Abstract
Tetracyclines, which represent one of the most commonly used antibiotics for poultry, are known to be deposited in bones, where they can remain, despite the observation of appropriate withdrawal times. The aim of the study was to determine the concentration of oxytretracycline (OTC) residues in the bone and muscle of chickens, following the oral administration of a commercially available liquid formulation, and to test their cytotoxic effects on an in vitro cell culture model. Seventy-two 1-day-old broiler chickens were randomly allotted into 2 groups (control and treated animals). OTC (40 mg/kg BW) was administered via drinking water during the 1 to 5 and 20 to 25 days of life periods. At the end of the trial, the birds were slaughtered and the OTC residues in the target tissues were measured by means of liquid chromatography (LC) - tandem mass spectrometry (MS/MS). Cytotoxicity was assessed by evaluating the pro-apoptotic effect of the bone residues on the K562 erythroleukemic line and on the peripheral blood mononuclear cells (PBMC). In all the animals, the OTC residues in the muscle were far below the established MRL of 100 μg/kg. The OTC levels in the bones of the treated animals were instead found in the parts per million (ppm) range. Cell cytotoxicity was assessed by evaluating the pro-apoptotic effect of OTC bone residues on the haematopoietic cell system. This in vitro system has revealed a significant pro-apoptotic effect on both the K562 cell line and PBMC cultures. This result suggests potential human and animal health risks due to the entry of tetracycline residues contained in the bones of treated livestock into the food-chain. This could be of concern, particularly for canine and feline diets, as meat, bone meal, and poultry by-products represent some of the main ingredients of pet foods, especially in the case of dry pet food. Further studies are needed to define the underlying mechanisms of cytotoxicity and to evaluate the in vivo toxicological implications due to the observed in vitro effects.
Keywords: broiler chicken, oxytetracycline, bone residue, cytotoxicity
INTRODUCTION
In intensive poultry production, environmental conditions often compromise animal health and immune responses by encouraging infectious diseases to develop and spread easily. For this reason, the group-level therapeutic use of antibiotics is very common. The tetracycline class of antimicrobial agents is one of the most commonly used antibiotics in poultry production because of its low cost, efficacy, and lack of side effects (Chopra and Roberts, 2001). Typical antimicrobial regimens used to treat gastrointestinal and respiratory diseases in broiler chickens include oral medication with oxytetracycline (OTC) for 3 to 5 d. Although OTC was one of the first tetracycline antibiotics ever produced, limited information is available on its pharmacokinetics in avian species (Black, 1977; Dyer, 1989; Serrano et al., 1999). It has been shown that oral administration results in low and variable systemic levels of the drug and that, among edible tissues, the kidneys and liver contain the highest concentrations (Black, 1977). In order to avoid the presence of drug residues in animal products and to preserve consumers’ health, the European Union has established maximum residue levels (MRL) for OTC in poultry products (European Union, 2010). The observance of appropriate withdrawal periods for each licensed veterinary product ensures the presence of OTC residues below the MRL. However, it is well-known that tetracyclines are able to deposit and persist in bones because they bind directly to the bone matrix or through a more complex binding that takes place between the bone matrix (especially in neonates), calcium ions, and the 4 rings of the basic tetracycline structure (Milch et al., 1957). Therefore, it is not surprising that a high incidence of tetracycline residues has been observed in bones taken from pig carcasses at slaughter houses (Kühne et al., 2000). According to the same authors, even the smallest recommended sub-therapeutical dosage of OTC administered orally to chickens leads to detectable residues in bones after the withdrawal of medicated feeds. As a consequence, the current withdrawal times, which are based on the detectable levels of drugs dropping below the MRL in edible tissues, do not seem able to guarantee that bone tissues are free of drug residues after the administration of tetracyclines. The toxicological implications of these residues are still not fully understood. Although in vitro and in vivo tetracycline cytotoxic effects have already been described, the exact underlying mechanism has not been identified yet (Fife and Sledge, 1998; Celik and Eke, 2011).
On the basis of these premises, the aim of the study was to investigate the concentration of OTC residues in the bone and muscle of broiler chickens, following oral administration of a commercially available liquid formulation, and to test their cytotoxic effects on an in vitro cell culture model.
MATERIALS AND METHODS
Animals and Housing
The study was supervised by the Department of Veterinary Sciences of the University of Turin (Italy) and performed in the animal farm of the Department of Agriculture, Forestry, and Food Science of the University of Turin (Italy). The experimental protocol was designed according to the guidelines of European and Italian laws pertaining to the care and use of experimental animals (European directive 86/609/EEC, put into practice in Italy by Legislative Decree 116/92).
