Abstract
The effect of commercial selection on the growth, efficiency, and yield of broilers was studied using 2 University of Alberta Meat Control strains unselected since 1957 and 1978, and a commercial Ross 308 strain (2005). Mixed-sex chicks (n = 180 per strain) were placed into 4 replicate pens per strain, and grown on a current nutritional program to 56 d of age. Weekly front and side profile photographs of 8 birds per strain were collected. Growth rate, feed intake, and measures of feed efficiency including feed conversion ratio, residual feed intake, and residual maintenance energy requirements were characterized. A nonlinear mixed Gompertz growth model was used to predict BW and BW variation, useful for subsequent stochastic growth simulation. Dissections were conducted on 8 birds per strain semiweekly from 21 to 56 d of age to characterize allometric growth of pectoralis muscles, leg meat, abdominal fat pad, liver, gut, and heart. A novel nonlinear analysis of covariance was used to test the hypothesis that allometric growth patterns have changed as a result of commercial selection pressure. From 1957 to 2005, broiler growth increased by over 400%, with a concurrent 50% reduction in feed conversion ratio, corresponding to a compound annual rate of increase in 42 d live BW of 3.30%. Forty-two-day FCR decreased by 2.55% each year over the same 48-yr period. Pectoralis major growth potential increased, whereas abdominal fat decreased due to genetic selection pressure over the same time period. From 1957 to 2005, pectoralis minor yield at 42 d of age was 30% higher in males and 37% higher in females; pectoralis major yield increased by 79% in males and 85% in females. Over almost 50 yr of commercial quantitative genetic selection pressure, intended beneficial changes have been achieved. Unintended changes such as enhanced sexual dimorphism are likely inconsequential, though musculoskeletal, immune function, and parent stock management challenges may require additional attention in future selection programs.
Keywords: broiler, genetic change, efficiency, yield dynamics
INTRODUCTION
A profound change in the productivity of the broiler chicken industry has been achieved via intentional genetic selection through traditional quantitative techniques (Hunton, 2006). Between 1960 and 2004, the US consumer price index for poultry products increased at half the rate of all other products (USDA, Economic Research Service, 2004), due to improvements in growth and efficiency. This has likely been a major factor contributing to higher per capita consumption of chicken meat between 1950 (9.4 kg) and 2005 (39.2 kg; USDA, Economic Research Service, 2014). Early on, limited statistical capabilities forced geneticists to focus on economically important parameters that were easily measured and highly heritable, such as BW, feed consumption, feed conversion ratio (FCR), and yield (Hunton, 2006). In response to changing consumer demands, product development has driven genetic selection, with concomitant unintended effects, including increased skeletal defects (Lilburn, 1994; Rath et al., 2000), metabolic disorders (Scheele, 1997; Olkowski, 2007), and altered immune function (Cheema et al., 2003). In 1962, 83% of broilers were marketed as whole birds, 15% as cut-up or parts, and 2% as further processed products (National Chicken Council, 2011). In 2005 only 11% of broilers were marketed as whole birds, 43% as cut-up or parts, and 46% as further processed products (National Chicken Council, 2011). As statistical capabilities expanded, more balanced selection programs became achievable (Emmerson, 1997).
Growth rate and efficiency (Sherwood, 1977; Marks, 1979; Chambers et al., 1981; Havenstein et al., 1994a, 2003b), and changes in the yield of specific, economically important portions (Chambers et al., 1981; Havenstein et al., 1994b, 2003a) have increased dramatically since the 1940s. Although some of these changes are due to environmental factors, 85 to 90% has been attributed to genetics (Sherwood, 1977; Havenstein et al., 1994a,b, 2003a,b).
Measuring both intended and unintended consequences can be aided by comprehensive new metrics and by looking at results differently. For example, efficiency parameters such as residual feed intake (RFI) and residual maintenance requirements (RMEm) better describe the biological efficiency of an animal than traditional FCR (Romero et al., 2009). Adapting an approach described by Motulsky and Ransnas (1987), the current project presents a new way to statistically compare nonlinear allometric development to provide a more comprehensive view of genetic change than traditional point-in-time comparisons. Finally, in addition to using traditional and new metrics to evaluate the effects of selection, a qualitative pictorial record was undertaken to supplement the quantitative record.
In the current experiment, 2 unselected meat control strains representative of broilers in 1957 or 1978 were compared with a commercial broiler (2005) to examine changes in growth, efficiency, and yield resulting from commercial selection pressures.
MATERIALS AND METHODS
Experimental Design
All experimental procedures were approved by the Animal Care and Use Committee–Livestock of the University of Alberta. The current study was conducted to determine the effect of commercial selection on the growth, efficiency, and yield of broilers. Three treatments (strains) were used in a completely randomized design.
Strains
The current experiment included 2 University of Alberta Meat Control strains unselected since 1957 and 1978 (AMC-1957 and AMC-1978, respectively) and a 2005 commercial Ross 308 broiler (Aviagen North America Inc., Huntsville, AL). The AMC-1957 and AMC-1978 strains have been maintained unselected at the University of Alberta Poultry Research Centre, Edmonton, Alberta, since 1989. Source parent flocks were all 46 wk of age.
The AMC-1957 strain was developed from 3 commercially available meat-type chickens and an experimental strain in 1957 at the Experimental Farm in Ottawa, Ontario, Canada, for research purposes by Ed Merritt, Robb Gowe, and Allan Grunder (Merritt and Gowe, 1962). This strain was the progenitor of the Athens-Canadian Randombred (ACRB) control strain maintained at the University of Georgia in Athens, Georgia (Hess, 1962). More than 100 peer-reviewed papers have been published using the Athens-Canadian strain as a representative of meat chickens of the 1950s. Nine broiler breeder companies contributed sire and dam strain chicks to develop 2 AMC-1978 lines (strain 20 = sire, strain 30 = dam; Chambers et al., 1984). The present study used the dam line.
Management
At hatch, mixed-sex broilers from each strain (n = 180 per strain) were individually wing banded and grown in 12 floor pens (n = 4 per strain) to 56 d of age. Birds were housed at a stocking density of 4.07 birds per m2 at the beginning of the experiment, decreasing to 1.90 birds per m2 at 56 d as birds were removed for sampling. All birds were fed nutritionally complete commercial-type diets ad libitum, based on contemporary recommendations for the Ross 308 (Aviagen Inc., 2003). A starter diet (3,068 kcal/kg; 23% CP) was fed from 0 to 14 d; a grower diet (3,152 kcal/kg; 20.15% CP) was fed from 15 to 28 d; and a finisher diet (3,196 kcal/kg 19% CP) was fed from 29 to 56 d.
Data Collection
Body weight and feed intake data were recorded weekly. Two birds per pen were randomly selected and identified at hatch for photographic representation of growth. Frontal and side aspects were photographed weekly for each bird. A single bird from each strain was ultimately chosen for the current paper on the basis of consistent photographic quality. A standard background was used in all photographs at each age to maintain a consistent perspective throughout the study, which was validated using ImageJ (National Institutes of Health, http://imagej.nih.gov/ij/). Twice per week from 21 to 56 d of age, 8 birds per treatment were randomly selected, placed in crates overnight, and after 11.5 h of feed withdrawal (range 10 to 13.5 h), were killed in a federally inspected processing plant using the method described by Schneider et al. (2012). Pectoralis major, pectoralis minor, drums, thighs, wings, abdominal fat pad (adhering to gizzard and abdominal wall, liver, gastrointestinal tract (1 cm above crop to the distal end of the colon, not including the bursa), and heart were dissected and weighed. Total breast muscle weight was calculated as pectoralis major + pectoralis minor weight. Sex was determined at dissection.
