Abstract
This is the first report providing estimates of the genetic basis of breast muscle myopathies (BMM) and their relationship with growth and yield in broiler chickens. In addition, this paper addresses the hypothesis that genetic selection for increase breast yield has contributed to the onset of BMM. Data were analyzed from ongoing recording of BMM within the Aviagen breeding program. This study focused on three BMM: deep pectoral myopathy (DPM; binary trait), white striping (WS; 4 categories) and wooden breast (WB; 3 categories). Data from two purebred commercial broiler lines (A and B) were utilized providing greater than 40,000 meat quality records per line. The difference in selection history between these two lines has resulted in contrasting breast yield (BY): 29% for Line A and 21% for Line B. Data were analyzed to estimate genetic parameters using a multivariate animal model including six traits: body weight (BW), processing body weight (PW), BY, DPM, WB, and WS, in addition to the appropriate fixed effects and permanent environmental effect of the dam. Results indicate similar patterns of heritability and genetic correlations for the two lines. Heritabilities (h2) of BW, PW and BY ranged from 0.271–0.418; for DPM and WB h2 <0.1; and for WS h2 ≤0.338. Genetic correlations between the BMM and BW, PW, or BY were ≤0.132 in Line A and ≤0.248 in Line B. This paper demonstrates the polygenic nature of these traits and the low genetic relationships with BW, PW, and BY, which facilitates genetic improvement across all traits in a balanced breeding program. It also highlights the importance of understanding the environmental and/or management factors that contribute greater than 65% of the variance in the incidence of white striping of breast muscle and more than 90% of the variance of the incidence of wooden breast and deep pectoral myopathy in broiler chickens.
Keywords: Broilers, breast muscle, meat quality, myopathy, heritability
INTRODUCTION
Globally, chicken meat is one of the most popular sources of animal protein for human consumption worldwide (OECD/FAO, 2015) and therefore consistency in product quality and food safety are of utmost importance. Through advances in genetic selection, farming practices and nutrition, the production of broiler chickens has become more efficient. Over the past 30 years, live weight has increased by 30.2 g per year and at the same time FCR has reduced yearly by around 0.036%. (National Chicken Council, 2015). Genetic selection has contributed significantly to the improvement in growth rate, biological efficiency, breast yield, longevity, and leg health (Havenstein et al., 2003a, 2003b; Fleming et al., 2007; Zuidhof et al., 2014). Balanced breeding goals have, in addition to growth, yield and biological efficiency, allowed for improvements in health traits and carcass quality. Over the past 15 years, broiler mortality has fallen by 0.05% per year, additionally carcass condemnation rate has also fallen from 1.79% to 0.24% (National Chicken Council, 2015; USDA, 2015). These figures show that it is possible to increase the productivity of the modern broiler without compromising bird health. The implementation of balanced breeding goals has allowed the simultaneous improvement of production and welfare related traits as shown by Kapell et al. (2012a, 2012b) for leg defects and contact dermatitis.
As with all body systems, the muscular system of a chicken is not exempt from pathology and any condition which impacts upon the quality of breast meat is of great importance to breeding companies and broiler producers. Carcasses affected by breast muscle myopathies (BMM) can be downgraded or in some cases condemned, resulting in economic losses for poultry meat producers (Mitchell, 1999; Mitchell and Sandercock, 2004).
One of the earliest myopathies reported in poultry was deep pectoral myopathy (DPM), initially identified in turkeys (Harper et al., 1975) but later also observed in broiler chickens (Richardson et al., 1980). This condition involves ischemic necrosis of supracoracoideus or pectoralis minor (P. minor) as a result of exertion (Jordan and Pattison, 1998). The P. minor muscle is responsible for the lifting of the wing. Due to the inelastic fascia surrounding the muscle and its tight location next to the sternum, the pectoralis minor is unable to expand with the influx of blood during exertion. As a consequence, the pressure within the muscle increases, which then compresses the venous return, resulting in a compartment syndrome (Siller et al., 1978a; Wight and Siller, 1980). Following the ischemia, there is rapid necrosis of the tissues and red blood cells in the muscle giving rise to hemorrhaging and eventually a greenish discoloration to the muscle (Bianchi et al., 2006); this discoloration is the basis of its more colloquial name “green muscle disease” (Figure 1). DPM can be induced in birds through surgical occlusion of the vascular supply (Orr and Riddell, 1977), electrical stimulation of the P. minor (Siller et al., 1978a; Wight et al., 1979), or by encouraging birds to flap (Siller et al., 1978b; Wight and Siller, 1980; Lien et al., 2012). Practical flock management recommendations are available to minimize the field incidence of deep pectoral myopathy by the industry (Bilgili and Hess, 2008).
Figure 1.
Breast muscles from 42 d old broiler exhibiting deep pectoral myopathy as shown by the green discoloration of the pectoralis minor muscle (see arrows).
