Abstract
There is preliminary data indicating that patients with generalized anxiety disorder (GAD) show impairment on decision-making tasks requiring the appropriate representation of reinforcement value. The current study aimed to extend this literature using the passive avoidance (PA) learning task, where the participant has to learn to respond to stimuli that engender reward and avoid responding to stimuli that engender punishment. Six stimuli engendering reward and six engendering punishment are presented once per block for 10 blocks of trials. Thirty-nine medication-free patients with GAD and 29 age-, IQ and gender matched healthy comparison individuals performed the task. In addition, indexes of social functioning as assessed by the Global Assessment of Functioning (GAF) scale were obtained to allow for correlational analyzes of potential relations between cognitive and social impairments. The results revealed a Group-by-Error Type-by-Block interaction; patients with GAD committed significantly more commission (passive avoidance) errors than comparison individuals in the later blocks (blocks 7,8, and 9). In addition, the extent of impairment on these blocks was associated with their functional impairment as measured by the GAF scale. These results link GAD with anomalous decision-making and indicate that a potential problem in reinforcement representation may contribute to the severity of expression of their disorder.
Keywords: GAD, Decision-making, Stimulus-reinforcement learning, Passive avoidance, Global assessment of functioning, Reward, Punishment
1. Introduction
Generalized anxiety disorder (GAD) is associated with significant disability and characterized by excessive, uncontrollable worry (e.g., Whittchen et al., 1994). Occupied by worry, GAD patients may show impaired social, occupational, and role functioning, reflected in high rates of unemployment, divorce, emotional problems, and self-reported interference with daily activities (e.g., Henning et al., 2007; Massion et al., 1993).
The avoidance model of worry explains symptoms of GAD focusing specifically on thought processes. This model asserts that worry is a linguistic activity that inhibits vivid mental imagery and associated somatic and emotional evaluation (e.g., Borkovec et al., 2004). This emphasis on thought shares features with other theories that focus both on thoughts and associated neural processes. Specifically, in such neuroscience-based theories, worry is viewed as a prefrontal-based process that leads to top-down priming of semantic threat representations, which disrupt appropriate processing of current environmental stimuli and lead to impaired decision-making (Blair and Blair, 2012).
Reinforcement-based decision-making involves the selection of response options according to the reinforcement (reward/punishment) associated with these options. Such decision-making has been receiving more attention with respect to anxiety recently. Several studies have examined reinforcement-based decision-making in individuals reporting heightened anxiety (e.g., Luhman et al., 2011; Maner et al., 2007; Raghunathan and Pham, 1999) though only one study has directly examined this issue in patients with GAD (Devido et al., 2009). In this study, the performance of patients with GAD, Generalized Social Phobia (GSP) and healthy controls (HC) was compared on the differential reward/punishment learning task (Devido et al., 2009). This task assesses the individual’s ability to learn the value of objects and select between these objects to maximize reward/minimize punishment (Blair et al., 2006). Patients with GAD showed impaired performance on this task compared to both patients with GSP and HCs (Devido et al., 2009). Similarly, work with subclinical participants reporting heightened anxiety or intolerance of uncertainty has, for the most part, reported indications of impaired decision-making; the choices of high anxious individuals result in lower winnings compared to lower anxious individuals (e.g., Luhman et al., 2011; Maner et al., 2007; Raghunathan and Pham, 1999). For example, Raghunathan and Pham (1999) asked participants to choose between high-probability, small reward and low-probability, large reward monetary options. Higher anxiety individuals were significantly more likely to choose the high-probability, small reward options relative to individuals reporting low anxiety. Similar results have been found using the balloon analog risk task (BART) (Maner et al., 2007). In this task, participants earn monetary rewards as they “pump up” a balloon but can lose their earnings if the balloon is pumped too many times. Higher anxiety individuals anxious made significantly fewer pumps than individuals reporting low anxiety (Maner et al., 2007). Further, in recent work Luhman et al. used a form of delay discounting task where participants chose between small, low-probability rewards available immediately at the beginning of each trial and large, high-probability rewards only available after some variable delay (Luhman et al., 2011). This study reported that higher levels of intolerance of uncertainty were associated with a tendency to select the immediately available, but less valuable and less probable rewards. However, not all studies report impaired decision-making performance. Undergraduates meeting GAD criteria according to the generalized anxiety disorder questionnaire (GADQ) -IV (GAD analogs) showed superior performance on the Iowa gambling task than undergraduates not meeting criteria (Mueller et al., 2010).