Empirical Models and Statistical Analysis
Feed intake, BW, and feed conversion data were analyzed as a 1-way ANOVA using the MIXED procedure (SAS Institute Inc., Cary, NC). Data were analyzed independently by age. Pairwise separation of means was conducted using the Diff option of the MIXED procedure, and means were reported as different when P < 0.05. The Bayesian information criterion was used to determine whether treatment-specific variance estimation was warranted. Standard errors of the mean were pooled unless the Bayesian information criterion was lower for models with treatment-specific variance estimates.
BW Model.
Coefficients describing BW curves were estimated with the NLMIXED procedure of SAS, using a nonlinear mixed Gompertz model (Wang and Zuidhof, 2004). The model had the form
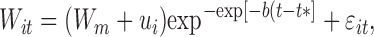 |
[1] |
where Wit was the expected BW (kg) of individual i at age t (d); Wm was the BW asymptote (mature weight) of all birds within a treatment; ui was a random deviation of mature BW of the individual i from the average mature BW [ui~N(0,σu2), and independent of ϵit]; b was a maturation rate (d−1); t* was the time (d) at which growth rate was maximum [t*=ln(−ln(W0/Wm)]/b; initial chick weight,W0, was measured]; and ϵit was the residual error of individual BW measurements.
Allometric Yield Model.
Allometric yield curves were estimated for each treatment to predict pectoralis minor, pectoralis major, total breast, drum, abdominal fat pad, liver, heart, and gut weights, using the equation
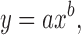 |
[2] |
where y was the weight of the carcass part (g), x was eviscerated BW (g), and a (scale variable) and b were coefficients estimated using the NLIN procedure of SAS. Biologically, the value of b is related to maturation rate. Values for b were <1 for early maturing tissues; ≈1 for tissues that matured at a rate similar to the body as a whole; and >1 for late maturing tissues.
Nonlinear Analysis of Covariance.
The null hypothesis of equality of allometric curves was evaluated using a paired F-test procedure described by Motulsky and Ransnas (1987). First, curves were estimated for 2 treatments (tmt) separately using the NLIN procedure of SAS. The overall sum of squares (SS) from this analysis was calculated: SSsep = SStmt1 + SStmt2. The total number of degrees of freedom was added: dfsep = dftmt1 + dftmt2. Next, data from the 2 treatments being compared were pooled and a single allometric curve was estimated. The total sum of squares (SSpool) was determined with dfpool degrees of freedom. To determine whether the separate fit was significantly better than the pooled fit, an F ratio was calculated:
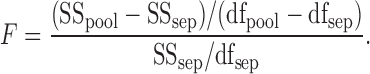 |
[3] |
A large F-value (corresponding low P-value) indicated that the separate fit explained variation in the data more appropriately than the pooled fit. Thus, where P < 0.05 the hypothesis that the curves for the 2 treatments were equal was rejected, and allometric curves were reported as different, or having shifted.
Efficiency.
Comparisons of efficiency were determined by FCR (feed intake/BW gain; g/g), residual feed intake (kcal/d), and residual maintenance requirements (kcal/kgb per d). A nonlinear mixed model with the form
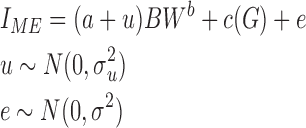 |
[4] |
was used to determine nutrient requirement coefficients for all strains together, where IME was intake of ME (kcal/d); BW was the average BW (kg) for the same period over which IME was measured; and G was gain in BW (g/d). The coefficient a was the average maintenance requirement (kcal/kgb) for all pens; u was the random pen deviation from the average maintenance requirement (kcal/kgb) such that a + u was the estimated maintenance ME requirement (MEm) for each pen; and c was the energy requirement for BW gain (kcal/g). Residual feed intake was calculated for each pen as RFI=IME − E, where E was the expected ME intake, based on the energy partitioning model. To account for the effect of feed intake on diet-induced thermogenesis, a source of bias for RFI estimates, a regression of MEm for each pen on feed intake was performed, and strain differences in the residual maintenance requirement (RMEm) were evaluated by ANOVA using the MIXED procedure of SAS.
RESULTS AND DISCUSSION
BW
A front profile pictorial record at 0, 28, and 56 d of age along with average mixed-sex BW for each strain is presented in Figure 1. Although all chicks were from breeder flocks of the same age, chick weights at hatch were lowest in the AMC-1957 strain and highest in the 2005 strain (Figure 2). The AMC-1978 strain was intermediate to, and different from both other strains. By 4 wk of age, the relative BW of the AMC-1978 strain reached a plateau at approximately 43% of the 2005 strain BW, and the relative BW of the AMC-1957 strain reached a plateau at approximately 21% of the 2005 strain BW (Figure 2). Although nutrient requirements for the 3 strains may be different, genetic selection accounted for 85 to 90% of the increase in BW from 1957 to 1991 (Havenstein et al., 1994a).
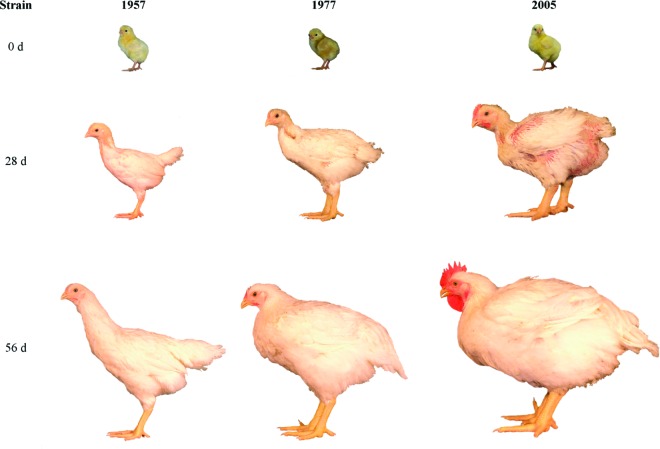
Figure 1.
Age-related changes in size (mixed-sex BW and front view photos) of University of Alberta Meat Control strains unselected since 1957 and 1978, and Ross 308 broilers (2005). Within each strain, images are of the same bird at 0, 28, and 56 d of age. Color version available in the online PDF.
Figure 2.