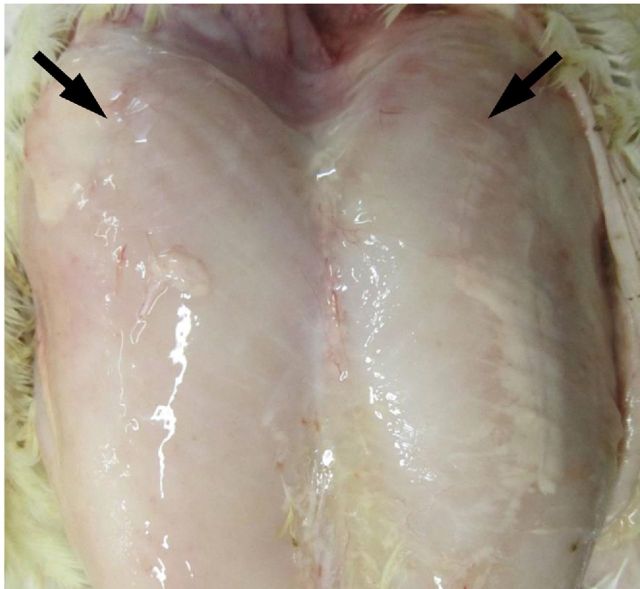
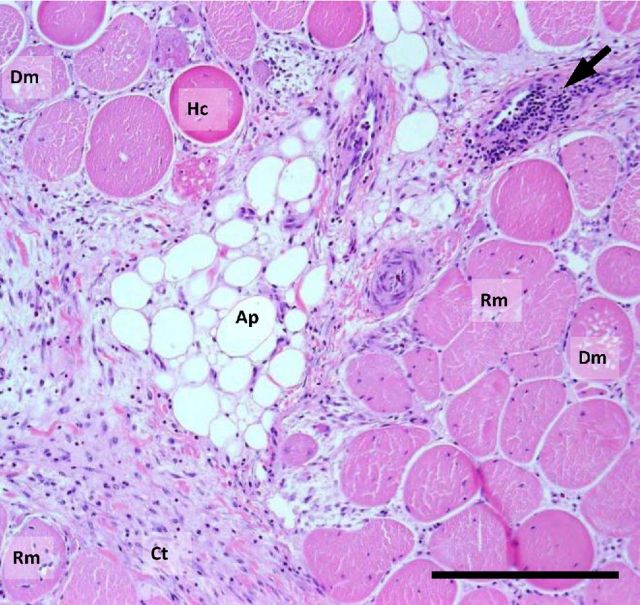
Recently there have been increased reports of two novel myopathies affecting the pectoralis major (P. major) muscle of broiler chickens which have been given the names “wooden breast” and “white striping” within the poultry industry vernacular (Petracci and Cavani, 2012). Current published research indicates that the myopathies can be observed in a number of commercial broiler strains (Kuttappan et al., 2012a,b,f; Petracci et al., 2013; Sihvo et al., 2013; Ferreira et al., 2014; Mudalal et al., 2015). Wooden breast (WB) is characterized by a hardening of the breast muscle typically in the proximal part of the fillet but the hardening can be found throughout the muscle in more severe cases. Depending on the severity of the condition, other macroscopic features of wooden breast include a paler color, surface hemorrhaging and the presence of a sterile exudate on the muscle surface (Figure 2). Histological analysis of the muscle shows active degeneration and regeneration of muscle fibers, and infiltration of immune cells with increased deposition of adipose and connective tissue (Figure 3), thus indicating that wooden breast can be characterized as a myodegeneration with fibrosis and regeneration (Sihvo et al., 2013).
Figure 2.
Breast muscle from 42-day-old broiler exhibiting wooden breast. Muscle is pale with an exudate over the surface. Arrows indicate where muscle is typically firm to the touch.
Figure 3.
Histomicrograph of breast muscle affected by wooden breast. Features of the muscle include degenerating muscle fibers (Dm), regenerating fibers (Rm), adipose tissue (Ap), hypercontracted fibers (Hc), increased connective tissue (Ct) and cellular infiltration (arrow). Black bar shows scale (100 μm).
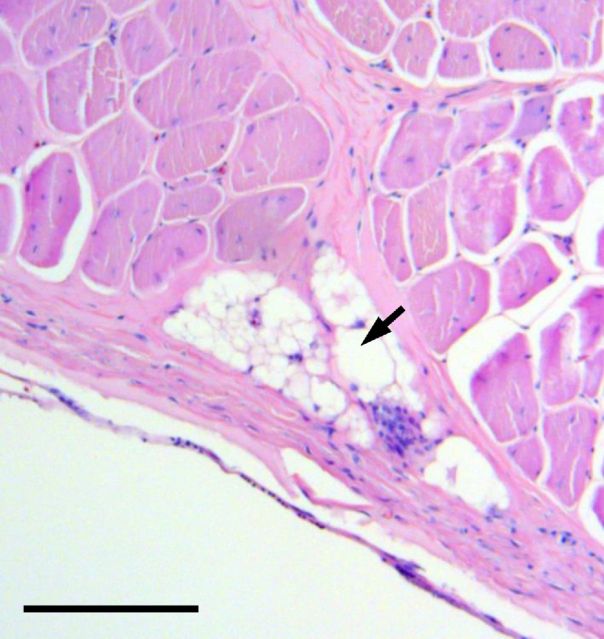
White striping (WS) is characterized by visible white lines parallel to the direction of the muscle fibers; the quantity and thickness of the white stripes can vary from bird to bird (Figure 4). Histological and chemical analysis of breast muscle displaying white striping showed that the white lines are composed of adipose tissue (Figure 5).
Figure 4.
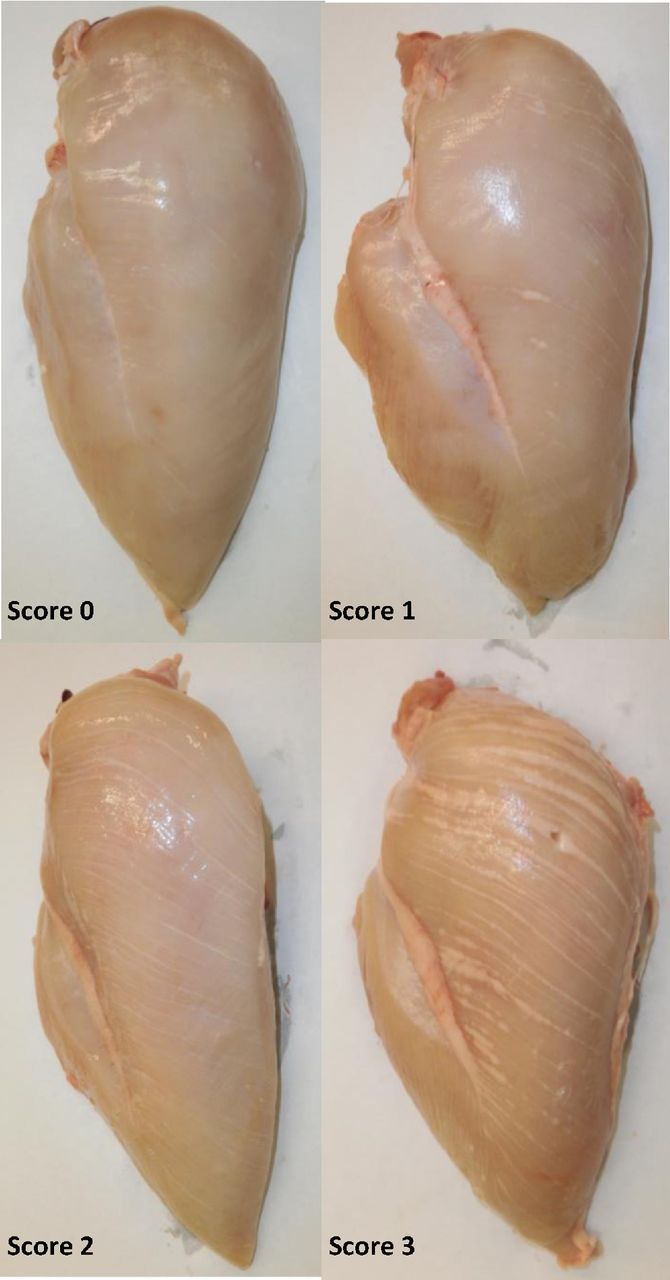
Breast fillets displaying different degrees of white striping. Score 0 indicates no white striping and score 3 indicates severe white striping.