Much of this literature has been interpreted as indicating that anxious individuals make decisions to avoid uncertain or risky consequences (Luhman et al., 2011; Mueller et al., 2010). However, it is worth considering an alternative explanation: individuals with GAD may be impaired in reinforcement-based decision-making. Considerable fMRI data has demonstrated that ventromedial prefrontal cortex (vmPFC), striatum and posterior cingulate cortex are importantly involved in the representation of reinforcement information (Clithero and Rangel, 2014). Lesions to vmPFC in particular have been shown to disrupt decision-making. Interestingly, with respect to impairments seen in patients with GAD, animal work indicates that orbital frontal cortex lesions (the region critically involved in the representation of reinforcement) promotes preference for the smaller and more immediate (as opposed to larger and delayed; c.f. Luhman et al., 2011) and the smaller and more certain (as opposed to larger and more probabilistic; cf. Raghunathan and Pham, 1999) of two reinforcers (Mobbini et al., 2002). Similarly, vmPFC atrophy in patients with frontal variant frontotemporal dementia is associated with a reduction of pumps on the Balloon Analog Risk Task (Strenziok et al., 2011). As such, it can be hypothesized that GAD involves impaired representation of reinforcement information within ventromedial prefrontal cortex (vmPFC) and associated structures and consequent decision-making impairment. Importantly, this second explanation has advantages over the avoidance hypothesis; it is unclear, for example, why a decision to avoid uncertain or risky consequences should result in increased errors when choosing between two stimuli associated with different levels of reward (cf. Devido et al., 2009).
Given the paucity of evidence on reinforcement-based decision-making in GAD, particularly work involving patients with DSM diagnoses of GAD, the goal of the current study was to examine the performance of patients on the passive avoidance (PA) learning task. The PA task was chosen because it allows a distinction between the vmPFC dependent reinforcement representation account of GAD briefly outlined above and hypotheses based on the suggestion that observed decision-making deficits in GAD reflect anxious individuals making decisions to avoid uncertain or risky consequences (Luhman et al., 2011; Mueller et al., 2010).
In the PA task, participants learn to respond to (approach) stimuli that engender reward and not respond to (passively avoid) stimuli that engender punishment (Finger et al., 2011). Not responding to a stimulus, passively avoiding it, results in neither reward nor punishment. Lesion and functional magnetic resonance imaging (fMRI) work suggest that vmPFC’s role in the representation of reinforcement is critical for successful performance on this task (Finger et al., 2011; Schoenbaum and Roesch, 2005). Deficits in reinforcement representation should increase commission errors (responding inappropriately to stimuli that engender punishment); the individual is less able to use the expected value of the stimulus to guide their decision-making. In contrast, if patients with GAD make decisions to avoid uncertain or risky consequences, then these individuals should make significantly more omission errors – they will be directly choosing to avoid uncertain or risky consequences by not responding to the stimuli. The current study tests these contrasting predictions.
2. Method
2.1. Patients
Thirty-nine patients with GAD and 29 healthy control individuals participated in the study and were paid for their participation. All patients met criteria for generalized anxiety according to the Structural Clinical Interview for DSM-IV (1994) Axis I disorders (SCID) conducted by a board-certified psychiatrist (First et al., 1997). Thirteen of the 39 patients with GAD were also co-morbid for GSP. All other co-occurring anxiety disorders as well as an ongoing diagnosis of major depressive disorder (MDD) were exclusory. All participants were required to be currently medication-free (no regular use of psychotropic medication within two weeks or fluoxetine/benzodiazepine within eight weeks of the study). Healthy comparison participants were in good physical health with no history of any psychiatric illness as confirmed by a physical exam and SCID.
Participants were matched on age, gender, and IQ as assessed by the verbal and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (see Table 1). Groups differed significantly on the Beck Anxiety Inventory (BAI) and the Inventory of Depressive Symptoms (IDS) (see Table 1). All participants provided written informed consent to participate in the study, which was approved by the NIMH Institutional Review Board. Recruitment occurred through NIMH Institutional Review Board (IRB) approved flyers and advertisements and through the NIH outpatient clinical services.