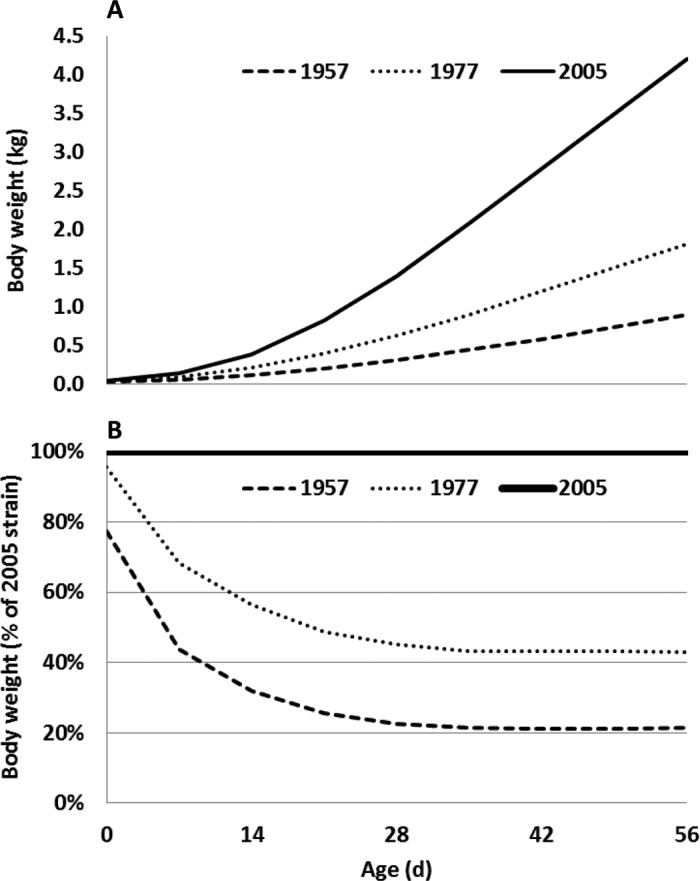
Absolute (panel A) and relative (panel B) BW of mixed sex University of Alberta Meat Control unselected since 1957 and 1978, and Ross 308 broilers (2005).
The 42-d BW of the AMC-1957 strain was 586 g, which was approximately 45 g less than the 1959 average of the male and female BW reported by Merritt and Gowe (1962). The 42-d BW of the AMC-1978 strain was 1,205 g, which was approximately 130 g less than the 1,336-g average of the male and female BW in the progenitor strain reported by Chambers et al. (1984). However, the 56-d weights of the AMC-1957 broilers in the present study were consistent with ACRB broiler weights reported by Havenstein et al. (2003b).
Gompertz growth parameters for a nonlinear mixed model (Table 1) predicted mature weights (Wm) of 2,373, 3,602, and 7,110 g for the AMC-1957, AMC-1978, and 2005 strains, respectively. Interestingly, in 1959, the mature prepubertal weight at 147 d of age reported for the AMC-1957 strain was 2,383 g (Merritt and Gowe, 1962). Conversely, rates of maturing (b) increased with selection over the last 50 yr. The SD in mature weights (σu) increased on an absolute basis since 1978, but σu as a percentage of mature weight decreased from 18% in 1957 to 12.3% in 2005. Average daily gain peaked in the AMC-1978 and 2005 strains from 43 to 49 d of age, at 43.7 and 104 g/d, respectively (Table 2). The highest ADG observed in the AMC-1957 strain was from 50 to 56 d of age, at 23.0 g/d. From 22 to 56 d of age, ADG relative to the 2005 strain in the AMC-1957 and AMC-1978 strains reached a plateau at approximately 20 and 41%, respectively. Peak average daily gains were predicted by the t* parameter of the Gompertz model to occur at 53.5, 44.4, and 40.4 d for AMC-1957, AMC-1978, and 2005, respectively (Table 1). The Gompertz parameters, complete with the estimates of variance, provide all the necessary information to facilitate subsequent stochastic simulation of growth.
Table 1. Parameter estimates and associated standard errors for nonlinear mixed Gompertz growth model1 for mixed sex University of Alberta Meat Control (AMC) strains unselected since 1957 and 1978, and Ross 308 broilers (2005).
| Parameter | AMC-1957 | SEM | AMC-1978 | SEM | 2005 | SEM |
|---|---|---|---|---|---|---|
| Wm, kg | 2.373 | 0.028 | 3.602 | 0.032 | 7.110 | 0.065 |
| σu, kg | 0.428 | 0.144 | 0.471 | 0.157 | 0.871 | 0.294 |
| b, d−1 | 0.0273 | 0.0002 | 0.0336 | 0.0002 | 0.0403 | 0.0002 |
| σ, kg | 0.0167 | 0.0035 | 0.0287 | 0.0058 | 0.0656 | 0.0136 |
| t*, calculated | 53.5 | 44.4 | 40.4 |
1The nonlinear mixed model was of the form Wit= (Wm+ui)exp−exp[-b(t−t*)]; t*=ln[−ln(W0/Wm)]/b, where Wit was the expected BW (kg) of individual i at age t (d); Wm was the BW asymptote of all birds within a treatment; ui was a random deviation of mature BW of the individual i from the average mature BW [ui~N(0,σu2), and independent of ϵit]; b was a maturation rate (d−1); t* was the time (d) at which growth rate was maximum {t*=ln[−ln(W0/Wm)]/b; initial chick weight, W0, was measured}; and ϵit was the residual error of individual BW measurements; W0 was 0.032, 0.042, and 0.044 kg for the AMC-1957 line, AMC-1978 line, and 2005 strains, respectively.
Table 2. Average daily gain (g/d) of University of Alberta Meat Control (AMC) strains unselected since 1957 and 1978, and Ross 308 broilers (2005).
| Age (d) | AMC-1957 | AMC-1978 | 2005 | SEM |
|---|---|---|---|---|
| 0 to 7 | 4.6c | 8.9b | 15.9a | 0.155 |
| 8 to 14 | 8.7c | 17.2b | 34.5a | 0.344 |
| 15 to 21 | 12.5c | 26.1b | 62.4a | 0.531 |
| 22 to 28 | 15.3c | 33.2b | 81.9a | 0.648 |
| 29 to 35 | 18.6c | 38.0b | 96.3a | 0.859 |
| 36 to 42 | 20.6c | 42.5b | 99.6a | 1.040 |
| 43 to 49 | 21.8c | 43.7b | 104.0a | 1.450 |
| 50 to 56 | 23.0c | 42.0b | 101.1a | 1.648 |
a–cMeans within rows with no common superscript differ significantly (P < 0.05).
A series of side profile images of each strain at 0, 28, and 56 d of age provides qualitative insight into changes in broiler posture over time (Figure 3). Generally, AMC-1957 birds had a more erect posture with a more extended neck. Similar to the changes described by Abourachid (1993) in broad-breasted turkeys, and Corr et al. (2003a) in modern broilers, posture has likely been altered by birds managing their center of gravity by retracting the head toward the body. Similar to Corr et al. (2003b) and Paxton et al. (2013), we qualitatively observed increased stance width in the 2005 broiler strain (Figure 1).
Figure 3.
Age-related changes in size of University of Alberta Meat Control strains unselected since 1957 and 1978, and Ross 308 broilers (2005). Within each strain, side profile images are of the same bird at 0, 28, and 56 d of age. Color version available in the online PDF.
