Abstract
Extensive research over the last couple of decades has made it obvious that mycotoxins are commonly prevalent in majority of feed ingredients. A worldwide mycotoxin survey in 2013 revealed 81% of around 3,000 grain and feed samples analyzed had at least 1 mycotoxin, which was higher than the 10-year average (from 2004 to 2013) of 76% in a total of 25,944 samples. The considerable increase in the number of positive samples in 2013 may be due to the improvements in detection methods and their sensitivity. The recently developed liquid chromatography coupled to (tandem) mass spectrometry allows the inclusion of a high number of analytes and is the most selective, sensitive, and accurate of all the mycotoxin analytical methods. Mycotoxins can affect the animals either individually or additively in the presence of more than 1 mycotoxin, and may affect various organs such as gastrointestinal tract, liver, and immune system, essentially resulting in reduced productivity of the birds and mortality in extreme cases. While the use of mycotoxin binding agents has been a commonly used counteracting strategy, considering the great diversity in the chemical structures of mycotoxins, it is very obvious that there is no single method that can be used to deactivate mycotoxins in feed. Therefore, different strategies have to be combined in order to specifically target individual mycotoxins without impacting the quality of feed. Enzymatic or microbial detoxification, referred to as “biotransformation” or “biodetoxification,” utilizes microorganisms or purified enzymes thereof to catabolize the entire mycotoxin or transform or cleave it to less or non-toxic compounds. However, the awareness on the prevalence of mycotoxins, available modern techniques to analyze them, the effects of mycotoxicoses, and the recent developments in the ways to safely eliminate the mycotoxins from the feed are very minimal among the producers. This symposium review paper comprehensively discusses the above mentioned aspects.
Keywords: occurrence, analysis, detoxification, mycotoxicosis, gut, immune function
INTRODUCTION
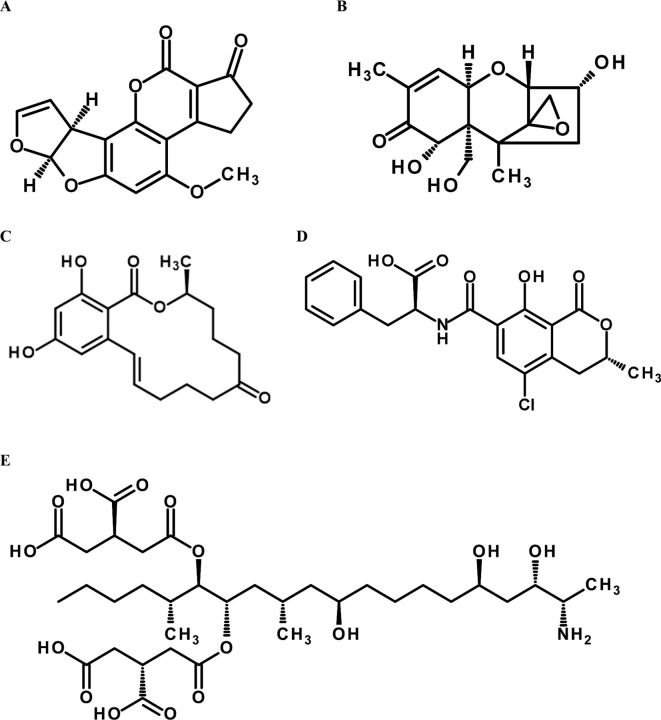
The term “mycotoxin” is derived from “mykes” meaning fungi and “toxicon” meaning poison. Mycotoxins are secondary metabolites of low molecular weight produced by a wide range of fungi, principally molds. There are over 200 species of molds that produce mycotoxins. Aflatoxins (AF), zearalenone (ZEN), ochratoxin A (OTA), fumonisins (FUM), trichothecenes such as deoxynivalenol (DON), and T-2 toxin are some of the mycotoxins that can significantly impact the health and productivity of poultry species (Figure 1). Fungal growth and subsequent mycotoxin formation is dependent on a range of factors including seasons, location of grain cultivation, drought and time of harvest. Long term analysis of grain and feed samples worldwide has indicated that it is possible to have grains with extremely high concentrations of mycotoxins, although the overall mycotoxin contamination is low, (Streit et al., 2013a). These data also revealed that mycotoxin contaminated grains typically contain more than just a single mycotoxin.
Figure 1.
Chemical structures of most prevalent mycotoxins. A: Aflatoxin B1, B: Deoxynivalenol, C: Zeralenone, D: Ochratoxin A, E: Fumonisin B1.
Mycotoxins produce a variety of diseases, collectively called “mycotoxicoses,” directly or in combination with other primary stressors such as pathogens (Raju and Devegowda, 2000). These diseases are exhibited by symptoms and lesions, which can be used to clinically diagnose the presence of mycotoxins although these symptoms are not just straightforward. When AF and OTA are co-contaminants of poultry feed, they interact in a synergistic manner (Huff and Doerr, 1981). During dual exposure of these toxins, OTA prevents the major effects of AF (i.e., fatty, yellow, enlarged and friable liver). This reduces the ability to diagnose aflatoxicosis in the field and the target organ in this interaction appears to be the kidney. The combination of AF and T2 toxin is the same as the interaction between AF and OTA, and exhibit synergistic toxicity (Huff et al., 1988a). Acute cases caused by ingestion of high levels of mycotoxins may result in mortality and a marked decline in the productivity of poultry characterized by obvious clinical signs and post-mortem lesions. However in most cases, mycotoxicoses is chronic and caused by low-level ingestion of fungal metabolites, resulting in measurable decline in performance and the occurrence of nonspecific changes, including subcutaneous hemorrhage in broilers and immunosuppression (D'mello et al., 1999). Under field conditions, suboptimal performance in the absence of an obvious infectious, environmental or management factor, or a nutritional deficiency suggest the possibility of mycotoxicoses. However, analysis of feed for mycotoxins is imperative in order to diagnose mycotoxicoses in these chronic cases, apart from the evaluation of history, clinical and post-mortem evaluation of flocks and microscopic examination of tissues (Schiefer, 1990).
The recognition that mycotoxins affect health and productivity of poultry has led to intensive research on counteracting methods over the last few decades, including detection and elimination or detoxification of mycotoxins. Although enzyme-linked immunosorbent assay (ELISA) based detection methods were used in the past, recent developments in the analysis and detection of mycotoxins in feed and feed ingredients improved the scenario considerably. The use of high performance liquid chromatography (HPLC) has been one such development which initiated the path to detect multiple mycotoxins simultaneously in a sample (Schumacher et al., 1997). The latest technique using liquid chromatography coupled to (tandem) mass spectrometry (LC-MS/MS) increased this potential phenomenally to detect hundreds of mycotoxins simultaneously in a sample (Malachova et al., 2014). This new development has also led to the detection of masked and emerging mycotoxins, which are neither routinely screened nor regulated by legislations (Berthiller et al., 2013).
The most well-known approach for detoxification of mycotoxins involves the use of nutritionally inert adsorbents with the capacity to bind and immobilize mycotoxins in the gastrointestinal tract of animals, thus reducing their bioavailability (Magnoli et al., 2011). Although this approach successfully eliminates the risk of certain mycotoxins such as the AF, it does not work comprehensively on all of the mycotoxins relevant to the poultry industry. Biotransformation has been one of the proven approaches for the detoxification of the non-adsorbable mycotoxins by altering their molecular structure into non-toxic metabolites which are excreted (Grenier et al., 2013). Therefore suppression of mycotoxicoses requires an integrated approach from detection to detoxification. The objective of this paper is to discuss in detail the determination and prevalence of mycotoxins, effects of mycotoxicoses and recent developments in the strategies to counteract mycotoxins.
MYCOTOXINS RELEVANT IN POULTRY NUTRITION
Aflatoxins
Aflatoxins, a class of mycotoxins produced by fungal species of the genus Aspergillus (flavus and parasiticus), are often found in feed ingredients used for poultry rations. Most prevalent forms of AF include B1, B2, G1, and G2, with aflatoxin B1 (AFB1) being the most common and biologically active component (Busby and Wogan, 1981). Aflatoxins cause a variety of effects in poultry, including decreased weight gain, poor feed efficiency, reduced egg production and egg weight, increased liver fat, changes in organ weights, reduction in serum protein levels, carcass bruising, poor pigmentation, liver damage, decreased activities of several enzymes involved in the digestion of starch, protein, lipids, and nucleic acids, and immunosuppression (Edds and Bortell, 1983; Leeson et al., 1995; Devegowda and Murthy, 2005). Evidence suggests that immunosuppression caused by AF results in many disease outbreaks, vaccination failures, and poor antibody titers (Devegowda and Murthy, 2005). At necropsy, livers are usually pale and enlarged, as a result of aflatoxicosis. Histologically, liver lesions include congestion of the hepatic sinusoids, focal hemorrhages, centrilobular fatty cytoplasmic vacuolation and/or necrosis, biliary hyperplasia, and nodular lymphoid infiltration (Leeson et al., 1995). Osborne et al. (1982) found that AF, at levels that did not affect growth, produced a malabsorption syndrome characterized by steatorrhea, hypocarotenoidemia, and decreased concentrations of bile salts and pancreatic lipase, trypsin, amylase, and RNase.
At a cellular level, chicks fed 1.0 mg/kg AFB1 had decreased hepatic gene expression of superoxide dismutase, glutathione S-transferase, and epoxide hydrolase and increased gene expression of Interleukin 6 and cytochrome p450 1A1 and 2H1 (Yarru et al., 2009a). In chicks fed 2.0 mg/kg AFB1, various hepatic genes associated with energy production and fatty acid metabolism (carnitine palmitoyl transferase), growth and development (insulin-like growth factor 1), antioxidant protection (glutathione S-transferase), detoxification (epoxide hydrolase), coagulation (coagulation factors IX and X), and immune protection (interleukins) were downregulated, whereas genes associated with cell proliferation (ornithine decarboxylase) were upregulated (Yarru et al., 2009b).
Ochratoxins
Ochratoxins are a group of structurally related metabolites that are produced by fungi belonging to the genera Aspergillus and Penicillium, and Ochratoxin A (OTA) is the most prevalent mycotoxin of this group. Signs of OTA toxicity in poultry include weakness, anemia, decreased feed consumption, reduced growth rate and egg production, poor feathering, and excessive mortality at high dietary concentrations (Hamilton et al., 1982; Gibson et al., 1989; Huff et al., 1988b). Pathophysiological changes include decreased urine concentration and glomerular filtration rate, impairment of proximal tubular function, and degeneration and ultrastructural alterations in renal integrity (Huff and Hamilton, 1975; Glahn et al., 1988, 1989). Increases in the relative weights of liver, spleen, pancreas, proventriculus, gizzard, and testes have also been reported in poultry fed OTA (Gibson et al., 1989; Huff et al., 1988b).
Ochratoxin A consists of an isocoumarin moiety linked through the 7-carboxy group to the amino acid L-β-phenylalanine. At a cellular level, OTA interferes with DNA, RNA, and protein synthesis by inhibiting the enzyme phenylalanine-tRNA synthetase (Marquardt and Frohlich, 1992). Ochratoxin A also affects renal carbohydrate metabolism through a reduction of the renal mRNA coding for phosphoenolpyruvate carboxykinase (PEPCK), a key enzyme in gluconeogenesis (Leeson et al., 1995). The effects of OTA on DNA, RNA, and protein synthesis are thought to be due to the phenylalanine moiety of the toxin competing with phenylalanine in the enzyme catalyzed reaction (Marquardt and Frohlich, 1992). Ochratoxin A also causes hypocarotenidemia in broilers (Huff and Hamilton, 1975) that is more severe than that caused by AF (Osborne et al., 1982; Schaeffer et al., 1987).
Fumonisins
The FUM are a group of mycotoxins that were first isolated from cultures of Fusarium verticillioides (moniliforme) and chemically characterized by Gelderblom et al. (1988). Six different FUM have been identified (A1, A2, B1, B2, B3, B4) and their structures elucidated (Bezuidenhout et al., 1988; Cawood et al., 1991; Plattner et al., 1992). However, fumonisin B1 (FB1) has been reported to be the predominant form produced by Fusarium verticillioides (Norred, 1993). Several other Fusarium species and a species of Alternaria have also been found to produce FB1 (Chen et al., 1992).
In comparison to horses and swine, 2 susceptible species, chicks and turkeys, are relatively resistant to the toxic effects of FB1. Mild to moderate toxicity was reported in chicks, ducks, and turkeys fed rations containing 75–400 mg FB1/kg for 21 days (Bermudez et al., 1995; Brown et al., 1992; Ledoux et al., 1992; Weibking et al., 1993, 1995). The primary changes in chicks, ducks, and turkeys were decreased body weight gain and liver pathology (Bermudez et al., 1995; Brown et al., 1992; Ledoux et al., 1992; Weibking et al., 1993, 1995). Hepatic changes in chicks were multifocal hepatic necrosis and biliary hyperplasia (Ledoux et al., 1992; Weibking et al., 1993). Hepatocellular hyperplasia and increased extramedullary hematopoiesis were also noted in 1 study (Weibking et al., 1993). The primary liver pathology observed in ducklings and turkeys fed FB1 was diffuse hepatocellular hyperplasia, with biliary hyperplasia evident in turkeys fed 150–300 mg FB1/kg (Weibking et al., 1995) and in ducklings fed 400 mg FB1/kg (Bermudez et al., 1995). In studies designed to evaluate the chronic effects of FB1, chick performance up to 7 weeks was not affected by up to 50 mg FB1/kg diet, whereas turkeys fed 50 mg FB1/kg diet had lower feed intakes than birds fed 0 or 25 mg FB1/kg diet (Broomhead et al., 2002).