Table 1.
Subject characteristics (standard deviations in brackets).
| Patients with GAD (N=29) | Healthy Comparison (N=39) | |||
|---|---|---|---|---|
| Characteristic | Mean | (SD) | Mean | (SD) |
| Age (years) | 35.8 | (10.74) | 31.3 | (9.92) |
| IQa | 117.3 | (13.46) | 117.8 | (10.60) |
| GAF | 61.0 | (4.57) | – | – |
| BAI | 11.5 | (7.60) | 2.4* | (2.58) |
| IDS | 19.5 | (7.75) | 5.2* | (4.38) |
| Gender | 12 male | 14 male | ||
Key to Table 1: GAF=Global assessment of functioning; BAI=Beck anxiety inventory; IDS=Inventory of depressive symptoms.
Assessed with the Wechsler abbreviated scale of intelligence (two-subtest form)
significantly different at p<0.001
2.2. Tasks and measures
2.2.1. The passive avoidance (PA) task
The PA task was a modified version of a task used previously (Finger et al., 2011; Kosson and Newman, 1986). Stimuli consisted of numbers (i.e. 6, 18, 26, 32, 48, 51, 54, 61, 73 74, 89, 93) that were associated with specific levels of reward or punishment (winning/losing 1, 400, 800, 1200, 1600, or 2000 points). The relationship between, for example, responding to a stimulus and winning 1 point was constant across the whole task with that participant. The relationship between a number and the reward/punishment associated with it was randomized across participants.
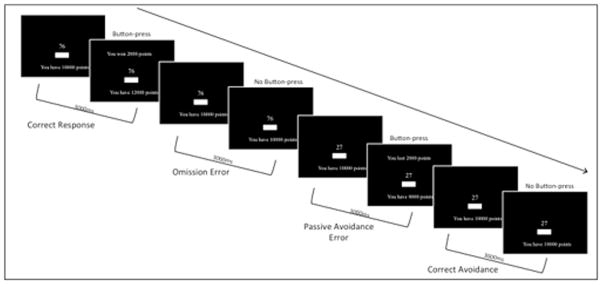
The stimuli were presented across ten blocks. In each block, each stimulus was presented to the participants in random order on a computer screen (i.e., there were 12 trials, one for each stimuli, within each block). Responding to half of the stimuli numbers engendered reward (gaining points) while responding to the other half engendered punishment (losing points). Not responding to a stimulus number elicited no reinforcement (no change in total points). All participants were allocated 10,000 points at the start of the test, and a running points total was visible on the screen throughout the task (see Fig. 1).
Fig. 1.
Illustration of the Passive Avoidance (PA) learning task.
The white stimuli numbers were each presented for 3000 ms on a black background. If a response was made during this time frame, a message appeared on the screen above the number, indicating how many points the participant had won (e.g., “You won 2000 points”) or lost (e.g., “You lost 2000 points). Participants were given the following instructions prior to starting the PA task: in this task, you are going to be presented with a series of numbers. Some of these numbers are good and will gain you points if you press the button when they are showing. Some are bad and will lose you points if you press the button when they are showing. If you do nothing you will neither gain nor lose points. Are you ready? Press the button to continue.
Participants were randomly assigned to two groups, and numbers that were associated with reward for one group were associated with punishment for the other group. Participants had to learn by trial and error to press the button on the screen in response to the reward-stimuli and to not respond to the punishment-stimuli.
Note that participants can make both commission (responding to “bad” stimuli associated with punishment) and omission (not responding to “good” stimuli associated with reward) errors. Further note that commission errors are the inverse of correct avoidances and omission errors are the inverse of correct hits; e.g., every extra “correct hit” means one less “omission errors.”
2.2.2. Beck anxiety inventory (BAI)
This measure assesses anxiety (Beck and Steer, 1993). It features 21 items, each describing a common symptom of anxiety. Each item is scored according to how bothersome the symptom is experienced by the subject (0 through 3).
