Abstract
Application of the CRISPR/Cas9 system to edit the genomes of human pluripotent stem cells (hPSCs) has the potential to revolutionize hPSC-based disease modeling, drug screening, and transplantation therapy. Here, we aim to provide a single resource to enable groups, even those with limited experience with hPSC culture or the CRISPR/Cas9 system, to successfully perform genome editing. The methods are presented in detail and are supported by a theoretical framework to allow for the incorporation of inevitable improvements in the rapidly evolving gene-editing field. We describe protocols to generate hPSC lines with gene-specific knock-outs, small targeted mutations, or knock-in reporters.
Keywords: CRISPR, pluripotent, stem cell, gene editing, knock-in
Human pluripotent stem cells (hPSCs) can theoretically be differentiated into any cell type in the body, making them a powerful tool for studying human differentiation, modeling disease, screening for drugs, and forming the basis of cellular therapies. The use of human embryonic stem cells (hESCs) derived from pre-implantation human embryos (Thomson et al., 1998) has been historically limited, but the advent of human induced pluripotent stem cells (hiPSC) (Nakagawa et al., 2008; Park et al., 2008; Takahashi et al., 2007; Yu et al., 2007) has enabled the wider research community to engage in hPSC research. In particular, the generation of patient-specific hiPSC lines opened the door to modeling a wide repertoire of human diseases in culture. Furthermore, cell banks of hESCs and hiPSCs and improved conditions for their culture (Beers et al., 2012; Chen et al., 2011; Ludwig and J, 2007; Soares et al., 2014) has made hPSC work accessible to non-specialist laboratories.
The utility of hPSCs can be further extended by gene editing, the process of making targeted, precise changes in their genomic DNA (Doudna and Charpentier, 2014). Genes can be knocked out to interrogate their role in biological processes. Alternatively, genetic variants of interest can be introduced or corrected to generate isogenic pairs of cell lines that can be compared to reveal cellular phenotypic differences attributable to the genetic variant (Ichida and Kiskinis, 2015; Merkle and Eggan, 2013). Since genotypic variability is a major driver of phenotypic variability, such isogenic models enable more subtle phenotypes to be detected than might be seen when comparing cells derived from two different individuals (Merkle and Eggan, 2013; Sandoe and Eggan, 2013). To permit these comparisons to be made in relevant cell types, the knock-in of reporter genes in loci that mark cell types of interest enables their isolation and manipulation.
Gene editing in hPSCs is facilitated by tailor-made nucleases that are targeted to DNA sequences of interest to produce double-strand breaks (DSBs). DSBs trigger the endogenous DNA repair machinery to repair the break via one of two pathways (Featherstone and Jackson, 1999). The error-prone non-homologous end-joining (NHEJ) pathway joins DNA ends in a manner that often introduces insertion and deletion (indel) mutations (Lieber, 2010). These indels can introduce frame-shifts and premature stop codons when targeted to open reading frames, thus disrupting gene function (Cho et al., 2013). Alternatively, DSBs can be resolved by homology directed repair (HDR), in which a template with sequence homology to the region containing the DSB is used to seamlessly repair the break (Jasin and Rothstein, 2013). HDR enables the targeted deletion or insertion of genetic sequences of interest. DSBs were first targeted to hPSCs using Zinc Finger Nucleases (ZFNs) (Urnov et al., 2010) and Transcription Activator-Like Effector Nucleases (TALENs) (Ding et al., 2013a). Although these technologies were breakthroughs at the time, the effort required for creating and validating these nucleases, whose DNA-binding specificity is mediated by protein domains, impeded their scalability and widespread adoption (Ding et al., 2013b; Hendriks et al., 2016).
In contrast, the prokaryotic Clustered Regularly-Interspaced Short Palindromic Repeat (CRISPR)/Cas9 system can be readily targeted to different DNA sequences (Jinek et al., 2012). This two-component system consists of a Cas9 nuclease and a CRISPR synthetic guide RNA (sgRNA), which contains a 20-base variable domain that mediates DNA-binding specificity. The sgRNA is a synthetic fusion of the naturally occurring bacterial CRISPR RNA (crRNA) that mediates binding specificity and a constant trans-activating CRISPR RNA (tracrRNA) that mediates the association of the crRNA/tracrRNA complex with Cas9 protein. As Cas9 scans the genome (Knight et al., 2015), the sgRNA base-pairs with complementary DNA sequences in the genome and when a perfect (or nearly perfect) match is detected and followed by a 3′ protospacer adjacent motif (PAM) (Sternberg et al., 2015), Cas9 will create a blunt-ended DSB three base pairs 5′ to the PAM (Jinek et al., 2012). The most commonly used Cas9 is isolated from the bacterium Streptococcus pyogenes (spCas9, referred to as Cas9 in this document) and has a PAM sequence of NGG. Cas9 orthologs isolated from different species and related proteins such as Cpf1 (Zetsche et al., 2015) have different PAM sequences, enabling virtually any genomic sequence to be targeted (Hendriks et al., 2016; Zhang et al., 2014b). The ease of changing this RNA sequence makes CRISPR/Cas9 a versatile and high-throughput tool for gene editing in hPSCs (Doudna and Charpentier, 2014; Hendriks et al., 2016).
This protocol is intended to serve as a reference for groups wishing to edit the genomes of hPSCs using the CRISPR/Cas9 system. While several excellent review articles and helpful protocols on this topic have recently been published (Anders and Jinek, 2014; Doudna and Charpentier, 2014; Gaj et al., 2013; Kime et al., 2016; Ran et al., 2013b; Song et al., 2014), we aim to provide all the crucial protocols in a single document to support groups with limited experience with hPSC culture or gene editing. Notably, since both the CRISPR/Cas9 system and tools and techniques for culturing hPSCs are rapidly evolving, the protocols described here are meant to provide a framework into which new advances can be incorporated. In particular, we describe protocols that enable the generation of gene knock-outs, small targeted mutations, and knock-in reporter hPSC lines. This document is organized into four sections:
-
Basic Protocol 1: Common procedures for CRISPR/Cas9-based gene editing in hPSCs
-
1.1)
sgRNA design
-
1.2)
sgRNA cloning into expression plasmids
-
1.3)
Plasmid DNA and PCR purification [Supporting protocol 1.1]
-
1.4)
sgRNA generation by in vitro transcription
-
1.5)
In vitro testing of sgRNA
-
1.6)
hPSC culture techniques for gene editing [Supporting protocol 1.2]
-
1.7)
CRISPR/Cas9 delivery into hPSCs
-
1.8)
Genomic DNA extraction [Supporting protocol 1.3]
-
1.9)
Barcoded deep sequencing
-
1.10)
PCR protocols [Supporting protocol 1.4]
-
1.1)
-
Basic Protocol 2: Generation of gene knock-out hPSC lines
-
2.1)
Sanger sequencing of mutant clones [Supporting protocol 2.1]
-
2.1)
-
Basic Protocol 3: Introduction of small targeted mutations into hPSCs
-
3.1)
Design of single-stranded oligodeoxynucleotides (ssODNs)
-
3.2)
3.2) Identification of targeted clones by ddPCR
-
3.2)
Identification of targeted clones by Sanger sequencing
-
3.1)
-
Basic Protocol 4: Generation of knock-in hPSC lines
-
4.1)
Gene targeting vector design
-
4.2)
Generation of the gene targeting vector
-
4.3)
Drug selection
-
4.4)
Confirmation of gene knock-in
-
4.5)
Excision of selection cassette
-
4.1)
Basic Protocol 1. Common procedures for CRISPR/Cas9-based gene editing in hPSCs
1.1. sgRNA design
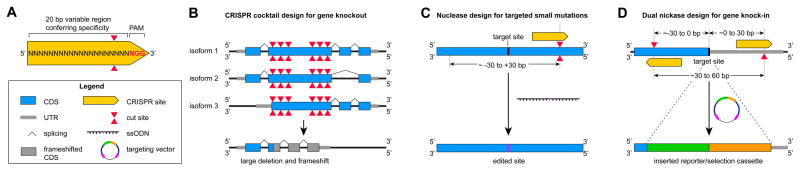
Gene targeting success largely depends on the design of the sgRNA (Fig. 1). The sgRNA should lead to high levels of on-target Cas9 activity, minimal off-target activity, and be located as close as possible to the site of gene targeting, generally within 30 bp (see also Critical Parameters). Most genomic loci will have suitable sgRNAs nearby, if not, alternatives to Streptococcus pyogenes Cas9 that have a different PAM, or designer nucleases such as TALENs, might enable efficient cutting closer to the target site. SgRNAs of interest can be cloned into an expression vector (protocol 1.2) to enable co-expression of the sgRNA, one of several Cas9 variants, and also a marker gene such as GFP or selectable marker such as puromycin to enable cells that have received CRISPR/Cas9 to be selected, if desired (Fig. 2). Alternatively, sgRNAs can be incorporated into a DNA template for in vitro transcription (protocol 1.4) enabling them to be tested in an in vitro cutting assay with Cas9 protein (protocol 1.5), and to be delivered to cells along with a Cas9 expression plasmid, Cas9 mRNA, or Cas9 protein to potentially reduce unwanted indel formation (Merkle et al., 2015; Ramakrishna et al., 2014). Alternative cloning or delivery strategies such as viral vectors for efficient gene knock-out (Sanjana et al., 2014) are discussed elsewhere (Arbab et al., 2015; Rahdar et al., 2015; Steyer et al., 2015; Xi et al., 2015).
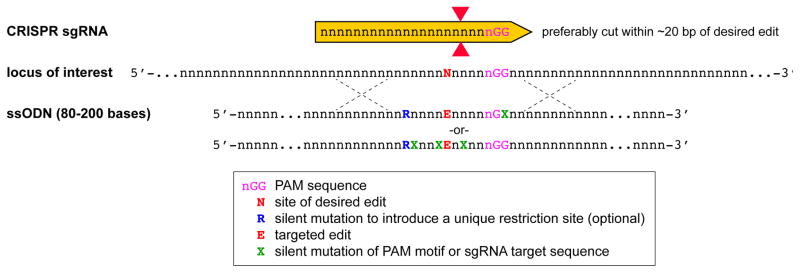
Figure 1.
CRISPR design for gene editing in hPSCs. A) Schematic DNA segment showing the 20-base binding site for a hypothetical sgRNA and the NGG protospacer adjacent motif (PAM) required for the Cas9 nuclease to introduce a DNA double-strand break three bases 5′ to the PAM. B) Efficient gene knock-out is achieved by targeting multiple sgRNAs to the same gene. For example, introducing multiple sgRNAs targeting the 5′ end of an exon and the 3′ end can increase the likelihood of recovering hPSC clones with large deletions. Since genes can have multiple splice isoforms and alternative start sites, it is advisable to target shared coding regions to ensure disruption of all isoforms. C) Small targeted mutations, such as single base changes or deletions or insertions of up to approximately 50-bp can be achieved by providing an ssODN template for homology directed repair. The CRISPR/Cas9 cut site should be designed to be as close as possible to the desired edit site. SsODNs carrying the desired mutation as well as silent mutations that mutate the PAM or disrupt the sgRNA binding region will prevent re-cutting by Cas9 after successful editing. D) The insertion of larger genetic elements such as reporter genes is mediated by gene targeting vectors. A dual nickase strategy enables the insertion of a reporter/selection cassette (green/orange) flanked by regions with sequence homology to the targeted genomic locus. Insertion of this reporter/selection cassette physically separates the sgRNA binding sites. Nicked DNA is repaired without indel formation, so this strategy protects the targeted locus from mutations due to continued Cas9 activity. CDS, coding sequence; ssODN, single-stranded oligodeoxynucleotide; UTR, untranslated region.
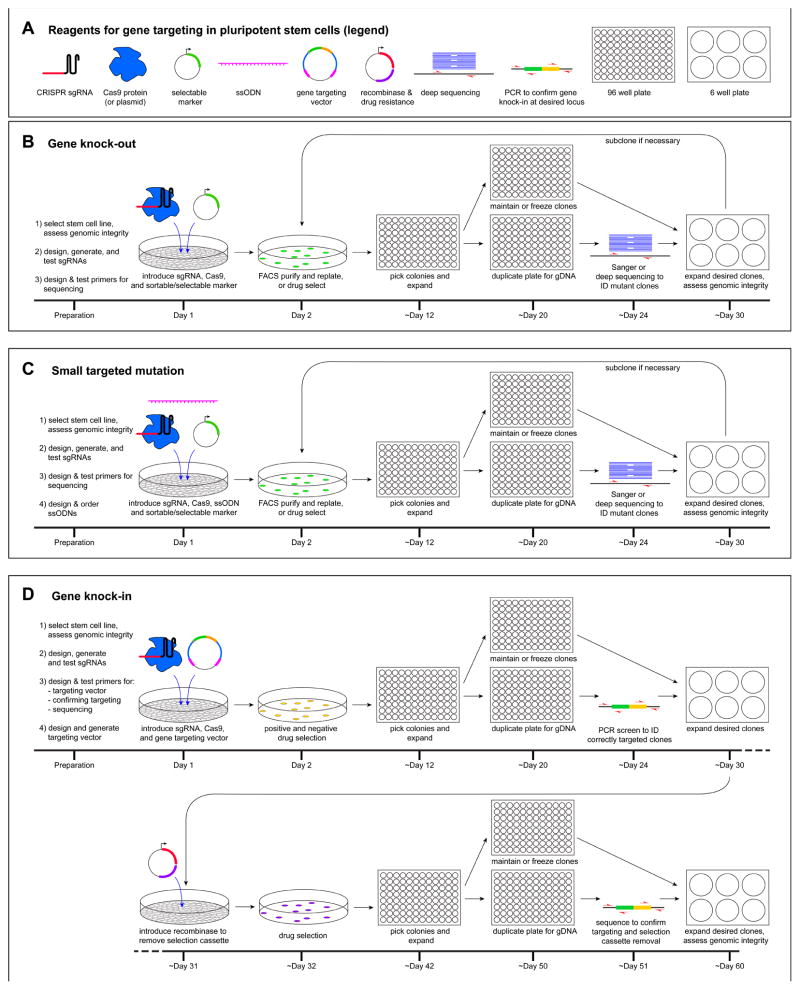
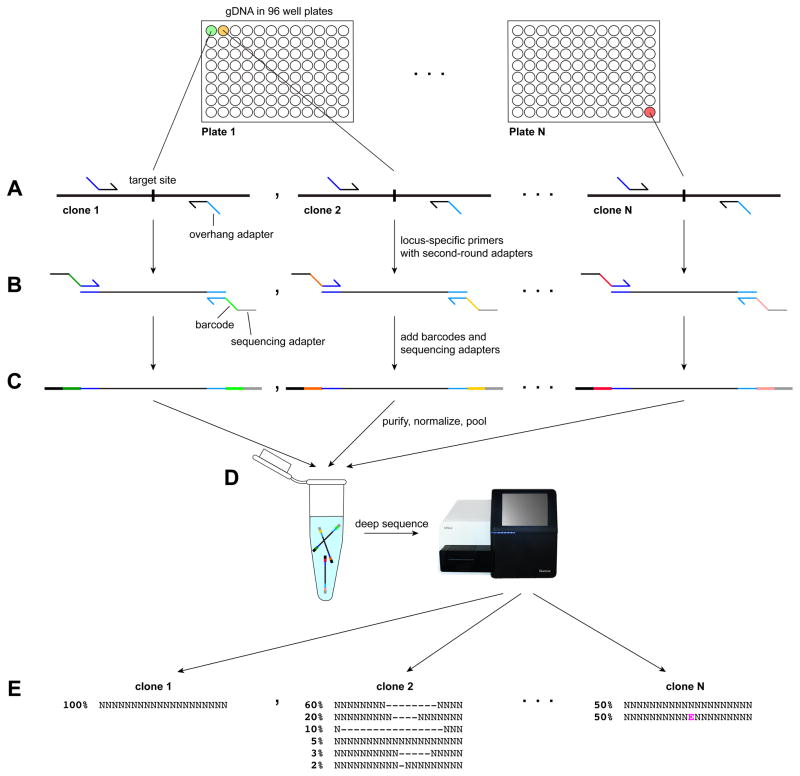
Figure 2.
Timeline and workflow for gene targeting in hPSCs. A) A subset of the essential reagents and steps required for gene targeting. B) Workflow for targeted gene knock-out. After designing and testing sgRNAs and PCR primers, sgRNAs and Cas9 are introduced into hPSCs by transfection or electroporation together with a selectable marker. Introduction of multiple sgRNAs targeting the same gene is advisable for gene knock-out. Co-introduction of a marker gene enables transfected cells to be purified by FACS or drug selection and then re-plated at clonal density. After colonies have reached a sufficient size, they are manually picked into 96-well plates, expanded, and split into two daughter plates. Genomic DNA is isolated from one daugther plate to enable screening by Sanger sequencing or barcoded deep sequencing to identify clones carrying desired mutations. These clones are then expanded from the second daughter plate and assessed for genomic integrity by karyotyping, SNP array, or sequencing. If the clone contains a heterogeneous mix of cells carrying different mutations, it can be subcloned to isolate the desired mutation. C) The workflow for introducing small targeted mutations is similar to that for gene knock-out, except that an ssODN is provided as a template for homology directed repair of the CRISPR/Cas9-induced DNA double-strand break. The rare cells that incorporate the desired mutation can be readily identified by Sanger sequencing or barcoded deep sequencing. D) The insertion of larger sequences such as reporter genes is relatively inefficient and requires a gene targeting vector and both positive and negative drug selection. Positive selection enables cells that have stably integrated the reporter to survive, whereas negative selection enriches for integration of the reporter in the desired genomic location. Correct targeting is confirmed by PCR screening. An additional subcloning step in which a recombinase is transiently introduced is required to remove the drug selection cassette. gDNA, genomic DNA; ID, identify; ssODN, single-stranded oligodeoxynucleotide.
Protocol Steps
Select an appropriate online tool for sgRNA design. There are multiple online bioinformatic tools to identify guide sequences with high predicted activity and minimal predicted off-target activity including CHOPCHOP (Montague et al., 2014), the CRISPR Design Tool (Hsu et al., 2013), the Broad Institute’s Genetic Perturbation Platform tool (Doench et al., 2016), and the Wellcome Trust Sanger Institute’s Genome Editing tool (Hodgkins et al., 2015). The tools from the Sanger Institute and from the Zhang laboratory score and rank pairs of dual nickases, eliminating the need to manually check that the requirements of overhang length and lack of off-target activity are met. Different gene targeting applications require distinct design approaches, as described in basic protocols 2, 3 and 4 (Fig. 1).
Using one of the online CRISPR design tools noted above, copy and paste the region to be targeted, along with about 100-bp upstream and downstream sequence, into the sequence field of the program. The tools will provide detailed instructions regarding the use of the online software. The output of each tool will be several target regions of 23-bases each, ranked by their predicted efficacy and lack of off-target sites (Fig. 1a).
Remove the PAM sequence from the guide sequence that has been chosen. This should leave you with a 20-bp guide sequence (N)20.
Since sgRNAs vary considerably in efficacy, and are readily designed, generated, and tested, it is advisable to design 2–3 sgRNAs per site of interest.
1.2. sgRNA cloning into expression plasmids
The generation of most sgRNA expression plasmids (Table 1) is based on a similar cloning strategy. The sgRNA sequence and its reverse complement are ordered as DNA oligos containing additional 5′ and 3′ sequences so that when annealed and phosphorylated, they generate a double-stranded oligo with sticky ends complementary to an expression plasmid digested with the restriction enzyme Bbs1 (Cong et al., 2013). After ligation, this expression plasmid containing the sgRNA sequence is transformed, amplified, sequence-verified, and can be used immediately for transfections. For the generation of gene knock-outs and targeted small mutations, plasmids containing Streptococcus pyogenes Cas9 nuclease and a marker gene such as GFP enable enrichment of transfected cells. For the generation of knock-in lines, a pair of plasmids should express Cas9 D10A nickase.
Table 1.
Plasmids and other available vectors for CRISPR/Cas9 delivery. 2A, 2A viral ribosomal skipping sequence; Puro, puromycin resistance gene; SpCas9, Streptococcus pyogenes Cas9; SpCas9n, Streptococcus pyogenes Cas9 D10A nickase.
| Plasmid | Addgene ID | Cas9 Version | Additional Elements | Reference |
|---|---|---|---|---|
| pX330 | 42230 | SpCas9 | N/A | (Cong et al., 2013) |
| pX335 | 42335 | SpCas9n | N/A | (Cong et al., 2013) |
| pX458 | 48138 | SpCas9 | 2A-EGFP | (Ran et al., 2013b) |
| pX459 | 62988 | SpCas9 | 2A-Puro | (Ran et al., 2013b) |
| pX460 | 48873 | SpCas9n | N/A | (Ran et al., 2013b) |
| pX461 | 48140 | SpCas9n | 2A-EGFP | (Ran et al., 2013b) |
| pX462 | 62987 | SpCas9n | 2A-Puro | (Ran et al., 2013b) |
| eSpCas9(1.1) | 71814 | eSpCas9 | N/A | (Slaymaker et al., 2016) |
| lentiCRISPR v2 | 52961 | SpCas9 | Lentiviral elements | (Sanjana et al., 2014) |
Materials
[Note: for supplier information and catalog numbers, see Table 2]
sgRNA oligos (two per sgRNA) (ordered on the smallest synthesis scale, 25 nM, purified by desalting)
sgRNA expression plasmid of choice
TOP10 chemically competent cells
Luria broth (LB) (see recipe)
Ampicillin (see recipe)
LB/Agar/Ampicillin plates (see recipe)
FastDigest BbsI or FastDigest BsmBI
FastAP
FastDigest Buffer (10x)
ddH2O
Gel loading dye, Purple (6x)
TAE (50x) (see recipe), diluted to 1x in H2O for use
Agarose powder
Ethidium bromide (see recipe)
Wizard SV Gel and PCR Clean-Up Kit (Promega)
sgRNA oligos (forward/reverse)
T4 DNA ligation buffer (10x)
T4 Polynucleotide kinase (PNK)
Quick ligation buffer (2x)
Quick ligase
Parafilm
QIAGEN Plasmid Plus Midiprep Kit
Sequencing primer (AGGGCCTATTTCCCATGATTCCTTCA)
PCR strips/tubes
1.5 mL microfuge tubes
Table 2.