The mechanism by which the FUM cause toxicity in animals appears to be due to the disruption of sphingolipid metabolism (Wang et al., 1991). Current evidence indicates that the FUM are specific inhibitors of ceramide synthase (sphinganine/sphingosine N-acyltransferase), a key enzyme required for the synthesis of ceramide and more complex sphingolipids. Inhibition of this enzyme system leads to an increase in tissue concentrations of the sphingolipids sphingosine (SO) and sphinganine (SA), and a change in the SA:SO ratio. An increase in the SA:SO ratio, has been observed in tissues of broilers, turkeys, and ducklings fed FB1 (Weibking et al., 1993; Bermudez et al., 1995; Ledoux et al., 1996; Broomhead et al., 2002; Tran et al., 2005).
Trichothecenes
Trichothecene mycotoxins are a group of fungal metabolites with the same basic backbone structure and include T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS), monoacetoxyscirpenol (MAS), neosolaniol, 8-acetoxyneosolaniol, 4-deacetylneosolaniol, nivalenol, 4-acetoxynivalenol (Fusarenone-X), DON (vomitoxin), and 3-acetyldeoxynivalenol (Leeson et al., 1995). Trichothecenes are the most potent small molecule inhibitors of protein synthesis known and the main toxic effect at the cellular level appears to be a primary inhibition of protein synthesis followed by a secondary disruption of DNA and RNA synthesis (Leeson et al., 1995). Toxic effects of trichothecenes include oral lesions, growth retardation, abnormal feathering, decreased egg production and egg shell quality, regression of the bursa of Fabricius, peroxidative changes in liver, abnormal blood coagulation, leucopoenia and proteinemia, and immunosuppression (Leeson et al., 1995; Danicke, 2002). Concentrations of T-2 that cause oral lesions are lower (0.4 mg/kg) than concentrations reported to decrease chick performance (3–4 mg/kg; Leeson et al., 1995). In a comprehensive review, Danicke (2002) concluded that broiler performance is affected at dietary concentrations of 3–4 mg/kg of T-2 toxin, whereas ducks were affected when the dietary concentration was as low as 0.4 mg/kg.
Deoxynivalenol is less toxic than T-2 toxin, and the level of DON that affects chick performance is still debated, with some researchers (Huff et al., 1986; Kubena et al., 1988, 1989) reporting toxic effects at 16 mg/kg diet, whereas others (Moran et al., 1982) report no toxic effect until dietary concentrations exceeded 116 mg/kg of DON. Danicke et al. (2001) summarized results of 49 studies with DON and concluded that a dietary concentration of 5 mg/kg had no negative effects on performance. Deoxynivalenol has also been reported to have both immunosuppressive and immunomodulating effects in poultry (Danicke, 2002). Recent studies indicate that DON at concentrations ranging from 1 to 7 mg/kg diet significantly alters several key functions of the intestinal tract including decreasing villus surface area available for absorption and altering the permeability of the intestinal tract (Awad et al., 2011; Osselaere et al., 2013).
Interactions among Mycotoxins
In general, contaminated feeds usually contain more than one mycotoxin. Grenier and Oswald (2011) recently conducted a meta-analysis of publications (>100) describing toxicological interactions among mycotoxins. In the analysis, the authors only explored experiments with a 2 × 2 factorial design where individual and combined effects of the mycotoxins were evaluated. Over half of the studies investigated the interactions between AF and other mycotoxins, and over half of the studies selected were poultry studies. Results indicated that most of the studies showed a synergistic or additive interaction on animal performance. However, results with respect to other response variables indicated that there were many types of interactions ranging from synergistic to antagonistic for a same association (Grenier and Oswald, 2011). Grenier and Oswald (2011) also observed from their review that a combination of mycotoxins, at concentrations that individually should not cause negative effects, may negatively affect animals.
CURRENT STATUS OF MYCOTOXIN ANALYTICAL TOOLS
Analytical Methods
Valid determination of mycotoxins and their metabolites is a crucial step in any intervention, mitigation, or remediation strategy to cope with the deleterious effects of mycotoxins to livestock. The determination of mycotoxins follows general trends in analytical chemistry. This includes — besides faster and more sensitive methods — the concurrent determination of a larger number of toxins in single measurements. Formerly, the most prominent mycotoxins such as AF, DON, FUM, OTA, or ZEN were monitored on a regular basis, the latest multi-mycotoxin analytical methods have enabled the determination of the so called “emerging” mycotoxins. Examples include the Fusarium toxins: moniliformin, fusaproliferin, or enniatins (Jestoi, 2008). The soluble plant metabolites of mycotoxins or the so called “masked” mycotoxins (Berthiller et al., 2013) can also be monitored. A prominent example is deoxynivalenol-3-glucoside (D3G), the most common plant metabolite of DON, which, in single cases, can even exceed the concentration of the native toxin in cereals and often results in high DON concentration in beers (Varga et al., 2013a).
In general, methods for mycotoxin determination can be divided into chromatographic methods, immunochemical methods and “other” methods, which include direct spectroscopic methods. The most selective, sensitive, and accurate of all these methods are often based on LC-MS/MS, which also allows the inclusion of a high number of analytes. Further reasons for multi-mycotoxin analytical methods are to quantify toxins in feed mixtures rather than single matrices, to use 1 method rather than many different ones and to monitor changes in regional mycotoxin patterns due to climate change. The power and necessity of such LC-MS/MS based multi-method has been highlighted by Streit et al. (2013b), showing the detection of 139 different (mostly fungal) secondary metabolites in a variety of feedstuffs. In a different experiment, 26 different mycotoxins were detected in a single hazelnut sample using the LC-MS/MS based multi-method (Varga et al., 2013b).
LC-MS/MS-Based Multi-Methods
In order to develop successful LC-MS/MS based multi-toxin detection methods, several obstacles have to be overcome. A major challenge is still the collection of a representative (cereal) sample taking into account the heterogeneous distribution of mycotoxins in a lot. However, the sample variability can be minimized through increasing the size of samples and subsamples and decreasing the particle size (Whitaker, 2006). Mycotoxins are chemically diverse substances, ranging from very polar (e.g., moniliformin, nivalenol) to non-polar (e.g., beauvericin, enniatins), while their solubility in liquid solvents also varies extensively. Typically acidified mixture of water with organic solvents such as methanol, acetone, or acetonitrile are used to extract a large amount of different toxins within a single procedure (Sulyok et al., 2006). It should be noted that the extraction of a multitude of different compounds with a single solvent mixture has to be a compromise as better suited solvents are available for the determination of single analytes. Also, extract clean-up options are restricted due to the chemical diversity of mycotoxins. While some methods rely on phase separation of acetonitrile-water mixtures by the addition of salts (QuEChERS-like methods; Desmarchelier et al., 2010), most modern methods simply dilute the raw extract before measurement to decrease the matrix load. As only ions can be detected in a mass spectrometer, the ionization process for neutral analytes is an important next step, usually achieved by electrospray interfaces. While powerful and constantly under development, electrospray devices are prone to matrix effects, which could lead to signal suppression or enhancement of analytes co-eluting with matrix compounds (Malachova et al., 2013). A very elegant, efficient, and cost-effective approach to compensate matrix effects is the use of stable isotope-labelled mycotoxins as internal standards (Varga et al., 2012). Further possibilities include matrix matched calibration, standard addition, or correction of the measured concentrations for the recovery after careful validation of each matrix. The most recent methods are capable of quantifying around 300 fungal metabolites in a variety of different food and feedstuffs (Malachova et al., 2014).
Analysis of Biomarkers for Mycotoxins
A different strategy to assess the exposure of individual animals to mycotoxins is the use of biomarker methods. Such methods are mainly based as well on LC-MS/MS and determine the concentration of mycotoxins, their metabolites (biomarkers of exposure), or other affected endogenous substances (biomarkers of effect) in biological fluids such as blood or urine. In addition to exposure assessment, biomarker methods are used in mycotoxin metabolization studies and to verify the effect of mycotoxin deactivation in vivo. For example, a multi-biomarker LC-MS/MS method was developed to assess human exposure to mycotoxins by assaying the urine (Warth et al., 2012). In urine samples obtained from Cameroon, biomarkers of exposure for up to 5 mycotoxins (OTA, DON, nivalenol, AF, and FB1) were detected simultaneously. Masked mycotoxins can be potentially reactivated by cleavage of the conjugate and liberation of the native toxin in the digestive tract of animals. Deoxynivalenol-3-glucoside was readily hydrolyzed to DON during digestion, thus proving its status as a masked mycotoxin. In this context, urine and feces from rats were analyzed by a validated LC–MS/MS biomarker method to study the metabolism of D3G, after application of the masked mycotoxin (Nagl et al., 2012). While in the case of rats most liberated DON was excreted in feces, very recently a higher amount of liberated DON was found in urine of piglets fed with D3G indicating species specific metabolism (Nagl et al., 2014). A good example for a biomarker of effect is the ratio of the sphingolipid compounds (SA:SO), which increases in the serum and tissue of animals after FUM exposure (Riley et al., 1993). This marker was used in serum along with the measurement of hydrolyzed FUM in urine and feces of treated pigs to prove the efficacy of FUMzyme (Biomin GmbH, Austria), an enzyme capable of degrading FUM and used as a feed additive (EFSA, 2014). The rise of mass spectrometry has led to many applications regarding mycotoxins over the last decade. The selectivity, sensitivity, and speed of modern LC-MS instruments not only results in the detection of novel toxins and quantification of known mycotoxins but is also a key to assess exposure to mycotoxins and the efficacy of mycotoxin deactivators.
PREVALENCE OF MYCOTOXINS
The conditions under which fungi and mycotoxins are produced in agricultural commodities depend highly on environmental factors such as water availability and temperature but also slightly elevated CO2 concentration may stimulation the growth of mycotoxigenic fungi (Magan et al., 2011). Extreme weather situations, precipitation, and drought lead to plant stress and hence they become more vulnerable for fungal infection (Wu et al., 2011). A primary challenge is that fungi, e.g., Fusarium species, are capable of producing different mycotoxins with diverse toxigenic potentials. The combination of multiple mycotoxins in feed can cause more adverse effects than a single mycotoxin due to additive or even synergistic interaction (CAST, 2003). Another challenge is the global trade of agricultural raw materials used as feed ingredients, which can result in the distribution of mycotoxins across the world (Bryden, 2012). Hence as part of a proper mycotoxin risk management, surveying the mycotoxin occurrence is very important to allow feed and animal producers to assess the risk of using certain feed ingredients or feeds from different regions.
Worldwide Mycotoxin Survey from 2004–2013
To evaluate the extent of mycotoxin contamination in feeds and feed ingredients on a global basis, an annual worldwide mycotoxin survey program was started in 2004. Results of this survey have been reported in a number of peer-reviewed publications (Binder et al., 2007; Griessler et al., 2010; Rodrigues et al., 2011; Rodrigues and Naehrer, 2012; Streit et al., 2013a; Schatzmayr and Streit, 2013). More than 85,000 individual analyses were conducted, on a total of 25,944 samples, for the most important mycotoxins in terms of agriculture and animal production: AF, DON, FUM, OTA, and ZEN. The details of sample collection and analytical procedures are reviewed elsewhere (Rodrigues and Naehrer, 2012; Schatzmayr and Streit, 2013).
Overall, 76% of the samples contained detectable amounts of at least 1 mycotoxin. Deoxynivalenol was the most dominant, as 57% of the samples tested positive, followed by FUM (55%), ZEN (38%), AF (27%), and OTA (26%) (Table 1). Clear yearly variations were observed in mycotoxin prevalence and contamination levels. In some cases, this was evident due to unusual weather conditions. Over the years, there were differences with regard to the prevalence of mycotoxins worldwide. For example, DON was most frequently detected in North Asia, North America, and Central Europe with the percentage of positive samples exceeding the global average of 57%. Exploring the results of only the finished feed samples, 69% of were found to be positive for FUM (Table 2). Other Fusarium toxins, DON and ZEN, were also highly prevalent at 59% and 52%, respectively.
Table 1.
Worldwide mycotoxin survey results in feed and feed ingredients from 2004–2013.1,2
| Parameters | AF | ZEN | DON | FUM | OTA |
|---|---|---|---|---|---|
| Number of tests | 15,614 | 20,696 | 23,625 | 14,919 | 10,832 |
| Positive samples | 4,230 | 7,912 | 13,577 | 8,143 | 2,770 |
| Average (μg/kg) | 13 | 94 | 553 | 863 | 3 |
| Median of positive (μg/kg) | 8.2 | 70 | 427 | 722 | 2.5 |
| Maximum (μg/kg) | 6,323 | 26,728 | 50,289 | 77,502 | 1,589 |
1Sample origin and sample type refer to the samples containing the highest detected concentration of the respective mycotoxin.
2AF = the sum of aflatoxin B1, aflatoxin B2, aflatoxin G1 and aflatoxin G2; ZEN = zearalenone; DON = deoxynivalenol; FUM = the sum of fumonisin B1 and fumonisin B2; OTA = ochratoxin A.
Table 2.
