Abstract
Introduction
Bacterial infections are a serious complication of cirrhosis, as they can lead to decompensation, multiple organ failure, and/or death. Preventing infections is therefore very relevant. Because gut bacterial translocation is their main pathogenic mechanism, prevention of infections is mostly based on the use of orally administered poorly absorbed antibiotics such as norfloxacin (selective intestinal decontamination). However, antibiotic prophylaxis leads to antibiotic resistance, limiting therapy and increasing morbidity and mortality.
Prevention of bacterial infections in cirrhosis should therefore move away from antibiotics.
Areas Covered
This review focuses on various potentially novel methods to prevent infections in cirrhosis focusing on non-antibiotic strategies. The use of probiotics, nonselective intestinal decontamination with rifaximin, prokinetics and beta-blockers or fecal microbiota transplant as means of targeting altered gut microbiota, bile acids and FXR agonists are all potential alternatives to selective intestinal decontamination. Prokinetics and beta-blockers can improve intestinal motility, while bile acids and FXR agonists help by improving the intestinal barrier. Finally, granulocyte colony stimulating factor (G-CSF) and statins are emerging therapeutic strategies that may improve immune dysfunction in cirrhosis.
Expert Opinion
Evidence for these strategies has been restricted to animal studies and proof-of concept studies but we expect this to change in coming years.
Keywords: cirrhosis, bacterial infections, bacterial translocation, selective intestinal decontamination, probiotics, rifaximin, fecal microbiota transplant, prokinetics, beta-blockers, bile acids, FXR agonist, statins, GCSF
1.0. Introduction
Bacterial infections are present in 25-40% of hospitalized patients with cirrhosis [1, 2]. They can lead to further decompensation of cirrhosis (recurrent variceal hemorrhage, hepatorenal syndrome) and are the main precipitant of multiorgan failure in cirrhosis, the so-called acute-on-chronic liver failure (AOCLF) [Figure 1]. Therefore, they are associated with a high mortality; with a four-fold increase in in-hospital mortality [3], and a post-discharge mortality rate of 28-30% [4, 5]. As such, prevention of bacterial infections in cirrhosis is crucial.
Figure 1.
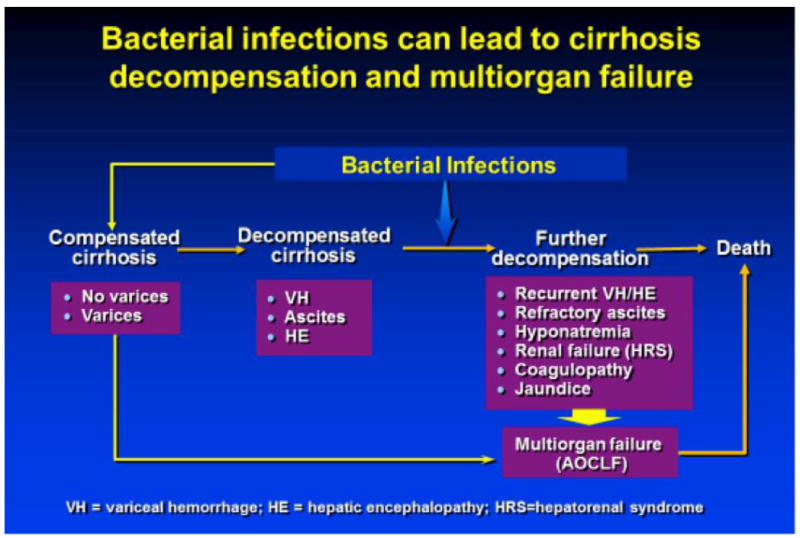
Bacterial infections can lead to decompensation of cirrhosis and multiorgan failure
Patients at higher risk of developing bacterial infections are those with poor liver function, upper gastrointestinal (GI) bleeding, low-protein ascites, and a prior episode of spontaneous bacterial peritonitis (SBP) [5, 6]. The most common infections in cirrhotic patients are SBP, urinary tract infections, pneumonia, and skin and soft tissue infections; any one of these may cause bacteremia and sepsis. SBP itself accounts for about 31% of the infections in patients with cirrhosis [2, 7]. Most infections are caused by gram-negative bacteria of intestinal origin; as such, bacterial translocation, defined as the passage of bacteria from the intestinal lumen to mesenteric lymph nodes or other extra-intestinal sites, has been implicated as a major mechanism in the development of these infections (particularly, spontaneous infections such as SBP). Perhaps more importantly, bacterial translocation is responsible for a pro-inflammatory state that worsens the hemodynamic status of patients with cirrhosis and leads to decompensation. This state of immune activation (“cytokine storm”) also leads to the development of cirrhosis-associated immune dysfunction [8, 9] that increases the susceptibility to other infections, including those due to Gram-positive or other bacteria not originating from the gut [Figure 2]. For example, patients with cirrhosis and pneumonia have a higher risk of developing bacteremia than patients without liver disease [10].
Figure 2.
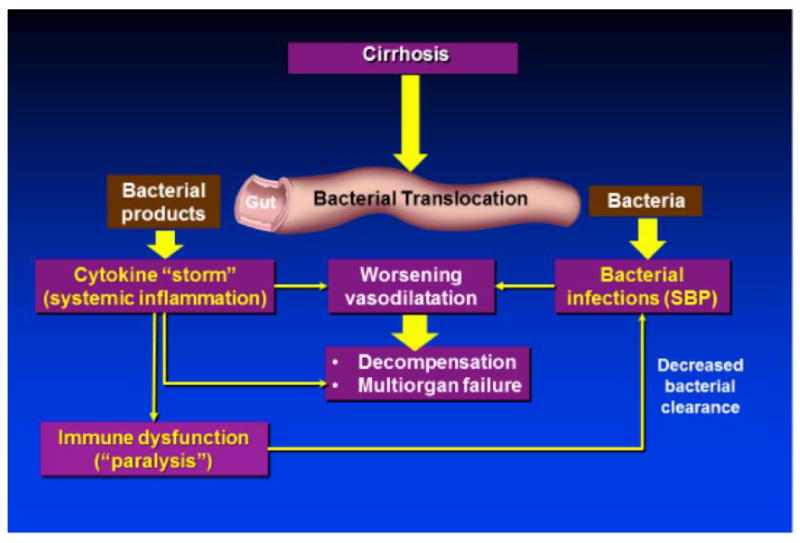
Effect of Bacterial Translocation in Cirrhosis
Therefore, preventing bacterial translocation will prevent infections and their deleterious consequences in patients with cirrhosis. Bacterial translocation occurs physiologically, but the healthy individual is able to eliminate translocating bacteria. In cirrhosis, a number of gastrointestinal abnormalities make it more likely for bacteria to translocate and spread to the systemic circulation; these abnormalities are as follows: 1) altered gut microbiota (changes in both the quantity and quality of gut bacteria); 2) altered intestinal permeability and 3) decreased phagocytosis. [Figure 3]. This review will focus on current and future novel strategies for preventing bacterial infections in cirrhosis, based on these mechanisms.
Figure 3.
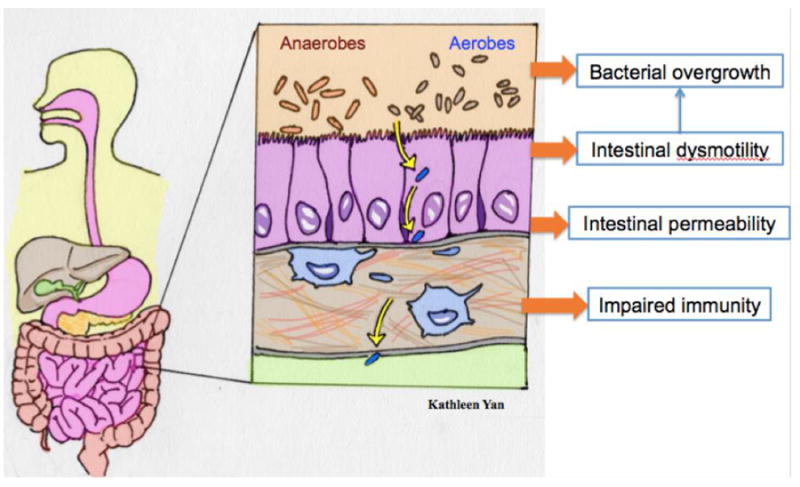
Mechanisms of Bacterial Translocation in Cirrhosis
2.0. Current strategy
Currently, the prevention of bacterial infections is focused solely on use of prophylactic antibiotics, usually norfloxacin, which target the most common organisms implicated in spontaneous infections in cirrhosis, Enterobacteriaceae and non-enterococcal streptococci. This is the strategy of “selective intestinal decontamination” [Figure 4], in which a poorly absorbable antibiotic such as norfloxacin changes the altered intestinal microbiome of cirrhosis to promote the growth of “good” anaerobic bacteria and suppress “bad” gram-negative bacteria[11]. In addition to the benefit of preventing infections, selective intestinal decontamination has also been shown to prevent the early recurrence of variceal hemorrhage [12], hepatorenal syndrome [13], and death [13, 14].