Reagent suppliers and catalog numbers.
| Reagent | Supplier | Catalog Number |
|---|---|---|
| 0.22 μm PES Filter | Corning | 431118 |
| 0.75 ml 2D Barcoded, V Bottom Tubes, Sterile | Thermo Fisher Scientific | 3732 |
| 1 Kb Plus DNA Ladder | Thermo Fisher Scientific | 10787018 |
| 10-cm Tissue Culture Treated Plate | Corning | 430167 |
| 100 bp DNA Ladder | NEB | N3231S |
| 15-cm Tissue Culture Treated Plate | Corning | 430599 |
| 6-well Tissue Culture Treated Plate | Corning | 3516 |
| 96-well Tissue Culture Treated Plate | Corning | 3916 |
| Adhesive Foil for 96-well Plates, Pierceable | VWR | 60941-126 |
| Agarose | Sigma-Aldrich | A9539 |
| Ampicillin Sodium Salt | Sigma-Aldrich | A9518 |
| Anti-Digoxigenin-AP, Fab Fragments | Sigma-Aldrich | 11093274910 |
| BAC DNA Miniprep Kit | Zymo Research | D4048 |
| Betamercaptoethanol (BME) | Life Technologies | 21985-023 |
| Fibroblast Growth Factor Basic Protein, Human Animal-Free Recombinant (bFGF) | EMD Millipore | GF003-AF |
| Boric Acid | Sigma-Alrich | B6768 |
| Bromophenol Blue | Sigma-Aldrich | B0126 |
| β-Nicotinamide Adenine Dinucleotide (NAD+) | NEB | B9007 |
| CDP-Star, ready-to-use | Sigma-Aldrich | 11685627001 |
| CellTrace Calcein Red-Orange, AM | Thermo Fisher Scientific | C34851 |
| CellTrace Calcein Green, AM | Thermo Fisher Scientific | C34852 |
| Chemiluminesence Detection Kit | GE Healthcare | |
| Cryogenic Vials, Internal Thread (2.0 mL) | Corning | 431386 |
| dATP Solution (100 mM) | Thermo Fisher Scientific | 10216-018 |
| dCTP Solution (100 mM) | Thermo Fisher Scientific | 10217-016 |
| dGTP Solution (100 mM) | Thermo Fisher Scientific | 10218-014 |
| ddPCR Supermix for Probes (no dUTP) | Bio-Rad | 1863023 |
| DIG Wash and Block Buffer Set | Sigma-Aldrich | 11585762001 |
| DirectPCR Lysis Reagent (Mouse Tail) | Viagen Biotech Inc | 102-T |
| DL-Dithiothreitol (DTT) | Sigma-Aldrich | D9779 |
| DMEM | Corning | 10-013 |
| DMEM:F12 | Life Technologies | 11320082 |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich | D2650 |
| DNA Plasmid Plus Midi Kit | QIAGEN | 12945 |
| DNeasy Blood and Tissue Kit | QIAGEN | 69504 |
| dNTPs Mix (10 mM each) | Thermo Fisher Scientific | R0192 |
| DpnI | NEB | R0176S |
| Droplet Oil | Biorad | 186-3030 |
| dTTP Solution (100 mM) | Thermo Fisher Scientific | 10219-012 |
| Ethylenediaminetetraacetic Acid Disoduim Salt Dihydrate (EDTA) | Sigma-Aldrich | E1644 |
| Elution Buffer | QIAGEN | 19086 |
| Ethanol | VWR | 89125-186 |
| Ethidium Bromide | Sigma-Aldrich | E7637 |
| E.Z.N.A PF miRNA Isolation Kit | OMEGA Bio-Tek | R7036-01 |
| Falcon 40 μm Cell Strainer | Corning | 352340 |
| Fast Antarctic Phosphotase (AP) | Thermo Fisher Scientific | EF0654 |
| FastDigest BpiI (BbsI) | Thermo Fisher Scientific | FD1014 |
| FastDigest Buffer (10X) | Thermo Fisher Scientific | B72 |
| FastDigest Esp3I (BsmBI) | Thermo Fisher Scientific | FD0454 |
| GC Buffer (5X) | NEB | B0519S |
| Gel Loading Dye, Purple (6X) | NEB | B7024S |
| Geltrex, hESC-Qualified, Ready-To-Use, Reduced Growth Factor Basement Membrane Matrix | Thermo Fisher Scientific | A1569601 |
| GeneArt Platinum Cas9 Nuclease (1 μg/μL) | Thermo Fisher Scientific | B25640 |
| Geneticin (G418) | Life Technologies | 10131035 |
| Acetic Acid, Glacial, Reagent ACS | Acros | 42322-0025 |
| GlutaMAX Supplement | Life Technologies | 35050-061 |
| Glycerol | Sigma-Aldrich | G5516 |
| HilyMax | Dojindo Molecular Technologies, Inc. | H357-10 |
| Hyclone Fetal Bovine Serum (FBS) (U.S.) Defined | Fisher Scientific | SH3007003 |
| KnockOut DMEM (KO-DMEM) | Life Technologies | 10829-018 |
| Knockout Serum Replacement (KOSR) | Life Technologies | 10828-028 |
| LB Broth (Miller) Powder Microbial Growth Medium | Sigma-Aldrich | L3522 |
| LB Broth with agar (Miller) Powder Microbial Growth Medium | Sigma-Aldrich | L3147 |
| Magnesium Chloride (MgCl2) | Sigma-Aldrich | M8266 |
| Matrigel, hESC-qualified | Thermo Fisher Scientific | 08774552 |
| Matrix SepraSeal (Sterile) cryogenic tube closure mat | Thermo Fisher Scientific | 4464 |
| MEGAscript SP6 Kit | Ambion | AM1330 |
| MEGAshortscript T7 Transcription Kit | Thermo Fisher Scientific | AM1354 |
| MEM, Non-Essential Amino Acids (NEAA), no glutamine | Life Technologies | 10370-088 |
| Microseal ‘B’ Adhesive Film | Bio-Rad | MSB1001 |
| mTeSR1 | Stemcell Technologies | 05850 |
| NEON Transfection System | Thermo Fisher Scientific | MPK10096 |
| NEON Transfection System Pipette | Thermo Fisher Scientific | MPP100 |
| Novex TBE Gel, 4–20%, 10 well | Thermo Fisher Scientific | EC6225BOX |
| Nylon Membrane, Positively Charged | Sigma-Aldrich | 11209299001 |
| One Shot TOP10 Chemically Competent E. coli | Thermo Fisher Scientific | C4040-03 |
| Parafilm | Sigma-Aldrich | P7543 |
| Phosphate Buffered Saline (PBS), without calcium and magnesium (1x) | Corning | 21-040-CV |
| PCR Digest Probe Synthesis Kit | Sigma-Aldrich | 11636090910 |
| Poly(ethylene glycol), PEG-8000 | Sigma-Aldrich | P5413 |
| Penicillin-Streptomycin Solution, 100x | Corning | 30-002-Cl |
| Phenol/Chlorophorm/Isoamyl Alcohol | Life Technologies | 5593031 |
| PhiX Control v3 | Illumina | FC-110-3001 |
| Phusion HF DNA Polymerase | NEB | M0530 |
| Phusion Hot Start II DNA Polymerase | Thermo Fisher Scientific | F549 |
| Potassium Chloride (KCl) | Sigma-Aldrich | P9541 |
| Protienase K, Recombinant, PCR Grade | Sigma-Aldrich | 3115887001 |
| Puromycin | Life Technologies | A1113803 |
| QIAprep Spin Midiprep Kit | QIAGEN | 12143 |
| Quick Ligase | NEB | M2200 |
| Quick Ligation Buffer (2X) | NEB | E6010 |
| RNase A (17,500 U) | QIAGEN | 19101 |
| ROCK Inhibitor (RI) | Stemgent | Y27632 |
| Sodium Dodecyl Sulfate (SDS) | Sigma-Aldrich | L3771 |
| Sodium Chloride (NaCl) | Sigma-Aldrich | S7653 |
| Sodium Hydroxide Solution (10N) | Sigma-Aldrich | 656054 |
| SURVEYOR Mutation Detection Kit | IDT | 706025 |
| SYBR Gold Nucleic Acid Gel Stain | Thermo Fisher Scientific | S-11494 |
| T4 DNA Ligation Buffer | NEB | B0202S |
| T4 Polynucleotide Kinase (PNK) | NEB | M0201S |
| T5 Exonuclease | NEB | M0363 |
| Taq DNA Ligase | NEB | M0208 |
| Taq PCR Buffer (10X) | NEB | B9014S |
| Taq PCR master mix (2X) | NEB | M0270L |
| TOP10 Chemically Competent Cells | Life Technologies | C404003 |
| Tris Base | Fisher Scientific | BP152-1 |
| Trizma hydrochloride (Tris-HCl) | Sigma-Aldrich | 93363 |
| TrypLE Express Enzyme (1X) | Life Technologies | 12604-039 |
| Trypsin-EDTA (0.25%), phenol red | Thermo Fisher Scientific | 25200-056 |
| UltraPure Phenol:Chloroform:Isoamyl Alcohol (25:24:1, v/v) | Thermo Fisher Scientific | 15593-049 |
| Wizard SV PCR and Gel Clean Up Kit | Promega | A9282 |
Water bath set at 42°C
Thermocycler
Microwave
Electrophoresis gel box and power source
UV transilluminator
NanoDrop or similar spectrophotometer
Bacterial incubator (shaking/non-shaking)
Tabletop centrifuge (up to 20,000 x g for microfuge tubes)
Centrifuge (up to 10,000 x g for 15/50 mL conical tubes)
Micropipette
Pipette Aid
Protocol steps
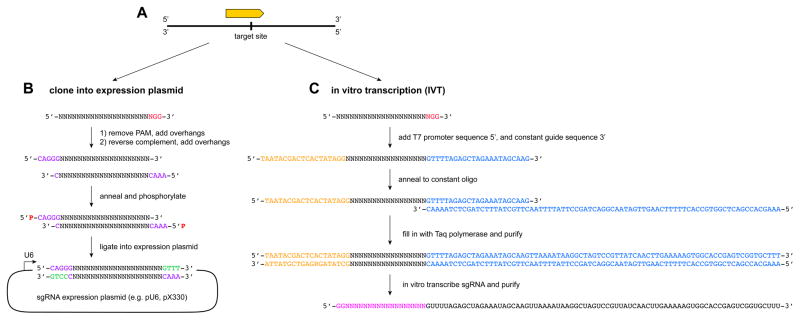
Choose the expression plasmid most suitable for your needs from the literature or from Table 1. Design sgRNAs as described in (protocol 1.1). The design software output will be a 23-bp sequence containing the 20-base variable region of the sgRNA and the 3′ PAM sequence (NGG). The sequence should then be modified according to the steps below (Fig. 3) to generate two oligos of 25-bp each.
Figure 3.
Workflow for sgRNA cloning. A) sgRNA variable regions are designed using available bioinformatics tools. B) If sgRNAs are to be cloned into an expression plasmid, the 20-base variable region is reverse complemented, and appropriate overhangs are added to variable sequence and its reverse complement. These are ordered as unmodified DNA oligos, which are then phosphorylated, annealed, and cloned into an appropriate expression plasmid cut with a restriction enzyme containing complementary sticky ends. The overhangs for a BbsI restriction site are illustrated. C) If sgRNAs are to be produced by in vitro transcription, the 5′-most base of the 20-base variable region is removed, and the T7 promoter sequence and a portion of the constant tracrRNA sequence are added to the 5′ and 3′ ends, respectively. This oligo is then annealed to a constant oligo, and the duplex is incubated with Taq polymerase to yield a double-stranded DNA template for in vitro transcription with T7 polymerase. The resulting RNA is purified and can be used for transfections or for in vitro cutting assays.
To the 5′-end of the 20-base guide sequence add 5′-CACCG-3′. The sequence of this oligo will be in the form of 5′-CACCG(N)20-3′ to generate an overhang compatible with a Bbs1 sticky end.
Reverse complement the 20-base guide sequence, add 5′-AAAC-3′ to the 5′ end, and add a C to the 3′ end, resulting in the following oligo: 5′-AAAC(N)20C-3′. This will generate an overhang compatible with a Bbs1 sticky end.
These two oligos should be ordered from your vendor of choice as desalted oligos at a standard synthesis scale (e.g. 25 nmol).
These oligos are then annealed, phosphorylated, and cloned into a BbsI-cut expression vector in a one-step protocol as previously described (Cong et al., 2013). It is critical to sequence the resulting clones. Digest/dephosphorylate 3 μg of expression plasmid with BbsI for 30 minutes at 37°C.
*Note: When cloning an sgRNA into LentiCRISPR v2 it will be necessary to digest the plasmid using FastDigest BsmBI as opposed to FastDigest BbsI. The design of the sgRNA oligos should be done in the same manner as all other sgRNA design.
| 3 μg | sgRNA expression plasmid |
| 2.5 μL | FastDigest BbsI* |
| 2.5 μL | FastAP |
| 5 μL | 10x FastDigest Buffer |
| x μL | ddH2O |
|
| |
| 50 L | total |
After digesting and dephosphorylating the plasmid, run the product on a 1% agarose gel in 1x TBE.
Cut the band out of the gel quickly, visualizing it using the longest wavelength setting under which the band can be visualized (i.e. 312 nm)
Dissolve the gel piece completely and purify it using the Promega Wizard SV Gel and PCR clean-up kit (protocol 1.3.2).
Dilute the purified plasmid backbone to 50 ng/μL in ddH2O. This purified plasmid is now ready for the ligation of the annealed oligos.
-
Phosphorylate and anneal each pair of sgRNA oligos.
1 μL Oligo 1 (100 μM) 1 μL Oligo 2 (100 μM) 1 μL 10x T4 DNA Ligase Buffer 6.5 μL ddH2O 0.5 μL T4 PNK
10 μL total -
Anneal this mixture in a thermocycler using the following conditions:
37°C 30 minutes 95°C 5 minutes 95°C to 25°C at a rate of −5°C/minute After annealing and phosphorylation, dilute 1 μl of the reaction product in 199 μl of ddH2O (1:200). The oligo duplex is now ready to be ligated into the expression plasmid.
-
Set up a ligation reaction, as indicated below, and incubate at room temperature for 10 minutes. As a control for incomplete BbsI digestion or dephosphorylation, set up and transform a parallel reaction in which the annealed oligos are replaced with water.
1 μL 50 ng BbsI-digested and dephosphorylated expression plasmid from step 1 (diluted to 50 ng/μL) 1 μL phosphorylated and annealed oligo duplex from step 2 (1:200 dilution) 5 μL 2x Quick Ligation Buffer 2 μL ddH2O 1 μL Quick Ligase
11 μL total -
Transform 1 μL of ligation reaction into chemically competent TOP10 E. coli (or other high-efficiency chemically competent strain) using a standard transformation protocol as shown below. Note that constructs containing repetitive elements such as viral LTRs are best transformed and maintained in a Stbl3 or similar strain.
Pre-warm LB/agar/ampicillin plate to 37°C
Thaw 50 μl chemically competent cells on ice.
Once thawed, add 1 μL ligation reaction and incubate on ice for 20–30 minutes.
Heat shock cells by placing them at 42°C in a water bath or heating block for 30 seconds.
Return cells to ice for 2 minutes.
Add 200 μL LB to cells and incubate, with shaking, for 1 hour at 37°C.
Plate 100 μL of recovered cells on a pre-warmed LB/agar/ampicillin plate.
Incubate overnight at 37°C.
-
The next day there should be dozens of colonies on the experimental plates and only a handful on the control plate. If so, amplify and purify the plasmid as follows.
NOTE: If control plates have many colonies, ensure that FastAP and FastDigest BbsI are fresh and active.In the morning, pick one colony into a 5 mL liquid LB starter culture containing 100 μg/ml ampicillin and incubate for 8 hours at 37°C under vigorous shaking (~300 RPM). Store the plate, wrapped in Parafilm, at 4°C until the sequence has been confirmed.
In the evening, inoculate a 50 mL liquid LB culture containing 100 μg/mL ampicillin with 50 μl of the 5 mL starter culture the 37°C under vigorous shaking (~300 RPM). At this point, it is advisable to make glycerol stocks from the starter cultures by removing 850 μL of bacterial suspension, adding 150 μL sterile glycerol, and snap freezing in labeled cryovials.
The following day, purify plasmid DNA from the liquid culture as described in (protocol 1.3.1).
-
Prepare the DNA for sequencing according to your sequencing facility’s recommendations with the following sequencing primer: AGGGCCTATTTCCCATGATTCCTTCA.
If the sequence is correct, the plasmid can be used for transfection into hESCs. If it is not, repeat steps 1–4.
1.3 [Supporting Protocol 1.1] Plasmid DNA and PCR purification
1.3.1. Plasmid DNA preparation for delivery to hPSCs
Human pluripotent stem cells are sensitive to bacterial endotoxins, so it is important that plasmids are prepared using a kit that removes endotoxins. Incomplete removal of wash buffer and other contaminants can dramatically increase the efficiency of downstream applications such as PCR and cloning. To ensure reproducible and efficient transfections, plasmids concentrations should be at least 1 μg/μL (preferably >2 μg/μL) for efficient transfection. Purification is performed using a QIAGEN Plasmid Plus Midi Kit and following manufacturer’s instructions with the following modifications:
Grow 50 mL LB liquid cultures containing the appropriate antibiotic overnight (12–16 hours).
Elute in 100 μL endotoxin-free elution buffer (Buffer EB).
1.3.2. Gel and PCR purification
The Promega Wizard SV gel and PCR purification kit is used to purify DNA from agarose gels and from PCR reactions according to manufacturer’s instructions with the following modifications:
Use an excess (350 μL) of membrane binding solution for all gel purifications.
For gel extractions, heat gel fragment and binding buffer to 65°C, flicking the tube every few minutes to mix until gel fragments are no longer visible. Then incubate for an additional 10 minutes before applying mixture to the column to ensure agarose is completely dissolved.
Perform two rinses with membrane wash soution, centrifuge the empty column, then remove it from the collection tube and air dry it for 5 minutes before placing it in a new collection tube for elution.
1.4. sgRNA generation by in vitro transcription
sgRNA, consisting of the 20-base variable region and 3′ constant tracrRNA region, can be directly transcribed in vitro with T7 polymerase from double-stranded DNA templates as previously described (Gagnon et al., 2014) (Fig. 3c). This approach enables the rapid testing of different sgRNAs by an in vitro cutting assay in combination with Cas9 protein (protocol 1.5.1) and provides sgRNA for direct transfection into cells. Wear gloves for all steps in this protocol, use fresh tips and trusted reagents, and take care to ensure that work surfaces and instruments are cleaned to remove potential sources of RNase.
CRISPR/Cas9 target sites should be designed with the help of online bioinformatic tools (protocol 1.1).
Delete the two most 5′ bases and the PAM from the 23-bp target site sequence to give an 18-bp core recognition motif. The two most 5′ bases will be replaced with GG to facilitate efficient T7 transcription.
-
To the 5′ end of this motif add the T7 promoter: TAATACGACTCACTATAGG, and to the 3′ end of the motif add part of the conserved tracrRNA domain: GTTTTAGAGCTAGAAATAGCAAG.
A modified sgRNA constant region thought to have increased stability and Cas9 affinity (Clontech) has a slightly different sequence, which would require the addition of the following 3′ sequence, with modified bases indicated in lower case (see also step 5 below): GTTTaAGAGCTAtgctgGAAATAG. For example, the oligo to order for a target sequence of interest would have the following sequence: TAATACGACTCACTATAGG(N)18GTTTTAGAGCTAGAAATAGCAAG
-
Order this oligo (desalting, smallest synthesis scale) and a constant oligo containing the remainder of the tracrRNA sequence (reverse complemented): AAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC.
The constant oligo for use with the enhanced sgRNA (Clontech) should have the following sequence, with modified bases indicated in lower case: AAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTaAACTTG CTAtgctgTTTCcagcaTAGCTCTtAAAC Reconstitute the oligos to 100 μM in ddH2O.
-
In a PCR strip, add 1 μL of the constant oligo and 1 μL of the T7-variable oligo together with 8 μL ddH2O (10 μL total). Anneal in a thermocycler with the following program:
95°C 5 minutes 95°C to 85°C −2°C per second 85°C to 25°C −0.1°C per second 4°C hold The annealed oligos will have single-stranded overhangs that can be filled in with Taq polymerase. To the annealed oligos, add 10 μL 2x Taq PCR master mix and incubate at 72°C for 15 minutes.
Purify the oligos using the Wizard SV Gel and PCR Clean-up System (protocol 1.3.2) and elute in 20 μL RNase-free water.
Analyze on a spectrophotometer to ensure a yield of approximately 100–200 ng/μL.
-
Run 5 μL of purified oligo on a 2% agarose gel with a 100-bp ladder.
The product is expected to have a size of approximately 120-bp -
For in vitro transcription, thaw T7 MEGAshortscript (Ambion) IVT components on ice and assemble reactions at room temperature in PCR strips, adding reagents in the order shown below. Incubate overnight at 37°C:
T7 10x Reaction Buffer 1 μL ATP Solution 1 μL GTP Solution 1 μL CTP Solution 1 μL UTP Solution 1 μL ddH2O 2 μL Oligo template 2 μL T7 Enzyme Mix 1 μL Add 19 μL ddH2O and 1 μL TURBO DNase (included in the MEGAshortscript IVT kit) and incubate at 37°C for 15 minutes.
-
Purify by Omega E.Z.N.A PF miRNA purification kit as follows:
To the 30 μL reaction, add 15 μL XD binding buffer and mix.
-
Add 54 μL (1.2 volumes) 100% RNase-free ethanol and mix.
Transfer the entire mixture to a HiBind MicroRNA column (take care not to use the HiBind X-Press column instead).
Centrifuge at 13,000 x g for 1 minute and discard the flow-through.
Add 500 μL XD Binding Buffer to the HiBind Micro RNA Column.
Centrifuge at 13,000 x g for 1 minute. Discard the filtrate and reuse the Collection Tube.