A total of seventy-two 1-day-old male and female broiler chickens (Ross 708) were randomly allotted into 2 groups (control and treated animals, n = 36) and raised in floor pens (3 pens/group). Each pen housed 12 chickens of a homogeneous weight and sex ratio. The birds were fed a commercial organic diet, based on corn and soybean (Abello FIN-IMM s.r.l., Verzuolo, CN, Italy), which was formulated to meet or exceed the requirements recommended by the National Research Council (1994) and adjusted according to the Ross 708 Broiler nutrition specification (Aviagen, 2014). The experiment was carried out in a 7-m wide × 50-m long × 7-m high poultry house, equipped with a waterproof floor and wall, covered completely by tiles, and with an automatic ventilation system. The chicks were distributed over 6 pens (3 pens/treatment) that were 1.0-m wide × 1.50-m long. Each pen was furnished with mixed sawdust and rice hulls as litter. Feeds and drinking water were provided ad libitum for the entire duration of the trial, until day 35, which corresponded to the slaughter day. The lighting schedule was 23L:1D during the first 3 d, followed by 18L:6D until the slaughter age. Ambient temperature was kept within the thermo-neutral zone, and during the first 3 wk, the birds were heated by means of infrared lamps. The chicks were vaccinated at hatching against Newcastle disease, Marek disease, infectious bronchitis, and coccidiosis. Health status and mortality were monitored daily during the entire experimental period. Chicken weight and feed consumption were recorded at 1, 21, and 35 d life using a high-precision scale (Sartorius, Signum), and the feed conversion ratio was calculated accordingly. Weight gain, ADG, daily feed consumption, and the feed conversion ratio were calculated for the 1 to 21, 21 to 35, and 1 to 35 d periods, on a pen basis. Three pens were assigned to the Control (C) group (i.e., the C group) and 3 pens were assigned to the OTC group (i.e., the OTC group); the mode of drug administration is described hereafter.
Treatment
Therapeutic treatments were applied, in accordance with the recommendations of the manufacturer, as far as the dose concentration, dosing period, and withdrawal time are concerned. Briefly, OTC (Ossitetraciclina liquida 20%, TreI, Reggio Emilia, Italy) was administered via drinking water at a dosage of 40 mg/kg live weight during the 1 to 5 and 20 to 25 d life periods. The amount of OTC dissolved in water was adjusted daily on the basis of the water intake and BW gain. The expected withdrawal time (10 d) was applied between the last OTC administration and slaughtering.
Slaughtering Procedures and Sample Collection
At the end of the trial (35 d), 6 chicks/pen, (3 males and 3 females), were randomly chosen from each pen, and sacrificed by CO2 gassing followed by neck-cutting. Immediately after slaughtering, the breast and leg were deboned, muscle was ground, and a pool was created. Samples were stored at −20°C pending analysis. The bones obtained from the breast and leg deboning (sternum, femur, tibia, and fibula) were dried overnight at 50°C, broken, cured in an autoclave (Alfa-10-plus) at 121°C and 1.0 bar over 30 min, dried at 50°C over the course of 36 h, and finely ground. Samples were stored at −20°C pending analysis.
Quantification of the OTC Residues
Acetonitrile, n-pentane, n-hexane, ethyl acetate, and methanol HPLC-grade, formic acid, EDTA disodium salt (Na2EDTA), citric acid, and hydrogen sodium phosphate were provided by Sigma–Aldrich (Milan, Italy). The high-purity water was obtained from Milli-Q purification system (Millipore, Bedford, MA, United States). A McIlvaine-Na2EDTA buffer 0.1 M was prepared by adding 12.9 g citric acid, 10.9 g hydrogen sodium phosphate, and 37.18 g EDTANa2 to 1 L Milli-Q water. The solid-phase extraction C18 cartridges (3 mL, 200 mg) were from Varian (Walnut, Creek, CA, United States). The analytical standards of OTC, 4-epioxytetracycline, and demethylchlortetracycline, used as internal standard, were provided by Sigma–Aldrich (Milan, Italy).
Standard stock solutions of OTC, 4-epioxy-tetracycline, or demethylchlortetracycline were prepared by dissolution in methanol to obtain a final concentration of 1.0 mg/mL. The standard stock solutions were stored at −20°C. The standard solutions were diluted in methanol to obtain a series of working standard solutions, which were stored at 4°C in the dark.