Indicators of Efficiency
Cumulative feed conversion ratios from 0 to 42 d of age were 2.88, 1.90, and 1.67 for the AMC-1957, AMC-1978, and 2005 strains, respectively (Table 3). By 42 d of age, production of 1 g of chicken by the 2005 broiler required 1.208 less g of feed than the AMC-1957 broiler. Cumulative FCR of the AMC-1957 strain followed a different trend than that of the AMC-1978 and 2005 strains. Small daily gains in the 1957 strain combined with high feed disappearance contributed to high feed conversion rates. From 8 to 14 d, FCR of the AMC-1957 broilers was 3.64, some of which was due to feed wastage. With a change in feed form from crumble to pellet at 14 d, FCR in the AMC-1957 strain decreased to 3.10 from 15 to 21 d, decreasing to 2.53 from 36 to 42 d, after which FCR increased. The observed feed wastage skewed the FCR values; the expected FCR pattern would be parallel to that of the other strains. Feed wastage by ACRB broilers, which are related to the AMC-1957 strain, has been previously reported (Havenstein et al., 1994a, 2003b). Genetic selection, in addition to increasing physiological efficiency, may have also inadvertently changed behaviors that contribute to feed wastage. In both the AMC-1978 and 2005 strains, cumulative FCR increased from week to week.
Table 3. Cumulative feed conversion ratio (g of feed:g of BW gain) of University of Alberta Meat Control (AMC) strains unselected since 1957 and 1978, and Ross 308 broilers (2005).
| Age (d) | AMC-1957 | SEM | AMC-1978 | SEM | 2005 | SEM | P-value |
|---|---|---|---|---|---|---|---|
| 0 to 7 | 2.553a | 0.258 | 1.382b | 0.030 | 1.108c | 0.026 | <0.0001 |
| 0 to 14 | 3.300a | 0.362 | 1.506b | 0.019 | 1.275c | 0.017 | <0.0001 |
| 0 to 21 | 3.188a | 0.170 | 1.608b | 0.013 | 1.379c | 0.006 | <0.0001 |
| 0 to 28 | 3.084a | 0.093 | 1.706b | 0.019 | 1.483c | 0.008 | <0.0001 |
| 0 to 35 | 3.003a | 0.118 | 1.832b | 0.030 | 1.573c | 0.012 | <0.0001 |
| 0 to 42 | 2.882a | 0.101 | 1.899b | 0.026 | 1.674c | 0.012 | <0.0001 |
| 0 to 49 | 2.871a | 0.103 | 2.018b | 0.017 | 1.808c | 0.018 | <0.0001 |
| 0 to 56 | 2.854a | 0.096 | 2.135b | 0.037 | 1.918c | 0.015 | <0.0001 |
a–cMeans within rows with no common superscript differ significantly (P < 0.05).
The reduction in FCR in the modern strain may be related to lower maintenance requirements because rapid growth increases the proportion of energy used for growth relative to maintenance (Latshaw and Moritz, 2009). At 56 d of age, gut weight as a percentage of live BW was 73% higher in the AMC-1957 broiler (9.0%) than the 2005 broiler (5.2%). Allometric analysis of gut yield data provided little evidence that commercial selection pressures have led to a change in gut yield (data not shown). Metabolically, the gut is a very active tissue, elevating energy expenditure (Spratt et al., 1990), and therefore contributes substantially to maintenance requirements. Modern broilers have greater absolute gut mass than unselected birds (Jackson and Diamond, 1996; Schmidt et al., 2009). Selection for increased growth and efficiency does not increase digestive function or nutrient uptake per unit of gut mass; however, increased digestive and absorptive capacity in modern broilers is a function of increased gut size (Jackson and Diamond, 1996).
From 22 to 56 d of age, breast conversion ratios (g of feed intake to g of breast meat) averaged 28.2, 17.0, and 9.4 g/g in the AMC-1957, AMC-1978, and 2005 broiler strains, respectively (Figure 4). This dramatic decrease was the synergistic result of increased growth rate, feed efficiency, and yield.
Figure 4.
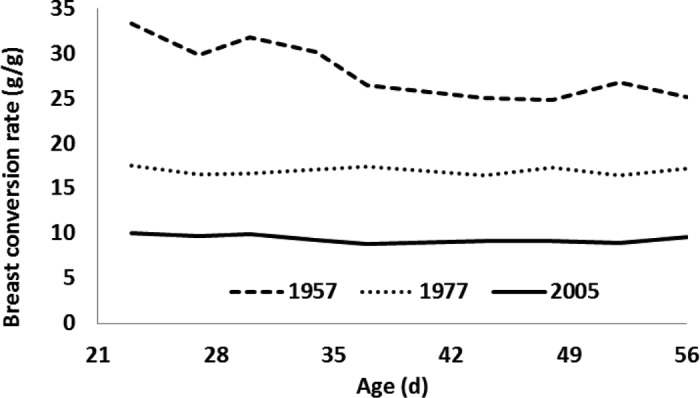
Breast conversion rate (g of feed/g of breast meat) of University of Alberta Meat Control unselected since 1957 and 1978, and Ross 308 broilers (2005).
Coefficients for the nonlinear mixed model for energy partitioning produced the following equation for predicting ME intake:
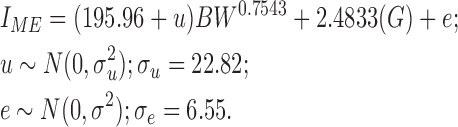 |
For every unit of metabolic BW (kg0.7543), the birds in the current study required 196 kcal of ME for maintenance. Maintenance ME requirements include the heat increment of feeding. The SD in this requirement among individual pens of birds was approximately 23 kcal/kg0.7543. At a pen level, the SD of the residual error was 6.55 kcal/kg0.7543. For individual broiler breeder hens, Romero et al. (2009) reported SD of maintenance requirements ranging from 21.3 to 58.3 kcal/kg0.54, which was very similar to the variance in maintenance requirements in the current analysis. Residual feed intake was higher in the AMC-1957 strain, meaning this strain was less biologically efficient than the AMC-1978 and 2005 strains, which did not significantly differ from each other (Table 4). Part of the reduced efficiency in the AMC-1957 strain was due to excessive feed wastage relative to the other strains. In the AMC-1978 strain, RMEm was lower than in the AMC-1957 strain, but the 2005 strain was not significantly different from either unselected strain. Both RFI and RMEm are measures of biological efficiency as opposed to feed efficiency. In contrast to FCR, RFI and RMEm account for maintenance and production energy requirements. The use of RMEm is a refinement on RFI because it accounts for the heat increment required to support high levels of production (Romero et al., 2009). These new efficiency metrics provide a means to illuminate differences in biological efficiency that may lead to investigation of underlying physiological mechanisms affecting efficiency, such as electron leak in mitochondria (Bottje et al., 2002).
Table 4. Residual feed intake (RFI) and residual maintenance requirements (RMEm) of mixed-sex University of Alberta Meat Control (AMC) strains unselected since 1957 and 1978, and Ross 308 broilers (2005).
| Efficiency measure | AMC-1957 | AMC-1978 | 2005 | SEM | P-value |
|---|---|---|---|---|---|
| RFI, kcal/d | 14.19a | −7.05b | −1.67b | 2.414 | 0.0004 |
| RMEm, kcal/kg0.75 | 2.831a | −3.610b | 0.779ab | 1.408 | 0.028 |
a,bMeans within rows with no common superscript differ significantly (P < 0.05).
