Summary
Electroreception is an ancient vertebrate sense with a fascinating evolutionary history involving multiple losses as well as independent evolution at least twice within teleosts. We review the phylogenetic distribution of electroreception and the morphology and innervation of electroreceptors in different vertebrate groups. We summarise recent work from our laboratory that has confirmed the homology of ampullary electroreceptors in non-teleost jawed vertebrates by showing, in conjunction with previously published work, that these are derived embryonically from lateral line placodes. Finally, we review hypotheses to explain the distribution of electroreception within teleosts, including the hypothesis that teleost ampullary and tuberous electroreceptors evolved via the modification of mechanosensory hair cells in lateral line neuromasts. We conclude that further experimental work on teleost electroreceptor development is needed to test such hypotheses.
Keywords: electroreception, electroreceptors, ampullary, tuberous, neuromast, hair cell, lateral line
The discovery of electroreception in weakly electric teleosts
The existence of strongly electric fishes, which use modified muscle cells in an “electric organ” to generate electric shocks for defence and/or to stun prey, has been known for centuries (Zupanc and Bullock, 2005): they include electric rays (over 60 species, including the genus Torpedo, in the batoid group of cartilaginous fishes), electric catfishes (the family Malapteruridae, in the siluriform teleost group of ray-finned bony fishes) and the electric eel (Electrophorus electricus, a gymnotiform teleost). In contrast, it is only 60 years since Lissman’s discovery that the mormyriform teleost Gymnarchus niloticus (the aba, or African knifefish) is weakly electric, i.e., uses a muscle-derived electric organ to generate a weak electric field, undetectable to us without amplification (Lissmann, 1951). The same paper also noted that the fish is sensitive to changes in the local electric field (Lissmann, 1951). Lissmann later described both electric organ discharges and electrolocation - the use of local distortions in the electric field to locate and identify objects - in G. niloticus as well as in other mormyriform and gymnotiform teleost species (Lissmann, 1958; Lissmann and Machin, 1958). His seminal work identified a previously unrecognised vertebrate sense: electroreception.
Electric organs have evolved independently multiple times within teleosts (Alves-Gomes, 2001; Kawasaki, 2009; Lavoué et al., 2012). Mormyriform and gymnotiform teleosts (Sullivan et al., 2000; Alves-Gomes, 2001; Lavoué and Sullivan, 2004; Kawasaki, 2009; Lavoué et al., 2012) are now known to use both passive electroreception (perception of low-frequency environmental electric fields) and active electroreception (perception of distortions in high-frequency self-generated electric fields) for electrolocation (von der Emde, 1999; Alves-Gomes, 2001; Caputi and Budelli, 2006; von der Emde, 2006). They also use high-frequency electroreception for social communication, including mate recognition and selection, by detecting the electric organ discharges of other fish (Feulner et al., 2009; Kawasaki, 2009).
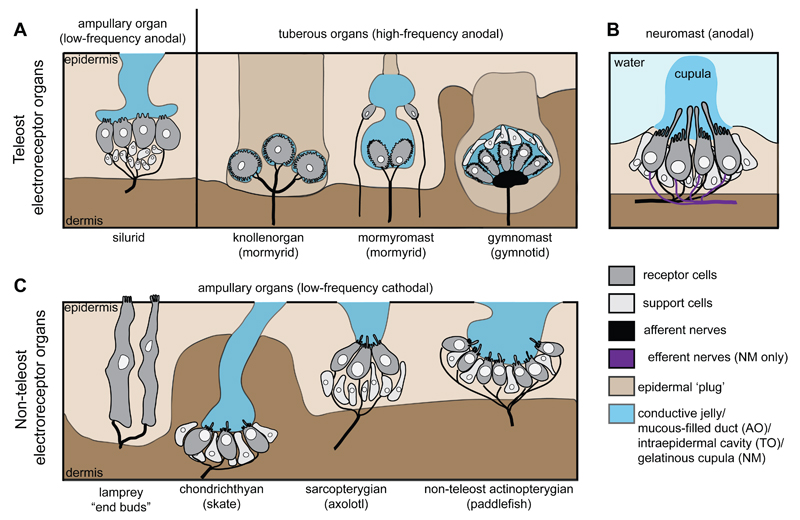
Two distinct types of electroreceptor organs mediate electroreception in both groups of weakly electric teleosts (Fig. 1A) (Gibbs, 2004; Jørgensen, 2005). “Ampullary” organs detect low-frequency environmental electric fields (passive electroreception); they comprise relatively few electroreceptor cells (generally with short, sparse apical microvilli) in epithelia at the base of mucous-filled ducts, which open to the surface via pores (Gibbs, 2004; Bodznick and Montgomery, 2005; Jørgensen, 2005). “Tuberous” organs of varying morphology detect high-frequency electric fields from electric organ discharges (self-generated and/or from other fish) for active electroreception; they lack ducts and are “plugged” by loosely packed epidermal cells, with the electroreceptor cells (which generally have numerous apical microvilli) surrounded by an intraepidermal cavity (Gibbs, 2004; Bodznick and Montgomery, 2005; Jørgensen, 2005; Kawasaki, 2005). Teleost electroreceptors are distributed on both head and trunk, and are part of the lateral line system: depending on their position, they are innervated by anterior (pre-otic) or posterior (post-otic) lateral line nerves, which project centrally to a special “electrosensory lateral line lobe” in the medulla (Bullock et al., 1983; Gibbs, 2004; Bell and Maler, 2005; Bodznick and Montgomery, 2005). The anterior and posterior lateral line nerves also innervate the mechanosensory hair cells of lateral line neuromasts (Fig. 1B), which are distributed in characteristic lines over the head and trunk and detect local water movement (Bleckmann and Zelick, 2009). Neuromast hair cells have a single cilium (kinocilium) flanked by a ‘hair bundle’, i.e., a characteristically stepped array of microvilli (stereocilia) (Gillespie and Müller, 2009). The neurons in pre-otic and post-otic cranial lateral line ganglia that give rise to the anterior and posterior lateral line nerves, respectively, and the neuromasts innervated by these nerves, are derived embryonically from lateral line placodes, i.e., paired patches of thickened neurogenic cranial ectoderm that elongate or migrate in characteristic lines over the head and trunk during embryonic development (Gibbs, 2004; Ghysen and Dambly-Chaudière, 2007; Ma and Raible, 2009; Sarrazin et al., 2010; Aman and Piotrowski, 2011).
Fig. 1.