Samples were prepared according to Oka et al. (1998) with minor modifications. Briefly, an aliquot of 1.0 g chicken muscle or 0.5 g ground bone pool was placed in a centrifuge tube, fortified with the standard solution, vortex shaken for 30 s, and left for 30 min at room temperature to ensure an appropriate distribution in the matrix. A volume of 4.0 mL McIlvaine–Na2EDTA 0.1 M buffer (pH 4) was added, the mixture was vortex shaken for 15 s and centrifuged at 2,600 rpm for 5 min at 10°C. The supernatant was transferred to a clean centrifuge tube, and the residue was re-extracted with 4.0 mL McIlvaine–Na2EDTA 0.1 M buffer and centrifuged. The combined supernatants were degreased with 4.0 mL n-pentane and centrifuged at 2,600 rpm for 15 min at 10ºC. The aqueous layer was loaded into a solid-phase extraction C18 cartridge, which had previously been activated with methanol and Milli-Q water. After sample loading, the solid-phase extraction C18 cartridge was washed with Milli-Q water. The analytes were eluted with a mixture of ethyl acetate and methanol (95:5). The solvent was removed under a 40°C stream of nitrogen, and the residue was dissolved in 100 μL mobile phase. An aliquot (10 μL) was injected into the liquid chromatography–electrospray interface (ESI)–mass spectrometry (MS)/MS system.
Analyses were performed with a 1,200-L Varian liquid chromatography–MS/MS triple quadrupole (Walnut, Creek, CA, United States). The mass spectrometer was equipped with an ESI, operating in the positive mode. The HPLC was equipped with 2 mobile-phase pumps (ProStar 210), a degassit on line, an autosampler (ProStar 410), and a column thermostat. The ESI interface was calibrated using a polypropylene glycol solution, and the ESI parameters were optimized for each analyte by direct infusion of the individual standard solution into the mass spectrometer.
The mass spectrometer parameters were: needle 5,000 V, shield 600 V, housing 50ºC, capillary voltage 50 V, and detector voltage 1,500 V. High-purity nitrogen was used at 25 psi as nebulizer gas and at 19 psi and 360ºC as drying gas. High-purity argon was used as the collision gas at 2.0 mTorr. The mass spectrometer was operated at selective reaction monitoring mode to confirm the identity of the analytes in the samples by selecting specific precursor-to-product ions for each analyte and by selecting the most abundant transition for the quantification.
Separations were conducted using a Pursuit C18 (100 × 2.0 mm inner diameter, 5 μm) column with a Polaris C18 (2.0 mm, 3 μm) guard column Varian (Walnut, Creek, CA, United States) at 25°C. Solvent A, the mobile-phase solvent, was water 0.1% formic acid, while Solvent B was acetonitrile 0.1% formic acid. The mobile phase was delivered to the liquid chromatography column at a flow rate of 0.3 mL/min. A gradient elution was performed: 0 to 3 min 10% B; 3.1 to 5 min 75% B; 5.1 to 15 min 10% B.
Calibration curves were prepared for both tissues, and good linearity was achieved over the tested concentration ranges (r2 > 0.99 and goodness-of-fit<10%). The limits of detection for both OTC and 4-epioxytetracycline in the muscle and bones were 0.5 and 4.5 μg/kg, respectively. The within-day precision and accuracy fell within the range of −20 to +10%. The analyses were performed in triplicate.
Bone Residue Cytotoxic Evaluation
In order to test the potential cytotoxic role of OTC, 2 different conditioned cell culture media were used as providers of OTC residues. Briefly, to obtain a cell culture medium (CCM), 10 mL RPMI 1640 cell culture medium was incubated and constantly shaken for 48 h at 37°C with 1 g ground bone (sterilized by autoclaving at 121°C in a steam pressure of 2 atm for 10 min) from chickens reared in the presence (OTC–CCM) or in the absence (C–CCM) of treatments with OTC (see the C-group and OTC-group in the Animals and Housing section). After incubation, the CCMs were recovered and filtered through 0.20-μm syringe filters (Sartorius Stedim Biotech, Goettingen, Germany) to remove any residual ground bone particles and microbial contamination. The CCMs were then diluted at 1: 1, 1: 2, 1: 4, 1: 8, and 1:16 ratios with an absolute RPMI 1640 growth medium, and the resulting mixtures were incubated with 5×105 cells/ml for 48 h at 37°C and 5% CO2 in a cell incubator (Thermo Scientific Heraeus, United States). The effect of OTC alone was evaluated by incubating the drug (2 μg/mL), as described above. Furthermore, 200 μM hydrogen peroxide (H2O2) was used as a standard positive control of apoptosis in the 5×105 cells/mL culture for 2 h at 37°C, 5% CO2.
The used cells were the K562 erythroleukemic line (Carbone et al., 1996) or the peripheral blood mononuclear cells (PBMC) from venous blood of healthy human donors (Terrazzano et al., 2007). The PBMCs were obtained by centrifugation on Lymphoprep (Nycomed Pharma) gradients of healthy donor buffy coats obtained from the Blood Bank of the Medical School of the Federico II University of Naples, as previously described (Terrazzano et al., 2007).