Yield Dynamics
Breast Yield.
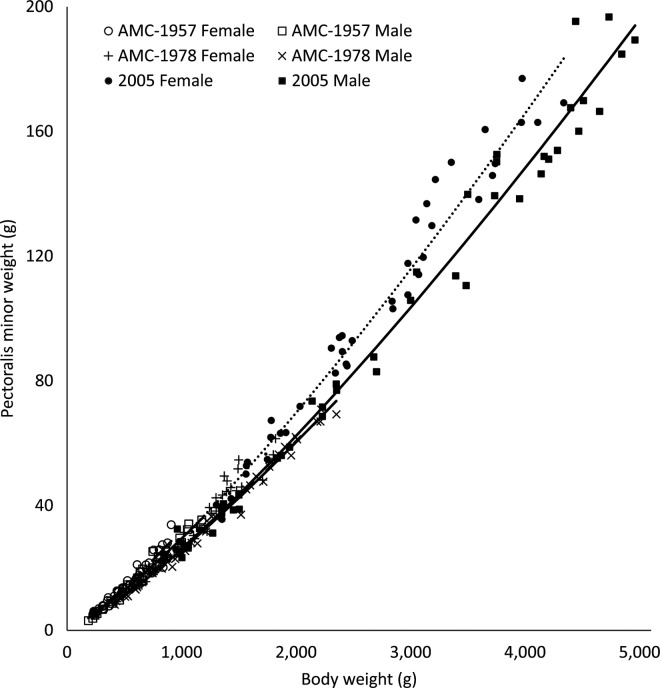
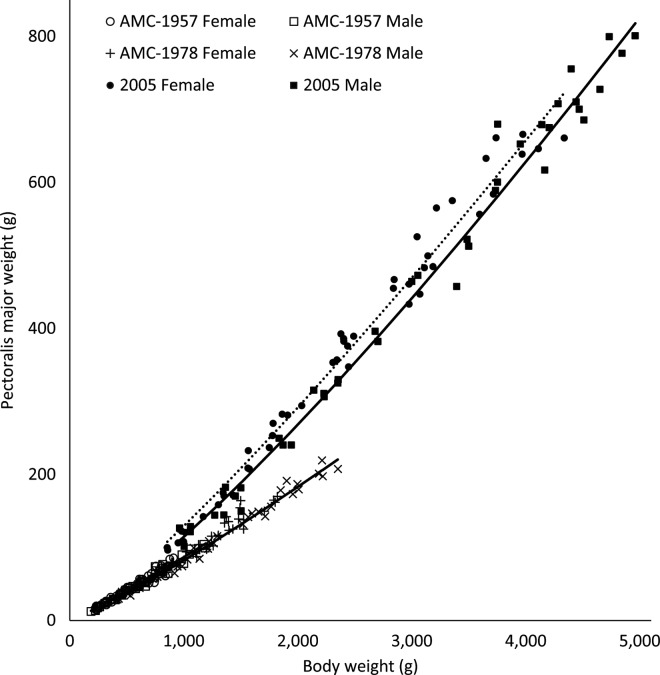
Expected weights of carcass parts can be calculated from the allometric coefficients presented in Table 5. Total breast meat yield was measured as the sum of weights of the pectoralis major and the pectoralis minor. Although the general shape of pectoralis minor allometric curves has not shifted dramatically as a result of commercial selection pressures (Figure 5), there has been a transformation in the genetic potential of total pectoralis major weights relative to BW in the 2005 strain relative to the AMC-1957 and AMC-1978 broiler strains (Figure 6). There was a downward shift in the pectoralis minor yield of AMC-1978 compared with AMC-1957 strain females (P = 0.0005) and males (P < 0.0001); no difference remained after further commercial selection during the period between 1978 and 2005. There was no increase in pectoralis major yield in the AMC-1978 strain compared with the AMC-1957 strain (P = 0.12 and P = 0.08 for females and males, respectively; Figure 6). In the 2005 strain, pectoralis major yield curves for both sexes were dramatically shifted upward relative to both the AMC-1957 and the AMC-1978 strains (P < 0.0005). Schmidt et al. (2009) reported a very similar change in the slope for breast yield in Ross 708 broilers relative to an experimental line unselected since the 1950s.
Table 5. Allometric1 yield coefficients of University of Alberta Meat Control (AMC) strains unselected since 1957 and 1978, and Ross 308 broilers (2005).
| AMC-1957 | AMC-1978 | 2005 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||||||||||||
| Item | a | b | a | b | a | b | a | b | a | b | a | b | |||||
| Major | 0.0402 | 1.115 | 0.0392 | 1.115 | 0.0267 | 1.169 | 0.0295 | 1.149 | 0.0398 | 1.171 | 0.0239 | 1.227 | |||||
| Minor | 0.0059 | 1.246 | 0.0076 | 1.196 | 0.0049 | 1.254 | 0.0055 | 1.224 | 0.0053 | 1.248 | 0.0047 | 1.249 | |||||
| Breast | 4.39E-5 | 1.147 | 4.56E-5 | 1.135 | 3.09E-5 | 1.189 | 3.42E-5 | 1.167 | 4.4E-5 | 1.186 | 2.85E-5 | 1.231 | |||||
| Drum | 0.042 | 1.11 | 0.0468 | 1.103 | 0.0469 | 1.092 | 0.0328 | 1.148 | 0.037 | 1.107 | 0.0444 | 1.09 | |||||
| Thigh | 0.0316 | 1.175 | 0.0493 | 1.107 | 0.0384 | 1.143 | 0.0307 | 1.171 | 0.0336 | 1.155 | 0.0277 | 1.177 | |||||
| Fat pad | 1.77E-7 | 2.64 | 6.6E-17 | 5.701 | 3.23E-5 | 1.916 | 0.0001 | 1.751 | 0.0001 | 1.668 | 0.0005 | 1.446 | |||||
| Gut | 0.3939 | 0.772 | 0.852 | 0.653 | 0.6952 | 0.686 | 0.5676 | 0.721 | 1.0935 | 0.631 | 0.8885 | 0.658 | |||||
| Heart | 0.0268 | 0.742 | 0.0379 | 0.705 | 0.0317 | 0.738 | 0.0275 | 0.776 | 0.0196 | 0.804 | 0.0312 | 0.763 | |||||
| Liver | 0.0609 | 0.823 | 0.078 | 0.783 | 0.127 | 0.725 | 0.176 | 0.678 | 0.0696 | 0.836 | 0.198 | 0.709 | |||||
1Allometric equation had the form y=axb, where y was the weight of the carcass part (g), x was eviscerated BW (g), and a and b were estimated coefficients.
Figure 5.
Allometric yield curves for pectoralis minor from University of Alberta Meat Control (AMC) unselected since 1957 and 1978, and Ross 308 (2005) broiler males (–) and females (···).
Figure 6.
Allometric yield curves for pectoralis major from University of Alberta Meat Control (AMC) unselected since 1957 and 1978, and Ross 308 (2005) broiler males (–) and females (···).
Sexual Dimorphism of Breast Yield.