Schematics illustrating the range of lateral line organ morphologies (not to scale). (A) Teleost ampullary organs (e.g. silurid, based on Northcutt et al., 2000), which respond to low-frequency anodal stimuli, contain electroreceptor cells with short, sparse microvilli, located at the base of mucous-filled ducts that open to the surface. Tuberous organs, which respond to high-frequency anodal stimuli, are morphologically varied but the electroreceptor cells (which have many microvilli) are generally located within an intraepidermal cavity plugged by epidermal cells. Both types of mormyrid tuberous organs (knollenorgan and mormyromast; adapted from Jørgensen, 2005) and a gymnotid tuberous organ (gymnomast; adapted from Cernuda-Cernuda and García-Fernández, 1996) are shown. (B) Neuromast receptor cells, which are mechanosensory but can also respond to large anodal stimuli, have a single cilium flanked by a stepped array of microvilli (the “hair bundle”). The cilia and hair bundles of all the receptor cells in the neuromast are encased together in a gelatinous cupula in contact with water. Unlike electroreceptors, which only receive afferent innervation, neuromast hair cells receive both afferent and efferent innervation. (C) Examples of non-teleost electroreceptor organs, which all respond to low-frequency cathodal stimuli: lamprey "end buds" containing multiple electroreceptor cells, each with multiple microvilli but no cilia (adapted from Jørgensen, 2005), and chondrichthyan (e.g. skate), sarcopterygian (e.g. axolotl) and non-teleost actinopterygian (e.g. paddlefish) ampullary organs, whose electroreceptor cells generally have a single cilium and variable numbers of microvilli. AO, ampullary organ; NM, neuromast; TO, tuberous organ.
Electroreception is phylogenetically widespread amongst living vertebrates
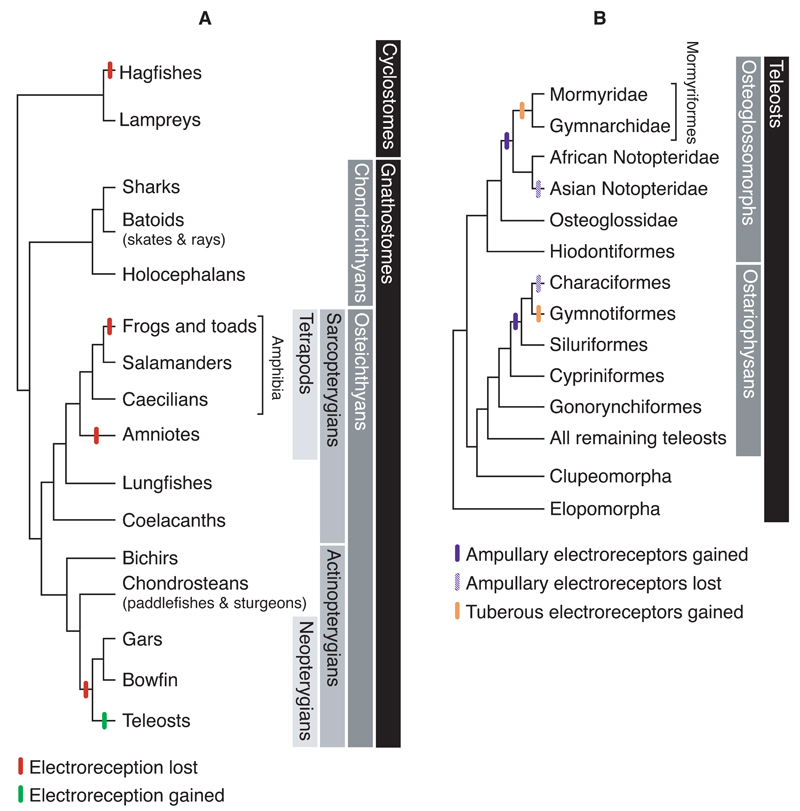
After electroreception was discovered in weakly electric teleosts, it was found to be phylogenetically widespread amongst living vertebrates (Fig. 2A) (Bullock et al., 1983; New, 1997; Northcutt, 1997; Schlosser, 2002). Within the cyclostomes, i.e., the only surviving jawless fishes (which recent molecular analyses have confirmed to be a monophyletic group, sister to the living jawed vertebrates, e.g. Delsuc et al., 2006; Mallatt and Winchell, 2007; Heimberg et al., 2010), there is no evidence for electroreception in hagfishes (Bullock et al., 1983; Braun and Northcutt, 1997). However, many ancestral characters have been lost within the hagfish lineage (e.g. Wicht and Northcutt, 1995; Ota et al., 2011). The lateral line system of eptatretid hagfish (Kishida et al., 1987; Wicht and Northcutt, 1995; Braun and Northcutt, 1997) has been characterised as secondarily simplified (Braun and Northcutt, 1997), while myxinoid hagfishes have lost the lateral line system altogether (Braun and Northcutt, 1997). In contrast, lampreys have mechanosensory lateral line neuromasts, which were recently shown to be functional at larval stages (Gelman et al., 2007), as well as epidermal “end bud” electroreceptor organs (Fig. 1C) on both head and trunk, containing up to 30 receptor cells, each with 80-90 apical microvilli (Bodznick and Northcutt, 1981; Jørgensen, 2005). Lamprey end buds respond to weak cathodal stimuli, i.e., negative potential relative to the interior of the animal (Bodznick and Preston, 1983), and are innervated by the anterior lateral line nerve (a recurrent branch of which innervates the end buds on the trunk), which projects to a dorsal octavolateral nucleus in the medulla (Bodznick and Northcutt, 1981; Bodznick and Preston, 1983; Ronan and Bodznick, 1986).
Fig. 2.
The phylogenetic distribution of electroreception among (A) vertebrates and (B) teleost fishes. Neopterygian and teleost phylogenies drawn after Near et al. (2012). (A) The distribution of electroreception among the vertebrates reveals it to be an ancient sense that was lost independently (red bar) in various lineages, including the lineage leading to neopterygian fishes (gars, bowfin and teleosts). Electroreception subsequently evolved independently within the teleosts (green bar). (B) The distribution of electroreception among teleost fishes suggests that ampullary electroreceptors (blue bar) evolved independently twice: once in the Osteoglossomorpha, along the lineage leading to the notopterids and mormyriforms (with subsequent loss in Asian notopterids); and once in the Ostariophysi, along the lineage leading to the siluriforms, gymnotiforms and characiforms (Near et al., 2012) (with subsequent loss in characiforms). Electric organs and tuberous electroreceptors (brown bar) subsequently evolved independently in the mormyriforms within the Osteoglossomorpha, and in the gymnotiforms within the Ostariophysi. Alternative hypotheses are discussed in the text.
Within the jawed vertebrates (gnathostomes), electrosensory “ampullary organs” are found in all cartilaginous fishes (chondrichthyans), i.e., sharks, batoids (rays, skates) and holocephalans, and in some lineages of non-teleost bony fishes (osteichthyans), both in the lobe-finned (sarcopterygian) clade - coelacanths, lungfishes, salamanders, caecilians; and in the ray-finned (actinopterygian) clade - bichirs, paddlefishes and sturgeons (Bullock et al., 1983; Northcutt and Bemis, 1993; New, 1997; Northcutt, 1997; Schlosser, 2002). Ampullary organs are so called because of their flask-like morphology (Fig. 1C), with a sensory epithelium at the base of an electrically conductive jelly-filled duct that opens to the surface via a pore (Jørgensen, 2005). The sensory epithelium contains supporting cells and electroreceptors with an apical kinocilium and variable numbers of apical microvilli (Jørgensen, 2005). Given their morphology, ampullary electroreceptors are sometimes described as modified hair cells, although they lack the hair bundle of stepped microvilli characteristic of mechanosensory hair cells (Gillespie and Müller, 2009).