Figure 5.
Histomicrograph of a breast fillet affected by white striping. The white stripe is composed of adipose tissue (arrow). Black bar shows scale (100 μm).
It has been reported that the breast tissue severely affected by white striping can exhibit an increase in connective tissue with varying degrees of myofibrilar degeneration and regeneration at the microscopic level (Ferreira et al., 2014; Russo et al., 2015). Kuttappan et al. (2012f) demonstrated that as the severity of white striping increased, the percentage fat as a proportion of dry matter of the muscle also increased, thus affirming the histological findings of increased adipogenesis in the tissues. The histological findings of wooden breast and white striping have some similarities (Kuttappan et al., 2012f; Ferreira et al., 2014); however, as they can be found both independently of each other and concurrently, they may represent two distinct myopathies.
There are a number of known causes of myodegeneration in poultry such as vitamin E and selenium deficiency, exertional myopathy, various toxicities, and tissue hypoxia. These have been suggested as potential causes of WB and WS due to similarities in histopathological morphology, however to date there is no clear etiology. There have been comparisons of WS and WB to muscular dystrophies found in other species due to the similarities in histological changes (Petracci et al., 2015). Vitamin E deficiency is well recognized across many species to cause nutritional muscular dystrophy (Van Vleet et al., 1976; Walsh et al., 1993), however attempts to reduce WS with supplemental vitamin E have not been successful (Guetchom et al., 2012; Kuttappan et al., 2012c) suggesting it is not associated with nutritional muscular dystrophy as a result of vitamin E deficiency. It has been proposed that there is an infectious component to the BMM; however, there have been no published reports yet of any pathogens associated with the myopathies. One investigation to characterize the hematological and serological profiles of birds with and without WS revealed no differences in leukocyte counts, suggesting there is no systemic infection or inflammation associated with white striping (Kuttappan et al., 2012d).
The most popular hypothesis suggests that genetic selection for increased growth rate and breast yield plays a role in the manifestation of the BMM in broiler chickens (Siller, 1985; Mitchell, 1999; Macrae et al., 2006; Kuttappan et al., 2012f; Petracci and Cavani, 2012; Sihvo et al., 2013; Petracci et al., 2015); however no information is published on the genetic estimates of BMM in broiler chickens. The aim of this study was to characterize the genetic basis of the myopathies by estimating the heritabilities for DPM, WB, and WS and their genetic relationship with growth rate and breast muscle yield in two pure broiler lines that exhibit contrasting breast meat yields.
MATERIALS AND METHODS
Birds, Housing, and Management
The data used in this study originates from the routine recording of breast meat production traits as part of the Aviagen (Newbridge, UK) breeding program. Two purebred commercial broiler lines with differing selection history and breast yield were used in this study; line A is a high-yielding chicken and line B is a moderate-yielding bird. The phenotypic data spans six generations collected over four years from 219 flocks of both lines of birds, with the inclusion of an extra generation of pedigrees for the estimation of the genetic parameters. The phenotypic traits of interest in this study were selection body weight (BW), processing body weight (PW) breast meat yield (BY), DPM, WB and WS (Table 1). The birds were all housed within environmentally controlled pedigree broiler farms in southern Scotland; a detailed description of environmental parameters can be found in Table 2. The birds were all housed in pens with wood shavings provided as the litter substrate with ad libitum access to food and water. The stocking densities for the birds were between 29 and 32 kg per m2 in line with the guidelines set down in the EU Council Directive 2007/43/EC. All birds were incubated in the same hatchery, where they also received the required vaccinations and are tagged with a barcoded wingband for identification. Once hatched, the birds are moved all together to the growing farms where they are placed in pens according to line.
Table 1.
Number of records for each trait used in the analysis for each of the two lines.
| Number of records | ||
|---|---|---|
| Trait | Line A | Line B |
| Body weight (BW) | 316,125 | 362,305 |
| Processing body weight (PW) | 49,071 | 64,994 |
| Breast yield (BY) | 49,071 | 64,994 |
| Deep pectoral myopathy (DPM) | 49,071 | 64,994 |
| Wooden breast (WB) | 41,702 | 55,797 |
| White striping (WS) | 42,578 | 56,837 |
Table 2.
Environmental parameters for all farms where birds were housed in this study.
| Environmental parameter | Target |
|---|---|
| Feed days: 0 to 10 | Starter (240 g CP/kg; 12.6 MJ ME/kg) |
| Feed days: 11 to 25 | Grower (210 g CP/kg; 13.3 MJ ME/kg) |
| Feed days: 25 to final weighing | Finisher (205 g CP/kg; 13.5 MJ ME/kg) |
| Stocking density | 29 to 32 kg bird weight per m2 |
| Temperature | Gradually reduced from 29 to 20°C |
| Photoperiod day 0 to 7 | 23L:1D |
| Photoperiod day 8 to final weighing | 18L:6D |
| Light intensity day 0 to 7 | 40 lux |
| Light intensity day 8 to final weighing | Gradually reduced from 20 to 10 lux |
Recording of Traits
All birds in this study were hatched in the same hatchery, fully pedigreed and uniquely tagged with a barcode wingband. The phenotypic data for the BMM and carcass traits were collected on a subset of birds taken from the overall population. This subset of birds consists of half and full siblings of the selection candidates and contribute the BMM and carcass data for breeding value prediction of selection candidates. All birds in the population (i.e., with or without BMM and carcass trait data) were individually weighed to obtain BW at 42 and 32 d for lines A and B, respectively, while only those contributing the BMM and carcass data were weighed at the slaughter plant to obtain PW (47 and 40 d for lines A and B, respectively). PW was recorded on an empty gut while BW was recorded on full gut. At slaughter, birds were stunned to kill in a brine-water bath with the electricity parameters set at 90V and 150 Hz. Following plucking and evisceration, the carcasses were blast chilled for 24 h. Following deboning, a trained team of individuals assessed for the presence of the myopathies based on in house scoring methods developed by Aviagen. The methods were designed to detect the phenotypic variation in severity of the conditions seen at the carcass inspection unit. Deep pectoral myopathy was recorded as present or absent, whereas WS and WB were scored based on severity. Wooden breast severity was based on a three-point scale, where score 0 is normal, score 1 is minor focal lesions of WB, and score 2 is extensive WB across the muscle. Similarly, WS was recorded on a 4-point scale of severity (Figure 4); a score of 0 represents no striping, score 1 (mild) is noticeable striping covering part of the breast, score 2 (moderate) is noticeable striping covering the breast surface extensively and score 3 (severe) is very thick stripes with extensive coverage over the breast surface.