Figure 4.
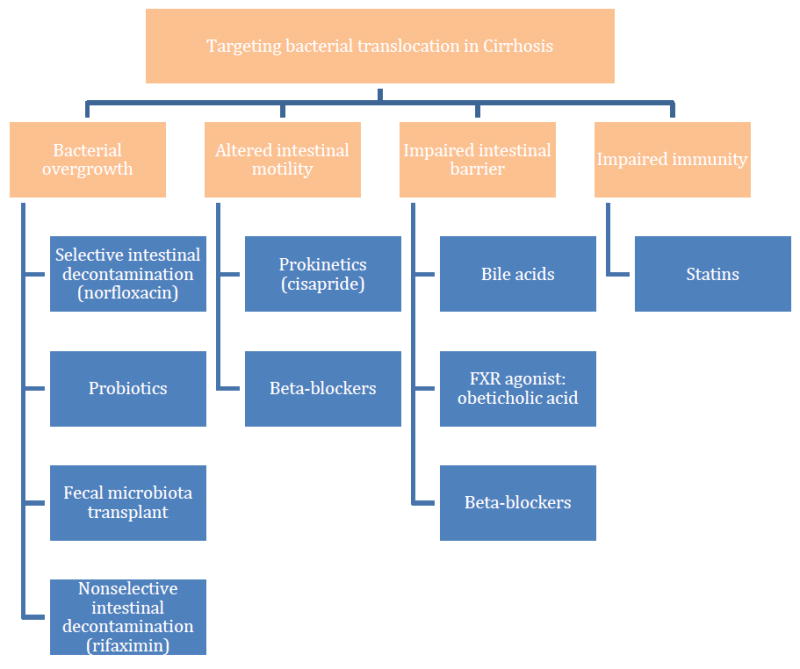
Strategies to target bacterial translocation in cirrhosis. Mechanisms are shown in orange, and treatment strategies in blue.
The major drawback of routine antibiotic prophylaxis is the emergence of multidrug resistant organisms [2, 15] which cause infections for which there are limited therapeutic antibiotics and are thereby associated with a greater incidence of septic shock and death [2]. Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE), and Extended-Spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae are the most prevalent multiresistant organisms reported in patients with cirrhosis [16]. Clostridium difficile infection is another infection on the rise, and a major risk factor for its development is antibiotic use; it causes higher mortality and longer hospitalizations in patients with cirrhosis [17].
Hence, routine prophylaxis should be limited to only patients specified below that are at the highest risk of developing a bacterial infection as is currently recommended in practice guidelines of both the American Association for the Study of Liver Diseases (AASLD) [18] and the European Association for the Study of the Liver (EASL) [19].
Patients with cirrhosis presenting with upper GI hemorrhage: Antibiotic prophylaxis with norfloxacin 400mg / 12 h PO for 7 days should be instituted from the time of admission, per Baveno VI recommendations [20]. Intravenous ceftriaxone at 1g/ 24h for 7 days should be considered in patients with advanced cirrhosis, in hospital settings with a high prevalence of quinolone-resistant bacterial infections and in patients on previous quinolone prophylaxis.
Patients who have survived an episode of SBP: Secondary prophylaxis with norfloxacin at 400mg/day PO is the recommended prophylaxis. Alternatively, since norfloxacin is no longer available in the U.S., ciprofloxacin at a dose of 500 mg/day can be used instead. Treatment should continue until liver transplant, death, or resolution of ascites or improvement in liver function to a compensated status.
Patients with ascitic fluid protein <1.5g/dL along with impaired renal function (creatinine ≥1.2, BUN ≥25, or serum Na ≤130) or liver failure (Child score ≥9 and bilirubin ≥3mg/dL): Primary prophylaxis with long-term norfloxacin 400mg/day PO or ciprofloxacin 500mg/day is recommended in these patients. This is particularly relevant in patients awaiting liver transplantation for whom the development of an infection would prompt removal from the transplant list.
3.0. Novel Strategies
As currently recommended antibiotic prophylaxis is associated with the development of infections due to multiresistant organisms, other methods for preventing bacterial translocation and bacterial infections are necessary. Novel therapies have been proposed based on their ability to alter bacterial translocation but the evidence for their use is limited and all require further studies. These strategies are outlined below together with their main purported mechanism of action (although many of them act at more than one level).
3.1. Prevention Strategies That Target the Altered Intestinal Microbiota
Though there are countless bacteria in the human gastrointestinal tract, most of them reside in the colon. There are hundreds of times more anaerobic bacteria than aerobic bacteria, with the latter being responsible for most cases of bacterial translocation [21]. Aspirates of the colon may reveal concentrations up to 1012 colony-forming units (CFU) /mL, whereas aspirates of the jejunum are much less concentrated at 103-104 CFU/mL [22]. The small intestine harbors significantly fewer bacteria than the colon due to its constant peristaltic motion and presence of antimicrobial gastric acid [23]. Bacterial overgrowth of the small intestine may occur in certain disease states, and is defined as at least 105 total CFU/mL in jejunal secretions. Small intestinal bacterial overgrowth is prominent in both cirrhotic rats with ascites and patients with cirrhosis [24-27], and is thought to be the most common site of bacterial translocation [28]. It is related to the severity of liver disease [27], and increases the risk of bacterial translocation and infection [24, 29, 30]. Proposed strategies to target this bacterial overgrowth involve decreasing the overall bacterial burden or changing the taxonomy of intestinal microbes to favor the growth of anaerobic bacteria [Figure 3].
3.1.1. Decreasing the overall burden/function of intestinal bacteria: Rifaximin
Rifaximin is an antibiotic with broad-spectrum activity that was thought to eliminate gut microbes non-selectively, hence reducing the overall burden of intestinal bacteria [31, 32]. However a recent study shows that, rather than changing the stool microbiome, it seems to have a direct effect on bacterial function by impairing their ability to translocate [33]. Rifaximin’s activity is specific to the gut as its absorption into the systemic circulation is practically nil, which limits systemic toxicity or side effects. The lack of systemic availability also limits the selective pressure for the development of widespread resistance that is seen with systemically available antibiotics; in addition, resistance to rifaximin is not efficiently transferred [34].There is already strong evidence for the use of rifaximin in maintaining remission from hepatic encephalopathy in cirrhosis [32, 35] and in this setting, it has not been associated with the development of infections due to multidrug resistant organisms [15]. The role of rifaximin in preventing infections is being investigated. Non-randomized studies have shown mixed results: there is both a positive effect and a lack of effect of rifaximin in preventing SBP or cirrhosis decompensation. Rifaximin was shown to lower the infection rate in cirrhotic patients compared to no treatment [35, 36], with as much as a 72% decrease in the risk of primary SBP [37], as well as lowering other complications of cirrhosis such as hepatorenal syndrome and variceal bleeding [36]. However, in a prospective cohort study comparing prophylaxis with rifaximin to prophylaxis with systemically absorbed antibiotic versus no prophylaxis, rifaximin did not reduce SBP occurrence in hospitalized cirrhotic patients compared to no treatment; only systemic antibiotic had an effect on reducing risk of SBP [38]. The major drawback is that none of these studies were randomized, placebo-controlled trials, which are needed to truly delineate the effect of rifaximin in preventing infections in cirrhosis.
3.1.2. Changing the taxonomy of intestinal microbes: Probiotics, prebiotics, and synbiotics
Probiotics are live bacteria that replace or add to the beneficial bacteria normally present in the gastrointestinal tract. Species such as Lactobacillus spp. have protective effects on the intestinal mucosa, such as lowering intestinal pH, preventing colonization by pathogenic species, and modulating the immune response; hence, they help to improve overall gut function [39]. Prebiotics are nondigestable food ingredients that promote the growth of the beneficial bacteria, such as fermentable fibers, which the bacteria break down for their nutrition and survival. Synbiotics are merely the combination of probiotics and prebiotics.
In rats with cirrhosis, administration of Lactobacillus johnsonii La1 with antioxidants reduced bacterial translocation and endotoxemia compared to control [40]. Other combinations of lactobacilli were also shown to be effective at reducing bacterial translocation and serum alanine aminotransferase levels in a rat model of acute liver injury [41]. An 8-species probiotic cocktail (3 bifidobacteria species and 5 lactobacilli species) called VSL#3 decreased bacterial translocation and improved intestinal permeability (measured by ileal occludin expression and oxidative damage) in rats with cirrhosis [42] [Table 1].
Table 1.