Apoptosis was assessed by staining of the cell membrane-exposed phosphatidylserine with fluorescein isothiocyanate-conjugated Annexin V, according to the manufacturer's instructions (BD Pharmingen), and as previously described (De Vitis et al., 2011). Samples were analyzed by means of flow cytometry, using a fluorescence-activated cell sorting Calibur (Beckman Instruments, Fullerton, CA, United States), equipped with CellQuest Analysis Software. The fluorescence-activated cell sorting analysis was based on Annexin V staining, and was conducted to evaluate the fluorescence intensity of the staining or the percentage of cells positive to Annexin V so as to have 2 measurements of the cells undergoing apoptosis.
Statistical Analysis
The statistical analysis for the growth performance parameters was performed with SPSS 17 for Windows (SPSS, 2008). The experimental unit was the pen. Before testing for group differences, normality of data distribution and homogeneity of variances were assessed using the Shapiro–Wilk test and the Levene test, respectively. Growth performance data from the C-group and OTC-group were compared by means of the Student's t-test. Results were considered statistically significant for P < 0.05. A statistical trend was considered for P < 0.20. The results are presented as mean values ± SD.
The analysis pertaining to the pro-apoptotic effect was performed using the Mann–Whitney test, and the results were considered significant when P < 0.05.
RESULTS
The birds remained healthy for the whole period, no signs of illness were observed, and the mortality rate was zero for both groups. Growth performance was not influenced by the treatment (Table 1), and a positive numerical trend was observed for the OTC group for the final individual BW (day 35), ADG (1 to 35 d), weight gain (1 to 35 d), and feed consumption over the 21 to 35 d period.
Table 1.
Growth performance parameters in control animals and in broiler chickens treated with oxytetracycline (OTC) (mean values ± SD, Student's t test, n = 3).
| Control group | OTC group | P-value | |
|---|---|---|---|
| Growth performance | |||
| Mortality rate (%) | - | - | - |
| Individual BW (g) | |||
| d 1 | 42.3 ± 1.2 | 42.1 ± 0.9 | 0.805 |
| d 21 | 834.5 ± 15.7 | 867.1 ± 57.0 | 0.395 |
| d 35 | 1,729.2 ± 11.2 | 1,771.89 ± 11.2 | 0.155 |
| ADG (g) | |||
| 1 to 21 d | 37.7 ± 0.7 | 39.3 ± 2.8 | 0.395 |
| 21 to 35 d | 63.9 ± 1.5 | 64.6 ± 2.0 | 0.646 |
| 1 to 35 d | 48.2 ± 0.3 | 49.4 ± 1.2 | 0.160 |
| Weight gain (g) | |||
| 1 to 21 d | 792.3 ± 14.6 | 825.0 ± 57.8 | 0.395 |
| 21 to 35 d | 894.7 ± 20.9 | 904.8 ± 28.6 | 0.646 |
| 1 to 35 d | 1,686.9 ± 11.6 | 1,729.8 ± 41.5 | 0.160 |
| Daily feed consumption (g) | |||
| 1 to 21 d | 59.8 ± 3.5 | 60.3 ± 5.3 | 0.898 |
| 21 to 35 d | 112.1 ± 2.0 | 115.8 ± 1.6 | 0.068 |
| 1 to 35 d | 80.7 ± 2.8 | 82.5 ± 3.1 | 0.503 |
| Feed conversion ratio | |||
| 1 to 21 d | 1.58 ± 0.06 | 1.53 ± 0.04 | 0.324 |
| 21 to 35 d | 1.76 ± 0.06 | 1.79 ± 0.05 | 0.461 |
| 1 to 35 d | 1.67 ± 0.06 | 1.67 ± 0.04 | 0.889 |
As shown in Table 2, the concentrations of OTC, expressed as the sum of the parent drug and its 4-epimer, were far below the established MRL (100 μg/kg) in the muscle of the treated birds. On the other hand, about 100-fold higher levels were measured in the bones. Neither the muscle nor the bone samples showed measurable concentrations of the drug in the control birds.
Table 2.
Oxytetracycline (OTC) concentrations (μg/kg), expressed as the sum of the parent drug and 4-epimer, in the bone and muscle of control and treated broiler chickens (μg/kg on DM basis, mean values ± SD, n = 3).