Statistical Analyses
The traits BW, PW, BY (percentage of PW), DPM (binary scale), WB (multinominal scale: 3 levels) and WS (multinominal scale: 4 levels) were analyzed in the following multivariate animal model to estimate genetic parameters for each of the two lines:
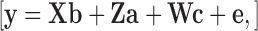 |
where: y is the vector of observations of the traits, b the vector of the fixed effect accounting for the interaction between the hatch-week, pen and contributing mating group. The vector of additive genetic effects is denoted by a, the vector of permanent environmental effects of the dam is denoted by c, and e represents the vector of residuals. X, Z, and W represent incidence matrices relating the vectors b, a, and c to y. The assumed (co)variance structure was:
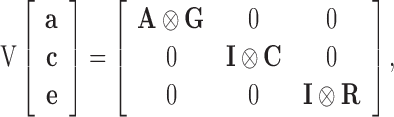 |
Where: A and I are the additive genetic relationship matrix and identity matrix, respectively. G, C, and R represent the variance and covariance matrices of additive genetic effects, permanent environmental effects of the dam and residual effects, respectively. All variance component analyses were performed by REML using VCE (Groeneveld et al., 2008). The inclusion of BW in the multivariate analysis, which was recorded in all birds in the dataset, allows unbiased estimates of BMM and BY, which were recorded on a subset of the overall population as described in the previous section.
RESULTS
Phenotypic Averages and Descriptive Statistics
Table 3 summarizes the production traits and the mean incidences of BMM for each of the lines analyzed. The incidences of BMM traits in this table are displayed as a mean of the percentage presence of the BMM (yes/no) in each flock analyzed. The BMM showed a greater total incidence in line A compared to line B, however the ranking of incidence of the myopathies was the same in both lines. White striping was the most common myopathy in both chicken lines, followed by DPM and then WB.
Table 3.
Descriptive statistics for the traits for each line. Breast yield is expressed as a percentage of processing body weight. The myopathies are expressed here as percentage incidence within the flocks.
| Line A | Line B | |||
|---|---|---|---|---|
| Trait | Mean | SD | Mean | SD |
| Body weight*kg (BW) | 2.33 | 0.29 | 1.91 | 0.23 |
| Processing body weight† (kg) (PW) | 2.47 | 0.30 | 2.39 | 0.29 |
| % Breast yield (BY) | 29.4 | 2.09 | 21.66 | 1.49 |
| % Deep pectoral myopathy (DPM) | 6.96 | 1.66 | 0.41 | 0.03 |
| % Wooden breast (WB) | 3.19 | 0.54 | 0.16 | 0.01 |
| % White striping (WS) | 49.6 | 8.68 | 14.46 | 3.08 |
*42 d of age Line A, 32 d of age Line B.
†47 d of age Line A, 40 d of age Line B.
The matrices of genetic and phenotypic correlations between the production traits and BMM are presented in Table 4. The phenotypic correlations (below the diagonal in Table 4) of the myopathies with the production traits of BY and BW were low in both lines of chicken. Similarly the phenotypic correlations between the individual myopathies were low across lines.
Table 4.
Estimates of heritabilities (bold, diagonal), genetic correlations (above diagonal) and phenotypic correlations (below diagonal) for body weight (BW), processing weight (PW), breast yield (BY), deep pectoral myopathy (DPM), wooden breast (WB) and white striping (WS). Standard errors are displayed in parentheses.
| BW | PW | BY | DPM | WB | WS |
|---|---|---|---|---|---|
| Line A | |||||
| 0.413(0.011) | 0.983(0.002) | –0.099(0.037) | 0.132(0.059) | –0.027(0.055) | 0.076(0.038) |
| 0.911 | 0.360(0.012) | –0.076(0.039) | 0.117(0.060) | –0.051(0.056) | 0.057(0.039) |
| 0.028 | 0.041 | 0.323(0.020) | 0.092(0.067) | 0.002(0.064) | 0.033(0.008) |
| 0.001 | –0.001 | –0.023 | 0.059(0.007) | 0.120(0.081) | –0.070(0.067) |
| 0.048 | 0.045 | 0.026 | 0.020 | 0.097(0.010) | 0.208(0.060) |
| 0.116 | 0.111 | 0.080 | –0.013 | 0.054 | 0.338(0.020) |
| Line B | |||||
| 0.355(0.010) | 0.971(0.003) | 0.066(0.030) | 0.037(0.070) | 0.160(0.072) | 0.228(0.037) |
| 0.836 | 0.271(0.010) | 0.080(0.032) | –0.007(0.071) | 0.171(0.073) | 0.222(0.039) |
| 0.216 | 0.254 | 0.418(0.018) | 0.190(0.069) | 0.141(0.072) | 0.248(0.041) |
| 0.011 | –0.007 | 0.011 | 0.021(0.003) | 0.060(0.016) | 0.180(0.079) |
| 0.020 | 0.016 | 0.020 | –0.002 | 0.024(0.004) | 0.350(0.074) |
| 0.148 | 0.156 | 0.022 | 0.025 | 0.038 | 0.185(0.012) |
The heritabilities for all the traits are displayed in Table 4. The heritabilities for BW, PW and BY were moderate for both lines. The estimated heritabilities for the myopathies ranged from low to moderate in both lines with WB and DPM being under 0.1 in both lines and WS being 0.338 and 0.185 in line A and B, respectively. There was a similar pattern of heritabilities for the six traits analyzed irrespective of the average breast yield of the individual line.
