Significance
Parkinson’s disease is the most common movement disorder. Here we describe the histopathological analysis of a unique patient with Parkinson’s disease who underwent unilateral cell transplantation in the putamen with human embryonic mesencephalic tissue at 24 y before death. The patient enjoyed major clinical benefits for at least a decade after transplantation. After a quarter of a century, complete graft-derived dopaminergic reinnervation was still evident in the transplanted putamen. α-Synuclein–positive inclusions, some with the appearance of typical Lewy bodies, were present in 11–12% of the grafted dopaminergic neurons, reflecting spread of pathology from the host brain to the transplant. The clinical improvements were gradually lost from 14 y posttransplantation, indicating that even extensive graft-derived dopaminergic reinnervation loses its efficacy in a severely degenerating brain.
Keywords: Parkinson’s disease, transplantation, autopsy, synucleinopathy, reinnervation
Abstract
Clinical trials using cells derived from embryonic ventral mesencephalon have shown that transplanted dopaminergic neurons can survive and function in the long term, as demonstrated by in vivo brain imaging using 18F-fluorodopa and 11C-raclopride positron emission tomography. Here we report the postmortem analysis of a patient with Parkinson’s disease who 24 y earlier underwent unilateral transplantation of embryonic dopaminergic neurons in the putamen and subsequently exhibited major motor improvement and recovery of striatal dopaminergic function. Histopathological analysis showed that a dense, near-normal graft-derived dopaminergic reinnervation of the putamen can be maintained for a quarter of a century despite severe host brain pathology and with no evidence of immune response. In addition, ubiquitin- and α-synuclein–positive inclusions were seen, some with the appearance of typical Lewy bodies, in 11–12% of the grafted dopaminergic neurons, reflecting the spread of pathology from the host brain to the transplants. Because the clinical benefits induced by transplantation in this patient were gradually lost after 14 y posttransplantation, our findings provide the first reported evidence, to our knowledge, that even a viable dopaminergic graft giving rise to extensive striatal reinnervation may lose its efficacy if widespread degenerative changes develop in the host brain.
A fundamental question for cell therapies in neurodegenerative disorders is whether transplanted neurons can survive and integrate their terminal network with the host brain for many years despite an ongoing disease process. In patients with Parkinson’s disease (PD) implanted with human embryonic mesencephalic tissue into the striatum, varying numbers of grafted dopaminergic neurons have survived for up to 2 decades (1–3); however, in some patients, characteristic Lewy bodies were detected in a small fraction (1–5%) of grafted dopaminergic neurons at 12–22 y after transplantation (2–5). Other studies have shown that grafted dopaminergic neurons remain healthy up to 14 y posttransplantation (1, 6). Whether the disease process affects the density and extension of a well-developed graft-derived dopaminergic innervation is unknown.
Here we report the histopathological analysis of a unique patient with PD who exhibited major clinical improvement for more than a decade after undergoing unilateral transplantation into the putamen, associated with complete restoration of putaminal dopaminergic function as demonstrated by positron emission tomography (7). We demonstrate, for the first time to our knowledge, that a dense graft-derived dopaminergic reinnervation of the putamen can be maintained for a quarter of a century despite widespread host brain pathology and α-synucleinopathy eventually affecting 11–12% of grafted neurons.
Results and Discussion
Patient History and Clinical Follow-Up.
In 1989, at age 59 y, the patient underwent transplantation with ventral mesencephalic tissue from four human embryos obtained at routine suction abortions (8). Using a computed tomography-guided stereotactic technique, dissociated tissue was implanted along three tracts in the anterior, middle, and posterior parts of the right putamen. The patient had developed typical PD in 1980 and was started on l-dopa treatment in 1982. He did well until 1986, when he developed “on–off” symptoms, with “off” periods characterized by resting tremor, hypokinetic movements, and rigidity, particularly on the left side of the body, and only mild parkinsonian symptoms during “on” periods.
Up to 10 y after transplantation, the patient was monitored according to the Core Assessment Program for Intracerebral Transplantations protocol (9) as part of a scientific program. He improved dramatically during the first 3 y, and l-dopa was withdrawn at 32 mo (8, 10). Immunosuppressive treatment (prednisolone, azathioprine, and cyclosporine) was slowly tapered and then stopped at 64 mo (11). Low-dose l-dopa (approximately one-third of the preoperative dose) was reintroduced at 74 mo owing to progression of symptoms axially and in the limbs ipsilateral to the graft (7). The patient responded well to this medication, and motor function and l-dopa dose remained unchanged thereafter. At 10 y after transplantation, he showed a marked clinical benefit, with virtually no rigidity, minor hypokinesia, intermittent and mild resting tremor, and no on–off fluctuations (7). Off-medication dyskinesias were not observed on clinical examinations during the first decade posttransplantation, except for a slight nonprogressive activity-related dyskinesia in the left foot that appeared during the third posttransplantation year (7, 8, 10, 11). Post hoc analysis of video recordings revealed nontroublesome, very mild off-medication dyskinesias on the left side of the body (contralateral to the graft) at 28 mo and 11 y after transplantation, but not at 12 mo after transplantation (12, 13).