There was no sex-related difference in pectoralis major yield in the AMC-1957 strain (P = 0.27). In contrast, the pectoralis major yield curve for females was shifted upward relative to males in both the AMC-1978 strain (P = 0.018) and the 2005 broiler strain (P = 0.0003). Most of the difference in total breast yield was due to changes in the genetic potential yield of the pectoralis major. From 1957 to 2005, pectoralis minor yield at 43 d of age increased by 30 and 37% in males and females, respectively; pectoralis major yield increased 79 and 85%. In 1991 versus 1957 ACRB broiler males at 43 d of age, there was a 15% increase in pectoralis minor yield and a 25% increase in pectoralis major; in females the differences were 23 and 38%, respectively (Havenstein et al., 1994b). Sexual dimorphism in breast yield is documented in modern broilers (Young et al., 2001; Kidd et al., 2004; Zuidhof, 2005), and the current data suggest sexual dimorphism in breast muscle has become more pronounced in modern strains as a result of divergent responses of females versus males to commercial selection pressures.
Leg Meat Yield.
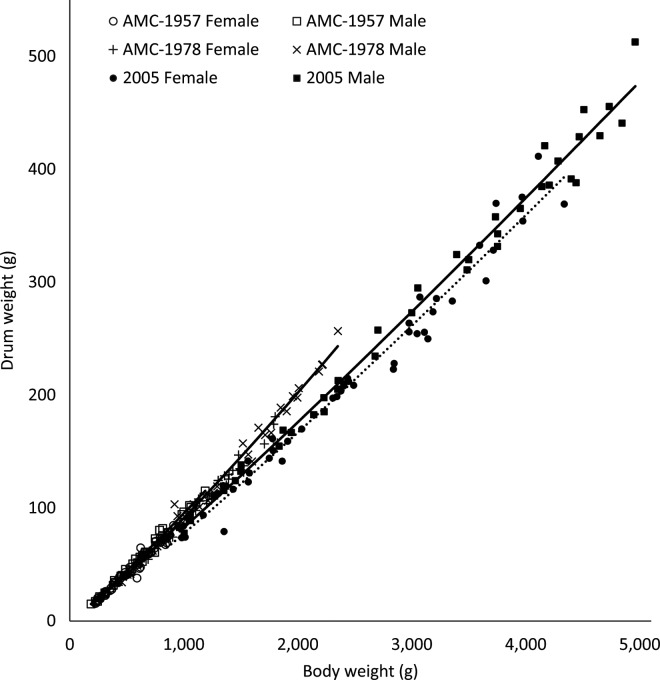
Yield curves for drums were lower in females compared with males (P < 0.0001), and in 2005 broilers compared with both AMC-1957 and AMC-1978 strain broilers (P < 0.0001; Figure 7). Sexual dimorphism in drum yield curves has been conserved.
Figure 7.
Allometric yield curves for drums from University of Alberta Meat Control (AMC) unselected since 1957 and 1978, and Ross 308 (2005) broiler males (–) and females (···).
Yield of Visceral Organs.
There was little change in gut yield as a result of commercial selection (data not shown). Whereas the gut yield of the 2005 female was shifted upward compared with the AMC-1978 (P = 0.03), overall gut yield did not differ between the AMC-1957 and 2005 strains (P = 0.07). Schmidt et al. (2009) investigated the complex relationship between gut length and mass relative to BW, and although age-related differences in gut weight were clear, there was little evidence for a shift in gut allometry. In the current study, allometric analysis showed that heart weights were higher in males compared with females (P < 0.0001). The heart yield curve in the AMC-1978 strain was shifted upward compared with the AMC-1957 strain (P = 0.004), but this difference did not persist in the 2005 broiler strain. Schmidt et al. (2009) reported a heart allometry shift in modern versus a heritage strain, with greater heart weights in the heritage strain, but only where BW was in the range of 502 to 1,207 g. In the current study, all data together contributed to a single test of the hypothesis, and the hypothesis that heart weight allometric curve had not shifted in the 2005 strain compared with the AMC-1957 strain could not be rejected (P = 0.4453).
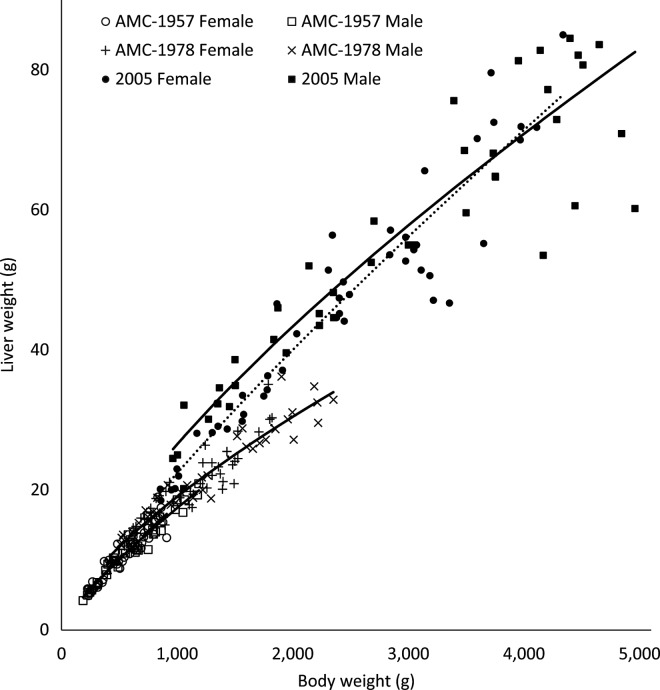
Whereas there were no sex differences in liver allometry, liver weights increased relative to BW in the AMC-1978 compared with the AMC-1957 strain, and again in the 2005 versus AMC-1978 strain (P < 0.0001; Figure 8). The liver is a major metabolic support organ, and it is not surprising that it has increased relative to body mass as a result of commercial selection pressure. An increase in proportional liver weight may facilitate and support increased growth rates in selected strains. The current result contrasts with Schmidt et al. (2009), who reported no change in liver allometry due to commercial selection pressure. However, that study compared Ross 708 broilers to the progeny of an unselected New Hampshire male line and a Columbian female line. Feed withdrawal for 12 h reduced liver weights by 0.1% of total BW, which was equivalent to a 4.5% shrinkage of the liver (Schettino et al., 2006). In the current study, the effect of feed withdrawal was assumed to affect all treatments similarly.
Figure 8.
Allometric yield curves for livers from University of Alberta Meat Control (AMC) unselected since 1957 and 1978, and Ross 308 (2005) broiler males (–) and females (···).