Non-antibiotic preventative strategies – studies in Animals
| Strategy | Author/Year | Model used | Number of animals | Endpoints | Results | Significance |
|---|---|---|---|---|---|---|
| Probiotics | Chiva 2002 [40] | Rats with CCl4-induced cirrhosis | N=29 rats (10 with probiotic + antioxidant, 11 with antioxidant, 8 control) | Intestinal flora, endotoxemia, and bacterial translocation | Rats treated with antioxidants + Lactobacillus or antioxidants only had reduced intestinal bacterial burden and bacterial translocation. Only the group treated with antioxidant + Lactobacillus had decreased endotoxemia. | Positive study |
| Probiotics | Adawi 2001 [41] | Rats with Acute liver injury induced through intraperitoneal injection of D-galactosamine | N=30 rats (6 rats in each of five groups) | Extent of liver injury, bacterial translocation, and intestinal microflora | All lactobacillus probiotics reduced the incidence of bacterial translocation, whereas Bifidobacterium increased the incidence of bacterial translocation. | Positive study |
| Probiotics | Sanchez 2015 [42] | Rats with CCl4-induced cirrhosis | N=46 rats (24 water only, 22 VSL#3+water) | Bacterial translocation, intestinal microbiota, gut barrier / inflammatory response | The probiotic combination VSL#3 decreases bacterial translocation, the pro-inflammatory state and ileal oxidative damage; it increases ileal occludin expression in rats with experimental cirrhosis | Positive study |
| Probiotics | Bauer 2002 [46] | Rats with CCl4-induced cirrhosis | N=34 rats with lactobacillus v. control, N=20 in norfloxacin + lactobacillus v. control | Bacterial translocation, ascitic fluid infection | Rats treated with Lactobacilli showed no difference in bacterial translocation or ascitic fluid infection compared to those with control (with or without norfloxacin pretreatment) | Negative study |
| Cisapride | Pardo 2000 [62] | Rats with CCl4-induced cirrhosis | Cirrhotic rats randomized to cisapride (N=15) or saline (N=15) | Intestinal bacterial overgrowth, bacterial translocation | Cisapride significantly reduced intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats | Positive study |
| Cisapride | Zhang 2003 [63] | Rats with CCl4-induced cirrhosis | Cirrhotic rats divided into no treatment (N=25), cisapride treatment (N=20), and saline treatment (N=20) | Intestinal bacterial overgrowth, bacterial translocation, intestinal transit and permeability | Compared with the placebo group, cisapride-treated rats had lower rates of bacterial/endotoxin translocation and intestinal bacterial overgrowth, which was closely associated with increased intestinal transit and improved intestinal permeability by cisapride. | Positive study |
| Beta-blockers | Perez-Paramo 2000 [61] | Rats with CCl4-induced cirrhosis | Cirrhotic rats randomized to propranolol (N=13) or placebo (N=12) | Intestinal bacterial load, transit and permeability of the bowel, and rate of bacterial translocation | Compared with the placebo group, propranolol-treated animals had significanctly faster intestinal transit, and lower rates of bacterial overgrowth and translocation. | Positive study |
| Bile acids | Ding 1993 [85] | Rats with bile-duct ligation (BDL) | BDL rats received saline (n=15) vs. cholic acid (n=9), deoxycholic acid (n=12), or whole bile (n=12) | Bacterial translocation; serum endotoxin | Rate of bacterial translocation was signficantly lower in bile-treated animals compared with saline-treated animals. Assays for endotoxin were negative in bile-treated animals and positive in saline-treated animals | Positive study |
| Conjugated bile acids | Lorenzo-Zuniga 2003 [79] | Rats with CCl4-induced cirrhosis | N=60 cirrhotic rats, randomized to cholylsarcosine (n=20), cholylglycine (n=20) or placebo (n=20). N=20 healthy non-cirrhotic controls | Bacterial overgrowth, bacterial translocation, and endotoxemia | Administration of conjugated bile acids reduced ileal bacterial content to normal levels in cirrhotic rats. Bacterial translocation was lower in cirrhotic rats who received conjugated bile acids (33% with cholylsarcosine, and 26% with cholylglycine) than in animals who received placebo (66%). | Positive study |
| FXR agonist: Obeticholic acid (OCA) | Verbeke 2015 [86] | Rats with bile-duct ligation (BDL) | N=28 untreated healthy controls; N=51 BDL (then randomized to vehicle, ursodeoxycholic acid, or obeticholic acid) | Gut permeability, inflammation, and bacterial translocation | After treatment with obeticholic acid, BDL-rats showed decreased markers of inflammation in the gut, normalized gut permeability, and a significant reduction in translocated bacterial strains. | Positive study |
| FXR agonist: Obeticholic acid | Ubeda 2014 [89] | Rats with CCl4-induced cirrhosis | N=22 rats with cirrhosis randomized to OCA or vehicle; N=14 controls | Gut bacterial translocation; ileal inflammation; hepatic fibrogenesis | In cirrhotic rats and compared with vehicle, OCA reduces bacterial translocation (83% vs. 20%, p<0.01), reduces markers of ileal inflammation (IL-17, IFNɣ, TLR4) and ameliorates hepatic expression of fibrosis markers | Positve study |
| FXR agonist: GW4064 | Inagaki 2006 [87] | Mice with bile-duct ligation (BDL) | BDL mice randomized to vehicle or FXR agonist GW4064 | Gut bacterial overgrowth; bacterial translocation; gut barrier integrity | Administration of GW4064 (FXR agonist) in BDL mice: reduced bacterial overgrowth in the ileum and cecum; reduced bacterial translocation; and restored the intestinal barrier (shown via occludin immunostaining and mucosal damage). | Positive study |
Randomized controlled trials (RCT) in liver transplant recipients demonstrated a significant reduction in infections in patients treated with Lactobacillus plantarum 299 and fiber compared to selective bowel decontamination with a mix of antibiotics [39], as well as in patients treated with a mixture of four lactic acid bacteria species and four fibers versus fibers only [43]. An RCT featuring administration of Escherichia coli Nissle improved the gut microbial profile and lowered endotoxemia, a marker of bacterial translocation, and mildly improved liver function [44]. A synbiotic preparation containing 4 species (Pediacoccus pentoseceus, Leuconostoc mesenteroides, Lactobacillus paracasei, Lactobacillus plantarum 2592) and 4 fibers (beta glucan, inulin, pectin, resistant starch) was effective at improving gut flora, reducing endotoxemia, and reversing minimal hepatic encephalopathy in 50% of patients with cirrhosis [45]. Together, these studies suggest a beneficial effect of probiotics in preventing infections in cirrhosis. [Table 2].
Table 2.
Non-antibiotic preventative strategies – studies in Humans
| Strategy | Author/Year | Type of study | Number of patients | Endpoints | Results | Significance |
|---|---|---|---|---|---|---|
| Probiotic | Rayes 2002 [39] | Randomized controlled trial | N=95 (32 SBD group, 31 Lactobacillus, 32 placebo) | Incidence of post-operative infections | Patients receiving living lactobacillus plus fiber developed significantly fewer bacterial infections (13%) compared to the group with SBD (48%) | Positive study |
| Probiotic | Rayes 2005 [43] | Randomized controlled trial | N=66 (33 probiotics+fibers group, 33 fibers-only group) | 30-day infection rate; length of hospital stay; duration of antibiotic therapy | Synbiotic treatment reduced the incidence of post-operative bacterial infections from 48% with only fibers to 3% with fibers and probiotic. The duration of antibiotic therapy was also shorter in the synbiotic group. | Positive study |
| Probiotic | Lata 2007 [44] | Randomized controlled trial | N=39 (22 probiotic, 17 placebo) | Serum endotoxin levels, biochemical analysis, stool microbiological analysis | Patients treated with E. coli Nissle demonstrated normalization of fecal flora, reduced endotoxemia and improved liver function | Some results not reaching statistical significance, but suggesting positive results |
| Probiotic | Liu 2004 [45] | Randomized controlled trial | N=55 (20 synbiotic, 20 fermentable fiber, 20 placebo) | Stool microbiological analysis, venous ammonia levels, serum endotoxin levels | Synbiotic treatment increased the fecal composition of Lactobacillus spp at the expense of other species and was associated with a significant reduction in blood ammonia levels, endotoxemia, and reversal of minimal hepatic encephalopathy in 50% of patients. | Positive study |
| Probiotic | Pande 2012 [47] | Randomized controlled trial | N=110 patients: 55 norfloxacin + probiotic, 55 norfloxacin + placebo | Occurrence of SBP within 6 months; mortality | No difference in primary or secondary prophylaxis of SBP or reducing mortality | Negative study |
| Rifaximin | Hanouneh 2012 [37] | Retrospective cohort | N=404 (49 of whom received rifaximin) | Incidence of SBP; transplant-free survival | There was a 72% reduction in the rate of SBP in the rifaximin group vs. controls (HR 0.28, 95% CI 0.11-0.71, p=0.007); as well as a transplant-free survival benefit (P=0.045) | Positive study |
| Rifaximin | Vlachogiannakos 2013 [36] | Retrospective cohort rifaximin, matched with 46 controls | N=23 patients who received | Survival; risk of variceal bleeding, HE, SBP, and HRS | Patients who received rifaximin had a significantly lower risk of developing SBP than controls (4.5% vs. 46%, p=0.027) | Positive study |
| Rifaximin | Mullen 2014 [35] | Phase 3, open-label maintenance study (nonrandomized) | N=392 | Rate of infections, complications, and hospitalizations | Infection event rates per person-years of exposure for rifaximin patients was 0.73, lower than that observed in the placebo group (1.33) or historical rifaximin group (1.12) | Positive study |
| Rifaximin | Lutz 2014 [38] | Prospective cohort | N=152 | Frequency of SBP | SBP rate in patients with rifaximin vs. no treatment were comparable (8/27 = 30% vs. 24/108=22%) and significantly higher than in those patients who received systemic antibiotic prophylaxis (0/17=0%). | Negative study |