Worldwide mycotoxin survey results in finished feed from 2004–2013.1,2
| Parameters | AF | ZEN | DON | FUM | OTA |
|---|---|---|---|---|---|
| Number of tests | 5,090 | 6,319 | 6,907 | 4,911 | 3,720 |
| Positive samples | 1,986 | 3,263 | 4,087 | 3,376 | 1,478 |
| Average (μg/kg) | 11 | 92 | 408 | 695 | 5 |
| Median of positive (μg/kg) | 8 | 55 | 328 | 567 | 2 |
| Maximum (μg/kg) | 2,454 | 5,791 | 32,893 | 77,502 | 1,589 |
1Sample origin and sample type refer to the samples containing the highest detected concentration of the respective mycotoxin.
2AF = the sum of aflatoxin B1, aflatoxin B2, aflatoxin G1 and aflatoxin G2; ZEN = zearalenone; DON = deoxynivalenol; FUM = the sum of fumonisin B1 and fumonisin B2; OTA = ochratoxin A.
Mycotoxin Occurrence in 2013
As part of the annual mycotoxin survey, the 2013 results revealed DON and FUM presence in more than half of finished feed and feed ingredient samples analyzed (Table 3). Over one-third of all tested samples were contaminated with ZEN and compared to the previous year while the number of samples positive for AF increased by 5% to a total of 30%. In 2013, 81% of all samples contained at least 1 mycotoxin and more than 1 mycotoxin were found in 45% of the samples. Asia was the region with maximum contamination for most of the tested mycotoxins (AF, ZEN, DON, and FUM), where the maximum concentration for all mycotoxins analyzed was 29,267 μg/kg of DON in a barley sample from China. The highest average AF concentration was observed in the samples from Europe. The incidence of OTA in Europe was lower compared to 2012; however, the average values were about 3 times higher (16 μg/kg). In North America, FUM remains the most common mycotoxin, although the incidence was 23% lower compared to the previous year. Samples from South America had the highest global average of ZEN at 221 μg/kg.
Table 3.
Worldwide mycotoxin survey results in feed and feed ingredients in 2013.1
| Parameters | AF | ZEN | DON | FUM | OTA |
|---|---|---|---|---|---|
| Number of tests | 2,839 | 3,470 | 3,931 | 2,699 | 2,459 |
| Percent positive (%) | 30 | 37 | 59 | 55 | 23 |
| Average (μg/kg) | 10 | 49 | 458 | 778 | 2 |
| Median of positive (μg/kg)2 | 4 | 41 | 351 | 665 | 2 |
| Maximum (μg/kg) | 1,563 | 5,324 | 29,267 | 26,828 | 595 |
1AF = the sum of aflatoxin B1, aflatoxin B2, aflatoxin G1 and aflatoxin G2; ZEN = zearalenone; DON = deoxynivalenol; FUM = the sum of fumonisin B1 and fumonisin B2; OTA = ochratoxin A.
2Median of all samples above the limit of detection.
In finished feeds, the major contaminant throughout all regions was FUM followed by DON (Table 4). In comparison with previous years, analyzed FUM and AF percent positives as well as average levels were slightly higher. A significant outcome to note is the maximum contamination levels found in samples from different regions: 1,165 ppb AF and 2,667 ppb ZEN in China; 9,903 ppb DON and 595 ppb OTA in Spain; and 10,282 ppb FUM in Italy. All these levels exceeded the EU recommendation and guideline levels for the presence of mycotoxins in animal feed for various species (EC 2002 and 2006). Out of more than 1,400 finished feed samples, 60% showed the presence of more than 1 different mycotoxin. Simultaneous exposure to these high levels of toxins represents an additional challenge for animals’ health due to the mycotoxins’ potential to synergistic interactions.
Table 4.
Worldwide mycotoxin survey results in finished feed in 2013.1
| Parameters | AF | ZEN | DON | FUM | OTA |
|---|---|---|---|---|---|
| Number of tests | 1,006 | 1,163 | 1,296 | 945 | 799 |
| Percent positive (%) | 40 | 48 | 60 | 72 | 36 |
| Average (μg/kg) | 7 | 43 | 280 | 687 | 5 |
| Median of positive (μg/kg)2 | 4 | 30 | 257 | 535 | 1,995 |
| Maximum (μg/kg) | 1,165 | 2,667 | 9,903 | 10,282 | 595 |
1AF = a sum of aflatoxin B1, aflatoxin B2, aflatoxin G1 and aflatoxin G2; ZEN = zearalenone; DON = deoxynivalenol; FUM = a sum of fumonisin B1 and fumonisin B2; OTA = ochratoxin A.
2Median of all samples above the limit of detection.
MYCOTOXICOSES
Mycotoxicoses on the Gastrointestinal Tract
Recent literature has implicated physiological and immunological effects of mycotoxins at lower and more common levels of contamination. As many of the mycotoxins and their metabolites inhibit protein synthesis, tissues with high levels of protein synthesis and turnover, such as those within the gastrointestinal tract (GIT) can be particularly susceptible to their toxic effects. In particular, the GIT is repeatedly exposed to mycotoxins at concentrations likely higher than other organ systems. However, there is increasing evidence that there are effects on the functionality of the GIT at realistic and occasional doses (Table 5) of mycotoxins (Grenier and Applegate, 2013).
Table 5.
Method used to categorize the experimental doses.
Meta-analyses of swine data suggests that mycotoxicoses can result in up to 30% reduction in growth, of which 15% is due to changes in maintenance with the bulk of reductions (85%) attributable to inefficient use of feed (Pastorelli et al., 2012 from studies including feed contaminated with AF, DON, FUM, and/or ZEN), whereas intestinal challenges (E. coli) resulted in 39.8% growth reduction, of which 74% is due to alterations in body maintenance and 26% from reductions in feed efficiency. Thus, while mycotoxicoses may not cause direct changes to GIT maintenance costs as readily as that of bacterial challenges, 4.5% of the 30% reduction in growth was attributable to maintenance costs of the animal, much of which can be linked to altered intestinal functionality and maintenance. With each of these challenges, it is important to realize that they have different effective nutritional and energy requirements due to tissues and systems they affect. Additionally, due to their physiological effects, time to recovery is inordinately different, in that recovery of feed intake behavior is nearly 25% longer for intestinal challenges versus that of mycotoxicoses (Pastorelli et al., 2012).
As reviewed by Grenier and Applegate (2013), mycotoxin absorption (and on occasion conversion to either active or inactive metabolites) varies considerably, but will ultimately determine systemic exposure and tissue distribution. Focusing on the predominate mycotoxins to which poultry are exposed to, AF is readily absorbed in the proximal GIT (greater than 80%; Agence Française de Sécurité Sanitaire des Aliments, 2009), while OTA is moderately absorbed (40%; Ringot et al., 2006), and DON and FUM are minimally absorbed (5 to 20 and 1%, respectively; Bouhet and Oswald, 2007; Osselaere et al., 2013). Notably, enterohepatic cycling of DON, FUM, and OTA can occur and increase the time and concentration of exposure along the intestine.
The intestine can also serve as a site of metabolic activation or deactivation for particular mycotoxins. For example, the activation of AFB1 to its toxic metabolite AFB1-exo-8, 9-epoxide (AFBO) takes place not only in the liver, but also in the intestinal tract (Sergent et al., 2008). Thus, rapidly dividing intestinal enterocytes with high protein turnover can become a major target of AFBO. Notably, AFBO can inhibit protein synthesis through interactions with RNA and can interact with DNA forming DNA adducts causing DNA breakage (Bbosa et al., 2013). AFBO can also have epigenetic effects including DNA methylation, histone modifications, maturation of miRNAs, and daily formation of single nucleotide polymorphisms (Bbosa et al., 2013). Thus, the question remains as to whether AFBO's effect on DNA, RNA, and protein synthesis in the GIT collectively affects enterocyte integrity, endogenous nutrient loss, nutrient digestion and absorption, or other intestinal functions.
Mycotoxicoses on Nutrient Digestion and Absorption
In order to put the breadth of scientific literature in perspective, it is important to categorize this research into realistic, occasional, and unrealistic doses (as described in Table 5; Grenier and Applegate, 2013) to realize any plausible influence of mycotoxins on intestinal functionality. Additionally, research on nutrient and energy usage by the animal must not be largely confounded by the effects of mold(s) (Aspergillus, Penicillium, and/or Fusarium spp.) on the nutrient content of the feed ingredients. For example, Dänicke et al. (2007) noted increased jejunal and ileal viscosity in broiler chicks fed wheat-based diets, whereas intestinal viscosity was substantially lowered when the wheat was inoculated with Fusarium spp, and was not different from the diets supplemented with an endo-1,4-β-xylanase. This suggested that the Fusarium contaminated wheat had altered non-starch polysaccharide content. Additionally, the Fusarium wheat had 10% greater CP concentration.
Beyond growth reductions, malabsorption was first implied in AF contamination of broilers (Osborne, 1975). Interestingly, the “malabsorption” was implied from increased excreta fat content when birds were fed unrealistic doses of 5 to 10 mg/kg. Concomitantly, they noted reductions in pancreatic lipase above 0.625 mg/kg and bile acid production above 2.5 mg/kg. Nevertheless, the thought of mycotoxicoses having an effect on intestinal functionality, nutrient and energy digestibility was introduced to the poultry science literature. Since then, others have noted reductions in specific pancreatic enzyme activity at occasional doses of AF (Osborne and Hamilton, 1981; Richardson and Hamilton, 1987), which is partially or completely overcome by pancreatomegaly (Osborne and Hamilton, 1981). Conversely, effects on specific maltase activity in the jejunal mucosa, dramatically increased when hens were exposed to occasional doses of AF (Applegate et al., 2009). Additional reductions in nutrient transporters have been noted, namely through realistic doses of DON reducing intestinal expression of SGLT1, GLUT2 (Awad et al., 2011), GLUT5, and palmitate transporters (Dietrich et al., 2012). At occasional doses of DON, (Awad et al., 2004, 2005) reductions were noted in epithelial short-circuit current, a measure of net ion transport, when glucose was added to the luminal side of intestinal explants.
These measured effects on pancreatic and intestinal functionality translate to reduced crude protein digestibility in ducks at realistic AF doses (Han et al., 2008), and reduced apparent nutrient and energy digestibility at occasional doses of AF (Kermanshahi et al., 2007, Verma et al., 2007; Applegate et al., 2009). Conflicting reports of Fusarium mycotoxins at occasional doses have been documented, wherein Dänicke et al. (2002) noted reductions in protein digestibility while the same researchers (Dänicke et al., 2003) noted increased protein digestibility and net protein utilization. Thus, direct effects on digestibility and nutrient absorption functionality have been mixed in studies. Nevertheless, alterations to intestinal functionality have been noted beyond just apparent nutrient utilization, in particular those of barrier and innate immune responsiveness have been noted that may compromise intestinal health.
Mycotoxicoses on Intestinal Barrier Function
Intestinal “health” encompasses both passive barriers (mucin and other non-specific barriers, commensal microflora, tight junction complexes between epithelia, epithelial sloughing/turnover, etc.), and active immunological processes (gut-associated lymphoid tissues, intra-epithelial lymphocytes, etc.) for self versus non-self recognition and pathogen clearance. Generally, barrier function has been assessed through numerous methods including passing of dextrans, evaluation of presence, functionality, and production of RNA for tight junction proteins, as well as through electrophysiological studies utilizing Ussing chambers (for review see Grenier and Applegate, 2013). The majority of the work with poultry has been conducted by Awad and co-workers from the University of Veterinary Medicine, Vienna, Austria using intestinal segments from birds in Ussing chambers. In their work, they have consistently noted that the basal epithelial resistance of either the intestinal tissues exposed to the equivalent of occasional doses of DON ex vivo (Awad et al., 2005, 2007, 2008, 2009) or the tissues from birds fed occasional doses of DON (Awad et al., 2004), was not altered with DON exposure, however functionality of active transport of glucose and amino acids were greatly inhibited. This inhibition is presumed to be through the effects of DON on protein synthesis and thus on nutrient transporters, as histomorphometry of the intestine to similar concentrations of DON is not substantially altered in the bird (Xu et al., 2011). Differential effects, however, are noted with AF exposure (ex vivo), wherein active glucose uptake is not readily affected in the bird, however, apical anion (presumably chloride) is dramatically inhibited.
Altered functionality has also been noted in cytokine expression within the intestine of mammals due to exposure to DON in vivo, ex vivo, or in cell culture models. Grenier and Applegate (2013) summarized a consistent trend in pro-inflammatory cytokine markers (IL-1β, Il-6, IL-8, and TNF-α) being up-regulated with or without exposure to a naïve antigen as well as up-regulation and/or steady production of Th1, Th2 and T-regulatory signature cytokines when not exposed to a naïve antigen (INF-γ, IL-12p40, IL-2, IL-4, IL-10, and TGF-β). Similar cytokine responses have been noted in pigs exposed to FUM (Grenier et al., 2011; Bracarense et al., 2012), plausibly due to FUM having lower absorption (and thus greater intestinal exposure) and a greater effect on barrier functionality in the pig (Pinton et al., 2012). The capacity to develop a similar synopsis for poultry has not been possible due to the paucity of reports with poultry as the experimental model. However, Xu et al. (2011) noted that although an intraperitoneal E. coli lipopolysaccharide (LPS) challenge up-regulated the expression of IL-8, MUC2, TGF-β, and TNFα-like factor 24 h after challenge, feeding of birds an “occasional” dose of DON did not result in altered responses to these cytokines with or without the LPS challenge. Thus, the question remains as to whether mycotoxicoses influences the bird's ability to mount an effective and timely immunological response to pathogen challenges.