Like lamprey end buds, non-teleost ampullary electroreceptors are excited by weak cathodal stimuli, which are thought to open voltage-gated Ca2+ channels in the apical membrane (Teeter et al., 1980; Münz et al., 1984; Lu and Fishman, 1995; Bodznick and Montgomery, 2005), and they are innervated by the anterior lateral line nerve, which projects to a dorsal octavolateral nucleus in the medulla (Bullock et al., 1983; Bell and Maler, 2005). In all non-teleost jawed vertebrates except lungfishes, ampullary organs are confined to the head; trunk ampullary organs in lungfishes, like trunk end buds in lampreys, are nevertheless innervated by a recurrent branch of the anterior lateral line nerve (Northcutt, 1986). Although lamprey end buds and non-teleost jawed vertebrate ampullary organs are morphologically different, their similarities - response to cathodal stimuli; innervation by the anterior lateral line nerve projecting to a dorsal octavolateral nucleus in the medulla - are so striking that they have long been assumed to be homologous, i.e., to have been inherited from the common ancestor of lampreys and jawed vertebrates (Bullock et al., 1983). [Note: Although monotreme mammals (Pettigrew, 1999) and dolphins (Czech-Damal et al., 2012) independently evolved electroreception via modified trigeminal nerve endings in the snout, this is entirely separate from ancestral lateral line-mediated electroreception, which was lost (together with the entire lateral line system) in the amniote ancestor. The trigeminal electroreceptive system will not be considered further here.]
Non-teleost ampullary organs develop from lateral line placodes
A key test of the hypothesis that all non-teleost electroreceptors are homologous is to show experimentally that these organs share a common embryonic origin. Unfortunately, the embryonic origin of lamprey electroreceptors is currently unknown. In larval lampreys (ammocoetes; about 70 days post-fertilisation; Richardson and Wright, 2003), the mechanosensory lateral line system is functional (Gelman et al., 2007) and the larvae respond to weak cathodal electric fields (Ronan, 1988). However, the end bud organs found in adult lampreys are not present in larval lampreys and newly metamorphosed adults: instead, the electroreceptors at these stages are thought to be cells with multiple microvilli (“microvillous cells”) found scattered in the epidermis of the branchial region and tail, which closely resemble the electroreceptor cells found in adult end buds (Whitear and Lane, 1983; Ronan, 1988; Jørgensen, 2005) and which seem to be innervated by lateral line nerves (Steven, 1951). As far as we are aware, neither neuromasts nor electroreceptors have been described during embryonic stages in the lamprey, although preliminary data from vital dye staining with FM 1-43, a fluorescent styryl dye taken up by mechanosensory hair cells (Nishikawa and Sasaki, 1996), suggest that neuromasts may be present by 20 days post-fertilisation in the sea lamprey, Petromyzon marinus (M.S.M., unpublished data). Experimental investigation of the embryonic origin of lamprey electroreceptors is needed to test further the hypothesis that all non-teleost ampullary electroreceptors are homologous. However, in conjunction with previously published work (Northcutt et al., 1995), we were recently able to confirm the homology of ampullary organs in all non-teleost jawed vertebrates, by showing that lateral line placodes give rise to ampullary organs in representatives of both the lobe-finned and ray-finned bony fish clades (Northcutt et al., 1995; Modrell et al., 2011a) and the cartilaginous fish clade (Gillis et al., 2012).
The first experimental data on the embryonic origin of non-teleost ampullary organs came from ablation and fate-mapping studies (performed by grafting tissue from pigmented wild-type embryos to albino host embryos) undertaken more than 15 years ago in a salamander, the Mexican axolotl Ambystoma mexicanum (a tetrapod, i.e., a derivative of the lobe-finned bony fish lineage) (Northcutt et al., 1995). This work built on an earlier descriptive study of axolotl lateral line organ development, which suggested that neuromasts differentiate within the central ridge of a given elongating lateral line primordium, and that ampullary organs differentiate later, from the flanks of the same elongating primordium (Northcutt et al., 1994). Before elongating, the lateral line placode also gives rise to the neurons that will innervate the neuromasts and ampullary organs arising from that placode (Northcutt et al., 1994). The subsequent experimental study demonstrated conclusively that individual lateral line placodes give rise to both ampullary organs and neuromasts in the axolotl (Northcutt et al., 1995).
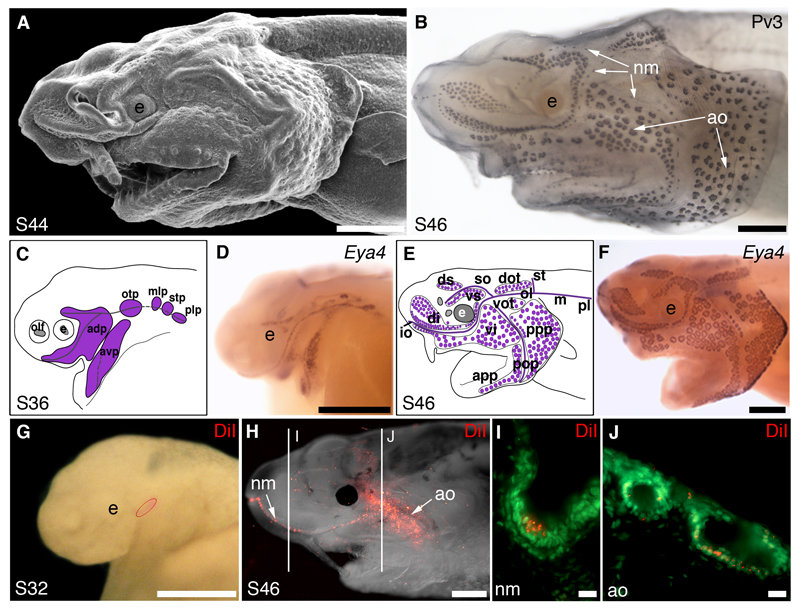
More recently, we investigated lateral line placode development in embryos of a basal ray-finned fish, the North American (Mississippi) paddlefish, Polyodon spathula (Fig. 3A; Modrell et al., 2011a). We had previously shown that Sox3, which encodes a member of the SoxB1 family of HMG domain transcription factors that is expressed in lateral line placodes and elongating lateral line primordia in the frog Xenopus (Schlosser and Ahrens, 2004), is also expressed in paddlefish lateral line placodes, neuromasts and ampullary organs (Modrell et al., 2011b). We found that parvalbumin-3 (Pv3), a Ca2+-binding protein that is thought to be the major Ca2+ buffer in mechanosensory hair cells of the inner ear and lateral line (Heller et al., 2002), is expressed in paddlefish electroreceptors as well as neuromast hair cells (Fig. 3B; Modrell et al., 2011a). We later found that Pv3 is also expressed in electroreceptors and neuromast hair cells in the axolotl (Modrell and Baker, 2012). The transcription co-factor gene Eya4, which we had previously shown to be specifically expressed in lateral line (and otic) placodes, neuromasts and ampullary organs in a shark, Scyliorhinus canicula (O'Neill et al., 2007), similarly proved to be expressed in lateral line (and otic) placodes, neuromasts and ampullary organs in the paddlefish (Fig. 3C-F; Modrell et al., 2011a). We later found similar expression of Eya4 in the axolotl (Modrell and Baker, 2012). Other Eya family genes, as well as Six1/2 and Six4/5 family transcription factor genes, were also expressed in multiple neurogenic placodes in paddlefish (including lateral line placodes), as well as in neuromasts and ampullary organs (Modrell et al., 2011a).