| Cisapride | Madrid 2001 [60] | Randomized controlled trial | N=34 (12 cisapride, 12 norfloxacin/neomycin, 10 placebo) | Small intestinal motor activity, orocecal transit time, bacterial overgrowth, liver function over 6 months | After 6 months, both cisapride and antibiotics significantly improved fasting cyclic activity, reduced duration of orocecal transit time, and decreased small intestinal bacterial overgrowth compared to placebo | Positive study |
| Cisapride | Pardo 2000 [62] | Cross-sectional study and pilot randomized study | N=46; of these, 10 were randomized to receive cisapride (5) or no treatment (5). | Intestinal bacterial overgrowth, orocecal transit time | Orocecal transit time was significantly decreased in patients who received cisapride therapy compared to no treatment; intestinal bacterial overgrowth was also significantly reduced in patients with cisapride treatment vs. no treatment (P<0.05). | Positive study |
| Cisapride | Sandhu 2005 [64] | Randomized controlled trial | N=94, of whom 48 received norfloxacin and 46 received norfloxacin + cisapride | Probability of developing SBP; mortality | The probability of developing SBP at 12 months was reduced from 56.8% in norfloxacin-only group to 21.7% in the norfloxacin + cisapride group (P=0.026). Probability of death at 18 months was 20.6% in norfloxacin-only group vs. 6.2% in norfloxacin + cisapride group (P=0.1) | Positive study |
| Beta blockers | Reiberger 2013 [67] | Prospective cohort study | N=50 (NSBB therapy initiated in 39 patients) | Intestinal permeability; serum levels of lipopolysaccharide-binding protein (LBP) and IL-6 | Under non-selective beta-blocker (NSBB) therapy, there was an amelioration of intestinal permeability and a significant decrease in bacterial translocation (measured by serum levels of LBP and IL-6), which was not limited to hemodynamic (HVPG) responders. | Positive study |
| Beta-blockers | Senzolo 2009 [68] | Meta-analysis | N=644 patients (257 treated with propranolol, 387 receiving no propranolol) | Occurrence of SBP | There was a significantly significant difference of 12.1%, P<0.001 in favor of propranolol in preventing SBP | Positive study |
| Beta-blockers | Merli 2015[69] | Prospective cohort study | N=400 | Occurrence of infections and risk factors | The use of beta-blockers was a protective factor against infection, with an OR 0.46, 95% CI 0.3-0.7, P=0.001. Cirrhotic patients with infection showed lower morbidity and mortality when taking beta-blockers | Positive study |
| G-CSF | Garg 2012 [96] | Randomized controlled trial | N=23 receiving G-CSF, N=24 receiving placebo | 60-day survival; Child-Turcot Pugh score, MELD score, SOFA, complications such as sepsis, HRS, and HE | Patients treated with G-CSF had a significantly higher 60-day survival than those with placebo (69.6% vs. 29%); fewer patients treated with G-CSF developed sepsis compared with those on placebo (14% vs. 41%, p=0.04). There was a significant impact / improvement in other endpoints as well. | Positive study; patients with acute-on- chronic liver failure |
| G-CSF | Duan 2013 [97] | Randomized controlled trial | N=27 receiving G-CSF, N=28 control | Peripheral CD34+ cell count, liver function, and 3-month survival / complications | Survival at 3 months was 48.1% in G-CSF treated patients vs. 21.4% in the control group (p=0.0181). More patients in the control group died of sepsis and HRS compared to those in the G-CSF group (Χ2 value 4.863, p=0.027). | Positive study; patients with Hep-B acute- on-chronic liver failure |
| G-CSF and EPO combination | Kedarisetty 2015 [98] | Randomized controlled trial | N=29 receiving G-CSF and darbopoietin, N=26 placebo | 12-month survival; Child-Pugh score, MELD score, need for large volume paracentesis, septic shock | Patients treated with G-CSF and Darbopoietin demonstrated a significant increase in 12-month survival compared to placebo (68.6% vs. 26.9%, p=0.003). The incidence of SBP was non-significant between the two groups, although there was a lower incidence of septic shock between G-CSF+EPO-treated and placebo-treated groups (6.9% vs. 38.5% respectively, p=0.005). | Positive study |
| Statins | Motzkus-Feagans 2013 [100] | Retrospective cohort study | N=19379 patients with compensated cirrhosis | Hospitalizations with infections | Compared with non-users, the rate of infection or death was significantly lower among statin users (adjusted HR 0.67, 95% CI 0.47-0.95) | Positive study |
However, there are also negative studies with probiotics, including case reports of Lactobacillus sepsis with probiotic therapy. In a rat model of cirrhosis, Lactobacillus rhamnosus strain GG proved ineffective at preventing bacterial translocation and ascitic fluid infection compared to control [46]. A randomized controlled trial in humans showed that addition of a 4-species probiotic (Enterococcus faecalis JPC, Clostridium butyricum, Bacillus mesentericus JPC, Bacillus coagulans) to norfloxacin did not reduce the occurrence of spontaneous bacterial peritonitis or mortality [47]. These negative results may be the result of various factors; the first of which is that not all probiotics are created equal, and different probiotics were used in all these studies; some species may be more effective than others, and the number of different species may make a difference as well. Also, the addition of norfloxacin to probiotic may have affected probiotic viability in the latter study. Further studies are needed to determine the optimal combination of probiotics and prebiotics, as well the dosing and duration of administration. These studies would need to compare pro/prebiotics to standard antibiotic prophylaxis and not in addition to it.
3.1.3. Changing the taxonomy of intestinal microbes: Fecal microbiota transplant
Fecal microbiota transplant (FMT) is an emerging therapy for the treatment of gastrointestinal dysbiosis, usually in Clostridium difficile infections [48]. It involves the transfer of feces from a healthy donor to the recipient via one of three routes: gastric, jejunal, or colonoscopic. It is capable of reestablishing intestinal homeostasis and preventing recurrent infections [48, 49]. However, the evidence for FMT outside of C. difficile infection is scant. In one case report, a 14-year-old girl colonized with highly resistant Klebsiella pneumonia leading to successive infections was successfully treated with fecal microbiota transplant, after which stool studies showed clearance of the organism that was sustained over several months [50]. In another case report, fecal microbiota transplant in a patient with cirrhosis and hepatic encephalopathy improved cognitive function and brought the gut flora taxonomy closer to normal, though this effect was not sustained beyond 7 weeks following transplant [51]. To date there are no studies examining the use of fecal microbiota transplant for preventing bacterial infections in patients with cirrhosis, though it remains a promising therapy.
3.2. Prevention Strategies that Target Abnormal Intestinal Motility in Cirrhosis
One of the causes of intestinal bacterial overgrowth in cirrhosis is that intestinal transit times are prolonged, particularly in patients with decompensated cirrhosis (the ones most prone to infection). The regular cyclical contractions of the gastrointestinal tract in the fasting state is due to waves of electrical activity known as migrating motor complexes (MMCs), which generate peristaltic waves that propel material through the intestinal lumen. It consists of three phases: phase I, the quiescent phase; phase II, characterized by a buildup of action potentials and contractility; and finally phase III, the peak of electrical and mechanical activity. Phase III of the MMC acts as the intestinal housekeeper, with its well-defined aborad migration that clears the gut, along with increased biliary secretions that act as a detergent [52, 53]. Intestinal peristalsis, gastric acid, and mucosal immunity work in concert to prevent bacterial overgrowth in the small intestine [54].
Abnormalities in small bowel motility in patients with cirrhosis include a prolonged MMC cycle duration, a prolonged phase II, and various changes in the contraction pattern of phase II in patients with cirrhosis compared to healthy controls, such as increased clustered contractions, less cyclic contractions, and more retrograde pressure waves [52, 55, 56]. The abnormalities in migrating motor complexes and increased clustered activity are more severe in Child-Pugh stage C cirrhotic patients compared to stage A patients; also, the extent of small bowel dysmotility is related to the degree of liver failure [52, 57, 58] and presence of portal hypertension [56]. Furthermore, these abnormalities in intestinal motility are reversed following liver transplantation [59].
These changes in intestinal motility in cirrhotic patients likely cause ineffective intestinal peristalsis, which delays the intestinal transit time and favors bacterial overgrowth. Indeed, decompensated cirrhotic patients have slower intestinal transit times than patients with compensated cirrhosis [58], with a significant correlation between small bowel transit time and Child-Pugh score (R=0.77, p=0.0003) [58]. These abnormalities in bowel motility and transit times are conducive to bacterial overgrowth, as patients with cirrhosis and a history of SBP have a significantly higher incidence of bacterial overgrowth compared to cirrhotic patients without SBP [52]. Other studies have also demonstrated the association of small intestinal bacterial overgrowth with intestinal dysmotility in both rats and patients with cirrhosis [24, 60, 61], and specifically in those cirrhotic patients with portal hypertension [56].