2.2.3. Inventory of depressive symptoms (IDS)
This measure assesses level of depressive symptomatology (Rush et al., 1986). It features 30 items, each describing a symptom of depression. Two sets of symptoms are mutually exclusive (weight loss/gain and appetitive loss/gain), resulting in 28 scored items. Each item is scored according to best fit (0 through 3).
2.2.4. Global assessment of functioning (GAF)
Scores (0 through 100) on this scale indicates clinician ratings of social, occupational and psychological functioning. A higher score indicates a higher level of functioning. The GAF is part of the SCID-IV.
3. Results
A 2 (Group: GAD vs. HC) × 2 (Error type: Commission vs. Omission) × 10 (Block) ANOVA was conducted on the accuracy data. This revealed a highly significant main effect of both Error Type (F(1,66)=74.6; p<0.001; ); participants made more commission (M=2.20; SEM=0.124) than omission errors (M=0.93; SEM=0.087). There was also a highly significant main effect of Block (F(9,594)=82.23; p<0.001; ); participants made less errors as block increased. There was also a significant Error Type-by-Block interaction (F(9,594)=113.98; p<0.001; ); the decline in error rates was particularly notable for Commission errors (responding to the “bad” stimuli).
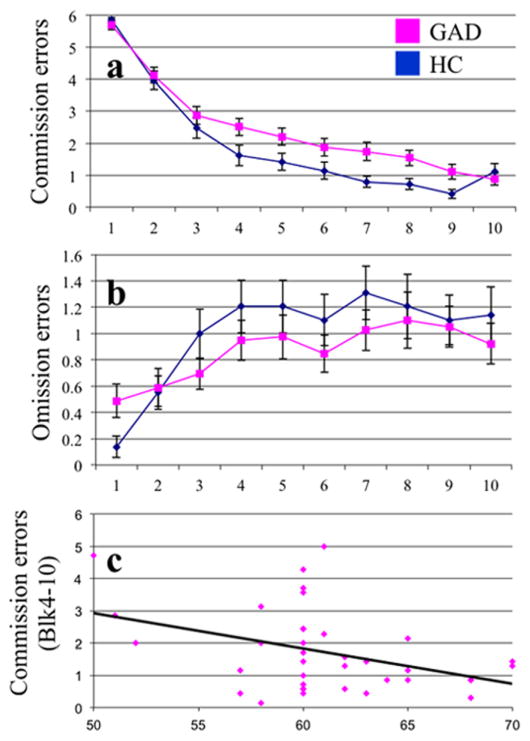
Importantly, there were a significant Group-by-Error Type interaction (F(1,66)=4.6; p<0.05 ). Patients with GAD showed significantly greater commission errors than HCs (M[GAD]=2.45; SEM=0.161; M[HC]=1.95; SEM=0.187) (F(1,66)= 4.12; p=0.046; ) but the groups did not differ in the number of omission errors (F(1,66)=0.76; p=0.449; ). There was also a significant Group-by-Error Type-by-Block interactions (F(9,594)=2.77; p=0.003 ). There were no group differences in omission errors. However, the group difference in commission errors emerged in the later blocks (blocks 7,8,9: F(1,66)=6.78 and 6.52 and 5.28 respectively; p=0.011 and 0.013 and 0.025; and 0.090 and 0.074); see Fig. 2.
Fig. 2.
Performance on the PA task by the GAD and HC groups. (A) Commission (passive avoidance) errors by block by group; (B) Omission errors by block by group; and (C) Correlation of commission errors (blocks 7–9) with GAF score.
Given that 13 of the 39 patients with GAD had comorbid GSP, we wanted to perform an additional analysis to determine whether the presence/absence of GSP would affect the behavioral profile on the task. Thus, a second ANOVA was conducted examining group differences between the 26 patients with GAD only and the HCs. This revealed comparable main effects of Error Type (F(1,53)=67.44; p<0.001; ) and Block (F(9,477)=70.13; p<0.001; ) as well as a comparable Error Type-by-Block interaction (F(9,477)=100.87; p<0.001; ). Critically, the Group-by-Error Type (F(1,53)=4.41; p=0.041; ) and Group-by-Error Type-by-Block (F(9,477)=2.80; p=0.003 ) interactions remained significant.