Fig. 3.
Lateral line placodes give rise to ampullary organs and neuromasts in a basal ray-finned bony fish, the North American (Mississippi) paddlefish, Polyodon spathula. Lateral views, anterior to the left, unless otherwise noted; staging according to Bemis and Grande (1992). All panels were previously published in Modrell et al. (2011a) and are reproduced here in accordance with the terms of the authors’ Licence to Publish agreement with Nature Publishing Group. (A) Scanning electron micrograph of a stage 44 embryo showing differentiated ampullary organ fields, particularly on the operculum. (B) Stage 46 embryo immunostained for the Ca2+-binding protein parvalbumin-3 (Pv3), which is strongly expressed in the sensory receptor cells of both neuromasts and ampullary organs (also see Modrell et al., 2011a). (C-F) Schematic diagrams and whole-mount in situ hybridisation for the transcription co-factor gene Eya4 at (C,D) stage 36, when Eya4 is expressed in developing neuromast canal lines and the ampullary organ fields flanking those lines (purple in C) and (E,F) stage 46, when Eya4 expression is maintained in both neuromasts and ampullary organs (purple in E). (G) Stage 32 embryo immediately following a focal DiI injection into the anterodorsal lateral line placode (injection site outlined in red). (H) The same embryo as in G, at stage 46. DiI-labelled cells are visible both in a neuromast canal line and ampullary organ fields. Lines indicate the plane of transverse sections showing DiI-labelled cells (red) in (I) a neuromast and (J) ampullary organs, both counterstained with the nuclear marker Sytox Green (green). Abbreviations: adp, anterodorsal placode; ao, ampullary organ; app, anterior preopercular ampullary field; avp, anteroventral placode; dot, dorsal otic ampullary field; di, dorsal infraorbital ampullary field; ds, dorsal supraorbital ampullary field; e, eye; epi, epibranchial placode region; io, infraorbital lateral line; m, middle lateral line; mlp, middle lateral line placode; ol; otic lateral line; otp, otic lateral line placode; plp, posterior lateral line placode; pll, posterior lateral line; pop, preopercular lateral line; ppp, posterior preopercular field; S, stage; stp, supratemporal placode; so, supraorbital lateral line; st, supratemporal lateral line; vi, ventral infraorbital field; vot, ventral otic field; vs, ventral supraorbital field. Scale bars: (A,B,D,G) 0.5mm, (F,H) 1mm, (I,J) 10μm.
These gene expression data were consistent with a lateral line placode origin for paddlefish ampullary organs and neuromasts. However, gene expression data cannot prove cell lineage, since the same gene could easily be expressed in cells of different lineages. Hence, we used focal injections of the vital lipophilic dye DiI to label individual lateral line placodes in paddlefish embryos (Fig. 3G; Modrell et al., 2011a). At later stages, DiI could be detected in ampullary organs, as well as in neuromasts and lateral line ganglia (Fig. 3H-J; Modrell et al., 2011a). Taken together with the previously published experimental data on the lateral line placode origin of ampullary organs in the axolotl (Northcutt et al., 1995), this work confirmed that ampullary organs are primitively lateral line placode-derived in bony fishes (Modrell et al., 2011a).
As described above, the homology of ampullary organs in bony and cartilaginous fishes is supported by several lines of evidence, primarily their response to cathodal stimuli and innervation by the anterior lateral line nerve projecting to a dorsal octavolateral nucleus in the medulla (to which we could also add expression of Eya4: O'Neill et al., 2007; Modrell et al., 2011a; Modrell and Baker, 2012). However, a descriptive study in the shark, S. canicula, had cast doubt on this assumed homology by suggesting that shark ampullary organs arise from neural crest cells (Freitas et al., 2006). Neural crest cells originate at the border of the neural plate, like neurogenic placodes, but they are a distinct cell population (see e.g. Schlosser, 2008). The proposed neural crest origin for shark electroreceptors (Freitas et al., 2006) was based on expression of the SoxE gene family member Sox8, which is not neural crest-specific, and cross-reaction with the HNK1 antibody, which recognises migrating neural crest cells (and other cell types) in some, but not all vertebrates (and which does not cross-react with neural crest cells in a related shark species, S. torazame; Kuratani and Horigome, 2000).
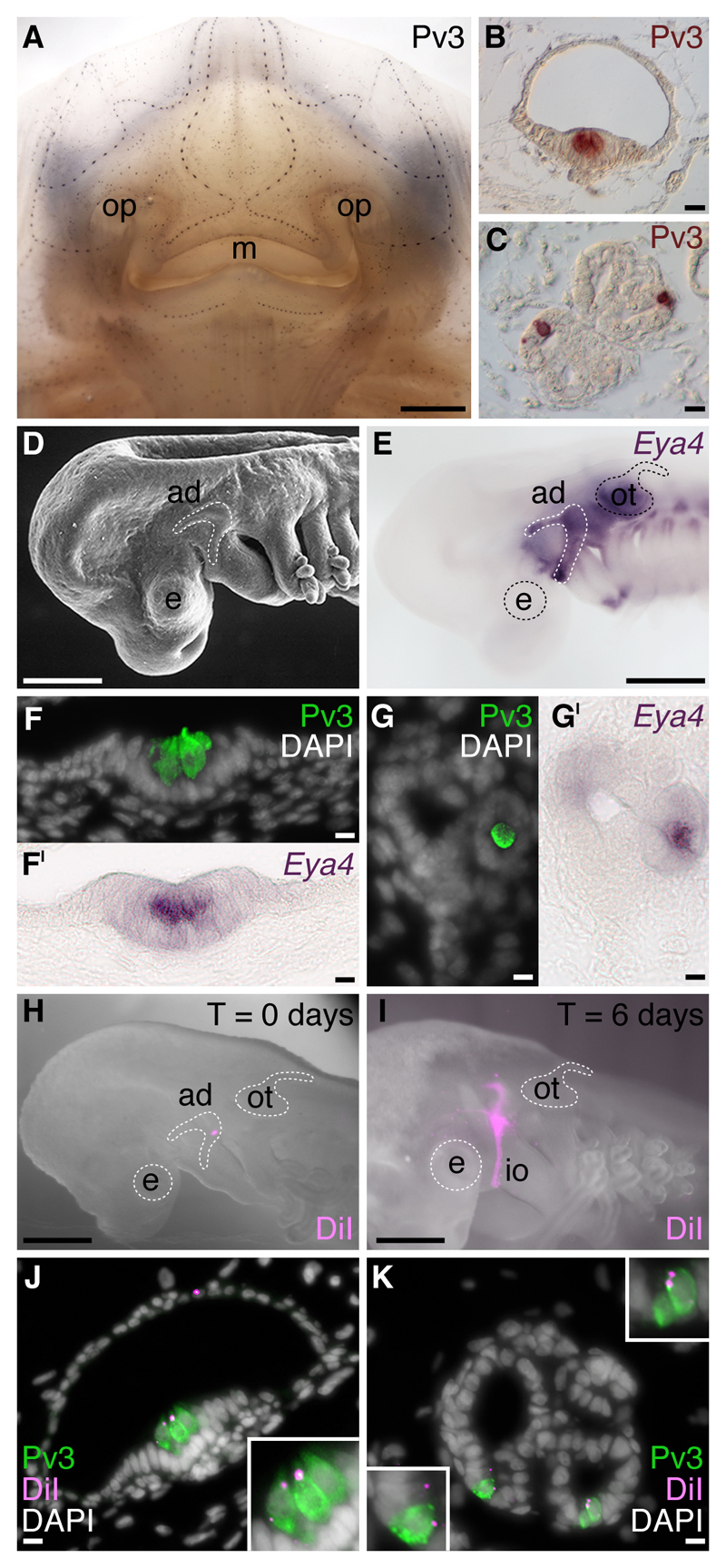
We recently investigated lateral line placode development in another cartilaginous fish, the little skate, Leucoraja erinacea (Fig. 4; Gillis et al., 2012). We found that Pv3 is expressed in skate neuromast hair cells and electroreceptors (Fig. 4A-C; Gillis et al., 2012), just as in paddlefish (Fig. 3B; Modrell et al., 2011a) and axolotl (Modrell and Baker, 2012), suggesting that Pv3 acts as a Ca2+ buffer for electroreceptors and mechanosensory hair cells in all jawed vertebrates. As expected from our previous data in shark (O'Neill et al., 2007), skate lateral line (and otic) placodes expressed Eya4 (Fig. 4D,E; Gillis et al., 2012), while co-labelling with Pv3 at later stages showed that Eya4 was maintained specifically in electroreceptors within ampullary organs, and hair cells within neuromasts (Fig. 4F-G’; Gillis et al., 2012). Crucially, in the first long-term in vivo fate-mapping study reported in any cartilaginous fish, we used the same focal DiI labelling approach as in the paddlefish to show that lateral line placodes give rise to ampullary organs and neuromasts in the skate (Fig. 4H-K; Gillis et al., 2012). Taken together with the previous fate-mapping studies in axolotl (Northcutt et al., 1995) and paddlefish (Modrell et al., 2011a), these data show that lateral line placodes give rise to ampullary organs (and neuromasts) in all jawed vertebrates. Overall, we can infer from these various studies (Northcutt et al., 1995; Modrell et al., 2011a; Modrell and Baker, 2012; Gillis et al., 2012) that the common ancestor of all jawed vertebrates (which a recent study suggests was more shark-like than previously thought; Davis et al., 2012) possessed a lateral line placode-derived system of electrosensory ampullary organs and mechanosensory neuromasts, which expressed Eya4 and most likely used Pv3 as a Ca2+ buffer.