| Control group | OTC group | |
|---|---|---|
| Muscle | <Limit of detection | 12.3 ± 6.9 |
| Bone | <Limit of detection | 1,286.3 ± 256.6 |
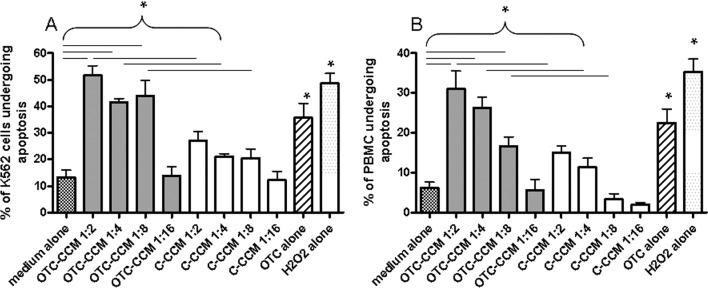
Figure 1 reports data from one representative experiment for apoptosis detection in the K562 cell line, in terms of peak overlay (Panel A) and individual peaks (Panel B). Forty-eight h incubation with OTC–CCM (ratio 1:2) (Peak 3) induced a significant increase in Annexin V staining, compared to the cell culture condition in the medium alone (Peak 2), which represents the basal level of the apoptosis that occurs in the K562 cell line without any incubation of CCM. Therefore, OTC–CCM seems able to induce apoptosis in the K562 cell line. C–CCM, instead, only induced slight Annexin V staining (Peak 4) at the same ratio. These results point out that C–CCM did not affect cell viability to any great extent. Interestingly, similar OTC–CCM and C–CCM effects were observed when PBMC was used instead of the K562 cell lines (Figure 2). The overall analysis of all the experiments suggest that a 48-h incubation with OTC–CCM induces significant increases in the percentage of cells undergoing apoptosis in both K562 and PBMC cell cultures (Figure 2, Panels A and B, respectively). The effect was observed to be significant at ratios of 1:2, 1:4, and 1:8 (P < 0.05), but not at the ratio of 1:16. The increase was also statistically significant after 24 h at the ratios of 1:2 and 1:4, but was only slightly detectable after 8 and 12 h incubation (data not shown). It should be noted that the incubation with 2 μg/mL pure OTC elicited similar effects on apoptosis to those obtained with OTC–CCM. Furthermore, the OTC–CCM and pure-OTC effects were quite similar to those elicited through the use of H2O2, which was used as a standard control of apoptosis induction. Despite the fact that apoptosis induction was evident, even after culture incubation with C–CCM at a ratio of 1:2 (P < 0.05), the increase was significantly lower than that obtained with OTC–CCM 1:2 (P < 0.05). It should be pointed out that CCM was used instead of direct incubation with ground bone, since the latter showed an extensive cytotoxic effect, which was probably due to direct contact with cells and oxygen subtraction from the system ascribable to the volume occupancy of the same ground particles in the culture medium (data not shown).
Figure 1.
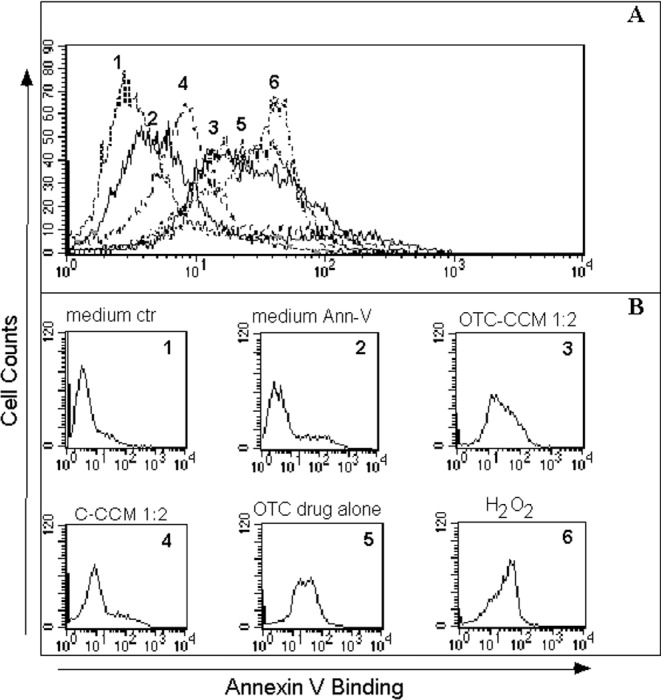
Apoptosis induction evaluated as fluorescence intensity of fluorescein isothiocyanate–Annexin V-staining in one representative experiment. The upper panel refers to the overlay of all the fluorescence peaks in the different conditions for the K562 cell line cultures. The lower panels represent the fluorescence peaks for each cell condition. The x-axis shows the fluorescence intensity of Annexin V binding on a logarithmic scale. The amplitude of the apoptosis induction is proportional to the right sliding of the peak on the x-axis towards higher values of fluorescence for Annexin-staining (to facilitate the reader's interpretation: Peak 1 is the one that shows the lowest intensity, while Peak 6 represents the peak at the highest intensity in the figure). In all the panels, the peaks correspond to the following different K562 cell culture conditions: 1 = in a growth medium alone without Annexin V staining, as a control of the cell natural fluorescence background; 2 = in a growth medium with Annexin V staining, as a control of the apoptosis that occurs in the K562 cell line maintained in a culture without any other incubation; 3 and 4 = peaks that represent the growth medium with the addition of a conditioned cell culture medium obtained from the ground bone of chickens reared in the presence [3 = oxytetracycline (OTC)–cell culture medium (CCM)] or in the absence [4 = control (C)–CCM] of a treatment with oxytetracycline, at a diluition of 1:2, stained with Annexin V; 4 = in a growth medium with the addition of C–CCM, at a diluition of 1:2, stained with Annexin V; 5 = in a growth medium with the addition of 2 μg/mL OTC, stained with Annexin V; 6 = in a medium with the addition of 100 μM H2O2, stained with Annexin V. The medium volumes for the different cell cultures were the same. See the Material and Methods section for the Annexin V staining.