Between 10 and 24 y after transplantation, the patient was followed as a regular patient at the neurology outpatient clinic. A routine neurologic examination, including assessments of rigidity, tremor, hypokinesia, and cognitive function, was performed by the same neurologist every 6–9 mo. Motor function remained unchanged until 12 y posttransplantation, when a low-dose dopamine agonist was added for 2 y and the l-dopa dose was increased to the preoperative level because of worsening hypokinesia. The increased l-dopa doses were accompanied by slight bilateral dyskinesias, but no off-medication dyskinesias were noted.
The patient initially responded well to this change in medication regimen, but starting in year 14 began to exhibit progressively increasing rigidity and hypokinesia and a gradual loss of the beneficial l-dopa response. Also in year 14, cognitive impairment was first noted, and progressive dementia ensued. His condition continued to deteriorate, and no graft-related motor improvement remained at 18 y posttransplantation. At that time, the patient was unable to walk and had lost the ability to swallow and speak. He died of cardiac insufficiency at 24 y after transplantation. Informed consent for an autopsy was obtained in June 2009.
Detailed descriptions of the preoperative history, the transplantation procedure, and the clinical course in this patient up to 10 y after surgery have been published previously (7, 8, 10).
Survival of Dopaminergic Neurons in the Grafts.
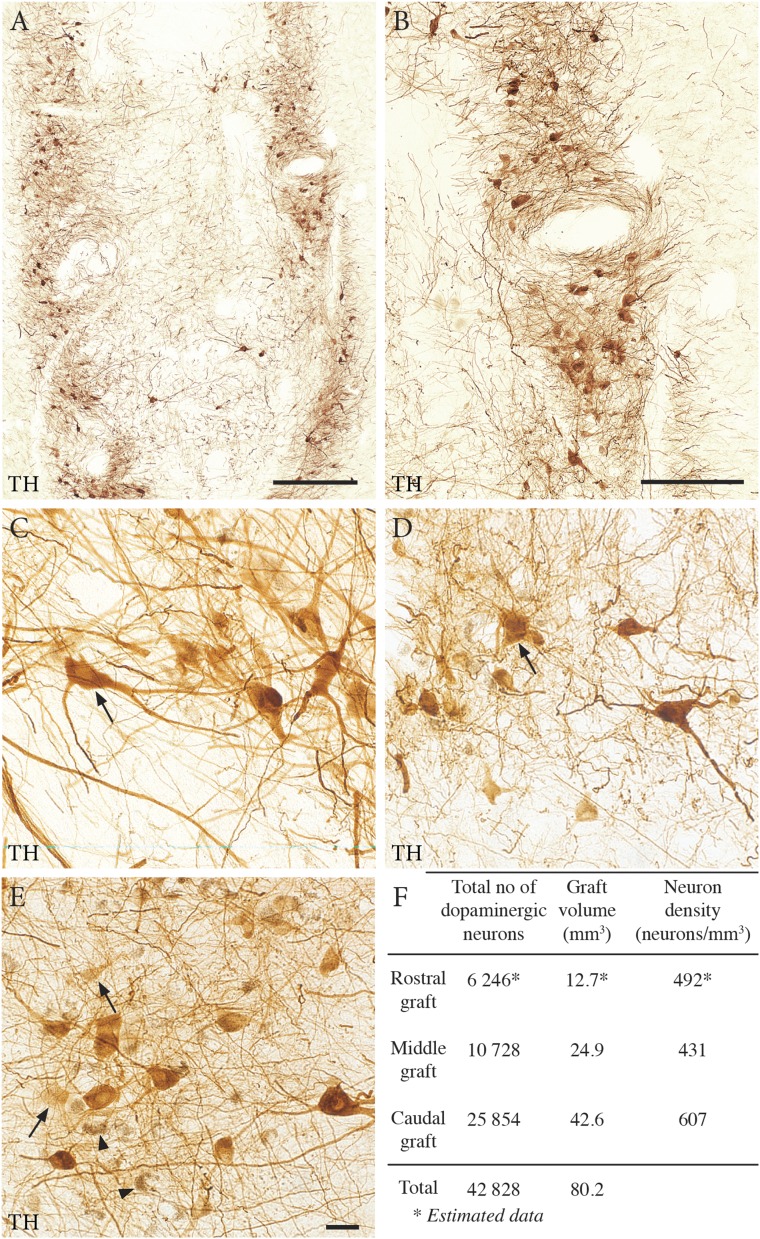
Postmortem analysis identified all three grafts in the dorsal putamen (Figs. 1 and 2A). A total of 6,246, 10,728, and 25,854 surviving dopaminergic neurons [tyrosine hydroxylase (TH)-immunoreactive and/or neuromelanin-containing] were found in the rostral, middle, and caudal grafts, respectively (Fig. 1F). The cells formed clusters in the periphery of each graft (Fig. 1 A and B) (3, 6). The majority (76%) of the neuromelanin-containing neurons within the graft were TH-immunoreactive (Fig. 1 C–E) and associated with dense networks of axonal/dendritic profiles. Approximately 24% of the neurons that contained distinct neuromelanin granules had no detectable TH immunoreactivity, suggesting down-regulation of TH (Fig. 1E).