Table 5 shows the proportion of phenotypic variance accounted for by environmental and maternal environment effects. For all the traits analyzed, the permanent maternal environment accounted for 2.8 to 3.9% of the phenotypic variance of the two body weight measurements and 2.5 to 3.0% of the phenotypic variance of BY in the two chicken lines. For the BMM, the amount of phenotypic variance attributed to the permanent maternal environment was under 1.1% in both lines. The residual variance is responsible for the majority of the phenotypic variance for all traits analyzed in this study. Residual variance accounts for between 55.9 to 69.7% of the phenotypic variance of BW, PW and BY; for the BMM residual variance accounts for between 89.7 to 97.9% of the phenotypic variance.
Table 5.
Phenotypic (PHEN), residual (RES) maternal permanent environmental (PEm) variances and proportions of phenotypic variance accounted for by RES (Prop RES) and PEm (Prop PEm) for body weight (BW), Processing body weight (PW), breast yield (BY), deep pectoral myopathy (DPM), wooden breast (WB), and white striping (WS).
| Line A | Line B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | PHEN | RES | PEM | Prop RES | Prop PEm | PHEN | RES | PEM | Prop RES | Prop PEm |
| BW | 361.44 | 202.06 | 10.00 | 0.559 | 0.028 | 230.71 | 139.79 | 9.03 | 0.606 | 0.039 |
| PW | 440.69 | 268.52 | 13.66 | 0.609 | 0.031 | 244.54 | 170.56 | 7.82 | 0.697 | 0.032 |
| BY | 570.52 | 369.33 | 16.99 | 0.647 | 0.030 | 416.11 | 231.68 | 10.51 | 0.557 | 0.025 |
| DPM | 739.22 | 691.11 | 4.26 | 0.935 | 0.006 | 495.82 | 485.52 | 7.9E-07 | 0.979 | 0.000 |
| WB | 508.42 | 456.09 | 2.92 | 0.897 | 0.006 | 461.73 | 450.79 | 1.7E-06 | 0.976 | 0.000 |
| WS | 628.86 | 409.22 | 7.22 | 0.651 | 0.011 | 340.00 | 275.42 | 1.85 | 0.810 | 0.005 |
The genetic correlations (Table 4, above the diagonal) between the two body weight measures and BY were low for both lines. Similarly the genetic correlations of BW and PW with the myopathies in line A and B were found to be low. Genetic selection for increased BY has been attributed to the manifestation of BMM, however the genetic correlations between the myopathies and breast yield were found to be low. The genetic correlations between the myopathies were low to moderate in both lines ranging from –0.070 to 0.208 in line A, and 0.060 to 0.350 in line B.
The phenotypic correlations of the BMM with the production traits of BY, BW, and PW were low in both lines of chicken with estimates ranging from –0.001 to 0.156. Similarly the phenotypic correlations between the individual myopathies were low across lines ranging from –0.002 to 0.054.
DISCUSSION
BMM can lead to downgrading or condemnation of carcasses and/or portions resulting in economic loss even though the myopathies are not a food safety issue but a product quality issue. Kuttappan et al. (2012e) showed that consumer acceptance of WS was negatively affected in the more severely affected breast fillets. This is the first report providing estimates of the genetic basis of BMM and their relationship with growth and yield in broiler chickens. Two contrasting genetic lines were used in this study; Line A, which had been selected for high breast yield, and Line B, which had been selected for moderate breast yield. The Line A showed a greater incidence of the myopathies in comparison to the Line B, which could indicate that BY has some relationship to the incidence of BMM. However, the genetic parameters were similar for both lines, which indicates that selection history for breast yield, while having some role, is not the driving force for the expression of myopathies. The low heritability estimates and large contribution of residual variance to the phenotypic variance indicate that non-genetic environmental factors play a greater role in the manifestation of the BMM.
Heritability is the ratio between the additive genetic variance and the environmental variance; it represents the proportion of the phenotypic variance of a trait under the influence of genetic effects. Hence, the greater the heritability estimate of a trait, the greater the influence of genetic effects. Conversely, those traits with lower estimates of heritability are more under the influence of environmental effects, thus the influences of genetics is much lower. In this study, BW, PW and BY showed estimates of heritability in the range of 0.27 to 0.42, similar in magnitude to those found in the literature (Le Bihan-Duval et al., 1999; Le Bihan-Duval et al., 2001; Alnahhas et al., 2014). The heritabilities for DPM and WB are very low (0.02 to 0.1) indicating a strong non-genetic basis explaining the variation in these traits. The low heritability for DPM was as predicted, as it is known that non-genetic factors such as excessive muscle exertion results in an increase in the incidence of DPM (Lien et al., 2012). Considering the underlying cause of WB, the low heritability indicates that environmental and/or flock management factors have the greatest influence on the incidence of this myopathy, therefore investigating and understanding the environmental triggers will be the most effective approach to reduce the field incidence of WB. Genetic progress in reducing WB will be slow but should be considered in a long-term, balanced breeding goal. The heritability for WS in both lines was low to moderate (0.19 to 0.34), meaning that there is a larger genetic component to the manifestation of the striping compared to the other myopathies, however WS is still predominately under the influence of non-genetic environmental factors. As with DPM and WB, understanding the non-genetic influences on WS will be vital to reducing the incidence in the field while genetic progress can be made through selection against WS. In this analysis incidence of the BMM were recorded on the observed scale: DPM was scored as a binary trait, WS was scored on a 4-point scale and WB scored on a 3-point scale. Heritabilities estimated on the observed scale depend upon the number of categories by which the trait is scored and typically discrete categories result in lower estimates compared to the continuous underlying scale (Gianola, 1982). Kapell et al. (2012b) demonstrated that genetic analysis using a 3-point observed scale resulted in slightly lower estimates of heritability for leg health traits compared to the estimates obtained if the data is transformed to a normal underlying scale as described by Dempster and Lerner (1950). As discussed by Kapell et al. (2012a), the number of categories in an observed scale can be increased for improved discrimination; however the practicality of a breeding program requires traits to be easily distinguishable. This is necessary to ensure accuracy of recording of traits and repeatability over time and between trait assessors.