Fig. 4.
Lateral line placodes give rise to ampullary organs and neuromasts in a cartilaginous fish, the little skate, Leucoraja erinacea. All panels except E, H and I were previously published in Gillis et al. (2012) and are reproduced here in accordance with the terms of the authors’ Licence Agreement with the Company of Biologists. (A) Whole-mount immunostaining for the Ca2+-binding protein parvalbumin-3 (Pv3) in an L. erinacea embryo at stage 33 (Maxwell et al., 2008) reveals superficial lines of cephalic mechanosensory neuromasts, as well as clusters of ampullary organs located deeper within the dermis. Immunohistochemical localisation of Pv3 in (B) neuromasts and (C) ampullary organs reveals small clusters of Pv3-positive sensory receptor cells nested among Pv3-negative supporting cells. To test the hypothesis that lateral line placodes give rise to neuromasts and ampullary organs, we fate-mapped the anterodorsal lateral line placode in L. erinacea, which is recognisable (D) as a horseshoe-shaped thickening of cranial ectoderm caudal to the eye and dorsal to the mandibular arch, and (E) by its expression of the transcription co-factor gene Eya4. Eya4 expression is maintained at later stages in the Pv3-positive sensory receptor cells of (F,FI) neuromasts and (G,GI) ampullary organs. (H) Example of an embryo immediately after focal labelling of the anterodorsal lateral line placode with the lipophilic vital dye DiI. (I) After 6 days of incubation, DiI-positive cells were observed migrating away from the placode, in the infraorbital sensory primordium. In embryos with DiI-labelled anterodorsal lateral line placodes, sensory receptor cells, support cells and canal cells of (J) neuromasts and (K) ampullary organs were DiI-positive, indicating their lateral line placodal origin. Abbreviations: ad, anterodorsal lateral line placode; ad, anterodorsal lateral line placode; e, eye; io, infraorbital sensory primordium; m, mouth; op, olfactory pit; ot, otic vesicle. Scale bars: (A) 2.5mm, (B-C) 10μm, (D,E,H) 0.5mm, (I) 0.4mm, (F-G’,J,K) 10μm.
Electroreception evolved independently at least twice within teleosts
Within the jawed vertebrates, electroreception was independently lost in the lineages leading to frogs, amniotes, and the neopterygian fishes, i.e., holosteans (gars, bowfin) and teleosts (Fig. 2A) (Bullock et al., 1983; New, 1997; Northcutt, 1997; Schlosser, 2002). Within teleosts, electroreception has evolved independently at least twice (Fig. 2B) (Bullock et al., 1983; New, 1997; Northcutt, 1997; Sullivan et al., 2000; Alves-Gomes, 2001; Lavoué and Sullivan, 2004; Kawasaki, 2009; Lavoué et al., 2012). Here, we review hypotheses for the evolution of teleost electroreceptors in light of the most recently published phylogeny of the ray-finned fishes (Near et al., 2012).
We consider the most parsimonious interpretation of the distribution of electroreception across teleosts to be that ampullary electroreceptors evolved independently twice, once in the Osteoglossomorpha and once in the Ostariophysi, with subsequent loss in some lineages, and evolution of electric organs and tuberous electroreceptors in a subset of the lineages retaining ampullary electroreceptors (Fig. 2B). On this interpretation, in the Osteoglossomorpha, ampullary electroreceptors evolved along the stem leading to the common ancestor of notopterids and mormyriforms (i.e., mormyrids and gymnarchids), with subsequent loss in the Asian notopterid lineage (Lavoué and Sullivan, 2004; Lavoué et al., 2012). An electric organ and tuberous electroreceptors subsequently evolved along the lineage leading to the mormyriforms. An alternative hypothesis is that ampullary electroreceptors, electric organs and tuberous electroreceptors evolved in mormyriforms, and that ampullary organs evolved independently in the African lineage of Notopteridae (Alves-Gomes, 2001).
Within the Ostariophysi, it has usually been proposed that ampullary electroreceptors evolved along the lineage leading to siluriforms (catfishes) and gymnotiforms, with an electric organ and tuberous electroreceptors subsequently evolving in gymnotiforms (Bullock et al., 1983; New, 1997; Northcutt, 1997; Sullivan et al., 2000; Alves-Gomes, 2001; Lavoué and Sullivan, 2004; Kawasaki, 2009; Lavoué et al., 2012). The most recent ray-finned fish phylogeny supports siluriforms as the sister group to a clade containing both gymnotiforms and characiforms (Near et al., 2012) (though see Lavoué et al., 2012). If this is correct, then ampullary electroreceptors must have been lost in characiforms (also supported by Lavoué et al., 2012). Alternatively, ampullary electroreceptors, electric organs and tuberous electroreceptors may have evolved along the lineage leading to the gymnotiforms, with ampullary organs evolving independently in siluriforms.