Fig. 1.
Survival of transplanted dopaminergic neurons in the three graft deposits. (A, B, and F) The graft deposits were rich in TH-positive neurons, arranged in clusters at the periphery of the grafts. (C–E) Cell counting revealed a total of ∼43,000 surviving dopaminergic neurons identified by their TH-positive immunostaining and/or neuromelanin content. The surviving neurons were categorized into three different populations: cells with very dense TH staining and an elaborate healthy morphology (arrow in C), cells with weaker TH staining and poorly stained processes (arrows in D and E), and cells with neuromelanin granules but no detectable TH staining (arrowheads in E). The first and second types accounted for 76% of the total number of surviving neurons. (Scale bars: 1 mm in A, 0.5 mm in B, 20 μm in E.)
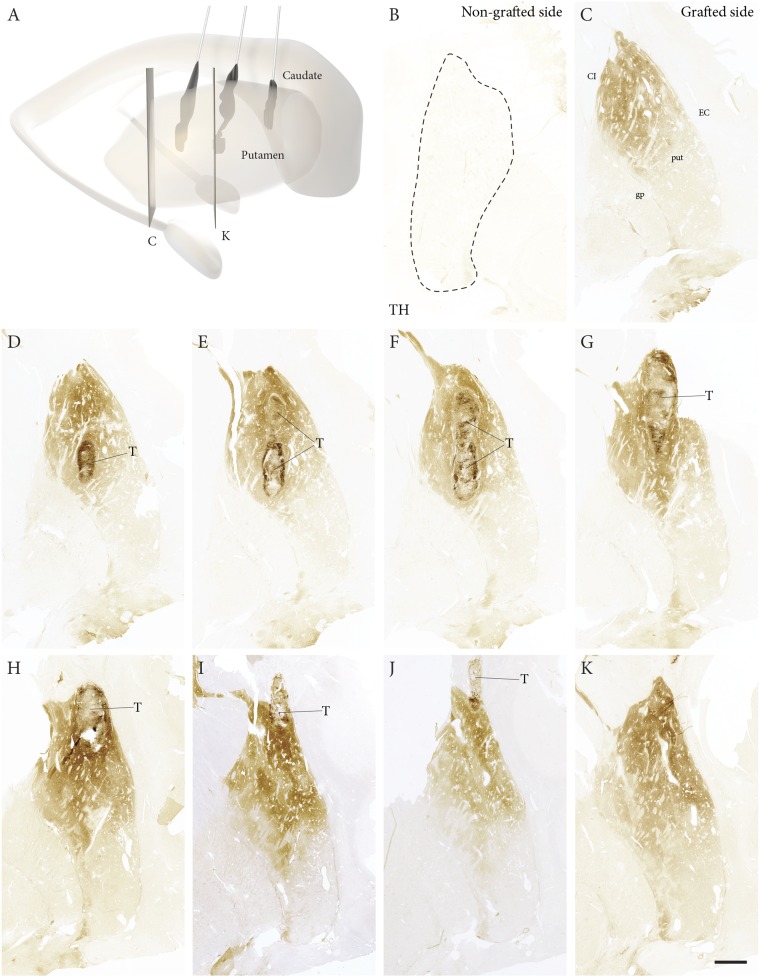
Fig. 2.