Mycotoxicoses on the Immune System
Aflatoxins are able to bind with both DNA and RNA and inhibit macromolecular synthesis by interfering with transcription and other aspects of protein synthesis. Inhibition of protein synthesis is also a “trademark” of trichothecenes including DON and T-2 toxin through the binding to eukaryotic ribosomes, and as well as OTA by blocking phenylalanine tRNA synthetase. Other most prevalent mycotoxins have structural similarity to biological compounds, such as FUM with the sphingoid bases SA and SO (disrupting the synthesis of sphingolipids-containing cell membrane) and ZEN with estradiol (the most important female sex hormone). Although considerable work has been done to correlate mechanisms of action and immunotoxicity, this aspect is not fully understood yet and deserves further research. However, it has been clearly demonstrated that rapidly dividing and activated cells with a high protein turnover (such as immune, intestinal, and hepatic cells) are predominantly affected by mycotoxins. Some recent work exemplifies this latter point where low doses of mycotoxins were able to impair the proliferation of specific lymphocytes primed and activated by an antigen (e.g., following vaccination), whereas no effect was observed on the total non-specific (i.e., that does not recognize the antigen) population of lymphocytes (Grenier et al., 2011). Mycotoxins do not possess immunogenic properties, meaning they are not able to induce an immune response unlike pathogens, but they do interfere with signaling pathways (MAPKs) that are implicated in cell growth, apoptosis or immune responses. As a consequence, the processes leading to the establishment of an efficient immune response are impaired and render the animal more susceptible to infection.
Subclinical Doses of Mycotoxins
Poultry species are considered to be less sensitive to mycotoxins, particularly toxins from Fusarium, compared to other species, such as the pig. Many experiments in poultry have reported toxic effects of mycotoxins but at doses not expected in the field (Table 5). However, recent research give evidence that at levels lower than those that would cause overt clinical mycotoxicoses (Table 5), mycotoxins modulate immune functions and may decrease resistance to infectious disease. In line with that, recent epidemiological data indicate high correlation between outbreaks of Newcastle disease and AF contamination of broiler rations (Yunus et al., 2011). Feeding broiler chickens 0.3 mg AF/kg of feed significantly reduced antibody titres against Newcastle disease and infectious bursal disease (review in Girish and Smith, 2008). Antibodies are produced by B-lymphocytes, which are programmed in the bursa of Fabricius. The reduced antibody concentration observed in poultry fed AF-contaminated diet is most likely related to lymphoid depletion and inhibition of development and functional maturation of the bursa of Fabricius, at doses as low as 0.1 mg AF/kg of feed. Ducks and broilers fed with concentrations of DON ranging from 3 to 12 mg/kg diet also had decreased antibody titers to common vaccines (Newcastle disease, infectious bronchitis) and a reduction in the mass of the bursa of Fabricius (Awad et al., 2013). For both DON and AF, the effects seen in the bursa of Fabricius, and the subsequent impact on antibody, might be a direct consequence of the inhibition of protein biosynthesis.
There is also growing evidence that, depending upon the level and length of exposure to the toxins, a biphasic response is expected. For instance, AF follows a pattern of hormesis, characterized by low-dose stimulation and high-dose inhibition with regard to bird performance (Diaz et al., 2008). Similarly, an initial increase followed by a decrease in humoral response (antibody response) with low doses of AF has been documented in poultry. The underlying mechanisms for this temporary increase are not known. In other animal models, DON at low doses promoted the expression of several cytokines and chemokines, whereas high doses exhibited immunosuppressive effects (Grenier and Applegate, 2013). There is therefore a need to pay closer attention to the effect of doses lower than those that would cause overt clinical symptoms.
Susceptibility to Infectious Diseases
Unlike pathogen exposure, there are no visible clinical signs of mycotoxin intoxication as most of the time these fungal metabolites are normally found at low levels. However, as previously mentioned, mycotoxins are able to affect activated and proliferating cells, damage epithelial tissue, increase intestinal permeability, and therefore may result in a weakened immune system. As a consequence, when a pathogen enters the organism, an appropriate and efficient immune response cannot be mounted, and eventually results in stronger clinical signs. In an experimental necrotic enteritis (caused by Clostridium perfringens) infection model, broiler chickens fed a diet contaminated with 5 mg DON/kg of feed were more prone to develop necrotic enteritis lesions compared to chickens on a control diet (Antonissen et al., 2014). In that case, DON acted as a predisposing factor by damaging the intestinal mucosa, leading to leakage of nutrients into the intestinal lumen, and therefore providing the necessary growth substrate for extensive proliferation of C. perfringens. Another predisposing factor to necrotic enteritis is mucosal damage caused by coccidial pathogens. The interaction of toxins from Fusarium with strains of Eimeria responsible for coccidiosis in poultry has been investigated. Realistic (Girgis et al., 2008, 2010a) and occasional (Girgis et al., 2010b) doses of Fusarium mycotoxins have also shown delayed intestinal recovery, up-regulation of IFN-γ, and delayed recruitment of CD4+ and CD8+ cells after Eimeria challenges in chickens. Similarly, chickens challenged with strains of Eimeria and fed with either individual dose of DON and FUM or in combination (1.5 mg DON/kg and 20 mg FUM/kg diet) showed higher occurrence of lesions in the GIT and more oocysts in the jejunum and excreta compared to only Eimeria challenged birds on the control diet (Grenier et al., unpublished data). Further, high (and unrealistic) doses of OTA to broilers (Koynarski et al., 2007a) and turkeys (Koynarsky et al., 2007b) have resulted in more severe lesion scores and greater incidence of bloody diarrhea after Eimeria challenge. Besides, typical upregulation of pro-inflammatory cytokines following coccidial infection was stronger in the jejunum of birds fed DON and FUM in combination, suggesting an exacerbation of the inflammatory response that might lead to tissue damage. Finally, it has been demonstrated that the effectiveness of lasalocid (a coccidiostat) was impaired when the levels of T-2 toxin exceeded 0.5 mg/kg in feed, as depicted by the development of clinical coccidiosis in birds (Varga and Vanyi, 1992).
MYCOTOXIN COUNTERACTING STRATEGIES
It is commonly known that mycotoxins vary in their chemical structures, which results in vast differences regarding their chemical, physical, and biochemical properties. While the biochemical properties define the toxicity of mycotoxins, chemical and physical properties determine the methods that can be used to detoxify them. Considering the great variety of mycotoxin structures it is very obvious that there is no single method, which can be used to deactivate mycotoxins in feed. Therefore, different strategies have to be combined in order to specifically target individual mycotoxins without impacting the quality of feed. The best known method for mycotoxin deactivation is “binding” with the use of binding agents, which are referred to as mycotoxin binders, adsorbents, or enterosorbents. They can be of organic (microbial) or inorganic (mainly clay minerals) nature. Another method is “bio-protection,” which uses different substances (algae, plant ingredients, etc.) that protect vulnerable organs such as the liver and strengthen the immune system of animals. Enzymatic or microbial detoxification, sometimes referred to as “biotransformation” or “biodetoxification” utilizes microorganisms or purified enzymes thereof to catabolize the entire mycotoxin or transform or cleave it to less or non-toxic compounds.
Mycotoxin Enterosorbents or Binders
The inclusion of binding agents or “enterosorbents” in the diet has been given considerable attention as a strategy to reduce foodborne exposures to mycotoxins. The use of clay-based materials for toxin binding is not new. For centuries, humans and animals have been reported to eat clay minerals, a process known as geophagy (Carretero, 2002). The consumption of edible clays for various purposes by people and animals in developing countries (and the United States) is common and in most cases is considered to be beneficial to health (Johns and Duquette, 1991; Diamond, 1999; Ferguson and Keaton, 1950; Loggi et al., 1992). Thus, the inclusion of non-nutritive clay minerals in the diet of animals has been widely adopted for reducing toxin bioavailability and exposure from contaminated feeds. In groundbreaking work in the 1980s, using multiple animal models and molecular assessment of sorption mechanisms, Phillips et al. (1988, 1995, 2002, 2008) reported that a calcium dioctahedral smectite clay (NovaSil, NS) significantly prevented the adverse effects of AF in animals via enterosorption in the GIT and a resulting decrease in toxin bioavailability. Clinical intervention studies indicate that refined NS (UPSN) can significantly reduce biomarkers of exposure for AFB1 as well as FB1 (Phillips et al., 2008; Robinson et al., 2012, Mitchell et al., 2014). The molecular mechanism for sorption of AF onto the surfaces of NS is thought to involve chemisorption of toxin onto interlamellar surfaces of the clay with the planar orientation of the AF molecule as the most stable configuration. The results also indicate a good correlation between the magnitude of partial positive charges on carbons C11 and C1 of the β-dicarbonyl system and the strength of adsorption of planar ligands. Other potential mechanisms of AFB1 sorption to NS surfaces may involve the chelation of interlayer cations (especially Ca2+) and various edge-site metals and/or the interaction with water molecules in the interlayer (Grant and Phillips, 1998; Phillips, 1999; Deng et al., 2010). NovaSil is a processed calcium montmorillonite clay. Its discovery as a high affinity and high capacity enterosorbent for AF, its chemical composition, and its sorption mechanism of AF at interlayer surfaces have been described in numerous publications in the scientific literature (Phillips et al., 2008; Robinson et al., 2012, Mitchell et al., 2014). NovaSil contains more calcium than sodium and swells less than sodium clay, hence it has restricted delamination upon hydration. This is thought to be one of the reasons for the preferential sorption of compounds such as AF. Recent studies have confirmed the ability of AF to be tightly adsorbed onto “dioctahedral smectite” clay surfaces (Phillips et al., 2002; Kannewischer et al., 2006; Marroquín-Cardona et al., 2009; Deng et al., 2010). This is not the case for other clay groups, such as kaolinites, attapulgites, zeolites, mica, alumina, and sand.
Due to low feed inclusion requirements and easy management of AF enterosorbents, the widespread acceptance of these products by the farm animal industry has led to the introduction of a variety of diverse materials and/or complex mixtures for AF binding. These have been labeled as mycotoxin enterosorbents, binders, sequestrants, interceptor molecules, trapping agents, adsorbents, toxin sorbents, and so on. These materials (and/or mixtures) are reported to contain smectite clays, zeolites, kaolinite, mica, silica, charcoal, and various biological constituents including chlorophyllins, yeast products, lactic acid bacteria, plant extracts, and algae. Some contain smectite or zeolite minerals that have been amended with natural or synthetic surfactants resulting in hydrophobic organoclays or organozeolites (Lemke et al., 1998; Dakovic et al., 2008; Kensler et al., 2013; El-Nezami et al., 2000; El-Nezami et al., 2006; Diaz et al., 2004; Baptista et al., 2002; Fruhauf et al., 2012; Avantaggiato et al., 2007; Cabassi et al., 2005; Piva et al., 2005; Miller et al., 2014). There is considerable evidence to indicate that smectite clays are the most effective AF enterosorbents as explained earlier.
In extensive studies in animals and humans, NS and similar montmorillonite clays have been reported to significantly decrease AF exposures and toxic effects following ingestion of doses up to 20 g/kg of diet. Research with NS and other materials suggest that potential AF enterosorbents should be rigorously evaluated in vitro and in vivo. These should meet the following criteria:
Favorable thermodynamic characteristics of sorption
Tolerable levels of priority metals, dioxins/furans, and other hazardous substances
Safety and efficacy in multiple animal species
Safety and efficacy in long-term rodent studies
Negligible interactions with vitamins, iron, and zinc
Need for Alternative Counteracting Strategies
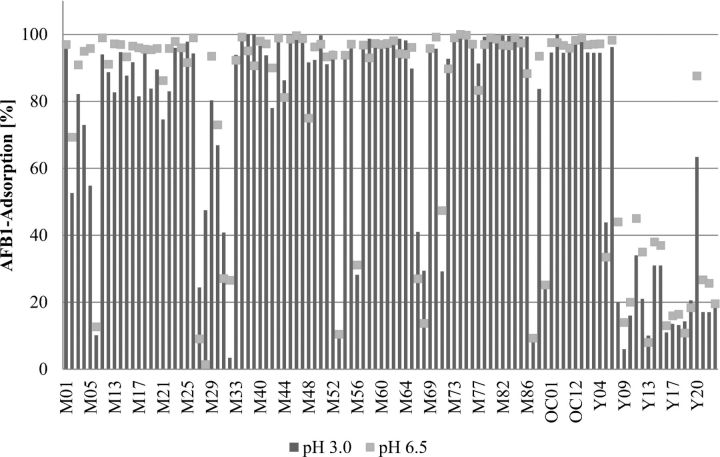
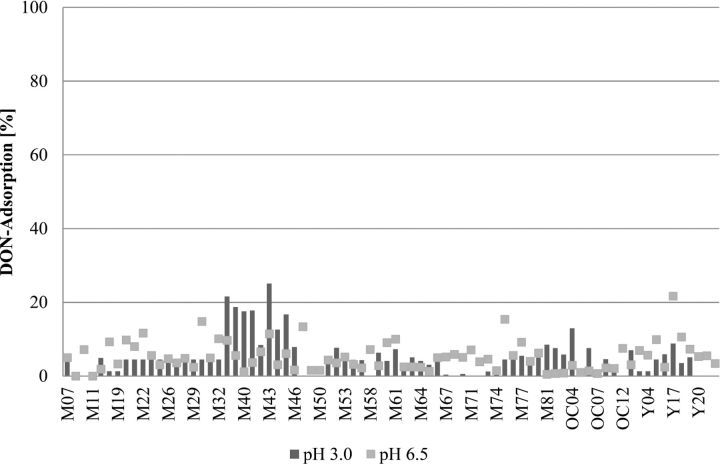
The adsorption efficacy of binding agents or enterosorbents is limited to only a few mycotoxins, such as AF, ergot alkaloids, and some other fungal toxins, while binders have been shown to be ineffective for trichothecenes (Huff et al., 1992; Kubena et al., 1993; Ramos et al., 1996; Scott, 1998; Huebner et al., 1999; Vekiru et al., 2007). These data are in line with results from the adsorption studies performed according to a standard protocol for mycotoxin adsorption using binder products sold in different markets around the world (Figures 2 and 3; Vekiru et al., 2007). While most of the clay minerals at 0.2% inclusion level bound more than 85% of 200 μg AF/L at pH 3.0 as well as pH 6.5, they resulted in less than 25% adsorption rate for 1,000 μg DON/L. On the other hand, organic materials such as yeast products (with ash content less than 15%) bound only less than 20% of AF and DON (Figures 2 and 3). Therefore, alternative approaches for efficient detoxification of mycotoxins are required.