Given the significant group differences in IDS scores, we reexamined the data as a 2 (Group: GAD vs HC)x2 (Error type: Commission vs. Omission)x10 (Block) ANCOVA with IDS as the covariate. However, the group differences on task performance could not be attributed to group differences in depression symptoms as identified by the IDS. The ANCOVA revealed comparable main effects of Error Type (F(1,62)=19.73; p<0.001; ) and Block (F(9,558)=17.17; p<0.001; ) as well as a comparable Error Type-by-Block interaction (F(9,558)=23.61; p<0.001; ). Critically, the Group-by-Error Type (F(1,62)= 4.20; p=0.045; ) and Group-by-Error Type-by-Block (F(9,558)=2.26; p=0.017; ) interactions remained significant. Indeed, IDS was not a significant covariate on its own or in interaction with other variables. For completion, a last 2 (Group: GAD vs HC)x2 (Error type: Commission vs. Omission)x10 (Block) ANCOVA with BAI as the covariate was performed on the data. This also revealed comparable results to the previous ANOVA and ANCOVA.
3.1. Relationship between performance and clinical features
Because group differences in commission errors were seen for blocks 7–9, we next examined whether performance predicted symptom severity in the patients with GAD. While there was no significance relationship between performance and scores on the IDS, there was a significant association between commission errors (blocks 7–9) and score on the GAF (r= −0.379; p=0.021); i.e., the greater the number of commission errors the more the severity in presentation.
4. Discussion
The current study examined reinforcement-based decision-making in patients with GAD using the PA learning task. The study revealed significant impairment in PA learning and that the severity of this impairment predicted level of impaired functioning as measured by the GAF (though it did not predict anxiety/depression severity as measured by the BAI or IDS).
The observation that patients with GAD showed significant impairment on the PA learning task is consistent with the previous study with patients with GAD reporting impaired reinforcement-based decision-making on the differential reward/punishment learning task (Devido et al., 2009). While both groups showed evidence of reinforcement-based learning across the task the patients with GAD were slower to learn to avoid the stimuli that engendered punishment than the comparison individuals. Moreover, it is compatible with previous findings that patients with GAD report poor problem orientation and pathological ‘conceptual planning’ (see Roemer et al., 2002). Previous fMRI work with the current task and the differential reward/punishment learning task has shown that it implicates the role of vmPFC in the representation of reinforcement expectancies (Blair et al., 2006; Finger et al., 2011). As noted above, vmPFC lesions promote preference for the smaller and more immediate (as opposed to larger and delayed) and the smaller and more certain (as opposed to larger and more probabilistic) of two reinforcers (Mobbini et al., 2002) as well as reduce pumps on the BART (Strenziok et al., 2011). These decision-making biases are also seen in individuals with high self-reported anxiety (e.g., Luhman et al., 2011; Maner et al., 2007; Raghunathan and Pham, 1999) – though it should be noted that vmPFC lesions increase selection of the risky decks on the Iowa Gambling Task (Bechara et al., 2000) rather than reduce them as has been reported in undergraduates who met GAD criteria according to the GADQ-IV (Mueller et al., 2010). Notably, several studies have indicated vmPFC dysfunction in patients with GAD at least in the context of emotional conflict adaptation (Etkin et al., 2010; Etkin and Schatzberg, 2011). In short, the current results, and previous findings on decision-making in anxious populations, are generally consistent with the suggestion of a disruption in reinforcement signaling – either as a primary impairment or as a secondary consequence of pathological worry.
Notably, the current results are not clearly compatible with suggestions that the decision-making deficit in GAD reflects an increased propensity to make decisions that will avoid uncertain or risky consequences (cf. Luhman et al., 2011; Mueller et al., 2010). This position would seem to predict increased omission errors. By responding, the individual exposes him or herself to the risk of losing. Thus, a decision-making bias away from actions with uncertain or risky consequences should have resulted in an increase in omission errors and a decrease in commission errors (by not responding, the individual would avoid the rewarded stimuli but also avoid the punished stimuli). In contrast, patients with GAD showed no significant increase in omission errors and instead an increase in commission errors. The patients were effectively more often making choices that exposed them to uncertain or risky consequences.