Overview of graft-derived TH-positive innervation. (A) A 3D reconstruction showing the location and size of three graft deposits in the caudal, middle, and rostral putamen. (C–K) Extent of the graft-derived TH-positive innervation throughout the postcommissural putamen (positions of the sections indicated in A, arranged in a caudal-to-rostral order). (B) In contrast, the nongrafted putamen was completely devoid of TH-positive fibers. (Scale bars: 1 mm in B, 0.5 mm in D, 20 µm in E–G, 5 mm in H–K.)
Complete Reinnervation of Grafted Putamen.
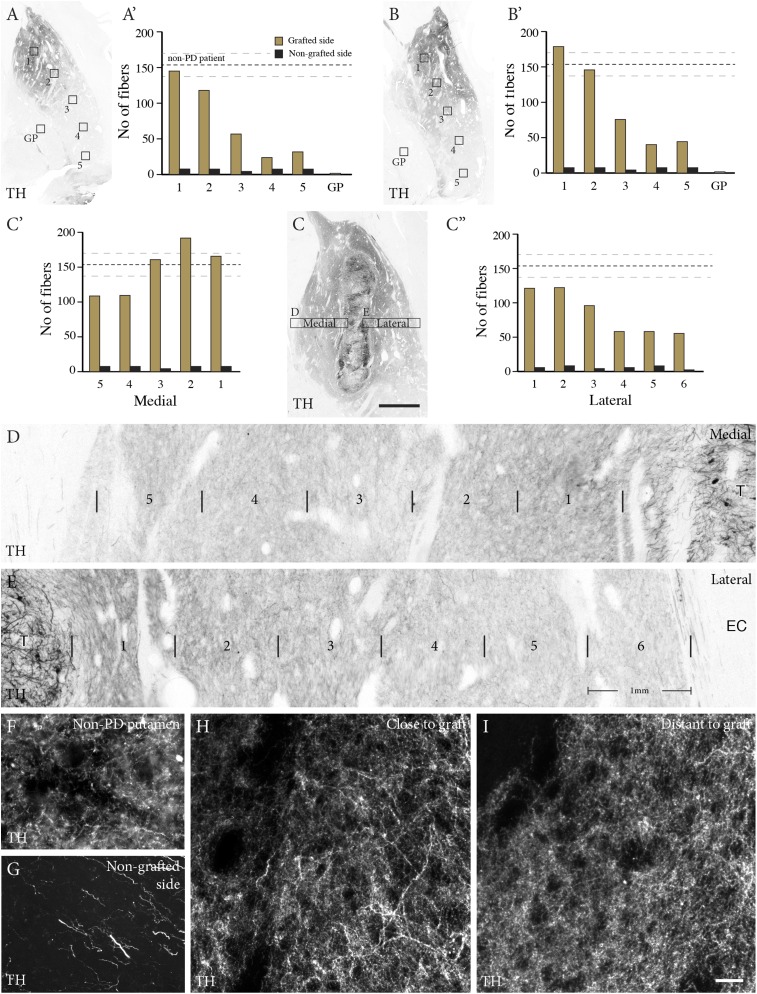
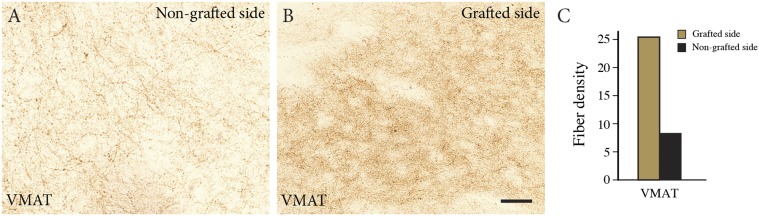
The nontransplanted putamen was devoid of TH-positive terminals (Figs. 2B and 3G), consistent with the presence of few neuromelanin-positive neurons bilaterally in the substantia nigra. In contrast, a rich network of TH-positive fibers extended throughout the postcommissural part of the transplanted putamen (Fig. 2 C–K). The TH-positive fiber density showed a gradient extending from the core of the grafts to the edge of the putamen (Fig. 3 A–C). In the area adjacent to the graft (Fig. 3 A–E), the density of the TH-positive innervation was comparable to that of the dopaminergic innervation in the putamen of an age-matched control subject (age 85 y) who died from a nonneurologic disease (Fig. 3 F, H, and I; dashed line in 3 A–C). A similar dense innervation was seen in sections stained for vesicular monoamine transporter 2 (VMAT2) (Fig. S1). The precommissural part of the putamen, containing the rostral graft, was paraffin-embedded. In the 4-µm-thick paraffin sections through this part, TH immunostaining allowed visualization of cells, but not of axons or terminals.