Figure 2.
Adsorption capacity of mycotoxin binder products of different origins at pH 3.0 and pH 6.5 on Aflatoxin. M: Mineral; OC: Organoclay; Y: Yeast.
Figure 3.
Adsorption capacity of mycotoxin binder products of different origins at pH 3.0 and pH 6.5 on deoxynivalenol (DON). M: Mineral; OC: Organoclay; Y: Yeast.
The approach to use microorganisms and their enzymes to detoxify specific mycotoxins not only works for non-adsorbable mycotoxins, but for all other toxins for which respective microbes can be isolated from the nature. This approach has been known for a long time, even longer than the binder concept. Within few years after the discovery of AF, the first report on a bacterium capable of detoxifying AF by catabolization was published (Ciegler et al., 1966). Since then, many microorganisms were isolated from different habitats such as the GIT of animals, soil, mycotoxin contaminated materials (e.g., grains) and insects feeding on such materials. The ability of various bacteria, yeast, fungi, and enzymes in detoxifying mycotoxins by transformation, cleavage and catabolization, has been recently reviewed (Karlovsky, 2011; McCormick, 2013). However, only a few of these organisms were useful or further investigated for practical applications in animal nutrition. Such microorganisms or enzymes have to fulfill many different requirements before they can be used for gastrointestinal detoxification of mycotoxin in animals such as:
The microorganism and its reaction products have to be non-toxic and safe
High detoxification reactivity
Good technological properties (fermentation, downstream processing, stabilization)
High stability in feed and during feed processing
No negative impact on feed (ingredients)
Compatibility and stability in the GIT
Detoxification reaction in the GIT has to be fast and as complete as possible
Recent Advances in Microbial and Enzymatic Mycotoxin Deactivation
One of the microorganisms which has been further developed into practical application is Trichosporon mycotoxinivorans, a yeast strain capable of detoxifying OTA and ZEN (Schatzmayr et al., 2003; Molnar et al., 2004; Vekiru et al., 2010). Application of this yeast in poultry diets has been proven to detoxify OTA (Politis et al., 2005). Another organism has been an anaerobic rumen bacterium BBSH 797 (Genus novus of family Coriobacteriacae, formerly Eubacterium) which was isolated and developed as a trichothecene detoxifying feed additive (Fuchs et al., 2002; Schatzmayr et al., 2006b). The BBSH 797 detoxifies trichothecenes by cleavage of the 12, 13 epoxide ring resulting in de-epoxy trichothecenes. Several microorganisms, mainly aerobic bacteria but also yeasts, with FUM degradation properties were also explored and isolated in order to detoxify FUM (Schatzmayr et al., 2006a). However for various reasons, none of these microorganisms were useful as a mycotoxin deactivating feed additive. Therefore, the catabolic pathway of FUM degradation was investigated and the gene coding for the key enzyme of FUM detoxification (FUMzyme) was identified, cloned and expressed in a yeast strain (Heinl et al., 2010; Hartinger and Moll, 2011). FUMzyme (carboxyl-esterase) was further developed and tested in swine for gastrointestinal detoxification of FUM by cleaving the tricarballylic side chains of FUM leading to the non-toxic metabolite hydrolyzed FUM (HFB1; Grenier et al., 2012; Grenier et al., 2013).
Regulatory Perspectives for Mycotoxin Binders and Deactivators
Many pellet binding products and flowing agents (clay minerals) or feed materials (yeast and their derivatives) with the claim of mycotoxin binding and or detoxification have been used in animal feeds worldwide. However, regulations for mycotoxin binders and deactivators have not been implemented in many parts of the world for various reasons. This negates the guarantee on the safety and efficacy of the product to the user. Therefore it is important to have guidelines in place which prove safety and efficacy of such additives under different in vitro and in vivo conditions. To overcome this unsatisfactory legal situation, recently the European Commission established a new group of technological feed additives for the reduction of mycotoxins in feed. In 2010, the European Food Safety Authority (EFSA) published guidelines with stringent requirements, e.g. the binding capacity must be demonstrated; mycotoxin degradation products must be safe for target animals and consumers; minimum 3 in vivo studies with significant efficacy at the lowest recommended dose; relevant biomarkers of each individual mycotoxin have to be used to demonstrate the efficacy of the product, for the evaluation of mycotoxin deactivating products (EFSA, 2010).
The bacterial strain BBSH 797 and the purified FUMzyme were the first ones for which dossiers were submitted to EFSA to get the approval as mycotoxin biotransforming agents in animal feeds. A comprehensive set of toxicity assays had to be performed for both components such as Repeated Dose 90-Day Oral Toxicity Study in Rodents (OECD 408; 1998), Acute Dermal Irritation/Corrosion (OECD 404; 2002), Acute Eye Irritation/Corrosion (OECD 405; 1997), Skin Sensitization (OECD 406; 1992), Mammalian Erythrocyte Micronucleus Test (OECD 474; 1997), Bacterial Reverse Mutation Test (OECD 471; 1997), In Vitro Mammalian Chromosome Aberration Test (OECD 473; 1997) and Tolerance test in target species up to 100-fold dose level. Furthermore, in vitro, ex vivo, and in vivo experiments were also carried out. The in vitro experiments comprised buffer tests and different cell based assays to prove the reduction of toxicity of the metabolites formed (Schatzmayr et al., 2006b). For the ex vivo experiments, pieces of different parts of the intestine were collected and inoculated with the bacterium and the enzyme, respectively, together with the mycotoxins for a short period, before samples were taken and analyzed for residual toxins and the non-toxic metabolites. Feeding experiments were conducted to demonstrate the mode of action of the bacterium and enzyme and biomarker analyses were performed to investigate the effect in animals. It has to be noted that performance parameters alone are not sufficient for this class of additives to prove effectiveness. In case of trichothecenes and the group's main representative DON, the biomarker of exposure is the mycotoxin itself (DON) and/or its metabolite (DOM-1) in blood serum, urine or faces of the animals. Also for FUM, the biomarker of exposure is FUM itself and the metabolites in the GIT of poultry while the biomarker of effect is the SA:SO ratio. Compared to a group of poultry only receiving mycotoxin contaminated diet, both, BBSH 797 and FUMzyme were able to significantly reduce the biomarkers of exposure as well as the biomarkers of effects when added together with the mycotoxins (unpublished data). Based on these results provided to EFSA, individual approvals were issued for BBSH 797 (EFSA, 2013) and FUMzyme (EFSA, 2014).
In conclusion, understanding the occurrence and prevalence of mycotoxins and their individual as well as additive negative effects on birds has become imperative. The usage of latest analytical techniques such as LC-MS/MS will increase the precision on the determination of the concentrations of multiple mycotoxins present in agricultural commodities, at once. Latest enzymatic deactivation technologies help to eliminate the mycotoxins that cannot be bound by using binder products.
Footnotes
Presented in the symposium titled “New Strategies to Counteract the Effects of Mycotoxins on Poultry Health and Performance,” held at the annual meeting of the Poultry Science Association at Corpus Christi, TX, on July 15, 2014.
REFERENCES
- Agence Française de Sécurité Sanitaire des Aliments. Maisons-Alfort, France: Published by AFSSA; 2009. Évaluation des risques liés à la presence de mycotoxines dans les chaînes alimentaires humaine et animale; Agence Française de Sécurité Sanitaire des Aliments; pp. 1–308. Maisons-Alfort, France. [Google Scholar]
- Antonissen G., Martel A., Pasmans F., Ducatelle R., Verbrugghe E., Vandenbroucke V., Li S., Haesebrouck F., Van Immerseel F., Croubels S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins. 2014;6:430–452. doi: 10.3390/toxins6020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate T. J., Schatzmayr G., Pricket K., Troche C., Jiang Z. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult. Sci. 2009;88:1235–1241. doi: 10.3382/ps.2008-00494. [DOI] [PubMed] [Google Scholar]
- Avantaggiato G., Havenaar R., Visconti A. Assessment of the multi-mycotoxin-binding efficacy of a carbon/aluminosilicate-based product in an in vitro gastrointestinal model. J. Agric. Food Chem. 2007;55:4810–4819. doi: 10.1021/jf0702803. [DOI] [PubMed] [Google Scholar]
- Awad W. A., Bohm J., Razzazi-Fazeli E., Hulan H. W., Zentek J. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poult. Sci. 2004;83:1964–1972. doi: 10.1093/ps/83.12.1964. [DOI] [PubMed] [Google Scholar]
- Awad W. A., Rehman H., Bohm J., Razzazi-Fazeli E., Zentek J. Effects of luminal deoxynivalenol and L-proline on electrophysiological parameters in the jejunums of laying hens. Poult. Sci. 2005;84:928–932. doi: 10.1093/ps/84.6.928. [DOI] [PubMed] [Google Scholar]
- Awad W. A., Aschenbach J. R., Setyabudi F. M. C. S., Razzazi-Fazeli E., Bohm J., Zentek J. In vitro effects of deoxynivalenol on small intestinal D-glucose uptake and absorption of deoxynivalenol across the isolated jejunal epithelium of laying hens. Poult. Sci. 2007;86:15–20. doi: 10.1093/ps/86.1.15. [DOI] [PubMed] [Google Scholar]
- Awad W. A., Razzai-Fazeli E., Bohm J., Zentek J. Effects of B-trichothecenes on luminal glucose transport across the isolated jejunal epithelium of broiler chicks. J. Anim. Physiol. Anim. Nutr. 2008;92:225–230. doi: 10.1111/j.1439-0396.2007.00709.x. [DOI] [PubMed] [Google Scholar]
- Awad W. A., Ghareeb K., Bohm J. Animal feed additive and the effect of the fusarium toxin deoxynivalenol on the electrophysiological measurement of transepithelial ion transport of young chickens with ussing chamber technique. Intl. J. Poult. Sci. 2009;8:25–27. [Google Scholar]
- Awad W. A., Hess M., Twaruzek M., Grajewski J., Kosicki R., Bohm J., Zentek J. The impact of the Fusarium Mycotoxin deoxynivalenol on the health and performance of broiler chicks. Intl. J. Mol. Sci. 2011;12:7996–8012. doi: 10.3390/ijms12117996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W., Ghareeb K., Böhm J., Zentek J. The toxicological impacts of the Fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins. 2013;5:912–925. doi: 10.3390/toxins5050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista A. S., Horii J., Calori-Domingues M. A., da Glória E. M., Salgado J. M., Vizioli M. R. Thermolysed and active yeast to reduce the toxicity of aflatoxin. Scientia Agricola. 2002;59:257–260. [Google Scholar]
- Bbosa G. S., Kitya D., Odda J., Ogwal-Okeng J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health. 2013;5:14–34. [Google Scholar]
- Bermudez A. J., Ledoux D. R., Rottinghaus G. E. Effects of Fusarium moniliforme culture material containing known levels of fumonisin B1 in ducklings. Avian Dis. 1995;39:879–886. [PubMed] [Google Scholar]
- Berthiller F., Crews C., Dall'Asta C., Saeger S. D., Haesaert G., Karlovsky P., Oswald I. P., Seefelder W., Speijers G., Stroka J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezuidenhout S. C., Gelderblom W. C. A., Gorst-Allman C. P., Horak R. M., Marasas W. F. O., Spiteller G., Vleggaar R. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. J. Chem. Soc. Chem. Commun. 1988. pp. 743–745.