Figure 2.
Apoptosis induction measured as a percentage of cells positive for the fluorescein isothiocyanate–Annexin V binding in the K562 cell line culture (Panel A) and of PBMC (Panel B). The graph bar-columns represent the mean values of the percentage of cells undergoing apoptosis in all the performed experiments. The different cell incubations and conditioned CCM dilutions are indicated on the x-axis. The abbreviations indicate the growth medium with the addition of a conditioned CCM obtained from the ground bone of chickens reared in the presence [oxytetracycline (OTC)–CCM] or in the absence [control (C)-CCM] of a treatment with OTC, a growth medium with the addition of 2 μg/mL OTC, or with 100 μM H2O2. All the cell cultures were stained with Annexin V (see the Material and Methods section). It should be noted that the bar-column of the medium alone indicates incubation in a growth medium with Annexin V staining, which has been used as a control of the apoptosis that occurs in the cells when in a culture without any other incubation is maintained. The statistical significance is indicated with an asterisk for each of the pairs of columns placed under the horizontal square brackets for the coupled-comparison.
DISCUSSION
Growth performance resulted to be within the range described in the Ross 708 broiler performance objectives (Aviagen, 2014), thus confirming that appropriate animal care and welfare conditions had been maintained throughout the study. The lack of influence of OTC on the growth performance parameters was an expected result as the drug had been administered at a therapeutic dosage regimen rather than for growth promoting purposes. Nevertheless, it cannot be excluded that an antibacterial action contributed to the positive trend of the BW and weight gain (Butaye et al., 2003).
Our findings confirm that the bones of broiler chickens treated with therapeutic dosages of oral OTC contain considerable amounts of OTC residues. The finding agrees with those of previous studies, thus demonstrating that bone represents a target tissue for tetracycline localization (Milch et al., 1957; Buyske et al., 1960; Kühne et al., 2000). The incidence of tetracycline antibiotic residues in the bones of slaughtered animals seems to vary from 18.8 to 100%, depending on the species. In a study performed by Kühne et al. (2000), the bones of chickens treated for 10 d with low oral dosages of OTC still contained detectable levels of the drug after a withdrawal time of 15 d. Interestingly, in the present study, the concentrations of OTC were below the MRL set by the European Community (100 μg/kg) in all the muscle samples. Similar results have been obtained in turkeys, in which the existence of a correlation between the percentage of positive results in the kidneys and liver and that in the bones has been pointed out (Kühne and Mitzscherling, 2003).
In the present study, CCM containing OTC residues induced a significant pro-apoptotic effect in both the K562 and PBMC cell cultures. It is worth noting that if one considers the CCM as a source of OTC, this in vitro system represents a useful model to test the cytotoxic effects of this drug.
A great deal of evidence supports the in vitro and in vivo cytotoxicity of tetracyclines (van den Bogert et al., 1981; Shao and Feng, 2013; Chi et al., 2014). Several possible mechanisms have been suggested, including the inhibition of mitochondrial protein synthesis and of the antioxidant defense system (van den Bogert et al., 1981; Chi et al., 2014). Moreover, the in vitro effects of tetracyclines have been related to the inhibition of lymphocyte proliferation and the negative modulation of neutrophil phagocytic functions (Thong and Ferrante, 1980).
As far as PBMC cell cultures are concerned, the effects of OTC–CCM appear to be dose-dependent, and comparable with that of hydrogen peroxide at a dilution ratio of 1:2. The effect of pure OTC on inducing apoptosis also appears to be rather relevant, since there has been no definitive observation on the toxic effects of this compound on mammalian cells and, in particular, on humans.