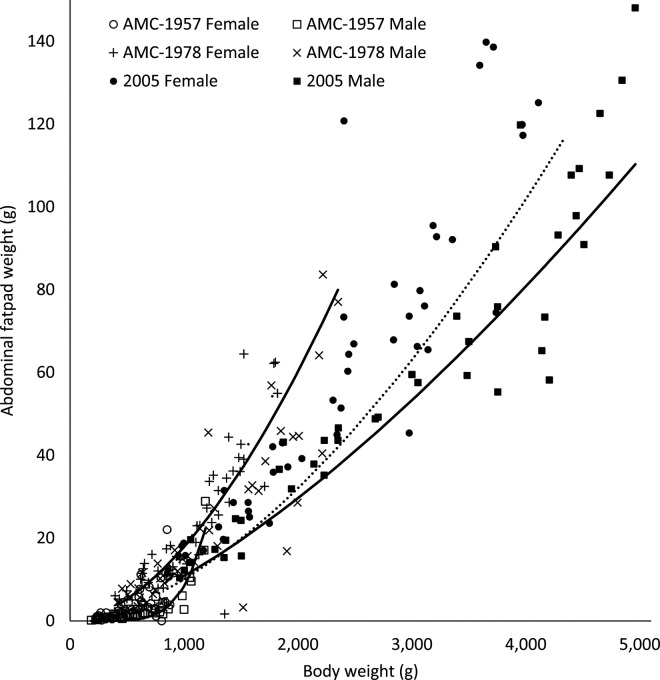
Abdominal Fat Pad.
Abdominal fat pad weights increased more quickly relative to BW in females than males in all 3 strains (P < 0.001; Figure 9). Relative to AMC-1957 broilers, abdominal fat pad yield curves were shifted upward in the AMC-1978 (P < 0.0001) and 2005 broilers (P = 0.03); however, abdominal fat pad yield curves were shifted downward in the 2005 broilers compared with the AMC-1978 strain (P = 0.05). Commercial selection programs have been implicated for making chicken meat a less healthy food for humans. Wang et al. (2010) claimed falsely that a rise in fat content in domestic livestock and high fat meat are due to confinement, selection for weight gain, and denial of exercise in enclosed spaces. In the body, however, fat deposition is energetically expensive, at approximately 9.2 kcal/g of BW gain, in contrast with lean tissue, which is composed of protein (4.1 kcal/g), and water (0 kcal/g). In sharp contrast, however, the current study provides reasonable evidence that commercial selection pressure has actually reduced fat deposition in broilers.
Figure 9.
Allometric yield curves for abdominal fat pads from University of Alberta Meat Control (AMC) unselected since 1957 and 1978, and Ross 308 (2005) broiler males (–) and females (···).
Allometric coefficients yielded insight directly into maturation rates. Higher values of the coefficient b indicated later maturation (higher relative weights at greater BW). As a result of commercial selection pressure, the maturation rate for breast meat increased, trading off with the maturation rate of the abdominal fat pad, which decreased. In dark meat, the rate of maturation relative to the rest of the body (allometric exponent b) did not change dramatically, the coefficient a decreased as a result of commercial selection pressure (Table 5), resulting from a decrease in dark meat yield and a corresponding increase in breast meat. Nonlinear analysis of covariance allowed comprehensive yield comparisons, leveraging the power of the entire data set rather than relying on age-specific analysis. Yield is dynamic; it changes over time because different parts of the body develop at different rates. The current study introduced a powerful and efficient method of statistically comparing yield, improving on the traditional approach of processing large numbers of birds at many ages. Comparisons at a specific age or BW may be better served by processing a large number of birds at a single time. However, nonlinear analysis of covariance provided a way to maintain a robust statistical comparison of treatments, while simultaneously reducing the number of birds required for research, and improving the comprehensiveness of yield prediction for any BW.
Benchmarking of genetic progress over 50 yr has been of interest and value to the commercial poultry industry, the research community, and society in a broader sense. Parts of the visual record of the current study have become sought after as a standard to illustrate the effect of genetic selection on broiler growth rates, efficiency, yield, immune function, and welfare. The current study also adds novel measures to the suite of studies that seek to objectively compare preserved genetic benchmarks in a comprehensive manner.
From 1957 to 2005, broiler growth rates increased by over 400%, with a concurrent 50% reduction in FCR. The claim that broiler 42-d live BW was increasing at a compounded rate of 3.1% (Barton, 1994) has held up to scrutiny. The current study confirms that the rate of increase in 42-d live BW from 1957 to 2005 was 3.30% per year, compounded for 48 yr. Similarly, FCR to 42 d of age has decreased by 2.55% per year, also in a compounding manner. The net result was that over a period of almost 50 yr, the broiler industry has been able to reduce the amount of feed required to produce chicken meat by one-half, and breast meat by 67%. Because feed accounts for approximately two-thirds of the cost of producing chicken, the resulting savings to consumers is substantial.
To counter a surprisingly widespread popular misunderstanding of the underlying mechanisms behind rapid broiler growth, it is important to note the basis for this transformative change in productivity. Modern chickens grow quickly because they have tremendous genetic potential to grow. Traditional selection methods—breeding efficient and robust birds with high growth rates—have been a particularly successful strategy in poultry because of high reproductive rates and short generation times. There have been lessons along the way. Unintended consequences to selection have proven challenging for the broiler industry, and will likely continue to emerge in spite of a high level of diligence manifested through comprehensive balanced selection programs. Many unintended changes such as increasing sexual dimorphism are not likely to become problematic, but musculoskeletal biomechanics, changes in immune response, and implications of huge growth potential for the welfare of breeding stock will undoubtedly challenge primary meat-type poultry breeders for the foreseeable future.