- Binder E. M., Tan L. M., Chin L. J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. [Google Scholar]
- Bouhet S., Oswald I. P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007;51:925–931. doi: 10.1002/mnfr.200600266. [DOI] [PubMed] [Google Scholar]
- Bracarense A.-P. F. L., Lucioli J., Grenier B., Drociunas Pacheco G., Moll W. D., Schatzmayr G., Oswald I. P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012;107:1776–1786. doi: 10.1017/S0007114511004946. [DOI] [PubMed] [Google Scholar]
- Broomhead J. N., Ledoux D. R., Bermudez A. J., Rottinghaus G. E. Chronic effects of fumonisin B1 in broilers and turkeys fed dietary treatments to market age. Poult. Sci. 2002;81:56–61. doi: 10.1093/ps/81.1.56. [DOI] [PubMed] [Google Scholar]
- Brown T. P., Rottinghaus G. E., Williams M. E. Fumonisin mycotoxicosis in broilers: Performance and pathology. Avian Dis. 1992;36:450–454. [PubMed] [Google Scholar]
- Bryden W. L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012;173(1–2):134–158. [Google Scholar]
- Busby W. F., Jr., Wogan G. N. Aflatoxins. In: Shank R. C., editor. Mycotoxins and N-Nitrosocompounds, Environmental Risks. Vol. 2. Boca Raton, FL: CRC Press Inc; 1981. pp. 3–27. [Google Scholar]
- Cabassi E., Miduri F., Cantoni A. M. Intoxication with fumonisin B1 (FB1) in piglets and supplementation with granulated activated carbon: Cellular-mediated immunoresponse. Vet. Res. Comm. 2005;29:225–227. doi: 10.1007/s11259-005-0048-7. [DOI] [PubMed] [Google Scholar]
- Carretero M. Clay minerals and their beneficial effects upon human health. Appl. Clay Sci. 2002;21:155–163. [Google Scholar]
- CAST. Council for Agricultural Science and Technology. Ames, IA: 2003. Mycotoxins: Risks in plant, animal, and human systems, Task Force Report No. 139; pp. 13–85. [Google Scholar]
- Cawood M. E., Gelderblom W. C. A., Vleggaar R., Behrend Y., Thiel P. G., Marasas W. F. O. Isolation of the fumonisin mycotoxins: A quantitative approach. J. Agric. Food Chem. 1991;39:1958–1962. [Google Scholar]
- Chen J., Mirocha C. J., Xie W., Hogge L., Olson D. Production of the mycotoxin fumonisin B1 by Alternaria alternata f. sp. Lycopersici. Appl. Environ. Microbiol. 1992;58:3928–3931. doi: 10.1128/aem.58.12.3928-3931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciegler A., Lillehoj B., Peterson E., Hall H. H. Microbial detoxification of aflatoxin. Appl. Microbiol. 1966;14:934–939. doi: 10.1128/am.14.6.934-939.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello J., Placinta C., Macdonald A. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999;80:183–205. [Google Scholar]
- Daković A., Matijašević S., Rottinghaus G. E., Ledoux D. R., Butkeraitis P., Ž. Sekulić Aflatoxin B1 adsorption by natural and copper modified montmorillonite. Colloid. Surface. B. 2008;66:20–25. doi: 10.1016/j.colsurfb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Danicke S., Gareis M., Bauer J. Orientation values for critical concentrations of deoxynivalenol and zearalenone in diets for pigs, ruminants and gallinaceous poultry. Proc. Soc. Nutr. Physiol. 2001;10:171–174. [Google Scholar]
- Danicke S. Prevention and control of mycotoxins in the poultry production chain: A European view. World. Poult. Sci. J. 2002;58:451–474. [Google Scholar]
- Dänicke S., Ueberschar K. H., Halle I., Matthes S., Valenta H., Flachowsky G. Effect of addition of a detoxifying agent to laying hen diets containing uncontaminated or Fusarium toxin-contaminated maize on performance of hens and on carryover of zearalenone. Poult. Sci. 2002;81:1671–1680. doi: 10.1093/ps/81.11.1671. [DOI] [PubMed] [Google Scholar]
- Dänicke S., Matthes S., Halle I., Ueberschar K. H., Doll S., Valenta H. Effects of graded levels of Fusarium toxin-contaminated wheat and of a detoxifying agent in broiler diets on performance, nutrient digestibility and blood chemical parameters. Brit. Poult. Sci. 2003;44:113–126. doi: 10.1080/0007166031000085300. [DOI] [PubMed] [Google Scholar]
- Dänicke S., Valenta H., Matthes S. On the interactions between fusarium toxin-contaminated wheat and nonstarch polysaccharide hydrolyzing enzymes in diets of broilers on performance, intestinal viscosity, and carryover of deoxynivalenol. Poult. Sci. 2007;85:291–298. doi: 10.1093/ps/86.2.291. [DOI] [PubMed] [Google Scholar]
- Deng Y., Velázquez A. B., Billes F., Dixon J. Bonding mechanisms between aflatoxin B1 and smectite. Appl. Clay Sci. 2010;50:92–98. [Google Scholar]
- Desmarchelier A., Oberson J.-M., Tella P., Gremaud E., Seefelder W., Mottier P. Development and comparison of two multiresidue methods for the analysis of 17 mycotoxins in cereals by liquid chromatography electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2010;58:7510–7519. doi: 10.1021/jf100891m. [DOI] [PubMed] [Google Scholar]
- Devegowda G., Murthy T. N. K. Mycotoxins: Their effects in poultry and some practical solutions. In: Diaz D. E., editor. The Mycotoxin Blue Book. Nottingham, UK: Nottingham University Press; 2005. pp. 25–56. [Google Scholar]
- Diamond J. Dirty eating for healthy living. Nature. 1999;400:120–121. doi: 10.1038/22014. [DOI] [PubMed] [Google Scholar]
- Diaz D., Hagler W., Jr., Blackwelder J., Eve J., Hopkins B., Anderson K., Jones F., Whitlow L. Aflatoxin binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia. 2004;157:233–241. doi: 10.1023/b:myco.0000020587.93872.59. [DOI] [PubMed] [Google Scholar]
- Diaz G. J., Calabrese E., Blain R. Aflatoxicosis in chickens (Gallus gallus): An example of hormesis? Poult. Sci. 2008;87:727–732. doi: 10.3382/ps.2007-00403. [DOI] [PubMed] [Google Scholar]
- Dietrich B., Neuenschwander S., Bucher B., Wenk C. Fusarium mycotoxin-contaminated wheat containing deoxynivalenol alters the gene expression in the liver and the jejunum of broilers. Animal. 2012;6:278–291. doi: 10.1017/S1751731111001601. [DOI] [PubMed] [Google Scholar]
- Edds G. T., Bortell R. R. Biological effects of aflatoxin in poultry. In: Diener U. L., Asquith R. L., Dickens J. W., editors. Aflatoxin and Aspergillus flavus in corn. Auburn University, AL: Alabama Agricultural Experiment Station; 1983. pp. 55–61. Southern Cooperative Services Bulletin. 279. [Google Scholar]
- EFSA. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Statement on the establishment of guidelines for the assessment of additives from the functional group ‘substances for reduction of the contamination of feed by mycotoxins’. EFSA Journal. 2010;8:8. [Google Scholar]
- EFSA. EFSA FEEDAP Panel: Scientific opinion on the safety and efficacy of micro-organism DSM 11798 when used as a technological feed additive for pigs. EFSA Journal. 2013;11:18. [Google Scholar]
- EFSA. EFSA Panel on Additives and Products or Substances used in Animal Feed; Scientific Opinion on the safety and efficacy of fumonisin esterase (FUMzyme®) as a technological feed additive for pigs. EFSA Journal. 2014;12:3667. [Google Scholar]
- El-Nezami H. S., Mykkänen H., Kankaanpää P., Suomalainen T., Ahokas J. T., Salminen S. The ability of a mixture of Lactobacillus and Propionibacterium to influence the faecal recovery of aflatoxins in healthy Egyptian volunteers: A pilot clinical study. Biosci. Microflora. 2000;19:41–45. [Google Scholar]
- El-Nezami H. S., Polychronaki N. N., Ma J., Zhu H., Ling W., Salminen E. K., Juvonen R. O., Salminen S. J., Poussa T., Mykkanen H. M. Probiotic supplementation reduces a biomarker for increased risk of liver cancer in young men from Southern China. Am. J. Clin. Nutr. 2006;83:1199–1203. doi: 10.1093/ajcn/83.5.1199. [DOI] [PubMed] [Google Scholar]
- European Commission (EC) 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed. Off. J. Eur. Union. 2002;140:10–22. Directive. [Google Scholar]
- European Commission (EC) Commission Recommendation (2006/576/EC) of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union. 229:7–9. [Google Scholar]
- Ferguson J., Keaton A. Studies of the diets of pregnant women in Mississippi: I. The ingestion of clay and laundry starch. New Orleans Med. Surg. J. 1950;102:460–463. [PubMed] [Google Scholar]
- Fruhauf S., Schwartz H., Ottner F., Krska R., Vekirua E. Yeast cell based feed additives: Studies on aflatoxin B1 and zearalenone. Food Addit. Contam. Part A. Chem. Anal. Control Expo. Risk. Assess. 2012;29:217–231. doi: 10.1080/19440049.2011.630679. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Binder E. M., Heidler D., Krska R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 2002;19:379–386. doi: 10.1080/02652030110091154. [DOI] [PubMed] [Google Scholar]
- Gelderblom W. C. A., Jaskiewicz K., Marasas W. F. O., Thiel P. G., Horak R. M., Vleggaar R., Kriek N. P. J. Fumonisins: Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988;54:1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. M., Bailey C. A., Kubena L. F., Huff W. E., Harvey R. B. Ochratoxin A and dietary protein. 1. Effects on body weight, feed conversion, relative organ weight, and mortality in three-week-old broilers. Poult. Sci. 1989;68:1658–1663. doi: 10.3382/ps.0681658. [DOI] [PubMed] [Google Scholar]
- Girgis G. N., Sharif S., Barta J. R., Boermans H. J., Smith T. K. Immunomodulatory effects of feed-borne Fusarium mycotoxins in chickens infected with coccidia. Exp. Biol. Med. 2008;233:1411–1420. doi: 10.3181/0805-RM-173. [DOI] [PubMed] [Google Scholar]
- Girgis G. N., Barta J. R., Brash M., Smith T. K. Morphologic changes in the intestine of broiler breeder pullets fed diets naturally contaminated with Fusarium mycotoxins with or without coccidial challenge. Avian Dis. 2010a;54:67–73. doi: 10.1637/8945-052809-Reg.1. [DOI] [PubMed] [Google Scholar]
- Girgis G. N., Barta J. R., Girish C. K., Karrow N. A., Boermans H. J., Smith T. K. Effects of feed-borne Fusarium mycotoxins and an organic mycotoxin adsorbent on immune cell dynamics in the jejunum of chickens infected with Eimeria maxima. Vet. Immunol. Immunopathol. 2010b;138:218–223. doi: 10.1016/j.vetimm.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Girish C. K., Smith T. Impact of feed-borne mycotoxins on avian cell-mediated and humoral immune responses. World Mycotoxin J. 2008;1:105–121. [Google Scholar]
- Glahn R. P., Shapiro R. S., Vena V. E., Wideman R. F., Jr., Huff W. E. Effects of chronic ochratoxin A and citrinin toxicosis on kidney function of single comb white leghorn pullets. Poult. Sci. 1989;68:1205–1212. doi: 10.3382/ps.0681205. [DOI] [PubMed] [Google Scholar]
- Glahn R. P., Wideman R. F., Jr., Evangelisti J. W., Huff W. E. Effects of ochratoxin A alone and in combination with citrinin on kidney function of single comb white leghorn pullets. Poult. Sci. 1988;67:1034–1042. doi: 10.3382/ps.0671034. [DOI] [PubMed] [Google Scholar]
- Grant P. G., Phillips T. D. Isothermal adsorption of Aflatoxin B1 on HSCAS clay. J. Agric. Food Chem. 1998;46:599–605. doi: 10.1021/jf970604v. [DOI] [PubMed] [Google Scholar]
- Grenier B., Oswald I. P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011;4:285–313. [Google Scholar]
- Grenier B., Loureiro-Bracarense A. P., Lucioli J., Pacheco G. D., Cossalter A. M., Moll W. D., Schatzmayr G., Oswald I. P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol. Nutr. Food Res. 2011;55:761–771. doi: 10.1002/mnfr.201000402. [DOI] [PubMed] [Google Scholar]
- Grenier B., Bracarense A. P. F. L., Schwartz H. E., Trumel C., Cossalter A. M., Schatzmayr G., Kolf-Clauw M., Moll W. D., Oswald I. P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B 1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012;83:1465–1473. doi: 10.1016/j.bcp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Grenier B., Bracarense A. P. F., Schwartz H. E., Lucioli J., Cossalter A.-M., Moll W.-D., Schatzmayr G., Oswald I. P. Biotransformation approaches to alleviate the effects induced by fusarium mycotoxins in swine. J. Agric. Food Chem. 2013;61:6711–6719. doi: 10.1021/jf400213q. [DOI] [PubMed] [Google Scholar]
- Grenier B., Applegate T. J. Invited review-Modulation of intestinal functions upon mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessler K., Rodrigues I., Handl J., Hofstetter U. Occurrence of mycotoxins in Southern Europe. World Mycotoxin J. 2010;3:301–309. [Google Scholar]
- Hamilton P. B., Huff W. E., Harris J. R., Wyatt R. D. Natural occurrences of ochratoxicosis in poultry. Poult. Sci. 1982;61:1832–1841. doi: 10.3382/ps.0611832. [DOI] [PubMed] [Google Scholar]