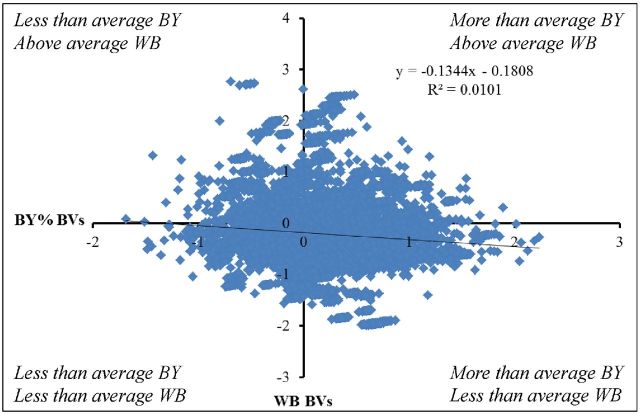
The genetic correlations between the myopathies and the production traits were low in this study, which has two implications. Firstly, it indicates that the genetic component influencing BW and BY is not influencing the genetic predisposition to express the BMM. This is important to bear in mind, as one of the current opinions for the cause of the increase in incidence of the BMM is that it is a consequence of genetic selection for increased breast yield and growth rates (Petracci et al., 2015; Russo et al., 2015). The genetic correlations between BMM, BW, and BY presented in this paper show that the relationship between them is very small. Furthermore, this indicates that future selection for breast yield and body weight does not necessarily represent an increased risk for the expression of BMM. This is illustrated in Figure 6 where the relationship between estimated breeding values for BY and WB is shown. It is worth noting that high breeding values for WB can be found across the whole range of breeding values for BY. In addition, there is a significant proportion of birds (46.4%) that have high genetic potential for BY and lower-than-average breeding value for WB. This shows that both traits can be selected in the desired direction in a balanced, multi-trait breeding program.
Figure 6.
XY plot of breeding values (BVs) for WB and % BY of line A (high yielding line). It shows that WB can occur in birds with both high and low BY. It can be seen that there are a proportion of birds with a high genetic potential for BY and below average WB in the bottom right quadrant of the plot. This proportion of birds represents those which can be selected for both traits in the desired direction.
The phenotypic correlations between the myopathies and production traits are also low, ranging from 0 to 0.16. This shows that the myopathies can occur in all sizes of birds with either high or low BY; this is important, as it indicates that the size and yield of the birds is not the driving force behind the manifestation of the myopathies. Both the genetic and phenotypic correlations between the individual myopathies are low to moderate, strengthening the hypothesis that they are independent conditions.
The data generated in this study shows a strong non-genetic component to the BMM and these effects must be investigated further. Growth rate has been proposed as a factor that influences the onset of BMM; while there has been genetic selection for increased growth rates, the findings in this study shows that the myopathies are under greater influence of environmental effects. It is well recognized in the poultry industry that growth of chickens is influenced by many factors such as nutrition (nutrient type, nutrient density, nutrient availability and feed form) and environmental factors within the chicken shed such as temperature and ventilation. Kuttappan et al. (2012a) investigated the impact of growth rate on the incidence of white striping and found an increase in white striping in birds fed a higher energy diet to achieve higher body weights and breast yield. Petracci et al. (2015) propose that increased muscle fiber size may, in certain circumstances, lead to an insufficient supply of oxygen and nutrients to the muscle cells, and additionally may be associated with a reduction in the removal of waste metabolites, which in turn could alter muscle function. This is possible, and further research into the vascularization of muscle tissue in relation to BMM is required to explore this possibility. It is clear that the mechanisms involved in muscle growth and development must be investigated further in relation to the onset of BMM.
One of the key cell types in the growth, development, and maintenance of muscle tissue are the satellite cells (i.e., resident stem cells). These cells are located on the periphery of muscle fibers and provide the myogenic precursors for the growth of the muscle after hatch (Moss and Leblond, 1970). During embryogenesis, the muscle progenitor cells undergo differentiation from embryonic myoblasts to fetal myoblasts to adult myoblasts (or satellite cells) (Ordahl et al., 2000). Increasing the temperature during incubation has been shown to influence myoblast activity and breast muscle yield (Piestun et al., 2009, 2011, 2013). Daily increases in incubation temperature for short periods (3 to 6 h) during the later stages of incubation have been found to increase the relative weight of the breast muscle which can be associated with larger muscle fibers in the post hatch bird (Piestun et al., 2009, 2011, 2013). Increased myoblast activity in terms of proliferation and differentiation can be seen in embryos exposed to thermal manipulation resulting in higher numbers of satellite cells in treated birds compared to controls (Piestun et al., 2009; Al-Musawi et al., 2012). It is clear that incubation temperature plays a pivotal role in the development of the chick and its muscular system, and its relationship to the development of BMM should be investigated further. Satellite cell activity is greatest immediately after hatch, but declines as the birds age, which potentially could have an impact on their ability to support growth of muscle fibers (Harthan et al., 2013) and/or their repair (Mann et al., 2011). The number of satellite cells and their subsequent fate can be influenced by the availability of feed and the level of key amino acids (Velleman et al., 2010, 2014; Powell et al., 2013). Satellite cell activity has also been found to be influenced by the availability of feed post hatch; Halevy et al. (2000, 2003) demonstrated that myoblast activity and muscle growth was reduced in broiler chicks and turkey poults that were delayed access to feed for 48 h. Similar findings were reported by Velleman et al. (2010), who showed that feed restriction during the first 2 wk of life resulted in a change in satellite cell activity compared to control birds, which was associated with an increase in muscle fiber necrosis and fat deposition in the P. major muscle. Further research on the impact of nutritional factors on satellite cells and their role on muscle fiber growth and development could contribute to developing strategies to reduce the risk of developing myopathies in the field.
This paper aimed to characterize the genetic basis of breast muscle myopathies found in broiler chickens, namely deep pectoral myopathy, wooden breast and white striping. The analysis of data from two pure-pedigree broiler lines with differing selection history for breast yield showed that there is a strong non-genetic component for all the breast muscle myopathy traits. Broad breeding goals including traits related to production, welfare, adaptability, liveability, and reproductive fitness are essential to achieve a balanced progress in pedigree broiler lines (Neeteson, 2010; Kapell et al., 2012a,b; Hocking, 2014). This approach has had and will continue to have benefits for the broiler industry as a whole. Addressing breast myopathies as part of overall balanced breeding goal should yield, albeit small to moderate, cumulative improvements, given the low genetic basis in these traits. More research is required to better understand the nature of the non-genetic components such as systematic environmental effects (e.g., management and nutrition) and how they influence muscle growth and development in broiler chickens.