3.2.1. Improving intestinal motility: Cisapride
Since altered intestinal motility is one of the factors predisposing to infections in patients with cirrhosis, prokinetic agents represent a therapeutic strategy to target this complication. Cisapride is the best studied agent for this purpose; it is unique among prokinetics as it does not have antidopaminergic properties, instead exerting its effect by increasing the physiologic release of acetylcholine from post ganglionic nerve endings of the myenteric plexus. It significantly reduces bacterial translocation in cirrhotic rats, namely by accelerating intestinal transit time and reducing intestinal bacterial overgrowth [62, 63]. Its administration in humans with cirrhosis improves fasting cyclical activity, reduces orocecal transit time and is associated with abolishment of bacterial overgrowth [60, 62]. A prospective randomized controlled trial in a heterogeneous group of cirrhotic patients with ascites showed that the combination of norfloxacin and cisapride significantly reduces the incidence of spontaneous bacterial peritonitis compared to norfloxacin alone [64]. However, the inclusion of patients at different risks for SBP and adding the prokinetic to norfloxacin rather than comparing it to norfloxacin, limit the validity of this study. Cisapride is associated with QT prolongation that has led to its discontinuation from the market. Further studies using other prokinetic agents, such as the 5-HT4 receptor antagonist prucalopride, would be worthwhile although there is no preliminary data.
3.2.2. Improving Intestinal Motility: Non-selective beta adrenergic-blockers
Cirrhosis is a state of increased adrenergic activity that results from vasodilatation (splanchnic and systemic), the hemodynamic hallmark of patients with decompensated cirrhosis. This increased sympathetic stimulation results in delayed intestinal transit, which may be reversed with beta-blockers. Since norepinephrine also increases the growth of gram-negative rods and increases intestinal permeability [65, 66], beta-blockers may also act as an antibacterial and target multiple mechanisms responsible for bacterial translocation in cirrhosis. Beta-blockers are already used to prevent variceal hemorrhage in cirrhosis, their main effect being a reduction in portal pressure.
In cirrhotic rats with ascites, propranolol significantly accelerated intestinal transit, reducing rates of bacterial overgrowth in the bowel and bacterial translocation [61]. In patients with cirrhosis, propranolol reduced intestinal permeability, measured by urinary sucrose levels, and bacterial translocation, measured by serum LPS-binding protein (LBP) and IL-6 [67]. A meta-analysis that included studies of beta-blockers in the prevention of hemorrhage (studies in which infection was not an outcome) suggested that patients with cirrhosis on propranolol have a lower risk of SBP and that this effect was independent on their portal pressure-reducing effect [68]. A recent prospective study also found a significant protective effect of beta-blockers against infection in cirrhotic patients; these patients had lower infection-related morbidity and mortality when taking beta-blockers [69]. Until more data is available beta-blockers should not be used with the objective of preventing infections. In fact, a recent controversial issue pertains to a potentially deleterious effect of beta-blockers in patients with cirrhosis and refractory ascites (the most prone to develop infections) [70]. Until this issue is resolved, and per Baveno recommendations, beta-blockers should not be discontinued in all patients with refractory ascites but only in those with a systolic blood pressure <90 mmHg, hyponatremia (<130 mEq/L) or increases in serum creatinine >0.3 mg/dL from baseline [20].
3.3. Prevention Strategies that Target the Impaired Intestinal Barrier in Cirrhosis
Normal intestine contains a mucosal barrier with secretory and physical components to prevent microbial translocation [71]. Mucins from epithelial goblet cells shield the microvillus membrane from bacteria [72]. Immunoglobulin A (IgA) antibodies is another important player in antimicrobial defense; it binds epitopes on pathogens and traps them in the mucus layer (immune exclusion), and neutralizes toxins [71, 73]. Bile acids also contribute by inhibiting bacterial overgrowth, promoting growth of the intestinal mucosa [74], and acting as a detergent to prevent bacterial adherence [75]. The intestinal epithelium itself maintains a critical barrier through the use of tight-junction complexes to maintain selective permeability, as well as through the active production of antimicrobial peptides and proteins [71].
This well-evolved intestinal barrier is compromised in cirrhosis. Markers of intestinal permeability and bacterial translocation are significantly correlated with [67]degree of liver dysfunction [76] in patients with cirrhosis. There are various factors involved in this breakdown in intestinal integrity, including altered expression of tight junction proteins occludin and claudin-1 [77], upregulation of tumor necrosis factor-α in the gut-associated lymphatic tissue [78], and deficiency of mucosal protective factors, such as bile acids [79], secretory IgA [80], and antimicrobial peptides [16, 81].
3.3.1. Protecting the Intestinal Barrier: Bile acids
Bile acids are bacteriostatic [82, 83], prevent bacterial overgrowth in the small intestine, and maintain intestinal barrier function. Decreased bile flow in cirrhosis results from bile duct obstruction, which fosters bacterial overgrowth and bacterial translocation, including leakage of endotoxin and bacterially-driven products from the gut into the systemic circulation [84]. The oral administration of bile acids cholic acid, deoxycholic acid, or whole bile) inhibits bacterial overgrowth and bacterial translocation in common bile duct ligated rats [85]. In a more relevant model of cirrhosis, the rat with carbon tetrachloride (CCl4)-induced cirrhosis, oral bile acids also reduced intestinal bacterial overgrowth and bacterial translocation, in addition to reducing mortality [79]. There are no relevant studies in humans but experimental studies suggest that bile acids, through both their antimicrobial effects and intestinal barrier effects, may prevent bacterial translocation and infections in cirrhosis.
3.3.2. Improving intestinal permeability
Farnesoid X Receptor (FXR) agonists Farnesoid X Receptor is a nuclear receptor and transcription factor activated by bile acids such as cholic and chenodeoxycholic acid. It is a chief regulator of the metabolism of bile acid, lipid, and carbohydrates. In the intestine, FXR induces genes involved in enteroprotection, from restoring intestinal permeability to reducing inflammation, and thus represents an enticing target for preventing bacterial translocation in cirrhosis. FXR-deficient rats demonstrate high rates of bacterial translocation and increased intestinal permeability [86]. In bile-duct ligated rats, administration of an FXR agonist GW4064 significantly reduced the number of bacteria both in the ileum and the mesenteric lymph nodes [87]. In humans with cirrhosis, a polymorphism in the FXR receptor gene, the rs56163822 genotype, significantly increased the risk of developing spontaneous bacterial peritonitis and was confirmed as a predictor of SBP [88].
Obeticholic acid (6-ethylchenodeoxycholic acid) is a potent semisynthetic bile acid and agonist of FXR, and was shown in bile duct-ligated rats to reduce intestinal inflammation and the number of bacterial strains that translocated to mesenteric lymph nodes, compared to animals treated with ursodeoxycholic acid (UDCA) [86]. In rats with CCl4-induced cirrhosis, administration of obeticholic acid significantly reduced bacterial translocation (from 83% to 20%, p<0.01) and improved markers of inflammation and fibrosis in the gut and liver (e.g, IL-17, TNF-α, TLR-4, and collagen) compared to placebo [89]. In humans, obeticholic acid was recently shown to improve liver fibrosis in patients with non-cirrhotic, non-alcoholic steatohepatitis, and was shown to be safe [90]. Even though it has not been specifically investigated in patients with cirrhosis, it is a promising therapeutic target that should be further explored regarding its capacity to prevent infections.
3.4.Prevention Strategies that Target Immune Dysfunction in Cirrhosis
The immune dysfunction in cirrhosis involves at once a state of immunodeficiency as well as a pro-inflammatory state [9, 91]. The pro-inflammatory state in cirrhosis is a result of continuous stimulation of immune cells by products of bacterial translocation that eventually leads a population of immune cells to become dysfunctional (“immune paralysis”) [92]. Not only is the quantity of lymphocytes, neutrophils, and other phagocytes reduced (partly because of pooling in the spleen resulting from portal hypertension), but these cells also demonstrate poor functional activity [93]. Additionally, the presence of porto-systemic shunting and liver dysfunction (with decreased hepatic production of complement) results in decreased clearance of bacteria by the liver [94].
3.4.1. Repopulating the liver with functional immune cells Granulocyte Colony-Stimulating Factor
One novel potential therapy that actually gets at the root cause of liver disease itself is the use of granulocyte colony-stimulating Factor (G-CSF). This 175-amino acid-long recombinant cytokine protein is the most potent agent available for mobilizing hematopoietic stem cells from the bone marrow. This therapy works by repopulating the liver [95] with both hepatocytes and non-parenchymal cells, which includes immune cells (e.g. neutrophils, T cells) that can help prevent infection. In recent randomized controlled trials, G-CSF was shown to decrease risk of infection and/or sepsis in patients with acute-on-chronic liver failure [96, 97] and, in combination with erythropoietin, to lower the incidence of septic shock to 7%, compared to 38% in the placebo group (P<0.005) [98]. Importantly, both trials showed a survival benefit in patients with advanced liver disease and therefore this strategy would appear to be particularly promising if confirmed by other groups.
