3- co-transport influences the spatial regulation of intracellular pH in rat ventricular myocytes. J Physiol 2013; 591:2287-306; PMID:23420656; http://dx.doi.org/ 10.1113/jphysiol.2012.249664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].De Giusti VC, Orlowski A, Villa-Abrille MC, de Cingolani GE, Casey JR, Alvarez BV, Aiello EA. Antibodies against the cardiac sodium/bicarbonate co-transporter (NBCe1) as pharmacological tools. British Journal of Pharmacology 2011; 164:1976-89; PMID:21595652; http://dx.doi.org/ 10.1111/j.1476-5381.2011.01496.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Giusti VC, Orlowski A, Aiello EA. Angiotensin II inhibits the electrogenic Na+/HCO3- cotransport of cat cardiac myocytes. J Mol Cell Cardiol 2010; 49:812-8; PMID:20692267; http://dx.doi.org/ 10.1016/j.yjmcc.2010.07.018 [DOI] [PubMed] [Google Scholar]
- [4].De Giusti VC, Garciarena CD, Aiello EA. Role of reactive oxygen species (ROS) in angiotensin II-induced stimulation of the cardiac Na+/HCO3- cotransport. J Mol Cell Cardiol 2009; 47:716-22; PMID:19646989; http://dx.doi.org/ 10.1016/j.yjmcc.2009.07.023 [DOI] [PubMed] [Google Scholar]
- [5].Villa-Abrille MC, Petroff MG, Aiello EA. The electrogenic Na+/HCO3- cotransport modulates resting membrane potential and action potential duration in cat ventricular myocytes. J Physiol 2007; 578:819-29; PMID:17138608; http://dx.doi.org/ 10.1113/jphysiol.2006.120170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aiello EA, Petroff MG, Mattiazzi AR, Cingolani HE. Evidence for an electrogenic Na+-HCO3- symport in rat cardiac myocytes. J Physiol 1998; 512 (Pt 1):137-48; PMID:9729624; http://dx.doi.org/ 10.1111/j.1469-7793.1998.137bf.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. American Journal of Physiology. Cell Physiol 2013; 304:C532-540; PMID:23283935; http://dx.doi.org/ 10.1152/ajpcell.00203.2012 [DOI] [PubMed] [Google Scholar]
- [8].Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension 2011; 57:442-51; PMID:21242460; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.110.161653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].De Giusti VC, Orlowski A, Ciancio MC, Espejo MS, Gonano LA, Caldiz CI, Vila Petroff MG, Villa-Abrille MC, Aiello EA. Aldosterone stimulates the cardiac sodium/bicarbonate cotransporter via activation of the g protein-coupled receptor gpr30. J Mol Cell Cardiol 2015; 89:260-7; PMID:26497404; http://dx.doi.org/ 10.1016/j.yjmcc.2015.10.024 [DOI] [PubMed] [Google Scholar]
- [10].Perez NG, Alvarez BV, Camilion de Hurtado MC, Cingolani HE. pHi regulation in myocardium of the spontaneously hypertensive rat. Compensated enhanced activity of the Na+-H+ exchanger. Circ Res 1995; 77:1192-1200; PMID:7586232; http://dx.doi.org/ 10.1161/01.RES.77.6.1192 [DOI] [PubMed] [Google Scholar]
- [11].Roos A, Boron WF. Intracellular pH. Physiol Rev 1981; 61:296-434; PMID:7012859 [DOI] [PubMed] [Google Scholar]
- [12].Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol 1999; 517 (Pt 1):159-80; PMID:10226157; http://dx.doi.org/ 10.1111/j.1469-7793.1999.0159z.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guo A, Song LS. AutoTT: automated detection and analysis of T-tubule architecture in cardiomyocytes. Biophys J 2014; 106:2729-36; PMID:24940790; http://dx.doi.org/ 10.1016/j.bpj.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Du GQ, Zhou L, Chen XY, Wan XP, He YY. The G protein-coupled receptor GPR30 mediates the proliferative and invasive effects induced by hydroxytamoxifen in endometrial cancer cells. Biochem Biophys Res Commun 2012; 420:343-9; PMID:22425989; http://dx.doi.org/ 10.1016/j.bbrc.2012.02.161 [DOI] [PubMed] [Google Scholar]
- [15].Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2010; 298:H16-23; PMID:19880667; http://dx.doi.org/ 10.1152/ajpheart.00588.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 2009; 297:H1806-1813; PMID:19717735; http://dx.doi.org/ 10.1152/ajpheart.00283.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PloS One 2010; 5:e15433; PMID:21082029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kang S, Liu Y, Sun D, Zhou C, Liu A, Xu C, Hao Y, Li D, Yan C, Sun H. Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PloS One 2012; 7:e48185; PMID:23110207; http://dx.doi.org/ 10.1371/journal.pone.0048185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lenhart PM, Broselid S, Barrick CJ, Leeb-Lundberg LM, Caron KM. G-protein-coupled receptor 30 interacts with receptor activity-modifying protein 3 and confers sex-dependent cardioprotection. J Mol Endocrinol 2013; 51:191-202; PMID:23674134; http://dx.doi.org/ 10.1530/JME-13-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovascular Res 2012; 94:96-104; PMID:22328091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Le Prigent K, Lagadic-Gossmann D, Mongodin E, Feuvray D. HCO3–dependent alkalinizing transporter in adult rat ventricular myocytes: characterization and modulation. Am J Physiol 1997; 273:H2596-2603; PMID:9435592 [DOI] [PubMed] [Google Scholar]
- [22].Vaughan-Jones RD, Villafuerte FC, Swietach P, Yamamoto T, Rossini A, Spitzer KW. pH-Regulated Na+ influx into the mammalian ventricular myocyte: the relative role of Na+-H+ exchange and Na+-HCO3- Co-transport. J Cardiovascular Electrophysiol 2006; 17 Suppl 1:S134-40; PMID:16686668; http://dx.doi.org/ 10.1111/j.1540-8167.2006.00394.x [DOI] [PubMed] [Google Scholar]
- [23].Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol 2009; 46:318-31; PMID:19041875; http://dx.doi.org/ 10.1016/j.yjmcc.2008.10.024 [DOI] [PubMed] [Google Scholar]
- [24].Rothstein EC, Byron KL, Reed RE, Fliegel L, Lucchesi PA. H2O2-induced Ca2+ overload in NRVM involves ERK1/2 MAP kinases: role for an NHE-1-dependent pathway. Am J Physiol Heart Circ Physiol 2002; 283:H598-605; PMID:12124207; http://dx.doi.org/ 10.1152/ajpheart.00198.2002 [DOI] [PubMed] [Google Scholar]
- [25].Bril A. [Ion transporters and cardiovascular diseases: pH control or modulation of intracellular calcium concentration]. Annales de Cardiologie et d'Angeiologie 2003; 52:41-51; PMID:12710294; http://dx.doi.org/ 10.1016/S0003-3928(02)00182-8 [DOI] [PubMed] [Google Scholar]
- [26].Aiello EA, Villa-Abrille MC, Dulce RA, Cingolani HE, Perez NG. Endothelin-1 stimulates the Na+/Ca2+ exchanger reverse mode through intracellular Na+ (Na+i)-dependent and Na+i-independent pathways. Hypertension 2005; 45:288-93; PMID:15611361; http://dx.doi.org/ 10.1161/01.HYP.0000152700.58940.b2 [DOI] [PubMed] [Google Scholar]