Fig. 3.
Complete reinnervation of grafted putamen. (A–E) From the core of the transplants, the graft-derived TH fibers extended throughout the postcommissural putamen, with an innervation density close to normal in the dorsal part, with a decreasing gradient dorsoventrally (A and B) and in the medial and lateral directions from the graft core (C–E). A′, B′, C′, and C′′ show quantification of TH-positive fiber density in the boxed regions (yellow bars), compared with the nongrafted side (black bars) and the innervation density recorded from an age-matched non-PD brain (dashed lines, ±SEM). (F–I) Dark-field images showing the dense varicose TH-positive innervation in the grafted putamen (H and I), similar in morphology and density to that seen in the non-PD brain (F). The nongrafted putamen was completely devoid of TH-positive terminals; only scattered neuritic profiles with the appearance of truncated preterminal axons remained (G). (Scale bars: 5 mm in C, 1 mm in D and E, 100 µm in F–I.)
Fig. S1.
Density of VMAT2-positive terminals in the grafted and nongrafted putamen. (A and B) Immunostaining for VMAT2 shows a markedly higher VMAT2-positive fiber density in the grafted putamen (B) compared with the nongrafted side (A). (C) The difference in VMAT2-positive innervation, as assessed by densitometry. (Scale bars: 200 µm in A, 20 µm in B and C.)
Microglia Reaction and Graft and Host Brain Pathology.
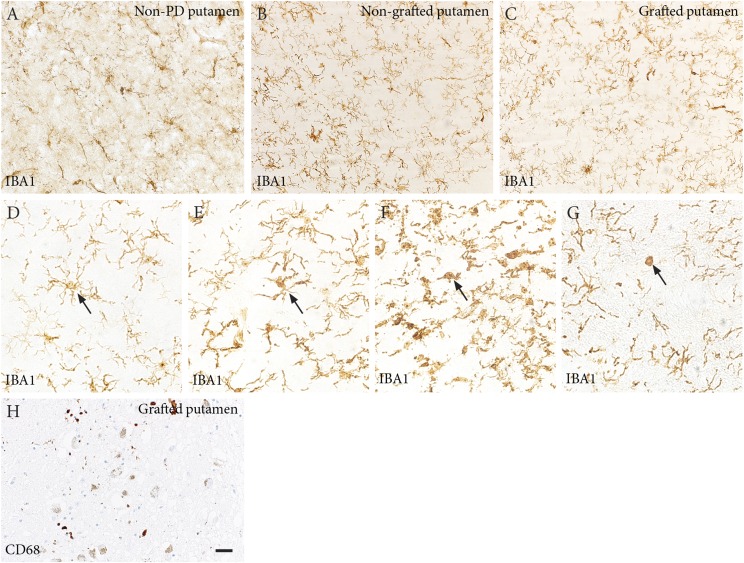
Although immunosuppression was terminated at 5 y posttransplantation, at 24 y there was no evidence of an ongoing immune/inflammatory response. Quantitative analyses showed no difference between transplanted putamen, nontransplanted putamen, and putamen in the control subject in terms of numbers of IBA-1–positive (recognizing both activated and nonactivated microglia) (Fig. S2 A–C) or CD68-positive cells (recognizing activated microglia) (Fig. S2H) or in the morphology of microglia (14) (Fig. S2 D–G).
Fig. S2.
(A–C) Microglial reaction to the transplants. IBA1 staining showing microglia cells in the putamen of an age-matched non-PD patient (A) and in the nongrafted (B) and grafted putamen (C) of the PD patient. Quantification of IBA1-positive cells showed no difference in microglia numbers (70 in the control brain, 77 in nongrafted putamen, and 78 in the grafted one on average in each counting field). (D–G) Microglia were classified into four different morphological phenotypes, believed to represent different steps of microglial activation (14): ramified (D), intermediate (E), amoeboid (F), and round (G). No major differences between grafted and nongrafted sides and normal putamen were observed. In control brain: ramified, 89%; intermediate, 7.4%; amoeboid, 2.8%; and round, 0.8%. In nongrafted putamen: ramified, 74%; intermediate, 18%; amoeboid, 7%; and round, 0.4%. In grafted putamen: ramified, 77%; intermediate, 16%; amoeboid, 6%; and round, 0%. In addition, staining with the activated microglia marker CD68 did not reveal any major difference in the number of immunoreactive cells between nongrafted and grafted putamen (62 vs. 82). (H) CD68 staining in the rostral graft. (Scale bars: 20 µm.)