Add 500 μL RNA Wash Buffer II (diluted with ethanol according to manufacturer’s instructions) to the HiBind Micro RNA Column.
Centrifuge at 13,000 x g for 1 minute. Discard the filtrate and reuse the Collection Tube.
Repeat previous two steps to perform a second wash with RNA Wash Buffer II.
Centrifuge at 13,000 x g for 2 minutes, then remove the HiBind Micro RNA Column and air dry for 5 minutes before transferring to an RNase-free 1.5 mL microfuge tube for sample collection.
Add 30 μL RNase-free water directly onto the column and incubate at room temperature for 2 minutes.
Centrifuge at 13,000 x g for 1 minute. Move eluted RNA to ice.
Analyze on a spectrophotometer. Expected yield is >400 ng/μL.
-
Remove 2 μL of purified RNA, heat to 70°C in loading dye for 5 minutes and run on a 2% agarose gel.
The expected result is a single sharp band of approximately 100 bases. If RNA bands on the gel appear as a smear, it may suggest degradation from RNase contamination. Carefully clean all surfaces, use only trusted RNase-free reagents, and redo the IVT. If the RNA gel shows two or more sharp bands, it could suggest RNA secondary structure or multimerization. This does not necessarily abolish cutting activity. Ensure that the RNA is heated to 70°C before the gel is run. In the unlikely event that RNA yield is low, the IVT reaction can be scaled up and run for up to 18 hours. Store RNA at −80°C long-term, or use immediately to test sgRNA activity in an in vitro cutting assay (protocol 1.5.1).
1.5. In vitro testing of sgRNAs
Before embarking on a costly and time-consuming gene targeting experiment, it is important to ensure that sgRNAs are efficient at inducing a DSB in the region of interest. Both assays noted below are useful to assess the relative cutting efficiencies of sgRNAs. The principal advantage of the in vitro cutting assay is that it is rapid, scalable, and reproducible. Empirically, the in vitro cutting efficiency correlates with in vivo cutting efficiencies of sgRNAs (Gagnon et al., 2014), but this may be somewhat locus dependent. sgRNAs that show only weak activity at the highest Cas9 concentration should be avoided, whereas sgRNAs that show activity at the lowest Cas9 concentration generally also work well in vivo, assuming efficient delivery. The principal advantages of the in vivo SURVEYOR assay is that it permits cutting efficiencies to be quantified in the context of the complex intracellular milieu (Qiu et al., 2004) and enables the assessment of off-target cutting elsewhere in the genome. However, cutting efficiency in a heterologous cell type (HEK293T cells) may differ from that in hPSCs, the protocol is more labor intensive, and variability in transfection efficiency can complicate the interpretation of sgRNA activity.
Materials
[Note: for supplier information and catalog numbers, see Table 2]
PCR strips/tubes
1.5 mL microfuge tubes
Forward/reverse primers (amplifying CRISPR/Cas9-targeted loci)
ddH2O
GC buffer (5x)
dNTPs (10 mM each)
Purified human genomic DNA
100% DMSO
Phusion Hot Start II
TAE (50x) (see recipe), diluted to 1x in H2O for use
Ethidium bromide
Agarose
2% agarose gel
GeneArt Platinum Cas9 Nuclease (1 μg/μL)
Purified IVT sgRNA
Assay Buffer (see recipe)
RNase A (4 μg/μL)
Reaction stop buffer (see recipe)
10-cm tissue culture treated plate
6-well tissue culture treated plate
Trypsin-EDTA (0.25%) solution
Cell counter
sgRNA expression plasmid (exact expression plasmid will be determined by downstream application, e.g. pX330)
HilyMax (or preferred transfection reagent)
Cell scraper
QIAGEN DNeasy Blood and Tissue Kit
IDT SURVEYOR Mutation Detection Kit
10x Taq PCR buffer
Promega Wizard SV Gel and PCR Clean-Up Kit
4–20% gradient polyacrylamide TBE gel
Gel loading dye, purple (6x)
TBE (10x) (see recipe), diluted to 1x in ddH2O for use
SYBR Gold
Thermocycler
Microwave
Electrophoresis gel box and power source
UV transilluminator
NanoDrop or similar spectrophotometer
Tabletop centrifuge (up to 20,000 x g for microfuge tubes)
1.5.1. In vitro cutting assay with Cas9 protein
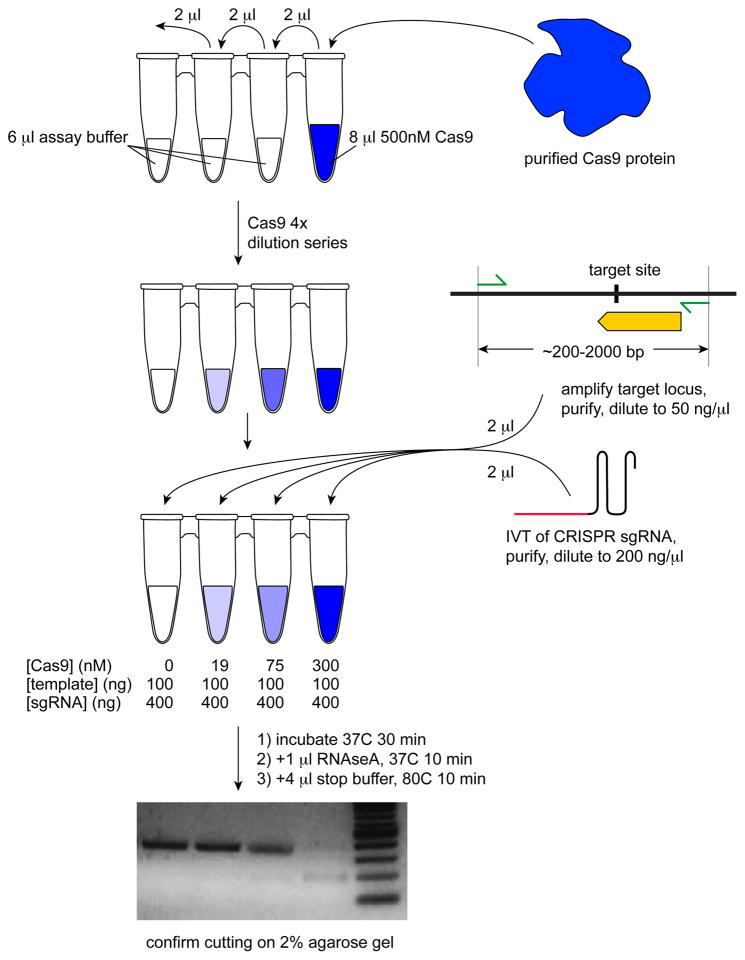
In vitro cutting is a quick and reliable method for testing the relative activity of sgRNAs prior to gene targeting in hPSCs. In this assay, sgRNA is provided in excess, and Cas9 protein is the rate-limiting reagent. Cas9 protein can be purchased from a number of commercial vendors. The genomic locus that will be tested for cutting should be PCR amplified from purified human genomic DNA (protocol 1.8.4), preferably from the hPSCs that will be targeted. The generation of sgRNA by in vitro transcription is described in protocol 1.4. The procedure for in vitro cutting is shown schematically in Figure 4.
Figure 4.
In vitro cutting assay to test sgRNA activity. Generate a 4x dilution series of Cas9 protein and add in vitro transcribed sgRNA and the DNA template containing the sgRNA target site. After a brief incubation, the sgRNA is digested away, and the reaction is visualized on a gel to confirm CRISPR/Cas9 activity and/or to compare the relative activities of different sgRNAs targeting the same locus.
Design primers so that the amplicon includes all targeted sites that will be interrogated to enable direct comparison. It is preferable to make cut site(s) slightly offset from the center of the amplicon and to avoid the extreme ends. Amplicons ranging in size from approximately 200–2000-bp work well.
Perform PCR amplification from purified genomic DNA with a high-fidelity polymerase such as Phusion Hot Start II (protocol 1.10.1) in reactions scaled to provide at least 1 μg of DNA.
Amplicons should be run on a 2% agarose gel to ensure they yield a single clean band of the expected size.
Purify amplicons with the Promega Wizard SV Gel and PCR Clean-up kit (protocol 1.3.2) and elute amplicons in 30 μL assay buffer.
Quantify purified amplicons by NanoDrop or similar spectrophotometer. Adjust concentration to 50 ng/μL with assay buffer.
Dilute Cas9 protein to 500 nM in assay buffer.
Dilute sgRNA to 200 ng/μL in RNase-free water.
In a strip of PCR tubes, add 6 μL assay buffer to tubes 1–3 of the strip and 8 μL 500 nM Cas9 protein to tube 4. Then add 2 μL 500 nM Cas9 from tube 4 to tube 3, mix, transfer 2 μL of this to tube 2, mix and remove and discard 2 μL of this mix. Since the final volume of the reaction is 10 μL, the final Cas9 concentrations are 0 nM, 19 nM, 75 nM, and 300 nM for tubes 1–4, respectively.
Add 2 μL sgRNA (200 ng/μL) and 2 μL purified PCR product (50 ng/μL) to each of the 4 tubes.
Incubate 30 minutes at 37°C.
Add 1 μL RNase A (4 μg/μL).
Incubate 10 minutes at 37°C.
Add 4 μL reaction stop buffer and incubate at 80°C for 10 minutes.
Run the full reaction (15 μL) on a 2% agarose gel and image. When comparing multiple sgRNAs, it may be helpful to group them by concentration.
More active sgRNAs will lead to more intense bands of smaller size than the uncut amplicon. Assuming that all tested sgRNAs have minimal predicted off-target activity, select the sgRNAs that show the greatest activity at the lowest Cas9 concentration.
In order to calculate the cutting efficiencies of each sample, the following equation can be used:
where a is the integrated intensity of the uncut PCR product and b and c are the integrated intensities of the cut PCR products.
1.5.2. SURVEYOR assay for CRISPR/Cas9 activity
The SURVEYOR assay has four major steps. First, a simple gene targeting experiment is undertaken in HEK293T cells after which genomic DNA is extracted. Second, a 500–2000-bp region around the site of targeting is amplified by PCR. It is important that the site of cutting is not centered on the PCR product as the assay relies on the ability to detect two unique cleavage products resulting from mismatches due to indel formation. This PCR product is then purified, denatured and re-annealed. This denaturing and re-annealing step will produce heteroduplexed DNA that can be recognized and cleaved by the SURVEYOR enzyme if a mismatch is present. The cleaved products are visualized on a gel to quantify cutting efficiency. When assaying cutting efficiency by the SURVEYOR assay it is important to include a sample of genomic DNA from mock transfected hPSC line to control for assay activity due to genomic SNPs rather than indels formed by Cas9-mediated NHEJ.
Expand HEK293T cells on a 10-cm plate and dissociate with Trypsin-EDTA (0.25%).
Count cells and plate ~600K cells per well of a 6-well plate. If you have multiple sgRNA expression plasmids to test, plate one well for each plasmid and leave one well for un-transfected cells.
The next day, transfect 1 μg of sgRNA expression plasmid per well using HilyMax or similar transfection reagent according to manufacturer’s recommendations.
Two days after transfection, harvest cells by scraping, pellet by centrifugation at 1000 RPM for 5 minutes, and extract genomic DNA using the DNeasy Blood and Tissue genomic DNA extraction kit (protocol 1.8.4), following manufacturer’s instructions.
Dilute genomic DNA to 250 ng/μL.
-
PCR amplify using Phusion Hot Start II polymerase (protocol 1.10.1) with the following components and cycling conditions:
ddH2O 31 μL GC Buffer 10 μL dNTPs 1.0 μL For Primer 2.5 μL (10 μM) Rev Primer 2.5 μL (10 μM) Human genomic DNA 1.0 μL (250 ng/μL) DMSO 1.5 μL Phusion Hot Start II* 0.5 μL 1. 98°C 30 seconds 2. 98°C 10 seconds 3. 65°C 30 seconds 4. 72°C 15 seconds 5. GOTO Step 2 29 more times 6. 72°C 5 minutes -
Run 5 μL of PCR product on a 1% agarose gel to check for single band products.
High-quality genomic DNA is critical for the success of the SURVEYOR assay. Following the PCR of the genomic region of targeted and untargeted cells, you should ensure that the PCR products yield bands of equal size and intensity on an agarose gel. If this is not the case, redo the PCR. If discrepancies remain, purify the genomic DNA using column purification or phenol/chloroform extraction and redo the PCR. -
In order to generate products that can be cleaved by SURVEYOR nuclease, the PCR products must be denatured and re-annealed in order to generate heteroduplexes with cleavable mismatches. Set up the annealing reaction as follows:
Taq PCR Buffer (10x) 2 μL Normalized PCR Product, 20 ng/μL 18 μL Total Volume 20 μL 1. 95°C 10 minutes 2. 95-85°C −2°C per second 3. 85°C 1 minute 4. 85-75°C −0.3°C per second 5. 75°C 1 minute 6. 75-65°C −0.3°C per second 7. 65°C 1 minute 8. 65-55°C −0.3°C per second 9. 55°C 1 minute 10. 55-45°C −0.3°C per second 11. 45°C 1 minute 12. 45-35°C −0.3°C per second 13. 35°C 1 minute 14. 35-25°C −0.3°C per second 15. 25°C 1 minute 16. 25-4°C −0.3°C per second 17. 4°C hold We have found that the addition of Taq PCR buffer to the reaction when generating the DNA heteroduplex can greatly improve the reliability of the assay, though this step deviates from the manufacturer’s protocol. -
Next, treat the heteroduplexes with SURVEYOR nuclease by setting up the following reaction on ice and incubating at 42°C for 30 min.
DNA Heteroduplex 20 μL MgCl2 (150 mM) 2.5 μL H2O 0.5 μL SURVEYOR Nuclease S 1 μL SURVEYOR Enhancer S 1 μL Total 25 μL Add 2 μL of Stop Solution if you do not intend to visualize the reaction product immediately.
For best resolution, products can be run on a 4–20% gradient polyacrylamide TBE gel. Load 15 μL of the product with 3 μL 6× purple dye and run the gel at 200 V for 45 min or until the dye front reaches the bottom of the gel. Include the DNA ladder and negative (untransfected) control on the same gel.
Stain the gel with SYBR Gold dye diluted 1:10,000 in 1× TBE for 15 minutes on a rocker at 50 RPM. Wash twice for 5 minutes each wash with 1× TBE on a rocker at 50 RPM.
-
Image the gel on an imaging system capable of visualizing SYBR Gold and quantify band intensities using Image Lab or equivalent software.
More active sgRNAs will lead to more intense bands of smaller size than the untransfected control. The size of these cleavage products is predictable based on the location of the cleavage site within the amplicon and the size of the amplicon.
1.6. [Supporting Protocol 1.2] hPSC culture techniques for gene editing
There are a number of different methods for culturing hPSCs that are compatible for use with gene editing (Merkle et al., 2015; Miyaoka et al., 2014; Ran et al., 2013a). Groups may wish to use their own preferred methods, but should be aware that conditions for routine hPSC maintenance may not be optimal for maintaining hPSC survival and pluripotency under the low densities and stressful conditions that cells experience during the gene editing protocols. In our hands, a 1:1 mixture of mTeSR1 and standard hPSC medium containing 20% knockout serum replacement (KOSR) and 100 ng/mL bFGF on a substrate of Matrigel or Geltrex robustly supports the culture techniques required for successful gene editing in multiple hESC and hiPSC lines (Merkle et al., 2015).
The methods described here cover the gene editing-specific variations of basic hPSC culture and assume mastery of basic tissue culture techniques and familiarity with hPSC culture. It is essential to use proper sterile technique. Lab coats and gloves should be worn at all times and all items coming into proximity of cells should be decontaminated liberally and often with 70% ethanol. Antibiotics should not be used for hPSC cultures (see Critical Parameters).
Groups with little to no hPSC experience are encouraged to consult useful reviews on the topic for more details (Chen et al., 2014; Vazin and Freed, 2010). Briefly, hPSCs should have a high nuclear:cytoplasmic ratio and prominent nucleoli when viewed under a phase-contrast microscope. They are grown under feeder-free conditions on Matrigel-coated plates and fed daily. Cultures are passaged non-enzymatically with 0.5 mM EDTA before cells reach 100% confluence and typically re-plated 1:10 in the presence of 10 μM Y27362 (RI). Cultures should have few to no cells with flat or elongated morphology, which are likely differentiated rather than pluripotent.
Differentiation in cultures can be a sign of a difficult cell line or sub-optimal growth conditions. If only a few cells are differentiated, they can be eliminated by gentle passaging since they tend to adhere more tightly to the plate than hPSCs. If differentiation is more widespread, circle the differentiated clusters with a marker under the microscope and aspirate those regions while changing media. In the case of widespread differentiation, circle un-differentiated colonies under the microscope and pick them into a fresh plate. Finally, it is worth noting that hPSCs are a sensitive cell type prone to genetic instability if handled improperly. Thus, we encourage groups to select cell lines at relatively low passage, if possible, and to characterize their genomic integrity before and after embarking on gene editing experiments by one of the following methods: karyotyping, SNP or CGH array, exome or whole genome sequencing (see Critical Parameters).
Materials
[Note: for supplier information and catalog numbers, see Table 2]
hESC/hiPSC line (low passage, karyotypically normal)
Matrigel or Geltrex hESC-qualified matrix
70% ethanol
DMEM:F12
Cryovials
10-cm tissue culture treated plate
15-cm tissue culture treated plate
96-well tissue culture treated plate
mTeSR1
hESC media (see recipe)
1:1 media (see recipe)
TrypLE express
10 mM Y27362 (ROCK Inhibitor, e.g. RI)
Tissue-culture grade Ca2+- and Mg2+-free PBS
Adhesive foil for 96-well plates
Proteinase K
DirectPCR (tail) lysis reagent
DirectPCR/ProK buffer
FBS
2× freezing media (see recipe)
0.5 mM EDTA
Water bath
Cell counter
Biosafety cabinet, class II or higher
Laminar flow hood
CO2 incubator
Hybridization oven capable of 50°C and 85°C
Vortexer
Mr. Frosty or other freezing container capable of cooling cells at a rate of −1°C per minute
1.6.1. Cell line selection and characterization
If possible, select a male cell line for gene editing to avoid the issue of erosion of X chromosome inactivation (Mekhoubad et al., 2012; Silva et al., 2008). The cell line should be karyotypically normal, low passage (<30), and previously been shown to remain pluripotent upon single cell passaging and to yield colonies from gene targeting. For disease modeling, preferably use cell lines that have been extensively SNP/CNP-typed or ideally whole genome sequenced. If this has not been performed for your cell line of interest, such as a patient-derived hiPSC line, it is worth considering given the time and expense that will be invested in gene editing experiments. See also Critical Parameters.
1.6.2. Coating plates with Matrigel
Matrigel is commonly used for hPSC feeder free culture. Geltrex is a suitable alternative that comes in a pre-diluted liquid format (Table 2).
Prepare Matrigel stock solution by thawing it slowly on ice. Different lots of Matrigel vary slightly in their protein concentration and therefore have slightly different dilution factors. Divide the volume of supplied Matrigel by the dilution factor (e.g. 5000 μL/250 = 20) to determine number of 600 μL aliquots that will be made. Calculate the total volume of diluted Matrigel based on the number of aliquots (e.g. 20*600 μL = 12,000 μL) and bring the Matrigel to this volume with ice-cold DMEM:F12. Make the calculated number of 600 μL aliquots and store at −20°C prior to use.
Thaw Matrigel aliquots slowly on ice and bring to 25 mL with ice-cold DMEM:F12.
To coat plates, add 1.5 mL ice-cold Matrigel solution to each well of a 6-well plate or add 8 mL per 10-cm plate. Shake from side-to-side and front-to-back to ensure an even coating.
Incubate at room temperature for at least 1 hour or at 37°C for at least 30 minutes for immediate use or store overnight at 4°C.
Prior to use, aspirate Matrigel, rinse plate with PBS, and add appropriate warm media to the plate.
1.6.3. Thawing cryovials
Prepare Matrigel-coated plates with 1:1 medium containing 10 μM Y27362 (1:1 + RI) and prepare additional 1:1 + RI (10 mL per cryovial) pre-warmed to 37°C.
Thaw cryovial in 37°C water bath until freezing medium containing the cell suspension has started to thaw and a small sphere of ice remains. Transfer the cells to 10 volumes of warm 1:1 + RI, taking care to limit the time cells are exposed to freezing medium.
Centrifuge thawed cell suspension at 1000 RPM for 5 minutes, re-suspend the pellet in warm 1:1 + RI and plate onto a Matrigel-coated 6-well plate or 10-cm plate containing 1:1 + RI, depending on the number of hPSCs in the vial.
Change medium to warm 1:1 without RI after 24 hours.
1.6.4. hPSC maintenance
Feed cells daily with mTeSR1 or 1:1 until colonies reach 1 mm in diameter (clearly visible to the naked eye) or the plate is 90% confluent.
Aspirate medium, wash cells briefly with PBS, and add 1 mL 0.5 mM EDTA per well of a 6-well plate, 5 mL per 10-cm plate, or 10 mL per 15-cm plate.
-
After 5 minutes at 37°C, cells should easily detach from the plate. This can be assessed under the microscope since cells should round up and take on a phase-bright appearance, and should easily lift off of the plate when media is pipetted onto the plate with a P1000. Aspirate EDTA (cells will remain attached), then gently wash cells off of the plate with 1:1 + RI using a P1000 (6-well plates) or a 5 mL serological pipette (10-cm plate) or 10 mL serological pipette (15-cm plate). Dilute cell suspension to the desired split ratio (typically 1:10 for maintenance) and plate onto Matrigel-coated plates in 1:1 + RI. If desired, the remainder of the cells can be frozen.
The preferred split ratio can be somewhat cell line-dependent. Some cell lines are best maintained by more frequent and gentle splits such as 1:4. Replace medium with 1:1 after 24 hours and continue daily feeding.
If expanding cells for transfection, a nearly confluent 10-cm plate yields approximately 1×107 cells, and a 15-cm plate yields approximately 3×107 cells, enough for approximately 10 transfections of 2.5×106 cell each.
1.6.5. Freezing cultures in cryovials
Grow the desired cell line to 80–90% confluence and ensure that there are very few, if any, differentiated cells.