Perhaps, the view that the decision-making deficit in GAD reflects an increased propensity to make decisions that will avoid uncertain or risky consequences (cf. Luhman et al., 2011; Mueller et al., 2010) needs revision. It could be suggested instead that rather than deciding to avoid actions with uncertain consequences, patients with GAD are driven by a need for certainty. The patients with GAD may have made more commission errors because they wished to collect more information to be certain about the stimulus value for future trials. This suggestion cannot be discounted. However, such a view would seem to suggest that patients with GAD should show increased risk taking; they should want to generate data regarding potential risk and uncertainty levels. This prediction would not seem to be supported.
Interestingly, in the current study, level of impairment predicted level of impaired functioning as measured by the GAF. Impairment in reinforcement-based decision-making will result in suboptimal decision-making in real world situations as well as the laboratory. This is perhaps most dramatically observed in patients with OFC lesions where the capacity to represent reinforcement can be severely compromised (e.g., Bechara et al., 2001). Indeed, it can be speculated, that this decision-making disruption may further exacerbate the patient’s worry. However, given that a measure of worry was not included in the current study, this speculation is in need or future testing.
Four caveats should be considered with respect to the current data. First, the current data cannot distinguish whether the difficulty with stimulus-reinforcement-based decision-making is primary or secondary to the pathophysiology associated with worry (i.e., the patients with GAD may have been attending to features of their foci of worry rather than representations necessary for successful task performance). Importantly, follow-up work can distinguish between these two positions. If patients with GAD show impairment in the representation of the expected value of a stimulus or in prediction error signaling (the signal, critical for stimulus-reinforcement learning, occurring when the individuals expected value does not match the value received; Rescorla and Wagner, 1972), we can predict reduced responsiveness to expected value within vmPFC and/or to prediction errors within caudate for example (cf. O’Doherty, 2012). Alternatively, if task performance is second to pathological worry, it can be predicted that the patients with GAD will show increased activity in regions previously implicated in worry that will not be differentially affected by expected value or prediction error. We are currently testing these predictions.
Secondly, the use of points as a secondary reinforcer in this study rather than other more salient reinforcers, such as money, could be criticized. However, fMRI work shows that very similar regions, in particular vmPFC (Kosson et al., 2006), are involved in the representation and anticipation of point gain as in financial gain (Knutson and Cooper, 2005). As such, although money may be a more salient secondary reinforcer than points gained, their computational implications are similar.
Third, the absence of a second population of patients with a different form of anxiety disorder means that we cannot be certain that the current results are specific to GAD rather than seen in anxiety more generally. However, it should be noted that a previous investigation of reinforcement-based decision-making did contrast the performance of patients with GAD with that of patients with GSP (Devido et al., 2009). This study reported no significant reinforcement-based decision-making impairment in patients with GSP at least. Importantly, though, the current results do suggest that this impairment is part of the pathophysiology of GAD even if we may later learn that it is not specific to GAD (as we now know that heightened amygdala responsiveness to fearful expressions is seen in both GSP and post traumatic stress disorder (PTSD) (Blair et al., 2008; Rauch et al., 2006) and that inadequate recruitment of top-down attentional systems important for emotional regulation is seen in GAD, GSP and PTSD (Blair et al., 2012, 2013).
Fourth, patients were excluded from the current study if, in addition to their disorder, they presented with common co-morbidities, including other anxiety disorders except GSP, substance use disorders, and severe mood disorders. Our goal was to determine the pathophysiology of GAD in a dataset where results could not be attributed to the presence of other comorbid conditions. However, this means that the patients studied were atypical to many anxiety patients presenting clinically and it may prove useful in future research to determine the extent to which these findings apply to patients presenting with significant co-morbidity.
In conclusion, the current study revealed impairments in stimulus-reinforcement-based decision-making in patients with GAD that related to the severity of their functional impairment as measured by the GAF. It now becomes important to determine whether this difficulty with stimulus-reinforcement-based decision-making is primary or secondary to the pathophysiology associated with worry.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health (Grant no. 1-ZIA-MH002860). The authors have no conflicts of interest or financial disclosures to report.