- Han X. Y., Huang Q. C., Li W. F., Jiang J. F., Xu Z. R. Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livestock Sci. 2008;119:216–220. [Google Scholar]
- Hartinger D., Moll W. D. Fumonisin elimination and prospects for detoxification by enzymatic transformation. World Mycotoxin J. 2011;4:271–283. [Google Scholar]
- Heinl S., Hartinger D., Thamhesl M., Vekiru E., Krska R., Schatzmayr G., Moll W. D., Grabherr R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010;145:120–129. doi: 10.1016/j.jbiotec.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Huebner H. J., Lemke S. L., Ottinger S. E., Mayura K., Phillips T. D. Molecular characterization of high affinity, high capacity clays for the equilibrium sorption of ergotamine. Food Addit. Contam. 1999;16:159–171. doi: 10.1080/026520399284118. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Hamilton P. B. Decreased plasma carotenoids during ochratoxicosis. Poult. Sci. 1975;54:1308–1310. doi: 10.3382/ps.0541308. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Doerr J. Synergism between aflatoxin and ochratoxin A in broiler chickens. Poult. Sci. 1981;60:550–555. doi: 10.3382/ps.0600550. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Harvey R. B., Kubena L. F., Rottinghaus G. E. Toxic synergism between aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1988a;67:1418–1423. doi: 10.3382/ps.0671418. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Kubena L. F., Harvey R. B. Progression of ochratoxicosis in broiler chicks. Poult. Sci. 1988b;67:1139–1146. doi: 10.3382/ps.0671139. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Kubena L. F., Harvey R. B., Hagler W. M., Jr., Swanson S. P., Phillips T. D., Creger C. R. Individual and combined effects of aflatoxin and deoxynivalenol (DON, vomitoxin) in broiler chickens. Poult. Sci. 1986;65:1291–1298. doi: 10.3382/ps.0651291. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Kubena L. F., Harvey R. B., Phillips T. D. Efficacy of hydrated sodium calcium aluminosilicate to reduce the individual and combined toxicity of aflatoxin and ochratoxin A. Poult. Sci. 1992;71:64–69. doi: 10.3382/ps.0710064. [DOI] [PubMed] [Google Scholar]
- Jestoi M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008;48:21–49. doi: 10.1080/10408390601062021. [DOI] [PubMed] [Google Scholar]
- Johns T., Duquette M. Detoxification and mineral supplementation as functions of geophagy. Am. J. Clin. Nutr. 1991;53:448–456. doi: 10.1093/ajcn/53.2.448. [DOI] [PubMed] [Google Scholar]
- Kannewischer I., Tenorio A., White G., Dixon J. Smectite clays as adsorbents of aflatoxin B1: Initial steps. Clay Sci. 2006;12:199–204. [Google Scholar]
- Karlovsky P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl. Microbiol. Biotechnol. 2011;91:491–504. doi: 10.1007/s00253-011-3401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler T. W., Groopman J. D., Egner P. A., Muñoz A., Qian G., Chen J. Chemoprevention of Hepatic Cancer in Aflatoxin Endemic Areas in Primary Liver Cancer. In: Gu J., editor. Berlin Heidelberg, Germany: Zhejiang University Press, Hangzhou, China, and Springer-Verlag; 2013. [Google Scholar]
- Kermanshahi H., Akbari M. R., Maleki M., Behgar M. Effect of prolonged low level inclusion of aflatoxin B1 into diet on performance, nutrient digestibility, histopathology and blood enzymes of broiler chickens. J. Anim. Vet. Adv. 2007;6:686–692. [Google Scholar]
- Koynarski V., Stoev S., Grozeva N., Mirtcheva T., Daskalov H., Mitev J., Mantle P. Experimental coccidiosis provoked by Eimeria acervulina in chicks simultaneously fed on ochratoxin A contaminated diet. Res. Vet. Sci. 2007a;82:225–231. doi: 10.1016/j.rvsc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Koynarsky V., Stoev S. D., Grozeva N., Mirtcheva T. Experimental coccidiosis provoked by Eimeria adenoeides in turkey poults given ochratoxin A. Veterinarski Archiv. 2007b;77:113–128. [Google Scholar]
- Kubena L. F., Huff W. E., Harvey R. B., Corrier D. E., Phillips T. D., Creger C. R. Influence of ochratoxin A and deoxynivalenol (DON) on growing broiler chicks. Poult. Sci. 1988;67:253–260. doi: 10.3382/ps.0670253. [DOI] [PubMed] [Google Scholar]
- Kubena L. F., Huff W. E., Harvey R. B., Phillips T. D., Rottinghaus G. E. Individual and combined toxicity of deoxynivalenol and T-2 toxin in broiler chicks. Poult. Sci. 1989;68:622–626. doi: 10.3382/ps.0680622. [DOI] [PubMed] [Google Scholar]
- Kubena L. F., Harvey R. B., Phillips T. D., Clement B. A. Effect of hydrated sodium calcium aluminosilicates on aflatoxicosis in broiler chicks. Poult. Sci. 1993;72:651–657. doi: 10.3382/ps.0720651. [DOI] [PubMed] [Google Scholar]
- Ledoux D. R., Bermudez A. J., Rottinghaus G. E. The effects of feeding Fusarium moniliforme culture material, containing known levels of fumonisin B1, in the young turkey poult. Poult. Sci. 1996;75:1472–1478. doi: 10.3382/ps.0751472. [DOI] [PubMed] [Google Scholar]
- Ledoux D. R., Brown T. P., Weibking T. S., Rottinghaus G. E. Fumonisin toxicity in broiler chicks. J. Vet. Diagn. Invest. 1992;4:330–333. doi: 10.1177/104063879200400317. [DOI] [PubMed] [Google Scholar]
- Leeson S., Diaz G. J., Summers J. D. Poultry Metabolic Disorders and Mycotoxins. Guelph, Ontario, Canada: University Books; 1995. [Google Scholar]
- Lemke S. L., Grant P. G., Phillips T. D. Adsorption of zearalenone by organophilic montmorillonite clay. J. Agric. Food Chem. 1998;46:3749–3796. [Google Scholar]
- Loggi D. G., Jr., Regenye G., Miles M. Pica and iron-deficiency anaemia: A case report. J. Oral Maxillofac. Surg. 1992;50:633–635. doi: 10.1016/0278-2391(92)90448-9. [DOI] [PubMed] [Google Scholar]
- Magan N., Medina A., Aldred D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011;60:150–163. [Google Scholar]
- Magnoli A., Monge M., Miazzo R., Cavaglieri L., Magnoli C., Merkis C., Cristofolini A., Dalcero A., Chiacchiera S. Effect of low levels of aflatoxin B1 on performance, biochemical parameters, and aflatoxin B1 in broiler liver tissues in the presence of monensin and sodium bentonite. Poult. Sci. 2011;90:48–58. doi: 10.3382/ps.2010-00971. [DOI] [PubMed] [Google Scholar]
- Malachova A., Sulyok M., Schuhmacher R., Berthiller F., Hajslova J., Veprikova Z., Zachariasova M., Lattanzio V. M. T., De Saeger S., Di Mavungu J. D., Malysheva S. V., Biselli S., Winkelmann O., Breidbach A., Hird S., Krska R. Collaborative investigation of matrix effects in mycotoxin determination by high performance liquid chromatography coupled to mass spectrometry. Qual. Assur. Saf. Crops Foods. 2013;5:91–103. [Google Scholar]
- Malachova A., Sulyok M., Beltrán Iturat E., Berthiller F., Krska R. Optimization and validation of a quantitative 1 liquid chromatography—Tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all relevant mycotoxins in four model food matrices. J. Chrom. A. 2014;1362:145–156. doi: 10.1016/j.chroma.2014.08.037. [DOI] [PubMed] [Google Scholar]
- Marroquín-Cardona A., Deng Y., Taylor J. F., Hallmark C. T., Johnson N. M., Phillips T. D. In vitro and in vivo characterization of mycotoxin binding additives used for animal feeds in Mexico. Food Addit. Contam. (A) 2009;26:733–743. doi: 10.1080/02652030802641872. [DOI] [PubMed] [Google Scholar]
- Marquardt R. R., Frohlich A. A. A review of recent advances in understanding ochratoxicosis. J. Anim. Sci. 1992;70:3968–3988. doi: 10.2527/1992.70123968x. [DOI] [PubMed] [Google Scholar]
- Masimango N., Remacale J., Ramaut J. L. The role of adsorption in the elimination of aflatoxin B1 from contaminated media. European J. Appl. Microbiol. Biotechnol. 1978;6:101–105. [Google Scholar]
- Mayura K., Abdel-Wahhab M., McKensie K., Sarr A., Edwards J., Naguib K., Phillips T. Prevention of maternal and developmental toxicity in rats via dietary inclusion of common aflatoxin sorbents: Potential for hidden risks. Toxicol. Sci. 1998;41:175–182. doi: 10.1006/toxs.1997.2405. [DOI] [PubMed] [Google Scholar]
- McCormick S. P. Microbial detoxification of mycotoxins. J. Chem. Ecol. 2013;39:907–918. doi: 10.1007/s10886-013-0321-0. [DOI] [PubMed] [Google Scholar]
- Miller J. D., Schaafsma A. W., Bhatnagar D., Bondy G., Carbone I., Harris L. J., Harrison G., Munkvold G. P., Oswald I. P., Pestka J. J., Sharpe L., Sumarah M. W., Tittlemier S. A., Zhou T. Mycotoxins that affect the North American agri-food sector: State of the art and directions for the future. World Mycotoxin J. 2014;7:63–82. [Google Scholar]
- Mitchell N. J., Xue K. S., Lin S., Marroquin-Cardona A., Brown K. A., Elmore S. E., Tang L., Romoser A., Gelderblom W. C., Wang J.-S., Phillips T. Calcium montmorillonite clay reduces AFB1 and FB1 biomarkers in rats exposed to single and co-exposures of aflatoxin and fumonisin. Toxicol. Appl. Pharmacol. 2014;34(7):795–804. doi: 10.1002/jat.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar O., Schatzmayr G., Fuchs E., Prillinger H. Trichosporon mycotoxinivorans sp. nov., a new yeast species useful in biological detoxification of various mycotoxins. Syst. Appl. Microbiol. 2004;27:661–671. doi: 10.1078/0723202042369947. [DOI] [PubMed] [Google Scholar]
- Moran E. T., Jr., Hunter B., Ferket P., Young L. G., McGirr L. G. High tolerance of broilers to vomitoxin from corn infected with Fusarium graminearum. Poult. Sci. 1982;61:1828–1831. doi: 10.3382/ps.0611828. [DOI] [PubMed] [Google Scholar]
- Nagl V., Schwartz H., Krska R., Moll W.-D., Knasmüller S., Ritzmann M., Adam G., Berthiller F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012;213:367–373. doi: 10.1016/j.toxlet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl V., Wöchtl B., Schwartz-Zimmermann H. E., Hennig-Pauka I., Moll W.-D., Adam G., Berthiller F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014;229:190–197. doi: 10.1016/j.toxlet.2014.06.032. [DOI] [PubMed] [Google Scholar]
- Norred W. P. Fumonisins – mycotoxins produced by Fusarium moniliforme. J. Toxicol. Environ. Health. 1993;38:309–328. doi: 10.1080/15287399309531720. [DOI] [PubMed] [Google Scholar]
- OECD. Test No. 406: Skin Sensitisation, OECD Guidelines for the Testing of Chemicals, Section 4. Paris, France: OECD Publishing; 1992. doi: 10.1787/9789264070660-en. [Google Scholar]
- OECD. Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4. Paris, France: OECD Publishing; 1997. doi: 10.1787/9789264071247-en. [Google Scholar]
- OECD. Test No. 473: In vitro Mammalian Chromosome Aberration Test, OECD Guidelines for the Testing of Chemicals, Section 4. Paris, France: OECD Publishing; 1997. doi: 10.1787/9789264071261-en. [Google Scholar]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4. Paris, France: OECD Publishing; 1997. doi: 10.1787/9789264071285-en. [Google Scholar]
- OECD. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4. Paris, France: OECD Publishing; 1998. doi: 10.1787/9789264070707-en. [Google Scholar]
- OECD. Test No. 404: Acute Dermal Irritation/Corrosion, OECD Guidelines for the Testing of Chemicals, Section 4. Paris, France: OECD Publishing; 2002. doi: 10.1787/9789264070622-en. [Google Scholar]
- Osborne D. J., Wyatt R. D., Hamilton P. B. Fat digestion during aflatoxicosis in chickens. Poult. Sci. 1975;54:1802. [Google Scholar]
- Osborne D. J., Hamilton P. B. Decreased pancreatic digestive enzymes during aflatoxicosis. Poult. Sci. 1981;60:1818–1821. doi: 10.3382/ps.0601818. [DOI] [PubMed] [Google Scholar]
- Osborne D. J., Huff W. E., Hamilton P. B., Burmeister H. R. Comparison of ochratoxin, aflatoxin, and T-2 toxin for their effects on elected parameters related to digestion and evidence for specific metabolism of carotenoids in chickens. Poult. Sci. 1982;61:1646–1652. doi: 10.3382/ps.0611646. [DOI] [PubMed] [Google Scholar]
- Osselaere A., Devreese M., Goossens J., Vandenbroucke V., de Baere S., de Backer P., Croubels S. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem. Toxicol. 2013;51:350–355. doi: 10.1016/j.fct.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Osselaere A., Santos R., Hautekiet V., De Backer P., Chiers K., Ducatelle R., Croubels S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE. 2013;8:e69014. doi: 10.1371/journal.pone.0069014. doi:10.1371/journal.pone.0069014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli H., van Milegen J., Lovatto P., Montagne L. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal. 2012;6:952–961. doi: 10.1017/S175173111100228X. [DOI] [PubMed] [Google Scholar]