Although a threshold for OTC in vitro toxicity could not be established, the most interesting result is that OTC residues in the bones of slaughtered animals maintain cytotoxic effects, in spite of mechanical and thermal treatments. In fact, the possible toxicological risks depend on the degree of biological activity maintained by the drug residues in fresh raw materials or following feed processing. Meat, bone meal, and poultry by-products are the main ingredients of pet foods, especially in the case of dry pet food. For the latter, the most frequently used processing technology to produce canine and feline diets or dinner ingredients is extrusion. The extrusion process usually involves the application of both relatively high temperatures (80 to 200°C) and short residence times (10 to 250 s) (Serrano and Agroturia, 1996). The thermal stability of tetracycline antibiotics in animal food products has been the subject of different studies (Ibrahim and Moats, 1994; Hassani et al., 2008). The variety of methodologies employed in the heat inactivation experiments and in the detection of the antibiotic residual concentrations could explain the differences in the results. However, according to Hassani et al. (2008), low-temperature–long-time treatments (conventional sterilization) could destroy >98% of the initial concentration of tetracycline residues, but high-temperature-short-time treatments would leave the residues in the 50 to 90% range unaltered. Finally, heat stability depends on the type of matrix. Although it is assumed that tetracyclines are not very heat-resistant, residues in bones seem to be more stable (Honikel et al., 1978). When considering an intermediate product from a rendering plant mixed with bone splinters containing bound tetracycline residues, a complete destruction during a heat treatment at 133°C for up to 45 min could not be demonstrated (Kühne et al., 2001). It should be noted that most heat stability studies have evaluated the degradation of parent drugs without considering the possible formation of breakdown products endowed with toxic effects. Little is known about the breakdown products formed from tetracycline and OTC during heat treatments (Gratacós-Cubarsí et al., 2007; Kühne et al., 2001). Therefore, it cannot be excluded that the cytotoxic effects observed on the K562 and PBM cells can be ascribed to different compounds from OTC.
Besides the fact that it is necessary to be cautious in drawing conclusions about in vivo toxicological implications due to the observed in vitro cytotoxic effects, it cannot be excluded that OTC residues in the bones of treated chickens can induce biological responses in pets and human consumers. Interestingly, higher OTC and doxycycline serum levels than the safety limits have been observed in gym-trained subjects with food intolerance symptoms (Di Cerbo et al., 2014). The authors speculated that tetracycline antibiotic residues transferred to the final consumers could act as haptens and induce specific intolerance to a wide variety of food. Further studies are needed to confirm this hypothesis.
Following oral treatment with therapeutic doses of OTC, residues in the parts per million range accumulate in the bones of treated animals. Although little is known about the biological activity of the residues contained in target organs of slaughtered animals, the present findings suggest in vitro pro-apoptotic effects on normal and cancer cells of humans. Further studies are needed to define the mechanisms responsible for the cytotoxic effect. Potential human and animal health risks, due to the entry of tetracycline residues contained in the bones of treated livestock into the food chain, should not be underestimated.
Acknowledgments
The authors are grateful to Paolo Montersino, Dario Sola, and Mario Colombano for the care of the birds, and to Chiara Bianchi and Lidia Sterpone for their technical support.
Footnotes
Contributed equally to this study.
REFERENCES
- Aviagen Ross 708 Broiler nutrition specification. 2014. ( http://en.aviagen.com/ross-708/), Accessed October 6, 2014.
- Black W. D. A study of the pharmacokinetics of oxytetracycline in the chicken. Poult. Sci. 1977;56:1430–1434. doi: 10.3382/ps.0561430. [DOI] [PubMed] [Google Scholar]
- Butaye P., Devriese L. A., Haesebrouck F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyske D. A., Eisner H. J., Kelly R. G. Concentration and persistence of tetracycline and chlortetracycline in bone. J. Pharmacol. Exp. Ther. 1960;130:150–156. [PubMed] [Google Scholar]
- Carbone E., Terrazzano G., Colonna M., Tuosto L., Piccolella E., Franksson L., Palazzolo G., Pérez-Villar J. J., Fontana S., Kärre K., Zappacosta S. Natural killer clones recognize specific soluble HLA class I molecules. Eur. J. Immunol. 1996;26:683–689. doi: 10.1002/eji.1830260326. [DOI] [PubMed] [Google Scholar]
- Celik A., Eke D. The assessment of cytotoxicity and genotoxicity of tetracycline antibiotic in human blood lymphocytes using CBMN and SCE analysis, in vitro. Int. J. Human Genet. 2011;11:23–29. [Google Scholar]