3.4.2. Modulating inflammation: Statins
Statins seem to have a protective effect against bacteremic infections, although they appear to have no effect on mortality. It is thought to work through its anti-inflammatory and immunomodulatory properties [99]. A retrospective cohort study in veterans with compensated cirrhosis showed a decrease in severe bacterial infections in statin users compared to non-statin users (HR 0.42, 95% CI 0.36-0.48) [100], suggesting that statin use may reduce the risk of infections in cirrhosis. However, these results must be taken with caution because of methodological issues, importantly its retrospective nature. Statin use has further benefits in cirrhotic patients in that it lowers portal pressure and portal hypertension, allowing improved liver perfusion and function [101] and has recently been shown to prevent decompensation and death in patients with compensated hepatitis C-related cirrhosis [102]. Overall, further studies are necessary to confirm these findings of the beneficial effects of statins in liver disease.
4.0.Conclusion
Prevention of bacterial infections in cirrhosis is currently limited to selective intestinal decontamination with antibiotics. Although effective, this management strategy is not ideal as it has led to the development of antibiotic resistance and should therefore be restricted to very specific populations of patients with cirrhosis with an especially high-risk of developing infections. Increasing knowledge regarding the mechanisms of infection, inflammation and immune deficiency in cirrhosis have led to potentially novel and useful strategies to prevent bacterial translocation and the development of infections in cirrhosis. Emerging alternatives to selective intestinal decontamination include: nonselective decontamination with rifaximin, probiotics, and fecal microbiota transplant, all of which target bacterial overgrowth; prokinetics like cisapride and beta-blockers to target intestinal dysmotility; bile acids and FXR agonists to target the impaired intestinal barrier; G-CSF and statins to restore the immune imbalance in cirrhosis. Ultimately, prevention of bacterial translocation and the resultant risk of immune activation and/or overt bacterial infection will result in prevention of decompensation and multiorgan failure and an improvement in survival. Hopefully, in the upcoming years the efficacy of many of these strategies and the specific population of patients with cirrhosis that would benefit from specific strategies will be clarified.
5.0.Expert Opinion
The research on antibiotic prophylaxis of bacterial infections in cirrhosis is solid in the settings of patients with gastrointestinal hemorrhage and in the prevention of recurrent spontaneous bacterial peritonitis (SBP). In these studies the key findings are that antibiotics not only prevent infections but could prevent recurrent variceal hemorrhage, recurrent SBP and death. It is not as solid in the area of primary prophylaxis of SBP/infections in general because further patient stratification is necessary. However, if restricted to patients with very severe liver disease, antibiotic prophylaxis can prevent hepatorenal syndrome and prolong survival. Research on non-antibiotic prophylaxis has mostly been restricted to animal studies and to some proof-of concept studies in patients with cirrhosis so, at this time, evidence is insufficient to recommend any of these non-antibiotic strategies.
The potential of this research is not only to prevent infections in cirrhosis but also to prevent the deleterious consequences of translocation of bacteria and its products from the gut by triggering a pro-inflammatory state that can lead to further immune dysfunction, creating a vicious cycle. Ultimately, the goal would be to prevent the development of decompensation, multiorgan failure and death in cirrhosis. Basic research to further define the mechanisms vis-à-vis different stages of cirrhosis is necessary so that an individualized approach can be applied to clinical trials. The potential strategies are many and it is unclear which one will be the best in general or, more importantly, which approach will be best for specific patient populations.
Hopefully, antibiotic stewardship and restriction of antibiotic prophylaxis to only those patients that really need it will lead to a decrease in infections due to multiresistant organisms. Concomitantly, further development of benign, non-antibiotic strategies may lead to randomized clinical trials that will prioritize among the different strategies outlined in this review.
Highlights.
Bacterial translocation is the main mechanism in the pathogenesis of spontaneous infection in cirrhosis, and results from the following physiologic alterations in cirrhosis: altered gut microbiota, intestinal dysmotility, gut barrier dysfunction, and immune dysfunction
Our current strategy for preventing infections is the method of selective intestinal decontamination, which uses an antibiotic such as norfloxacin to change the taxonomy of gut microbiota. However, it is associated with the development of antibiotic-resistant organisms
Novel approaches to preventing bacterial infections include the following strategies: targeting altered gut microbiota using probiotics and rifaximin; targeting intestinal dysmotility with prokinetics, and beta-blockers; improving the intestinal barrier with bile acids and FXR agonists; and targeting immune dysfunction with G-CSF and statins.
Of these approaches, there is most evidence in human studies for probiotics, rifaximin, and beta-blockers.
There is insufficient evidence to officially recommend any of these strategies at this point.
Acknowledgments
The authors were supported by Yale Liver Center NIH P30 DK34989; and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH T35DK104689.
List of abbreviations
- AOCLF
Acute-on-chronic liver failure
- SBP
spontaneous bacterial peritonitis
- MDR
multidrug-resistant
- ESBL
extended spectrum β–lactamase
- CDI
Clostridium difficile infection
- CFU
colony-forming units
- RCT
randomized controlled trial
- FMT
fecal microbiota transplant
- LPS
lipopolysaccharide
- LBP
LPS-binding protein
- MELD
Model for End-Stage Liver Disease
- FXR
Farnesoid X-Receptor
- CCl4
carbon tetrachloride
- UDCA
ursodeoxycholic acid
- OCA
obeticholic acid
- TNF
tumor necrosis factor
- TLR
toll-like receptor
- Ig
Immunoglobulin
Footnotes
Declaration of interest: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
References
- 1.Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33(1):41–48. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 2.Fernández J, Acevedo J, Castro M, Garcia O, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology. 2012;55(5):1551–1561. doi: 10.1002/hep.25532. **This is a seminal prospective study on the epidemiology of bacterial infections.
- 3.Arvaniti V, D’Amico G, Fede G, Manousou P, et al. Infections in Patients With Cirrhosis Increase Mortality Four-Fold and Should Be Used in Determining Prognosis. Gastroenterology. 2010;139(4):1246–1256.e5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis: in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol. 2001;96(4):1232–1236. doi: 10.1111/j.1572-0241.2001.03708.x. [DOI] [PubMed] [Google Scholar]
- 5.Merli M, Lucidi C, Giannelli V, Giusto M, et al. Cirrhotic Patients Are at Risk for Health Care Associated Bacterial Infections. Clinical Gastroenterology and Hepatology. 2010;8(11):979–985.e1. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. Journal of Hepatology. 2012;56(Supplement 1):S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 7.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18(3):353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 8.Dirchwolf M, Podhorzer A, Marino M, Shulman C, et al. Immune dysfunction in cirrhosis: Distinct cytokines phenotypes according to cirrhosis severity. Cytokine. 2016;77:14–25. doi: 10.1016/j.cyto.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghoner A, et al. Patients with acute on chronic liver failure display ‘sepsis-like’ immune paralysis. Journal of Hepatology. 2005;42(2):195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Falguera M, Trujillano J, Caro S, Menendez R, et al. A Prediction Rule for Estimating the Risk of Bacteremia in Patients with Community-Acquired Pneumonia. Clinical Infectious Diseases. 2009;49(3):409–416. doi: 10.1086/600291. [DOI] [PubMed] [Google Scholar]
- 11.Fernández J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology. 2015 doi: 10.1002/hep.28330. Epub ahead of print. **Provides an in-depth discussion on antibiotic strategies in cirrhosis.