Using a label maker (not by hand), label the desired number of sterile screw-top 2 mL cryovials (approximately 1–5×106 cells per vial) with your initials, the date, the cell line name and passage number, leaving space to write in the cell number.
Make 2× freezing medium and chill to 4°C.
Warm an appropriate volume of 1:1 + RI to 37°C.
Aspirate medium, wash with PBS, add 0.5 M EDTA, and dissociate cells in 1:1 + RI as described in (protocol 1.6.4), using 0.5 mL of 1:1 + RI for each cryovial.
Gently dissociate cells, taking care to avoid bubbles, until a uniform cell suspension is achieved.
Slowly but steadily over the course of 60 seconds under steady mixing, add an equal volume of ice-cold 2× freezing medium to the cell suspension.
Pipette out 1 mL of the cell suspension into each cryovial.
Rapidly cap vials, taking care to keep lids sterile, move them to a Mr. Frosty or similar device to ensure even freezing and immediately move cells to a −80°C freezer.
After 24 hours, transfer frozen vials to liquid nitrogen for long-term storage.
1.6.6. Plating of hPSCs at clonal density
When targeting hPSCs for gene knock-out or to introduce small targeted mutations, it is necessary to re-plate transfected cells at a low density to enable individual clones to be picked. Transfected cells can be enriched by drug selection if a selectable marker was introduced, or by fluorescence activated cell sorting (FACS) if a fluorescent marker was used. This procedure is also useful when sub-cloning cell lines to eliminate unwanted heterogeneity, in which case drug selection or FACS is unnecessary, so those steps can be omitted. The specifics for drug selection vary depending on the cell plating density and type of drug used, so the protocol below assumes that a fluorescent marker has been used to visualize transfected cells.
24–48 hours after transfection of necessary targeting components (protocol 1.7) and a fluorescent marker, dissociate cells to a single-cell suspension. Aspirate medium, wash cells briefly with PBS, and add 5 mL TrypLE Express per 10-cm plate.
After 3 minutes at 37°C, cells should easily detach from the plate. This can be assessed under the microscope since cells should round up and take on a phase-bright appearance, and should easily lift off of the plate when media is pipetted onto the plate with a P1000. Without removing TrypLE, gently wash cells off the plate with 1:1 + RI + 2% FBS using a 15 mL serological pipette and collect in a 50 mL Falcon tube.
Add a fluorescent live-cell marker other than the florescent marker used (e.g. calcein red AM or calcein green AM) to the dilution recommended by the manufacturer, then centrifuge at 1000 RPM for 5 minutes.
Re-suspend pellet in warm 1:1 + RI, pass the cell suspension through a 40 μm cell strainer, and bring to a FACS facility.
Perform FACS on a sorter capable of gentle sorting with a large nozzle (100 μm) and low sheath pressures (20–25 PSI) such as Beckman Coulter MoFlo or BD Aria-III to minimize mechanical stress to the hPSCs (Pruszak et al., 2007). Sort for the live-cell marker and fluorescent marker, and collect live cells in the top quartile of the fluorescent population into warm 1:1 + RI. Record the number of sorted cells.
Plate 9,000 cells per Matrigel-coated 10-cm plate containing warm 1:1 + RI. Since the surface area is 60 cm2, this corresponds a “clonal” plating density of 150 cells/cm2.
Replace medium with 1:1 after 24 hours and continue daily feeding until colonies have appeared that are visible to the naked eye (approximately 10 days).
1.6.7. Colony picking
Colonies are picked following drug selection or the plating of hPSCs at low (clonal) density of approximately 150 cells/cm2. The risk of contamination is greatest during colony picking. During this procedure, wear disposable sleeves, clean the area thoroughly with 70% ethanol, and use a fresh box of tips.
Once hPSC colonies are approximately 300 μm in diameter or are large enough to be visible to the naked eye they are ready to be picked. This will take roughly 7 – 10 days after plating at clonal density (Fig. 5). In advance of picking, identify colonies to be picked under a phase-contrast microscope or EVOS and circle with a marker for later identification. Mark and pick only round, well-isolated colonies to minimize the chance of cross-contamination.
Prepare a 96-well plate by coating it with Matrigel (50 μL per well) for 1 hour at 37°C using a multichannel pipette, then rinse with PBS and fill with 100 μL 1:1 + RI using a multichannel pipette.
Rinse the plate containing colonies with PBS and replace with 1:1 + RI.
Using a P200 pipette and fresh box of tips, pick colonies under a dissection scope with a heated stage in a laminar flow hood or EVOS microscope in a biosafety cabinet. Using the edge of the pipette tip, slice the colony into strips (perpendicular to axis of pipette), and then scrape up these strips (along axis of pipette) while sucking up the pieces, roughly 10–30 per colony. (Fig. 5). Transfer pieces and medium into the 96-well plate, keeping track of which wells have already received cells.
Figure 5.
Procedure for colony picking. A) Example of an hPSC colony of suitable size for picking. A colony of this size should be visible to the naked eye. Colonies are picked under sterile conditions. B, C) To ensure the successful propagation of a hPSC colony, use the edge of a fresh aerosol-barrier P200 pipette tip (bottom right) to cut the colony into strips spaced approximately 100 microns apart (B). Then push the tip perpendicular to these strips to scrape chunks of the colony off of the plate, gently aspirating them into the pipette tip as you go. Transfer these pieces into a fresh 96-well plate.
1.6.8. hPSC culture in 96-well plates
hPSC culture in 96-well plates is a quick and economical way to culture hundreds of clones in parallel. Cells are fed and dissociated with multichannel pipettes compatible with aerosol-barrier 200 μL tips, and old medium is aspirated using multichannel aspirators compatible with barrier-free 200 μL tips. This enables the use of fresh tips for each well, limiting the risk of cross-contamination.
Prepare 96-well plates by coating them with Matrigel (50 μL per well) for 1 hour at 37°C using a multichannel pipette, then aspirate, add 100 μL PBS, aspirate, and add 100 μL 1:1 + RI using a multichannel pipette.
Feed cells daily with 100 μL 1:1 medium using a multichannel pipette, changing tips between wells when aspirating and feeding. When cells are sparse, it is sufficient to feed every other day with 200 μL 1:1 medium. Clones will likely expand at slightly different rates. Once colonies become visible to the naked eye in a majority of wells, they should be dissociated on-plate with EDTA. To do so, aspirate medium, add 100 μL PBS with a multichannel pipette, aspirate, and add 50 μL EDTA with a multichannel pipette. Incubate at 37°C for 5 minutes or until cells start to adopt a phase-bright appearance under phase-contrast microscopy. Then aspirate EDTA from one row using fresh tips, and feed with 200 μL 1:1 + RI, pipetting up-and-down 5 times with a multichannel pipette to dissociate and disperse cells. They will re-attach and seed new colonies. Repeat for the remaining rows. The following day, feed with 1:1 as normal.
To split cells from one 96-well plate onto two plates, coat two plates with Matrigel and prepare them with 100 μL 1:1 + RI as described in step 1. Incubate cells with EDTA as described in step 3, but after dissociating cells from the plate with 200 μL 1:1 + RI transfer 100 μL to each of the two new plates, taking care to keep track of which wells were seeded with cells.
1.6.9. Freezing hPSC clones in 96-well format
There are two options to freeze cells in 96-well format: 96-well tissue culture plates and individual tubes in 96-place tube racks. Freezing cells in plates is simple and inexpensive, but the entire plate must be thawed in order to access clones of interest. Freezing cells in tubes enables individual clones of interest to be retrieved and thawed and is compatible with 2D barcoding to track samples. This approach is more expensive. Neither approach enables long-term storage in liquid nitrogen, so clones of interest should be thawed, expanded, and re-frozen in cryovials within a few months of targeting to ensure durable storage.
To freeze cells in plates, add EDTA and dissociate cells in 100 μL 1:1 + RI as described in (protocol 1.6.8). Once all wells have been dissociated, add an equal volume (100 μL) of 2x freezing medium to each well, mixing gently to minimize osmotic shock but working quickly enough to complete the plate within 5 minutes. Seal the plate with sterile foil, replace the lid, transfer it to a clean Styrofoam box, and place the box at − 80°C to freeze.
To freeze cells in 96-well tube racks, add EDTA and dissociate cells in 100 μL 1:1 + RI as described in (protocol 1.6.8) and transfer cell suspensions to 96-well tube rack filled with sterile 0.75 mL 2D-barcoded tubes. Add 100 μL of 2x freezing medium to each tube, mixing gently to minimize osmotic shock but working quickly enough to complete the plate within 5 minutes. Cap the tubes by firmly pressing in sterile SepraSeal caps, transfer the tube rack to a Styrofoam box, and place the box at −80°C to freeze. Cells frozen in tube racks can be moved to LN2 the following day. Note that cells frozen in plates can be stored at −80C for up to 6 months, but cannot be moved to LN2.
1.7. CRISPR/Cas9 delivery into hPSCs
Human pluripotent stem cells are relatively difficult to transfect, so efficiently delivering CRISPR/Cas9 and other gene targeting components such as ssODNs or gene targeting vectors into cells is a major determinant of gene editing success. This protocol describes the delivery of plasmid-encoded sgRNA and Cas9 with the NEON electroporation system. The advantages of this approach are that expression plasmids are well characterized and electroporation parameters are optimized, reliably delivering transfection efficiencies of 50–70%. However, plasmid-encoded Cas9 and sgRNA persist in cells for several days, increasing the likelihood of off-target mutations and heterogeneous clones that have multiple mutations at the two alleles of the targeted site (Merkle et al., 2015). Electroporation of in vitro transcribed sgRNA, which has a much shorter half-life (Kim et al., 2014), may address this concern, as may replacing plasmid-encoded Cas9 with Cas9 RNA or protein. Gene editing with Cas9 mRNA is efficient (Wang et al., 2013), and Cas9 protein is now available from a wide variety of commercial sources and is readily delivered into hPSCs where it mediates efficient gene editing with minimal off-target activity (Kim et al., 2014; Ramakrishna et al., 2014; Zuris et al., 2015). Furthermore, conditional (Davis et al., 2015) or inducible (Chen et al., 2015; Gonzalez et al., 2014) Cas9 systems may also be suitable choices for investigators, depending on their experimental needs. A growing range of commercially available lipid-based and other CRISPR/Cas9 delivery methods are becoming available and the user should select the preferred delivery method. For more detail, we refer readers to several recent reviews (Doudna and Charpentier, 2014; Hendriks et al., 2016). The protocol below is for the delivery of Cas9 and sgRNA via a single expression plasmid by electroporation with the NEON system.
Groups interested in using an electroporation system other than NEON, delivering Cas9 RNA or protein or in vitro transcribed sgRNA, or using different cell numbers or DNA concentrations should first optimize electroporation conditions. To do so, keep constant the cell number (e.g. 2.5×106) and the duration and number of pulses (e.g. 20 ms, 1 pulse) and adjust DNA concentration (e.g. 1–10 μg) and electroporation voltage (e.g. 1000–2000 V). Include a constant amount of a fluorescent marker plasmid (500 ng) to track transfection efficiency. Quantify the absolute number and percentage of fluorescent cells by FACS or fluorescence microscopy. Select the condition with the highest percentage of transfected cells in which cell survival is not dramatically reduced compared to mock transfected cells.
Materials
[Note: for supplier information and catalog numbers, see Table 2]
100 μL NEON pipette tips
R buffer
Electrolytic buffer (E2)
10-cm tissue culture plate 1:1 media
Matrigel
ROCK inhibitor
sgRNA/Cas9 expression plasmid(s) (nuclease or nickase)
ssODN (point mutant generation only)
Targeting plasmid (knock-in generation only)
1.5 mL microfuge tubes
Electroporation cuvette
Tissue-culture grade Ca2+- and Mg2+-free
TrypLE express
DMEM:F12
FBS
50 mL conical tube
NEON electroporation system
Cell counter such as Countess (Life Technologies)
Centrifuge
Water bath
Biosafety cabinet, class II or higher
Laminar flow hood
CO2 incubator
Before starting, assemble components to be electroporated. These include the CRISPR expression plasmid and depending on the application, an ssODN, fluorescent marker plasmid, or gene targeting plasmid. All DNA components should be purified using endotoxin free kits (protocol 1.3.1) and should have a concentration of at least 1 μg/μL, preferably 2 μg/μL or higher. For expression plasmids in which the sgRNA and Cas9 are encoded on the same plasmid, the following amounts should be used when electroporating 2.5×106 hPSCs:
Gene knock-out with up to 6 sgRNAs: 1 μg each sgRNA/Cas9 expression plasmid.
Small targeted mutation: 1 μg sgRNA/Cas9 expression plasmid(s), 4 μL of a 100 μM ssODN
Gene knock-in with dual nickases: 1 μg each sgRNA/Cas9 expression plasmid and 4 μg targeting vector.
For the electroporation conditions used, the total volume of all components should be kept under 5 μL and the total concentration of nucleic acids should be kept under 8 μg. Cells should spend as little time in resuspension (R) buffer as possible and should be plated into pre-warmed 1:1 + RI and returned to the incubator immediately after electroporation. Pre-labeling all plates and assembling all components helps minimize experimental time and increases the likelihood of success.
Prepare for electroporation by warming R buffer to room temperature, preparing 10-cm plates with Matrigel and 1:1 + RI as described in (protocol 1.6.2), and ensuring that components to be electroporated are at sufficient concentration (at least 1 μg/μL for DNA), and adding 3 mL electrolytic buffer (E2) to the electroporation chamber.
Wash plate of cells to be targeted with PBS. Add a sufficient volume of TrypLE Express to coat the plate and incubate at 37°C for 5 minutes.
-
After 5 minutes, remove plate from incubator and wash cells off the plate with 20 mL warm DMEM:F12 + 2% FBS + RI. Collect in a 50 mL conical tube.
Cells should dissociate easily and show no evidence of clumping. If they do, they were likely over-digested or triturated roughly. Repeat with a fresh plate and adjust digestion time accordingly. Take a sample of cells to count and spin cells down at 1000 RPM for 5 minutes.
-
After spinning, aspirate supernatant and re-suspend in warm R buffer to a density of 25 million cells/mL.
Cells should form a loose pellet that is easily re-suspended. Add 120 μL of cell suspension to the DNA mixture.
-
Aspirate the DNA and cell suspension with a 100 μL electroporation pipette. It is critical that no bubbles are present.
-
The following conditions should be used for the electroporation:
1600 V
20 millisecond
-
1 pulse
Some groups have also had success with conditions of 1050 V, 30 milliseconds, and 2 pulses. It may also be possible to scale down the electroporation to 10 μl tips.
-
After electroporation, transfer the 100 μL cell suspension to pre-warmed 1:1 + RI in a Matrigel-coated plate and ensure even distribution.
-
The next day, examine the plate to assess survival.
Typically, cells should be ~20% confluent the day after electroporation, but survival can be cell line dependent. Occasionally, cell survival is low after electroporation. This could be due to endotoxins or low DNA concentration, harsh dissociation or electroporation conditions or a cell line that is not well-adapted to feeder free culture and single-cell dissociation. As a negative control, include mock-transfected cells (electroporated without DNA), which should survive well.
1.8 [Supporting Protocol 1.3] Genomic DNA extraction
Genomic DNA is frequently extracted during hPSC gene targeting for a number of purposes, some of which require purified, high-quality DNA and some of which are designed for simplicity and scale. Protocol 1.8.1 should be used to generate DNA for targeting vector assembly, Southern blotting, or analysis of genomic integrity, and protocols 1.8.2 and 1.8.3 should be used to screen 96-well plates of clones for desired modifications. Protocol 1.8.4 can be used as template for the SURVEYOR assay and the in vitro cutting assay with Cas9 protein.
Materials
[Note: for supplier information and catalog numbers, see Table 2]
0.5M EDTA
TrypLE Express
Tissue-culture grade Ca2+- and Mg2+-free PBS
1.5 mL microfuge tubes
SDS Lysis Buffer (see recipe)
25:24:1 phenol:chloroform:isoamyl alcohol solution
100% Ethanol
70% Ethanol
TE (see recipe)
DirectPCR/ProK buffer (see recipe)
Microseal-B Adhesive Film
HotShot Component 1 (see recipe)
HotShot Component 2 (see recipe)
Centrifuge (capable of 4°C and room temperature)
NanoDrop or similar spectrophotometer
1.8.1. Genomic DNA extraction by phenol:chloroform:isoamyl alcohol
This protocol is used to extract genomic DNA to be used as a template for targeting vector assembly, Southern blotting, or analysis of genomic integrity by sequencing or SNP array. Take adequate precautions when working with phenol. Label all tubes and plates ahead of time to avoid mislabeling errors during transfers. Alternatively, genomic DNA can be extracted with commercially available kits such as the QIAGEN DNeasy Blood and Tissue Kit.
Dissociate 1–5 x 106 hPSCs using EDTA or TrypLE, centrifuge at 1000 RPM for 5 minutes, carefully remove supernatant and re-suspend pellet in 500 μl PBS
Transfer cells to a 1.5 mL microfuge tube, centrifuge at 300 x g for 3 minutes, carefully remove supernatant and re-suspend pellet in 500 μL of SDS lysis buffer.
Incubate at 50°C overnight.
In a chemical fume hood, add 500 μL of 25:24:1 phenol:chloroform:isoamyl alcohol solution. Mix the solutions by inverting the tubes vigorously for 1 minute. Spin the tubes in a microfuge at 14,000 x g for 5 min at room temperature.
-
Carefully transfer the top layer of solution into a new pre-labeled tube and add 1 mL of 100% ethanol. Mix the solutions by vigorously inverting the tube several times, then centrifuge at 4 °C at 14,000 x g for 20 minutes.
When transferring the phenol:chloroform-extracted supernatant, take care not to transfer the lower (organic) phase. It is better to leave some of the aqueous phase behind, especially if it is viscous and makes pipetting difficult. After centrifugation, a white pellet should be clearly visible. Carefully remove the supernatant with a P1000 and rinse the pellet with 1 mL of 70% ethanol. Centrifuge at 4°C at 14,000 x g for 10 min.
Carefully remove the supernatant with a P1000 and remove any residual 70% alcohol with a P20 and air-dry the pellet until no visible traces of liquid remain (about 10 minutes).
-
Add 100 μL TE to the pellet, flick the tube to dislodge the pellet, and incubate 2 hours at room temperature. Gently pipette up-and-down 5x with a P200 to mix, if necessary.
If reconstitution of DNA pellets is difficult, incubate the DNA and TE at 50°C for 1 hour and periodically flick the tube. If reconstituted DNA is highly viscous, add an additional 100 μL TE. Determine DNA concentration and purity by NanoDrop or similar spectrophotometer and store genomic DNA at 4°C for up to 1 week or at −20°C long-term. Avoid frequent freeze-thawing of genomic DNA.
1.8.2. Genomic DNA extraction from 96-well plates with DirectPCR/ProK buffer
This protocol is a rapid and high-throughput method of generating genomic DNA of sufficient quality to enable screening for small modifications and knock-ins (protocols 2.1, 4.3.1), as well as barcoded deep sequencing (protocol 1.9).
Culture hPSCs in 96-well plates as described in (protocol 1.6.8). Once most wells have become confluent, aspirate media with a multichannel pipette and rinse once with 100 μL PBS.
Aspirate PBS and add 50 μL of DirectPCR/ProK buffer.
Seal the top of the 96-well plate well with Microseal-B adhesive film and incubate plates overnight at 50°C in a hybridization oven.
The following morning, raise the temperature to 85°C for 2 hours to completely inactivate the Proteinase K.
After the overnight incubation, plates should be centrifuged for 1 minute at 2000 RPM.
Let plates cool to room temperature and mix well by pipetting up-and-down 8x with a P200 multichannel pipette changing tips as you go. Store at 4°C for up to one week, or at −20°C longer term. Avoid frequent freeze-thawing of genomic DNA. A 1:10 dilution of this purified DNA in water can be directly used as PCR template.
1.8.3. Genomic DNA extraction from 96-well plates with HotShot buffer
This protocol is a quick and easy method to generate genomic DNA for PCRs with small amplicons, such as ddPCR (protocol 3.2) and barcoded deep sequencing (protocol 1.9).
Culture hPSCs in 96-well plates as described in (protocol 1.6.8). Once most wells have become confluent, aspirate media with a multichannel pipette and rinse once with 100 μL PBS.
Aspirate PBS, add 50 μL HotShot component 1, seal the plate well with Microseal-B adhesive film, and incubate at 95°C for 30 min.
Cool plates to room temperature, spin down any condensation, and add 50 μL component 2, mixing well by pipetting up-and-down 8x with a P200 multichannel pipette.
Store at 4°C for up to two weeks, or at −20°C for up to 1 year. A 1:10 dilution of this mixture in water can be directly used as PCR template.
1.8.4. Genomic DNA extraction using QIAGEN DNeasy Blood and Tissue Kit
Genomic DNA purified from this spin column-based kit can be used as a template for the SURVEYOR assay (protocol 1.5.2) and is isolated following manufacturer’s instructions. We recommend that following the addition of 200 μL Buffer AL and vortexing, samples should be incubated at 56°C for 10 minutes.
1.9. Barcoded Deep Sequencing
A major challenge when performing gene editing in hPSCs is to identify correctly targeted clones, which can be rare or heterogeneous (see also Critical Parameters). A number of strategies to address this issue have emerged, including ddPCR (Hindson et al., 2011; Miyaoka et al., 2014), SMRT sequencing (Hendel et al., 2014), deconvolution of Sanger sequencing reads (Brinkman et al., 2014; Hill et al., 2014), and barcoded deep sequencing (Bell et al., 2014; Merkle et al., 2015; Quail et al., 2012) to allow for the efficient screening and identification of correctly targeted clones. The relative merits of these techniques are discussed elsewhere (Hendriks et al., 2016). Barcoded deep sequencing is a powerful technique since it provides hundreds to thousands of unique sequencing reads per clone and enables thousands of clones to be interrogated in parallel. This data enables the nature and frequency of mutations within clones to be accurately determined, and enables the identification of rare clones with desired edits. The data also provides insight into the level of sub-cloning that will be necessary to remove unwanted heterogeneity. A drawback of barcoded deep sequencing is that read lengths are short, requiring amplicons to be short. This technique therefore does not accommodate large deletions or insertions, which might be analyzed better by Sanger sequencing or gel electrophoresis.