Regardless of how many times ampullary electroreceptors evolved within the teleosts, it is clear that they are not homologous with non-teleost ampullary electroreceptors, since teleost ampullary electroreceptors are all excited by anodal stimuli (i.e., those which make the exterior of the animal positive with respect to the interior), rather than cathodal stimuli as in all non-teleosts, and the voltage sensor is the basal membrane, rather than the apical membrane (Bodznick and Montgomery, 2005). It has been proposed that teleost ampullary electroreceptors independently evolved in both Osteoglossomorpha and Ostariophysi via the modification of mechanosensory lateral line neuromast hair cells, which seems plausible given that neurotransmitter release is triggered in mechanosensory hair cells by the opening of voltage-gated Ca2+ channels in the basal membrane (Bullock et al., 1983; Bodznick and Montgomery, 2005). This hypothesis is also supported by the fact that lateral line mechanosensory hair cells, like teleost electroreceptors, are excited by anodal stimuli, although they are 2-3 orders of magnitude less sensitive than electroreceptors (Murray, 1956; Bodznick and Preston, 1983; Bullock et al., 1983; Münz et al., 1984; Tong and Bullock, 1984; Baumann and Roth, 1986; Barry et al., 1988). It is perhaps also suggestive that the ampullary electroreceptors of the notopterid Xenomystus nigri (African knifefish, in the sister group to the mormyriforms; Fig. 2B) have an apical kinocilium as well as microvilli (Jørgensen, 2005). The different types of tuberous electroreceptors, on the other hand, could have evolved independently within the two weakly electric teleost groups (i.e., mormyriforms within the Osteoglossomorpha, and gymnotiforms within the Ostariophysi) either as a specialisation of ampullary electroreceptors, or via a second independent modification of neuromast hair cells.
Currently, there is no experimental evidence to support any of these hypotheses. If teleost electroreceptors (ampullary and/or tuberous) evolved via the modification of neuromast hair cells, then they must be lateral line placode-derived. However, their embryonic origin currently remains unclear (Northcutt, 2005). It has been suggested that ampullary electroreceptors in siluriforms (catfishes), and both ampullary and tuberous electroreceptors in gymnotiforms, are induced to form in local surface ectoderm by lateral line nerves (Vischer et al., 1989; Roth, 2003). However, gymnotiform tuberous electroreceptors can develop in the absence of innervation (Bensouilah and Denizot, 1994; Weisleder et al., 1994; Weisleder et al., 1996). Furthermore, siluriform ampullary electroreceptors initially develop in the lateral zones of lateral line placode-derived sensory primordia, flanking the lines of differentiating neuromasts (Northcutt, 2003), just like lateral line placode-derived ampullary organs in non-teleosts (Northcutt et al., 1995; Modrell et al., 2011a). Similarly, in the gymnotiform Eigenmannia, the first electroreceptor primordia appear on the lateral edges of the neuromast lines, several days after the first appearance of neuromasts (Vischer, 1989), which would also be consistent with origin from the flanks of a lateral line primordium. As noted by Northcutt (2005), apart from the posterior lateral line placode, which migrates down the trunk (see e.g. Haas and Gilmour, 2006), lateral line placodes in teleosts could not be identified before the introduction of molecular markers such as Eya1 (Sahly et al., 1999). Posterior lateral line placode migration and development is being intensively studied in the zebrafish Danio rerio (Ghysen and Dambly-Chaudière, 2007; Ma and Raible, 2009; Sarrazin et al., 2010; Aman and Piotrowski, 2011). This cypriniform species is the standard laboratory model for teleost developmental biology: however, cypriniforms lack electroreceptors (Fig. 2B). Overall, we conclude that hypotheses about teleost electroreceptor evolution cannot be tested until further experimental work, ideally involving in vivo fate-mapping, is undertaken to determine the embryonic origins and molecular characteristics of ampullary and tuberous electroreceptors in representatives of the different electroreceptive teleost groups.
Outlook
The massive reduction in cost of next-generation transcriptome sequencing (“RNA-Seq”; Wang et al., 2009) has transformed molecular approaches to species without a sequenced genome, while the ability to perform targeted mutagenesis using custom-designed transcription activator-like effector nucleases (TALENs; reviewed in Joung and Sander, 2013) seems set to herald a revolution in evolutionary developmental biology. As we move into the seventh decade of research into electroreception, the prospects are very bright for a much deeper understanding of the mechanisms underlying electroreceptor development in multiple vertebrate taxa, and hence for our understanding of electroreceptor evolution.
Acknowledgments
The scanning electron micrograph in Figure 2, previously published in Modrell et al. (2011a), was produced by Glenn Northcutt and Willy Bemis. The scanning electron micrograph in Figure 3, previously published in Gillis et al. (2012), was produced by Glenn Northcutt, Ken Catania and Carl Luer.
Funding
The work of M.S.M. on paddlefish embryos was funded by the Biotechnology and Biological Sciences Research Council (BB/F00818X/1 to C.V.H.B.). The work of J.A.G. on skate embryos was funded by a Royal Society Newton International Fellowship (to J.A.G.), Marine Biological Laboratory Spiegel and Colwin Endowed Summer Research Fellowships (to J.A.G.) and the Biotechnology and Biological Sciences Research Council (BB/F00818X/1 to C.V.H.B.).