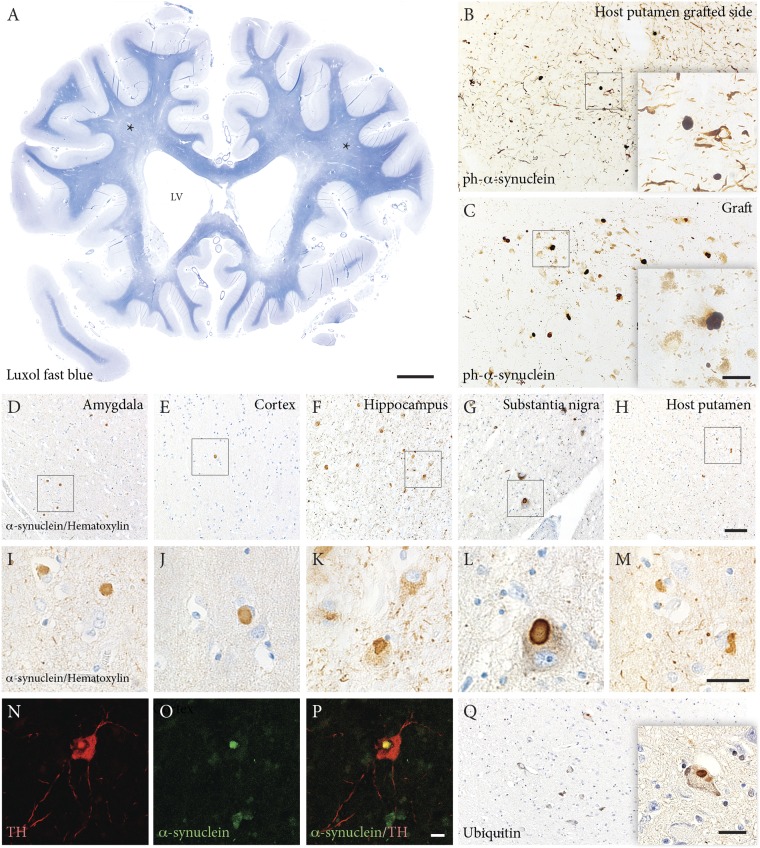
The patient exhibited a dense, near-normal dopaminergic reinnervation derived from the grafts at 24 y posttransplantation. Two main mechanisms likely underlie the gradual loss of the l-dopa response and dopaminergic graft effects on motor function, as well as the progressive cognitive decline starting at 14 y posttransplantation. First, a considerable number of synucleinopathy-related phenotypes were detected in the transplanted neurons, reflecting pathology spread from the host brain to the transplant, which could contribute to the decreasing functional efficacy of a portion of the grafted dopaminergic neurons. Quantitative analyses revealed ubiquitin- and α-synuclein–positive Lewy bodies in 11% and 12% of transplanted neurons, respectively (Fig. 4 B, C, and Q), higher than the percentages reported previously (1.2–5% of transplanted neurons) in 12- to 22-y-old transplants (2, 3, 5).
Fig. 4.
White matter atrophy and Lewy body pathology. (A) Luxol fast blue staining of a whole brain slice from the rostral part of the brain showing white matter atrophy (asterisks) and enlarged lateral ventricles (LV). (B and C) Phosphorylated (S129) α-synuclein staining showing the presence of Lewy bodies in the grafted cells (C) and in the host putamen surrounding the graft (B). (D–M) Quantification of phosphorylated α-synuclein showing that up to 12% of neuromelanin-containing cells contain Lewy bodies. Extensive Lewy pathology appears in different brain regions, including amygdala (D and I), cortex (E and J), hippocampus (F and K), substantia nigra (G and L) and the nongrafted putamen (H and M). (N–P) Confocal images illustrating a host nigral dopaminergic neuron (red, TH positive) containing a typical Lewy body (green, α-synuclein positive). (Q) Ubiquitin-positive inclusions also appeared in the grafted cells. Up to 11% of the neuromelanin-containing cells contained ubiquitin-positive aggregates. (Scale bars: 10 mm in A, 20 µm in B and C, 50 µm in D–H, 20 µm in I–Q.)