- 12.Hou MC, Lin HC, Liu TT, Kuo B, et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: A randomized trial. Hepatology. 2004;39(3):746–753. doi: 10.1002/hep.20126. [DOI] [PubMed] [Google Scholar]
- 13.Fernández J, Navasa M, Planas R, Montoliu S, et al. Primary Prophylaxis of Spontaneous Bacterial Peritonitis Delays Hepatorenal Syndrome and Improves Survival in Cirrhosis. Gastroenterology. 2007;133(3):818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 14.Bernard B, Grange JD, Khac EN, Amiot X, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: A meta-analysis. Hepatology. 1999;29(6):1655–1661. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 15.Tandon P, DeLisle A, Topal JE, Garcia-Tsao G. High Prevalence of Antibiotic-Resistant Bacterial Infections Among Patients With Cirrhosis at a US Liver Center. Clinical Gastroenterology and Hepatology. 2012;10(11):1291–1298. doi: 10.1016/j.cgh.2012.08.017.*
- 16.Jalan R, Fernandez J, Wiest R, Schnabl B, et al. Bacterial infections in cirrhosis: A position statement based on the EASL Special Conference 2013. Journal of Hepatology. 2014;60(6):1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, Ananthakrishnan AN, Hafeezullah M, Zadvornova Y, et al. Clostridium difficile Is Associated With Poor Outcomes in Patients With Cirrhosis: A National and Tertiary Center Perspective. Am J Gastroenterol. 2009;105(1):106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 18.Runyon BA. Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. AASLD Practice Guidelines. 2012 [Google Scholar]
- 19.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of Hepatology. 2010;53(3):397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 20.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. Journal of Hepatology. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Steffen EK, Berg RD, Deitch EA. Comparison of Translocation Rates of Various Indigenous Bacteria from the Gastrointestinal Tract to the Mesenteric Lymph Node. Journal of Infectious Diseases. 1988;157(5):1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- 22.Marteau P, Pochart P, Doré J, Béra-Maillet C, et al. Comparative Study of Bacterial Groups within the Human Cecal and Fecal Microbiota. Applied and Environmental Microbiology. 2001;67(10):4939–4942. doi: 10.1128/AEM.67.10.4939-4942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley EMM, Quera R. Small Intestinal Bacterial Overgrowth: Roles of Antibiotics, Prebiotics, and Probiotics. Gastroenterology. 2006;130(2, Supplement):S78–S90. doi: 10.1053/j.gastro.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Casafont Morencos F, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, et al. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Digestive Diseases and Sciences. 1996;41(3):552–556. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- 25.Guarner C, Runyon BA, Young S, Heck M, et al. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. Journal of Hepatology. 1997;26(6):1372–1378. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- 26.Bauer TM, Steinbruckner B, Brinkmann FE, Ditzen AK, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96(10):2962–2967. doi: 10.1111/j.1572-0241.2001.04668.x. [DOI] [PubMed] [Google Scholar]
- 27.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Alimentary Pharmacology & Therapeutics. 2009;29(12):1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 28.Koh IH, Guatelli R, Montero EF, Keller R, et al. Where is the site of bacterial translocation - small or large bowel? Transplant Proc. 1996;28(5):2661. [PubMed] [Google Scholar]
- 29.Llovet JM, Bartoli R, March F, Planas R, et al. Translocated intenstinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: molecular epidemiologic evidence. Journal of Hepatology. 1998;28(2):307–313. doi: 10.1016/0168-8278(88)80018-7. [DOI] [PubMed] [Google Scholar]
- 30.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. Journal of Hepatology. 2014;60(1):197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 31.Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26(1):17–25. doi: 10.1097/MOG.0b013e328333dc8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bass NM, Mullen KD, Sanyal A, Poordad F, et al. Rifaximin Treatment in Hepatic Encephalopathy. New England Journal of Medicine. 2010;362(12):1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, et al. Modulation of the Metabiome by Rifaximin in Patients with Cirrhosis and Minimal Hepatic Encephalopathy. PLoS ONE. 2013;8(4):e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupont HL. Biologic properties and clinical uses of rifaximin. Expert Opinion on Pharmacotherapy. 2011;12:293–302. doi: 10.1517/14656566.2011.546347. [DOI] [PubMed] [Google Scholar]
- 35.Mullen KD, Sanyal AJ, Bass NM, Poordad FF, et al. Rifaximin Is Safe and Well Tolerated for Long-term Maintenance of Remission From Overt Hepatic Encephalopathy. Clinical Gastroenterology and Hepatology. 2014;12(8):1390–1397.e2. doi: 10.1016/j.cgh.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28(3):450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 37.Hanouneh MA, Hanouneh IA, Hashash JG, Law R, et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012;46(8):709–715. doi: 10.1097/MCG.0b013e3182506dbb. [DOI] [PubMed] [Google Scholar]
- 38.Lutz P, Parcina M, Bekeredjian-Ding I, Nischalke HD, et al. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PLoS One. 2014;9(4):e93909. doi: 10.1371/journal.pone.0093909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rayes N, Seehofer D, Hansen S, Boucsein K, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74(1):123–127. doi: 10.1097/00007890-200207150-00021.*
- 40.Chiva M, Soriano G, Rochat I, Peralta C, et al. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. Journal of Hepatology. 2002;37(4):456–462. doi: 10.1016/s0168-8278(02)00142-3. [DOI] [PubMed] [Google Scholar]
- 41.Adawi D, Ahrne S, Molin G. Effects of different probiotic strains of Lactobacillus and Bifidobacterium on bacterial translocation and liver injury in an acute liver injury model. Int J Food Microbiol. 2001;70(3):213–220. doi: 10.1016/s0168-1605(01)00550-5. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez E, Neito JC, Boullosa A, Vidal S, et al. VSL#3 probiotic treatment decreases bacterial translocation in rats with carbon tetrachloride-induced cirrhosis. Liver International. 2015;35(3):735–745. doi: 10.1111/liv.12566. [DOI] [PubMed] [Google Scholar]
- 43.Rayes N, Seehofer D, Theruvath T, Schiller RA, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation - a randomized, double-blind trial. Am J Transplant. 2005;5(1):125–130. doi: 10.1111/j.1600-6143.2004.00649.x.*
- 44.Lata J, Novotny I, Pribramska V, Jurankova J, et al. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastro Hepatol. 2007;19(12):1111–1113. doi: 10.1097/MEG.0b013e3282efa40e. [DOI] [PubMed] [Google Scholar]
- 45.Liu Q, Duan ZP, Ha DK, Bengmark S, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39(5):1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 46.Bauer TM, Fernandez J, Navasa M, Vila J, Rodes J. Failure of Lactobacillus spp. to prevent bacterial translocation in a rat model of experimental cirrhosis. J Hepatol. 2002;36(4):501–506. doi: 10.1016/s0168-8278(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 47.Pande C, Kumar A, Sarin SK. Addition of probiotics to norfloxacin does not improve efficacy in the prevention of spontaneous bacterial peritonitis: a double-blind placebo-controlled randomized-controlled trial. Eur J Gastro Hepatol. 2012;24(7):831–839. doi: 10.1097/MEG.0b013e3283537d61. [DOI] [PubMed] [Google Scholar]
- 48.Konturek PC, Haziri D, Brzozowski T, Hess T, et al. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J Physiol Pharmacol. 2015;66(4):483–491. [PubMed] [Google Scholar]
- 49.Smits LP, Bouter KEC, de Vos WM, Borody TJ, Neiuwdorp M. Therapeutic Potential of Fecal Microbiota Transplantation. Gastroenterology. 2013;145(5):946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 50.Freedman A, Eppes S. 1805 Use of Stool Transplant to Clear Fecal Colonization with Carbapenem-Resistant Enterobacteraciae (CRE): Proof of Concept. Open Forum Infectious Diseases. 2014;1(suppl 1):S65. [Google Scholar]
- 51.Kao D, Roach B, Park H, Hotte N, et al. Fecal microbiota transplantation (FMT) in the management of hepatic encephalopathy (HE) Hepatology. 2015 doi: 10.1002/hep.28121. [DOI] [PubMed] [Google Scholar]
- 52.Chang CS, Chen G, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28(5):1187–1190. doi: 10.1002/hep.510280504.*
- 53.Code CF. The interdigestive housekeeper of gastrointestinal tract. Propect Biol Med. 1987;22:549–555. [Google Scholar]
- 54.Sarker SA, Gyr K. Non-immunological defence mechanisms of the gut. Gut. 1992;33(7):987–993. doi: 10.1136/gut.33.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chesta J, Defilippi C, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology. 1993;17(5):828–832. [PubMed] [Google Scholar]
- 56.Gunnarsdottir SA, Sadik R, Shev S, Simren M, et al. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98(6):1362–1370. doi: 10.1111/j.1572-0241.2003.07475.x. [DOI] [PubMed] [Google Scholar]
- 57.Madrid AM, Cumsille F, Defilippi C. Altered small bowel motility in patients with liver cirrhosis depends on severity of liver disease. Digestive Diseases and Sciences. 1997;42(4):738–742. doi: 10.1023/a:1018899611006. [DOI] [PubMed] [Google Scholar]
- 58.Chander Roland B, Garcia-Tsao G, Ciarleglio MM, Deng Y, Sheth A. Decompensated cirrhotics have slower intestinal transit times as compared with compensated cirrhosis and healthy controls. J Clin Gastroenterol. 2013;47(10):888–893. doi: 10.1097/MCG.0b013e31829006bb. [DOI] [PubMed] [Google Scholar]