References
- Alves-Gomes JA. The evolution of electroreception and bioelectrogenesis in teleost fish: a phylogenetic perspective. J Fish Biol. 2001;58:1489–1511. [Google Scholar]
- Aman A, Piotrowski T. Cell-cell signaling interactions coordinate multiple cell behaviors that drive morphogenesis of the lateral line. Cell Adh Migr. 2011;5:499–508. doi: 10.4161/cam.5.6.19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MA, White RL, Bennett MV. The elasmobranch spiracular organ. II. Physiological studies. J Comp Physiol A. 1988;163:93–98. doi: 10.1007/BF00612000. [DOI] [PubMed] [Google Scholar]
- Baumann M, Roth A. The Ca++ permeability of the apical membrane of neuromast hair cells. J Comp Physiol A. 1986;158:681–688. doi: 10.1007/BF00603825. [DOI] [PubMed] [Google Scholar]
- Bell CC, Maler L. Central neuroanatomy of electrosensory systems in fish. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 68–111. [Google Scholar]
- Bemis WE, Grande L. Early development of the actinopterygian head. I. External development and staging of the paddlefish Polyodon spathula. J Morphol. 1992;213:47–83. doi: 10.1002/jmor.1052130106. [DOI] [PubMed] [Google Scholar]
- Bensouilah M, Denizot JP. Formation of new sensory cells in deafferented tuberous organs of the gymnotid fish Eigenmannia virescens. J Neurosci Res. 1994;39:545–555. doi: 10.1002/jnr.490390506. [DOI] [PubMed] [Google Scholar]
- Bleckmann H, Zelick R. Lateral line system of fish. Integr Zool. 2009;4:13–25. doi: 10.1111/j.1749-4877.2008.00131.x. [DOI] [PubMed] [Google Scholar]
- Bodznick D, Northcutt RG. Electroreception in lampreys: evidence that the earliest vertebrates were electroreceptive. Science. 1981;212:465–467. doi: 10.1126/science.7209544. [DOI] [PubMed] [Google Scholar]
- Bodznick D, Montgomery JC. The physiology of low-frequency electrosensory systems. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 132–153. [Google Scholar]
- Bodznick D, Preston DG. Physiological characterization of electroreceptors in the lampreys Ichthyomyzon unicuspis and Petromyzon marinus . J Comp Physiol A. 1983;152:209–217. [Google Scholar]
- Braun CB, Northcutt RG. The lateral line system of hagfishes (Craniata: Myxinoidea) Acta Zool (Stockh) 1997;78:247–268. [Google Scholar]
- Bullock TH, Bodznick DA, Northcutt RG. The phylogenetic distribution of electroreception: evidence for convergent evolution of a primitive vertebrate sense modality. Brain Res. 1983;287:25–46. doi: 10.1016/0165-0173(83)90003-6. [DOI] [PubMed] [Google Scholar]
- Caputi AA, Budelli R. Peripheral electrosensory imaging by weakly electric fish. J Comp Physiol A. 2006;192:587–600. doi: 10.1007/s00359-006-0100-2. [DOI] [PubMed] [Google Scholar]
- Cernuda-Cernuda R, García-Fernández JM. Structural diversity of the ordinary and specialized lateral line organs. Microsc Res Tech. 1996;34:302–312. doi: 10.1002/(SICI)1097-0029(19960701)34:4<302::AID-JEMT3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Czech-Damal NU, Liebschner A, Miersch L, Klauer G, Hanke FD, Marshall C, Dehnhardt G, Hanke W. Electroreception in the Guiana dolphin (Sotalia guianensis) Proc R Soc B. 2012;279:663–668. doi: 10.1098/rspb.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SP, Finarelli JA, Coates MI. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature. 2012;486:247–250. doi: 10.1038/nature11080. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Feulner PG, Plath M, Engelmann J, Kirschbaum F, Tiedemann R. Electrifying love: electric fish use species-specific discharge for mate recognition. Biol Lett. 2009;5:225–228. doi: 10.1098/rsbl.2008.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Albert JS, Evans DH, Cohn MJ. Developmental origin of shark electrosensory organs. Evol Dev. 2006;8:74–80. doi: 10.1111/j.1525-142X.2006.05076.x. [DOI] [PubMed] [Google Scholar]
- Gelman S, Ayali A, Tytell ED, Cohen AH. Larval lampreys possess a functional lateral line system. J Comp Physiol A. 2007;193:271–277. doi: 10.1007/s00359-006-0183-9. [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudière C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- Gibbs MA. Lateral line receptors: where do they come from developmentally and where is our research going? Brain Behav Evol. 2004;64:163–181. doi: 10.1159/000079745. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Müller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JA, Modrell MS, Northcutt RG, Catania KC, Luer CA, Baker CVH. Electrosensory ampullary organs are derived from lateral line placodes in cartilaginous fishes. Development. 2012;139:3142–3146. doi: 10.1242/dev.084046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–680. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Heimberg AM, Cowper-Sal Lari R, Semon M, Donoghue PC, Peterson KJ. MicroRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Natl Acad Sci USA. 2010;107:19379–19383. doi: 10.1073/pnas.1010350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller S, Bell AM, Denis CS, Choe Y, Hudspeth AJ. Parvalbumin 3 is an abundant Ca2+ buffer in hair cells. J Assoc Res Otolaryngol. 2002;3:488–498. doi: 10.1007/s10162-002-2050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen JM. Morphology of electroreceptive sensory organs. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 47–67. [Google Scholar]
- Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M. Physiology of tuberous electrosensory systems. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 154–194. [Google Scholar]
- Kawasaki M. Evolution of time-coding systems in weakly electric fishes. Zoolog Sci. 2009;26:587–599. doi: 10.2108/zsj.26.587. [DOI] [PubMed] [Google Scholar]
- Kishida R, Goris RC, Nishizawa H, Koyama H, Kadota T, Amemiya F. Primary neurons of the lateral line nerves and their central projections in hagfishes. J Comp Neurol. 1987;264:303–310. doi: 10.1002/cne.902640303. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Horigome N. Developmental morphology of branchiomeric nerves in a cat shark, Scyliorhinus torazame, with special reference to rhombomeres, cephalic mesoderm, and distribution patterns of cephalic crest cells. Zool Sci. 2000;17:893–909. [Google Scholar]
- Lavoué S, Miya M, Arnegard ME, Sullivan JP, Hopkins CD, Nishida M. Comparable ages for the independent origins of electrogenesis in African and South American weakly electric fishes. PLoS One. 2012;7:e36287. doi: 10.1371/journal.pone.0036287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoué S, Sullivan JP. Simultaneous analysis of five molecular markers provides a well-supported phylogenetic hypothesis for the living bony-tongue fishes (Osteoglossomorpha: Teleostei) Mol Phylogenet Evol. 2004;33:171–185. doi: 10.1016/j.ympev.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Lissmann HW. Continuous electrical signals from the tail of a fish, Gymnarchus niloticus Cuv. Nature. 1951;167:201–202. doi: 10.1038/167201a0. [DOI] [PubMed] [Google Scholar]
- Lissmann HW. On the function and evolution of electric organs in fish. J Exp Biol. 1958;35:156–191. [Google Scholar]
- Lissmann HW, Machin KE. The mechanism of object location in Gymnarchus niloticus and similar fish. J Exp Biol. 1958;35:451–486. [Google Scholar]
- Lu J, Fishman HM. Ion channels and transporters in the electroreceptive ampullary epithelium from skates. Biophys J. 1995;69:2467–2475. doi: 10.1016/S0006-3495(95)80117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Raible DW. Signaling pathways regulating zebrafish lateral line development. Curr Biol. 2009;19:R381–6. doi: 10.1016/j.cub.2009.03.057. [DOI] [PubMed] [Google Scholar]
- Mallatt J, Winchell CJ. Ribosomal RNA genes and deuterostome phylogeny revisited: more cyclostomes, elasmobranchs, reptiles, and a brittle star. Mol Phylogenet Evol. 2007;43:1005–1022. doi: 10.1016/j.ympev.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Maxwell EE, Fröbisch NB, Heppleston AC. Variability and conservation in late chondrichthyan development: ontogeny of the winter skate (Leucoraja ocellata) Anat Rec (Hoboken) 2008;291:1079–1087. doi: 10.1002/ar.20719. [DOI] [PubMed] [Google Scholar]