- Chi Z., Liu R., You H., Ma S., Cui H., Zhang Q. Probing the in vitro cytotoxicity of the veterinary drug oxytetracycline. PloS ONE. 2014;9:e102334. doi: 10.1371/journal.pone.0102334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vitis S., Treglia A. Sonia, Ulianich L., Turco S., Terrazzano G., Lombardi A., Miele C., Garbi C., Beguinot F., Di Jeso B. Tyr phosphatase-mediated P-ERK inhibition suppresses senescence in EIA + v-raf transformed cells, which, paradoxically, are apoptosis-protected in a MEK-dependent manner. Neoplasia. 2011;13:120–30. doi: 10.1593/neo.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cerbo A., Canello S., Guidetti G., Laurino C., Palmieri B. Unusual antibiotic presence in gym trained subjects with food intolerance: A case report: A preliminary study. Nutr. Hosp. 2014;30:395–398. doi: 10.3305/nh.2014.30.2.7594. [DOI] [PubMed] [Google Scholar]
- Dyer D. C. Pharmacokinetics of oxytetracycline in the turkey: Evaluation of biliary and urinary excretion. Am. J. Vet. Res. 1989;50:522–524. [PubMed] [Google Scholar]
- European Union. Commission Regulation EU/37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. J. Eur. Union. 2010;L15:1–72. [Google Scholar]
- Fife R. S., Sledge G. W., Jr Effects of doxycycline on cancer cells in vitro and in vivo. Adv. Dent. Res. 1998;12:94–96. doi: 10.1177/08959374980120012801. [DOI] [PubMed] [Google Scholar]
- Gratacós-Cubarsí M., Fernandez-García A., Picouet P., Valero-Pamplona A., García-Regueiro J. A., Castellari M. Formation of tetracycline degradation products in chicken and pig meat under different thermal processing conditions. J. Agric. Food Chem. 2007;55:4610–4616. doi: 10.1021/jf070115n. [DOI] [PubMed] [Google Scholar]
- Hassani M., Lazaro R., Perez C., Condon S., Pagan R. Thermostability of oxytetracycline, tetracycline, and doxycycline at ultrahigh temperatures. J. Agric. Food Chem. 2008;56:2676–2680. doi: 10.1021/jf800008p. [DOI] [PubMed] [Google Scholar]
- Honikel K. O., Schmidt U., Woltersdorf W., Leistner L. Effect of storage and processing on tetracycline residues in meat and bones. J. AOAC Int. 1978;61:1222–1227. [PubMed] [Google Scholar]
- Ibrahim A., Moats W. A. The effect of cooking procedures on oxytetracycline in lamb muscle. J. Agric. Food Chem. 1994;72:2561–2563. [Google Scholar]
- Kühne M., Körner U., Wenzel S. Tetracycline residues in meat and bone meals. Part 2: The effect of heat treatments on bound tetracycline residues. Food Addit. Contam. 2001;18:593–600. doi: 10.1080/02652030118164. [DOI] [PubMed] [Google Scholar]
- Kühne M., Mitzscherling A. T. The entry of bound residues of tetracyclines into the food chain–A contribution to hazard identification. Berl. Munch. Tierarztl. Wochenschr. 2003;117:201–206. [PubMed] [Google Scholar]
- Kühne M., Wegmann S., Kobe A., Fries R. Tetracycline residues in bones of slaughtered animals. Food Control. 2000;11:175–180. [Google Scholar]
- Milch R. A., Rall D. P., Tobie J. E. Bone localization of the tetracyclines. J. Natl. Cancer Inst. 1957;19:87–93. [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry. 9th rev. ed. Washington, DC: Natl. Acad. Press; 1994. [Google Scholar]
- Oka H., Ito Y., Ikai Y., Kagami T., Harada K. Mass spectrometric analysis of tetracycline antibiotics in foods. J. Chromatogr. A. 1998;812:309–319. doi: 10.1016/s0021-9673(97)01278-8. [DOI] [PubMed] [Google Scholar]
- Serrano J. M., Moreno L., Rosado I., Guimera E., Escudero E. Biliary elimination kinetics and tissue concentrations of oxytetracycline after intravenous administration in hens. J. Vet. Pharmacol. Ther. 1999;22:148–152. doi: 10.1046/j.1365-2885.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- Serrano X., Agroturia S. A. The extrusion-cooking process in animal feeding: Nutritional implications. In: Morand-Fehr P., editor. Feed Manufacturing in Southern Europe: New Challenges. Spain: Zaragoza; 1996. pp. 107–114. [Google Scholar]
- Shao J., Feng G. Selective killing effect of oxytetracycline, propafenone and metamizole on A549 or Hela cells. Chin. J. Cancer Res. 2013;25:662–670. doi: 10.3978/j.issn.1000-9604.2013.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. Statistical Package for the Social Sciences. New York, New York, United States: McGraw–Hill; 2008. Version 17.0. [Google Scholar]
- Terrazzano G., Sica M., Gianfrani C., Mazzarella G., Maurano F., De Giulio B., de Saint-Mezard S., Zanzi D., Maiuri L., Londei M., Jabri B., Troncone R., Auricchio S., Zappacosta S., Carbone E. Gliadin regulates the NK-dendritic cell cross-talk by HLA-E surface stabilization. J. Immunol. 2007;179:372–381. doi: 10.4049/jimmunol.179.1.372. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A. Effect of tetracycline treatment on immunological responses in mice. Clin. Exp. Immunol. 1980;39:728–732. [PMC free article] [PubMed] [Google Scholar]
- van den Bogert C., Dontje B. H., Wybenga J. J., Kroon A. M. Arrest of in vivo proliferation of Zajdela tumor cells by inhibition of mitochondrial protein synthesis. Cancer Res. 1981;41:1943–1947. [PubMed] [Google Scholar]