- 59.Madrid AM, Brahm J, Buckel E, Silva G, Defilippi C. Orthotopic liver transplantation improves small bowel motility disorders in cirrhotic patients. Am J Gastroenterol. 1997;92(6):1044–1045. [PubMed] [Google Scholar]
- 60.Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96(4):1251–1255. doi: 10.1111/j.1572-0241.2001.03636.x. [DOI] [PubMed] [Google Scholar]
- 61.Perez-Paramo M, Munoz J, Albillos A, Freile I, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31(1):43–38. doi: 10.1002/hep.510310109.*
- 62.Pardo A, Bartoli R, Lorenzo-Zuniga V, Planas R, et al. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31(4):858–863. doi: 10.1053/he.2000.5746.*
- 63.Zhang SC, Wang W, Ren WY, He BM, et al. Effect of cisapride on intestinal bacterial and endotoxin translocation in cirrhosis. World J Gastroenterol. 2003;9(3):534–538. doi: 10.3748/wjg.v9.i3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandhu BS, Gupta R, Sharma J, Singh J, et al. Norfloxacin and cisapride combination decreases the incidence of spontaneous bacterial peritonitis in cirrhotic ascites. J Gastroenterol Hepatol. 2005;20(4):599–605. doi: 10.1111/j.1440-1746.2005.03796.x. [DOI] [PubMed] [Google Scholar]
- 65.Freestone PPE, Haigh RD, Williams PH, Lyte M. Stimulation of bacterial growth by heat stable norepinephrine-induced autoinducers. FEMS Microbiol Lett. 1999;172(1):53–60. doi: 10.1111/j.1574-6968.1999.tb13449.x. [DOI] [PubMed] [Google Scholar]
- 66.Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992;50(3):203–212. doi: 10.1016/0024-3205(92)90273-r. [DOI] [PubMed] [Google Scholar]
- 67.Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. Journal of Hepatology. 2013;58(5):911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Senzolo M, Cholongitas E, Burra P, Leandro G, et al. Beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver International. 2009;29(8):1189–1193. doi: 10.1111/j.1478-3231.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 69.Merli M, Lucidi C, Di Gregorio V, Giannelli V, et al. The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver International. 2015;35(2):362–369. doi: 10.1111/liv.12593. [DOI] [PubMed] [Google Scholar]
- 70.Abraldes J, Tandon P. The Use of Beta-Blockers in Advanced Cirrhosis Where Do We Stand? Current Hepatology Reports. 2015;14(1):46–52. [Google Scholar]
- 71.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41(3):422–433. doi: 10.1002/hep.20632. **Seminal review article on the mechanisms of bacterial translocation
- 72.Aranow JS, Fink MP. Determinants of intestinal barrier failure in critical illness. British Journal of Anaesthesia. 1996;77(1):71–81. doi: 10.1093/bja/77.1.71. [DOI] [PubMed] [Google Scholar]
- 73.Spaeth G, Gottwald T, Specian RD, Mainous RD, et al. Secretory immunoglobulin A, intestinal mucin, and mucosal permeability in nutritionally induced bacterial translocation in rats. Annals of Surgery. 1994;220(6):798–808. doi: 10.1097/00000658-199412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levi AC, Borghi F, Petrino R, Bargoni A, et al. Modifications of the trophism of intestinal mucosa after intestinal and bilio-pancreatic diversion in the rat. Ital J Gastroenterol. 1991;23:202–207. [PubMed] [Google Scholar]
- 75.Bertok L. Bile acids and endotoxins: physico-chemical defense of the body. Orvosi Hetil. 1999;140(1):3–8. [PubMed] [Google Scholar]
- 76.Pascual S, Such J, Esteban A, Zapater P, et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50(53):1482–1486. [PubMed] [Google Scholar]
- 77.Assimakopoulos SF, Tsamandas AG, Tsiaoussis GI, Karatza E, et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. European Journal of Clinical Investigation. 2012;42(4):439–446. doi: 10.1111/j.1365-2362.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- 78.Genescà J, Marti R, Rojo F, Campos F, et al. Increased tumour necrosis factor α production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut. 2003;52(7):1054–1059. doi: 10.1136/gut.52.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lorenzo-Zuniga V, Bartoli R, Planas R, Hofmann A, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37(3):551–557. doi: 10.1053/jhep.2003.50116. [DOI] [PubMed] [Google Scholar]
- 80.Saitoh O, Sugi K, Kojima K, Matsumoto H, et al. Increased prevalence of intestinal inflammation in patients with liver cirrhosis. World J Gastroenterol. 1999;5(5):391–396. doi: 10.3748/wjg.v5.i5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teltschik Z, Wiest R, Beisner J, Nuding S, et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised paneth cell antimicrobial host defense. Hepatology. 2012;55(4):1154–1163. doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 82.Sung JY, Shaffer E, Costerton JW. Antibacterial activity of bile salts against common pathogens. Effects of hydrophobicity of the molecule and the presence of phospholipids. Dig Dis Sci. 1993;38:2104–2112. doi: 10.1007/BF01297092. [DOI] [PubMed] [Google Scholar]
- 83.Floch MH, Gershengoren W, Elliott S, Spiro HM. Bile acid inhibition of the intestinal microflora - a function for simple bile acids. Gastroenterology. 1971;61:228–233. [PubMed] [Google Scholar]
- 84.Slocum MM, Sittig KM, Specian RD, Deitch EA. Absence of intestinal bile promotes bacterial translocation. Am Surg. 1992;58(5):305–310. [PubMed] [Google Scholar]
- 85.Ding JW, Andersson R, Soltesz V, Willen R, Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 1993;25(1):11–19. doi: 10.1159/000129252. [DOI] [PubMed] [Google Scholar]
- 86.Verbeke L, Farre R, Verbinnen, Covens K, et al. The FXR Agonist Obeticholic Acid Prevents Gut Barrier Dysfunction and Bacterial Translocation in Cholestatic Rats. The American Journal of Pathology. 2015;185(2):409–419. doi: 10.1016/j.ajpath.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 87.Inagaki T, Moschetta A, Lee YK, Peng L, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(10):3920–3925. doi: 10.1073/pnas.0509592103. **Elucidates the central role of FXR in protecting the gut from bacterial invasion
- 88.Lutz P, Berger C, Langhans B, Grunhage F, et al. A farnesoid X receptor polymorphism predisposes to spontaneous bacterial peritonitis. Digestive and Liver Disease. 2014;46(11):1047–1050. doi: 10.1016/j.dld.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Ubeda M, Borrero MJ, Lario M, Munoz L, et al. O153 The Farnesoid X Receptor Agonist, Obeticholic Acid, improves intestinal antibacterial defense and reduces gut bacterial translocation and hepatic fibrogenesis in CCl4-cirrhotic rats with ascites. Journal of Hepatology. 2014;60(1, Supplement):S63. [Google Scholar]
- 90.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malik R, Mookerjee RP, Jalan R. Infection and inflammation in liver failure: Two sides of the same coin. Journal of Hepatology. 2009;51(3):426–429. doi: 10.1016/j.jhep.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 92.O’Brien AJ, Fullerton JN, Massey KA, Auld G, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20(5):518–523. doi: 10.1038/nm.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tritto G, Bechlis Z, Stadlbauer V, Davies N, et al. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. Journal of Hepatology. 2011;55(3):574–581. doi: 10.1016/j.jhep.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 94.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune Dysfunction and Infections in Patients With Cirrhosis. Clinical Gastroenterology and Hepatology. 2011;9(9):727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 95.Gaia S, Olivero A, Smedile A, Ruella M, et al. Multiple courses of G-CSF in patients with decompensated cirrhosis: consistent mobilization of immature cells expressing hepatocyte markers and exploratory clinical evaluation. Hepatology International. 2013;7(4):1075–1083. doi: 10.1007/s12072-013-9473-9. [DOI] [PubMed] [Google Scholar]
- 96.Garg V, Garg H, Khan A, Trehanpati N, et al. Granulocyte Colony Stimulating Factor Mobilizes CD34+ Cells and Improves Survival of Patients With Acute-on-Chronic Liver Failure. Gastroenterology. 2012;142(3):505–512.e1. doi: 10.1053/j.gastro.2011.11.027.*
- 97.Duan XZ, Liu FF, Tong JJ, Yang HZ, et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2013;19(7):1104–1110. doi: 10.3748/wjg.v19.i7.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kedarisetty CK, Anand L, Bhardwaj A, Bhadoria AS, et al. Combination of Granulocyte Colony-Stimulating Factor and Erythropoietin Improves Outcomes of Patients With Decompensated Cirrhosis. Gastroenterology. 2015;148(7):1362–1370.e7. doi: 10.1053/j.gastro.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 99.Jain MK, Ridker PM. Anti-Inflammatory Effects of Statins: Clinical Evidence and Basic Mechanisms. Nat Rev Drug Discov. 2005;4(12):977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 100.Motzkus-Feagans C, Pakyz AL, Ratliff SM, Bajaj JS, Lapane KL. Statin use and infections in Veterans with cirrhosis. Aliment Pharmacol Ther. 2013;38(6):611–618. doi: 10.1111/apt.12430. [DOI] [PubMed] [Google Scholar]
- 101.Abraldes JG, Albillos A, Banares R, Turnes J, et al. Simvastatin Lowers Portal Pressure in Patients With Cirrhosis and Portal Hypertension: A Randomized Controlled Trial. Gastroenterology. 2009;136(5):1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 102.Mohanty A, Tate J, Garcia-Tsao G. Statins are Associated with a Decreased Risk of Decompensation and Death in Veterans with Hepatitis C-related Compensated Cirrhosis. Gastroenterology. doi: 10.1053/j.gastro.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