REFERENCES
- Al-Musawi S. L., Stickland N. C., Bayol S. A. M. In ovo temperature manipulation differentially influences limb musculoskeletal development in two lines of chick embryos selected for divergent growth rates. J. Exp. Biol. 2012;215:1594–1604. doi: 10.1242/jeb.068791. [DOI] [PubMed] [Google Scholar]
- Alnahhas N., Berri C., Boulay M., Baeza E., Jego Y., Baumard Y., Chabault M., Le Bihan-Duval E. Selecting broiler chickens for ultimate pH of breast muscle: analysis of divergent selection experiment and phenotypic consequences on meat quality, growth, and body composition traits. J. Anim. Sci. 2014;92:3816–3824. doi: 10.2527/jas.2014-7597. [DOI] [PubMed] [Google Scholar]
- Bianchi M., Petracci M., Franchini A., Cavani C. The occurrence of deep pectoral myopathy in roaster chickens. Poult. Sci. 2006;85:1843–1846. doi: 10.1093/ps/85.10.1843. [DOI] [PubMed] [Google Scholar]
- Bilgili S. F., Hess J. B. Aviagen Brief, March. Huntsville, AL: Aviagen; 2008. Green Muscle Disease - Reducing the incidence in broiler flocks; p. 4. 35811. [Google Scholar]
- Dempster E. R., Lerner I. M. Heritability of threshold characters. Genetics. 1950;35:212–236. doi: 10.1093/genetics/35.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira T. Z., Casagrande R. A., Vieira S. L., Driemeier D., Kindlein L. An investigation of a reported case of white striping in broilers. J. App. Poult. Res. 2014;23:1–6. [Google Scholar]
- Fleming E. C., Fisher C., McAdam J. Southport, UK: Proc. Br. Soc. Anim. Sci.; 2007. Genetic progress in broiler traits—Implications for welfare; p. 050. [Google Scholar]
- Gianola D. Theory and analysis of threshold characters. J. Anim. Sci. 1982;54:1079–1096. [Google Scholar]
- Groeneveld E., Kovac M., Mielenz N. VCE User's Guide and Reference Manual Version 6.0 (Version 6.0) 2008. [Google Scholar]
- Guetchom B., Venne D., Chenier S., Chorfi Y. Effect of extra dietary vitamin E on preventing nutritional myopathy in broiler chickens. J. Appl. Poult. Res. 2012;21:548–555. [Google Scholar]
- Halevy O., Geyra A., Barak M., Uni Z., Sklan D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000;130:858–864. doi: 10.1093/jn/130.4.858. [DOI] [PubMed] [Google Scholar]
- Halevy O., Nadel Y., Barak M., Sklan D. Early posthatch feeding stimulates satellite cell proliferation and skeletal muscle growth in turkey poults. J. Nutr. 2003;133:1376–1382. doi: 10.1093/jn/133.5.1376. [DOI] [PubMed] [Google Scholar]
- Harper J. A., Bernier P. E., Helfer D. H., Schmitz J. A. Degenerative myopathy of the deep pectoral muscle in the turkey. J. Hered. 1975;66:362–366. doi: 10.1093/oxfordjournals.jhered.a108648. [DOI] [PubMed] [Google Scholar]
- Harthan L. B., McFarland D. C., Velleman S. G. The effect of nutritional status and myogenic satellite cell age on turkey satellite cell proliferation, differentiation, and expression of myogenic transcriptional regulatory factors and heparan sulfate proteoglycans syndecan-4 and glypican-1. Poult. Sci. 2013;93:174–86. doi: 10.3382/ps.2013-03570. [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003a;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003b;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Hocking P. M. Unexpected consequences of genetic selection in broilers and turkeys: problems and solutions. Br. Poult. Sci. 2014;55:1–12. doi: 10.1080/00071668.2014.877692. [DOI] [PubMed] [Google Scholar]
- Jordan F. T. W., Pattison M. Poultry Diseases. London, UK: Saunders; 1998. Deep Pectoral Myopathy of Turkeys and Chickens; pp. 398–399. [Google Scholar]
- Kapell D. N., Hill W. G., Neeteson A. M., McAdam J., Koerhuis A. N., Avendano S. Genetic parameters of foot-pad dermatitis and body weight in purebred broiler lines in 2 contrasting environments. Poult. Sci. 2012a;91:565–574. doi: 10.3382/ps.2011-01934. [DOI] [PubMed] [Google Scholar]
- Kapell D. N., Hill W. G., Neeteson A. M., McAdam J., Koerhuis A. N., Avendano S. Twenty-five years of selection for improved leg health in purebred broiler lines and underlying genetic parameters. Poult. Sci. 2012b;91:3032–3043. doi: 10.3382/ps.2012-02578. [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Brewer V. B., Apple J. K., Waldroup P. W., Owens C. M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012a;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Brewer V. B., Mauromoustakos A., McKee S. R., Emmert J., Meullenet J. F., Owens C. M. Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012b;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Goodgame S. D., Bradley C. D., Mauromoustakos A., Hargis B. M., Waldroup P. W., Owens C. M. Effect of different levels of dietary vitamin E (DL-alpha-tocopherol acetate) on the occurrence of various degrees of white striping on broiler breast fillets. Poult. Sci. 2012c;91:3230–3235. doi: 10.3382/ps.2012-02397. [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Huff G. R., Huff W. E., Hargis B. M., Apple J. K., Coon C., Owens C. M. Comparison of hematologic and serologic profiles of broiler birds with normal and severe degrees of white striping in breast fillets. Poult. Sci. 2012d;92:339–345. doi: 10.3382/ps.2012-02647. [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Lee Y. S., Erf G. F., Meullenet J. F., McKee S. R., Owens C. M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012e;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Shivaprasad H. L., Shaw D. P., Valentine B. A., Hargis B. M., Clark F. D., McKee S. R., Owens C. M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2012f;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Millet N., Remignon H. Broiler meat quality: effect of selection for increased carcass quality and estimates of genetic parameters. Poult. Sci. 1999;78:822–826. doi: 10.1093/ps/78.6.822. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Berri C., Baeza E., Millet N., Beaumont C. Estimation of the genetic parameters of meat characteristics and of their genetic correlations with growth and body composition in an experimental broiler line. Poult. Sci. 2001;80:839–843. doi: 10.1093/ps/80.7.839. [DOI] [PubMed] [Google Scholar]