The process of targeted amplicon deep sequencing involves an initial gene-specific PCR with overhang adaptors to generate an ~200-bp product around the region of interest (Fig. 6a). Barcoding primers and sequencing adapters are then ligated to the ends of the amplicon in a second short PCR reaction (Fig. 6b). Following this, the barcoded amplicons are normalized by approximate concentration, pooled, and gel purified (Fig. 6c). The resulting library is then sequenced on a MiSeq (Fig. 6d). A number of software packages are available to analyze the resulting data (Fig. 6e).
Figure 6.
Barcoded deep sequencing of gene-edited hPSC clones. A) Genomic DNA extracted from hPSCs is used as the template for PCRs to amplify the targeted region. These locus-specific PCR primers carry adapters that enable a second-round PCR. B) A brief second-round PCR adds unique barcodes and sequencing adapters to each amplicon. The schematic shown is for sequencing using dual-indexed, paired-end reads. C–D) Amplicons are gel-purified, concentration-normalized (C), and pooled into an amplicon library that can contain thousands of clones representing the same targeted locus or many different loci (D). PhiX is often spiked in to add diversity to the library and improve sequencing quality. E) Sequencing reads from each clone are aligned to a reference sequence and analyzed for mutations. In this example, clone 1 has no mutations and clone 2 is heterogeneous and would likely have to be subcloned. Clone N is an example of a successful heterozygous targeted editing experiment, in which 50% of the reads contain the desired single base change.
Materials
[Note: for supplier information and catalog numbers, see Table 2]
Genomic DNA (DirectPCR/ProK buffer-extracted)
GC buffer
dNTPs
Gene-specific forward/reverse primers (with overhang adaptors)
100% DMSO
Phusion Hot Start II Polymerase
Agarose
1% agarose gel
1.5% agarose gel
TAE (50x) (see recipe), diluted to 1× in H2O for use
Ethidium bromide
96-well PCR plates
Forward/reverse primers for adding Illumina sequencing adaptors and indices
Promega Wizard SV Gel and PCR Clean-Up Kit
PhiX Control V3
Thermocycler
Microwave
Electrophoresis gel box and power source
UV transilluminator
NanoDrop or similar spectrophotometer
Access to Illumina Miseq System and consumables
1.9.1 First round PCR with target gene-specific primers
This PCR amplifies the genomic region surrounding the targeted site and adds adapter overhangs.
-
Design primers to yield a 150–200-bp product (excluding the extension sequences) and have 5′ extensions that will enable the second round PCR. Note that amplicons of this size are designed assuming 150-base paired end reads. If longer reads are required, there are available sequencing kits and the primers can be spaced further from each other as appropriate. The extension sequences are:
Forward primer: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-3′
Reverse primer: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-3′
In a 96-well PCR plate, set up a 10 μL reaction with the PCR conditions given in (protocol 1.10.1) using 1 μL of genomic DNA (diluted 1:10) extracted with DirectPCR/ProK buffer (protocol 1.8.2) as template.
To confirm the PCR worked, select four clones from each targeted locus and run 2 μL of the product on a 1% agarose gel. If this PCR fails or gives multiple bands, re-design the primers.
1.9.2 Second PCR with barcoding primers
This PCR attaches sequencing adaptors and barcodes to the amplicons. Depending on the mode of barcoding chosen, there will either be a single reverse primer along with multiple unique forward primers (single indexing) or multiple unique forward primers with multiple unique reverse primers (dual indexing). For reference on primer design see the Illumina Customer Sequence Letter (https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/experiment-design/illumina-adapter-sequences_1000000002694-00.pdf) or the Microsynth Appnote on amplicon deep sequencing (http://www.microsynth.ch/download.php?file_id=615&download=true).
Dilute the first round PCR product 1:4 with ddH2O.
Pipette all components of a 10 μL PCR reaction (protocol 1.10.1) except primers and template into a 96-well plate.
Add barcoding primers from a stock plate, then add 1 μL of the diluted template, again taking care to prevent cross-contamination.
-
Run the following PCR protocol:
1. 98°C 30 seconds 2. 98°C 10 seconds 3. 66°C 20 seconds 4. 72°C 20 seconds 5. GOTO step 2, 20 times 6. 72°C 3 minutes 7. 4°C forever
1.9.3 Pool and gel-extract amplicons
Following the second round barcoding PCR, run 2 μL of each PCR product on a large gel and visualize on a gel imager.
Estimate DNA concentration from the band intensity to permit amplicon normalization for pooling.
Pool together amplicons produced from the same genomic primers. These should have the same size and roughly similar intensities. Add a larger volume for the weaker bands (e.g. 8 μl) and a smaller volume for the strongest bands (e.g. 1 μl).
The desired product should be approximately 300-bp in size. In some cases, prominent primer dimer approximately 175-bp in size can form. It is important to eliminate these by gel purification in a 1.5% agarose gel run long enough to clearly resolve the primer dimer and PCR product. In some instances, the volume of sub-pools will be too large to fit in a single gel lane. In this case, PCR purify the pooled products as described in (protocol 1.3.2) and elute in a smaller volume prior to loading them on the gel.
Cut the amplicons out of the gel and purify them as described in (protocol 1.3.2). Analyze all sub-pools by NanoDrop or similar spectrophotometer to ensure DNA is high quality and to determine concentration. Calculate the volume of each sub-pool based on its concentration and the number of samples represented to ensure an even representation of all amplicons, and combine into a single pool.
Prior to sequencing, spike in PhiX to a final concentration of 5% to improve sequencing quality of the relatively low-complexity amplicon pool.
1.9.4 Sequencing and analysis
Submit purified/pooled amplicons for sequencing according to the recommendations of your sequencing facility. Data can then be analyzed using a number of bioinformatics tools such as CRISPResso (https://github.com/lucapinello/CRISPResso) or Outknocker (http://www.outknocker.org) following software instructions. Note that it will be important to correlate barcodes in sequencing files with clones on the plate so that clones of interest can be expanded for downstream experiments.
1.10. [Supporting Protocol 1.4] PCR protocols
All PCRs are performed using Phusion Hot Start II DNA Polymerase. Reactions should be assembled on ice, adding reagents in the indicated order. For PCR reactions in 96-well plates, make a master mix of water, buffer, DMSO, dNTPs, polymerase, and primers, and distribute to each well with a multichannel pipette. Then add template DNA with a multichannel pipette, keeping track of which wells received template and making sure to change tips to prevent cross-contamination.
1.10.1. Genomic PCR for short amplicons
This reaction is used to screen for mutants in gene knock-out experiments, to confirm correct targeting in gene knock-in experiments, and to generate amplicons for barcoded deep sequencing and Sanger sequencing.
| 5.4 μL | ddH2O |
| 2.0 μL | GC Buffer (5x) |
| 0.3 μL | DMSO |
| 0.2 μL | dNTPs (10 mM) |
| 0.1 μL | Phusion Hot Start II DNA Polymerase |
| 0.5 μL | Forward Primer (10 μM) |
| 0.5 μL | Reverse Primer (10 μM) |
| 1.0 μL | genomic DNA (protocol 1.8.2), diluted 10-fold in ddH2O |
Run the following program:
Incubate at 98°C for 30 seconds
Incubate at 98°C for 10 seconds
-
Incubate at 72°C for 20 seconds
Decrease by 0.5°C every cycle
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 2 for 23 more times
Incubate at 98°C for 10 seconds
Incubate at 60°C for 30 seconds
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 6 for 11 more times
Incubate at 72°C for 5 minutes
Incubate at 4°C forever
1.10.2 Genomic PCR for larger amplicons
This reaction is used to test primers to amplify the 2–4 kb genomic locus that includes the target site as a first step in targeting vector design. Once a suitable primer pair has been identified, scale the reaction up to 100 μL and add an additional 8 cycles in step 9 of the PCR program to generate sufficient quantities of DNA for purification. The program can also be used to generate template DNA for in vitro cutting assay or SURVEYOR assay.
| 5.4 μL | ddH2O |
| 2.0 μL | GC Buffer (5x) |
| 0.3 μL | DMSO |
| 0.2 μL | dNTPs (10 mM) |
| 0.5 μL | Forward Primer (10 μM) |
| 0.5 μL | Reverse Primer (10 μM) |
| 1.0 μL | Purified genomic DNA (protocol 1.8.1) from the hPSC line to be targeted (100 ng/μL) |
| 0.1 μL | Phusion Hot Start II DNA Polymerase |
Run the following program:
Incubate at 98°C for 30 seconds
Incubate at 98°C for 10 seconds
-
Incubate at 72°C for 20 seconds
Decrease by 0.5°C every cycle
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 2 for 23 more times
Incubate at 98°C for 10 seconds
Incubate at 60°C for 30 seconds
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 6 for 11 more times
Incubate at 72°C for 5 minutes
Incubate at 4°C forever
1.10.3 Homology arm PCR (10 μL)
This reaction is used to test primers to amplify the homology arms for gene targeting vectors. Once a suitable primer pair has been identified, scale the reaction up to 100 μL and add an additional 8 cycles in step 9 of the PCR program to generate sufficient quantities of DNA for purification and Gibson assembly.
| 5.4 μL | ddH2O |
| 2.0 μL | GC Buffer (5x) |
| 0.3 μL | DMSO |
| 0.2 μL | dNTPs (10 mM) |
| 0.1 μL | Phusion Hot Start II DNA Polymerase |
| 0.5 μL | Forward Primer (10 μM) |
| 0.5 μL | Reverse Primer (10 μM) |
| 1.0 μL | Template DNA (1 ng purified genomic PCR product) |
Run the following program:
Incubate at 98°C for 30 seconds
Incubate at 98°C for 10 seconds
-
Incubate at 72°C for 20 seconds
Decrease by 0.5C every cycle
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 2 for 23 more times
Incubate at 98°C for 10 seconds
Incubate at 60°C for 30 seconds
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 6 for 11 more times
Incubate at 72°C for 5 minutes
Incubate at 4°C forever
1.10.4. PCR and purification for targeting vector backbone and reporter/selection cassette
This reaction is used to PCR amplify the gene targeting vector backbone or the reporter/selection cassette to provide the raw materials for targeting vector assembly.
| 54 μL | ddH2O |
| 20 μL | GC Buffer (5x) |
| 3 μL | DMSO |
| 2 μL | dNTPs (10 mM) |
| 1 μL | Phusion Hot Start II DNA Polymerase |
| 5 μL | Forward Primer (10 μM) |
| 5 μL | Reverse Primer (10 μM) |
| 10 μL | Plasmid DNA diluted to 1 ng/uL |
Run the following program:
Incubate at 98°C for 30 seconds
Incubate at 98°C for 10 seconds
-
Incubate at 72°C for 20 seconds
Decrease by 0.5°C every cycle
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 2 for 23 more times
Incubate at 98°C for 10 seconds
Incubate at 60°C for 120 seconds
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 6 for 19 more times
Incubate at 72°C for 5 minutes
Incubate at 4°C forever
Add 5 μL DpnI restriction enzyme to digest away template DNA and incubate overnight at 37°C. Run 2 μL of the product on a gel to ensure amplification of a single band of the expected size. Purify the PCR product as described (protocol 1.3.2). A control in which this purified product is transformed should be performed for all Gibson assembly reactions to gauge background colonies due to incomplete Dpn1 digestion.
1.10.5 Colony Screen PCR (10 μL)
To screen for correct assembly of a targeting vector, pick a colony from a plate containing appropriate antibiotics with a sterile 200 μL tip into a 8-well PCR strip or 96-well plate containing 50 μL LB and mix well. This solution will provide the template for a colony screen PCR as well as live bacterial culture to be seeded into an overnight culture should the results of the PCR screen indicate likely correct assembly.
| 4.4 μL | ddH2O |
| 2.0 μL | GC Buffer (5x) |
| 0.3 μL | DMSO |
| 0.2 μL | dNTPs (10 mM) |
| 0.1 μL | Phusion Hot Start II DNA Polymerase |
| 0.5 μL | Forward Primer (10 μM) |
| 0.5 μL | Reverse Primer (10 μM) |
| 2.0 μL | DNA (pick a single colony into 50 μL LB) |
Run the following program:
Incubate at 98°C for 30 seconds
Incubate at 98°C for 10 seconds
-
Incubate at 72°C for 20 seconds
Decrease by 0.5°C every cycle
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 2 for 23 more times
Incubate at 98°C for 10 seconds
Incubate at 60°C for 30 seconds
Incubate at 72°C for 15 seconds per 1000-bp
Cycle to step 6 for 11 more times
Incubate at 72°C for 5 minutes
Incubate at 4°C forever
Basic Protocol 2. Generation of knock-out hPSC lines
Due to the imprecise nature of NHEJ, the simplest form of gene targeting in hPSCs is the generation of frameshift mutations that disrupt gene function. However, targeting genes with multiple sgRNAs can result in larger deletions that remove entire functional regions of a gene (Shalem et al., 2014; Varshney et al., 2015) (Fig. 1b). Even in the presence of large deletions that should introduce frameshifts, gene products are sometimes still produced due to poorly annotated alternative start sites or splice variants. It is therefore important to confirm gene knockout not just by sequencing of genomic DNA but also at the RNA and protein level. The steps involved in gene knock-out in hPSCs are as follows (Fig. 1b, 2b):
Select an appropriate hESC or hiPSC line that has been confirmed to be karyotypically normal, grows well under feeder-free conditions, remains pluripotent when passaged as single cells, and differentiates well into the cell type of interest (protocol 1.6.1). Expand this line in preparation for gene targeting (protocol 1.6.4).
Design multiple sgRNAs (protocol 1.1) to target 5′ constitutive exons (common to most or all splice isoforms) to maximize the likelihood of gene disruption by large deletion and frameshift (Fig. 1b). Note that alternative start sites are not always well annotated, so it is advisable to simultaneously target cells with multiple sgRNAs to maximize the likelihood of a large deletion that removes a functional domain.
Clone sgRNAs into desired expression vector (protocol 1.2), (Table 1) or generate by IVT (protocol 1.4).
Test sgRNAs to identify most active ones to use in gene targeting (protocol 1.5.1 or protocol 1.5.2).
Introduce sgRNA, Cas9, and a marker gene by electroporation (protocol 1.7).
48 hours after transfection, purify transfected cells by FACS and plate 9,000 cells per 10-cm plate in 1:1 + RI. Feed the following day with 1:1 medium and culture until colonies are large enough to pick (protocol 1.6.6).
Pick colonies onto 96-well plates (protocol 1.6.7), expand, and duplicate plates (protocol 1.6.8)
Freeze down one plate (or keep in culture) (protocol 1.6.9) and extract genomic DNA from the second plate (protocol 1.8.2 or protocol 1.8.3).
Amplify the targeted locus with appropriate primers. If a single sgRNA was used and indels are expected to be small, screen clones by deep sequencing (protocol 1.9). If multiple sgRNAs were used and indels are expected to be large, screen for mutations by PCR and Sanger sequencing (protocol 2.1).
Retrieve promising clones (protocol 1.6.3), expand (protocol 1.6.6), and subclone (protocol 1.6.4) if the colony contains a mixture of different indels. If a homozygous knock-out is desired and only heterozygous knock-outs were observed, expand the clone, confirm it is karyotypically normal, and repeat transfection (steps 5–10).
Assess the genomic integrity of the edited clone (protocol 1.6.1).
2.1. [Supporting Protocol 2.1] Sanger sequencing of mutant clones
If large deletions (or insertions) are expected following gene targeting, the nature of mutations can be determined by SMRT sequencing (Hendel et al., 2014), or standard PCR followed by gel electrophoresis and Sanger sequencing of promising clones. Since many groups will not have ready access to SMRT sequencing, the procedure for PCR and Sanger sequencing is given below. This protocol can also be used to screen clones targeted with ssODNs.
Design forward and reverse genomic primers that bind at least 50 base pairs 5′ and 3′ to any predicted cut sites that were tested, respectively. If hPSCs were targeted with an ssODN, design the PCR primers such that they reside outside of the 200-bp ssODN. Test these primers on untargeted DNA using a touchdown PCR program appropriate for the size of the expected amplicon (protocol 1.10.1) to identify a suitable pair.
Extract genomic DNA from targeted clones and an untargeted control (protocol 1.8.2), dilute it 1:10, and use this as template for a genomic PCR (protocol 1.10.1).
-
Run the PCR products on a 1% agarose gel and image.
A range of outcomes are possible, but a likely outcome is that individual clones will yield multiple bands, most of which will be smaller than the control band, especially if multiple sgRNAs were used. Clones in which the control band is absent are more likely to include homozygous knock-outs. -
PCR bands from clones of interest can be gel purified (protocol 1.3.2), submitted for Sanger sequencing, and aligned to a reference sequence. This approach is most useful when analyzing complex band patterns from large deletions. If PCR products are similar in size, PCR products can be purified for sequencing en masse. To do so, run 2 μL of a 10 μL PCR (protocol 1.10.1) on a 1% agarose gel to ensure success of the PCR reaction. Then purify the remaining 8 μL using AMPureXP magnetic beads following manufacturers instructions. After the final ethanol wash and prior to the final elution, place the PCR plate with beads in a thermocycler set to 37°C for ~5 minutes with the lid open. This will evaporate much of the remaining ethanol and result in much cleaner DNA. This plate of purified PCR products can then be sent for sequencing with an internal sequencing primer according to the guidelines set forth by your sequencing facility. Forward and reverse reads should indicate indel boundaries clearly.
Clones with large deletions that introduce frame-shifts in both alleles are most likely to have a complete loss of gene function. Loss of gene function should be confirmed by RT-qPCR and/or Western blotting in hPSCs or an hPSC-derived cell type that normally expresses the gene of interest. If more than two bands are present in the gel, it may be necessary to gel purify bands prior to sequencing.
Basic Protocol 3. Introduction of small targeted mutations into hPSCs
Small targeted mutations are a powerful method of genome modification (Fig. 1c). They can be used to introduce or correct candidate disease-causing mutations or regulatory variants, to introduce recombinase recognition sites such as LoxP to enable conditional gene knock-out or long-range recombination, or to introduce tags enabling genes of interest to be visualized or biochemically purified (Ran et al., 2013a). These targeted mutations are created by introducing a DNA double-strand break (DSB) near the site of interest using the CRISPR/Cas9 system and providing a template for homology directed repair that contains the modification(s) of interest flanked by short homology arms. This homologous template is provided in the form of an ssODN (80–200-bp) that can be ordered from a commercial oligo supplier. The relevant steps are as follows (Fig. 1c, 2c):
Select an appropriate hESC or hiPSC that has been confirmed to be karyotypically normal, grows well under feeder-free conditions, remains pluripotent when passaged as single cells, differentiates well into the cell type of interest (protocol 1.6.1) and expand in preparation for gene targeting (protocol 1.6.4).
Design sgRNAs (protocol 1.1) to target as close to the desired edit site as possible (Fig. 1b).
Clone sgRNAs into desired expression vector (protocol 1.2), (table 1) or generate by IVT (protocol 1.4).
Test sgRNAs to identify most active ones to use in gene targeting (protocol 1.5.1 or protocol 1.5.2).
Design and order ssODNs to introduce the desired variants (protocol 3.1).
Introduce sgRNAs, Cas9, ssODN, and a marker gene by electroporation (protocol 1.7).
48 hours after transfection, purify transfected cells by FACS and plate 9,000 cells per 10-cm plates in 1:1 + RI (protocol 1.6.6). Feed the following day with 1:1 medium and culture until colonies are large enough to pick.
Pick colonies onto 96-well plates (protocol 1.6.7), expand, and duplicate plates (protocol 1.6.8).
Freeze down one plate (or keep in culture) (protocol 1.6.9) and extract genomic DNA from the second plate (protocol 1.8.2 or protocol 1.8.3).
Amplify the targeted locus with appropriate primers and screen clones by barcoded deep sequencing (protocol 1.9), ddPCR (protocol 3.2) or Sanger sequencing.
Retrieve promising clones, expand, and sub-clone if the colony contains a mixture of different indels. If a homozygous edit is desired and only heterozygous edits were observed, expand the clone, confirm it is karyotypically normal, and repeat transfection (steps 6–11).
Assess the genomic integrity of the edited clone (protocol 1.6.1).
3.1. Design of single-stranded oligodeoxynucleotides (ssODNs)
ssODNs carrying a desired mutation flanked on either side by homology arms of 40–100 bases can introduce small targeted mutations by homology directed repair (Miyaoka et al., 2014; Ran et al., 2013a; Yang et al., 2013b) (Fig. 7). In order to prevent the ssODN from being recognized and cut prior to targeting and to protect the genomic allele that has successfully used the ssODN to repair its DSB from being cut again and mutated by NHEJ, the ssODN should be designed to either mutate the PAM or mutate 2–4 residues of the 20-base variable region of the sgRNA, preferably close to the PAM. If the region being targeted is protein coding, these mutations should be designed so that they do not produce any coding changes or produce codons that are rarely used in humans. Finally, these silent substitutions can be designed in such a way so that they introduce or remove restriction sites to facilitate screening for targeted clones by PCR and restriction digest. After being designed and ordered, the ssODN should be diluted to a concentration of 100 μM or higher. Some groups have shown that the use of an anti-sense ssODN for genome editing yields higher targeting efficiencies (Bonner and Kmiec, 2009; Olsen et al., 2005), while others find that this is an artifact of the assay used for analysis of targeting efficiencies and, in fact, sense ssODNs provide a higher targeting efficiency (Aarts and te Riele, 2010).
Figure 7.
Schematic for ssODN design. The CRISPR site should be designed so that the cut site (red triangles) is in close proximity to the site of the desired edit (red N). ssODNs should be designed to carry the desired edit with 40 to 100 bases of homologous sequence on either side. In addition, these ssODNs should carry silent substitutions (assuming a coding region is being targeted) that either mutates the PAM or the recognition domain so that the ssODN itself is not targeted and so that the modified locus is protected from further DSBs. In addition, it may be useful to introduce (or remove) a unique restriction site so that targeted clones can be screened by PCR and restriction digest as a backup method for sequencing. The sequences shown are for general reference and relative position of the sgRNA and the bases to be modified will vary depending on experimental needs.