Acknowledgments
The authors thank the visionary scientists at Agriculture Canada (Ottawa, Ontario, Canada) who recognized the rapid rate of change in broilers in response to commercial selection pressures and synthesized the unselected strains used in this study, and the Poultry Research Centre (Edmonton, Alberta) for continuing the expensive conservation efforts. Thanks to the staff of the Poultry Research Centre for excellent technical support. Thanks to the Egg Farmers of Alberta (Calgary, Alberta) and the Alberta Chicken Producers (Edmonton, Alberta) for quota exemptions and permits that allow us to conserve these lines, and to all the Adopt a Heritage Chicken supporters in the Edmonton, Alberta, region and beyond.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
REFERENCES
- Abourachid A. Mechanics of standing in birds: Functional explanation of lameness problems in giant turkeys. Br. Poult. Sci. 1993;34:887–898. doi: 10.1080/00071669308417649. [DOI] [PubMed] [Google Scholar]
- Aviagen Inc. Ross x ross 308 North American broiler performance objectives. Huntsville, AL: Aviagen Inc.; 2003. [Google Scholar]
- Barton N. Breeding meat type poultry for the future targets for selection, limits to performance and market requirements for chicken.; Proc. 9th European Poultry Conference; UK Branch, Andover, Hants, UK: World's Poultry Science Association; 1994. pp. 33–38. [Google Scholar]
- Bottje W., Iqbal M., Tang Z. X., Cawthon D., Okimoto R., Wing T., Cooper M. Association of mitochondrial function with feed efficiency within a single genetic line of male broilers. Poult. Sci. 2002;81:546–555. doi: 10.1093/ps/81.4.546. [DOI] [PubMed] [Google Scholar]
- Chambers J. R., Bernon D. E., Gavora J. S. Synthesis and parameters of new populations of meat-type chickens. Theor. Appl. Genet. 1984;69:23–30. doi: 10.1007/BF00262532. [DOI] [PubMed] [Google Scholar]
- Chambers J. R., Gavora J. S., Fortin A. Genetic changes in meat-type chickens in the last twenty years. Can. J. Anim. Sci. 1981;61:555–563. [Google Scholar]
- Cheema M., Qureshi M., Havenstein G. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1519–1529. doi: 10.1093/ps/82.10.1519. [DOI] [PubMed] [Google Scholar]
- Corr S. A., Gentle M. J., Mccorquodale C. C., Bennett D. The effect of morphology on the musculoskeletal system of the modern broiler. Anim. Welf. 2003a;12:145–157. [Google Scholar]
- Corr S. A., Gentle M. J., Mccorquodale C. C., Bennett D. The effect of morphology on walking ability in the modern broiler: A gait analysis study. Anim. Welf. 2003b;12:159–171. [Google Scholar]
- Emmerson D. Commercial approaches to genetic selection for growth and feed conversion in domestic poultry. Poult. Sci. 1997;76:1121–1125. doi: 10.1093/ps/76.8.1121. [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003a;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003b;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Scheideler S. E., Larson B. T. Growth, livability, and feed conversion of 1957 vs 1991 broilers when fed typical 1957 and 1991 broiler diets. Poult. Sci. 1994a;73:1785–1794. doi: 10.3382/ps.0731785. [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Scheideler S. E., Rives D. V. Carcass composition and yield of 1991 vs 1957 broilers when fed typical 1957 and 1991 broiler diets. Poult. Sci. 1994b;73:1795–1804. doi: 10.3382/ps.0731795. [DOI] [PubMed] [Google Scholar]
- Hess C. W. Randombred populations of the southern regional poultry breeding project. Worlds Poult. Sci. J. 1962;18:147–152. [Google Scholar]
- Hunton P. 100 years of poultry genetics. World's Poult. Sci. J. 2006;62:417–428. [Google Scholar]
- Jackson S., Diamond J. Metabolic and digestive responses to artificial selection in chickens. Evolution. 1996;50:1638–1650. doi: 10.1111/j.1558-5646.1996.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Kidd M. T., Mcdaniel C. D., Branton S. L., Miller E. R., Boren B. B., Fancher B. I. Increasing amino acid density improves live performance and carcass yields of commercial broilers. J. Appl. Poult. Res. 2004;13:593–604. [Google Scholar]
- Latshaw J. D., Moritz J. S. The partitioning of metabolizable energy by broiler chickens. Poult. Sci. 2009;88:98–105. doi: 10.3382/ps.2008-00161. [DOI] [PubMed] [Google Scholar]
- Lilburn M. S. Skeletal growth of commercial poultry species. Poult. Sci. 1994;73:897–903. doi: 10.3382/ps.0730897. [DOI] [PubMed] [Google Scholar]
- Marks H. Growth rate and feed intake of selected and nonselected broilers. Growth. 1979;43:80–90. [PubMed] [Google Scholar]
- Merritt E., Gowe R. Development and genetic properties of a control strain of meat-type fowl.; Proc. 12th World's Poultry Congress; Sydney: 1962. pp. 66–70. [Google Scholar]
- Motulsky H. J., Ransnas L. A. Fitting curves to data using nonlinear regression: A practical and nonmathematical review. FASEB J. 1987;1:365–374. [PubMed] [Google Scholar]
- National Chicken Council. 2011. How broilers are marketed. Accessed Jun. 26, 2014. http://www.nationalchickencouncil.org/about-the-industry/statistics/how-broilers-are-marketed/.
- Olkowski A. A. Pathophysiology of heart failure in broiler chickens: Structural, biochemical, and molecular characteristics. Poult. Sci. 2007;86:999–1005. doi: 10.1093/ps/86.5.999. [DOI] [PubMed] [Google Scholar]
- Paxton H., Daley M. A., Corr S. A., Hutchinson J. R. The gait dynamics of the modern broiler chicken: A cautionary tale of selective breeding. J. Exp. Biol. 2013;216:3237–3248. doi: 10.1242/jeb.080309. [DOI] [PubMed] [Google Scholar]
- Rath N. C., Huff G. R., Huff W. E., Balog J. M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000;79:1024–1032. doi: 10.1093/ps/79.7.1024. [DOI] [PubMed] [Google Scholar]
- Romero L. F., Zuidhof M. J., Renema R. A., Naeima A., Robinson F. E. Characterization of energetic efficiency in adult broiler breeder hens. Poult. Sci. 2009;88:227–235. doi: 10.3382/ps.2008-00141. [DOI] [PubMed] [Google Scholar]
- Scheele C. W. Pathological changes in metabolism of poultry related to increasing production levels. Vet. Q. 1997;19:127–130. doi: 10.1080/01652176.1997.9694756. [DOI] [PubMed] [Google Scholar]
- Schettino D. N., Cancado S. V., Baiao N. C., Lara L. J. C., Figueiredo T. C., Santos W. L. M. Effect of pre-slaughter fasting periods on the carcass yielding of commercial broilers. Arq. Bras. Med. Vet. Zootec. 2006;58:918–924. [Google Scholar]
- Schmidt C. J., Persia M. E., Feierstein E., Kingham B., Saylor W. W. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult. Sci. 2009;88:2610–2619. doi: 10.3382/ps.2009-00055. [DOI] [PubMed] [Google Scholar]
- Schneider B. L., Renema R. A., Betti M., Carney V. L., Zuidhof M. J. Effect of holding temperature, shackling, sex, and age on broiler breast meat quality. Poult. Sci. 2012;91:468–477. doi: 10.3382/ps.2010-00952. [DOI] [PubMed] [Google Scholar]
- Sherwood D. H. Modern broiler feeds and strains: What two decades of improvement have done. Feedstuffs. 1977;49:70. [Google Scholar]
- Spratt R. S., Mcbride B. W., Bayley H. S., Leeson S. Energy metabolism of broiler breeder hens: 2. Contribution of tissues to total heat production in fed and fasted hens. Poult. Sci. 1990;69:1348–1356. doi: 10.3382/ps.0691348. [DOI] [PubMed] [Google Scholar]
- USDA, Economic Research Service. 2004. Poultry yearbook (89007). Accessed Jun. 26, 2014. http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1367.
- USDA, Economic Research Service. 2014. Food availability (per capita) data system: Poultry (chicken and turkey). Accessed Jun. 26, 2014. http://www.ers.usda.gov/data-products/food-availability-(per-capita)-data-system/.aspx.
- Wang Y., Lehane C., Ghebremeskel K., Crawford M. A. Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public Health Nutr. 2010;13:400–408. doi: 10.1017/S1368980009991157. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zuidhof M. J. Estimation of growth parameters using a nonlinear mixed Gompertz model. Poult. Sci. 2004;83:847–852. doi: 10.1093/ps/83.6.847. [DOI] [PubMed] [Google Scholar]
- Young L. L., Northcutt J. K., Buhr R. J., Lyon C. E., Ware G. O. Effects of age, sex, and duration of postmortem aging on percentage yield of parts from broiler chicken carcasses. Poult. Sci. 2001;80:376–379. doi: 10.1093/ps/80.3.376. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J. Mathematical characterization of broiler carcass yield dynamics. Poult. Sci. 2005;84:1108–1122. doi: 10.1093/ps/84.7.1108. [DOI] [PubMed] [Google Scholar]