- Lien R. J., Bilgili S. F., Hess J. B., Joiner K. S. Induction of deep pectoral myopathy in broiler chickens via encouraged wing flapping. J. Appl. Poult. Res. 2012;21:556–562. [Google Scholar]
- Macrae V. E., Mahon M., Gilpin S., Sandercock D. A., Mitchell M. A. Skeletal muscle fiber growth and growth associated myopathy in the domestic chicken (Gallus domesticus) Br. Poult. Sci. 2006;47:264–272. doi: 10.1080/00071660600753615. [DOI] [PubMed] [Google Scholar]
- Mann C. J., Perdiguero E., Kharraz Y., Aguilar S., Pessina P., Serrano A. L., Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. A. Muscle abnormalities-pathophysiological mechanisms. In: Richardson R. I., Mead G. C., editors. Poultry Meat Science. Wallingford. UK: 1999. pp. 65–98. [Google Scholar]
- Mitchell M. A., Sandercock D. A. Spontaneous and stress induced myopathies in modern meat birds: A cause for quality and welfare concerns. Proc. Aust. Poult. Sci. Symp. 2004:100–107. [Google Scholar]
- Moss F. P., Leblond C. P. Nature of dividing nuclei in the skeletal muscle of growing rats. J. Cell Biol. 1970;44:459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- National Chicken Council. U.S. Broiler Performance Statistics. 2015. Accessed May 2015. http://www.nationalchickencouncil.org/about-the-industry/statistics/u-s-broiler-performance/ [Google Scholar]
- Neeteson A. M. Striking the balance. J. Anim. Breed. Genet. 2010;127:85–86. doi: 10.1111/j.1439-0388.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- OECD-FAO. OECD-FAO Agricultural Outlook 2015. Paris: OECD Publishing; 2015. doi: 10.1787/agr_outlook-2015-en. [Google Scholar]
- Ordahl C. P., Williams B. A., Denetclaw W. Determination and morphogenesis in myogenic progenitor cells: an experimental embryological approach. Curr. Top. Dev. Biol. 2000;48:319–367. doi: 10.1016/s0070-2153(08)60761-9. [DOI] [PubMed] [Google Scholar]
- Orr J. P., Riddell C. Investigation of the vascular supply of the pectoral muscles of the domestic turkey and comparison of experimentally produced infarcts with naturally occurring deep pectoral myopathy. Am. J. Vet. Res. 1977;38:1237–1242. [PubMed] [Google Scholar]
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurance of white striping under commercial condition and its impact of breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds. Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Piestun Y., Harel M., Barak M., Yahav S., Halevy O. Thermal manipulations in late-term chick embryos have immediate and longer term effects on myoblast proliferation and skeletal muscle hypertrophy. J. Appl. Physiol. 2009;106:233–240. doi: 10.1152/japplphysiol.91090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piestun Y., Halevy O., Shinder D., Ruzal M., Druyan S., Yahav S. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. J. Thermal Biol. 2011;36:469–474. [Google Scholar]
- Piestun Y., Druyan S., Brake J., Yahav S. Thermal manipulations during broiler incubation alter performance of broilers to 70 days of age. Poult. Sci. 2013;92:1155–1163. doi: 10.3382/ps.2012-02609. [DOI] [PubMed] [Google Scholar]
- Powell D. J., McFarland D. C., Cowieson A. J., Muir W. I., Velleman S. G. The effect of nutritional status on myogenic gene expression of satellite cells derived from different muscle types. Poult. Sci. 2013;93:2278–2288. doi: 10.3382/ps.2013-03810. [DOI] [PubMed] [Google Scholar]
- Richardson J. A., Burgener J., Winterfield R. W., Dhillon A. S. Deep pectoral myopathy in seven-week-old broiler chickens. Avian Dis. 1980;24:1054–1059. [PubMed] [Google Scholar]
- Russo E., Drigo M., Longoni C., Pezzotti R., Fasoli P., Recordati C. Evaluation of white striping prevalence and predisposing factors in broilers at slaughter. Poult. Sci. 2015;94:1843–1848. doi: 10.3382/ps/pev172. [DOI] [PubMed] [Google Scholar]
- Sihvo H. K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2013;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Siller W. G. Deep pectoral myopathy: A penalty for successful selection for muscle growth. Poult. Sci. 1985;64:1591–1595. doi: 10.3382/ps.0641591. [DOI] [PubMed] [Google Scholar]
- Siller W. G., Wight P. A. L., Martindale L. Exercise-induced deep pectoral myopathy in broiler fowls and turkeys. Vet. Sci. Com. 1978a;2:331–336. [PubMed] [Google Scholar]
- Siller W. G., Wight P. A. L., Martindale L., Bannister D. W. Deep pectoral myopathy: An experimental simulation in the fowl. Res. Vet. Sci. 1978b;24:267–268. [PubMed] [Google Scholar]
- U.S.D.A. United States Department of Agriculture. Poultry Slaughter Annual Summary. 2015. Accessed May 2015. http://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Poultry_Slaughter/index.asp. [Google Scholar]
- Van Vleet J. F., Ruth G., Ferrans V. J. Ultrastructural alterations in skeletal muscle of pigs with selenium-vitamin E deficiency. Am. J. Vet. Res. 1976;37:911–22. [PubMed] [Google Scholar]
- Velleman S. G., Nestor K. E., Coy C. S., Harford I., Anthony N. B. Effect of posthatch feed restriction on broiler breast muscle development and muscle transcriptional regulatory factor gene and heparan sulfate proteoglycan expression. Int. J. Poult. Sci. 2010;9:417–425. [Google Scholar]
- Velleman S. G., Coy C. S., Emmerson D. A. Effect of the timing of posthatch feed restrictions on the deposition of fat during broiler breast muscle development. Poult. Sci. 2014;93:2622–2627. doi: 10.3382/ps.2014-04206. [DOI] [PubMed] [Google Scholar]
- Walsh D. M., Kennedy D. G., Goodall E. A., Kennedy S. Antioxidant enzyme activity in the muscle of calves depleted of vitamin E or selenium or both. Br. J. Nutr. 1993;70:621–630. doi: 10.1079/bjn19930153. [DOI] [PubMed] [Google Scholar]
- Wight P. A. L., Siller W. G., Martindale L., Filshie J. H. The induction by muscle stimulation of deep pectoral myopathy in the fowl. Avian. Path. 1979;8:115–121. doi: 10.1080/03079457908418332. [DOI] [PubMed] [Google Scholar]
- Wight P. A. L., Siller W. G. Pathology of deep pectoral myopathy of broilers. Vet. Pathol. 1980;17:29–39. doi: 10.1177/030098588001700103. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Schneider B. L., Carney V. L., Korver D. R., Robinson F. E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;94:1389–1397. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]