References
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. The Psychological Corporation Harcourt Brace & Company; San Antonio, TX: 1993. [Google Scholar]
- Blair KS, Blair RJR. A cognitive neuroscience approach to generalized anxiety disorder and social phobia. Emot Rev. 2012;4:133–138. [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, DeVido J, Otero M, Blair RJR, Pine DS. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Marsh AA, Morton J, Vythilingham M, Jones M, Mondillo K, Pine DS, Drevets WC, Blair RJR. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate cortex in object choice. J Neurosci. 2006;26:11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJR, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, Scaramozza M, Mondillo K, Pine DS, Charney DS, Blair RJ. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2013;43:85–95. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine O, Behar ES. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg R, Mennin D, Turk C, editors. Generalized Anxiety Disorder: Advances in Research and Practice. Guildford Press; New York: 2004. pp. 77–108. [Google Scholar]
- Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 2014;9:1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devido G, Jones M, Geraci M, Hollon N, Blair RJ, Pine DS, Blair K. Stimulus-reinforcement-based decision making and anxiety: impairment in generalized anxiety disorder (GAD) but not in generalized social phobia (GSP) Psychol Med. 2009;39:1153–1161. doi: 10.1017/S003329170800487X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, Pine DS, Blair RJR. Disrupted reinforcement signaling in the orbital frontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am J Psychiatry. 2011;168:834–841. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Henning ER, Turk CL, Mennin DS, Fresco DM, Heimberg RG. Impairment and quality of life in individuals with generalized anxiety disorder. Depression Anxiety. 2007;24:342–349. doi: 10.1002/da.20249. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Budhani S, Nakic M, Chen G, Saad ZS, Vythilingam M, Pine DS, Blair RJ. The role of the amygdala and rostral anterior cingulate in encoding expected outcomes during learning. Neuroimage. 2006;29:1161–1172. doi: 10.1016/j.neuroimage.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Newman JP. Psychopathy and the allocation of attentional capacity in a divided-attention situation. J Abnorm Psychol. 1986;95:257–263. [PubMed] [Google Scholar]
- Luhman CC, Ishida K, Hajcak G. Intolerance of uncertainty and decisions about delayed, probabilistic rewards. Behav Ther. 2011;42:378–386. doi: 10.1016/j.beth.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Maner JK, Richey JA, Cromer K, Mallott M, Lejuez CW, Joiner TE, Schmidt NB. Dispositional anxiety and risk-avoidant decision making. Personal Individ Differ. 2007;42:665–675. [Google Scholar]
- Massion AO, Warshaw MG, Keller MB. Quality of life and psychiatric morbidity in panic disorder and generalized anxiety disorder. Am J Psychiatry. 1993;150:600–607. doi: 10.1176/ajp.150.4.600. [DOI] [PubMed] [Google Scholar]
- Mobbini S, Brody S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Nguyen J, Ray WJ, Borkovec TD. Future oriented decision-making in generalized anxiety disorder is evident across different versions of the Iowa Gambling Task. J Behavioral Ther Exp Psychiatry. 2010;41:165–171. doi: 10.1016/j.jbtep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Beyond simple reinforcement learning: the computational neurobiology of reward-learning and valuation. Eur J Neurosci. 2012;35:987–990. doi: 10.1111/j.1460-9568.2012.08074.x. [DOI] [PubMed] [Google Scholar]
- Raghunathan R, Pham MT. All negative moods are not equal: motivational influences of anxiety and sadness on decision making. Organ Behavior Hum Decis Process. 1999;79:56–77. doi: 10.1006/obhd.1999.2838. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research – past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II. Century-Crofts; Appleton: 1972. pp. 64–99. [Google Scholar]
- Roemer L, Orsillo SM, Barlow DH. The Nature and Treatment of Anxiety and Panic. Guilford Press; New York, NY: 2002. Anxiety and its Disorders. [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The inventory for depressive symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M, Pulaski S, Krueger F, Zamboni G, Clawson D, Grafman J. Regional brain atrophy and impaired decision making on the balloon analog risk task in behavioral variant frontotemporal dementia. Cogn Behav Neurol. 2011;24:59–67. doi: 10.1097/WNN.0b013e3182255a7c. [DOI] [PubMed] [Google Scholar]
- Whittchen HU, Zhao S, Kessler RC, Eaton WW. DSM-III-R generalized anxiety disorder in the national comorbidity survey. Arch Gen Psychiatry. 1994;51:355–364. doi: 10.1001/archpsyc.1994.03950050015002. [DOI] [PubMed] [Google Scholar]