Second, because the majority of the more than 40,000 grafted neurons maintained TH immunoreactivity and provided extensive dopaminergic reinnervation of the putamen, it is likely that the complete lack of responsiveness to l-dopa and the concomitant loss of the graft effect were caused mainly by the severe pathological changes postsynaptically, i.e., in the host brain outside the graft. The patient displayed substantial general atrophy, with considerable widening of the lateral ventricles and severe medial temporal lobe atrophy (Fig. 4A). Neuropathologically, PD with dementia was diagnosed, owing to characteristics of extensive Lewy pathology in all brain regions including the limbic system and neocortex, corresponding to Braak stage 6 (15) (Fig. 4 D–P). Quantitative analysis showed the occurrence of Lewy bodies in 20% of remaining pigment granule-containing neurons in the host substantia nigra, higher than the percentage of Lewy bodies in the grafted dopaminergic neurons (11–12%). α-Synuclein–positive neurites were widespread in the brain, commonly involving limbic and neocortical regions, accompanied by degeneration of white matter, which also was macroscopically atrophic.
Conclusions
Our results demonstrate a complete graft-derived dopaminergic reinnervation of the postcommissural putamen maintained for 24 y after transplantation in a patient with PD. This finding is consistent with observations in the same patient at 10 y posttransplantation, when positron emission tomography revealed normalization of 18F-dopa uptake and 11C-raclopride binding in the grafted putamen (7). These findings provide the first evidence, to our knowledge, that a rich dopaminergic innervation that develops over the first 3 y after transplantation and gives rise to major motor improvement can be preserved for a quarter of a century in PD. However, the clinical outcome in this patient demonstrates that even such a viable graft may lose its efficacy if widespread α-synucleinopathy and major degenerative changes develop in the host brain.
Materials and Methods
The study protocol was approved by the Regional Ethical Review Board of Lund University (nr2014/631).
Postmortem Brain Preparation.
The patient’s brain (obtained at 4 d postmortem) was fixed in 6% buffered formaldehyde solution for 1 mo and then transferred to 20% sucrose solution. Regions were cut into coronal whole brain sections and small specimens. The brain regions in sucrose were sliced into blocks containing basal ganglia (four blocks from rostral to caudal), mesencephalon, pons, cerebral cortex, hippocampus, and some other brain regions. The most rostral block of the basal ganglia was paraffin-embedded, and the other three blocks were used for frozen section preparation. All brain regions not set aside for quantitative assessments were used for diagnostic analysis, including assessment of synuclein pathology and viability of the tissue.
Immunohistochemistry.
Basal ganglia blocks were cut into 40-µm-thick free-floating frozen sections and 4-µm-thick slide-mounted paraffin sections. For diaminobenzidine (DAB, Vector Laboratories) staining, free-floating sections were quenched with 3% H2O2 in 10% methanol for 15 min before blocking (5% normal horse or goat serum and 0.25% Triton X-100 in 0.1 M PBS, pH 7.4) for 1 h. Sections were incubated in primary antibodies (diluted in 3% serum and 0.25% Triton X-100 in PBS) overnight at 4 °C or room temperature. The antibodies used in these experiments are listed in Table S1. After rinsing, biotinylated secondary antibodies (horse anti-mouse, horse anti-rabbit, goat anti-rat; Jackson ImmunoResearch Laboratories) were applied for 2 h at room temperature, followed by incubation with ABC complex (Vector Laboratories) for 30 min, rinsing, and then incubation with DAB (Vector Laboratories) for 30 s or longer.
Table S1.
Antibodies used in this study
| Antibody name | Species | Source | Working dilution |
| Tyrosine hydroxylase (TH) | Rabbit | Pel-Freeze | 1:500 |
| α-Synuclein | Mouse | Invitrogen | 1:500 |
| Ser-129 phosphorylated α-synuclein | Rabbit | Abcam | 1:1,000 |
| Ubiquitin | Rabbit | Dako | 1:1,000 |
| IBA1 | Rabbit | Wako | 1:500 |
| CD68 | Mouse | Dako | 1:200 |
| VMAT2 | Rabbit | Abcam | 1:3,000 |
| GFAP | Rabbit | Dako | 1:500 |
| Alexa Fluor 488 anti-mouse | Donkey | Jackson | 1:200 |
| Cy3-conjugated anti-rabbit | Donkey | Jackson | 1:200 |
| Biotinylated anti-rat | Goat | Jackson | 1:200 |
| Biotinylated anti-mouse | Horse | Jackson | 1:400 |
| Biotinylated anti-rabbit | Horse | Jackson | 1:400 |
Paraffin sections were deparaffinized with xylene and then sequential decreasing concentrations of ethanol before use in the immunohistochemistry procedure described above.