- Phillips T. Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol. Sci. 1999;52:118–126. doi: 10.1093/toxsci/52.suppl_1.118. [DOI] [PubMed] [Google Scholar]
- Phillips T., Clement B., Park D. Approaches to reduction of aflatoxins in foods and feeds. In: Eaton D. L., Groopman J. D., editors. The Toxicology of Aflatoxins. San Diego (CA): Academic Press; 1994. pp. 383–406. [Google Scholar]
- Phillips T., Kubena L., Harvey R., Taylor D., Heidelbaugh N. Hydrated sodium calcium aluminosilicate: A high affinity sorbent for aflatoxin. Poult. Sci. 1988;67:243–247. doi: 10.3382/ps.0670243. [DOI] [PubMed] [Google Scholar]
- Phillips T., Lemke S., Grant P. Characterization of clay based enterosorbents for the prevention of aflatoxicosis. In: DeVries J. W., Trucksess M. W., Jackson L. S., editors. Mycotoxins and Food Safety: Advances in Experimental Medicine and Biology. New York (NY): Kluwer Academic/Plenum Publishers; 2002. pp. 157–171. [DOI] [PubMed] [Google Scholar]
- Phillips T., Sarr A., Grant P. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Nat. Toxins. 1995;3:204–213. doi: 10.1002/nt.2620030407. [DOI] [PubMed] [Google Scholar]
- Phillips T. D., Afriyie-Gyawu E., Williams J. H., Huebner H. J., Ankrah N.-A., Ofori-Adjei D., Jolly P. E., Johnson N., Taylor J. F., Marroquin-Cardona A., Xu L., Tang L., Wang J. S. Reducing human exposure to aflatoxin through use of clay. Food Addit. Contam. 2008;25:134–145. doi: 10.1080/02652030701567467. [DOI] [PubMed] [Google Scholar]
- Pinton P., Tsybulskyy D., Lucioli J., Laffitte J., Callu P., Lyazhri F., Grosjean F., Bracarense A.P., Kolf-Clauw M., Oswald I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins and MAPKinases. Toxicol. Sci. 2012;130:180–190. doi: 10.1093/toxsci/kfs239. [DOI] [PubMed] [Google Scholar]
- Piva A., Casadei G., Pagliuca G., Cabassi E., Galvano Solfrizzo M., Riley R. T., Diaz D. E. Activated carbon does not prevent the toxicity of culture material containing fumonisin B1 when fed to weanling piglets. J. Anim. Sci. 2005;83:1939–1947. doi: 10.2527/2005.8381939x. [DOI] [PubMed] [Google Scholar]
- Plattner R. D., Weisleder D., Shackleford D. D., Peterson R., Powell R. G. A new fumonisin from solid cultures of Fusarium moniliforme. Mycopathologia. 1992;117:23–28. doi: 10.1007/BF00497275. [DOI] [PubMed] [Google Scholar]
- Politis I., Fegeros K., Nitsch S., Schatzmayr G., Kantas D. Use of Trichosporon mycotoxinivorans to suppress the effects of ochratoxicosis on the immune system of broiler chicks. Brit. Poult. Sci. 2005;46:58–65. doi: 10.1080/00071660400023904. [DOI] [PubMed] [Google Scholar]
- Raju M., Devegowda G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin) Brit. Poult. Sci. 2000;41:640–650. doi: 10.1080/713654986. [DOI] [PubMed] [Google Scholar]
- Richard J. Union, MO: Romer Labs; 2000. Romer Labs Guide to Mycotoxins. Vol. 2: Sampling and Sample Preparation for Mycotoxin Analysis. [Google Scholar]
- Richardson K. E., Hamilton P. B. Enhanced production of pancreatic digestive enzymes during aflatoxicosis in egg-type chickens. Poult. Sci. 1987;66:640–644. doi: 10.3382/ps.0660640. [DOI] [PubMed] [Google Scholar]
- Riley R. T., An N. H., Showker J. L., Yoo H. S., Norred W. P., Chamberlain W. J., Wang E., Merrill A. H., Motelin G., Beasley V. R., Haschek W. M. Alteration of tissue and serum sphinganine to sphingosine ratio: An early biomarker of exposure to fumonisin-containing feeds in pigs. Toxicol. Appl. Pharmacol. 1993;118:105–112. doi: 10.1006/taap.1993.1015. [DOI] [PubMed] [Google Scholar]
- Ramos A. J., Hernández E., Plá-Delfina J. M., Merino M. Intestinal absorption of zearalenone and in vitro study of non-nutritive sorbent materials. Intl. J. Pharm. 1996;128:129–137. [Google Scholar]
- Ringot D., Chango A., Schneider Y. J., Larondelle Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006;159:18–46. doi: 10.1016/j.cbi.2005.10.106. [DOI] [PubMed] [Google Scholar]
- Robinson A., Johnson N. M., Strey A., Taylor J. F., Marroquin-Cardona A., Mitchell N. J., Afriyie-Gyawu E., Ankrah N. A., Williams J. H., Wang J. S., Jolly P. E., Nachman R. J., Phillips T. D. Calcium montmorillonite clay reduces urinary biomarkers of fumonisin B(1) exposure in rats and humans. Food Addit. Contam. A. Chem. Anal. Control Expo. Risk Assess. 2012;29:809. doi: 10.1080/19440049.2011.651628. doi: 10.1080/19440049.2011.651628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues I., Handl J., Binder E. M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food. Addit. Contam. B. 2011;4:168–179. doi: 10.1080/19393210.2011.589034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues I., Chin L. J. A comprehensive survey on the occurrence of mycotoxins in maize dried distillers’ grain and solubles sourced worldwide. World Mycotoxin J. 2012;5:83–88. [Google Scholar]
- Rodrigues I., Naehrer K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins. 2012;4:663–675. doi: 10.3390/toxins4090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent T., Ribonnet L., Kolosova A., Garsou S., Schaut A., de Saeger S., van Peteghem C., Larondelle Y., Pussemier L., Schneider Y. J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol. 2008;46:813–841. doi: 10.1016/j.fct.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Schaeffer J. L., Tyczkowski J. J., Hamilton P. B. Alterations in carotenoid metabolism during ochratoxicosis in young broiler chickens. Poult. Sci. 1987;66:318–324. doi: 10.3382/ps.0660318. [DOI] [PubMed] [Google Scholar]
- Schatzmayr G., Heidler D., Fuchs E., Nitsch S., Mohnl M., Täiubel M., Loibner A. P., Braun R., Binder E. M. Investigation of different yeast strains for the detoxification of ochratoxin A. Mycotoxin Res. 2003;19:124–128. doi: 10.1007/BF02942950. [DOI] [PubMed] [Google Scholar]
- Schatzmayr G., Täubel M., Vekiru E., Moll D., Schatzmayr D., Binder E. M., Krska R., Loibner A. P. Detoxification of mycotoxins by biotransformation. In: Barug D., Bhatnagar D., van Egmond H. P., van der Kamp J. W., van Osenbruggen W. A., Visconti A., editors. The Mycotoxin Factbook, Food and Feed Topics. Wageningen, The Netherlands: Wageningen Academic Publishers; 2006a. pp. 363–375. [Google Scholar]
- Schatzmayr G., Zehner F., Täubel M., Schatzmayr D., Klimitsch A., Loibner A. P., Binder E. M. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 2006b;50:543–551. doi: 10.1002/mnfr.200500181. [DOI] [PubMed] [Google Scholar]
- Schatzmayr G., Streit E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013;6(3):213–222. [Google Scholar]
- Schiefer H. Mycotoxicoses of domestic animals and their diagnosis. Can. J. Physiol. Pharmacol. 1990;68:987–990. doi: 10.1139/y90-150. [DOI] [PubMed] [Google Scholar]
- Schumacher R., Ksrka R., Weingaertner J., Grasserbauer M. Interlaboratory comparison study for the determination of the Fusarium mycotoxins deoxynivalenol in wheat and zearenone in maize using different methods. Fresenius J Anal. Chem. 1997;359:510–515. [Google Scholar]
- Scott P. M. Industrial and farm detoxification processes for mycotoxins. Revue de Médecine Vétérinaire. 1998;149:543–548. [Google Scholar]
- Streit E., Naehrer K., Rodrigues I., Schatzmayr G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agri. 2013a;93:2892–2899. doi: 10.1002/jsfa.6225. [DOI] [PubMed] [Google Scholar]
- Streit E., Schwab C., Sulyok M., Naehrer K., Krska R., Schatzmayr G. Multi-mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins. 2013b;5:504–523. doi: 10.3390/toxins5030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulyok M., Berthiller F., Krska R., Schuhmacher R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006;20:2649–2659. doi: 10.1002/rcm.2640. [DOI] [PubMed] [Google Scholar]
- Tran S. T., Auvergne A., Benard G., Bailly J. D., Tardieu D., Babile R., Guerre P. Chronic effects of fumonisin B1 on ducks. Poult. Sci. 2005;84:22–28. doi: 10.1093/ps/84.1.22. [DOI] [PubMed] [Google Scholar]
- Varga E., Glauner T., Köppen R., Mayer K., Sulyok M., Schuhmacher R., Krska R., Berthiller F. Stable isotope dilution assay for the accurate determination of mycotoxins in maize by UHPLC-MS/MS. Anal. Bioanal. Chem. 2012;402:2675–2686. doi: 10.1007/s00216-012-5757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga E., Malachova A., Schwartz H., Krska R., Berthiller F. Survey of deoxynivalenol and its conjugates deoxynivalenol-3-glucoside and 3-acetyl-deoxynivalenol in 374 beer samples. Food Addit. Contam. A. 2013a;30:137–146. doi: 10.1080/19440049.2012.726745. [DOI] [PubMed] [Google Scholar]
- Varga E., Glauner T., Berthiller F., Krska R., Schuhmacher R., Sulyok M. Development and validation of a (semi-)quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013b;405:5087–5104. doi: 10.1007/s00216-013-6831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga I., Vanyi A. Interaction of T-2 fusariotoxin with anticoccidial efficacy of lasalocid in chickens. Int. J. Parasit. 1992;22:523–525. doi: 10.1016/0020-7519(92)90154-d. [DOI] [PubMed] [Google Scholar]
- Vekiru E., Fruhauf S., Sahin M., Ottner F., Schatzmayr G., Krska R. Investigation of various adsorbents for their ability to bind Aflatoxin B1. Mycotoxin Res. 2007;23:27–33. doi: 10.1007/BF02946021. [DOI] [PubMed] [Google Scholar]
- Vekiru E., Hametner C., Mitterbauer R., Rechthaler J., Adam G., Schatzmayr G., Krska R., Schuhmacher R. Cleavage of zearalenone by Trichosporon mycotoxinivorans to a novel nonestrogenic metabolite. Appl. Env. Microbiol. 2010;76:2353–2359. doi: 10.1128/AEM.01438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma J., Johri T. S., Swain B. K. Effect of aflatoxin, ochratoxin and their combination on protein and energy utilisation in white leghorn laying hens. J. Sci. Food Agric. 2007;87:760–764. [Google Scholar]
- Warth B., Sulyok M., Fruhmann P., Mikula H., Berthiller F., Schuhmacher R., Hametner C., Abia W. A., Adam G., Fröhlich J., Krska R. Development and validation of a rapid multi-biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Commun. Mass Spectrom. 2012;26:1533–1540. doi: 10.1002/rcm.6255. [DOI] [PubMed] [Google Scholar]
- Wang E., Norred W. P., Bacon C. W., Riley R. T., Merrill A. H., Jr Inhibition of sphingolipid biosynthesis by fumonisins: Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- Weibking T. S., Ledoux D. R., Bermudez A. J., Turk J. R., Rottinghaus G. E., Wang E., Merrill A. H., Jr Effects of feeding Fusarium moniliforme culture material, containing known levels of fumonisin B1, on the young broiler chick. Poult. Sci. 1993;72:456–466. doi: 10.3382/ps.0720456. [DOI] [PubMed] [Google Scholar]
- Weibking T. S., Ledoux D. R., Bermudez A. J., Turk J. R., Rottinghaus G. E. Effects on turkey poults of feeding Fusarium moniliforme M-1325 culture material grown under different environmental conditions. Avian Dis. 1995;39:32–38. [PubMed] [Google Scholar]
- Whitaker T. B. Sampling foods for mycotoxins. Food Addit. Contam. 2006;23:50–61. doi: 10.1080/02652030500241587. [DOI] [PubMed] [Google Scholar]
- Wu F., Bhatnagar D., Bui-Klimke T., Carbone I., Hellmich R., Munkvold G., Paul P., Payne G., Takle E. Climate change impacts on mycotoxin risks in US maize. World Mycotoxin J. 2011;4:79–93. [Google Scholar]
- Xu L., Eicher S., Applegate T. J. Effect of increasing dietary concentrations of corn naturally-contaminated with deoxynivalenol on broiler and turkey poult performance and response to LPS. Poult. Sci. 2011;90:2766–2774. doi: 10.3382/ps.2011-01654. [DOI] [PubMed] [Google Scholar]
- Yarru L. P., Settivari R. S., Gowda N.K.S., Antoniou E., Ledoux D. R., Rottinghaus G. E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult Sci. 2009a;88:12, 2620–2627. doi: 10.3382/ps.2009-00204. [DOI] [PubMed] [Google Scholar]
- Yarru L. P., Settivari R. S., Antoniou E., Ledoux D. R., Rottinghaus G. E. Toxicological and gene expression analysis of the impact of aflatoxin B1 on hepatic function of male broiler chicks. Poult Sci. 2009b;88:360–371. doi: 10.3382/ps.2008-00258. [DOI] [PubMed] [Google Scholar]
- Yunus A. W., Razzazi-Fazeli E., Bohm J. Aflatoxin B1 in affecting broilers performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins. 2011;3:566–590. doi: 10.3390/toxins3060566. [DOI] [PMC free article] [PubMed] [Google Scholar]