3.2. Identification of targeted clones by ddPCR
Genome editing with ssODNs is relatively inefficient, and typically only a few percent of transfected cells carry the desired edit. A high-throughput method for identifying these edited clones is therefore important. Depending on the type of targeting experiment carried out (knock-out/knock-in/single base substitution) different methods of screening can be employed. Knock-ins can be identified by a PCR assay or Southern blot, but knock-outs or single base substitutions are best identified by barcoded deep sequencing (protocol 1.9), RFLP analysis, Sanger sequencing, or ddPCR. Since RFLP is a common technique and Sanger sequencing (protocol 2.1) and barcoded sequencing (protocol 1.9) are described above, only the ddPCR assay is detailed below.
A ddPCR TaqMan assay can be used to detect rare editing events. The targeted locus is first PCR amplified with primers that fall outside of the ssODN. The reaction product is then annealed to two different TaqMan probes designed to carry different fluorophores and preferentially bind either the edited or reference allele and will fluoresce when quenchers are removed upon PCR amplification. ddPCR enables rare editing events to be detected and the percent editing to be quantified (Miyaoka et al., 2014). This is particularly useful for determining which concentrations and combinations of CRISPR/Cas9 and ssODNs give good editing before embarking on an experiment. For example, transfect cells with ssODN, CRISPR/Cas9, and fluorescent plasmid, sort fluorescent cells, plate down half of the cells at clonal density (9,000 cells per 10-cm plate) and extract genomic DNA from the other half. Run a ddPCR assay on the sorted populations from the different conditions and identify conditions that had the highest rates of editing and then only pick colonies from those conditions. The percent editing observed also provides a reference for how many colonies need to be picked. The ddPCR method requires some up-front work in terms of probe design and assay optimization (Miyaoka et al., 2014), but the major advantage is that it gives results more quickly than barcoded deep sequencing.
Materials
[Note: for supplier information and catalog numbers, see Table 2]
Forward/reverse genomic loci primers
Internal sequencing primer
Genomic DNA (DirectPCR/ProK-extracted)
GC buffer (5x)
dNTPs
ddH2O
100% DMSO
Phusion Hot Start II Polymerase
Agarose
1% agarose gel
TAE (50x) (see recipe), diluted to 1× in H2O for use
Ethidium bromide
96-well PCR plates
AMPure XP magnetic beads
Thermocycler
Microwave
Electrophoresis gel box and power source
UV transilluminator
NanoDrop or similar spectrophotometer
Biorad XQ200 droplet digital PCR machine and droplet generator, or similar system
Design TaqMan probes to bind either the reference (unedited) or edited sequence and maximize differences in binding affinity (melting temperature) between the two probes (Hughesman et al., 2015). Avoid a guanine at the 5′ end, as this markedly quenches the fluorophore post-cleavage). Design probes so that the reference probe has a 5′ HEX fluorophore, the edit probe has a 5′ FAM fluorophore, and both have a 3′ quencher (Iowa Black with Zen). These probes can be ordered from IDT.
-
Extract genomic DNA on-plate from 96-well plates or from cell pellets using the HotShot protocol (protocol 1.8.3). Use this extracted genomic DNA to set up a PCR reaction:
1. 12.5 μL ddPCR supermix 2. 0.225 μL Forward primer (100 μM) 3. 0.225 μL Reverse primer (100 μM) 4. 0.08 μL Reference probe (100 μM) 5. 0.08 μL Alternate (edit-specific) probe (100 μM) 6. x μL Sample (this is typically 1 μl for a confluent well of a 96-well plate digested with 50–100 μl HotShot or DirectPCR/ProK buffer) 7. Bring to 25 μL ddH2O To generate droplets for the BioRad QX200, add 20 μL of PCR mix to the central wells of the droplet chamber (all 8 wells must be full).
Add 50 μL droplet oil to each oil chamber, place in holder, add rubber gasket, put in droplet generator, and activate.
-
Once droplets are generated, very gently remove entire volume of droplets to a fresh 96-well plate. Seal the plate with a heat sealable foil lid and run program shown below.
95°C for 10 minutes
94°C for 30 seconds
Annealing temp for 60 seconds (this depends on how the probes were designed. The annealing temperature should maximize differences in probe binding. Typically, this is at 55°C or 60°C.)
72°C for 30 seconds
GOTO Step 2, 49 more times
98°C for 10 minutes
12°C forever
-
Analyze droplets on a QX200.
The assay should show clear separation of the two fluorescent channels. As a positive control, it is useful to have template DNA containing the desired edit. If clear separation is not observed, it may be necessary to alter the PCR conditions by increasing the annealing temperature or increasing the number of cycles, or by re-designing the probes.
Basic Protocol 4. Generation of knock-in cell lines
One of the most promising applications of CRISPR/Cas9 technology is the knock-in of sequences of interest such as reporter genes into the desired genomic locus. The protocol given here is suited for an insert of 0.1 – 10 kb accompanied by a drug selection cassette to enrich for the rare cells that incorporate the insert by homologous recombination at the targeted locus. Shorter sequences might be more easily introduced by ssODNs. The efficient insertion of longer sequences requires longer homology arms (Byrne et al., 2015) or delivery vectors such as HDAdVs (Aizawa et al., 2012). The protocol involves the generation of a pair of CRISPR/Cas9 nickases, which act cooperatively to generate a DSB in the region of the genome where the desired knock-in should occur (Mali et al., 2013a; Ran et al., 2013a) (Fig. 1d). This approach is an effective way to maximize gene targeting efficiency while minimizing unwanted indels (Merkle et al., 2015). A targeting plasmid must be generated in which the sequence to be knocked-in will be flanked by homology arms that will direct the HDR leading to the introduction of the desired knock-in sequence. Gene knock-in is achieved by the co-introduction of the CRISPR/Cas9 nickase pair and the targeting vector, followed by drug selection, colony picking, verification of successful knock-in, and removal of the selection cassette (Fig. 1d, 2d):
Select an appropriate hESC or hiPSC line that has been confirmed to be karyotypically normal, grows well under feeder-free conditions, remains pluripotent when passaged as single cells, differentiates well into the cell type of interest (protocol 1.6.1) and expand in preparation for gene targeting (protocol 1.6.4).
Design sgRNAs for use with Cas9 dual nickases (protocol 1.1) that flank the desired edit site (Fig. 1d).
Clone sgRNAs into desired expression vector (protocol 1.2), (table 1) or generate by IVT (protocol 1.4).
Test sgRNAs to identify most active ones to use in gene targeting (protocol 1.5.1 or protocol 1.5.2).
Amplify genomic locus to be targeted from the hPSC line to be used for targeting (protocol 4.1.1).
Amplify homology arms (protocol 4.1.2) and assemble and sequence verify the gene targeting vector (protocol 4.1.3).
Introduce sgRNAs, Cas9, and gene targeting vector by transfection (protocol 1.7).
Perform drug selection and culture cells until drug-resistant colonies are large enough to pick (protocol 4.3).
Pick colonies onto 96-well plates (protocol 1.6.7), expand, and duplicate plates (protocol 1.6.8).
Freeze down one plate (or keep in culture) (protocol 1.6.9) and extract genomic DNA from the second plate (protocol 1.8.2 or protocol 1.8.3).
Amplify the targeted locus with appropriate primers, screen clones by PCR (protocol 4.3.1) and confirm by Southern blot, if desired.
Retrieve correctly targeted clones, expand, and transfect with recombinase to remove the selection cassette and select clones lacking the selection cassette (protocol 4.4).
Assess the genomic integrity of the edited clone (protocol 1.6.1).
4.1. Gene targeting vector design
The targeted knock-in of a reporter/selection cassette by homologous recombination is achieved by providing the cell with a template for homology directed repair that contains the reporter/selection cassette flanked by homology arms. It is important that these homology arms do not contain PCR-induced mutations, since these reduce targeting efficiency or alter the function of the targeted locus. PCR reactions used in the assembly of targeting vectors should be performed with a high-fidelity polymerase, and genomic DNA from the cell line to be targeted should be used as the template for homology arm cloning. The entire genomic locus is first PCR amplified, and then 5′ and 3′ homology arms are PCR amplified from this template, finally the gene targeting vector is generated by Gibson assembly of the two homology arms (Gibson, 2011; Gibson et al., 2009), the gene targeting vector backbone (pDTA-TK), and the reporter/selection cassette. This strategy is versatile, since only the homology arms need to be varied to target the insertion to different regions of the genome, enabling the parallel generation of many gene-targeting vectors. One drawback of this strategy is that it may result in homology arms derived from different parental alleles (e.g. 5′ arm maternal, 3′ arm paternal). If the maternal and paternal alleles have significantly different sequences, knock-in efficiency may be reduced. To avoid this possibility, TOPO clone the genomic locus amplicon prior to homology arm amplification.
After extracting high-quality genomic DNA from the hPSC to be targeted (protocol 1.8.1), there are two sets of primers that need to be designed:
Genomic primers to amplify the locus of interest
Homology arm primers to amplify 5′ and 3′ homology arms with overhangs for subsequent Gibson reaction
4.1.1 Genomic locus amplification
While homology arms could theoretically be directly amplified from genomic DNA, the constraints on primer design imposed by the site of desired knock-in (for example the STOP codon of a protein coding gene) make this process inefficient. It is therefore desirable to first PCR amplify the genomic region of interest (Fig. 6a), which will provide a purified template for homology arm PCR (Fig. 6b). The added benefit of this approach is that it identifies primers known to work on genomic DNA that can later be used to screen for appropriate gene knock-in (protocol 4.3.1), (Fig. 6d).
Design three forward and three reverse primers that are at least 1000-bp but no more than 2000-bp away from the site of targeting. These six different primers can be combined in a total of nine unique combinations, which will be used to amplify the region of interest. Because of the way the primers were designed, the pairs should give amplicons of at least 2000-bp, and at most, 4000-bp.
Test all 9 possible combinations of these primers in 10 μL PCR reactions with Phusion HotStart II polymerase and genomic DNA from the hPSC line to be targeted using a touchdown PCR program (protocol 1.10.1).
Run products on a 1% agarose gel.
-
In most cases, several primer pairs will yield a PCR product for a given locus. Record which primer pairs worked and store these, since they will be used to later screen for correctly targeted stem cell clones. Select the primer pair that gave the largest product that is a single clear band of the expected size, and run a 100 μL PCR using the same set of conditions.
Should no amplicons be generated, confirm the purity of the genomic DNA template, adjust the template concentration and/or annealing temperature, and re-run the PCR. If this also fails, a BAC may provide a better PCR template or it may be possible to have homology arms synthesized. Purify the PCR product as described in protocol 1.3.2.
4.1.2 Homology arm cloning
Homology arms should be between 600 and 2000-bp in length. When designing the homology arm primers, ensure that the reporter/selection cassette will not delete or introduce any undesired sequences that could compromise the function of the gene or the reporter. For example, for C-terminal fusions, the reporter gene should be inserted in-frame immediately 5′ to the endogenous stop codon of the targeted gene and carry its own stop codon. To ensure expression of the reporter gene upon removal of the selection cassette from the targeting vector, minimize extraneous vector sequence between the end of the reporter gene and start of the gene’s endogenous 3′ UTR and gene’s polyadenylation sequence. Alternatively, an exogenous polyadenylation signal can be introduced along either the reporter gene in the targeting construct.
Homology arms are cloned by PCR amplification, using the purified genomic locus amplicon (protocol 4.1.1) as a template. The PCR primers used to amplify them should contain an additional 20-bases of 5′ sequence corresponding to overhangs complementary to the targeting vector backbone and reporter/selection cassette to facilitate the generation of the targeting vector by Gibson assembly (protocol 4.2), (Fig. 6c) (Gibson et al., 2009; Merkle et al., 2015). The sequence of these overhangs will differ depending on the reporter/selection strategy used and the targeting vector backbone as described below.
Note that if a dual nicksase strategy is used, it should not be necessary to alter sgRNA binding sites in the homology arms. However, if wild-type Cas9 is employed and the sgRNA binding site is present on one of the homology arms, it is likely that the targeting plasmid will be cut at that site, which might compromise targeting efficiencies and introduce unwanted mutations. In this case, it is best to mutate the sgRNA binding site in the homology arm during the assembly of the targeting vector.
-
Order the following primers:
Three unique 5′ homology arm (HA) forward primers (at least 600-bp but no more than 2000-bp away from 5′ HA reverse primer).
One 5′ HA reverse primer.
Three unique 3′ HA reverse primers (at least 600-bp but no more than 2000-bp away from 3′ HA forward primer).
-
One 3′ HA forward primer.
The 5′ HA reverse and 3′ HA forward primers should have Gibson overhang sequences complementary to the 5′ and 3′ ends of the reporter/selection cassette and are strategy-specific. The 5′ HA forward primers and 3′ HA reverse primers should have a 5′ sequence corresponding to complementary sequences in the targeting vector backbone. For pDTA-TK, these sequences correspond to:
5′ HA forward: TGTCTGGATCGTAGTTCTAGAG
3′ HA reverse: GGTACCCTAGAGAATTCCTAGA
Using the purified genomic locus amplicon (protocol 4.1.1) as a template, set up 6 different 10 μL PCR reactions, three for the 5′ HA (3 different forward and one reverse 5′ homology arm primers) and three for the 3′ HA (3 different reverse and one forward 3′ homology arm primers) using a touchdown PCR program (protocol 1.10.1).
Run products on a 1% agarose gel.
-
Select the primer pair(s) that gave a single clear band of the expected size, and run a 100 μL PCR using the same set of conditions as above.
If the genomic locus PCR succeeded, it is very likely that the HA PCRs will also succeed unless it is impossible to design suitable primers in the region of gene insertion. In this case, it may be possible to have homology arms synthesized. Purify the PCR product as described in protocol 1.3.2.
4.1.3 PCR amplification of the targeting vector backbone and reporter/selection cassette
To enable Gibson assembly, the gene targeting vector backbone and reporter/selection cassette should be PCR amplified, generally from plasmid templates. The primers to use for the pDTA-TK backbone are: CTCTAGAACTACGATCCAGACATGAT and TCTAGGAATTCTCTAGGGTACCTC. The primers for the reporter/selection cassette are strategy-dependent and should designed with the help of software such as Primer3 following general guidelines such as a melting temperatures of approximately 60°C, approximately 50% GC content, and low self-homology. Design at least two forward and two reverse primers that include the entire reporter/selection cassette and minimize the inclusion of vector backbone sequence, and test these in 10 μL PCR reactions to identify pairs that generate clean amplicons of the expected size. Once suitable primers have been identified, set up 100 μL PCR reactions as described in protocol 1.10.4 and purify PCR products.
4.2. Generation of the gene targeting vector
Targeting efficiencies can vary widely depending on sequence of the targeted locus and the size and sequence of the sequence to be inserted. While it is not easy to predict the exact frequency of targeting, shorter inserts will be inserted with higher efficiency. Factors that can severely compromise targeting efficiency include homology between sequences in the reporter/selection cassette and the sgRNA. These sequences are usually considered for potential off-target sites during sgRNA design.
The gene targeting vector is generated via a Gibson reaction in which four fragments are ligated to one another to generate a circular plasmid DNA. These four fragments include the targeting plasmid backbone, reporter/selection cassette, and the 5′ and 3′ homology arms. Following the Gibson reaction (~2 hours), the targeting vector is transformed, expanded and purified using a low endotoxin midiprep kit, and sequenced.
Materials
[Note: for supplier information and catalog numbers, see Table 2]
Targeting vector backbone (PCR amplified for Gibson assembly)
Reporter/Selection Cassette (PCR amplified for Gibson assembly)
Genomic locus primers
Homology arm primers with appropriate Gibson overhangs
PCR strips
1.5 mL microfuge tubes
ddH2O
GC buffer (5x)
dNTPs
100% DMSO
Phusion
DpnI
TAE (50x) (see recipe), diluted to 1x in H2O for use
Agarose
1% agarose gel
Ethidium bromide
Gibson assembly mix (see recipe)
Chemically competent E. coli (TOP10)
Thermocycler
Microwave
Electrophoresis gel box and power source
UV transilluminator
NanoDrop or similar spectrophotometer
Protocol Steps
Ensure that the targeting vector backbone and reporter/selection cassette have been PCR amplified, DpnI digested and purified (protocols 1.10.4, 4.1.3), that 5′ and 3′ homology arms have been generated and purified with the appropriate Gibson overhangs (protocol 4.1.2), and that all components are at a concentration of 100 ng/μL or higher.
-
Perform Gibson assembly as follows and incubate at 50°C for 2 hours:
15 μL Gibson Assembly Mix 100 ng Vector Backbone 100 ng Reporter/Selection Cassette 100 ng 5′ Homology Arm 100 ng 3′ Homology Arm x μL ddH2O
20 μL Total Following this reaction, transform 2 μL of the ligation reaction into 50 μL of chemically competent TOP10 E. coli, recover for 2 hours at 37°C following heat shock, and plate on plates containing appropriate antibiotics. As a negative control, set up a reaction that replaces the homology arms with water.
Because the Gibson reactions are not perfect and there could be some carryover of targeting vectors used to amplify the vector backbone or selection cassette, screen for correctly assembled plasmids by colony PCR (protocol 1.10.5). Pick 8 colonies into 8 wells of a PCR strip containing 50 μL LB. Using 2 μL of this suspension as template, set up a PCR using the 5′ HA forward primer used for the generation of the 5′ HA PCR product and a reverse primer inside of the reporter/selection cassette. A band should be present if the construct was correctly assembled.
Two of the colonies that have been determined to contain the correctly assembled plasmid should be grown up for a midiprep/maxiprep in appropriate antibiotics (protocol 1.3.1).
Sequence verify the homology arms and reporter/selection cassette from one of the clones prior to use in gene targeting.
4.3. Drug selection
Drug selection is used to identify those rare cells that have genomically integrated the gene targeting vector. A positive selection cassette introduced between the homology arms, either 5′ or 3′ to the insertion sequence of interest such as a reporter gene, confers drug resistance to cells carrying the cassette. Commonly used positive selection cassettes confer resistance to the drugs neomycin, puromycin, or hygromycin. The protocol below is designed for neomycin-resistance cassettes driven by the PGK promoter (Merkle et al., 2015). The selection timeline (concentration and duration of drug) depends on cell density and the type of drug used, so groups wishing to use puromycin or hygromycin for drug selection should first optimize these conditions based on published values (Moore et al., 2010).
The pDTA-TK contains two negative selection cassettes, DTA (diphtheria toxin subunit A) and TK (herpes simplex virus thymidine kinase) driven by strong promoters flanking the homology arms. DTA is toxic on its own, and the TK gene product phorphorylates the drug ganciclovir to produce a toxic guanosine analog (Yanagawa et al., 1999). A homologous recombination event in which crossover occurs within the 5′ and 3′ homology arms (Fig. 6c, 6d) would exclude the DTA and TK cassettes from the targeted locus, so these cassettes select against the random insertion of the entire gene targeting vector at a random genomic location.
Materials
[Note: for supplier information and catalog numbers, see Table 2]
Geneticin (G418) (see recipe)
1:1 media
When 2.5 x 106 cells are transfected (protocol 1.7) and plated onto 10-cm plates, plates are typically about 20% confluent the following day. Survival rate varies somewhat by cell line, DNA concentration, and transfection method. Feed with 1:1 medium until cells have reached approximately 60% confluence.
-
For hPSCs transfected with a neomycin selection cassette, add 1:1 medium containing 50 μg/mL of G418. Continue treatment until most cells have died and only a few drug-resistant clones remain and are large enough to be picked (approximately 10 days).
G418 is a relatively mild drug that will result in gradual cell death over the course of approximately 1 week. If hPSC cultures reach confluence, drug selection may not be effective. In this case, re-plate cells at a lower density and begin drug selection again. Following selection, drug-resistant clones can be manually picked into 96-well plates containing 1:1 + RI (protocol 1.6.7). G418 selection is discontinued upon picking.
-
48 hours after picking, feed cells with 1:1 media containing 5 μM ganciclovir to kill colonies that have randomly incorporated the selection cassette.
Only a minority of colonies should be killed by ganciclovir. Negative selection is not performed at the same time as positive selection to minimize stress to hPSCs.
4.4. Confirmation of gene knock-in
Following positive and negative selection, it is necessary to confirm targeting of the reporter/selection cassette to the locus of interest. The gene targeting plasmid could be integrated randomly in the genome in such a way that the negative selection cassettes are disrupted or silenced. Furthermore, during homologous recombination at the targeted locus, it is possible that the 5′ arm, 3′ arm, or both arms are not integrated seamlessly. Since a typical gene targeting experiment will yield dozens of drug-resistant clones, it is advisable to first screen for desired gene knock-in by PCR amplification across both the 5′ and 3′ homology arms (protocol 4.3.1). These results can be confirmed by Southern blot, the procedure which is described elsewhere (Southern, 2006). Note that for Southern blotting, it will first be necessary to expand hPSCs on 6-well plates to obtain sufficient quantities of genomic DNA (10 μg).
To screen for correct gene knock-in by PCR, the validated primers used to amplify the genomic locus (protocol 4.1.1) are paired with primers within the reporter/selection cassette (Fig. 6d). Since the genomic primers fall outside of the homology arms, amplicons of 1–2 kb are expected only if the reporter/selection cassette is targeted to the correct locus. Since both the 5′ and 3′ arms are screened, partially targeted clones can be distinguished from ones in which homologous recombination has seamlessly resolved both arms (Merkle et al., 2015).
Materials
[Note: for supplier information and catalog numbers, see Table 2]
Forward/reverse primers for 5′ and 3′ homology arm screening (described below)
Genomic DNA (DirectPCR/ProK-extracted)
GC buffer (5x)
dNTPs
ddH2O
100% DMSO
Phusion Hot Start II Polymerase
Agarose
1% agarose gel
TAE (50x) (see recipe), diluted to 1x in H2O for use
Ethidium bromide
96-well PCR plates
Thermocycler
Microwave
Electrophoresis gel box and power source
UV transilluminator
NanoDrop or similar spectrophotometer
Extract genomic DNA from targeted drug-resistant hPSC clones (protocol 1.8.2).