For immunofluorescence double labeling, two different primary antibodies raised in rabbit and mouse followed by Alexa Fluor 488 anti-mouse and Cy3-conjugated anti-rabbit secondary antibodies raised in donkey (Jackson ImmunoResearch Laboratories) were added. Autofluorescence Eliminator reagent (EMD Millipore) was applied before mounting.
Before blocking, both free-floating frozen sections and slide-mounted paraffin sections were treated with 10 mM citrate buffer (pH 6.4) to achieve effective antigen retrieval. Free-floating sections were incubated in citrate buffer at 80 °C for 30 min. Paraffin sections were boiled in citrate buffer in a microwave oven for 14 min at 800 W. Sections were rinsed in PBS before staining.
Imaging and Quantification.
Bright-field images were captured using an Olympus DP73 camera, driven by CellSens software. Dark-field images were obtained using an Olympus AX70 camera, driven by Leica DMI 6000B software.
The numbers of surviving neuromelanin- and/or TH-positive grafted neurons were assessed along each injection track in both paraffin and frozen TH-stained sections. The Abercrombie formula was used to estimate the number of cells in each graft. In this formula, cell diameter is assessed from an average of 45 neurons in each graft (16).
The density of the TH-positive innervation was assessed using a modified “sphere counting” technique of Mouton et al. (17, 18). Using a 100× oil-immersion objective, in a bright-field microscope (Olympus AX70), a z-stack was captured throughout the entire thickness of the section at 1-µm intervals, using Velocity v5.4.2 image analysis software (PerkinElmer). Next, a 19-µm-diameter sphere (the “probe”) was created to measure fiber density within the z-stack, and the points at which fibers passed through the circumference of the sphere at each z-level were counted. A score of 1 was assigned for each fiber crossing. For each probe, the total number of fibers passing through the sphere was expressed as a measure of fiber density within the measured volume.
To quantify the VMAT2-positive fibers, staining intensity was measured using ImageJ. Ten images from both grafted and nongrafted putamen were chosen for analysis. Images were adjusted to gray scale, and a threshold was set to exclude the background area. Intensity measurements were performed within regions of interest and within the range of threshold limit (19).
For quantification of microglia, eight image fields were chosen from sections of grafted and nongrafted putamen and from the putamen of a normal subject immunostained with IBA1 antibody. The cell numbers in each field were counted, and the IBA1-positive cells classified into four different morphological phenotypes. In addition, five images were chosen in CD68-stained sections and the total cell numbers in all fields were counted.
Acknowledgments
We thank A. Persson and A. Flasch for their excellent technical support, and A. C. McCourt for the language editing. This work was supported by grants from the Swedish Research Council, BAGADILICO-Excellence in Parkinson and Huntington Research, the Swedish Parkinson Foundation, the Torsten Söderberg Foundation, and the Strategic Research Area MultiPark (multidisciplinary research focus on Parkinson’s disease at Lund University).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 6332.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605245113/-/DCSupplemental.
References
- 1.Hallett PJ, et al. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Reports. 2014;7(6):1755–1761. doi: 10.1016/j.celrep.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurowska Z, et al. Signs of degeneration in 12- to 22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson’s disease. J Parkinsons Dis. 2011;1(1):83–92. doi: 10.3233/JPD-2011-11004. [DOI] [PubMed] [Google Scholar]
- 3.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 4.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 5.Li JY, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25(8):1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- 6.Mendez I, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128(Pt 7):1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccini P, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2(12):1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 8.Lindvall O, et al. Transplantation of fetal dopamine neurons in Parkinson’s disease: One-year clinical and neurophysiological observations in two patients with putaminal implants. Ann Neurol. 1992;31(2):155–165. doi: 10.1002/ana.410310206. [DOI] [PubMed] [Google Scholar]
- 9.Langston JW, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 10.Lindvall O, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann Neurol. 1994;35(2):172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- 11.Wenning GK, et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann Neurol. 1997;42(1):95–107. doi: 10.1002/ana.410420115. [DOI] [PubMed] [Google Scholar]
- 12.Hagell P, et al. Dyskinesias following neural transplantation in Parkinson’s disease. Nat Neurosci. 2002;5(7):627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 13.Piccini P, et al. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain. 2005;128(Pt 12):2977–2986. doi: 10.1093/brain/awh649. [DOI] [PubMed] [Google Scholar]
- 14.Thored P, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57(8):835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 16.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 17.Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microsc. 2002;206(Pt 1):54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- 18.Grealish S, et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15(5):653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 2013;296(3):378–381. doi: 10.1002/ar.22641. [DOI] [PubMed] [Google Scholar]