- Modrell MS, Baker CVH. Evolution of electrosensory ampullary organs: conservation of Eya4 expression during lateral line development in jawed vertebrates. Evol Dev. 2012;14:277–285. doi: 10.1111/j.1525-142X.2012.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Bemis WE, Northcutt RG, Davis MC, Baker CVH. Electrosensory ampullary organs are derived from lateral line placodes in bony fishes. Nat Commun. 2011a;2:496. doi: 10.1038/ncomms1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Buckley D, Baker CVH. Molecular analysis of neurogenic placode development in a basal ray-finned fish. Genesis. 2011b;49:278–294. doi: 10.1002/dvg.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz H, Claas B, Fritzsch B. Electroreceptive and mechanoreceptive units in the lateral line of the axolotl Ambystoma mexicanum. J Comp Physiol A. 1984;154:33–44. [Google Scholar]
- Murray RW. The response of the lateralis organs of Xenopus laevis to electrical stimulation by direct current. J Physiol. 1956;134:408–420. doi: 10.1113/jphysiol.1956.sp005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL. Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sc USA. 2012;109:13698–13703. doi: 10.1073/pnas.1206625109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New JG. The evolution of vertebrate electrosensory systems. Brain Behav Evol. 1997;50:244–252. doi: 10.1159/000113338. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Sasaki F. Internalization of styryl dye FM1-43 in the hair cells of lateral line organs in Xenopus larvae. J Histochem Cytochem. 1996;44:733–741. doi: 10.1177/44.7.8675994. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Lungfish neural characters and their bearing on sarcopterygian phylogeny. J Morph. 1986;(Suppl.1):277–297. [Google Scholar]
- Northcutt RG. Evolution of gnathostome lateral line ontogenies. Brain Behav Evol. 1997;50:25–37. doi: 10.1159/000113319. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Development of the lateral line system in the channel catfish. In: Browman HI, Skiftesvik AB, editors. The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference. Bergen, Norway: Institute of Marine Research; 2003. pp. 137–159. [Google Scholar]
- Northcutt RG. Ontogeny of electroreceptors and their neural circuitry. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 112–131. [Google Scholar]
- Northcutt RG, Bemis WE. Cranial nerves of the coelacanth, Latimeria chalumnae [Osteichthyes: Sarcopterygii: Actinistia], and comparisons with other craniata. Brain Behav Evol. 1993;42:1–76. doi: 10.1159/000114175. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brändle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev Biol. 1995;168:358–373. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Catania KC, Criley BB. Development of lateral line organs in the axolotl. J Comp Neurol. 1994;340:480–514. doi: 10.1002/cne.903400404. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Holmes PH, Albert JS. Distribution and innervation of lateral line organs in the channel catfish. J Comp Neurol. 2000;421:570–592. [PubMed] [Google Scholar]
- O'Neill P, McCole RB, Baker CVH. A molecular analysis of neurogenic placode and cranial sensory ganglion development in the shark, Scyliorhinus canicula. Dev Biol. 2007;304:156–181. doi: 10.1016/j.ydbio.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota KG, Fujimoto S, Oisi Y, Kuratani S. Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish. Nat Commun. 2011;2:373. doi: 10.1038/ncomms1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew JD. Electroreception in monotremes. J Exp Biol. 1999;202:1447–1454. doi: 10.1242/jeb.202.10.1447. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Wright GM. Developmental transformations in a normal series of embryos of the sea lamprey Petromyzon marinus (Linnaeus) J Morphol. 2003;257:348–363. doi: 10.1002/jmor.10119. [DOI] [PubMed] [Google Scholar]
- Ronan M. Anatomical and physiological evidence for electroreception in larval lampreys. Brain Res. 1988;448:173–177. doi: 10.1016/0006-8993(88)91115-8. [DOI] [PubMed] [Google Scholar]
- Ronan MC, Bodznick D. End buds: non-ampullary electroreceptors in adult lampreys. J Comp Physiol A. 1986;158:9–15. doi: 10.1007/BF00614515. [DOI] [PubMed] [Google Scholar]
- Roth A. Development of catfish lateral line organs: electroreceptors require innervation, although mechanoreceptors do not. Naturwissenschaften. 2003;90:251–255. doi: 10.1007/s00114-003-0424-5. [DOI] [PubMed] [Google Scholar]
- Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev Genes Evol. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- Sarrazin AF, Nuñez VA, Sapède D, Tassin V, Dambly-Chaudière C, Ghysen A. Origin and early development of the posterior lateral line system of zebrafish. J Neurosci. 2010;30:8234–8244. doi: 10.1523/JNEUROSCI.5137-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. Do vertebrate neural crest and cranial placodes have a common evolutionary origin? BioEssays. 2008;30:659–672. doi: 10.1002/bies.20775. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians. II. Evolutionary diversification. Zoology (Jena) 2002;105:177–193. doi: 10.1078/0944-2006-00062. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Steven DM. Sensory cells and pigment distribution in the tail of the ammocoete. Quart J Micr Sci. 1951;92:233–247. [Google Scholar]
- Sullivan JP, Lavoué S, Hopkins CD. Molecular systematics of the African electric fishes (Mormyroidea: teleostei) and a model for the evolution of their electric organs. J Exp Biol. 2000;203:665–683. doi: 10.1242/jeb.203.4.665. [DOI] [PubMed] [Google Scholar]
- Teeter JH, Szamier RB, Bennett MVL. Ampullary electroreceptors in the sturgeon Scaphirhynchus platorynchus (Rafinesque) J Comp Physiol A. 1980;138:213–223. [Google Scholar]
- Tong S, Bullock TH. Physiological properties of the electro- and mechanoreceptors in catfish Ictalurus nebulosus. Sci Sinica B. 1984;27:1023–1028. [PubMed] [Google Scholar]
- Vischer HA. The development of lateral-line receptors in Eigenmannia (Teleostei, Gymnotiformes). II. The electroreceptive lateral-line system. Brain Behav Evol. 1989;33:223–236. doi: 10.1159/000115930. [DOI] [PubMed] [Google Scholar]
- Vischer HA, Lannoo MJ, Heiligenberg W. Development of the electrosensory nervous system in Eigenmannia (Gymnotiformes): I. The peripheral nervous system. J Comp Neurol. 1989;290:16–40. doi: 10.1002/cne.902900103. [DOI] [PubMed] [Google Scholar]
- von der Emde G. Active electrolocation of objects in weakly electric fish. J Exp Biol. 1999;202:1205–1215. doi: 10.1242/jeb.202.10.1205. [DOI] [PubMed] [Google Scholar]
- von der Emde G. Non-visual environmental imaging and object detection through active electrolocation in weakly electric fish. J Comp Physiol A. 2006;192:601–612. doi: 10.1007/s00359-006-0096-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder P, Lu Y, Zakon HH. Effects of denervation upon receptor cell survival and basal cell proliferation in tuberous electroreceptor organs of a weakly electric fish. J Comp Neurol. 1994;347:545–552. doi: 10.1002/cne.903470406. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Lu Y, Zakon HH. Tuberous electroreceptor organs form in denervated regenerating skin of a weakly electric fish. J Comp Neurol. 1996;367:563–574. doi: 10.1002/(SICI)1096-9861(19960415)367:4<563::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Whitear M, Lane EB. Multivillous cells: epidermal sensory cells of unknown function in lamprey skin. J Zool. 1983;201:259–272. [Google Scholar]
- Wicht H, Northcutt RG. Ontogeny of the head of the Pacific hagfish (Eptatretus stouti, Myxinoidea): development of the lateral line system. Philos Trans R Soc Lond B. 1995;349:119–134. doi: 10.1098/rstb.1995.0098. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH, Bullock TH. From electrogenesis to electroreception: an overview. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 5–46. [Google Scholar]