-
Generate pools of genomic DNA from up to 24 targeted clones, dilute this genomic DNA 1:10 in ddH2O, and set up 10 μL PCR reactions (protocol 1.10.1) with combinations of all validated genomic primers and reporter/selection cassette primers (Fig. 6d). Run products on a 1% agarose gel and visualize.
Since the targeting efficiency is sometimes quite low and there are no good positive controls, this strategy maximizes the likelihood that targeted clones will be observed by PCR. Once primer pairs that yield amplicons of the expected size have been identified, screen through individual clones by PCR as described above. Clones that yield single PCR products of the expected size for both the 5′ and 3′ arms are considered to be correctly targeted.
4.5. Excision of the selection cassette
Drug selection cassettes are often flanked with recognition sites for a transposase or recombinase (such as loxP or Frt for Cre and FlpO recombinase, respectively) to facilitate their excision following gene targeting. To excise these cassettes, correctly targeted clones are transfected with a transposase or recombinase along with a selectable marker such as a drug resistance cassette. After selecting for transfected cells, clones are picked into 96-well plates, duplicated, and screened for drug sensitivity (Fig. 2d).
Expand correctly targeted clones in 1:1 medium.
Introduce the appropriate recombinase and marker gene, such as a plasmid expressing FlpO and puromycin.
Enrich for transfected cells by FACS (protocol 1.6.6) or drug selection (protocol 4.2).
Culture cells until colonies are of sufficient size for picking into 96-well plates (protocol 1.6.7).
-
Expand cells in 96-well plates, duplicate plates (protocol 1.6.8), and challenge one of the two plates with the drug used for positive selection.
Clones in which the cassette was successfully removed should die within 7 days. Expand clones lacking the selection cassette and re-confirm correct targeting and selection cassette excision by PCR and Sanger sequencing (protocol 4.3).
REAGENTS AND SOLUTIONS
Luria broth (LB)
Dissolve 25 g of LB powder in 800 mL of H2O and bring the volume up to 1 L with H2O. Shake well to dissolve as much of the powder as possible an autoclave on a liquid cycle. After autoclaving, allow LB to cool to room temperature before use.
Ampicillin
Dilute ampicillin sodium salt to 100 mg/mL in sterile water to make stock solution. After dissolving, sterile-filter through a 0.22 μm PES filter, aliquot and store at −20°C for up to 1 year. Use at a working concentration of 100 μg/mL.
LB/agar/antibiotic bacterial plates
Dissolve 40 g of LB/agar powder in 800 mL of H2O and bring the volume up to 1 L with H2O. Shake well and autoclave on a liquid cycle. After autoclaving, allow LB/agar to cool until warm to the touch (~50°C), add appropriate antibiotic, mix, and pour into petri dishes. Allow the plates to set overnight at room temperature and store at 4°C up to 3 months.
50x TAE buffer
Dissolve 242 g Tris into 600 mL H2O. Then add 57.2 mL glacial acetic acid and 100 mL 500 mM EDTA (pH 8.0). Adjust volume to 1 L with H2O and store at room temperature. To make working stock, dilute 50x TAE to 1x in H2O.
1%/1.5%/2% agarose gel
Dissolve 1/1.5/2 g of agarose powder into 100 mL of 1X TAE (can be scaled as necessary). Microwave this solution for about 3 minutes or until all of the powder is dissolved. Allow the solution to cool and add ethidium bromide to a final concentration of 10 μg/mL. Pour the solution into a gel box and allow the gel to set.
Ethidium bromide
Dissolve ethidium bromide powder into H2O to a final concentration of 10 mg/mL. Store ethidium bromide stock solution, light-protected, for up to 1 year. Use caution when handling, as ethidium bromide is a known carcinogen. Use at a final concentration of 10 μg/mL. Store at room temperature.
Assay buffer (in vitro cutting assay)
Dilute KCl to 200 mM, MgCl2 to 10 mM, Tris pH 8.0 to 20 mM in molecular biology grade H2O and store at room temperature.
Reaction stop buffer (in vitro cutting assay)
To 10 mL of molecular biology grade H2O add 6 mL of 0.5 M EDTA pH 8.0, 1 g SDS (2% final w/v), 15 g glycerol (30% final w/v) and a dash of bromophenol blue. Bring the volume up to 50 mL with H2O and store at room temperature.
10x TBE
Dissolve 108 g Tris and 55 g boric acid in 800 mL molecular biology grade H2O. Once Tris and boric acid have gone into solution add 40 mL of 500 mM EDTA pH 8.0. Adjust volume to 1 L with H2O. To make working stock, dilute 10x TAE to 1x in H2O. Store at room temperature.
hESC medium (human embryonic stem cell medium)
Combine 389.5 mL KO-DMEM, 100 mL KOSR, 5 mL non-essential amino acids (NEAA), 5 mL glutamax and 0.5 mL beta-mercaptoethanol (BME) to make 500 mL hESC media. Sterile-filter and store at 4°C for up to 2 weeks. Add bFGF to a final concentration of 100 ng/μl.
1:1 Media
Combine 250 mL of mTeSR1 with 250 mL of hESC media. Store at 4°C for up to 2 weeks.
2x freezing media
Add 50 mL 100% DMSO to 200 mL FBS and sterile-filter to make 2x freezing media. Store at 4°C for up to 1 year.
SDS lysis buffer
Dilute Tris pH 8.0 to 10 mM, NaCl to 200 mM, EDTA to 10 mM in ddH2O and SDS to a final concentration of 0.5% to make SDS lysis buffer. Store at room temperature for up to one year. Prior to each use, add Proteinase K to a final concentration of 100 μg/mL. Once ProK has been added, the solution cannot be stored.
DirectPCR/ProK buffer
Add Proteinase K solution DirectPCR (Tail) Lysis Buffer to a final concentration of 100 μg/mL immediately prior to use. This solution cannot be stored.
TE
In molecular biology grade H2O, dilute Tris, pH 8.0 to 10 mM and EDTA to 0.5 mM. Store at room temperature for up to one year.
HotShot component 1
Add 125 μL 10N NaOH and 20 μL 0.5 mM EDTA to 50 mL molecular biology grade H2O. Store at room temperature for up to one year.
HotShot component 2
Dissolve 325 mg Tris-HCl in 50 mL of molecular biology grade H2O. Store at room temperature for up to one year.
Gibson assembly mixture
From (Gibson et al., 2009). Prepare Assembly master mix (1.2 mL) by combining the following reagents. Make 15 μL aliquots and store at −20°C for up to one year
320 μL 5x isothermal reaction buffer (see recipe)
0.64 μL of 10U/μL T5 exonuclease
20 μL of 2U/μL Phusion DNA Polymerase
160 μL of 40U/μL Taq DNA Ligase
700 μL molecular biology grade ddH2O
5x isothermal reaction buffer
From (Gibson et al., 2009).
3 mL 1M Tris-HCl pH 7.5
150 μL of 2M MgCl2
60 μL of 100 mM dGTP
60 μL of 100 mM dATP
60 μL of 100 mM dTTP
60 μL of 100 mM dCTP
300 μL of 1 M DTT
1.5 g PEG-8000
300 μL of 100 mM NAD+
Bring volume to 6 mL with molecular biology grade ddH2O
Geneticin (G418)
Dilute geneticin to 50 mg/mL in ddH2O, pass through a 0.22 μm syringe filter, aliquot and store at −20°C for up to one year. Use at 50 μg/mL (1,000x).
CATALOG NUMBERS
The catalog numbers and suppliers for reagents used in this protocol are given in Table 2.
COMMENTARY
Background Information
In the past two decades, there have been profound advances in the field of hPSC culture and gene editing. The generation of iPSCs from somatic cells have made hPSC research broadly accessible (Malik and Rao, 2013), and improved methods for their culture (Beers et al., 2012; Chen et al., 2011; Ludwig and J, 2007) have made hPSCs amenable to manipulation. In parallel, the gene-editing field has progressed rapidly. Meganucleases and homing endonuclease-based approaches provided an important proof of principle (Silva et al., 2011), but these systems lacked flexibility and were difficult to target to desired genomic sites. The engineering of customizable zinc finger nucleases (ZFNs) (Kim et al., 1996) led to great excitement about genome engineering but the difficulty of designing and testing ZFNs limited their widespread adoption. Another wave of excitement came with the development of custom transcription activator-like (TAL) effector nucleases (TALENs) (Christian et al., 2010), which, like ZFNs, can be targeted to specific genomic regions to induce DSBs, but are more readily designed than ZFNs. The DNA binding specificity of TALENS is mediated by protein-DNA interaction, requiring considerable cloning to generate each TALEN. The real breakthrough came with the description of the CRISPR/Cas9 system, in which the DNA binding specificity of a nuclease is mediated by an easily modifiable crRNA/tracrRNA (Jinek et al., 2012), or sgRNA, as reviewed elsewhere (Doudna and Charpentier, 2014).
The confluence of advances in hPSC generation and culture and gene editing technologies have spawned a revolution in hPSC gene editing, which promises to influence the fields of human development and cell type specification, disease modeling, drug screening, and cell transplantation. In the context of hPSCs, one of the first descriptions of CRISPR/Cas9-based gene editing was at the “safe harbor” AAVS1 locus (Mali et al., 2013b). Following the initial finding that CRISPR/Cas9 can mediate genome modification in stem cells, many groups began showing that the technology could be used for generation of gene knock-out cell lines (Cong et al., 2013; Mali et al., 2013b), small targeted mutations (Miyaoka et al., 2014; Ran et al., 2013a), and knock-in stem cell lines (Mali et al., 2013a; Merkle et al., 2015; Ran et al., 2013a). While, there were initial concerns that off-target effects were a significant issue in CRISPR/Cas9 gene targeting, several groups showed that the mutational load observed in CRISPR/Cas9 or TALEN targeted cell lines was no different than that observed through the routine culturing of stem cells (Suzuki et al., 2014; Veres et al., 2014). Several other resources for the use of CRISPR/Cas9 technology have since been described. These include libraries for genome-wide knock-out screens (Sanjana et al., 2014; Shalem et al., 2014), CRISPR/Cas9-mediated gene activation, (Maeder et al., 2013; Perez-Pinera et al., 2013), and gene silencing (Qi et al., 2013). We hope this protocol will enable other groups to successfully incorporate CRISPR/Cas9-based gene editing into their research plans.
Critical Parameters and Troubleshooting
As there are a large number of steps in this protocol, the critical parameters and approaches for troubleshooting are given throughout. However, there are a few variables that bear repeating since they are common mistakes that can result in experimental failure. We also discuss practical strategies for tracking the many steps and long time frames of hPSC gene editing experiments.
1. sgRNA Design Considerations
When designing sgRNAs for the targeting of Cas9 to the desired loci, there are several important considerations. The sgRNA should have high levels of on-target activity with minimal off-target activity. The location of the cut site should be as close as possible to the site of targeting, ideally within 30-bp and preferably within 50-bp since the efficiency of gene editing by homology directed repair (HDR) drops rapidly with increasing distance from the cut site. There will be certain instances when no high-quality cut sites are within 50-bp of the target site. In this case, investigators should consider whether targeting a different location within the locus of interest could achieve the same goals and adjust the targeting strategy as necessary. If this is not possible, alternatives to Streptococcus pyogenes Cas9 that have a different PAM, or designer nucleases such as TALENs, might enable efficient cutting closer to the target site.
2. sgRNA Expression Plasmid Choice
When designing a targeting strategy, the choice of expression plasmid will be determined by the application. In general, when attempting to disrupt the genome in a gross manner (such as in the case of generating a knock-out cell line) a nuclease should be used. In contrast, when a precise targeting event is desired (such as in the case of generating a point mutation) a nickase should be used. Other important considerations are the use of a plasmid co-expressing GFP or puromycin to allow for selection of cells that have been successfully transfected with the expression plasmid. Protocol 1.1 can be referenced for more detailed notes on expression plasmid choice.
3. Low sgRNA Cloning Effeciencies
While the cloning of sgRNA into the expression plasmid is typically a very high efficiency reaction, low cloning efficiencies can occur. This could be due to poor gel purifications of the expression plasmid. Ensure that that gel is dissolved completely by incubating with membrane binding solution at 65°C for at least 10 minutes and occasionally flicking the tube to ensure it is well mixed.
4. Plasmid DNA Preparations
Human pluripotent stem cells are sensitive to bacterial endotoxins, so it is important that plasmids are prepared using a kit that removes endotoxins. Complete removal of wash buffer and other contaminants can dramatically increase the efficiency of downstream applications such as PCR and cloning.
5. In vitro Cutting Assay Failure
If, at the highest concentration of Cas9, cutting is not observed, ensure that calculations and buffers are correct. If possible, include a positive control sgRNA and amplicon to ensure that the Cas9 protein is active. If a very bright band is seen on the gel at ~120-bp, RNase digestion was likely incomplete.
6. Use of Antibiotics in Cell Culture Media
Antibiotics are not included in any media used so that when contamination arises, it can be immediately detected and eliminated since antibiotics can provide a constant low level of stress to cells.
7. Carefully select and culture hPSC lines
Some hPSC lines are prone to spontaneous differentiation and are less likely to yield pluripotent colonies when grown at clonal density. If possible, avoid these lines in favor of karyotypically normal lines with high clonal plating efficiencies.
Feed cultures daily with fresh media, split cultures regularly, never allow them to become over-confluent, and vigilantly eliminate differentiated cells. Cells should be adapted to feeder-free conditions and passaged with EDTA or TrypLE for at least three passages prior to the initiation of gene targeting experiments. Also consider how well a cell line performs in experimentally relevant assays, such as the generation of a disease-relevant cell type. When culturing cells, take care that colonies do not get too large or that plates get over-confluent or go for more than 48 hours between feeding. It is better to go back to a freeze of cells at an earlier passage rather than work with a cell line with a sub-optimal culture history.
8. Confirm the integrity of the targeted locus and hPSC genome before and after gene targeting
Human embryonic and induced pluripotent stem cells frequently contain sub-populations of cells that are aneuploid or carry large copy number variants (CNVs) that can emerge over time in culture (Draper et al., 2004; International Stem Cell et al., 2011; Mayshar et al., 2010; Nguyen et al., 2014; Spits et al., 2008). If these mutations confer a growth or survival advantage, they will likely become fixed during a gene targeting experiment that takes cells through a clonal bottleneck, often under stressful conditions of drug selection or low-density culture. The sudden change of growth kinetics of a culture following gene editing is a clear indication that a structural variant conferring this behavior has been acquired. To reduce the risk of acquiring such undesirable mutations, ensure that hPSCs are obtained from a reliable source and have not been passaged extensively, preferably 30 times of fewer. Finally, screen for aneuploidy and CNVs prior to embarking on a gene editing experiment and after clones carrying the desired edits have been identified using karyotyping, SNP genotyping, or whole genome sequencing. Karyotyping is a widely used technique, but is only sensitive to structural events 5 Mb or larger and present in at least 5% of cells. Whole genome sequencing is comprehensive but costly, so SNP arrays present a good compromise for the routine analysis of hPSC genomic integrity.
9. Test sgRNAs prior to use in gene targeting
The success of a gene targeting experiment depends heavily on the efficient generation of DSBs at the locus of interest. Assuming that DNA delivery is efficient, the critical factor becomes the activity of the CRISPR/Cas9 system at the targeted locus. It is therefore advisable to design and test the activity of multiple sgRNAs for each locus of interest. Relative to the time and expense of gene editing experiments, these assays are fast and cheap.
10. Design the targeting strategy to maximize the yield of useful clones
Gene targeting experiments are likely to generate many edited hPSC clones, but only a subset of these are likely to be experimentally useful. In knock-out experiments, deletions that do not result in frame-shifts or that target exons that are not in conserved splice isoforms may not effectively disrupt gene function. Also, it may be necessary to perform a second round of targeting to ensure that both alleles are disrupted. Similarly, hPSCs that have been engineered to carry small targeted mutations or larger knock-in genes may carry unwanted mutations. These can be present at the targeted allele due to imperfect homology directed repair or continued CRISPR/Cas9 activity. The use of dual nickases for gene knock-in and/or the mutation of the PAM can mitigate the latter effect. However, clones often carry unwanted mutations at the other allele of the targeted locus (Merkle et al., 2015) or may carry off-target mutations elsewhere in the genome. It is important to screen for these mutations by sequencing since they may affect experiments performed with gene-edited hPSCs in unanticipated ways.
11. Use pure, concentrated DNA
hPSC are sensitive to the presence of endotoxins, residual ethanol and other impurities. We recommend purifying plasmid DNA using the QIAGEN Plasmid Plus midiprep kit and eluting in buffer TE or EB. DNA concentrations should be quantified by NanoDrop or similar spectrophotometer. DNA should be concentrated, ideally >2000 ng/μL. If impurities exist or DNA is too dilute, DNA should be ethanol precipitated and re-suspended in a smaller volume of buffer TE or EB. All buffers should be sterile and endotoxin-free.
12. Keep detailed and accurate records
Gene editing in hPSCs has many steps to be performed over 1–2 months. Careful record keeping is critical to ensure that gene targeting is successful and that the correct clones are selected and carried forward into downstream assays. Give all primers unique names and annotate which ones generated useful amplicons. This is particularly important after clones are picked into 96-well plates, since often only a few clones contain the desired edits. In addition to labeling plates with unique names and changing tips to prevent cross-contamination, it is helpful to develop an electronic record keeping system to track primers, PCR products, clone name and location, and sequencing reads. Ideally, a barcoding system could be employed for the labeling and identification of clones. If this is not possible, a naming scheme that is both clear and easy to identify should be put in place.
Anticipated results
The success of a gene targeting experiment depends on a number of factors, most critically the location and activity of the sgRNAs, the transfection efficiency, the characteristics of the cell line, and the design of the gene targeting strategy. This makes it difficult to anticipate results accurately, but best estimates are given below.
1) sgRNA design, generation, and activity
The abundance of bioinformatics tools has made sgRNA design straightforward. Many loci will have multiple CRISPR target sites within 50-bp of the desired mutation site, and most will have such sites within 100-bp. The generation of sgRNA expression vectors is highly efficient, though clones should be carefully screened since DNA oligos sometimes carry mutations. Most sgRNA sequences bioinformatically predicted to have high activity will display measurable activity in cutting assays. Since in silico predictions are not always accurate, users should compare multiple sgRNAs and select the most active ones for gene targeting experiments.
2) Efficiency of gene knock-out
Since the introduction of CRISPR/Cas9-mediated DSBs and the error-prone process of non-homologous end-joining (NHEJ) are both relatively efficient, it is relatively easy to knock out gene function. The efficiency of this process is strongly dependent on sgRNA activity, but generally ranges from about 25% of the clones having heterozygous mutations to about 75% of the clones having mixed homozygous mutations.
3) Efficiency of small targeted mutations
The generation of small targeted mutations is relatively inefficient since it relies on the relatively inefficient process of homology directed repair, and in most cases it is not possible to enrich for correctly-edited cells prior to screening. Successful editing ranges in efficiency from fractions of a percent to approximately 20% with a median of approximately 2–5%, depending on sgRNA activity, the targeted locus, and transfection conditions. It is advisable to gauge the likely editing efficiency by deep sequencing or ddPCR prior to picking colonies, or to pick at least 200 colonies prior to screening.
4) Gene targeting vector generation
The generation of gene targeting vectors is a multi-step but relatively straightforward process. PCR amplification of genomic loci succeeds for approximately 90% of loci. The amplification of homology arms from these genomic PCR products virtually never fails. Homology arms for the remaining loci might be possible to order as a commercially synthesized gene fragments. The assembly of homology arms, the targeting vector backbone, and reporter/selection cassette by Gibson cloning is a remarkably efficient process, provided that components are of sufficient purity and concentration. In our hands, if 8 bacterial clones are screened by colony PCR per assembly, at least one of these clones will have a correctly assembled targeting vector in well over 90% of experiments.
5) Efficiency of gene knock-in
The efficiency of gene knock-in depends largely on the in vivo activity of the sgRNAs, but also somewhat on the length of the sequence to be inserted which is inversely correlated with targeting efficiency. Positive and dual negative drug selection strongly enrich for the extremely rare cells (about 1 in 10,000) that stably insert the gene targeting cassette. In our hands, over half of all drug-resistant colonies are correctly targeted, and approximately half of those do not have unwanted mutations on the other allele. A typical gene targeting experiment performed with the pDTA-TK backbone yields several dozen drug-resistant clones, so given an approximately 25% yield of correctly targeted clones lacking unwanted mutations, the success of a gene knock-in experiment is very likely. If only a handful of colonies survive drug selection, it is advisable to redo the targeting experiment with different sgRNAs, improved DNA delivery methods, or higher DNA concentrations.
6) Undesired mutations and heterogeneity
Initial concerns about likely widespread off-target mutations from CRISPR/Cas9 have not materialized. Although they are detectable, off-target mutations following gene targeting in hPSCs are rare (Cho et al., 2014; Fu et al., 2013; Hsu et al., 2013; Pattanayak et al., 2013; Suzuki et al., 2014; Veres et al., 2014; Yang et al., 2013a). However, ‘on-target’ mutations at the targeted locus are common, since either allele can be cut in a gene targeting experiment. This may be desirable in gene knock-out experiments, but for small targeted mutations and knock-ins, the goal is typically to edit one locus and leave the other one intact. CRISPR/Cas9 can introduce a DSB in one allele that is repaired by HDR, but a DSB in the other allele is often repaired by NHEJ, introducing indels. If CRISPR/Cas9 activity persists, these unwanted mutations are likely to arise over time in some cells of an expanding hPSC colony, leading to the formation of heterogeneous clones (Merkle et al., 2015). It is important for groups to be aware that just because a clone appears to be correctly targeted, it may contain a substantial fraction of unwanted cells that might make the clone unsuitable for downstream experiments. To eliminate the unwanted cells, it will be necessary to sub-clone the hPSC line. To reduce the likelihood of generating mixed clones, it may be best to choose a method that reduces the time window in which CRISPR/Cas9 is active, such as delivering Cas9 as RNA or protein.
Time considerations
Primers, sgRNAs, and ssODNs can be designed and generated in a matter of days, and CRISPRs can be designed and tested within a week. Targeting vector design and cloning can be performed in approximately 1–2 weeks. Once these components are assembled, the approximate timelines for gene editing following the generation of reagents for gene targeting are given in Figure 2. Notably, cell lines vary in their rates of growth, so these figures are meant to provide only a guideline. The time frame of barcoded deep sequencing depends on sequencing library generation (approximately 1 week), sequencing turnaround times (variable) and bioinformatics analysis (variable).
Figure 8.
Schematic of workflow for gene targeting vector assembly and confirmation of gene targeting. A) PCR amplify the genomic locus to be targeted from genomic DNA isolated from the hPSC line to be targeted. Design three genomic forward and reverse primers (green) positioned 1–2kb from the target site and test them in all 9 possible combinations. This approach successfully produces amplicons for the vast majority of loci. B) The resulting amplicon is purified and used as a PCR template for the amplification of 5′ and 3 homology arms. These homology arm (HA) primers contain overhangs to enable Gibson assembly. Some groups have found that HA up to 2kb in length can improve gene targeting efficiencies. The strategy shown here is generalizable for longer homology arms. C) Homology arm amplicons with appropriate Gibson overhangs are assembled to the reporter/selection cassette and pDTA-TK plasmid backbone. The resulting gene targeting vector is sequence verified and introduced to hPSCs along with CRISPR/Cas9 to serve as the template for homologous recombination. D) To test for reporter/selection cassette insertion into the desired locus, primers binding to the reporter/selection cassette are paired with genomic primers (green) previously validated to yield amplicons from genomic DNA (A).
Acknowledgments
We thank Feng Zheng, Jack Sandoe, Lindy Barrett, James Gagnon, Summer Thyme, Belinda von Niederhäusern, and Werner Neuhausser for their assistance with developing methodologies for CRISPR/Cas9-based gene editing. KE and FTM were supported by grants from the National Institutes of Health (HL109525, 5P01GM099117, 5K99NS083713). FTM is supported by funds from the Wellcome Trust, the Academy of Medical Sciences, and the Medical Research Council (MR/P501967/1). EK and DS are supported by grants from the Les Turner ALS Foundation, Target ALS, and the Muscular Dystrophy Association.
Footnotes
KEY REFERENCE
An excellent history of gene targeting, the history of the CRISPR/Cas9 system and the future of CRISPR/Cas9 mediated genome engineering (Doudna and Charpentier, 2014). Several protocol papers have been published over the last several years providing detailed instructions for carrying out gene targeting experiments (Dambournet et al., 2014; Ran et al., 2013b; Song et al., 2014). For a review on TALEN and CRISPR/Cas9 mediated gene targeting and the advantages and disadvantages of each method, we refer readers to a recent review article (Hendriks et al., 2016). Several other reviews provide a basic overview of the CRISPR/Cas9 system and its future applications (Hsu et al., 2014; Zhang et al., 2014a).
INTERNET RESOURCES
CRISPR Design Tool (Feng Zhang Laboratory) http://crispr.mit.edu/
This tool is useful for the design of CRISPR sgRNAs. It allows for the design of both nucleases and paired nickases. For detailed instructions of use, refer to the help tab.
CHOPCHOP CRISPR Design Tool (Alex Schier laboratory) https://chopchop.rc.fas.harvard.edu/
Resource for the design of sgRNAs that provides a helpful visual interface. It also provides PCR primers that can be used for the SURVEYOR assay, Sanger sequencing of the targeted region or the in vitro cutting assay with Cas9 protein for a wide range of organisms.
sgRNA Design Tool (Broad Institute Genetic Perturbation Platform) http://www.broadinstitute.org/rnai/public/analysis-tools/sgrna-design
This sgRNA Design tool uses a distinct algorithm to predict on-target and off-target activity for S. pyogenes Cas9 in human and mouse genomes.
WGE (Wellcome Trust Sanger Institute Genome Editing) tool http://www.sanger.ac.uk/htgt/wge/
Convenient and visually oriented tool for finding CRISPR binding sites in the mouse or human genome using recent assemblies for up-to-date gene annotations and off-target prediction.
Addgene CRISPR/Cas9 Plasmids and Resources: http://www.addgene.org/CRISPR/?gclid=CjwKEAiA__C1BRDqyJOQ8_Tq230SJABWBSxnsp4uXzZ7H-iFnJ5QRhQ8dphNCpPvuwYI0fl08tu03hoC5u7w_wcB
This is Addgene’s page dedicated to the CRISPR/Cas9 technology. It provides basic protocols and the various versions of plasmids dozens of labs have deposited over the past several years.
Google Groups: Genome Engineering using CRISPR/Cas9 Systems Forum https://groups.google.com/forum/#!forum/crispr
This Google groups forum enables users to ask questions and see other questions that others using the CRISPR/Cas9 technology have asked.
CRISPR Genome Engineering Resources (Feng Zhang Laboratory) http://www.genome-engineering.org/crispr/
This page contains protocols, tools, troubleshooting tips and other resources pertaining to CRISPR/Cas9 genome engineering.
References
- Aarts M, te Riele H. Subtle gene modification in mouse ES cells: evidence for incorporation of unmodified oligonucleotides without induction of DNA damage. Nucleic acids research. 2010;38:6956–6967. doi: 10.1093/nar/gkq589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa E, Hirabayashi Y, Iwanaga Y, Suzuki K, Sakurai K, Shimoji M, Aiba K, Wada T, Tooi N, Kawase E, Suemori H, Nakatsuji N, Mitani K. Efficient and accurate homologous recombination in hESCs and hiPSCs using helper-dependent adenoviral vectors. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:424–431. doi: 10.1038/mt.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders C, Jinek M. In vitro enzymology of Cas9. Methods in enzymology. 2014;546:1–20. doi: 10.1016/B978-0-12-801185-0.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab M, Srinivasan S, Hashimoto T, Geijsen N, Sherwood RI. Cloning-free CRISPR. Stem cell reports. 2015;5:908–917. doi: 10.1016/j.stemcr.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nature protocols. 2012;7:2029–2040. doi: 10.1038/nprot.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Magor GW, Gillinder KR, Perkins AC. A high-throughput screening strategy for detecting CRISPR-Cas9 induced mutations using next-generation sequencing. BMC genomics. 2014;15:1002. doi: 10.1186/1471-2164-15-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner M, Kmiec EB. DNA breakage associated with targeted gene alteration directed by DNA oligonucleotides. Mutation research. 2009;669:85–94. doi: 10.1016/j.mrfmmm.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic acids research. 2014;42:e168. doi: 10.1093/nar/gku936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SM, Ortiz L, Mali P, Aach J, Church GM. Multi-kilobase homozygous targeted gene replacement in human induced pluripotent stem cells. Nucleic acids research. 2015;43:e21. doi: 10.1093/nar/gku1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nature methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KG, Mallon BS, McKay RD, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell stem cell. 2014;14:13–26. doi: 10.1016/j.stem.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cao J, Xiong M, Petersen AJ, Dong Y, Tao Y, Huang CT, Du Z, Zhang SC. Engineering Human Stem Cell Lines with Inducible Gene Knockout using CRISPR/Cas9. Cell stem cell. 2015;17:233–244. doi: 10.1016/j.stem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature biotechnology. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae S, Kim JS. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome research. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186:757–761. doi: 10.1534/genetics.110.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambournet D, Hong SH, Grassart A, Drubin DG. Tagging endogenous loci for live-cell fluorescence imaging and molecule counting using ZFNs, TALENs, and Cas9. Methods in enzymology. 2014;546:139–160. doi: 10.1016/B978-0-12-801185-0.00007-6. [DOI] [PubMed] [Google Scholar]
- Davis KM, Pattanayak V, Thompson DB, Zuris JA, Liu DR. Small molecule-triggered Cas9 protein with improved genome-editing specificity. Nature chemical biology. 2015;11:316–318. doi: 10.1038/nchembio.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT, Trevisan M, Gupta RM, Moisan A, Banks E, Friesen M, Schinzel RT, Xia F, Tang A, Xia Y, Figueroa E, Wann A, Ahfeldt T, Daheron L, Zhang F, Rubin LL, Peng LF, Chung RT, Musunuru K, Cowan CA. A TALEN genome-editing system for generating human stem cell-based disease models. Cell stem cell. 2013a;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Regan SN, Xia Y, Oostrom LA, Cowan CA, Musunuru K. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell stem cell. 2013b;12:393–394. doi: 10.1016/j.stem.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, Virgin HW, Listgarten J, Root DE. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nature biotechnology. 2016;34:184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Draper JS, Moore HD, Ruban LN, Gokhale PJ, Andrews PW. Culture and characterization of human embryonic stem cells. Stem cells and development. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Jackson SP. DNA double-strand break repair. Current biology: CB. 1999;9:R759–761. doi: 10.1016/S0960-9822(00)80005-6. [DOI] [PubMed] [Google Scholar]
- Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nature biotechnology. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon JA, Valen E, Thyme SB, Huang P, Akhmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PloS one. 2014;9:e98186. doi: 10.1371/journal.pone.0098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in biotechnology. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG. Enzymatic assembly of overlapping DNA fragments. Methods in enzymology. 2011;498:349–361. doi: 10.1016/B978-0-12-385120-8.00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Zhu Z, Shi ZD, Lelli K, Verma N, Li QV, Huangfu D. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell stem cell. 2014;15:215–226. doi: 10.1016/j.stem.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel A, Kildebeck EJ, Fine EJ, Clark JT, Punjya N, Sebastiano V, Bao G, Porteus MH. Quantifying genome-editing outcomes at endogenous loci with SMRT sequencing. Cell reports. 2014;7:293–305. doi: 10.1016/j.celrep.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks WT, Warren CR, Cowan CA. Genome Editing in Human Pluripotent Stem Cells: Approaches, Pitfalls, and Solutions. Cell stem cell. 2016;18:53–65. doi: 10.1016/j.stem.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JT, Demarest BL, Bisgrove BW, Su YC, Smith M, Yost HJ. Poly peak parser: Method and software for identification of unknown indels using sanger sequencing of polymerase chain reaction products. Developmental dynamics: an official publication of the American Association of Anatomists. 2014;243:1632–1636. doi: 10.1002/dvdy.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC, Kitano TK, Hodel MR, Petersen JF, Wyatt PW, Steenblock ER, Shah PH, Bousse LJ, Troup CB, Mellen JC, Wittmann DK, Erndt NG, Cauley TH, Koehler RT, So AP, Dube S, Rose KA, Montesclaros L, Wang S, Stumbo DP, Hodges SP, Romine S, Milanovich FP, White HE, Regan JF, Karlin-Neumann GA, Hindson CM, Saxonov S, Colston BW. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Analytical chemistry. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkins A, Farne A, Perera S, Grego T, Parry-Smith DJ, Skarnes WC, Iyer V. WGE: a CRISPR database for genome engineering. Bioinformatics. 2015;31:3078–3080. doi: 10.1093/bioinformatics/btv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nature biotechnology. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughesman C, Fakhfakh K, Bidshahri R, Lund HL, Haynes C. A new general model for predicting melting thermodynamics of complementary and mismatched B-form duplexes containing locked nucleic acids: application to probe design for digital PCR detection of somatic mutations. Biochemistry. 2015;54:1338–1352. doi: 10.1021/bi500905b. [DOI] [PubMed] [Google Scholar]
- Ichida JK, Kiskinis E. Probing disorders of the nervous system using reprogramming approaches. The EMBO journal. 2015;34:1456–1477. doi: 10.15252/embj.201591267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Stem Cell. Amps I, Andrews K, Anyfantis PW, Armstrong G, Avery L, Baharvand S, Baker H, Baker J, Munoz D, Beil MB, Benvenisty S, Ben-Yosef N, Biancotti D, Bosman JC, Brena A, Brison RM, Caisander D, Camarasa G, Chen MV, Chiao J, Choi E, Choo YM, Collins AB, Colman D, Crook A, Daley JM, Dalton GQ, De Sousa A, Denning PA, Downie C, Dvorak J, Montgomery P, Feki KD, Ford A, Fox A, Fraga V, Frumkin AM, Ge T, Gokhale L, Golan-Lev PJ, Gourabi T, Gropp H, Lu M, Hampl G, Harron A, Healy K, Herath L, Holm W, Hovatta F, Hyllner O, Inamdar J, Irwanto MS, Ishii AK, Jaconi T, Jin M, Kimber Y, Kiselev S, Knowles S, Kopper BB, Kukharenko O, Kuliev V, Lagarkova A, Laird MA, Lako PW, Laslett M, Lavon AL, Lee N, Lee DR, Li JE, Lim C, Ludwig LS, Ma TE, Maltby Y, Mateizel E, Mayshar I, Mileikovsky Y, Minger M, Miyazaki SL, Moon T, Moore SY, Mummery H, Nagy C, Nakatsuji A, Narwani N, Oh K, Oh SK, Olson SK, Otonkoski C, Pan T, Park F, Pells IH, Pera S, Pereira MF, Qi LV, Raj O, Reubinoff GS, Robins B, Robson A, Rossant P, Salekdeh J, Schulz GH, Sermon TC, Sheik Mohamed K, Shen J, Sherrer H, Sidhu E, Sivarajah K, Skottman S, Spits H, Stacey C, Strehl GN, Strelchenko R, Suemori N, Sun H, Suuronen B, Takahashi R, Tuuri K, Venu T, Verlinsky P, Ward-van Oostwaard Y, Weisenberger D, Wu DJ, Yamanaka Y, Young SL, Zhou Q. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nature biotechnology. 2011;29:1132–1144. doi: 10.1038/nbt.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M, Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harbor perspectives in biology. 2013;5:a012740. doi: 10.1101/cshperspect.a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim D, Cho SW, Kim J, Kim JS. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome research. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kime C, Mandegar MA, Srivastava D, Yamanaka S, Conklin BR, Rand TA. Efficient CRISPR/Cas9-Based Genome Engineering in Human Pluripotent Stem Cells. In: Haines Jonathan L, et al., editors. Current protocols in human genetics. Vol. 88. 2016. pp. 21 24 21–21 24 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SC, Xie L, Deng W, Guglielmi B, Witkowsky LB, Bosanac L, Zhang ET, El Beheiry M, Masson JB, Dahan M, Liu Z, Doudna JA, Tjian R. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science. 2015;350:823–826. doi: 10.1126/science.aac6572. [DOI] [PubMed] [Google Scholar]
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annual review of biochemistry. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, JAT Defined, feeder-independent medium for human embryonic stem cell culture. Current protocols in stem cell biology. 2007;Chapter 1(Unit 1C):2. doi: 10.1002/9780470151808.sc01c02s2. [DOI] [PubMed] [Google Scholar]
- Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nature methods. 2013;10:977–979. doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nature biotechnology. 2013a;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013b;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N, Rao MS. A review of the methods for human iPSC derivation. Methods in molecular biology. 2013;997:23–33. doi: 10.1007/978-1-62703-348-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, Plath K, Lowry WE, Benvenisty N. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell stem cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Mekhoubad S, Bock C, de Boer AS, Kiskinis E, Meissner A, Eggan K. Erosion of dosage compensation impacts human iPSC disease modeling. Cell stem cell. 2012;10:595–609. doi: 10.1016/j.stem.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell stem cell. 2013;12:656–668. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Neuhausser WM, Santos D, Valen E, Gagnon JA, Maas K, Sandoe J, Schier AF, Eggan K. Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the targeted locus. Cell reports. 2015;11:875–883. doi: 10.1016/j.celrep.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka Y, Chan AH, Judge LM, Yoo J, Huang M, Nguyen TD, Lizarraga PP, So PL, Conklin BR. Isolation of single-base genome-edited human iPS cells without antibiotic selection. Nature methods. 2014;11:291–293. doi: 10.1038/nmeth.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic acids research. 2014;42:W401–407. doi: 10.1093/nar/gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JC, Atze K, Yeung PL, Toro-Ramos AJ, Camarillo C, Thompson K, Ricupero CL, Brenneman MA, Cohen RI, Hart RP. Efficient, high-throughput transfection of human embryonic stem cells. Stem cell research & therapy. 2010;1:23. doi: 10.1186/scrt23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature biotechnology. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Geens M, Mertzanidou A, Jacobs K, Heirman C, Breckpot K, Spits C. Gain of 20q11.21 in human embryonic stem cells improves cell survival by increased expression of Bcl-xL. Molecular human reproduction. 2014;20:168–177. doi: 10.1093/molehr/gat077. [DOI] [PubMed] [Google Scholar]
- Olsen PA, Randol M, Luna L, Brown T, Krauss S. Genomic sequence correction by single-stranded DNA oligonucleotides: role of DNA synthesis and chemical modifications of the oligonucleotide ends. The journal of gene medicine. 2005;7:1534–1544. doi: 10.1002/jgm.804. [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nature biotechnology. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, Thakore PI, Glass KA, Ousterout DG, Leong KW, Guilak F, Crawford GE, Reddy TE, Gersbach CA. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nature methods. 2013;10:973–976. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem cells. 2007;25:2257–2268. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P, Shandilya H, D’Alessio JM, O’Connor K, Durocher J, Gerard GF. Mutation detection using Surveyor nuclease. BioTechniques. 2004;36:702–707. doi: 10.2144/04364PF01. [DOI] [PubMed] [Google Scholar]
- Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, Bertoni A, Swerdlow HP, Gu Y. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC genomics. 2012;13:341. doi: 10.1186/1471-2164-13-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahdar M, McMahon MA, Prakash TP, Swayze EE, Bennett CF, Cleveland DW. Synthetic CRISPR RNA-Cas9-guided genome editing in human cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E7110–7117. doi: 10.1073/pnas.1520883112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna S, Kwaku Dad AB, Beloor J, Gopalappa R, Lee SK, Kim H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome research. 2014;24:1020–1027. doi: 10.1101/gr.171264.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013a;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature protocols. 2013b;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nature neuroscience. 2013;16:780–789. doi: 10.1038/nn.3425. [DOI] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nature methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva G, Poirot L, Galetto R, Smith J, Montoya G, Duchateau P, Paques F. Meganucleases and other tools for targeted genome engineering: perspectives and challenges for gene therapy. Current gene therapy. 2011;11:11–27. doi: 10.2174/156652311794520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SS, Rowntree RK, Mekhoubad S, Lee JT. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4820–4825. doi: 10.1073/pnas.0712136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares FA, Sheldon M, Rao M, Mummery C, Vallier L. International coordination of large-scale human induced pluripotent stem cell initiatives: Wellcome Trust and ISSCR workshops white paper. Stem cell reports. 2014;3:931–939. doi: 10.1016/j.stemcr.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Kim YH, Kim JS, Kim H. Genome engineering in human cells. Methods in enzymology. 2014;546:93–118. doi: 10.1016/B978-0-12-801185-0.00005-2. [DOI] [PubMed] [Google Scholar]
- Southern E. Southern blotting. Nature protocols. 2006;1:518–525. doi: 10.1038/nprot.2006.73. [DOI] [PubMed] [Google Scholar]
- Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K. Recurrent chromosomal abnormalities in human embryonic stem cells. Nature biotechnology. 2008;26:1361–1363. doi: 10.1038/nbt.1510. [DOI] [PubMed] [Google Scholar]
- Sternberg SH, LaFrance B, Kaplan M, Doudna JA. Conformational control of DNA target cleavage by CRISPR-Cas9. Nature. 2015;527:110–113. doi: 10.1038/nature15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer B, Carlson-Stevermer J, Angenent-Mari N, Khalil A, Harkness T, Saha K. High content analysis platform for optimization of lipid mediated CRISPR-Cas9 delivery strategies in human cells. Acta biomaterialia. 2015 doi: 10.1016/j.actbio.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yu C, Qu J, Li M, Yao X, Yuan T, Goebl A, Tang S, Ren R, Aizawa E, Zhang F, Xu X, Soligalla RD, Chen F, Kim J, Kim NY, Liao HK, Benner C, Esteban CR, Jin Y, Liu GH, Li Y, Izpisua Belmonte JC. Targeted gene correction minimally impacts whole-genome mutational load in human-disease-specific induced pluripotent stem cell clones. Cell stem cell. 2014;15:31–36. doi: 10.1016/j.stem.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nature reviews. Genetics. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- Varshney GK, Pei W, LaFave MC, Idol J, Xu L, Gallardo V, Carrington B, Bishop K, Jones M, Li M, Harper U, Huang SC, Prakash A, Chen W, Sood R, Ledin J, Burgess SM. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome research. 2015;25:1030–1042. doi: 10.1101/gr.186379.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restorative neurology and neuroscience. 2010;28:589–603. doi: 10.3233/RNN-2010-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres A, Gosis BS, Ding Q, Collins R, Ragavendran A, Brand H, Erdin S, Cowan CA, Talkowski ME, Musunuru K. Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell stem cell. 2014;15:27–30. doi: 10.1016/j.stem.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi L, Schmidt JC, Zaug AJ, Ascarrunz DR, Cech TR. A novel two-step genome editing strategy with CRISPR-Cas9 provides new insights into telomerase action and TERT gene expression. Genome biology. 2015;16:231. doi: 10.1186/s13059-015-0791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y, Kobayashi T, Ohnishi M, Kobayashi T, Tamura S, Tsuzuki T, Sanbo M, Yagi T, Tashiro F, Miyazaki J. Enrichment and efficient screening of ES cells containing a targeted mutation: the use of DT-A gene with the polyadenylation signal as a negative selection maker. Transgenic research. 1999;8:215–221. doi: 10.1023/a:1008914020843. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013a;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Guell M, Byrne S, Yang JL, De Los Angeles A, Mali P, Aach J, Kim-Kiselak C, Briggs AW, Rios X, Huang PY, Daley G, Church G. Optimization of scarless human stem cell genome editing. Nucleic acids research. 2013b;41:9049–9061. doi: 10.1093/nar/gkt555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY) 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Human molecular genetics. 2014a;23:R40–46. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ge X, Yang F, Zhang L, Zheng J, Tan X, Jin ZB, Qu J, Gu F. Comparison of non-canonical PAMs for CRISPR/Cas9-mediated DNA cleavage in human cells. Scientific reports. 2014b;4:5405. doi: 10.1038/srep05405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nature biotechnology. 2015;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]